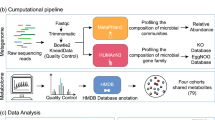
Abstract
Indian immigration to westernized countries has recently surged, increasing their risk of Inflammatory Bowel Disease (IBD) post-migration. While crucial for understanding IBD risk, the gut microbiome remains understudied in Indians. This cross-sectional study examines the impact of westernization on the gut microbiomes of Indians residing in India, Indo-Immigrants, and Indo-Canadians compared to Euro-Canadian and Euro-Immigrant controls. Stool samples for 16S rRNA and shotgun sequencing assessed microbial taxa and functional profiles, alongside dietary and demographic data to evaluate lifestyle patterns. Indians and Indo-Immigrants had distinct microbiotas from controls, with high abundances of Prevotella spp. and CAZymes reflecting their high complex carbohydrate diet. Indo-Canadians exhibited a transitional microbiome towards westernization, mirroring increasing dietary acculturation. Considering 44% of Canadians are first- and second-generation immigrants and the global adoption of westernized practices, future research should investigate the health implications of such microbiome transitions in immigrant populations and newly industrialized nations.
Similar content being viewed by others
Introduction
The growing immigrant population in North America is rapidly westernizing through the adoption of practices like reduced physical activity, changes in hygiene/medical practices, and acculturation of the westernized diet, commonly characterized by caloric dense, highly processed foods with low fiber intake. Amidst this backdrop are health disparities emerging in these populations. Furthermore, the global spread of industrialization raises concern for the increasing prevalence of modern diseases1. For instance, inflammatory bowel disease (IBD) is increasing in prevalence globally, and alarming evidence reveals a specifically increased risk in young Indian immigrants and Indians born in several westernized countries2,3,4. In 2019, India had an incidence rate of 2.34, whereas South Asians in Canada showed an incidence rate of 14.6 per 100,000 person-years, which was comparable to the general population in Canada5,6. A likely contributor to this elevated risk in westernized countries like Canada may stem from changes in the gut microbiome as a response to living in an industrialized environment.
The industrialized microbiome has been previously characterized by the decline of VANISH (volatile and/or associated negatively with industrialized societies of humans) taxa, frequently found in populations adhering to traditional lifestyles, and the rise of BloSSUM (bloom or selected in societies of urbanization/modernization) taxa, which appear in modern, industrialized individuals7,8. Furthermore, health consequences following the assimilation into westernized culture have been previously documented. For example, non-industrialized Irish Travellers who adopted modern practices and Hmong and Karen migrants from Thailand who relocated to the United States (US) both experienced gut microbiome shifts that were associated with an increased risk for metabolic disorders9,10. Currently, there is only one study published on the Indian immigrant gut microbiome, highlighting the gap in non-western/non-European microbiome research. Indeed, over 71% of all microbiome research comes from westernized cohorts, skewing our interpretation of a healthy human gut11. Here, we aimed to investigate the distinction between the Indian and westernized gut microbiomes and the differences in the gut of Indians who migrate to Canada, alongside the adoption of the westernized diet.
Results
Participant Characteristics
The study included Indians (n = 61), Indo-Immigrants (n = 32), Indo-Canadians (n = 17), Euro-Canadians (n = 41) and Euro-Immigrants (n = 23) (Figure S1). Baseline demographics were comparable across groups, though Indians were older and consumed significantly less alcohol (pBONF = 3.29e-09) (Table 1). Indo-Immigrants exhibited more of a change towards westernization (p = 0.002) and higher immigration-related stress (p = 0.04), with no significant differences in age at or years since immigration (Table 2).
Indian Microbiota is Distinct from Westernized Groups
To examine the taxa and complexity of the microbial ecosystem in Indians compared to westerners, alpha diversity revealed the lowest diversity in Indians and highest in Euro-Canadians (Fig. 1A, B). Average percentage of unclassified reads per sample in each cohort can be found in Table S1. Bray Curtis dissimilarity of shotgun sequence data showed significant differences across groups, with 26.3% of variation explained on the first 2 axes (Fig. 1C). Indians and Indo-Immigrants’ microbiotas differed from westernized cohorts (Table 3), and each other (pseudo-F = 9.132, pBONF = 0.01). Weighted UniFrac also demonstrated distinctions in the Indian and Indo-Immigrant microbiomes (pseudo-F = 25.98, pBONF = 0.01), with 61.8% of the variation explained on the first 2 axes (Fig. 1D) with similar findings from 16S rRNA gene amplicon sequence data (Figure S2).
A Pielou’s Evenness (H = 85.7, p = 1.07e-17). B Shannon’s Diversity (H = 79.8, p = 1.89e-16). Beta diversity was explored with Bray Curtis dissimilarity and Weighted UniFrac, using Pairwise Permutational Multivariate Analysis of Variance (PERMANOVA) to test differences between groups. C Bray Curtis principal coordinate analysis (PCoA) plot shows 26.3% of variation was captured on the first two axes. D Weighted UniFrac PCoA plot shows 61.8% of the variation was captured on the first two axes. Pairwise comparisons results can be found in Table S1. E LEfSe (Linear discriminate analysis Effect Size) cladogram results, depicting differentially abundant bacteria across cohorts, with genus set as lowest taxonomic rank. *** = q \(\le\) 0.001, ** = q \(\le\) 0.01, * = q \(\le\) 0.05. Boxes represent the interquartile range (IQR) between the first and third quartiles, the horizontal line indicates the median, and whiskers are the upper and lower values within 1.5 times the IQR.
Linear Discriminate Analysis (LDA) Effect Size (LEfSe) identified dominant taxa in each group, with Bacteroidota (formerly Bacteroidetes) and Pseudomonadota (formerly Proteobacteria) in Indians and Firmicutes in Euro-Canadians (Fig. 1E, Table S1). Indo-Immigrants displayed highest abundances of Eubacterium and Erysipelotrichaceae (a VANISH taxon)12, both previously found to be enriched in non-industrialized populations13, though abundance plots of both taxa reveal low abundances in Indians (Figure S4). As expected, genera common in the industrialized microbiome were enriched in the westernized European cohorts such as Alistipes, Bacteroides, and Clostridia, all previously noted at high abundances in industrialized groups13,14. Nonetheless, average relative abundances for Alistipes and Bacteroides were higher in Indians than Indo-Immigrants. Indo-Canadians represented a transitional gut microbiota, harbouring both taxa found in non-industrialized, such as Collinsella, and industrialized populations, such as Blautia and Anaerostipes (both noted as BloSSUM taxa)13,14. Similar results were found in 16S rRNA gene sequence data (Figure S3, Table S2).
When assessing differences in bacterial species abundances among groups, LEfSe showed that Indians harboured higher abundances of Phocaeicola plebeius, Megasphaera sp., Dialister hominis, and Escherichia coli (Fig. 2). Among Indo-Immigrants, Dorea longicatena, Faecalibacterium prausnitizii and Lachnospiraceae bacterium were notably abundant, while Indo-Canadians had increased abundances of two Ruminoccocus torques SGBs, Blautia wexlerae, Eubacterium rectale, Blautia massiliensis, and Anaerostipes hadrus. Taxa enriched in Euro-Canadians included Phocaeicola vulgatus, Lachnospiraceae bacterium, Clostridium sp., and Blautia faecis, and Euro-Immigrants showed enrichment of Fusicatenibacter saccharivorans, Clostridia bacterium, and Blautia obeum. A key distinguishing feature from Indians to Indo-Canadians was the decline in Prevotella spp., with Prevotella copri (clade A) being over five times more abundant in Indians (Table 4). P. copri Clade A relative abundances were 10.8 for Indians, 5.89 for Indo-Immigrants, and 2.75 for Indo-Canadians (Fig. 2C). A similar pattern of decreasing abundance across Indian cohorts was also observed for Clades B, C and D. All clades were lowest in abundance in Euro-Canadians. Overall, these findings demonstrate that the gut microbiome in Indians are distinct from the West, and Indo-Canadians exhibit a transition towards the industrialized microbiome.
