Abstract
The diverse utility of acyclic vinylsilanes has driven the interest in the synthesis of enantioenriched vinylsilanes bearing a Si-stereogenic center. However, the predominant approaches for catalytic asymmetric generation of Si-stereogenic vinylsilanes have mainly relied on transition metal-catalyzed reactions of alkynes with different silicon sources. Here we successfully realize the enantioselective synthesis of linear silicon-stereogenic vinylsilanes with good yields and enantiomeric ratios from simple alkenes under rhodium catalysis. The significance of this transformation lies in its ability to achieve regioconvergent and enantioconvergent conversion, efficiently transforming petroleum-derived isomeric mixtures of olefin feedstocks into a single regio- and stereoisomer product. The practicality of this method is further exemplified by the diverse downstream transformations of these enantioenriched silicon-stereogenic vinylsilanes leveraging the olefin functionality and the leaving group nature of the aryl substituent on silicon as well as the development of chiral π-conjugated double bond systems.
Similar content being viewed by others
Introduction
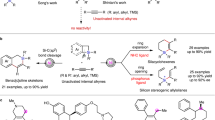
The continuous growth in the application of enantiopure silicon-bearing compounds with Si-stereogenic centers across myriad domains including chemical synthesis and technical materials highlights their rising significance1,2,3,4,5,6,7,8,9,10,11. However, the fabrication of silicon-stereogenic centers via asymmetric catalysis remains an enduring challenge12,13,14,15,16,17,18,19,20,21,22,23,24. This is primarily attributed to the inherently elongated C−Si bond compared to the C−C bond, and the inherent instability of Si-containing unsaturated bonds. Acyclic vinylsilanes are highly versatile synthons that have garnered considerable use in organic synthesis, the pharmaceutical industry, and the silicone industry25,26,27,28,29,30,31,32,33. Consequently, the quest to develop efficient methodologies for the synthesis of enantioenriched vinylsilanes bearing a Si-stereogenic center, has garnered significant attention. Despite extensive research efforts, the predominantly reported strategies for the catalytic asymmetric synthesis of silicon-stereogenic vinylsilanes have relied on transition metal-catalyzed reactions using alkynes as the substrates including hydrosilylation of alkynes with dihydrosilanes34,35,36,37,38,39 and ring-opening reaction of silacyclobutanes with alkynes (Fig. 1A)40. However, the enantioselective hydrosilylation of alkynes typically yields enantioenriched triorgano-substituted Si-stereogenic alkenylsilanes, yet fall short of providing tetraorgano-substituted Si-stereogenic alkenylsilanes41. The enantioselective ring-opening reaction of alkynes with silacyclobutanes is not compatible with terminal alkynes and fails to control regioselectivity in the case of unsymmetric internal alkynes, which diminishes its practical utility. In 2022, Zhang and Xiong et al. made an important breakthrough in the catalytic asymmetric construction of tetraorgano-substituted silicon-stereogenic alkenylsilanes, which underwent the desymmetrization of prochiral divinyl-substituted silanes by enantioselective protoboration (Fig. 1B)42. However, its scope is limited to aryl-substituted divinylorganosilanes and these prochiral divinyl-substituted silanes generally need multi-step synthesis. Recently, our group developed a palladium-catalyzed enantioselective 1,5-migration/carbene coupling reaction to deliver highly enantioenriched silicon-stereogenic vinyl silane. However, the products are still limited to silicon-stereogenic styrylsilanes (Fig. 1C)43. Therefore, there remains a pressing need to devise efficient methods that utilize readily accessible substrates to construct tetraorgano-substituted Si-stereogenic alkenylsilanes with high enantioselectivity in a straightforward manner.
A Asymmetric construction of Si-stereogenic vinylsilanes from alkynes. B Desymmetrizing protoboration of divinyl-substituted silanes (Xiong & Zhang’s work). C Enantioselective 1,5-migration/carbene coupling reaction toward highly enantioenriched silicon-stereogenic vinyl silane. D Asymmetric construction of Si-stereogenic vinylsilanes from simple alkenes (This work).
Considering that simple alkenes represent a rich and prolific class of chemical feedstocks widely utilized as keystones in organic synthesis44,45,46,47,48,49, there exists a strong impetus to forge an alternative pathway for the creation of enantioenriched Si-stereogenic alkenylsilanes from simple, readily available olefins. Such transformations would not only boast exceptional functional group tolerance but also vastly broaden the scope of chemical and structural variants of enantioenriched Si-stereogenic alkenylsilanes that could be synthesized with ease. However, the enantioselective synthesis of enantiopure alkenylsilanes from simple alkene precursors has not yet been realized. Buoyed by the benefits observed in Ni-catalyzed silylation of terminal alkenes with silacyclobutanes50, which have become a key member of sila-synthons in the organosilicon synthetic arena51,52,53,54,55,56, for the synthesis of vinylsilanes such as wide substrate applicability and obviating the need for hydrogen acceptors, we became interested in control of the stereochemical outcome and overcoming the limitations intrinsic to internal alkene substrates for this transformation. We aimed to develop a robust chiral catalytic system, one that enables asymmetric construction of structurally varied, enantioenriched tetraorgano-substituted Si-stereogenic alkenylsilanes in a manner that is both highly effective and exquisitely atom-economical, complete with an extensive functional group tolerance. Herein, we have developed a Rh-catalyzed system, which has the capability of adeptly steering the enantioselectivities of silylation of both terminal and internal alkenes with silacyclobutanes, delivering sought-after linear alkenylsilanes bearing a quaternary silicon stereocenter which are inaccessible from alkynes (Fig. 1D). Mechanistic studies suggest that this innovative Rh-catalytic system developed in our work operates through a distinct rate-determining step and catalytic cycle, differing from the Ni-catalyzed system previously reported by Yorimitsu and Oshima50.
