Abstract
Sepsis-associated acute kidney injury (SA-AKI) portends severe health burden due to significant morbidity and mortality, while early diagnosis remains challenging. In this study, proximity-dependent barcoding assay (PBA) is established to profile the surface proteome of single urinary extracellular vesicle (uEV). Principle uEV clusters with unique function and origination are profiled in SA-AKI in a screening cohort. Complement receptor CD35 on single uEV (CD35-uEV) displays high diagnostic accuracy for SA-AKI (AUC-ROC 0.89 in validation cohort, n = 134). Besides, CD35-uEV enables identification of subclinical AKI (AUC-ROC 0.84 in prospective cohort, n = 72). Moreover, CD35-uEV correlates closely with AKI severity which also predicts persistent AKI (AUC-ROC 0.77), mortality risks (AUC-ROC 0.70) and progression to AKD (AUC-ROC 0.66). Multi-omics profiling reveals that CD35-uEV are predominantly released from injured podocytes exhibiting diminished CD35 expression. Overall, this study identifies a single uEV biomarker related to injured podocyte for early diagnosis and risk stratification of SA-AKI.
Similar content being viewed by others

Introduction
Sepsis-associated acute kidney injury (SA-AKI) is a prevalent complication in septic patients and a leading cause of in-hospital mortality1. Approximately 30–50% of sepsis patients develop AKI, with mortality rates reaching up to 50%2. At present, the diagnosis of SA-AKI relies on serum creatinine levels and urine output, which are insensitive and nonspecific for kidney injury. In the last decade, several biomarkers are in developing to identify patients at risk of SA-AKI3. However, early injury markers are still lacking, leading to the challenge of timely recognition and intervention in clinic practice.
Extracellular vesicles (EVs) are membrane-bound structures secreted by nearly all cell types, playing pivotal roles in diverse pathophysiological processes through mediating intercellular communication4. EVs are ubiquitously present in body fluids such as blood, urine, and cerebrospinal fluid, rendering them invaluable sources for disease biomarker discovery5,6,7. The non-invasive feature of urinary extracellular vesicles (uEVs) collection, coupled with their specific cargo from parent cells such as proteins, RNAs, underscores the significant potential in biomarker research8. A notable study from Miranda et al. identified the presence of mRNAs encoding proteins from all regions of the nephron and the collecting duct in uEVs indicating the rationality of uEVs as non-invasive injury biomarker of kidney disease9. Accordingly, uEVs biomarkers reflecting the severity of histological inflammation and fibrosis were reported in recent years10,11,12,13,14.
However, the heterogeneity of uEV and complex cargo hinders the development of accurate biomarkers for clinic translation. This diversity makes it challenging to search for potential biomarkers from mixed clusters, especially for those rare-event related information. Hence, discrimination of subcluster constitution is crucial to minimize interference from irrelevant EVs and to realize the full potential of uEVs for precise disease diagnosis. The advancements in single-vesicle analysis have made it possible to facilitate the development of useful biomarkers on level of single EV15. Recently, it was reported that droplet digital immuno-PCR could be employed to analyze surface proteins on individual vesicle, allowing for the subcluster analysis of plasma EVs to identify biomarker of breast cancer16. Therefore, single uEV analysis may provide a promising approach for delineating the composition of uEVs to identify critical subcluster as biomarker of renal injury.
Proximity barcoding assay (PBA) technology captures EVs with subsequent single-round rolling circle amplification (RCA) and DNA barcodes sequencing to detect proteome of individual EV. This approach provides unique potential to discriminate uEV heterogeneity, which meanwhile elucidates the function and origin of specific subclusters17. In this work, we aim to leverage PBA technology to delineate the subcluster composition of uEVs during SA-AKI. We identified a uEV subpopulation predominantly marked by complement receptor CD35, which significantly decreases during SA-AKI and demonstrates accurate diagnostic performance in the early detection and prognostic assessment of SA-AKI. Multi-omics analysis revealed that CD35 was localized to injured podocytes, which may represent an injury marker for early diagnosis and intervention of SA-AKI.
Results
Study design and patients
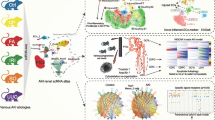
To screening urinary biomarkers for SA-AKI at the single uEVs level, uEV from 8 SA-AKI and 8 sepsis non-AKI patients were applied for PBA proteome assay and the main uEV subpopulations with proportional difference were identified. Then, the protein biomarkers on single EVs were analyzed from the main differential subclusters which were subsequently validated in two independent cohorts: patients with clinically diagnosed SA-AKI (SA-AKI cohort, n = 134) and prospective cohort of sepsis patients who had not yet been clinically diagnosed with AKI (E-AKI cohort, n = 72), assessing the potential of this biomarker for early diagnosis and prognosis of SA-AKI. The origin of this biomarker and its relevance to renal injury was explored through integrative single-cell RNA sequencing and spatial transcriptomics analysis. Finally, diagnostic performance was evaluated through combination of the biomarker with the well-established biomarker TIMP2*IGFBP7. A detailed workflow is provided in Fig. 1.
To explore urinary biomarkers for sepsis-associated AKI (SA-AKI) at the single uEV level, surface proteome was detected by using proximity barcoding assay (PBA) assay in the screening cohort (SA-AKI and non-AKI sepsis patients, n = 8 for each group). Next, the main differential uEV subpopulations in SA-AKI compared to non-AKI was characterized. Protein biomarker on single uEV was then identified and validated in two independent cohorts: a SA-AKI cohort (n = 134) and a subclinical E-AKI cohort (n = 72). By integrating single-cell RNA sequencing and spatial transcriptomics, the cellular origin and its capacity to reflect renal cellular injury of the biomarker was explored. Finally, diagnostic performance was evaluated through combination of the useful biomarker with the well-established biomarker TIMP2*IGFBP7. (Image was Created in BioRender. Tang, T. (2025) https://BioRender.com/su096gu).
uEVs characteristics and clustering
To profile the composition of uEVs, small EVs were purified from a screening set of sepsis patients, both with and without AKI, and subjected to PBA analysis. The basic clinical characteristics of these patients were presented in Supplementary Table 1. The presence of uEVs was validated through the analysis of established EV protein markers, including Alix, TSG101, and CD81, as detected by WB analysis with EV samples purified from equal volume of urine. Significantly increased levels of EV-related protein markers were observed in the AKI group compared to non-AKI patients, indicating enhanced EV secretion into the urine in related to the same urine volume (Fig. 2A). This finding was further supported by quantification of uEVs by acetylcholinesterase activity measurement (Fig. 2B). Transmission electron microscopy (TEM) images showed typical cup-shaped morphology of the membrane structures (Fig. 2C). Nanoparticle tracking analysis (NTA) revealed a typical size distribution of uEVs, with most of the vesicles ranging from 50 to 200 nm in diameter (Fig. 2D).
A Western blot analysis confirming the presence of uEV markers Alix, TSG101, and CD81 (Non_AKI, n = 3, AKI, n = 3). uEVs samples purified from equal volume of 24-h urine were loaded. Data are presented as mean with SD (Paired two-tailed t-test); B uEV particle counts in the non-AKI and AKI groups of sepsis patients detected by SBI EV quantitation kit (Non_AKI, n = 6, AKI, n = 6, paired two-tailed t-test); Morphology and size characterization of uEV from AKI patients were characterized with transmission electron microscopy (TEM) (C) and nanoparticle tracking analysis (NTA) (D), the experiment was repeated three times; E Dimensionality reduction clustering analysis identifying 32 distinct uEV subclusters; F Distribution patterns of the 32 uEV subclusters in SA-AKI and non-AKI groups; G Protein characterization of the top two proteins for the main uEV subclusters, highlighting their unique protein signature. Source data are provided as a Source Data file.
Through establishing a target proteome analysis via PBA technology (protein tags and captured protein readings are shown in Supplementary Fig. 1), dimensionality reduction and clustering of uEVs were performed to characterize the landscape of uEVs. Visualization of uEVs by t-SNE identified 32 subgroups, displaying significant differences in the composition between the AKI and non-AKI groups (Fig. 2E, F). Among the 32 subclusters, clusters 0–10 were recognized as the main clusters, with proportions varied 1-30% among the total uEV populations. The principal proteins ranked top two were shown to display the molecular feature of each cluster, such as siglec10/CD33, CD35/CD21, and AQP1/AQP2, indicating a tubular origin or functions related to immune regulation for the specific cluster (Fig. 2G). Therefore, PBA assay was successfully established for delineating the subcluster composition of uEVs.
