Abstract
Systemic lipid homeostasis requires hepatic autophagy, a major cellular program for intracellular fat recycling. Here, we find melanocortin 3 receptor (MC3R) regulates hepatic autophagy in addition to its previously established CNS role in systemic energy partitioning and puberty. Mice with Mc3r deficiency develop obesity with hepatic triglyceride accumulation and disrupted hepatocellular autophagosome turnover. Mice with partially inactive human MC3R due to obesogenic variants demonstrate similar hepatic autophagic dysfunction. In vitro and in vivo activation of hepatic MC3R upregulates autophagy through LC3II activation, TFEB cytoplasmic-to-nuclear translocation, and subsequent downstream gene activation. MC3R-deficient hepatocytes had blunted autophagosome-lysosome docking and lipid droplet clearance. Finally, the liver-specific rescue of Mc3r was sufficient to restore hepatocellular autophagy, improve hepatocyte mitochondrial function and systemic energy expenditures, reduce adipose tissue lipid accumulation, and partially restore body weight in both male and female mice. We thus report a role for MC3R in regulating hepatic autophagy and systemic adiposity.
Similar content being viewed by others
Introduction
Proper hepatic autophagy is needed for active energy redistribution, stimulating both the formation and recycling of cellular lipid-droplets (LDs)1,2,3. Through dynamic fat repartitioning, activation of autophagy can prevent obesity-associated complications and liver steatosis in mice and humans3,4,5. Loss of autophagy has been linked to the development of metabolic dysfunction-associated fatty liver disease (MAFLD)6,7. Altered hepatic autophagy has been observed previously in obese mouse models5,8. Together with MC4R, MC3R is one of the two melanocortin G protein coupled receptors that are mainly expressed in the central nervous system (CNS) and modulate energy expenditure and systemic adiposity in humans9,10,11,12. Knockout (KO) mouse studies13,14,15 and human association analyses for MC3R variants16,17,18,19,20,21 support its role in regulating body weight, adiposity, and puberty22. In mice, global Mc3r deficiency obesity is a mild, late-onset form of obesity, that increases body weight moderately because it increases fat mass while reducing in lean mass23,24. Despite the relatively mild overall obesity phenotype, hepatic triglyceride (TG) accumulation is markedly increased in Mc3rTB/TB; the floxed transcriptionally blocked Mc3r deficient mouse23. Liver microarray analysis, which was confirmed using qPCR, showed more fatty acid synthesis gene upregulation and lower serum TG23 and reduced fasting induced lipolysis in white adipose tissue24, driving nutrient partitioning towards liver steatosis and systemic adiposity.
Previous studies have demonstrated that Mc3r global knockout mice do not have excessive food intake; rather they are described as being hypophagic relative to controls13,15,23. Additionally, some studies show evidence for reduced energy expenditure, respiratory exchange ratio, and locomotion in Mc3rTB/TB mice23. However, other studies have demonstrated normophagia and normal global metabolism in Mc3r deficiency24, leaving the mechanism of commonly observed increased adiposity undetermined14,23,25,26.
Interestingly, unlike other monogenic obesity models with defective hypothalamic signaling (e.g., Mc4rKO mice), recent studies suggest Mc3rKO mice develop an obese phenotype in part via MC3R insufficiency outside of the CNS8,23. Indeed, neither nervous system-specific rescue of Mc3r with nestin-cre nor hypothalamic rescue with steroidogenic factor (SF1)-cre fully reverses the abnormal fat mass gain in Mc3rKO mice on a normal chow diet, and neither CNS-Mc3r recovery model has a significant effect on body weight for mice given a high-fat diet23. Regardless of nutritional state, cellular mechanisms for lipid mobilization and nutrient partitioning play important roles in loss of function (LoF) MC3R-associated obesity. In our humanized MC3R knock-in mouse model27, a coding sequence variant (C17A + G241A) that impairs MC3R activity and appears to bias stem cell differentiation towards more adipogenic phenotypes, seems to cause obesity at least in part through peripheral mechanisms.
The liver has a central role in lipid transport and metabolism, including but not limited to the synthesis and catabolism of lipoproteins. Dietary short-chain fatty acids from the portal circulation, adipocyte-derived fatty acids, and excess triglycerides in chylomicron remnants are all transported to the liver28. Intrahepatic fatty acids are then reassembled as triglycerides in the endoplasmic reticulum, and combined with phospholipids, cholesterol, cholesteryl esters, and apoB molecules29 to form VLDL that is generally released into the circulation, where other tissues will utilize triglycerides for energy or will store them for later use. When VLDL secretion is not well matched with its intrahepatic synthesis, hepatic steatosis results30.
After identifying that Mc3r transcripts are expressed in liver tissue, we sought to examine if liver Mc3r is important for hepatic and/or systemic fat accumulation in Mc3r deficiency, and if so, to investigate the underlying mechanisms. Given that proper hepatic autophagy is needed for active energy redistribution, stimulating both formation and recycling of cellular LDs1,2,3 we aimed to investigate the liver-specific role of Mc3r in LD-autophagy, finding that activation of MC3R signaling induces autophagy in wild-type but not Mc3r-deficient mice and that autophagy flux is dysregulated in Mc3r deficiency, with defective lysosomal turnover. Further, our data show liver-specific Mc3r rescue in the context of global Mc3r deficiency restored liver autophagy and reduced adiposity due to changes in metabolic processes affecting energy expenditure.
Results
Mc3r expression regulates systemic and hepatic adiposity
Prior work using RNAseq approaches indicated tissue-specific regulation of Mc3r expression in both mouse31,32 and axolotl liver33. Although transcriptome databases reported no Mc3r transcripts in mouse liver, we examined Mc3r transcript levels from control and Mc3rTB/TB mice (MC3R-deficient due to insertion of a transcription blocker) using the more sensitive droplet digital PCR (ddPCR) method34, and found murine hepatic Mc3r expression (Fig. 1a). Although the estimated total Mc3r transcript level was lower in liver compared to hypothalamus (Fig. 1b), it was significantly higher in livers from control mice compared to those of Mc3rTB/TB mouse livers at Zeitgeber time (ZT) 12 and not at ZT2 (Fig. 1a). Notably, we found that expression of Mc3r transcripts in the control liver (Fig. 1a) was high at nighttime (ZT12), when MC3R ligands circulate in rodent plasma35,36.
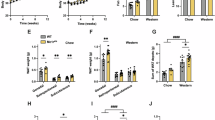
a Normalized droplet digital PCR Mc3r transcript levels from Mc3r+/+ liver (n = 37) at ZT12 (6:00 PM) and from Mc3r+/+ liver (n = 35) at ZT2 (8:00 AM) and Mc3rTB/TB liver (n = 18) at ZT12, and from Mc3rTB/TB liver (n = 19) at ZT2. b Normalized Mc3r transcript levels from Mc3r+/+ liver (n = 21) and hypothalamus (n = 4, with each RNA extraction sample combining 6 pooled hypothalamus isolations); c Body weight measured from Mc3r+/+ (n = 8), Mc3rTB/TB (n = 10), MC3RhWT/hWT (n = 12) and MC3RhDM/hDM (n = 11) independent observations. d Total body % fat from Mc3r+/+ (n = 9), Mc3rTB/TB (n = 8), MC3RhWT/hWT (n = 7) and MC3RhDM/hDM (n = 7) independent observations. e Liver triglycerides (TG) from Mc3r+/+ (n = 6), Mc3rTB/TB (n = 5), MC3RhWT/hWT (n = 7) and MC3RhDM/hDM (n = 6) independent observations. f Oil Red O images of livers (n = 2/genotype from two independent observations) g Transmission Electron Microscope (TEM) images of livers from mice in the fed state (n = 1–2/genotype from two independent experiments) at 8–12 weeks of age (h) Quantification of lipid droplet (LD) number from TEM images (n = 16–28 images/genotype from two independent observations) shown in (g). Asterisks indicate LD. Data are represented as mean ± SEM. Groups were compared by one-way ANOVA followed by Tukey’s HSD (a), paired two-tailed Student’s t-test (b, h), two-way ANOVA followed by Tukey’s HSD (c–e). * p < 0.05; ** p < 0.01; *** p < 0.001; male mice (8–12 weeks of age) under chow-fed diet were used unless otherwise indicated. Scale bar, 50 µm (f) and 5 µm (g). Source data are provided as a Source data file.
To understand Mc3r’s peripheral functions during obesogenesis, we began by examining systemic and hepatic adiposity in mouse genetic models with either no Mc3r activity (Mc3rTB/TB) or reduced Mc3r activity due to knock-in of a human mutated MC3R (MC3RhDM/hDM)27 that is linked to childhood and adult obesity18,37. With regular chow diet, we found progressive weight gain (Fig. 1c) in Mc3rTB/TB and MC3RhDM/hDM mice, with significantly increased total adiposity (Fig. 1d) and liver TG accumulation (Fig. 1e) by 12 weeks of age, compared to control wild-type mice (i.e., Mc3r+/+, C57BL/6) or knock-in mice expressing the intact human MC3R (i.e., MC3RhWT/hWT). Oil Red O staining from Mc3rTB/TB and MC3RhDM/hDM mice confirmed elevated hepatic fat deposition (Fig. 1f). The elevated liver TG of Mc3r insufficiency was associated with increased LD deposition, as detected by transmission electron microscopy (TEM) in liver cells in the fed condition (Fig. 1g, h). Since no significant differences were seen in liver or systemic fat development between Mc3r+/+ and MC3RhWT/hWT mice (Fig. 1), we refer to both such mice as controls. Our control mice, Mc3r+/+ and MC3RhWT/hWT showed no significant differences in liver or systemic fat development (Fig. 1).
Altered hepatic lipid droplet recycling and autophagy in MC3R-insufficient mice
Under chow diet, both Mc3rTB/TB and MC3RhDM/hDM livers displayed significantly increased TG levels compared to the levels seen in the control livers (Fig. 1e), suggesting potential defects in fat metabolism in the livers due to defective MC3R activity. Previously, we found hepatic TG levels were not different in mice with defective Mc3r compared to the control mice during high-fat diet27. We examined liver LD quantity (the major hepatocyte TG storage) from chow diet fed Mc3rTB/TB and MC3RhDM/hDM mice after overnight (O/N) fasting, which normally stimulates hepatic fat uptake and TG production. With TEM, we found that a similar number of LDs formed in Mc3r defective livers upon O/N fasting (Supplementary Fig. 1a, b), confirming there was no defect in hepatic fatty acid uptake or TG deposition in Mc3r-insufficient mice.
