Abstract
Peptidoglycan recognition proteins (PGLYRPs) are implicated in the control of the intestinal microbiota; however, molecular requirements for peptidoglycan (PGN) binding and receptor signaling mechanisms remain poorly understood. Here we show that PGLYRP1 is a receptor for the disaccharide motif of lysine N-acetylglucosamine N-acetylmuramic tripeptide (GMTriP-K). PGLYRP1 is required for innate immune activation by GMTriP-K but not muramyl dipeptide (MDP). In macrophages, intracellular PGLYRP1 complexes with NOD2 and GEF-H1, both of which are required for GMTriP-K-regulated gene expression. PGLYRP1 localizes to the endoplasmic reticulum and interacts at the Golgi with NOD2 upon GMTriP-K stimulation. PGLYRP1 and dependent gene expression signatures are induced in both mouse intestinal inflammation and human ulcerative colitis. Importantly, PGLYRP1 activation by GMTriP-K can result in the protection of mice from TNBS-induced colitis. Mammalian PGLYRPs can function as intracellular pattern recognition receptors for the control of host defense responses in the intestine.
Similar content being viewed by others
Introduction
The innate immune system recognizes molecular patterns of microorganisms to trigger protective immune responses and genetic variants that impair immune recognition of peptidoglycans (PGNs) have been linked to inflammatory bowel disease1. Peptidoglycan recognition proteins (PGRPs) play an important role in innate immunity by recognizing PGN and they are evolutionarily conserved pattern recognition receptors in both invertebrates and vertebrates2,3. Humans and mice have four PGRPs, namely PGLYRP1, PGLYRP2, PGLYRP3, and PGLYRP42,3. PGLYRPs, 1, 3, and 4 are bactericidal and PGLYRP2 has amidase activity when secreted4. How PGRPs modulate immune function is incompletely understood5,6 and no direct signaling mechanism have been established for vertebrate PGRPs compared to invertebrate PGRPs7,8. Instead, NOD-like receptors (NLRs) NOD19 and NOD210,11 have been linked to the recognition of PGN fragments and were found to activate innate immune responses through mechanisms that require guanine nucleotide exchange factor (GEF)-H1 for signaling12,13,14.
The PGN core repeating unit includes a disaccharide unit (GlcNAc-MurNAc) with short peptides linked to the lactyl group of the muramic acid (Fig. 1a). PGNs incorporates diversified elements across species, including glycan modifications, use of rare amino acids and variable cross-linking chemistries connecting adjacent peptide chains15. Furthermore, PGNs can be broken down by host/bacteria lysozymes and transglucosylases and the resulting PGN repertoire diversity poses challenges for immune recognition requiring specific receptors to initiate the host defenses16. To examine the role of PGN chemical structure on immune activation, we established synthetic strategies for PGN fragments with a variety of pathogenic relevant features17. These studies identified N-acetylglucosamine N-acetylmuramic tripeptide (GMTriP-K) as a unique immune effector inducing host defense responses distinct from the model compound muramyl dipeptide (MDP)17. However, it remains unclear how GMTriP-K and MDP, which differ from each other in one sugar unit (GlcNAc) and one peptide (lysine), could induce such distinct immune responses. We hypothesized that additional PGN receptors may be involved in specifying immune responses to these diverse PGN derivatives. To identify such PGN host receptor(s) and determine their binding specificities, we developed a small-fragment microarray incorporating a wide variety of existing and novel glycan and peptide chain modifications into the PGN backbone. Here, we show that PGLYRP1 functions as an intracellular receptor for the disaccharide motif of GMTriP-K and is responsible for regulating transcriptional responses in macrophages that can control intestinal inflammation.
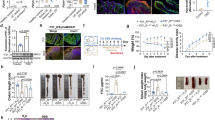
a Peptidoglycan fragment library: GMTriP-K (1) and MDP (2); synthetic peptidoglycan fragments (1a-b, 2a-h and 3a-d) were all prepared with amine linkage points for attachment to the array surface via NHS-chemistry. b General workflow for the printing, incubation, and analysis of the PGN small fragment microarray (Created in BioRender. Putnik, R. (2025) https://BioRender.com/x50o125). c Microarray binding of PGLYRPs. PGLYRP1 binds to the disaccharide components of the array, whereas PGLYRP3 and PGLYRP4 show no association with the compounds on the array. d Determination of Apparent Dissociation Constant for PGLYRP1 to GMTriP-K (3 independent experiments were carried out with each experiment done in technical replicates). e Alphafold protein structure prediction of human PGLYRP1. f–i Docking prediction of GMTriP-K to human PGLYRP1. j Docking-prediction of PGLYRP3 and GMTriP-K demonstrating shallow GlcNAc preventing interaction. For synthetic procedures and compound characterization (NMRs, HRMS), please see the SI. For (c), each condition was screened in at least biological triplicate and technical replicated (see SI for raw image files and other biologically replicate binding data). Data are presented as mean ± SEM. All experiments were repeated twice and yielded consistent results.
Results
PGLYRP1 binds the disaccharide motif of GMTriP-K
GMTriP-K represents the main disaccharide fragment generated via lysozyme digestion of the cell wall17 and 1,6-anhydro disaccharide is the major lytic product produced by bacteria during bacterial growth and division18. To identify receptors mediating the recognition of GMTriP-K, we synthesized an expanded library of PGN derivatives and printed them on a microarray surface using NHS-activation chemistry (Fig. 1a, b). The dynamic assay surface was optimized for downstream screening applications and contained biologically relevant modifications of mono- and disaccharides in different orientations, positioning around the carbohydrate core and extending peptides. As the focus of this study was disaccharide fragments, GMTriP-K was immobilized to the array surface through the free amine on the extending lysine sidechain, exposing the GlcNAc-MurNAc disaccharides. In addition, the canonical 1,6-anhydro disaccharide motif found in the pertussis-derived tracheal cytotoxin was included with an ornithine modification on the third peptide19. Prior to use, the array was fully validated using a variety of antibodies and lectins (see Supplementary Data File 1 for a full list of array components; Figs. S8–10 and supporting information for a complete description of the validation methods).
The validated PGN array was then used to evaluate the binding preferences of three bactericidal PGLYRPs: PGLYRP1, PGLYRP3, and PGLYRP4 (Fig. 1c). When PGLYRP1 (10 μg/mL) was applied to the microarray, the data showed that it bound to GMTriP-K and anhydro-containing disaccharide tripeptides (Figs. 1c and S9, 10). However, no binding was observed for monosaccharide-containing units (Fig. 1c). When a concentration gradient of PGLYRP1 was applied to the array (1–200 μg/mL), affinity to the disaccharide fragments was determined as low as 2 µg/mL, suggesting that the receptor is a µM binder for GMTriP-K (Fig. 1d). Fitting these data using a saturation binding experiment, the apparent kDa for GMTriP-K to bind PGLYRP1 was estimated to be 5.7–42.2 μM (Fig. 1d, Supplementary text). Furthermore, no binding was observed when PGLYRP3 and PGLYRP4 were applied to the array at these concentrations (1–200 μg/mL) (Fig. 1c). It should be noted that the array presented PGN fragments in multiple orientations, with some fragments anchored to the peptide (compounds 1a-c, 2g–h, and 3a-d), and others anchored to the carbohydrate (2a-f) (Supplementary Data File 1). However, PGLYRP1 was only bound to the components of the array when the disaccharide moiety was present and exposed, suggesting that PGLYRP1 binds preferentially to disaccharide moieties over the monosaccharide-only residues.