The complement receptor-related uEV subcluster demonstrates potential in diagnosing SA-AKI among the top 10 subclusters, 5 subclusters that exhibited the most pronounced proportional differences between the AKI and non-AKI groups were identified, which showed distinct distributions as indicated by UMAP plots (Fig. 3A, B). These clusters were defined as endothelial cell-derived expressing PECAM1, EMCN, and VCAM1 (cluster 0, EC-EV), tubular epithelial cell-derived expressing tight junction proteins CLDN1, CLDN10, and CLDN19 (cluster 6, TEC-EV), complement receptor-related cluster expressing CD35 and CD21(cluster2, CMR-EV), immune response-related EVs with CD38, CD26, TICAM2 (cluster1, IMR-EV) and lysosome-related cluster expressing LAMP1, LAMP2 (cluster4, LYS-EV) (Fig. 3C).
A UMAP plot distribution of the 5 uEV subclusters with the most significant proportional differences (endothelial cell derived EV: EC-EV, Tubular epithelial-derived EV: TEC-EV, Complement receptor related EV: CMR-EV, Lysosome related EV: LYS-EV, immune response related EVs: IMR-EV); B UMAP plot distribution of these 5 subclusters between AKI and non-AKI groups; C Key characteristic proteins of the 5 uEV subclusters; D Proportional distribution patterns of the 5 uEV subclusters across AKI stage (stage 1 vs. stages 2-3) were resolved through sample-specific proportional weighting normalization; E Proportional distribution of the 5 uEV subclusters in transient and persistent AKI; F Radar chart highlighting CMR-uEV as the most significantly different cluster between SA-AKI and non-AKI groups (Mann–Whitney U two-sided test, SA-AKI, n = 8, Non-AKI, n = 8); G, H Second polynomial distribution revealed significant trends (ptrend = 0.047) in the proportional distribution of CMR-uEV in SA-AKI patients with different duration (G) (Non-AKI, n = 8; Transient-AKI, n = 3, Persistent-AKI, n = 5) and stages (H) (Non-AKI, n = 8; AKI-stage1, n = 2, AKI-stage2-3, n = 6). Data are presented as box plots showing the median (middle line), the 25th and 75th percentiles (box limits), the minimum and maximum values (whiskers), and outliers (individual points) Source data are provided as a Source Data file.
To determine whether the proportions of these 5 clusters are associated with the severity and prognosis of AKI, the proportions of each cluster across different AKI stages and durations were analyzed (Fig. 3D). By assigning proportional weights to each sample, we quantified uEV subcluster variations across different groups, providing a comprehensive overview of their trends in transient and persistent AKI (Fig. 3E). We observed that the proportions of the TEC-EV and EC-EV clusters characterized with normal cellular markers were reduced in stages 2-3 (severe AKI) and persistent AKI, probably due to the reduced expression of these markers in healthy renal tubule epithelial and endothelial cells as previously demonstrated in single-cell transcriptome studies18,19. Notably, the proportions of the CMR-EV showed a remarkable decrease in SA-AKI patients compared to Non-AKI. In contrast, the IMR-EV cluster displayed upregulated proportion in severe and persistent SA-AKI, likely reflecting the aberrant immune activation20 (Fig. 3D, E).
To eliminate statistical bias caused by individual variation, we conducted a Mann–Whitney U test to analyze the proportion differences of these 5 uEV clusters between AKI and non-AKI sepsis patients. The results showed that the CMR-EV cluster exhibited a significant difference between the two groups (Fig.3F). Although the IMR-EV subcluster was markedly upregulated in severe and persistent SA-AKI, it showed no significant difference when comparing SA-AKI to non-AKI. To further elucidate the association between CMR-EVs and the severity and prognosis of AKI, a quadratic polynomial model demonstrated stage-dependent (R² = 0.69, trend test p < 0.05) and duration-sensitive associations (R² = 0.69, trend test p < 0.05) of CMR-EV proportions with AKI progression trajectories (Fig. 3G, H). Hence, the characterization of the main differential subclusters of uEVs was delineated, identifying the complement receptor-related uEV subcluster with diagnostic potential for SA-AKI.
Complement receptor CD35 on single uEV reduced remarkably in SA-AKI
Through the analysis of uEV cluster proportions, we identified a significant reduction in the CMR-EV cluster in AKI patients. This finding prompted us to explore whether proteins on individual CMR-EV cluster could serve as biomarkers of SA-AKI, providing an alternative to CMR-EV proportion for easy application. To verify this hypothesis, we used the limma package to identify differentially expressed proteins between the AKI and non-AKI groups, normalized to EVs count. The results showed that 44 proteins exhibited differential expression at the single EV level, with CD35 and CD21 ranking as the top ones, which were indeed the signature proteins of the CMR-EV cluster (Fig. 4A). Principal component analysis (PCA) demonstrated that these differential proteins could effectively distinguish SA-AKI from non-AKI individuals (Fig. 4B). Two unsupervised clustering algorithms, consensus clustering and non-negative matrix factorization (NMF) were then applied to validate this finding. The results revealed that consensus clustering achieved 100% accuracy in patient grouping, whereas NMF misclassified only two AKI patients (AKI05 and AKI08) (Supplementary Fig. 2). Additionally, KEGG enrichment analysis revealed that the upregulated single EV proteins were primarily enriched in inflammation and cell adhesion pathways, while the top downregulated proteins were involved in complement and coagulation pathway (Supplementary Fig. 3).
A Heatmap revealing differentially expressed proteins on single uEV between SA-AKI and non-AKI groups; B PCA plot illustrating the discrimination between AKI and non-AKI based on differentially expressed proteins on single uEV; C Consensus bar chart identifying CD21 and CD35 as the most characteristic differential proteins across 5 algorithms; D Single-molecule fluorescence microscopy confirming colocalization of CD21 (red) and CD35 (red) with uEV markers (CD81, green; CD63, yellow) at single vesicle; E Western blot showing significantly decreased CD35 expression in the SA-AKI compared to non-AKI group. CD21 and CD35 expression was normalized to EV marker, CD9 (n = 3 for Healthy control (HC), AKI and Non-AKI group respectively, pairwise comparisons between groups were performed using paired two-tailed t-test); F CD21 and CD35 were normalized to uEV protein concentration (c(uEV)) detected by BSA assay, data are presented as mean with SD (AKI n = 3, Non_AKI n = 3, HC n = 3, pairwise comparisons between groups were performed using paired two-tailed t-test). Source data are provided as a Source Data file.
To determine the reliability of CD35 and CD21 as single-vesicle biomarkers, we employed 4 machine learning algorithms in conjunction with the threshold from the limma package. Across all models, CD35 and CD21 were consistently identified as candidate biomarkers among the 44 differentially expressed proteins (Fig. 4C, Supplementary Fig. 4A–F). The AUC-ROC for CD35 and CD21 reached 0.92 and 0.91, respectively (Supplementary Fig. 4G, H). These findings suggest that differential proteins on single EV possess a strong discriminatory capability for AKI.
To confirm the presence of CD35 and CD21 on uEVs, single-molecule imaging was applied to verify their colocalization with CD63 and CD81 at single-vesicle level (Fig. 4D). The results revealed that the proportions of CD35+ cluster (CD63+CD35+, 8.5%; CD81+CD35+, 6.5%; CD63+CD81+CD35+, 1.6%) were much higher than CD21+ cluster (CD63+CD21+,0.7%; CD81+CD35+, 0.5%; CD63+CD81+CD35+, 0.1%). Therefore, we observed variant percentage of subpopulations characterized with CD35 and distinct combinations of EV markers, which is consistent with the EV heterogeneity with distinct surface marker profiles21. Moreover, the overall count of CD21+ cluster appeared to be relatively lower compared to CD35+ cluster. To further validate protein expression, total CD35 and CD21 were measured by western blot analysis and normalized to exosome marker CD9. Decreased CD35 was observed despite the increased number of EVs in AKI group isolated from the same volume of 24-hour urine samples compared to healthy controls and sepsis non-AKI. In contrast, CD21 at single-vesicle showed no significant differences across the groups (Fig. 4E). To eliminate potential biases arising from variations in individual EV marker, CD35 and CD21 levels were also normalized to total uEV protein concentration of each sample, which consistently demonstrated the reduction of CD35 expression in SA-AKI patients (Fig. 4F).