Next, due to liver autophagy’s essential role in hepatic LD recycling and fat catabolism1,3,38, we aimed to determine if defective Mc3r leads to TG accumulation by impairing LD recycling and degradation by liver autophagy. Using TEM to examine autophagosome (AP) organization, we found similar AP production in the control versus Mc3rTB/TB mice after O/N fasting (Fig. 2a, b), suggesting the capability for AP biogenesis itself is unchanged in Mc3r defective livers. However, unlike AP from the control cells that contained electron-dense structures that likely are degradative debris of intracellular components, many AP from Mc3rTB/TB or MC3RhDM/hDM mice contained intact membrane structures (Fig. 2a and c, see arrows and arrowheads indicating mitochondrial outer and inner membranes). This suggests inefficient AP degradation in the Mc3r defective livers. In addition, liver TEM images from Mc3rTB/TB or MC3RhDM/hDM mice often showed multiple AP structures in very close proximity, unlike the discrete and isolated AP structures observed in control cells (Fig. 2a and c, asterisks). These AP aggregates with undegraded contents in the livers of Mc3rTB/TB or MC3RhDM/hDM mice led us to hypothesize that excessive hepatic TG accumulation in MC3R defective mice results from compromised hepatic TG degradation. Thus, we studied if disrupting the hepatic Mc3r pathway dysregulates the autophagic recycling process.
a Transmission Electron Microscope images showing liver autophagosomes (AP) from overnight (O/N) fasted Mc3r+/+ and Mc3rTB/TB (n = 1–2/genotype from two independent experiments). b Quantification of AP number from TEM images from both Mc3r+/+ (n = 15–16 images/genotype from two independent observations) shown in (a). c TEM images showing liver AP from overnight (O/N) fasted Mc3rhWT/hWT and MC3RhDM/hDM. d Western blot images of liver lysates from Mc3r+/+, Mc3rTB/TB, Mc3rhWT/hWT and MC3RhDM/hDM. e Quantification of LC3II normalized by actin from both fed-fasted Mc3r+/+ (n = 4) independent observations, Mc3rTB/TB (n = 5) independent observations, MC3RhWT/hWT (n = 10) independent observations, and MC3RhDM/hDM (n = 9 (fed) and 10 (fasted) independent observations) in (d). f Western blot images of primary hepatocytes from Mc3r+/+, Mc3rTB/TB and Mc4r+/− after 1 h serum starvation or 30 min of 10 mM chloroquine (CQ) treatment. g Quantification of AP flux estimated in (f) and Supplementary Fig. 1c from Mc3r+/+ (n = 3), Mc3rTB/TB (n = 3), MC3RhWT/hWT (n = 4) and MC3RhDM/hDM (n = 3). h Fluorescent images of primary hepatocytes from Mc3r+/+, Mc3rTB/TB and MC3RhDM/hDM transgenic mice carrying GFP-LC3 after staining with Lysotracker Red. i Quantification of AP volume in (h) (n = ~1500/2 mice/genotype from two independent experiments). Asterisks indicate AP. Arrows and arrowheads indicate mitochondrial outer and inner membranes, respectively. Data are represented as mean ± SEM. Groups were compared by unpaired two-tailed Student’s t-test (b), ordinary one-way ANOVA followed by Kruskal–Wallis test followed by Dunn’s multiple comparisons test or Tukey’s HSD test (e, g, i). * p < 0.05; ** p < 0.01; *** p < 0.001, Scale bar, 1 µm (a, c) and 10 µm (h). Source data are provided as a Source data file.
Defective autophagosome turnover in MC3R deficient liver
Impaired lysosomal AP clearance could accumulate APs with undegraded subcellular compartments. To examine the liver AP recycling program, we monitored LC3II expression in the livers of MC3RhDM/hDM and Mc3rTB/TB mice. Since LC3II is converted from LC3I by lipidation during autophagy induction and constantly undergoes recycling after autophagolysosome formation, the total level of cellular LC3II (normalized to beta-actin) was examined as an indicator of overall cellular autophagy activity. We found that under fed conditions (when mice maintain basal autophagy activity in the liver), both Mc3rTB/TB and MC3RhDM/hDM mice exhibited much greater basal LC3II protein levels in liver compared to control mice (Fig. 2d, e) due to defective Mc3r, leading to LC3II accumulation. As expected, under fasting conditions, there was significant induction of liver LC3II levels in control mice (Fig. 2d, e), confirming our fasting condition enhances hepatic autophagy. However, the similarly fasted Mc3rTB/TB and MC3RhDM/hDM mice failed to increase liver LC3II (Fig. 2d, e), suggesting impaired autophagic regulation in Mc3r-deficient livers.
To examine which functions of liver autophagy are dysregulated in Mc3rTB/TB and MC3RhDM/hDM mice, we studied AP turnover kinetics (AP flux) in primary hepatocytes by using chloroquine, a well-known AP-lysosome fusion blocker39. To minimize pleiotropic effects, we designed an acute AP flux assay by treating with chloroquine for only 30 min39. Blockade of AP degradation by chloroquine marginally increased LC3II in the control hepatocytes under fed or serum starved conditions without showing significant cytotoxic phenotypes (Fig. 2f and Supplementary Fig. 1c). Our estimated AP flux data suggested that under serum starvation, 10 to 20% of AP undergo lysosomal digestion in control hepatocytes (Fig. 2g). In contrast, the AP flux was perturbed in the similarly treated cells from Mc3rTB/TB or MC3RhDM/hDM mice (Fig. 2f, g). Furthermore, supporting dysregulated AP degradation in the hepatocytes with defective Mc3r, we also observed accumulated p62/SQSTM1 (a key cargo protein that normally undergoes autophagic degradation40) during serum starvation in Mc3r-insufficient hepatocytes (Fig. 2f and Supplementary Fig. 1c). As an obesity control, we also tested autophagy flux in Mc4r+/− mice, as they display a comparable adiposity with Mc3rTB/TB mice, though not as severe an obesity phenotype as is seen in Mc4r−/− mice13,41,42. Importantly, upon serum starvation, hepatocytes isolated from Mc4r+/− mice could maintain AP flux to a similar extent as seen in control hepatocytes, as evidenced by normal activity of p62 degradation (Fig. 2f, g). The dysregulated autophagy observed in Mc3rTB/TB and MC3RhDM/hDM liver is thus not due solely to the obesity phenotype, rather these data indicate a distinct role of Mc3r in autophagy regulation.
To further monitor hepatic autophagy processes, we generated transgenic GFP-LC3 mice with Mc3r insufficiency. We found that hepatocytes carrying intact Mc3r displayed dispersed cytoplasmic GFP-LC3 with very few discrete LC3 vesicle structures during fed conditions (Fig. 2h). Conversely, hepatocytes from Mc3rTB/TB or MC3RhDM/hDM mice displayed many GFP-LC3 vesicles that were aggregated even without serum starvation (Fig. 2h, Inlay). Similar LC3 aggregates were formed in control hepatocytes when AP degradation was blocked by chloroquine (Supplementary Fig. 1d). Additional 3D reconstruction of GFP-LC3 structures further revealed augmented AP volumes in Mc3r-insufficient hepatocytes compared to control cells (Fig. 2i). These results clearly support impaired AP turnover caused by Mc3r deficiency in mouse liver.
Liver-specific role of MC3R in controlling adiposity and glucose metabolism
To interrogate potential hepatic functions of Mc3r, we selectively re-expressed liver Mc3r in Mc3rTB/TB mice by crossing them with mice expressing Cre-recombinase only in hepatocytes (Supplementary Fig. 2a). Using the resulting progeny (Mc3rHep/Hep mice expressing Mc3r only in the liver), we found liver-specific Mc3r recovery significantly improved percentage of fat and lean mass relative to Mc3rTB/TB but lean mass percentage was still significantly reduced, and fat mass percentage still significantly increased, relative to control mice (Fig. 3a, b). The abnormalities of Mc3rTB/TB mice in serum insulin (Fig. 3c) and fasting glucose concentrations (Fig. 3d), and consequently in insulin sensitivity by glucose and insulin tolerance test area under curve (AUC) analyses (Fig. 3f, h) were also ameliorated in Mc3rHep/Hep mice. Restoring Mc3r expression only in the liver led to partially improved overall body weight regulation during chow diet compared to the Mc3r null mice in both male (Fig. 3i) and female (Fig. 3j) mice. We also compared weight gains in contemporaneously studied Mc4r+/− mice, the far less severely affected heterozygotes of the severe monogenetic obesity Mc4r−/− mouse (Supplementary Fig. 3a, b). Our data finding Mc3rTB/TB homozygotes gain similar body weight compared to Mc4r heterozygotes confirm that Mc3r insufficiency is a moderate form of obesity that can be, to a certain extent, improved by restoring hepatic Mc3r expression.
a % Fat mass from Mc3r+/+ (n = 10), Mc3rTB/TB (n = 14) and Mc3rHep/Hep (n = 10) independent observations. b % Lean mass from Mc3r+/+ (n = 10), Mc3rTB/TB (n = 14) and Mc3rHep/Hep (n = 10) independent observations. c Fasting serum insulin from Mc3r+/+, Mc3rTB/TB and Mc3rHep/Hep (n = 7) independent observations/genotype. d Fasting glucose from Mc3r+/+ (n = 7), Mc3rTB/TB (n = 6) and Mc3rHep/Hep (n = 6) independent observations. e Insulin tolerance test (ITT) measured from Mc3r+/+ (n = 7), Mc3rTB/TB (n = 6) and Mc3rHep/Hep (n = 7) independent observations. * p < 0.05; ** p < 0.01; *** p < 0.001 group compared to Mc3r+/+ or #p < 0.05 group compared to Mc3rTB/TB. f ITT AUC measured from (e). g Glucose tolerance test (GTT) measured from Mc3r+/+ (n = 7), Mc3rTB/TB (n = 6) and Mc3rHep/Hep (n = 6) independent observations. h GTT AUC measured from (g). i Body weight measured from male Mc3r+/+ (n = 8), Mc3rTB/TB (n = 9) and Mc3rHep/Hep (n = 7) independent observations under chow diets. j Body weight measured from female Mc3r+/+ (n = 14), Mc3rTB/TB (n = 8) and Mc3rHep/Hep (n = 9) independent observations under chow diets. Data are represented as mean ± SEM. Groups were compared by ordinary one-way ANOVA followed by Tukey’s HSD test (a–d, f, h), two-way ANOVA followed by Tukey’s HSD test (e, g, i, j). * p < 0.05; ** p < 0.01; *** p < 0.001. Source data are provided as a Source Data file.
Liver-specific role of MC3R in controlling hepatic fat accumulation and fatty acid metabolism
To examine tissue fat accumulation and histology in liver-specific Mc3r recovery, we observed elevated hepatic fat deposition in Mc3rTB/TB that was reversed in the Mc3rHep/hep mice as assessed by H&E staining (Fig. 4a) and Oil Red O staining (Fig. 4b). Tissue TG content, markedly increased in Mc3rTB/TB was returned towards normal in Mc3rHep/Hep, suggesting restored hepatic lipid homeostasis (Fig. 4c). Importantly, hepatic Mc3r reactivation fully reversed the increased liver weight (Fig. 4d), but only partially reduced epididymal white adipose tissue (eWAT) weight (Fig. 4e) compared to Mc3rTB/TB and control mice.
a H&E staining (b) Oil Red O staining for liver tissue from Mc3r+/+, Mc3rTB/TB and Mc3rHep/Hep. c Fold change of liver TG levels from Mc3r+/+ (n = 8), Mc3rTB/TB (n = 8) and Mc3rHep/Hep (n = 7) independent observations. d Liver weight (g) from Mc3r+/+ (n = 36), Mc3rTB/TB (n = 11) and Mc3rHep/Hep (n = 24) independent observations (e) eWAT weight (g) from Mc3r+/+ (n = 33), Mc3rTB/TB (n = 15) and Mc3rHep/Hep (n = 24) independent observations (f) Serum TG measured from Mc3r+/+ (n = 6), Mc3rTB/TB (n = 7) and Mc3rHep/Hep (n = 5) independent observations. g Serum non-esterified fatty acids (NEFA) from Mc3r+/+ (n = 6), Mc3rTB/TB (n = 9) and Mc3rHep/Hep (n = 9) independent observations. h Serum glycerol from Mc3r+/+ (n = 6), Mc3rTB/TB (n = 9) and Mc3rHep/Hep (n = 9) independent observations. i Serum TC from Mc3r+/+ (n = 6), Mc3rTB/TB (n = 9) and Mc3rHep/Hep (n = 6) independent observations. j qPCR mRNA measurements for fatty acid metabolism from Mc3r+/+ (n = 8 fed) and (n = 7 fasted) independent observations, Mc3rTB/TB (n = 8), and Mc3rHep/Hep (n = 6) independent observations from both fed-fasted conditions. Data are represented as mean ± SEM. Groups were compared by one-way ANOVA followed by Tukey’s HSD test (c–f), one-way ANOVA followed by Fisher’s LSD test (g–i), and two-way ANOVA followed by Tukey’s HSD test (j). * p < 0.05; ** p < 0.01; *** p < 0.001, Scale bar, 50 µm (a, b). Source data are provided as a Source Data file.
