Abstract
Food digestion is essential for nutrient absorption, supporting protein synthesis and maintaining endoplasmic reticulum (ER) homeostasis. However, whether animals can sense ER stress and suppress digestion to reduce ER overload remains unclear. Here, we show that Caenorhabditis elegans can sense ER stress and shut down digestion as a protective response. Food intake activates the unfolded protein response in the ER, and loss of its central regulator, XBP-1, impairs digestion, highlighting the importance of ER homeostasis in food digestion. We identify FDR-1, a food-induced protein, as a key factor that promotes digestion by preserving ER balance through its interaction with DPY-11. Disruption of FDR-1 triggers the innate immune p38/PMK-1 pathway, leading to a protective shutdown of digestion to mitigate ER stress. These findings reveal an adaptive mechanism by which animals limit digestion under ER stress and suggest that modulating nutrient intake may offer therapeutic strategies for diseases related to ER dysfunction.
Similar content being viewed by others
Introduction
Food provides essential nutrients crucial for maintaining healthy physiological functions. The primary nutrients in food—carbohydrates, proteins, and fats—cannot be absorbed directly and must be broken down into smaller molecules in the small intestine through digestion. The functionality of cells depends on protein homeostasis, and the process of food digestion is accompanied by increased nutrition uptake, which regulates the protein translation process of cells1. Conversely, under dietary restriction, protein synthesis is reduced due to GCN2 activation2. Thus, food digestion is fundamental to nutrient acquisition and may impact protein homeostasis in animals.
When animals detect food, their bodies undergo a series of physiological changes, including an increased heart rate3 and heightened saliva and digestive enzyme production4. At the subcellular level, mitochondria play a key role in metabolic adaptations to changing nutritional states, with food perception rapidly inducing mitochondrial fragmentation in the liver5. The endoplasmic reticulum (ER) acts as a quality-control organelle for protein homeostasis, or proteostasis. This is regulated by the unfolded protein response (UPR), a signaling pathway that monitors the accuracy of protein folding within the ER lumen6. Research in mouse models has demonstrated that food cues transiently activate hepatic mTOR and ER stress signaling pathways to prepare the ER for incoming nutrients7. While food-induced ER stress signaling is essential for maintaining protein homeostasis, it remains unclear whether ER homeostasis is critical for sustaining food digestion in animals.
When Caenorhabditis elegans hatch in the absence of food, they arrest development at the L1 larval stage and enter a stable diapause in which many cellular stress–response pathways are suppressed8,9. Feeding for 4 h from L1 arrest animals induces an extensive reprogramming of gene expression and alternative mRNA splicing by acting through mTORC110. The set of genes upregulated by both feeding and mTORC1 is strongly enriched for translation, protein folding, post-transcriptional regulation, and mRNA splicing10, implying that feeding may induce proteostasis. However, it remains unclear whether the burst of protein synthesis induced by feeding is itself responsible for activating the unfolded protein response in the ER (UPRER) to meet the increased folding demand.
In this work, using the established digestive research system in C. elegans, we explore the relationship between ER homeostasis and food digestion. We found that food intake triggers an adaptive UPR to restore proteostasis. Disruption of ER homeostasis, via mutation of xbp-1—a key regulator of unfolded protein response of the endoplasmic reticulum (UPRER)—impairs digestion. Through transcriptome analysis and RNAi screening, we identified FDR-1, a food-responsive protein, and elucidated its role in regulating digestion by maintaining ER balance. Our findings reveal an adaptive mechanism in animals where a shutdown of food digestion due to ER homeostasis disruption acts as a protective strategy to alleviate ER overload and maintain ER protein homeostasis.
Results
Feeding induces protein synthesis and UPRER
When an organism encounters food through smell, sight, or ingestion, it triggers various physiological responses. Previous research has shown that food cues transiently activate hepatic mTOR and ER stress signaling pathways to prepare the ER for incoming nutrients7. The ER plays a central role in protein biosynthesis, suggesting that protein synthesis may also respond to food digestion.
To investigate whether protein synthesis changes upon feeding or during food digestion, we utilized C. elegans to examine alterations in protein translation and transcriptome levels during feeding (Fig. 1a). The L1-stage was selected to synchronize animals and eliminate variability in feeding history. We employed the Surface Sensing of Translation (SUnSET) assay, which detects ongoing protein translation by measuring puromycin incorporation into newly synthesized proteins11. We observed a significant increase in puromycin levels in animals under feeding conditions, indicating that feeding enhances protein translation (Fig. 1b).
a Schematic overview of the methods used to measure protein synthesis and gene expression in response to feeding. b Western blot analysis showing puromycin incorporation in animals with or without food. The SUnSET assay was used to monitor ongoing protein translation, detected by puromycin incorporation into newly synthesized proteins. A significant increase in puromycin levels was observed in L1-stage animals fed with OP50 for 10 h (*p < 0.05, two-sided t-test). Error bars represent the mean ± SD of three independent biological replicates. c Gene Ontology enrichment analysis of food-induced genes in L1-stage animals. Animals were grown on NGM plates for 4 h with or without OP50 feeding. The raw p-values were adjusted to obtain the padj values. d Heatmap displaying the clustering of translation-related gene expression in animals with or without food. Colors indicate the relative intensity of gene mRNA levels (Red: high; Blue: low) in the presence or absence of OP50. e Quantification of hsp-4p::gfp fluorescence in L1 animals after varying durations of feeding (L1 + 0.5 h, L1 + 1.5 h, L1 + 3 h, L1 + 6 h, L1 + 8 h, L1 + 24 h). Mean ± SD; ns denotes p > 0.05, ****p < 0.0001 via two-sided t-test. f Fluorescent micrographs and quantification of GFP in L1 transgenic animals expressing hsp-4p::gfp after 24 h of feeding. Scale bar, 20 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. g Schematic illustration depicting the relationship between food digestion and ER homeostasis. Food digestion enhances protein synthesis, which may activate the UPRER to mitigate ER overload and maintain ER protein homeostasis. However, it remains unclear whether disruptions in ER protein homeostasis could feedback into the food response system, potentially leading to the inhibition of food digestion to reduce ER stress. For all panels, n = number of animals which were scored. Representative data shown is one of three biological replicates. Source data are provided as a Source Data file. See also Supplementary Figs. 1 and 2 and Supplementary Data 1.
To specifically capture early transcriptional changes induced by feeding (rather than developmental progression), we focused on the L1 + 4 h time point for RNA-seq. This short timeframe minimizes confounding effects of the L1 developmental program, as demonstrated in prior studies10. RNA-seq enrichment analysis identified translation-related genes as the most significantly upregulated category during feeding (Fig. 1c and Supplementary Data 1). Heatmap clustering further confirmed the upregulation of translation-related genes under feeding conditions (Fig. 1d and Supplementary Data 1). Ogawa et al.10 showed that feeding for 4 h post-L1 arrest induces robust upregulation of translation-related genes, aligning with our observations (Fig. 1c). These results demonstrate that feeding induces protein synthesis in C. elegans.
To rule out the possibility that prolonged fasting (10 h) might trigger developmental arrest or diapause in L1-stage worms—potentially confounding our observations—we conducted complementary experiments in adult animals, which do not enter developmental diapause under food deprivation. Specifically, we repeated the puromycin incorporation assay in adults under fed, food-deprived, and refeeding conditions (Supplementary Fig. 1a–c). Consistent with L1-stage data (Fig. 1b), adult worms exhibited significantly lower protein synthesis rates during food deprivation and rapid recovery of synthesis rates upon refeeding (Supplementary Fig. 1a–c). These results confirm that feeding induces protein synthesis independently of developmental progression.
To further confirm the role of feeding, we tested the eat-2(ad1116) mutant, which exhibits defective pharyngeal pumping and reduced food intake12. Under standard feeding conditions, eat-2 mutants showed reduced protein synthesis compared to wild-type controls (Supplementary Fig. 1d). This finding directly supports our hypothesis that nutrient ingestion drives protein synthesis.
Given the increase in protein synthesis during feeding, this suggests that a heightened protein folding capacity in the ER might activate its quality-control pathways, particularly the unfolded protein response of the ER (UPRER). We used the transgenic strain zcIs4(hsp-4p::gfp), a reporter for UPRER, to monitor this response. We observed that UPRER (hsp-4p::gfp) was induced in L1 animals after 6 h of feeding (Fig. 1e). By 24 h, the UPRER response was significantly elevated (Fig. 1e, f).
To test whether all food sources universally activate ER stress, we performed experiments using the hsp-4p::GFP reporter strain and fed animals distinct bacterial diets: Escherichia coli (standard food), heat-killed E. coli (HK-E. coli, low-quality food)13, Bacillus subtilis (digestible alternative)14, and Staphylococcus saprophyticus (S. saprophyticus, a non-digestible food source)14,15. Notably, digestible foods (E. coli, HK-E. coli, and B. subtilis) robustly activated hsp-4p::GFP, while non-digestible S. saprophyticus failed to induce ER stress (Supplementary Fig. 2a). These results demonstrate that ER stress activation is not a generic consequence of food intake but rather depends on nutrient utilization and proteostatic demand associated with digestible diets. This aligns with our model that feeding-induced protein synthesis and folding requirements drive UPRER activation.