CD35 expression on single uEV as biomarker of SA-AKI in an independent validation cohort
To explore the diagnostic potential of CD35 expression on single uEV (CD35-uEV), urine samples were collected from 70 SA-AKI patients, 44 non-AKI sepsis patients, and 20 healthy controls across multicenters (Fig. 5A). The basic clinical characteristics were shown in Table 1. SA-AKI patients exhibited higher SOFA scores, elevated levels of procalcitonin and high-sensitivity CRP, increased frequency of catecholamine use, and significantly reduced 24-h urine output. Additionally, these patients showed a greater predisposition toward bacterial and fungal infections. To account for the potential influence of urine concentration, CD35 on single uEV was calculated by normalizing the total CD35 measured by ELISA to the number of EVs detected by EV quantification kit. The results showed no significant difference in CD35-uEV between the non-AKI and healthy control groups (p = 0.235), while a significant reduction was observed in AKI (p < 0.001) compared to non-AKI group (Fig. 5B). The AUC-ROC for CD35-uEV in distinguishing AKI from non-AKI in sepsis patients was 0.89 (95% confidence interval (CI), 0.83–0.95) (Fig. 5C). Moreover, to minimize methodological bias from EV quantification, single CD35-uEV levels were also normalized to the uEVs count detected by nano-flow cytometry. The results consistently demonstrated that CD35-uEV levels significantly reduced in the AKI group (p < 0.001) (Supplementary Fig. 5A), with comparable diagnostic capacity (AUC-ROC: 0.88) (Supplementary Fig. 5C).
A Sample processing and detection workflow of SA-AKI cohort (n = 134, collected within 24 h after clinical diagnosis of AKI) (Image was created in BioRender. Li, N. (2025) https://BioRender.com/d94efgw); B Expression differences of CD35-uEV among various groups (Mann–Whitney U two-sided statistics). CD35 at single EV (CD35-uEV) was calculated by total CD35 measured by ELISA normalized to EV account; C ROC curve illustrating the ability of CD35-uEV to discriminate AKI from non-AKI sepsis patients (AUC-ROC 0.89, 95% CI 0.83–0.95); D CD35-uEV levels across AKI severity stages were analyzed using Mann–Whitney U two-sided statistics (Compared in pairs) (Non-AKI: n = 44, Stage1: n = 29, Stage2: n = 31, Stage3: n = 10); E Comparative analysis of CD35-uEV concentrations between transient versus persistent AKI was conducted with Mann–Whitney U two-sided statistics (p = 6.1E−5, Transient-AKI: n = 27, Persistent-AKI: n = 43); F ROC curve for CD35-uEV in predicting persistent AKI; G Correlation between CD35-uEV and median recovery time from AKI (log-rank test, p-value: 0.037). The blue and pink translucent bands indicate the 95% confidence intervals (error bands) for the low-risk and high-risk groups, respectively; H–J Correlation (general linear regression) between CD35-uEV and peak serum creatinine (Max_Scr) (r = −0.44, 1.5E−6) (H), procalcitonin levels (r = −0.26, p = 0.009) (I) and hypersensitive C-reactive protein (hs-CRP) levels (r = −0.27, p = 0.006) (J); K The General linear regression based restricted cubic spline curve revealed that CD35-uEV are an independent predictor of the lowest eGFR. Source data are provided as a Source Data file.
To investigate whether CD35-uEV correlate with the severity and adverse outcome of AKI, we analyzed their expression across different stages and durations of AKI patients. The results showed that CD35-uEV progressively decreased with advancing AKI stages (Fig. 5D). Besides, CD35-uEV levels were also significantly lower (p < 0.001, Fig. 5E) in the persistent AKI group compared to transient AKI (AUC-ROC: 0.77, 95% CI of 0.65–0.90) (Fig. 5F).
To assess whether CD35-uEV is associated with the recovery time of AKI patients, we calculated the median expression level of CD35-uEV in the AKI group (12.05 ×10−11 ng/EV) and divided the patients into high-risk (<12.05 ×10−11 ng per EV) and low-risk (≥12.05 ×10−11 ng per EV) groups. The KM curve showed that the median recovery time for the low-risk group was significantly shorter than that for the high-risk group (p = 0.037) (Fig. 5G). Correlation analysis revealed a significant negative correlation between CD35-uEV levels and the highest serum creatinine after admission (r = −0.44, p < 0.001), procalcitonin (r = −0.26, p = 0.009), and serum hs-CRP (r = −0.27, p = 0.006) (Fig. 5H–J).
To confirm whether CD35-uEV is an independent predictor of kidney function deterioration, we performed linear regression to examine the association between CD35-uEV and the highest serum creatinine, adjusting for age, gender, history of hypertension, diabetes, and cardiovascular disease, as well as urine output, SOFA scores, pathogen identification, and catecholamine use. The results were visualized using restricted cubic splines. After adjusting for these confounding factors, CD35-uEV remained significantly associated with the highest serum creatinine (p < 0.001), demonstrating that CD35-uEV is independently associated with renal function decline for SA-AKI (Fig. 5K). Hence, CD35 expression on single uEV was a reliable biomarker for the diagnosis of SA-AKI, which meanwhile was associated with the severity and recovery time.
CD35-uEV can serve as predictors for the risk of death and AKD in patients with SA-AKI
Given the capacity of CD35-uEV in the diagnosis and prognosis of AKI, we sought to determine whether this marker could also predict short and long-term adverse outcomes in AKI patients. Correlation analysis (Fig. 6A) showed no significant correlation between CD35-uEV and ICU length of stay (p = 0.79), neither clear association with renal replacement therapy was observed (p = 0.503) (AUC-ROC: 0.57, 95% CI: 0.38–0.76) (Fig. 6B, C). During the 30-day follow-up period, 10 patients died. In the deceased group, CD35-uEV expression was significantly lower (p = 0.043) (Fig. 6D), with an AUC-ROC of 0.70 for discriminating from survivals (95% CI: 0.54–0.86) (Fig. 6E). Based on the median CD35-uEV level (2.89 × 10−11 ng/EV, death patients were divided into high-risk (<2.89 × 10−11 ng/per EV) and low-risk (≥2.89 × 10−11 ng/per EV) groups. The KM curve indicated that the median survival tended to be lower in the high-risk group compared to the low-risk group, although without reaching statistically significance (p = 0.12) (Fig. 6F). Intriguingly, patients progressing to AKD exhibited significantly attenuated CD35-uEV expression (p = 0.045; Fig. 6G), demonstrating moderate predictive capacity for disease progression (AUC-ROC: 0.66, 95% CI: 0.53–0.79) (Fig. 6H). However, no clear predictive ability was observed for the outcome progressing towards CKD (p = 0.234, AUC-ROC: 0.59, 95% CI: 0.45–0.72) (Fig. 6I, J). Overall, CD35-uEV could serve as a useful biomarker for predicting the risk of death and progression to AKD for SA-AKI.
A Correlation analysis (general linear regression) between CD35-uEV levels and ICU length of stay (r = −0.035, p = 0.79); B Comparison of CD35-uEV expression between the renal replacement therapy (RRT) and non-RRT groups revealed no significant difference (p = 0.503, Mann–Whitney U two-sided statistics; RT: n = 14, No-RT: n = 51); C. ROC curve for CD35-uEV predicting the need for renal replacement therapy (AUC-ROC 0.57, 95% CI 0.38–0.76); D CD35-uEV expression was significantly reduced in patients who died during the study (p = 0.043, Mann–Whitney U two-sided statistics; Death group: n = 11, Alive group: n = 53); E ROC curve for CD35-uEV predicting mortality (AUC-ROC 0.70, 95% CI: 0.54–0.86); F Kaplan–Meier survival analysis (log-rank test) for patients stratified by high and low CD35-uEV levels revealed a trend towards longer survival in the high CD35-uEV group, though this did not reach statistical significance (p = 0.12). The blue and pink translucent bands indicate the 95% confidence intervals (error bands) for the low-risk and high-risk groups, respectively; G CD35-uEV expression was markedly reduced in patients who progressed to acute kidney disease (AKD) (p = 0.045, Mann–Whitney U two-sided statistics; AKD group: n = 27, Non-AKD group: n = 39); H ROC curve for CD35-uEV predicting AKD (AUC-ROC 0.66, 95% CI 0.53–0.79); I Comparison of CD35-uEV expression in SA-AKI patients who progressed to chronic kidney disease (CKD, n = 23) and who did not (non-CKD, n = 54) (p = 0.234) (Mann–Whitney U two-sided statistics); J ROC curve for CD35-uEV to predict CKD (AUC-ROC 0.59, 95% CI 0.45–0.72). Data are presented as box plots showing the median (middle line), the 25th and 75th percentiles (box limits), the minimum and maximum values (whiskers), and outliers (individual points). Source data are provided as a Source Data file.