We next modeled the binding of human PGLYRP1 (Fig. 1e) with GMTriP-K. The minimized structure of GMTriP-K was modeled after the known crystal structure of PGLYRP3 complexed with a MTP ligand (pdb code 1TWQ) as PGLYRP3 is 44% identical and 68% similar to PGLYRP1. Using these coordinates, the GMTriP-K was docked using AutoDock Vina and minimized into a model of PGLYRP1 (obtained from AlphaFold) using the Webina and YASARA servers20. The full GMTriP-K ligand was constructed using the programs PyMOL and ChemDraw3D. The final modeled structure aligned well with our experimental data for PGLYRP1 ligand interactions. We found the lysine solvent exposed multiple hydrogen bonds both in the peptide and the MurNAc portion of the fragment, and a perfectly sized binding pocket for the GlcNAc, permitting many van der Waals interactions (Fig. 1f–h). Particular attention was given to the stereochemistry of the reducing end of the disaccharide, assuring that the hydroxy group was in the minimal beta confirmation (Fig. 1f–h). PGLYRP1 binding was prevented when the alternative chirality at the C1 position was used, removing a hydrogen bond and rotating the neighboring GlcNAc residue backwards into a sterically crowded region of the protein (Fig. 1i). Alternatively, in PGLYRP3 models, the pocket for the GlcNAc was too shallow to dock GMTriP-K (Fig. 1j), agreeing with the array data, which did not yield a GMTriP-K-PGLYRP3 interaction. Together, the PGN fragment array data and ligand receptor modeling analysis indicated that PGLYRP1 can specifically bind to the disaccharide units of GMTriP-K.
PGLYRP1 is required for GMTriP-K regulated gene expression
To determine whether PGLYRP1 is required for the induction of innate immune responses by GMTriP-K, we performed RNA-seq analysis of wild type and PGLYRP1 deficient bone marrow-derived macrophages (BMDMs). GMTriP-K stimulation regulated significantly 3435 (p < 0.05) (Supplementary Data File 2) genes in wild type macrophages and 2500 (p < 0.05) genes in Pglyrp1-/- macrophages after 18-h stimulation (Supplementary Data File 3). Compared to wild type macrophages, Pglyrp1-/- macrophages expressed significantly less GMTriP-K induced immune regulatory genes such as Il1a, Il1b, Il12b, Saa3, Cx3cr1, Ccl2 (Fig. 2a, Supplementary Data File 4) indicating that PGLYRP1 was required for the transcriptomic response to GMTriP-K. Remarkably, compared to wild type BMDMs, the expression of same gene signature including Il1a, Il1b, Saa3 and Il12b was also significantly reduced in Nod2-/- macrophages upon GMTriP-K stimulation, indicating that PGLYRP1 might function as a GMTriP-K receptor in the NOD2 pathway (Fig. 2b, Supplementary Data File 5).
a RNA-seq and volcano plot analysis of gene expression in BMDMs from wild type and Pglyrp1-/- mice after 18 h of stimulation with 25 µM GMTriP-K. b Volcano plot analysis of gene expression in BMDMs from wild type and NOD2-/- mice after 18 h of stimulation with 25 µM GMTriP-K. c Volcano plot analysis of gene expression in BMDMs from wild type mice after 18 h of stimulation with 25 µM GMTriP-K or 25 µM MDP. d Hierarchal cluster analysis of genes induced by either stimulus in wild type or Pglyrp1-/- BMDMs. e Hierarchical cluster analysis of genes induced by GMTriP-K in BMDMs from wild type, Pglyrp1-/-, Nod2-/-, or Arhgef2-/- mice. f qPCR analysis of gene expression in BMDMs from indicated mouse strains stimulated with 25 µM GMTriP-K or 25 µM GMDiP stimulation on BMDMs from wild type and Pylyrp1-/- mice for 18 h (n = 3). g, h Gene set enrichment analysis of the differentially expressed genes induced by indicated stimulus in wild type BMDM. g Top 25 enriched signaling pathways for the differentially expressed gene sets. h Gene set enrichment analysis summarized in mountain plots representing significantly enriched (left) or depleted (right) of genes for the indicated gene sets and collections. Data are presented as mean ± SEM with indicated P values analyzed by one-way ANOVA. All experiments were repeated twice and yielded consistent results.
We next determined whether GMTriP-K induced immune responses differed from those induced by MDP in wild type, Pglyrp1-/- Nod2-/- and Arhgef2-/- macrophages. We found that GMTriP-K induced a different gene expression signature with significantly higher expression of inflammatory genes such as Cxcl2, Il1a, and Il1b compared to MDP in wild type macrophages (Fig. 2c). To better understand PGLYRP1-dependent gene regulation, we performed hierarchical clustering of differentially regulated genes (4-fold difference) by GMTriP-K or MDP in wild type and Pglyrp1-/- macrophages (Fig. 2d). We found that PGLYRP1 was required for GMTriP-K but not MDP-induced gene expression (Fig. 2d, Supplementary Data File 6). Three main clusters emerged from this analysis indicating that PGLYRP1 was required for gene induction by GMTriP-K. Cluster 1 represented genes, such as Il1a, Il12a, Cd14, Stk40, Itga1, and Foxp4, which depended on PGLYRP1 expression and were specifically induced by GMTriP-K but not by MDP (Fig. 2d). Cluster 2 contained genes, such as Ifnb, Acod1, Cxcl16, Nos2, and Gbp5, whose expression was increased in PGLYRP1-deficient compared to wild type macrophages in response to MDP. Cluster 3 contained genes associated with inflammation, such as Il6, Il1b, Tnf, Relb, and Il12b, which required PGLYRP1, and were induced at significantly higher levels with GMTriP-K stimulation compared to MDP (Fig. 2d).
We next determined whether PGLYRP1 dependent proinflammatory gene expression required NOD2 and GEF-H1. Among genes uniquely induced by GMTriP-K (vs. MDP) in wild type BMDMs, we selected genes with a z-score >2 (Supplementary Data File 7). This gene list was used for hierarchical clustering in stimulated wild type and PGLYRP1-, NOD2-, and GEF-H1-deficient BMDMs (Fig. 2e). This approach revealed that the GMTriP-K-induced gene expression required PGLYRP1, NOD2 and GEF-H1 for the induction of inflammatory regulators such as Tnf, Il6, Il1a, Il1b, Il12a, and Il12b (Fig. 2e). We also confirmed in independent experiments that the induction of key immune and metabolic regulators, such as Il6, Il12b, Cxcl3, Cxcl10, Acod1 and Isg15, by GMTriP-K required both PGLYRP1 and NOD2 (Fig. 2f).
Functional enrichment analysis confirmed that GMTriP-K induced immune responses were significantly different from those induced by MDP. GMTriP-K-induced signatures were significantly enriched for “positive regulation of cytokine production” and “response to molecules of bacterial origin” (Fig. 2g). In contrast, MDP responses were associated with type I interferon responses, as indicated by the “response to interferon beta” and “response to virus” association (Fig. 2g). Enrichment analysis showed a correlation between GMTriP-K responses and the regulation of an inflammatory response, while the MDP response was best characterized as a response to IFN-β (Fig. 2h).
To better understand MDP specific transcriptional response in PGLYRP1 deficient macrophages, we selected genes with a z-score >2 preferentially induced by MDP (vs. GMTriP-K) in wild type BMDMs (Supplementary Data File 8). MDP responses were indeed characterized by a type I interferon signature that included members of the GBP and SLFN gene families, Oasl1/2, Rsad1, Cxcl10, Isg15, Irf7, Stat2, and Zbp1 (Fig. 3a). Hierarchical clustering revealed that MDP-induced gene expression was independent of PGLYRP1 but required NOD2 and GEF-H1 (Fig. 3a). Surprisingly, MDP-induced gene expression increased significantly in the absence of PGLYRP1 compared to wild type BMDMs (Fig. 3a).
a Hierarchal clustering of MDP induced genes in BMDMs from wild-type, Pglyrp1-/-, Nod2-/-, or Arhgef2-/- mice. b, c BMDMs from wild type, Pglyrp1-/, Nod2-/-, or Arhgef2-/- mice were stimulated with 25 μM GMTriP-K or 25 μM MDP and analyzed via western blotting with the indicated antibodies. d BMDMs from wild type or Ifnar1-/- mice were stimulated with 25 μM GMTriP-K or 25 μM MDP for 18 h and analyzed for STAT1 pathway activation with phospho-specific antibodies as indicated. e Wild type or GEF-H1-deficient BMDMs were treated with 100 ng/mL of IFNγ or IFNβ for 18 h and analyzed via western blotting with the indicated antibodies. f BMDMs from wild type, Pglyrp1-/, and Nod2-/- mice were stimulated with 25 μM GMTriP-K or 25 μM MDP, lysed, and analyzed via western blotting with the indicated antibodies. g qPCR analysis of gene expression in BMDMs from indicated mouse strains stimulated with 25 µM GMTriP-K or 25 µM GMDiP for 18 h. Data are presented as mean ± SEM with indicated P values analyzed by one-way ANOVA. All experiments were repeated twice and yielded consistent results.
