Abstract
Humans must obtain vitamin B9 (folate) from plant-based diet. The sources as well as the effect of food processing are discussed in detail. Industrial production, fortification and biofortification, kinetics, and physiological role in humans are described. As folate deficiency leads to several pathological states, current opinions toward prevention through fortification are discussed. Claimed risks of increased folate intake are mentioned as well as analytical ways for measurement of folate.

Similar content being viewed by others
Introduction
Vitamin B9 is one of the crucial vitamins whose physiological function and consequence of its deficiency are well known, however, there is a lack of a comprehensive paper summarizing essential aspects of this vitamin encompassing its natural occurrence, the impact of different factors on its stability and absorption as well as its further fate in the human organism in the context of its possible deficiency with its causes, and consequences, as well as current discussion on possible risks of high dose folate supplementation. This paper aims to offer such a complex review.
Vitamin B9, or folate, is a generic term given to a group of chemically related molecules based on the folic acid structure (Fig. 1). These molecules contain a pteridine heterocycle that can be in a reduced or oxidized form; a p-aminobenzoic acid bridge and a mono-/polyglutamate chain of variable length. Additionally, one carbon unit can be bound to either the pteridine ring, p-aminobenzoic moiety, or both. Folic acid is the most oxidized folate form. Folic acid can be reduced at nitrogen-8 to produce dihydrofolate. Further reduction at nitrogen-5 generates the active coenzyme form: tetrahydrofolate (THF). Both reductive steps are catalysed by the enzyme dihydrofolate reductase.
Reduced tetrahydrofolate may serve as an acceptor of one-carbon units via nitrogen-5 and nitrogen-10. These carbon units can bind in different oxidation states and generate different forms of tetrahydrofolate cofactors which have distinct physiological functions: 5-methyl-THF; 5,10-methylene-THF (methylene-THF) and 10-formyl-THF. A synthetic folate molecule, 5-formyl-THF (folinic acid), is often used in medications.
Sources of vitamin B9
Folate – vitamin B9
Plants, fungi, certain protozoa, several archaea, and many bacteria can synthesize folate de novo. Animals and humans are unable to synthesize folate and entirely depend on an adequate and constant intake of the vitamin from exogenous sources1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32. Folate occurs in a wide variety of foods. Dark green vegetables (e.g., spinach, broccoli, Brussels sprouts, and romaine lettuce), cereals (especially whole grains), fruits (e.g., oranges, papaya, and avocado), and legumes (e.g., chickpea, soybean, and lentil) are the major sources1,13,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89. Liver, yeast, and egg yolk contain also very high amounts of folate1,33,50,60,75,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105. Logically, the contribution of every dietary source to meet the daily folate requirements depends on its consumption in the general population. For example, yeast, liver, and pulses, which are rich in folate, contribute less to folate supply due to their low consumption, in contrast to vegetables and fruits with lower folate contents but higher consumption106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123. In the United Kingdom, Ireland, and Sweden, bread as a typical cereal product provides, on average, 9–14%, 14%, and 13%, resp., of dietary folate daily124,125,126,127,128. Similarly, bread and rolls contribute to the folate intake in the average Polish diet by 17%129. Pseudocereals such as quinoa and amaranth are suitable alternatives to gluten-containing cereals (wheat, barley, and rye), in terms of folate content, for people with coeliac disease88,130,131,132,133,134. Meat and meat products, except for the liver, which is the folate storage organ in mammals, contain little folate75,135. Folate status tends to be higher in people eating plant-based diets as compared to meat-eaters (i.e., omnivores), with the highest levels of folate being in vegans115,136,137,138,139,140,141,142,143,144,145. Milk and fermented dairy products, which are not considered rich in folate, constitute an important dietary source of folate because they are consumed in relatively large quantities; milk is responsible for 10–15% of the daily folate intake in European countries. This is particularly important for high milk and dairy products consuming countries such as the Netherlands, Sweden, and Spain1,34,50,98,107,115,117,146,147,148,149. Potatoes do not have high folate content but are also the major source of the vitamin due to the high frequency of their consumption; potatoes supply about 10% of the total folate intake of people in European countries such as the Netherlands, Ireland, Norway, and Finland67,98,115,117,128,141,148,150,151. Younger potato tubers (‘new potatoes’) are richer in folate than mature ones; higher consumption of new or baby potatoes could significantly increase folate intake152,153. Some wild vegetables and fruits contain amounts of folate comparable to those in conventional ones and may serve for the diversification of our current diet to increase folate intake154,155,156,157. Similarly, some microalgae, e.g., Chlorella, but not Arthrospira (Spirulina), seaweeds, and yeasts, such as Yarrowia lipolytica, could represent an additional source of folate in the human diet109,119,158,159,160,161,162,163,164,165,166. Several mushrooms that are higher in folate (e.g., oyster and enoki) could enhance natural folate intake as well109,162,167,168,169,170. Edible insects, such as mealworms and crickets, may also enrich the human diet with folate171,172,173.
Data on the folate content in foods vary. Variations, especially in foods of plant origin, could be attributed to factors such as plant varieties and cultivars, growing conditions (e.g., season and climate), and agronomic practices (e.g., harvest time and postharvest handling). A microbiological assay is a widely accepted official method for folate measurement in many countries. Differences in the analytical methodology may also affect the measured folate content41,56,98,106,174,175,176,177,178,179,180,181,182,183. The contents of folate in some selected foodstuffs are shown in Table 1.
Based on several human studies, food folate (a mixture of natural reduced pteroylmono- and polyglutamates) has a lower bioavailability than synthetic monoglutamate folic acid added to foods for supplementation and food fortification purposes. Folic acid is absorbed almost completely when taken without simultaneous consumption of food, whereas its bioavailability from fortified foods or supplements ingested during a meal is about 85%. The bioavailability of food folate is estimated to be around 50%, i.e., half that of folic acid taken with water on an empty stomach, due to losses during digestion and absorption. In general, folic acid in fortified products or taken with foods is 85/50 or 1.7 times more bioavailable than food folate. Several factors may hinder the absorption of natural food folate, e.g., partial release from the food matrix (incomplete liberation from cellular structures), destruction within the gastrointestinal tract, and incomplete hydrolysis of polyglutamates to monoglutamates (possibly mediated by partial inhibition of deconjugation enzymes by other dietary constituents such as organic acids). On the contrary, such factors are negligible in the case of added folic acid, which does not require the release from cellular structures, is more stable and less susceptible to destruction within the lumen than natural food folate, and exists as a monoglutamate, i.e. the form necessary for normal absorption in the small intestine (see Absorption section below). The bioavailability of supplemental 5-methyl-THF has been reported to be similar or higher compared to folic acid at equimolar doses. A typical diet would contain a combination of food folate and folic acid provided in fortified products or supplements; the dietary folate (‘dietary folate equivalents’) would then be computed as follows: μg food folate + (1.7 x μg folic acid). Although there is a broad agreement that naturally occurring food folate is not as bioavailable as folic acid, uncertainties still exist in relation to the extent of these differences, particularly in the context of a whole diet. Some studies indicate that the bioavailability of food folate is underestimated and is higher than the generally assumed value of 50%. Therefore, more research is needed for a better understanding of folate bioavailability from food and influencing factors1,37,75,98,120,146,147,149,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247.
There is a paucity of data on the possible contribution of folate, which is produced by microorganisms in the colon, to the overall human body’s needs for folate. It might be a complementary endogenous source of folate to that derived from the diet248,249. A part of the microbiota in the human large intestine is capable of synthesizing folate (folate prototrophs); the rest microbiome members lacking the ability, however, are consumers (folate auxotrophs) and rely on those folate producers to provide folate, which may limit the vitamin availability for the host including humans248,250,251,252,253,254,255,256,257,258,259. It is known that the human gut microbiome is different and stratified, not continuous, in the population. It may be clustered into three enterotypes according to the species composition and functional properties. Although all vitamin metabolic pathways were represented in all microbiome samples, enterotypes 1 and 2 are enriched in genes involved in the biosynthesis of different vitamins, those for thiamine and folate being in enterotype 2. It may be beneficial to the human host260,261. The abundance of folate biosynthetic genes in human colon microbiome may change with the age262,263,264. Moreover, microbial folate production in the colon may be influenced by diet. Intake of soluble, fermentable dietary fibres enhanced plasma folate concentrations in rats and humans, bacterial load, and total folate content in the colon, but not the whole body’s folate status in piglets265,266,267. A positive effect of supplementation with folate-producing bifidobacteria on folate plasma levels has been observed in a rat experiment as well as in a human trial268,269, but not in a mouse experiment270. In another rat experiment, it was found that folate derived from caecal bacteria is not absorbed and does not increase the liver folate stores271. It has been shown that microbially synthesized folate can be partly absorbed across the large intestine in piglets272. Absorption of isotopically labelled 5-formyl-THF across the colon at a considerably lower rate (about one-fiftieth) than across the small intestine has been reported. However, the difference in the net absorption was estimated to be smaller (approximately one-tenth) due to much longer transit in the colon than in the small intestine273,274,275. The existence of a folate transporter in the human colonic cells has been demonstrated; it is expressed at much lower levels in the cells in the colon than in the small intestine, where folate absorption primarily occurs276. Thus, in situ produced microbial folate may favourably influence the cellular nutrition of the local colonocytes and may be important in maintaining intestinal homeostasis and modulating gut microbiome function, e.g., through regulation of colon mucosal proliferation (i.e., colorectal cancer prevention) and its anti-inflammatory effects248,249,256,257,277,278,279,280,281,282,283,284,285,286. However, there are still a lot of questions that remain to be answered about the relationship between folate levels in the colonic mucosa and the systemic circulation and the colorectal cancer risk, and about the role of folate derived from the diet and that from local microbial production287,288,289,290. Regardless, it is still unknown whether folate synthesized by the human colonic microbiota can substantially affect the body’s general folate status as this has never been sufficiently quantified to date90,211,233,248,251,277,286,291.
Impact of food processing and storage on folate contents
Food processing and storage can greatly affect the folate content75,85,106,292,293,294,295,296,297,298,299,300,301,302,303.
Milling of cereals
Primary processing of cereals, particularly milling processes transforming cereals into more palatable and shelf-stable food ingredients, gives rise to significant folate losses because folates are not evenly distributed in grain fractions. The outer layers of the grain (the bran and the aleurone layer, the outermost layer of the endosperm, remaining attached to the bran during milling) and the germ are rich in folate, and they are generally separated during milling from the starchy endosperm, which is ground into flour304,305,306,307,308,309,310,311,312,313,314,315,316,317,318. Amounts of folate in refined wheat and rye flours decline by 21–89.5% and 27.7–83%, resp., in comparison to the whole grain ones126,319,320,321,322. Similarly, the folate levels in various barley and maize milled products, compared to whole cereals, decrease by 43.8–61.1% and 33–67%, resp.319,320,323,324,325,326. Commonly used oat flakes contain only 16% less folate compared to whole grains327. Folate losses are 46–79% and 27.3–55% in non-parboiled and parboiled white rice, resp., compared to brown rice. The folate decline in parboiled rice is generally lower, in contrast to the non-parboiled one, because a part of the vitamin diffuses from the vitamin-rich outer bran layer into the endosperm during the parboiling process, in which raw rice is soaked in water and partially steamed before drying and milling, and so it is retained during the following milling313,319,325,328,329,330,331,332,333. Considering the folate content, foods containing all components of the cereal grain (the ‘whole grain concept’) are more suitable for nutrition than those containing highly refined cereal products304,305,306,334.
Folate properties and stability; mechanisms of folate losses during food processing
Folates are water soluble and more stable in alkaline conditions with the lowest stability, unfortunately, in the pH range commonly encountered in plant foods (pH 4–6). Folates are sensitive to heat, atmospheric oxygen, UV radiation (e.g., present in sunlight), electron-beam radiation, and reducing sugars (such as fructose)66,79,86,90,106,123,292,295,306,318,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355. Folic acid and 5-methyl-THF in aqueous solutions are photostable in the absence of oxygen106,337,340,356,357,358. Another B-vitamin, vitamin B2, riboflavin, as a photosensitizing compound359, gives rise to the oxidative cleavage of folic acid and 5-methyl-THF by visible light, which is absorbed by and yields excited states of riboflavin356,360,361. The degradation rate of folic acid in the presence of riboflavin depends on the pH, achieving the highest values at the pH around 6.2362. Iron and copper ions leaking from process equipment are prooxidative and hence catalyse the oxidation of folates66,223. Sulfite and nitrite, used as food preservatives, can cause degradation of folates, too223,301,336. Folates can resist heat degradation in anaerobic conditions, while they are degraded in the presence of oxygen; with folic acid, 10-formylfolate, and 5-formyl-THF being relatively stable vitamers and 5-methyl-THF and THF being very thermolabile ones. Folic acid is the most stable form. Hence, in general, the stability of formyl- and/or oxidized forms is much higher than that of methyl- and/or reduced ones, and the acidic environment accelerates the thermal decomposition66,106,234,346,363,364,365,366,367,368,369,370,371,372,373,374.
Two main mechanisms are involved in folate losses during food processing. The first one is leaching into the surrounding liquid, and the vitamin will be lost in any soaking or cooking water that is not consumed in the whole dish. The second one is oxidative degradation during heat treatment. The vitamin retention highly depends on the type of food, the method used, temperature, and process duration102,106,130,134,162,180,295,296,363,365,375,376,377,378,379,380,381,382,383,384,385,386,387,388,389,390.
Processing of vegetables and fruits
Boiling, steaming, and frying are estimated to cause average folate losses of 40–50%, 40%, and 15–30%, resp., in vegetables solely, and those of 25–30%, 30%, 30%, resp., in the total dish when cooking liquids are not thrown out380,381. Folate decline in vegetables during baking is estimated to be 20–35%380,381,382. For example, boiling, steaming, microwaving, and sous-vide resulted in folate losses of 36–62% and 25–56%, 9–57% and 10–30%, 51% and 17%, 41% and 23% in spinach and broccoli, resp.102,162,379,384,386,391,392. Non-leafy vegetables retain more folate during their boiling than do leafy ones379,386,393. Leeks, cauliflower, and green beans lost 26% and 28%, 8% and 10%, and 10% and 21% folate during steaming and blanching, resp.394. Similar changes concerning the relation between folate retention and processing methods (boiling, pressure cooking, steaming, and microwaving) were reported in frozen vegetables used for domestic cooking393. Freezing and thawing successively lead to tissue disruption and hence to a better release of folate. For instance, boiling fresh green beans and spinach caused no significant and 47% folate losses, resp., while that of frozen vegetables led to losses of 15% and 59%, resp., predominantly due to easier diffusion into the boiling water379. Likewise, blanching of fresh leeks, cauliflower, and green beans or frozen and thawed ones gave rise to folate losses of 28% or 85%, 10% or 65%, and 21% or 79%, resp., owing to leakage into the liquid during blanching394. Blanching of fresh vegetables before freezing reduced folate content by 12–35% in peas, 40% in cauliflower, 61% in cabbage, and 70% in spinach395. In another study, folate losses during vegetable blanching before freezing amounted to about 10% in broccoli, cauliflower, and green beans, 20% in peas, 26% in spinach, and only 1% in yellow beans396. Compared to fresh spinach, the folate amount declined by 38% in the frozen one, mostly due to the washing step and without any effect of the blanching step during the industrial freezing processing chain397. The total content of folate in vacuum-packed broccoli (crushed and mixed with water) decreased after heating at the higher temperature for a shorter time (90 °C, 4 min) less than after that at the lower temperature for a longer time (40 °C, 40 min), i.e., by 12% and 24%, resp.234. Folate levels in sweet corn cobs without bracts were reduced by 55%, 23%, and 20% by boiling, steaming, and microwaving, resp., compared to uncooked fresh corn398. Steaming in preference to boiling could be promoted as a means of saving the folate content of cooked green vegetables. Consumers choosing to boil vegetables should be strongly discouraged from doing so for prolonged periods if they would like to keep folate. In addition, minimalization of the cooking water and consumption it as soup or gravy will decrease the vitamin losses384,399. Likewise, other forms of cooking that minimize the direct contact with cooking water, such as steam blanching (instead of water blanching), steam pressure cooking, microwaving, and sous-vide are preferable to boiling in terms of folate retention292,384,385,393. Frying caused folate losses of 1–31% in drumstick, taro, bele, amaranth, and ota leaves, predominantly due to thermal destruction, while boiling caused those of 10–47%, mainly due to leaching, i.e., most lost folate was saved in the boiling water. Therefore, in terms of folate intake, boiling may be a healthier choice for cooking vegetables than frying, provided the cooking water is consumed together with the cooked vegetables387. Dried laver lost 8% of folate after toasting for 10 s162.
Sous-vide cooked, oven-baked, and boiled potatoes lost no, 37%, and 18–41% of their folate content, resp., compared to raw ones384,385. The presence or absence of potato skin had no significant impact on folate retention during boiling384,385. In other studies, folate content in boiled potatoes was reduced by 9% and 23% when they were unpeeled and by 23% and 39% when they were peeled390,400.
Retention of folates in green peas, broccoli, and potatoes cooked by different methods, stored, and reheated for use in modern large-scale service systems (e.g., hospitals) was also investigated. After-cooking storage at various temperatures (directly cooled or held warm and then cooled) and different periods followed by reheating caused no significant losses of folate385. On the other hand, folate content in three frozen vegetable-based ready meals declined by 7–37%, 11–45%, and 8–50% after reheating on a stove, in a microwave oven, and in a baking oven, resp. The study demonstrated that it is difficult to predict which reheating method is preferable regarding folate stability because no clear pattern in folate retention between different heating methods was seen401.
Folate losses could be expected during the production of fruit and vegetable juices. It includes various technological steps, among others, separation of pomace and pasteurization or, in the case of juice concentrates, also thermo-vacuum evaporation. The production process of sea buckthorn juice and juice concentrate resulted in folate losses of 19% and 25%, resp., compared to berries402. Berry juices (golden raspberry, red raspberry, blackberry, blueberry, cherry, and strawberry) contained 7–22% less folate than berries37. Folate contents in fresh, non-pasteurized juices were reduced by 11–40% in leafy vegetables (beet greens, turnip greens, Romaine lettuce, and carrot greens), by 32–49% in root vegetables (beet, turnip, and carrot), and by 49% in broccoli compared to the initial vegetables403.
Rosehips, rich in folate and ascorbic acid, have traditionally been used as a health food supplement in many European countries. They are not often consumed fresh, and therefore, air drying to produce a stable product is a crucial step. The degradation of folate was shown to be affected by temperature and dependent on the drying time – shorter drying time at a higher temperature can limit vitamin decomposition mediated by thermal degradation. The cutting of rosehips into slices reduced the required drying time from 11 h to 100 min and decreased average folate losses from 27% to 18% for whole rosehips and slices, resp., compared to fresh rosehips. When sliced rosehips were dried, an increase in temperature from 70 °C to 90 °C shortened the necessary drying time from 160 min to 105 min and lowered folate losses from 21% to 13%. The levels of ascorbic acid seemed to follow the same pattern as the folate levels during drying; a high content of ascorbic acid could provide possible protection of folate from degradation404. Folate content was determined in sultanas after rack, ground, trellis, or natural drying of vine fruits. The folate levels differed, depending on the drying method, the highest being in emulsion-rack dried sultanas70.
Processing of legumes
Legumes are usually processed before consumption, and their processing may cause losses of folate405,406,407,408. Folate content in boiled (heated to boiling temperature and then simmered for 2 h) soaked peas and chickpeas decreased by 55% and 47%, and that in pressure-cooked (for 20 min) ones by 49% and 38%, resp., compared to raw legumes. Leaching was the main reason for the vitamin loss because nearly all the lost folate was found in the water used for soaking and heat processing. Higher folate retention in pressure-cooked legumes can be attributed to the shorter exposure to heat409. On the other hand, in navy beans, pressure cooking caused higher folate reduction than ordinary cooking. Folate stability was higher in the beans cooked in a water-oil mixture than in water. Folate declines were lower in non-soaked than in soaked navy beans during the following cooking410. Fresh kidney beans boiled for 10 min and dried red beans boiled for 30 min without soaking lost 14% and 19% of folate, resp.162. Effects of boiling on folate retention were evaluated in soaked mung beans, adzuki beans, cowpeas, faba beans, peas, and common beans. Folate losses in the boiled pulses due to heating degradation and leaching depended on the pulse variety and ranged from 18% to 36%, with an average of 24%, compared to unprocessed ones411. Boiling of soaked lentils and soybeans for 25 min resulted in a folate decline of 57% and 5%, resp.102. Blanching before canning decreased folate amounts by 10% and 21% in soaked faba beans and chickpeas, resp. The folate content in the germinated faba beans declined by 32% after boiling, mainly due to the leaching and not degradation, as approximately 90% of the lost folate occurred in the cooking medium (which is also consumed as nabet soup). After deep-frying falafel balls made from the soaked faba bean paste, folate losses of 10% due to the heat treatment were observed412. In West Africa, cowpea seeds are usually prepared by using two different methods. The first consists of directly boiling the seeds in water for 1 h, and the second involves a pre-soaking step for one night followed by boiling in water for 25 min. Both methods resulted in a similar decrease in total folate concentration, the former by 38% and the latter by 43%. However, the latter is recommended because of improved folate bioavailability. During a pre-soaking step, enzymatic interconversion of folate vitamers takes place in favour of 5-methyl-THF, which is considered the most bioavailable of all the vitamers present in seeds413. Folate losses of 26% and 29%, compared to raw soybeans, occurred during the preparation of tempeh, involving soaking, dehulling, boiling, and fermentation, and that of soymilk, involving soaking, blanching, milling, and homogenization, resp. Deep-frying of tempeh and ultra-heat treatment of soymilk caused a folate decline of 21% and 14%, resp., in comparison to unprocessed products414. Likewise, the preparation of tofu, involving soaking, milling, boiling, coagulation, and pressing, led to 60% losses of folate compared to raw soybeans. Most of the lost folate was found in the whey after pressing415. Similar folate reduction during the processing of raw soybeans into tempeh and tofu was also observed in another study416. In one study, tempeh contained 68% more folate than the starting raw soybeans, apparently owing to using a different fungus strain in the fermentation step with much higher folate synthesis capability than in other cases417. Due to naturally high folate amounts, soybeans, tempeh, tofu, and soymilk are good dietary sources of folate, despite the losses during preparation414,415,416.
