Abstract
Environmental factors profoundly affect the brain’s sensory processing network, optimizing adaptive strategies for growth and survival. We hypothesized that sensory-specific functional responses and/or functional networks could be altered by manipulating environmental conditions. To elucidate how the environment influences brain development, we exposed male mice to two - socially isolated and enriched environments - during early life (from 4 to 11 weeks) and investigated their effects on mouse brain functionality using sensory stimulus-evoked BOLD fMRI and resting-state fMRI. Here, we show that social isolation leads to reduced segregation of brain networks, notably affecting the olfactory and visual networks. Conversely, the enriched environment maintains network segregation while enhancing higher order sensory and visual cortical functions. Additionally, enriched mice exhibited an improved sensorimotor functional response. Our combined use of sensory stimulus-evoked BOLD fMRI and resting-state fMRI revealed changes at local and global levels, providing a comprehensive perspective. This expands our understanding of how environmental factors influence brain functionality and highlights the importance of physical and social cues - such as tactile, visual, and olfactory - that may provide therapeutic insights for psychiatric disorders.
Similar content being viewed by others
Introduction
The environment includes not only natural factors (e.g., terrain, climate) but also social factors in colonial animals, which do not play a passive role but have an enormous impact on life. Individuals grow according to the blueprint in their genes, but development can be stimulated or suppressed by the presence or absence of stimulating factors in the external environment. Survival is also highly dependent on an animal’s ability to adapt to its environment; information about the external environment is received by different senses, such as visual, auditory, tactile, and olfactory receptors, and these signals are then processed in the sensory regions of the brain, allowing animals to adapt to their environment. It is there that sensory processing is pivotal, facilitating appropriate responses for both survival and growth.
Environmental conditions play a crucial role in shaping sensory processing1, as shown by the studies of nocturnal vs. diurnal rodents2 for natural factors, and densely vs. sparsely populated geographies for social factors3. Indeed, many animals are born with “plastic” brains that allow them to respond flexibly to their environment. Studies have shown that environmental factors have profound effects on brain neurochemistry2,3, brain weight4, synaptic strengthening5 and resulting behavioral changes6,7 in laboratory animals, and a link between geographic factors and brain structure has been reported in human studies8. However, these observations are primarily based on isolated brain regions and specific behavioral paradigms, and the precise mechanisms by which environmental factors modulate global functional brain network dynamics and sensory functions remain largely unexplored. While environmental conditions are linked to brain structure and behavior, it is less clear how they affect network-level changes, particularly in terms of cortico-cortical and cortico-thalamic communication. Research could investigate how specific brain regions, when influenced by sensory stimuli or social/environmental factors, reorganize or strengthen their connections to adapt to new challenges or stimuli.
The traditional view of sensory processing is that primary sensory cortices process information from their primary sensory organs and any multisensory integration is performed by higher order cortices9. However, evidence for connectivity between primary sensory cortices is emerging, showing that they are either depolarizing or hyperpolarizing, depending on the direction of connectivity. For example, whisker stimulation was found to hyperpolarize visual cortex, whereas visual stimulation depolarized the whisker somatosensory cortex10. These interactions were found to increase sensory perception and sensitivity in a multisensory environment. Therefore, proper segregation of sensory systems is required in addition to the integration of multisensory information for proper behavior. For this reason, much of the focus on multisensory integration has been on sensory loss and the resulting cross-modal plasticity, where the loss of a sensation is compensated for by a spared sensory cortex11. However, there are few studies investigating how the enhancement or impairment of a sensation, without its loss, affects multisensory integration and brain reorganization. Such findings would help to link how animals perceive the environment to their well-being.
Environmental enrichment (EE) in the laboratory aims to create an environment in which caged animals can have a range of physical experiences by adding a variety of objects, such as toys, running wheels, and tunnels. This in turn increases sensory, perceptual, and motor stimulation, which is known to promote neural plasticity in various sensory areas of the brain12,13,14,15,16,17. Indeed, animals raised in EE show improved learning and memory, reduced anxiety, reduced age-related cognitive decline, and altered levels of immediate early genes and synaptic changes in the brain18,19,20. These studies have mostly been limited to region-specific chemical, anatomical, and behavioral manifestations, with few functional studies at the whole-brain level. For example, rodents raised in EE showed increased volumetric changes in the hippocampus and sensorimotor cortex as measured by volumetric MRI21 and increased signal enhancement in prefrontal, sensory, and limbic structures as measured by manganese-enhanced MRI22. Resting-state functional MRI (fMRI) studies have demonstrated increased connectivity between hippocampal and sensory cortical regions23. In addition, EE has been found to alleviate neurological disorders such as stroke, traumatic brain injury24,25, progression of tau pathology26, Alzheimer’s disease27, and other degenerative diseases in animal studies28.
Social isolation (SI), a form of severe social deprivation, is commonly used to model stress, anxiety, and depression and has been shown to counteract the neurochemical, anatomical, and behavioral improvements observed with EE29,30,31,32. Lack of a social environment has been shown to mimic chronic stress, autism, attention-deficit/hyperactivity disorder, and schizophrenia in rodents33. Human studies of SI have found a positive association with mortality, decreased visual acuity and contrast, impaired odor discrimination, and impaired auditory perception34,35,36. However, these studies are generally confounded by the perception of loneliness and the older age of the subjects. In addition, there are few studies investigating how SI during early life affects the development of sensory systems, with most focusing on cognitive, social and verbal impairments. Studies in these children have reported thinner cortices, less segregated brain networks and altered white matter tracts37,38,39. In rodents, impaired resting-state cortical functional connectivity and abnormal structural connectivity have been reported40,41. As with EE studies, most studies of SI have focused on local brain changes, with none investigating multisensory integration and the global brain network as a result of these changes.
fMRI has become an increasingly popular tool for investigating in vivo brain-wide functional changes due to normal aging, disease, or tasks for translational neuroscience in humans and rodents42,43,44,45. Stimulus-evoked fMRI measures how specific sensory stimuli elicit brain activation, providing insights into sensory-specific functional responses and activation patterns of specific brain regions46,47,48. In contrast, resting-state fMRI provides insight into intrinsic brain activity and the functional connectivity between different brain regions when the animal is at rest, in the absence of specific external tasks providing information on how well the brain segregates into specific functional networks and the connectivity between them49,50,51,52.
The combination of stimulus-evoked fMRI (e.g. visual, tactile, olfactory) with resting-state fMRI provides a more comprehensive approach to understanding brain function. By employing multimodal stimuli together, we can study how the brain processes and integrates different types of sensory information and how these sensory systems interact across different brain regions. This multimodal approach also allows us to investigate cross-modal influences (i.e. how one sensory input can affect the processing of another), providing insight into how sensory processing might interact within a broader brain network. While similar methods have been used in humans49, the combination of multimodal stimuli with rs-fMRI is relatively new in rodents. By elucidating the mechanisms by which early life experiences shape sensory processing and brain network organization, this research will provide valuable insights into neural plasticity, adaptive strategies for growth and survival, and potential therapeutic approaches for neurological disorders associated with sensory processing abnormalities and developmental disorders. Ultimately, this study aims to deepen our understanding of the complex relationship between environmental factors and brain development, with implications for both clinical and non-clinical contexts.
Results
Environmental conditions
Three different housing environments are shown in Fig. 1a and described in detail in Methods. Mice were placed in their environments at 28 d post-weaning (P28) for 7 weeks. fMRI experiments were performed the following week (Fig. 1b). The following environmental conditions were used to observe the effects of social isolation and enriched environment (Fig. 1c): single isolated mouse in standard cage (Standard Single, SS); grouped mice in standard cage (Standard Group, SG); and grouped mice in enriched cage (Enriched Group, EG). SG mice are control mice in typical laboratory housing conditions, SS mice represent the social isolation model, and EG mice represent the enriched physical environment model. Thus, significant differences between SS and SG would show the effects of social isolation, while EG and SG would show the effects of enriched environment (Fig. 1d). In addition, differences between EG and SS would show the combination of both physical enrichment and social environment. Body weight was measured weekly for all mice during the 7-week housing period. Four-week-old mice, initially weighing approximately 14 g, were subjected to different housing environments for 7 weeks to 11 weeks of age, resulting in significant differences in weight gain between groups. In particular, the SS group showed a significant increase in body weight from week 2, and this difference between the SS and EG groups persisted through week 7 (p < 0.05). (Fig. 1e).
a Housing conditions for each group. b Experimental schedule. Mice were placed in their environment at 28 d post-weaning (P28) for 7 weeks. Histology was performed immediately after the fMRI experiment. c Presence or absence of environmental conditions for each group. d Group comparisons and hypothesized effects between each housing condition. e Body weight measurements indicated a significantly greater increase in SS (n = 20; blue) than in SG (n = 20; red), and EG (n = 20; green) during 7-week change housing conditions (p < 0.05). Data are presented as the mean ± SEM (two-way ANOVA corrected with Tukey’s test; *p < 0.05, **p < 0. 01, ***p < 0. 001, ****p < 0. 0001).
The vasculature and physiology did not change from the environmental conditions
At the end of the housing period, to determine whether different housing environments would have vascular effects, we used ultrasound imaging to measure both vessel wall thickness and blood flow velocity in the common carotid artery (CCA). No significant differences in CCA diameter and peak velocity were observed between groups (Fig. S1), suggesting that there would be no vascular effect on BOLD fMRI responses.
To assess the physiological effects on the BOLD fMRI signal between groups, heart rate (HR) and respiratory rate (RR) were measured at pre-stimulation and during stimulation for the fMRI experiment. No significant differences in HR and RR were observed between groups at pre-stimulation and during stimulation, indicating no effects of different housing environments on basal physiological conditions and no effects of stimulation on physiological conditions (Fig. S2).
Whisker-pad stimulation evokes an enhanced response in the enriched group
The right whisker pad (WP) was electrically stimulated (Fig. 2a), resulting in a brain-wide response of the whisker somatosensory circuit (Fig. 2b). The stimulation-evoked BOLD fMRI response was observed in areas related to the whisker network, such as primary whisker and secondary somatosensory cortex (S1BF/S2), primary and secondary motor cortex (M1/M2), ventral posterior medial and posterior medial nucleus of the thalamus (VPM/PO), and intermediate ventral lateral caudate putamen (CPivl). Response was also observed in the dorsal retrosplenial cortex (RSCd), anterior posterior parietal cortex (PPCa), anteromedial visual area (V2am), globus pallidus externalis (GPe), substantia nigra pars reticulata (SNr), ventral-antero-lateral thalamus (VAL), zona incerta (ZI), and midbrain reticular nucleus (MRN). The response in S1BF, the main responding area, was similar amongst the three groups (Χ2 = 0.466, p = 0.806) (Fig. 2c). AUC values for ROIs related to the contralateral sensorimotor, association, and visual-auditory areas were calculated and plotted (Fig. 2d). Areas of significant change are shown in bold and italics. Although S1BF responses were similar between groups, S2 response was increased in EG compared to SS (p = 0.054). EG had a significantly higher response in CPivl (p = 0.023) and VPM (p = 0.002) compared to SS and in PO (p = 0.01) and VAL (p = 0.013) compared to SG. Therefore, PO and VAL showed an enrichment effect. EG showed significantly lower primary somatosensory hindlimb response (S1HL) compared to SS (p = 0.038) (Fig. 2e). Interestingly, V2am, which is considered an area of association, showed a significantly elevated response in SS (p = 0.025) and a subtly elevated response in EG (p = 0.178) compared to SG. This U-trend (SS and EG both higher than SG, but significance is only observed between either SS vs. SG or EG vs. SG) was also observed in RSCd (SS vs. SG, p = 0.057; SS vs. EG, p = 0.133; EG vs. SG, p = 0.053), which is strongly connected to the visual network. WP stimulation increased respiration in the early part of the stimulation (Fig. S2d). However, this increase in respiration during stimulation did not lead to motion artifacts (Fig. S3a) or activation of off-target areas due to increased oxygen flow to the brain by increased respiration (Fig. S3b). Regardless, motion regression did not change our observed trend or significant findings (Fig. S3c, g).
a fMRI experimental design. b Group-averaged BOLD response maps in SS, SG, and EG from right whisker pad stimulation (two-tailed one-sample t-test, p < 0.01, cluster-corrected with defining threshold Z > 2.3). Scale bar = 2 mm. c BOLD responses from whisker primary somatosensory cortex from SS (n = 6, blue), SG (n = 8, red), and EG (n = 8, green). The orange bar represents the stimulation duration. d Area under the curve (AUC) values from the contralateral somatomotor, polymodal, and visual/auditory areas. Significant areas after two-way ANOVA with Tukey’s correction are shown in bold and italics in e. Data are presented as the mean ± SEM (Kruskal-Wallis test, corrected with Dunn’s test; *p < 0.05, **p < 0.01) (S1BF whisker primary somatosensory cortex, S1FL forelimb primary somatosensory cortex, S1HL hindlimb primary somatosensory cortex, S2 secondary somatosensory cortex, M1 primary motor cortex, M2 secondary motor cortex, CPivl intermediate ventral lateral caudate putamen, VPM ventral posterior medial thalamus, PO posterior thalamus, VAL ventral anterolateral thalamus, ACCd dorsal anterior cingulate cortex, RSCd dorsal retrosplenial cortex, PPCa posterior parietal cortex, V2am anteromedial visual area, V1 primary visual cortex, V2al higher-order lateral visual area, V2lm higher-order lateral visual area, A1 primary auditory cortex, A2d dorsal secondary auditory cortex, LG lateral geniculate nucleus, LP lateral posterior nucleus, LD lateral dorsal thalamus, SCo superior optical colliculus, SCm superior motor-related colliculus, ZI zona incerta). Exact p-values are provided in the source data. Source data are provided as a Source Data file.
