Abstract
In March 2024, clade 2.3.4.4b highly pathogenic avian influenza A(H5N1) viruses were first detected in U.S. dairy cattle. Similar viruses have since caused 70 zoonotic human infections. To assess changes to zoonotic potential, we characterized A(H5N1) clade 2.3.4.4b viruses isolated from cows’ milk and birds. Bovine-derived viruses are lethal in mice and ferrets and transmit to direct but not airborne contact ferrets. All viruses replicate in human bronchial epithelial cells despite preferentially binding avian virus-like receptors. The bovine-derived viruses remain susceptible to FDA-approved antivirals, and they are inhibited by sera from ferrets vaccinated with WHO-recommended candidate vaccine viruses (CVV) or human sera from clade 2.3.4.4c vaccinees. While 2.3.4.4b viruses induce severe disease in mammalian models, they retain many avian virus-like characteristics. Combined, we conclude that the risk of contemporary bovine-derived viruses to humans not in contact with affected animals is low. However, heightened vigilance remains essential to promptly detect and respond to any changes.
Similar content being viewed by others
Introduction
Since its initial identification in 1996, the A/goose/Guangdong/1/1996 (gs/Gd) lineage of highly pathogenic avian influenza (HPAI) A(H5N1) virus has undergone dramatic genetic diversification with the emergence of multiple genetic clades, some of which spread globally. Viruses of this lineage pose a significant public health threat and have a laboratory-confirmed case-fatality rate of around 48% following zoonotic infection1. The true case fatality rate is likely substantially lower than 48% based on seroprevalence studies, including a recent finding in which 2% of U.S. bovine veterinarians were H5 seropositive despite no recollection of recent influenza like illness, and a study showing 4.6% of Egyptian live bird market workers were H5 seropositive2,3. Their impact on avian species has been particularly dramatic, with roughly 170 million poultry culled in the United States (U.S.) alone since 20224,5. Between 2020–2021, viruses with hemagglutinin (HA) clade 2.3.4.4b [A(H5N1) 2.3.4.4b] dominated in many countries6,7. Following widespread circulation in Africa, Asia, and Europe, 2.3.4.4b viruses entered North America in late 2021, representing only the second introduction of gs/Gd-lineage viruses into the continent. Soon after introduction into the Americas, the viruses quickly diversified and acquired internal gene segments from endemic low-pathogenicity avian influenza viruses through reassortment8,9. Further, there was an increase in the number of identified hosts, particularly non-poultry avians and terrestrial and aquatic mammals10,11,12,13,14. The diversification of hosts coupled with a steady isolation of this lineage since 2021 is a concerning pattern with the potential to alter viral evolution and host range.
On March 25, 2024, an A(H5N1) virus-positive milk sample was collected from a dairy cow in the U.S. with subsequent confirmation in cats and wild birds associated with this index farm15,16. The virus was identified as HPAI A(H5N1) 2.3.4.4b, genotype B3.13. To date, the virus has been reported on 1075 dairy farms spanning 17 states. High infectious viral loads, with some samples exceeding 108 TCID50/ml, were reported in raw milk17,18 while the presence of non-infectious viral RNA has been detected in pasteurized commercial milk products sourced from at least 15 states19,20. Seventy laboratory-confirmed human cases in the U.S. have been reported, in California, Colorado, Iowa, Louisiana, Michigan, Missouri, Nevada, Ohio, Oregon, Texas, Washington, Wisconsin, and Wyoming with a majority occurring as a result of occupational exposure to infected cattle or poultry21,22,23. Among these, one death occurred after exposure to backyard poultry in Louisiana24. These zoonotic infections have primarily manifested with conjunctivitis, accompanied by limited respiratory symptoms. Though at least two cases have led to severe disease in patients with potential co-morbidities. As the outbreak among dairy cows continues to spread across the U.S., there is concern the viruses may accumulate mammalian adaptive markers, increasing their risk to humans.
In this study, we compare the in vitro and in vivo phenotypic properties of five bovine viruses and two avian influenza A(H5N1) 2.3.4.4b viruses. The panel of viruses was expanded for antigenic and antiviral studies to include a total of 17 A(H5N1) 2.3.4.4b viruses (Table 1), with an overarching goal to address the potential risks they pose to human health. We investigate various viral characteristics, including replication kinetics in primary human cells, receptor binding preferences, pathogenesis in mice, pathogenesis and transmission dynamics in ferrets, and levels of population immunity. Our findings indicate that these viruses retain avian-like features despite sustained transmission through dairy cattle.
Results
Replication of A(H5N1) 2.3.4.4b viruses in vitro
Replication capacity of emerging influenza viruses in mammalian cells can be an early indicator of viral fitness in non-avian hosts. Accordingly, we assessed viral replication kinetics of A(H5N1) 2.3.4.4b viruses detected in cows (genotype B3.13) and avian hosts (genotypes Minor60-goose/KS/930F and B3.7-goose/LA/957) in two different mammalian cell models, the Madin Darby canine-kidney cell line (MDCK) and primary differentiated normal human bronchial epithelial (NHBE) cells. The avian viruses were selected based on HA phylogeny, with goose/KS/930F being basal to the bovine viruses, and goose/LA/957 sharing a common ancestor but is slightly divergent. All viruses replicated efficiently in both cell types. A(H5N1) 2.3.4.4b viruses grew to high titers in MDCK cells, peaking by 36 h post-infection (hpi) with median titers of 8.1 to 9.4 log10TCID50/mL, higher (P ≤ 0.01) than those of the seasonal CA/09 (H1N1)pdm09 (Fig. 1a). In differentiated NHBE cell cultures, all bovine viruses exhibited productive replication reaching peak titers by ≈72 hpi (8.0 to 8.9 log10TCID50/mL). However, no significant differences were observed in replication kinetics between avian-, bovine- or the human-origin influenza viruses in these cells (Fig. 1b).
a MDCK. b NHBE cells were inoculated at MOI of 0.005 with three representative bovine A(H5N1) 2.3.4.4b, concurrently circulating avian A(H5N1), and human A(H1N1)pdm09 viruses and incubated at 37 °C. The supernatants from MDCK virus-infected cells and apical surface washes from NHBE inserts were collected at the indicated time points and virus titers were determined by TCID50 assay. The data are shown as the mean ± SD from quadruplicate wells. Statistical significance was determined by two-way ANOVA with Tukey’s post-test for multiple comparisons.
