Abstract
Unintentional weight loss is common among patients with Parkinson’s disease (PD) and is associated with poor quality of life and accelerated disease progression. To explore how early α-synuclein pathology contributes to metabolic dysregulation leading to weight loss in PD, transgenic mice overexpressing human wild-type α-synuclein (α-Syn) and controls were fed a high-fat diet (HFD) chow for 4 months. Compared with controls on HFD, α-Syn mice on HFD exhibited a dramatically leaner phenotype, improved glucose tolerance, a major decrease in fat mass, an increase in energy expenditure, a decrease in insulin signaling in the olfactory bulb, aggravated olfactory and motor dysfunctions, and an increase in mortality. Our results show that high-fat diet in α-Syn mice provides a sensitive tool for assessing the underlying mechanism of metabolic dysfunction and its impact on weight loss and disease progression in PD. Moreover, a role is proposed for olfactory dysfunction in PD-related unintentional weight loss.
Similar content being viewed by others
Introduction
Although Parkinson’s disease (PD) is generally associated with its characteristic movement dysfunction, non-motor symptoms add substantially to the overall level of disability and can seriously impact quality of life1. For example, unintentional weight loss, primarily due to the loss of fat mass rather than muscle mass2, is common among patients with PD3,4,5,6,7,8. Risks associated with this non-motor feature include malnutrition, frailty, falls, fractures, and death. Many PD patients with unintentional weight loss are particularly troubled by the combination of bradykinesia, postural instability, tremor, and urinary urgency, which increases their chances of falls and fractures, thus significantly impacting quality of life, risk of early institutionalization, and health economic status. Unintentional weight loss often predates PD motor symptoms by several years3,9, perhaps comprising an unrecognized manifestation of prodromal PD. Weight loss in PD patients does not appear to be caused by reduced energy intake. In fact, in a cross-sectional study, despite equivalent dietary intake, PD patients had lower whole-body fat mass and percentage compared with controls, even after accounting for the effects of age and gender6. Longitudinal data from 1673 participants in the National Institute of Neurological Disorders and Stroke (NINDS) Exploratory Trials in Parkinson’s Disease Long-term Study 1 showed that patients with a decreasing body mass index (BMI) had higher motor and total Unified Parkinson’s Disease Rating Scale (UPDRS) scores than the patients with a stable BMI (despite optimal treatment), whereas those with an increasing BMI had lower motor and total UPDRS scores than patients with a stable BMI10. This indicates that strategies aimed at increasing body weight/BMI would likely improve the quality of life and prognosis for people living with PD. However, the pathophysiological mechanisms underlying weight loss and its relationship to other PD symptoms are poorly understood. Importantly, despite the impact of weight loss on worsening UPDRS scores, the nature of the link between weight loss and dopaminergic neurodegeneration itself remains unknown. Finally, we do not know how the α-synuclein accumulation that is central to PD pathogenesis impacts metabolism and energy homeostasis, ultimately leading to weight loss.
Male transgenic mice (Line 61) overexpressing human wild-type α-synuclein have been reported to be leaner than littermate control male mice11, indicating that α-synuclein has a causal role in weight loss in PD. Since dysregulation of glucose metabolism is a very prominent early manifestation of PD12,13 and diabetes, a disorder of glucose metabolism, is a major risk factor for PD14, we reasoned that high-fat diet-induced diabetes/obesity in transgenic mice overexpressing human wild-type α-synuclein (henceforth, α-Syn mice) may serve as a tool for discovering how early α-synuclein pathology contributes to metabolic dysregulation, leading to weight loss in PD. Thus, both male and female α-Syn and age-matched control mice were fed a high-fat diet chow (60% fat calories) ad libitum. Our results show that α-Syn mice, when challenged with diet-induced obesity, reveal a clear profile of robust metabolic dysfunction, thus providing a sensitive tool for assessing the underlying mechanism of metabolic dysfunction and its impact on weight loss and disease progression in PD. We also provide a theoretical explanation for weight loss in PD by linking metabolism and energy homeostasis with olfactory dysfunction in PD, based on the following: (1) the olfactory system is a metabolic sensor of brain insulin and glucose levels, playing a role in controlling energy homeostasis in response to both sensory (external) and hormonal (internal) signals15,16; (2) α-synuclein pathology in PD likely first appears in the olfactory bulb17,18; and (3) impaired sense of smell is very often the first manifestation of prodromal PD19.
Results
α-Syn mice are leaner compared with littermate controls
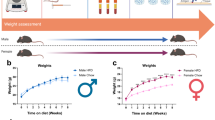
Starting at 1 month of age (post-weaning), the body weights of α-Syn mice and controls were recorded monthly until 20 months of age. The body weights were measured cross-sectionally and randomly across multiple generations over a period of 7 years. Only male mice were used for this initial body weight analysis. The α-Syn mice were significantly (P < 0.005) leaner compared with littermate controls across all age groups (Fig. 1). At 1 month of age, the α-Syn mice weighed, on average, 9.4% less than the age-matched controls. Both strains of mice gained weight at a somewhat rapid pace until 5 months of age, with α-Syn mice maintaining the initial percentage of weight difference. At 5 months of age body weight was somewhat stabilized in both strains, but over time the difference in their body weight slowly increased, with 20-month-old α-Syn mice being, on average, 17.1% lighter than controls.
α-Syn mice are resistant to high-fat diet-induced weight gain
We hypothesized that high-fat diet (HFD)-induced type 2 diabetes (a major PD risk factor) may be informative regarding the linkages between α-synucleinopathy and metabolic dysfunction/weight loss in PD. Thus, three separate groups of male α-Syn mice and age-matched controls were fed a high-fat chow diet (60% fat calories) ad libitum starting at 1, 4, and 12 months of age to assess the impact of aging and/or disease severity. Similarly, another three separate groups of male α-Syn mice and age-matched controls were fed a regular chow diet (RGD). To assess sex-specific effects, a group of female mice was similarly fed a high-fat chow diet starting at 4 months of age. Compared with controls on HFD (control-HFD), α-Syn mice on HFD (α-Syn-HFD) were dramatically leaner (Fig. 2), despite only modest differences in calorie (Fig. 3) and water intake (Fig. 4) in the HFD groups. Note that some of the subjects died (see Fig. 6) at various time points before the analysis time window ended and were excluded. The weight difference between α-Syn-HFD and control-HFD groups was apparent within 1 week post-HFD in the 12-month male group, whereas in the remaining groups the difference was evident within 3–4 weeks post-HFD (Fig. 2). Although the 1-month male and 4-month female α-Syn mice on regular chow diet in general weighed less than controls on the same diet, unlike the cross-sectional body weight analysis represented in Fig. 1, the differences were not statistically significant (P = 0.2127 for 1-month male and P = 0.3355 for 4-month female), possibly due to the amount of variation inherent in measurement of body weight for this small sample size. Similarly, the water intake for the younger (1- and 4-month) control mice on regular chow diet was much higher (Fig. 4a, b, d), compared with other groups; however, body weights are unlikely to be influenced by the higher water intake in these younger groups since the correlation between fluid consumption and body weight is small20.