Results and Discussion
Condition screening
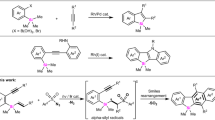
To realize the catalytic construction of enantioenriched Si-stereogenic alkenylsilanes from simple and widely available olefins, we selected styrene (1a) and 1-phenyl-1-t-butyl-silacyclobutane (2a) as the model substrates (Fig. 2 and Supplementary Tables 1-6). In light of the literature work, we first tried a lot of different chiral ligands under Ni-catalyzed condition, unfortunately these conditions were generally found to be ineffective, leading to low yields and enantiomeric ratios (er). Encouraged by the recent advances in Rh-catalyzed reactions involving C−Si bond cleavage of silacyclobutanes57,58,59,60,61, we wonder if the Rh-catalyzed condition is also efficient for the reaction of styrene (1a) and 1-phenyl-1-t-butyl-silacyclobutane (2a). Following this idea, we carried out this reaction by use of PPh3 as the ligand in the presence of [Rh(cod)Cl]2 (2.5 mol%) in toluene at 120 °C for 24 h. Gratifyingly, the reaction worked smoothly to deliver the racemic Si-stereogenic alkenylsilane 3aa in 54% yield. Then, a range of different chiral P-ligands were evaluated as shown in Fig. 2 to try to control the enantioselectivity. (Rp, R)-FOXAP ligands were found to be optimal in terms of enantioselectivities albeit with moderate yield. The diastereoisomeric ligand (Rp, S)-tBu-FOXAP led to a significant decrease in both the enantioselectivity and yield. Next, we further optimized the reaction outcome by varying the different moieties of this ligand skeleton. The steric hindered (Rp, R)-tBu-FOXAP (L16) ligand gave 38% yield and 76% ee. The further results disclosed that modification of the phosphine group in the FOXAP framework also led to an obvious change in enantioselectivity. The enantioselectivity can be obviously improved by the introduction of sterically-hindered substituents at the 3,5-position of the phenyl ring on the phosphine group. The ligand L25 gave 40% yield and 87% ee. To improve the conversion efficiency and enantioselectivity, we have then performed extensive optimizations on solvents, Rh(I) precursors, bases, additives, reaction temperature, and time. The use of n-Heptane as the solvent resulted in an improvement of ee (42% yield, 90% ee) Employment of [Rh(cod)OMe]2 as the precatalyst slightly improved the yield to 45%. The addition of LiOtBu is capable of further improving the yield to 57%. We speculated that LiOtBu might have interaction with Si-atom of SCBs due to its Lewis acidity. This interaction would facilitate the oxidative addition of C−Si bond onto the rhodium(I). Lowering the reaction temperature to 100 °C led to a slight improvement in the enantioselectivity (92% ee) without significant changes to the yield. Finally, we found that slowly adding 1a via syringe into the reaction mixture over 2 h along with extended reaction time and higher reaction concentration (Supplementary Fig. 1), the yield of 3aa was improved to 86% (isolated yield: 82%) with the maintenance of satisfied enantiomeric excess (92% ee). In addition, the employment of L26 as the ligand under the optimized condition could further improve the yield of the reaction with a slight decrease in enantioselectivity (89% yield and 94:6 er).
Unless otherwise specified, all reactions were carried out in a N2-filled glove box by using [Rh(cod)Cl]2 (2.5 mol%), ligand (5.0–10.0 mol%), 1a (0.2 mmol) and 2a (0.1 mmol) in toluene (0.1 M) in 120 °C for 24 h. Yields were determined by GC-MS analysis of the crude reaction mixture. Enantiomeric ratio (er) was determined by chiral HPLC on commercial columns. The isolated yield of 3aa is given in parentheses.
Substrate scope
Equipped with the fine-tuned reaction conditions, we proceeded to examine the substrate scope of this asymmetric transformation (Fig. 3). A range of styrene derivatives, encompassing monosubstituted phenyl rings with electron-neutral, electron-rich, or electron-deficient substituents located in ortho, meta, or para positions, proved amenable to our methodology. These substrates delivered the anticipated products 3ba–3ka in good to excellent yields and high enantiomeric ratios. Our reaction conditions displayed compatibility with various functional groups, like CF3 (3ca), F (3da, 3ia, and 3ka), TMS (3ea), Bpin (3fa), and OMe (3ha). Additionally, polycyclic aryl-substituted alkenes seamlessly partook in the reaction, yielding compounds 3la–3ma with good yields and exemplary enantioselectivity. Heteroaryl alkenes are also compatible along with a slight drop of enantioselectivity as evidenced by 3na. Our reaction protocol also successfully converted a ferrocene-substituted alkene into the corresponding product, 3oa, achieving an impressive 88% yield and an enantiomeric ratio of 99.5:0.5. Moving beyond terminal aryl alkenes, our disclosed chiral Rh-catalytic system demonstrated exceptional prowess in discerning the prochiral faces of terminal alkyl alkenes, furnishing the desired products with good enantioselectivity. Commercially accessible feedstock terminal alkenes with varying carbon chain lengths were converted into the corresponding products 3pa–3sa in good yields (71–81%), with high enantiomeric ratios (94:6 to 95:5). The silylation of vinylcyclohexane was also performed with ease, leading to chiral product 3ta in an 80% yield and 95:5 er. Common alkenes featuring amino and Bpin groups were equally well-suited to the reaction, yielding products 3ua and 3va in moderate yields and respectable enantioselectivities (47% yield and 94:6 er for 3ua; 43% yield and 92:8 er for 3va).
a Reactions were carried out by using [Rh(cod)OMe]2 (2.5 mol%), L25 (5.2 mol%), 1 (0.4 mmol), 2 (0.2 mmol), LiOtBu (3.0 eq) in n-Heptane (0.3 M) for 48 h. Isolated yields after chromatography are shown. Enantiomeric ratio (er) was determined by chiral HPLC on commercial columns. b L26 instead of L25. c [Rh(cod)OMe]2 (5 mol%), L26 (11.0 mol%). d L29 instead of L25. e L28 instead of L25.