To further evaluate the impact of reactivating hepatic Mc3r on circulatory lipids at the systemic level, we measured serum triglyceride concentrations, which were elevated in Mc3rTB/TB mice and restored to values not different from those of Mc3r+/+ in Mc3rHep/Hep (Fig. 4f). Serum non-esterified fatty acid (NEFA) concentrations were significantly higher in Mc3r deficient mice; hepatic Mc3r recovery similarly normalized NEFA (Fig. 4g) without a significant change in serum glycerol concentrations (Fig. 4h), suggesting improving insulin sensitivity and hepatic fatty acid metabolism. Elevated serum total cholesterol was also normalized in Mc3rHep/hep (Fig. 4i), which is consistent with the observed improvements in systemic obesity and liver steatosis.
We also examined hepatic mRNA expression of fatty acid metabolic genes (Fig. 4j). Cd36, the primary transporter responsible for uptake prior to fatty acid esterification process, was significantly increased in Mc3rTB/TB mice in the fasted state, though not in the fed state. Mc3rHep/Hep hepatic recovery mice showed significantly reduced Cd36 in the fasted state compared to Mc3rTB/TB with values similar to control mice (Fig. 4f). These findings suggest that Mc3r deficiency leads to an overall increase in total fatty acid esterification. Liver lipid droplet surface binding protein Cidec (cell death inducing DFFA like effector C), was found to be upregulated in Mc3rTB/TB, as described previously23, consistent with the excessive lipid droplet formation in Mc3rTB/TB. Mc3rHep/Hep returned Cidec to control levels compared to Mc3rTB/TB, suggesting improvement in insulin sensitivity. In both wild-type control and hepatic recovery groups, the liver showed increased Cidec expression levels when mice were fasted. At the same time, Mc3r global deficiency did not respond to fasting and exhibited lower Cidec expression compared to the fed state, consistent with a defect in lipid droplet formation and lipid droplet autophagy. We observed no significant differences between Mc3rTB/TB and controls for hepatic expression of the key rate-limiting enzymes of lipogenesis (fatty acid synthase; Fasn) and of Lipolysis (Lipe). In the fasted state, hepatic Ppargc1a was notably lower in Mc3rTB/TB than control mice but returned to a normal level in the hepatic recovery mice. This suggests that Mc3r deficiency may inhibit the oxidation of fatty acids, which in turn affects the breakdown of excess fatty acids. The reversal of a significant systemic adiposity phenotype could potentially be explained by the altered regulation of hepatic fat accumulation and fatty acid metabolism in Mc3rHep/Hep.
Effect of MC3R deficiency in regulating feeding behavior
We monitored food intake for 15 days in single-housed mice in our vivarium, which was not significantly different in Mc3rTB/TB and Mc3rHep/Hep mice compared to Mc3r+/+ (Supplementary Fig. 3c), even after adjusting for body weight or lean mass. Additionally, feeding efficiency (FE) did not differ between groups (Supplementary Fig. 3d). However, when food intake monitoring was conducted using CLAMS metabolic chambers (Fig. 5a–c), food intake differences emerged. Daily energy intake was significantly reduced in Mc3rTB/TB and Mc3rHep/Hep mice compared to Mc3r+/+ at 22 °C (Fig. 5b). After adjusting energy intake for body weight, lean & fat mass, or lean mass, Mc3rTB/TB and Mc3rHep/Hep remained significantly lower than Mc3r+/+ (Supplementary Table 1). At thermoneutrality (30 °C), energy intake was significantly reduced in Mc3rTB/TB vs. Mc3r+/+ only during the dark phase (Fig. 5c). This reduction in energy intake is consistent with previously reported findings that global transcriptionally blocked Mc3r-deficient mice are relatively hypophagic23,25. Here, re-expression of Mc3r only in the liver did not restore energy intake, suggesting that feeding behavior alterations in Mc3r deficiency are centrally (CNS) regulated and not via hepatic regulation23.
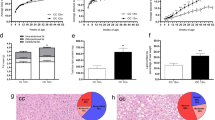
a Cumulative energy intake (kcal) monitored for light and dark phase at ambient 22 °C and thermoneutral temperature 30 °C. b Daily energy intake (kcal) for light, dark, and total (24 h) at 22 °C from Mc3r+/+, Mc3rTB/TB and Mc3rHep/Hep (n = 20) independent observations. c Daily energy intake (kcal) for light, dark, and total (24 h) at 30 °C from Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep (n = 20) independent observations. d Total energy expenditure (kcal/mouse/h) for light and dark phase e TEE (kcal/mouse/h) for light, dark, and total (24 h) at 22 °C from Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep (n = 20) independent observations. f TEE (kcal/mouse/h) for light, dark, and total (24 h) at 30 °C from Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep (n = 20) independent observations. g Oxygen consumption (VO2) (ml/mouse/h) for light and dark phase h VO2 (ml/mouse/h) for light, dark, and total (24 h) at 22 °C from Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep (n = 20) independent observations. i VO2 (ml/mouse/h) for light, dark, and total (24 h) at 30 °C from Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep (n = 20) independent observations. j Respiratory exchange ratio (RER) (VCO2/VO2) for light and dark phase (k) RER (VCO2/VO2) for light, dark, and total (24 h) at 22 °C from Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep (n = 20) independent observations. l RER (VCO2/VO2) for light, dark, and total (24 h) at 30 °C from Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep (n = 20) independent observations. In (a, d, g, and j), data from the first day of adaptation are not shown. Chow-fed, n = 20 mice/group (10 males and 10 females) mice 3–4 months of age. Data are represented as mean ± SEM. Groups were compared using Friedman’s test by Dunn’s paired analysis (b, c, e, f, h, i, k, l) and post hoc test for adjusted estimated marginal means in Supplementary Table 1. * p < 0.05; ** p < 0.01; *** p < 0.001. Source data are provided as a Source Data file.
Effect of hepatic Mc3r reactivation on energy expenditure, locomotion, and brown adipose tissue thermogenesis
To examine whether differences in total energy expenditure (TEE) contribute to the altered energy balance seen in Mc3rHEP/HEP, we performed indirect calorimetry at ambient 22 °C and thermoneutral temperature 30 °C during chow feeding using Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep mice (Fig. 5d). TEE was significantly reduced in Mc3rTB/TB mice compared to Mc3r+/+ in both light and dark phases. Notably, with Mc3r reactivation, Mc3rHep/Hep mice exhibited a significant increase in TEE compared to Mc3rTB/TB in both light and dark phases, which also translated to total 24-h energy expenditure (EE) at ambient 22 °C (Fig. 5e, f). For TEE at a thermoneutral temperature of 30 °C, Mc3rTB/TB was significantly lower compared to both Mc3r+/+ and Mc3rHep/Hep groups in the light phase and for total 24-h EE (Fig. 5e, f). Recovery from reduced 24-h total energy expenditure in the Mc3rTB/TB phenotype indicates that the role of hepatic Mc3r in autophagy is a key mechanism involves in lipid metabolism, energy expenditure and body weight. For Mc3rTB/TB mice, TEE was also reduced significantly compared to both Mc3r+/+ or Mc3rHep/Hep groups after adjusting for body weight and lean & fat mass at both temperature conditions (Supplementary Table 1). At a thermoneutral temperature of 30 °C, total energy expenditure (TEE), adjusted for body weight and lean and fat mass, showed a trend for an intermediate phenotype in Mc3rHep/Hep; however, it was not statistically significant compared to Mc3rTB/TB.
Compared to control mice (Fig. 5g–i), total oxygen consumption (VO2) was significantly reduced in Mc3rTB/TB mice, but not in Mc3rHep/Hep mice, at ambient 22 °C and 30 °C. Total VO2 was significantly increased in Mc3rHep/Hep versus Mc3rTB/TB mice at ambient 22 °C (Fig. 5g–i). After adjusting VO2 for body weight, and total lean & fat mass, Mc3rTB/TB also significantly differed from Mc3r+/+at both temperatures. At a thermoneutral temperature of 30 °C, Mc3rHep/Hep mice’s total VO2 exhibited a significant recovery relative to Mc3rTB/TB, even after adjustment for body weight and total lean & fat mass, confirming the significant recovery phenotype (Supplementary Table 1). This difference in VO2 consumption is a key indicator of improvement in metabolic activity.
Respiratory exchange ratio (RER) appeared to show lower total RER in Mc3rTB/TB compared to Mc3r+/+, while Mc3rHep/Hep mice were significantly increased compared to Mc3rTB/TB mice at 22 °C in the dark phase and 24-h RER at 30 °C (Fig. 5j–l). Restored RER in Mc3rHep/Hep implies an altered substrate preference (from fat to carbohydrates) for generating energy, as might be expected in a mouse with less adipose tissue.
We also monitored locomotion activity by beam breaks in the metabolic cages during the light and dark phase cycles at 22 °C and 30 °C using Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep mice. Total activity and ambulatory activity (physical activity) were all similar between groups during both light and dark phases (Supplementary Fig. 4a–f), suggesting that locomotion (by beam breaks) is not a contributing factor in the change in the obesity phenotype of Mc3r deficiency.
We checked brown adipose tissue (BAT) UCP-1 activity to monitor thermogenesis after studying mice either at ambient 22 °C or after cold exposure (6 °C) for 6 h. UCP-1 staining increased after cold exposure compared to ambient temperature in all groups without notable differences in the signal intensity between Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep in either temperature condition (Supplementary Fig. 4g). Immunoblotting results for UCP-1 protein expression were also similar between the groups (Supplementary Fig. 4h, i). Mc3r deficiency did not significantly alter BAT UCP-1 activity or brown adipose tissue thermogenesis, one of the alternative mechanisms for dissipating energy as heat.
Hepatic Mc3r reactivation restores adaptative energy expenditure, cellular respiration, and lipid droplet autophagy
To further investigate the critical role of hepatic MC3R in energy balance, we studied how Mc3r deficiency affects adaptative energy expenditure in response to cold exposure, when the body requires energy for thermogenesis. Adaptive energy expenditure (EE) in response to cold exposure was significantly blunted in Mc3rTB/TB compared to Mc3r+/+ mice, even within the first few hours (Fig. 6a, b). In contrast, Mc3rHep/Hep mice normally responded to cold exposure EE compared to Mc3r deficient mice after 6 h at 6 °C (Fig. 6a, b). Corroborative results were found from oxygen consumption measurements. Oxygen consumption (VO2) was significantly reduced in Mc3rTB/TB mice and significantly different from Mc3r+/+ and Mc3rHep/Hep at 6 °C (Fig. 6c, d). These results suggest that hepatic Mc3r is essential for regulating adaptive energy expenditure in response to thermogenic energy demands.
a Energy expenditure (kcal/mouse/h) at 6 °C from Mc3r+/+(n = 15), Mc3rTB/TB (n = 16), and Mc3rHep/Hep (n = 12) independent observations. b EE (kcal/mouse/h) at 6 °C from Mc3r+/+ (n = 15), Mc3rTB/TB (n = 16), and Mc3rHep/Hep (n = 12) independent observations. c Oxygen consumption (VO2) (ml/mouse/h) at 6 °C from Mc3r+/+ (n = 15), Mc3rTB/TB (n = 16), and Mc3rHep/Hep (n = 12) independent observations. d VO2 (ml/mouse/h) at 6 °C from Mc3r+/+ (n = 15), Mc3rTB/TB (n = 16), and Mc3rHep/Hep (n = 12) independent observations. e Oxygen consumption rate (OCR) from cultured primary hepatocytes from Mc3r+/+, Mc3rTB/TB and Mc3rHep/Hep from 3 independent experiments. f Basal respiration OCR (pmol/min/5000 cells) from 3 independent experiments. g Maximal respiration OCR (pmol/min/5000 cells) from 3 independent experiments. h Bulk RNA-Sequencing analysis of mouse liver samples from Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep genotypes, all on a normal chow diet (n = 4) independent observations. Pairwise scatter plot illustrates the Log2 fold change (Log2FC) values for all significantly differentially expressed genes across three genotype comparisons. The Log2FC for Mc3rTB/TB vs Mc3r+/+ is plotted against Mc3rHep/Hep vs Mc3r+/+ (blue-filled circles), and linear regression fit lines are shown for the comparisons between Mc3rTB/TB vs. Mc3r+/+ and Mc3rHep/Hep vs Mc3r+/+ (blue line). i The Log2FC for Mc3rTB/TB vs Mc3r+/+ is plotted against Mc3rHep/Hep vs Mc3rTB/TB (orange-filled circles). Linear regression fit lines are shown for the comparisons between Mc3rTB/TB vs. Mc3r+/+ and Mc3rHep/Hep vs Mc3rTB/TB (orange line), with the black line shows best fit perfect correlation lines included for visualization. j Heatmap depicting significant gene ontology (GO) biological pathways related to lipid droplet autophagy, lipid metabolism, and energy homeostasis. The negative log10 P values for these pathways were compared across the Mc3rTB/TB vs. Mc3r+/+, Mc3rHep/Hep vs Mc3r+/+ and Mc3rHep/Hep vs Mc3rTB/TB comparisons (adjusted p-value ≤ 0.05). k Western blot images of liver lysates from Mc3r+/+, Mc3rTB/TB and Mc3rHep/Hep. l Quantification of LC3II normalized by β-actin in (g) from Mc3r+/+ (n = 12), Mc3rTB/TB (n = 10), and Mc3rHep/Hep (n = 11) independent observations. m Quantification of LC3II normalized by LC3I in (g) from Mc3r+/+(n = 12), Mc3rTB/TB (n = 10), and Mc3rHep/Hep (n = 11) independent observations. Data are represented as mean ± SEM. Groups were compared by one-way ANOVA followed by Fisher’s LSD test (b, d), one-way ANOVA followed by Tukey’s HSD test (f, g), two-tailed simple linear regression (h, i). p < 0.05; ** p < 0.01; *** p < 0.001. Source data are provided as a Source Data file.