Mean change from initial body weights of 1-month male (a), 4-month male (b), 12-month male (c), and 4-month female (d) mice, expressed ± standard error. The number of mice for each group is shown in parentheses. Statistical analysis was performed separately for the high-fat diet (P < 0.0001 for a–d) and regular diet groups, using two-way repeated measures ANOVA. For the regular diet groups, the weight difference did not reach statistical significance for the 1-month male (P = 0.2127) and 4-month female (P = 0.3355) groups, whereas the differences were statistically significant for the 4- and 12-month male groups (P < 0.0001).
Average daily calorie intake was measured weekly. Although the differences in the levels of calorie intake between α-Syn and control mice in the high-fat and regular diet groups were only modest, they were significant (P < 0.0001; Time × Genotype for each diet; b–d), except for that of the 1-month male (a) regular diet sub-group (P = 0.3351). The number of mice for each group is shown in parentheses. Statistical analysis was performed separately for the high-fat diet and regular diet groups in a–d, using two-way repeated measures ANOVA.
α-Syn mice are resistant to high-fat diet-induced impaired glucose tolerance
In line with the body weight data, α-Syn mice on HFD exhibited lower fasting blood glucose levels and a more rapid clearance of blood glucose during the intraperitoneal glucose tolerance test (IPGTT) than control-HFD mice (Fig. 5). There was also a striking influence of age (disease progression) and sex on the temporal dynamics of glucose clearance from the blood. Although the 4-month α-Syn-HFD males had better (P = 0.0176) glucose tolerance than age-matched control-HFD males, the former’s glucose levels were still significantly (P = 0.0413) higher than those of α-Syn-RGD males (Fig. 5e). However, in 4-month α-Syn-HFD and α-Syn-RGD females, glucose levels (Fig. 5g) were not significantly different (P = 0.2770). Unlike the 4-month α-Syn-HFD males, the glucose tolerance of 12-month α-Syn-HFD males (Fig. 5c, f) was indistinguishable from that of control-RGD males. Note that in the 1-month males, we measured only fasting glucose levels, which indeed were lower in α-Syn-HFD mice than in control-HFD mice (Fig. 5a).
Fasting blood glucose levels in 1-month male group (a) and IPGTT in 4-month male (b), 12-month male (c), and 4-month female (d) groups after 14–16 weeks of high-fat diet treatment. The area under the curve (AUC) for IPGTT curves (b–d) is shown in (e–g). The number of mice for each group is shown in parentheses. Statistical analysis was performed separately for the high-fat diet (**P = 0.0085) and regular diet sub-groups (P = 0.2181) in the 1-month male group (a) using unpaired t-tests. For the IPGTT of male groups, analysis was performed using three-way repeated measures ANOVA due to equal sample sizes (P < 0.0001). Mixed effects model (P < 0.0001) was used for the IPGTT of female group due to unequal sample sizes. Statistical significance for each time point during the IPGTT (b–d) was calculated using unpaired t-test; those time points for which the differences in glucose levels between control-HFD and α-Syn-HFD were significant (P < 0.05) are marked with bicolor asterisks in (b–d). AUC analysis was performed using two-way ANOVA with Tukey’s multiple comparison test. ** in (f), P = 0.0017; * in (g), P = 0.0493. ns nonsignificant.
High-fat diet-induced increased mortality in α-Synuclein mice
Despite the leaner phenotype and better glucose tolerance, the death rate in male α-Syn mice on HFD was much higher in all age groups, albeit less stark in 1-month-old males; in the 1-month-old male group, which exhibited increased mortality only after 15 weeks into HFD treatment (Fig. 6). By the end of HFD treatment (15–21 weeks), about 55–60 percent of the male α-Syn mice had died in all age groups. However, unlike the males, female α-Syn-HFD mice did not exhibit increased mortality (Fig. 6d), likely due to reduced human α-synuclein protein levels in the brains of female α-Syn mice (Fig. 7).
High-fat diet exacerbates olfactory and motor dysfunction in α-Synuclein mice
The 4-month male group was tested for olfactory dysfunction (general anosmia) using the buried food test paradigm on the 13th week of high-fat diet. The α-Syn-RGD mice exhibited a moderate olfactory dysfunction as they took significantly (P < 0.0001) more time than control-RGD mice to retrieve the hidden cereal (Fig. 8a). High-fat diet significantly (P = 0.0001) aggravated olfactory dysfunction in α-Syn mice, as only one of the α-Syn-HFD mice retrieved the hidden cereal within (and only narrowly so) the maximum time of 600 s (Fig. 8a). High-fat diet did not influence olfactory function in control mice (P = 0.8336). As with olfactory dysfunction, high-fat diet significantly (P = 0.0101) exacerbated motor dysfunction in 4-month male α-Syn mice but not in control mice (Fig. 8b). We used the hindlimb extension reflex score to assess motor dysfunction as it is not affected by body weight and is sensitive to dopamine levels. As observed for the 4-month α-Syn males, high-fat diet significantly (P = 0.0278) exacerbated hindlimb extension reflex scores in 12-month α-Syn males (Fig. 8c). We reasoned that if there is an association between increased mortality and severe olfactory/motor dysfunction in α-Syn-HFD males, α-Syn-HFD females should exhibit either milder or no olfactory/motor dysfunction, as increased mortality was not observed in α-Syn-HFD females. Indeed, none of the female groups exhibited motor dysfunction as assessed by hindlimb extension reflex score (Fig. 8d). Since the results of the exhibited buried food (olfactory) tests were confounded by excessive digging behaviors in all females except those in the control-RGD subgroup, those data are not included. Despite the confounding, the α-Syn-HFD females retrieved the hidden cereal purposefully and started eating by 231 s, whereas the control-RGD females took on average 118 s to retrieve the hidden cereal. The results indicate that female α-Syn-HFD mice developed only modest olfactory dysfunction.
a Histogram plots of the retrieval time of a hidden cereal in buried food pellet retrieval test for general anosmia. Hindlimb extension reflex scores of 4-month male (b), 12-month male (c), and 4-month females (d). e Representative photographs of 4-month male mice showing hindlimb extension reflex scores: absence of hindlimb extension or the mouse gripping its tail = 0; extension of only one hindlimb or extension of both hindlimbs without splayed toes = 1; extension of both hindlimbs with splaying of toes (normal reflex) = 2. The number of mice for each group is shown in parentheses. Statistical analysis for buried food pellet retrieval test was performed using two-way ANOVA with Tukey’s multiple comparison test, whereas Mann-Whitney test was used (separately for α-Syn and control groups) for hindlimb extension reflex scores, which were by design not normally distributed. *** in (a), P = 0.0001; **** in (a), P < 0.0001; * in (b), P = 0.0101; * in (c), P = 0.0278.
