Abstract
Emerging evidence highlights the importance of gastrointestinal (GI) dysfunction in Parkinson’s disease (PD). While inflammatory bowel disease (IBD) has been linked to PD, the association with other GI conditions remains unclear. This study analyzed data from 501,483 participants, including 907 PD cases. Cox models revealed that digestive diseases were significantly associated with an increased PD risk (HR = 1.43). Eleven digestive diseases were linked to PD, with lifestyle factors such as sleep patterns and diet reducing PD risk. Notably, interactions between Non-alcoholic Fatty Liver Disease (NAFLD) and sleep (P-int = 0.0119), and Crohn’s disease and dietary diversity (P-int = 0.0081) were observed. Population attributable fraction (PAF) analysis indicated that eliminating exposure to gastroesophageal reflux disease (GERD), gastritis and duodenitis, and gallbladder and biliary diseases could reduce PD cases by approximately 8.9%, 7.9%, and 3.8%, respectively. These findings emphasize the role of digestive diseases and lifestyle in PD risk.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) ranks as the second most prevalent neurodegenerative disorder globally, marked by a progressive decline in both motor and nonmotor functions, which profoundly affects patients’ quality of life1. Since 19902,3, the global prevalence, disability, and mortality rates associated with PD have risen rapidly, highlighting its significance as a major public health issue. In light of these troubling trends, it is crucial to identify modifiable risk factors and to develop effective prevention strategies aimed at reducing the impact of this disease.
Nonmotor symptoms, particularly gastrointestinal issues such as constipation and dysphagia4, can manifest 10–20 years before PD onset5. Furthermore, digestive diseases may act as early indicators of broader health concerns, influenced by factors such as lifestyle choices, immune regulation6, and various systemic conditions. These digestive disorders can disrupt GI motility and alter the gut microbiome, which may interact with the gut–brain axis7. Moreover, the GI tract could play a significant role in the pathogenesis of PD8. Studies show that when α-synuclein aggregates in the enteric nervous system, it can spread to the central nervous system through structures like the dorsal motor nucleus of the vagus (DMV)9. In addition, gut diseases such as inflammatory bowel disease (IBD) trigger systemic inflammation, which allows inflammatory factors to enter the brain, activate microglia, interfere with neuronal function, and damage nerve cells10. While certain conditions, including gastroesophageal reflux disease (GERD)11, Crohn’s disease12, ulcerative colitis13, and irritable bowel syndrome (IBS)14, have documented associations with PD, the influence of other digestive diseases—such as gastritis, celiac disease, peptic ulcers, pancreatitis, gallbladder and biliary diseases, nonalcoholic fatty liver disease (NAFLD), chronic cirrhosis, appendicitis, and gastrointestinal cancers—on PD risk remains largely unclear. Moreover, the extent to which each of these diseases contributes to PD risk, as indicated by population-attributable fraction (PAF) weights, has yet to be established. Given the diverse array of digestive diseases, significant clarification is still needed regarding their association with PD risk. To inform clinical treatment and preventive strategies, well-designed prospective cohort studies are crucial for validating these associations and generating robust evidence concerning the impact of digestive diseases on PD risk.
In this study, we utilized the extensive sample size and comprehensive covariate data from the UK Biobank to perform a prospective analysis examining the association between 14 digestive diseases and PD risk. Our primary innovation involves a thorough evaluation of how multiple digestive diseases affect PD incidence, as well as the calculation of the PAF, which quantifies the proportion of PD cases that could be theoretically prevented by eliminating specific digestive diseases. We also examined the combined effects of digestive diseases and lifestyle factors—specifically, sleep patterns and dietary diversity —on the risk of developing PD. To delve into potential mechanisms, we categorized conditions such as gastritis, duodenitis, pancreatitis, and appendicitis into acute and chronic subgroups. These findings offer valuable insights for the development of targeted prevention and treatment strategies.
Results
Characteristics of study participants
During a median follow-up of 12.6 years (interquartile range = 11.7–13.2), 2906 cases of PD were identified. Table 1 outlines the baseline characteristics of participants who developed PD compared to those who did not. Patients with PD were more likely to be older, male, and have higher body mass index, lower educational attainment, recent histories of smoking and drinking, lower levels of physical activity, higher blood pressure, and a family history of PD.
Association between digestive diseases and PD
When examining the combined impact of multiple digestive diseases (Fig. 1), the presence of any digestive disease was associated with an elevated risk of developing PD compared to participants without digestive conditions [hazard ratio (HR) = 1.43; 95% confidence interval (CI) = 1.33, 1.54; adjusted P < 0.001)]. Additionally, there was a positive association between the number of digestive diseases present at baseline and PD risk, with each additional digestive disease increasing PD risk (HR per additional disease = 1.17; 95% CI = 1.13, 1.20; adjusted P < 0.001).