To elaborate the mechanism for altered energy expenditure at the hepatocyte level, we cultured primary hepatocytes to study cellular mitochondrial respiration using the Seahorse Mito Stress Test. Compared to Mc3r+/+ and Mc3rHep/Hep group, Mc3rTB/TB cells showed reduced basal level respiration (Fig. 6e). We observed restoration in basal respiration, measured by the oxygen consumption rate, in Mc3rHep/Hep hepatocytes compared to the Mc3rTB/TB group (Fig. 6f). Injection of the mitochondrial uncoupler FCCP allows a measurement of maximum respiration which reflects mitochondrial ability to respond to increased energy demands (Fig. 6e). Maximum respiration was also restored to normal in Mc3rHep/Hep, which was significantly greater than Mc3rTB/TB group and similar to the Mc3r+/+ control group (Fig. 6g). These Seahorse assay results in hepatocytes align with the energy expenditure data observed in the mouse models, further validating the importance of liver Mc3r for energy regulation. These findings strongly support the conclusion that hepatic Mc3r plays a crucial role in adapting to external stimuli, contributing significantly to systemic obesity through its effects on peripheral tissues.
To understand the role of Mc3r using an unbiased approach, bulk RNA-sequencing analysis was performed on liver samples from Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep genotypes. Log2 fold change (Log2FC) values for all significantly differentially expressed genes were plotted in a scatter plot. Linear regression fit line comparisons between Mc3rTB/TB vs. Mc3r+/+ and Mc3rHep/Hep vs Mc3r+/+ (blue line, r = 0.189, p < 0.0001) suggest that Mc3rTB/TB and Mc3rHep/Hep are drastically different (Fig. 6h). Linear regression fit lines for the comparisons between Mc3rTB/TB vs. Mc3r+/+ and Mc3rHep/Hep vs Mc3rTB/TB (orange line, r = 0.726, p < 0.0001) slightly overlapped with the best-fit perfect correlation line, indicate that Mc3r+/+ and Mc3rHep/Hep are largely similar (Fig. 6i). These data suggest that the hepatic changes in differentially expressed genes caused by Mc3r global knockout are largely reversed by the recovery of hepatic Mc3r.
Next, we performed gene ontology (GO) biological pathway analysis for the comparisons between Mc3rTB/TB vs. Mc3r+/+, Mc3rHep/Hep vs Mc3r+/+ and Mc3rHep/Hep vs Mc3rTB/TB. A heatmap of negative adjusted log10 P values shows that the Mc3rHep/Hep and Mc3r+/+ groups had greater similarity (Fig. 6j). In contrast, Mc3rTB/TB shows much lower p-values that are significantly different compared to Mc3r+/+ and Mc3rHep/Hep, particularly in pathways related to lipid droplet autophagy and lipid metabolism (Fig. 6j). These findings reinforce the notion that liver autophagy is a predominant mechanism influencing fat accumulation and lipid partitioning in MC3R deficiency.
In line with a hepatic role for MC3R and the role of autophagy in lipid metabolism3,8, Mc3rHep/Hep mice indeed had a diminution of the augmented hepatic LC3II signals to the level seen in control livers (Fig. 6k, l). The liver ratio of LC3II to LC3I was also normalized in Mc3rHep/Hep mice (Fig. 6m), supporting the restored hepatic LC3 development.
Hepatic MC3R activation and circulating plasma neuropeptides in autophagy regulation
Gamma-melanocortin stimulating hormone (γ-MSH), derived from proopiomelanocortin (POMC), has specific affinity to MC3R over MC4R43. Therefore, we examined if the circulating γ-MSH pool is altered in mice with deficiency for Mc3r. Using an enzyme-linked immunosorbent assay (ELISA) specific to gamma 2 MSH, we found that plasma γ-MSH significantly increased at nighttime (ZT 15) compared to daytime (ZT 3) in control mice (Supplementary Fig. 5a). Mc3rTB/TB mice exhibited impaired plasma γ-MSH regulation during the circadian cycle, although fasting could elevate plasma γ-MSH at ZT15 in wildtype mice (Supplementary Fig. 5a). These data suggested altered circadian control of circulating γ-MSH signaling in Mc3r-insufficient mice. Further, the primary hepatocytes with defective MC3R pathway signaling failed to produce cAMP, compared to the control cells, in response to the MC3R-specific (versus MC4R)43 agonist, [D-Trp8]-γ-MSH (Supplementary Fig. 5b). Thus, our data suggest that Mc3rTB/TB mice have defective MC3R signaling in liver cells, possibly due to loss of both the receptor (Fig. 1b) and ligand functions (Supplementary Fig. 5a).
To examine how activation of the MC3R pathway affects hepatic autophagy, we assessed AP organization in primary hepatocytes that carry transgenic GFP-LC3 with the control or deficient Mc3r after stimulation with [D-Trp8]-γ-MSH. In control cells, [D-Trp8]-γ-MSH significantly increased cytosolic GFP-LC3 structures to a similar extent as seen in the cells exposed to rapamycin, a well-known pharmacological activator of liver autophagy44,45 (Fig. 7a). Using live cell imaging, we found dispersed cytoplasmic GFP-LC3 molecules rapidly develop into multiple vesicles (or tubular structures) at 30 min of [D-Trp8]-γ-MSH treatment (Fig. 7b). These GFP-LC3 vesicles further expanded, eventually fusing to lysotracker red containing subcellular compartments (i.e., lysosomes). Soon after lysosomal fusion, LC3 fluorescent signals became dimmer, possibly due to degradation, recycling, and dispersal of LC3 molecules (Fig. 7b, See Inlay). Thus, MC3R activation by γ-MSH serves as a signal for reorganizing hepatic LC3 in autophagosomes. To confirm the observed results, we have included representative data from time-lapse live cell imaging of primary hepatocytes treated with the MC3R agonist, gamma MSH for both Mc3r-deficient and wild-type mice (Supplementary Fig. 6), again showing greater autophagosome development after hormone stimulation in control mice but not in Mc3rTB/TB.
a Representative fluorescent images of primary hepatocytes from Mc3r+/+ transgenic mice carrying GFP-LC3 under 2 nM D-Trp8-γ-MSH (γ-MSH) or 10 nM rapamycin (Rap) after staining with Lysotracker Red and DAPI. b Time-lapse images of primary hepatocytes from Mc3r+/+ transgenic mice carrying GFP-LC3 under 2 nM γ-MSH after staining with Lysotracker Red. c Representative western blot images of primary hepatocytes from Mc3r+/+ and Mc3rTB/TB after 2 nM γ-MSH treatment. d Representative super resolution microscope images of primary hepatocytes from Mc3r+/+ transgenic mice carrying GFP-LC3 under 100 nM MC3R-specific (versus MC4R) agonist (NDP42) for 60 min after staining with Lysotracker Red compared to control. e Measurements of total area of GFP-LC3 after 60 min of stimulation (d). f Representative western blot images of primary hepatocytes treated with serum starvation (HBSS) for 60 min, 1 µM Torin for 60 min, or 100 nM NDP42 (MC3R-specific agonist versus MC4R) for 30 min. g Quantification of LC3II normalized by β-actin in (f). Groups were analyzed between Control (n = 5), Starvation (n = 3), Torin (n = 5), and NDP42 (n = 5) independent experiments. h Representative western blot images of liver lysates from Mc3r+/+ with intraperitoneal γ-MSH injection. i Quantification of LC3II normalized by β-actin in (h) (n = 10) independent observations. j Representative western blot images of nuclear fractions (top panels) and cytosolic fractions (bottom panels) from primary Mc3r+/+ hepatocytes after 1 h starvation (HBSS) or 2 nM γ-MSH treatment. k qPCR measurement from Mc3r+/+ and Mc3rTB/TB hepatocytes after 2 nM γ-MSH (n ≥ 5 independent observations). l Heatmap of Log10 normalized counts for TFEB target genes and lysosomal genes in mouse liver RNAseq analysis across Mc3r+/+, Mc3rTB/TB, and Mc3rHep/Hep genotypes on a normal chow diet (n = 3) independent observations. Data are represented as mean ± SEM. Groups were compared by paired two-tailed Student’s t-test (e), unpaired two-tailed Student’s t-test (i, k), one-way ANOVA followed by Fisher’s LSD test (g). p < 0.05; ** p < 0.01; *** p < 0.001, Scale bar, 10 µm (a, b, d). Source data are provided as a Source Data file.
Similar to our imaging data, cultured hepatocytes carrying intact Mc3r increased LC3II upon [D-Trp8]-γ-MSH exposure in a time-dependent manner (Fig. 7c). We also confirmed these findings using a synthetic specific ligand for MC3R (NDP-MSH compound-42, KAF4098-32 (sequence Ac-Val-Gln-(pI)DPhe-DTic-NH2))46 thus indicating specific involvement of hepatic MC3R signaling in controlling LC3II production in primary hepatocytes (Fig. 7d–g). Importantly, no such increase of LC3II was evident in either Mc3rTB/TB (Fig. 7c) or MC3RhDM/hDM hepatocytes (Supplementary Fig. 5c), indicating perturbed hepatic autophagy in the absence of intact Mc3r.
Similarly, imaging of MC3RhDM/hDM hepatocytes revealed perturbed GFP-LC3 vesicle development and interaction with lysosomes in response to MC3R agonists (Supplementary Fig. 5d). To further test if in vivo activation of MC3R can modulate hepatic autophagy, liver LC3II was examined in vivo in Mc3r+/+ mice (fed state) after intraperitoneal administration of [D-Trp8]-γ-MSH (Fig. 7h, i and Supplementary Figs. 5e and 7f). Compared to saline injection, γ-MSH significantly increased liver LC3II (Fig. 7i) in mice with intact MC3R. Therefore, both in vitro and in vivo, activation of MC3R appeared to modulate mouse liver autophagy.
TFEB signaling affected by MC3R pathway in hepatic autophagy regulation
Several signaling mechanisms that are key to liver autophagy regulation have been proposed. Inhibition of mechanistic target of rapamycin kinase complex I (MTORC1) or activation of AMP-activated protein kinase (AMPK) can trigger liver autophagy47,48. In addition, for more sustainable autophagy, the mechanism may involve the activation of TFEB, a master regulator of autophagy and lysosome biogenesis, for which nuclear-cytoplasmic shuttling is required for the upregulation of autophagy- and lysosomal-related genes49,50.