We thus investigated type I interferon receptor activation after GMTriP-K and MDP stimulation. We found that GMTriP-K as well as MDP induced activation of STAT1 (Fig. 3b). However, PGLYRP1 was specifically required for the induction of STAT1 phosphorylation by GMTriP-K, but not by MDP (Fig. 3b). In the absence of PGLYRP1 we noted an increased phosphorylation of STAT1 by MDP (Fig. 3b). GMTriP-K and MDP required NOD2 and GEF-H1 for STAT1 activation (Fig. 3b, c). This was dependent on the induction of type I interferons because Ifnar1-/- mice did not respond with STAT1 activation when stimulation with either GMTriP-K or MDP (Fig. 3d). GEF-H1 was required for GMTriP-K or MDP-induced type I interferon induction but not for IFN receptor signaling, as IFNβ or IFNγ mediated STAT1 phosphorylation was absent in Arhgef2-/- deficient macrophages (Fig. 3e).
We also confirmed that the regulation of PGLYRP1 and NOD2 dependent mRNA expression corresponded to protein expression in macrophages. The induction of IL1β, OASL1, ZBP-1 and STAT2 by GMTri-P-K was dependent on PGLYRP1 and NOD2 (Fig. 3f). In contrast, MDP induced IL1β, OASL1, ZBP-1 and STAT2 expression in PGLYRP1- but not NOD2-deficient macrophages (Fig. 3f).
To confirm the specificity and requirement of PGLYRP1 for specific GMTriP-K induced signaling, we also stimulated macrophages with N-acetylglucosamine N-acetylmuramic dipeptide (GMDiP). Indeed, PGLYRP1 was not required for the induction of Il1a, Il6, Il12b, and Cxcl10 mRNA expression by GMDiP, consistent with the inability of GMDiP to bind to PGLYRP1 in our microarrays (Fig. 3g).
Phosphorylation of muramyl peptides by N-acetylglucosamine kinase (NAGK) is required for NOD2 activation21; therefore, we assessed if NAGK had a role in GMTriP-K activation. We found that NAGK did not phosphorylate disaccharides but was able to phosphorylate monosaccharide controls (Fig.S11, Supplementary Information 8).
Finally, to determine the contribution of PGLYRP1 to immune signaling in response to complete PGNs we stimulated wild type, Pglyrp1-/- or Nod2-/- macrophages with heat-inactivated Listeria monocytogenes, a Gram-positive bacteria that does not contain LPS. We found that the induction of mRNA expression of key regulators like Il1β Il12β, Il6, Cxcl3, and Acod1 was significantly reduced in PGLYRP1 and NOD2 deficient macrophages compared to wild type macrophages 6 h after stimulation (Supplementary Fig. 12). The response to heat-inactivated Listeria monocytogenes was most significantly reduced in Nod2-/- macrophages (Supplementary Fig. 12).
In summary, the gene expression and signaling analyses identified PGLYRP1 as a specific receptor for GMTriP-K and GMTriP-K mediated transcriptional responses required the expression of PGLYRP1, NOD2 and GEF-H1 in macrophages.
PGLYRP1 is expressed in the ER and Golgi apparatus
We next aimed to identify the subcellular localization of PGLYRP1 and associated signaling components. Surprisingly, PGLYRP1 colocalized with SEC61β in the endoplasmic reticulum (ER) throughout the cell (Fig. 4a–c). SEC61β is a subunit of the SEC61 protein translocon complex expressed in the ER and Golgi. PGLYRP1 was also identified in Golgi-associated structures near the cell nucleus positive for the cis-Golgi matrix protein 130 (GM130/GOLGA2) (Fig. 4a, b, d). In addition, PGLYRP1 was localized to a vesicular compartment but not to cell surface (Fig. 4a–c). We analyzed the colocalization of PGLYRP1 with ER and Golgi markers by confocal microscopy in 10 cells with one representative analysis shown (Fig. 4e–k). Analysis of the co-localization found a significant overlap between PGLYRP1 and the ER/Golgi markers in both the microscopy images and in volume analysis (Fig. 4i–k). 59.44 ± 3.59% (Mean ± SEM%) of the detected PGLYRP1 overlapped with 53.99 ± 4.73% of detected SEC61β in the cells with a mean Pearson’s coefficient of 0.779. 12.95 ± 2.74% of the PGLYRP1-positive compartment colocalized with 20.31 ± 3.66% of the GM130-positive compartment with a mean Pearson’s coefficient of 0.45 (Fig. 4e–k, Tab. S1). We also generated colocalization channels based on volume analysis to better visualize the ER- and Golgi-associated subcellular compartments that contain PGLYRP1 (Fig. 4i–k).
a–k Representative confocal microscopy images of HEK 293T cells expressing untagged (UT) or Flag-tagged PGLYRP1, orange fluorescent protein (OFP)-tagged SEC61β and GFP-tagged NOD2. Cells were transfected with PGLYRP1-UT (a–d) or PGLYRP1-Flag (e–k) and SEC61β-OFP and then probed with PGLYRP1 and GM130 antibodies. PGLYRP1, SEC61β-OFP, and GM130 were detected in Alexa Fluor 488 (green pseudo-color), mOrange (red), and Alexa Fluor 647 (blue) channels, respectively. l–y Cells were transfected with PGLYRP1-UT and NOD2-GFP and stained for PGLYRP1 and GM130. NOD2-GFP, PGLYRP1, and GM130 were detected in EGFP (green pseudo-color), Rhodamine Red-X (red), and Alexa Fluor 647 (blue) channels, respectively. All channels were scanned sequentially, and images are pseudo-colored independent of channel wavelengths with nuclear counterstain in greyscale (Scale bars indicate 2 μm in (a–d, l–r) and 5 μm in (e–k, s–y)). Typically, 10-12 cells were analyzed for each condition. Data are presented as mean ± SEM Pearson’s coefficients were calculated in Imaris 10.1.1 image analysis software. All experiments were repeated twice and yielded consistent results.
We next determined the colocalization of NOD2 with cellular compartments that contained PGLYRP1 or GM130 (Fig. 4l–r). In these experiments, we expressed untagged PGLYRP1 and GFP-tagged NOD2 in HEK 293 T cells and stained for PGLYRP1 and GM130 (Fig. 4l–r). NOD2 was found to be expressed throughout the cytoplasm but enriched around the Golgi (Fig. 4l–o). Confocal image and volume analysis showed significant overlap of the aggregation of NOD2 with PGLYRP1 in a GM130-positive compartment close to the nucleus (Fig. 4l–o). We found that 17.99 ± 2.38% of NOD2 overlapped with 51.06 ± 5.34% of the PGLYRP1-positive compartment with a mean Pearson’s coefficient of 0.5 (n = 12) (Fig. 4m, Tab. S2). In contrast, 2.15 ± 0.31% of NOD2 co-localized with 46.36 ± 5.98% of the GM130-positive compartment with a mean Pearson’s coefficient of 0.21 (Fig. 4s–y). The colocalization channels based on the confocal volume analysis for PGLYRP1, NOD2 and GM130 showed that PGLYRP1 and NOD2 enriched at the GM130-positive Golgi compartment (Fig. 4w–y). Together, the image analyses indicate that PGLYRP1 is expressed throughout the ER and colocalizes with NOD2 at a Golgi-associated compartment.
GMTriP-K induces interaction of PGLYRP1 with GEF-H1 and NOD2
To better understand the interaction of PGLYRP1 with the NOD2-GEF-H1 signaling system, we analyzed the expression of PGLYRP1, NOD2 and GEF-H1 in BMDMs from wild type, Pglyrp1-/-, Nod2-/-, and Arhgef2-/- mice (Fig. 5a). The GEF-H1 antibody detected a protein of 120 kDa and three less-expressed isoforms between 90 and 110 kDa in wild type and PGLYRP1- and NOD2-deficient BMDMs (Fig. 5a). As expected, the proteins detected by the GEF-H1, PGLYRP1 or NOD2 antibodies were absent in the corresponding gene deficient macrophages (Fig. 5a).
a Western blot analysis of protein expression in BMDMs from wildtype, Arhgef2-/-, Pglyrp1-/-, and Nod2-/- mice. b Time course of protein interactions with GEF-H1 after GMTriP-K stimulation of BMDMs. c Analysis of protein interactions with PGLYRP1 or d NOD2 after GMTriP-K stimulation of BMDMs. e Input assessment of protein expression for the IPs in (c, d). f Assessment of protein interactions with GEF-H1 after stimulation with indicated immune stimuli. Representative experiments of at least three repeats are shown.