A Taxonomic stacked bar plots depicting relative abundances of top 18 most abundant bacteria across all samples from shotgun sequence data (N = 174; Indian (n = 61), Indo-Immigr (n = 32), Indo-Can (n = 23), Euro-Can (n = 41), Euro-Immigr (n = 23). B Heatmap generated in MicrobiomeAnalyst 2.0, displaying taxa identified by Random Forest as key features that contribute to the predictive accuracy of classifying samples into their respective groups (Figure S5). For the heatmap, features were filtered for minimum 4 counts in 20% or more samples, and low variance filter of 10%. Bars on the top represent the cohorts that group the individual sample columns displayed. Taxa are labelled in rows, with taxonomic rank noted before the bacteria name. Colours on heatmap represent the relative abundances of each bacteria in a given sample. C Heatmap generated in R Studio, displaying the average relative abundances of P. copri clades in each cohort. D LEfSe results of top differentially abundant bacteria. A Kruskal-Wallis test was performed with a significance (α) at 0.05 for one-against-all comparisons. Differentially abundant bacteria were detected using a Linear Discriminate Analysis (LDA) score (equal to or greater than 3.5). E Abundance plot of notable differentially abundant species identified by LEfSe, displaying average relative abundance per cohort.
Indian Gut Microbiome Predicted to be More Pathogen-Tolerant
To understand potential functionality of the gut microbiota in Indians, we used BugBase predictions (Fig. 3), which estimated that Indians appeared to demonstrate higher stress tolerance driven by Pseudomonadota (Proteobacteria). The Indian cohorts had the highest potentially pathogenic bacteria (pFDR = 2.07e-18), mostly dominated by Gram negative bacteria driven mainly by a combination of Pseudomonadota, Bacteroidota (Bacteroidetes), and Firmicutes. In addition, BugBase predicted that the Indian gut microbiome had the highest potential for biofilm formation. Overall, these results suggest the Indian gut microbiome may be more pathogen-tolerant, as healthy individuals were predicted to harbour microbes with higher pathogenic potential and stress tolerance. However, it is important to note that results from BugBase are only predictions made using an older GreenGenes database, therefore these findings should be substantiated in future studies.
A Kruskal-Wallis test was performed in BugBase followed by Mann-Whitney-Wilcoxon tests for pairwise comparisons with adjusted P values shown. Relative abundance is presented on the y-axis. Indian (n = 61), Indo-Immigr (n = 32), Indo-Can (n = 23), Euro-Can (n = 41), Euro-Immigr (n = 23). (A) Stress tolerance (B) Potentially pathogenic bacteria (C) Gram negative bacteria (D) Containing mobile elements (E) Aerobic bacteria (F) Anaerobic bacteria (G) Facultatively Anaerobic bacteria (H) Biofilm formation. Taxonomic contributions are also displayed for each phenotype prediction. All figures were generated using BugBase. *** = p \(\le\) 0.001, ** = p \(\le\) 0.01, * = p \(\le\) 0.05.
Gut Functional Potential in Indians is Distinctive from Westernized Groups
To further understand functional attributes of the Indian gut microbiome, pathway analysis using MetaCyc was used and revealed pathways enriched in lipopolysaccharide (LPS) production, peptidoglycan synthesis, microbial growth/metabolism, protein synthesis and DNA synthesis/repair (Fig. 4). In Indo-Canadians, indications of increased glucose storage were detected. Euro-Canadians had increased pathways related to fatty acid biosynthesis, and Euro-Immigrants expressed pathways indicating sugar metabolism (Table S3). CAZy gene family analysis revealed distinct profiles in the Indian gut including glycoside hydrolases (GH)43, GH51, and GH10, all of which are known for their degradation of plant-based polysaccharides (Table S4). P. copri was the top taxa contributing to these CAZy families (Fig. 5A). In addition, KEGG identities K00561 and K18220 were enriched in Indians and Indo-Immigrants, respectively, both recognized for antimicrobial resistance genes (Fig. 5B). Overall, these results suggest that both diet and drugs were influencing the gut microbiome of Indians.
Pathway abundances were annotated using MetaCyc, then normalized to account for gene length and sampling depth. LEfSe (Linear discriminate analysis Effect Size) was used to test for differentially abundant microbial metabolic pathways using unstratified data. A Kruskal-Wallis test was performed with a significance (α) at 0.05 for one-against-all comparisons. Discriminate features were identified using a Linear Discriminate Analysis (LDA) score, with an LDA score equal to or greater than 3.0. Using MicrobiomeAnalyst 2.0, a heatmap was generated to display the differentially abundant pathways identified from LEfSe. Each column is a sample, and the coloured bars on top represent the cohorts. Indian (n = 61), Indo-Immigr (n = 32), Indo-Can (n = 23), Euro-Can (n = 41), Euro-Immigr (n = 23). Metabolic pathways are labelled in rows and annotated with a colour that describes their general function. Colours on the heatmap represent the relative abundances of each pathway. * L-orthinine biosynthesis I is indirectly linked with protein synthesis and also involved with urea cycle; * CMP-3-deoxy-D-manno-octulosonate biosynthesis is directly involved in lipopolysaccharide (LPS) production.
Normalized gene family abundance table was regrouped to CAZy database using human_regroup_table. Barplots for stratified taxa contributions for each CAZy gene family were generated using human_barplot. Indian (n = 61), Indo-Immigr (n = 32), Indo-Can (n = 23), Euro-Can (n = 41), Euro-Immigr (n = 23). Prevotella copri is the top contributor to CAZyme families (A) GH10, GH43, GH51, all which were enriched in Indians. (B) Top taxa contributions to antimicrobial resistance-related KEGG orthologues abundant in Indians and Indo-Immigrants. Normalized gene family abundance table was regrouped to KEGG database using human_regroup_table. Barplots for stratified taxa contributions for each KEGG ortholog was generated using human_barplot. Prevotella copri AM42 24 is the top contributor to K00561, enriched in Indians and Coprococcus comes is the top contributing taxa to K18220, enriched in Indo-Immigrants.
Westernized Dietary Acculturation Observed in Indo-Canadians
To understand the role of diet associated with the gut microbiome in our Indian cohort, we collected and analyzed dietary recalls using both validated software from North America and from India to account for Indian foods. Indo-Canadians had the highest daily intake of NOVA Group 4 Ultra-Processed Food (UPF), constituting 61% of their caloric intake (95% CI = 467.9 – 780.5 kcal, per 1000 kcal), while Indians consumed significantly less than all other cohorts, reporting 12% of their daily intake (95% CI = 63.66–127.1 kcal per 1000 kcal) (Fig. 6). Consistent with high UPF consumption, total fiber was also found to be the lowest in Indo-Canadians, opposite in Indians who consumed more fiber than Indo-Canadians (p = 0.0028) and Euro-Canadians (p = 0.0110). The highest proportion of non-meat eaters were Indians at 60% (n = 36/61, p = <0.0001), with 30% vegetarian and 30% pescetarian. In Indo-Immigrants, 19% (n = 6/32), 12% (n = 2/17) of Indo-Canadians, 10% (n = 4/41) Euro-Canadians and 39% (n = 9/23) of Euro-Immigrants were non-meat eaters. Cooking oil usage highlighted sunflower oil, ghee and mustard oil as the top choices of Indians, whereas Indo-Immigrants mainly used olive oil, butter and ghee, with olive oil and butter being the primary fats used by the three westernized groups (Figure S6, Table 5). These results highlight the westernized diet adopted by Indo-Canadians, including high levels ofUPFs and low levels of fiber, are potential drivers in the westernized gut microbiota.