Next we tested whether feeding induces UPR depended on developmental stage. We found that in well-fed L1 larvae (5 h of feeding with E. coli OP50) or adults, hsp-4p::GFP expression exhibited basal GFP levels. Upon starvation (4 h of food deprivation), hsp-4p::GFP expression significantly decreased in both L1 larvae and adults (Supplementary Fig. 2b, c). However, refeeding starved animals for 4 h restored UPRER activation to levels comparable to initial feeding in both L1 larvae and adults (Supplementary Fig. 2b, c). This indicates that feeding directly activates UPRER to manage ER overload, independent of developmental stage.
In summary, our study indicates that feeding stimulates protein synthesis, which in turn activates UPRER to manage ER overload and maintain protein homeostasis (Fig. 1g). Food digestion enhances protein synthesis, which may activate the UPRER to mitigate ER overload and maintain ER protein homeostasis. However, it remains unclear whether disruptions in ER protein homeostasis could feedback into the food response system, potentially leading to the inhibition of food digestion to reduce ER stress.
ER protein homeostasis disruption shuts down food digestion
Previous research has shown that food cues transiently activate hepatic ER stress signaling to prepare the ER for incoming nutrients7. Our findings in C. elegans also demonstrate that feeding induces the unfolded protein response of the ER (UPRER) (Fig. 1e, f), which acts as a protein quality control system to maintain ER protein homeostasis. When the ER becomes overloaded, it is possible that animals may shut down food digestion to reduce protein synthesis and alleviate ER stress. However, whether disruptions in ER protein homeostasis affect the food response system, leading to a shutdown of digestion to counteract ER overload, remains unclear (Fig. 1g).
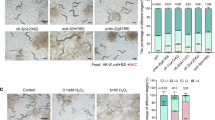
In our previous work14, we demonstrated that C. elegans fed S. saprophyticus (S. saprophyticus) exhibited three key phenotypes: (i) developmental arrest at early larval stages; (ii) accumulation of intact S. saprophyticus within the intestinal tract, indicating a failure to degrade the bacteria; and (iii) pronounced distension of the intestinal lumen, consistent with impaired processing of live S. saprophyticus. These findings suggested that C. elegans lacks the capacity to digest viable S. saprophyticus. Building on this, we have developed a food digestion research system where low-quality food (heat-killed E. coli) triggers the digestion of indigestible food S. saprophyticus in C. elegans14,15. Animal growth is used as an indicator of their ability to digest indigestible food, S. saprophyticus14,15. To explore the role of UPRER in maintaining digestion efficiency, we examined the impact of disrupting UPRER on food digestion. The UPRER pathway consists of three main branches: PERK, IRE1, and ATF6, which promote the transcription of target genes to maintain ER protein homeostasis6. We assessed digestion efficiency in animals with mutations in these pathways, including xbp-1(zc12), xbp-1(tm2482), atf-6(ok551), and pek-1(ok275). In our food digestion system, where C. elegans were fed heat-killed E. coli (HK-E. coli) along with S. saprophyticus, xbp-1 mutants showed slower growth and significantly shorter body length compared to wild-type animals (Fig. 2a), indicating reduced food digestion capacity in xbp-1 mutants. Although feeding induced a UPRER response (hsp-4p::gfp) in wild-type animals, this response was diminished in xbp-1 mutants (Fig. 2b).
a Developmental progression of N2, xbp-1(tm2482), xbp-1(zc12), atf-6(ok551) and pek-1(ok275) animals grown on heat-killed E. coli + S. saprophyticus at 20 °C for 4 days. Scale bar, 200 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. b Microscope images and quantification of GFP expression in wild-type and xbp-1(zc12) mutants carrying the hsp-4p::gfp reporter, with L1-stage animals fed on heat-killed E. coli OP50 for 24 h. Scale bar, 50 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. c Representative images and quantification of YFP expression in the CPL-1(W32A Y35A)::YFP reporter strain at day 1 of adulthood, treated with control or xbp-1 RNAi. Scale bar, 200 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. For all panels, n = number of animals which were scored. Representative data shown is one of three biological replicates. Source data are provided as a Source Data file.
XBP-1 is crucial for maintaining UPRER. Accumulation of misfolded proteins in the ER triggers the UPRER, which reduces translation and enhances ER chaperones and ER-associated degradation (ERAD) to restore ER homeostasis6. To investigate the role of XBP-1 in ER quality control, we examined the ERAD substrate protein CPL-1(W32A Y35A)::YFP in C. elegans. CPL-1(W32A Y35A), a misfolded form of the lysosomal cathepsin L-like peptidase, accumulates in the ER lumen when ERAD is impaired16,17,18. We found that misfolded CPL-1 protein accumulated in animals treated with xbp-1 RNAi (Fig. 2c), suggesting that XBP-1 positively regulates ERAD to restore ER homeostasis under normal feeding conditions.
In summary, our results indicate that food intake enhances protein synthesis and activates the XBP-1-mediated UPRER pathway, which is crucial for maintaining protein homeostasis and efficient food digestion in C. elegans. Disruption of the UPRER pathway, particularly through xbp-1 mutations, impairs food utilization and digestion, highlighting the essential role of UPRER in supporting digestion.
Identifying food response proteins that regulate digestion and maintain ER protein homeostasis
The process of food digestion in animals necessitates the maintenance of protein homeostasis, which is mediated by XBP-1 (Fig. 2). XBP-1 is a well-known regulator of protein homeostasis in the ER. To identify additional host factors that respond to food and regulate digestion through influencing the UPRER, we conducted RNA-seq analyses (Fig. 3a, Supplementary Data 2) under various food conditions (E. coli, heat-killed E. coli, and S. saprophyticus) compared to no food conditions (NGM). By comparing gene expression levels between the presence and absence of food, we identified genes that were upregulated in response to food. Enrichment analysis revealed 94 overlapping genes, of which 18 were expressed in the intestine (Fig. 3a). We then performed RNAi screening of these candidate genes to examine their effects on UPRER and food digestion (Fig. 3a).
a Schematic illustration of the screening process for food-responsive genes that regulate UPRER and food digestion. RNA-seq was conducted to identify food-induced genes under various feeding conditions (E. coli, heat-killed E. coli, and S. saprophyticus) compared to no food (NGM) conditions. A Venn diagram shows the total number of food-induced genes across different conditions and their overlap of 94 genes. Out of these, 18 genes expressed in the intestine were selected as candidates for RNAi screening to assess their effects on UPRER and food digestion phenotype. b Fluorescent micrographs and quantification of GFP in transgenic animals (L1 + 24 h stage) expressing hsp-4p::gfp following control or C49C8.5 RNAi treatment. Scale bar, 20 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. c The ability of animals to digest food was measured by their developmental progression under heat-killed E. coli + S. saprophyticus feeding conditions. Developmental progression was observed in animals treated with control or C49C8.5 RNAi and grown on heat-killed E. coli + S. saprophyticus at 20 °C for 4 days. Scale bar, 200 μm. Mean ± SD; **p < 0.01, via two-sided t-test. d Fluorescent micrographs and quantification of hsp-4p::gfp expression in wild-type or C49C8.5 mutant animals (L1 + 24 h stage) under OP50 feeding conditions. Scale bar, 20 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. e Developmental progression of animals grown on heat-killed E. coli + S. saprophyticus at 20 °C for 4 days. Scale bar, 20 μm. Mean ± SD; ns denotes p > 0.05, ****p < 0.0001 via two-sided t-test. f Microscope images and bar graph show the relative width of the intestinal lumen between N2 and C49C8.5 (ok3369) mutants when fed HK-E. coli + S. saprophyticus. Scale bar, 10 µm. Mean ± SD; ****p < 0.0001 via two-sided t-test. g Pumping rate of WT N2 and the C49C8.5(ok3369) mutant fed HK-E. coli + S. saprophyticus at the L4 + 1 day stage. Mean ± SD; ns denotes p > 0.05 via two-sided t-test. For all panels, n = number of animals which were scored. Representative data shown is one of three biological replicates. Source data are provided as a Source Data file. See also Supplementary Figs. 3, 4 and Supplementary Data 2.