To explore potential signaling mechanisms that interplay with MC3R for liver autophagy regulation, we investigated MTORC1 and AMPK signaling as well as nuclear-cytoplasmic TFEB signaling. Using primary hepatocytes from control and Mc3rTB/TB mice, we found that under serum starvation, 4E-BP1 phosphorylation at Thr37/46 (indicative of MTORC1 activation) was clearly inhibited in both control and Mc3r defective hepatocytes (Supplementary Fig. 7a). This suggests a similar degree of autophagy initiation by MTORC1 inhibition occurs in both groups. Serum starvation also did not alter AMPKα phosphorylation (indicative of AMPK activation) in the MC3R defective hepatocytes (Supplementary Fig. 7a), indicating unchanged liver AMPK signaling. However, unlike stimulation of nuclear TFEB localization in the control cells or Mc3rHep/Hep cells, Mc3rTB/TB hepatocytes failed to trigger TFEB’s nuclear delivery during serum starvation (Fig. 7j). Thus, TFEB shuttling to the nucleus could be triggered by [D-Trp8]-γ-MSH in hepatocytes with intact Mc3r, but not in the cells from Mc3rTB/TB mice (Fig. 7j), concurrently there was no change in TFEB protein expression in whole cell protein lysates (Supplementary Fig. 2b). These results suggest a potential interplay between MC3R pathway and TFEB signaling for hepatic autophagy.
Finally, to reveal TFEB activity in the liver, we monitored TFEB-activated downstream genes using primary hepatocytes from control and Mc3rTB/TB mice. Upon treatment with [D-Trp8]-γ-MSH, the transcription of autophagy-related genes and genes for lysosomal homeostasis were significantly upregulated in the hepatocytes with intact Mc3r but were dysregulated in cells with insufficient MC3R activity (Fig. 7k). To further investigate precise candidate mechanisms underlying the hepatic Mc3r reactivation model, we performed DESeq2 analysis on bulk RNA-sequencing data from liver tissue. Notably, TFEB-driven autophagy-related genes, including Mcoln1 (mucolipin 1), Neu1 (neuraminidases), and Sqstm1 (p62 protein), showed increased expression in Mc3rHep/Hep mice compared to Mc3rTB/TB mice (Fig. 7l). Interestingly, lysosomal-associated membrane proteins, Lamp1 and Lamp2 showed expression was elevated in Mc3rTB/TB mice compared to both Mc3r+/+ and Mc3rHep/Hep groups (Fig. 7l). Therefore, our data suggest MC3R-dependent transcriptional activation of TFEB signaling and downstream events in the liver.
Discussion
We provide evidence identifying a previously unknown regulator of hepatocellular autophagy. We show in two mouse models of MC3R insufficiency that autophagic flux is disrupted in a unique manner; we also demonstrate that, when administered in concentrations similar to those that circulate endogenously and that can initiate MC3R-dependent signaling, γ-MSH can activate the hepatic TFEB signaling program. MC3R activation by γ-MSH drives TFEB signaling, leading to the activation of genes related to autophagy or lysosomal homeostasis. A specific MC3R agonist, NDP-42, also activated hepatic LC3II in Mc3r+/+ hepatocytes but did not further activate LC3II in Mc3rTB/TB, which showed blunted autophagosome-lysosome docking and LD-clearance, leading to defective lipid autophagy, thus confirming the role of MC3R pathway in hepatocytes and not through the activation of other receptors. Liver RNA-seq analysis revealed that differentially expressed genes (DEGs) were significantly altered in global knockout mice, while the DEG profile of hepatic reactivation mice was closer to that of the control group. Gene ontology (GO) pathway analysis suggests that the affected pathways are related to lipid droplet autophagy, macroautophagy, and lipid metabolism, indicating that Mc3r deficiency leads to distinct changes compared to either Mc3r reactivation or wild-type mice. In line with this notion, recent studies using thermal proteome profiling revealed that the top reactome enriched after MC3R activation is that of autophagy proteins51. Importantly, liver-specific MC3R reactivation (Mc3rHep/Hep mouse) was sufficient to reestablish the dysregulated TFEB signaling and improve (though not completely restore) systemic lipid metabolism of Mc3r null mice. Thus, the current study elucidates a previously unknown function of hepatic MC3R in regulating body weight in mice. These data do not, however, indicate that the MC3R is involved in the stimulation of hepatic autophagy by fasting or starvation. Indeed, it appears that peripheral ACTH concentrations (and therefore likely peripheral gamma-MSH concentrations) are reduced in mice during prolonged food restriction or fasting52,53.
Mc3r expression in the lateral hypothalamic area and ventromedial hypothalamic area play crucial roles in regulating feeding, rheostasis, body weight maintenance, and metabolism23,25,54,55. Our data confirmed relative hypophagia in transcriptionally blocked global Mc3r deficient mice studied in metabolic chambers (using real-time monitoring feeder hangers)23. We found that the recovery of liver-specific Mc3r did not reverse lowered energy intake (as indicated in Fig. 5a–c), and thus, hepatic re-expression of Mc3r did not restore feeding behavior, supporting the previous finding that Mc3r deficiency is related to appetite regulation by hypothalamic MC3R neurons23. However, total energy expenditure, oxygen consumption and RER were reduced in global Mc3r deficient mice even after adjusting for body weight and total lean plus fat mass. Total energy expenditure measured using indirect calorimetry was lower in Mc3rTB/TB in the mid-dark period and even after adjustment for body weight23. Hepatic Mc3r reactivation improved total energy expenditure, oxygen consumption, and RER. These differences in total energy expenditure, oxygen consumption, and RER, as observed here, may contribute to the less obese body composition of Mc3rHep/Hep. RER is an indicator of oxidizing fatty acids and is significantly lower in Mc3rTB/TB. The increased RER in Mc3rHep/Hep suggests a shift in substance preference. Hepatic reactivation of Mc3r restored lipid recycling and shifted metabolism towards using carbohydrates for fuel. Improvement in energy expenditure-related parameters suggests a potential mechanism to improve body weight, fat mass, and systemic adiposity.
In a similar line, we assessed cellular respiration in primary hepatocytes. Mc3r deficient mice exhibited reduced oxygen consumption, suggesting a reduced metabolic demand and a decrease in their maximal electron transport capacity, as these cells are not fully utilizing their mitochondrial capacity under normal conditions. Notably, in red oxidative muscle isolated from gastrocnemius, Mc3rKO mice were previously shown to have a decrease in fatty acid oxidation and citrate synthase activity, consistent with reduced mitochondrial content14.
Primary hepatocytes isolated from mice exposed to a 60% high-fat diet (HFD) for 12 weeks (who generally develop diabetes) have been reported to have reduced basal respiration as well as reduced ATP-linked respiration (oxidative phosphorylation), and their response to the uncoupler FCCP was also decreased when mitochondrial energy metabolism assessed via extracellular flux analysis56. However, mitochondria isolated from mice with liver steatosis from a 35% high-fat and high-sugar diet for 5 months (who mostly show insulin resistance without diabetes) have normal levels of mitochondrial respiratory chain complex I–V proteins and do not demonstrate adversely impacted mitochondrial respiration57. These data suggest that hepatic mitochondria may initially activate compensatory mechanisms to counteract liver damage associated with severe hepatic steatosis. However, persistent fat accumulation can eventually surpass this adaptive capacity, leading to impaired mitochondrial function due to an overload of free fatty acids58. Indeed, primary human hepatocytes exposed in vitro to FFAs showed suppressed maximal respiration and maximum fatty acids beta-oxidation, further indicating compromised mitochondrial function59. Consequently, reductions in liver mitochondrial State 3 respiration can be found60, particularly in mice with high histological grade MAFLD61. However, there are also rodent models, e.g., the Otsuka Long-Evans Tokushima fatty rat62 and mice with heterozygous inactivation of the mitochondrial trifunctional protein63, where mitochondrial dysfunction is present before insulin resistance and steatosis develops, and is believed to contribute etiologically to the development of MAFLD.
Dietary fats absorbed by the gut are transported to the liver for proper processing, packaging into lipoproteins, and circulation. In the liver, autophagy is the primary mechanism for the catabolism of lipoproteins, although lipolysis also occurs, though to a lesser extent. Reactivation of Mc3r leads to improved lipid droplet autophagy; here, we showed how Mc3r insufficiency affected cellular and molecular mechanisms of autophagy. Due to defective Mc3r-mediated lipid droplet autophagy, hepatocytes do not efficiently metabolize triglycerides. By restoring defective autophagy, we observed reductions in liver triglyceride content and liver weight and partial improvements in eWAT weight. Our results demonstrated the restoration of normal circulating non-esterified fatty acid concentrations, total cholesterol, and triglycerides in Mc3rHep/Hep mice. These suggest that Mc3r reactivation largely restores lipid metabolism in hepatocytes and leads to reduced excess circulating fats that would be stored by extrahepatic tissues (e.g., eWAT). The partial recovery of body weight, fat mass, and total energy expenditure at the systemic level underscores how hepatic Mc3r reactivation can modulate a systemic obesity phenotype.
The restoration of hepatic Mc3r resulted in improvements in adiposity, metabolic dysfunction-associated steatotic liver disease, and insulin sensitivity. Our observations from Mc3rTB/TB and MC3RhDM/hDM livers lead us to hypothesize that the MC3R pathway functions specifically in determining lysosomal-AP interaction and degradation processes, based on the: (1) prevalent AP accumulation with lumenal compartments containing undegraded organellar membranes (Fig. 2a–c and h, i), (2) impaired AP flux and p62 clearance upon serum starvation (Fig. 2f, g and Supplementary Fig. 1c), and (3) increased basal LC3II in both hepatic tissues and isolated hepatocytes (Fig. 2d, e). In addition, phopho-4E-BP1 degradation confirms the LC3II activation and change in MTORC1 might be possible upstream autophagy mediators (Supplementary Fig. 7a). Moreover, the findings of MC3R-dependent nuclear TFEB allocation (Fig. 7j) with subsequent autophagy and lysosomal gene activations (Fig. 7k) further support MC3R’s specific role in the regulation of autophagy.
Given hepatic TFEB’s central importance in lysosomal biogenesis49,64,65,66, the pathogenic outcome of defective MC3R signaling in the liver might have been a result of impaired lysosome development. However, our fluorescent imaging data indicates no apparent defect in lysosome structures in MC3R defective livers (Fig. 2h). In addition, the data obtained from monitoring cathepsin D (a lysosomal aspartyl protease) maturation shows intact lysosomal trafficking and development without MC3R activity in hepatocytes (Supplementary Fig. 7b). Therefore, MC3R likely plays a role in fine-tuning lysosomal activity, rather than driving overall lysosomal biogenesis. Since loss of Mc3r leads to TFEB accumulation in the nucleus while reducing γ-MSH-induced TFEB nuclear localization (Fig. 7j), data shown here that MC3R signaling possibly modulates hepatic lysosome activity through the control of TFEB nuclear import and export activities. We also showed that expression of TFEB downstream target genes for autophagy-related and lysosomal-associated membrane proteins is restored in hepatic Mc3r re-expression. Further research is needed to determine whether TFEB is the sole regulator responsible for mediating autophagy regulation in MC3R deficiency. In the future, more comprehensive research on MC3R-autophagy-TFEB regulation is warranted.
Finally, our findings of Mc3r’s peripheral role in hepatic autophagy opens opportunities in both translational and basic research. Despite the beneficial effect of liver autophagy in preventing human liver metabolic diseases39,67, enhancing hepatocellular autophagy by non-physiological agonists (e.g., rapamycin) or gene manipulation has limited merit due to side effects or limited clinical applicability. Thus, the demand for developing tools for liver autophagy regulation with physiologically approachable and effective means is high, and this makes peripheral modulation of the MC3R a conceivable strategy. In order to delve deeper into the impact of MC3R on hepatic lipid droplet autophagy, it will be necessary to utilize a mouse model with a tissue-specific knockout. Furthermore, the dual roles of MC3R in the neuronal and peripheral tissues may also provide a model system to study biological impacts and mechanisms of the brain-peripheral tissue communication in metabolic regulation.