Receptor binding specificity of A(H5N1) 2.3.4.4b viruses
To determine whether a shift in receptor preference from the avian virus-like α2,3 linked sialic acids to the human virus-like α2,6 linkage followed transmission in dairy cattle, we evaluated the binding of the bovine viruses to both receptor ligands in solid phase binding assays25. All bovine A(H5N1) 2.3.4.4b viruses tested bound exclusively to the avian virus-like α2,3 linked sialic acid receptors and not to human virus-like receptors (Fig. 2a–e). As expected, the human CA/09 (H1N1)pdm09 virus preferentially bound to α2,6 sialic acids (Fig. 2g) and the avian goose/KS/930F (H5N1) was restricted to α2,3 sialic acids (Fig. 2f). Consistent with the observed binding patterns, the bovine and avian A(H5N1) viruses maintained the avian virus preferred glutamine at HA position 226 (H3 numbering), a position recently shown to be important for receptor preference of the bovine A(H5N1) 2.3.4.4b viruses26. Acquiring or losing N-glycosylation in the globular head of the HA protein can directly influence or alter the affinity of influenza virus towards specific receptors. One of our tested viruses (TX/97794) had an amino acid substitution suggesting potential additional N-glycosylation (caused by HA-A156T), but it retained patterns similar to the other bovine viruses lacking the putative glycosylation site (Supplementary Table 1).
a–e The solid-phase binding assay with five representative bovine A(H5N1) 2.3.4.4b viruses. f concurrently circulating avian A(H5N1) virus. g and human A(H1N1)pdm09 viruses to biotinylated sialylglycopolymers (Neu5Acα3’Lac-Gly-PAA, and 3’SLN-C3-PAA) that are the avian influenza virus preferred receptors and biotinylated sialylglycopolymers (Neu5Acα6’Lac-C2-PAA, and 6’SLN-C3-PAA) that are the human influenza virus preferred receptors. The data are shown as the mean ± SD from duplicate wells and represent one of two independent experiments.
Pathogenicity of A(H5N1) 2.3.4.4b viruses in mice
The high viral loads detected in raw milk from infected cows present a potential risk of human exposure. To investigate pathogenicity of genotype B3.13 of A(H5N1) 2.3.4.4b viruses that were detected in milk as well as the effect of the glycosylation of HA at position 156 (HA-A156T), we determined the 50% mouse lethal dose (MLD50) via intranasal inoculation of mice with serial infectious doses for both viruses (n = 3/virus dose). BALB/c mice inoculated with either bovine/OH/439 (H5N1) or bovine/TX/98638 (H5N1) at doses ranging from 101 to 106 TCID50 per mouse showed that both viruses caused 100% mortality at doses of >101 TCID50 (Fig. 3a, b). Signs of morbidity were observed starting 2–3 days post-inoculation (dpi), including ruffled fur, loss of body weight (Supplementary Fig. 1), and neurologic symptoms associated with movement including ataxia, paralysis, and tremors. All mouse mortality events occurred between 5 to 8 dpi. Bovine/OH/439 (H5N1) and bovine/TX/98638 (H5N1) viruses were lethal to mice with MLD50 values of 1.25 and 1.5 log10TCID50, respectively (Fig. 3a, b). Both A(H5N1) viruses spread systemically in mice, with viral detection in nasal turbinates, lungs, brains, intestines, and livers, at 3 and 4 dpi (Fig. 3c).
a, b BALB/c mice (6-8 weeks old) were lightly anesthetized and inoculated IN with 10-fold-serial dilutions of bovine/OH/439 (H5N1) and bovine/TX/98638 (H5N1) viruses to determine MLD50 for each (n = 3/virus dose). c Virus titer in tissues from infected mice was determined by TCID50 assay in MDCK cells shown as the mean ± SD with individual mouse data represented by shapes. Immunohistochemistry of fixed tissues from A(H5N1) 2.3.4.4b virus-infected mice (n = 3/group) showed virus antigen in nasal epithelium (d, scale bar, 50 µm), lung (e, scale bar, 200 µm), brain (f, scale bar, 2 mm), spinal cord (g, scale bar, 1 mm), liver (h, scale bar, 100 µm), and brown adipose tissue (BAT) (i, scale bar, 100 µm).
Infected mice exhibited widespread and disseminated microscopic lesions consistent with severe viral infection. The upper respiratory tract displayed extensive infection of nasal respiratory epithelia characterized by antigen staining and loss of cilia (Fig. 3d) but surprisingly limited inflammatory cell infiltrates or nasal exudates (Fig. 3e). The lower respiratory tract exhibited extensive necrotizing bronchiolitis and diffuse alveolar damage characterized by necrotic cell debris, septal thickening and necrosis, alveolar collapse, alveolar edema, and fibrin. Antigen-positive cells included both type I and type I pneumocytes (Fig. 3e). Virus dissemination to neural or neural-adjacent tissues was evident by abundant viral antigen in the olfactory epithelia (Supplementary Fig. 2a), randomly distributed throughout the brain (Fig. 3f), proximal spinal cord (Fig. 3g), ganglia, and liver (Fig. 3h). Examination of whole sections of the mouse heads revealed viral antigen staining in other epithelial areas, including the maxillary sinus and Eustachian tubes of the inner ear (Supplementary Fig. 2b–d). Antigen staining was also observed in brown adipose tissues (Fig. 3i), eyes, pituitary gland, incisor teeth, and bone marrow (Supplementary Fig. 2e–i). These data suggest that bovine viruses are highly virulent in mice due to extrapulmonary dispersion and efficient replication soon after inoculation.
Pathogenicity and transmission of A(H5N1) 2.3.4.4b virus in ferrets
To understand if the A(H5N1) 2.3.4.4b viruses are transmissible in mammals, donor ferrets (n = 3) were inoculated with 104 TCID50/0.5 mL of bovine/OH/439. At 1 dpi, each donor ferret was housed with a naïve direct contact (DC) ferret (n = 3) and one ferret was placed in an adjacent cage separated by a perforated barrier as an airborne contact (AC). Donor ferrets displayed elevated temperatures with a mean temperature increase of 1.7 °C (4.4% increase) at 2–3 dpi and infected DC ferrets had a mean temperature increase of 2.7 °C (7.1% increase) at 5–7 dpi, which coincided with the onset of weight loss. Humane endpoints for donors were met by 8 dpi (Fig. 4a, b). Donor ferrets shed virus in nasal washes with peak titers ranging from 6.3–6.7 log10TCID50/mL (Fig. 4c). Two of three naive DC ferrets also became infected, met human endpoints (8-10 dpi), and shed virus in nasal washes to peak titers of 3.5–4.3 log10TCID50/mL between 5–7 days post-contact (dpc) (Fig. 4c). The third DC ferret did not show clinical signs, and the virus was not detected in nasal washes. Virus was not detected in any of the AC ferrets, and they displayed no signs of disease.
Ferrets (n = 3) were lightly anesthetized and IN inoculated with 104 TCID50 units of bovine/OH/439 (H5N1) virus in 500 μl of PBS. a Body weights (% starting weight) and b survival were monitored daily. c Virus titers in nasal washes and d various tissues were determined by TCID50 assay in MDCK cells. At 24 hpi, donor ferrets were placed in direct contact (DC) or aerosol contact (AC) with naïve uninfected ferrets (n = 3). Shapes and colors differentiate between inoculated, DC and AC ferrets. a data are represented as lines for individual ferrets. The data in panels (c) and (d) are shown as the mean ± SD with individual ferret data represented by shapes. The dotted line indicates limit of detection for the assay (1.0 log10TCID50/mL). Weight loss data for AC animals was excluded from panel a to improve visualization of data points for infected animals in the Donor and DC groups.
Tissues were taken from donor and DC ferrets that had reached humane endpoints (6 to 10 dpi) for virus titration. Infectious virus was detected in all tissues collected, including the nasal turbinate, trachea, lungs, brain, liver, and duodenum portion of the small intestine, from two of the three infected donors and two infected DCs. One donor ferret did not have any viable virus detected in the liver or trachea, but virus was recovered from the other tissues (Fig. 4d). Thus, bovine/OH/439 (H5N1) 2.3.4.4b virus spread systemically in multiple tissues of donor ferrets and transmitted to two out of the three naive ferrets by contact route but failed to transmit via the airborne route.