Then, a number of SCBs bearing diverse substituents on silicon were tested using 1a as the alkene partner under the optimized condition. Installation of electron-donating group at the para-position of the phenyl ring on silicon led to a slight decrease in yield along with a neglectable effect of enantioselectivity, offering an array of chiral silicon-stereogenic vinylsilanes (3ab–3ae) in a 53 − 75% yield and 94:6 er to 96:4 er. Introduction of the substituents on the meta-position of the phenyl ring did not significantly affect the reaction efficiency and enantioselectivity as evidenced by 3ag (62% yield and 96:4 er) and 3ah (81% yield and 96:4 er). 3,4-Disubstituted aryl group on silicon was also well tolerated, giving the desired product 3ai in 73% yield with 94.5:5.5 er. Switching of the phenyl substituent on silicon of SCB 2a to 5-indolyl group did not significantly affect the performance. It proceeds smoothly to afford the product 3aj in 72% yield and 96:4 er. Replacement of the t-butyl group on silicon of SCB 2a by less steric hindered groups such as methyl, i-propyl would lead to an obvious drop in enantioselectivity with an improvement of reactivity. Extending the π-conjugation of the phenyl ring on silicon of SCB is benefit for improving chiral control as illustrated by the high enantioselectivity of 3am (97:3 er). Increasing the steric hindrance of the phenyl ring on silicon has little effect on the reaction efficiency and enantioselectivity as evidenced by 3an–3ao. The absolute stereochemistry of products 3oa and 3ch has been confirmed as S by single crystal X-ray crystallography (Supplementary Tables 7,8).
Encouraged by the recent advances in the Rh-catalyzed distant functionalization of internal alkenes through a chain-walking mechanism that yields linear functionalized entities62,63,64, we embarked on the reaction of internal alkenes with silacyclobutanes (SCBs) to potentially achieve terminally silylated products. Such advancements would broaden the method’s utility for the enantioselective synthesis of acyclic Si-stereogenic alkenylsilanes. Yet, the direct combination of internal alkenes with SCBs had remained uncharted territory. To our satisfaction, after thorough optimization of reaction variables, the Rh-catalyzed isomerization/silylation of 2-octene (4a) with SCB 2a progressed smoothly, exclusively yielding the linear Si-stereogenic alkenylsilane 5a in a 73% yield and 94:6 er, with L26 starring as the chiral ligand and commonly utilized Et3SiH as the additive, which assited the Rh-catalyzed isomerization of C=C double bond. We next turned our focus to the scope with respect to the internal alkene partner within the refined optimized conditions (Fig. 4). We were pleased to observe that internal alkenes sporting the C=C double bond at varying junctures yet with an identical carbon chain length, including 2-octene, 3-octene, and 4-octene, were amenable substrates, affording 5a with comparable yields and enantioselectivities. Furthermore, both E (4b, 4c and 4 d) and Z (4e and 4 f) alkenes, as well as E/Z mixtures (4a and 4g–4k), underwent the process seamlessly, achieving excellent terminal regioselectivity and enantioselectivity, independent of the original position of the olefin’s C=C bond. Further exploration disclosed a preference for migration towards the less hindered primary C(sp3)–H site, retaining good enantioselectivity when multiple methyls were present on the alkyl chain (4 h and 4j). Notably, when alkenes harbored both trisubstituted and disubstituted internal C=C bonds (4 h), the reaction selectively targeted the less substituted C=C bond for isomerization and subsequent enantioselective silylation, producing the coveted product (5f, with a 43% yield and 94.5:5.5 er). We are also able to isolate terminally silylated products 5g with high enantioselectivity (95:5 er) from internal alkene bearing a phenyl ring at the other terminus of the alkyl chain (4i), albeit in lower yield (20%). Additionally, akin to terminal alkenes, diverse functional groups within internal alkenes like ether (4j) and amine (4k) proved to be compatible under standard reaction conditions, leading to the desired products 5h and 5i with moderate yields and respectable enantioselectivities. Given that isomeric mixtures of unactivated alkenes, easily obtainable via alkane dehydrogenation and generally more economical than purified isomers, are readily accessible in large quantities, we assessed the potential for remote silylation of these mixtures to yield a singular isomer. To our gratification, our methodology facilitated the regioconvergent production of the enantiopure terminal Si-stereogenic vinylsilane 5a in a 66% yield with a 94:6 enantiomeric ratio from mixed octene isomers.
a Reactions were carried out by using [Rh(cod)OMe]2 (5 mol%), L26 (11 mol%), 1 (0.4 mmol), 2 (0.2 mmol), Et3SiH (50 mol%) and LiOtBu (3.0 eq) in n-Heptane (0.3 M) for 48 h. Isolated yields after chromatography are shown. The E/Z were determined by 1HNMR. Enantiomeric ratio (er) was determined by chiral HPLC on commercial columns. b NaBH4 (1.0 eq) instead of Et3SiH (50 mol%). c L26-Rh-H (5 mol%) was used.