Middle bands are the median values, top and bottom boxes display the first and third quartiles, and whiskers are the min and max values. Indian (n = 61), Indo-Immigr (n = 32), Indo-Can (n = 23), Euro-Can (n = 41), Euro-Immigr (n = 23). (A) Calories from Group 4 Ultra-Processed Food (UPF) was counted for each subject from the ESHA dietary reports then compared to total average daily caloric intake. A Kruskal-Wallis test was performed, followed by Dunn’s multiple comparisons, with adjusted p values displayed. (B) Fiber intake was normalized per 1000 kcal for each subject. An ordinary one-way ANOVA was performed, followed by Tukey’s multiple comparisons, with adjusted p values displayed. Graphs (A) and (B) were generated in Prism. (C) Distance-based redundancy analysis (dbRDA) plot was generated using Weighted UniFrac distance matrix from 16S rRNA gene amplicon data. This dbRDA plot depicts the distribution of samples and their associations with lifestyle factors. Each dot is a sample, colour coded by cohort. Variance inflation factor values ranged between 1.14 (vegetarian) – 1.38 (pescetarian), indicating each variable uniquely contributed to the model.
To understand what dietary factors could be driving differences in microbial taxa, the distance-based redundancy analysis (dbRDA) model, accounting for demographics and dietary patterns elucidated 16.62% of the microbial variance on the first 2 axes (PERMANOVA F = 5.9923, p = 0.001) (Fig. 6C). After false discovery rate (FDR) adjustment, UPF and alcohol intake were principal determinants in differentiating the microbiota in westernized cohorts (pFDR = 0.003). The pescetarian diet (pFDR = 0.070) and fiber intake (pFDR = 0.2268) appeared to influence clustering of Indian samples, albeit not significant. Advancing age (pFDR = 0.044) influenced another clustering, whereas vegetarianism showed no significant effect (pFDR = 0.371). Overall, these results suggest that UPF and alcohol intake were driving westernization of the gut microbiome in Indian migrants, with this effect most prominent in Indo-Canadians. Furthermore, the higher fiber intake in Indians correlated with high Prevotella spp. and CAZy gene families tailored to the traditional Indian diet.
Given the above data indicated that the Indo-Canadian group had adopted a westernized diet, further analysis of diet was examined including absolute macronutrient and micronutrient intakes (Tables 6,7; Table S5-S6). Total energy intake was significantly higher in Euro-Canadian males vs. Indo-Immigrant males (p = 0.0144, 95% CI = -1461 – -110.0 kcal). Median percentage of protein intake was highest in Euro-Canadians (16.1%, 95% CI = 13.2 – 17.5%) and lowest protein intake was in Indians (12.5%, 95% CI = 11.7 – 13.3). Indians consumed the highest percentage of carbohydrates in their diet (56.5%, 95% CI = 54.7% - 59.0%) and lowest carbohydrate diet intake was in Indo-Canadians (44.0%, 95% CI = 40.6% – 50.6%). The highest percentage of fat consumption was in Indo-Canadians (39.7%, CI 95% 33.8% – 42.2%) and the lowest was in Indians (31.1%, 95% CI = 28.5% – 34.4%). Software comparisons between North American ESHA and Indian EpiNu revealed no macronutrient composition differences however, EpiNu calculated a significantly higher intake of PUFAs and MUFAs and lower intake of SFA amongst Indians, highlighting discrepancy in dietary fat types between dietary software, which could skew diet analysis between the countries (Fig. 7A, B). When the type of oils consumed were examined, Indo-Canadians had the least diversity with no traditional fats added to their diet like ghee (Fig. 7C).
A Percentages of fat, protein and carbohydrates were calculated in ESHA and EpiNu for each participant, then mean values were calculated for each cohort and an ordinary one-way Analysis of Variance (ANOVA) was used. B Significant differences in types of fat consumed by Indians (n = 61) and Indo-Immigrants (n = 32) were calculated between EpiNu vs. ESHA. Dietary fats displayed in grams per 1000 Calories (kcal). Kruskal-Wallis tests were conducted to determine differences between groups. The middle band inside of the boxes is the median, the bottom and top boxes are the first and third quartiles, and the whiskers are the min to max values. All graphs were generated in Prism. C This plot displays the number of subjects who reported to use each of the listed oils/fats when cooking, per cohort. Oils that were reported less than 5 times across all cohorts were grouped into the "other" category. A total of 70% (n = 43/61) of Indians, 56% (n = 18/32) of Indo-Immigrants, 53% (n = 9/17) Indo-Canadians, 85% (n = 35/41) Euro-Canadians, and 78% (n = 18/23) of Euro-Immigrants reported the types of fats they use when cooking.
Discussion
Earlier studies identified that the Indian gut microbiota is distinct, being enriched in Prevotella spp., Dialister spp., and Megasphaera spp.15,16,17,18,19,20,21, unlike westernized groups dominated by Bacteroides spp.22 and Phocaeicola vulgatus23. The taxonomic heterogeneity within Indians also highlights how generalizing the “Indian microbiome” may inaccurately represent this population, given the cultural variations that exist within the country. Contrary to associations between low alpha diversity and disease in western populations, healthy Indians showed lower alpha diversity than westernized cohorts, suggesting this diversity measure alone may not always be a reliable marker of westernization or health status24. However, the dominance of Prevotella spp. in the Indian gut may have masked the detection of rarer species, potentially underestimating richness estimates in Indians.
Taxonomic data revealed Indians and Indo-Immigrants harbour higher abundances of bacteria characteristic of pre-industrialized societies, including some previously characterized as VANISH taxa. In contrast, Caucasian cohorts possess microbes typical of industrialized populations, including BloSSUM taxa noted in previous studies. However, Indo-Canadians exhibited a transitional microbiota that embodied a blend of both lifestyles, a trend previously detected in infants living in transitional societies (intermediate between non-industrialized and industrialized lifestyles)25. A key genus that is distinctive in this transition in immigrants is the loss of Prevotella spp.10,26. In fact, this contrast in Prevotella spp. abundance in non-westernized versus westernized groups has sparked interest both in its relation to lifestyle and disease, previously being linked to IBD27,28, rheumatoid arthritis29, and obesity30. Yet, contradictory studies also showed its correlation with reduced risks of cardiovascular disease (CVD)31 diabetes32, and obesity33, illustrating the current contradictory understanding around Prevotella spp.-dominance in the gut and its role in diseases.
Globally, Indians have some of the highest abundances of Prevotella spp. in their gut34, as reflected in our data, emphasizing the importance of context when investigating the role of microbes in our intestines, as host-microbe interactions may divergently evolve depending on the host’s genetics, geography, and lifestyle. In fact, these factors may have already driven evolutionary changes in Prevotella spp. strains: isolated P. copri strains from Indian/non-western populations showed traits that aid in digestion of plant-based carbohydrates using carbohydrate-active enzymes (CAZymes), whereas P. copri strains from westernized populations were enriched with virulence factors and resistance genes34. In our study, Indians had a significantly higher average abundance of P. copri Clade A, along with notable abundances of Clades B and C, which subsequently declined in Indo-Immigrants and Indo-Canadians. All P. copri clades have previously been documented at higher abundances in non-westernized individuals than westernized35, and our results highlight this effect of westernization on clade abundances using groups of the same ethnicity. These findings demonstrate how specific strains can evolve with its host, cautioning against broad associations between bacterial species and diseases.