High-fat diet-induced altered body composition in α-Syn mice
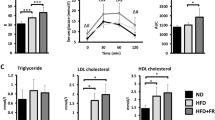
The body composition analysis, by weighing adipose tissues and internal organs, was performed at the end of the study, 21 (1-month male), 15 (4-month male), 19 (12-month male), and 15 (4-month female) weeks post high-fat diet treatment. The slow weight gain of α-Syn-HFD mice reflected a dramatically reduced fat mass, compared with control-HFD mice. In particular, the subcutaneous inguinal fat (iWAT) showed the biggest difference between the two groups (Fig. 9a–d). Similar to the white adipose tissue, high-fat diet increased brown adipose tissue (BAT) mass in both strains of mice, compared with regular diet; however, the BAT mass of control-HFD mice was significantly (P = 0.0214, P = 0.0008, P = 0.0185, and P = 0.0012 for 1-, 4-, and 12-month males, and 4-month females, respectively) greater than that of α-Syn-HFD mice (Fig. 9a–d). Since the calorie intake was similar among HFD groups, the results indicate that α-Syn mice have enhanced fat-burning capacity. In addition, since the food intake was similar and the body weight was only modestly, albeit significantly (P < 0.0001; Fig. 1), different between the α-Syn and control mice on regular diet, it is unlikely that the α-Syn mice had a deficit in nutrient absorption. In addition, there was a near doubling of liver weight in 4- and 12-month-old male control-HFD mice but none in α-Syn-HFD mice (Fig. 9f, g). The influence of HFD on liver weight was sex-selective in that only a modest increase was observed in female control-HFD mice (Fig. 9h). Since most of the mice with lower body weight died before we were able to assess fat mass, the difference in fat mass between the two 1-month male groups on HFD was not large and their liver weight showed only a modest contrast. Using a new cohort of 4-month-old males, we assessed body composition using quantitative MRI (qMRI) 17 weeks post high-fat diet treatment; the data confirm that the resistance to weight gain in α-Syn-HFD mice was due to reduced fat mass rather than reduced lean mass (Fig. 10). The high-fat diet did not significantly (P = 0.4679, control-HFD vs. control-RGD and P = 0.8759, α-Syn-HFD vs. α-Syn-RGD) affect the lean mass of either strain of mice. However, the average lean mass of control-HFD mice was modestly, albeit significantly (P = 0.0005), higher than that of α-Syn-HFD mice (Fig. 10).
Fat composition was measured by weighing, subcutaneous inguinal white adipose tissue (iWAT), gonadal white adipose tissue (gWAT), and brown adipose tissue (BAT) (a–d). The weights of organs other than liver were unaffected (e–h). The number of mice for each group is shown in parentheses. Statistical analysis for iWAT, gWAT, and BAT were performed using two-way ANOVA with Tukey’s multiple comparison test. Statistical analysis for organs was performed separately for the high-fat diet and regular diet sub-groups using unpaired t-tests. *P < 0.0444; **P < 0.0041; ***P < 0.0009, and ****P < 0.0001.
The high-fat diet did not affect significantly (P = 0.4679, control-HFD vs. control-RGD and P = 0.8759, α-Syn-HFD vs. α-Syn-RGD) the lean mass of either genotype. The number of mice for each group is shown in parentheses. Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparison test. * in Body weight plot, P = 0.0131; * in Fat mass plot, P = 0.0345; * in Lean mass plot, P = 0.0251; ** in Lean mass plot, P = 0.0080; ***P = 0.0005; ****P < 0.0001.
Evidence of altered insulin signaling in α-Syn mice on high-fat diet
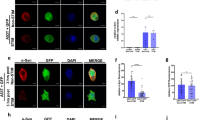
Since the olfactory system is a metabolic sensor of brain insulin and glucose levels, participating actively in the modulation of peripheral metabolism in response to both external and internal cues15,16, we assessed, in the 4-month male group, insulin signaling in the olfactory bulb using the Insulin/IGF-1 Signaling Pathway Antibody Kit (Cell Signaling Cat# 42022). Levels of Type I insulin-like growth factor receptor β (IGF-IRβ), IGF-IRβ phosphorylated at tyrosine 1131 (p IGF-IRβ), insulin receptor phosphorylated at tyrosine 972 (p IR), Akt phosphorylated at threonine 308 (p Akt), glycogen synthase kinase-3β (GSK-3β) phosphorylated at serine 9, and mechanistic target of rapamycin (mTOR) phosphorylated at serine 2448 were significantly (P = 0.0095, P = 0.0008, P = 0.0359, P = 0.0001, P = 0.0053, and P = 0.0006 for IGF-IRβ, p IGF-IRβ, p IR, p Akt, p GSK, and p mTOR, respectively) lower in α-Syn-HFD mice (Fig. 11). However, the diet did not alter total protein levels of IR, Akt, mTOR, and GSK in α-Syn or control mice (Fig. 11). It has been shown that induction of insulin signaling with IGF-1 suppresses α-synuclein aggregation and toxicity21. Accordingly, the decrease in IGF-IRβ was accompanied by an increase in α-synuclein levels in α-Syn-HFD mice (Fig. 11). GSK-3β and mTOR regulate synaptic plasticity by controlling synapse density and regulation of postsynaptic density protein 95 (PSD-95)21,22,23. As predicted, we observed a significant (P = 0.0293) decrease in the levels of PSD-95 in the olfactory bulb of α-Syn-HFD mice (Fig. 11). A decrease in PSD-95 can lead to an increase in presynaptic protein synaptophysin levels, considered to be a compensatory mechanism in deficient synaptic transmission24, which is proposed to be an early event in PD-related neurodegeneration. Therefore, we assessed synaptophysin levels in the olfactory bulb, the proposed induction/initial seeding site of α-synuclein pathology, and as expected the levels increased in α-Syn-HFD mice (Fig. 11).
Western blot analysis of regulators of insulin signaling and synaptic plasticity in the olfactory bulb of the 4-month male group. Statistical analysis was performed using unpaired t-tests, comparing α-Syn and control mice separately for each diet group. P = 0.0095 (IGF-IRβ), P = 0.0008 (p IGF-IRβ), P = 0.0359 (p IR), P = 0.0001 (p Akt), P = 0.0053 (p GSK), P = 0.0006 (p mTOR), P = 0.0293 (PSD-95), P = 0.0340 (synaptophysin), and P = 0.0001 (human α-synuclein).