We next examined the interactions between PGLYRP1, the NOD2/GEF-H1 system and ER and Golgi proteins SEC61β and GM130 during GMTriP-K stimulation. We performed immunoprecipitations of endogenously expressed GEF-H1 at different timepoints after stimulation with 25 μM GMTriP-K (Fig. 5b, c). Complexes that contained PGLYRP1, NOD2 and full length GEF-H1 formed within 1 h of stimulation and were maintained over 18 h (Fig. 5b). Over the stimulation period, GM130 and SEC61β association increased in the complex (Fig. 5b). We were also able to confirm the formation of these complexes after GMTriP-K stimulation by immunoprecipitations with antibodies against PGLYRP1 or NOD2. (Fig. 5c, d, e).
Finally, we found that PGLYRP1/GEF-H1/NOD2 complexes that contain SEC61β and GM130 formed specifically in the presence of GMTriP-K (Fig. 5f). In these experiments, we stimulated macrophages with 25 μg/mL bis-(3′,5′)-cyclic dimeric guanosine monophosphate (cdi-GMP), 100 ng/mL LPS or 25 μM GMTriP-K and used the N-terminus GEF-H1 antibody to pull down protein complexes. GEF-H1 complexes that contained PGLYRP1/NOD2 and SEC61β only formed in the presence of GMTriP-K (Fig. 5f). GEF-H1 interacted with GM130 in unstimulated, cdi-GMP and LPS stimulated macrophages and only GMTriP-K stimulation significantly increased GM130 in the complex (Fig. 5f).
Together, our data indicates a new mechanism of GMTriP-K recognition in which intracellular PGLYRP1 and NOD2 complexes interact with GEF-H1 at the ER and Golgi compartments. GEF-H1 may function as a signaling hub that facilitates PGLYRP1/NOD2 signaling at ER to Golgi associated membranes.
PGLYRP1 mediated immune responses regulate intestinal inflammation
To determine the importance of GMTriP-K detection by PGLYRP1 in the regulation of intestinal inflammation, we studied hapten induced colitis after administration of trinitrobenzene sulfonic acid (TNBS). As shown in Fig. 6a, GMTriP-K administration for 3 days prior to the TNBS challenge protected mice from the weight loss seen in wild type mice during the development of TNBS colitis. Remarkably, Pglyrp1-/- mice developed more severe colitis than wild type mice in response to TNBS administration and losing weight more rapidly compared to wild type mice (Fig. 6a). Importantly, pretreatment with GMTriP-K was unable to protect Pglyrp1-/- mice from TNBS induced colitis (Fig. 6a).
a Weight development of wild type and Pglyrp1-/- mice during TNBS-induced colitis (n = 4 per group). Immunofluorescence staining of PGLYRP1 and macrophages (F4/80) in colon sections of wild type and Pglyrp1-/- mice before (b), after TNBS induced colitis (c, d) TNBS colitis induction after 3 days of i.p. pretreatment with 100 μg of GMTriP-K in 50 μL PBS at day 9. e Quantitation of F4/80+ macrophages and percentage of PGLYRP1 expressing F4/80+ macrophages during TNBS colitis. Macrophages were counted in 4 independent regions per section and quantitated using Imaris 10.1.1 image analysis software. f Staining for PGLYRP1 and SEC61β in the colon of wild type mice with TNBS colitis. g qPCR analysis of gene expression in mesenteric lymph nodes of wild type and Pglyrp1-/- mice on day 9 of the TNBS and GMTPriP-K treatment protocol (n = 3–5). Scale bars represent 20 μm in (b, c, d) and 2 μm in (g). Data are presented as mean ± SEM with indicated P values analyzed by one-way ANOVA. All experiments were repeated twice and yielded consistent results.
The assessment of PGLYRP1 expression in colon sections identified F4/80 positive macrophages in the colon lamina propria (Fig. 6b). The antibody was specific as the PGLYRP1 staining was absent in Pglyrp1-/- mice (Fig. 6b). Upon the induction of TNBS colitis, the lamina propria of wild type and Pglyrp1-/- mice showed inflammatory cell infiltration with increased recruitment of F4/80 positive macrophages (Fig. 6c, e). The pretreatment of mice with GMTriP-K before the induction of TNBS did not change the recruitment of macrophages into the inflamed lamina propria (Fig. 6d, e). PGLYRP1 expressing macrophages were found distributed at the base of colon crypts and between muscle layers in wild type mice (Fig. 6d). Regardless of pretreatment with GMTriP-K, Pglyrp1-/- mice developed severe TNBS colitis with transmural macrophage infiltration (Fig. 6d). While macrophages were recruited during TNBS colitis, the percentage of PGLYRP1 expressing macrophages was not significantly different between the wild type and TNBS treatment groups. GMTriP-K pretreatment did not further increase the recruitment of macrophages when compared to TNBS treatment alone (Fig. 6e). Mice that received TNBS and GMTriP-K demonstrated a significantly higher percentage of PGLYRP1 expressing macrophages compared to wild type but not TNBS treated mice (Fig. 6e). We also stained for SEC61β in PGLYRP1 expressing cells in the lamina propria in colon tissues from wild type mice that received TNBS (Fig. 6f). These experiments revealed the co-localization of PGLYRP1 and SEC61β positive subcellular compartments in lamina propria cells that expressed PGLYRP1 (Fig. 6f).
We also investigated the immune system response to TNBS colitis in mesenteric lymph nodes (MLN). The gene expression in MLNs during colitis can represent regulation of immune cells due to antigen transport to MLNs but also includes gene expression of immune cells recruited from the intestines22,23. Pretreatment of mice with GMTriP-K prevented the induction of PGLYRP1 expression that increased 10-fold during TNBS colitis in MLNs suggesting a ligand-receptor feedback loop (Fig. 6g). Remarkedly, Pglyrp1-/- mice expressed significantly more NOD2 in MLNs compared to wild type mice during TNBS colitis (Fig. 6g). Induction of inflammatory genes during TNBS colitis was significantly alleviated after pretreatment with GMTriP-K for 3 days in wild type mice (Fig. 6g). We found that PGLYRP1 expression was significantly increased during TNBS induced colitis (Fig. 6g). Expression of inflammatory chemokines, IL6 and IL12b, as well as immune regulators, such TNFSF15, CXCL3, CXCL10 and ISG15, were significantly reduced (Fig. 6g). In contrast, Pglyrp1-/- mice showed an enhanced expression of these inflammatory factors and this enhanced expression pattern did not significantly change after GMTriP-K pretreatment (Fig. 6g). This is consistent with their increased susceptibility to TNBS induced colitis that was not mitigated by GMTriP-K pretreatment. Together, these data suggest that GMTriP-K challenge can provide immune protection to TNBS induced colitis and this protection requires PGLYRP1.
We next investigated whether extracellular PGLYRP1 could (1) regulate GMTriP-K responses in wild type mouse or (2) restore GMTriP-K detection/signaling in Pglyrp1-/- BMDMs. We found that PGLYRP1 inhibited GMTriP-K-induced Il6 and Acod1 expression in wild type BMDMs (Fig. S13). However, PGLYRP1 could not re-establish GMTriP-K detection/signaling in PGLYRP1 deficient BMDMs (Fig. S13), suggesting that extracellular PGLYRP1 bound to GMTriP-K cannot initiate signaling in macrophages. Taking these data together, GMTriP-K facilitated mucosal recovery in colitis through PGLYRP1-dependent immune regulation.