Indians, who traditionally consume a high-complex-carbohydrate diet, have been shown to exhibit increased expression of carbohydrate metabolism genes for complex polysaccharides in the gut15,19,21. Our findings reflected this relationship, as we detected enrichment of CAZy families that aid in the digestion of xylan and xyloglucan, with P. copri as the top contributing taxa. A similar pattern was in fact detected in another study on immigrants who migrated from Thailand to the US, where a decrease in Prevotella spp. abundance corresponded with a loss of CAZymes that break down plant fiber10. The subsequent loss of P. copri in our Indo-Immigrant and Indo-Canadian cohorts aligned with their reduction of a high carbohydrate, high fiber diet, which was a trend previously explored among Hadza and Nepali groups36. Plant-derived microbiota-accessible carbohydrates (MACs) are required to maintain abundances of P. copri, whereas other species such as Bacteroides thetaiotaomicron can utilize both plant and animal-derived MACs, allowing for persistence in the intestines36. This relationship suggests that Prevotella spp. abundance in the Indian gut is highly linked to their specific dietary patterns. Furthermore, the generational loss of P. copri in Indian migrants prompts questions about gut health implications, meriting further research on strain-specific P. copri-host interactions within this demographic.
A previous study found Dialister succinatiphilus and Megasphaera more abundant in Indo-Immigrants versus Indo-Canadians, who had significantly higher D. invisus. Our data align with this trend, with D. invisus enriched in Indo-Immigrants, D. succinatiphilus in Indo-Canadians, and Megasphaera in our Indian cohort. While little is known about these taxa, a relationship between a carbohydrate-rich diet and succinate-utilizing bacteria has been previously discussed37, with D. succinatiphilus being a well-known species in this category. Additionally, Ruminococcus torques, a known mucin degrader38, previously linked to IBD risk39 and identified as a significant contribution in a Crohn’s Disease microbiome-risk score40, was notably enriched in Indo-Canadians. Deeper investigations should explore the role of specific R. torques strains in the gut and its implications in IBD should be explored further.
India’s industrialization is reshaping dietary habits, as reflected in our data showing increased use of sunflower oil over traditional fats like ghee. This trend is likely influenced by globalization41 and dietary guidelines recommending a reduction in saturated fat intake and increase in PUFA-rich vegetable oils. Overtime, these vegetable oils became inexpensive and widely accessible in India, contributing to a shift away from traditional fats42. Coinciding with increased PUFA consumption is the rising epidemic of several modern diseases in India such as IBD43, type-2 diabetes, and metabolic syndrome44. India is now in an accelerating incidence phase of IBD1, doubling the number of patients from 1990 to 201945. While dietary studies in Indian IBD patients are limited, previous research in other populations has shown that increased intake of linoleic (n-6 PUFA) was associated with an increased risk for UC46, whereas increased intake of α-linolenic acid (n-3 PUFA) was associated with reduced UC-risk47. These patterns highlight the importance of further investigating the potential role of vegetable oil consumption in relation to the rising incidence of IBD in India.
In contrast, the westernized cohorts in our study reported cooking mainly with olive oil and butter, and few reported to cook with vegetable oils rich in omega-6 PUFAs. While these self-reports are subject to limitations, it is worth highlighting that while India has adopted the use of white oils like sunflower oil and canola oil, North America may currently be shifting away from them. Historically, the westernized diet has been characterized by a much higher intake of omega-6 PUFA, leading to omega-6:omega-3 ratios of 20:1, which is far from the estimated ratio of 1:1 of our ancestors48. Excessive omega-6 PUFAs consumption has been associated with increased risks of CVD49, cancer50, and autoimmune diseases51. Furthermore, with large-scale studies and meta-analyses refuting the link between saturated fatty acids and CVD52,53 and the growing popularity of the Mediterranean Diet54, a cultural shift in the North America may be occurring, once again preferring olive oil and butter55, which was an observation also recently reported from an Australian cohort56.
Another factor of industrialization that is common in India is the overuse of antibiotics, with India having one of the highest threats for antimicrobial resistance57. Our results showed enriched KEGG identities in Indians and Indo-Immigrants that contain signatures for antibiotic resistance genes, a pattern previously documented in the Indian gut15,58. Despite an exclusion criteria of antibiotic use three months prior to stool collection, we could not gather comprehensive data on our subjects’ long-term antibiotic usage, hence we cannot directly link these enriched antibiotic resistance signatures to persistent antibiotic exposure. The widespread use of antibiotics in India not only increases the prevalence of antibiotic resistance, but also accelerates microbiome turnover, which was indicative in the pathways enriched in our Indian cohort including peptidoglycan synthesis, microbial growth/metabolism, and DNA synthesis/repair. While common usage of antibiotics is a likely catalyst for cell turnover, the higher risk for infectious disease via water contamination and inadequate sanitation in India may also serve as environmental triggers. Our findings highlight India’s unique transition phase, balancing western influences with traditional lifestyle practices, all of which are shaping their gut microbiome. Future research should delve deeper into how westernization is currently influencing the gut microbiome in Indians, as this will likely pose future health challenges.
Coinciding with previous findings, we observed dietary acculturation from the traditional high-complex-carbohydrate Indian diet to an increased intake of NOVA Group 4 UPFs26,59,60, which are characterized by industrially made ingredients with non-nutritive additives like emulsifiers, flavouring and sweeteners61. This dietary shift might explain the enrichment of pathways tapping into glycogen storage in Indo-Canadians, suggesting microbes had inadequate nutrient availability from the host diet62. In our study, Indo-Canadians showed a 49% rise in Group 4 UPF intake and a significant reduction in fiber consumption, raising concerns regarding their gut health and chronic disease risks.
High UPF consumption has been linked to increased risks of CVD, metabolic disorders, micronutrient deficiencies, and anxiety and depression61, which may partly be due to imbalances in the gut microbiome that are a triggered from UPFs. While there are several potential culprits involved in the ultra-processing of foods that may have mechanistic interactions with the gut, it is likely an interplay of several factors surrounding UPF consumption. First, despite being affordable and accessible, UPFs are often high in calories and palatability, but low in essential nutrients, leading to overconsumption and displacement of nutritious meals63. Additionally, emulsifiers, used to mix components in UPF, have been extensively studied in murine models, showing their ability to modulate the gut microbiota, reduce mucus thickness and increase intestinal permeability, bacterial translocation and inflammation64. While studies translating these same mechanistic effects of emulsifiers in humans are scarce, a short-term prospective study (N = 588) found a positive association between dietary emulsifiers and the inflammatory biomarker glycoprotein acetyls65. Moreover, a randomized double-blind controlled-feeding study (N = 16) with the emulsifier carboxymethylcellulose (CMC) found varied responses in the treatment group, with two subjects exhibiting significant alterations in gut composition and microbial encroachment, whereas other participants seemed non-sensitive to CMC66,67. Although constrained by a small sample size, these findings prompt the need to further investigate in large-scale cohorts, as this may in part explain the inconsistent outcomes observed when transitioning from murine to human studies. Overall, these findings underscore that emulsifiers may be one of many components in UPF that influence the gut microbiome, but further human studies are necessary to fully understand the complex role of UPFs on the gut microbiome and inflammation. Future research should also consider examining the synergistic effects of multiple food additives to mirror the composition of UPFs typically consumed by humans.