Substrate utilization and energy expenditure in α-Syn mice
The 4-month male group was subjected to 48-h indirect calorimetry to calculate respiratory exchange ratio (RER) and energy expenditure. An RER of about 0.7 indicates that fats are being used as the main fuel for the body, whereas an RER of 1 indicates that carbohydrates are being used. An RER between 0.7 and 1 indicates a mix of fat and carbohydrates. The energy substrates of HFD groups were predominantly fat during both the light and dark (active) cycles, whereas RGD groups switched from predominantly fat during the light cycle to predominantly carbohydrates during the dark cycle (Fig. 12a). Interestingly, during the dark cycle, α-Syn-RGD mice tended to utilize substantially more carbohydrates than control-RGD mice. There was an effect of body weight on energy expenditure; consistent with resistance to weight gain, the energy expenditure in α-Syn-HFD mice was significantly higher (P = 0.0003), compared with control-HFD mice, when normalized to total body weight (Fig. 12b). Although a similar trend was observed when energy expenditure was normalized to lean mass (Fig. 12c), the difference was not significant (P = 0.2325), possibly because it was insufficiently powered statistically. However, the energy expenditure, normalized to lean mass, in α-Syn-HFD was significantly higher (P = 0.0433) than that of the regular chow diet group, indicating that the increased energy expenditure contributed to resistance to weight gain in α-Syn-HFD mice. To validate the increase in energy expenditure, we also measured mitochondrial uncoupling protein 1 (UCP1) and UCP2 mRNA levels in BAT. As expected, UCP1 (Fig. 12d) and UCP2 (Fig. 12e) mRNA levels were significantly (P = 0.0007 and P = 0.0011 for UCP1 and UCP2, respectively) higher in α-Syn-HFD mice than in control-HFD mice, with UCP2 mRNA levels increasing the most. In line with increased UCP1 and UCP2 levels, histological analysis revealed significantly (P = 0.0001) more brown adipocyte density (Fig. 12j) and strikingly fewer fat vacuoles (Fig. 12g) in the BAT tissue of a-Syn-HFD mice than in that of control-HFD mice.
Indirect calorimetry data showing 48-h respiratory exchange ratio (a). Energy expenditure, represented as heat, is normalized to both total body weight (b) and lean mass (c). Relative mRNA expressions of UCP1 (d) and UCP2 (e) in BAT. f–i Representative images of hematoxylin and eosin staining of frozen section of BAT from 4-month male group. Density of brown adipocytes (j) was assessed semi-quantitatively by manually counting the number of hematoxylin-stained nuclei within a 5 × 5 mm reticle, using a 10× objective lens. The number of mice for each group is shown in parentheses. Statistical analysis was performed using a mixed effects model (P < 0.0001), due to unequal sample sizes, for respiratory exchange ratio (a), and using two-way ANOVA with Tukey’s multiple comparison test, for histogram (b–e, j). **** in (d), P < 0.0001; * in (j), P = 0.0285; **in (j), P = 0.0098.
Discussion
We demonstrate here that α-Syn transgenic mice, when challenged with high-fat diet, clearly reveal a profile of robust metabolic dysfunction, indicating a relationship between abnormal α-synuclein accumulation and the dysregulation of energy homeostasis in this well-studied model of Parkinson’s disease (PD). α-Syn mice fed with high-fat diet could thus serve as models for understanding the metabolic dysfunction and associated weight loss that significantly impact the quality of life of PD patients.
Unintentional weight loss, primarily due to the loss of fat mass rather than muscle mass2, is common among patients with PD3,4,5,7,8, often predating the development of clinical PD by several years3. In line with these findings, dysregulation of glucose metabolism has been found to be a very prominent early manifestation of PD12,13. Despite their heavy toll on patients and caregivers, metabolic dysfunction, unintentional weight loss, and disproportionate fat loss in PD are not well understood, and their cause-and-effect relationship with α-synuclein pathology is unclear.
Based on available literature and the data presented here, we propose a hypothesis linking metabolic dysfunction and unintentional weight loss with olfactory dysfunction in PD. Sense of smell is often the first casualty of PD, predating the development of clinical PD by as much as 20 years19. Over 90% of PD patients exhibit olfactory dysfunction25 and there is strong evidence that α-synucleinopathy, one of the hallmarks of PD, first appears in the olfactory system17,18. Recent evidence shows that, in addition to sensing external chemical cues, the olfactory system is a metabolic sensor of brain insulin and glucose levels, playing a key role in controlling energy homeostasis in response to both sensory (external) and hormonal (internal) signals15,16. The exact mechanisms by which olfaction influences metabolism are unknown. Riera et al.26 recently demonstrated that upon inducing hyposmia (reduced olfaction) in mice, the animals become resistant to diet-induced obesity, and conversely, that enhancing their sense of smell leads to increased adiposity. The researchers further showed that the leaner phenotype associated with hyposmia was accompanied by increased lipolysis via upregulation of uncoupling protein 1 (UCP1)26. Consistent with this report, a recent study27 showed that female olfactory cues reduced body weight in males by enhancing fat burning via UCP1 upregulation. Lastly, in a human study, the use of a novel self-administered nasal device to reduce olfactory sensitivity (induced hyposmia) resulted in significant weight loss28. Whether it is food odor (main olfactory system26) or social odor (vomeronasal27), these reports corroborate the fact that olfaction plays a role in modulating body weight by regulating lipolysis.
A reciprocal communication exists between olfactory sensitivity and energy homeostasis, as sensitivity increases in a fasted state and decreases in a sated state16,26. This suggests that hyposmia/loss of smell (as seen in PD) could skew the brain’s homeostatic response towards a “satiety state-like” metabolic response, thus leading to catabolic energy utilization/lipolysis. This could partly explain unintentional weight loss in PD, as well as resistance to diet-induced weight gain in α-Syn mice, which also exhibit olfactory dyfunction and olfactory bulb α-synuclein pathology similar to that seen in PD patients29. Indeed, a significant correlation was found between olfaction and BMI in human PD patients, indicating that hyposmia, but not hypogeusia, contributes to weight loss in PD30. Further demonstrating a link between olfactory dysfunction and energy homeostasis, a recent 9-year population-based study showed that evolution of olfactory dysfunction increased the risk of weight loss in PD patients31. Although the data from the current study reveal that loss of fat mass, increased energy expenditure, and upregulation of thermogenic genes in BAT were accompanied by severe olfactory dysfunction, a limitation is that we could not analyze hypothalamic interoceptive neurons to assess whether severe olfactory dysfunction did indeed skew the brain’s homeostatic response towards a “satiety state-like” metabolic state in α-Syn-HFD mice. A reciprocal communication exists between olfactory sensitivity and energy homeostasis through the agouti-related protein (AgRP) and proopiomelanocortin (POMC) interoceptive neurons in the arcuate nucleus of the hypothalamus32,33,34. AgRP and POMC neurons have opposing functions: AgRP neurons are activated by energy deficit (fasted state), while POMC neurons are activated by energy surfeit (sated state). Olfactory detection of food in a hungry mouse has been shown to reset the activity of its AgRP and POMC neurons from a pattern associated with energy deficit to that associated with satiety, even if no food is consumed33. How olfactory signals are communicated to the AgRP and POMC neurons as well as which downstream molecular pathways are involved in the AgRP and POMC neuronal control of energy homeostasis are both unknown34.