We also measured AUC values from the ipsilateral ROIs (Fig. S4a). The ipsilateral S2 and PO were also significantly higher in EG (Fig. S4b). In conclusion, WP stimulation showed altered responses within sensorimotor areas (enhanced PO and VAL from enrichment) and association areas (enhanced V2am and RSCd from enrichment and/or isolation), while the response in the main response area to WP stimulation (S1BF) was not altered.
Forepaw stimulation activates cross-modal areas in both enriched and isolated groups
Given the enhanced sensorimotor area activity from WP stimulation, we performed forepaw stimulation to determine if the enhanced activity could be found in another tactile modality. The left forepaw (FP) was electrically stimulated (Fig. 3a), resulting in a BOLD fMRI response within the forepaw somatosensory network, such as forelimb primary somatosensory cortex (S1FL), hindlimb primary somatosensory cortex (S1HL), S2, M1, M2, intermediate dorsal caudate putamen (CPid), ventral posterolateral nucleus of the thalamus (VPL), and PO (Fig. 3b). Similar to WP stimulation, no group differences were observed in the forelimb somatosensory cortex (S1FL), the main responding area (Χ2 = 0.801, p = 0.685) (Fig. 3c). However, in contrast to WP stimulation, significantly different group responses were observed mainly in the contralateral association and visual-auditory areas, such as the dorsal anterior cingulate cortex (ACCd) (Χ2 = 6.149, p = 0.041), RSCd (Χ2 = 6.053, p = 0.042), V2am (Χ2 = 6.854, p = 0.026), primary visual cortex (V1) (Χ2 = 8.117, p = 0.012), and dorsal higher order auditory area (A2d) (Χ2 = 6.045, p = 0.043) (Fig. 3d, e). EG mice showed significantly enhanced V1 (p = 0.013), V2am (p = 0.041), and RSCd (p = 0.042) responses compared to SG, indicating an enrichment effect. A similar U-trend as seen with WP stimulation was observed for V2am (SS vs. SG, p = 0.162; EG vs. SG, p = 0.042), A2d (SS vs. SG, p = 0.048; EG vs. SG, p = 0.371), and ACCd (SS vs. SG, p = 0.058; EG vs. SG, p = 0.211). Although no significant differences were observed in M1 (Χ2 = 4.497, p = 0.104), SS showed a significantly higher response compared to EG in caudal M1 (M1c) (p = 0.02), which corresponds to the hindlimb motor cortex. No significant changes in AUC values were observed from the ipsilateral ROIs (Fig. S4a). Our data showed that FP stimulation recruits from both association (enhanced V2am and RSCd from enrichment) and visual-auditory areas (enhanced V1 from enrichment and enhanced A2d from isolation) with little change in the forepaw network. Thus, FP stimulation induced changes in cross-modal functions as a function of environmental conditions.
a fMRI experimental design. b Group-averaged BOLD response maps in SS, SG, and EG in response to left forepaw stimulation (two-tailed one-sample t-test, p < 0.01, cluster-corrected with defining threshold Z > 2.3). Scale bar = 2 mm. c BOLD response from the primary somatosensory cortex of the forelimb from SS (n = 6; blue), SG (n = 8; red), and EG (n = 8; green). The Orange bar represents the stimulation duration. d Area under the curve (AUC) values from the contralateral somatomotor, polymodal, and visual/auditory areas. Significant areas after two-way ANOVA with Tukey’s correction are shown in bold and italics in e. Data are presented as mean ± SEM (Kruskal-Wallis test, corrected with Dunn’s test, *p < 0.05). See the caption for Fig. 2 for abbreviations (CPid intermediate dorsal caudate putamen, M1c caudal primary motor cortex). Exact p-values are provided in the source data. Source data are provided as a Source Data file.
Visual stimulation results in a reduced visual response in isolated groups
Given the enhanced V2am activity observed in both SS and EG with WP and FP stimulation, we performed visual stimulation (VIS) to determine whether the higher-order response of the visual network was enhanced. Binocular VIS stimulation (Fig. 4a) elicited a strong bilateral BOLD fMRI response within the visual network, including V1/V2, superior colliculus (SCo), lateral geniculate (LG) (dorsal and ventral), and lateral posterior (LP) of the thalamus, in addition to association areas such as ACCd, RSCd, PPCa, and medial entorhinal cortex (MEC) (Fig. 4b). The SS group showed reduced activity in V1, but this did not reach significance (Χ2 = 4.267, p = 0.118) (Fig. 4c, e). However, EG showed a significantly enhanced response in higher-order lateral visual cortex (V2lm) compared to SG (p = 0.037) and SS (p = 0.049), suggesting an enrichment effect. Interestingly, V2am, which was enhanced in EG and SS as a form of U-trend by WP and FP stimulation, did not show an enhanced response to VIS stimulation. SS presented significantly reduced activities in PPCa (p = 0.033) compared to SG, suggesting a social isolation effect. SS also presented significantly reduced activities in RSCd (p = 0.038), VP (VPM + VPL) (p = 0.049), and PO (p = 0.034) compared to EG, indicating a mixed effect of enrichment and social isolation (Fig. 4e). Interestingly, the polarities of S1FL and M1 responses differed between SS and SG/EG. SS showed a small average positive response, whereas SG and EG showed negative responses. We also looked at whether the left and right hemisphere responses were similar or asymmetric and found the brain generally responds symmetrically to binocular visual stimulation (Fig. S5). Our data suggest that VIS stimulation induced either an enhanced response from EG or a reduced response from SS in V2lm, RSCd, VP, and PO with little change in V1, the main responding area, to VIS stimulation. Moreover, in contrast to somatosensory stimulation, VIS stimulation induced reduced responses in cortical areas with association function such as PPCa in SS.
a fMRI experimental design. b Group-averaged BOLD response maps in SS, SG, and EG in response to binocular visual stimulation (two-tailed one-sample t-test, p < 0.01, cluster-corrected with defining threshold Z > 2.3). Scale bar = 2 mm. c BOLD response from the primary visual cortex in SS (n = 6; blue), SG (n = 8; red), and EG (n = 8; green). The orange bar represents the stimulation duration. d Area under the curve (AUC) values from bilateral somatomotor, polymodal, and visual/auditory areas. Significant areas after two-way ANOVA with Tukey’s correction are shown in bold and italics in e. Data are presented as the mean ± SEM (Kruskal-Wallis test corrected with Dunn’s test, *p < 0.05). See the caption for Fig. 2 for abbreviations (CPidt intermediate dorsal tip caudate putamen, VPN ventral posterior nucleus). Exact p-values are provided in the source data. Source data are provided as a Source Data file.
Olfactory stimulation results in hyperactivity in isolated groups
The olfactory system is the most developed and separated from the somatosensory and visual systems in rodents. Amyl acetate odor was delivered through the nose cone (Fig. 5a), which resulted in a brain-wide response of the olfactory limbic system: main olfactory bulb (MOB); dorsal taenia tecta (TTd); piriform cortex (PIR); bed nuclei of the stria terminalis (BNST); nucleus accumbens (NAc); lateral preoptic area (LPO); mediodorsal thalamus (MD); habenula (Hb); CA1 Subfield of the hippocampus (CA1); and periaqueductal gray (PAG) (Fig. 5b). Limbic areas located ventrally, such as amygdala, were not analyzed due to distortion of EPI images from the ear canal. Although not significant, an increased response in PIR, the primary olfactory cortex, was observed in the SS group (SS vs. SG, p = 0.163) (Fig. 5c). As the main areas involved in olfaction were very different from those of WP, FP and VIS, we categorized the ROIs as olfactory, limbic and association-polymodal (Fig. 5d). Compared to SG, SS showed significantly elevated responses from TTd (p = 0.015) in the olfactory area, from BNST (p = 0.004) in the limbic area, and from ACCd (p = 0.022), RSCd (p = 0.029) in the association area, and LP (p = 0.034), and LD (p = 0.018) in the thalamus, indicating a social isolation effect in these areas. Interestingly, compared to EG, SS presented with significantly increased responses in LP (p = 0.031), LD (p = 0.005), S1BF (p = 0.048), V2am (p = 0.014), habenula (Hb) (p = 0.026), and PO (p = 0.037), suggesting a mixed effect (Fig. 5d, e). Enrichment only enhanced the TTd response (EG vs. SG, p = 0.004). Taken together, our results indicate that social isolation leads to an enhanced olfactory response in the whole brain. Like WP stimulation, we observed increase in respiration, yet these did not result in motion- or respiration-related BOLD activations (Fig. S3d–g).
a fMRI experimental design. b Group-averaged BOLD response maps in SS, SG, and EG in response to olfactory stimulation (two-tailed one-sample t-test, p < 0.01, cluster-corrected with defining threshold Z > 2.3). Scale bar = 2 mm. c BOLD response from piriform cortex in SS (n = 6; blue), SG (n = 8; red), and EG (n = 8; green). The orange bar represents the stimulation duration. d Area under the curve (AUC) values from bilateral olfactory limbic and polymodal areas. Significant areas after two-way ANOVA with Tukey’s correction are bold and italics in e. Data are presented as the mean ± SEM (Kruskal-Wallis test corrected with Dunn’s test, *p < 0.05, **p < 0.01) (MOB main olfactory bulb, AON anterior olfactory nucleus, TTd dorsal tenia tecta, PIR piriform cortex, BNST bed nucleus of the stria terminalis, NAc nucleus accumbens, PVH paraventricular hypothalamus, AHN anterior hypothalamus, LPO lateral preoptic area, VTA ventral tegmental area, PAG periaqueductal gray, RSC retrosplenial cortex, PPC posterior parietal cortex, LP lateral posterior thalamus, PO posterior thalamus, LD lateral dorsal thalamus, Hb habenula, MD, mediodorsal thalamus, S1BF whisker primary somatosensory cortex, V2am higher order anteromedial visual area). Exact p-values are provided in the source data. Source data are provided as a Source Data file.
Elevated c-Fos in isolated groups after sensory stimulation
To access the neural effects on BOLD fMRI signal between groups, c-Fos immunochemical staining was performed after each stimulation and in the absence of stimulation (Fig. S6). Due to low expression levels in subregions, only major structures (e.g. somatosensory (SS), somatomotor cortex (SM), hippocampus, caudate putamen, piriform area, amygdala, thalamus, hypothalamus) were considered for analysis. Due to the small sample size (n = 3 ~ 4 per stimulation for each group) and high individual variability, statistical significance was rarely observed. Tactile stimuli (WP and FP) induced higher c-Fos expression trends in S1, piriform cortex, hippocampus and amygdala in SS compared to EG and SG, with a notable difference in amygdala expression between SS and EG during WP stimulation (p = 0.073). VIS stimulation induced increased c-Fos expression in the amygdala and hypothalamus for SS. OLF stimulation resulted in increased expression in multiple regions (hippocampus, piriform cortex, amygdala, caudate putamen, and hypothalamus), with SS showing significantly higher hypothalamic expression compared to both SG (p = 0.0013) and EG (p = 0.0075). Overall, SS showed higher c-Fos expression than SG in specific ROIs and whole brain during WP, FP and VIS stimulation, with significantly higher expression during VIS stimulation (p = 0.0311).
Resting-state functional connectivity and network segregation
Somatosensory stimulation-evoked BOLD fMRI showed an enhanced response within the visual circuitry in both SS and EG (e.g., U-trend), as seen with FP stimulation (Fig. 3), suggesting altered basal connectivity. We analyzed resting-state fMRI data to address whether basal connections between known mouse networks were altered in SS and EG. Group independent component analysis (ICA) was performed to find networks common to all three groups. Ten components were extracted, resulting in reported networks including parts of the medial network, lateral cortical network (LCN), posterior lateral network (PLN), hippocampus (HPC), thalamus (TH), olfactory (OLF), and striatum (STR) networks53,54 (Fig. 6a). Two components comprised the medial network (ACC and RSC), and three components comprised the LCN (orofacial (ORF), whisker/forelimb (Wh/FL), and hindlimb/trunk (HL/tr)). Rather than combining the components into one large network (e.g., medial or LCN), we kept the components separate because our ORF, Wh/FL, and HL/tr components are spatially consistent with the reported mouse somatic networks55. Each of the somatic networks is composed of its respective primary and secondary sensory and motor areas. The PLN includes PPCa, V1, all higher order visual areas (V2am/al/pm/lm), A1, and A2d. The HPC network is restricted to the dorsal hippocampus and includes the DG and CA1 subfields, the subiculum, and the medial entorhinal cortex. The TH network includes the higher order thalamic nuclei: anterior thalamus; mediodorsal (MD), LD, LP, and PO. The OLF network is composed of the MOB, PIR, medial and lateral orbitofrontal cortex (OFCm/OFCl), and anterior insula (AI). Finally, the STR network is composed of the rostral lateral, intermediate dorsal, intermediate tip, intermediate ventral lateral, and caudal intermediate areas, which were segmented using an enhanced and unified atlas56 (Table S1).
a Group-averaged independent component analysis (ICA) maps of all three groups (SS, SG, and EG) are presented as individual networks, along with the location of the prominent seeds of each network (defining threshold Z > 2.3). b A functional connectivity (FC) matrix (56 × 56) with regions of interest (ROIs) based on the observed networks for SS (n = 6; blue), SG (n = 8; red), and EG (n = 8; green). The bottom triangle is unthresholded, and the top triangle is symmetrically thresholded at |r| > 0.2. c Graph theory and network segregation metrics of the whole brain. d Mean within-diagonal functional connectivity (FC) within the PLN and TH networks. e Network segregation between two specific networks. Higher values indicate segregated networks. Data are presented as the mean ± SEM (Kruskal-Wallis test, corrected with Dunn’s test; *p < 0.05, **p < 0.01). (ACC anterior cingulate, RSC retrosplenial, ORF orofacial, Wh/FL whisker/forelimb, HL/tr hindlimb/trunk, PLN posterior lateral network, HPC hippocampal network, TH thalamic, OLF olfactory, STR striatum). Source data are provided as a Source Data file.