In the multivariable Cox proportional hazards model (Fig. 2), after adjusting for potential confounders, the digestive diseases with the highest PD risk were celiac disease (HR = 2.02, 95% CI = 1.49, 2.74; adjusted P < 0.001), Crohn’s disease (HR = 1.77, 95% CI = 1.25, 2.51; adjusted P = 0.003), and NAFLD (HR = 1.77, 95% CI = 1.43, 2.19; adjusted P < 0.001). Although no significant association was found between appendicitis and PD in the multivariable Cox model, a significant positive association was observed when classifying appendicitis as acute, chronic, or unspecified. Specifically, chronic appendicitis was linked to an increased risk of PD (HR = 4.71; 95% CI = 1.77, 12.55; P = 0.002), whereas acute and unspecified appendicitis did not show a significant impact on PD risk.
Further analyses of gastritis indicated that chronic gastritis was significantly associated with PD (HR = 1.29; 95% CI = 1.03–1.61; P = 0.026). Other forms of gastritis also demonstrated a significant association with PD (HR = 1.32; 95% CI = 1.19, 1.47; P < 0.001). Additionally, the analysis of pancreatitis revealed that acute pancreatitis was significantly linked to PD, with an HR of 1.44 (95% CI = 1.08, 1.93; P = 0.014) (Table 2).
PAF of PD for digestive diseases
The PAF analysis indicated that the three digestive diseases with the most substantial potential impact on PD incidence were as follows: eliminating GERD could lead to an estimated 8.9% reduction in PD cases. Additionally, the removal of gastritis and duodenitis from the population could result in a 7.9% decrease in PD cases, while addressing gallbladder and biliary diseases could contribute to a 3.8% reduction in PD incidence (Fig. 3).
x-Axis (PAF value %): Shows the percentage of Parkinson’s disease cases that could potentially be prevented if these digestive diseases were eliminated. y-Aaxis (Disease): Lists the 11 digestive diseases significantly associated with Parkinson’s disease (gastroesophageal reflux disease (GERD), gastritis and duodenitis, celiac disease, Crohn’s disease, ulcerative colitis, irritable bowel syndrome (IBS), peptic ulcer, pancreatitis, gall-bladder and biliary diseases, nonalcoholic fatty liver dis-ease (NAFLD), chronic liver cirrhosis).
Subgroup analysis
In the subgroup analysis (Table 3, Supplementary Table 6), which was stratified by sex, race, alcohol consumption, education level, smoking status, and hypertension, the results remained largely consistent. When stratified by race (P-int = 0.0006) and educational attainment (P-int = 0.0086), a significant association was observed in the peptic ulcer group. Among participants with peptic ulcer onset, those of nonwhite ethnicity exhibited a heightened risk of PD (HR = 2.96; 95% CI = 1.86, 4.70; P < 0.001), suggesting a synergistic effect of peptic ulcer onset and nonwhite ethnicity on the incidence of PD. Furthermore, when stratified according to hypertension levels, ulcerative colitis (P-int = 0.0505) and IBS (P-int = =0.0060) demonstrated significant interactions regarding their impact on PD risk.
Combined impact of digestive diseases and lifestyle factors on PD
Lifestyle factors, such as sleep patterns and dietary diversity, are significantly associated with PD (Supplementary Table 4). Participants who maintain ideal sleep patterns and enjoy high dietary diversity demonstrate a reduced risk of developing PD in most scenarios. Figure 4 depicts the combined impact of digestive diseases and lifestyle factors on PD risk, using participants without digestive diseases and those adhering to ideal lifestyle choices as the reference group. In many subgroups, digestive diseases significantly influenced PD risk when considered alongside sleep patterns and dietary habits (Supplementary Fig. 2).
Notably, significant interactions were identified between NAFLD and sleep quality (P-int = 0.0119), between celiac disease and dietary diversity (P-int = 0.0410), and between Crohn’s disease and dietary diversity (P-int = 0.0081) during PD onset (Fig. 4). Specifically, participants with NAFLD who also experienced poor sleep quality faced an elevated risk of PD (HR = 2.60; 95% CI = 1.96, 3.44; adjusted P < 0.001). In contrast, those with celiac disease and inadequate dietary diversity exhibited an even higher risk (HR = 5.32; 95% CI = 2.62, 10.82; adjusted P < 0.001). The combined HR for the concurrence of poor sleep quality and NAFLD, as well as poor dietary diversity, exceeded the sum of the individual HRs associated with these factors. However, when stratifying the data by the onset of Crohn’s disease and poor dietary diversity, the influence on PD risk was not statistically significant (adjusted P = 0.880).