Breadmaking and other processing of cereals and cereal products
Breadmaking, a common process to prepare cereals for consumption, involves many variable factors, which can affect the folate levels in the end-product418. It is presumed that folates in bread derive not only from flour but also from yeast. A bread made using yeast usually contains more folate than the flour from which it was made, even though some folate losses occur during baking126,321,372,419,420,421. Yeast has a high content of folate, but also the ability to synthesize the vitamin during fermentation, and this may compensate for folate losses during baking. The sourdough fermentation is a traditional practice, especially in rye bread making, to improve the sensory quality and shelf-life of bread. A sourdough starter consists of lactic acid bacteria, whose contribution to enhanced folate levels, in contrast to yeast, is negligible372,418,422,423,424. Folate amounts in white bread differed up to 3.2-fold depending on a combination of various factors, such as the wheat flour extraction rate, leavening agents (baker’s yeast or baker’s yeast with sourdough), and prebaking and baking conditions (different sets of time and temperature)425. The white wheat bread had an 11% lower folate amount than the whole-grain one195. The folate content was 80% and 40% higher in the dough after fermentation and in the wholemeal rye bread, resp., compared to the starting flour, and similarly, 109% and 38%, resp., in the wheat bread. About 22–25% and 25–34% of the folate in the fermented dough was lost during the baking of rye and wheat bread, resp.316,372,419,426. Rye bread baked using lactic acid bacteria fermentation contained 31% less folate than those using yeast alone or yeast with lactic acid bacteria for fermentation372. The use of baker’s yeast during the baking procedure considerably increased (2.1–2.5-fold) the folate content in the wheat bread in comparison to the use of baking powder as a leavening agent195,372. Steamed whole-grain wheat bread contained 16% more folate than the oven-baked one195. During breadmaking, there was a decrease of added folic acid from fortified flour to bread stage by about 20% and 22% for wheat and rye bread, resp.420,427. Folic acid losses in fortified wheat breakfast rolls, Baladi bread, white pan bread, wholemeal pan bread, white baguette, and brown soda bread due to baking amounted to 19–25%, 15%, 24%, 32%, 22%, and 26%, resp. Consequently, folic acid averages of around 10–25% in the flour are necessary to compensate for the losses during baking and to achieve the required folic acid values in fortified bakery products302,426,428,429. The effect of breadmaking on the retention of two fortificants, folic acid and 5-methyl-THF, in wholemeal rye bread was compared. Breadmaking resulted in losses of 24% and 65% for folic acid and 5-methyl-THF resp. Retention of 5-methyl-THF fortificant during breadmaking varied depending on the bread size (so on baking time) and it was only 50% of that of folic acid in breads of the same size127. The amounts of folic acid and 5-methyl-THF used as fortificants in wheat bread were reduced by 10% and 47%, respectively due to breadmaking430. A loss of 5-methyl-THF fortificant in wheat bread baked in a commercial bakery amounted to 71%431. The influence of breadmaking on the folate content in white and whole-grain bread fortified with 20 g/100 g and 40 g/100 g fresh vegetables, either Swiss chard or spinach, as a natural source of folate, were studied. Although the magnitude losses of folate content of raw materials (wheat flour, wheat bran, dough, baker’s yeast, and vegetables) due to the heat treatment during breadmaking were about 45%, the fortification increased the total folate content by up to 190% and 100% in white and whole-grain bread, resp., without adverse effects on sensory properties, such as odour and taste, or overall consumer acceptance of the vegetable-fortified bread432. Injera is an Ethiopian fermented flatbread usually made from the whole grain gluten-free cereal tef. Both main processing steps during traditional injera preparation, i.e., fermentation mostly by lactic acid bacteria and baking, led to folate reduction. The folate content in injera was, on average, 32% lower compared to tef433.
Tarhana, a traditional Turkish dried soup based on a fermented mixture of wheat flour and yoghurt, is prepared through lactic acid fermentation, initiated by yoghurt or sour milk. The fermentation for 2–4 days resulted in a folate increase of 21–26%. Drying of tarhana brought about folate losses of 6%, 10%, and 17% at temperatures of 50 °C, 60 °C, and 70 °C, resp.434.
Nixtamalization is a process for the preparation of maize, which is used for the production of tortillas. It involves cooking and steeping dried corn in hot water with calcium hydroxide, discarding steeping liquids, and washing with subsequent removal of the pericarp (hulls). The resulting product is called nixtamal. Fresh nixtamal is wet-milled to make masa (nixtamal dough), which is formed into tortillas and baked (the traditional method), or it can also be dried and ground to make corn masa flour (nixtamalized corn flour), which is mixed with water to prepare masa that is used for baking tortillas. Nixtamal and corn masa flour can be fortified with vitamins and minerals435,436. Fortified tortillas made from masa produced through the traditional nixtamalization and wet-milling process, where folic acid was added to nixtamal before milling, contained 15%, 33%, and, in one study, even 80% less folic acid compared to the folic acid amount added to nixtamal (theoretical fortified level). No significant differences in folate levels were found in prebaked masa and baked tortillas. Baking as a high temperature/short time process (usually 290–300 °C for 42–50 s) had a minimal effect on folate content. It was observed that the commercial production step resulting in the greatest folate loss was the holding of hot, freshly ground, fortified masa (for 0.5–4 h) before baking. The losses in commercial masa increased significantly with prebake masa holding time. It was supposed that folic acid losses could be owing to utilization by lactic acid bacteria, which are naturally present in masa and whose count increased in masa during storage435,436,437. This assumption was not confirmed in an experiment with bacteria isolated from the dough (corn masa) samples from six commercial tortilla mills. Sterile fortified masa inoculated with bacteria, held at 56 °C for 3 and 6 h, replicating the conditions of freshly milled masa as held before baking in commercial tortillerias, showed folic acid losses of 66–79% in the first 3 h of incubation. Losses to the same extent were found in control non-inoculated sterile masa incubated under identical conditions after 3 and 6 h. In addition, the losses were comparable to those reported in the above-mentioned studies for the time between masa fortification and tortilla baking438. The decline in folic acid was not owing to bacteria. The traditional method produces substantial heat during the grinding of nixtamal to masa and involves the holding of hot masa until it is used. The combination of the high moisture content of masa and high masa holding temperatures before baking is the likely cause of folic acid chemical degradation when using the traditional method. Encapsulation of folic acid may help mitigate the problems435,438,439. On the other hand, fortified tortillas and tortilla chips prepared from masa made by mixing water with fortified corn masa flour lost 13% and no folic acid during tortilla baking and chip frying, resp., compared to fortified masa flour439.
During roasting barley malt for 20 min, the folate content was not affected at a temperature of 100 °C but declined continuously with increasing temperature up to 200 °C, at which folate was completely degraded. Barley malt may be used in several food products, and therefore, it would be beneficial to apply its pale form, as the coloured types are treated at higher temperatures resulting in lower folate content440. Extrusion decreased folate amounts by 26% and 28% in non-germinated and germinated rye grains, resp., compared to unprocessed grains441. Extrusion processing of corn and wheat flour blend and rice flour alone fortified with folic acid led to a folic acid decrease of 10% and 63%, resp., in extruded rice-shaped kernels442.
The average folate losses caused by cooking brown and white rice reached about 40% and 48%, respectively330,443. Rinsing before cooking had almost no effect on folate levels in brown rice but removed 73% and 88% of folate in fortified parboiled and non-parboiled white rice. Rinsing did not reduce detrimental inorganic arsenic in whole grain (brown) rice and eliminated 5–13% and 13–19% of arsenic in parboiled and non-parboiled white rice, resp. Cooking in variable amounts of water decreased folate contents, with increasing water excess, by up to 65–70% at a water-to-rice ratio of 10:1 for all three rice types (less in brown rice), but at the same time, efficiently reduced the quantity of inorganic arsenic by up to 60%, 70–83%, and 45–54% in cooked rinsed brown, parboiled, and non-parboiled white rice, resp.444. Losses of folic acid in fortified rice cooked by different methods (e.g., stir-frying and boiling, microwaving, and boiling) amounted to 8–66%, on average. The retention of folic acid seems to be more affected by the type of fortification (e.g., coating, cold extrusion, hot extrusion, parboiling, and sonication) than by the cooking method445,446,447,448,449,450,451,452.
Folate declines due to boiling were 36%, 15–30%, and 4–6% in spaghetti, white and yellow Asian noodles, and instant Asian noodles, resp.162,453. Preparation of fortified white and yellow Asian noodles, including dough kneading, cutting, and drying, led to minimal (1.3%) folic acid losses compared to fortified wheat flour. Preparation of fortified instant Asian noodles, involving additional processing steps (steaming, frying, and draining), led to a loss of 32%. Compared to the starting fortified flour, total folic acid losses after boiling all three styles of fortified Asian noodles amounted to about 41% in white and yellow and around 43% in instant noodles454. In other studies, no changes in the content of folic acid, which was used for the fortification of wheat flour, during the main four stages of instant fried Asian noodle manufacturing (mixing, sheeting and cutting, steaming, and frying) were observed455,456. A comparison of retention of folic acid and 5-methyl-THF used for fortification of flour during the noodle-making and the following boiling showed a very low, no significant losses in folic acid during both processes whereas a loss of 28% was found during the noodle-making, compared to fortified flour, and that of 57% during the noodle boiling, compared to fresh noodles, was observed when 5-methyl-THF was used as the fortificant. Compared to the fortified flour, the boiled noodles contained 69% less 5-methyl-THF fortificant457. Commercial unfortified durum wheat pasta lost 51% folate after boiling. In commercial durum wheat pasta fortified with folic acid, folic acid content declined by 72% after boiling458. The effects of two preparation methods on folate losses in rice noodles fortified with folic acid were examined – raw noodles (i.e., extruded kneaded dough) were boiled or steamed. The folate losses observed after the boiling (process A) or steaming (process B) of raw noodles, drying of fresh noodles prepared by either of the two types of noodle processing methods, and cooking (boiling) of dried noodles prepared using processes A or B were 42% or 20%, 0%, and 72% or 53%, resp., compared to the initial content of added folic acid in fortified rice459. In another study, the influence of rice flour particle size (≤63, 80, 100, 125, and 140 μm) on the retention of folic acid fortificant during rice noodle processing was analysed. Compared to 100% of folic acid in fortified rice flour, the amount of folic acid in the five types of rice noodles decreased by 50–56% after boiling the raw noodles and by 7–13% after cooking (boiling) the dried noodles before consumption. The reduction in the particle size of rice flour led to a decline in the losses of the fortificant460.
Processing of eggs, milk, and meat
Eggs lose 0%, 19%, 2–39%, 11–47%, and 10–50% folate, resp., due to poaching, scrambling, boiling, frying, and baking92,100,102,104,162,329,375,376,382,383,443. In milk, heat-induced folate decrease amounted to 8–10%, 4–20%, and 42–45% during pasteurization, ultra-heat treatment, and sterilization, resp. Modern technologies reducing oxygen levels in the milk before ultra-heat treatment increase folate retention in the processed milk75,149,228,461,462,463. Folate content declined by 27%, 35%, 41%, and 52–63% in the beef after boiling, in the pork loin after pan-broiling, in the chicken breast after boiling, and in the mackerel after shallow-frying, resp.162. Steamed mackerel (i.e., the common form sold) lost 24% of folate during frying in soybean oil; the estimated total loss of folate in the mackerel by steaming and frying was 74%443. The influence of stewing and roasting on folate content in white and dark, fresh or frozen, chicken meat was also studied464. Sous-vide (60 °C/75 min) and steaming (100 °C/30 min) did not significantly affect folate amounts in chicken liver, whereas another sous-vide (75 °C/45 min), grilling without oil addition (200–220 °C/4 min), grilling with oil addition (170–200 °C/6 min), and baking (180 °C/30 min) decreased them by 16%, 9%, 22%, and 42%, resp., compared to raw liver465. Manufacturing of fortified sausages, including cooking in a steam oven at 72 °C, did not influence the content of added folic acid466.
Food preservation techniques – canning, ionizing irradiation, and high pressure processing
The effects of industrial canning on folate content in green beans were investigated. Compared to fresh vegetables, folate content lessened by 10% in green bean cans (30% in beans alone, but most of the lost folate was retained in the covering liquid), mainly owing to the sterilization step with no significant impact of washing and blanching steps during the canning chain397. Canning reduced folate by up to 40% in table beets with increasing processing time and temperature, while it did not cause any significant folate amount changes in green beans, compared to unprocessed vegetables467. Industrial canning, including soaking, blanching, and autoclaving, resulted in losses of 0–20% and 24% in faba beans and chickpeas, resp., in comparison with raw legumes. Soaking of legumes brought about folate increase (probably due to enzymatic de novo synthesis from initiated germination), blanching, and mainly autoclaving led to folate decline. The folate lost from legumes during autoclaving was recovered in the canning medium412,468. In cans, folate concentrations are usually equilibrated between the vegetables and the covering liquid379,397,469. The folate content in strawberry jams was 9–16% less than in the initial frozen fruit49.
Ionizing radiation (accelerated electrons, gamma rays, and X-rays) is used as a non-thermal preservation technology for extending shelf life and increasing the safety of food348,470. Electron-beam irradiation (2 kGy) decreased folic acid levels by about 20–30% in hamburgers and sausages fortified with folic acid471,472. Wheat flour fortified with folic acid showed no significant loss in its folic acid content following electron beam irradiation at doses of up to 1 kGy (doses required for disinfestation). Around 30% of folic acid was degraded when fortified flour was irradiated at doses of 5 and 10 kGy. The higher stability of folic acid in flour than in meat products is explained by differences in moisture. Non-solubilized folic acid in dry materials is not sensitive to irradiation treatment, while it is easily degraded in aqueous solutions348,349. Gamma-irradiation at doses of 1, 2, and 5 kGy did not influence the folate amount in watercress, whereas at a dose of 2 kGy, folate content declined by 34% in buckler sorrel. Different sensitivities were likely because of the plant matrix effect179. Folate amounts in gamma-irradiated baby-leaf spinach declined with increasing dose of irradiation from 0.5 to 2 kGy reaching losses of about 24% at the highest dose, irrespective of whether the treatment took place in the air or nitrogen atmosphere470.
High (hydrostatic) pressure processing is a novel technique for the preservation of food products in a gentle way, allowing better retention of food sensory and nutritional quality; it inactivates microorganisms in foods due to permeabilization of cell membranes394,473,474,475,476,477. Effects of high pressure processing on folate stability were investigated in model solutions as well as in vegetables (carrot, asparagus, green beans, yardlong beans, winged beans, leeks, cauliflower, and broccoli) and fruits (orange, kiwi, and papaya). Depending on processing conditions (pressure-temperature-time combinations), various, sometimes marked, folate losses were observed365,369,374,394,473,475,478,479,480,481. Folates during that processing were shown to be more stable, e.g., in fresh-cut papaya, freshly squeezed orange juice, and kiwi puree; all those fruits are naturally rich in ascorbic acid, which may protect folates against pressure and heat degradation365,477,479. Folate stability during high pressure processing is comparable to that during heat treatments. Though high pressure processing is generally considered to lead to better preservation of vitamins, compared with thermal treatment, this obviously is not the case for folates106.
Storage
Folate losses can occur during the storage of foods, depending on the storage conditions and duration. Green beans, leeks, and cauliflower lost no, 15%, and 25% of folate, resp., during storage in a refrigerator at 4 °C for 24 h394. No folate losses occurred in untreated green beans, yardlong beans, and winged beans during storage in a refrigerator at 4 °C for 10 days, while after high-pressure treatment preceding the storage, profound folate degradation happened, which was positively proportional to the increase in pressure and extending of holding time during treatment473. Fresh spinach commercially packaged in polyethylene plastic bags was stored at 4 °C, 10 °C, and 20 °C °C for 8, 6, and 4 days, resp. Based on the visual colour and appearance, spinach was commercially unacceptable after those storage times (shelf-life values). Folate levels decreased with increasing storage time at approximately the same rate for each temperature, reaching a loss of about 47% at each temperature and shelf life compared to the initial folate amount. Therefore, producers and retailers should maintain storage temperatures as low as possible to minimize the vitamin losses in fresh spinach. Consumers should keep fresh spinach refrigerated and use it as close as possible to the time at which it was purchased482. Folate content in frillice, rocket, and iceberg lettuce was reduced by 2–40% after storage at room temperature (22 °C) in regular light after 2–4 h to simulate the conditions in lunchtime restaurants, depending on whether samples were stored as whole leaves, or small torn or cut pieces. Storage of lettuce in a refrigerator at 4 °C for 8 days led to folate losses of 14%69. No significant changes in folate content occurred in choy sum during storage at 4 °C in the dark for 3 weeks182,483. Storage of watercress and bucker sorrel in polyethylene bags at 4 °C for 7 and 12 days, resp., gave rise to a loss of 37% in the former and no alteration in the latter in folate content179. Storage of fresh sweet corn cobs in bracts at room temperature (25 °C) or in a refrigerator (+4 °C) caused folate reduction of 32% and 24% in 3 days, and that of 54% and 55% in 7 days, resp.398. The percentage of folate losses in strawberries during refrigerated storage at 4 °C amounted to 21%, 42%, 55%, 78%, 88%, and 93% on days 1, 2, 3, 4, 5, and 6, resp., compared to fresh fruits (day 0). Therefore, strawberries should be consumed within a day or two after harvest before the folate losses reach more than 50%178. In another study, the folate content in fresh strawberries declined by 16% and 29% during 3 and 9 days of storage, resp., at 4 °C in the dark; after 9 days, strawberries were considered not fit to be eaten. On the other hand, the storage at room temperature (20–25 °C) in daylight, mimicking the procedure of commercial retailing, led to folate losses of 27% and 38% after 1 and 3 days, respectively49. Strawberry puree lost no, 13%, 43%, and 84% of initial folate content after 1, 2, 3, and 4 days, resp., storage at 7 °C in the dark484. Potatoes are often stored at low temperatures for several months before processing. Folate concentrations increased in tubers stored in the cold. The extent of the increase, which seems to be genotype dependent, was about 2-fold at 9 °C after 4 months or up to 1.8-fold at 4 °C after 7 months151,152.
Storage of blanched vegetables at −20 °C for 12 months did not affect folate content in peas, cauliflower, cabbage, and spinach and that for 6 months in green faba beans395,468. In another study, the 5-methyl-THF content in blanched vegetables decreased with the time of frozen storage at −18 °C by 98% in cauliflower, 24% in broccoli, 39% in peas and spinach just after 3 months, and by 82–98% in all of them after 6 months. In green and yellow beans, significant losses of 75% and 95%, resp., were observed no earlier than after 9 months of frozen storage396. No loss of 5-methyl-THF was detected in spinach, broccoli, potatoes, strawberries, apples, oranges, and bananas frozen at −60 °C after storage for 12 months485. The fresh kernels of sweet corn stripped from the cobs stored at −20 °C lost 62% of folate after 4 months398. Frozen products can lose folate during storage due to oxidation, in contrast to canned products, which can lose more during the initial thermal treatment, but then are relatively stable because of the lack of oxygen293.
Folate losses reached values of 76.4–79.7% in glass-bottled tomato juice after storage in the dark for twelve months, irrespective of storage temperature (8, 22, and 37 °C)486. Folic acid degradation in fortified vitamin juices during long-term storage was studied. The juices were stored in the dark and light (500 lux for 10 h/day) in light-transmissive (clear PET and glass) and non-transmissive (brown PET and cardboard) packaging at 18 °C, reflecting common storage conditions, e.g., at a supermarket. Average decreases in folic acid concentrations of 36% (dark) and 39% (light) after 6 months and 47% (dark) and 50% (light) after 12 months of storage were observed487. Natural folates and added folic acid in fortified orange juice stored below 8 °C in the dark were stable during shelf life for 35 days (best before date) and during one-week simulated household consumption. The high endogenous ascorbic acid content in the juice might have prevented oxidative degradation of natural folates and added folic acid. This suggests that orange juice may be considered a good source of natural folate regarding content and stability during storage and a suitable vehicle for folic acid fortification488. Sea buckthorn juice was stored in the dark under two household storage conditions (6 °C and 25 °C) and accelerated aging conditions (40 °C) for up to 7 days. The folate content was almost unchanged during the storage at 6 °C after 7 days. The juice showed folate losses of 5% at 25 °C and 17% at 40 °C after 7 days of storage402.
When wheat grains and whole-grain powder were stored in closed paper bags at room temperature for 8 months, the folate loss occurred earlier in powder (after 2 months of storage) than in the grains (after 4 months of storage). The average folate losses in grains and powder after 6 months of storage were 17% and 28%, resp., indicating that folates were more stable in the grains than in powder up to 6 months of storage. The 8-month storage led to a more extensive folate reduction both in the wheat grains (26%) and the whole-grain powder (30%)321. Storage of cereal and pseudocereal wholemeal flours in paper bags at 20 °C and 50% relative humidity for 3 months caused a folate decrease of 45%, 37%, 19–38%, 41%, and 23% in wheat, rye, amaranth, buckwheat, and quinoa, resp.130. Factors influencing folic acid content in fortified wheat flour were studied too: packaging (paper bags or multilayer aluminium/PET bags), temperature (25 °C or 40 °C), relative humidity (65% or 85%), and duration (6 months). In flour packed in multilayer bags (non-permeable to oxygen and humidity), no significant folic acid losses were observed after 6 months, irrespective of temperature and relative humidity. In flour packed in permeable paper bags, folic acid content decreased by 17–19% after 3 months when flour was stored at 65% relative humidity, regardless of storage temperature. At 85% relative humidity, folic acid decreases of 21–22% at 25 °C and 40–49% at 40 °C were found after 3 months of storage. In flour packed in paper bags and stored for 6 months, folic acid losses of 15–20% at 25 °C and 20–22% at 40 °C during storage at 65% relative humidity and those of 22–27% at 25 °C and 47–53% at 40 °C during storage at 85% relative humidity were observed. The observed folic acid losses in fortified flour packed in paper bags were most likely due to oxidative degradation. Therefore, the choice of suitable flour packaging, which is not permeable to both oxygen and moisture, is of critical importance in limiting losses of added folic acid, and it must be taken into account when planning a fortification program in countries with a tropical environment. Co-fortification with or without ferrous sulfate did not have any significant effect on the folate retention in wheat flour fortified with folic acid, irrespective of storage conditions and packaging489. There was no significant decrease in folic acid fortificant content during the six-month shelf life of fortified corn masa flour439. The average folate losses in rice (brown and milled) due to storage in paper bags for 1 year reached nearly 23%330. Storage of fortified rice under accelerated conditions (fluorescent light at 40 °C) in different packaging for 3 months caused no significant changes in folic acid content446. Folic acid losses of 0–18% and 24–43% after 3 and 9 months of storage under typical tropical conditions (40 °C and 60% relative humidity), resp., were observed in rice extruded products prepared from rice flour fortified with folic acid and various iron compounds. Increased iron concentration levels resulted in faster degradation and more loss of folic acid490.