Our screening results showed that RNAi targeting the gene C49C8.5 led to a significant increase in hsp-4p::gfp expression (Fig. 3b and Supplementary Fig. 3a), indicating that C49C8.5 plays a role in maintaining ER protein homeostasis. Additionally, C49C8.5 RNAi caused growth inhibition in the food digestion model (HK-E. coli + S. saprophyticus), suggesting a decrease in food digestion ability in C49C8.5 RNAi animals (Fig. 3c). To confirm these findings, we used the C49C8.5(ok3369) knockout mutant to assess UPRER and food digestion phenotype. We observed that UPRER is activated in C49C8.5(ok3369) mutant animals (Fig. 3d). The C49C8.5(ok3369) mutant animals also exhibited defects in food digestion, showing slow growth under HK-E. coli + S. saprophyticus conditions (Fig. 3e). These defects were rescued by reintroducing C49C8.5 into the mutant animals (Fig. 3e). Moreover, to determine if the regulation of UPRER by C49C8.5 is specific, we performed RNAi targeting C49C8.5 and found no changes in mitochondrial unfolded protein response (UPRmt) (hsp-6p::GFP) or cytosolic heat shock response (hsp-16.2p::GFP) reporters (Supplementary Fig. 3b, c).
hsp-4p::GFP activation occurs in response to ER stress, which can either be beneficial or detrimental. To investigate the physiological role of C49C8.5 in maintaining organismal health, we assessed survival in C49C8.5 mutant animals under ER stress induced by tunicamycin-a compound that inhibits N-linked glycosylation, thereby causing misfolded protein accumulation in the ER19. We observed a significant reduction in survival in C49C8.5 mutants compared to controls (Supplementary Fig. 4a–c), suggesting that loss of C49C8.5 exacerbates ER dysfunction under proteotoxic stress. To directly assess C49C8.5’s functional impact, we generated transgenic C. elegans overexpressing C49C8.5 (driven by the rpl-28p promoter) in the hsp-4p::GFP background. Under tunicamycin-induced ER stress (25 ng/μL), C49C8.5 overexpression significantly suppressed hsp-4p::GFP fluorescence compared to controls (Supplementary Fig. 4d), which indicates that elevated FDR-1 levels mitigate ER dysfunction, directly supporting its role in maintaining ER proteostasis. These findings imply that C49C8.5 is critical for mitigating ER stress and maintaining cellular homeostasis.
To assess digestive function, we measured intestinal lumen width—a marker known to increase when digestion is impaired, as previously reported14,20,21. C49C8.5 mutants exhibited significant intestinal enlargement (Fig. 3f), yet showed no differences in feeding behavior (pumping rates, Fig. 3g). This suggests that impaired digestion in these mutants is caused by defects in nutrient processing rather than reduced food intake.
Overall, these results suggest that the food response protein C49C8.5 facilitates digestion by maintaining ER protein homeostasis. C49C8.5 is the C. elegans ortholog of human ANGPT2 (angiopoietin-2) and ANGPT4 (angiopoietin-4), as annotated in WormBase. As C49C8.5 is an uncharacterized protein, therefore, we named C49C8.5 as food digestion regulation protein (fdr-1).
FDR-1 response to food
To investigate the role of FDR-1 in regulating food digestion through the maintenance of ER homeostasis, we explored its expression and localization, as well as its response to food.
We first constructed a transcriptional reporter strain, fdr-1p::gfp, and observed that fdr-1p::gfp exhibited strong expression in intestinal cells, with minimal signal detected in neuronal cells (Fig. 4a). Additionally, using CRISPR-Cas9 technology, we generated fdr-1p::fdr-1::gfp::flag knock-in worms (Supplementary Fig. 5a, b), which also showed that FDR-1 is localized in the intestine (Fig. 4b).
a Bright-field and fluorescence images showing the expression pattern of fdr-1 in transgenic animals carrying fdr-1p::gfp. Scale bar, 100 μm (up), 10 μm (down). b Bright-field and fluorescence images displaying the expression pattern of fdr-1 in transgenic animals with a single-copy insertion of fdr-1p::fdr-1::gfp::flag, constructed via CRISPR/Cas9. Scale bar, 100 μm (left), 10 μm (right). c Microscope images showing co-localization of FDR-1::GFP with the ER marker (vha-6p::SEL-1(1-79)::mCherry::HDEL). Scale bar, 10 μm. d Microscope images and bar graph illustrating GFP expression in fdr-1p::fdr-1::gfp::flag transgenic L1-stage animals after 24 h of feeding with different food sources (E. coli, heat-killed E. coli, heat-killed E. coli + S. saprophyticus, or S. saprophyticus). NGM without food was used as a control. Scale bar, 10 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. e Western blot analysis of FDR-1 protein levels in fdr-1p::fdr-1::gfp::flag transgenic L1-stage animals after 24 h of feeding with different food sources (E. coli, heat-killed E. coli, heat-killed E. coli + S. saprophyticus, or S. saprophyticus). NGM without food served as a control. The relative intensity of FDR-1::GFP::FLAG versus tubulin is quantified from four biological replicates and shown. Mean ± SD; *p < 0.05, **p < 0.01, ***p < 0.001, via two-sided t-test. For all panels, n = number of animals which were scored. Representative data shown is one of three biological replicates. Source data are provided as a Source Data file. See also Supplementary Figs. 5 and 6.
To determine whether FDR-1 is localized to the ER, we crossed the FDR-1::GFP knock-in strain with an mCherry-HDEL marker22, which labels the ER. We found that FDR-1::GFP colocalizes with the ER marker, mCherry-HDEL, confirming that FDR-1 is localized to the ER (Fig. 4c).
To assess FDR-1 response to food, we exposed FDR-1::GFP knock-in worms to various food conditions (E. coli, heat-killed E. coli, heat-killed E. coli + S. saprophyticus, and S. saprophyticus). We observed that both E. coli and heat-killed E. coli significantly induced FDR-1 expression (Fig. 4d), with Western blot results supporting this finding (Fig. 4e). Notably, heat-killed E. coli induced a more pronounced increase in FDR-1 expression compared to live E. coli, suggesting that E. coli cell wall components strongly stimulate FDR-1 expression.
To determine whether fdr-1 responds to specific bacterial components, we tested its activation using purified peptidoglycan (PGN) and Lipopolysaccharide (LPS). In transgenic worms expressing fdr-1p::fdr-1::GFP or fdr-1p::GFP, PGN extracted from OP50 robustly induced GFP fluorescence, whereas LPS had no significant effect (Supplementary Fig. 6a, b). This aligns with our earlier findings that heat-killed E. coli (enriched in cell wall components) activates fdr-1, and supports the hypothesis that PGN is a key components from cell wall for activating fdr-1.
In summary, FDR-1 is localized to the ER in the intestine, is induced by food, and plays a role in maintaining UPRER homeostasis and regulating food digestion.
XBP-1 regulates FDR-1 expression to maintain ER homeostasis for food digestion
HSP-4, the C. elegans homolog of the mammalian ER chaperone BiP (Hsp70)23, is transcriptionally upregulated in response to ER stress through the IRE-1 kinase/RNase and the XBP-1 transcription factor24. To explore the relationship between XBP-1 and FDR-1, we investigated several aspects (Fig. 5a):
-
1).
Whether fdr-1 mutation increases the accumulation of misfolded proteins, thereby inducing UPRER (significant upregulation of hsp-4p::gfp).
-
2).
Whether fdr-1 mutation activates UPRER in an XBP-1-dependent manner.
-
3).
Whether XBP-1 regulates fdr-1 expression.
-
4).
Whether the XBP-1-FDR-1 axis regulates food digestion.
a Schematic illustration depicting the potential relationship between XBP-1 and FDR-1 in maintaining ER homeostasis and regulating food digestion. b Representative images and quantification of GFP expression in CPL-1(W32A Y35A)::YFP transgenic animals at day 1 of adulthood, following treatment with control or fdr-1 RNAi. Scale bar, 100 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. c Representative images and quantification of GFP expression in wild-type and mutant animals carrying the hsp-4p::gfp reporter, with L1-stage animals fed on heat-killed E. coli for 24 h. Scale bar, 20 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. d qRT-PCR analysis showing the expression levels of xbp-1 and xbp-1s in wild-type N2 and fdr-1 mutants, with L1-stage animals fed on E. coli for 24 h. Mean ± SD; ** P < 0.01, *** P < 0.001, via two-sided t-test. e qRT-PCR analysis showing the expression level of C49C8.5 in wild-type N2 and xbp-1 mutants, with L1-stage animals fed on heat-killed E. coli for 24 h. Mean ± SD; ****p < 0.0001 via two-sided t-test. f Microscope images and bar graph showing GFP expression in wild-type and xbp-1(tm2482) mutants carrying fdr-1p::gfp, with L1-stage animals fed on heat-killed E. coli for 24 h. Scale bar, 20 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. g Microscope images and bar graph showing GFP expression in wild-type and xbp-1(tm2482) mutants carrying fdr-1p::fdr-1::gfp, with L1-stage animals fed on heat-killed E. coli for 24 h. Scale bar, 20 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. h Developmental progression of N2, fdr-1(ok3369), xbp-1(zc12), and fdr-1(ok3369);xbp-1(zc12) animals grown on heat-killed E. coli + S. saprophyticus at 20 °C for 4 days. Scale bar, 200 μm. Mean ± SD; ns denotes p > 0.05,****p < 0.0001 via two-sided t-test. For all panels, n = number of animals which were scored. Representative data shown is one of three biological replicates. Source data are provided as a Source Data file. See also Supplementary Fig. 7.
ERAD is a core downstream effector of XBP-1s and directly addresses ER luminal proteostasis by clearing misfolded proteins via the ubiquitin-proteasome system25,26. To assess whether fdr-1 mutation activates UPRER through the accumulation of misfolded proteins (Fig. 5a-1), we examined the ERAD substrate protein CPL-1(W32A Y35A)::YFP in animals with fdr-1 RNAi. CPL-1(W32A Y35A)::YFP is a misfolded mutant that accumulates in the ER lumen when ERAD is impaired. In wild-type animals, this substrate is efficiently degraded, yielding low fluorescence, whereas ERAD dysfunction leads to marked fluorescence increases. We found that misfolded CPL-1 protein accumulates in fdr-1 RNAi animals (Fig. 5b and Supplementary Fig. 7a), suggesting that FDR-1 positively regulates ERAD to restore ER homeostasis under normal feeding conditions.