Synthetic utility
To showcase the utility of our Rh-catalyzed system, we first conducted a scaled-up reaction of 1a with 2a to obtain approximately 1.1 grams of 3aa in a 72% yield and a 95:5 er. We then executed a suite of late-stage transformations to unveil the synthetic versatility of these enantiopure Si-stereogenic vinylsilanes as platform molecules toward diverse chiral silicon molecules (Fig. 5a). Initially, we successfully demonstrated that the vinyl group of 3aa could be converted into a spectrum of functional groups with negligible loss in enantioselectivity. For instance, the bromination of 3aa proceeded smoothly, yielding the product 6 with a 75% yield and a 93.5:6.5 er. The vinyl group of 3aa was also amenable to highly selective epoxidation, delivering the chiral epoxide 7 in a 95% yield and 94:6 er. Additionally, the geometrical isomerization from E to Z configuration of the alkene was achieved with a 58% yield and 95:5 er under photochemical conditions. Moreover, the comprehensive hydrogenation of all unsaturated bonds, including the phenyl groups on 3aa, was accomplished using Yu’s arene hydrogenation65, rendering the product 9 in a 97% yield with a 92:8 er. We further showed that the C = C bond of 3aa could be oxidized to the enantioenriched silicon-stereogenic acylsilane 10, a structure otherwise challenging to access. The site selectivity of the hydroboration of 3aa was modifiable by employing various catalytic systems and boron reagents, producing products 11 and 12, respectively. Moreover, we successfully proved that the phenyl group on silicon of 3aa can be directly transformed to hydroxyl group to yield the chiral silanol 13 without remarkable loss of enantiopurity. Additionally, we illustrated that the hydroxyl group of product 13 could be selectively reduced to the chiral hydrosilane 15 while forming the silyl ether 14 without affecting the C=C bond. The selective reduction of the C=C bond of 3aa via hydrogenation yielded the product 16 in a 93% yield with a 95:5 er.
a the diverse down-stream transformations of product 3aa. (b) the synthesis of chiral π-conjugated double bond systems 17, 18, 19 and 20 bearing quaternary silicon stereocenters. c the images of 17, 18, 19 and 20 in both solution and solid state. d the fluorescence emission spectra of 17, 18, 19 and 20 in DCM and solid state. e CD spectra of 17, 19 and 20 and their enantiomers in CHCl3 (1.0 × 10−4 M) at room temperature.
Another fascinating application would be the expedient access to chiral π-conjugated double bond systems. Because the π-conjugated arylvinyl derivatives hold great importance in functional materials66,67,68 and the value of chirality in optoelectronics has been well acknowledged69,70. However, to date, the synthesis and characterization of enantioenriched π-conjugated arylvinyl derivatives have been sparsely studied to date. Thus, we synthesized four enantioriched π-conjugated double bond systems 17, 18, 19 and 20 tailing quaternary silicon stereocenters by the reaction of dienes and trienes such as 1,4-divinylbenzene as well as 1,3,5-trivinylbenzene with SCB 2a under our optimized Rh-catalyzed conditions (Fig. 5b) and examined their photophysical behaviors in both solution and the solid state. All four compounds exhibited fluorescence with distinct emission wavelengths and intensities (Fig. 5c, d). Compound 19 had the longest maximum emission wavelength and the highest quantum yields (Φf) of 0.73 and 0.87 in the solid state and in solution, respectively. It is presumably attributed to the twisted conformations of the piperidyl and the Si-stereogenic vinylsilane moiety, which is capable of restricting intermolecular interactions. We further investigated the chiroptical properties of these π-conjugated systems through circular dichroism (CD) and circularly polarized luminescence (CPL) spectra in CHCl3. The CD spectra are mirror images of each other, in which 17, 19, 20 and their enantiomers exhibited pronounced Cotton effects (Fig. 5e). Compound 19 possesses the highest absorption dissymmetry factor (gabs = 9.0 × 10-3). Finally, the CPL measurement which reflected the chirality of the excited states was also conducted. Unfortunately, these compounds are CPL-inactive in both solution and solid state.
Mechanistic studies
Stimulated by the insights into the Rh-catalyzed intramolecular C−H silylation with silacyclobutanes (SCBs) detailed by He and Yu71,72 as well as DFT calculation on Rh-catalyzed transformation of SCBs with alkynes73, we surmised that the [Rh]−H species could likewise serve as the catalytic powerhouse in our current system. Pursuing this concept, we meticulously forged the chiral [Rh]−H catalyst L26-Rh-H (Fig. 6a and Supplementary Fig. 2-6) and eagerly employed it in the reaction of styrene 1a with SCB 2a (Fig. 6b). Much to our satisfaction, the reaction proceeded effortlessly under [Rh]−H catalysis, delivering the product 3aa with an 86% yield and a 94:6 er, corroborating our supposition regarding the active catalytic species. Additionally, we carried out the reaction using 2,2′-di-deuterated styrene 1a-D2 with SCB 2a under both Ni-catalyzed and Rh-catalyzed conditions (Fig. 6c). Under Ni-catalyzed conditions, consistent with literature reports, one deuterium atom on 1a-D2 was selectively transferred to the terminal position of the propyl group. Contrastingly, in the product 3aa obtained under the Rh-catalyzed condition, deuterium was detected at both the β-position of the styryl group and the terminal position of the propyl group. This was substantiated by both 1H and 2H NMR spectroscopy, with the β-position deuteration in product 3aa noticeably diminished. These findings signify that the reaction mechanism under our Rh-catalyzed system may diverge from that of Yorimitsu’s nickel catalyst system. Moreover, we charted a linear correlation between the enantiopurity of the product 3aa and the associated chiral ligand L25, suggesting that a singular chiral ligand is implicated in each enantiodetermining transition state (Fig. 6d).