Storage of Baladi bread in polyethylene bags at ambient room temperature (about 20 °C) in the dark (cupboard) according to household practice or chilled (about 5 °C) for 48 h (i.e., shelf-life) did not significantly affect folate content, compared to bread after baking421. Storage of different rye breads at −18 °C for 2 weeks did not influence folate contents. However, during prolonged storage, folate contents gradually dropped, reaching 25% and 38% losses in the bread leavened with baker’s yeast and in the bread fermented with sourdough, resp., after 16 weeks, likely due to air oxidation. Higher folate content reduction in the bread made using sourdough was explained by its acidic pH, which is less favourable for folate stability, as mentioned above424. Losses of fortificants in fortified wheat bread stored in paper bags at room temperature (21 °C) for 7 days amounted to 3% for folic acid and 82% for 5-methyl-THF430. Folic acid was stable in fortified wheat breakfast rolls for 90-day storage at −20 °C429. Storage of fortified tortillas and tortilla chips in sealed low-density polyethylene bags at 22 °C and 65% relative humidity for 2 months, common shelf life for these products, led to a folic acid decrease of 13% and 9% in the respective products439.
The vacuum-packaged tortillas and the vacuum-packed freeze-dried broccoli au gratin were stored either on Earth or aboard the International Space Station at room temperature for 880 days. The folate contents declined and were not significantly different in flight and ground samples during the storage. Folic acid levels in tortillas were about 15% and 45% lower after 13 and 880 days, resp., compared to the initial analysis. A folate decrease in broccoli amounted to about 15% and 22% after 13 and 880 days of storage491.
Folate was stable in cold stored eggs (4 °C) for four weeks492. Similarly, no changes in the folate content were observed in eggs stored at refrigerator temperature (4–7 °C) or room temperature (18–20 °C) for 27 days (i.e., from the date of laying to the best before date). The same was confirmed for novel eggs enriched with natural folate through the addition of supplemental folic acid to the hen’s feed493. The folic acid level in sausages fortified with folic acid was retained after 3 months of refrigerated storage (4 °C)466. No alteration or a decline of 81% occurred in folate amounts in whole-milk powder during storage in the nitrogen or oxygen atmosphere, resp., for 57 days. Similarly, in skimmed milk powder stored at 37 °C, folate content decreased by 13% and 30% in nitrogen and by 86% and 88% in oxygen atmosphere after 25 and 105 days, resp. Exclusion of oxygen from the package is necessary to prevent folate degradation during the long-term storage of milk powders494. Folate losses in ultra-heat treated milk packed in Tetra Pak stored at 24 °C amounted to 11% and 32% after 12 and 20 weeks, respectively463.
Enhancement of folate content through processing
There are food process techniques that can elevate the content of folate. Before cooking pulses, soaking is a common processing step employed to soften and make the seeds more digestible. Soaking, probably due to enzymatic de novo synthesis activated upon the initiation of germination, increased folate content by 46%, 28%, 16%, 65%, 81%, and 13% in mung beans, adzuki beans, cowpeas, faba beans, peas, and common beans, compared to raw pulses. In addition, some folate diffused into the soaking water; it represented, on average, 15% of the total folate enhancement during soaking411. In another study, an increase in folate content during soaking in faba beans and chickpeas by 39–51% and 51–66%, resp., was observed412. Folate levels increased in soybeans by 10–15% after soaking for 12 h and then declined likely owing to dissolution in water495. The behaviour of folate during soaking depends on various factors, e.g., duration, seed-to-water ratio, temperature, and to a great extent on the legume species, which differ in their germination capacity413.
Germination could be more beneficial than soaking to enhance the production of folates in seeds for human consumption407,496. Germination of plant seeds is a biological process used to obtain a typical flavour and texture in foods and a natural way to increase folate levels. It has been applied for a long time302,497,498. Germination of faba beans, chickpeas, brown lentils, white beans, black-eyed peas, soybean, mungbean, and cowpea resulted in an up to 1.77, 2.4, 3.1, 2.8, 2.6, 3.7, 4.3, and 2-fold increase in folate content406,412,496,499,500,501,502. Therefore, germination of legumes can be recommended to produce foods with enhanced folate content. For example, household preparation increased the folate levels in germinated faba bean soup (nabet soup) by 100% and in bean stew (foul) by 20%, compared to raw beans412. The novel industrial canning process for dried faba beans, which newly involved pre-germination of soaked dried seeds, led to a 52% higher folate content in the novel product compared to the conventional canned beans468. In germinated rye, wheat, and barley, the folate increased by up to 5.3, 5.7, and 7-fold, resp.316,421,423,440,441,497,503,504. Germinated cereal grains could serve as functional ingredients for the breadmaking industry. It was shown that oven-drying of germinated wheat grains at 50 °C did not affect the folate content, so it did not decrease the improved nutritional value of germinated grains421,503. By the addition of germinated wheat flour to the native one, bread with 66% more folate compared to conventional Egyptian baladi bread could be prepared421. Germination enhanced folate content in pseudocereals, namely by 21% and 26% in amaranth and buckwheat, resp.134. Increased folate levels were also observed during the germination of maize seeds505,506.
Beers contain various amounts of folate owing to the differences in the brewing process and the choice of raw materials, which influence not only the sensorial profile but also the level of health-positive compounds, including folate. In small- and large-scale brewing, the folate content increased during mashing, decreased after wort boiling, and increased during fermentation. Large-scale brewing showed a decline in folate between the end of maturation and the final bottled beer because of operations that do not occur in small-scale brewing, such as filtration, pasteurization, and dilution to the desired gravity with deoxygenated water302,440,507,508,509,510,511,512,513,514,515.
In wines, folate amounts vary, like in beers. There was no significant difference between red and white wines in the folate content range. The chemical composition of wine is determined by two factors: the initial grape must and the fermentation by yeast. The folate content of wine is generated primarily by the yeast during fermentation rather than being present at appreciable levels in the starting grape must. There is a large variability in the ability of the different yeast strains to produce folate516.
Owing to fermentation, folate content rises not only during breadmaking, as reported in this paper, but also during the production of fermented dairy products. For example, yoghurt usually contains 2-fold higher amounts of folate compared to the original milk, dependent on starter cultures used (bacteria species and strains)302,517,518,519,520.
Folate content in plants may be increased by stimulation of folate biosynthesis. Enhanced folate accumulation stimulated by red light irradiation and amino acid addition in wheat seedlings, phenylalanine addition in hydroponically cultivated spinach, cool and warm white light in Lamb’s lettuce leaves, and salicylic acid in coriander foliage and foxtail millet panicles were reported504,521,522,523,524,525,526.
Changes in folate content during ripening (i.e., different maturity stages) were studied in corn398,527,528, cowpea leaves399, winged beans529, potato tubers530, faba beans468, tomato4,5,6,181,486,531,532,533,534, avocado531, strawberries49, banana531, Australian green plum535, and papaya38,531. Treatment by exogenous ethylene, as a common postharvest practice to trigger the ripening of mature green fruits before placing them on the shelf, caused a 24% and 51% folate increase in tomatoes and bananas, resp., a 26% folate decrease in papayas, and no change in avocados, compared to non-treated fruits531.
The content of folate in eggs was affected by the rearing system; eggs from the organic farming system contained significantly more folate (by about 36%) than those from the free range, barn, and cage systems, in which the folate contents were comparable92. In another study, significantly higher folate levels were found in eggs from the free range system than from the barn one (by 58%)493. There was no significant difference in amounts of folate in eggs from three different breeds of hens raised on farms fed with three different feeds (one organic and two conventional)104. Supplementation of laying hens by feeding with folic acid brought about a 2–3-fold increase in egg folate content. Moreover, folic acid from feed was converted to natural folate vitamers, especially 5-methyl-THF492,536,537,538,539,540,541,542. Folic acid in total egg folate content represented at most 10%, a level which would be converted into biologically active folates by humans after ingestion. Folate-enriched eggs produced in this way could offer an alternative without the safety concerns related to folic acid-fortified foods493,536,542,543.
Food ingredients influencing folate stability
Some food ingredients and natural compounds may influence the stability of folates. Ascorbic acid (vitamin C) protected folates, naturally present in foods or folic acid and 5-methyl-THF added as fortificants, against degradation by heat, oxidation, and ultraviolet radiation during processing and storage in model systems and food products. The addition of ascorbic acid could be considered as a strategy for preventing folate degradation during processing193,346,365,371,430,431,457,475,477,544,545,546,547,548,549. Vitamin C and, to a higher extent, vitamin E added to egg yolk preserved 5-methyl-THF from thermal oxidative degradation during yolk thermal pasteurization or spray-drying347. The thermal stability of 5-methyl-THF increased in skim milk due to the presence of casein and folate binding protein, and in soymilk due to the presence of phenolic antioxidant compounds545. Tannic acid, a polyphenolic compound used as a food additive, improved the photostability of folic acid against ultraviolet light in solution and in gummy, a common delivery system for vitamins in supplements550. Similarly, the photodecomposition of folic acid by ultraviolet radiation was inhibited or delayed in varying degrees by natural phenolic compounds, such as hydroxycinnamic acids (e.g., caffeic acid, ferulic acid, and p-coumaric acid), flavonoids (e.g., quercetin and epigallocatechin gallate), stilbenes (e.g., resveratrol), etc., with caffeic acid being the most effective. The findings are useful for the protection of food and beverages against undesired effects of light exposure, i.e., for preventing premature quality loss and for the co-encapsulation of folic acid with those antioxidants as an effective way to protect the vitamin B9551,552,553. Also using green tea-enriched extracts containing epigallocatechin gallate and epigallocatechin would be a simple and relatively inexpensive method to preserve 5-methyl-THF against air oxidation554.
Folic acid loss occurs in solutions upon heating in the presence of reducing sugars, such as fructose, glucose, lactose, and mannose, via the nonenzymatic glycation reaction (a Maillard-like reaction). The reaction can be expected during thermal food processing, particularly in dairy products such as heated milk, milk powder, and infant formula, containing an excess of lactose, in cereal-derived products such as biscuits and breakfast cereals, containing maltose, and in heat-treated fruits, e.g., pasteurized fruit juices, rich in fructose and glucose555. In baked model cookies, made from wheat flour fortified with folic acid and different carbohydrates, the reducing monosaccharides glucose and fructose were most effective in depleting folic acid by about 50% of its initial content, the reducing disaccharide lactose decreased folic acid by 23%, and non-reducing disaccharide sucrose did it by about 15% only at the end of baking likely due to the cleavage into glucose and fructose. Therefore, baked products should be made from sucrose rather than from glucose and fructose when a maximum of folic acid has to be retained. In particular, heated products for diabetics made from fructose or heat-treated foods, sweetened with corn syrup or high-fructose corn syrup, may contain lower amounts of folates due to glycation reaction556. Fructose significantly accelerated the thermal degradation of the solution of 5-methyl-THF, but glucose did not. Ascorbic acid addition to folate with fructose before heating prevented 5-methyl-THF degradation557. The importance of folate glycation in fruits and vegetables remains unclear, given that antioxidants, such as ascorbic acid and phenolic compounds, are inherently present. There is no data regarding fruits and vegetables on the balance between protection by antioxidants and degradation by reducing sugars. Moreover, ascorbic acid is often added to processed products. The added amount of ascorbic acid and its own degradation rate might therefore determine whether and when glycation of folates can take place106.
A food constituent of particular interest is folate-binding protein (FBP) occurring in milk. It possesses different affinities to various folate vitamers, with the highest for synthetic folic acid. Its binding affinity is also influenced by the pH of the environment. Like all proteins, FBP is heat-sensitive, and denaturation affects its folate binding capacity. Raw milk retains its native FBP content whereas ultra-heat treatment of milk inactivates FBP. Data on pasteurization are inconsistent. FBP is destroyed by heat beyond the temperature of 72 °C. In pasteurized milk, FBP is only partly denatured by heating, and folate remains bound to FBP. Ultra-high-temperature milk (UHT, heated for 145 °C/5 s) and yoghurt (heated for 90 °C/10 min before inoculation) lose their FBP through denaturation due to high processing temperatures. Cottage cheese and whey products contain FBP, while hard cheese contains negligible amounts, probably due to the separation of the whey proteins during manufacturing. Freezy-drying or spray-drying for the manufacture of milk powder seems to retain most of the FBP in an active state. FBP increases the stability of folates against degradation over a range of temperatures and pH conditions. On the other hand, human in vitro and in vivo studies revealed that FBP decreases the absorption of folates from the gastrointestinal tract. This effect of FBP is dose-dependent, and it also depends on the folate form. Folic acid is more affected than 5-methyl-THF owing to the different affinities of FBP for various vitamers. The bioavailability of folates from dairy products declined with increasing amounts of FBP, in order, UHT milk, fermented milk, and pasteurized milk. For example, the bioavailability of folic acid from fortified pasteurized milk was non-significantly 6-26% less relative to that of folic acid from fortified UHT milk. It may be of importance in infants when milk formulas and gruels are the main dietary source of folate. Producers of those products should consider either denaturing the FBP or replacing folic acid with 5-methyl-THF as fortificant. The effect of bovine FBP on folate absorption for adults should be negligible, since the daily intake of FBP originating from dairy products in a mixed diet is low, probably less than 10% of the total folate intake. Exceptions could be consumers with high intakes of cottage cheese and whey products which seem to be quite rich in active FBP75,147,149,188,189,224,227,228,558,559. The presence of FBP in plants has recently been reported560. The role of FBP in the stability and bioavailability of folates is still unclear and requires further research.
Increasing fortificant stability by encapsulation
Encapsulation may increase the stability of folic acid, commonly used for food fortification, during food processing and storage561,562,563,564,565,566. Folic acid encapsulated in zein fibres and nanocapsules showed resistance to thermal treatment and ultraviolet irradiation exposure in contrast to unencapsulated folic acid567. Folic acid incorporated in edible alginate/chitosan nanolaminates was more stable under ultraviolet light exposure than non-encapsulated folic acid568. The influence of processing and storage on the stability of encapsulated folic acid in apple and orange juices was studied. Folic acid encapsulated by using mesoporous silica particles was more stable, compared to free folic acid, when the apple or orange juices were sterilized, exposed to visible or ultraviolet light, and stored at 4 °C for 28 days. Thermal, light, and storage stability of free and encapsulated folic acid was much higher in orange juice, which is rich in ascorbic acid, in contrast to apple juice, likely due to the above-mentioned protective effect of ascorbic acid546. The stability of encapsulated folic acid (two different matrices: whey protein concentrate and resistant starch, and two encapsulation techniques: electrospraying and nanospray drying) during storage in water solution and in dry conditions under natural light and darkness was investigated. Greater encapsulation efficiency was observed for the protein-based capsules. The best results in terms of folic acid stabilization in the different conditions assayed were also obtained for the protein-based capsules, although both materials and encapsulation techniques led to improved folic acid stability569. Entrapment in β-lactoglobulin and lactoferrin coacervates showed good protection for folic acid against degradation during storage treatments, such as freezing and freeze-drying570,571. Microencapsulation of 5-methyl-THF, a mentioned less stable alternative fortificant, in pectin-alginate gel enhanced its thermal stability during extrusion processing of starch, particularly at elevated extrusion temperatures373. 5-methyl-THF encapsulated with modified starch used for fortification of wheat flour had higher stability than the free compound during the breadmaking, the following storage of bread slices in polyethylene bags for 3 and 7 days at room temperature, and the toasting. The losses of the fortificant were further markedly decreased when it was co-encapsulated along with sodium ascorbate, which enhances resistance of 5-methyl-THF to thermal oxidative degradation as reported above431. Similar results were obtained after baking and 7 days of storage in wheat bread fortified with free or microencapsulated 5-methyl-THF, with or without sodium ascorbate. Skim milk powder was used for encapsulation430. The binding of folic acid to proteins, such as whey protein isolate, casein, β-lactoglobulin, α-lactalbumin, and bovine serum albumin decreased folic acid losses due to photodegradation induced by ultraviolet radiation. All those proteins may be considered carrier materials suitable for folic acid delivery in functional foods572,573,574,575,576,577. The stability of folic acid may be improved not only by encapsulation but also by the synthesis of some derivatives. A novel derivative, 6-deoxy-6-[1-(2-amino)ethylamino)folate]-β-cyclodextrin, showed enhanced photostability against ultraviolet light compared to free folic acid and may provide a more stable source of folate as a food additive in both the solid state and aqueous solution578.
Industrial production of folate
Folic acid, which does not occur naturally in foods, is industrially produced by chemical synthesis. It is used not only in fortified foods but also in dietary supplements1,34,98,139,222,579,580,581,582,583,584,585,586,587,588,589,590,591,592,593,594,595,596,597,598,599,600,601,602,603,604,605,606,607,608,609,610,611,612. The pharmaceutical industry offers folic acid for therapeutic and prophylactic use. The major part, about 75%, is used for feed enrichment in animal nutrition86,245,353,613,614,615,616,617. All commercial syntheses are based on the concept of a three-component, one-pot reaction of triamino-pyrimidinone with a three-carbon compound of variable structure (e.g., halogen derivatives of propanal, propanone, and propane) and p-aminobenzoyl-L-glutamic acid to yield folic acid. There are some alternative approaches for the synthesis of folic acid. In a two-step procedure, 2-hydroxymalondialdehyde is firstly condensed with p-aminobenzoyl-L-glutamic acid, forming a diimine, which subsequently reacts with triamino-pyrimidinone to obtain folic acid. Another viable method starts from 6-formylpterin. Condensation of 6-formylpterin with the diester of p-aminobenzoyl-L-glutamic acid, followed by reduction of the Schiff base with sodium borohydride and hydrolysis, leads to folic acid1,86,353,618. The synthetic yield of folic acid is around 84%618,619,620,621,622,623,624. 5-methyl-THF, which may be used as an alternative to folic acid for food fortification and dietary supplementation, is produced synthetically from folic acid1,353,625,626,627.
Attempts have been made to develop a biotechnological method of folate production for a future switch from current chemical manufacturing to a sustainable fermentative one. Folate production capacity has been studied in various strains of the yeast Saccharomyces cerevisiae and yeast species isolated from environments such as marine and tropic milieus, including fruits, vegetables, fish, and insects, as well as in some bacteria103,628. Recently, the yeast Scheffersomyces stipitis has been shown to produce folate at concentrations of 3.4 mg/L under optimized cultivation conditions, the highest value obtained during fermentation in microorganisms with natural production ability629,630. Genetically modified folate overproducing strains of some fungi and bacteria have been constructed, e.g., Ashbya gossypii, Escherichia coli, and Bacillus subtilis, the first being the best folate producer reported to date with folate titers of 6.6 mg/L (i.e., 146-fold higher than the wild strain)631,632,633,634. However, despite the improvements in folate production by microorganisms that have been achieved, the industrial microbial production of folate is still far from being economically feasible due to very low yields. The fermentation process is not competitive with low-cost industrial chemical synthesis as yet. Thus, more efforts are needed to increase folate production levels through metabolic engineering1,631,632,634,635.
Fortification
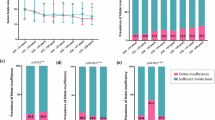
Clinical and epidemiological data show that folate deficiency is widespread in many populations. Limited bioavailability and loss of folate during food processing and storage, and false nutrition or malnutrition, make the possibilities of reaching recommended targets for folate intake through food folates alone still rather uncertain. Fortification, the process of adding micronutrients to an appropriate food vehicle in order to correct or prevent community-wide deficiencies, has been proposed as one way to enhance folate intake. The advantage of food fortification is, compared with supplementation, that there is no need to change dietary habits121,122,194,233,333,580,581,596,631,636,637,638,639,640,641,642,643,644,645,646,647,648,649,650,651,652,653,654,655,656,657,658,659,660,661,662,663,664,665,666,667,668,669,670,671,672,673,674,675,676,677,678,679,680,681,682,683,684,685,686,687,688. Over 70 countries, including countries of North America, South America, West, East, and Southern Africa, Central and Southeast Asia, Australia, and New Zealand, have implemented mandatory folic acid fortification of foods until 2022, starting with the United States of America in 1998680,689,690,691,692. In Europe, only Moldova, Kosovo, and, most recently, the United Kingdom mandate fortification of food with folic acid693. Voluntary fortification of food products with folic acid takes place in a lot of countries (in some of them also along with the mandatory one), e.g., Canada, the U.S.A., the Dominican Republic, Sierra Leone, Sudan, Eswatini, Saudi Arabia, Kuwait, Iraq, India, Bangladesh, Myanmar, China, and many European countries458,587,612,614,642,654,660,688,694,695,696,697,698,699,700,701,702,703,704,705,706,707,708,709. An interesting economic analysis of possible folic acid food fortification is available from the Netherlands710.
The most common food vehicles for mandatory folic acid fortification are wheat flour, maize flour, and rice328,331,333,637,653,680,689,711,712,713,714,715,716,717,718,719,720,721,722,723,724. On a voluntary basis, foods such as breakfast cereals, cereal bars, cereal snacks, crisp bread, pasta, baby foods, biscuits, buns, cakes, pastries, milk, milk powder, dairy products, sweets, fruit juices, coffee, cocoa, soft-drinks, soy products, dried soups, margarine, fat spreads, and table salt, are fortified with folic acid98,122,174,224,300,458,559,614,642,643,654,660,666,671,688,692,694,695,696,697,698,701,725,726,727,728,729,730,731,732,733,734,735,736. Further strategies for fortification have been investigated, e.g., fortification of salt737,738,739,740,741,742,743, sugar738, tea744,745, mineral water746,747, and bouillon cubes748,749.