The UPRER is activated when the basal machinery of the ER is overwhelmed by unfolded or misfolded proteins. Upon activation, IRE1 splices xbp-1u to produce xbp-1s24, which functions as a transcription factor regulating genes such as chaperones, including HSP-4. We observed that hsp-4p::gfp and the mRNA level of xbp-1s are elevated in fdr-1 mutant animals (Fig. 5c, d), indicating that fdr-1 mutation increases the accumulation of misfolded proteins, which in turn induces UPRER.
To determine if fdr-1 mutation activates UPRER through XBP-1 (Fig. 5a-2), we created the xbp-1(zc12);fdr-1(ok3369) double mutant and assessed changes in hsp-4p::gfp fluorescence. We found that the double mutant showed reduced hsp-4p::gfp fluorescence compared to fdr-1(ok3369) mutants under both heat-killed E. coli (HK-E. coli) and E. coli feeding conditions (Fig. 5c and Supplementary Fig. 7b). This suggests that fdr-1 mutation induces UPRER in an XBP-1-dependent manner.
To examine whether XBP-1 regulates FDR-1 (Fig. 5a-3), we performed qPCR and found reduced fdr-1 mRNA levels in xbp-1(tm2482) mutants (Fig. 5e). Furthermore, the expression of the fdr-1p::gfp transcriptional reporter is also diminished in xbp-1 mutant animals fed with HK-E. coli or E. coli (Fig. 5f and Supplementary Fig. 7c). Additionally, FDR-1::GFP fluorescence is reduced in xbp-1 mutant animals fed with HK-E. coli (Fig. 5g). These results indicate that XBP-1 positively regulates FDR-1 expression.
Mutations in xbp-1 result in the accumulation of misfolded proteins, disrupting ER protein homeostasis and negatively impacting the animal’s ability to utilize food (Fig. 2a, c). Similarly, fdr-1 mutations lead to the accumulation of misfolded proteins and a food digestion defect due to inefficient food utilization (Figs. 5b and 3e). To investigate the relationship between FDR-1 and XBP-1 in food digestion (Fig. 5a-4), we assessed the food digestion ability of the double mutant (fdr-1; xbp-1) in the food digestion model (HK-E. coli + S. saprophyticus) and found growth inhibition and food digestion defects (Fig. 5h). This suggests that FDR-1 and XBP-1 are part of the same pathway regulating food usage.
In summary, XBP-1 regulates FDR-1 expression to maintain ER protein homeostasis, which supports efficient food digestion.
FDR-1 interacts with DPY-11, which is critical for regulating UPRER
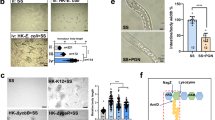
To further elucidate the molecular mechanism by which FDR-1 regulates ER homeostasis and promotes food digestion, we conducted immunoprecipitation (IP) in C. elegans expressing fdr-1p::fdr-1::gfp::flag to identify its interacting proteins (Fig. 6a). Mass spectrometry (MS) analysis revealed 27 candidate proteins potentially interacting with FDR-1 (Supplementary Data 3).
a Immunoprecipitation of FDR-1::GFP::FLAG from control (N2) and fdr-1p::fdr-1::gfp::flag transgenic animals. b Microscope images showing co-localization of FDR-1 (GFP) with DPY-11 (mCherry) in the C. elegans gut. Scale bar, 10 µm. c In vivo immunoprecipitation (IP) experiment validating the interaction between FDR-1 and DPY-11 using transgenic animals expressing fdr-1p::fdr-1::gfp::flag (#1) and fdr-1p::fdr-1::gfp::flag + dpy-11p::dpy-11::myc::mCherry (#2). Transgenic animals (#1) were used as a negative control. For co-immunoprecipitation, lysates from transgenic worms (#1 and #2) were incubated with anti-FLAG beads to pull down FDR-1::GFP::FLAG binding proteins. The binding proteins were then eluted and detected by Western blot (anti-myc). The top panel shows FDR-1::GFP::FLAG and DPY-11::myc::mCherry. Adult animals cultured under standard E. coli OP50 conditions were collected for these experiments. d Representative images and quantification of GFP expression in the CPL-1(W32A Y35A)::YFP reporter strain at day 1 of adulthood, treated with control or dpy-11 RNAi. Scale bar, 100 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. e Representative images and quantification of GFP expression in wild-type animals carrying the hsp-4p::gfp reporter at the young adult stage, treated with control RNAi or dpy-11 RNAi. Scale bar, 100 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. f Developmental progression of N2, dpy-11(CB207)and dpy-11(CB1180) animals grown on heat-killed E. coli + S. saprophyticus at 20 °C for 4 days. Scale bar, 200 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. g, h Microscope images (g) and Western blot analysis (h) showing GFP expression in the fdr-1p::fdr-1::gfp reporter strain at the young adult stage, treated with control RNAi or dpy-11 RNAi. Scale bar, 100 μm (g). The relative intensity of FDR-1::GFP::FLAG versus tubulin is quantified and shown in (h). Mean ± SD; ** P < 0.01, ****p < 0.0001 via two-sided t-test. For all panels, n = number of animals which were scored. Representative data shown is one of three biological replicates. Source data are provided as a Source Data file. See also Supplementary Fig. 8 and Supplementary Data 3.
Among these candidates, DPY-11, an ortholog of human TMX1, stands out. TMX1 encodes a member of the disulfide isomerase (PDI) family, which is involved in protein folding and thiol-disulfide interchange reactions within the ER27. This suggests that FDR-1-DPY-11 interaction might play a role in regulating ER homeostasis. To validate this interaction, we generated transgenic animals expressing dpy-11p::dpy-11::myc::mCherry in fdr-1::gfp::flag animals. We observed that FDR-1 and DPY-11 co-localized in the intestine (Fig. 6b) and confirmed their interaction through FDR-1::GFP::FLAG pull-down, which identified DPY-11::myc::mCherry as an interacting partner (Fig. 6c). These results indicate that FDR-1 interacts with DPY-11.
If the DPY-11-FDR-1 interaction is critical for regulating ER homeostasis and food digestion, then disrupting dpy-11 should affect ER homeostasis and food digestion similarly to fdr-1 mutants. We found that knocking down dpy-11 resulted in the accumulation of misfolded CPL-1 protein (Fig. 6d) and an induced UPRER response (Fig. 6e), similar to the effects seen with fdr-1 RNAi. Furthermore, in the food digestion model (HK-E. coli + S. saprophyticus), dpy-11 mutants exhibited growth inhibition and defective food digestion (Fig. 6f). This suggests that DPY-11, interacting with FDR-1, positively regulates ERAD to maintain ER homeostasis and promote food digestion.
Given that human TMX1 (a homolog of DPY-11) functions in protein folding, we investigated whether DPY-11 affects FDR-1 folding. In Safra et al. study17, ire-1 mutants showed reduced DAF-28::GFP fluorescence despite elevated total DAF-28::GFP protein levels (as quantified by Western blot). This paradox arises because Western blotting detects DAF-28::GFP regardless of its folding state, whereas fluorescence depends on proper protein folding. The authors proposed that misfolded DAF-28::GFP loses fluorescent capacity while accumulating intracellularly due to impaired proteostasis. This observation established a model in which fluorescence intensity inversely correlates with misfolded protein burden, even when total protein levels are unchanged or elevated17. We found that RNAi against dpy-11 reduced FDR-1::GFP fluorescence (Fig. 6g) and increased FDR-1::GFP protein levels in Western blot analysis (Fig. 6h). This suggests that DPY-11 facilitates the proper folding of FDR-1. To rule out transcriptional effects, we measured fdr-1p::GFP fluorescence in dpy-11(RNAi) animals and observed no changes (Supplementary Fig. 8). If DPY-11 solely affected FDR-1 turnover, reduced fluorescence would correlate with decreased total protein (via degradation). Instead, the inverse relationship—reduced fluorescence (Fig. 6g) with elevated total protein (Fig. 6h)—supports a folding defect.
In summary, our results indicate that DPY-11, an interacting protein of FDR-1, influences the correct folding of FDR-1 and plays a crucial role in regulating UPRER and maintaining food digestion.