In addition to inherent limitations of self-reported food diaries (e.g. accuracy of participant reporting), our study also faced limitations in representing cultural diets, primarily due to inadequacies in the existing North American databases for Indian cuisine. This led to the use of the EpiNu® Nutritional software, an Indian dietary tool that is tailored for Indian dishes. Another limitation was the shallow depth of shotgun sequencing and taxonomic classification methods used restricted our ability to identify strain-level differences in bacteria like P. copri. Uneven sample sizing across groups also presented a limitation; specifically, recruiting Indo-Canadians was considerably more difficult, which may reflect a combination of cultural, social and geographical barriers.
As this project focused on the relative abundance of Prevotella spp., quantitative PCR could also help to determine total biomass and absolute abundances of specific taxa present in the gut, as this may also be a key factor influencing disease risk68. Additionally, while metagenomics can provide important insights into the genetic content present within the gut, these data only represent the functional potential and does not provide information regarding genetic expression and actual function of microbiome communities. The increased signatures for antibiotic resistance identified through KEGG identities in Indians and Indo-Immigrants also would have been further supported with data on their long-term antibiotic usage. Furthermore, BugBase analyzes 16S data against an older version of the Greengenes database, which may influence the accuracy of the predictions, especially in relation to the newer Greengenes2 and MetaPhlAn4 databases used. Future work should also aim to analyze parasite communities in the microbiomes of these populations, as this may differ in those living in India versus Canada. Lastly, since this was a cross-sectional study, these data provide associations between westernization and gut microbiome changes, and further investigation is needed to understand the mechanistic interactions of specific factors of westernization on the gut and IBD risk.
Overall, consistent with previous studies, our research reveals that the Indian gut microbiome, enriched in Prevotella spp., is adapted to their high carbohydrate, high fiber diet. We discovered that the Indian microbiome exhibits characteristics of higher bacterial cell turnover, pathogenic potential and stress tolerance, which may reflect a chronic alteration in the response to a higher load of pathogen exposure. However, since our subjects were healthy and not displaying GI-related ailments, this may suggest that Indians have a higher resilience against stressors posed on the gut (Fig. 8). These findings highlight the importance of studying diverse gut microbiomes, particularly in non-westernized and transitional populations, to better predict and manage diseases like IBD. Our study provides a snapshot on the gut microbiome changes in a population undergoing westernization, revealing a drastic transition within the gut microbiomes of Indo-Immigrants and Indo-Canadians, extending our knowledge of the strong effect of immigration and westernization on the gut microbiome. This transition likely reflects microbial adaptation to the dietary acculturation observed, providing insight into the dynamic nature within our gut. Future investigations should examine the health effects of microbiome changes amidst global westernization, as this transition will likely redefine health outcomes for both migrating populations and those living in rapidly modernizing nations.
Graphical representation highlighting the main findings from our study. As Indo-Immigrants and Indo-Canadians subsequently increased ultra-processed food intake, their fiber consumption dramatically reduced. This change in dietary pattern was observed alongside a transition away from high Prevotella spp., which were found abundant in the Indian gut. Instead, Indo-Canadians adopted BloSSUM (Bloom or Selected in Societies of Urbanization/Modernization) taxa, which are commonly found in the westernized microbiome. High Prevotella copri in the Indian gut contributed to enriched carbohydrate-active enzymes (CAZymes) that are specialized to degrade complex carbohydrates common in their diet, which decline in Indian migrant cohorts. Additionally, the Indian microbiome displayed characteristics of a more robust gut, with predictive functions of higher stress tolerance and increased microbial cell turnover. Figure was drawn by hand in Procreate.
Methods
Study Design
A cross-sectional study was conducted including three Indian groups (Indian ancestry was confirmed in each group): (1) Indians residing in India (“Indians”), (2) Indians who migrated to Canada (“Indo-Immigrants”) and (3) Canadians born in Canada from Indian ancestry (“Indo-Canadians”). Caucasian individuals born in Canada with European ancestry (“Euro-Canadian”) were used as a control, and immigrants with European ancestry from a westernized country (“Euro-Immigrants”) as a westernized immigrant control. Healthy participants between the ages of 17–55 years were recruited at three sites: (1) Kolkata, India (2) Manipal, India (3) Kelowna, Canada. In Canada, participants were recruited from the University of British Columbia Okanagan campus, along with religious temples in Kelowna and Vancouver. Posters and pamphlets were handed out to the community, with recruitment material tailored for both Punjabi and Indian/Bengali communities. Exclusion criteria included pregnancy, diagnosis of any chronic inflammatory condition, or travel to India within three months prior to stool collection. Subjects were also not able to provide a sample if they had been on antibiotics less than 2 months prior. Participants completed a demographic questionnaire to assess baseline characteristics. Immigrants also completed a lifestyle survey to assess changes in lifestyle and immigration-related stress.
All research involving human participants was conducted in accordance with the Declaration of Helsinki. Written informed consent to participate was obtained from all participants prior to enrollment. In Canada, the study was approved by the University of British Columbia Clinical Research Ethics Board (UBC CREB) (H21-01555). Approval for sample collection in India was approved under Institutional Ethics Committee (IEC) No 411/2018 and by UBC CREB (H17-01324).
Lifestyle Analysis
To determine immigration-related stress from Indo-Immigrants and Euro-Immigrants, a survey was provided that asked questions regarding their experiences since migration to Canada, such as stress involving documentation, financial insecurity, discrimination, etc. Questions were adopted from Sternberg 2016, in which they administered a survey to Mexican immigrants in the United States69, with questions removed/modified to be applicable for Canadian immigrants in our study (see Supplementary Material, pg. 18). While it can be assumed that since Euro-Immigrants were already westernized, they would experience less of a change in lifestyle in Canada compared to Indo-Immigrants. We wanted to confirm this through the completion of a survey in which subjects were provided a list of statements of lifestyle changes towards westernization, and they were instructed to score how relevant these statements were to their experience (see Supplementary Material, pg. 17).
Stool Collection & DNA Extraction
Stool samples were collected from India (Kolkata and Manipal) and Canada (Kelowna) and processed on site as previously described70. Participants were given at-home stool collection kits consisting of a large plastic container (Medline, 320-DYND36500), a stool collection hat (Medline, DYND36600), gloves, a facemask, and a stool collection guide. Subjects stored their sample in their freezer for no longer than 3 days. Samples were transported on dry ice to the lab, then stored in a −80 °C freezer until homogenization. In a biosafety cabinet, stool was homogenized in liquid nitrogen, then stored in -80 °C. DNA was extracted from stool samples using the QIAamp PowerFecal Pro DNA Kit (Qiagen, Cat. No. 51804) following the manufacturer’s instructions. All samples except those collected in India had an additional wash step (C5) to improve DNA purity. DNA samples were sent to Gut4Health Microbiome Core Facility (BC Children’s Hospital Research Institute, Vancouver, British Columbia) for 16S sequencing on the Illumina MiSeq platform (V4-V4 region amplified with 515 f and 806r primers) ( ~ 75,800 reads per sample). For shotgun sequencing, DNA concentration was normalized to 30μL in nuclease free water, then sent to the Center for Health Genomics and Informatics (University of Calgary, British Columbia) for shotgun sequencing on the Illumina NovaSeq platform ( ~ 9.9 M reads per sample).