PGLYRPs are upregulated in ulcerative colitis
To determine the involvement of PGLYRP1 in human inflammatory bowel disease, we analyzed the expression of PGLYRP1 in healthy colon and ulcerative colitis (UC) by immunostaining. We found that a subset of CD68 positive macrophages expressed PGLYRP1 in the healthy colon lamina propria (Fig. 7a). In active UC we found a significantly increased recruitment of PGLYRP1 expressing macrophages into the inflamed colon lamina propria with some of these cells infiltrating and crossing the crypt epithelium (Fig. 7a, b).
a Immunofluorescence staining of PGLYRP1 and macrophages (CD68) in tissue sections of healthy human colon and active ulcerative colitis (arrow indicates immune cells that crossed the epithelial barrier, cr, Crypt.). b Quantitation of CD68+ macrophages and percentage of PGLYRP1 expressing CD68+ macrophages in the normal colon and ulcerative colitis lamina propria. Macrophages were counted in 4 independent regions per sample and quantitated using Imaris 10.1.1 image analysis software. c Hierarchal cluster analysis of genes that were significantly regulated in active, inactive ulcerative colitis and healthy control colons. d Volcano plot analysis of gene expression comparing gene expression in healthy control and active ulcerative colitis. e qPCR analysis of expression of indicated genes in healthy control (n = 6), inactive (n = 8), and active ulcerative colitis (n = 6) samples. Scale bars represent 25 μm. Data are presented as mean ± SEM with indicated P values analyzed by one-way ANOVA. Student’s test was used to compare control and UC samples. All experiments were repeated at least twice and yielded consistent results.
To understand the regulation of PGLYRPs and NOD2 in inflammatory bowel diseases, we analyzed transcriptional responses in biopsies from active (n = 6), inactive (n = 8) UC and healthy controls (n = 6). The RNAseq analysis revealed 8785 (p < 0.05, FDR < 0.05) genes differentially regulated in active UC compared to healthy controls (Supplementary Data File 9). Unsupervised clustering of genes that where significantly regulated in active UC vs healthy and inactive UC vs healthy resulted in 4 clusters of genes that were down regulated in active UC (Cluster I), or upregulated in UC compared to healthy controls (Cluster II), genes that were upregulated in active UC compared to healthy controls with a further decrease in inactive UC (Cluster III) and genes that were significantly downregulated in inactive UC compared to active UC and healthy colon (Cluster IV) (Fig. 7c, Supplementary Data File 10). The volcano plot in Fig. 6d showed that genes that were significantly upregulated in active UC compared to healthy control samples. These genes are associated with antimicrobial host defense such as DUOXA2, DUOX2, REG1, REG3a, DEFA5, and DEFA6. Active UC was also characterized by the significant induction of IL1a, IL1b, IL6, SAA1/2, CXCL3/8/10, IFNG, MMP7/10, ISG15, IRF7 and ACOD1, indicating the activation of mucosal immune system (Fig. 7d).
Among the genes in cluster II, we found that the expression of PGLYRP1, PGLYRP2, PGLYRP4 and NOD2 was significantly increased in active UCs compared to inactive UC and healthy colon (Fig. 7e). Active UC samples were also characterized by significantly enhanced IL6, IL1b, IFNG, CXCL3 and NFKB2 expression when compared to inactive UC and healthy control samples (Fig. 7e).
In conclusion, these data indicated that PGLYRPs are regulated during mucosal immune responses in ulcerative colitis. Importantly, a subset of CD68 positive macrophages expressed PGLYRP1 in normal and inflamed human colons.
Discussion
We found that PGLYRP1 can function as an ER and Golgi-associated peptidoglycan receptor for GMTriP-K. Among the human PGRPs tested on the small fragment microarray, only PGLYRP1 specifically bound to the disaccharide motif of GMTriP-K. Our study demonstrates that monomers such as GMTriP-K and its analogs, derived through amidation of lysine and anhydration of muramic acid, can bind with PGLYRP1. Our microarray approach enables the quantification of glycan-protein binding parameters. Other in vitro studies suggest binding specificities of PGN to the four mammalian PGLYRPs; these studies have largely focused on the third amino acid residue of PGNs, which generally differs between Gram-positive and Gram-negative bacteria, with the former using lysine and the latter using diaminopimelic acid24. Binding of PGLYRP1 to monosaccharide PGN units has been suggested; however, in these studies, the PGLYRP1 protein was bound to a surface in unknown orientation and surface plasmon resonance was used to detect binding25. In agreement with our work, PGLYRP1 has been suggested to potentially accommodate binding of GMTetP-K26 and showed some specificity for GMTriP-K containing dimers over GMTetP-K dimers27,28. Other PGN arrays focused primarily on large oligosaccharides (larger than 1.5 kDa) where all saccharides were bound via the anomeric position of the reducing end of the carbohydrate, omitting glycan recognition29. In our experiments, PGLYRP1 bound to the array with µM affinity and showed no binding to monosaccharide or peptide fragments, suggesting that this receptor prefers disaccharide moieties over monosaccharide components.
Importantly, here we uncovered that PGLYRP1 was required for the GMTriP-K but not for GMDiP or MDP induced immune responses. PGLYRP1 dependent transcriptional programming was significantly different from that induced by MDP although both pathways required downstream NOD2 and GEF-H1. We did not observe any phosphorylated product when we assayed NAGK for its ability to phosphorylate GMTriP-K, suggesting that the NOD2 response to this compound was a result of PGLYRP1 signaling rather than the canonical NAGK pathway.
PGLYRP1 was also required for immune responses to heat-inactivated Listeria monocytogenes. Genes that were PGLYRP1 dependently induced also showed significant reduction of expression in Nod2-/- macrophages. It should be noted that immune responses to live Listeria monocytogenes can include the activation of additional transcriptional programs in different cell types requiring STING and TLR2 pathways30,31,32.
Microscopy and protein enrichment assays indicate that PGLYRP1 is part of an ER and Golgi-based signaling system, in which PGLYRP1 interacts with NOD2 and GEF-H1. PGLYRP1, GEF-H1, and NOD2 formed complexes with SEC61β and GM130 in the presence of GMTriP-K. SEC61β connects ER membranes to the microtubule (MT) network by directly binding MTs33, indicating a close relationship between MT and ER. SEC61β was also observed in the post-ER compartment33,34. GM130 is a Golgi-forming protein and has multiple functions in autophagy, apoptosis, cell polarity, cell migration, intracellular protein transport and MT formation35.
Our data now indicates that PGLYRP1 forms complexes that include GEF-H1 as a requirement for signaling. We have previously identified GEF-H1 as an important signaling interactor for the activation of innate immune kinases in the MAVS and NOD2 pathways14,36. The ER is strongly associated with MT through ER tubules, which are constantly formed from existing ER membranes by associating with the growing plus end of MTs or interacting with MT molecular motors37. It will be important to understand the cell type specific molecular mechanism of MT associated GEF-H1 isoforms and cleavage products in membrane trafficking and assembly of PGLYRP1/GEF-H1/NOD2 complexes and subsequent immune regulation.
It needs to be determined whether PGLYRP1 signaling involves the sequential transition/translocation of PGLYRP1 from the ER, ER–Golgi intermediate compartment (ERGIC), and Golgi. The mechanisms of PGLYRP1 transport may require additional interactors and activation events similar to the STING signaling pathway that requires transition of STING from ER > ERGIC > Golgi38.
Remarkably, GMTriP-K pretreatment protected the mucosa from TNBS induced colitis in wild type mice. A similar function has been proposed for MDP although the underlying mechanisms were unclear39. PGLYRP1 dependent genes that were induced by GMTriP-K included major immune regulatory cytokines such as IL1a, IL1b, IL6, IL12b, and CXCL3, and regulators such as ACOD1 and ISG15. In addition, deletion of PGLYRP1 resulted in a significant upregulation of NOD2, suggesting that feedback mechanisms exist between different PGN recognition pathways. In this context, an elevated response to MDP may contribute to the severe TNBS colitis in Pglyrp1-/- mice. We suggest that PGLYRP1-mediated signaling is crucial for mucosal protection, expanding the previous identification of PGLYRP1 as a scavenger for PGN6. These findings are consistent with previous work that demonstrated that PGLYRPs protected mice from DSS-induced colitis6. We found that extracellular PGLYRP1 can inhibit the response of macrophages to GMTriP-K but cannot restore the signaling in PGLYRP1 deficient macrophages, indicating that uptake of GMTriP-K is required for signaling by PGLYRP1.