Activated immune pathway due to ER homeostasis disruption shuts down food digestion, benefiting animal survival
Studies have demonstrated that inhibition of the conserved innate immune p38/PMK-1 pathway (TIR-1-NSY-1-PMK-1) can activate the food digestion system in C. elegans14. The fdr-1 mutant exhibits food digestion defects in a food digestive model using HK-E. coli and S. saprophyticus (Fig. 3e). Therefore, we investigated whether the levels of innate immunity, specifically p38/PMK-1, are elevated in fdr-1 mutants. Our results show that p38/PMK-1 levels are significantly increased in fdr-1 mutants when fed HK-E. coli + S. saprophyticus and HK-E. coli (Fig. 7a and Supplementary Fig. 9a), but remain unchanged under E. coli conditions (Supplementary Fig. 9b). Using the PMK-1 downstream immune reporter gene sysm-1p::gfp to assess immune activation28, we observed a significant increase in sysm-1p::gfp fluorescence in the fdr-1(ok3369) mutant under HK-E. coli + S. saprophyticus conditions (Fig. 7b). This suggests that fdr-1 mutation activates p-PMK-1 in animals fed HK-E. coli + S. saprophyticus, which may inhibit the digestion of S. saprophyticus.
a Western blot images showing the levels of p-PMK-1 in L1 animals fed on heat-killed E. coli + S. saprophyticus for 4 days. The relative intensity of p-PMK-1 versus tubulin is quantified and shown. b Representative images and quantification of GFP expression in wild-type and fdr-1(ok3369) mutants carrying the sysm-1p::gfp reporter, with L1-stage animals fed on heat-killed E. coli + S. saprophyticus for 24 h. Scale bar, 100 μm. Mean ± SD; ****p < 0.0001 via two-sided t-test. c Developmental progression of N2, fdr-1 (ok3369), nsy-1(ag3)and nsy-1(ag3); fdr-1 (ok3369) animals grown on heat-killed E. coli + S. saprophyticus at 20 °C for 4 days. Scale bar, 200 μm. Mean ± SD; ns denotes p > 0.05, ****p < 0.0001 via two-sided t-test. d Survival curves of animals grown on heat-killed E. coli + S. saprophyticus at 20 °C. See Supplementary Data 4 for summary statistics. The Log-rank (Mantel–Cox) test was used for statistical analysis of lifespan. ns denotes p > 0.05, ****p < 0.0001 via two-sided t-test. For all panels, n = number of animals which were scored. Representative data shown is one of three biological replicates. Source data are provided as a Source Data file. See also Supplementary Fig. 9 and Supplementary Data 4.
To determine whether reducing p38/PMK-1 levels in the fdr-1 mutant could alleviate its digestion defects, we created a fdr-1(ok3369);nsy-1(ag3) double mutant by crossing it with the nsy-1 mutant, which abolishes p-PMK-1 activity. In the food digestion model (HK-E. coli + S. saprophyticus), the double mutant showed restored growth and alleviated digestion defects compared to the fdr-1(ok3369) mutant (Fig. 7c). This result indicates that disruption of ER homeostasis by fdr-1 mutation activates the PMK-1 innate immune pathway, leading to the shutdown of food digestion.
To further investigate whether UPRER dysfunction disrupts digestive physiology depends on innate immunity (via PMK-1), we examined the digestive phenotype in enpl-1 (ER chaperone) or ostb-1(oligosaccharyltransferase subunit) RNAi animals. In wild-type (N2) animals, RNAi targeting enpl-1 (ER chaperone) or ostb-1(oligosaccharyltransferase subunit)—both induce UPRER (Supplementary Fig. 9c)—significantly reduced animals’ growth, implying impaired food digestion (Supplementary Fig. 9d). Strikingly, in pmk-1 mutants, the digestion defects caused by enpl-1 or ostb-1 RNAi were substantially suppressed (Supplementary Fig. 9d). This demonstrates that the p38 MAPK pathway (pmk-1) is required for the digestion impairment triggered by UPRER disruption. These data provide direct genetic evidence that UPRER dysfunction disrupts digestive physiology through PMK-1.
When the ER becomes overloaded, animals may shut down food digestion to reduce protein synthesis and alleviate ER stress, which could be beneficial for their survival. To test this hypothesis, we examined the survival rates of wild-type (N2), fdr-1(ok3369), nsy-1(ag3), and fdr-1(ok3369);nsy-1(ag3) double mutant animals fed HK-E. coli + S. saprophyticus (Fig. 7d and Supplementary Data 4). The results showed that the fdr-1(ok3369);nsy-1(ag3) double mutant had the shortest survival rate. This suggests that while reducing p38/PMK-1 levels temporarily improves food utilization, it results in decreased survival rates, thus affecting overall longevity. These findings indicate that activation of the immune pathway due to ER homeostasis disruption leads to the shutdown of food digestion, which benefits the animals’ survival.
Discussion
Our study using the model organism C. elegans has demonstrated that food intake triggers the unfolded protein response of the endoplasmic reticulum (UPRER), a critical process for maintaining ER homeostasis. Conversely, disruption of ER homeostasis through XBP-1 mutations, a pivotal component of the UPRER pathway, impairs food utilization and digestion. We have found that C49C8.5, a protein responsive to food intake, is essential for optimizing food utilization and digestion by preserving ER homeostasis. Mutations in fdr-1 lead to an accumulation of misfolded proteins, which activates UPRER in an XBP-1-dependent manner. Furthermore, fdr-1 mutations also trigger the innate immune pathway p38/PMK-1, resulting in a shutdown of food digestion (Fig. 8). This shutdown acts as a protective mechanism, enabling the animals to survive by reducing ER stress.
The study uncovers an adaptive mechanism in which disruptions in ER homeostasis trigger a shutdown of digestion as a protective strategy to prevent ER overload and preserve protein balance. This reveals a sophisticated survival tactic used by C. elegans. The key findings are: (1) Food intake induces ER stress, activating the unfolded protein response (UPRER) to manage protein folding and maintain balance. (2) FDR-1 is crucial for maintaining ER homeostasis and ensuring efficient digestion. When FDR-1 is disrupted, digestion is impaired, and ER stress increases. (3) FDR-1 interacts with the protein DPY-11, working together to regulate ER function and support digestive efficiency. (4) Disruption of ER homeostasis activates the PMK-1 innate immune pathway, which shuts down digestion to protect the organism, reducing protein overload and allowing the ER to recover.
Food intake has been shown to activate UPRER responses across various species. For instance, in mouse models, nutrient detection by POMC neurons leads to the upregulation of mTOR in the liver, which subsequently increases XBP-1s levels to maintain ER homeostasis7. Similarly, our study demonstrates that in C. elegans, food intake triggers the upregulation of the UPRER pathway, with XBP-1 playing a conserved role in this process. But why does UPRER respond to food intake? During digestion and nutrient absorption, there is a concomitant increase in protein synthesis (translation), which raises the demand on the ER to manage protein folding. UPRER is essential for handling the accumulation of unfolded or misfolded proteins that occurs under these conditions. By activating UPRER in response to food, the organism mitigates ER stress through mechanisms such as reducing protein translation, enhancing chaperone expression, and promoting ER-associated protein degradation. These responses help maintain ER homeostasis and support the organism’s survival.
Food intake induces UPRER, but it was previously unclear whether disruptions in ER homeostasis directly impact food digestion. In this study, we discovered that when ER homeostasis is disrupted—such as in xbp-1 or fdr-1 mutants that exhibit impaired food utilization—the organism also shows defective digestion. Our previous research has shown that UPRER functions as a physiological food evaluation system, promoting aversion behavior in response to low-quality food13. UPRER is activated when animals consume low-quality food, aiding in their avoidance of such food to enhance survival. Therefore, the shutdown of food digestion or avoidance behavior may serve as a protective strategy to alleviate ER overload by reducing food intake, which in turn decreases protein synthesis.
In our study, we found that ER homeostasis is disrupted in animals with a fdr-1 mutation, leading to impaired food utilization. We also observed that the innate immune pathway p38/PMK-1 is activated in these fdr-1 mutant animals. Reducing the activity of the p38/PMK-1 pathway in fdr-1 mutants improves their food utilization capacity, but this comes at the expense of reduced survival rates. Our previous research has also shown that UPRmt activation induces PMK-1 to inhibit food digestion20. This suggests that the innate immune pathway PMK-1 may play a role in sensing disruptions in both ER and mitochondrial protein homeostasis. When this homeostasis is compromised, PMK-1 is activated to shut down food digestion, thereby reducing protein overload by limiting food-induced protein synthesis. This mechanism appears to enhance survival by mitigating the stress associated with protein homeostasis disruptions.
In summary, our study reveals an adaptive mechanism in C. elegans where the disruption of ER homeostasis prompts a shutdown of food digestion as a protective strategy. This response may help alleviate ER overload and maintain ER protein homeostasis, showcasing a sophisticated survival strategy employed by these organisms. Our work opens avenues for investigating how cellular stress—specifically ER stress—can be sensed at the whole-organism physiological level to shutdown digestion. By demonstrating that ER stress can lead to a shutdown of digestion as a protective mechanism, our study suggests potential therapeutic strategies for alleviating ER overload in ER stress-related diseases.
Methods
C. elegans strains and maintenance
Nematode stocks were maintained on nematode growth medium (NGM) plates seeded with bacteria (E. coli OP50) at 20 °C. The following strains/alleles were obtained from the Caenorhabditis Genetics Center (CGC) or as indicated:
-
1).