Sensitivity analysis
The competing risk model (Supplementary Table 5) revealed a persistent significant linear association between ten digestive diseases and PD. After excluding participants who developed PD within the first 2 years of follow-up, the results mirrored those of the original analysis. Furthermore, when the analysis was limited to participants over the age of 55 years at recruitment, the significant linear association between the 10 digestive diseases and PD remained intact (Supplementary Table 5). Considering the routine prescription of proton pump inhibitors and nonsteroidal anti-inflammatory drugs among patients with digestive diseases, both of which are known to be associated with PD, we adjusted for nonsteroidal anti-inflammatory drug and proton pump inhibitor usage in our analysis. Even after these adjustments, a significant linear association between the ten digestive diseases and PD persisted for both medications.
Discussion
In this large-scale prospective cohort study, we identified a longitudinal causal association between various digestive system diseases and the risk of PD. Our findings revealed that conditions such as celiac disease, Crohn’s disease, NAFLD, cirrhosis, ulcerative colitis, GERD, IBS, pancreatitis, gallbladder disease, gastritis and duodenitis, and peptic ulcers were significantly associated with an increased risk of developing PD. In contrast, we found no significant associations for intestinal diverticular disease, appendicitis, or overall gastrointestinal cancer. The PAF analysis indicated that eliminating GERD, gastritis and duodenitis, and gallbladder and biliary diseases could substantially lower the incidence of PD, positioning these conditions as key contributors to PD risk.
Beyond digestive diseases, we also examined the combined effects of lifestyle factors and digestive diseases on PD risk. Our analysis demonstrated that these lifestyle factors—such as sleep patterns and dietary diversity in conjunction with digestive diseases, had a significant impact on the risk of developing PD. Across various subgroups, we observed consistent associations, with no significant interaction effects identified in most cases, indicating that the association between digestive diseases and PD applies to the general population.
Our study builds on the limited body of research exploring the connection between digestive diseases and PD. While prior studies have noted associations between PD and specific digestive disorders, including GERD11, Crohn’s disease12, ulcerative colitis13, and IBS14, these investigations often focused on single-disease analyses with smaller cohorts. Research on other digestive conditions—such as gastritis, celiac disease, peptic ulcers, pancreatitis, gallbladder and biliary diseases, NAFLD, chronic cirrhosis, appendicitis, and gastrointestinal cancers—has been scarce, leaving many causal associations unclear. Our study, which involved 2906 incident PD cases and comprehensively assessed 14 digestive diseases, provides essential clarity regarding these associations, highlighting the importance of digestive health in understanding and potentially mitigating PD risk.
We observed notable associations between PD and various chronic digestive diseases, including chronic gastritis, duodenitis, and chronic appendicitis. Notably, chronic appendicitis may heighten the risk of PD by promoting α-synuclein aggregation15. Additionally, intestinal inflammation may play a pivotal role in PD development by activating immune cells16 and generating inflammatory factors17. Recent studies have shown that IBD (especially Crohn’s disease) and PD have genetic risk factors in common18,19. A genome-wide association study has identified substantial genetic overlap between PD and inflammatory bowel diseases, with genes such as LRRK2, NOD2, and FCGR2A demonstrating effects on disease risk20. Although data constraints precluded our ability to examine associations between monogenic Parkinson’s disease and digestive diseases, the potential contribution of genetic factors to these relationships cannot be overlooked20. Our findings also indicated a connection between acute pancreatitis and an increased risk of PD, although further research is needed to elucidate the underlying mechanisms.
In our analysis, we found no significant interactions between digestive diseases and lifestyle factors in most strata, indicating that the influence of digestive diseases on PD risk remains relatively consistent regardless of lifestyle variations. However, we did identify significant interactions between NAFLD and sleep quality, as well as between celiac disease and Crohn’s disease, with dietary diversity. Specifically, short sleep duration and prolonged daytime napping are recognized as risk factors for NAFLD, with poor sleep quality strongly correlated with a higher risk of developing this condition21. Furthermore, conditions such as celiac disease and Crohn’s disease frequently disrupt gut microbiota balance, which exacerbates inflammation and leads to immune dysregulation22. A high-fiber diet can reduce inflammatory markers, such as C-reactive protein23, and protect against colitis in animal studies. Conversely, inadequate fiber intake may deplete the mucus layer, compromise the intestinal barrier, and increase permeability24. Enhancing gut health through dietary interventions has the potential to mitigate neuroinflammation associated with neurodegenerative diseases, including PD25.