CD35-uEV enables early identification and severity prediction of subclinical SA-AKI
To investigate whether CD35-uEV enables early identification of AKI, we collected uEVs samples from 72 sepsis patients within 12 h after sepsis onset, who had not yet been diagnosed with AKI and followed them to observe their risk of developing AKI defined as E-AKI prospective cohort (Fig. 7A, Table 2). Patients with subclinical AKI exhibited reduced urine output, elevated levels of procalcitonin and high-sensitivity CRP, and a higher frequency of catecholamine use. However, no significant differences were observed between the groups in terms of infection agents or SOFA scores. During the follow-up period, 26 patients developed AKI. After normalizing for EV counts, single CD35-uEV levels were significantly lower in patients who progressed to AKI compared to those who did not (p < 0.001, AUC-ROC: 0.84, 95% CI: 0.74–0.94) (Fig. 7B, C). Notably, similar results were observed when normalized to uEV counts quantified by nano-flow cytometry (p < 0.001, AUC-ROC: 0.82, 95% CI: 0.71–0.93) (Supplementary Fig. 5B, D). Regarding AKI stages, patients who developed stage 1 AKI showed significantly lower baseline CD35-uEV levels compared to those who did not develop AKI. Additionally, patients who developed stage 2 AKI had lower CD35-uEV levels compared to those with stage 1 (Fig. 7D). The AUC-ROC for distinguishing AKI stage 1 from stages 2-3 (severe AKI) was 0.69 (95% CI: 0.49–0.89) (Fig. 7E), suggesting the predictive ability for severe AKI. Similarly, baseline CD35-uEV levels also demonstrated predictive value for patients progressing to persistent AKI (p = 0.04, AUC-ROC: 0.72, 95% CI: 0.52–0.92) (Fig. 7F, G).
A Workflow for sample collection and analysis of E-AKI cohort (n = 72), with samples collected 12 h post diagnosis of sepsis, prior to a clinical diagnosis of AKI (Image was created in BioRender. Li, N. (2025) https://BioRender.com/6zj1ctq); B CD35-uEV levels were significantly reduced in subclinical AKI compared to non-AKI sepsis patients (p = 6.0E−7, Mann–Whitney U two-sided statistics); C ROC curve illustrating the predictive accuracy of CD35-uEV for subclinical AKI (AUC-ROC 0.84, 95% CI 0.74–0.94); D CD35-uEV levels decreased progressively with increasing AKI stages (Mann–Whitney U two-sided statistics (Compared in pairs), Non-AKI: n = 46, Stage1: n = 11, Stage2: n = 11, Stage3: n = 4); E ROC curve for CD35-uEV predicting AKI severity in subclinical AKI patients (AUC-ROC 0.69, 95% CI 0.49–0.89); F Significant reduction of CD35-uEV in patients progressing to persistent AKI compared to those with transient AKI (p = 0.04, Paired two-tailed t-test, Transient-AKI: n = 11, Persistent-AKI: n = 15); G ROC curve demonstrating the prediction of CD35-uEV for persistent AKI in subclinical AKI patients (AUC-ROC 0.72, 95% CI 0.52–0.92); H Logistic regression-based restricted cubic spline analysis identified CD35-uEV as an independent diagnostic factor for subclinical AKI (p < 0.001) (adjusting for age, gender, history of hypertension, diabetes, and cardiovascular disease, as well as urine output, SOFA scores, pathogen identification, and catecholamine use), the pink translucent band represents the 95% confidence interval (error bands); I Correlation (general linear regression) of CD35-uEV levels with the peak serum creatinine (Max_Scr) (r = −0.32, p = 0.008) in subclinical AKI patients post-admission. Source data are provided as a Source Data file.
To further validate whether CD35-uEV is an independent predictive biomarker for subclinical AKI, we used logistic regression (adjusting for age, gender, history of hypertension, diabetes, and cardiovascular disease, as well as urine output, SOFA scores, pathogen identification, and catecholamine use) to model the association between early CD35-uEV levels within 12 h after sepsis and subsequent clinical diagnosis of AKI. The restricted cubic spline analysis showed that as CD35-uEV levels decreased, the risk of AKI significantly increased (p < 0.001), confirming that CD35-uEV is an independent risk factor for AKI (Fig. 7H). Correlation analysis revealed that, baseline CD35-uEV levels significantly correlated with the highest serum creatinine (r = −0.32, p = 0.008) (Fig. 7I). Therefore, early detection of CD35-uEV provides a valuable biomarker for identifying subclinical AKI and predicting the occurrence of severe AKI as well.
Multi-omics analysis reveals the origination of CD35 from injured podocytes
To trace the origin of CD35-uEV, regional transcriptomics data from the KPMP database was analyzed. We found that CD35 is primarily localized to the glomerulus, with decreased expression in the AKI group (Fig. 8A). To further clarify the cellular changes of CD35, single-cell transcriptomics data from biopsies of pneumonia-related AKI patients (GSE21062) were analyzed, including 4 patients with AKI and 3 non-AKI patients. After normalizing and clustering the data, we identified 27 distinct cell populations (Fig. 8B). Key annotation proteins for each cluster were shown in Supplementary Fig. 6. Cellular localization analysis of CD35 revealed that it was predominantly expressed in podocytes, with remarkably reduced levels in the injured population (Fig. 8C). Differential analysis showed that CD35 as well as podocyte marker NPHS1 were downregulated in the AKI group, whereas inflammation and injury markers such as SPP1, GPX3, and C1R were upregulated (Fig. 8D). KEGG pathway enrichment analysis indicated upregulation of inflammation-related pathways, including NK cell-mediated cytotoxicity, antigen presentation, and oxidative phosphorylation. Additionally, pathways related to ferroptosis and necroptosis were significantly upregulated (Fig. 8E). Meanwhile, CytoTRACE analysis revealed a lower differentiation state in injured podocytes compared to normal podocytes population (Fig. 8F), which was consistent with previous studies showing that podocytes undergo dedifferentiation following injury22. Pseudotime analysis was performed to further investigate the differentiation trajectory of podocytes. Interestingly, podocytes population transitioned to injured podocytes subgroups, accompanied by a remarkable reduction in CD35 expression (Fig. 8G, H).
A Regional proteomic analysis revealed CD35 localization within the glomeruli, with a marked decrease in expression in AKI groups (Kidney Precision Medicine Project database: https://atlas.kpmp.org/); B Single-cell clustering analysis of pneumonia-associated AKI identified distinct cell populations, including injured podocytes; C Localization of CD35 in podocytes which reduced in the injured population; D Differential gene expression analysis demonstrated a significant reduction of CD35 in podocytes from AKI patients (based on limma package); E KEGG pathway enrichment analysis highlighted the upregulation of inflammatory and injury-related pathways in AKI podocytes (KEGG pathway enrichment was performed via hypergeometric testing with Benjamini-Hochberg FDR correction, threshold: FDR ≤ 0.05); F Cytotrace analysis identified injured podocytes in a dedifferentiated state; G Pseudotime analysis traced the trajectory of normal podocyte differentiated into injured states, H characterized with decline of CD35 during AKI progression; I Spatial transcriptomic mapping showed spatial distribution of injured podocytes (Spatial mapping of single-cell transcriptomic annotations onto spatially resolved transcriptomic profiles). J Raincloud plot illustrating significantly reduced CD35 expression in injured podocytes at the spatial transcriptomic level (Injury podocyte, n = 7 data mapped cells; normal podocyte, n = 7 data mapped cells, Mann–Whitney U statistics, p = 0.012). Data are presented as box plots showing the median (middle line), the 25th and 75th percentiles (box limits), the minimum and maximum values (whiskers), and outliers (individual points). Podo podocyte, Podo_inj injured podocyte. Source data are provided as a Source Data file.
To observe spatial distribution of injured podocytes, we collected additional spatial transcriptomics data from AKI patients in the KPMP database. Using Seurat’s integration method, we mapped cell clusters onto the spatial transcriptomics data, scoring each spatial transcriptomics label for cell characteristics. The results demonstrated that podocytes and injured podocytes were located in the glomerulus, supporting the accuracy of our spatial cell annotations (Fig. 8I). Moreover, injured podocytes were widely distributed in multiple glomeruli rather than confined to a limited area. Further analysis revealed a general downregulation of CD35 in injured podocytes compare to normal podocytes (p = 0.012) (Fig. 8J). Therefore, multi-omic data demonstrated that podocytes are injured in SA-AKI, characterized by reduced CD35 expression. The unique population of uEVs with reduced CD35 expression may originate from these injured podocytes.
CD35 is primarily localized to fraction of podocyte-derived EVs in urine
Next, the source of urinary CD35 was further investigated. In normal renal tissue obtained from patients undergoing nephrectomy adjacent to renal carcinoma, CD35 expression exhibited predominant glomerular localization, demonstrating strong spatial concordance with the podocyte-specific marker synaptopodin (Supplementary Fig. 7A). Correspondingly, super-resolution imaging demonstrated high colocalization of CD35 with Nephrin on individual vesicles. Among the total vesicles, approximately 13.57% were CD35+, decreasing to 3.1% in the AKI group. While Nephrin+ vesicles accounted for 15.78% in the non-AKI group and 13.35% in the AKI group. Notably, Nephrin co-expression was observed in 94.4% of CD35+ vesicles in the non-AKI group and 87.5% in the AKI group (Supplementary Fig. 7B). These findings suggest that CD35-uEV is predominantly derived from podocyte.