The following strains were obtained from the CGC:
N2 Bristol (wild-type control strain);
RB2446:[C49C8.5(ok3369) IV];
SJ17: [xbp-1(zc12) III; zcIs4 V];
xbp-1(tm2482) III;
RB772:[atf-6(ok551) X];
RB545:[pek-1(ok275) X];
SJ4005:[zcIs4(hsp-4p::gfp) V];
hjSi158:[vha-6p::SEL-1(1-79)::mCherry::HDEL::let-858 30 UTR] I;
vkEx1879: [nhx-2p::cpl-1(W32A Y35A)::YFP + myo-2p::mCherry];
AU3: [nsy-1(ag3) II];
CB1180: [dpy-11(e1180) V];
CB207:[dpy-11(e207) V];
AU78: [agIs219 (T24B8.5p::GFP::unc-54 3’ UTR + ttx-3p::GFP::unc-54 3’ UTR) III];
DA1116: [eat-2(ad1116) II];
SJ4100: [zcIs13 (hsp-6p::gfp)];
CL2070: [dvIs70 (hsp-16.2p::gfp)].
-
2).
The following strains were constructed in this study:
YNU37: C49C8.5 {ylf5[C49C8.5p::C49C8.5::gfp::3 × flag (knock-in)]};
YNU401: [nsy-1(ag3); C49C8.5(ok3369)];
YNU40:ylfEx140[(C49C8.5p::C49C8.5::gfp)+myo-2p::mCherry];C49C8.5(ok3369);
YNU346:[agIs219 (T24B8.5p::GFP::unc-54 3′ UTR + ttx-3p::GFP::unc-54 3′ UTR) III; C49C8.5(ok3369)];
YNU496: [nsy-1(ag3); zcIs4(hsp-4p::gfp)];
YNU497: ylf5[C49C8.5p::C49C8.5::gfp::3 × flag (knock-in); hjSi158[vha-6p::sel-1(1-79)::mcherry::hdel::let-858 3′utr];
YNU48: {ylf5[C49C8.5p::C49C8.5::3 × flag::gfp(knock-in)];xbp-1(tm2482)};
YNU343: [C49C8.5(ok3369); zcIs4(hsp-4p::gfp)];
YNU344: [C49C8.5(ok3369); xbp-1(zc12); zcIs4(hsp-4p::gfp)];
YNU47: [C49C8.5(ok3369); xbp-1(tm2482)];
YNU442: [ylfEx237(C49C8.5p::GFP)+ myo-2p::mCherry];
YNU493: ylfEx265{ylfEx237 [(C49C8.5p::GFP)+ myo-2p::mCherry]; xbp-1(tm2482)};
YNU423:ylfEx219{ylf5[C49C8.5p::C49C8.5::gfp::3 × flag-knock-in)];dpy-11p::dpy-11::myc::mcherry+odr-1p::GFP};
YNU499: ylfEx264[(rpl-28p::C49C8.5::mcherry+ odr-1p::mcherry); zcIs4(hsp-4p::gfp)];
YNU443:[nsy-1(ag3); C49C8.5(ok3369); zcIs4(hsp-4p::gfp)];
YNU498: ylfEx263(rpl-28p::C49C8.5::mcherry+ odr-1p::gfp).
Bacterial strains
E. coli OP50 (from CGC), E. coli HT115 (Dharmacon), B. subtilis (from ATCC#6051) and S. saprophyticus (from ATCC#15305) were cultured at 37 °C in LB medium. A standard overnight cultured bacteria was then spread onto each Nematode growth media (NGM) plate.
Generation of transgenic lines
Generation of YNU37: C49C8.5 {ylf5[C49C8.5p::C49C8.5::gfp::3 × flag (knock-in)]} by CRISPR/Cas9
The single-guide RNA (sgRNA) targeting sequences were designed using the online CRISPR design tool (http://crispor.tefor.net/) and cloned into the pPD162 vector29 to generate sgRNA-expressing vectors. Building repair template: 500–700 bp homology arms for C49C8.5 target were PCR amplified from N2 genomic DNA and inserted into the mNG^SEC^3 × Flag vector pDD268 using Gibson assembly (New England BioLabs). pCFJ90 (Pmyo-2:mCherry; Addgene #19327) was used as a co-injection marker. The sgRNA vector (50 ng/mL) for the target gene and C49C8.5 repair template (10 ng/mL) was co-injected with the co-injection marker pCFJ90 (2.5 ng/mL) into gonads of young adult animals. Injected animals were transferred to new OP50 plates (three animals per plate) and allowed to lay eggs for 2–3 days at 25 °C in the absence of selection. Then, hygromycin was added to a final concentration of 250 mg/mL and the plates were returned to 25 °C for an additional 3–4 days. Candidate knock-in animals (those that survived hygromycin selection, were Rollers (Rol) and lacked the red fluorescent extrachromosomal array markers) were singled to establish lines. Only one line from each injection plate was kept30. All lines were confirmed by PCR and Western blot.
The primer used for creating this strain is:
ylf5-268 sgRNA: TAAGTATAGCAACGATGAAT,
ylf5-267 sgRNA: AAGTATAGCAACGATGAAT.
Generation of YNU423
To construct the C. elegans plasmid for overexpression of dpy-11, 3× Myc tag and genomic DNA of dpy-11 was first insert to pPD49.26:mCherry, which was digested with SphI and named resultant vector. 3-kb of the dpy-11 promoter was then inserted into the resultant vector digested by PstI. The construct was verified by sequencing.
To generate the strains YNU423: ylfEx219{ylf5[C49C8.5p::C49C8.5::gfp::3 × flag (knock-in)];dpy-11p::dpy-11::myc::mcherry+odr-1p::gfp}, a DNA plasmid mixture containing dpy-11p::dpy-11::myc::mcherry (25 ng/µL) and odr-1p::gfp (25 ng/µL) was injected into the gonads of adult ylf5 [C49C8.5p::C49C8.5::gfp::3 × flag] hermaphrodite worms by standard methods31.
Generation of YNU442
To construct the C. elegans plasmid for expression 3536 bp promoter of C49C8.5 was inserted into the pPD49.26-GFP vector. DNA plasmid mixture containing C49C8.5p::gfp (10 ng/µL) and myo-2p::mCherry (20 ng/µL) was injected into the gonads of adult N2.
Generation of YNU40
To construct the C. elegans plasmid for expression of C49C8.5, 3536 bp promoter and 4966 bp genomic DNA of C49C8.5 was inserted into the pPD49.26-GFP vector. DNA plasmid mixture containing C49C8.5p::C49C8.5::gfp (10 ng/µL) and myo-2p::mCherry (20 ng/µL) was injected into the gonads of adult C49C8.5(ok3369).
Generation of YNU498
To construct the C. elegans plasmid for expression of C49C8.5, 1437 bp promoter of rpl-28 and 4966 bp genomic DNA of C49C8.5 was inserted into the pPD49.26-mcherry vector. DNA plasmid mixture containing C49C8.5p::C49C8.5::mcherry (10 ng/µL) and odr-1p::gfp (20 ng/ µL) was injected into the gonads of adult N2.
Generation of YNU499
To construct the C. elegans plasmid for expression of C49C8.5, 1437 bp promoter of rpl-28 and 4966 bp genomic DNA of C49C8.5 was inserted into the pPD49.26-mcherry vector. DNA plasmid mixture containing C49C8.5p::C49C8.5::mcherry (10 ng/µL) and odr-1p::mcherry (20 ng/µL) was injected into the gonads of adult zcIs4(hsp-4p::gfp).
Food preparation
Heat-killed E. coli preparation
Heat-killed E. coli (HK) was prepared by an established protocol32. A standard overnight bacterial (50 mL E. coli) culture was concentrated to 1/10 vol (5 mL E. coli) and was then heat-killed at 80 °C for 2 h.
Preparation of S. saprophyticus (ATCC 15305)
S. saprophyticus was prepared by an established protocol14. A standard overnight culture of S. saprophyticus (37 °C in LB broth) was diluted into fresh LB broth (1:100 ratio), which was then spread onto each NGM plate when the diluted bacteria grew to OD600 = 0.5.
Preparation of HK-E. coli + S. saprophyticus
S. saprophyticus and HK-E. coli + S. saprophyticus were prepared by following our established protocol. HK-E. coli (100 µL) and S. saprophyticus (100 µL) were mixed at a 1:1 ratio, then 200 µL of the mixture was spread onto 60 mm NGM plates.
SUnSET assay
Synchronized L1 worms were seeded on the NGM plate (no food, as control) and E. coli feeding plate (food) for 10 h culturing at 20 °C. The C. elegans on the plates of different treatments were then washed off with M9 and collected in 1.5 mL tubes (aspirated the M9 as much as possible). Adding 100 mL puromycin working solution (1 mg/mL) to soak worms at 20 °C for 1 h. The worms were then lysed by boiling and further processed for Western blot analysis with antibodies against puromycin (Merck Millipore) and α-tubulin (Sigma-Aldrich). 3 independent experiments were performed and quantified in each condition.
RNAi treatment
All feeding RNAi experiments used bacterial clones from the MRC RNAi library33 or the ORF-RNAi Library34. RNAi plates were prepared by adding IPTG with final concentration of 1 mM to NGM. Overnight, 37 °C shakily cultured RNAi strains (LB broth containing 100 µg/mL ampicillin) and the control strain (HT115 strain with empty L4440 vector) were seeded into RNAi feeding plates and cultured at room temperature for 2 days before use.