ROI-to-ROI correlation analysis was performed for each group and is shown in Fig. 6b. The bottom triangle displays the unthresholded correlations, while the top triangle shows symmetrically thresholded correlations (|r| > 0.2). This absolute threshold was chosen to preserve and balance the network sparsity and density with the inclusion of negative weights57,58. The SS group presented with denser connections within the sensory networks in addition to denser between-network connections compared to both SG and EG. Three graph theory metrics were calculated for unbiased ICA-derived signed network analysis with symmetrically thresholded networks: clustering coefficient, positive participation coefficient, and modularity57 (Fig. 6c). The SS network presented with higher clustering coefficient compared to EG (p = 0.037), indicating increased local hyperconnectivity within neighboring ROIs within the same network. Similarly, the SS network showed a significantly higher positive participation coefficient compared to EG, reflecting greater inter-network connectivity. In contrast, modularity, a measure of how well the brain organizes itself into distinct networks with dense within-module and sparse between-module connectivity, was not significantly different, although a trend toward lower modularity in the SS network compared to SG and EG was observed. Our computed graph metrics suggest that social isolation leads to reduced segregation, with ROIs hyperconnected to other networks (higher positive participation coefficient) and within-network hyperconnectivity (higher clustering coefficient). In contrast, the EG network likely exhibits enhanced segregation at rest.
To investigate which specific network connectivity was altered, we used network segregation using our observed ICA networks, measuring the difference within and between networks as a proportion of the within network (Fig. 6c). SS showed reduced whole-brain network segregation (SS vs. SG, p = 0.089), but significance was only reached when compared to EG (p = 0.03). The mean between-network connectivity of SS was significantly increased compared to SG and EG (p = 0.027 and p = 0.021, respectively). These results are consistent with our findings of low modularity (lower network segregation) and high positive participation coefficient of SS (higher between-network connectivity).
We also examined which specific network connectivity was altered by calculating network segregation between all networks (Fig. 6d, Fig. S7). SS showed increased within-network PLN connectivity compared to SG (p = 0.053). In addition, SS showed significantly increased within-network TH connectivity compared to SG (p = 0.025) and EG (p = 0.019) (Fig. 6d). Segregation between specific networks was also calculated (Fig. 6e, Fig. S7). Segregation between all three somatosensory networks (ORF, Wh/FL, and HL/tr) to PLN and TH was significantly reduced in SS compared to EG (with an exception for Wh/FL to PLN, which is significant for SG), suggesting that social isolation leads to hyperconnectivity between these areas. Interestingly, when the within (main) and between (inter) networks were reversed (e.g., PLN to Wh/FL instead of Wh/FL to PLN), network segregation was not significantly different (SS vs. SG, p = 0.595; SS vs. EG, p = 0.798) (Fig. S7), suggesting asymmetric changes in hyperconnectivity between these areas. The majority of these changes were observed between SS vs EG suggesting mixed effects. These results could be attributed to a subtle improvement due to enrichment in EG and a subtle detriment due to social isolation in SS. Overall, the current analysis of resting-state fMRI data revealed similar resting-state network connectivity between SG and EG, while SS networks were less modular, less segregated, and hyperconnected, indicating aberrant brain connectivity in socially isolated mice.
Functional network segregation and connectivity from sensory stimulation-evoked BOLD fMRI
Next, we attempted to characterize functional segregation from our sensory stimulation-evoked BOLD fMRI data. This would allow us to measure the activation of other networks by specific stimulation modalities. Using the networks detected from the resting-state fMRI data, we assigned the following networks as the main network for each stimulation modality: Wh/FP for both WP and FP; PLN for VIS; and OLF for OLF. The main networks for each stimulation are reported as their mean AUC values and are outlined in boxes in Fig. 7. Similar to the resting state fMRI connectivity results, we observed reduced segregation of sensory responses in SS. Segregation of the HL/tr network was significantly reduced by FP stimulation in SS compared to SG (p = 0.021) and EG (p = 0.013), which coincides with the significantly elevated M1c response from FP stimulation (Fig. 3e).
Network segregation was calculated using area under the curve (AUC) values within the resting state networks observed during evoked sensory stimulation from SS (n = 6; blue), SG (n = 8; red), and EG (n = 8; green). The main network for each stimulation is outlined in a black box and shown as the mean AUC within the network. The main network is used to normalize response differences between networks for segregation values. Lower values indicate greater activation of the network relative to the main network, implying reduced segregation. Data are presented as mean ± SEM (Kruskal-Wallis test, corrected with Dunn’s test; *p < 0.05, **p < 0.01). (ORF: orofacial; Wh/FL: whisker/forelimb; HL/tr: hindlimb/trunk; PLN: posterior lateral network; TH: thalamic; OLF: olfactory). Source data are provided as a Source Data file.
VIS stimulation resulted in reduced segregation of Wh/FL, OLF, STR, and ACC networks in SS compared to SG, in addition to lower PLN activity (SS vs. EG, p = 0.046) compared to EG. This reduced activity in the PLN is likely due to the significantly increased V2lm response in EG and the nonsignificantly reduced V1 response in SS (Fig. 4e). Lastly, OLF stimulation resulted in reduced segregation of PLN and RSC in SS. Taken together, our data suggest that SS results in exaggerated non-primary sensory network responses to somatosensory and visual stimulation, which is similar to the less segregated resting-state networks in SS group. Sensory network responses to somatosensory and visual stimulation were similar between SG and EG.
Negative BOLD and negative correlation influences on segregation
We next investigated whether impaired inhibitory modulation contributed to reduced segregation in the SS group. Impaired negative BOLD responses were observed in S1FL and M1 regions of the Wh/FL network in SS mice from VIS stimulation (Fig. 4e). This aligns with the reduced segregation during resting-state (Wh/FL – PLN) and evoked states (increased Wh/FL activity from VIS). Negative BOLD responses were found in S1FL, S2c, M1w, M2rdm, and CPivl from VIS stimulation and V1, A1, and A2v from OLF stimulation in both SG and EG mice (Fig. 8a). However, SS mice exhibited significantly diminished negative BOLD responses in S2c, M1w, and CPivl compared to SG and in A1 and CPivl compared to EG when comparing the AUC of the negative BOLD response (Fig. 8b). Of particular note is the ROIs presented here are local seeds used with resting-state analysis, while the ROIs presented in Fig. 4 are from anatomical ROIs covering the whole region. This is likely why positive BOLD response is observed in S2, A1, and A2 (Fig. 4), while a negative response is observed with local specific ROIs.
a BOLD time course for stimulation in SS (n = 6; blue), SG (n = 8; red), and EG (n = 8; green). The gray shade represents 20 s of stimulation. b Area under the curve (AUC) of the negative BOLD response. c Negative weights calculated via Brain Connectivity Toolbox from resting-state data within the corresponding areas where negative BOLD responses were observed. d Number of negative weights connected to each ROI before and after thresholding. Data are presented as mean ± SEM (Kruskal-Wallis test, corrected with Dunn’s test; *p < 0.05). (S1FL primary somatosensory forelimb, S2c caudal secondary somatosensory area, M1w whisker primary motor area, M2rdm rostral dorsal medial secondary motor area, CPivl caudate putamen intermediate ventral lateral area, V1 primary visual area, A1 primary auditory area, A2v ventral secondary auditory area). Source data are provided as a Source Data file.
To quantify inhibitory processes, we next calculated the negative strength, which is the sum of all negative weights, of these areas from symmetrically thresholded resting-state. SS mice exhibited significantly lower negative strength in CPivl and A2v compared to EG, with trends of reduced strength in S2c and M2rdm (p = 0.0530 and p = 0.0645, respectively) (Fig. 8c). The number of negative weights in these areas did not significantly differ except for in A2v, although a general trend of lower number of negative weights is observed in SS group (Fig. 8d). Reduced total negative strength in SS mice further highlights the role of negative weights in maintaining proper network segregation (Fig. S9b). This effect was accentuated when an asymmetric threshold, preserving more negative weights, was applied. Additionally, the negative participation coefficient, which measures inter-network inhibitory interactions, was significantly lower in SS compared to EG (Fig. S9c). In contrast, when a proportional threshold was applied, maintaining only positive weights, no significant differences in graph theory metrics were observed (Fig. S9d). These findings reveal that diminished negative BOLD response and lower negative strength contribute to impaired inhibitory modulation, which underpins the reduced segregation observed in SS mice.
Environmental influences on sensory functions of mouse behavior
To evaluate the effects of a 7-week environmental change on sensory function in mice, a comprehensive battery of behavioral tests was conducted. These tests included the Optomotor Vision Test for visual contrast, the Object Preference Test for multisensory integration, the Olfactory Habituation Test for olfactory function and working memory, the Hot Plate Pain and Tactile Test for somatosensory function, the Open Field test for locomotion and anxiety, the Elevated Plus Maze Test for anxiety, and the Interactive Behavior Test for social interaction.
Visual contrast in mice was assessed using an optomotor apparatus presenting rotating cylindrical patterns at three different contrast levels (Fig. 9a). The highest frequency of head tracking responses was observed for the standard contrast pattern, with no significant differences between groups. Lower contrast patterns (dark and light) elicited fewer head-tracking responses in all groups, with no significant differences between groups. SG showed uniform visual contrast ability across all contrasts, with no significant differences in their responses. In contrast, EG showed reduced ability to perceive dark contrasts compared to standard contrasts (p = 0.0275), while SS showed reduced head-tracking responses to light contrasts compared to standard contrasts (p = 0.0264).
a The visual contrast, as measured by the optomotor test, showed no group differences across the three contrast conditions b The object preference test shows that the EG had a higher preference for the visual (V) object than the SS. There was no significant difference between groups in their preference for the tactile (T) object or the combined visual and tactile (V & T) object. c SS failed to show olfactory habituation responses, indicating impaired olfactory function compared to the EG. d The hot plate test showed no significant difference in tactile pain sensitivity between groups. e SG exhibited greater locomotion in the open field than the other groups. f Anxiety levels, as assessed by the elevated plus maze test, showed that SS exhibited greater anxiety than EG. g Interactive behavior exhibited exhibited that SS showed more nose-to-nose contact behavior than SG. Data are presented as mean ± SEM (Kruskal-Wallis test corrected with Dunn’s test; *p < 0.05, **p < 0.01). Note that the sample size for behavioral measures (a) through (e) was n = 12 per group, while measures (f) and (g) used n = 8 per group. The mouse image used is from TogoTV (© 2016 DBCLS TogoTV, CC-BY-4.0 https://creativecommons.org/licenses/by/4.0/. Source data are provided as a Source Data file.
To evaluate the effect of environmental conditions on the sensory processing of unimodal versus multimodal stimuli in mice, we conducted an object preference test using visual (V), tactile (T), and combined visual-tactile objects (V&T) (Fig. 9b). Analysis of exploration time ratios (calculated as the proportion of time spent exploring each object relative to the total exploration time) revealed no significant group difference for multimodal (combined visual-tactile) stimuli. However, we observed clear preferences for unimodal stimuli across groups. EG showed significantly higher exploration rates for the visual stimulus object compared to SS (p = 0.0478). Notably, only EG showed a significant preference for the visual object above the chance level of 33.3%, suggesting an increased affinity for visual stimuli in enriched environments. Conversely, although not reaching statistical significance, SS appeared to prefer tactile over visual objects, possibly indicating a shift in sensory preference due to social isolation. These results suggest that environmental conditions may differentially modulate preferences for specific sensory modalities, rather than affecting overall preference for multimodal versus unimodal stimuli.
An olfactory habituation test was administered to assess olfactory function and working memory. The test measured the number of sniffs in response to repeated presentations of a limonene odor stimulus over four trials. Both SG and EG showed a habituation pattern, with sniffing frequency subsequently decreasing in trials 2–4. In contrast, SS failed to show habituation and maintained elevated sniffing frequencies across all trials. Notably, SS exhibited significantly higher sniffing frequencies in the fourth trial compared to EG (p = 0.0137), suggesting that SS may have less sensitive olfactory function or impaired working memory (Fig. 9c).
In the hot plate test, which measures pain sensitivity, most mice tolerated pain for a maximum of 30 seconds with no significant group differences (Fig. 9d, p = 0.303). In a 5-min open field test, SG exhibited significantly greater locomotion compared to the other two groups, as evidenced by increased total distance traveled (SG > SS, p = 0.0175; SG > EG, p = 0.0112) (Fig. 9e). However, analysis of time spent in the central area, a measure of anxiety-like behavior, showed no significant differences between the groups (p = 0.559). However, in a 10 min elevated plus maze test, a more specific anxiety test, SS showed significantly fewer entries into the open arm compared to EG (Fig. 9f, p = 0.0316). However, total distance traveled was not significantly different between groups. These results suggest that while SS and EG exhibited comparable locomotor activity, SS exhibited more anxiety-like behavior than EG.
Using RFID tags on mice, we studied interactive behavior by placing four mice at a time in an open field. The total contact time between mice appeared to be higher in EG and SS compared to SG, although the difference was not statistically significant. However, nose-to-nose contact behavior was significantly more frequent in SS compared to SG (Fig. 9g, p = 0.0308). This increased nose-to-nose contact in the SS mice may be due to their housing conditions, as they were housed one per cage, unlike the other groups. Thus, this trial likely provided their first opportunity to interact with unfamiliar mice, which may explain the higher frequency of this particular behavior.