Next, we explored the presence of CD35 in other urine fractions obtained through differential centrifugation. Interestingly, the results showed that CD35 was not detectable in the fraction of neither large EV nor supernatant, proving that CD35 is exclusively localized to sEV (p < 0.0001) (Supplementary Fig. 7C). To rule out the possibility of its origin from circulation, serum from SA-AKI and non-AKI patients were collected and no significant differences (p = 0.46) in circulating CD35 were noted (Supplementary Fig. 7D). Collectively, these results suggest that CD35 likely originates predominantly from podocyte-derived EVs, rather than existing in soluble form or deriving from circulatory source.
Combination of tubular and glomerular injury markers enhances diagnosis accuracy of SA-AKI
Currently, several biomarkers have been validated for the early diagnosis of AKI, with TIMP2*IGFBP7 being the only FDA-approved test23. The ADQI guidelines recommend using a combination of multiple biomarkers to improve the accuracy of early AKI diagnosis in sepsis24. TIMP2*IGFBP7 is a well-established marker of tubular cell cycle arrest due to stress injury25. We aimed to explore whether the combination of TIMP2*IGFBP7 with CD35-uEV could further enhance the diagnostic accuracy for SA-AKI.
Impressively, we identified that CD35-uEV displayed higher accuracy compared to TIMP2*IGFBP7 (AUC-ROC: 0.84 vs 0.75) in our sepsis cohort, while the combined biomarker yielded the best accuracy, with an AUC-ROC of 0.87 (Supplementary Fig. 8A, B). The calibration curve also showed that the predicted values closely matched the actual values when CD35-uEV were combined with TIMP2*IGFBP7 (Supplementary Fig. 8C). Decision curve analysis further demonstrated that the net benefit was highest when both CD35-uEV and TIMP2*IGFBP7 were used together (Supplementary Fig. 8D). To visualize the combined diagnostic capacity, the scores of CD35-uEV and TIMP2*IGFBP7 to calculate the probability of AKI occurrence in sepsis patients was presented in nomogram (Supplementary Fig. 8E). Besides, the risk of severe AKI could be predicted through the combined biomarker score (p = 0.02, AUC-ROC: 0.78, 95% CI: 0.59–0.96) (Supplementary Fig. 8F, G), although predicting persistent AKI was not significantly achieved (Supplementary Fig. 8H, I) (p = 0.9754; AUC-ROC: 0.52, 95% CI: 0.26–0.78).
Furthermore, to determine whether the combined score is an independent early diagnostic factor for AKI, a multivariable logistic regression model was established to evaluate the association with subsequent AKI diagnosis in the E-AKI cohort. The restricted cubic spline analysis showed that the risk of subsequent AKI diagnosis significantly increased with the increasing level of the combined score (p < 0.001) (Supplementary Fig. 8J). Therefore, combination of tubular and glomerular injury markers with TIMP2*IGFBP7 and CD35-uEV enhances diagnosis accuracy of SA-AKI, which also shows potential in predicting severe AKI.
CD35-uEV as a specific biomarker for podocyte injury in SA-AKI other than ischemic AKI
To evaluate whether CD35-uEV also serves as a biomarker for AKI caused by other etiologies, 30 patients who underwent cardiac surgery with a risk of developing ischemic AKI were enrolled. The results demonstrated no statistically significant difference in CD35-uEV levels between the AKI and non-AKI groups (AUC-ROC: 0.57, 95% CI: 0.36–0.79) (Supplementary Fig. 9A, B). Given that CD35-uEV is proposed as a non-invasive biomarker of podocyte injury, we further explore podocyte damage in cardiac surgery-associated AKI. Single-cell RNA sequencing data from urine cells of patients following cardiac surgery (GSE19932) was analyzed through dimensionality reduction and clustering. The analysis revealed no significant changes in the expression of canonical podocyte markers, including NPHS1, NPHS2, WT1, and CD35, between the groups (Supplementary Fig. 9C). Moreover, podocyte differentiation scores were comparable between AKI and non-AKI groups (Supplementary Fig. 9D), indicating no substantial podocyte injury occurred. These findings suggest that CD35-uEV may represent a specific and non-invasive biomarker for podocyte injury in SA-AKI other than ischemic AKI.
Discussion
SA-AKI carries a high mortality risk and is often accompanied by severe adverse outcome. Timely intervention is crucial for improving patient outcomes. However, early and accurate biomarkers for diagnosis of AKI are still lacking. In this study, we employed single-vesicle analysis to delineate the heterogeneous populations of uEVs in sepsis patients. Notably, we identified a specific subcluster characterized by CD35 expression with excellent potential for discriminating SA-AKI. Further studies identified that CD35 on single uEV (CD35-uEV) not only enable early diagnosis of subclinical SA-AKI but also provide a reliable prediction of progressing to severe and/or persistent AKI. Impressively, CD35-uEV represents a non-invasive histological biomarker originated exclusively from the injured podocytes for SA-AKI.
Utilizing PBA technology, we successfully captured surface proteome on individual uEV, allowing a complete profiling of the uEV landscape. By comparing the top 5 clusters with remarkable proportional difference, we found that proportion of complement receptor CD21+ CD35+ cluster (CMR-EV) could clearly distinguish SA-AKI from non-AKI patients. Given the key roles of CD21 and CD35 in complement system regulation, the reduced proportion of this cluster may be linked to abnormal complement activation and dysfunction in sepsis26. We also identified a distinct subcluster, IMR-EV, characterized by the expression of inflammation-regulating markers such as CD38, CD26, and TICAM2. The proportion of this subcluster was elevated in severe and persistent AKI indicating its involvement in the development of these conditions. Therefore, using PBA technology, uEV clusters was profiled, a unique uEV cluster characterized with complement receptor expression as potential biomarker of SA-AKI was identified.
Next, the diagnostic potential of CD35 on single vesicles of this unique subcluster was explored in the multicenter independent validation cohorts. CD35-uEV is demonstrated as accurate diagnostic biomarker of SA-AKI, associating with the severity and duration as well. Subclinical AKI was proposed by Dialysis Quality Initiative (ADQI) group which was characterized by elevated levels of biomarkers in the absence of an increase in serum creatinine24. In this study, CD35-uEV showed remarkable ability to identify the sub-clinical AKI patients and to assess the risk of severe clinical AKI in the prospective cohort. In the past decades, the diagnostic potential of tubular injury markers was evaluated in SA-AKI. It turned out that urinary [TIMP-2]·[IGFBP7] outperformed other biomarkers for prediction of AKI with area under the curve of 0.80, while data for KIM-1 and NGAL specifically used for SA-AKI are not sufficient23. It is noteworthy that elevations of those early biomarkers for AKI occur in only one-third of subclinical AKI patients27. This presents challenges for the early and accurate diagnosis of SA-AKI. Herein we identified a useful single uEV biomarker that is capable of identifying clinical and subclinical AKI patients with good performance.
Persistent AKI is associated with higher mortality and poor prognosis28,29, and patients progressing to AKD are prone to multiple organ complications30. The 23rd ADQI [23] conference recommended using biomarkers to predict the duration and recovery of AKI. Currently, only CCL14 was reported to predict persistent AKI31, and biomarkers for predicting AKD are still lacking. Impressively, in our study, CD35-uEV demonstrated potential in predicting both persistent AKI and AKD. AKI patients with higher levels of CD35-uEV had significantly shorter median recovery times. However, no significant differences were observed regarding CKD outcomes, indicating CD35-uEV is well-suited for monitoring short-term SA-AKI progression via reflecting the acute renal injury conditions. Interestingly, in the prospective E-AKI clinical cohort, CD35-uEV at 12 h after sepsis onset predicted the risk of persistent AKI, indicating its potential application in early AKI screening, stratification of high-risk patients and clinical decision-making. CD35-uEV as a useful diagnostic biomarker may provide a promising approach for prognostic assessment of SA-AKI.