We found that PGLYRP1 was expressed in macrophages in both mouse and human colon and upregulated during intestinal inflammation. Unraveling the role of the PGLYRP1-based GMTriP-K recognition system in inflammatory bowel disease will be important in the context of diseases associated with NOD2 and GEF-H1 variants. The interdependency of PGLYRP1 signaling, NOD2, and GEF-H1 will require more detailed understanding of this mechanism specifically in Crohn’s disease and other chronic inflammatory conditions where NOD2 is a major risk factor1. It should be noted that ARHGEF2, the gene encoding GEF-H1, maps to a human chromosome 1 locus associated with susceptibility to inflammatory bowel disease40,41.
Our identification of PGLYRP1 as a receptor for GMTriP-K does not exclude ligand independent functions of PGLYRP1 in regulating immune responses. Previous studies have shown that deletion of PGLYRP1 in mice resulted in reduced tumor growth accompanied by an enhanced activation/effector phenotype in CD8+ T cells42. PGLYRP1 deficient mice were also protected from experimental autoimmune encephalomyelitis (EAE) with defects in antigen presentation and alterations in expression profiles of myeloid cells42. Notably, PGLYRP1 plays a role in Lyme disease and immune dysregulation19.
In summary, we show that PGLYRP1 is located at the ER and Golgi apparatus and functions as receptor for monomeric GMTriP-K in conjunction with NOD2 and GEF-H1 (Fig. 8). Our results show that macrophages expressing PGLYRP1 are part of immune responses in human colitis and that ligand-dependent PGLYRP1 signaling promotes mucosal protection in an animal model of the disease.
PGLYRP1 is required for detection of GMTriP-K. PGLYRP1 localizes to the endoplasmic reticulum and Golgi and interacts with NOD2 and GEF-H1 to induce the expression of immune regulators that regulate intestinal inflammation (Created in BioRender. Reinecker, H. (2025) https://BioRender.com/i55t156).
Methods
The research complies with all relevant ethical regulations. Human colon tissue samples were collected in the Division of Digestive and Liver Diseases at the University of Texas Southwestern Medical Center under the IRB protocol number STU-112010-130,” Registry and Biorepository for the study of Gastrointestinal Inflammatory Diseases”. Animal experimental protocols were approved by the Committee on Research Animal Care at the UT Southwestern Medical Center, Dallas under protocol number 2020-102946.
Please see key resources list for antibodies, primers and chemicals used (Table S3).
Glycan microarray fabrication, experimentation, and data analysis
Glycan microarray fabrication validation, experimentation, and data analysis were performed (see Supplementary Methods). For the microarrays used herein, a total of 110 array components were printed in 16 replicate blocks on 3-D hydrogel NHS-activated slides (3-D Hydrogel Coating(H), Schott Minifab, Phoenix, AZ). Following the printing, slides were vacuum sealed and stored at −20 °C until the time of use. Unreacted NHS groups were blocked before use by immersion in blocking buffer solution (50 mM ethanolamine in 100 mM borate buffer, pH 8.5) for 1 h. The slides were rinsed trice with PBST (PBS + 0.05% v/v Tween 20) and then submerged in water for 5 min before drying by centrifugation (284 × g for 5 min) then used immediately for incubation. The slides were enclosed in a ProPlate 16-well module (Grace Bio-Labs, Bend, OR) to separate the 16 identical subarrays for different experimental conditions. Recombinant human PGLYRP1 (R&D Systems, Minneapolis, MN) was applied to the subarrays at various concentrations (typically 10 μg/mL) in PBST, and the slide was sealed with adhesive film and incubated at RT for 18 h. Arrays were washed thoroughly with PBST and patted dry. Primary anti-PGLYRP1 (R&D Systems, Minneapolis, MN) was diluted 100-fold in PBST and applied to the subarrays. The arrays were sealed and incubated at RT for 4 h, followed by washing and drying. Fluorescently labeled secondary antibody (Cy3-IgG, Invitrogen) was diluted 500-fold and applied to each subarray. The slides were sealed and incubated at RT for 1 h. Arrays were rinsed with PBST, and the 16-well module was removed, and the slide was fully submerged in water for 5 min. The slide was dried by centrifugation (284 × g for 5 min) prior to scanning. Slides were scanned using a GenePix 4000B microarray scanner (Molecular Devices, Sunnyvale, CA). Data analysis was performed using GenePix Pro 7 software (Molecular Devices, Sunnyvale, CA). Missing spots were flagged and excluded from analysis. Background fluorescence was subtracted from median fluorescence, and values were averaged for duplicate spots. Data was processed using Excel and GraphPad Prism 9 software. The supporting information provides full data and displays representative images and graphs.
Mice
All experimental mice were sex-matched and used at 6–12 weeks of age. Arhgef2-/- mice were previously described36. C57BL/6 wild type, Pglyrp1-/-, and Nod2-/- animals were from Jackson Laboratory (Bar Harbor, ME, USA). All animals were bred and housed in a pathogen-free animal facility according to the institutional guidelines.
BMDMs culture
BMDM cells were generated by flushing bone marrow cells from femurs and tibia of wild type or Arhgef2-/-, PGLYRP1-/-, and Nod2-/- male mice aged between 6–8 weeks. Red blood cells were removed by using ACK lysing buffer (Gibco, #A1049201). Then, 70 µm cell strainers were used to filter BMDM cells, followed by resuspension in complete DMEM supplemented with 10% FBS and 0.5% penicillin/streptomycin mixture, and 200 ng M-CSF. BMDM cells were cultured in 10 cm petri dish at 37 °C, 5% CO2 for 6 days before experimentation. BMDMs were detached with 0.25% trypsin solution treatment and 1,000,000 cells/well were seeded in a 6-well plate. 25 µM GMTriP-K, GMDiP, or MDP (Invivogen; Cat# tlrl-mdp), 100 ng/mL IFNγ (Biolegend #575304), 100 ng/mL IFNβ (Biolegend #581302), or 500 ng/mL PGLYRP1 (R&D system #2590-PGB) were used to stimulate BMDM cells for indicated duration up to 18 h. For heat inactivation, Listeria monocytogenes (Microbiologics Cat#01249 UV-V) were suspended in PBS, pH 7.4, and centrifuged at 10,000 × g for 10 min. The resulting pellets were washed twice and resuspended in PBS to a specific OD600 value equivalent to 1 × 107 cfu/mL. The suspensions were heated at 100 °C for 1 h, washed twice by centrifugation and resuspended in PBS, thoroughly mixed and stored at −80 °C until use. 106 macrophages were stimulated with 1 × 107 cfu/mL for 6 or 18 h. Proteins and RNA were harvested with RIPA lysis buffer or the Qiagen RNeasy Mini Plus kit, respectively. HEK 293T cells were purchased from the American Type Culture Collection and grown in DMEM supplemented with 10% fetal bovine serum and 0.5% penicillin/streptomycin.
RNA sequencing and analysis
Total RNA was isolated from BMDMs derived from wild type, Arhgef2-/-, Pglyrp1-/-, and Nod2-/- mice, or human colon tissue biopsies using a RNeasy Micro kit (Qiagen). Libraries were synthesized using TruSeq Stranded mRNA sample preparation kit from 500 ng of purified total RNA and indexed adapters according to the manufacturer’s protocol (Illumina). The final libraries were quantified using a Qubit fluorometer (Agilent Technologies), and RT-qPCR was performed using the Kapa Biosystems library quantification kit according to the manufacturer’s protocol. Pooled libraries were subjected to 35 bp paired-end sequencing according to the manufacturer’s protocol (Illumina NextSeq 500). The targeted sequencing depth was set at 30 million paired end reads per sample. Blc2fastq2 Conversion software (Illumina) was used to generate demultiplexed Fastq files. The adapters were trimmed using Trim Galore (v0.6.4). The trimmed reads were aligned to the human genome (GRCH38) or mouse genome (mm10) using STAR (2.7.3a). Subsequently, the mapped reads were quantified using the featureCounts of the Subread (v1.6.3) package. Genes with low expression (genes with an expression value of zero in more than 30% of the samples) were removed before subsequent analysis. Gene expression was normalized using the Voom method in the R package “limma” (v 3.50.3). Differentially expressed genes were also identified using this software. Gene set enrichment analysis of differentially expressed genes was performed using the R package “clusterProfiler” (v4.2.2). Volcano plots were generated using R package ggplot2 (v3.5.1). SeqMonk Mapped Sequence Data Analyzed 1.48.1 was used for DEseq2 analysis and initial visualization. Heat maps were generated using R package pheatmap (v1.0.12).