In further support of a role for olfaction in PD-related metabolic dysfunction and weight loss, we provide evidence of altered insulin signaling in the olfactory bulb of α-Syn-HSD mice. Levels of IGF-IRβ, p IGF-IRβ, p IR, p Akt, p GSK, and p mTOR were significantly lower in α-Syn-HFD mice. However, total protein levels of these insulin signaling molecules, except IGF-IRβ, were not altered. Both GSK-3β and mTOR regulate several elements downstream of IGF-IRβ that are typically disrupted in PD, including apoptosis, autophagy, neuronal metabolism, protein synthesis, and synaptic plasticity21. Indeed, GSK-3β and mTOR signaling are altered in PD35,36. GSK-3β and mTOR regulate synaptic plasticity by controlling synapse density and regulation of postsynaptic density protein 95 (PSD-95)21,22,23. As anticipated, there was a significant decrease in the levels of PSD-95 in the olfactory bulbs of α-Syn-HFD mice, and this was accompanied by an increase in synaptophysin levels. A decrease in PSD-95 can lead to an increase in presynaptic protein synaptophysin levels, considered a compensatory mechanism in deficient synaptic transmission24, which is proposed to be an early event in PD-related neurodegeneration. Taken together, these data indicate that GSK-3β and mTOR are likely two common denominators that link metabolic dysfunction with weight loss and neurodegeneration, and thus offer a molecular basis for the association between weight loss and disease severity seen in PD patients10.
Several of our observations support the notion that resistance to high-fat diet-induced weight gain in α-Syn mice is due to enhanced fat-burning capacity and energy expenditure:
-
(1)
Body composition analysis using qMRI confirmed that the resistance to weight gain in α-Syn-HFD mice was due to reduced fat mass rather than reduced lean mass. Interestingly, weight loss in PD occurs primarily due to fat rather than muscle loss6.
-
(2)
Fat composition analysis by weighing BAT, iWAT, and gWAT at the end of the study not only confirmed the qMRI data but further revealed that the biggest reduction occurred for iWAT in 4- and 12-month-old male α-Syn-HFD mice, whereas both iWAT and gWAT were significantly reduced in female α-Syn-HFD mice, compared with control-HFD mice.
-
(3)
The resistance to weight gain and reduced fat mass in α-Syn-HFD mice was accompanied by a more rapid clearance of blood glucose during the intraperitoneal glucose tolerance test. Similar to the composition of fat mass, there was an effect of sex on the temporal dynamics of blood glucose clearance during the intraperitoneal glucose tolerance test. Although the 4-month-old male α-Syn-HFD mice had better glucose tolerance than age-matched control-HFD mice, the glucose levels of the male α-Syn-HFD mice did not return to those of the regular diet group at 120 min. However, the glucose levels of the equivalent female mice did return to the levels of the equivalent regular diet group at this time. Although the exact reason for this sex difference is unclear, it probably reflects the difference in the composition of fat mass in male vs. female α-Syn-HFD mice. Most men accumulate excess calories in visceral adipose tissue (VAT), whereas women in general equivalently expand VAT and subcutaneous adipose tissue (SAT) in response to over-nutrition37,38. SAT produces more leptin and is less inflamed and less insulin-resistant than VAT, likely explaining why generally women are more insulin sensitive. Interestingly, along with the improved glucose tolerance, subcutaneous iWAT was the largest in female α-Syn-HFD mice, as opposed to visceral gonadal WAT (gWAT) in age-matched males.
-
(4)
High-fat diet typically leads to a dramatic increase in fat accumulation in hepatocytes; accordingly, there was a near doubling of liver weight in 4- and 12-month-old male control-HFD mice, and this increase in liver weight was absent in α-Syn-HFD mice; a similar effect was also seen in the 1-month-old male and 4-month-old female mice, albeit to a lesser degree. Hepatocytes accumulate fat when the cellular input of fatty acids via either ingestion (here high-fat diet) or hepatic lipogenesis surpasses fatty acid output via oxidation or export39. Although the α-Syn-HFD mice were protected against the increase in liver weight, the α-Syn-HFD groups had a relatively higher fat mass, compared with regular diet chow groups. This, along with the fact that ingestion of high-fat diet chow was similar in control and α-Syn mice, indicates that a combination of fatty acid oxidation and export prevented hepatic fat accumulation and thus the increase in liver weight in α-Syn-HFD mice.
-
(5)
Lastly, calorimetry data revealed that energy expenditure normalized to body weight was significantly higher in α-Syn-HFD mice than in control-HFD mice. However, the increase in energy expenditure in α-Syn-HFD mice did not reach statistical significance when normalized to lean mass. It is noteworthy that energy expenditure is influenced by physical activity levels. Since our Oxymax system was not equipped for concomitant measurement of activity levels, the effect of activity on energy expenditure could not be determined. Despite this limitation, the fact that energy expenditure normalized to lean mass in α-Syn-HFD was significantly higher than that of the regular chow diet group indicates that the increased energy expenditure contributed to resistance to weight gain in α-Syn-HFD mice. In support of this notion, there was an increase in UCP1 and UCP2 mRNA levels, and strikingly fewer fat vacuoles in the BAT of α-Syn-HFD mice than in that of control-HFD mice. We ruled out the possibility of potential influence of tremor on energy expenditure given that: (1) tremor is not documented in α-Syn mice. (2) Based on our preliminary observation, high-fat diet treatment does not lead to onset of tremor in α-Syn mice. (3) The lower fat mass in α-Syn mice was associated with lower body weight and, as shown in Fig. 1, α-Syn mice were consistently leaner starting at 1 month of age, when they exhibit no motor deficits.
Although the α-Syn-HFD mice exhibited a leaner phenotype and better glucose tolerance, the death rate in male α-Syn-HFD was much higher in all age groups. The exact reason for this increased mortality is unclear. However, a survey of clinical studies indicates an association among olfactory dysfunction, weight loss, disease severity/progression, and mortality in PD patients that may be important here: (1) An association between weight loss and increased risk of death has been reported in PD40. (2) Severe hyposmia is one of the early predictors of mortality in PD41. (3) A significant correlation was found between olfaction and BMI in PD30. (4) The severity of olfactory dysfunction in PD increases with disease progression42,43. (5) A recent 9-year population-based study showed that evolution of olfactory dysfunction increased the risk of weight loss in PD patients31. (6) Underscoring a relationship between weight loss and disease severity, longitudinal data from 1673 participants in NINDS Exploratory Trials in Parkinson’s Disease Long-term Study 1 showed that patients with a decreasing body mass index (BMI) had higher motor and total Unified Parkinson’s Disease Rating Scale (UPDRS) scores than patients with a stable BMI (despite optimal treatment), whereas those with an increasing BMI had lower scores (than patients with a stable BMI)10. (7) One can see why an “olfaction-weight-dyskinesia” phenotype has been proposed to exist in PD5. Taking these findings together, olfactory dysfunction may serve as a “canary in a coal mine,” heralding weight loss, faster disease progression, and increased risk of death. Based on this possibility, we reasoned that male α-Syn mice on high-fat diet should exhibit increased olfactory and motor dysfunctions, along with increased mortality, and, conversely, that females should exhibit either milder or no olfactory/motor dysfunctions, as increased mortality is not associated with α-Syn-HFD females. As expected, high-fat diet significantly exacerbated olfactory and motor dysfunctions in α-Syn males but not in females.