Given the promising potential of CD35-uEV in diagnosing SA-AKI, the cellular source of this biomarker and its implication to renal damage was further explored. Integrative analysis of multi-omic data collectively confirmed that CD35 is predominantly expressed in renal podocytes. Besides, extensive podocyte damage was noticed in SA-AKI, aligning with a early study showing podocytes present severely damaged morphology32. Despite of this finding, most previous studies have focused on vascular endothelial damage33,34 or tubular cell injury35,36 which consequently yielded biomarkers mostly derived from the injured tubule epithelial cells for SA-AKI. However, the dissociation between structural and functional changes was consistently noted in postmortem human and animal models37,38,39. Herein, we identified a notable decrease of CD35 expression in the damaged podocytes, closely paralleling the extent of podocyte injury. The dominant proportion of CD35+ Nephrin+ uEVs exclusively presented in the small EV fraction of urine collectively proved this unique cluster originated from injured podocytes. These findings were indeed consistent with previous studies showing the presence of membrane-association of complement receptor 1, CD35 in urine40. Interestingly, membrane-bound complement regulatory proteins were increasingly recognized as critical component of the complement system41,42. It is reasonable to speculate that the podocyte derived complement receptor CD35 on EVs may play an important role in the dysregulated immune responses for SA-AKI. Thus, CD35-uEV may serve as a practical non-invasive marker for podocyte injury and complement related immune dysregulation. Importantly, our findings may suggest an unrecognized pathophysiology mechanism of SA-AKI. However, podocyte injury appears to be uncommon in AKI caused by other etiologies, which may explain the lack of detectable changes in CD35-uEV levels observed in cardiac surgery-associated AKI.
The ADQI guidelines recommend using multiple biomarkers to improve the accuracy of early diagnosis of SA-AKI24. TIMP2 and IGFBP7 are cell cycle arrest markers of tubular epithelial cell injury and have been used for early diagnosis of various AKIs43. A meta-analysis23 confirmed that TIMP2*IGFBP7 significantly outperforms other biomarkers for early AKI diagnosis. Comparatively, we identified that CD35-uEV outperformed TIMP2*IGFBP7 in terms of early diagnosis of SA-AKI. This superiority may be attributed to the property of uEVs derived from injured renal cells which better reflect cellular damage compared to traditional soluble biomarkers. Further analysis identified that the combination of CD35-uEV with TIMP2*IGFBP7 improved the accuracy for early AKI diagnosis and prediction of subsequent risk of severe AKI. Therefore, the combination of tubular and glomerular injury markers may provide an approach to enhance the diagnostic accuracy of SA-AKI. Further high-quality, multicenter studies are needed to evaluate the external applicability and efficacy of this biomarker across different races, countries, and healthcare settings.
Overall, through proteomic characterization of single uEV, this study identified CD35-uEV derived from injured podocyte as a practical biomarker of SA-AKI to realize early diagnosis and prognostic assessment. Integration of tubular and glomerular injury markers based on CD35-uEV and TIMP2*IGFBP7 offers a useful diagnostic approach with improving accuracy. With CD35-uEV we describe the useful marker that specifically detects podocyte injury to identify the risk, severity and prognosis of SA-AKI.
Methods
Study population and endpoint outcomes
This study adhered to the ethical principles outlined in the Declaration of Helsinki and other relevant ethical guidelines. The research protocol received approval from the Institutional Review Boards (IRBs) of Zhongda Hospital, Southeast University; Jiangsu Province Hospital of Traditional Chinese Medicine; and The Second Affiliated Hospital of Anhui Medical University. Written informed consent was obtained from all participants prior to their involvement in the study. This study enrolled ICU patients primarily diagnosed with sepsis, defined by an increase of ≥2 points from baseline in the SOFA score in the presence of infection. Between June 2022 and February 2024, we collected data from individuals of SA-AKI and E-AKI cohorts across 3 centers: Zhongda Hospital Southeast University, Jiangsu Provincial Hospital of Traditional Chinese Medicine, and the Second Affiliated Hospital of Anhui Medical University.
Inclusion criteria for the SA-AKI cohort were sepsis patients who developed AKI within seven days of sepsis diagnosis, based on the KDIGO criteria44 (a sudden decrease in kidney function within 48 h with an absolute increase in serum creatinine of ≥26.5 μmol/L, a ≥50% increase from baseline within seven days, or urine output <0.5 ml/kg/h for at least six hours). Patients who did not develop AKI within 7 days post-sepsis diagnosis and healthy individuals served as controls. Participants were required to be 18 years or older. Exclusion criteria included (1) end-stage renal disease requiring dialysis, (2) kidney transplantation, (3) a history of chronic kidney disease (CKD, defined as structural and functional kidney abnormalities persisting for over three months), (4) end-stage cancer of the urinary system, (5) unwillingness to participate, and (6) urinary tract infections. Urine samples were collected within 24 h of admission.
The E-AKI cohort included patients within 12 h of sepsis admission, who were subsequently monitored for the risk of developing AKI. The exclusion criteria were the same as the SA-AKI cohort. Urine samples were collected within 12 h of admission.
To validate biomarker expression across various AKI subtypes, we additionally enrolled post-cardiac surgery patients (AKI: n = 15; non-AKI: n = 15). Urine samples were collected within 24 h of AKI diagnosis, the same inclusion/exclusion criteria were applied as in the SA-AKI cohort.
For each patient, we recorded serum creatinine levels, glomerular filtration rate (eGFR), AKI stage (AKI Stage 1 is defined by an increase in serum creatinine of ≥0.3 mg/dL or a relative increase of ≥50%, or a urine output of <0.5 mL/kg/h for 6–12 h. AKI Stages 2–3 are defined by an increase in serum creatinine of >200% or a urine output of <0.5 mL/kg/h for ≥12 h), and basic clinical information. We also monitored multiple outcomes, including the risk of persistent AKI (defined as AKI lasting more than 2 days), length of ICU stay, renal replacement therapy (RT), 30-day mortality, progression to acute kidney disease (AKD, defined as AKI lasting more than 7 days45), and the risk of developing chronic kidney disease (CKD, defined as chronic renal structural and functional impairment (history of renal impairment greater than 3 months46)).
Urine sample collection and preparation
24-hour urine samples were collected from each patient within the designated timeframe (SA-AKI cohort: 24 h within diagnosis AKI; E-AKI cohort: within 12 h of admission). Samples were immediately centrifuged at 4 °C and 3000 × g for 20 min to remove cell debris. The supernatant was further centrifuged at 13,500 × g for 30 min to precipitate large vesicles and keep the supernatant. Ultracentrifugation was performed at 200,000 × g for 2 h to pellet the small extracellular vesicles (sEVs), which were then resuspended in PBS. Post-ultracentrifugation EV suspension was processed through a size exclusion chromatography (SEC) (Izon, Christchurch, New Zealand) column to yield purified EV suspension according to the manufacturer’s protocol.
Single extracellular vesicle analysis with PBA
In this study, we employed PBA technology17 to detect surface proteome on individual uEV. The PBA reagent kit employed in this study was ExoSeek® Panel 260 (Secretech, Shanghai, China). Briefly, uEVs were captured through GM1 gangliosides on the membrane of uEVs using the B subunit of cholera toxin (CTB). The uEVs samples (5 µL) were added in 96 well plate with CTB coating and incubated at room temperature for one hour. The plate was then washed three times with PBS containing 0.05% Tween 20 (PBST) to remove unbound materials. Specific DNA-labeled antibody probes were then added to the captured uEVs and incubated at room temperature for two hours. The plate was then washed with PBST for 3 times. Individual RCA products were added to the wells. The next step involved annealing the DNA-labeled antibody probes to their complementary sequences on the RCA products. The single EV barcode was added via extension reaction. Then DNA sequences they carried were amplified via PCR, providing labels for protein expression levels in individual uEVs. PCR amplification of these probes and adjacent RCA sequences was conducted to increase the signal strength. After amplification, sequencing was performed to read the unique barcodes representing individual probes and RCA products. Sequencing was carried out on the Illumina NovaSeq S4 platform using a PE150 sequencing strategy, which provides high-throughput, paired-end reads of 150 base pairs. The sequencing data was then processed via Evisualizer decoding package (Secretech, Shanghai, China) to decode the barcodes, allowing for the identification and quantification of protein levels in individual uEVs. This process enabled a detailed analysis of the protein composition and provided insights into the heterogeneity of the uEV population.
Single EV data was processed by clustering analysis using the Seurat package. Initially, we normalized the data to balance the features, followed by dimension reduction through principal component analysis (PCA). We then visualized the data spatially using t-distributed Stochastic Neighbor Embedding (t-SNE) and Uniform Manifold Approximation and Projection (UMAP) based on the PCA results. Through Seurat’s FindNeighbors and FindClusters functions, we clustered the vesicles according to their expression patterns to identify distinct subclusters. To characterize the functional features of these subclusters, we identified signature proteins for each uEV cluster using the FindAllMarkers function and displayed the top signature proteins of each subgroup through a dot plot. Subgroups were categorized based on AKI staging and persistent AKI to assess the association between individual uEV cluster and AKI. Differential expression of individual uEV proteins between AKI groups was screened using the limma package. Furthermore, protein biomarkers on individual uEV were jointly selected through the integration of four supervised machine learning and regularization algorithms (Random Forest, XGBOOST, LASSO, SVM-RFE).