Replication and transmission of A(H5N1) 2.3.4.4b viruses in chickens
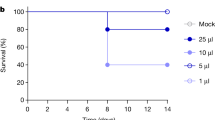
While avian HPAI A(H5N1) 2.3.4.4b viruses cause 100% mortality in chickens27, the pathogenicity of bovine-origin A(H5N1) viruses in experimentally infected poultry is unknown; therefore, we investigated the replication and transmissibility of bovine/OH/439 (H5N1) and goose/KS/930F (H5N1) viruses in chickens to determine if mammalian passage had impacted the viruses’ ability to infect and transmit in poultry. All donor chickens (n = 6/group) died or reached humane endpoints at 2 dpi. In the avian virus group, five contact chickens died or were euthanized at 2 dpc (3 dpi for donors), and the remaining four chickens at 3 dpc. In the bovine virus group, two contact chickens died or were euthanized at 2 dpc, four chickens likewise at 3 dpc, and two chickens survived until 6 dpc (Fig. 5). Oral and cloacal swabs to determine viral titers were collected from live birds at 2, 4, and 6 dpi. These animals rapidly succumbed to infection, and thus, no discernable viral titer trends could be observed (Supplementary Table 5). Therefore, the bovine A(H5N1) 2.3.4.4b virus was highly lethal to chickens and was comparable in pathogenicity to the avian-origin virus.
Chickens (6 weeks old) were inoculated intra-tracheally with 106 EID50/0.3 mL of goose/KS/930F (H5N1) or bovine/OH/439 (H5N1) viruses (n = 6/group). After 16 hpi, two donor chickens from each experimental group were placed in contact with three naive direct contact chickens. The chickens were monitored twice daily for disease symptoms and survival.
Antiviral susceptibility of A(H5N1) 2.3.4.4b viruses
To assess the potential efficacy of available therapeutic options for the control of bovine A(H5N1) 2.3.4.4b infections in humans, we determined the frequencies of genetic markers associated with reduced or highly reduced inhibition (RI/HRI) by FDA-approved influenza neuraminidase (NA) inhibitors (NAIs, oseltamivir, zanamivir, peramivir) or the cap dependent endonuclease inhibitor (CENI, baloxavir). Sequence-based analysis of NA and PA proteins available in public databases revealed low frequencies of bovine A(H5N1) influenza viruses with NAI (0.07%, 1/1489) and CENI (0.21%, 3/1406) RI/HRI-associated substitutions (Supplementary Table 2). A single bovine A(H5N1) virus had NA-T438I, which mediates zanamivir/peramivir RI28. One bovine A(H5N1) virus had PA-A37T that was shown to cause baloxavir reduced susceptibility in human A(H3N2) viruses29. Two viruses had PA-A36T, which has been recently shown to confer baloxavir reduced susceptibility in avian influenza A viruses30. Phenotypic testing confirmed that all bovine A(H5N1) viruses tested were susceptible to NAIs and CENI baloxavir at sub-nanomolar concentrations (Table 2). The results were consistent between bovine viruses from Texas and Ohio. Oseltamivir IC50s of bovine A(H5N1) viruses were slightly lower than contemporary A(H5N1) 2.3.4.4.b viruses circulating in 2022-2023 in birds28. Overall, highly pathogenic influenza A(H5N1) viruses isolated from dairy cattle retain susceptibility to FDA-approved antiviral drugs.
Antigenic relationship of A(H5N1) viruses to WHO candidate vaccine viruses
Analysis of the antigenic relationships between the bovine A(H5N1) 2.3.4.4b viruses and WHO-recommended candidate vaccine viruses (CVVs) was accomplished using post-infection ferret antiserum generated against three clade 2.3.4.4b CVVs [Astrakhan/3212 (H5N8), ck/Ghana/39, and wigeon/SC/345], a well-characterized representative avian clade 2.3.4.4b virus8 [eagle/FL/W22], and the clade 2.3.4.4c CVV [gyrfalcon/41088 (H5N8)] (Fig. 6a, Supplementary Table 3). All viruses tested, including those predicted to have an additional putative glycosylation site in HA (TX/38161, TX/42041, TX/43134, and TX/97794), reacted to within two-fold of homologous titers to at least one of the three clade 2.3.4.4b CVVs. However, it should be noted that 8 of the 16 viruses reacted to within 8-fold of the ferret antiserum raised against the stockpiled gyrfalcon/41088 clade 2.3.4.4c CVV. These data suggest that vaccines made using available clade 2.3.4.4b CVVs may protect against bovine A(H5N1) viruses in the event of a pandemic.
a Antigenic properties of 16 bovine HPAI A(H5N1) 2.3.4.4b viruses were determined in HI assay with post-infection ferret antisera generated against the WHO-recommended CVVs of clade 2.3.4.4b [Astrakhan/3212 (H5N8), ck/Ghana/21 (H5N1), wigeon/SC/345 (H5N1),], and 2.3.4.4c [gyrfalcon/41088 (H5N8)] and representative avian eagle/FL/W22 (H5N1) virus. The data are shown as the mean ± SD with HI titers for each virus as individual circles. The dotted line indicates limit of detection for the assay (HI titer = 10). (★) indicates the endpoint titer of homologous ferret sera to the indicated CVV. b The presence of cross-reactive HA antibodies against bovine A(H5N1) 2.3.4.4b viruses was examined in a set of human sera obtained from a Phase 1 clade 2.3.4.4c H5 vaccine trial. Individuals (aged 18 to 50 years) were vaccinated with adjuvanted 15 µg of the HA derived from gyrfalcon/41088 (H5N8) 2.3.4.4c antigen (n = 20). The data are shown as the mean ± SD. c A set of human sera (n = 24) was obtained from BioIVT (individuals, aged 18 to 46 years, vaccination history unknown). NA activity inhibiting (NI) antibody levels in human serum samples against N1 NA protein derived from CA/09 (H1N1)pdm09, bovine/OH/439 (H5N1), and eagle/FL/W22 (H5N1) viruses (as measured by ELLA assay). The data are shown as geometric mean titer (line) of the individual IC50 NI titers (dots). Statistical significance was determined by two-way ANOVA with Tukey’s post-test for multiple comparisons.****p < 0.0001.
H5 HA subtype neutralizing antibodies in human sera
To further evaluate the utility of existing stockpiled vaccines, we tested a set of human sera obtained from a Phase 1 A(H5N8) clade 2.3.4.4c vaccine trial with donors aged 18 to 50 years31. We examined the presence of cross-reactive HA antibodies against bovine A(H5N1) 2.3.4.4b viruses. Previous studies have shown that HI antibody titers of ≥40 are protective against disease32,33. Antibodies induced by vaccination of individuals with an adjuvanted vaccine (either MF59 or AS03 as described previously by Neuzil, et al.31) containing 15 µg of the HA derived from gyrfalcon/41088 (H5N8) 2.3.4.4c antigen reacted with GMTs ranging from 45.9 to 88.8 to the bovine A(H5N1) viruses (Fig. 6b). Twenty of 20 individuals achieved a seroprotective HI titer of at least 40 against bovine/OH/342, 13 of 20 against bovine/OH/368, 18 of 20 against bovine/TX/40106, and 15 of 20 against bovine/TX/97794 (Supplementary Table 4). Thus, vaccination of some humans with an adjuvanted vaccine using an A(H5N8) 2.3.4.4c antigen induces sufficient HA antibody responses that could offer protection against HA clade-mismatched virus.