Discussion
In this work, we sought to measure brain-wide altered sensory responses and functional network due to different environmental conditions (social isolation or environmental enrichment) offered to mice from adolescence (4 weeks) to early adulthood (11 weeks) using multisensory (WP, FP, VIS, and OLF) stimulus-evoked BOLD and resting-state fMRI. The effects of the physical and social environment on brain function are summarized in Supplementary Table 2 by comparing three different groups. First, we assessed enrichment effects by comparing EG to SG groups that had different physical housing enrichment conditions (e.g., cage size, nest, running wheel, igloo, etc.). Second, we examined the combined effects of physical housing enrichment and social engagement by contrasting EG and SS groups. Finally, we examined the effect of social isolation by comparing SS to SG groups (i.e., single versus group housing).
Physical enrichment enhanced sensorimotor responses to tactile stimulation. WP stimulation produced stronger BOLD fMRI responses in PO and VAL thalamus in EG, which are the higher order and motor thalamic nuclei of the somatosensory and motor pathways, respectively, that propagate tactile/motor signal to the cortex59,60. PO projects to S1BF and whisker M1, while also receiving reciprocal corticothalamic inputs, and is thought to fine-tune computations between ascending sensory signals and motor commands61. The VAL is considered an integrative hub of cortical, cerebellar, and basal ganglionic projections involved in postural maintenance, control of general movement, and learning of motor-related tasks62. Therefore, enhanced PO and VAL are most likely due to increased activities, such as movement and object exploration, in the enriched environment provided to EG mice.
Interestingly, stimulation of FP, another tactile sensation, produced larger BOLD fMRI signals in cross-modal cortical areas (e.g., S1HL, V1 and V2am of the EG group (Fig. 3e). Although direct cortical projections between V1 and S1FL do not exist, evidence for multisensory interplay between the forepaw somatosensory and primary visual systems has been reported55. Functional cross-modal sensory studies have primarily been conducted between visual, auditory, or whisker somatosensory modalities, with none, according to our relatively thorough literature review, involving the forepaw sensory system11,63,64. Nevertheless, human fMRI studies have shown activity in visual areas during a tactile task involving their arms, suggesting that there may be functional interaction between these areas in rodents65,66. The hindlimb somatosensory cortex (S1HL) in the SG group showed a significantly smaller response compared to EG (and SS, although not significant), indicating that changes in either the physical or social environment could alter the functional connectivity between the forelimb and hindlimb.
Physical enrichment alone did not appear to enhance VIS stimulated responses in visual areas such as V1, PPCa, V2am, or V2al, suggesting that these areas are unchanged when stimulated by their dominant modality (Fig. 4d). In contrast, an elevated response was detected in V2lm, which has the second largest projections from V1 after V2al67. This trend is similar to WP stimulation, where there are no differences between primary cortices (S1BF/V1), but an elevated response in secondary cortices (S2/V2lm) (Fig. 2e, Fig. 4e). V2lm is thought to be the gateway to the ventral stream, which is involved in memory and object identification68,69. Therefore, elevated V2lm activity could be due to the presence of more objects in the environment and recruitment of the ventral stream function to recognize and remember the locations of the objects. However, we did not detect increased activity within the hippocampal formation with VIS stimulation, which could be due to our stimulation modality or anesthesia (Fig. S10). In our multisensory object task, EG mice significantly preferred the visual object compared to SS mice (Fig. 9b). This preference for visual cues may be due to the enhanced V2lm leading to better object identification and recognition. In addition, we found no difference in visual contrast between the groups (Fig. 9a). Several studies have shown that primary visual cortex activity does not correlate with visual contrast tasks in mice or primates70,71. Although not significant, SS mice had a reduced V1 response but preserved visual contrast. In contrast, we saw significantly elevated V2lm in EG mice and reduced V2pm in SS mice (Fig. 4d, Fig. S10b), suggesting that these areas may also not be involved in standard visual contrast. However, our visual stimulation for fMRI was a flashing white LED light, thus it may not accurately represent the areas involved in visual contrast.
Physical enrichment increased OLF-stimulated responses only in the dorsal tenia tecta (TTd). The TTd is involved in olfactory information processing with large inputs from the piriform cortex and lateral entorhinal cortex and relatively little input from the olfactory bulb72. Enhancement in this area may be due to increased input from the lateral entorhinal cortex as running was shown to increase hippocampal neurogenesis73. However, we did not detect increased activity within the hippocampal formation (Fig. S10); thus further studies investigating whether improved TTd activity is due to odor perception or extra cortical function will be required.
However, as shown in Supplementary Table 2, while EG mice showed clear enrichment effects compared to SG, contrasting EG to SS led to stark differences in numerous other brain regions such as VPM, CPivl, GPe, MRN and S2 for the case of WP stimulation (Fig. 2 and Fig. S10) and ventral posterior nucleus (VPN; VPL + VPM), PO and RSCd for the case of VIS stimulation (Fig. 4). These differences likely stem from the subtle effects of both EG and SS compared to SG, but become significant when compared to each other, suggesting that the combination of physical enrichment and social engagement has a greater effect than either alone.
VPM is the primary sensory relay for ascending tactile signals61,74. The enriched environment may have subtly enhanced VPM sensitivity due to the various physical objects and the presence of littermates, whereas social isolation leads to slightly reduced VPM activation. In addition to enhanced thalamic sensory processing due to enrichment, we also found elevated response in the intermediate ventral lateral caudate putamen (CPivl), a subregion of the caudate putamen (CP) that receives prominent projections from the whisker somatosensory network75. CPivl and external globus pallidus (GPe) are part of the basal ganglia, which is known for its control in locomotion, multisensory integration, and decision-making76,77. GPe is traditionally considered part of the indirect pathway of the basal ganglia, which functions to stop locomotion by inhibiting VAL78. In the indirect pathway, CP inhibits GPe by disinhibiting the subthalamic nucleus (STN), which excites the internal globus pallidus (GPi) and the substantia nigra reticular (SNr). These in turn inhibit VAL. However, we observed an enhanced CP and VAL response, suggesting enhanced activation of the direct pathway. Enrichment promoted synaptic plasticity associated with vesicle trafficking and exocytosis in the striatum, supporting our enhanced CP activity79. Our enhanced GPe activity may be due to the hyperdirect pathway, in which the cortex directly stimulates the STN, which then stimulates the GPe. Alternatively, whisker deflection was found to activate a subpopulation of GPe cells in addition to being stimulated by direct pathway cells of the CP, which also supports our BOLD fMRI findings80.
MRN is part of the mesencephalic locomotor region that is thought to relay locomotion signals from the basal ganglia to the spinal cord81,82. Stimulation of glutamatergic neurons innervated by the basal ganglia elicited locomotion in the MRN, whereas GABAergic neurons abolished locomotion in the MRN83. The increased MRN activity, combined with our increased activity in the basal ganglia, suggests potentiated circuits involved in sensorimotor integration, movement initiation function, and locomotion due to either the more complex physical environment or the use of the running wheels, whereas single housed mice show a slight reduction in these areas suggesting that these mice may have spent more time inactive rather than active which may have altered their sensitivity to sensory stimulation84.
In addition to enhanced V2lm activity upon physical enrichment alone by VIS stimulation, EG mice showed elevated RSCd along with thalamic nuclei (PO and VPN) compared to SS. RSCd is primarily involved in spatial memory; however, it is also a multisensory processor and is well connected to V155,85,86,87. Although significance was reached comparing EG to SS, our data suggest that rather than an enrichment effect, we see a reduced response in RSCd due to social isolation because EG has similar response as SG (Fig. 4e). To our surprise, PO and VPN responses were significantly increased when comparing EG to SS. Although PO and VPN are predominantly part of the somatosensory pathway, which had an increased response to WP stimulation, they also appear to be involved in visual processing. PO in particular has also been found to have a multisensory function with a specific subset of PO cells contributing to the reflex light response88. VPM was found to process and relay visual inputs in which simultaneous whisker and visual stimulation enhanced information flow to S1 compared to whisker stimulation alone89,90. The visual presence of various objects and cage mates in the environment may have enhanced PO and VPN function to coordinate with the whisker somatosensory system for navigation.
Although most brain regions showing significant group differences responded to their specific single sensory stimulus, some brain regions, such as the aforementioned PO and V2am, exhibited significantly different responses to more than one type of sensory stimulation. While PO showed an increased response in EG to WP stimulation, V2am exhibited greater response to both tactile stimulations in EG or SS compared to SG, which we consider as a U-trend (Fig. 2d, Fig. 3d). The U-trend is particularly prominent within the association areas (ACCd, RSCd, and V2am) to FP stimulation. This suggests that these areas are involved in cross-modal functional changes, with the forepaw sensory system being the most sensitive to environmental and social changes. Recent work has classified V2am as part of the posterior parietal cortex (PPC), rather than a functionally specific visual area, in mice91,92. The PPC is an integral area that processes different sensory signals for spatial processing and movement planning in the environment93,94. As such, V2am is classified as part of the dorsal stream of visual processing with reciprocal connections to M2 and V2al55,95. The dorsal stream is involved in visually guided actions and recognition when objects are present in the environment and is thought to have two branches, one of which is involved in object grasping96. Thus, our data suggest that V2am is an integral part of tactile processing plasticity, with increased activity due to both physical enrichment and social isolation.
The effect of social isolation was particularly pronounced in the OLF stimulation with enhanced BOLD fMRI responses in the olfactory region (TTd), limbic system (BNST, LPO), and association polymodal areas (ACCd, RSCd, V2am, LD) by social isolation alone, in addition to other association polymodal areas such as PO and Hb by the combined effect of physical enrichment and social engagement. The BNST is a central region implicated in anxiety in which lesions to it abolish anxiety-like responses97. The Hb, particularly the lateral portion, is thought to mediate information between the basal ganglia and the limbic system and has been implicated in both depression and schizophrenia98. In addition, LPO is known to regulate feeding behavior and the reward system99. Studies have shown increased LPO-Hb activity after foot shock administration, suggesting that our SS mice may perceive the OLF stimulation as aversive100. This may be due to the fact that SS mice had no other olfactory cues for proper olfactory perception development, whereas both SG and EG mice had their cage mates and physical objects. Interestingly, TTd response was elevated in EG mice as well as in SS. TTd was found to be involved in driving sympathetic and emotional stress response to the hypothalamus101. Therefore, the enhanced TTd response in SS, in addition to the enhanced Hb and LPO response, could be related to stress due to isolation whereas the elevated response in EG may be related to increased olfactory or hippocampal function due to enriched environment. Consistent with this, we observed EG mice with preserved olfactory habituation while SS mice had impaired olfactory habituation (Fig. 9c). In addition, OLF stimulation resulted in significantly increased c-Fos expression in the hypothalamus of SS mice (Fig. S6f), which is indicative of social isolation-induced stress29. We also observed increased activity in association areas (ACCd, RSCd, V2am) and higher order thalamic nuclei (LP, PO). V2am was elevated by both tactile and olfactory stimulation, further supporting its function as a cross-modal plasticity area. These areas may also contribute to impaired olfactory perception and thus processing102,103. SS mice showed increased anxiety as indicated by the elevated plus maze test (Fig. 9f), which is supported by increased ACCd response, which is known to be involved in attention, sensory processing, and emotion104,105. Interestingly, both SS and EG mice moved significantly less than SG mice, while center time was not different when placed in an open field (Fig. 9e). This may be related to learning (in EG) and anxiety (in SS) rather than a locomotor effect. Enriched rats were found to move less than standard housed rats when tested multiple times in the open field106. The simpler open field compared to the enriched environment may have reduced motivation and/or facilitated environmental learning in EG mice, resulting in reduced interest or locomotion. In contrast, the reduced locomotion in SS mice, in addition to less time spent in the open arm of the elevated plus maze, suggests an anxiety effect. We also observed few social isolation effects with FP and VIS stimulation. FP stimulation of SS enhanced S1HL and hindlimb M1 cortices compared to EG mice, indicating altered cross-modal connectivity, and increased V2am and A2d response compared to SG mice, similar to the aforementioned U-trend. However, VIS stimulation impaired BOLD fMRI responses in PPCa and V2pm compared to SG mice, indicating reduced higher order visual function due to the lack of visual social cues. Other than reduced visual response, we see SS results in increased activity in many cross-modal areas unrelated to the sensory modality, which can be indicative of altered connectivity.