Molecular biology technique
Characterization of uEVs
Purified uEVs samples were placed on a 200-mesh nickel grid, stained with 2% phosphotungstic acid for 5 min, air-dried, and then subjected to morphological analysis using Transmission Electron Microscopy (TEM). The particle size distribution of sEVs was determined using nanoparticle tracking analysis with the ZetaView PMX 110 (Particle Metrix)47.
Visualizing single EV with super-resolution microscopy
To visualize single EV, dSTORM imaging was performed with super-resolution microscopy (Oxford Nanoimaging, ONI). uEVs were immobilized on a chip surface and stained with anti-CD35 (1:50, CL488-68033, Proteintech), CD21 (1:50, Abcam, ab315160), anti-Nephrin (Abcam, ab216341), anti-CD63 (Abcam, ab134045), and anti-CD81 (Abcam, ab79559) following the protocol provided by the EV Profiler Kit (EV-MAN-1.0, ONI, Oxford). For indirect staining, secondary antibodies, including anti-Ms-FITC, anti-Rab-Cy3, and anti-Rab-AF488, were incubated for 30 min, followed by a wash with 40 µL of W1 buffer. Fresh imaging buffer was then applied to the chip, which was immediately imaged in dSTORM mode using NimOS software (version 1.19, ONI). A minimum of six images were captured per sample and analyzed using the EV profiling App on the ONI online platform CODI. The analysis workflow encompassed drift correction, filtering by frame index and localization precision to 20 nm, and cluster filtering with a circularity threshold ranging from 0.7 to 1.
Nanoflow cytometry
Purified uEVs were incubated overnight at 4 °C with anti-CD35 FITC antibody (BD Pharmingen, 555452) and CD21 APC antibody (BD Pharmingen, 555422) in 100 µL PBS, alongside a separate aliquot incubated with FITC-conjugated and APC-conjugated isotype control antibodies Following PBS washes to remove unbound antibodies, the uEVs were pelleted by ultracentrifugation at 200,000 × g for 2 h. Before measurement, calibration was performed using standard samples. Data signals were collected using Apogee analyzer, with automated discrimination of positive and negative gating for CD35 (488 nm) and CD21 (638 nm) using the isotype control.
Western blot
The extracted pure uEVs were mixed with RIPA lysis buffer (Beyotime) at a 1:1 ratio and supplemented with protease inhibitor (Beyotime) at a 1:50 dilution. The mixture was incubated on ice for 2 h for lysis. After lysis, the mixture was centrifuged at 12,000 × g for 15 min, and the supernatant was collected to obtain pure protein. The extracted proteins were mixed with loading buffer (Biosharp) at one-fifth of the volume and heated at 95 degrees Celsius for 5 min to denature the proteins. The denatured proteins were loaded at 25 µg per well and subjected to SDS-PAGE at 160 V for 45 min. After electrophoresis, the polyacrylamide gel was assembled in a sandwich configuration with sponges, the gel, a polyvinylidene difluoride, and another sponge, and transferred using a fast transfer device (GenScript). The membrane was then blocked with 5% milk in PBS for 2 h. It was incubated overnight with primary antibodies at 4 °C and with secondary antibodies at room temperature for 1 h, followed by three washes with Tris Buffered Saline with Tween-20. Exposure was conducted under Electrochemiluminescence developing solution.47. EV proteins were loaded from samples extracted from equal urine volumes (50 mL) of 24-h urine. The antibodies used were as follows: Rabbit anti-CD9 (1:1000, ab92726, Abcam), anti-CD21 (1:2000, ab75985, Abcam), Rabbit anti-TSG101 (1:1000, ab125011, Abcam), Mouse anti-CD81 (1:1000, ab79559, Abcam), mouse anti-Alix (1:1000, sc-53540, Santa Cruz), and anti-CD35 (1:2000, CSB-PA822164ESR1HU, Huamei). Unprocessed scans of the western blot can be referenced in the source data.
ELISA
CD35, TIMP2, and IGFBP7 expression was measured using an ELISA kit (AB277439, Abcam, Cambridge (CD35); E-EL-H1453, Elabscience, (TIMP2); E-EL-H6176, Elabscience, (IGFBP7)). Standards and test samples were added to a 96-well plate and incubated at room temperature for 3 h. The wells were washed three times with PBST. 100 µL of prepared biotinylated antibody was added to each well and incubated at room temperature for 1 h. The washing step was repeated three times. Next, 100 µL of prepared streptavidin solution was added, followed by incubation at room temperature for 45 min. After three times washes, 100 µL of TMB substrate was added and incubated at room temperature for 30 min before the addition of stop solution, and absorbance was immediately measured using a spectrophotometer at 450 nm.
Quantification of uEVs
uEVs quantification was performed using an exosome quantification kit (SBI, FluoroCet, FCET96A-1). According to the kit instructions, 50 µL of standards and test samples were added to each well of a 96-well polystyrene plate, followed by 50 µL each of Buffer A and Buffer B, to make a total reaction volume of 150 µL. The mixture was gently agitated and incubated at room temperature for 20 min. Fluorescence was measured using a fluorometer at an excitation wavelength of 570 nm and an emission wavelength of 590 nm. uEVs were extracted from equal volume of urine (50 mL) from 24-hour urine. The standard curve for quantification was in units of 1 × 10^7EVs per 250 ng of EVs. The total number of EVs applied for detection varied between 2 × 10^10 and 4 × 10^10EVs.
Single-cell transcriptome combined with spatial transcriptome analysis
10X single-cell RNA-sequencing data (scRNA-seq) were obtained from GEO database (GSE210622; GSE199321). Quality control (QC) is performed to filter out low-quality cells and potential doublets. This involves removing cells with unusual gene counts, high mitochondrial gene expression, and low UMI counts. After QC, data is normalized to account for differences in sequencing depth across cells using the “NormalizeData” function in Seurat. Highly variable genes are identified with the “FindVariableFeatures” function. Dimensionality reduction using PCA simplifies the data, and visualization is done using t-SNE or UMAP. Cells are clustered with algorithms like Louvain using the “FindNeighbors” and “FindClusters” functions in Seurat. Clusters are annotated based on known marker genes to identify cell types or states. Differential expression analysis with the “FindMarkers” function identifies marker genes for each cluster. Pathway and functional enrichment analyses are performed on these marker genes using tools of clusterProfiler, or GSEA to understand the biological processes and pathways enriched in each cluster. Pseudotemporal analysis and cytotrace analysis are used to evaluate the differentiation trajectory of various cells. Spatial transcriptomics data was obtained from the KPMP database (V10S14-087_XY01_21-0061). KMPM enables the visualization of gene expression directly within the database, allowing users to explore the expression patterns and distribution of genes in spatially resolved transcriptomics data online (http://www.huayingtangkyoto.com/202576/web_summary.html). Using Seurat’s integration method, anchors were identified and integrated with annotated single-cell data. Mapping techniques were then employed to localize the regions of high expression within the spatial transcriptomics data.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism 7.00. Continuous variables were expressed as mean ± SD or median with interquartile range, while categorical variables were represented as percentages or ratios. The unpaired t-test or Mann–Whitney U test was utilized to evaluate significant differences between two groups. Differences among three groups were assessed using one-way analysis of variance or the Kruskal–Wallis test, with subsequent multiple comparisons. Categorical variables were analyzed using the Chi-square test. The receiver operating characteristic (ROC) curve was employed to evaluate the accuracy of biomarkers. Kaplan–Meier (KM) curves were used to assess survival times. Multivariate logistic regression combined with restricted cubic splines was performed to visualize the association between biomarkers and clinical information. All tests were two-tailed, and P values < 0.05 were considered statistically significant.
Data availability
Individual EV protein expression data can be downloaded from Figshare public database (https://figshare.com/articles/dataset/Expression_csv/29356076). Single-cell RNA-sequencing (10X) data were downloaded from the GEO database (GSE210622; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE210622, GSE199321; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE199321). Spatial transcriptomics data were obtained from the Kidney Precision Medicine Project (KPMP) database (accession: V10S14-087_XY01_21-0061). Interactive visualizations of spatial transcriptomics data are publicly accessible at: http://www.huayingtangkyoto.com/202576/web_summary.html. All remaining data are available within the Article and Supplementary Information files. Source data are provided with this paper.
References
Poston, J. T. & Koyner, J. L. Sepsis associated acute kidney injury. BMJ Brit. Med. J. 364, k4891 (2019).