These findings highlight the necessity for individualized management strategies, as optimizing sleep and dietary habits may be particularly advantageous for patients with specific digestive conditions. Lifestyle factors, including sleep patterns and dietary diversity, also play a significant role in the management of PD26. Sufficient sleep is essential for alleviating nonmotor symptoms of PD, such as cognitive impairments and mood disorders, and it may mitigate neurodegenerative risks by enhancing overall brain function27. Similarly, a diet rich in fiber and low in added sugars has been associated with a reduced incidence of PD26. Healthy dietary habits may further influence gut microbiota diversity, potentially decreasing inflammation and neurodegenerative processes26. Studies have also shown that healthy dietary patterns such as the Mediterranean Diet (MD), Mediterranean-DASH Intervention for Neurodegenerative Delay Diet (MIND), and high-fiber diet have demonstrated positive effects in improving digestive health and reducing the risk of PD28, thus providing a synergistic advantage in PD management24,26. Further exploration of these interactions could deepen our understanding of the complex association between PD and the combined effects of digestive diseases and lifestyle factors, ultimately guiding targeted interventions aimed at improving patient outcomes.
This study boasts several notable strengths, primarily stemming from its use of the large-scale, well-characterized UK Biobank cohort. This extensive dataset provides comprehensive information across a wide range of covariates, allowing for an in-depth analysis of the associations between 14 distinct digestive diseases and PD. The substantial sample size, combined with the prospective study design, significantly bolsters the reliability of our findings. Additionally, detailed insights into lifestyle factors—including sleep patterns and dietary diversity—enable the identification of interactions that could lead to targeted lifestyle interventions for individuals suffering from digestive diseases. Such approaches hold the potential to effectively lower the risk of developing PD within this demographic. Moreover, our calculation of the PAF quantifies the potential public health impact that could arise from eliminating specific digestive diseases, reinforcing their status as modifiable risk factors. Early diagnosis and timely treatment of Helicobacter pylori (HP) infection can effectively reduce the risk of neurodegenerative diseases associated with gastritis and gastric ulcers29. Additionally, for IBD, the use of antibiotics and immunosuppressants to modulate inflammatory responses may also help reduce the risk of PD associated with these conditions30. This information is vital for public health initiatives aimed at disease prevention, as it helps policymakers and healthcare professionals prioritize interventions that could offer substantial benefits. Our study also differentiates between chronic and acute manifestations of conditions like gastritis, duodenitis, pancreatitis, and appendicitis, providing new perspectives on the distinct roles these diseases may play in the pathogenesis of PD.
Despite these strengths, several limitations warrant consideration. As an observational cohort study, our ability to draw causal associations is limited by the potential for residual confounding, even with comprehensive adjustments for known risk factors. To reduce reverse causality, we excluded participants who developed PD within the first 2 years of follow-up; however, unmeasured or unknown confounders may still have impacted the results. Second, although we utilized validated diagnostic codes for digestive diseases, there remains a risk of misclassification. While the prospective nature of the study reduces the likelihood of differential misclassification, nondifferential misclassification could still occur. Additionally, the research did not fully account for factors such as the chronicity, severity, or duration of digestive diseases. Third, the statistical power to detect associations with rarer digestive diseases may also be limited due to their low prevalence in the cohort, potentially resulting in weaker or undetected associations. Furthermore, recent studies have suggested that alterations in the gut microbiota, particularly changes in bacteria involved in the production of short-chain fatty acids (SCFAs), and the increase in gut-derived pathogenic factors, may play a role in the pathogenesis of PD31. However, due to the lack of relevant data in the UK Biobank, we were unable to conduct a more in-depth exploration of the gut microbiota. Fourth, our study did not stratify participants by genetic PD subtypes (sporadic vs. monogenic), which may exhibit different associations with digestive diseases. While monogenic PD represents a minority of cases, future research should consider genetic stratification, particularly given known associations between certain PD genes (e.g., LRRK2) and inflammatory conditions like Crohn’s disease18. Lastly, the predominantly European ancestry of UK Biobank participants raises questions about the generalizability of our findings to more ethnically diverse populations is limited. Future studies should focus on diverse cohorts to confirm these findings across different demographic groups.