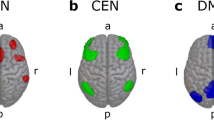
Social isolation not only alters olfactory-evoked responses, but also drastically modifies the connectivity and segregation of brain networks. Proper brain function and development requires the maturation of distinct functional systems that result in modular and specialized networks. These networks should be segregated from each other and integrate only when needed (e.g., during a task)52. Reduced segregation has been reported in aging brains, low SES children, and schizophrenia37,42,43. We calculated whole-brain network segregation between networks, in which the SS group resulted in impaired resting-state network segregation, indicating altered basal connectivity (Fig. 6). In addition, segregation analysis with stimulation evoked BOLD fMRI data showed aberrant recruitment of somatosensory networks from visual stimulation (Fig. 7). Fig. 10 shows the resting-state networks we observed on the cortical surface, indicated by their respective colors. Networks with blurred colors indicate more activity or less segregation within that network compared to the main network of its respective stimulation modality. The SS group presented with hyperconnected resting-state networks, resulting in a “blurring” of the networks due to lower network segregation, while EG and SG are similar. In particular, blurring was observed between the whisker/forelimb network (Wh/FL) and the posterior lateral network (PLN), which is composed of visual and auditory areas (Fig. 10a). Our results are consistent with a rat model of schizophrenia, which can be modeled via post-weaning social isolation, that showed hyperconnectivity between posterior visual areas and somatomotor areas, similar to our Wh/FL-PLN hyperconnectivity29,107. We also observed a hyperconnected thalamic (TH) network, which resulted in reduced segregation with the hippocampus (HPC) and TH network (Fig. 6d, Fig. S7). The TH is composed of higher order thalamic nuclei ATN108, MD109, PO110, LD111, and LP, which have memory, emotion, and sensory modulation functions. Socially isolated rats showed higher connectivity within the thalamic, visual, hippocampal, and olfactory networks, which was attenuated by antidepressant treatment112. Although not significant, we also detected increased connectivity within the visual (p = 0.063) and hippocampal (p = 0.174) networks in SS mice.
a Our observed resting-state networks are displayed on the cortical surface and are indicated by their respective colors. SG and EG did not differ in network segregation and are therefore shown as the control group. The SS group showed reduced network segregation, as indicated by the blurring of the networks. b Evoked sensory network segregation, calculated from sensory-evoked data, is shown. Using the observed RSN, SS had reduced segregation. Networks with blurred colors indicate greater activity within that network compared to the main network of its respective stimulation modality. Areas with significantly increased or decreased blood oxygen level-dependent (BOLD) responses from their respective stimulations are marked with their respective labels. Note that the EG group had an increased BOLD response, but network segregation remained unchanged, suggesting increased integration. Cortical surface image adapted with permission from Allen Brain Atlas 3D Brain Explorer 2148.
As shown in Fig. 10, network blurring appears to be unidirectional from rostral to caudal. Hyperconnectivity is commonly measured and reported as the increased mean difference between networks. Network segregation normalizes hyperconnectivity in proportion to a main network, which can provide directionality. In our case, we found a significant decrease when calculating segregation from Wh/FL to PLN (treating Wh/FL as the main network), but not when calculating from PLN to Wh/FL (treating PLN as the main network) in SS (Fig. S7). This suggests that in isolation, somatosensory networks reorganize the less used PLN in a process of cross-modal plasticity due to a lack of social visual and auditory cues in their environment. Two ideas may explain this asymmetry in cross-modal plasticity: intrinsic development and the influence of external experience. The critical period of a sensory system is an intrinsic period during which brain networks are most susceptible to change in response to sensory experience for proper network development113. Typically, this critical period occurs within the first few weeks of postnatal life; however, recent findings, in addition to our results, show that plasticity persists beyond this period well into adulthood114. The visual and auditory critical periods occur later in development and follow the whisker sensory critical period, whereas olfactory plasticity is maintained throughout adulthood113,115. This suggests a hierarchy of sensory importance, with olfaction being the most important and visual/auditory being the least important, which may explain the rewiring of the PLN by the Wh/FL network (Fig. 10a).
Visual deprivation, such as dark rearing or enucleation, has been shown to induce neural and vascular changes within the visual network beyond the critical period116,117. In this work, SS mice with VIS stimulation appear to have a reduced response within V1, suggesting that the lack of social cues can also induce plastic changes well past the critical period (Fig. 4). Similar findings have been reported in the somatosensory network after whisker trimming, but only during the critical period118. We hypothesized that SS mice would also be deprived of tactile sensation due to the lack of social touch; however, our results suggest that most of the somatosensory network is spared (Fig. 7). This may be because they use their somatosensory sensation for navigation within the cage, keeping it stimulated, or because the somatosensory network is more resilient to changes in the post-critical period. This may explain why SS mice preferred tactile cues in our multisensory object test as well (Fig. 9b).
Interestingly, when calculating segregation with sensory stimulation data, we see reduced segregation primarily from VIS stimulation in SS, which closely matches the blurred resting-state segregation in reverse order (Fig. 10b). Compared to PLN activation, we found increased activity of the Wh/FL, OLF, and STR networks. One reason for the altered PLN-Wh/FL connectivity may be due to the diminished negative BOLD response in S1FL and forepaw M1 observed in SS to VIS stimulation (Fig. 4d, Fig. 8b). Both SG and EG exhibited a negative BOLD response, which is likely due to active neuronal inhibition119,120. It is unlikely that our negative BOLD response is due to a vascular steal effect because VIS stimulation resulted in negative BOLD in a functionally specific (S1FL and whisker-related areas) and not in all adjacent somatosensory areas (e.g., mouth, hindlimb, or trunk)121. Furthermore, these areas, in addition to S2c, M2rdm, and CPivl, also tended to have reduced negative strength at rest suggesting a lack of inhibitory modulation going into or out of these areas (Fig. 8c). Although the nature of negative weights is not fully understood, studies suggest GABAergic modulation and their importance in the stability and segregation of brain networks122,123. Negative weights may be due to anticorrelation or phase shift of the BOLD signal between areas. These are most likely due to inhibitory regulation, as such observations are found in areas with high inhibitory connectivity and between areas with opposing functions (task on vs task off)124,125. The reduced segregation and negative participation coefficient in SS mice further supports the importance of negative weights in maintaining proper networks (Fig. S9). Taken together, our resting-state data suggest a unidirectional rewiring of sensory systems that appears to follow the sensory hierarchy based on intrinsic critical period ordering. Our evoked sensory data further support this rewiring, indicating that the loss of functional specialization in a “blurred” network is compensated by the other networks that rewired it in a social isolation setting.
The EG group did not show improved segregation compared to SG, despite the increased activity in many areas from WP, FP and VIS stimulation. All three stimulations did not result in significant differences in network segregation within the sensory networks. Nevertheless, the higher activity observed in the cortex and thalamus from WP, FP or VIS stimulation and the unchanged network segregation compared to SG suggest an improved integration of the brain network, where enhancement in one network is properly reciprocated by another network. This suggests that enrichment symmetrically improves connectivity and modulation between networks which may be a key mechanism for its ability to alleviate neurological disorders126.
The rewiring of the brain network could be due to altered white matter tracts and/or altered regional neural activity leading to changes in connectivity40. In a separate cohort of mice, we measured c-Fos, a marker of neural activity, after each stimulation, similar to the fMRI conditions (Fig. S6). Interestingly, we observed, although not statistically significant, higher c-Fos expression in SS mice compared to EG and SG mice across all stimulations (Fig. S6a); higher c-Fos expression in primary sensory cortices for each stimulation, e.g. S1BF from WP stimulation, S1FL from FP stimulation, and PIR from OLF stimulation, and in somatosensory (SS) from VIS stimulation (which may support the finding of impaired network segregation). The higher expression in SS is likely due to social isolation stress causing increased glutamatergic response and inhibiting GABAergic effects127. Environmentally enriched mice were found to have more localized and specific neural activity in the barrel cortex compared to the salt-and-pepper dispersion of neural activity in standard cage mice128. This may explain the lower c-Fos expression we observed in EG mice in both S1BF and S1FL from WP and FP stimulation, respectively. Several studies have also shown attenuated c-Fos expression in the hippocampus or amygdala in enriched rodents when presented with a novel environment or task compared to standard cage rodents, suggesting that enrichment may differentially alter c-Fos expression in the same area depending on the task or stimulation129,130.
Interestingly, these differences in c-Fos expression did not translate into our fMRI results, as all groups showed similar BOLD fMRI responses within the primary cortices of their respective sensory modality. Our data suggest that more neural activity is required to elicit the same BOLD fMRI response in SS mice, whereas less activity is required in EG mice, suggesting that environmental exposure alters neurovascular coupling. Neuronal nitric oxide synthase (nNOS) is the predominant mediator of neurovascular coupling and is expressed predominantly in GABAergic interneurons in the cortex131,132. In addition, inhibited neurons do not express c-Fos. Since proper network segregation requires GABAergic modulation, the higher c-Fos expression by sensory stimulation and impaired network segregation by resting state fMRI suggest that reduced inhibitory processes may be a major factor in altered network segregation in SS mice. In addition to altered brain connectivity, vascular and physiological changes due to environmental exposures may also affect the BOLD fMRI signal. However, in this work, ultrasound imaging of the common carotid artery (CCA) in anesthetized mice showed no significant environmental influence on basal CCA wall thickness and blood flow velocity after the housing period (Fig. S1), and HR and RR measured before and during fMRI stimulation were not altered, indicating no effects on BOLD fMRI signals (Fig. S2).
In summary, we used sensory stimulus-evoked BOLD fMRI and resting-state fMRI experiments to investigate the effects of environmental enrichment and social isolation at the whole-brain level. The physically enriched environment appeared to improve tactile and visual subcortical and association cortical function, as the tunnel, running wheel, and igloo nest provided rich tactile and visual stimulation, while maintaining proper brain segregation. Also, the combination of physically and socially enriched environment (EG group) provides a stronger effect compared to non-enriched isolated environment (SS group). On the other hand, social isolation experienced during adolescence and young adulthood profoundly influenced neurodevelopment, presumably by modulating emotional states (e.g., stress and anxiety) and inhibitory processes which significantly impaired brain network connectivity and segregation. Interestingly, heightened olfactory brain response with impaired olfactory perception emerged as a hallmark of social isolation, while tactile and visual sensations show comparatively minimal changes within their main modality. By utilizing resting-state and sensory stimulus-evoked BOLD fMRI with behavior and c-Fos measurements, we showed significant changes at the network level and potential neural and region targets that are altered due to environmental conditions.
There are several limitations to our interpretations. First, the use of anesthesia may confound the interpretation and translation of our study. Anesthesia can alter metabolic, neuronal, and vascular responses in the brain133,134,135. However, under ketamine/xylazine anesthesia, we have previously shown that the BOLD fMRI response closely follows neural activity in the somatosensory network136. Second, our analysis of network segregation relies on the reliability of our observed resting-state networks. Our reported networks are in close agreement with those reported under both anesthetic and awake conditions54,135. A key difference is our segmentation of certain networks into smaller subnetworks (e.g., LCN into ORF, Wh/FL, and HL/tr). Our observed networks may be due to anesthesia, higher spatial resolution, or conservative smoothing (1.5x voxel size); however, they closely resemble anatomically traced neural networks in mice55. One difference, however, is that our network combines the whisker and forepaw networks, whereas they were reported separately in the aforementioned study. In addition, the resting state of mice under anesthesia is known to closely follow the underlying structural connectivity135,137,138, and thus we believe that our interpreted segregation is representative of altered brain connectivity. Lastly, we were unable to characterize key limbic areas such as the amygdala in our analysis due to image distortion of the ventral regions. As these areas are key modulators and involved in environmental cues, we were not able to link the changes in the sensory network to amygdala activity.
Methods
Animals
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Sungkyunkwan University and Daegu Gyeongbuk Medical Innovation Foundation, and were conducted in accordance with the guidelines and regulations approved by the IACUC. Adolescent male C57BL/6 mice (Orient Bio, Korea, n = 92) were used to study the effects of enriched group housing (EG, n = 32), standard group housing (SG, n = 32), and standard solitary housing (SS, n = 28). Female mice were not utilized due to confounds of the estrous cycle on physiology and brain state. Two different cohorts of mice were used: for fMRI (total n = 32, EG: n = 12, SG: n = 12, SS: n = 8) and for behavioral testing, vascular measurements and c-Fos immunostaining (total n = 60, EG: n = 20, SG: n = 20, SS: n = 20). Mice were separated into their respective environments after weaning (P28) and housed there for 6 weeks prior to behavioral experiments and for 7 weeks prior to fMRI experiments. Mouse body weights were recorded one day prior to the start of the experiment and weekly thereafter while the animals were housed in different environmental conditions. All environments were maintained in a temperature- and humidity-controlled facility with standard laboratory chow and water ad libitum under a 12-hour dark-light cycle.
Environmental housing setup
Three different housing environments are shown in Fig. 1a. EG housing mice (n = 32; 4 mice per environment) were housed in a 50 × 50 cm environment where mice had free access to a 30 cm tunnel, an igloo nest, and a running wheel. SS housing mice (n = 28; one mouse per cage) were isolated in standard 30 × 22 cm mouse cages. SG housing mice (n = 32; 4 mice per cage) were housed in a standard laboratory mouse cage (30 × 22 cm). All mice were weaned at 4 weeks of age into their respective environments and housed for 7 weeks prior to the fMRI experiment. For each housing environment in cohort 1, a live mouse tracking system (LMT)139 was placed to monitor the social interaction of the mouse 24 h before fMRI experimentation (Supplementary Information).
fMRI experiments
Animal handling
Brain imaging was performed under self-breathing i.v. continuous ketamine/xylazine (45/2.25 mg/kg/h) anesthesia as previously described140. Mice were initially anesthetized under 4% isoflurane, followed by an i.p. induction bolus of ketamine/xylazine (100/10 mg/kg), after which isoflurane was discontinued. After confirmation of loss of the righting reflex, the tail vein was cannulated with a 31-G needle for i.v. administration of ketamine/xylazine. The mice were secured by bite and ear bars to a custom MRI cradle, which was then transferred to the magnet and connected to a small animal ventilator for spontaneous breathing with a 20/80% O2/air mixture at a rate of 90 breaths/min through the cradle nose cone. Respiratory rate and heart rate were monitored by a pressure transducer on the ventral chest and a pulse oximeter on the tail, respectively. Temperature was maintained at 37.5 °C with a warm water circulating pad and a feedback rectal thermometer. Continuous i.v. infusion was started 40 min after induction.