Taking cues from these experimental outcomes, we embarked on density functional theory (DFT) calculations at the MN15(SMD)/SDD&6-311 + G(d,p)//B3LYP-D3(BJ)/SDD&6-31G(d) level to gain deeper insights into the detailed reaction mechanism and the contributing factors to the enantioselectivity (Fig. 6e-f, Supplementary Fig. 7-10 and Source data), with the silylation of alkene 1a with SCB 2a as the model reaction. The calculated energy profile, initiating from the L26-Rh-H active catalyst form, is illustrated in Fig. 6e and Supplementary Fig. 7. The process starts with the Si−C oxidative addition of SCB 2a through the transition state (S)-TS1, culminating in the five-membered rhodacycle intermediate (S)-IM1. This intermediate then undergoes C−H reductive elimination via transition state (S)-TS2, spawning the Rh-silyl intermediate (S)-IM2. The coordination of the incoming styrene 1a to the Rh center of (S)-IM2 could afford intermediate (S)-IM3, from which the selective 1,2-insertion of the C=C bond into the Rh−Si bond via transition state (S)-TS3 leads to intermediate (S)-IM4. An alternative 2,1-insertion was also considered but proved kinetically less favorable than the 1,2-insertion (Supplementary Fig. 8). Finally, the catalytic sequence closes with β-H elimination through transition state (S)-TS4, emancipating product 3aa and regenerating the active catalyst species.
The computations show that the initial Si−C oxidative addition is the rate-determining step of the reaction, which also constitutes the enantioselectivity-determining step. The calculated energy difference between (S)-TS1 and (R)-TS1 is 2.3 kcal/mol (22.4 versus 24.7 kcal/mol), which corresponds to a predicted ratio of 96:4 at 373.15 K, in excellent agreement with the experimentally-observed enantioselectivity (Fig. 6e and Supplementary Fig. 9). The optimized geometries (Fig. 6f) unveil the presence of the Rh···Ph interaction in both (S)-TS1 and (R)-TS1 (2.63 and 2.69 Å). However, the relative orientation of the SCB moiety differs significantly. In (S)-TS1, the SCB moiety is distanced from the ligand, whereas in (R)-TS1, the SCB moiety is closer to the ligand to sustain the Rh···Ph interaction. Consequently, the steric repulsion between the SCB moiety and ligand is more pronounced in (R)-TS1 than in (S)-TS1 (2.06 and 2.16 versus 2.14 Å), which thus makes (R)-TS1 higher in energy than (S)-TS1, enabling the excellent enantioselectivity observed in experiments. It should be pointed out here that apart from the Si−C oxidative addition, the possibility of the reaction that initiated by the insertion of styrene into the Rh−H bond was also evaluated (Supplementary Fig. 10). The computations show that the insertion of styrene into the Rh−H bond to form Rh-alkyl intermediate is kinetically feasible. However, the ensuing Si−C oxidative addition was found to be accompanied by high energy barriers, thereby ruling out this possibility. Nonetheless, the reversibility of the insertion into the Rh−H bond indeed aligns well with the deuterium-labeling experiments (Fig. 6c).
In conclusion, we have pioneered a highly enantioselective intermolecular silylation of terminal alkenes and the remote silylation of internal alkenes by the development of a chiral Rh-catalytic system. This process constitutes an efficient and general route for the construction of an array of enantioenriched silicon-stereogenic vinylsilanes with excellent functional group tolerance and linear selectivity that are otherwise difficult to prepare. Unlike the reaction pathway observed in Ni-catalyzed systems, our mechanistic investigations indicate that this reaction may operate through distinct mechanisms under the Rh-catalyzed system. Furthermore, the synthesized enantioenriched Si-stereogenic vinylsilanes have proven to be versatile precursors, readily convertible into diverse chiral structures, including silanols, acylsilanes, and monohydrosilane compounds. We anticipate that this advancement will significantly enrich the repertoire of chiral silicon compounds in synthetic chemistry, offering broad implications and utility.
Methods
General procedure for enantioselective silylation of terminal alkene with silacyclobutanes
If terminal alkene 1 is an oil, the 25 mL Schlenk tube was added [Rh(cod)OMe]2 (2.5–5.0 mol%), Ligand (5.0–10.0 mol%), and n-Heptane (0.4 M) in a nitrogen-filled glovebox. The mixture was stirred at rt for 0.5 h. Then the starting material 2 (0.2 mmol, 1.0 equiv.), LiOtBu (0.6 mmol, 3.0 equiv.) were added in the Schlenk tube. The formed mixture was stirred at 100 °C under N2, a solution of 1 (0.4 mmol, 2.0 equiv.) in n-Heptane (0.5 mL) was added by syringe pump for 2 h. The solution was stirred at 100 °C under N2 for 48 h. The solution was then cooled to room temperature, filtered with diatomaceous earth and the solvent was removed under vacuum. The crude product was purified by column chromatography on reversed phase column (C18(ODS)) (eluent: H2O/MeCN) to afford the pure product.
If terminal alkene 1 is a solid, the 25 mL Schlenk tube was added [Rh(cod)OMe]2 (2.5–5.0 mol%), Ligand (5.0–10.0 mol%), and n-Heptane (0.2 M) in a nitrogen-filled glovebox. The mixture was stirred at rt for 0.5 h. Then the starting material 1 (0.4 mmol, 2.0 equiv.), 2 (0.2 mmol, 1.0 equiv.), LiOtBu (0.6 mmol, 3.0 equiv.) were added in the Schlenk tube. The formed mixture was stirred at 100 °C under N2 for 48 h. The solution was then cooled to room temperature, filtered with diatomaceous earth and the solvent was removed under vaccum. The crude product was purified by flash chromatography on reversed-phase column (C18(ODS)) (eluent: H2O/MeCN) to afford the pure product.