Biofortification
Biofortification refers to a strategy where conventional plant breeding techniques, genetic engineering, and agronomic interventions are used to enhance the nutrient content of food crops. Biofortification has the advantage of being more sustainable by eliminating the need to fortify each batch of food, as is the case with fortification653,750,751. Biofortification, i.e., the enhancement of natural folate content in plants, holds the potential to reach the required folate levels, which are low, particularly in staple crops, such as rice, potato, maize, wheat, and cassava752,753,754,755. Biofortification by conventional breeding relies on an inheritance of favourable quantitative trait loci from sexually compatible parental lines. It is constrained by the natural variation of the desired trait present in the available collection of crop germplasm, as well as by being time-consuming. On the other hand, conventional breeding, though limited in its potential for folate level improvement, is promising, as it might face lower regulatory restrictions compared with genetic engineering, hence allowing a more rapid implementation in agriculture, reaching the populations in need756. Breeding approaches focus on the pursuit of sufficient folate variation in target plant germplasm. Screening vast collections of germplasm may reveal greater diversity and thereby favour the applicability of breeding strategies25,752,757,758. Variation in folate levels has been analysed in different wheat131,759,760,761,762,763,764,765,766, barley458,762,767, rye131,762,768,769, oat762,770, rice330,452,765,766,771,772,773, maize766,774,775, foxtail millet776,777, potato151,152,778,779, lentil63,780,781, soybean782,783,784, faba bean411,785, common bean411,781,786,787,788, adzuki bean, mung bean, cowpea411, winged bean529, pea63,411,781,789, chickpea63,781, strawberry49,74,484, tomato181,486,533, pak choi790, lettuce64, spinach45, and coriander523 accessions. A lot of efforts have been made on folate biofortification in plants by genetic engineering approaches. The possible folate enhancement is not restricted by limited natural variation in the folate content of a particular plant species, as is the case of plant breeding. Genetic engineering makes use of fundamental knowledge on the complex matter of folate biosynthesis and its regulation, part of which remains to be elucidated. The main goal is to design an effective folate enhancement strategy, considering both folate accumulation and stability, adaptable to the specific metabolism of target tissues in different crops because different biosynthetic steps need to be engineered in each one to result in a substantial folate increase. Manipulation of genes encoding enzymes for, e.g., folate biosynthetic and salvage pathway, polyglutamylation, and folate binding proteins, has been carried out with some achievements2,10,25,34,303,310,560,752,754,776,791,792,793,794,795,796,797,798,799,800,801,802. Compared with non-genetically modified plants, folate content increased 0.17–150-fold in rice grains, 2–25-fold in tomato, 2–12-fold in potato tubers, 2.3-fold in wheat grains, 2.1–8.5-fold in lettuce, 2–4.2-fold in maize grains, 3-fold in Mexican common bean, and 1.3–4-fold in Arabidopsis leaves after genetic modification532,752,754,791,803,804,805,806,807,808,809,810,811,812. The maximum level of folate biofortification reached in rice seeds exceeds the recommended daily allowance for an adult person (400 μg) more than fourfold. Cooking experiments demonstrated around 45% folate losses after 30 min of boiling. Assuming an average bioavailability of natural folates in a mixed diet of about 50%, it is very likely that 100 g of the biofortified rice grains can satisfy the daily folate requirement for an average adult person or at least supply most of it812. None of the folate biofortified crops has been approved for commercial release.
In addition to biofortification, another strategy for increasing folate content in foods is its in situ production during fermentation by folate-synthesizing microorganisms. Lactic acid bacteria, e.g., lactococci, streptococci, and lactobacilli, widely used as starter cultures for the fermentation of a large variety of foods, have been intensively investigated. Most lactococci and streptococci, such as Lactococcus lactis and Streptococcus thermophilus, have the ability to synthesize folate de novo. This was already discussed above with fermented dairy products, such as yoghurt. On the other hand, many lactobacilli are not capable of producing folate de novo because some genes coding for enzymes involved in folate biosynthesis are lacking in their genome; this is the case for, e.g., Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus reuteri. However, they can often synthesize folate if some precursors, e.g., p-aminobenzoic acid, are available in their environment (the culture medium or food). Some lactobacilli are folate auxotrophs dependent on folate intake from exogenous sources. The ability of microbial cultures to produce or consume folate varies considerably, being a strain‐dependent trait and influenced by fermentation conditions. Proper selection of strains and their combination for starter cultures is essential to develop fermented foods with increased folate content1,123,302,434,469,518,519,813,814,815,816,817,818,819,820,821,822,823,824,825,826,827,828,829,830,831. It has been shown that different substrates, such as milk (e.g., cow and goat), legumes (e.g., soy, and white beans), cereals (e.g., wheat, oat, barley, maize, sorghum, tef, and pearl millet), pseudocereals (e.g., amaranth, chia, and quinoa), and vegetables (e.g., cabbage, beetroot, turnip, potato, oca, papalisa) are suitable to be fermented by lactic acid bacteria and hence to improve the folate content469,819,823,824,825,832,833,834,835,836,837,838,839,840,841,842,843. Similarly, bifidobacteria synthesize folate de novo or from precursors or, on the contrary, do not synthesize but utilize available folate depending on the respective strain and medium composition. Folate-producing bifidobacteria may be used for in situ fortification of fermented dairy products. For example, the addition of Bifidobacterium bifidum to a common yoghurt starter culture (Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus) brought about higher folate content in the yoghurt compared with the yoghurt obtained by fermentation of milk with the common yoghurt starter1,146,258,517,815,817,824,828,831,844,845,846. Propionibacteria, e.g., Propionibacterium freudenreichii, have been demonstrated as folate-producing bacteria. High differences in folate production can be found between different strains. The advantage of propionibacteria is their ability to synthesize vitamin B12 as well. For example, co-inoculation of kefir grains, used to prepare kefir, with a folate and vitamin B12 producing Propionibacterium freudenreichii strain resulted in an increased content of both important vitamins in kefir. Similarly, the co-cultivation of a folate producer, Lactobacillus plantarum, with a vitamin B12 producer, Propionibacterium freudenreichii, in a whey permeate medium led to a natural dietary supplement with an optimal ratio of folate and vitamin B12146,847,848,849,850,851,852. Yeasts, e.g., Saccharomyces cerevisiae and Candida glabrata, have a high capability of producing folate. Careful selection of strain opens possibilities for optimizing folate content in yeast-fermented foods, such as bread and African types of porridges103,835,838,853,854,855. A combination of the lactic acid bacteria and yeasts may be useful in increasing folate through fermentation, e.g., in pearl millet fermented gruel (Lactobacillus fermentum and Pichia kudriavzevii)856, in ogi, a fermented maize gruel, (Lactobaccilus plantarum and Candida tropicalis)841, and in idli, a steamed cake based on fermented mixture of rice and black gram, (Lactococcus lactis and Saccharomyces boulardii)857. In the case of idli, levels not only of folate but also of riboflavin were enhanced857.
Searching for native strains of folate-producing microbes from different niches (ethnic foods, fruits, vegetables, cereals, vegetation, animals, soil, and so on) is very important for the development of novel in situ fortified products103,146,258,818,827,832,834,837,843,847,852,853,855,856,858,859,860,861,862,863,864,865. For example, folate levels were 3–5-fold higher in white wheat bread leavened with a Saccharomyces cerevisiae strain, originally isolated from the Rainbow trout intestine, compared to white wheat bread leavened with a commercial Baker’s yeast strain866. A yoghurt with a starter culture consisting of Streptococcus thermophilus, a high folate producer, and Lactobacillus delbrueckii subsp. bulgaricus, a folate consumer, usually contains 2-fold higher amounts of folate compared to the original milk. A new Lactobacillus delbrueckii subsp. bulgaricus strain, capable of producing folate, was isolated from artisanal yoghurts of the northwestern region of Argentina. The fermentation of milk with a starter composed of two folate producing Streptococcus thermophilus strains and a folate producing Lactobacillus delbrueckii subsp. bulgaricus strain resulted in the yoghurt containing 4.5-fold more folate compared with the non-fermented milk826,861. Another way to obtain folate high producing microorganisms for in situ fortification is genetic modification. Genetic engineering was successfully used to increase folate production by Lactococcus lactis and to transform a folate consumer, Lactobacillus gasseri, into a folate producer867,868,869,870,871,872. Use of genetically modified wine yeasts for wine fermentation resulted in elevated folate content in wine516,873. However, despite the approach being efficacious in improving folate levels, the selection of natural overproducers has not gained favour due to the legislative limitations and negative perception of genetic modification by consumers1,814,835.
The folate content of in situ fortified fermented food products enriched by folate-producing microorganisms is still low. Such products would provide economic benefits to food manufacturers since increased “natural” folate concentrations would be an important value-added effect without increasing production costs. Consumers would benefit from such products since they could increase their folate intake while consuming products that form part of their normal diet1,146,814,842,874.
Kinetics in humans and homeostasis
Compartmentalization
Different forms of folate have different abilities to be transported through the biological membranes, which results in compartmentalization of folate between extracellular space, cytosol, and mitochondria, with smaller folate amounts located in the nucleus875. The most important determinant of this compartmentalization is the polyglutamate tail. Monoglutamate, but not polyglutamate forms are substrates to several folate-transporting proteins and are able to cross plasma membranes275,876 or mitochondrial membranes877,878. Without being metabolized, polyglutamate forms are therefore not absorbed from the intestine or are trapped within the intracellular compartment where the polyglutamate tail is added879. Another factor contributing to this compartmentalization is the presence of the one-carbon units. Folates with bound carbon units (i.e., methyl-THF, methylene-THF, formyl-THF) are unable to cross the mitochondrial membrane880.
Folate transporters
Folate molecules have hydrophilic properties and passive diffusion across cell membranes is minimal. Specific transport proteins are required to mediate folate transfer across cell membranes, either during intestinal absorption or distribution into tissues. Several folate transporters have been identified and characterized: the reduced folate carrier, the folate receptors, and the proton-coupled folate transporter881. Reduced folate carrier is expressed ubiquitously in all tissues, but it is selective only for the reduced folate forms882,883. Proton-coupled folate transporter seems to be the major transporter at low pH levels and in intestine884. Both reduced folate carrier and proton-coupled folate transporter are the most common ways used by folates to reach tissues. The membrane-bound folate receptor has the highest affinity for folate (Kd ∼0.1–1 nmol/l), characteristically binds folic acid, reduced folates, many antifolate drugs, and folate conjugates, and transports them by a non-classical endocytic mechanism881,885. Furthermore, folates are substrates for less-selective transport proteins like organic anion transporter OATP1B1, multidrug resistance-associated proteins (MRPs), and the breast cancer resistance protein275,886.
Absorption
The most common form of folate used in nutrient supplementation is folic acid, even though it is not normally present in food or in nature as aforementioned. Folic acid has high bioavailability, it is stable, and can be quickly converted into the active tetrahydrofolate forms. Dietary folate exists in polyglutamate form which must be converted into monoglutamate before absorption. This reaction is catalysed by the enzyme folate conjugase on the brush border of enterocytes in the proximal small intestine887,888. Along the brush border folate conjugase, enterocytes express intracellular folate conjugase, an enzyme with similar activity but different properties888,889. The absorption of monoglutamate forms occurs mostly by a saturable mechanism via the reduced folate carrier, folate receptor, and especially proton-coupled folate transporter with Km of 1–3 μM881,890,891. The pH optimum for the active saturable transport is 5.5–6.0, which explains why antacids seem to reduce folate absorption. An additional passive, unsaturable absorption pathway exists that is used when the intraintestinal folate concentrations exceed 10 μM209.
Oxidized and reduced forms of folate are absorbed to a similar degree; however, the reported bioavailability values range from 10 to 98%192,199,211. 5-methyl-THF is absorbed unchanged. Other forms, including 5-formyl-THF, are converted to 5-methyl-THF by intestinal mucosa (as the organ most responsible for adding the methyl group and reducing the vitamin) or in a small degree by the liver887,892.
Improving folate bioavailability through processing
Because intestinal deconjugation of polyglutamates to monoglutamates is the rate-limiting step in intestinal folate absorption, an increase of folate monoglutamate portion in foods may improve the bioavailability of dietary folate1,37,216. The tissue and cell disruption during processing (e.g., mixing, cutting, crushing, freezing/thawing, and high pressure treatment) makes native polyglutamyl folates accessible for endogenous conjugases (γ-glutamyl hydrolases) and results in the hydrolysis to monoglutamyl folates in vegetables (e.g., leeks, cauliflower, broccoli, spinach, soybeans, green beans, cowpea leaves, turnip, and carrot) and fruits (e.g., orange, papaya, sweet cherry, strawberry, and blackberry). Deconjugation could be affected by several factors, such as differences in native conjugase activity, the presence of endogenous conjugase inhibitors, and the use of heat during processing. Heating, e.g., during blanching and steaming, largely inactivates conjugases such that long-chain polyglutamyl folates are preserved. Food processing may by itself increase folate bioavailability. On the other hand, higher losses of total folate after treatment may occur because of leakage due to matrix disruption and oxidative degradation of monoglutamate forms due to their lower stability37,75,117,180,234,394,399,403,414,473,474,475,477,480,533,893. Changes in folate glutamylation profiles during maturation were observed in cowpea leaves, winged beans, tomato, avocado, banana, and papaya38,399,529,893.
Distribution and tissue retention
After absorption, the monoglutamate folates are distributed to tissues and converted to the polyglutamate form by the enzyme folylpolyglutamate synthetase. The majority of folate entering tissue cells is in the form of 5-methyl-THF monoglutamate or is quickly metabolized to this form, which has a low affinity for folylpolyglutamate synthetase894. For the polyglutamylation to be effective and to achieve tissue retention, 5-methyl-THF needs to be first metabolized to THF via the methionine cycle. However, the passage of 5-methyl-THF through the methionine cycle is limited, especially in situations with high intracellular 5-methyl-THF levels. Under such conditions, the newly absorbed folate is not retained by the tissue and is released back into the systemic circulation, mostly as 5-methyl-THF.
The largest pool of folates is in the liver which can accumulate 50% of the total body folate content895,896. Folates in the liver may be directed into three metabolic pathways. 1) The folate monoglutamates can be converted to polyglutamate forms to be retained; 2) these polyglutamate stores can be hydrolysed to monoglutamates by the enzyme glutamate carboxypeptidase II and released to meet the body’s requirements; and 3) the folate monoglutamates can be partially secreted into bile and excreted to the duodenum and small intestine, undergoing enterohepatic folate circulation897. After the liver, the pancreas is the second largest store of folate898.
Elimination and excretion
Any folate excess not retained by tissues is excreted in the urine and faeces, in an intact form or as metabolites. Daily excretion of folates in humans is estimated to be <1% of the total folate body pool. Only about 5% of ingested folate is excreted with urine in unchanged form at physiological doses899. The mechanism of folate breakdown is incompletely understood but happens in most tissues and primarily involves the irreversible oxidative cleavage of the C9-N10 bond, forming various pterins and folate-derived amines (p-aminobenzoyl-polyglutamates). The pterin moiety is excreted in bile and appears in faeces. Faeces usually contain high concentrations of folates, but most of them originate from the bacterial synthesis in the lower gut. The p-aminobenzoyl-polyglutamates are further hydrolysed to monoglutamates by lysosomal glutamylhydrolase and acetylated, forming the main metabolites p-aminobenzoylglutamate and its acetylated form, p-acetamidobenzoylglutamate900. This metabolic pathway is present in all tissues, with the highest activity being detected in liver and kidney 901. p-Acetamidobenzoylglutamate and p-aminobenzoylglutamate are subsequently excreted in urine.
Enterohepatic circulation
Folates are subject to enterohepatic circulation which limits the loss of the pterin moiety in faeces. After excretion in bile, the folates are rapidly reabsorbed for redistribution to the liver and tissues. The importance of this process has been demonstrated in animal studies897 which have shown that bile drainage leads to the rapid decrease in folate serum concentrations by 60–70% within 6 h. The enterohepatic cycle seems to play a significant role in maintaining folate homeostasis and its interruption may affect folate availability more than dietary deficiency.
Physiological function
Even though folates are distributed and enter cells as monoglutamates, the functional cofactors are in the form of polyglutamates. The polyglutamate tail not only helps with vitamin retention in cells but also increases the folate affinity for folate-dependent enzymes by as much as 1000-fold275,902.
Folate coenzymes are involved in three major metabolic cycles: the purine cycle, the thymidylate cycle, and the methionine cycle. The folates enter these cycles with the bound one-carbon unit in different oxidation states (5-methyl-THF, 5,10-methylene-THF, 10-formyl-THF) which are cleaved off during biochemical reactions. The cofactors then exit the cycles as dihydrofolate or THF. The central pathway interconnecting these different biochemical functions of folates is the regeneration of the one-carbon units to THF – the one-carbon folate metabolism or cycle.
One-carbon folate cycle
The main source of one-carbon units for folate-mediated methylation in humans is serine. In a reversible reaction catalysed by serine hydroxymethyltransferase (SHMT), the serine β-carbon is transferred to tetrahydrofolate to form methylene-THF. Mammals contain two distinct isoforms of SHMT encoded by different genes: cytosolic SHMT1 and mitochondrial SHMT2903. It has been shown in healthy volunteers904, in cell culture905 and in most cancer cells906 that the majority of one-carbon units transferred to methionine originates in the mitochondria (i.e., SHMT2). Thus, the one-carbon folate cycle may be thought to start with the mitochondrial SHMT2-catalysed demethylation of serine in the presence of tetrahydrofolate to produce glycine and methylene-THF (Fig. 2). Next stage is the two-step oxidation of methylene-THF to methenyl-THF (CH+-THF) and 10-formyl-THF. These oxidative reactions are catalysed by isoenzymes methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) or MTHFD2-like (MTHFD2L) and consume oxidized nicotinamide adenine dinucleotide (NAD+), which is reduced NADH. The final mitochondrial step is the cleavage of 10-formyl-THF into formate and free THF by the enzyme methylenetetrahydrofolate dehydrogenase 1 like (MTHFD1L). Formate is a critical intermediate metabolite. Along with free THF, it is able to cross the mitochondrial membrane and its availability determines the direction of the reversible cytosolic pathway907.
In the mitochondrial compartment, one-carbon units are attached to tetrahydrofolate, oxidized, and transported into cytoplasm, where they are available for the three principal folate biochemical pathways. See text for detailed description. DHF dihydrofolate, THF tetrahydrofolate, SHMT serine hydroxymethyltransferase, CH3-THF 5-methyl-THF, CH2=THF 5,10-methylene-THF, CHO-THF 10-formyl-THF, CH+-THF methenyl-THF, MTHFR methylenetetrahydrofolate reductase, MTHFD1 methylenetetrahydrofolate dehydrogenase 1, MTHFD1L methylenetetrahydrofolate dehydrogenase 1 like, MTHFD2 methylenetetrahydrofolate dehydrogenase 2, MTHFD2L methylenetetrahydrofolate dehydrogenase 2 like.
In the cytosol, the formate is incorporated back into tetrahydrofolate to form 10-formyl-THF (catalysed by methylenetetrahydrofolate dehydrogenase 1; MTHFD1). 10-formyl-THF is required for the de novo purine synthesis in the purine cycle. Alternatively, 10-formyl-THF can be sequentially reduced to methylene-THF by MTHFD1. The further role of methylene-THF depends on the cellular demands: it can complete the folate cycle by remethylation of glycin to serin by SHMT1; it can enter the thymidylate cycle to generate dTMP from dUMP; or it can undergo final reduction to 5-methyl-THF by methylenetetrahydrofolate reductase (MTHFR) and enters the methionine cycle. It is important to note that this last reduction is physiologically irreversible, and the methionine cycle is the only pathway that utilizes 5-methyl-THF. Disturbance of the methionine cycle, especially of the first enzymatic step catalysed by methionine synthase, can lead to folates being trapped in the 5-methyl-THF form and the inability of the cell to produce purines and dTMP.
The folate compartmentalization leads to the separation of cytosolic and mitochondrial one-carbon metabolic pathways which remain connected only through specific metabolites. Even though the majority of one-carbon demand is in the cytosol, almost all THF substrates to meet this demand are produced in the mitochondria. Why such a parallel set of biochemical pathways exists is not clear. However, it has been shown that the mitochondrial 10-formyl-THF production accounts for almost 50% of NAD+/NADP+ consumption and NADH/NADPH production in cell880. One-carbon oxidation might be localized in mitochondria to uncouple it from glycolytic and other metabolic reactions in the cytosol, which might be disrupted by NAD+ depletion that would happen if one-carbon oxidation should take place in cytosol359,877.
Nucleotide synthesis – thymidylate and purine cycles
Human cells produce purines in the cytosol both via salvage and de novo biosynthesis pathways. The de novo synthetic pathways consist of 10 steps which convert phosphoribosyl pyrophosphate into inosine monophosphate and further to adenosine and guanosine908. The conversion into inosine monophosphate requires two one-carbon units from 10-formyl-THF, which are incorporated at the 2’- and 8’-positions of the purine ring. These reactions release free THF and are catalysed by glycinamide ribonucleotide transformylase (GARTF) and 5-amino-4-imidazolecarboxamide ribonucleotide (AICAR) transformylase909.
Methylene-THF is also required for the synthesis of DNA, specifically for the production of dTMP. Enzyme thymidylate synthase catalyses the transfer of one carbon from methylene-THF to the 5’-position of dUMP. The THF molecule also serves as the reducing agent to reduce this one-carbon to methyl group to form dTMP. THF is oxidized to dihydrofolate and needs to be reduced back to THF before it can be reutilized in the folate cycle. This THF regeneration step is catalysed by dihydrofolate reductase. Significant thymidylate synthase activity was detected only in replicating, especially fast-growing cells, and thymidylate synthase activity and protein levels are subject to cell cycle variations, associated with the onset of the S-phase910. Some evidence suggests that thymidylate synthase is active only as part of the replitase complex in the nucleus (see below) and not in cytosol911. Dihydrofolate reductase inhibiting drugs (e.g., methotrexate and others, see later) are therefore selective inhibitors of rapidly growing cells and have proven to be effective antineoplastic agents912,913.
Methionine cycle
5-methyl-THF is a substrate for methionine synthase (one of only two vitamin B12-dependent enzymes in mammals) and the methionine cycle914,915. 5-methyl-THF acts as a methyl donor for homocysteine methylation, forming methionine and free THF. Methionine can be further metabolized to S-adenosylmethionine, which acts as a methyl donor for a broad spectrum of reactions, including methylation of histones, DNA, neurotransmitters, phospholipids, and synthesis of glutathione, phosphotidylcholine, and creatine. These reactions are critical for the regulation of gene expression, development, and genomic stability (the role of vitamin B9 and B12 in genomic stability is complexly reviewed in ref. 916). The methionine cycle is very sensitive to inadequate folate levels. When folate concentrations are low, the remethylation of intracellular homocysteine is disrupted which leads to increased plasmatic homocysteine concentrations. Total plasma homocysteine can be considered an indirect indicator of folate insufficiency917,918 but it should be stressed that hyperhomocysteinemia can be mediated by other causes as well919.
These cytosolic pathways show different relative importance in various cell types. In quickly proliferating cells, such as stem cells, hematopoietic cells, or cancer cells, the majority of one-carbon units are incorporated into nucleotides (purines and dTMP)877,920. In slowly proliferating cells (such as cultured fibroblasts) most of one-carbon flows through thymidylate and methionine cycle921. In non-proliferating cells, however, one-carbon groups almost exclusively enter the methionine cycle. In the liver and kidney – tissues with the highest activity of one-carbon anabolic reactions – S-adenosylmethionine is required for the synthesis of creatine, which accounts for almost 80% of all methylation reactions in the body922.