In conclusion, this extensive prospective cohort study has established a significant association between digestive system diseases and an increased risk of developing PD. The analysis of PAF highlights that the elimination of key digestive conditions—particularly GERD, gastritis and duodenitis, as well as gallbladder and biliary diseases—could lead to a substantial decrease in PD incidence. This underscores their critical relevance to public health as major contributors to the disease burden. Our findings also elucidate the interactions between digestive diseases and lifestyle factors, indicating that targeted lifestyle interventions may effectively mitigate PD onset in individuals with pre-existing gastrointestinal conditions. Notably, factors such as sleep patterns and dietary diversity appear to play a significant role in influencing PD risk. This study enriches our understanding of PD etiology and emphasizes the necessity of an integrated approach that addresses both digestive health and lifestyle modifications. The insights gained from this research provide valuable guidance for the development of targeted prevention strategies aimed specifically at reducing PD risk among individuals with digestive diseases.
Methods
Data sources and research design
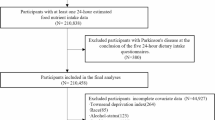
The UK Biobank is a large-scale prospective study that recruited over 500,000 middle-aged participants from April 2007 to December 2010. For this analysis, we included 501,483 participants, of whom 907 had a baseline diagnosis of PD (Supplementary Fig. 1). Ethical approval for the UK Biobank study was granted by the North West Multicenter Research Ethics Committee.
Definition of the digestive diseases
This study focused on 14 specific diseases of the digestive system, which are commonly encountered and widely studied in clinical research. In addition to these individual diseases, we defined a compound outcome, termed overall digestive diseases, which included individuals diagnosed with any of the aforementioned conditions at the baseline. Diagnoses were identified with reference to a combination of diagnostic codes sourced from national inpatient datasets, primary care datasets, cancer registries, and self-reported medical conditions. Detailed diagnostic codes are provided in Supplementary Table 1. According to the annual audit committee report, the accuracy of these diagnostic codes is >89% (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/319355/PbR_DAF_costing_briefing_June_2014.pdf).
Ascertainment of PD
The date of PD onset was determined using the algorithm recommended by UK Biobank (https://biobank.ndph.ox.ac.uk/ukb/ukb/docs/alg_outcome_main.pdf). Disease information was obtained via linkage to inpatient electronic health records and death registries, including data from Hospital Episode Statistics (HES) in England, the Scottish Morbidity Record, and the Patient Episode Database for Wales. Mortality data, including date and cause of death, were sourced from NHS Digital (England and Wales) and the NHS Central Register (Scotland). Follow-up time was defined as the interval from baseline assessment to the earliest of PD diagnosis, death, loss to follow-up, or censoring. Censoring dates were October 31, 2022 (England), August 31, 2022 (Scotland), and May 31, 2022 (Wales). PD cases were identified using the ICD-10 code G20. Further details on diagnostic criteria and associated codes are provided in Supplementary Table 2.
Covariate assessment
The analysis incorporated several covariates, including the age at the time of recruitment, sex (categorized as female and male), BMI, Townsend Deprivation Index (TDI), ethnicity (classified as White, Asia, Black, and Other), alcohol consumption (Never, Previous and Current), physical activity (whether a person met the UK Physical activity guide lines of 150 min of walking or moderate activity per week or 75 min of vigorous activity), healthy diet, educational attainment (categorized as any school degree, college or university degree, vocational, and other), smoking status (never, adolescence, adulthood, and childhood), hypertension (normal, evaluated, stage 1 hypertension, and stage 2 hypertension), and family history of PD.
Definition of nonsteroidal anti-inflammatory drugs (NSAIDs) and proton pump inhibitors (PPIs)
During the baseline assessment, the routine use of NSAIDs and PPIs among participants was initially assessed via a touchscreen questionnaire and subsequently confirmed during an oral interview conducted by trained staff. In the touchscreen questionnaire assessing NSAID use, participants were asked whether they regularly consumed any of the following medications: Aspirin, Ibuprofen (e.g., Nurofen), Paracetamol, Ranitidine (e.g., Zantac), Omeprazole (e.g., Zanprol), Laxatives (e.g., Dulcolax, Senokot), None of the above, Do not know, or Prefer not to answer32.
For the assessment of PPI use, participants were queried with the question, “Do you regularly take any prescription medications?” “Regular use” was defined as taking the medication on most days of the week over the past 4 weeks. If participants selected “Yes” or “Unsure,” they were then asked by the interviewer, “In the touchscreen questionnaire, you indicated that you are taking regular prescription medications. Could you please specify which medications these are?” Information regarding PPI use was recorded in free text format, with the types of PPIs documented including omeprazole, lansoprazole, pantoprazole, rabeprazole, and esomeprazole33. Detailed definitions and descriptions of the covariates are provided in Supplementary Table 2.