16S rRNA Taxonomic Analysis
Paired-end demultiplexed reads were imported into QIIME 2 (Version 2022.2)71. Quality control was completed with DADA2, which included filtering, chimera removal, dereplication, denoising and merging paired-end reads72. For taxonomic classification, the q2-feature-classifer73 was trained using the GreenGenes2 database (10.28.22)74. Amplicon sequence variants (ASVs) were filtered for unclassified ASVs (i.e., identified only to phyla level), sequence counts below 1000, and mitochondrial/chloroplast DNA. Using q2-alignment, ASVs were aligned with mafft75 to construct a phylogeny with fastree via q2-phylogeny76. Alpha diversity was calculated with Shannon77 and Pielou’s Evenness (Kruskal-Wallis), both diversity metrics were also calculated with rarefied data at a sampling depth of 12053, but no major differences were observed78. Beta diversity metrics were calculated using rarefied data with Bray Curtis79 and Weighted UniFrac (PERMANOVA)80. To determine if there were differentially abundant bacteria across cohorts, the LEfSe algorithm was used via the Huttenhower Lab Galaxy Hub81, using a one-against-all strategy with a threshold of 3.5 on the logarithmic LDA score and an alpha of 0.05 for Kruskal-Wallis test among classes. BugBase was used to predict microbiome phenotypes such as Gram-negative, Gram-positive, potentially pathogenic and stress-tolerant bacteria82,83,84,85,86. To understand associations between the distinctions in beta diversity from the taxonomic data with the dietary patterns and baseline characteristics that were significantly different, a dbDRA was conducted in R statistical software (4.2.2)87, using the Weighted UniFrac plot generated from 16S amplicon data in QIIME 2.
Shotgun Taxonomic Analysis
Paired-end demultiplexed raw sequence reads were first assessed for quality using FastQC and all samples were compiled into a MutliQC report to determine parameters for trimming. The input sequence data had an average of 9.9 million reads per sample, with an average length of 127 base pairs. Quality control was performed using KneadData (Version 0.10), with reads trimmed based on the minimum average quality threshold of 25 with a search-window size of 4 bases (SLIDINGWINDOW:4:25). To additionally improve quality, a fixed number of 10 base pairs were trimmed from the ends of reads, and any sequences exceeding a length of 120 base pairs were trimmed down to this maximum length. Reads shorter than the minimum threshold of 60 base pairs were removed. Additionally, host contamination, primers and sequencing adapters were also removed using KneadData. Human host sequences were removed by aligning reads against a host genome database and eliminating perfectly mapped reads.
Taxonomic profiling was conducted using MetaPhlAn4, then each sample was normalized to relative abundances. The normalized relative abundance output table was then imported into R statistical software for downstream analysis including alpha diversity metrics Shannon77 and Pielou’s Evenness (Kruskal-Wallis)78, as well as beta diversity metrics Bray Curtis79 and Weighted UniFrac (PERMANOVA)80. Differentially abundant bacteria were determined using LEfSE with a 3.5 threshold and alpha 0.0581. The normalized abundance output table was imported into MicrobiomeAnalyst 2.0 to apply a Random Forest algorithm ranked according to their Mean Decrease Accuracy score, indicating the extent to which each taxon’s presence or absence influences the overall accuracy of classifying samples into their respective cohort88. These taxa were then visualized in a heatmap generated in MicrobiomeAnalyst 2.0, displaying the highest abundant taxa across cohorts. For the heatmap, a low count filter was applied to remove features less than 4 in fewer than 20% of samples, and a low variance filter was set to remove the lowest 10% of features with minimal variability, as determined by an inter-quantile range threshold.
Functional Profiling
Shotgun reads were analyzed for functional potential using HUMAnN (Version 3.6)89. Gene family and pathway abundances were annotated using the UniRef9090 and MetaCyc91 databases, respectively, then normalized to reads per kilobase to account for gene length. Both tables were further normalized to relative abundance to account for sampling depth. The normalized gene family abundance output was then regrouped to the CAZy and KEGG databases. To determine differentially abundant genes/pathways across cohorts, tables were collapsed to perform LEfSe analysis on the unstratified data using a one-against-all strategy with a threshold of 3.0 on the logarithmic LDA score and an alpha of 0.05 for Kruskal-Wallis test among classes81. Differentially abundant features were then plotted as a heatmap using MicrobiomeAnalyst 2.088.
Nutritional Analysis
Participants filled out a food log for 3 consecutive days prior to their stool collection. Food logs were then entered in the ESHA Food Processor® software, which generated a table for each participant with their nutrient intake for each day. Several data cleaning steps were taken to ensure food intake was inputted into ESHA to accurately reflect the participant’s food log (i.e. calories, proportions, recipes, etc.). One researcher had conducted 4 rounds of data cleaning and three additional researchers reviewed ESHA data for outliers such as abnormally high caloric or nutrient intake. Each nutrient was averaged over 3 days, and results were reported both as absolute values (males and females separate) and as values normalized to 1000 calories. To account for potential biases in using a North American nutritional software, we inputted food logs into the Madras Diabetes Research Foundation (MDRF) EpiNu® Nutritional software, which was designed to represent the Indian diet. If subjects did not specify the type of cooking oils, the cooking oil type was adjusted to reflect the dominant cooking oil used in the subject’s region in India.
To calculate the percentage of daily caloric intake from UPFs, dietary data for each individual was examined in ESHA to flag any products that were classified as UPF according to the NOVA classification. Calories from UPFs were added up for each participant, averaged over 3 days, then divided by the average caloric intake of each participant x 100. To determine participants who were vegetarian or pescetarian, participants were asked in the demographic questionnaire if they had any dietary restrictions. In addition to the subject’s reported diet, the food logs themselves were scanned for validation. Pescatarians were counted if their food logs reported to only be eating fish, but not meat. Whereas vegetarians were counted if their food records did not contain any meat, fish, but did include eggs (ovo-vegetarian) and/or dairy (lacto-vegetarian).
Power Analysis
To estimate the required sample size per group, beta diversity distance matrix scores were used92. Due to the nonparametric nature of our data, median and interquartile range (IQR) values were used instead of mean and standard deviation. The required sample size was determined using the following formula (Eq. 1).
Median Bray Curtis distances of 0.8273044 and 0.608826 for the Indian and Indo-Immigrant cohorts were used, respectively, and the larger IQR (0.1767818) was chosen. Therefore, with a 5% alpha error and a 20% beta error, the total estimated sample size required per group was 10 participants.
Statistical Analysis
Statistical analyses for demographics and dietary data were performed using both R statistical software and GraphPad Prism (Version 10.0.3). We applied the Kruskal-Wallis test coupled with Dunn’s post hoc analysis for nonparametric data. For data that passed the Shapiro-Wilk test of normality, an ordinary one-way ANOVA followed by Tukey’s post hoc test was performed. Categorical data were analyzed using either a Chi-Square, Fisher’s Exact or Fisher’s Exact with Monte Carlo simulation, depending on the data structure. Results were reported as median values and IQR, unless otherwise specified.
Data availability
Data supporting this study’s findings are available within the paper and its Supplementary Information. The 16S rRNA gene sequences files and metagenomic sequence files of fecal samples are publicly available on NCBI under accession number PRJNA1082632. The underlying code for this study is available on GitHub and can be accessed via this link: https://github.com/leahdaloisio/India-Microbiome-Project.
References
Kaplan, G. G. & Windsor, J. W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 18, 56–66 (2021).