Nuclear folate metabolism
Most of the folate one-carbon cycle with connected biochemical pathways occurs in mitochondria and cytosol, however, evidence suggests that at least some of these reactions are functional in the nucleus as well. In the nuclei of S-phase cells, but not during the G1-phase, a multienzyme complex was detected which contains enzymes required for the salvage pathway, de novo synthesis of dTMP, and DNA replication923. This complex was termed replicase. It is localized to the sites of DNA replication via nuclear lamina anchors924. The formation of replicase starts in the S-phase with increased activity of small ubiquitin-like modifier (SUMO) ligase, SUMOylation of cytosolic SHMT1, thymidylate synthase, dihydrofolate reductase and MTHFD1, and the translocation of these enzymes into the nucleus. There, these SUMOylated proteins directly bind to other nuclear enzymes and form an active functional replicase complex. Both serine (via SHMT1 or nuclear isoform SHMT2α) and formate (via MTHFD1) serve as one-carbon donors for the production of methylene-THF, which enters the nuclear thymidylate cycle to provide dTMP for DNA synthesis (Fig. 3). Impaired nuclear folate metabolism leads to suppression of dTMP production and increases dUTP production and incorporation into DNA. Such uracil misincorporation may induce DNA breaks925,926. It has been suggested that DNA uracil levels could present a biomarker for insufficient folate status925, however, a significant inverse correlation was detected only for vitamin B12 status927.
SUMOlyated cytosolic enzymes are transported into the nucleus where they form a multienzyme complex (replitase), responsible for local production of dTMP required for DNA synthesis. CH2=THF 5,10-methylene-THF, DHF dihydrofolate, THF tetrahydrofolate, DHFR dihydrofolate reductase, dT deoxythymidine, dTMP deoxythymidine monophosphate, dTTP deoxythymidine triphosphate, dU deoxyuridine, dUMP deoxyuridine monophosphate, dUTP deoxyuridine triphosphate, MTHFD1 methylenetetrahydrofolate dehydrogenase 1, SMHT serine hydroxymethyltransferase, TYMS thymidylate synthase.
There is no strong evidence that either folate-dependent de novo purine biosynthesis or methionine cycle exists in the nucleus. Enzymes required for de novo purine biosynthesis localize exclusively to the cytosol928,929. Likewise, homocysteine remethylation to methionine also appears to be a cytosolic process, as the enzymes that compose this cycle are exclusively cytosolic930,931,932.
Folate deficiency, folate supplementation and claimed risks
Folate deficiency and related disorders
Folate deficiency can be caused by various factors. The most common are dietary insufficiency or malabsorption. Deficient absorption of folates is present in many pathological conditions, e.g., in alcoholism, celiac disease, amyloidosis, short bowel syndrome, or gastric bypass933. Elevated pH, which occurs in achlorhydria, or as aforementioned after extensive use of antacids, can also decrease folate absorption. Many drugs (e.g., methotrexate, phenytoin, trimethoprim, and possibly sulfasalazine) can antagonize folate utilization or inhibit its conversion to active forms934,935. Situations with increased requirements for folates (typically in pregnancy) can also cause symptoms of folate deficiency. Furthermore, genetic polymorphisms or mutations of the proteins involved in one-carbon metabolism (e.g., MTHFR) or folate uptake (e.g., reduced folate carrier) also increase the risk of folate-associated diseases936.
Given the broad functional importance of folate-connected metabolism in the body, it is unsurprising that the symptoms and dysfunctions caused by folate deficiency vary considerably. A “classical” symptom is megaloblastic anaemia: the formation of large abnormal nucleated erythrocytes, caused by inhibited maturation of erythropoietic precursor cells. Hypersegmented neutrophils are also pathognomonic of the disease. Poor folate status can cause abnormalities in DNA synthesis, protein synthesis and posttranslational modifications, and in gene expression, which may result in chronic diseases such as cardiovascular diseases, neuropsychiatric disorders (cognitive dysfunction, depression, psychosis, memory impairment), or cancer. Several cancers have been associated with folate deficiency, such as colorectal, prostate, and breast cancer937. The causes of carcinogenesis in low folate status are hypothesised to be the uracil misincorporation938 and hypomethylation of DNA causing dysregulated gene expression916,934.
The methods for folate detection are summarized in Table 2 with details in the Supplementary Table S1.
Pregnancy, current opinions on folate fortifications with claimed risks
Neural tube defects are relatively common congenital abnormalities with complex but incompletely understood aetiology, which include anencephaly, encephalocele, and spina bifida. Global prevalence of live births ranges from 0.8/1000 (USA), 1/1000 (EU) to 5/1000 (China)939,940,941. Folic acid supplementation has been shown to reduce the risk of neural tube defects942,943; however, the mechanism by which folate reduces this risk remains obscure.
Achieving optimal folate status is challenging, however, in Europe, the recommendations of folic acid supplementation during the periconceptional period (400 μg/day folic acid from preconception until the end of the first trimester of pregnancy) have been largely ineffective in reducing the neural tube defects incidence944. The reason seems to be poor compliance of women who start taking folic acid only after the period of neural tube closure (day 17–28 post-fertilization), as many pregnancies may go unnoticed during this timeframe.
Mandatory folic acid fortification is an effective intervention to reduce the prevalence of neural tube defects (spina bifida, anencephaly, and encephalocele)128,585,596,599,657,670,680,682,684,685,690,691,725,726,944,945,946,947,948,949,950,951,952,953,954,955,956,957,958,959,960,961,962,963,964,965,966,967,968,969,970,971,972,973,974,975,976,977,978,979,980,981,982,983,984,985,986,987,988,989,990,991,992,993. In the absence of population-wide fortification and given the generally poor compliance with current folic acid recommendations, optimising the folate status of mothers in very early pregnancy for protection against neural tube defects remains challenging679,691,994,995. Optimal folate status also has possible preventative roles in, e.g., cardiovascular disease, in particular stroke, several types of cancer, and age-related cognitive impairment77,128,233,300,671,680,916,944,995,996,997,998,999,1000,1001,1002,1003,1004,1005,1006,1007,1008,1009,1010,1011,1012,1013,1014,1015,1016,1017,1018,1019,1020,1021,1022,1023,1024,1025,1026,1027,1028.
Many countries have not implemented mandatory folic acid fortification owing to concerns about potential harmful effects that might be caused by the increased intake of folic acid from fortified foods90,128,300,614,679,688,702,986,989,990,1028,1029,1030,1031,1032,1033,1034,1035,1036,1037,1038,1039,1040,1041,1042,1043,1044,1045. Worldwide, countries with mandatory policies of folate food fortification have reported significant reductions (by 27–50%) in neural tube defects951,965,1046. European countries have been reluctant to introduce mandatory folate food fortification. There is a large body of literature with observational studies, clinical trials, meta-analyses, reviews, hypotheses, and speculations on the potential association between folic acid and adverse health outcomes. The main issues are 1. masking of vitamin B12 deficiency primarily in the elderly674,677,680,683,967,973,1047,1048,1049,1050,1051,1052; 2. a risk of cognitive impairment in elderly individuals with suboptimal vitamin B12 status98,678,680,690,973,995,1030,1047,1053,1054,1055,1056,1057,1058,1059,1060,1061,1062,1063,1064,1065,1066,1067,1068,1069,1070,1071; 3. a risk of cancer98,581,979,1017,1025,1072,1073,1074,1075,1076,1077,1078,1079,1080,1081,1082,1083,1084,1085,1086,1087,1088 with special attention to colorectal cancer581,680,987,1006,1014,1015,1024,1086,1089,1090,1091,1092,1093,1094,1095,1096,1097,1098,1099,1100,1101,1102,1103,1104,1105,1106,1107,1108,1109,1110,1111,1112,1113,1114,1115,1116, breast cancer581,1078,1117,1118,1119,1120,1121, and prostate cancer581,1078,1122,1123,1124,1125,1126,1127; 4. negative health outcomes in offspring, such as hypersensitivity-related outcomes (e.g., asthma and eczema)604,986,1128,1129,1130,1131,1132,1133,1134,1135,1136,1137,1138,1139,1140,1141, autism1142,1143,1144,1145,1146,1147, child neurocognitive development1019,1148,1149,1150,1151, and others128,603,605,606,609,680,1023,1152,1153,1154,1155,1156; and 5. presence of unmetabolized folic acid in the circulation98,600,670,677,972,978,1036,1047,1105,1145,1157,1158,1159,1160,1161,1162,1163,1164,1165,1166,1167,1168. The concerns have arisen mostly from high-dose supplementation studies that have claimed to link folic acid supplementation and adverse effects. However, there is comparatively less data on the effects that can be specifically attributed to food fortification due to numerous potential confounding factors691. At present, given the heterogeneity and inconsistency in the findings among studies, there is an insufficient body of evidence to support human adverse health outcomes that are a result of high amounts of folate or folic acid intake1031,1043. Unequivocal and credible evidence to support the purported associations is lacking246,983,987,995,1015.
Regarding masking of vitamin B12 deficiency, it refers to the fact that both folate and vitamin B12 deficiency gives rise to the same type of anaemia, and, in the 1940s, before the recognition that vitamin B12 is a cause of pernicious anaemia, folic acid used at high doses (≥5 mg) for its treatment restored normal blood values, but did not prevent the vitamin B12 deficiency related neuropathy, which remained progressive and could lead to irreversible neurological damage without treatment with vitamin B12, and so masked vitamin B12 deficiency and delayed its diagnosis. Folate and vitamin B12 deficiencies could not be diagnostically distinguished based on haematological symptoms at that time233,690,1169. However, current medical practice does not rely on the presence of anaemia for the diagnosis of vitamin B12 deficiency, which frequently presents without anaemia614,973,986,1007,1033,1035,1052,1061. It is estimated that it happens in about 30% of patients973,1039,1049,1055. Today, blood levels of vitamin B12 and related metabolites are directly measured as a first-line test599,691,1035,1039,1055. The experience of mandatory fortification of foods with folic acid in the US showed no evidence of a higher prevalence of vitamin B12 deficiency in the absence of anaemia or macrocytosis128,671,988,1052. To address the issue of masking, based on case reports from the 1940s and 1950s, a tolerable upper intake level for folic acid from fortified foods or supplements was later set as 1 mg per day, an amount which would not mask haematological signs of vitamin B12 deficiency98,991,1035,1036,1039,1055,1169,1170,1171. Therefore, the risk of masking vitamin B12 deficiency and delaying vitamin B12 deficiency diagnosis resulting from mandatory folic acid fortification is considered unlikely233,581,671,678,687,691,990,1039,1169,1172.
As for cognitive impairment due to folic acid in elderly individuals with suboptimal vitamin B12 status, the paucity of clear data provides insufficient evidence of an increased risk of causing or accelerating cognitive impairment resulting from vitamin B12 deficiency98,246,1173. Folic acid had no significant effect on the cognitive decline of older individuals671,691,988,991,1033,1035,1066,1169. Considering the potential harmful health impacts, if there are, of high folic acid and low vitamin B12 intake, suggestions have been made to include both folic acid and vitamin B12 in food fortification policies. That could prevent potential adverse outcomes of imbalance of both vitamins and address a public health issue of vitamin B12 deficiency, widespread in all age groups, particularly among the elderly128,333,614,637,677,690,944,953,982,1030,1051,1061,1174,1175,1176. In addition, vitamin B12 deficiency itself may be a risk factor for neural tube defects. Adding vitamin B12 to folic acid might further reduce the risk of neural tube defects1059,1061,1062,1177,1178. Fortification with both vitamins would increase the benefits and reduce the risks, but more evidence on efficacy, dosage, and feasibility is required before this could be considered128,678,1174.
Concerning the relationship between folic acid and the risk of cancer, the incidence of several common cancers (e.g., colorectal, breast, and prostate cancer) and total cancer in the US, Canada, and Australia has mostly remained stable or decreased since the introduction of mandatory fortification685,691,1077,1179. A large meta-analysis of data on 50,000 patients showed that folic acid supplementation does not significantly increase the incidence of site-specific cancer1077.
The Australian Health Ministers’ Advisory Council (AHMAC) found that meta-analyses of randomized control trials for colorectal, prostate, other cancer sites, and total cancer consistently demonstrated no increase in cancer risk associated with supplementation at a population level685,691. The European Food Safety Authority (EFSA) review similarly found no consistent association of folate or folic acid with cancer risk, and it noted that potentially adverse effects tended to manifest at intake levels in excess of the tolerable upper intake level of 1 mg daily98. Currently, an expert panel for the EFSA concluded that meta-analyses indicated no association between folate and colorectal cancer. Evidence from intervention studies on the relationship between folic acid supplementation and the risk of adenomas is mixed from protective effects over the null association to elevated risk. Too few studies with mixed results prevented any clear conclusion on total folate intake and risk of prostate cancer246. The Scientific Advisory Committee on Nutrition (SACN) summarized that findings from the different study types are inconsistent, and the evidence is inconclusive but overall does not suggest an adverse association. Meta-analyses of randomized control trials reported no effect of folic acid supplementation on colorectal cancer risk. Meta-analyses of observational studies are heterogeneous but suggested a protective association of folate intakes above about 400 µg/day. Observational studies of serum or plasma folate concentration provide no clear evidence of an association with colorectal cancer risk. Findings do not suggest a detrimental effect of folic acid/folate on overall cancer risk. Meta-analyses of randomized control trials of folic acid supplementation show no effect of folic acid on prostate cancer risk. Genetic studies suggested that higher blood folate concentrations are associated with an increased risk of prostate cancer988. According to an expert panel for the US National Toxicology Program (NTP), inadequate dietary folate intake increases colorectal cancer risk in humans, but there is no benefit for cancer reduction from supplements among people whose baseline folate status is adequate. There is suggestive evidence that folic acid has an adverse effect on the development of prostate cancer. Such data coming from human studies justify the need for further research1173. The Prime Minister’s Chief Science Advisor stated that findings from genetic studies suggested that higher blood folate is weakly associated with increased risks of colorectal and prostate cancer, whereas with decreased risks of breast and total cancer. The associations seen in the genetic studies are not necessarily causal, and their public health significance remains uncertain691. There is strong evidence that low folate status promotes cancer, especially colorectal cancer. However, evidence demonstrating a dose-response relationship between folate status and/or folate/folic acid intake within the normal human exposure ranges and increased rates of tumour growth in vivo is lacking. In general, there is no clear evidence from randomized controlled trials that supplementation/fortification with folic acid increases the cancer risk1034,1035. Most recent observational studies from 2021 revealed that the introduction of mandatory folic acid fortification of bread flour has not adversely affected colorectal cancer incidence in Australia1180 and that there was no evidence that high folate intake, both total and from synthetic forms, in the post-fortification period was related to increased colorectal cancer risk in this US female population1181. The latest meta-analysis of 24 cohort studies, mostly from the USA and Europe, involving 37,280 patients and 6,165,894 individuals has shown that high folate intake may be protective against colon cancer1182.
As regards folic acid maternal supplementation and health outcomes in children, there is no or limited evidence that children are at increased risk of atopy, asthma, wheezing, eczema, susceptibility to respiratory infection, childhood cancer, and autism spectrum disorders246,671,691,1035,1173,1183,1184,1185,1186.
In respect of unmetabolized folic acid in circulation, it has been pointed out that it is unlikely to be a new phenomenon. It is known that oral intake of folic acid above a certain threshold level (around 200 μg) results in an appearance of unmetabolized folic acid in the blood due to saturation of dihydrofolate reductase capacity. A number of studies conducted in countries with either mandatory or voluntary folic acid food fortification have reported detectable amounts of unmetabolised folic acid in the circulation in considerable proportions of adults and children. Dietary supplement use has increased in the U.S.A., while pregnant women have been prescribed folic acid tablets for nearly half a century, suggesting millions of person-years exposure to unmetabolized folic acid. The appearance of unmetabolized folic acid with a dose of 200 µg suggests that prior to fortification, any user of folic acid supplements would already have measurable unmetabolized folic acid, and any potential adverse effects would have been experienced. Biological and health consequences, if any, of unmetabolized folic acid are not established. Currently, there is no consistent evidence of adverse health effects causatively associated with circulating unmetabolized folic acid128,671,680,691,944,982,988,995,1031,1035,1060,1133,1134,1139,1187,1188,1189.
Besides folic acid, 5-methyl-THF has also been allowed for food fortification in the European Union and other countries626,627,1190,1191,1192. It is an important research question whether or not 5-methyl-THF is an effective and safer alternative to folic acid in providing supplemental levels of folate190,197,235,236,674,680,1015,1190,1193. Evidence for the efficacy of 5-methyl-THF in preventing neural tube defects is lacking at present190,192,197,680,691,1152. The utility of 5-methyl-THF is limited because it is less stable than folic acid in foods that undergo thermal processing211,1022. Using 5-methyl-THF may be advantageous for individuals with defects in the methylenetetrahydrofolate reductase enzyme who could have difficulty metabolizing folic acid from supplements or fortified foods to 5-methyl-THF by going straight to the next step in the metabolic pathway of vitamin B9192,580,680,1034,1081,1152,1193,1194,1195,1196. Use of 5-methyl-THF prevents the occurrence of unmetabolized folic acid in the peripheral circulation192.
Overall, regarding folic acid fortification, there is an inherent degree of uncertainty in nearly any aspect of scientific research. That is pertinent to the complex biological role of folate and its potential for both beneficial and adverse health effects depending on the dose and timing of exposure. The nature of science is that it cannot prove a negative. That is, there is no experimental design or methodology that can prove with 100% certainty that folic acid is completely ‘safe’691. Although the risk-benefit debate surrounding food fortification with folic acid continues among policymakers as well as researchers, the balance of available scientific evidence at this time indicates that the proven benefits of mandatory folic acid fortification outweigh the potential risks128,651,679,687,691,1022,1034. There are no established risks for adverse consequences resulting from existing mandatory folic acid fortification programs that have been implemented in many countries. Current folic acid fortification programs have been shown to support public health in populations1035. The effectiveness and safety of folic acid fortification programs have withstood the test of time671,680. There is also an interesting integrated risk-benefit analysis suggesting that a modest fortification with vitamin B9 will be very likely safe and suitable for prevention1197. However, additional research is needed to assess the health effects of folic acid supplement use, given the occurrence of some individuals and population groups exceeding the current tolerable upper intake level for folic acid. It is critical to evaluate all evidence for each of the concerns about potential harmful effects and to determine if there is a causal relation680,1022,1031,1035. Continued, careful, and effective monitoring should remain a key aspect of policy in this area, both to ensure that the target folic acid levels for beneficial effects are reached and to avoid any risk of overexposure in the population and potentially at-risk groups128,614,1034,1066,1073.
Antifolate drugs
Folate metabolic reactions are essential for the proper function of all living cells but are especially critical for rapidly growing and dividing cells. Inhibition of folate-mediated biochemical reactions has been therefore successfully used in the therapy of pathological states involving such cells, where the predominant effect of antifolate medication facilitates a selective inhibitory effect: cancer, bacterial, and protozoal infections.
The mechanism of antifolate drugs differs depending on the target enzyme. Even if folate biochemistry comprises numerous enzymes, several of them have specific and crucial roles in the folate cycles which makes them relevant therapeutical targets (Fig. 4).
Summarized pathways of folate metabolism. The blue area marks de novo folate synthesis exclusive to bacteria and some protozoa. Principal enzymes targeted by antifolate drugs are highlighted in red. Protozoa express a bifunctional enzyme with dihydrofolate reductase and thymidylate synthase activity on a single protein. GTP guanosine triphosphate, DHPPP 6-hydroxymethyl-7,8-dihydropterin pyrophosphate, DHP 7,8-dihydropteroate, DHF dihydrofolate, THF tetrahydrofolate, CH3-THF 5-methyl-THF, CH2=THF 5,10-methylene-THF, DHPS dihydropteroate synthase, DHFS dihydrofolate synthase, DHFR dihydrofolate reductase, TYMS thymidylate synthase.
Dihydrofolate reductase inhibitors
Dihydrofolate reductase is one of the most known and studied enzymes in folate metabolism. It has an important function in the THF regeneration from the thymidylate cycle and in THF production from dietary folate molecules. There are several differences between human, bacterial, and protozoal dihydrofolate reductase and folate biosynthetic pathways. A) Bacteria and some protozoa possess and use an endogenous folate biosynthetic pathway; however, certain parasitic protozoa like Plasmodium sp. and Cryptosporidium sp. have also salvage pathway that allows them to use exogenous folates1198,1199; while humans do not have the ability to synthesize folates de novo; B) Human and bacterial dihydrofolate reductase share high sequence homology, but structural differences are present that allow drugs selectively target the bacterial enzyme1200; C) Protozoa (e.g., Plasmodium, Toxoplasma, Trypanosoma, Leishmania sp.) have a bifunctional enzyme called dihydrofolate reductase-thymidylate synthase (DHFR-TYMS) in which dihydrofolate reductase and thymidylate synthase are two domains of a single homodimeric protein; in humans and bacteria, dihydrofolate reductase and thymidylate synthase occur as two separate, monofunctional proteins1201.
Antifolate drugs that act as dihydrofolate reductase inhibitors have been in therapeutical use for decades; the general overview is provided in Table 3. Novel candidate drugs were developed that inhibit not only dihydrofolate reductase but also thymidylate synthase and other enzymes in the thymidylate or purine cycles (AICAR formyl transferase, GARFT). Several drugs of this class have been investigated to their clinical effectiveness and safety. From the structural point of view, the “classical antifolates” are analogues of folate with pterin moiety: methotrexate, raltitrexed, pralatrexate, and pemetrexed. They do not passively cross the plasma membrane but use reduced folate carrier transporter to enter cells1202,1203, and they possess and require (poly)glutamate tail to utilize this active transport mechanism1204. “Non-classical” antifolates (piritrexim, trimetrexate, talotrexin, and nolatrexed) are lipophilic molecules that passively diffuse across cell membranes and do not require a specific transport mechanism. However, clinical studies showed satisfactory profiles for only a few candidate molecules that were approved for therapeutical use.
Dihydropteroate synthase
In bacteria and some protozoa, dihydropteroate synthase is the first step in de novo synthesis of THF. Dihydropteroate synthase catalyses the production of 7,8-dihydropteroate from 6-hydroxymethyl-7,8-dihydropterin pyrophosphate (DHPPP) and p-aminobenzoic acid. Subsequently, dihydrofolate synthase adds glutamate to 7,8-dihydropteroate (DHP) and produces dihydrofolate, which enters the folate cycle when reduced to tetrahydrofolate via dihydrofolate reductase. Antifolate drugs that act as dihydropteroate synthase inhibitors cause a selective, very pronounced reduction in folate levels.
Current clinical research on vitamin B9
In addition to above-mentioned issues, folic acid and derived drugs have been investigated in other pathological states both as possible drugs/preventive agents and as diagnostic tools.