Assessment of sleep patterns and dietary diversity
The sleep patterns were evaluated using five key sleep behaviors: sleep duration, circadian preference, insomnia, snoring, and daytime sleepiness, as assessed through a touchscreen questionnaire34. Each behavior was scored as either 1 (healthy) or 0 (unhealthy) based on previously established health criteria. The cumulative sleep health score ranged from 0 to 5, with higher scores indicating healthier sleep patterns. The participants were then classified into two categories: “ideal sleep pattern” and “poor sleep pattern,” based on the median sleep health score.
Dietary diversity was assessed by examining the frequency of food intake and calculating the Shannon Diversity Index. This index quantifies dietary diversity by calculating the richness of food types and the balance of their consumption frequencies. The higher the index, the more diverse the diet35,36. The formula for calculating the metric is as follows:
Foods were categorized based on the consumption frequency, with integer codes assigned (where 1 represented the lowest frequency and N the highest). The Shannon Index was used to quantify dietary diversity. Based on this analysis, the participants were categorized into high and low dietary diversity groups.
Statistical analysis
The UK Biobank dataset contains a substantial amount of missing data, which could reduce the power to detect associations and introduce bias. To address this concern, we applied random forest imputation37, a machine learning-based multivariate technique, thereby incorporating all covariates to improve the prediction of missing values. Missing data related to observed variables can lead to greater bias in complete case analysis38; thus, multiple imputation provides more reliable estimates. Considering the significant amount of missing data in our sample, the use of multiple imputations was deemed essential to mitigate any potential bias. The results of the imputation analysis are detailed in the supplementary materials (Supplementary Table 3).
We performed chi-square (χ2) tests for categorical variables and the analysis of variance (ANOVA) for continuous variables for the evaluation of the baseline characteristic differences across digestive diseases. We then applied Cox proportional hazards regression models to estimate the associations between overall digestive diseases and the risk of PD. Given the potential for co-occurrence among digestive diseases, we also assessed the association between the number of digestive diseases and PD risk. In addition, we applied Cox models to estimate the associations of individual digestive diseases with PD risk. Three analytical models were applied in the Cox regression analysis: (1) Model 1 adjusted for age and sex; (2) Model 2 further adjusted for ethnicity, Townsend deprivation index, body mass index, education attainment, healthy diet, smoking status, alcohol consumption, physical activity, and hypertension; (3) Model 3 was additionally adjusted for family history of PD. The false discovery rate (FDR) procedure was used to adjust P-values39.
To estimate the proportion of PD cases that could potentially be prevented by eliminating the 11 digestive diseases significantly associated with PD, we employed the causal PAF package to calculate the Population Attributable Fraction (PAF) for these conditions. The PAF calculation was based on Levin’s formula40, which requires relative risk (RR) estimates and the prevalence of each risk factor41.
We further examined the associations between PD risk and specific digestive diseases—gastritis, duodenitis, pancreatitis and appendicitis—within both acute and chronic subgroups using Cox proportional hazards models.
Subgroup analyses and interaction analyses were conducted to explore the potential effect modifications by sex, ethnicity, alcohol consumption, educational attainment, smoking status, and hypertension.
In addition, we investigated the joint effects of lifestyle factors (such as sleep patterns and dietary diversity) and digestive diseases on PD risk42. Initially, we employed Cox proportional hazards regression to evaluate associations between these lifestyle factors and digestive diseases. Next, we integrated the 11 digestive diseases significantly associated with PD with various lifestyle factors to form new variables and categorized them into four groups based on a 2 × 2 matrix of digestive disease exposure (no or yes) and lifestyle factor risk (low-risk or high-risk). Low-risk lifestyle factors were defined as “ideal sleep patterns,” and “high dietary diversity,”. Likelihood ratio tests were performed to examine the interaction between these combined variables and PD risk, thereby providing further insights into their collective role in PD pathogenesis.
All analyses were two-tailed, and p < 0.05 was considered to indicate statistical significance. All statistical analyses were conducted using R version 4.2.2.
Sensitivity analysis
To evaluate the robustness of our findings, we performed a series of sensitivity analyses as detailed: (1) we restricted the analysis to participants aged >55 years at recruitment (n = 290,625) so as to control for potential immortal time bias, which occurs when follow-up periods without event risk are incorrectly included43. (2) To minimize reverse causation, we excluded participants who developed PD within the first 2 years following baseline assessment. (3) We employed a competing risk model to account for death and loss to follow-up as competing events, thereby providing a more comprehensive assessment of the PD risk44. (4) We then adjusted for the use of NSAIDs and PPIs for their potential confounding effects. NSAIDs are commonly used among PD patients and may influence disease risk, while PPIs are frequently prescribed for digestive diseases and have been linked to PD45.
Data availability
The datasets presented in this study can be found in online repositories (UK Biobank(dnanexus.com)). This research has been conducted using the UK Biobank resource under application number 94166.