RNAi screen
Food digestion (developmental) screening on HK-E. coli + S. saprophyticus: Synchronized L1 worms were treated by feeding candidate RNAi for the second generation and grew to adult. They were then bleached and allowed to hatch in M9 buffer for 12 h–16 h. The synchronized L1 worms were seeded on the feeding plate (HK-E. coli + S. saprophyticus). The food digestion (development) phenotype (body length) was measured after culturing 4 days at 20 °C.
Host factors regulated UPRER screening: Synchronized L1 hsp-4p::gfp transgenic animals were treated by feeding candidate RNAi for the second generation and grew to adult. They were then bleached and allowed to hatch in M9 buffer for 12 h–16 h. The synchronized L1 nematodes were then fed candidate RNAi + HK-E. coli (1:1) and the hsp-4p::gfp fluorescence level was observed after culturing 1 days at 20 °C.
C49C8.5 expression assay with bacterial induction
Synchronized L1 animals (C49C8.5p::C49C8.5::gfp::flag) were seeded onto E. coli, HK-E. coli, HK-E. coli + S. saprophyticus or S. saprophyticus (NGM no food as control) feeding plates and cultured at 20 °C 1 days before observing the fluorescence and testing by Western blot.
Microscopy and imaging analysis
Plate prototypes were observed using an Olympus MVX10 dissecting microscope with a DP80 camera. Analysis of fluorescence, worms were mounted on 3% agar pads in M9 buffer with 5 mM levamisole, which was performed with an Olympus BX53 microscope with a DP80 camera (hsp-4p::gfp, hsp-6p::gfp, hsp-16.2p::gfp, C49C8.5p::C49C8.5::gfp, C49C8.5p::gfp, nhx-2p::CPL-1 (W32A Y35A)::YFP, sysm-1p::gfp). To quantify fluorescent intensity, the entire intestine regions were outlined and quantified using ImageJ software. The fluorescence intensity was then normalized to the body area.
Confocal fluorescence images (C49C8.5p::C49C8.5::gfp::3xflag, C49C8.5p:: gfp, dpy-11p::dpy-11::myc::mcherry;C49C8.5p::C49C8.5::gfp::3xflag, mCherry::HDEL; C49C8.5::gfp) were captured using an inverted Zeiss LSM 900 confocal microscope system equipped, and processed and analyzed with ZEN imaging software (v.2.3)
Lifespan analysis
Lifespan studies were conducted on NGM plates at 20 °C as described previously35. Briefly, on day zero, healthy L4 stage hermaphrodites were placed onto NGM plates seeded with HK-E. coli + S. saprophyticus. To prevent self-progeny accumulation, animals were transferred to fresh HK-E. coli + S. saprophyticus seeded plates during the reproductive period (approximately the first ten days) and then every 2 days thereafter. Survival was monitored daily. Statistical analysis was performed using Prism8 software. All survival data can be found in Supplementary Data 4.
Worm total protein extraction
Total proteins were extracted using our established method36. Worms were lysed by freeze-thaw cycles performed three times in liquid nitrogen, with a protein lysis buffer containing PMSF (1 mg/mL). The lysed worms were then ground in a tissue grinding tube. The resulting mixture was centrifuged at 10,625 × g for 10 min at 4 °C, and the supernatant was collected as the total protein extract. Protein concentration was measured using the Pierce BCA Protein Assay Kit (ThermoFisher, 23,227).
IP-MS and coimmunoprecipitation
For immunoprecipitation-mass spectrometry (IP-MS), 5 mg of total protein from YNU37 [C49C8.5p::C49C8.5::GFP::3 × FLAG] worm lysate was incubated with 30 µl of ANTI-FLAG beads (Sigma, #A2220) at 4 °C overnight. The mixture was then washed five times with wash buffer (50 mM Tris, 150 mM NaCl, pH 7.4). Proteins were eluted by boiling in 1% SDS and detected using silver staining and Western blotting before being subjected to MS analysis.
The MS proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD066847.
For coimmunoprecipitation, the lysate from transgenic worms YNU423 (carrying dpy-11p::dpy-11::Myc::mCherry and C49C8.5p::C49C8.5::GFP::3 × FLAG) was processed similarly. The samples were analyzed by Western blot using anti-FLAG mouse monoclonal antibody (Sigma) and anti-Myc mouse monoclonal antibody.
Western blot
The targeting proteins were analyzed by standard Western blot methods and probed with following antibodies: anti-flag M2, monoclonal antibody (dilution = 1:5000, Sigma, F3165), Monoclonal anti-a-tubulin antibody (dilution = 1:5000, Sigma, T5168), Anti Myc-Tag (19C2) Mouse mAb, monoclonal antibody (dilution = 1:5000, abmart, M20002), anti-p-p38 (dilution = 1:5000; Cell Signaling, 4511S), Anti-Puromycin, mouse monoclonal (dilution = 1:3000, Merck Millipore, MABE343), β actin monoclonal antibody (dilution = 1:5000, proteintech, 66009-1-lg), mCherry Polyclonal antibody (dilution = 1:5000, proteintech, 26765-1-AP), Peroxidase-conjugated Affinipure Goat Anti-Rabbit IgG(H + L) (dilution = 1:10,000, Jackson, 111-035-003), Peroxidase-conjugated Affinipure Goat Anti-Mouse IgG(H + L) (dilution = 1:10,000, Jackson, 115-035-003).
Observation the width of intestinal lumen
Synchronized L1 animals (N2 or fdr-1(ok3369) mutants) were seeded onto HK-E. coli + S. saprophyticus at 20 °C for 3 days and examined by microscope. Animals that cannot digest bacteria will develop an accumulation of bacteria in the lumen, which will cause the lumen to swell.
Pharyngeal pumping rates
Pharyngeal pumping rates were measured at the L4 + 1 day stage. Using an Olympus MVX10 dissecting microscope equipped with a DP80 camera, pharyngeal pumping was counted at 15-s intervals.
ER stress survival assay
Age-synchronized day 1 adults were transferred to NGM plates containing 1% DMSO (vehicle control; BioFox, #1084ML500), 25 μg/mL tunicamycin (Sangon Biotech, #A611129-0005), or 50 μg/mL tunicamycin to induce ER stress. Survival was assessed daily under standard conditions (20 °C), with animals scored as dead if unresponsive to gentle platinum wire prodding. To mitigate confounding by progeny, adults were transferred to fresh drug-containing plates every 24 h. Statistical significance between survival curves was determined using the log-rank test in GraphPad Prism 8.0.
Tunicamycin treatment to induce ER stress
Eggs were plated on NGM agar seeded with E. coli OP50 and cultured at 20 °C until reaching the L4 larval stage. L4 animals were harvested using sterile M9 buffer, washed once with M9, and resuspended in M9 containing either 0.1% DMSO (vehicle control) or 25 ng/μl tunicamycin (ER stress inducer). Suspensions were incubated at 20 °C for 3 h on a rotary shaker (120 rpm) to ensure uniform drug exposure. Post-treatment, animals were washed twice with sterile M9, transferred to fresh OP50-seeded NGM plates, and allowed to recover at 20 °C prior to downstream analyses.
Peptidoglycan (PGN) extraction
PGN was isolated from E. coli OP50 using an established protocol37 with the following modifications. Bacterial pellets from overnight cultures were resuspended in 1/10 volume of 1 M NaCl and incubated at 100 °C for 30 min in a water bath to disrupt membranes. Following three rounds of washing with ddH2O, the insoluble cell fragments were crushed by ultrasound for one hour. Next, they were gradually digested using DNase (50 μg/mL), RNase (60 μg/mL), and trypsin (50 µg/mL) at 37 °C for one hour. Bacterial pellets were collected, cleaned three times with ddH2O, and then heated to 100 °C for five minutes to inactivate the enzymes. The purified PGN was pelleted (12,000 × g, 10 min), resuspended in 20 mM HEPES buffer (pH 7.5), and stored at −20 °C.
Real-time PCR
Sample preparation
Synchronized L1 worms (N2, C49C8.5(ok3369), xbp-1(tm2482) were cultured in M9 (M9 liquid culture) adding different bacteria (E. coli or HK-E. coli) for 24 h at 20 °C.
RNA isolation
The prepared samples were quickly collected and 500 µL Trizol (Invitrogen) was added. Liquid nitrogen freeze-thaw was repeated three times. Added chloroform (200 µL) to extract RNA. Followed by isopropanol precipitation (400 µL) of the aqueous phase, and a single wash of the resulting pellet with 75% ethanol. Finally, RNA pellets were dried in a tissue culture hood and resuspended in RNase-free water.
cDNA synthesis
The cDNA was reversed according to the reverse transcription kit (Takara) protocols.
qPCR reaction
qPCR was performed by using PowerUpTM SYBRTM Green (Thermo Fisher A25742) on a real-time PCR machine (ABI-QuantStudioI). Relative gene expression levels were calculated using the 2-ΔΔCt method.
Primers
act-3 (Forward): GGAGTCATGGTCGGTATGGG,
act-3 (Reverse): GGACTGGGTGCTCTTCTGG;
C49C8.5 (Forward): TGACTGGTGGAGTTGCAGC,
C49C8.5 (Reverse): CGATTATACCACCATCGGCAG;
xbp-1 (Forward): CCGATCCACCTCCATCAAC,
xbp-1 (Reverse): ACCGTCTGCTCCTTCCTCAATG;
xbp-1s (Forward): TGCCTTTGAATCAGCAGTGG,
xbp-1s (Reverse): ACCGTCTGCTCCTTCCTCAATG.