N1 NA subtype neutralizing antibodies in human sera
Using a commercially available panel of 24 human sera with unknown vaccination or infection status were screened and selected based on existing A(H1N1)pdm09 virus HA antibody titers (HI ≥ 40), we investigated the extent of cross-reactivity of NA antibodies generated against seasonal influenza virus/vaccine exposure against A(H5N1) 2.3.4.4b NA (Fig. 6c). As expected, all individuals demonstrated neutralization of the CA/09 (H1N1)pdm09 NA with a mean NI titer of 29.0. While mean NI titers were lower for both bovine/OH/439 and eagle/FL/W22 viruses, 17.6 and 14.6, respectively, 14 of 24 sera inhibited NA activity of the bovine N1 from these viruses to roughly 1.5 times above assay background (NI titer of 9), while 13 of 24 sera inhibited NA activity of the avian N1 (Fig. 6c). The N1 NA proteins of the A(H5N1) 2.3.4.4b and A(H1N1)pdm09 viruses have 89.6% amino acid identity, with considerable conservation at some antigenic sites8. Although the levels of protective NA antibodies have not been established, these data suggest that some level of cross-protection against infection with bovine A(H5N1) viruses may be achieved based on NA antibodies generated against seasonal A(H1N1)pdm09 virus in people who have been previously infected and/or vaccinated to seasonal H1N1 influenza viruses.
Discussion
The introduction of HPAI A(H5N1) 2.3.4.4b viruses into U.S. dairy cattle, followed by their spread and zoonotic infections, is unprecedented34,35. The primary objective of our study was to understand if, and to what extent, these viruses have acquired properties suggestive of increased zoonotic risk following replication in dairy cattle. We acknowledge this study could have been more thorough by inclusion of a zoonotic virus isolate, unfortunately, there were none available at the time of study.
Critically, while the bovine A(H5N1) 2.3.4.4b viruses tested were pathogenic and highly virulent in inoculated mice and ferrets, we observed some contact transmission but no airborne transmission in a ferret model. This transmission profile may be partially attributed to the strict preference for avian-like α2,3 linked sialic acid ligands observed in the receptor binding assay. The lack of binding to α2,6 linked sialic acids that we observed contrasts with a study of an early bovine virus, A/dairy cattle/New Mexico/A24920343-93/2024 (H5N1), that demonstrated some degree of human-receptor binding36. These differences may in part be due to assay variations, which measure a limited number of glycans, as studies utilizing large glycan array panels continue to show a clear preference for avian-like ligands37,38,39. Further, the bovine and avian A(H5N1) viruses that have been used in this study maintained the avian virus preferred glutamine and glycine at HA position 226, and 228 (H3 numbering), respectively, as previously described26. Further evidence of avian host preference of the bovine A(H5N1) viruses was demonstrated by the poultry inoculation studies shown here, lack of cow-to-cow transmission in experimentally infected calves40, and the robust presence of avian-like receptor ligands in the bovine mammary gland41 which are readily bound by contemporary H5 proteins42. Despite the remaining restriction to avian-type cellular receptors, the bovine and avian A(H5N1) viruses studied replicated more efficiently in mammalian and differentiated NHBE cells than the human A(H1N1)pdm09 virus. Similar viruses have shown efficient replication potential in several other mammalian-derived cell lines43.
The degree of airborne transmission of the bovine A(H5N1) viruses to exposed ferrets has varied between viruses and studies. While we were unable to detect airborne transmission, studies of the human isolate A/Texas/37/2024 (H5N1) have demonstrated an ability to transmit in ferrets, albeit inefficiently, via this route36,37,44. Nevertheless, there has been no evidence for human-to-human transmission. The acquisition of the mammalian adaptation marker PB2-E627K in some human isolates, along with a recent severe human infection with no poultry or bovine epidemiological link45 does, however, raise concern and highlights the importance of continued laboratory testing of these and future isolates.
The pathogenesis of the bovine A(H5N1) viruses mirror those previously reported36,44. Gu and colleagues34 demonstrated that A/Texas/37/2024 (H5N1) was pathogenic in mice and was able to cause systemic infection similarly to what we observed. The ferret pathogenesis of the bovine/OH/439 A(H5N1) virus used in our study was also like that of A/Texas/37/2024 as reported by others36,37. The major differences between these A/Texas/37/2024 studies and our data was the demonstration of airborne transmission, albeit inefficient, as detailed above. Importantly, A/Texas/37/2024 also contained polymerase basic protein 2 marker of mammalian adaptation PB2-E627K, whereas our animal viruses did not. The severe phenotypes of the A(H5N1) 2.3.4.4b viruses seen in animal models are fortunately not manifesting in the current zoonotic cases. This phenomenon is not well understood but could be attributed to multiple factors including but not limited to route of exposure, pre-existing immunity (discussed below), comorbidities, or mammalian adaptation markers in polymerase protein(s). The exposure dose in zoonotic infections could be high based on viral titers in milk from infected cows being >108 TCID50/ml in some samples, but dairy workers are likely being exposed through milk splashes or fomites which may play a role in why a large proportion of cases are experiencing conjunctivitis over respiratory symptoms.