General procedure for enantioselective silylation of internal alkenes with silacyclobutanes
The 25 mL Schlenk tube was added [Rh(cod)OMe]2 (5.0 mol%), L26 (10.0 mol%), n-Heptane (0.2 M), and Et3SiH (0.5 equiv.) in a nitrogen-filled glovebox. The mixture was stirred at rt for 0.5 h. Then the internal alkene 4 (0.4 mmol, 2.0 equiv.), 2a (0.2 mmol, 1.0 equiv.), LiOtBu (0.6 mmol, 3.0 equiv.) were added in the Schlenk tube. The formed mixture was stirred at 100 °C under N2 for 48 h. The solution was then cooled to room temperature, filtered with diatomaceous earth and the solvent was removed under vacuum. The residue was purified by flash chromatography on reversed-phase column (C18(ODS)) (eluent: H2O/MeCN) to afford the pure product.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information Files as well as from the corresponding authors on request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2368782 (3oa) and CCDC 2368779 (3ch). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Cartesian coordinates of the calculated structures are available from Source data. Source data are provided with this paper.
References
Shimada, M. et al. Multifunctional Octamethyltetrasila[2.2]cyclophanes: Conformational variations, circularly polarized luminescence, and organic electroluminescence. J. Am. Chem. Soc. 139, 11214–11221 (2017).
Koga, S. et al. Access to chiral silicon centers for application to circularly polarized luminescence materials. J. Org. Chem. 82, 6108–6117 (2017).
Tacke, R. et al. Syntheses and pharmacological characterization of achiral and chiral enantiopure C/Si/Ge-analogous derivatives of the muscarinic antagonist cycrimine: a study on C/Si/Ge bioisosterism. J. Organomet. Chem. 640, 140–165 (2001).
Tacke, R. & Heinrich, T. Syntheses of enantiopure Si-centrochiral silicon-based muscarinic antagonists using an enantioselective enzymatic esterification as the key step. Silicon Chem. 1, 35–39 (2002).
Luo, G. et al. Asymmetric total synthesis and antidepressant activity of (−)-sila-mesembranol bearing a silicon stereocenter. Org. Chem. Front. 8, 5941–5947 (2021).
Kawakami, Y. & Li, Y. Stereochemical study on polymers with stereogenic silicon atoms. J. Polym. Res. 7, 63–72 (2000).
Kawakami, Y. et al. Control of stereochemical structures of silicon-containing polymeric systems. Polym. Int. 58, 279–284 (2009).
Bai, X.-F. et al. Lewis-base-mediated diastereoselective silylations of alcohols: synthesis of silicon-stereogenic dialkoxysilanes controlled by chiral aryl BINMOLs. Chem. Asian J. 12, 1730–1735 (2017).
Chang, X., Ma, P.-L., Chen, H.-C., Li, C.-Y. & Wang, P. Asymmetric synthesis and application of Chiral Spirosilabiindanes. Angew. Chem. Int. Ed. 59, 8937–8940 (2020).
Zhang, H. & Zhao, D. Synthesis of silicon-stereogenic silanols involving iridium-catalyzed Enantioselective C–H silylation leading to a new ligand scaffold. ACS Catal. 11, 10748–10753 (2021).
Yang, B., Gao, J., Tan, X., Ge, Y. & He, C. Chiral PSiSi-ligand enabled Iridium-catalyzed atroposelective intermolecular C−H silylation. Angew. Chem. Int. Ed. 62, e202307812 (2023).
Chan, T. H. & Wang, D. Chiral organosilicon compounds in asymmetric synthesis. Chem. Rev. 92, 995–1006 (1992).
Oestreich, M. Silicon-stereogenic silanes in asymmetric catalysis. Synlett 11, 1629–1643 (2007).
Xu, L.-W. Desymmetrization catalyzed by transition-metal complexes: enantioselective formation of silicon-stereogenic silanes. Angew. Chem. Int. Ed. 51, 12932–12934 (2012).
Shintani, R. Catalytic asymmetric synthesis of silicon-stereogenic compounds by enantioselective desymmetrization of prochiral Tetraorganosilanes. J. Synth. Org. Chem. Jpn. 76, 1163–1169 (2018).
Shintani, R. Recent progress in catalytic enantioselective desymmetrization of prochiral Organosilanes for the synthesis of silicon-stereogenic compounds. Synlett 29, 388–396 (2018).
Wu, Y.-C. & Wang, P. Silicon-stereogenic monohydrosilane: synthesis and applications. Angew. Chem. Int. Ed. 61, e202205382 (2022).
Ge, Y.-C., Huang, X.-F., Ke, J. & He, C. Transition-metal-catalyzed enantioselective C−H silylation. Chem Catal. 2, 2898–2928 (2022).
Wu, Y., Zheng, L., Wang, Y. & Wang, P. Catalytic asymmetric synthesis of silicon-stereogenic organosilanes. Chem 10, 3461–3514 (2023).
Xu, L.-W., Li, L., Lai, G.-Q. & Jiang, J.-X. The recent synthesis and application of silicon-stereogenic silanes: A renewed and significant challenge in asymmetric synthesis. Chem. Soc. Rev. 40, 1777–1790 (2011).
Ye, Z.-T., Wu, Z.-W., Zhang, X.-X., Zhou, J. & Yu, J.-S. Organocatalytic enantioselective construction of Si-stereocenters: recent advances and perspectives. Chem. Soc. Rev. 53, 8546–8562 (2024).
Jin, C. et al. Axial chirality reversal and enantioselective access to Si-stereogenic silylallene. Chem 9, 2956–2970 (2023).
Zhang, X.-X., Gao, Y., Zhang, Y.-X., Zhou, J. & Yu, J.-S. Highly Enantioselective construction of multifunctional silicon-stereogenic silacycles by asymmetric Enamine catalysis. Angew. Chem. Int. Ed. 62, e202217724 (2023).
Hu, T. et al. Lewis base-catalyzed dynamic kinetic asymmetric transformation of racemic Chlorosilanes en route to Si-stereogenic silylethers. J. Am. Chem. Soc. 146, 23092–23102 (2024).