One of the currently very intensively investigated targets is the folate receptor α. Pafolacianine, a modified folic acid conjugate with indocyanine green dye, binds to this receptor, which is prominently expressed in some cancers, and enables intraoperative tumour tissue imaging without serious adverse reactions1205. The same folate receptor is the target of the novel anticancer drug mirvetuximab soravtansine1206,1207,1208; also another conjugate antibody farletuzumab with eribulin has been tested1209. There are also attempts to use the receptor as a base for vaccination1210 in cancer immunotherapy as well as to employ IgE antibodies against this receptor1211 for cancer treatment.
There are some recent clinical trials on the effect of folic acid or its close derivatives: Oral folinic acid supplementation might be beneficial in children with autism spectrum disorder1212,1213,1214. The addition of folic acid increased the hypotensive effect of amlodipine1215. Similarly, patients suffering from hyperhomocysteinemia and hypertension administered with different doses of folic acid according to the genotype had a more pronounced decrease in arterial blood pressure when treated with another calcium channel blocker levamlodipine1216. Folic acid administration improved sexual function in postmenopausal women1217. A combination of folic acid with vitamin B12 could improve cognitive impairment in patients with Alzheimer´s disease1218 while the same combination had in general no effect on cognitive conditions in children aged 6–9 years1219. Interestingly, the effect of folic acid on cognition might be dependent on plasma levels of ω-3 fatty acids1220. In fact, folic acid combined with docosahexaenoic acid had a better effect on cognition than both compounds given in monotherapies1221.
The combination of vitamins B9 and B12 did not modify fracture risk1222. The combination of folic acid with vitamin B12 improved treatment outcomes in patients with type 2 diabetes mellitus, but the effect seems to be driven mostly by vitamin B121223. Treatment with folic acid and zinc neither improved semen quality and live birth rates in couples seeking infertility treatment1224 nor modified sperm DNA methylation pattern1225. In patients with methylenetetrahydrofolate reductase gene 677 TT genotype, folic acid however improved seminal parameters1226.
Conclusion
Vitamin B9 exists in several forms which are differently present in nature, have different stability, and different physiological functions. As this vitamin is crucial for humans and humans are not able to synthesize it, it must be taken from the diet or food supplements. Various ways of food preparation and storage impact in different ways its stability. There are several situations when a lack of this vitamin can be encountered. Its deficiency can lead to several severe consequences, including foetal malformation. For this reason, sufficient intake from food should be assured. The politics of different countries are not uniform as several countries have obligatory folate fortification while others are reluctant to this approach. Regardless, it seems that its fortification surpasses the claimed risks associated with a high intake of folates. Last but not least, given the differences in use and synthesis between humans and pathogens, healthy and tumour cells, there are several clinically used drugs targeting folate-dependent pathways.
References
Rossi, M., Raimondi, S., Costantino, L. & Amaretti, A. Folate: Relevance of chemical and microbial production. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants (eds Vandamme, E. J. & Revuelta, J. L.) 5, 103–128 (Wiley-VCH Verlag GmbH & Co. KGaA, 2016).
Gorelova, V. et al. Folates in plants: research advances and progress in crop biofortification. Front. Chem. 5, 21 (2017).
Gorelova, V. et al. Evolution of folate biosynthesis and metabolism across algae and land plant lineages. Sci. Rep. 9, 5731 (2019).
Basset, G. et al. Folate synthesis in plants: the first step of the pterin branch is mediated by a unique bimodular GTP cyclohydrolase I. Proc. Natl. Acad. Sci. 99, 12489–12494 (2002).
Basset, G. J. et al. Folate synthesis in plants: the p-aminobenzoate branch is initiated by a bifunctional PabA-PabB protein that is targeted to plastids. Proc. Natl. Acad. Sci. 101, 1496–1501 (2004).
Basset, G. J. C. et al. Folate synthesis in plants: the last step of the p‐aminobenzoate branch is catalyzed by a plastidial aminodeoxychorismate lyase. Plant J. 40, 453–461 (2004).
Noiriel, A., Naponelli, V., Gregory, J. F. III & Hanson, A. D. Pterin and folate salvage. Plants and Escherichia coli lack capacity to reduce oxidized pterins. Plant Physiol. 143, 1101–1109 (2007).
Orsomando, G. et al. Evidence for folate‐salvage reactions in plants. Plant J. 46, 426–435 (2006).
Hanson, A. D. & Gregory, J. F. III Synthesis and turnover of folates in plants. Curr. Opin. Plant Biol. 5, 244–249 (2002).
Hanson, A. D. & Gregory, J. F. III Folate biosynthesis, turnover, and transport in plants. Annu. Rev. Plant Biol. 62, 105–125 (2011).
Swarbrick, J., Iliades, P., Simpson, J. S. & Macreadie, I. Folate biosynthesis–reappraisal of old and novel targets in the search for new antimicrobials. Open Enzym Inhib. J. 1, 12–33 (2008).
Vickers, T. J. & Beverley, S. M. Folate metabolic pathways in Leishmania. Essays Biochem. 51, 63–80 (2011).
Ravanel, S., Douce, R. & Rébeillé, F. Metabolism of folates in plants. In Advances in Botanical Research 59 (eds Rébeillé, F. & Douce, R.) Ch. 3, 67–106 (Academic Press, 2011).
Liu, Z. et al. B vitamin supply in plants and humans: the importance of vitamer homeostasis. Plant J. 111, 662–682 (2022).
Levin, I. et al. An alternative pathway for reduced folate biosynthesis in bacteria and halophilic archaea. Mol. Microbiol. 54, 1307–1318 (2004).
Cantwell-Jones, A. et al. Global plant diversity as a reservoir of micronutrients for humanity. Nat. Plants 8, 225–232 (2022).
Molina-Venegas, R., Morales-Castilla, I. & Rodríguez, M. Á. Unreliable prediction of B-vitamin source species. Nat. Plants 9, 31–33 (2023).
Verhoef, H., Veenemans, J., Mwangi, M. N. & Prentice, A. M. Safety and benefits of interventions to increase folate status in malaria‐endemic areas. Br. J. Haematol. 177, 905–918 (2017).
Maynard, C., Cummins, I., Green, J. & Weinkove, D. A bacterial route for folic acid supplementation. BMC Biol. 16, 1–10 (2018).
Kordus, S. L. & Baughn, A. D. Revitalizing antifolates through understanding mechanisms that govern susceptibility and resistance. Med. Chem. Commun. 10, 880–895 (2019).
Braakman, R. & Smith, E. The emergence and early evolution of biological carbon-fixation. PLoS Comput. Biol. 8, e1002455 (2012).
de Crécy-Lagard, V. et al. Comparative genomics of bacterial and plant folate synthesis and salvage: predictions and validations. BMC Genom. 8, 1–15 (2007).
Falb, M. et al. Metabolism of halophilic archaea. Extremophiles 12, 177–196 (2008).
Sousa, F. L. & Martin, W. F. Biochemical fossils of the ancient transition from geoenergetics to bioenergetics in prokaryotic one carbon compound metabolism. Biochim. Biophys. Acta Bioenerg. 1837, 964–981 (2014).
Blancquaert, D. et al. Folates and folic acid: from fundamental research toward sustainable health. Crit. Rev. Plant Sci. 29, 14–35 (2010).
Krishnan, A., Kloehn, J., Lunghi, M. & Soldati-Favre, D. Vitamin and cofactor acquisition in apicomplexans: Synthesis versus salvage. J. Biol. Chem. 295, 701–714 (2020).
Perli, T. et al. Vitamin requirements and biosynthesis in Saccharomyces cerevisiae. Yeast 37, 283–304 (2020).
Akhtar, T. A. et al. A central role for gamma‐glutamyl hydrolases in plant folate homeostasis. Plant J 64, 256–266 (2010).
Gambonnet, B. et al. Folate distribution during higher plant development. J. Sci. Food Agric. 81, 835–841 (2001).
Meir, Z. & Osherov, N. Vitamin biosynthesis as an antifungal target. J. Fungus 4, 72 (2018).
Goyer, A. et al. 5-Formyltetrahydrofolate is an inhibitory but well tolerated metabolite in Arabidopsis leaves. J. Biol. Chem. 280, 26137–26142 (2005).
de Crécy-Lagard, V. R. et al. Comparative genomics guided discovery of two missing archaeal enzyme families involved in the biosynthesis of the pterin moiety of tetrahydromethanopterin and tetrahydrofolate. ACS Chem. Biol 7, 1807–1816 (2012).
Jakobsen, J., Melse-Boonstra, A. & Rychlik, M. Challenges to quantify total vitamin activity: how to combine the contribution of diverse vitamers? Curr. Dev. Nutr. 3, nzz086 (2019).
Saini, R. K., Nile, S. H. & Keum, Y.-S. Folates: Chemistry, analysis, occurrence, biofortification and bioavailability. Food Res. Int. 89, 1–13 (2016).
Striegel, L. et al. Durian fruits discovered as superior folate sources. Front. Nutr. 5, 114 (2018).
Striegel, L. et al. Promising tropical fruits high in folates. Foods 8, 363 (2019).
Zou, Y. et al. Quantification of polyglutamyl 5-methyltetrahydrofolate, monoglutamyl folate vitamers, and total folates in different berries and berry juice by UHPLC–MS/MS. Food Chem. 276, 1–8 (2019).
Ramos-Parra, P. A., García-Salinas, C. & Hernández-Brenes, C. & Díaz de la Garza, R. o. I. Folate levels and polyglutamylation profiles of papaya (Carica papaya cv. Maradol) during fruit development and ripening. J. Agric. Food Chem. 61, 3949–3956 (2013).
Thomas, P. M., Flanagan, V. P. & Pawlosky, R. J. Determination of 5-methyltetrahydrofolic acid and folic acid in citrus juices using stable isotope dilution− mass spectrometry. J. Agric. Food Chem. 51, 1293–1296 (2003).
Ringling, C. & Rychlik, M. Analysis of seven folates in food by LC–MS/MS to improve accuracy of total folate data. Eur. Food Res. Technol. 236, 17–28 (2013).
Ringling, C. & Rychlik, M. Origins of the difference between food folate analysis results obtained by LC–MS/MS and microbiological assays. Anal. Bioanal. Chem. 409, 1815–1825 (2017).
Zhang, H. et al. Improved folate monoglutamate extraction and application to folate quantification from wild lentil seeds by ultra-performance liquid chromatography-selective reaction monitoring mass spectrometry. J. Chromatogr. B 1121, 39–47 (2019).
Zhang, H. et al. Folate stability and method optimization for folate extraction from seeds of pulse crops using LC-SRM MS. J. Food Compos. Anal. 71, 44–55 (2018).
Shohag, M. et al. Folate content and composition of vegetables commonly consumed in China. J. Food Sci. 77, H239–H245 (2012).
Shohag, M. et al. Natural variation of folate content and composition in spinach (Spinacia oleracea) germplasm. J. Agric. Food Chem. 59, 12520–12526 (2011).
Shohag, M. et al. A rapid method for sensitive profiling of folates from plant leaf by ultra-performance liquid chromatography coupled to tandem quadrupole mass spectrometer. J. Chromatogr. B 1040, 169–179 (2017).
Wang, C., Riedl, K. M. & Schwartz, S. J. A liquid chromatography–tandem mass spectrometric method for quantitative determination of native 5-methyltetrahydrofolate and its polyglutamyl derivatives in raw vegetables. J. Chromatogr. B 878, 2949–2958 (2010).
Strålsjö, L., Åhlin, H., Witthöft, C. M. & Jastrebova, J. Folate determination in Swedish berries by radioprotein-binding assay (RPBA) and high performance liquid chromatography (HPLC). Eur. Food Res. Technol. 216, 264–269 (2003).
Strålsjö, L. M., Witthöft, C. M., Sjöholm, I. M. & Jägerstad, M. I. Folate content in strawberries (Fragaria× ananassa): effects of cultivar, ripeness, year of harvest, storage, and commercial processing. J. Agric. Food Chem. 51, 128–133 (2003).
Islam, M. S., Mehmood, S., Zhang, C. & Liang, Q. Identification of the prepared foods promising for dietary folate intake in Beijing, China. Food Sci. Nutr. 8, 6557–6567 (2020).
Hall, C., Hillen, C. & Garden Robinson, J. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 94, 11–31 (2017).
Vishnumohan, S., Arcot, J. & Pickford, R. Naturally-occurring folates in foods: method development and analysis using liquid chromatography–tandem mass spectrometry (LC–MS/MS). Food Chem. 125, 736–742 (2011).
Vishnumohan, S. et al. Determination of folate contents in selected Indian foods using the tri-enzyme extraction and estimated folate intakes of the population based on 24-h recall. Int. J. Food Sci. Nutr. 60, 170–180 (2009).
Vishnumohan, S., Pickford, R. & Arcot, J. Naturally occurring folates in selected traditionally prepared foods in Southern India. J. Food Sci. Technol. 54, 4173–4180 (2017).
Meng, Z. et al. Optimized extraction and characterization of folates from date palm fruits and their tracking during fruits wine fermentation. Front. Nutr. 8, 699555 (2021).
Fajardo, V., Alonso-Aperte, E. & Varela-Moreiras, G. Lack of data on folate in convenience foods: should ready-to-eat products be considered relevant for folate intake? The European challenge. J. Food Compos. Anal. 28, 155–163 (2012).
Fajardo, V., Alonso-Aperte, E. & Varela-Moreiras, G. Total folate content in ready-to-eat vegetable meals from the Spanish market. J. Food Compos. Anal. 64, 223–231 (2017).
López, A. et al. Chemical composition and antioxidant capacity of lettuce: Comparative study of regular-sized (Romaine) and baby-sized (Little Gem and Mini Romaine) types. J. Food Compos. Anal. 33, 39–48 (2014).
Yang, X., Gil, M. I., Yang, Q. & Tomás‐Barberán, F. A. Bioactive compounds in lettuce: Highlighting the benefits to human health and impacts of preharvest and postharvest practices. Compr. Rev. Food Sci. Food Saf. 21, 4–45 (2022).
Devi, R., Arcot, J., Sotheeswaran, S. & Ali, S. Folate contents of some selected Fijian foods using tri-enzyme extraction method. Food Chem. 106, 1100–1104 (2008).
Chew, S., Loh, S. & Khor, G. Determination of folate content in commonly consumed Malaysian foods. Int. Food Res. J. 19, 189–197 (2012).
Tornero, E. M., Espinosa-Mansilla, A. & Merás, I. D. High-performance liquid chromatography with fast-scanning fluorescence detection and post-column on-line photoderivatization for the analysis of folic acid and its metabolites in vegetables. Microchem. J. 133, 333–345 (2017).
Sen Gupta, D. et al. Lentils (Lens culinaris L.), a rich source of folates. J. Agric. Food Chem. 61, 7794–7799 (2013).
Kim, D.-E. et al. Metabolite profiling of green, green/red, and red lettuce cultivars: Variation in health beneficial compounds and antioxidant potential. Food Res. Int. 105, 361–370 (2018).
Kim, M. J. et al. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 49, 19–34 (2016).
Strandler, H. S., Patring, J., Jägerstad, M. & Jastrebova, J. Challenges in the determination of unsubstituted food folates: impact of stabilities and conversions on analytical results. J. Agric. Food Chem. 63, 2367–2377 (2015).
Alfthan, G. et al. Folate intake, plasma folate and homocysteine status in a random Finnish population. Eur. J. Clin. Nutr. 57, 81–88 (2003).
Bogers, R. P. et al. Effect of increased vegetable and fruit consumption on plasma folate and homocysteine concentrations. Nutr 23, 97–102 (2007).
Johansson, M., Jägerstad, M. & Frølich, W. Folates in lettuce: a pilot study. Scand. J. Food Nutr. 51, 22–30 (2007).
Bennett, L. E., Singh, D. P. & Clingeleffer, P. R. Micronutrient mineral and folate content of Australian and imported dried fruit products. Crit. Rev. Food Sci. Nutr. 51, 38–49 (2011).
Rychlik, M. Revised folate content of foods determined by stable isotope dilution assays. J. Food Compos. Anal. 17, 475–483 (2004).
Paucean, A. et al. Folic acid, minerals, amino-acids, fatty acids and volatile compounds of green and red lentils. Folic acid content optimization in wheat-lentils composite flours. Chem. Cent. J. 12, 1–9 (2018).
Jamieson, J. A., Viana, L. & English, M. M. Folate content and chemical composition of commercially available gluten-free flour alternatives. Plant Foods Hum. Nutr. 75, 337–343 (2020).
Tulipani, S. et al. Folate content in different strawberry genotypes and folate status in healthy subjects after strawberry consumption. BioFactors 34, 47–55 (2008).
Witthöft, C. M., Forssén, K., Johannesson, L. & Jägerstad, M. Folates-food sources, analyses, retention and bioavailability. Food Nutr. Res. 43, 138–146 (1999).
Öhrvik, V. E., Olsson, J. C., Sundberg, B. E. & Witthöft, C. M. Effect of 2 pieces of nutritional advice on folate status in Swedish women: a randomized controlled trial. Am. J. Clin. Nutr. 89, 1053–1058 (2009).
Rampersaud, G. C., Kauwell, G. P. & Bailey, L. B. Folate: a key to optimizing health and reducing disease risk in the elderly. J. Am. Coll. Nutr. 22, 1–8 (2003).
Angeles-Agdeppa, I. et al. Food sources, energy and nutrient intakes of adults: 2013 Philippines National Nutrition Survey. Nutr. J. 18, 59 (2019).
Jägerstad, M. & Jastrebova, J. Occurrence, stability, and determination of formyl folates in foods. J. Agric. Food Chem. 61, 9758–9768 (2013).
Hefni, M., Ӧhrvik, V., Tabekha, M. & Witthöft, C. Folate content in foods commonly consumed in Egypt. Food Chem. 121, 540–545 (2010).
Allen, L. H. Causes of vitamin B12 and folate deficiency. Food Nutr. Bull. 29, S20–S34 (2008).
Han, J.-Y. & Tyler, R. T. Determination of folate concentrations in pulses by a microbiological method employing trienzyme extraction. J. Agric. Food Chem. 51, 5315–5318 (2003).
Iwatani, Y., Arcot, J. & Shrestha, A. K. Determination of folate contents in some Australian vegetables. J. Food Compos. Anal. 16, 37–48 (2003).
Martin, H. et al. Quantification of folate in fruits and vegetables: a fluorescence-based homogeneous assay. Anal. Biochem. 402, 137–145 (2010).
Bationo, F., Savadogo, B. & Goubgou, M. Folates in various African foods: contents, food processing and matrix effects. Int. J. Vitam. Nutr. Res. 93, 459–470 (2022).
Rawalpally, T. R. Folic acid. Kirk-Othmer Encycl. Chem. Technol., 1–18, https://doi.org/10.1002/0471238961.0615120918012301.a01.pub2 (2014).
Pajari, A. M. et al. Bioactive compounds in whole grains and their implications for health. In Whole Grains and Health (ed. Rikard Landberg, N. S.) Ch. 16, 301–336 (John Wiley & Sons Ltd., 2021).
Hager, A.-S. et al. Nutritional properties and ultra-structure of commercial gluten free flours from different botanical sources compared to wheat flours. J. Cereal Sci. 56, 239–247 (2012).
Mudryj, A. N., Yu, N. & Aukema, H. M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 39, 1197–1204 (2014).
Naderi, N. & House, J. D. Recent Developments in Folate Nutrition. In Advances in Food and Nutrition Research 83 (ed. Eskin, M.) Ch. 5, 195–213 (Academic Press, 2018).
Yazynina, E., Johansson, M., Jägerstad, M. & Jastrebova, J. Low folate content in gluten-free cereal products and their main ingredients. Food Chem. 111, 236–242 (2008).
Czarnowska-Kujawska, M. et al. Folate content and yolk color of hen eggs from different farming systems. Molecules 26, 1034 (2021).
Czarnowska-Kujawska, M., Gujska, E. & Michalak, J. Folate determination in livers of different animal species. Czech J. Food Sci. 38, 43–48 (2020).
Gmelch, L. et al. Comprehensive vitamer profiling of folate mono-and polyglutamates in baker’s yeast (Saccharomyces cerevisiae) as a function of different sample preparation procedures. Metabolites 10, 301 (2020).
Patring, J. D. et al. Development of a simplified method for the determination of folates in baker’s yeast by HPLC with ultraviolet and fluorescence detection. J. Agric. Food Chem. 53, 2406–2411 (2005).
Jastrebova, J., Strandler, H. S., Patring, J. & Wiklund, T. Comparison of UPLC and HPLC for analysis of dietary folates. Chromatographia 73, 219–225 (2011).
Nojavan, Y. et al. Ion pair-based dispersive liquid–liquid microextraction followed by high performance liquid chromatography as a new method for determining five folate derivatives in foodstuffs. Talanta 137, 31–37 (2015).
EFSA Panel on Dietetic Products Nutrition and Allergies Scientific opinion on dietary reference values for folate. EFSA J. 12, 3893 (2014).
Réhault-Godbert, S., Guyot, N. & Nys, Y. The golden egg: nutritional value, bioactivities, and emerging benefits for human health. Nutrients 11, 684 (2019).
Roe, M., Church, S., Pinchen, H. & Finglas, P. Nutrient analysis of eggs: analytical report, 1–44 (Institute of Food Research, 2013).
Williams, P. Nutritional composition of red meat. Nutr. Diet. 64, S113–S119 (2007).
Bassett, M. & Sammán, N. Folate content and retention in selected raw and processed foods. Arch. Latinoam. Nutr. 60, 298–305 (2010).
Hjortmo, S., Patring, J., Jastrebova, J. & Andlid, T. Inherent biodiversity of folate content and composition in yeasts. Trends Food Sci. Technol. 16, 311–316 (2005).
Strandler, H. S., Jastrebova, J. & Mattisson, I. Folate content in Swedish eggs: influence of breed, feed and processing. Eur. Food Res. Technol. 233, 923–930 (2011).
Ložnjak, P. et al. Quantification of folate in food using deconjugase of plant origin combined with LC-MS/MS: A method comparison of a large and diverse sample set. Food Chem. 305, 125450 (2020).
Delchier, N., Herbig, A. L., Rychlik, M. & Renard, C. M. Folates in fruits and vegetables: contents, processing, and stability. Compr. Rev. Food Sci. Food Saf. 15, 506–528 (2016).
Partearroyo, T. et al. Dietary sources and intakes of folates and vitamin B12 in the Spanish population: Findings from the ANIBES study. PLoS One 12, e0189230 (2017).
Park, J. Y. et al. Comparison of standardised dietary folate intake across ten countries participating in the European Prospective Investigation into Cancer and Nutrition. Br. J. Nutr. 108, 552–569 (2012).