Unintentional weight loss in PD is primarily due to fat loss2; similarly, our body composition analysis indicates that resistance to weight gain in α-Syn mice is due to loss of fat mass. It is possible that the fat loss is the direct consequence of increased energy expenditure. Interestingly, PD patients tend to increase their energy intake as their weights decrease3, hinting at increased energy expenditure as seen in SNCA mice. Increased energy expenditure may also lead to dysregulation in the neurometabolic coupling that ensures that the brain’s high energy demands are met during sensory, motor, and cognitive function. Dysfunctional neurometabolic coupling could lead to a cellular energetics deficit that aggravates oxidative stress, neuroinflammation, and neurodegeneration, ultimately increasing risk of death. Although our study does not address this, it is interesting to note that an altered neurometabolic profile has been reported for PD44. This could ultimately help explain why α-Syn mice on high-fat diet, as well as PD patients with severe hyposmia, have a shorter lifespan.
A related question on the increased mortality in α-Syn mice is whether it is associated specifically with a high-fat diet. To our knowledge, there are no previous reports of α-Syn mice on any diet aside from regular chow diet. The impact of high-sugar diet appears to be substantially different from that of high-fat diet, with pronounced sex-specific differences. For example, a 10-week high-fat diet led to weight gain in both sexes of C57BL6/J mice (same genetic background as α-Syn mice), while a high-sugar diet did not affect weight in females but decreased weight gain in males45. High-sugar diet in wild-derived mice has been reported to decrease the survival of females and the competitive ability of males46. Since weight loss in PD has prognostic significance (see above), and targeted dietary interventions to prevent weight loss has the potential to improve long-term outcomes40, future studies on the impact of various diet types in α-Syn mice are warranted.
Male α-Syn (Line 61) mice on regular chow diet have previously been reported to be relatively leaner with no significant change in glucose or insulin response in IPGTT, compared with wild-type male controls11, suggesting a modest metabolic alteration in α-Syn mice. The data from the current study not only corroborate the previous report11 but further show that high-fat diet is an effective way to enhance mild metabolic phenotypes in α-Syn mice in order to elucidate the relationship between pathological α-synuclein aggregation and the dysregulation of energy homeostasis that leads to weight loss and accelerated disease progression in PD. To this end, we provide a novel hypothesis linking metabolic dysfunction in PD with olfactory dysfunction.
Methods
Mice
Transgenic mice overexpressing human wild-type α-synuclein under the direction of a mouse Thy-1 promoter (mThy1-hSNCA; Line 61)47 in C57BL/6J background and non-transgenic littermate controls, both male and female, were used. The mice were housed in standard plexiglass cages with 7099 TEK-Fresh animal bedding (Envigo, Indianapolis, IN) and were fed either a high-fat diet chow (60% fat calories; Cat # D12492; Research Diets, New Brunswick, NJ) or a standard Teklad irradiated LM-485 mouse diet (Cat # 7912; Envigo, Indianapolis, IN) ad libitum and with full-time access to acidified drinking water. The mice were maintained in a 12/12 h light/dark cycle at 24 °C room temperature and 50-55% humidity. Animal husbandry was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Society for Neuroscience Policies on the Use of Animals and Humans in Research. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the South Texas Veterans Health Care System, Audie L. Murphy Division, and the University of Texas Health Science Center, San Antonio. All live animal testing was performed during the light cycle with 30-40 lux light intensity maintained throughout the tests. The smallest possible number of mice was used (based on power calculations) and all efforts were made to minimize suffering. All experiments were randomized and performed blind-coded whenever feasible, although for all live animal testing weight gain made it obvious to the experimenter which animals was fed with high-fat diet chow.
Body weight
Body weight was measured between 10 AM and noon once a week. For the purpose of clarity, cumulative changes in body weight are represented in Fig. 2.
Food and water intake
Twenty-four-hour food intake was measured manually as the difference between the pelleted chow put into the cage and that remaining at the end of 24 h. Measurements were taken between 5 PM and 6 PM, and the chow crumbs that had fallen into the bedding were accounted for. Water intake was measured by weighing the water bottle.
Intraperitoneal glucose tolerance test
Mice were fasted for 5 h and then their tails were bled to measure fasting glucose concentrations using a glucometer (Contour next EZ). Immediately after measuring fasting glucose concentrations, the mice were injected, intraperitoneally, with 500 μl of 10% Dextrose USP (B. Braun Medical Inc., Bethlehem, PA) and blood glucose concentrations were determined 15, 30, 60, and 120 min later.
Indirect calorimetry
Indirect calorimetry studies were performed using an eight-chamber Oxymax-Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) at the Integrative Physiology and Aging Core of the San Antonio Nathan Shock Center, UTHSCSA. The mice were acclimated to the system for 24 h prior to collecting various measurements of indirect calorimetry for 48 h. Food and water were provided ad libitum.
Test for general anosmia
General anosmia was assessed by the buried food retrieval paradigm using our previously published protocol48. After a 3-day acclimation and confirmation of the palatability of a sweetened cereal pellet (Cap’n Crunch; Quaker Oats Company), overnight fasted mice were placed in a testing cage in which the sweetened cereal pellet was buried 0.5 cm below the bedding so that it was not visible. The testing cage consisted of a clean mouse cage that was filled ~3 cm with fresh bedding. The retrieval time was recorded from the instant the mouse was released in the center of the testing cage until the cereal pellet was found (maximum 10 min). Retrieval time for an unscented glass marble and time to find an exposed (vs. hidden) cereal pellet were determined similarly to assess any potential confounding of the buried cereal test by deficits in motor function or anxiety-related excessive digging behaviors (data not shown). Any animal exhibiting excessive digging or sedentary behavior was excluded from the analysis.
Test for hindlimb extension reflex
Extension reflex of the hindlimb was tested by suspending the mice by tail in midair and observing the pattern of hindlimb extension reflex for about 5 s. The pattern was scored as follows: absence of hindlimb extension or the mouse gripping its tail = 0; extension of only one hindlimb or extension of both hindlimbs without splayed toes = 1; extension of both hindlimbs with splaying of toes (the normal response) = 248,49.
Body composition
Body composition was assessed by quantitative MRI (EchoMRI LLC, Houston, TX), and total fat mass, lean mass, and free water were calculated. At the end of the study the mice were euthanized and BAT, iWAT and gWAT, liver, spleen, heart, and kidneys were carefully dissected out and weighed.