Stimulation protocols for evoked BOLD fMRI
The right whisker pad (WP) and left forepaw (FP) were electrically stimulated as previously described140. A three-pin electrode pad with two 2-mm-apart anodes and a cathode center was placed on the right whisker, and two 30-G needle electrodes were inserted between the left forepaw digits for WP and FP stimulation, respectively. Stimulation was delivered at a frequency of 4 Hz, a pulse width of 0.5 ms, and a current of 0.4 mA for WP or 0.6 mA for FP. Binocular visual stimulation (VIS) was performed with white LED lights at 4 Hz and 10 ms pulse width, as previously described46. Olfactory stimulation (OLF) was delivered through the nose cone of the cradle. A container containing a mixture of 5% amyl acetate in mineral oil was connected to a separate air tube connected to the nose cone. At the onset of stimulation, a solenoid switch switched the flow of fresh oxygen/air mixture through the amyl acetate container. All stimulations were randomized in a block design of 40-s baseline, 20-s stimulation, 60-s interstimulus interval, 20-s stimulation, and 60-s recovery. Eight repeats of the block design were averaged for each stimulation. A 6-minute resting scan was performed at the end of all sensory stimulations. Two exclusion criteria were conducted. For the first, mice without response in the primary sensory cortex from each sensory stimulation (S1BF:WP, S1FL:FP, V1:VIS, PIR:OLF) were excluded from analysis (Fig. S11b). From this, 3 mice from EG and SG, and 2 mice from SS were excluded, resulting in a sample size of 6/8 SS, 9/12 SG, and 9/12 EG. The second exclusion criteria from this sample is explained in Evoked fMRI data analysis section below.
fMRI acquisition parameters
Images were acquired with a 15.2 T/11 Bruker Biospec system (Billerica, MA, U.S.A.) using a 15 mm i.d. surface coil for radiofrequency transmission/reception and Paravision 6.0.1 software. Image acquisition began after the mouse brain was placed in the isocenter of the magnet, followed by correction for field inhomogeneity using the MAPSHIM protocol. Anatomical images were acquired using the Fast Low Angle Shot (FLASH) sequence with the following parameters TR/TE = 3000/45 ms; matrix size = 256 × 128; field of view = 15.80 × 7.65 mm2; 20 0.5-mm slices; and four averages. Functional scans were acquired using gradient-echo planar imaging: TR/TE = 1000/11.5 ms; matrix size = 120 × 58; field of view = 15.84 × 7.65 mm2 (0.13 × 0.13 mm2 in-plane resolution); 20 continuous 0.5-mm slices (interleaved acquisition); 50° flip angle; 10 dummy scans; and 200 (evoked) or 360 (resting-state) repetitions.
fMRI data analysis
fMRI pre-processing
Images were processed and analyzed using analysis of functional neuroimaging (AFNI), FMRIB software library (FSL), advanced normalization tools (ANTs), and statistical parametric mapping (SPM12). All images were converted to NIFTI format and scaled 10x its resolution to work with preprocessing software. Functional images were corrected for spikes (3dDespike, AFNI), slice time (3dTshift, AFNI), and motion (3dvolreg, AFNI), after which the images were temporally averaged (fslmaths, FSL) to create an EPI template for registration. Anatomical images were bias field corrected (ANTs), skull stripped (bet, FSL), and coregistered to the EPI template (SPM12). Linear affine parameters and nonlinear regularization were estimated between the coregistered anatomical image and the Australian Mouse Brain Mapping Consortium (AMBMC) template, after which the deformations were applied to the functional images at their native resolution (SPM12, normalize).
Evoked fMRI data analysis
The general linear model (GLM) was used to statistically map the evoked response. The preprocessed images were smoothed to 1.5x voxel size, and a design matrix consisting of a linear detrend and our ketamine/xylazine hemodynamic response function convolved with the two stimulation blocks was used as regressors for individual GLM analysis (3dDeconvolve, AFNI). Four ANOVA tests (one for each stimulation) were performed on group response maps (3dANOVA, AFNI). Voxels were cluster-corrected by finding the first-nearest-neighbor clusters (N = 1) that pass the p < 0.01 threshold uncorrected and correcting them with a family-wise error correction to p = 0.05 (3dClustsim, AFNI).
BOLD signal quantification was performed by normalizing the unsmoothed images to the first 40 s of baseline to calculate percent change. Percent signal change was then extracted using regions-of-interest (ROIs) based on the AMBMC and enhanced/unified atlas56. Voxels adjacent to different areas were excluded for each ROI to limit partial voxel spread. The area under the curve (AUC) was calculated by first temporally smoothing the time series with a 6 sec window and integrating the area of the two stimulation blocks. For WP, FP, and VIS stimulation, the area from stimulus onset to 30 s after onset was used. For OLF, 40 s after onset was used due to a prolonged BOLD response. For the second exclusion, exclusion criteria were the interquartile range of primary sensory cortex AUCs for each stimulation modality (S1BF/WP, S1FL/FP, V1/VIS, PIR/OLF). Mice with AUCs outside the outer fence were excluded. From this, 0 SS, 1 SG, and 1 EG were further excluded, resulting in the final sample size of SS (n = 6/8), SG (n = 8/12), and EG (n = 8/12). Group statistical tests were performed using GraphPad Prism 9.
Resting-state fMRI data analysis
After preprocessing as described above, 24 motion-related regressors were regressed followed by denoising using independent component analysis (ICA) via melodic (FSL). Automatic dimension estimation was used to find the signal and noise components of each mouse. For mice with noise components (ventricular, vascular, or motion), the respective components were regressed out, which is akin to a manual ICA-FIX method (Fig. S12a)141. The cleaned images were linearly detrended, passband filtered between 0.01 - 0.25 Hz, and spatially smoothed to 1.5x voxel size (3dTproject, AFNI). Frame-wise displacement (FD) was calculated by assuming the sphere with radius 4.63 mm. Any frames with a FD of 0.009 mm were censored. From this, 1 SS, 4 SG, and 3 EG mice had FD of less than 5 frames (Fig. S13c). For group ICA, ten components were analyzed by temporally concatenating resting-state scans from all three groups to find common spatial patterns across all three groups. A 21-voxel seed was placed on ROIs (n = 56) belonging to networks identified by group ICA, to extract the mean time course for seed-based functional connectivity (FC) analysis (Table S1). The Pearson correlation between ROIs was calculated, resulting in a 56 × 56 matrix for each mouse. The matrices were Fisher z-transformed for group averaging, statistical testing, and graph theory metric analysis. Negative z values were retained, and all metrics were calculated using a symmetrical threshold of ∣r∣ > 0.2. Results were transformed back to r for presentation.
Four graph theory metrics were calculated using the brain connectivity toolbox (BCT): modularity, clustering coefficient, participation coefficient, and strength57. All metrics were calculated using weighted and signed functions for each metric. Modularity was calculated with the Louvain algorithm with gammas ranging from 0.1 to 2.0 in 0.1 increments. The area-under-curve (AUC) of the resulting modularity x gamma curve was calculated for each group. Participation coefficient was similarly calculated, as calculation requires the modularity value. Additionally, the positive and negative coefficients were calculated separately. The clustering coefficient calculates the clustering coefficient for each ROI. For each mouse, clustering coefficient was reported as the average clustering coefficient of all ROIs. Strength calculates the sum of all positive and negative weights connected to a ROI. Like the participation coefficient, negative and positive weight strengths are reported.
Functional segregation analysis
Within-network connectivity was calculated as the mean ROI-ROI z-value of all ROIs within a designated network (e.g., the mean of the z-values of all ROIs in the PLN network to all other PLN ROIs). Between-network connectivity was calculated as the mean ROI-ROI z-value of one network to the mean z-value of another network (e.g., mean z-value of PLN to mean z-value of ORF). Network segregation measures the difference in mean within-network and mean between-network correlations as a proportion of mean within-network correlation, which is expressed by the following formula:
where \(\overline{{Z}_{w}}\) is the mean z-value between ROIs within the same network; and \(\overline{{Z}_{b}}\) is the mean z-value between ROIs of one network and either all other ROIs or ROIs of another network. For evoked data, the AUC values of each ROI within a network were averaged to calculate network segregation, rather than the mean z-values.
Behavior tests
In the Optomotor Vision Test, Object Preference Test, Olfactory Habituation Test, Hot Plate Test, and Open Field Test, a total of 36 mice were divided into three groups (EG: n = 12, SG: n = 12, SS: n = 12). For the Elevated Plus Maze Test and Interactive Behavior Test, 24 mice were used, with 8 mice per group.
Optomotor vision test
Visual contrast sensitivity and motion perception were evaluated using an Optomotor Drum device142,143,144. A cylindrical visual drum (30 cm diameter, 50 cm height) with an inner surface featuring vertical black and white stripes (2 cm wide) of varying contrast levels was employed. The mouse was positioned centrally within the rotating drum. The 3 contrast patterns were as follows; dark grey & black (dark contrast), white & black (standard contrast), and white & light grey (light contrast) as used in Felgerolle et al.’s144 study. Each test consisted of three consecutive two-minute phases: (1) stationary adaptation, (2) clockwise rotation, and (3) counterclockwise rotation. This sequence was repeated for each of the three contrast patterns. The mouse’s tracking behavior was observed and recorded as it responded to the stationary and moving visual stimuli.
Object preference test
This test aimed to assess mice’s perception of various sensory inputs and their preference for multisensory information. The test was conducted with modifications to the protocol described by Cloke et al.145. A cylindrical chamber was used, with three objects (V, T, and V&T) placed at equidistant intervals. Object preference was quantified by measuring contact time. Each object, measuring approximately 3 × 3 × 10 cm, was created from a rectangular bottle with its surface modified to represent different sensory modalities. Object V, representing visual information, was covered with colored stickers. Object T, designed to provide tactile information, was fitted with large Velcro. Object V&T, representing multisensory input, was covered with both colored stickers and large Velcro. This setup allowed for the simultaneous presentation of visual, tactile, and combined visual-tactile stimuli. Mice were placed in the center of a chamber, and their behavior was recorded for 10 min using a ceiling-mounted camera.
Olfactory habituation test
The olfactory test was conducted following modifications to protocols described by Sundberg et al. and Trinh and Storm146,147. Naive animals were initially pretrained with distilled water cotton swabs for four 60-second presentations at 2-minute intervals to mitigate novelty responses. Next, the main test involved presentation of limonene odorant four times for 60 seconds with 2-minute intervals. Odor habituation was indicated by a significant decrease in investigation time during subsequent presentations of the same odorant.
Hot plate test
A thermal nociception test was conducted using a 50 °C hot plate to assess mice’s tactile sensory response to heat stimuli. Mice were placed on the heated surface for a maximum of 30 s, and the test was terminated when they exhibited hind paw shaking or licking. The latency to these pain responses was recorded as a quantitative measure of the mouse’s pain sensitivity.
Open field test
The open field test was conducted using an acrylic glass apparatus measuring 50 cm × 50 cm × 50 cm. Each mouse was placed in the center of the apparatus and allowed to explore freely for 5 min, during which their behavior was recorded on video. The total distance traveled by each mouse and the time spent in the central area of the apparatus were calculated from the recorded video.
Elevated plus maze test
The test is administered using a device shaped like a plus sign, consisting of two open arms and two closed arms arranged in a cross pattern. The open arms measure 25 × 5 × 0.5 cm and face each other, while the closed arms measure 25 × 5 × 16 cm and have walls 16 cm high. There is a small platform (5 × 5 × 0.5 cm) in the middle of the labyrinth. The open arms have a low wall (0.5 cm) to prevent falling, while the closed arms have high walls to enclose the space. The entire apparatus is suspended 50 cm above the floor. The mice are allowed to move freely in the maze for 10 min. Ethovision XT was used to analyze the distance traveled, the number of entries into each arm, the time spent in each arm, and the percentage of entries into the open arms during the elevated plus maze test.
Interactive behavior test
This test was designed to monitor the social behavior of mice and was developed by139. The Live Mouse Tracking System (LMT) consisted of a camera attached to the top frame above a Plexiglas box measuring 50 × 50 × 35 cm. Beneath the box were 16 RFID antennas to identify and track the movements of each mouse. For the LMT test, each mouse was previously implanted with an RFID probe under its abdominal skin. The mice were then placed in the LMT for 24 h and their behavior was recorded and analyzed using a Python script developed by de Chaumont et al.
Ultrasound imaging of CCA vasculature
Carotid ultrasound evaluation was performed at week 11, and the internal diameters of the blood vessels in systolic and diastolic states in longitudinal view and the blood flow velocity from longitudinal ultrasound images were measured (Supplementary Method).
Systemic physiologic parameter measurements
To investigate potential differences in systemic basal physiological conditions among mice in the three housing environments and to test whether each stimulation induced physiological changes, pulsatile signals (heart rate) and respiratory signals were measured using an MR-compatible optical pulse oximeter attached to the tail and a pressure-sensitive sensor placed under the abdomen, respectively (Supplementary Method).
Quantification of c-Fos active cells
After the fMRI scan of each stimulus, brain sections immune-stained with c-Fos antibody were prepared and the spatial distribution of c-Fos activation within whole brain ROIs, the total number of c-Fos activated cells within those annotated regions was counted (Supplementary method).
Statistical analysis
Statistical analyses were performed using SPSS (behavior) or GraphPad Prism 9.5.1 (fMRI). Group differences per region were analyzed using the nonparametric Kruskal-Wallis test and corrected for multiple comparisons using Dunn’s test. Kruskal-Wallis results are presented as (chi-squared, exact p-value) with df = 2. Significance after multiple comparisons is presented with its adjusted p-value. Significance is based on p < 0.05. All data are presented as mean ± standard error of mean (SEM).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The EG data generated in this study have been deposited in the Mendeley Data database under the accession code (https://doi.org/10.17632/n5m4rpn448.1). The SG data generated in this study have been deposited in the Mendeley Data database under the accession code (https://doi.org/10.17632/xwhw59k5zc.1). The SS data generated in this study have been deposited in the Mendeley Data database under the accession code (https://doi.org/10.17632/nnd959fbf7.1). Source data for graphs of the BOLD signal and behavioral results in the main figures are provided with this paper in the Source Data file. Source data are provided with this paper.