Taken together, our study provides insight into the role of peripheral MC3R in regulating body weight and adiposity in mice. Given the similar obesogenic mechanism seen in our humanized MC3R mutant mouse model, insufficient peripheral activation of MC3R appears to likely play a part in explaining obesity in humans with MC3R deficiency.
Methods
Materials and reagents
Information on reagents, antibodies, primers are provided in Supplementary Table 2. Datasets are publicly available68.
Ethics statement
This research complies with all relevant ethical regulations. All animal studies were reviewed and approved for the accepted standards of humane animal care under protocols approved by the NICHD Animal Care and Use Committee.
Animals
Studies were performed in males and female C56BL/6 background mice with the following genotypes: Mc3rTB/TB, MC3RhDM/hDM, MC3RhWT/hWT, MC3RHep/Hep, Mc4r+/- and wild-type (BL/6). GFP-LC3 mice that were cross bred with MC3RTB/TB, MC3RhDM/hDM, MC3RhWT/hWT, and WT mice were a gift from Dr. Noboru Mizushima (RIKEN Bio, Japan), who first developed this model in 2008, and the breeding pair was received from Dr. Sergey Leikin of the NICHD, an investigator who currently has a colony of GFP-LC3 mice. Albumin-Cre mice were purchased from Jackson Laboratory (B6.Cg-Speer6-ps1Tg(Alb-cre)21Mgn/J, stock#:003574) and crossed with MC3RTB/TB to generate MC3RHep/Hep (Supplementary Table 3). Mice were housed and maintained on a 14-h light, 10-h dark cycle and studied at 21–25 °C. We monitored body weight from 4 to 24 weeks of age. Mice were fed a regular chow NIH-07 diet containing metabolizable 3.05 kcal/g (Lab Diet, Arden Hills, MN), and tissue samples were collected from mice that were either at 12 or 22 weeks of age. Mice were randomly selected for different groups or different treatments.
Mouse body composition
Body composition was determined using a PIXImus dual-energy X-ray absorptiometer (DEXA, Lunar, Madison, WI). Mice were either euthanized with CO2 or anesthetized with ketamine and xylazine (100/10 mg/kg body weight) by intraperitoneal injection.
Insulin tolerance test
Mice were fasted for 5 h before an i.p. administration of 0.75 U/kg body weight of insulin. The load glucose of each mouse was measured using a human glucometer with blood drops from the tail vein after tail snipping. Basal glucose was determined at the 0 time point, and blood glucose was measured 30, 60, 90, and 120 min after insulin was injected.
Glucose tolerance test
Mice were fasted for 16 h (overnight) prior to glucose tolerance testing. Baseline blood glucose was measured from a tail snip blood droplet collection using a Contour NEXT EZ meter with Contour NEXT Test strips (Ascensia Diabetes, Parsippany, NJ). Mice were then given 2.5 g/kg body weight by intraperitoneal glucose (Sigma-Aldrich, St. Louis, MO, #G7021) injection. Blood glucose measurements were taken every 30 min for 2 h.
Food intake and feeding efficiency monitoring
12 week old mice were single-housed for 15 days, with food weight and body weight measured every 3 days. Total food intake was recorded for the 15 day period and then divided by total change in body weight to determine the feeding efficiency. After monitoring was complete, mice were then returned to their original cagemates.
Energy expenditure, energy intake, and locomotion activity
Total energy expenditure (TEE), energy intake (calculated using metabolizable energy of diet 3.05 kcal/g), oxygen consumption rate (VO2), respiratory exchange ratio (RER), and physical activity (infrared beam breaks as total activity, 1-inch spacing) were measured with an indirect calorimetry system (CLAMS-HC Oxymax v5.52 software, Columbus Instruments) as previously described69. Prior to the metabolic measurements, mice were acclimated in the metabolic chambers at room temperature for 2 days, so the data recorded on the second day were excluded from the daily energy expenditure final calculations. On day 3, metabolic parameters were recorded (260 s intervals) continuously at 22 °C for 24 h. On day 4, the chamber temperature was elevated to 30 °C at 6 am and recorded for additional 24 h (the first hour after temperature change was excluded from analysis). During the second week, mice were acclimated to 22 °C in metabolic chambers for 24 h, followed by cold exposure at 6 °C for 6 h, recorded from morning to noon. Mice were housed individually with ad libitum access to food (hanging feeder) and water in Tecniplast 1284 cages with ~95 g of wood chip bedding (7090 Teklad sani-chips, Envigo, Indianapolis, IN) with measured physical activity (infrared beam breaks as total activity, 1 inch spacing) and continual monitoring of total activity in each cage. Calorimetry parameters are: 7.75 L volume, 0.9 L/min flow rate, 0.6 L/min sampling flow, 15 s settle time, 5 s measure time, with each chamber sampled every 260 s. Thus, the physical activity was measured per 260 s interval, giving 14 sampling cycles per 61 min interval. All 12 calorimetry chambers were housed in a single temperature-controlled environmental chamber.
Liver perfusion and primary hepatocyte culture
Mice were anesthetized at 8 weeks of age for a terminal surgery to collect hepatocytes following hepatic perfusion. The hepatic portal vein and inferior vena cava were exposed by carefully moving the viscera to the right, outside of the abdominal cavity. A 20-gauge catheter was inserted into the inferior vena cava and the perfusion tubing was connected to the needle. Perfusion was initiated at a 4 ml/min flow rate with about 25–30 ml of pre-warmed (37 °C) Liver Perfusion Medium (GIBCO 17701-038). Once successful cannulation was confirmed, the portal vein was cut to allow efflux and the superior vena cava was clamped. The liver became pale in color after 5 to 8 min of perfusion, and the solution was switched to Liver Digest Medium (GIBCO 17703-034), containing type I collagenase for 10 min at a 4 ml/min flow rate. Pressure was applied periodically (5–10 times during the procedure) with a swab to the portal vein for 5-s intervals so that the liver swelled, leading to enhanced hepatic cell dissociation, which in turn increased final yield. After collagenase perfusion, when the liver became porous and spongy in texture, the liver was harvested and placed in a pre-chilled sterile petri dish with Hepatocyte Wash Medium (GIBCO 17704-024) followed by tissue cell culture using Waymouth medium (GIBCO 11200-035) containing 3% FBS, 1% Insulin-Transferrin-Selenium and 1% Penicillin-Streptomycin. Cultured hepatocytes were treated for further assay after 24–48 h.
Seahorse mitochondria stress test
Primary hepatocytes were imaged and de-gassed using the BioTek Cytation 5 Imaging Reader. Seahorse XF Cell Mito Stress Test (Cat#103015-100) was performed with approximately 5000 cells per well using the Agilent Seahorse XF Pro with our optimized concentrations of 1 μM oligomycin, then 1 μM Carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP), then 0.5 μM rotenone and 0.5 μM antimycin A, and finally 2.5 μM Hoechst. Data analysis was performed using Seahorse Wave Desktop Software, which adjusts for differences in cell number within each well studied.
Total protein isolation and protein subcellular fractionation
Protein was isolated from liver tissues or cultured hepatocytes using RIPA buffer containing a proteinase inhibitor. Tissues or cells were sonicated and centrifuged at 13,000 × g for 15 min at 4°. Supernatant was collected for total protein and protein concentrations were measured by BCA assay. For subcellular fractionation, primary hepatocytes were harvested after the treatment and washed with 1×PBS with protease-phosphatase inhibitor cocktail on ice. Hepatocytes were lysed in buffer A [10 mM HEPES buffer (Ph 7.8), 0.34 M sucrose, 10% glycerol, 10 mM KCl, 1.5 mM MgCl2, 1 mM PMSF, protease and phosphatase inhibitors, and 0.1% Triton-X100] by repeated pipetting and kept on ice for 7 min to generate the cytoplasmic proteins were separated from nuclei by centrifuging at 2000 × g for 5 min at 4 °C. The supernatant was collected in separate tubes as a cytosolic fraction and the pellet was washed with buffer A and centrifuged at 1500 × g for 5 min. The resultant nuclear pellet was suspended in nuclear lysis buffer B [50 mM Tris-HCl, pH 7.8, 420 mM NaCl, 0.5% IGEPAL, 0.34 M sucrose, and protease and phosphatase inhibitors]. Nuclei were kept on ice for 30 min and then spun at 16,000 × g for 30 min at 4 °C to separate the nucleoplasm.
Western blot assay
Protein samples were separated using 4–12% NuPAGE gels (Invitrogen, Carlsbad, CA) and the membranes were blotted with antibodies for rabbit polyclonal anti-LC3I/II, rabbit polyclonal anti-p4E-BP, rabbit polyclonal MTORC1, rabbit polyclonal anti-pAMPK, rabbit polyclonal anti-AMPK, mouse monoclonal anti-β-actin, rabbit polyclonal anti-p62, rabbit polyclonal anti-TFEB, rabbit polyclonal anti-H3, rabbit anti-UCP-1, and rabbit anti-vinculin antibodies (Supplementary Table 2). Primary antibodies were diluted to a 1:1000 concentration, and secondary antibodies were diluted to a 1:2500 concentration. Protein levels were quantified by image density scanning using Image J analysis (NIH, Bethesda, MD). The values were adjusted for β-actin expression.
Brown adipose tissue (BAT) immunofluorescence procedure
Standard protocols for immunofluorescence of BAT were followed with some adaptations. Mice were housed at either 22 °C or exposed to 6 °C for 6 h. After temperature exposure, mice were perfused using 1× PBS followed by 4% paraformaldehyde (PFA); then, BAT samples were fixed overnight in 4% PFA at 4 °C. Cryoprotection of BAT was achieved by immersion in 10%, 20%, and 30% (w/v) sucrose overnight, followed by embedding in OCT and freezing using Tissue-Tek Cryo 3 Flex Cryostat for sectioning. For UCP-1 expression detection, 10 μm frozen sections were airdried for 30 min at room temperature. Sections were blocked with 5% horse serum and incubated in a humidified chamber with UCP-1 (1:250) and perilipin (1:500) antibodies overnight at 4 °C (Supplementary Table 2). Following washing steps, anti-rabbit Alexa Fluor 488 (Thermo Fisher Scientific #A1100) and mouse Alexa Fluor 647 (Thermo Fisher Scientific # A21236) secondary antibodies (1:500) were incubated for 30 min. Following washing steps, slides were treated with DAPI solution (300 nM in 0.1% TBS-T) for 3 min at room temperature to visualize cell nuclei and mounted with VectoMount express mounting medium (Vector Laboratories, Inc). Imaging was performed using a fluorescence microscope at 20× magnification.
Tissue Hematoxylin and Eosin staining
At 22 weeks of age, mice were anesthetized with ketamine-xylazine mix and perfused with 1× PBS followed by 4% PFA. After 24 h 4% PFA fixation tissued transfer to 70% ethanol. Paraffin sections of liver and eWAT were prepared on slides at 5–10 µm. Stain with hematoxylin and eosin according to standard protocols (Tissue TEK Prisma Stain K, SKU#6190).
Tissue Oil Red O staining
Frozen sections of liver tissue were prepared on slides at 5–10 µm. The sections were fixed in cold 10% formalin for 10 min followed by rinsing and drying 3 times. The slides were incubated with propylene glycol for 5 min to avoid carrying water into Oil Red O. Slides were stained in a pre-warmed Oil Red O solution for 10 min in 60 °C, then moved to an 85% propylene glycol solution for 5 min. After washing 3 times, the slides were placed in distilled water and covered with a mounting medium. The sections were then examined under the light microscope.