Imaeda, N. et al. Folate intake and food sources in Japanese female dietitians. Environ. Health Prev. Med. 7, 156–161 (2002).
Yoshino, K. et al. Trends in dietary intake of folate, vitamins B6, and B12 among Japanese adults in two rural communities from 1974 through 2001. J. Epidemiol. 15, 29–37 (2005).
Iglesia, I. et al. Foods contributing to vitamin B 6, folate, and vitamin B 12 intakes and biomarkers status in European adolescents: the HELENA study. Eur. J. Nutr. 56, 1767–1782 (2017).
Steluti, J., Martini, L. A., Peters, B. S. & Marchioni, D. M. Folate, vitamin B6 and vitamin B12 in adolescence: serum concentrations, prevalence of inadequate intakes and sources in food. J. Pediatr. 87, 43–49 (2011).
Krishnaswamy, K. & Nair, K. M. Importance of folate in human nutrition. Br. J. Nutr. 85, S115–S124 (2001).
Pravst, I. et al. Dietary intake of folate and assessment of the folate deficiency prevalence in Slovenia using serum biomarkers. Nutrients 13, 3860 (2021).
Brevik, A. et al. Plasma concentration of folate as a biomarker for the intake of fruit and vegetables: the Hordaland Homocysteine Study. Am. J. Clin. Nutr. 81, 434–439 (2005).
Zekovic, M. et al. Validity of the food frequency questionnaire assessing the folate intake in women of reproductive age living in a country without food fortification: application of the method of triads. Nutrients 9, 128 (2017).
Konings, E. J. et al. Folate intake of the Dutch population according to newly established liquid chromatography data for foods. Am. J. Clin. Nutr. 73, 765–776 (2001).
Planells, E. et al. Vitamins B6 and B12 and folate status in an adult Mediterranean population. Eur. J. Clin. Nutr. 57, 777–785 (2003).
Kim, Y.-N. & Cho, Y.-O. Folate food source, usual intake, and folate status in Korean adults. Nutr. Res. Pract. 12, 47–51 (2018).
Finglas, P. M. & Wright, A. J. Folate bioavailability and health. Phytochem. Rev. 1, 189–198 (2002).
Palchetti, C. Z. et al. Prevalence of inadequate intake of folate in the post-fortification era: data from the Brazilian National Dietary Surveys 2008–2009 and 2017–2018. Br. J. Nutr. 128, 1638–1646 (2022).
Evans, S. E. et al. Effect of increasing voluntary folic acid food fortification on dietary folate intakes and adequacy of reproductive-age women in New Zealand. Public Health Nutr. 17, 1447–1453 (2014).
Saubade, F., Hemery, Y. M., Guyot, J.-P. & Humblot, C. Lactic acid fermentation as a tool for increasing the folate content of foods. Crit. Rev. Food Sci. Nutr. 57, 3894–3910 (2017).
Shewry, P. R. & Hey, S. J. The contribution of wheat to human diet and health. Food Energy Secur. 4, 178–202 (2015).
Lockyer, S. & Spiro, A. The role of bread in the UK diet: An update. Nutr. Bull. 45, 133–164 (2020).
Patring, J., Wandel, M., Jägerstad, M. & Frølich, W. Folate content of Norwegian and Swedish flours and bread analysed by use of liquid chromatography–mass spectrometry. J. Food Compos. Anal. 22, 649–656 (2009).
Öhrvik, V., Öhrvik, H., Tallkvist, J. & Witthöft, C. Folates in bread: retention during bread-making and in vitro bioaccessibility. Eur. J. Nutr. 49, 365–372 (2010).
McNulty, H., Ward, M., Caffrey, A. & Pentieva, K. Contribution of folic acid to human health and challenges of translating the science into effective policy: a call to action for the implementation of food fortification in Ireland. Proc. Nutr. Soc. 82, 91–103 (2023).
Laskowski, W. et al. How important are cereals and cereal products in the average polish diet? Nutrients 11, 679 (2019).
Schoenlechner, R., Wendner, M., Siebenhandl-Ehn, S. & Berghofer, E. Pseudocereals as alternative sources for high folate content in staple foods. J. Cereal Sci. 52, 475–479 (2010).
Gujska, E. & Kuncewicz, A. Determination of folate in some cereals and commercial cereal-grain products consumed in Poland using trienzyme extraction and high-performance liquid chromatography methods. Eur. Food Res. Technol. 221, 208–213 (2005).
Caselato‐Sousa, V. M. & Amaya‐Farfán, J. State of knowledge on amaranth grain: a comprehensive review. J. Food Sci. 77, R93–R104 (2012).
Martínez-Villaluenga, C., Peñas, E. & Hernández-Ledesma, B. Pseudocereal grains: Nutritional value, health benefits and current applications for the development of gluten-free foods. Food Chem. Toxicol. 137, 111178 (2020).
Motta, C. et al. Folates in quinoa (Chenopodium quinoa), amaranth (Amaranthus sp.) and buckwheat (Fagopyrum esculentum): Influence of cooking and malting. J. Food Compos. Anal. 64, 181–187 (2017).
Ahmad, R. S., Imran, A. & Hussain, M. B. Nutritional composition of meat. In Meat Science and Nutrition (ed. Arshad, M. S.) Ch. 4, 61–75 (IntechOpen Limited, 2018).
Neufingerl, N. & Eilander, A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients 14, 29 (2022).
Gilsing, A. M. et al. Serum concentrations of vitamin B12 and folate in British male omnivores, vegetarians and vegans: results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur. J. Clin. Nutr. 64, 933–939 (2010).
Koebnick, C. et al. Folate status during pregnancy in women is improved by long-term high vegetable intake compared with the average western diet. J. Nutr. 131, 733–739 (2001).
Öhrvik, V. et al. Dietary intake and biomarker status of folate in Swedish adults. Eur. J. Nutr. 57, 451–462 (2018).
Brouwer, I. A. et al. Dietary folate from vegetables and citrus fruit decreases plasma homocysteine concentrations in humans in a dietary controlled trial. J. Nutr. 129, 1135–1139 (1999).
Hatzis, C. M. et al. Dietary and other lifestyle correlates of serum folate concentrations in a healthy adult population in Crete, Greece: a cross-sectional study. Nutr. J. 5, 1–10 (2006).
Davey, G. K. et al. EPIC–Oxford:lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 6, 259–268 (2003).
Larsson, C. L. & Johansson, G. K. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am. J. Clin. Nutr. 76, 100–106 (2002).
Cade, J. E., Burley, V. J., Greenwood, D. C. & Group, U. Ws. C. S. S. The UK Women’s Cohort Study: comparison of vegetarians, fish-eaters and meat-eaters. Public Health Nutr. 7, 871–878 (2004).
Cosgrove, M., Flynn, A. & Kiely, M. Consumption of red meat, white meat and processed meat in Irish adults in relation to dietary quality. Br. J. Nutr. 93, 933–942 (2005).
Iyer, R. & Tomar, S. Folate: a functional food constituent. J. Food Sci. 74, R114–R122 (2009).
Verwei, M. et al. The binding of folic acid and 5-methyltetrahydrofolate to folate-binding proteins during gastric passage differs in a dynamic in vitro gastrointestinal model. J. Nutr. 134, 31–37 (2004).
Buttriss, J. L. Folate status in the UK. Nutr. Bull. 40, 153–157 (2015).
Forssén, K. M., Jagerstad, M. I., Wigertz, K. & Witthöft, C. M. Folates and dairy products: a critical update. J. Am. Coll. Nutr. 19, 100S–110S (2000).
Ezekiel, R., Singh, N., Sharma, S. & Kaur, A. Beneficial phytochemicals in potato-a review. Food Res. Int. 50, 487–496 (2013).
Goyer, A. & Navarre, D. A. Determination of folate concentrations in diverse potato germplasm using a trienzyme extraction and a microbiological assay. J. Agric. Food Chem. 55, 3523–3528 (2007).
Goyer, A. Maximizing the nutritional potential of potato: the case of folate. Potato Res. 60, 319–325 (2017).
Goyer, A. & Navarre, D. A. Folate is higher in developmentally younger potato tubers. J. Sci. Food Agric. 89, 579–583 (2009).
Morales, P. et al. Optimization and application of FL-HPLC for folates analysis in 20 species of Mediterranean wild vegetables. Food Anal. Methods 8, 302–311 (2015).
Ogle, B. M., Johansson, M., Tuyet, H. T. & Johannesson, L. Evaluation of the significance of dietary folate from wild vegetables in Vietnam. Asia Pac. J. Clin. Nutr. 10, 216–221 (2001).
Fyfe, S. et al. Future flavours from the past: sensory and nutritional profiles of green plum (Buchanania obovata), red bush apple (Syzygium suborbiculare) and wild peach (Terminalia carpentariae) from East Arnhem Land, Australia. Future Foods 5, 100136 (2022).
Fyfe, S. A. et al. Buchanania obovata: an Australian indigenous food for diet diversification. Nutr. Diet. 75, 527–532 (2018).
Woortman, D. V. et al. Microalgae a superior source of folates: quantification of folates in halophile microalgae by stable isotope dilution assay. Front. Bioeng. Biotechnol. 7, 481 (2020).
Fujii, K., Nakashima, H. & Hashidzume, Y. Isolation of folate‐producing microalgae, from oligotrophic ponds in Yamaguchi, Japan. J. Appl. Microbiol. 108, 1421–1429 (2010).
Edelmann, M. et al. Riboflavin, niacin, folate and vitamin B12 in commercial microalgae powders. J. Food Compos. Anal. 82, 103226 (2019).
Bito, T., Okumura, E., Fujishima, M. & Watanabe, F. Potential of Chlorella as a dietary supplement to promote human health. Nutrients 12, 2524 (2020).
Han, Y., Yon, M. & Hyun, T. Folate intake estimated with an updated database and its association to blood folate and homocysteine in Korean college students. Eur. J. Clin. Nutr. 59, 246–254 (2005).
Yon, M. & Hyun, T. H. Folate content of foods commonly consumed in Korea measured after trienzyme extraction. Nutr. Res. 23, 735–746 (2003).
De Quirós, A. R.-B., De Ron, C. C., López-Hernández, J. & Lage-Yusty, M. Determination of folates in seaweeds by high-performance liquid chromatography. J. Chromatogr. A 1032, 135–139 (2004).
Jach, M. E. & Malm, A. Yarrowia lipolytica as an alternative and valuable source of nutritional and bioactive compounds for humans. Molecules 27, 2300 (2022).
Jach, M. E. et al. Production of enriched in B vitamins biomass of Yarrowia lipolytica grown in biofuel waste. Saudi J. Biol. Sci. 28, 2925–2932 (2021).
Phillips, K. M., Ruggio, D. M. & Haytowitz, D. B. Folate composition of 10 types of mushrooms determined by liquid chromatography–mass spectrometry. Food Chem. 129, 630–636 (2011).
Nakalembe, I., Kabasa, J. D. & Olila, D. Comparative nutrient composition of selected wild edible mushrooms from two agro-ecological zones, Uganda. Springerplus 4, 1–15 (2015).
Raman, J. et al. Cultivation and nutritional value of prominent Pleurotus spp.: an overview. Mycobiology 49, 1–14 (2021).
Mattila, P. et al. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 49, 2343–2348 (2001).
Weber, N. et al. Folate contents in insects as promising food components quantified by stable isotope dilution. Front. Nutr. 9, 970255 (2022).
Nowak, V., Persijn, D., Rittenschober, D. & Charrondiere, U. R. Review of food composition data for edible insects. Food Chem. 193, 39–46 (2016).
Alagappan, S. et al. Nutritional analysis, volatile composition, antimicrobial and antioxidant properties of Australian green ants (Oecophylla smaragdina). Future Foods 3, 100007 (2021).
Bouckaert, K. P. et al. Critical evaluation of folate data in European and international databases: recommendations for standardization in international nutritional studies. Mol. Nutr. Food Res. 55, 166–180 (2011).
Arcot, J. & Shrestha, A. Folate: methods of analysis. Trends Food Sci. Technol. 16, 253–266 (2005).
Nicolas, G. et al. Compilation of a standardised international folate database for EPIC. Food Chem. 193, 134–140 (2016).
Westenbrink, S., Jansen-van der Vliet, M. & van Rossum, C. Updated folate data in the Dutch Food Composition Database and implications for intake estimates. Food Nutr. Res. 56, 5449 (2012).
Octavia, L. & Choo, W. S. Folate, ascorbic acid, anthocyanin and colour changes in strawberry (Fragaria× annanasa) during refrigerated storage. LWT 86, 652–659 (2017).
Pinela, J. et al. Stability of total folates/vitamin B9 in irradiated watercress and buckler sorrel during refrigerated storage. Food Chem. 274, 686–690 (2019).
Munyaka, A. W. et al. Influence of Thermal Processing on Hydrolysis and Stability of Folate Poly-γ-glutamates in Broccoli (Brassica oleracea var. italica), Carrot (Daucus carota) and Tomato (Lycopersicon esculentum). J. Agric. Food Chem. 58, 4230–4240 (2010).
Upadhyaya, P. et al. Natural variation in folate levels among tomato (Solanum lycopersicum) accessions. Food Chem. 217, 610–619 (2017).
Houlihan, A. et al. Folate content of Asian vegetables (Rural Industries Research and Development Corporation, 2011).
Puwastien, P., Pinprapai, N., Judprasong, K. & Tamura, T. International inter-laboratory analyses of food folate. J. Food Compos. Anal. 18, 387–397 (2005).
EFSA Panel on Nutrition Novel Foods and Food Allergens Conversion of calcium‐l‐methylfolate and (6S)‐5‐methyltetrahydrofolic acid glucosamine salt into dietary folate equivalents. EFSA J. 20, e07452 (2022).
Witthöft, C. M., Straålsjoö, L., Berglund, G. & Lundin, E. G. A human model to determine folate bioavailability from food: a pilot study for evaluation. Food Nutr. Res. 47, 6–18 (2003).
Seyoum, E. & Selhub, J. Properties of food folates determined by stability and susceptibility to intestinal pteroylpolyglutamate hydrolase action. J. Nutr. 128, 1956–1960 (1998).
Bailey, L. B. Dietary reference intakes for folate: the debut of dietary folate equivalents. Nutr. Rev. 56, 294–299 (1998).
Nygren-Babol, L. & Jägerstad, M. Folate-binding protein in milk: a review of biochemistry, physiology, and analytical methods. Crit. Rev. Food Sci. Nutr. 52, 410–425 (2012).
Nygren-Babol, L., Sternesjö, Å., Jägerstad, M. & Björck, L. Affinity and rate constants for interactions of bovine folate-binding protein and folate derivatives determined by optical biosensor technology. Effect of stereoselectivity. J. Agric. Food Chem. 53, 5473–5478 (2005).
Cochrane, K. M. et al. Is natural (6 S)-5-methyltetrahydrofolic acid as effective as synthetic folic acid in increasing serum and red blood cell folate concentrations during pregnancy? A proof-of-concept pilot study. Trials 21, 1–12 (2020).
Buffière, C. et al. Food matrix structure (from biscuit to custard) has an impact on folate bioavailability in healthy volunteers. Eur. J. Nutr. 60, 411–423 (2021).
Scaglione, F. & Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 44, 480–488 (2014).
Ringling, C. & Rychlik, M. Simulation of food folate digestion and bioavailability of an oxidation product of 5-methyltetrahydrofolate. Nutrients 9, 969 (2017).
Marchetta, C. M. et al. Assessing the association between natural food folate intake and blood folate concentrations: a systematic review and Bayesian meta-analysis of trials and observational studies. Nutrients 7, 2663–2686 (2015).
Liu, F., Edelmann, M., Piironen, V. & Kariluoto, S. 5-Methyltetrahydrofolate is a crucial factor in determining the bioaccessibility of folate in bread. J. Agric. Food Chem. 70, 13379–13390 (2022).
Liu, F., Edelmann, M., Piironen, V. & Kariluoto, S. The bioaccessibility of folate in breads and the stability of folate vitamers during in vitro digestion. Food Funct. 13, 3220–3233 (2022).
Henderson, A. M. et al. L-5-methyltetrahydrofolate supplementation increases blood folate concentrations to a greater extent than folic acid supplementation in Malaysian women. J. Nutr. 148, 885–890 (2018).
Rychlik, M. et al. Application of stable isotope dilution assays based on liquid chromatography–tandem mass spectrometry for the assessment of folate bioavailability. J. Chromatogr. B 792, 167–176 (2003).
Hannon-Fletcher, M. P. et al. Determining bioavailability of food folates in a controlled intervention study. Am. J. Clin. Nutr. 80, 911–918 (2004).
Mönch, S. et al. Folate bioavailability from foods rich in folates assessed in a short term human study using stable isotope dilution assays. Food Funct. 6, 241–247 (2015).
Mönch, S. et al. Pilot study on folate bioavailability from a Camembert cheese reveals contradictory findings to recent results from a human short-term study. Front. Nutr. 3, 9 (2016).
Konings, E. J. et al. Intestinal absorption of different types of folate in healthy subjects with an ileostomy. Br. J. Nutr. 88, 235–242 (2002).
Hiolle, M. et al. In vitro digestion of complex foods: how microstructure influences food disintegration and micronutrient bioaccessibility. Food Res. Int. 128, 108817 (2020).
Winkels, R. M. et al. Bioavailability of food folates is 80% of that of folic acid. Am. J. Clin. Nutr. 85, 465–473 (2007).
Brouwer, I. A., van Dusseldorp, M., West, C. E. & Steegers-Theunissen, R. P. Bioavailability and bioefficacy of folate and folic acid in man. Nutr. Res. Rev. 14, 267–294 (2001).
Wright, A. et al. Single oral doses of 13C forms of pteroylmonoglutamic acid and 5-formyltetrahydrofolic acid elicit differences in short-term kinetics of labelled and unlabelled folates in plasma: potential problems in interpretation of folate bioavailability studies. Br. J. Nutr. 90, 363–371 (2003).
Wright, A. J., Dainty, J. R. & Finglas, P. M. Folic acid metabolism in human subjects revisited: potential implications for proposed mandatory folic acid fortification in the UK. Br. J. Nutr. 98, 667–675 (2007).
Gregory, J. III, Quinlivan, E. & Davis, S. Integrating the issues of folate bioavailability, intake and metabolism in the era of fortification. Trends Food Sci. Technol. 16, 229–240 (2005).
Gregory, J. III Case study: folate bioavailability. J. Nutr 131, 1376s–1382s (2001).
Öhrvik, V. E. et al. Folate bioavailability from breads and a meal assessed with a human stable-isotope area under the curve and ileostomy model. Am. J. Clin. Nutr. 92, 532–538 (2010).
Öhrvik, V. E. & Witthöft, C. M. Human folate bioavailability. Nutrients 3, 475–490 (2011).
Bhandari, S. & Gregory, J. III Inhibition by selected food components of human and porcine intestinal pteroylpolyglutamate hydrolase activity. Am. J. Clin. Nutr. 51, 87–94 (1990).
Wei, M.-M., Bailey, L. B., Toth, J. P. & Gregory, J. F. III Bioavailability for humans of deuterium-labeled monoglutamyl and polyglutamyl folates is affected by selected foods. J. Nutr. 126, 3100–3108 (1996).
Wei, M.-M. & Gregory, J. F. III Organic acids in selected foods inhibit intestinal brush border pteroylpolyglutamate hydrolase in vitro: potential mechanism affecting the bioavailability of dietary polyglutamyl folate. J. Agric. Food Chem. 46, 211–219 (1998).
Picciano, M. F. et al. Effect of cow milk on food folate bioavailability in young women. Am. J. Clin. Nutr. 80, 1565–1569 (2004).
Caudill, M. A. Folate bioavailability: implications for establishing dietary recommendations and optimizing status. Am. J. Clin. Nutr. 91, 1455S–1460S (2010).
Sanderson, P. et al. Folate bioavailability: UK food standards agency workshop report. Br. J. Nutr. 90, 473–479 (2003).
McNulty, H. & Pentieva, K. Folate bioavailability. Proc. Nutr. Soc. 63, 529–536 (2004).
McKillop, D. J. et al. The rate of intestinal absorption of natural food folates is not related to the extent of folate conjugation. Am. J. Clin. Nutr. 84, 167–173 (2006).
Pietrzik, K., Bailey, L. & Shane, B. Folic acid and L-5-methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 49, 535–548 (2010).
Chan, Y.-M., Bailey, R. & O’Connor, D. L. Folate. Adv. Nutr. 4, 123–125 (2013).
Brandon, E. et al. Bioaccessibility of vitamin A, vitamin C and folic acid from dietary supplements, fortified food and infant formula. Int. J. Food Sci. Nutr. 65, 426–435 (2014).
Yaman, M. et al. The bioaccessibility of water-soluble vitamins: a review. Trends Food Sci. Technol. 109, 552–563 (2021).
Yaman, M., Mızrak, Ö. F., Catak, J. & Sargın, H. S. In vitro bioaccessibility of added folic acid in commercially available baby foods formulated with milk and milk products. Food Sci. Biotechnol. 28, 1837–1844 (2019).
Etcheverry, P., Grusak, M. A. & Fleige, L. E. Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D, and. E. Front. Physiol. 3, 317 (2012).
Hughes, J. & Buttriss, J. An update on folates and folic acid: contribution of MAFF‐funded research. Nutr. Bull. 25, 113–124 (2000).
Verwei, M. et al. Folic acid and 5-methyltetrahydrofolate in fortified milk are bioaccessible as determined in a dynamic in vitro gastrointestinal model. J. Nutr. 133, 2377–2383 (2003).
Arkbåge, K. Vitamin B12, Folate and Folate Binding Proteins in Dairy Products: Analysis, Process, Retention and Bioavailability (Swedish University of Agricultural Sciences, 2003).
Melse-Boonstra, A. Bioavailability of micronutrients from nutrient-dense whole foods: zooming in on dairy, vegetables, and fruits. Front. Nutr. 7, 101 (2020).
Melse-Boonstra, A., Verhoef, P. & West, C. Quantifying folate bioavailability: a critical appraisal of methods. Curr. Opin. Clin. Nutr. Metab. Care 7, 539–545 (2004).
Melse-Boonstra, A. et al. A dual-isotope-labeling method of studying the bioavailability of hexaglutamyl folic acid relative to that of monoglutamyl folic acid in humans by using multiple orally administered low doses. Am. J. Clin. Nutr. 84, 1128–1133 (2006).
Prinz‐Langenohl, R. et al. [6S]‐5‐methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild‐type 677C→ T polymorphism of methylenetetrahydrofolate reductase. Br. J. Pharmacol. 158, 2014–2021 (2009).