While current data suggests A(H5N1) 2.3.4.4b viruses do not spread efficiently between humans, they do meet two other criteria that are broadly accepted as requirements for the generation of pandemic viruses: productive replication in humans and antigenic disparity from circulating viruses leading to a lack of robust population immunity to the novel virus antigens, especially in the context of HA46. It is therefore important to understand the effectiveness of pharmacological interventions against 2.3.4.4b viruses in terms of both prophylaxis and treatment. The WHO Global Influenza and Response System (GISRS) routinely consider CVVs for zoonotic influenza, including A(H5N1). In some countries, A(H5N1) vaccines have been stockpiled. A recent study identified some degree of heterologous antibody recognition of Astrakhan/3212 by sera from clade 1 or 2.1 vaccinated individuals47 indicating these earlier stockpiled vaccines may have some efficacy against currently circulating 2.3.4.4b viruses. Supporting this conclusion are studies using serum from clade 2.3.4.4c adjuvanted H5 vaccinees that showed some degree of cross reactivity to clade 2.3.4.4b viruses31. Additionally, it has been reported that a limited number of individuals born prior to 1970 exhibited neutralizing titers against a bovine A(H5N1) virus but the titers were relatively low48. However, a closely matched CVV is likely to provide better protection. Our data indicate that ferret post-infection antisera raised against three 2.3.4.4b CVVs, including Astrakhan/3212, effectively inhibited the HA activity of a majority of bovine A(H5N1) viruses we tested, while ferret post-infection antisera raised against gyrfalcon/41088 2.3.4.4c CVV only reacted with half of the viruses. Further, sera from individuals receiving gyrfalcon/41088 (H5N8) clade 2.3.4.4c adjuvanted vaccine also broadly inhibited 2.3.4.4b bovine viruses, with minor exceptions, including seven participants who had HI titers <40 to bovine/OH/368, which has a novel mutation in antigenic site B. However, most participants had HI titers ≥ 40, which has long been the standard correlate for protection against seasonal influenza viruses33. Humoral protective immunity is dominated by antibody responses to the HA surface glycoprotein, but evidence suggests that some protective immunity may be generated against the lesser abundant NA glycoprotein. While NA-based immunity may still be permissive to viral infection, it may lessen disease severity, decrease viral loads in tissues, and reduce viral shedding49. Importantly, seasonal A(H1N1)pdm09 viruses have an N1 NA as do circulating A(H5N1) viruses, although the genes encoding the two proteins are genetically distinct. It has also recently been reported that ferrets with prior A(H1N1)pdm09 immunity exhibit no mortality and reduced symptoms when challenged with a lethal dose of A(H5N1)48. Correspondingly, we observed neutralization of avian and bovine 2.3.4.4b virus NAs with serum from healthy adults, similar to our previous studies of avian 2.3.4.4b viruses8. While this suggests that N1-based antibody immunity may be effective against circulating A(H5N1) viruses, further in vivo testing is urgently required to fully address this hypothesis. Collectively, current seasonal vaccination strategies, stockpiled pre-pandemic vaccines, and WHO CVVs appear to provide cross-reactivity against currently circulating bovine HPAI A(H5N1) 2.3.4.4b viruses and may be useful if vaccination efforts are deemed necessary for high-risk groups such as those with direct exposure to affected agricultural species. Additionally, our phenotypic testing indicated that circulating bovine viruses remained susceptible to currently available antiviral therapies at sub-nanomolar concentrations, and genotypic testing revealed a low frequency of substitutions associated with reduced drug efficacy. Many of the initial human 2.3.4.4b human infections were treated with the NAI oseltamivir, but current in vitro data show higher oseltamivir IC50 values for A(H5N1) 2.3.4.4b viruses as compared to viruses circulating before 202130,50. It is not understood if this translates to decreased drug efficacy in humans, but it may increase the importance of alternative drug classes. This includes CENI baloxavir marboxil, which targets PA protein and has the potential to treat severe human infections if A(H5N1) 2.3.4.4b acquires genetic signatures of RI by oseltamivir36,37,40,44,51.
Based on the data generated through this assessment, the risk to human health posed by the bovine A(H5N1) viruses in their current form is low, especially for those not exposed to dairy cows, their raw milk, or culling infected poultry flocks. The viruses show inefficient transmission within laboratory models and more importantly, no human-to-human transmission has been reported. We have also demonstrated that currently available antiviral drugs remain effective, and there are vaccines available that offer cross-protection in the event they are needed. Despite this work, the A(H5N1) 2.3.4.4b viruses continue circulating in wild and domestic animals at a large-scale. New A(H5N1) 2.3.4.4b genotypes have since emerged including D1.1, causing a severe infection in British Columbia, Canada, in a teenager with a history of mild asthma and a BMI >35 with no link to poultry or other affected species45 as well as a fatal case in Louisiana in an elderly male with exposure to sick and dying poultry52. Additionally, the D1.1 genotype resulted in a fatal infection in a 3-year-old from Durango, Mexico with no known exposure to infected poultry53. Limited data are available for these cases and virus characteristics. It is imperative that we maintain increased surveillance within wild birds, domestic animals, and humans for potential genetic and phenotypic changes that could have a profound impact on human health.
Methods
Ethics statement
All animal studies were approved by the St. Jude Children’s Research Hospital Institutional Animal Care and Use Committee (IACUC, protocol number 428) in accordance with the guidelines established by the Institute of Laboratory Animal Resources, approved by the Governing Board of the US National Research Council. All animal experiments were carried out by trained personnel working in a U.S. Department of Agriculture (USDA)-inspected Animal Biosafety Level 3+ animal facility in accordance with all applicable regulations. Animal holding rooms were on a 12 h light/12 h dark cycle, with dry-bulb temperatures set below each species lower critical temperature to minimize potential heat stress (71 °C ± 1 °C with alarm points beyond this value). Room humidity was set to 45%, with active humidification of each room allowing ≥40% humidity during winter months. This study utilized A(H5N1) viruses and are subject to the guidelines of, and compliance with, requirements discussed in Title 9 (CFR Parts 121 [Possession, Use, and Transfer of Select Agent Toxins] and 122 [Importation and Transportation of Controlled Organisms and Vectors]).
Cells
Madin–Darby Canine Kidney cells (MDCK, ATCC #CCL-34) were maintained in complete growth medium [MEM (CellGro), 5% FBS (HyClone), 1 mM L-glutamine, 1× penicillin/streptomycin/amphotericin B (Gibco)] at 37 °C, 5% CO2. Differentiated primary normal human bronchial epithelial (NHBE) cells (Mattek, AIR-100) were obtained from one donor (healthy male, 23 years old) and cultured at an air-liquid interface in manufacturer provided media at 37 °C, 5% CO2.
Virus isolation
A total of 15 influenza A(H5N1) 2.3.4.4b genotype B3.13 viruses were isolated from raw milk samples collected on two geographically distinct farms, one in Ohio and one in Texas (Table 1)54. Primary samples were inoculated into the allantoic cavities of 10-day-old embryonated chicken eggs (eggs), incubated at 35 °C for ≤48 h, harvested, and stored at −80 °C. Seasonal human CA/09 (H1N1)pdm09 was propagated in MDCK cells at 37 °C for 48 h. CVVs [Astrakhan/3212 (H5N8) and wigeon/SC/345 (H5N1), a recombinant 6 + 2 ck/Ghana/21 (H5N1) AVL-763 (all kindly shared by CDC)], and an early North American reassortment 6 + 2 clade 2.3.4.4b virus eagle/FL/W22 (H5N1) was generated by reverse genetics with A/Puerto Rico/8/1934 (H1N1) internal gene segments and all were propagated in eggs at 37 °C for 48 h. 50% tissue culture infectious dose (TCID50) or 50% egg infectious dose (EID50) were calculated by Reed and Muench method55 using limiting dilution in MDCK cells or eggs.
Replication kinetics
Multi-round replication curves were performed in MDCK cells (5 × 105 cells/well, 12-well plates), primary NHBE cell (≈1.2 × 106 cells/insert) at MOI of 0.005 of indicated viruses. Monolayer supernatants or a 200 µL apical layer wash for airway cultures were collected at indicated timepoints and titrated by TCID50 assay in MDCK cells.
Receptor binding assay
Fetuin coated immunoassay plates were incubated with 32 HA units of A(H5N1) 2.3.4.4b viruses in blocking buffer (PBS, 1% BSA) then the serially diluted biotinylated sialylglycopolymers [Neu5Acα3’Lac-Gly-PAA, 3’SLN-C3-PAA, Neu5Acα6’Lac-C2-PAA, and 6’SLN-C3-PAA (Sigma-Aldrich)] in the reaction buffer [PBS, 0.02% Tween-80, 0.02 % BSA, 5 µM oseltamivir carboxylate (MedChem Express)] were added and incubated for 2 h at 4 °C. After washing, HRP-conjugated streptavidin (Invitrogen; 1:2000) was added for 1 h at 4 °C. Plates were washed and TMB (3,3′, 5,5′ tetramethylbenzidine dihydrochloride, Sigma-Aldrich) substrate was added for 10 min at room temperature (RT). Reactions were stopped with 1 N H2SO4 and absorbances were measured at 450 nm using a Synergy H1 microplate reader (BioTek Instruments).