Real-time quantitative-PCR
Total RNA of BMDMs from wild type, Arhgef2-/-, Pglyrp1-/-, and Nod2-/- mice was isolated using the RNeasy Mini Plus kit (Qiagen). cDNA was prepared from RNA using a PrimeScript RT Reagent cDNA Synthesis Kit (Takara). Real-time qPCR was performed using PowerTrack SYBR Green master mix (Applied Biosystems) with gene specific primers and and relative expressions were calculated using the ΔΔCT method43. Gene expression was normalized to GAPDH. All experiments were repeated at least twice. The primer sequences are listed in Tab. S3.
Transfection and confocal microscopy
HEK 293 T cells were seeded on 8-well-chambered coverslip slides (ibidi GmbH, cat# 80806) at 2.5 × 104 per well, and cells at 60-70% confluency were transfected the next day using Lipofectamine 3000 reagent (Invitrogen #L3000-008) according to the manufacturer’s protocol. Typically, 50–200 ng of total plasmid DNA was transfected into each well without any cytotoxicity. All expression plasmids encoded for full-length proteins, either untagged or C-terminally tagged with Flag epitope or fluorescent proteins. On the day after transfection, the cells were either used for live cell imaging or fixed and stained for immunofluorescence microscopy. For immunocytochemistry, the cells were fixed with paraformaldehyde (Thermo Scientific Chemicals, cat# 047377.9, methanol-free) which was freshly diluted to 4% with concentrated PBS and water. Essentially, cells were fixed for 10 min at 37 °C, washed with PBS, and permeabilized with 0.1% Triton X-100 in PBS for 90 s. The cells were washed with wash buffer (0.01% Triton/PBS) and blocked with blocking buffer (5% donkey serum 0.01% Triton/PBS) for 1 h at room temperature. The cells were sequentially incubated with specific primary antibodies and highly cross-adsorbed secondary antibodies in the blocking buffer for a minimum of 1 h at room temperature. Thorough washes (3 × 5 min) were performed between and after primary and secondary antibody incubation. Optionally, 2–5 min of nuclear staining with DAPI was performed after secondary antibody incubation. Confocal images were captured using the DMi8 microscope system (Leica Microsystems) with a 63X oil lens, with each image having at least 2048 × 2048-pixel resolution. Multicolor imaging channels (four-color) were typically well separated as DAPI, Alexa Fluor 488/EGFP, mOrange/mRFP/Rhodamine Red-X, and Alexa Fluor 647. In addition, each channel was scanned strictly sequentially to avoid crosstalk between the fluorophores. Colocalization studies were carried out in Imaris image analysis software.
Western blots and immunoprecipitations
BMDM cell extracts were harvested using RIPA lysis buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 0.5 mM EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl, diluted with dH2O) containing protease and phosphatase single-use inhibitor cocktail (Thermo Scientific, #78430). Standard protocols for western blotting were used for SDS-PAGE and wet transfer onto PVDF membranes. Primary antibodies were diluted in blocking buffer (5% milk or 5% BSA) and incubated overnight at 4 °C. Secondary Anti-rabbit-Ig G-HRP (Cell Signaling, #7074,) or Anti-mouse-Ig G-HRP (Cell Signaling, #7076) antibodies were used at 1/3000 dilution and incubated for 1 h at room temperature. Proteins were visualized using enhanced chemiluminescence (Immobilon Western; Millipore #WBKLS0100). The following antibodies were used in this study: rabbit antibodies against GEF-H1 (ab155785; Abcam,1/1000 dilution), sheep anti-mouse GEF-H1 (Exalpha Biological; #X1089S, 1/1000 dilution), Rabbit anti-SEC61β (D5Q1W) (Cell Signaling,#14648,1:1000 dilution), Mouse anti-GM130 (BD biosciences #610822, 1/1000 dilution), GAPDH (Cell signaling, #2118, 1:500 dilution), PGLYRP1(R&D system, #AF2696, 1:500 dilution), NOD2 (Invitrogen, #PA5-104317, 1:500 dilution), RB anti-phospho-stat1(Tyr701) (58D6) (Cell signaling, #9197,1/1000 dilution), RB anti-stat1 (D1K9Y) (Cell signaling, # 9172, 1/1000 dilution), and mouse anti-β-actin (8H10D10) (Cell signaling, #3700, 1/10000 dilution), Anti-FLAG (Millipore Sigma, #F7425, 1/3000 dilution). For immunoprecipitations BMDMs were lysed on ice for 20 min in Pierce IP lysis buffer (Thermo Scientific, #87787). Cell debris was pelleted by centrifugation and the supernatant was then incubated and rotated for 60 min at 4 °C with protein G plus agarose (Pierce Thermo Scientific, #20423). Precleared lysates were incubated and rotated at 4 °C overnight with immunoprecipitation antibodies. The protein G agarose beads were added, and the incubation continued at 4 °C for 4 h. Following three washes with the lysis buffer, the agarose beads were mixed with 1 × SDS sample buffer and incubated at 95 °C for 10 min prior to immunoblotting analysis.
TNBS induced colitis model
Mice were divided into 6 groups: (1) Wild type control; (2) Wild type TNBS; (3) Wild type TNBS plus GMTriP-K; (4) Pglyrp1-/- control; (5) Pglyrp1-/- TNBS; (6) Pglyrp1-/- TNBS plus GMTriP-K. Each mouse was anesthetized with isoflurane and administered with 3.75 mg TNBS (MilliporeSigma, #P2297) in 100 µL 45% ethanol solution by intra-rectum injection via a 3.5 French catheter with a 1 mL syringe. Control mice were administered with the ethanol solution without TNBS using the same technique. Mice received 100 μg of GMTriP-K in 50 μL PBS i.p for 3 days prior to TNBS colitis induction. Colon and mesenteric lymph node were harvested for histology, immune staining, and qPCR at the 6th day post TNBS injection.
Immunofluorescence staining of mouse and human colon sections
Tissue sections were baked for 20 min, deparaffinized, and rehydrated. Slides were washed with PBS three times for 5 min each wash. Citrate buffer pH6 was used as antigen retriever solution. Tissue sections were permeabilized with 0.25% Triton X-100 and blocked with 5% normal donkey serum). After washing the slides with PBS, the sections were incubated overnight in a primary antibody against PGLYRP1 (R&D Systems, #AF2696; 1:500 dilution), CD68 (Cell Signaling Technology, #76437; 1:1000 dilution), SEC61β (Cell Signaling Technology, # 14648 1:1000) and F4/80 (Cell Signaling Technology, #70076; 1:1000) in 4 degrees C in a humidified shielded chamber. After one wash with 0.05% Tween/PBS (0.05% PBST) followed by two washes with PBS, the slides were incubated with secondary antibodies, anti-goat Alexa Fluor 488 (Life Technologies, #A32814TR) and anti-rabbit Alexa Fluor 594 (Life Technologies, #A11037) sequentially. Secondary antibodies were diluted in blocking buffer and incubated for 1 h in shielded humid chamber. Slides were washed with 0.05% PBST and PBS. Sections were counterstained separately with DAPI. Slides were mounted with Prolong Gold Antifade reagent with DAPI (Invitrogen, #P36931). Confocal images were captured using the DMi8 microscope system (Leica Microsystems) with a 63X oil lens, with each image having at least 2048 × 2048-pixel resolution. Multicolor imaging channels (four-color) were typically well separated as DAPI, Alexa Fluor 488, and Alexa Fluor 594. In addition, each channel was scanned strictly sequentially to avoid crosstalk between the fluorophores. Colocalization studies were carried out in Imaris image analysis software.