Immunoblotting
Following euthanasia by CO2 inhalation, the mice were decapitated. The olfactory bulbs were quickly dissected out and snap frozen in liquid nitrogen; the tissues were stored at -80°C temporarily and then processed for protein extraction. The olfactory bulbs were homogenized in protein extraction buffer prepared in ultrapure water (50 mM HEPES, 10% v/v glycerol, 1 mM EDTA, 10 mM NaF, 1 mM activated Na3VO4, 150 mM NaCl, 1% v/v Triton X 100, 0.1% w/v SDS), supplemented with cOmplete Mini EDTA-free Protease Inhibitor Cocktail tablets (cat # 11836170001; Sigma-Aldrich, Inc. St. Louis, MO) and PhosSTOP mini tablets (cat #4906837001; Sigma-Aldrich). Protein concentrations were determined through Bradford assay using Pierce BCA Protein Assay Kit (cat # 23227; ThermoFisher Scientific, Waltham, MA), measuring absorbance at 540 nm. Samples (20 mg) were loaded on Invitrogen Novex 12% Tris-Glycine Plus Midi Gels (cat # WXP01226BOX; ThermoFisher Scientific) and ran for 50 min at 150 volts, and were then transferred onto nitrocellulose membranes at 4 °C (25 volts for 60 min). The membranes were stained with Ponceau S to confirm equal loading. The membranes were blocked with 5% nonfat dried milk in 1X TBST buffer and then incubated overnight with one of the following antibodies, prepared in 5% nonfat dried milk, at 4 °C: (1) Insulin/IGF-1 Signaling Pathway Antibody Kit (cat# 42022; Cell Signaling Technology, Danvers, MA), (2) insulin receptor phosphorylated at tyrosine 972 (Cat # 44-800G; Invitrogen/ThermoFisher Scientific), (3) human α-synuclein (cat # ab138501; abcam, Waltham, MA), (4) PSD-95 (cat # ab18258; abcam), (5) Synaptophysin (cat # S5768; Sigma-Aldrich), or (6) β-actin (JLA20; cat # AB_528068; DSHB, University of Iowa). The bands were visualized using Pierce ECL Western Blotting Substrate (Cat # 32209; ThermoFisher Scientific). Optical density measurements of the bands were performed using NIH ImageJ software, and the optical density values were normalized to that of β-actin.
Reverse transcription-quantitative real-time PCR (RT-qPCR) analysis
RNA from snap-frozen BATs was isolated using TRIzol/chloroform extraction and RNeasy Mini Kit (cat# 74106; QIAGEN Science, Germantown, MD). RNA was converted into cDNA using the SuperScript VILO cDNA Synthesis Kit (cat# 11754250; Invitrogen/ThermoFisher Scientific) and qPCR was performed using pre-designed TaqMan probes (ThermoFisher Scientific) for UCP1 (Mm00494069_m1), UCP2 (Mm00627599_m1) and GAPDH (Mm99999915_g1) on QuantStudio 3 (ThermoFisher Scientific). Transcriptional levels were determined using ∆∆Ct method and mRNA fold change relative to control-RGD was calculated.
Histology
BATs, fixed in 4% paraformaldehyde, were cryoprotected in 30% sucrose solution, and then embedded in Tissue-Tek O.C.T. for cryosectioning at 15 μm thickness. These sections were stained with hematoxylin and eosin using standard methods and then imaged with a Keyence BZ-X800 microscope. Semi-quantitative assessment of the number of brown adipocytes was performed by manually counting the number of hematoxylin-stained nuclei within a 10 × 10 mm reticle, using a 10× objective lens.
Statistics
Statistical analyses of the data were performed in R version 3+ (Vienna, Austria) or GraphPad Prism 10.2.3 (GraphPad Software, Inc.). As specified in the legend for each figure, the following statistical tests were used: independent unpaired t-tests, two-way and three-way repeated measures ANOVA, two-way ANOVA with Tukey’s multiple comparison test, mixed effects model, and Log-rank (Mantel-Cox). All sets of continuous data were tested for normality using the Shapiro-Wilk test, and fewer than 5% of the tests concluded that the set was non-normal at the 0.05 significance level, confirming that the data sets met the assumption of a normal distribution. The hindlimb reflex scores are discrete integer values from 0 to 2, which does not satisfy normality. For these non-Gaussian measures, we used Mann-Whitney test. Results were expressed as mean ± SEM, and differences in mean were considered significant at P < 0.05.
Data availability
All raw data and any additional information on methodology or data analyses used in the current study will be available upon request to the corresponding authors.
References
Zis, P., Erro, R., Walton, C. C., Sauerbier, A. & Chaudhuri, K. R. The range and nature of non-motor symptoms in drug-naive Parkinson’s disease patients: a state-of-the-art systematic review. NPJ Parkinsons Dis. 1, 15013 (2015).
Markus, H. S., Tomkins, A. M. & Stern, G. M. Increased prevalence of undernutrition in Parkinson’s disease and its relationship to clinical disease parameters. J. Neural Transm. Park. Dis. Dement. Sect. 5, 117–125 (1993).
Chen, H., Zhang, S. M., Hernan, M. A., Willett, W. C. & Ascherio, A. Weight loss in Parkinson’s disease. Ann. Neurol. 53, 676–679 (2003).
Cheshire, W. P. Jr. & Wszolek, Z. K. Body mass index is reduced early in Parkinson’s disease. Parkinsonism Relat. Disord. 11, 35–38 (2005).
Sharma, J. C. & Lewis, A. Weight in Parkinson’s disease: phenotypical significance. Int. Rev. Neurobiol. 134, 891–919 (2017).
Tan, A. H. et al. Altered body composition, sarcopenia, frailty, and their clinico-biological correlates, in Parkinson’s disease. Parkinsonism Relat. Disord. 56, 58–64 (2018).
Uc, E. Y. et al. Predictors of weight loss in Parkinson’s disease. Mov. Disord. 21, 930–936 (2006).
van der Marck, M. A. et al. Body mass index in Parkinson’s disease: a meta-analysis. Parkinsonism Relat. Disord. 18, 263–267 (2012).
Logroscino, G., Sesso, H. D., Paffenbarger, R. S. Jr. & Lee, I. M. Body mass index and risk of Parkinson’s disease: a prospective cohort study. Am. J. Epidemiol. 166, 1186–1190 (2007).
Wills, A. M. et al. Association between change in body mass index, unified Parkinson’s disease rating scale scores, and survival among persons with Parkinson disease: secondary analysis of longitudinal data from NINDS Exploratory Trials in Parkinson Disease Long-term Study 1. JAMA Neurol. 73, 321–328 (2016).
Cuvelier, E. et al. Overexpression of wild-type human alpha-Synuclein causes metabolism abnormalities in Thy1-aSYN transgenic mice. Front. Mol. Neurosci. 11, 321 (2018).
Borghammer, P. et al. Cortical hypometabolism and hypoperfusion in Parkinson’s disease is extensive: probably even at early disease stages. Brain Struct. Funct. 214, 303–317 (2010).
Dunn, L. et al. Dysregulation of glucose metabolism is an early event in sporadic Parkinson’s disease. Neurobiol. Aging 35, 1111–1115 (2014).
Pagano, G. et al. Diabetes mellitus and Parkinson disease. Neurology 90, e1654–e1662 (2018).
Garrison, J. L. & Knight, Z. A. Linking smell to metabolism and aging. Science 358, 718–719 (2017).
Tucker, K. et al. The olfactory bulb: a metabolic sensor of brain insulin and glucose concentrations via a voltage-gated potassium channel. Results Probl. Cell Differ. 52, 147–157 (2010).
Adler, C. H. & Beach, T. G. Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov. Disord. 31, 1114–1119 (2016).