Code availability
Codes are available from the corresponding author upon reasonable request.
References
Kalia, L. V. & Lang, A. E. Parkinson’s disease. Lancet 386, 896–912 (2015).
Dorsey, E. R. et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68, 384–386 (2007).
Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. https://doi.org/10.1016/s1474-4422(18)30295-3 (2018).
Sharma, A., Voigt, R. M., Goetz, C. G. & Keshavarzian, A. Parkinson’s disease and the gut: a primer for gastroenterologists. Am. J. Gastroenterol. https://doi.org/10.14309/ajg.0000000000003508 (2025).
Warnecke, T., Schäfer, K. H., Claus, I., Del Tredici, K. & Jost, W. H. Gastrointestinal involvement in Parkinson’s disease: pathophysiology, diagnosis, and management. NPJ Parkinsons Dis. 8, 31 (2022).
Corsello, A., Pugliese, D., Gasbarrini, A. & Armuzzi, A. Diet and Nutrients in gastrointestinal chronic diseases. Nutrients https://doi.org/10.3390/nu12092693 (2020).
Tait, C. & Sayuk, G. S. The brain-gut-microbiotal axis: a framework for understanding functional GI illness and their therapeutic interventions. Eur. J. Intern. Med. 84, 1–9 (2021).
Higinbotham, A. S. & Kilbane, C. W. The gastrointestinal tract and Parkinson’s disease. Front. Cell Infect. Microbiol. 13, 1158986 (2023).
Chen, M. & Mor, D. E. Gut-to-brain α-synuclein transmission in Parkinson’s disease: evidence for prion-like mechanisms. Int. J. Mol. Sci. 24, 7205 (2023).
Holcomb, M. et al. Probiotic treatment induces sex-dependent neuroprotection and gut microbiome shifts after traumatic brain injury. J. Neuroinflamm. 22, 114 (2025).
Clarrett, D. M. & Hachem, C. Gastroesophageal reflux disease (GERD). Mo Med 115, 214–218 (2018).
Ridler, C. Parkinson disease: LRRK2 variants linked to PD and Crohn’s disease. Nat. Rev. Neurol. 14, 126 (2018).
Pellicano, R., Ianiro, G., Fagoonee, S., Settanni, C. R. & Gasbarrini, A. Review: extragastric diseases and Helicobacter pylori. Helicobacter 25, e12741 (2020).
Zhang, X. et al. Association between irritable bowel syndrome and risk of Parkinson’s disease: a systematic review and meta-analysis. Front. Neurol. 12, 720958 (2021).
Chen, Y. et al. Increased accumulation of α-synuclein in inflamed appendices of Parkinson’s disease patients. Mov. Disord. 36, 1911–1918 (2021).
Shen, J. et al. Plasma MIA, CRP, and albumin predict cognitive decline in Parkinson’s disease. Ann. Neurol. 92, 255–269 (2022).
Zhang, H. et al. Activated Schwann cells and increased inflammatory cytokines IL-1β, IL-6, and TNF-α in patients’ sural nerve are lack of tight relationship with specific sensory disturbances in Parkinson’s disease. CNS Neurosci. Ther. 26, 518–526 (2020).
Herrick, M. K. & Tansey, M. G. Is LRRK2 the missing link between inflammatory bowel disease and Parkinson’s disease?. NPJ Parkinsons Dis. 7, 26 (2021).
Lee, H. S., Lobbestael, E., Vermeire, S., Sabino, J. & Cleynen, I. Inflammatory bowel disease and Parkinson’s disease: common pathophysiological links. Gut 70, 408–417 (2021).
Witoelar, A. et al. Genome-wide pleiotropy between Parkinson disease and autoimmune diseases. JAMA Neurol. 74, 780–792 (2017).
Bu, L. F. et al. Non-alcoholic fatty liver disease and sleep disorders. World J. Hepatol. 16, 304–315 (2024).
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Zhang, F., Fan, D., Huang, J. -l & Zuo, T. The gut microbiome: linking dietary fiber to inflammatory diseases. Med. Microecol. 14, 100070 (2022).
Silveira, A. L. M. et al. Preventive rather than therapeutic treatment with high fiber diet attenuates clinical and inflammatory markers of acute and chronic DSS-induced colitis in mice. Eur. J. Nutr. 56, 179–191 (2017).
Zheng, S. Y. et al. Potential roles of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res. Rev. 69, 101347 (2021).
Kwon, D. et al. Diet and the gut microbiome in patients with Parkinson’s disease. NPJ Parkinsons Dis. 10, 89 (2024).