Peerapornratana, S., Manrique-Caballero, C. L., Gómez, H. & Kellum, J. A. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 96, 1083–1099 (2019).
Balkrishna, A. et al. Sepsis-mediated renal dysfunction: pathophysiology, biomarkers and role of phytoconstituents in its management. Biomed. Pharmacother. 165, 115183 (2023).
van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018).
Mori, M. A., Ludwig, R. G., Garcia-Martin, R., Brandão, B. B. & Kahn, C. R. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 30, 656–673 (2019).
Kim, K. M., Abdelmohsen, K., Mustapic, M., Kapogiannis, D. & Gorospe, M. RNA in extracellular vesicles. Wires RNA 8, e1413 (2017).
Stremersch, S., De Smedt, S. C. & Raemdonck, K. Therapeutic and diagnostic applications of extracellular vesicles. J. Control Rel. 244, 167–183 (2016).
Merchant, M. L., Rood, I. M., Deegens, J. & Klein, J. B. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat. Rev. Nephrol. 13, 731–749 (2017).
Miranda, K. C. et al. Massively parallel sequencing of human urinary exosome/microvesicle RNA reveals a predominance of non-coding RNA. Plos One 9, e96094 (2014).
Almeida, A. et al. Urinary extracellular vesicles contain mature transcriptome enriched in circular and long noncoding RNAs with functional significance in prostate cancer. J. Extracell. Vesicles 11, e12210 (2022).
Yang, Q. et al. Metabolomic investigation of urinary extracellular vesicles for early detection and screening of lung cancer. J. Nanobiotechnol. 21, 153 (2023).
Bertolone, L. et al. Proteomic analysis of urinary extracellular vesicles highlights specific signatures for patients with primary aldosteronism. Front. Endocrinol. 14, 1096441 (2023).
Jeanmard, N. et al. Proteomic profiling of urinary extracellular vesicles differentiates breast cancer patients from healthy women. PLOS One 18, e291574 (2023).
Barreiro, K. et al. Urinary extracellular vesicles: Assessment of pre-analytical variables and development of a quality control with focus on transcriptomic biomarker research. J. Extracell. Vesicles 10, e12158 (2021).
Wang, S. et al. Recent advances in single extracellular vesicle detection methods. Biosens. Bioelectron. 154, 112056 (2020).
Liu, C. et al. Profiling of single-vesicle surface proteins via droplet digital immuno-PCR for multi-subpopulation extracellular vesicles counting towards cancer diagnostics. Chem. Eng. J. 471, 144364 (2023).
Wu, D. et al. Profiling surface proteins on individual exosomes using a proximity barcoding assay. Nat. Commun. 10, 3854 (2019).
Hinze, C. et al. Single-cell transcriptomics reveals common epithelial response patterns in human acute kidney injury. Genome Med. 14, 103 (2022).
Klocke, J. et al. Urinary single-cell sequencing captures kidney injury and repair processes in human acute kidney injury. Kidney Int. 102, 1359–1370 (2022).
LaFavers, K. Disruption of kidney-immune system crosstalk in sepsis with acute kidney injury: lessons learned from animal models and their application to human health. Int. J. Mol. Sci. 23, 1702 (2022).
Spitzberg, J. D. et al. Multiplexed analysis of EV reveals specific biomarker composition with diagnostic impact. Nat. Commun. 14, 1239 (2023).
May, C. J., Saleem, M. & Welsh, G. I. Podocyte dedifferentiation: a specialized process for a specialized cell. Front. Endocrinol. 5, 148 (2014).
Kashani, K. et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 17, R25 (2013).
Ostermann, M. et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Netw. Open 3, e2019209 (2020).
La, A. M. et al. Real-world use of AKI biomarkers: a quality improvement project using urinary tissue inhibitor metalloprotease-2 and insulin-like growth factor binding protein 7 ([TIMP-2]*[IGFBP7]). Am. J. Nephrol. 54, 281–290 (2023).
Stasi, A. et al. PMMA-based continuous hemofiltration modulated complement activation and renal dysfunction in LPS-induced acute kidney injury. Front. Immunol. 12, 605212 (2021).
Boutin, L. et al. Subclinical and clinical acute kidney injury share similar urinary peptide signatures and prognosis. Intens. Care Med. 49, 1191–1202 (2023).
Darmon, M., Truche, A. S., Abdel-Nabey, M., Schnell, D. & Souweine, B. Early recognition of persistent acute kidney injury. Semin. Nephrol. 39, 431–441 (2019).
Zarbock, A. et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat. Rev. Nephrol. 19, 401–417 (2023).
Wang, H. et al. Patient outcomes following AKI and AKD: a population-based cohort study. BMC Med. 20, 229 (2022).
Bagshaw, S. M. et al. External validation of urinary C-C motif chemokine ligand 14 (CCL14) for prediction of persistent acute kidney injury. Crit. Care 25, 185 (2021).
Adembri, C. et al. Sepsis induces albuminuria and alterations in the glomerular filtration barrier: a morphofunctional study in the rat. Crit. Care 15, R277 (2011).
Privratsky, J. R. et al. A macrophage-endothelial immunoregulatory axis ameliorates septic acute kidney injury. Kidney Int. 103, 514–528 (2023).
Atreya, M. R. et al. Prognostic and predictive value of endothelial dysfunction biomarkers in sepsis-associated acute kidney injury: risk-stratified analysis from a prospective observational cohort of pediatric septic shock. Crit. Care 27, 260 (2023).
Wang, B. et al. Fatty acid-binding protein 4 is a therapeutic target for septic acute kidney injury by regulating inflammatory response and cell apoptosis. Cell Death Dis. 13, 333 (2022).
Hu, B. C. et al. Auto- and paracrine rewiring of NIX-mediated mitophagy by insulin-like growth factor-binding protein 7 in septic AKI escalates inflammation-coupling tubular damage. Life Sci. 322, 121653 (2023).
Kuwabara, S., Goggins, E. & Okusa, M. D. The pathophysiology of sepsis-associated AKI. Clin. J. Am. Soc. Nephro. 17, 1050–1069 (2022).
Chang, Y.M., Chou, Y.T., Kan, W.C. & Shiao, C.C. Sepsis and acute kidney injury: a review focusing on the bidirectional interplay. Int. J. Mol. Sci. 23, 9159 (2022).
Luxen, M. et al. Sepsis induces heterogeneous transcription of coagulation- and inflammation-associated genes in renal microvasculature. Thromb. Res. 237, 112–128 (2024).
Pascual, M. et al. Identification of membrane-bound CR1 (CD35) in human urine: evidence for its release by glomerular podocytes. J. Exp. Med. 179, 889–899 (1994).
Miwa, T. & Song, W. C. Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int. Immunopharmacol. 1, 445–459 (2001).
Jacobson, A. C. & Weis, J. H. Comparative functional evolution of human and mouse CR1 and CR2. J. Immunol. 181, 2953–2959 (2008).
Huang, F. et al. Predictive value of urinary cell cycle arrest biomarkers for all cause-acute kidney injury: a meta-analysis. Sci. Rep.13, 6037 (2023).
Thomas, M. E. et al. The definition of acute kidney injury and its use in practice. Kidney Int. 87, 62–73 (2015).
Chawla, L. S. et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 13, 241–257 (2017).
Levey, A. S. et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 67, 2089–2100 (2005).
Zhong, X. et al. Tubular epithelial cells-derived small extracellular vesicle-VEGF-A promotes peritubular capillary repair in ischemic kidney injury. NPJ Regen. Med. 7, 73 (2022).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 82241045, 82122011, 82230022, and 82200772), National Key R&D Program of China (2022YFC2502503), Natural Science Foundation of Jiangsu Province grant (BK20220828), and Postgraduate Research& Practice Innovation Program of Jiangsu Province (KYCX25_0522).
Author information
Authors and Affiliations
Contributions
L.L.L., L.B.C., X.J.F., T.T.T. contributed study design. Z.L., L.N., G.M.L., Z.T.T., T.T.T., H.R.B., Q.W.W., Z.T. and F.Y.Q. contributed participant recruitment. M.N.N. and L.N. contributed sample collection. M.N.N., L.N. and S.A.R. contributed experimental operation. L.N., S.A.R. and F.Y.Q. contributed data analysis. L.N., T.T.T. and L.L.L. contributed manuscript writing. All authors contributed manuscript review and final approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Bendetta Bussolati and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, N., Tang, TT., Gu, M. et al. Single urinary extracellular vesicle proteomics identifies complement receptor CD35 as a biomarker for sepsis-associated acute kidney injury. Nat Commun 16, 6960 (2025). https://doi.org/10.1038/s41467-025-62229-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62229-4