Pathogenicity in mice
Six to eight-week-old female BALB/c mice (Jackson Laboratory) were lightly anesthetized with isoflurane and intranasally (IN) inoculated with 10-fold serial dilutions containing 101 to 106 TCID50/mouse of bovine/OH/439 (H5N1) and bovine/TX/98638 (H5N1) viruses (n = 3/virus dose) in a total volume of 20 µL PBS. Mice were weighed and monitored daily for clinical signs for 14 dpi. The 50% mouse lethal dose (MLD50) was calculated for both tested A(H5N1) viruses56. To determine viral replication in BALB/c mice, a group of mice (n = 6/virus) was lightly anesthetized with isoflurane and inoculated intranasally with 104 TCID50 in a total volume of 20 µL PBS. At 3 and 4 dpi, three mice from each tested A(H5N1) virus were euthanized, and tissues (nasal turbinates, lungs, intestines, livers, and brains) were collected, and virus titration was determined by TCID50 assay in MDCK cells.
Histopathology and immunohistochemistry
BALB/c mice (n = 3/group) were sacrificed at 4 dpi and tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4–6 μm thickness and stained with hematoxylin and eosin (H&E). Immunohistochemical (IHC) staining for viral antigen detection was performed using the Ventana Discovery Ultra Autostainer (Roche Ventana). Sections were initially heated for 4 min at 72 °C and placed in EZ prep solution (Roche Ventana) for deparaffinization. Antigen retrieval was performed for 56 min at 95 °C in Cell Conditioning Solution 1 (Roche Ventana). A rabbit primary monoclonal antibody (GeneTex) raised against NP protein of A/Kansas/14/2017(H3N2) virus was applied at 1:8000, and the OmniMap anti-rabbit HRP (per manufacturer instructions, Roche Life Science) and ChromoMap DAB detection kits (Roche Ventana) were used to label virus-positive cells. Lung sections were then counterstained with hematoxylin and examined by a pathologist blinded to the experimental group assignments.
Pathogenicity and transmission in ferrets
Six-month-old influenza-seronegative male ferrets (Triple F Farms) were IN inoculated with 104 TCID50 units of bovine/OH/439 (H5N1) virus in 500 µL of PBS. One direct contact ferret was placed in the same cage as each inoculated animal 24 hpi. To model aerosol transmission, one ferret was placed in an adjacent cage with a perforated barrier allowing airflow and excluding contact. This setup was performed in triplicate. Animals were monitored daily for clinical illness (temperature, weight loss, relative inactivity indices, ataxia, respiratory symptoms, neuropathologic signs)57. Body temperature was measured using subcutaneous implantable transponders (Avidity Science). Nasal washes were collected by intramuscular (IM) ketamine injection (25 mg/kg) and IN instillation of 1 mL PBS to induce sneezing starting 1 dpi and alternating days thereafter for a total of 7 collections. Any ferret reaching the humane endpoint or found dead had tissues collected for virus isolation and pathology. Humane endpoints were predetermined based on a scoring guide considering weight loss, evidence of neurologic activity, behavioral changes, body temperature, and overt clinical signs and approved by the St. Jude IACUC. Tissues (nasal turbinate, trachea, lung, brain, liver, and small intestine) were homogenized and virus titers were determined by TCID50 in MDCK cells.
Experimental infection of chickens
White leghorn chickens (AVS Bio) were inoculated with 106 EID50/0.3 mL via intra-tracheal route (IT) with goose/KS/930F (H5N1) or bovine/OH/439 (H5N1) viruses (n = 6/group). After 16 hpi, two donor chickens from each experimental group were placed in contact with three naive direct contact chickens in replicates of three cages, except for one replicate of the avian virus, for which only two contact chickens were available. Chickens were monitored twice daily for disease symptoms and euthanized according to IACUC approved humane endpoint protocols if disease signs were observed.
Antiviral susceptibility
Sequence data for NA (n = 1489) and PA (n = 1406) proteins of bovine influenza viruses available publicly through the Global Initiative on Sharing All Influenza Data (GISAID) database (https://gisaid.org, accessed November 8, 2024) were screened for genetic markers associated with RI/HRI by NAIs and CENI baloxavir according to the World Health Organization (WHO) Global Influenza Program 2024 guidelines58,59. Nucleotide sequences were aligned using progressive (FFT-NS-2) and iterative (FFT-NS-i) algorithms of Multiple Alignment Fast Fourier Transform60. Amino acid sequences were analyzed with BioEdit (v.7.7.1) software. Phenotypic susceptibility to NAIs (oseltamivir carboxylate [oseltamivir] and zanamivir [MedChem Express]) was assessed with a fluorescence-based assay with 2′-(4-methylumberlliferyl)-α-D-N-acetylneuraminic acid (MUNANA) substrate (Sigma-Aldrich)61. NA activity of each virus was standardized to relative fluorescent unit equivalents of 10 µM 4-methylumbelliferone (4-MU)62. After 30 min of incubation with NAI (5 pM - 50 µM) at 37 °C, fluorescent NA-cleaved MUNANA substrate was measured with a Synergy 2 multimode microplate reader (BioTek Instruments) at Ex/Em 360/460 nm. The concentration of drug required to inhibit 50% of the NA activity signal of non-drug containing samples (IC50s) were estimated from dose–response curves by using the sigmoidal, four-parameter logistic non-linear regression equation (GraphPad Prism v.10.1.2). Phenotypic susceptibility to CENI baloxavir marboxil active metabolite baloxavir acid (baloxavir [MedChem Express]) was determined by Influenza Replication Inhibition NA-based Assay (IRINA) in MDCK cells63. Virus inoculum was standardized to the fluorescent signal equivalent to 1.9 nM/well of 4-MU and incubated with baloxavir (6 pM - 111 nM) on cell monolayer (96-well microplates, 8 h at 37 °C) without TPCK-treated trypsin to achieve a single cycle of virus replication. NA activity of the infected cells was measured, and baloxavir half-maximal effective concentrations (EC50s) were calculated as described for NAI assay.
Serologic testing and virus antigenicity
Antigenic cross-reactivity and/or seroconversion of post-challenge ferret sera was assessed using the cattle-derived viral antigens with post-infection ferret antisera (Supplementary Table 3) in an HI assay. Human sera were obtained from a Phase 1 A(H5N8) clade 2.3.4.4c vaccine trial. Individuals (aged 18 to 50 years) were vaccinated with adjuvanted (15 µg) of the HA derived from gyrfalcon/41088 (H5N8) 2.3.4.4c antigen in (n = 20). Briefly, sera samples were treated with receptor-destroying enzyme II (Denka Seiken Co.) and were serially diluted and incubated with 4 HA units of respective virus for 45 min at RT before the addition of 0.5% chicken erythrocytes (Rockland Immunochemicals). HI titers were recorded after 30 min incubation at RT as the reciprocal of the highest serum dilution with complete inhibition of hemagglutination.