Statistics and reproducibility
Statistical analyses were performed using Prism (GraphPad 9) or R (Rstudio). Statistical significance was evaluated by two-tailed unpaired Student’s t-test when comparing two groups; two-tailed paired Student’s t-test for group comparisons; one-way ANOVA for comparing more than two groups; and the Wilcoxon rank sum test for RNA-seq data. Correction methods were labeled in figure legend. p values are indicated. Bar graphs show the entire range of values denoting the median. Data are presented as mean ± SEM. All experiments were repeated as stated to ensure reproducibility.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNAseq data generated in this study have been deposited in the GEO database under accession code GSE279626. Biochemistry and original western blots generated in this study are provided in the Supplementary Information. Source data are provided with this paper.
References
Caruso, R., Warner, N., Inohara, N. & Nunez, G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity 41, 898–908 (2014).
Werner, T. et al. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 97, 13772–13777 (2000).
Dziarski, R. & Gupta, D. Review: mammalian peptidoglycan recognition proteins (PGRPs) in innate immunity. Innate Immun. 16, 168–174 (2010).
Dziarski, R., Platt, K. A., Gelius, E., Steiner, H. & Gupta, D. Defect in neutrophil killing and increased susceptibility to infection with nonpathogenic gram-positive bacteria in peptidoglycan recognition protein-S (PGRP-S)-deficient mice. Blood 102, 689–697 (2003).
Saha, S. et al. PGLYRP-2 and Nod2 are both required for peptidoglycan-induced arthritis and local inflammation. Cell Host Microbe 5, 137–150 (2009).
Saha, S. et al. Peptidoglycan recognition proteins protect mice from experimental colitis by promoting normal gut flora and preventing induction of interferon-gamma. Cell Host Microbe 8, 147–162 (2010).
Kaneko, T. et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat. Immunol. 7, 715–723 (2006).
Chang, C. I., Chelliah, Y., Borek, D., Mengin-Lecreulx, D. & Deisenhofer, J. Structure of tracheal cytotoxin in complex with a heterodimeric pattern-recognition receptor. Science 311, 1761–1764 (2006).
Girardin, S. E. et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300, 1584–1587 (2003).
Girardin, S. E. et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278, 8869–8872 (2003).
Inohara, N. et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implic. Crohn’s. Dis. J. Biol. Chem. 278, 5509–5512 (2003).
Fukazawa, A. et al. GEF-H1 mediated control of NOD1 dependent NF-kappaB activation by Shigella effectors. PLoS Pathog. 4, e1000228 (2008).
Barnich, N., Aguirre, J. E., Reinecker, H. C., Xavier, R. & Podolsky, D. K. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor-kappaB activation in muramyl dipeptide recognition. J. Cell Biol. 170, 21–26 (2005).
Zhao, Y. et al. Microbial recognition by GEF-H1 controls IKKepsilon mediated activation of IRF5. Nat. Commun. 10, 1349 (2019).
Vollmer, W., Blanot, D. & de Pedro, M. A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 32, 149–167 (2008).
Vollmer, W., Joris, B., Charlier, P. & Foster, S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 32, 259–286 (2008).
Bersch, K. L. et al. Bacterial peptidoglycan fragments differentially regulate innate immune signaling. ACS Cent. Sci. 7, 688–696 (2021).
Schaub, R. E., Lenz, J. D. & Dillard, J. P. Analysis of peptidoglycan fragment release. Methods Mol. Biol. 1440, 185–200 (2016).
Gupta, A. et al. A human secretome library screen reveals a role for peptidoglycan recognition protein 1 in Lyme borreliosis. PLoS Pathog. 16, e1009030 (2020).
Kochnev, Y., Hellemann, E., Cassidy, K. C. & Durrant, J. D. Webina: an open-source library and web app that runs AutoDock Vina entirely in the web browser. Bioinformatics 36, 4513–4515 (2020).
Stafford, C. A. et al. Phosphorylation of muramyl peptides by NAGK is required for NOD2 activation. Nature 609, 590–596 (2022).
Mowat, A. M. & Agace, W. W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685 (2014).
Park, S. M. et al. T cell fate following Salmonella infection is determined by a STING-IRF1 signaling axis in mice. Commun. Biol. 2, 464 (2019).
Royet, J. & Dziarski, R. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat. Rev. Microbiol 5, 264–277 (2007).
Kumar, S. et al. Selective recognition of synthetic lysine and meso-diaminopimelic acid-type peptidoglycan fragments by human peptidoglycan recognition proteins Ialpha and S. J. Biol. Chem. 280, 37005–37012 (2005).
Dziarski, R. & Gupta, D. The peptidoglycan recognition proteins (PGRPs). Genome Biol. 7, 232 (2006).
Inamura, S. et al. Synthesis of peptidoglycan fragments and evaluation of their biological activity. Org. Biomol. Chem. 4, 232–242 (2006).
Cho, J. H. et al. Human peptidoglycan recognition protein S is an effector of neutrophil-mediated innate immunity. Blood 106, 2551–2558 (2005).
Wang, N., Hirata, A., Nokihara, K., Fukase, K. & Fujimoto, Y. Peptidoglycan microarray as a novel tool to explore protein-ligand recognition. Biopolymers 106, 422–429 (2016).
Hansen, K. et al. Listeria monocytogenes induces IFNbeta expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J. 33, 1654–1666 (2014).
Soria-Castro, R. et al. TLR2 Regulates Mast Cell IL-6 and IL-13 Production During Listeria monocytogenes Infection. Front. Immunol. 12, 650779 (2021).
Anand, P. K. et al. TLR2 and RIP2 pathways mediate autophagy of Listeria monocytogenes via extracellular signal-regulated kinase (ERK) activation. J. Biol. Chem. 286, 42981–42991 (2011).
Zhu, Y. et al. Sec61beta facilitates the maintenance of endoplasmic reticulum homeostasis by associating microtubules. Protein Cell 9, 616–628 (2018).
Greenfield, J. J. A. & High, S. The Sec61 complex is located in both the ER and the ER-Golgi intermediate compartment. J. Cell Sci. 112, 1477–1486 (1999).
Nakamura, N. Emerging new roles of GM130, a cis-Golgi matrix protein, in higher order cell functions. J. Pharm. Sci. 112, 255–264 (2010).
Chiang, H. S. et al. GEF-H1 controls microtubule-dependent sensing of nucleic acids for antiviral host defenses. Nat. Immunol. 15, 63–71 (2014).
Friedman, J. R. & Voeltz, G. K. The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol. 21, 709–717 (2011).
Hopfner, K. P. & Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521 (2020).
Watanabe, T. et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J. Clin. Investig. 118, 545–559 (2008).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Olivier, J. F. et al. CCDC88B interacts with RASAL3 and ARHGEF2 and regulates dendritic cell function in neuroinflammation and colitis. Commun. Biol. 7, 77 (2024).
Schnell, A. et al. Targeting PGLYRP1 promotes antitumor immunity while inhibiting autoimmune neuroinflammation. Nat. Immunol. 24, 1908–1920 (2023).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Acknowledgements
H.C.R. was supported by NIH/NIAID R01AI113333 and NIH/GM R01GM138599, C.L.G. was supported by NIH/GM R01GM138599 and NIH/NIAID 1P01AI172525. R.P. and S.H. were supported by T32GM133395. We thank Neal Silverman for critically reading the manuscript.
Author information
Authors and Affiliations
Contributions
H.C.R. conceptualized, designed, interpreted experiments, analyzed RNA sequencing, and wrote the manuscript, C.L.G. conceptualized and designed peptidoglycan synthesis routes and microarrays, S.C. developed methodology and carried out western blots, immunoprecipitations, RNA isolations and mouse work. R.P. carried out peptidoglycan synthesis and established microarrays, X.L. designed carried out imaging experiments and edited the manuscript, S.L. provided expertise and methodology for immunoprecipitations and mouse characterizations. L.X. and L.G. provided bioinformatics analysis, K.B. S.G. and J.Z. provided expertise for peptidoglycan synthesis, J.C.G. and S.T. provided critical expertise and methodology for the establishment of microarrays and screening. B.J.B. and M.V. contributed to the docking studies with GMTriP-K. A.D. developed and carried out immunostaining of tissue sections. J.Y. and E.R. contribute UC sections and RNAseq data sets.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Vijay Kuchroo, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, S., Putnik, R., Li, X. et al. PGLYRP1-mediated intracellular peptidoglycan detection promotes intestinal mucosal protection. Nat Commun 16, 1864 (2025). https://doi.org/10.1038/s41467-025-57126-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-57126-9