Luh, T.-Y., & Liu, S.-T. Synthetic Applications of Allylsilanes and Vinylsilanes, Wiley (1998).
Fleming, I., Dunogues, J., & Smithers, R. The Electrophilic Substitution of Allylsilanes and Vinylsilanes, John Wiley & Sons, Inc. (2004).
Hiyama, T., & Oestreich, M. Organosilicon Chemistry: Novel Approaches and Reactions, Wiley-VCH, Weinheim (2019).
Blumenkopf, T. A. & Overman, L. Vinylsilane- and alkynylsilane-terminated cyclization reactions. Chem. Rev. 86, 857–873 (1986).
Luh, T.-Y. From vinylsilanes to organic/inorganic hybrid materials. Pure Appl. Chem. 77, 2083–2090 (2005).
Curtis-Long, M. J. & Aye, Y. Vinyl-, Propargyl-, and Allenylsilicon reagents in asymmetric synthesis: a relatively untapped resource of environmentally benign reagents. Chem. Eur. J. 15, 5402–5416 (2009).
Pawluć, P., Prukała, W., & Marciniec, B. Silylative coupling of Olefins with Vinylsilanes in the synthesis of π-conjugated double bond systems. Eur. J. Org. Chem. 219–229 (2010).
Riant, O., Indukuri, K. & Cornelissen, L. Recent developments in the chemistry of Vinylsiloxanes. Synthesis 48, 4400–4422 (2016).
Wang, D. et al. Inverse vulcanization of Vinyltriethoxysilane: A novel interfacial coupling agent for silica-filled rubber composites. Macromolecules 55, 8485–8494 (2022).
Igawa, K., Yoshihiro, D., Ichikawa, N., Kokan, N. & Tomooka, K. Catalytic enantioselective synthesis of alkenylhydrosilanes. Angew. Chem. Int. Ed. 51, 12745–12748 (2012).
Xu, J.-X. et al. Platinum-catalyzed multicomponent alcoholysis/hydrosilylation and Bis-hydrosilylation of alkynes with dihydrosilanes. ChemCatChem 9, 3111–3116 (2017).
Wen, H., Wan, X. & Huang, Z. Asymmetric synthesis of silicon-stereogenic Vinylhydrosilanes by Cobalt-catalyzed regio- and enantioselective alkyne hydrosilylation with dihydrosilanes. Angew. Chem. Int. Ed. 57, 6319–6323 (2018).
Xu, J.-L., Wang, Z.-L., Zhao, J.-B. & Xu, Y.-H. Enantioselective construction of Si-stereogenic linear alkenylhydrosilanes via copper-catalyzed hydrosilylation of alkynes. Chem. Catal. 4, 100887 (2024).
Xie, J.-L. et al. Palladium-catalyzed hydrosilylation of ynones to access silicon-stereogenic silylenones by stereospecific aromatic interaction assisted Si–H activation. Sci. China Chem. 64, 761–769 (2021).
Ling, F.-Y. et al. An unusual autocatalysis with an air-stable Pd complex to promote enantioselective synthesis of Si-stereogenic enynes. Chem. Sci. 14, 1123–1131 (2023).
Wang, X.-C., Li, B., Ju, C.-W. & Zhao, D. Nickel(0)-catalyzed divergent reactions of silacyclobutanes with internal alkynes. Nat. Commun. 13, 3392–3403 (2022).
Zeng, Y. et al. Rhodium-catalyzed dynamic kinetic asymmetric hydrosilylation to access silicon-stereogenic center. Angew. Chem. Int. Ed. 61, e202214147 (2022).
Zhang, G. et al. Asymmetric synthesis of silicon-stereogenic silanes by copper-catalyzed desymmetrizing protoboration of Vinylsilanes. Angew. Chem. Int. Ed. 59, 11927–11931 (2020).
Shi, Y. et al. Divergent synthesis of Enantioenriched Silicon-stereogenic Benzyl-, Vinyl- and Borylsilanes via asymmetric Aryl to Alkyl 1,5-Palladium migration. Angew. Chem. Int. Ed. 63, e202405520 (2024).
McDonald, R. I., Liu, G. & Stahl, S. S. Palladium (ii)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications. Chem. Rev. 111, 2981–3019 (2011).
Yin, G., Mu, X. & Liu, G. Palladium (ii)-catalyzed oxidative difunctionalization of alkenes: bond forming at a high-valent palladium center. Acc. Chem. Res. 49, 2413–2423 (2016).
Dhungana, R. K., Kc, S., Basnet, P. & Giri, R. Transition metal-catalyzed dicarbofunctionalization of unactivated olefins. Chem. Rec. 18, 1314–1340 (2018).
Coombs, J. R. & Morken, J. P. Catalytic enantioselective functionalization of unactivated terminal alkenes. Angew. Chem. Int. Ed. 55, 2636–2649 (2016).
Wang, Z.-X., Bai, X.-Y. & Li, B.-J. Metal-catalyzed substrate-directed enantioselective functionalization of unactivated alkenes. Chin. J. Chem. 37, 1174–1180 (2019).
Li, Z.-L., Fang, G.-C., Gu, Q.-S. & Liu, X.-Y. Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 49, 32–48 (2020).
Hirano, K., Yorimitsu, H. & Oshima, K. Nickel-catalyzed regio- and stereoselective silylation of terminal alkenes with silacyclobutanes: facile access to vinylsilanes from alkenes. J. Am. Chem. Soc. 129, 6094–6095 (2007).
Hirano, K., Yorimitsu, H. & Oshima, K. Nickel-catalysed reactions with trialkylboranes and silacyclobutanes. Chem. Commun. 44, 3234–3241 (2008).