Enzyme-linked lectin assay
The presence of NA-specific antibodies in human was determined in enzyme-linked lectin assays (ELLAs) as previously described64. The influenza A(H6N1) viruses used in ELLA assay were generated by reverse genetics, all having the same HA gene from A/Teal/Hong Kong/w312/1997 (H6N1) virus, six internal segments from A/Puerto Rico/8/1934 (H1N1) virus and the NA gene of bovine/OH/439 (H5N1) virus, eagle/FL/W22 (H5N1), or CA/09 (H1N1)pdm09 viruses. The flat-bottom, 96-well plates (Thermo Scientific) were coated with fetuin (Sigma-Aldrich) at 25 g/mL in 0.1 M PBS at 4 °C for 48 h. Heat-inactivated human sera (56 °C for 1 h) that were commercially obtained from BioIVT (individuals, aged 18 to 46 years, vaccination history unknown) were titrated by serially diluted in Dulbecco’s phosphate-buffered saline (DPBS), 1% BSA, 0.5% Tween-20 and added to plates. An optimized amount of rg–derived H6N1 virus was added, and the plates were incubated for 18 h at 37 °C. After three washing, horseradish peroxidase–conjugated peanut agglutinin (Sigma-Aldrich) was added, and the plates were incubated for 2 h at RT. After washing, 3,3’,5,5’-tetramethylbenzidine (Sigma-Aldrich) substrate was added. Reactions were stopped after 10 min by adding 1 N H2SO4 and the plates were read at 490 nM using a Synergy H1 microplate reader. The NI titers were defined as the reciprocal of the last dilution that resulted in at least 50% inhibition using nonlinear regression analysis of GraphPad Prism software (version 10.1.2).
Statistical analysis
Data were analyzed using two-way Anova with multiple comparisons in GraphPad Prism v.10.1.2. Replicates, group comparisons, and P values are listed in each figure legend.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data generated in this study are provided in the main manuscript, figures, supplemental figures, and in source data files. Source data are provided with this paper.
References
WHO. Avian Influenza Weekly Update # 990: 21 March 2025. 4 (2025).
Leonard, J. H. et al. Notes from the field: seroprevalence of highly pathogenic avian influenza a(H5) virus infections among bovine veterinary practitioners — United States, September 2024. MMWR and Morbidity and Mortality Weekly Report 74 https://doi.org/10.15585/mmwr.mm7404a2 (2025).
Gomaa, M. et al. We are underestimating, again, the true burden of H5N1 in humans. Bmj Glob. Health 8, e13146 (2023).
WHO. Global Influenza Program, (https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who-2003-2024, 2024).
CDC. H5N1 Bird Flu Detections across the United States in Backyard and Commercial Poultry, https://www.cdc.gov/bird-flu/situation-summary/data-map-commercial.html (2024).
Caliendo, V. et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci. Rep. 12, 11729 (2022).
Pohlmann, A. et al. Has epizootic become enzootic? Evidence for a fundamental change in the infection dynamics of highly pathogenic avian influenza in Europe, 2021. mBio 13, e0060922 (2022).
Kandeil, A. et al. Rapid evolution of A(H5N1) influenza viruses after intercontinental spread to North America. Nat. Commun. 14, 3082 (2023).
Youk, S. et al. H5N1 highly pathogenic avian influenza clade 2.3.4.4b in wild and domestic birds: introductions into the United States and reassortments, December 2021-April 2022. Virology 587, 109860 (2023).
Plaza, P. I., Gamarra-Toledo, V., Eugui, J. R. & Lambertucci, S. A. Recent changes in patterns of mammal infection with highly pathogenic avian influenza A(H5N1) virus worldwide. Emerg. Infect. Dis. 30, 444–452 (2024).
National Wildlife Health Centre, U. Highly pathogenic avian influenza H5N1 virus (HPAIv) has been present in North America since its first detection in wild birds in November 2021 (Newfoundland and Labrador, Canada, and has subsequently spread across the Americas. (2023).
Puryear, W. et al. Highly pathogenic avian influenza A(H5N1) virus outbreak in New England Seals, United States. Emerg. Infect. Dis. 29, 786–791 (2023).
Harvey, J. A., Mullinax, J. M., Runge, M. C. & Prosser, D. J. The changing dynamics of highly pathogenic avian influenza H5N1: next steps for management & science in North America. Biol. Conserv 282, 110041 (2023).
Murawski, A. et al. Highly pathogenic avian influenza A(H5N1) virus in a common bottlenose dolphin (Tursiops truncatus) in Florida. Commun Biol 7 ARTN 476 https://doi.org/10.1038/s42003-024-06173-x (2024).
Burrough, E. R. et al. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Emerg. Infect. Dis. 30, 1335–1343 (2024).
Hu, X. et al. Genomic characterization of highly pathogenic avian influenza A H5N1 virus newly emerged in dairy cattle. Emerg. Microbes Infect. 13, 2380421 (2024).
Caserta, L. C. et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 634, 669–676 (2024).
Guan, L. et al. Cow’s milk containing avian influenza A(H5N1) virus - heat inactivation and infectivity in mice. N. Engl. J. Med 391, 87–90 (2024).
Spackman, E. et al. Characterization of highly pathogenic avian influenza virus in retail dairy products in the US. J. Virol. 98, e0088124 (2024).
FDA. Updates on Highly Pathogenic Avian Influenza (HPAI), https://www.fda.gov/food/alerts-advisories-safety-information/updates-highly-pathogenic-avian-influenza-hpai#secondtesting (2024).
Uyeki, T. M. et al. Highly pathogenic avian influenza A(H5N1) virus infection in a dairy farm worker. New Engl. J. Med. 390, 2028–2029 (2024).
CDC. CDC Confirms Second Human H5 Bird Flu Case in Michigan; Third Case Tied to Dairy Outbreak, https://www.cdc.gov/media/releases/2024/p0530-h5-human-case-michigan.html (2024).
CDC. CDC Reports Fourth Human Case of H5 Bird Flu Tied to Dairy Cow Outbreak, https://www.cdc.gov/media/releases/2024/p-0703-4th-human-case-h5.html#:~:text=This%20is%20the%20fourth%20case,for%20A(H5N1)%20virus (2024).
CDC. H5 Bird Flu: Current Situation, https://www.cdc.gov/bird-flu/situation-summary/index.html (2024).
Rogers, G. N. & Paulson, J. C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127, 361–373 (1983).
Lin, T. H. et al. A single mutation in bovine influenza H5N1 hemagglutinin switches specificity to human receptors. Science 386, 1128–1134 (2024).
Swayne, D. E. & Suarez, D. L. Highly pathogenic avian influenza. Rev. Sci. Tech. 19, 463–482 (2000).
Andreev, K. et al. Antiviral susceptibility of highly pathogenic avian influenza A(H5N1) viruses circulating globally in 2022-2023. J. Infect. Dis. 229, 1830–1835 (2023).
Omoto, S. et al. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci. Rep. 8, 9633 (2018).
Andreev, K. et al. Genotypic and phenotypic susceptibility of emerging avian influenza A viruses to neuraminidase and cap-dependent endonuclease inhibitors. Antivir. Res 229, 105959 (2024).
Neuzil, K. M. et al. Safety and Immunogenicity of influenza A/H5N8 virus vaccine in healthy adults: durability and cross-reactivity of antibody responses. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciac982 (2023).
Hobson, D., Curry, R. L., Beare, A. S. & Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. (Lond). 70, 767–777 (1972).
Coudeville, L. et al. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med. Res. Methodol. 10, 18 (2010).
Kozlov, M. & Mallapaty, S. Bird flu outbreak in US cows: why scientists are concerned. Nature 628, 484–485 (2024).