Total RNA preparation and qPCR assay
Total RNA was isolated from liver tissue or cultured hepatocytes and homogenized with Trizol (Invitrogen, Carlsbad, CA) and RNeasy kit (QIAGEN Cat No: 74106). Complementary DNA was synthesized using SuperScript III first-strand (Invitrogen Cat No: 18080051) and quantitative real-time PCR was performed using a 7900HT fast real-time PCR system (Applied Biosystems, Foster City, CA). Primers used for SYBR assay (4367659, Life Technologies, Grand Island, NY) and Taqman PrimeTime® Std qPCR Assays (Integrated DNA Technologies, Inc. Coralville, IA) for fat metabolism genes are listed in the Supplementary Table 2.
Bulk RNA-sequencing from mouse liver
For each animal, RNA-Seq libraries were constructed using TruSeq RNA Library Preparation Kit (Illumina, San Diego, CA) and sequenced on NovaSeq 6000 System (Illumina) generating roughly 60 million clusters each read as 100 bp paired end reads. Reads were trimmed (trimming of adapters and low quality was doing with cutadapt (switches used for cutadapt -a AGATCGGAAGAGCACACGTCTGAACTCCAGTCA -A AGATCGGAAGAGCGTCGTGTAGGGAAAGAGTGT --overlap 6 -q 20 –minimum-length 25) and aligned to GRCm38 (GENCODE release 27) using STAR (v2.7.3a) 2-pass alignment and quantitated using subread feature counts (v1.6.4) against GENCODE v32 gene annotation. Differential expression between four biological replicates for each condition was tested using DESeq2. Within comparisons, mRNAs demonstrating a log2 fold change greater than 0.5 in each direction and adjusted p-value under 0.05 were evaluated for GO enrichment using package clusterProfiler (6).
Serum metabolite measurements
Blood was collected from mice at 22 weeks of age via cardiac puncture immediately after euthanasia, and centrifuged for 10 min at 2000 × g to collect serum. Mouse serum lipids were quantified using enzymatic colorimetric assay kits from Fujifilm Wako Pure Chemical Corporation (Osaka, Japan): Wako Cholesterol E for total cholesterol (TC) and Wako L-Type Triglyceride M for triglycerides (TG). Serum non-esterified free fatty acids (NEFA) levels were measured using the mouse Fujifilm Wako HR Series NEFA-HR (2) (Fujifilm Heathcare American Corporation, Lexington, MA # 991-34891). Serum glycerol levels were measured using the glycerol assay kit (Sigma-Aldrich, St. Louis, MO, #MAK117).
Triglyceride measurement
Small pieces (~ 0.3 g) of liver were taken for triglyceride measurement. A 3:2 mixture of hexane and 2-propanol solvent was added to each sample followed by homogenization with a tissue grinder. The lipid-containing layer was transferred to a new tube. After evaporation, the dried extract was reconstituted with 2-propanol. Triglycerides were measured using the L-type TG M Microtiter Procedure (Wako Diagnostics, Richmond, VA, reagents 461-08992, 461-09092, 464-01601).
Confocal microscopy
Isolated hepatocytes carrying pEGFP-N1-TFEB were cultured in Nunc Lab-Tek 1.5 Chambered 4 well Coverglass (Thermo Fisher Scientific Inc., Waltham, MA) prior to imaging experiments. Cells were treated with chloroquine, rapamycin, [D-Trp8]-γ-MSH or NDP-42 for various times. Live images were acquired during [D-Trp8]-γ-MSH or NDP-42 treatment by using Zeiss LSM 880 Airyscan microscopy (Carl Zeiss Inc., Thornwood, NY). Cells were fixed using 4% PFA in PBS at 37 C for 15 min and incubated with DAPI or LysoTracker (Life Technologies, Grand Island, NY) for 5 min for nucleus or lysosome staining, respectively. Image J Fiji (NIH, Bethesda, MD) was used to process and analyze image data.
Electron microscopy
Mouse livers were immersed and fixed in 2.5% glutaraldehyde made in 0.1 M sodium cacodylate buffer (pH 7.4) for 1 h at room temperature. Tissues were then rinsed in 0.1 M sodium cacodylate buffer. The following processing steps were carried out using the variable wattage Pelco BioWave Pro microwave oven (Ted Pella, Inc., Redding, CA.): post-fixed in 1% osmium tetroxide made in 0.1 M sodium cacodylate buffer, rinsed in double distilled water (DDW), 2% (aq.) uranyl acetate enhancement, DDW rinse, ethanol dehydration series up to 100% ethanol, followed by a Embed-812 resin (Electron Microscopy Sciences, Hatfield, PA.) infiltration series up to 100% resin. The epoxy resin was polymerized for 20 h in an oven set at 60 °C. Ultra-thin sections (90 nm) were prepared on a Leica EM UC7 ultramicrotome. Thin sections were picked up and placed on 200-mesh copper grids (Electron Microscopy Sciences, Hatfield, PA) and post-stained with uranyl acetate and lead citrate. Imaging was accomplished using a JEOL-1400 Transmission Electron Microscope operating at 80 kV and images were acquired on a Gatan UltraScan 1000XP camera with the assistance of the NICHD Microscopy & Imaging Core.
Droplet digital PCR (ddPCR)
Liver tissue was collected from Mc3r+/+ and Mc3rTB/TB mice at ZT2 and ZT12 and RNA was isolated as previously described. RNA (diluted to 70 ng/uL) was DNAse treated and incubated following the iScript gDNA Clear Synthesis Kit protocol with adaptations for ddPCR application. Following DNase treatment, extracted RNA was used as a template to synthesize cDNA using the cDNA Synthesis Kit (Bio Rad: 1725035). DNase-treated RNA samples were treated separately with both iScript reverse transcriptase supermix and No-RT control and reverse transcribed PCR reaction. NRT samples were used to account for background genomic DNA for each replicate. ddPCR was performed on each RT and NRT sample, using beta-actin as a control gene for comparison, according to the ddPCR Supermix for probes (no dUTP) protocol (Bio Rad: #1863024). Probes were obtained from Applied Biosystems for beta-actin (FAM) (Assay ID: Mm02619580_g1) and Mc3r (FAM) (Assay ID: Mm00434876_s1). Droplets were generated using the Automated Droplet Generator (Bio Rad: #1864101) and PCR was performed to amplify DNA within each droplet (1. 94 °C for 10 min, 2. 94 °C for 30 s, 3. 60 °C for 1 min, 4. Step 2–3 repeated 40 times, 5. 98 °C for 10 min, 6. 4 °C hold). The amplified droplets were read using the QX200 Droplet Reader (Bio Rad: #1864003) and analyzed using QuantaSoft to determine Mc3r expression relative to beta-actin expression.
Plasma γ-MSH measurement
Plasma samples were collected using EDTA and apoprotein as an anticoagulant and protease inhibitor from fed or 24 h fasted mice at 9 AM and 9 PM and were acidified by adding equal volume of buffer A (Phoenix Pharmaceuticals Cat. No. RK-BA-1). Samples were then mixed and spun at 17,000 × g for 20 min (4 °C). A C-18 SEP-COLUMN (Phoenix Pharmaceuticals RK-SEPCOL-1) was equilibrated by washing with 1 mL buffer B once (Phoenix Pharmaceuticals RK-BB-1) and 3 mL of buffer A three times. The acidified plasma solution was loaded onto the C-18 SEP-Column then washed with 3 mL buffer A twice before eluting in 750 μL buffer B. The eluted samples were then concentrated by speed vacuum and lyophilized. The samples were resuspended in equal volume buffer A before γ-MSH plasma concentration was measured by ELISA (EK-043-01).
In vivo γ-MSH administration
Singly housed 12-week-old C57BL/6J mice in the fed state were injected intraperitoneally with saline or [D-TRP8] γ-MSH (Phoenix Pharmaceuticals 043-10) at a dosage of 200 μg/kg bodyweight and then sacrificed at 30, 60, 90, or 120 min post-injection. Liver samples were collected, and proteins were freshly prepared. Hepatic expression of LC3I/II was examined via western blot analysis. Primary LC3 antibodies were diluted 1:1000 and β-actin 1:5000. The absolute absorbance of LC3II and β-actin were quantified using Amersham software and the values of LC3II (corrected for β-actin) were used to determine activation of autophagy.
Statistics analysis and Reproducibility
Sample sizes were based on prior animal studies, suggesting meaningful results from 5–20 animals per group. We mentioned exact independent observations in legends and exact p values in data file. We performed three time independent experiments for representative experiments, such as micrographs for tissue histology and western blots. For electron microscopy and super-resolution confocal images, two independent experiments had the analysis for subcellular analysis using multiple images. Data are expressed as mean ± S.E.M. unless otherwise indicated. Throughout the study, *p < 0.05; **p < 0.01; ***p < 0.001. GraphPad Prism 9.2.0 software (GraphPad Software, San Diego, CA) was used for Student’s t-tests (two-tailed), one-way ANOVA followed by Tukey’s HSD test or Fisher’s LSD test, and two-way analysis of variance followed by Tukey’s HSD or Bonferroni/Dunn’s post-hoc test or Friedman’s test by Dunn’s paired analysis. IBM SPSS 27 software (Armonk, New York) was used to perform analysis of covariance for serum data adjusted for fat mass. Energy expenditure data was examined using analysis of covariance (ANCOVA) adjusted for body weight, lean & fat mass, and lean mass, and post hoc tests for estimated marginal means were calculated using SPSS 28.0.2. Differences were considered significant at p = 0.05. Data met assumptions of the statistical tests, including requirements for similar variance across groups.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets analyzed for the current study are deposited at https://doi.org/10.6084/m9.figshare.23900223. RNAseq data are deposited as PRJNA1180604 [https://www.ncbi.nlm.nih.gov/bioproject/1180604]. Source data are provided with this paper.
References
Liu, K. & Czaja, M. J. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 20, 3–11 (2013).
Singh R. Autophagy and regulation of lipid metabolism. In: Sensory and Metabolic Control of Energy Balance (eds Meyerhof W., Beisiegel U., Joost H. G.) Ch. 4, 5–46 (Springer-Verlag Berlin, 2010).
Singh, R. et al. Autophagy regulates lipid metabolism. Nature 458, 1131–1135 (2009).
Lin, C. W. et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J. Hepatol. 58, 993–999 (2013).
Yang, L., Li, P., Fu, S., Calay, E. S. & Hotamisligil, G. S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 11, 467–478 (2010).
Kim, K. H. et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 19, 83–92 (2013).
Wang, K. W. Molecular mechanism of hepatic steatosis: pathophysiological role of autophagy. Expert Rev. Mol. Med. 18, 12 (2016).
Girardet, C., Begriche, K., Ptitsyn, A., Koza, R. A. & Butler, A. A. Unravelling the mysterious roles of melanocortin-3 receptors in metabolic homeostasis and obesity using mouse genetics. Int. J. Obes. Suppl. 4, S37–S44 (2014).
Benoit, S. C. et al. A novel selective melanocortin-4 receptor agonist reduces food intake in rats and mice without producing aversive consequences. J. Neurosci. 20, 3442–3448 (2000).
Butler, A. A. et al. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat. Neurosci. 4, 605–611 (2001).
Lee, Y. S., Poh, L. K. & Loke, K. Y. A novel melanocortin 3 receptor gene (MC3R) mutation associated with severe obesity. J. Clin. Endocrinol. Metab. 87, 1423–1426 (2002).
Li, W. D. et al. Melanocortin 3 receptor (MC3R) gene variants in extremely obese women. Int. J. Obes. Relat. Metab. Disord. 24, 206–210 (2000).
Chen, A. S. et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 26, 97–102 (2000).
Sutton, G. M. et al. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology 147, 2183–2196 (2006).
Butler, A. A. et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141, 3518–3521 (2000).
Aris, I. M. et al. MC3R gene polymorphisms are associated with early childhood adiposity gain and infant appetite in an Asian population. Pediatr. Obes. 11, 450–458 (2016).
Demidowich, A. P., Jun, J. Y. & Yanovski, J. A. Polymorphisms and mutations in the melanocortin-3 receptor and their relation to human obesity. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 2468–2476 (2017).
Feng, N. et al. Co-occurrence of two partially inactivating polymorphisms of MC3R is associated with pediatric-onset obesity. Diabetes 54, 2663–2667 (2005).