Code availability
Figures and results were analyzed using custom MATLAB and unix code based on AFNI, FSL, and ANTs programs. Scripts can be shared upon request.
References
Greven, C. U. et al. Sensory processing sensitivity in the context of environmental sensitivity: A critical review and development of research agenda. Neurosci. Biobehav Rev. 98, 287–305 (2019).
Campi, K. L. & Krubitzer, L. Comparative studies of diurnal and nocturnal rodents: differences in lifestyle result in alterations in cortical field size and number. J. Comp. Neurol. 518, 4491–4512 (2010).
Ronnback, A., Dahlqvist, P., Bergstrom, S. A. & Olsson, T. Diurnal effects of enriched environment on immediate early gene expression in the rat brain. Brain Res 1046, 137–144 (2005).
Diaz, J. L. Brain weights correlate with behavioral parameters in individual inbred mice housed in a common and enriched environment. Behav. Neural Biol. 50, 164–183 (1988).
Nakamura, H., Kobayashi, S., Ohashi, Y. & Ando, S. Age-changes of brain synapses and synaptic plasticity in response to an enriched environment. J. Neurosci. Res. 56, 307–315 (1999).
Leger, M. et al. Environmental enrichment improves recent but not remote memory in association with a modified brain metabolic activation profile in adult mice. Behav. Brain Res. 228, 22–29 (2012).
Simpson, J. & Kelly, J. P. The impact of environmental enrichment in laboratory rats—behavioural and neurochemical aspects. Behav. Brain Res 222, 246–264 (2011).
Kuhn, S. et al. In search of features that constitute an “enriched environment” in humans: Associations between geographical properties and brain structure. Sci. Rep. 7, 11920 (2017).
Henschke, J. U., Noesselt, T., Scheich, H. & Budinger, E. Possible anatomical pathways for short-latency multisensory integration processes in primary sensory cortices. Brain Struct. Funct. 220, 955–977 (2015).
Iurilli, G. et al. Sound-driven synaptic inhibition in primary visual cortex. Neuron 73, 814–828 (2012).
Teichert, M. & Bolz, J. How senses work together: Cross-modal interactions between primary sensory cortices. Neural Plast. 2018, 5380921 (2018).
Liu, J. et al. Enriched environment attenuates pyroptosis to improve functional recovery after cerebral ischemia/reperfusion injury. Front Aging Neurosci. 13, 717644 (2021).
Zheng, J. et al. Adult hippocampal neurogenesis along the dorsoventral axis contributes differentially to environmental enrichment combined with voluntary exercise in alleviating chronic inflammatory pain in mice. J. Neurosci. 37, 4145–4157 (2017).
Bengoetxea, H. et al. Enriched and deprived sensory experience induces structural changes and rewires connectivity during the postnatal development of the brain. Neural Plast. 2012, 305693 (2012).
Nithianantharajah, J. & Hannan, A. J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697–709 (2006).
Sale, A. et al. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat. Neurosci. 10, 679–681 (2007).
Cai, R. et al. Environmental enrichment improves behavioral performance and auditory spatial representation of primary auditory cortical neurons in rat. Neurobiol. Learn Mem. 91, 366–376 (2009).
Ali, A. E., Wilson, Y. M. & Murphy, M. A single exposure to an enriched environment stimulates the activation of discrete neuronal populations in the brain of the fos-tau-lacZ mouse. Neurobiol. Learn Mem. 92, 381–390 (2009).
Franklin, T. B., Murphy, J. A., Myers, T. L., Clarke, D. B. & Currie, R. W. Enriched environment during adolescence changes brain-derived neurotrophic factor and TrkB levels in the rat visual system but does not offer neuroprotection to retinal ganglion cells following axotomy. Brain Res. 1095, 1–11 (2006).
van Praag, H., Kempermann, G. & Gage, F. H. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 1, 191–198 (2000).
Scholz, J., Allemang-Grand, R., Dazai, J. & Lerch, J. P. Environmental enrichment is associated with rapid volumetric brain changes in adult mice. Neuroimage 109, 190–198 (2015).
Li, R. et al. Mapping accumulative whole-brain activities during environmental enrichment with manganese-enhanced magnetic resonance imaging. Neuroimage 210, 116588 (2020).
Manno, F. A. M. et al. Environmental enrichment leads to behavioral circadian shifts enhancing brain-wide functional connectivity between sensory cortices and eliciting increased hippocampal spiking. Neuroimage 252, 119016 (2022).
Schreiber, S. et al. Enriched environment improves the cognitive effects from traumatic brain injury in mice. Behav. Brain Res. 271, 59–64 (2014).
Lippert-Gruener, M., Maegele, M., Garbe, J. & Angelov, D. N. Late effects of enriched environment (EE) plus multimodal early onset stimulation (MEOS) after traumatic brain injury in rats: Ongoing improvement of neuromotor function despite sustained volume of the CNS lesion. Exp. Neurol. 203, 82–94 (2007).
Mate, V. et al. Enriched environment ameliorates propagation of tau pathology and improves cognition in rat model of tauopathy. Front Aging Neurosci. 14, 935973 (2022).
Li, J. Z. et al. An enriched environment delays the progression from mild cognitive impairment to alzheimer’s disease in senescence-accelerated mouse prone 8 mice. Exp. Ther. Med. 22, 1320 (2021).
Berlet, R., Galang Cabantan, D. A., Gonzales-Portillo, D. & Borlongan, C. V. Enriched environment and exercise enhance stem cell therapy for stroke, parkinson’s disease, and huntington’s disease. Front Cell Dev. Biol. 10, 798826 (2022).
Fone, K. C. & Porkess, M. V. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav Rev. 32, 1087–1102 (2008).
Lapiz, M. D. et al. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci. Behav. Physiol. 33, 13–29 (2003).
Lukkes, J. L., Watt, M. J., Lowry, C. A. & Forster, G. L. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav. Neurosci. 3, 18 (2009).
Xiong, Y., Hong, H., Liu, C. & Zhang, Y. Q. Social isolation and the brain: effects and mechanisms. Mol. Psychiatry 28, 191–201 (2023).
Meyer-Lindenberg, A. & Tost, H. Neural mechanisms of social risk for psychiatric disorders. Nat. Neurosci. 15, 663–668 (2012).
Umberson, D. & Montez, J. K. Social relationships and health: a flashpoint for health policy. J. Health Soc. Behav. 51, S54–S66 (2010).
Holt-Lunstad, J., Smith, T. B., Baker, M., Harris, T. & Stephenson, D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect. Psychol. Sci. 10, 227–237 (2015).
Brandt, L., Liu, S., Heim, C. & Heinz, A. The effects of social isolation stress and discrimination on mental health. Transl. Psychiatry 12, 398 (2022).
Tooley, U. A. et al. Associations between neighborhood ses and functional brain network development. Cereb. Cortex 30, 1–19 (2020).
Lawson, G. M., Duda, J. T., Avants, B. B., Wu, J. & Farah, M. J. Associations between children’s socioeconomic status and prefrontal cortical thickness. Dev. Sci. 16, 641–652 (2013).
Eluvathingal, T. J. et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics 117, 2093–2100 (2006).
Liu, C. et al. Altered structural connectome in adolescent socially isolated mice. Neuroimage 139, 259–270 (2016).
Kim, G. S., Lee, H. & Jeong, Y. Altered dorsal functional connectivity after post-weaning social isolation and resocialization in mice. Neuroimage 245, 118740 (2021).
Yang, G. J. et al. Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc. Natl Acad. Sci. USA 113, E219–E228 (2016).
Chan, M. Y., Park, D. C., Savalia, N. K., Petersen, S. E. & Wig, G. S. Decreased segregation of brain systems across the healthy adult lifespan. Proc. Natl Acad. Sci. USA 111, E4997–E5006 (2014).
Chae, S. Y. et al. Physical exercise enhances adult cortical plasticity in a neonatal rat model of hypoxic-ischemic injury: Evidence from BOLD-fMRI and electrophysiological recordings. Neuroimage 188, 335–346 (2019).
Jung, W. B. et al. Neuroplasticity for spontaneous functional recovery after neonatal hypoxic ischemic brain injury in rats observed by functional MRI and diffusion tensor imaging. Neuroimage 126, 140–150 (2016).
Dinh, T. N. A., Jung, W. B., Shim, H. J. & Kim, S. G. Characteristics of fMRI responses to visual stimulation in anesthetized vs. awake mice. Neuroimage 226, 117542 (2021).
Tsurugizawa, T. et al. Awake functional MRI detects neural circuit dysfunction in a mouse model of autism. Sci. Adv. 6, eaav4520 (2020).
Jung, W. B., Shim, H. J. & Kim, S. G. Mouse BOLD fMRI at ultrahigh field detects somatosensory networks including thalamic nuclei. Neuroimage 195, 203–214 (2019).
van den Heuvel, M. P. & Hulshoff Pol, H. E. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 20, 519–534 (2010).
Scholvinck, M. L., Maier, A., Ye, F. Q., Duyn, J. H. & Leopold, D. A. Neural basis of global resting-state fMRI activity. Proc. Natl Acad. Sci. USA 107, 10238–10243 (2010).
Wig, G. S. Segregated systems of human brain networks. Trends Cogn. Sci. 21, 981–996 (2017).
Tononi, G., Sporns, O. & Edelman, G. M. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc. Natl Acad. Sci. USA 91, 5033–5037 (1994).
Coletta, L. et al. Network structure of the mouse brain connectome with voxel resolution. Sci. Adv. 6, eabb7187 (2020).
Zerbi, V., Grandjean, J., Rudin, M. & Wenderoth, N. Mapping the mouse brain with rs-fMRI: An optimized pipeline for functional network identification. Neuroimage 123, 11–21 (2015).
Zingg, B. et al. Neural networks of the mouse neocortex. Cell 156, 1096–1111 (2014).
Chon, U., Vanselow, D. J., Cheng, K. C. & Kim, Y. Enhanced and unified anatomical labeling for a common mouse brain atlas. Nat. Commun. 10, 5067 (2019).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069 (2010).
van den Heuvel, M. P., Stam, C. J., Kahn, R. S. & Hulshoff Pol, H. E. Efficiency of functional brain networks and intellectual performance. J. Neurosci. 29, 7619–7624 (2009).
Casas-Torremocha, D., Clasca, F. & Nunez, A. Posterior thalamic nucleus modulation of tactile stimuli processing in rat motor and primary somatosensory cortices. Front Neural Circuits 11, 69 (2017).
Tlamsa, A. P. & Brumberg, J. C. Organization and morphology of thalamocortical neurons of mouse ventral lateral thalamus. Somatosens. Mot. Res. 27, 34–43 (2010).
Ahissar, E. & Oram, T. Thalamic relay or cortico-thalamic processing? Old question, new answers. Cereb. Cortex 25, 845–848 (2015).
Bosch-Bouju, C., Hyland, B. I. & Parr-Brownlie, L. C. Motor thalamus integration of cortical, cerebellar and basal ganglia information: implications for normal and parkinsonian conditions. Front Comput Neurosci. 7, 163 (2013).
Lopez-Bendito, G., Anibal-Martinez, M. & Martini, F. J. Cross-modal plasticity in brains deprived of visual input before vision. Annu Rev. Neurosci. 45, 471–489 (2022).
Bavelier, D. & Neville, H. J. Cross-modal plasticity: where and how? Nat. Rev. Neurosci. 3, 443–452 (2002).
Zhang, M. et al. Tactile discrimination of grating orientation: fMRI activation patterns. Hum. Brain Mapp. 25, 370–377 (2005).
Zangaladze, A., Epstein, C. M., Grafton, S. T. & Sathian, K. Involvement of visual cortex in tactile discrimination of orientation. Nature 401, 587–590 (1999).
Froudarakis, E. et al. The visual cortex in context. Annu Rev. Vis. Sci. 5, 317–339 (2019).
Glickfeld, L. L. & Olsen, S. R. Higher-order areas of the mouse visual cortex. Annu Rev. Vis. Sci. 3, 251–273 (2017).
Wang, Q., Gao, E. & Burkhalter, A. Gateways of ventral and dorsal streams in mouse visual cortex. J. Neurosci. 31, 1905–1918 (2011).
Busse, L. et al. The detection of visual contrast in the behaving mouse. J. Neurosci. 31, 11351–11361 (2011).
Chen, Y., Geisler, W. S. & Seidemann, E. Optimal decoding of correlated neural population responses in the primate visual cortex. Nat. Neurosci. 9, 1412–1420 (2006).
Haberly, L. B. & Price, J. L. Association and commissural fiber systems of the olfactory cortex of the rat. J. Comp. Neurol. 178, 711–740 (1978).
Bednarczyk, M. R., Aumont, A., Decary, S., Bergeron, R. & Fernandes, K. J. Prolonged voluntary wheel-running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus 19, 913–927 (2009).
Adibi, M. Whisker-mediated touch system in rodents: from neuron to behavior. Front Syst. Neurosci. 13, 40 (2019).
Hintiryan, H. et al. The mouse cortico-striatal projectome. Nat. Neurosci. 19, 1100–1114 (2016).
Reig, R. & Silberberg, G. Multisensory integration in the mouse striatum. Neuron 83, 1200–1212 (2014).
Ding, L. & Gold, J. I. The basal ganglia’s contributions to perceptual decision making. Neuron 79, 640–649 (2013).
Milardi, D. et al. The cortico-basal ganglia-cerebellar network: past, present and future perspectives. Front Syst. Neurosci. 13, 61 (2019).