Nguyen, T.-Q. et al. Emergence and interstate spread of highly pathogenic avian influenza A(H5N1) in dairy cattle. Science 388, eadq0900 (2025).
Gu, C., et al. A human isolate of bovine H5N1 is transmissible and lethal in animal models. Nature 636, 711–718 (2024).
Pulit-Penaloza, J. A. et al. Transmission of a human isolate of clade 2.3.4.4b A(H5N1) virus in ferrets. Nature 636, 705–710 (2024).
Santos, J. J. S. et al. Bovine H5N1 influenza virus binds poorly to human-type sialic acid receptors. preprint at bioRxiv https://doi.org/10.1101/2024.08.01.606177 (2024).
Chopra, P. et al. Receptor Binding Specificity of a Bovine A(H5N1) Influenza Virus. preprint at bioRxiv https://doi.org/10.1101/2024.07.30.605893 (2024).
Halwe, N. J. et al. H5N1 clade 2.3.4.4b dynamics in experimentally infected calves and cows. Nature 637, 903–912 (2024).
Nelli, R. K. et al. Sialic acid receptor specificity in the mammary gland of dairy cattle infected with highly pathogenic avian influenza A(H5N1) virus. Emerg. Infect. Dis. 30, 1361–1373 (2024).
Rios Carrasco, M., Grone, A., van den Brand, J. M. A. & de Vries, R. P. The mammary glands of cows abundantly display receptors for circulating avian H5 viruses. J. Virol. 98, e0105224 (2024).
Meliopoulos, V. et al. Susceptibility of bovine respiratory and mammary epithelial cells to avian and mammalian-derived clade 2.3.4.4b H5N1 highly pathogenic avian influenza viruses. preprint at bioRxiv https://doi.org/10.1101/2025.01.09.632235 (2025).
Eisfeld, A. J. et al. Pathogenicity and transmissibility of bovine H5N1 influenza virus. Nature 633, 426–432 (2024).
Jassem, A. N. et al. Critical illness in an adolescent with influenza A(H5N1) virus infection. N. Engl. J. Med. 392, 927–929 (2024).
McCullers, J. A. Preparing for the next influenza pandemic. Pediatr. Infect. Dis. J. 27, S57–S59 (2008).
Khurana, S. et al. Licensed H5N1 vaccines generate cross-neutralizing antibodies against highly pathogenic H5N1 clade 2.3.4.4b influenza virus. Nat. Med. 30, 2771–2776 (2024).
Le Sage, V. et al. Influenza A(H5N1) immune response among ferrets with influenza A(H1N1)pdm09 immunity. Emerg. Infect. Dis. 31, 477–487 (2025).
Zhang, X. & Ross, T. M. Anti-neuraminidase immunity in the combat against influenza. Expert Rev. Vaccines 23, 474–484 (2024).
Nguyen, H. T. et al. Antiviral susceptibility of clade 2.3.4.4b highly pathogenic avian influenza A(H5N1) viruses isolated from birds and mammals in the United States, 2022. Antivir. Res 217, 105679 (2023).
Baker, A. L. et al. Dairy cows inoculated with highly pathogenic avian influenza virus H5N1. Nature 637, 913–920 (2024).
LDH. LDH reports first US. H5N1-related human death, https://ldh.la.gov/news/H5N1-death (2025).
WHO. Avian Influenza A(H5N1) - Mexico, https://www.who.int/emergencies/disease-outbreak-news/item/2025-DON564 (2025).
Scott Krauss, D. W. & Webster, R. G. In influenza virus 865 Methods in Molecular Biology (ed Gabriele Neumann Yoshihiro Kawaoka) Ch. 2, 234 (Humana Totowa, 2012).
Reed, L. J. & Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 27, 493–497 (1938).
REED, L. J. & MUENCH, H. A simple method of estimating fifty per cent endpoints12. Am. J. Epidemiol. 27, 493–497 (1938).
Maher, J. A. & DeStefano, J. The ferret: an animal model to study influenza virus. Lab Anim. 33, 50 (2004).
WHO. Summary of polymerase acidic (PA) protein amino acid substitutions analysed for their effects on baloxavir susceptibility, https://www.who.int/publications/m/item/summary-of-polymerase-acidic-(pa)-protein-amino-acid-substitutions-analysed-for-their-effects-on-baloxavir-susceptibility (2024).
WHO. Summary of neuraminidase (NA) amino acid substitutions associated with reduced inhibition by neuraminidase inhibitors (NAIs) among avian influenza viruses of Group 1 and Group 2 NAs, https://www.who.int/publications/m/item/summary-of-neuraminidase-(na)-amino-acid-substitutions-associated-with-reduced-inhibition-by-neuraminidase-inhibitors-(nais)-among-avian-influenza-viruses-of-group-1-and-group-2-nas (2024).
Katoh, K., Misawa, K., Kuma, K. & Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Leang, S. K. & Hurt, A. C. Fluorescence-based neuraminidase inhibition assay to assess the susceptibility of influenza viruses to the neuraminidase inhibitor class of antivirals. J. Vis. Exp. https://doi.org/10.3791/55570 (2017).
Marathe, B. M., Lévêque, V., Klumpp, K., Webster, R. G. & Govorkova, E. A. Determination of Neuraminidase Kinetic Constants Using Whole Influenza Virus Preparations And Correction For Spectroscopic Interference By A Fluorogenic Substrate. Plos ONE 8, e71401 (2013).
Patel, M. C. et al. An optimized cell-based assay to assess influenza virus replication by measuring neuraminidase activity and its applications for virological surveillance. Antivir. Res. 208, 105457 (2022).
Couzens, L. et al. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. J. Virol. Methods 210, 7–14 (2014).
Acknowledgments
This project was funded in whole or in part by federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract 75N93021C00016 and by St. Jude ALSAC. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We gratefully acknowledge the efforts of the GISAID Data Science Initiative and associated public databases that contributed to this study, and the U.S. CDC for reagents including Astrakhan/3212 (H5N8) and wigeon/SC/345 (H5N1), and recombinant 6 + 2 ck/Ghana/21 (H5N1) AVL-763.
Author information
Authors and Affiliations
Contributions
Each author participated in scientific discussion of this manuscript and provided comments, critiques, and/or approvals prior to submissions. R.J.W., E.A.G., T.P.F., A.K., C.S.D., R.L.P., and A.S.B. conceptualized the research project(s). T.P.F., A.K., W.N.H., J.C.J., T.J., K.A., P.S., J.F., M.L.D., J.C.C., J.F., J.D., P.V., and C.S.D. conceived methodologies and/or acquired data. W.N.H., T.P.F., A.K., P.S., K.A., and J.C.J. provided data visualization. RJW and EAG administered the projects. R.J.W., E.A.G., T.P.F., A.K., and J.C.J. wrote the original draft, and all other authors reviewed and edited subsequent drafts. Funding was acquired by R.J.W., A.S.B., and R.L.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fabrizio, T.P., Kandeil, A., Harrington, W.N. et al. Genotype B3.13 influenza A(H5N1) viruses isolated from dairy cattle demonstrate high virulence in laboratory models, but retain avian virus-like properties. Nat Commun 16, 6771 (2025). https://doi.org/10.1038/s41467-025-61757-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61757-3