Beach, T. G. et al. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 117, 169–174 (2009).
Fereshtehnejad, S. M. et al. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: a prospective study. Brain 142, 2051–2067 (2019).
Ramirez, I. & Fuller, J. L. Genetic influence on water and sweetened water consumption in mice. Physiol. Behav. 16, 163–168 (1976).
Athauda, D. & Foltynie, T. Insulin resistance and Parkinson’s disease: a new target for disease modification?. Prog. Neurobiol. 145-146, 98–120 (2016).
Collingridge, G. L., Peineau, S., Howland, J. G. & Wang, Y. T. Long-term depression in the CNS. Nat. Rev. Neurosci. 11, 459–473 (2010).
Nelson, C. D., Kim, M. J., Hsin, H., Chen, Y. & Sheng, M. Phosphorylation of threonine-19 of PSD-95 by GSK-3beta is required for PSD-95 mobilization and long-term depression. J. Neurosci. 33, 12122–12135 (2013).
Vaz, A. R. et al. Overexpression of miR-124 in motor neurons plays a key role in ALS pathological processes. Int. J. Mol. Sci. 22, 6128 (2021).
Wu, Y., Le, W. & Jankovic, J. Preclinical biomarkers of Parkinson disease. Arch. Neurol. 68, 22–30 (2011).
Riera, C. E. et al. The sense of smell impacts metabolic health and obesity. Cell Metab. 26, 198–211.e5 (2017).
Garratt, M. et al. Sensory detection of female olfactory cues as a central regulator of energy metabolism and body weight in male mice. iScience 26, 106455 (2023).
Dicker, D. et al. Weight loss, dietary preferences, and reduction in the sense of smell with the use of a novel nasal device. Obes. Facts 13, 473–486 (2020).
Chesselet, M. F. & Richter, F. Modelling of Parkinson’s disease in mice. Lancet Neurol. 10, 1108–1118 (2011).
Roos, D. S., Oranje, O. J. M., Freriksen, A. F. D., Berendse, H. W. & Boesveldt, S. Flavor perception and the risk of malnutrition in patients with Parkinson’s disease. J. Neural Transm.125, 925–930 (2018).
Kristiansen, I., Hiorth, Y. H., Ushakova, A., Tysnes, O. B. & Alves, G. Risk factors and evolution of weight loss in Parkinson’s disease: a 9-year population-based study. Parkinsonism Relat. Disord. 129, 107181 (2024).
Betley, J. N. et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015).
Chen, Y., Lin, Y.-C., Kuo, T.-W. & Knight, Z. A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015).
Jovanovic, P. & Riera, C. E. Olfactory system and energy metabolism: a two-way street. Trends Endocrinol. Metab. 33, 281–291 (2022).
Procaccini, C. et al. Role of metabolism in neurodegenerative disorders. Metabolism 65, 1376–1390 (2016).
Wills, J. et al. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp. Neurol. 225, 210–218 (2010).
Jensen, M. D. Gender differences in regional fatty acid metabolism before and after meal ingestion. J. Clin. Investig. 96, 2297–2303 (1995).
SantaCruz-Calvo, S. et al. Adaptive immune cells shape obesity-associated type 2 diabetes mellitus and less prominent comorbidities. Nat. Rev. Endocrinol. 18, 23–42 (2022).
Lian, C. Y., Zhai, Z. Z., Li, Z. F. & Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem. Biol. Interact. 330, 109199 (2020).
Cumming, K., Macleod, A. D., Myint, P. K. & Counsell, C. E. Early weight loss in parkinsonism predicts poor outcomes: evidence from an incident cohort study. Neurology 89, 2254–2261 (2017).
Backstrom, D. et al. Early predictors of mortality in Parkinsonism and Parkinson disease: a population-based study. Neurology 91, e2045–e2056 (2018).
Berendse, H. W., Roos, D. S., Raijmakers, P. & Doty, R. L. Motor and non-motor correlates of olfactory dysfunction in Parkinson’s disease. J. Neurol. Sci. 310, 21–24 (2011).
Roos, D. S., Twisk, J. W. R., Raijmakers, P., Doty, R. L. & Berendse, H. W. Hyposmia as a marker of (non-)motor disease severity in Parkinson’s disease. J. Neural Transm.126, 1471–1478 (2019).
Klietz, M. et al. Altered neurometabolic profile in early Parkinson’s disease: a study with short echo-time whole brain MR spectroscopic imaging. Front. Neurol. 10, 777 (2019).
Church, J. S., Renzelman, M. L. & Schwartzer, J. J. Ten-week high fat and high sugar diets in mice alter gut-brain axis cytokines in a sex-dependent manner. J. Nutr. Biochem. 100, 108903 (2022).
Ruff, J. S. et al. Human-relevant levels of added sugar consumption increase female mortality and lower male fitness in mice. Nat. Commun. 4, 2245 (2013).
Rockenstein, E. et al. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J. Neurosci. Res. 68, 568–578 (2002).
Biju, K. C., Shen, Q., Hernandez, E. T., Mader, M. J. & Clark, R. A. Reduced cerebral blood flow in an alpha-synuclein transgenic mouse model of Parkinson’s disease. J. Cereb. Blood Flow Metab. 40, 2441–2453 (2020).
Jaworski, D. M., Soloway, P., Caterina, J. & Falls, W. A. Tissue inhibitor of metalloproteinase-2(TIMP-2)-deficient mice display motor deficits. J. Neurobiol. 66, 82–94 (2006).
Acknowledgements
This work was supported by a Merit Review Grant from the Veterans Health Administration (1 I01 BX 003157) and a grant from the William and Ella Owens Medical Research Foundation, both awarded to R.A.C., as well as pilot and career development grants from the Perry & Ruby Stevens Parkinson’s Disease Center of Excellence, awarded to K.C.B. R.A.C. is supported by the NIH-funded Clinical and Translational Science Award (UM1-TR004538). We thank Dr. Randy Strong at UTHSCSA for providing the original breeding pairs of transgenic mice overexpressing human wild-type α-synuclein (Line 61). The calorimetry and qMRI analyses were performed at the Integrated Physiology and Aging Core of the NIH-funded San Antonio Nathan Shock Center (P30-AG013319), UTHSCSA.
Author information
Authors and Affiliations
Contributions
R.A.C. and K.C.B. conceived and designed the study. L.N. provided expertise on the design and interpretation of all metabolic analyses. E.T.H., A.M.S., and A.C.F.-O. performed the behavioral tests. A.M.S. and S.K.H. analyzed the immunoblot, qMRI, calorimetry, and body weight data. M.J.M. and E.T.H. designed and performed the statistical analysis. All authors were involved in the overall data analysis. K.C.B. and R.A.C. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Biju, K.C., Torres Hernandez, E., Stallings, A.M. et al. Metabolic dysregulation and resistance to high-fat diet-induced weight gain in mice overexpressing human wild-type α-synuclein. npj Parkinsons Dis. 11, 90 (2025). https://doi.org/10.1038/s41531-025-00961-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-00961-w