Song, S. Y. et al. Environmental enrichment upregulates striatal synaptic vesicle-associated proteins and improves motor function. Front Neurol. 9, 465 (2018).
Johansson, Y. & Ketzef, M. Sensory processing in external globus pallidus neurons. Cell Rep. 42, 111952 (2023).
Shik, M. L., Severin, F. V. & Orlovskii, G. N. [Control of walking and running by means of electric stimulation of the midbrain]. Biofizika 11, 659–666 (1966).
Sherman, D. et al. Anatomical location of the mesencephalic locomotor region and its possible role in locomotion, posture, cataplexy, and parkinsonism. Front Neurol. 6, 140 (2015).
Roseberry, T. K. et al. Cell-type-specific control of brainstem locomotor circuits by basal ganglia. Cell 164, 526–537 (2016).
Medendorp, W. E. et al. Altered behavior in mice socially isolated during adolescence corresponds with immature dendritic spine morphology and impaired plasticity in the prefrontal cortex. Front Behav. Neurosci. 12, 87 (2018).
Keene, C. S. & Bucci, D. J. Involvement of the retrosplenial cortex in processing multiple conditioned stimuli. Behav. Neurosci. 122, 651–658 (2008).
Robinson, S., Keene, C. S., Iaccarino, H. F., Duan, D. & Bucci, D. J. Involvement of retrosplenial cortex in forming associations between multiple sensory stimuli. Behav. Neurosci. 125, 578–587 (2011).
Vann, S. D., Aggleton, J. P. & Maguire, E. A. What does the retrosplenial cortex do? Nat. Rev. Neurosci. 10, 792–802 (2009).
Allen, A. E., Procyk, C. A., Howarth, M., Walmsley, L. & Brown, T. M. Visual input to the mouse lateral posterior and posterior thalamic nuclei: photoreceptive origins and retinotopic order. J. Physiol. 594, 1911–1929 (2016).
Bieler, M., Xu, X., Marquardt, A. & Hanganu-Opatz, I. L. Multisensory integration in rodent tactile but not visual thalamus. Sci. Rep. 8, 15684 (2018).
Allen, A. E., Procyk, C. A., Brown, T. M. & Lucas, R. J. Convergence of visual and whisker responses in the primary somatosensory thalamus (ventral posterior medial region) of the mouse. J. Physiol. 595, 865–881 (2017).
Gilissen, S. R. J., Farrow, K., Bonin, V. & Arckens, L. Reconsidering the border between the visual and posterior parietal cortex of mice. Cereb. Cortex 31, 1675–1692 (2021).
Hovde, K., Gianatti, M., Witter, M. P. & Whitlock, J. R. Architecture and organization of mouse posterior parietal cortex relative to extrastriate areas. Eur. J. Neurosci. 49, 1313–1329 (2019).
Bolognini, N. & Maravita, A. Proprioceptive alignment of visual and somatosensory maps in the posterior parietal cortex. Curr. Biol. 17, 1890–1895 (2007).
Whitlock, J. R. Posterior parietal cortex. Curr. Biol. 27, R691–R695 (2017).
Wang, Q., Sporns, O. & Burkhalter, A. Network analysis of corticocortical connections reveals ventral and dorsal processing streams in mouse visual cortex. J. Neurosci. 32, 4386–4399 (2012).
Rizzolatti, G. & Matelli, M. Two different streams form the dorsal visual system: anatomy and functions. Exp. Brain Res 153, 146–157 (2003).
Lee, Y. & Davis, M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J. Neurosci. 17, 6434–6446 (1997).
Hikosaka, O., Sesack, S. R., Lecourtier, L. & Shepard, P. D. Habenula: crossroad between the basal ganglia and the limbic system. J. Neurosci. 28, 11825–11829 (2008).
Fakhoury, M., Salman, I., Najjar, W., Merhej, G. & Lawand, N. The lateral hypothalamus: An uncharted territory for processing peripheral neurogenic inflammation. Front Neurosci. 14, 101 (2020).
Lecca, S. et al. Aversive stimuli drive hypothalamus-to-habenula excitation to promote escape behavior. Elife 6, e30697 (2017).
Kataoka, N., Shima, Y., Nakajima, K. & Nakamura, K. A central master driver of psychosocial stress responses in the rat. Science 367, 1105–1112 (2020).
Hoenen, M., Wolf, O. T. & Pause, B. M. The impact of stress on odor perception. Perception 46, 366–376 (2017).
Raynaud, A., Meunier, N., Acquistapace, A. & Bombail, V. Chronic variable stress exposure in male Wistar rats affects the first step of olfactory detection. Behav. Brain Res. 291, 36–45 (2015).
Kim, J., Wasserman, E. A., Castro, L. & Freeman, J. H. Anterior cingulate cortex inactivation impairs rodent visual selective attention and prospective memory. Behav. Neurosci. 130, 75–90 (2016).
Bush, G., Luu, P. & Posner, M. I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222 (2000).
Rojas-Carvajal, M., Fornaguera, J., Mora-Gallegos, A. & Brenes, J. C. Testing experience and environmental enrichment potentiated open-field habituation and grooming behaviour in rats. Anim. Behav. 137, 225–235 (2018).
Kaneko, G. et al. Hypofrontality and posterior hyperactivity in early schizophrenia: imaging and behavior in a preclinical model. Biol. Psychiatry 81, 503–513 (2017).
Aggleton, J. P., Hunt, P. R., Nagle, S. & Neave, N. The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behav. Brain Res. 81, 189–198 (1996).
Mitchell, A. S. & Chakraborty, S. What does the mediodorsal thalamus do? Front Syst. Neurosci. 7, 37 (2013).
Mo, C. & Sherman, S. M. A sensorimotor pathway via higher-order thalamus. J. Neurosci. 39, 692–704 (2019).
van Groen, T., Kadish, I. & Wyss, J. M. The role of the laterodorsal nucleus of the thalamus in spatial learning and memory in the rat. Behav. Brain Res. 136, 329–337 (2002).
Li, J. et al. Effects of stress on behavior and resting-state fMRI in rats and evaluation of Telmisartan therapy in a stress-induced depression model. BMC Psychiatry 18, 337 (2018).
Hensch, T. K. Critical period regulation. Annu Rev. Neurosci. 27, 549–579 (2004).
Fu, M. & Zuo, Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. 34, 177–187 (2011).
Pedrosa, L. R. R., Coimbra, G. D. S., Correa, M. G., Dias, I. A. & Bahia, C. P. Time window of the critical period for neuroplasticity in S1, V1, and A1 sensory areas of small rodents: A systematic review. Front Neuroanat. 16, 763245 (2022).
Argandona, E. G. & Lafuente, J. V. Effects of dark-rearing on the vascularization of the developmental rat visual cortex. Brain Res. 732, 43–51 (1996).
Cragg, B. G. Changes in visual cortex on first exposure of rats to light. Effect on synaptic dimensions. Nature 215, 251–253 (1967).
Shoykhet, M., Land, P. W. & Simons, D. J. Whisker trimming begun at birth or on postnatal day 12 affects excitatory and inhibitory receptive fields of layer IV barrel neurons. J. Neurophysiol. 94, 3987–3995 (2005).
Shmuel, A. et al. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 36, 1195–1210 (2002).
Moon, H. S. et al. Contribution of excitatory and inhibitory neuronal activity to BOLD fMRI. Cereb. Cortex 31, 4053–4067 (2021).
Smith, A. T., Williams, A. L. & Singh, K. D. Negative BOLD in the visual cortex: evidence against blood stealing. Hum. Brain Mapp. 21, 213–220 (2004).
Saberi, M., Khosrowabadi, R., Khatibi, A., Misic, B. & Jafari, G. Topological impact of negative links on the stability of resting-state brain network. Sci. Rep. 11, 2176 (2021).
Cassady, K. et al. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. Neuroimage 186, 234–244 (2019).
Fox, M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl Acad. Sci. USA 102, 9673–9678 (2005).
Liang, Z., King, J. & Zhang, N. Anticorrelated resting-state functional connectivity in awake rat brain. Neuroimage 59, 1190–1199 (2012).
Han, Y. et al. The role of enriched environment in neural development and repair. Front Cell Neurosci. 16, 890666 (2022).
Mumtaz, F., Khan, M. I., Zubair, M. & Dehpour, A. R. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed. Pharmacother. 105, 1205–1222 (2018).
LeMessurier, A. M. et al. Enrichment drives emergence of functional columns and improves sensory coding in the whisker map in L2/3 of mouse S1. Elife 8, e46321 (2019).
Leger, M. et al. Environmental enrichment enhances episodic-like memory in association with a modified neuronal activation profile in adult mice. PLoS One 7, e48043 (2012).
Matsuda, W., Ehara, A., Nakadate, K., Yoshimoto, K. & Ueda, S. Effects of environmental enrichment on the activity of the amygdala in micrencephalic rats exposed to a novel open field. Congenit. Anom. (Kyoto) 58, 16–23 (2018).
Irikura, K., Maynard, K. I. & Moskowitz, M. A. Importance of nitric oxide synthase inhibition to the attenuated vascular responses induced by topical L-nitroarginine during vibrissal stimulation. J. Cereb. Blood Flow. Metab. 14, 45–48 (1994).
Valtschanoff, J. G. et al. Neurons in rat cerebral cortex that synthesize nitric oxide: NADPH diaphorase histochemistry, NOS immunocytochemistry, and colocalization with GABA. Neurosci. Lett. 157, 157–161 (1993).
Franceschini, M. A. et al. The effect of different anesthetics on neurovascular coupling. Neuroimage 51, 1367–1377 (2010).
Masamoto, K. & Kanno, I. Anesthesia and the quantitative evaluation of neurovascular coupling. J. Cereb. Blood Flow. Metab. 32, 1233–1247 (2012).
Gutierrez-Barragan, D. et al. Unique spatiotemporal fMRI dynamics in the awake mouse brain. Curr. Biol. 32, 631–644.e636 (2022).
Jung, W. B., Im, G. H., Jiang, H. & Kim, S. G. Early fMRI responses to somatosensory and optogenetic stimulation reflect neural information flow. Proc. Natl Acad. Sci. USA 118, e2023265118 (2021).
Chuang, K. H., Li, Z., Huang, H. H., Khorasani Gerdekoohi, S. & Athwal, D. Hemodynamic transient and functional connectivity follow structural connectivity and cell type over the brain hierarchy. Proc. Natl Acad. Sci. USA 120, e2202435120 (2023).
Whitesell, J. D. et al. Regional, layer, and cell-type-specific connectivity of the mouse default mode network. Neuron 109, 545–559.e548 (2021).
de Chaumont, F. et al. Real-time analysis of the behaviour of groups of mice via a depth-sensing camera and machine learning. Nat. Biomed. Eng. 3, 930–942 (2019).
You, T., Im, G. H. & Kim, S. G. Characterization of brain-wide somatosensory BOLD fMRI in mice under dexmedetomidine/isoflurane and ketamine/xylazine. Sci. Rep. 11, 13110 (2021).
Grandjean, J. et al. Common functional networks in the mouse brain revealed by multi-centre resting-state fMRI analysis. Neuroimage 205, 116278 (2020).
Benkner, B., Mutter, M., Ecke, G. & Munch, T. A. Characterizing visual performance in mice: an objective and automated system based on the optokinetic reflex. Behav. Neurosci. 127, 788–796 (2013).
Cowey, A. & Franzini, C. The retinal origin of uncrossed optic nerve fibres in rats and their role in visual discrimination. Exp. Brain Res 35, 443–455 (1979).
Felgerolle, C. et al. Visual behavior impairments as an aberrant sensory processing in the mouse model of fragile X syndrome. Front Behav. Neurosci. 13, 228 (2019).
Cloke, J. M. et al. A novel multisensory integration task reveals robust deficits in rodent models of schizophrenia: converging evidence for remediation via nicotinic receptor stimulation of inhibitory transmission in the prefrontal cortex. J. Neurosci. 36, 12570–12585 (2016).
Sundberg, H., Doving, K., Novikov, S. & Ursin, H. A method for studying responses and habituation to odors in rats. Behav. Neural Biol. 34, 113–119 (1982).
Trinh, K. & Storm, D. R. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat. Neurosci. 6, 519–525 (2003).
Oh, S. W. et al. A mesoscale connectome of the mouse brain. Nature 508, 207–214 (2014).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korea government (No. NRF-2020R1A2C2012416 and NRF-2023R1A2C2003798) to J. H. Lee, and a grant to S. Chung (NRF-2022R1A2C1007252) and the Basic Research Program through Korea Brain Research Institute funded by the Ministry of Science and ICT (Grant No. 25-BR-03-02 and 25-BR-02-02). All fMRI data were acquired from the 15.2 T MRI system that was supported by IBS grant (IBS-R015-D1).
Author information
Authors and Affiliations
Contributions
J.H.L., T.L., and S.C. designed and supervised the study. T.Y. and T.L. performed the data analysis, conducted the behavioral study, and drafted the manuscript. G.I., S.G.K., and J.H.L. designed the fMRI protocols and acquired the fMRI data. M.S.J., K.S.K., J.L., and C.S. provided the mouse breeding, and S.L. & C.S. conducted the behavioral study. W.B.J. analyzed physiological data. W.B.J. & S.L. analyzed c-fos data. J.H.L., T.L., S.C., and T.Y. wrote the manuscript, which all authors commented on and edited.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Joanes Grandjean, Yong Jeong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
You, T., Lee, T., Im, G.H. et al. Differential impacts of social isolation and enriched environment on multi-sensory brain-wide functionality and network segregation. Nat Commun 16, 7036 (2025). https://doi.org/10.1038/s41467-025-62253-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62253-4
This article is cited by
-
Brain connectivity benefits from enriched environments
Nature Reviews Neuroscience (2025)