RNA-seq
Preparation of animal samples for RNA-seq
RNA-seq was done with three biological replicates that were independently generated, collected, and processed. A large number of synchronized L1 wild-type (N2) worms were obtained by bleaching adult worms. L1 worms were separately cultured into the different bacteria prepared in advance (E. coli, HK-E. coli or S. saprophyticus) with NGM plates (as food), NGM plates without adding bacteria as controls (as no food) for 4 h at 20 °C. L1 worms were then collected into 1.5 mL tube for sequencing.
RNA sequencing and data processing
A total amount of 1 mg RNA per sample was used as input material for the RNA sample preparations. cDNA libraries were constructed, and single-end libraries were sequenced using the Illumina platform (Novogene, Beijing, China). HISAT238 was used to map the clean reads to the reference gene sequence (Species: Caenorhabditis_elegans; Source: NCBI; Reference Genome Version: GCF_000002985.6_WBcel235), and then “featureCounts” tool in subread software39 was used to calculate the gene expression level of each sample. Read counts were inputted into DESeq240 to calculate differential gene expression and statistical significance.
Quantification
ImageJ software was used for quantifying relative fluorescence intensity of various reporters, which was normalized to the body area. And analysis of the developmental body length of nematodes was measured by ImageJ software.
Statistical analysis was performed with Prism 8 (Graphpad Software Inc.).
Statistical analysis
All statistical analyses were preformed using Student’s t-test. Two-tailed unpaired t-test was used for statistical analysis of two groups of samples. Data are presented as Mean ± SD, and p < 0.05 was considered a significant difference. The Log rank (Mantel–Cox) test was used for statistical analysis of lifespan.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data in main Manuscript and Supplementary information are listed in the Source data file. RNA-seq data are shown in Supplementary Data 1 and 2. RNA-Sequencing data have been deposited in CNCB under accession codes PRJCA041472 and PRJCA042026. The raw mass spectrometry data from this study was deposited at the ProteomeXchange Consortium with the identifier PRIDE: PXD066847. All reagents and strains generated by this study are available through request to the corresponding author with a completed Material Transfer Agreement. Source data are provided with this paper.
References
Sung, Y., Yu, Y. C. & Han, J. M. Nutrient sensors and their crosstalk. Exp. Mol. Med. 55, 1076–1089 (2023).
Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Bio. 23, 56–73 (2022).
LeBlanc, J. & Cabanac, M. Cephalic postprandial thermogenesis in human subjects. Physiol. Behav. 46, 479–482 (1989).
Feldman, M. & Richardson, C. T. Role of thought, sight, smell, and taste of food in the cephalic phase of gastric acid secretion in humans. Gastroenterology 90, 428–433 (1986).
Henschke, S. et al. Food perception promotes phosphorylation of MFFS131 and mitochondrial fragmentation in liver. Science 384, 438–446 (2024).
Hetz, C., Zhang, K. Z. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Bio. 21, 421–438 (2020).
Brandt, C. et al. Food perception primes hepatic ER homeostasis via melanocortin-dependent control of mTOR activation. Cell 175, 1321 (2018).
Baugh, L. R. & Hu, P. J. Starvation responses throughout the Caenorhabditis elegans life cycle. Genetics 216, 837–878 (2020).
Baugh, L. R. To grow or not to grow: nutritional control of development during Caenorhabditis elegans L1 arrest. Genetics 194, 539–555 (2013).
Ogawa, T. et al. Nutrient control of growth and metabolism through mTORC1 regulation of mRNA splicing. Mol. Cell 84, 4558–4575 e4558 (2024).
Schmidt, E. K., Clavarino, G., Ceppi, M. & Pierre, P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 6, 275–277 (2009).
Kumar, S. et al. Lifespan extension in C. elegans Caused by bacterial colonization of the intestine and subsequent activation of an innate immune response. Dev. Cell 49, 100–117 e106 (2019).
Liu, P., Liu, X. & Qi, B. UPRER–immunity axis acts as physiological food evaluation system that promotes aversion behavior in sensing low-quality food. eLife 13, RP94181 (2024).
Geng, S. et al. Gut commensal E. coli outer membrane proteins activate the host food digestive system through neural-immune communication. Cell Host Microbe 30, 1401–1416 e1408 (2022).
Liu, H. & Qi, B. Protocol for investigating the effect of food digestion in C. elegans on development by feeding the inedible bacteria Staphylococcus saprophyticus. STAR Protoc. 4, 101990 (2023).
Miedel, M. T. et al. A pro-cathepsin L mutant is a luminal substrate for endoplasmic-reticulum-associated degradation in C. elegans. PLoS ONE 7, e40145 (2012).
Safra, M. et al. The FOXO transcription factor DAF-16 bypasses requirement to promote endoplasmic reticulum homeostasis. Cell Metab. 20, 870–881 (2014).
Efstathiou, S. et al. ER-associated RNA silencing promotes ER quality control. Nat. Cell Biol. 24, 1714–1725 (2022).
Heifetz, A., Keenan, R. W. & Elbein, A. D. Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry 18, 2186–2192 (1979).
Hao, F. R., Liu, H. M. & Qi, B. Bacterial peptidoglycan acts as a digestive signal mediating host adaptation to diverse food resources in C. elegans. Nat. Commun. 15, 3286 (2024).
Liu, Y. et al. Neuronal PRDX-2-mediated ROS signaling regulates food digestion via peripheral UPR(mt) activation. Nat. Commun. 15, 10582 (2024).
Klemm, R. W. et al. A conserved role for atlastin GTPases in regulating lipid droplet size. Cell Rep. 3, 1465–1475 (2013).
Shen, J. S., Chen, X., Hendershot, L. & Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3, 99–111 (2002).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the mRNA. Nature 415, 92–96 (2002).
Smith, M. H., Ploegh, H. L. & Weissman, J. S. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science 334, 1086–1090 (2011).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Guerra, C. & Molinari, M. Thioredoxin-related transmembrane proteins: TMX1 and little brothers TMX2, TMX3, TMX4 and TMX. Cells 9, 2000 (2020).
Yuan, W. et al. Modulating p38 MAPK signaling by proteostasis mechanisms supports tissue integrity during growth and aging. Nat. Commun. 14, 4543 (2023).
Dickinson, D. J., Ward, J. D., Reiner, D. J. & Goldstein, B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10, 1028–1034 (2013).
Dickinson, D. J., Pani, A. M., Heppert, J. K., Higgins, C. D. & Goldstein, B. Streamlined genome engineering with a self-excising drug selection cassette. Genetics 200, 1035–1049 (2015).
Mello, C. C., Kramer, J. M., Stinchcomb, D. & Ambros, V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991).
Qi, B., Kniazeva, M. & Han, M. A vitamin-B2-sensing mechanism that regulates gut protease activity to impact animal’s food behavior and growth. Elife 6, e26243 (2017).
Kamath, R. S. et al. Systematic functional analysis of the genome using RNAi. Nature 421, 231–237 (2003).
Rual, J. F. et al. Toward improving phenome mapping with an ORFeome-based RNAi library. Genome Res 14, 2162–2168 (2004).
Kimura, K. D., Tissenbaum, H. A., Liu, Y. & Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–946 (1997).
He, Y. J. et al. N-glycosylated intestinal protein BCF-1 shapes microbial colonization by binding bacteria via its fimbrial protein. Cell Rep. 42, 111993 (2023).
Tian, D. & Han, M. Bacterial peptidoglycan muropeptides benefit mitochondrial homeostasis and animal physiology by acting as ATP synthase agonists. Dev. Cell 57, 361–372 e365 (2022).
Mortazavi, A., Williams, B. A., Mccue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 (2008).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Acknowledgements
We thank the Caenorhabditis Genetics Center (CGC) (funded by NIH P40OD010440) for strains; We thank Dr. Bin Liang (Yunnan University) for sharing strain: hjSi158. This work was supported by the Yunnan Provincial Science and Technology Project at Southwest United Graduate School (202302AP370005 to B.Q.), Yunnan Applied Basic Research Projects (202101AT070022, 202001AW070006 to Z.S., 202201AT070196 to B.Q.), Yunnan Revitalization Talent Support Program (C619300A086 to Z.S., K264202230211 to B.Q.), National Natural Science Foundation of China (32071129 to Z.S.; 32170794 to B.Q.), a grant (2023KF003 to Y.J.H) from YNCUB, 2023 Yunnan Province Postdoctoral Innovation Project (Y.J.H).
Author information
Authors and Affiliations
Contributions
B.Q. and Y.J.H. conceived the study, designed the experiments and analyzed data. Z.S. and Z.L.H. designed the experiments for providing some key ideas. Y.J.H. performed most of experiments. H.J.X. and Y.Q.Z. performed some experiments. B.Q., Z.S., and Y.J.H. wrote and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, Y., Zhang, Y., Xie, H. et al. The shutdown of food digestion due to endoplasmic reticulum homeostasis disruption acts as a protective mechanism in C. elegans. Nat Commun 16, 8417 (2025). https://doi.org/10.1038/s41467-025-63712-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63712-8