Benchimol, E. et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am. J. Gastroenterol. 110, 553–563 (2015).
Carroll, M. W. et al. Pediatric inflammatory bowel disease among South Asians living in British Columbia, Canada: a distinct clinical phenotype. Inflamm. Bowel Dis. 22, 387–396 (2016).
Pinsk, V. et al. Inflammatory bowel disease in the South Asian pediatric population of British Columbia. Am. J. Gastroenterol. 102, 1077–1083 (2007).
Dhaliwal, J. et al. Incidence of Inflammatory Bowel Disease in South Asian and Chinese People: A Population-Based Cohort Study from Ontario, Canada. Clin. Epidemiol. 13, 1109 (2021).
Giri, S. et al. Burden of inflammatory bowel disease in India: analysis of the Global Burden of Disease study from 1990 to 2019. Intest Res. (2025).
Fragiadakis, G. K. et al. Links between environment, diet, and the hunter-gatherer microbiome. Gut Microbes. 10, 216–227 (2019).
Sonnenburg, E. D. & Sonnenburg, J. L. The ancestral and industrialized gut microbiota and implications for human health. Nat. Rev. Microbiol. 17, 383–390 (2019).
Parizadeh, M. & Arrieta, M. C. The global human gut microbiome: genes, lifestyles, and diet. Trends Mol. Med. 29, 789–801 (2023).
Vangay, P. et al. US immigration westernizes the human gut microbiome. Cell. 175, 962–972. e910 (2018).
Abdill, R. J., Adamowicz, E. M. & Blekhman, R. Public human microbiome data are dominated by highly developed countries. PLoS Biol. 20, e3001536 (2022).
Carter, M. M. et al. Ultra-deep sequencing of Hadza hunter-gatherers recovers vanishing gut microbes. Cell. 186, 3111–3124 (2023).
Sonnenburg J. L., Sonnenburg E. D. Vulnerability of the industrialized microbiota. Science. 366 (2019).
Jha, A. R. et al. Gut microbiome transition across a lifestyle gradient in Himalaya. PLoS Biol. 16, e2005396 (2018).
Bhute, S. et al. Molecular characterization and meta-analysis of gut microbial communities illustrate enrichment of prevotella and megasphaera in Indian subjects. Front Microbiol. 7, 660 (2016).
Kumbhare, S. V. et al. A cross-sectional comparative study of gut bacterial community of Indian and Finnish children. Sci. Rep. 7, 10555 (2017).
Jain, A., Li, X. H. & Chen, W. N. Similarities and differences in gut microbiome composition correlate with dietary patterns of Indian and Chinese adults. AMB Express. 8, 104 (2018).
Tandon, D. et al. A snapshot of gut microbiota of an adult urban population from Western region of India. PLoS ONE. 13, e0195643 (2018).
Dhakan, D. B. et al. The unique composition of Indian gut microbiome, gene catalogue, and associated fecal metabolome deciphered using multi-omics approaches. GigaScience. 8, 01 (2019).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature. 473, 174–180 (2011).
Kulkarni, A., Kumbhare, S. V., Dhotre, D. P. & Shouche, Y. S. Mining the Core Gut Microbiome from a Sample Indian Population. Indian J. Microbiol. 59, 90–95 (2019).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature. 486, 222–227 (2012).
Bodykevich, G., Ibtisam, A., Mao, L. The gut microbiome belonging to Colombian adults of younger generations has shifted to a more westernized composition compared to older generations. Undergraduate J Exp Microbiol Immunol. 9 (2023).
Fanfan, D. et al. The intersection of social determinants of health, the microbiome, and health outcomes in immigrants: a scoping review. Am. J. Biol. Anthropol. 183, 3–19 (2023).
Olm, M. R. et al. Robust variation in infant gut microbiome assembly across a spectrum of lifestyles. Science. 376, 1220–1223 (2022).
Copeland, J. K. et al. The impact of migration on the gut metagenome of South Asian Canadians. Gut Microbes. 13, 1–29 (2021).
Hertz, S. et al. Microscopic colitis patients possess a perturbed and inflammatory gut microbiota. Dig. Dis. Sci. 67, 2433–2443 (2022).
Iljazovic, A. et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 1-12 (2020).
Scher, J. U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. elife. 2, e01202 (2013).
Moreno-Indias, I. et al. Insulin resistance is associated with specific gut microbiota in appendix samples from morbidly obese patients. Am. J. Transl. Res. 8, 5672 (2016).
Wang, Y. et al. High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front Microbiol. 7, 129 (2016).
Bibbò, S., Dore, M. P., Pes, G. M., Delitala, G. & Delitala, A. P. Is there a role for gut microbiota in type 1 diabetes pathogenesis? Ann. Med. 49, 11–22 (2017).
Borgo, F. et al. Relative abundance in bacterial and fungal gut microbes in obese children: a case control study. Child Obes. 13, 78–84 (2017).
Prasoodanan, P. K. V. et al. Western and non-western gut microbiomes reveal new roles of Prevotella in carbohydrate metabolism and mouth–gut axis. NPJ Biofilms Microbiomes. 7, 1–17 (2021).
Tett, A. et al. The Prevotella copri complex comprises four distinct clades underrepresented in westernized populations. Cell Host Microbe. 26, 666–679. e667 (2019).
Gellman, R. H. et al. Hadza Prevotella require diet-derived microbiota-accessible carbohydrates to persist in mice. Cell Rep. 42, (2023).
Nakayama, J. et al. Impact of westernized diet on gut microbiota in children on Leyte island. Front Microbiol. 8, 197 (2017).
Png, C. W. et al. Mucolytic bacteria with increased prevalence in IBD mucosa augmentin vitroutilization of mucin by other bacteria. Am. J. Gastroenterol. 105, 2420–2428 (2010).
Ali, F. et al. S3194 Metformin: A Therapeutic Adjunct for Patients With Inflammatory Bowel Disease? Am. J. Gastroenterol. 115, S1676 (2020).
Garay, J. A. R. et al. Gut microbiome composition is associated with future onset of Crohn’s Disease in healthy first-degree relatives. Gastroenterology. 165, 670–681 (2023).
USDA. The Role of Policy and Industry Structure in India’s Oilseed Markets. USDA Economic Research Service. (2007).
Mani, I. & Kurpad, A. V. Fats & fatty acids in Indian diets: Time for serious introspection. Indian J. Med Res. 144, 507 (2016).
Snell, A., Segal, J., Limdi, J. & Banerjee, R. Inflammatory bowel disease in India: challenges and opportunities. Frontline Gastroenterol. 12, 390–396 (2020).
Lakshmipriya, N. et al. Type of vegetable oils used in cooking and risk of metabolic syndrome among Asian Indians. Int J. Food Sci. Nutr. 64, 131–139 (2013).
Dharni, K. et al. Trends of inflammatory bowel disease from the Global Burden of Disease Study (1990-2019). Indian J. Gastroenterol. 43, 188–198 (2024).
Investigators IiES. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case–control study within a European prospective cohort study. Gut. 58, 1606–1611 (2009).
Ananthakrishnan, A. N. et al. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut. 63, 776–784 (2014).
Simopoulos, A. P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 8, 128 (2016).
Simopoulos, A. P. Omega-6/omega-3 essential fatty acid ratio and chronic diseases. Food Rev. Int. 20, 77–90 (2004).
De Lorgeril, M. & Salen, P. New insights into the health effects of dietary saturated and omega-6 and omega-3 polyunsaturated fatty acids. BMC Med. 10, 1–5 (2012).