Lee, Y. S., Poh, L. K., Kek, B. L. & Loke, K. Y. The role of melanocortin 3 receptor gene in childhood obesity. Diabetes 56, 2622–2630 (2007).
Lembertas, A. V. et al. Identification of an obesity quantitative trait locus on mouse chromosome 2 and evidence of linkage to body fat and insulin on the human homologous region 20q. J. Clin. Invest. 100, 1240–1247 (1997).
Savastano, D. M. et al. Energy intake and energy expenditure among children with polymorphisms of the melanocortin-3 receptor. Am. J. Clin. Nutr. 90, 912–920 (2009).
Lam, B. Y. H. et al. MC3R links nutritional state to childhood growth and the timing of puberty. Nature 599, 436–441 (2021).
Begriche, K. et al. Genetic dissection of the functions of the melanocortin-3 receptor, a seven-transmembrane G-protein-coupled receptor, suggests roles for central and peripheral receptors in energy homeostasis. J. Biol. Chem. 286, 40771–40781 (2011).
Renquist, B. J. et al. Melanocortin-3 receptor regulates the normal fasting response. Proc. Natl. Acad. Sci. USA 109, E1489–E1498 (2012).
Ghamari-Langroudi, M. et al. Regulation of energy rheostasis by the melanocortin-3 receptor. Sci. Adv. 4, eaat0866 (2018).
Zhang, Y. et al. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB J. 19, 1482–1491 (2005).
Lee, B. et al. A mouse model for a partially inactive obesity-associated human MC3R variant. Nat. Commun. 7, 10522 (2016).
Tiwari, S. & Siddiqi, S. A. Intracellular trafficking and secretion of VLDL. Arterioscler Thromb. Vasc. Biol. 32, 1079–1086 (2012).
Pan, M., Liang, Js. J. S., Fisher, E. A. & Ginsberg, H. N. The late addition of core lipids to nascent apolipoprotein B100, resulting in the assembly and secretion of triglyceride-rich lipoproteins, is independent of both microsomal triglyceride transfer protein activity and new triglyceride synthesis. J. Biol. Chem. 277, 4413–4421 (2002).
Minehira, K. et al. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J. Lipid Res. 49, 2038–2044 (2008).
Li, B. et al. A comprehensive mouse transcriptomic BodyMap across 17 tissues by RNA-seq. Sci. Rep. 7, 4200 (2017).
Gunewardena, S. S. et al. Deciphering the developmental dynamics of the mouse liver transcriptome. PLoS ONE 10, e0141220 (2015).
Wang, X. et al. Pharmacological evaluation of melanocortin 2 receptor accessory protein 2 on Axolotl neural melanocortin signaling. Front. Endocrinol. (Lausanne) 13, 820896 (2022).
Wong, A. M. et al. Characterization of the adiponectin promoter + Cre recombinase insertion in the Tg(Adipoq-cre)1Evdr mouse by targeted locus amplification and droplet digital PCR. Adipocyte 10, 21–27 (2021).
Pedersen, R. C., Ling, N. & Brownie, A. C. Immunoreactive gamma-melanotropin in rat pituitary and plasma: a partial characterization. Endocrinology 110, 825–834 (1982).
Ni, X. P., Pearce, D., Butler, A. A., Cone, R. D. & Humphreys, M. H. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J. Clin. Invest. 111, 1251–1258 (2003).
Demidowich, A. P. et al. Associations of the melanocortin 3 receptor C17A + G241A haplotype with body composition and inflammation in African-American adults. Ann. Hum. Genet. 83, 355–360 (2019).
Seo A. Y. et al. AMPK and vacuole-associated Atg14p orchestrate μ-lipophagy for energy production and long-term survival under glucose starvation. Elife 6, e21690 (2017).
Wang, J. H. et al. Autophagy suppresses age-dependent ischemia and reperfusion injury in livers of mice. Gastroenterology 141, 2188–2199.e2186 (2011).
Bjorkoy, G. et al. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 452, 181–197 (2009).
Butler, A. A. & Cone, R. D. The melanocortin receptors: lessons from knockout models. Neuropeptides 36, 77–84 (2002).
Butler, A. A. & Cone, R. D. Knockout studies defining different roles for melanocortin receptors in energy homeostasis. Ann. N. Y Acad. Sci. 994, 240–245 (2003).
Joseph, C. G. et al. gamma(2)-Melanocyte stimulation hormone (gamma(2)-MSH) truncation studies results in the cautionary note that gamma(2)-MSH is not selective for the mouse MC3R over the mouse MC5R. Peptides 31, 2304–2313 (2010).
Harada, M., Hanada, S., Toivola, D. M., Ghori, N. & Omary, M. B. Autophagy activation by rapamycin eliminates mouse Mallory-Denk bodies and blocks their proteasome inhibitor-mediated formation. Hepatology 47, 2026–2035 (2008).
Zhou, W. & Ye, S. Rapamycin improves insulin resistance and hepatic steatosis in type 2 diabetes rats through activation of autophagy. Cell Biol. Int. 42, 1282–1291 (2018).
Fleming, K. A. et al. Discovery of polypharmacological melanocortin-3 and -4 receptor probes and identification of a 100-Fold selective nM MC3R agonist versus a μM MC4R partial agonist. J. Med. Chem. 62, 2738–2749 (2019).
Han, J. B. & Wang, Y. G. mTORC1 signaling in hepatic lipid metabolism. Protein Cell 9, 145–151 (2018).
Fullerton, M. D. AMP-activated protein kinase and its multifaceted regulation of hepatic metabolism. Curr. Opin. Lipidol. 27, 172–180 (2016).
Settembre, C. & Ballabio, A. Lysosome: regulator of lipid degradation pathways. Trends Cell Biol. 24, 743–750 (2014).
Pastore, N. et al. TFE3 regulates whole-body energy metabolism in cooperation with TFEB. Embo Mol. Med. 9, 605–621 (2017).
Sandbaumhuter, F. A., Nezhyva, M., Andren, P. E. & Jansson, E. T. Label-free quantitative thermal proteome profiling reveals target transcription factors with activities modulated by MC3R signaling. Anal. Chem. 95, 15400–15408 (2023).
Han, E. S., Evans, T. R. & Nelson, J. F. Adrenocortical responsiveness to adrenocorticotropic hormone is enhanced in chronically food-restricted rats. J. Nutr. 128, 1415–1420 (1998).
Lee, M. et al. Effects of selective modulation of the central melanocortin-3-receptor on food intake and hypothalamic POMC expression. Peptides 29, 440–447 (2008).
Dunigan, A. I., Swanson, A. M., Olson, D. P. & Roseberry, A. G. Whole-brain efferent and afferent connectivity of mouse ventral tegmental area melanocortin-3 receptor neurons. J. Comp. Neurol. 529, 1157–1183 (2021).
Pei, H. et al. Lateral hypothalamic Mc3R-expressing neurons modulate locomotor activity, energy expenditure, and adiposity in male mice. Endocrinology 160, 343–358 (2019).
Liu, Z. et al. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS ONE 10, e0128274 (2015).
Franko, A. et al. Liver adapts mitochondrial function to insulin resistant and diabetic states in mice. J. Hepatol. 60, 816–823 (2014).
Takeichi, Y. et al. Non-alcoholic fatty liver disease in mice with hepatocyte-specific deletion of mitochondrial fission factor. Diabetologia 64, 2092–2107 (2021).
Kwon Y. et al. Induction of steatosis in primary human hepatocytes recapitulates key pathophysiological aspects of metabolic dysfunction-associated steatotic liver disease. J. Hepatol. 82, 18–27 (2025).
Mantena, S. K. et al. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem. J. 417, 183–193 (2009).
Zhao, Q. Y. et al. Assessment of mitochondrial function in metabolic dysfunction-associated fatty liver disease using obese mouse models. Zool. Res 41, 539–551 (2020).
Rector, R. S. et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J. Hepatol. 52, 727–736 (2010).
Ibdah, J. A. et al. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology 128, 1381–1390 (2005).
Settembre, C. et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol. 15, 647–658 (2013).
Napolitano, G. et al. mTOR-dependent phosphorylation controls TFEB nuclear export. Nat. Commun. 9, 3312 (2018).
Zhang, H. et al. Dynamic MTORC1-TFEB feedback signaling regulates hepatic autophagy, steatosis and liver injury in long-term nutrient oversupply. Autophagy 14, 1779–1795 (2018).
Amir, M. & Czaja, M. J. Autophagy in nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 5, 159–166 (2011).
Patel, T. et al. Melanocortin 3 receptor regulates hepatic autophagy and systemic adiposity. figshare. Dataset. https://doi.org/10.6084/m9.figshare.23900223 (2024).
Skop, V. et al. Mouse thermoregulation: introducing the concept of the thermoneutral point. Cell Rep. 31, 107501 (2020).
Acknowledgements
We thank Drs. Noboru Mizushima (RIKEN Bio http://www.brc.riken.jp/lab/animal/en/dist.shtml) and Sergey Leikin (Cell Biology and Metabolism Program, NICHD) for the kind gift of GFP-LC3 transgenic mice. We thank Dr. Jennifer Lippincott-Schwartz (Janelia Research Campus) for providing resources for imaging analysis. We also thank the NICHD Microscopy and Imaging Core for their assistance with performing transmission electron microscopy. We acknowledge the NIDDK Mouse Metabolism Core for their assistance performing mouse indirect calorimetric studies and serum-free fatty acid assay. We thank Oksana Gavrilova for review and help in interpreting energy expenditure results. We acknowledge the Molecular Genomics Core at NICHD for Bulk RNAseq experiments. We gratefully acknowledge the assistance of the NICHD animal care facility for the research support of animal breeding and housing. This research was supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), grant ZIAHD00641 (to J.A.Y.) with supplemental funding from an NICHD Early Career Investigator Award (to T.P.P.), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant 1R01DK124504 (to C.H.L.) with supplemental support from an NIH Bench-to-Bedside award (to J.A.Y.) made possible by the NIH Office of Clinical Research. The NICHD and co-funders had no role in the design and conduct of the study, collection, management, analysis, or interpretation of the data, preparation of the manuscript for publication, or decision to submit the manuscript for publication. NICHD did review the manuscript and approve its submission. T.P.P, J.Y.J., A.Y.S., and J.A.Y. had access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the National Institutes of Health or the US Department of Health and Human Services.
Funding
Open access funding provided by the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
T.P.P., J.Y.J., A.Y.S. and J.A.Y. designed, carried out and interpreted the experiments. T.P.P., J.Y.J., A.Y.S. and J.A.Y. wrote the manuscript. T.P.P, A.Y.S. and J.A.Y. prepared figures. T.P.P., N.J.L. carried out the liver-specific recovery study, and T.P.P. performed the cell fractionation and cell biology experiments. A.M.W., D.E., J.C., N.T., E.K.A., D.B.G., S.M.J. and P.P. helped with cell and animal experiments. T.C.D. acquired energy expenditure data. M.E. and C.H.L. synthesized MC3R specific agonist, NDP-42 compound. A.C. helped perform RNAseq analysis. A.W. performed serum lipid measurements. All authors provided critical review of the manuscript and gave final approval of the submitted version.
Corresponding author
Ethics declarations
Competing interests
J.A.Y. receives grant support unrelated to this article for pharmacotherapy trials for human obesity from Hikma Pharmaceuticals, Inc., Soleno Therapeutics, Inc., and Rhythm Pharmaceuticals, Inc., and reagents (anti-activin receptor antibodies) from Versanis Bio for mouse studies. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jong-Woo Sohn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patel, T.P., Jun, J.Y., Seo, A.Y. et al. Melanocortin 3 receptor regulates hepatic autophagy and systemic adiposity. Nat Commun 16, 1690 (2025). https://doi.org/10.1038/s41467-025-56936-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56936-1
This article is cited by
-
Effect of a partial loss of function MC3R mutation on MC3R+GHSR-1A heterodimer activity and RNF11 regulation
Journal of Endocrinological Investigation (2025)