Gabbert, C. et al. Lifestyle factors and clinical severity of Parkinson’s disease. Sci. Rep. 13, 9537 (2023).
Tosefsky, K. N. et al. The role of diet in Parkinson’s disease. J. Parkinsons Dis. 14, S21–s34 (2024).
Huang, T. T., Cao, Y. X. & Cao, L. Novel therapeutic regimens against Helicobacter pylori: an updated systematic review. Front. Microbiol. 15, 1418129 (2024).
Seyedian, S. S., Nokhostin, F. & Malamir, M. D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 12, 113–122 (2019).
Li, Z. et al. Gut bacterial profiles in Parkinson’s disease: a systematic review. CNS Neurosci. Ther. 29, 140–157 (2023).
Yuan, S. et al. Associations between the use of common nonsteroidal anti-inflammatory drugs, genetic susceptibility and dementia in participants with chronic pain: a prospective study based on 194,758 participants from the UK Biobank. J. Psychiatr. Res. 169, 152–159 (2024).
Yang, M. et al. Regular use of proton-pump inhibitors and risk of stroke: a population-based cohort study and meta-analysis of randomized-controlled trials. BMC Med. 19, 316 (2021).
Fan, M. et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur. Heart J. 41, 1182–1189 (2020).
Yap, C. X. et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 184, 5916–5931.e5917 (2021).
Konopiński, M. K. Shannon diversity index: a call to replace the original Shannon’s formula with unbiased estimator in the population genetics studies. PeerJ 8, e9391 (2020).
Li, J. et al. Comparison of the effects of imputation methods for missing data in predictive modelling of cohort study datasets. BMC Med. Res. Methodol. 24, 41 (2024).
Sterne, J. A. et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Br. Med. J. 338, b2393 (2009).
Rothwell, J. A. et al. Circulating amino acid levels and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition and UK Biobank cohorts. BMC Med. 21, 80 (2023).
Mulligan, M. D. et al. Population attributable fraction of hypertension for dementia: global, regional, and national estimates for 186 countries. EClinicalMedicine 60, 102012 (2023).
Dragioti, E. et al. Global population attributable fraction of potentially modifiable risk factors for mental disorders: a meta-umbrella systematic review. Mol. Psychiatry 27, 3510–3519 (2022).
Chen, L. H. et al. Physical activity and sleep pattern in relation to incident Parkinson’s disease: a cohort study. Int. J. Behav. Nutr. Phys. Act. 21, 17 (2024).
Yadav, K. & Lewis, R. J. Immortal time bias in observational studies. J. Am. Med. Assoc. 325, 686–687 (2021).
Shi, Y. et al. Association of pro-inflammatory diet with increased risk of all-cause dementia and Alzheimer’s dementia: a prospective study of 166,377 UK Biobank participants. BMC Med. 21, 266 (2023).
Giuliano, S. et al. Should evidence of an autolysosomal de-acidification defect in Alzheimer and Parkinson diseases call for caution in prescribing chronic PPI and DMARD?. Autophagy 19, 2800–2806 (2023).
Acknowledgements
We are grateful to all the participants of UK Biobank and all the people involved in building the UK Biobank study. This study was supported by the National Natural Science Foundation of China (82171402) and the Clinical Research Center for Precision Diagnosis and Treatment of Neurological Diseases of Fujian Province (2022Y2005).
Author information
Authors and Affiliations
Contributions
Kaitai Yang: Formal analysis, Resources, Writing - original draft, Data curation. Ruitian Zeng: Writing -original draft, Software, Methodology. Yiling Zheng: Writing -original draft, Visualization, Software. Siqi Zhong: Methodology, Investigation. Jiani Wang: Methodology, Investigation. Xinxi Yu: Visualization, Software. Huilin Zhong: Investigation. Xuanjie Chen: Software. Yisen Shi: Data curation. Fabin Lin: Visualization. Qinyong Ye: Writing -review & editing, Methodology, Conceptualization. Ning Sun: Writing - review &editing, Conceptualization. Guoen Cai: Writing -review & editing, Supervision, Methodology, Conceptualization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The UK Biobank study has been approved by the North West Multi-centre Research Ethics Committee as a Research Tissue Bank and all UK Biobank participants provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, K., Zeng, R., Zheng, Y. et al. Associations of digestive diseases exposure and lifestyle factors with Parkinson’s disease. npj Parkinsons Dis. 11, 245 (2025). https://doi.org/10.1038/s41531-025-01098-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-01098-6
This article is cited by
-
Cognitive reserve, frailty status, and risk of neurodegenerative diseases: a prospective cohort study
npj Parkinson's Disease (2025)