Simopoulos, A. P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed. Pharmacother. 60, 502–507 (2006).
Dehghan, M. et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 390, 2050–2062 (2017).
Siri-Tarino, P. W., Sun, Q., Hu, F. B. & Krauss, R. M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 91, 535–546 (2010).
Vetrani, C. et al. Planeterranea: An attempt to broaden the beneficialeffects of the Mediterranean diet worldwide. Front Nutr. 9, 973757 (2022).
Fernandes, J. et al. Is olive oil good for you? A systematic review and meta-analysis on anti-inflammatory benefits from regular dietary intake. Nutrition. 69, 110559 (2020).
Wilson, N. A., Villani, A. & Mantzioris, E. Preferences for dietary oils and fats in cooking and food preparation methods: a cross-sectional analysis of Australian adults. Proc. Nutr. Soc. 82, E72 (2023).
Gandra, S., Joshi, J., Trett, A., Lamkang, A. S., Laxminarayan, R. Scoping report on antimicrobial resistance in India. Center for Disease Dynamics, Economics & Policy: Washington, DC, USA (2017).
Gupta, M. et al. Antibiotic-resistant Enterobacteriaceae in healthy gut flora: A report from north Indian semiurban community. Indian J. Med Res. 149, 276–280 (2019).
Lesser, I. A., Gasevic, D. & Lear, S. A. The association between acculturation and dietary patterns of South Asian immigrants. PloS ONE. 9, e88495 (2014).
Noor, S. et al. Relationship between diet and acculturation among South Asian children living in Canada. Appetite. 147, 104524 (2020).
Tristan Asensi, M., Napoletano, A., Sofi, F. & Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients. 15, 1546 (2023).
Esteban-Torres, M., Ruiz, L., Rossini, V., Nally, K. & van Sinderen, D. Intracellular glycogen accumulation by human gut commensals as a niche adaptation trait. Gut Microbes. 15, 2235067 (2023).
Dinu, M. et al. Consumption of ultra-processed foods is inversely associated with adherence to the Mediterranean diet: a cross-sectional study. Nutrients. 14, 2073 (2022).
Bancil, A. S. et al. Food additive emulsifiers and their impact on gut microbiome, permeability, and inflammation: mechanistic insights in inflammatory bowel disease. J. Crohns Colitis. 15, 1068–1079 (2021).
Um, C. Y. et al. Association of emulsifier and highly processed food intake with circulating markers of intestinal permeability and inflammation in the cancer prevention study-3 diet assessment sub-study. Nutr. Cancer. 74, 1701–1711 (2022).
Chassaing, B. et al. Randomized controlled-feeding study of dietary emulsifier carboxymethylcellulose reveals detrimental impacts on the gut microbiota and metabolome. Gastroenterology. 162, 743–756 (2022).
Daniel, N. et al. Human intestinal microbiome determines individualized inflammatory response to dietary emulsifier carboxymethylcellulose consumption. Cell Mol. Gastroenterol. Hepatol. 17, 315–318 (2024).
Vandeputte, D. et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 551, 507–511 (2017).
Sternberg, R. M. et al. Development of the stress of Immigration Survey (SOIS): a field test among mexican immigrant women. Fam. Community Health. 39, 40 (2016).
Gorzelak, M. A. et al. Methods for improving human gut microbiome data by reducing variability through sample processing and storage of stool. PloS ONE. 10, e0134802 (2015).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 13, 581–583 (2016).
Bokulich, N. A. et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome. 6, 1–17 (2018).
McDonald, D. et al. Greengenes2 enables a shared data universe for microbiome studies. bioRxiv 2022-12 (2022).
Katoh, K., Misawa, K., Ki, Kuma K. & Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2–approximately maximum-likelihood trees for large alignments. PloS ONE. 5, e9490 (2010).
Shannon, C. E. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423 (1948).
Pielou, E. C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 13, 131–144 (1966).
Sorenson, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content, and its application to analysis of vegetation on Danish commons. Biol. Skr. 5, 1–5 (1948).
Lozupone, C. A., Hamady, M., Kelley, S. T. & Knight, R. Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl Environ. Microbiol. 73, 1576–1585 (2007).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, 1–18 (2011).
Ward, T. et al. BugBase predicts organism-level microbiome phenotypes. bioRxiv (2017).
Langille, M. G. et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31, 814–821 (2013).
Chen, I.M. A. et al. IMG/M: integrated genome and metagenome comparative data analysis system. Nucleic Acids Res (2016).
Snyder, E. et al. PATRIC: the VBI pathosystems resource integration center. Nucleic Acids Res. 35, D401–D406 (2007).
Kanehisa, M., Goto, S., Sato, Y., Furumichi, M. & Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 40, D109–D114 (2012).
R Core Team R. R: A language and environment for statistical computing. 2018. In: R Foundation for Statistical Computing, Vienna, Austria; 2018.
Lu, Y. et al. MicrobiomeAnalyst 2.0: comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res. 51, W310–W318 (2023).
Beghini, F. et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. elife. 10, e65088 (2021).
Suzek, B. E., Huang, H., McGarvey, P., Mazumder, R. & Wu, C. H. UniRef: comprehensive and non-redundant UniProt reference clusters. Bioinformatics. 23, 1282–1288 (2007).
Caspi, R. et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res. 42, D459–D471 (2014).
Jiang, L. Beta-diversity distance matrices for microbiome sample size and power calculations-How to obtain good estimates. Comput. Struct. Biotechnol. J. 20, 2259–2267 (2022).
Acknowledgements
We thank all the individuals who took part in this study. We thank the entire Ghosh family for support. For dietary analysis, we thank Nadia Anvari. For lab support, we thank Mekenna Smith, Hephzibah Bomide, Carson McComb, Ayva Lewis, Erik Kaila, Andrea Verdugo Meza and Jessica Josephson. This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and a UBC Killam Research Award to support D.L.G. on her sabbatical in India.
Author information
Authors and Affiliations
Contributions
L.D.D. wrote the manuscript and prepared all figures and tables under the supervision and review of D.L.G. (Principal Investigator). In India, the study was initiated by D.L.G. and S.G. Sample collections and DNA extractions in India were performed by M.B., V.S., M.P., U.B. and D.R.H. Upon arrival to Canada, samples from India were organized and quality control was completed by J.B. Participant recruitment, sample collections and DNA extractions in Canada were led by L.D.D. Microbiome analysis was conducted by L.D.D. with guidance/training from N.A. and S.P., and R.K. assisted with processing MetaPhlAn4/HUMAnN3 commands and uploading data to NCBI. Diet data entry and cleaning was primarily performed by C.L. and L.D.D. completed the analysis. Dietary data was inputted into EpiNu by P.S., L.N. and S.V and L.D.D. completed the analysis. Lastly, extensive guidance and contributions were provided by N.H. for the dietary component of this study, along with guidance in the laboratory in Canada. MB, SG, SP and DLG supervised with MB and SG supporting the study in India. D.L.G. managed and administered funding for the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no Competing Non-Financial Interests but the following Competing Interests: DLG is on the Board of Directors for Crohn’s and Colitis Canada and is also the co-founder and Chief Scientific Officer of Melius MicroBiomics Inc. where LDD is the Medical Science Writer.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
D’Aloisio, L.D., Ballal, M., Ghosh, S. et al. The adoption of a westernized gut microbiome in Indian Immigrants and Indo-Canadians is associated with dietary acculturation. npj Biofilms Microbiomes 11, 151 (2025). https://doi.org/10.1038/s41522-025-00778-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41522-025-00778-8