Li, L. J., Zhang, Y. B., Gao, L. & Song, Z. L. Recent advances in C–Si bond activation via a direct transition metal insertion. Tetrahedron Lett. 56, 1466–1473 (2015).
Mu, Q. C., Chen, J., Xia, C. G. & Xu, L. W. Synthesis of silacyclobutanes and their catalytic transformations enabled by transition-metal complexes. Coord. Chem. Rev. 374, 93–113 (2018).
Huang, J., Liu, F., Wu, X., Chen, J.-Q. & Wu, J. Recent advances in the reactions of silacyclobutanes and their applications. Org. Chem. Front. 9, 2840–2855 (2022).
Huang, W. S., Wang, Q., Yang, H. & Xu, L. W. State-of-the-art advances in enantioselective transition-metal-mediated reactions of Silacyclobutanes. Synthesis 54, 5400–5408 (2022).
Liu, M., Qi, L. & Zhao, D. Recent advances in transition metal-catalyzed C–Si bond cleavage of Silacyclobutanes. Chin. J. Org. Chem. 43, 3508–3525 (2023).
Zhang, Q.-W. et al. Rhodium-catalyzed intramolecular C-H Silylation by Silacyclobutanes. Angew. Chem. Int. Ed. 55, 6319–6323 (2016).
Chen, H. et al. Rhodium-catalyzed reaction of Silacyclobutanes with unactivated alkynes to afford Silacyclohexenes. Angew. Chem. Int. Ed. 58, 4695–4699 (2019).
Zhu, W.-K. et al. Rhodium-catalyzed hydrolytic cleavage of the silicon–carbon bond of Silacyclobutanes to access Silanols. Org. Lett. 25, 7186–7191 (2023).
Sun, Y., Zhou, K., Ma, C., Li, Z., & Zhang, J. Rhodium/Ming-Phos-catalyzed asymmetric annulation reaction of silacyclobutanes with terminal alkynes. Green Synth. Catal. https://doi.org/10.1016/j.gresc.2023.09.001 (2023).
Wang, X. et al. Multifunctional P-ligand-controlled “silicon-centered” selectivity in Rh/Cu-catalyzed Si–C bond cleavage of silacyclobutanes. Org. Chem. Front. 8, 6577–6584 (2021).
Borah, A. J. & Shi, Z. Rhodium-catalyzed, remote terminal hydroarylation of activated Olefins through a long-range deconjugative isomerization. J. Am. Chem. Soc. 140, 6062–6066 (2018).
Wagner-Carlberg, N. & Rovis, T. Rhodium(III)-catalyzed anti-Markovnikov hydroamidation of unactivated alkenes using Dioxazolones as amidating reagents. J. Am. Chem. Soc. 144, 22426–22432 (2022).
Li, H.-L. et al. Highly Diastereoselective Hydrosilane-assisted Rhodium-catalyzed spiro-type cycloisomerization of Succinimide and Pyrazolone-based functional 1,6-Dienes. Chem. Asian J. 16, 1730–1734 (2021).
Li, H.-X. & Yu, Z.-X. Arene reduction by Rh/Pd or Rh/Pt under 1 atm hydrogen gas and room temperature. Org. Lett. 26, 3458–3462 (2024).
Martin, R. E. & Diederich, F. Linear monodisperse π-conjugated oligomers: model compounds for polymers and more. Angew. Chem. Int. Ed. 38, 1350–1377 (1999).
Villarln, D. & Wezenberg, S. J. Stiff-Stilbene photoswitches: from fundamental studies to emergent applications. Angew. Chem. Int. Ed. 59, 13192–13202 (2020).
Müllen, K. & Scherf, U. Conjugated polymers: where we come from, where we stand, and where we might go. Macromol. Chem. Phys. 224, 2200337 (2023).
Ahn, J. et al. Chiral organic semiconducting materials for next-generation optoelectronic sensors. Device 1, 100176 (2023).
Li, X., Xie, Y. & Li, Z. The progress of circularly polarized luminescence in chiral purely organic materials. Adv. Photonics Res. 2, 2000136 (2021).
Zhang, L. et al. A combined computational and experimental study of Rh-catalyzed C-H silylation with silacyclobutanes: insights leading to a more efficient catalyst system. J. Am. Chem. Soc. 143, 3571–3582 (2021).
An, K. et al. Rhodium hydride enabled enantioselective intermolecular C–H silylation to access acyclic stereogenic Si–H. Nat. Commun. 13, 847–857 (2022).
Li, J., Chen, W. & Qu, S. DFT study of rhodium-catalyzed transformation of silacyclobutane with alkyne or H2O: Si–Cl bond reductive elimination vs. alkyne insertion. Org. Chem. Front. 11, 3663–3674 (2024).
Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (22022103, 22221002, 22371132, 22071114, 22188101, 22073066, and 22471191), the Natural Science Foundation of Tianjin (22JCZDJC00090, 23JCJQJC00130), the National Key Research and Development Program of China (2019YFA0210500, 2021YFF0701700), the “Frontiers Science Center for New Organic Matter”, Nankai University (Grant Number 63181206), Haihe Laboratory of Sustainable Chemical Transformations and the Fundamental Research Funds for the Central Universities and Nankai University.
Author information
Authors and Affiliations
Contributions
K. Y., J. Z., S.-H. C., S. C. & Y. S. performed the experiments. D. P. & G. H. performed the DFT calculation. D. Z. & G. H. conceived the concept, directed the project and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jin-Sheng Yu and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yin, K., Zhang, J., Pan, D. et al. Enantioselective construction of silicon-stereogenic vinylsilanes from simple alkenes. Nat Commun 16, 797 (2025). https://doi.org/10.1038/s41467-025-56232-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56232-y