West, A. A. et al. Folate. In Present Knowledge in Nutrition (eds Marriott, B. P., Birt, D. F., Stallings, V. A. & Yates, A. A.) Ch. 14, 239–255 (Academic Press, 2020). https://www.sciencedirect.com/science/article/abs/pii/B9780323661621000147, https://www.sciencedirect.com/book/9780323661621/present-knowledge-in-nutrition#book-info.
Munyaka, A. W. et al. Acidification, crushing and thermal treatments can influence the profile and stability of folate poly-γ-glutamates in broccoli (Brassica oleracea L. var. italica). Food Chem 117, 568–575 (2009).
Pentieva, K. et al. The short-term bioavailabilities of [6S]-5-methyltetrahydrofolate and folic acid are equivalent in men. J. Nutr. 134, 580–585 (2004).
Green, T. J. et al. Wheat rolls fortified with microencapsulated L-5-methyltetrahydrofolic acid or equimolar folic acid increase blood folate concentrations to a similar extent in healthy men and women. J. Nutr. 143, 867–871 (2013).
Jia, X. et al. Association between tea drinking and plasma folate concentration among women aged 18–30 years in China. Public Health Nutr. 24, 4929–4936 (2021).
Alemdaroglu, N. C. et al. Influence of green and black tea on folic acid pharmacokinetics in healthy volunteers: potential risk of diminished folic acid bioavailability. Biopharm. Drug Dispos. 29, 335–348 (2008).
Liu, J. et al. Tea consumption is not associated with reduced plasma folate concentration among Chinese pregnant women. Birth Defects Res. A Clin. Mol. Teratol. 103, 747–753 (2015).
Umegaki, K. et al. Effect of tea catechins on folate analysis in green tea by microbiological assay. J. Nutr. Sci. Vitaminol. 62, 134–138 (2016).
Shiraishi, M. et al. Association between the serum folate levels and tea consumption during pregnancy. BioSci. Trends 4, 225–230 (2010).
Otake, M., Sakurai, K., Watanabe, M. & Mori, C. Association between serum folate levels and caffeinated beverage consumption in pregnant women in Chiba: the Japan environment and children’s study. J. Epidemiol. 28, 414–419 (2018).
Moretti, D. et al. Bioavailability of iron, zinc, folic acid, and vitamin A from fortified maize. Ann. N. Y. Acad. Sci. 1312, 54–65 (2014).
Chandra-Hioe, M. V. et al. Transport of folic acid across Caco-2 cells is more effective than 5-methyltetrahydrofolate following the in vitro digestion of fortified bread. Food Res. Int. 53, 104–109 (2013).
Bailey, S. W. & Ayling, J. E. The pharmacokinetic advantage of 5-methyltetrahydrofolate for minimization of the risk for birth defects. Sci. Rep. 8, 4096 (2018).
Åkesson, A. et al. Preparatory work for the update of the tolerable upper intake levels for folic acid/folate. EFSA Support. Publ. 20, 7940E (2023).
Gregory, J. III Accounting for differences in the bioactivity and bioavailability of vitamers. Food Nutr. Res. 56, 5809 (2012).
Strozzi, G. P. & Mogna, L. Quantification of folic acid in human feces after administration of Bifidobacterium probiotic strains. J. Clin. Gastroenterol. 42, S179–S184 (2008).
Chan, Y.-M., Aufreiter, S., O’Keefe, S. J. & O’Connor, D. L. Switching to a fibre-rich and low-fat diet increases colonic folate contents among African Americans. Appl. Physiol. Nutr. Metab. 44, 127–132 (2019).
Kim, T. H., Yang, J., Darling, P. B. & O’Connor, D. L. A large pool of available folate exists in the large intestine of human infants and piglets. J. Nutr. 134, 1389–1394 (2004).
Biesalski, H. K. Nutrition meets the microbiome: micronutrients and the microbiota. Ann. N. Y. Acad. Sci. 1372, 53–64 (2016).
Engevik, M. A. et al. Microbial metabolic capacity for intestinal folate production and modulation of host folate receptors. Front. Microbiol. 10, 2305 (2019).
Rowland, I. et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 57, 1–24 (2018).
Magnusdottir, S., Ravcheev, D., de Crecy-Lagard, V. & Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 6, 148 (2015).
Rodionov, D. A. et al. Micronutrient requirements and sharing capabilities of the human gut microbiome. Front. Microbiol. 10, 1326 (2019).
Soto-Martin, E. C. et al. Vitamin biosynthesis by human gut butyrate-producing bacteria and cross-feeding in synthetic microbial communities. mBio 11, e0088620 (2020).
Das, P., Babaei, P. & Nielsen, J. Metagenomic analysis of microbe-mediated vitamin metabolism in the human gut microbiome. BMC Genom. 20, 1–11 (2019).
D’Aimmo, M. R. et al. The potential of bifidobacteria as a source of natural folate. J. Appl. Microbiol. 112, 975–984 (2012).
Rosario, D. et al. Systematic analysis of gut microbiome reveals the role of bacterial folate and homocysteine metabolism in Parkinson’s disease. Cell Rep. 34, 108807 (2021).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature 473, 174–180 (2011).
Costea, P. I. et al. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol. 3, 8–16 (2018).
Kurokawa, K. et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 14, 169–181 (2007).
Bäckhed, F. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Christian, T., R, F. L. & TJ, G. Citrus pectin and oligofructose improve folate status and lower serum total homocysteine in rats. Int. J. Vitam. Nutr. Res. 73, 403–409 (2003).
Houghton, L. A. et al. Association between dietary fiber intake and the folate status of a group of female adolescents. Am. J. Clin. Nutr. 66, 1414–1421 (1997).
Aufreiter, S., Kim, J. H. & O’Connor, D. L. Dietary oligosaccharides increase colonic weight and the amount but not concentration of bacterially synthesized folate in the colon of piglets. J. Nutr. 141, 366–372 (2011).
Pompei, A. et al. Administration of folate-producing bifidobacteria enhances folate status in Wistar rats. J. Nutr. 137, 2742–2746 (2007).
Valentini, L. et al. Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota–The “RISTOMED project”: Randomized controlled trial in healthy older people. Clin. Nutr. 34, 593–602 (2015).
Sugahara, H. et al. Differences in folate production by bifidobacteria of different origins. Biosci. Microbiota Food Health 34, 87–93 (2015).
Sepehr, E. et al. Folate derived from cecal bacterial fermentation does not increase liver folate stores in 28-d folate-depleted male Sprague-Dawley rats. J. Nutr. 133, 1347–1354 (2003).
Asrar, F. M. & O’Connor, D. L. Bacterially synthesized folate and supplemental folic acid are absorbed across the large intestine of piglets. J. Nutr. Biochem. 16, 587–593 (2005).
Aufreiter, S. et al. Folate is absorbed across the colon of adults: evidence from cecal infusion of 13C-labeled [6S]-5-formyltetrahydrofolic acid. Am. J. Clin. Nutr. 90, 116–123 (2009).
Lakoff, A. et al. Folate is absorbed across the human colon: evidence by using enteric-coated caplets containing 13C-labeled [6S]-5-formyltetrahydrofolate. Am. J. Clin. Nutr. 100, 1278–1286 (2014).
Visentin, M., Diop-Bove, N., Zhao, R. & Goldman, I. D. The intestinal absorption of folates. Annu. Rev. Physiol. 76, 251–274 (2014).
Dudeja, P. et al. Mechanism of folate transport across the human colonic basolateral membrane. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G54–G60 (2001).
Zhan, Q. et al. Unveiling of dietary and gut-microbiota derived B vitamins: metabolism patterns and their synergistic functions in gut-brain homeostasis. Crit. Rev. Food Sci. Nutr. 64, 4046–4058 (2024).
Klaassen, M. A. et al. Anti-inflammatory gut microbial pathways are decreased during Crohn’s disease exacerbations. J. Crohns Colitis 13, 1439–1449 (2019).
Liu, M. et al. Probiotic potential of a folate-producing strain Latilactobacillus sakei LZ217 and its modulation effects on human gut microbiota. Foods 11, 234 (2022).
Gholami, H., Chmiel, J. A., Burton, J. P. & Maleki Vareki, S. The role of microbiota-derived vitamins in immune homeostasis and enhancing cancer immunotherapy. Cancers 15, 1300 (2023).
O’Keefe, S. J. et al. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J. Nutr. 139, 2044–2048 (2009).
Hossain, K. S., Amarasena, S. & Mayengbam, S. B vitamins and their roles in gut health. Microorganisms 10, 1168 (2022).
Crott, J. W. et al. Moderate folate depletion modulates the expression of selected genes involved in cell cycle, intracellular signaling and folate uptake in human colonic epithelial cell lines. J. Nutr. Biochem. 19, 328–335 (2008).
Thomas, C. M. et al. FolC2‐mediated folate metabolism contributes to suppression of inflammation by probiotic Lactobacillus reuteri. Microbiologyopen 5, 802–818 (2016).
Said, H. M. Intestinal absorption of water-soluble vitamins in health and disease. Biochem. J. 437, 357–372 (2011).
Said, H. M. Recent advances in transport of water-soluble vitamins in organs of the digestive system: a focus on the colon and the pancreas. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G601–G610 (2013).
Kok, D. E. et al. Bacterial folate biosynthesis and colorectal cancer risk: more than just a gut feeling. Crit. Rev. Food Sci. Nutr. 60, 244–256 (2020).
Williams, E. A. et al. Systemic folate status, rectal mucosal folate concentration and dietary intake in patients at differential risk of bowel cancer (The FAB2 Study). Eur. J. Nutr. 52, 1801–1810 (2013).
McGlynn, A. P. et al. Low colonocyte folate is associated with uracil misincorporation and global DNA hypomethylation in human colorectum. J. Nutr. 143, 27–33 (2013).
Flood, A. et al. Concentration of folate in colorectal tissue biopsies predicts prevalence of adenomatous polyps. Gut 60, 66–72 (2011).
Barkhidarian, B. et al. Probiotic supplementation and micronutrient status in healthy subjects: a systematic review of clinical trials. Nutrients 13, 3001 (2021).
Berry Ottaway, P. Stability of vitamins during food processing and storage. In Chemical Deterioration and Physical Instability of Food and Beverages (eds Skibsted, H. L., Risbo, J. & Andersen, L. M.) Ch. 19, 539–560 (Woodhead Publishing, 2010).
Godoy, H. T., Amaya-Farfan, J. & Rodriguez-Amaya, D. B. Degradation of vitamins. In Chemical Changes During Processing and Storage of Foods (eds Rodriguez-Amaya, D. B. & Amaya-Farfan, J.) Ch. 8, 329–383 (Academic Press, 2021).
Bationo, F. et al. Total folate in West African cereal-based fermented foods: bioaccessibility and influence of processing. J. Food Compos. Anal. 85, 103309 (2020).
Wusigale & Liang, L. Folates: stability and interaction with biological molecules. J. Agric. Food Res. 2, 100039 (2020).
Delchier, N. et al. Mechanisms of folate losses during processing: diffusion vs. heat degradation. Food Chem. 157, 439–447 (2014).
Saubade, F. et al. Influence of fermentation and other processing steps on the folate content of a traditional African cereal-based fermented food. Int. J. Food Microbiol. 266, 79–86 (2018).
Wahengbam, E. D. et al. Effect of iron and folic acid fortification on in vitro bioavailability and starch hydrolysis in ready-to-eat parboiled rice. Food Chem. 292, 39–46 (2019).
Jastrebova, J. et al. HPLC determination of folates in raw and processed beetroots. Food Chem. 80, 579–588 (2003).
Witthöft, C. M. & Hefni, M. Folic acid and folates: Physiology and health effects. In Encyclopedia of Food and Health (eds Caballero, B., Finglas, P. M. & Toldrá, F.) 724-730 (Academic Press, 2016).
Lešková, E. et al. Vitamin losses: Retention during heat treatment and continual changes expressed by mathematical models. J. Food Compost. Anal. 19, 252–276 (2006).
Jägerstad, M. et al. Increasing natural food folates through bioprocessing and biotechnology. Trends Food Sci. Technol. 16, 298–306 (2005).
Scott, J., Rébeillé, F. & Fletcher, J. Folic acid and folates: the feasibility for nutritional enhancement in plant foods. J. Sci. Food Agric. 80, 795–824 (2000).
Giordano, D., Reyneri, A. & Blandino, M. Folate distribution in barley (Hordeum vulgare L.), common wheat (Triticum aestivum L.) and durum wheat (Triticum turgidum durum Desf.) pearled fractions. J. Sci. Food Agric. 96, 1709–1715 (2016).
Boz, H. Effect of processing on cereal folates. J. Cereal Sci. 99, 103202 (2021).
Henry, C. & Heppell, N. Nutritional losses and gains during processing: future problems and issues. Proc. Nutr. Soc. 61, 145–148 (2002).
Buri, R. C., von Reding, W. & Gavin, M. H. Description and characterization of wheat aleurone. Cereal Foods World 49, 274 (2004).
Hemery, Y. et al. Potential of dry fractionation of wheat bran for the development of food ingredients, part II: electrostatic separation of particles. J. Cereal Sci. 53, 9–18 (2011).
Blandino, M. et al. Distribution of bioactive compounds in maize fractions obtained in two different types of large scale milling processes. J. Cereal Sci. 77, 251–258 (2017).
McIntosh, S. R., Brushett, D. & Henry, R. J. GTP cyclohydrolase 1 expression and folate accumulation in the developing wheat seed. J. Cereal Sci. 48, 503–512 (2008).
Suri, D. J. & Tanumihardjo, S. A. Effects of different processing methods on the micronutrient and phytochemical contents of maize: from A to Z. Compr. Rev. Food Sci. Food Saf. 15, 912–926 (2016).
Slavin, J. L., Jacobs, D. & Marquart, L. Grain processing and nutrition. Crit. Rev. Food Sci. Nutr. 40, 309–326 (2000).
Thielecke, F., Lecerf, J.-M. & Nugent, A. P. Processing in the food chain: do cereals have to be processed to add value to the human diet? Nutr. Res. Rev. 34, 159–173 (2021).
Lebert, L., Buche, F., Sorin, A. & Aussenac, T. The wheat aleurone layer: optimisation of its benefits and application to bakery products. Foods 11, 3552 (2022).
Meziani, S. et al. Wheat aleurone layer: A site enriched with nutrients and bioactive molecules with potential nutritional opportunities for breeding. J. Cereal Sci. 100, 103225 (2021).
Liukkonen, K.-H. et al. Process-induced changes on bioactive compounds in whole grain rye. Proc. Nutr. Soc. 62, 117–122 (2003).
Kamal-Eldin, A. et al. Physical, microscopic and chemical characterisation of industrial rye and wheat brans from the Nordic countries. Food Nutr. Res. 53, 1912 (2009).
MacDonald, R. & Reitmeier, C. Food processing. In Understanding Food Systems: Agriculture, Food Science, and Nutrition in the United States (eds MacDonald, R. & Reitmeier, C.) Ch. 6, 179–225 (Academic Press, 2017).
Hegedüs, M., Pedersen, B. & Eggum, B. F. O. The influence of milling on the nutritive value of flour from cereal grains. 7. Vitamins and tryptophan. Plant Foods Hum. Nutr. 35, 175–180 (1985).
Călinoiu, L. F. & Vodnar, D. C. Whole grains and phenolic acids: a review on bioactivity, functionality, health benefits and bioavailability. Nutrients 10, 1615 (2018).
Liang, Q. et al. Folate content and retention in wheat grains and wheat-based foods: Effects of storage, processing, and cooking methods. Food Chem. 333, 127459 (2020).
Jiang, Z. et al. Effect of milling on nutritional components in common and zinc-biofortified wheat. Nutrients 15, 833 (2023).
Dunn, M. L., Jain, V. & Klein, B. P. Stability of key micronutrients added to fortified maize flours and corn meal. Ann. N. Y. Acad. Sci. 1312, 15–25 (2014).
Edelmann, M., Kariluoto, S., Nyström, L. & Piironen, V. Folate in barley grain and fractions. J. Cereal Sci. 58, 37–44 (2013).
Garg, M. et al. Vitamins in cereals: a critical review of content, health effects, processing losses, bioaccessibility, fortification, and biofortification strategies for their improvement. Front. Nutr. 8, 586815 (2021).
Gwirtz, J. A. & Garcia‐Casal, M. N. Processing maize flour and corn meal food products. Ann. N. Y. Acad. Sci. 1312, 66–75 (2014).
Edelmann, M., Kariluoto, S., Nyström, L. & Piironen, V. Folate in oats and its milling fractions. Food Chem. 135, 1938–1947 (2012).
Tiozon, R. J. N., Fernie, A. R. & Sreenivasulu, N. Meeting human dietary vitamin requirements in the staple rice via strategies of biofortification and post-harvest fortification. Trends Food Sci. Technol. 109, 65–82 (2021).
DGExpert. DGE Deutsche Gesellschaft für Ernährung, http://www.nutrisurvey.de/info/interaktives/search.htm (2018).
Dong, W. et al. Determination of folate content in rice germplasm (Oryza sativa L.) using tri-enzyme extraction and microbiological assays. Int. J. Food Sci. Nutr. 62, 537–543 (2011).
de Pee, S. Proposing nutrients and nutrient levels for rice fortification. Ann. N. Y. Acad. Sci. 1324, 55–66 (2014).
Monks, J. L. F. et al. Effects of milling on proximate composition, folic acid, fatty acids and technological properties of rice. J. Food Compos. Anal. 30, 73–79 (2013).
WHO. Guideline: Fortification of rice with vitamins and minerals as a public health strategy (World Health Organization, 2018).
Sumczynski, D. et al. Preparation of non-traditional Dickkopf and Richard wheat flakes: Phenolic and vitamin profiles and antioxidant activity. LWT 90, 31–37 (2018).
Akhtar, M. J., Khan, M. A. & Ahmad, I. Photodegradation of folic acid in aqueous solution. J. Pharm. Biomed. Anal. 19, 269–275 (1999).
Bergström, L. Nutrient Losses and Gains in the Preparation of Foods (National Food Administration, 1994).
Schnellbaecher, A., Binder, D., Bellmaine, S. & Zimmer, A. Vitamins in cell culture media: Stability and stabilization strategies. Biotechnol. Bioeng. 116, 1537–1555 (2019).
Riaz, M. N., Asif, M. & Ali, R. Stability of vitamins during extrusion. Crit. Rev. Food Sci. Nutr. 49, 361–368 (2009).
Yessaad, M. et al. Development of a stability indicating method for simultaneous analysis of five water-soluble vitamins by liquid chromatography. Pharm. Technol. Hosp. Pharm. 3, 207–218 (2018).
Lorente, C. & Thomas, A. H. Photophysics and photochemistry of pterins in aqueous solution. Acc. Chem. Res. 39, 395–402 (2006).
Patro, B. S., Adhikari, S., Mukherjee, T. & Chattopadhyay, S. Possible role of hydroxyl radicals in the oxidative degradation of folic acid. Bioorg. Med. Chem. Lett. 15, 67–71 (2005).
Steindal, A. H., Juzeniene, A., Johnsson, A. & Moan, J. Photodegradation of 5‐methyltetrahydrofolate: biophysical aspects. Photochem. Photobiol. 82, 1651–1655 (2006).
Off, M. K. et al. Ultraviolet photodegradation of folic acid. J. Photochem. Photobiol. B Biol. 80, 47–55 (2005).
Martin, C. B., Walker, D. & Soniat, M. Density functional theory study of possible mechanisms of folic acid photodecomposition. J. Photochem. Photobiol. A Chem. 208, 1–6 (2009).
Gazzali, A. M. et al. Stability of folic acid under several parameters. Eur. J. Pharm. Sci. 93, 419–430 (2016).
Liang, X. S., Zhao, F. Q. & Hao, L. X. Research on stability of synthetic folic acid. Adv. Mater. Res. 781, 1215–1218 (2013).
Yang, Y. et al. Degradation of 5-methyltetrahydrofolate in model and egg yolk systems and strategies for its stabilization. J. Food Sci. Technol. 58, 3473–3481 (2021).
Araújo, M. et al. LC/MS/MS identification of some folic acid degradation products after E-beam irradiation. Radiat. Phys. Chem. 81, 1166–1169 (2012).
Araújo, M. M. et al. Irradiation stability of folic acid in powder and aqueous solution. J. Agric. Food Chem. 59, 1244–1248 (2011).
Araújo, M. M. et al. Mechanism of folic acid radiolysis in aqueous solution. LWT 63, 599–603 (2015).
Vora, A., Riga, A., Dollimore, D. & Alexander, K. Thermal stability of folic acid in the solid-state. J. Therm. Anal. Calorim. 75, 709–717 (2004).
Neves, D. A. et al. Thermal and in vitro digestion stability of folic acid in bread. J. Food Compos. Anal. 84, 103311 (2019).
Mair, P. et al. Vitamins, 12. Vitamin B9. In Ullmann’s Encyclopedia of Industrial Chemistry, 1–15 (Wiley‐VCH Verlag GmbH & Co. KGaA, 2019).
Fitzpatrick, T. B. et al. Vitamin deficiencies in humans: can plant science help? Plant Cell 24, 395–414 (2012).
Wu, Z., Li, X., Hou, C. & Qian, Y. Solubility of folic acid in water at pH values between 0 and 7 at temperatures (298.15, 303.15, and 313.15) K. J. Chem. Eng. Data 55, 3958–3961 (2010).
Juzeniene, A., Tam, T. T. T., Iani, V. & Moan, J. 5-methyltetrahydrofolate can be photodegraded by endogenous photosensitizers. Free Radic. Biol. Med. 47, 1199–1204 (2009).
Vorobey, P. et al. Influence of human serum albumin on photodegradation of folic acid in solution. Photochem. Photobiol. 82, 817–822 (2006).
Tam, T. T. T. et al. Photodegradation of 5-methyltetrahydrofolate in the presence of uroporphyrin. J. Photochem. Photobiol. B Biol. 94, 201–204 (2009).
Hrubša, M. et al. Biological properties of vitamins of the B-complex, part 1: vitamins B1, B2, B3, and B5. Nutrients 14, 484 (2022).
Steindal, A. H. et al. 5-Methyltetrahydrofolate is photosensitive in the presence of riboflavin. Photochem. Photobiol. Sci. 7, 814–818 (2008).
Scurachio, R. S., Skibsted, L. H., Metzker, G. & Cardoso, D. R. Photodegradation of folate sensitized by riboflavin. Photochem. Photobiol. 87, 840–845 (2011).
Akhtar, M. J., Khan, M. A. & Ahmad, I. Effect of riboflavin on the photolysis of folic acid in aqueous solution. J. Pharm. Biomed. Anal. 23, 1039–1044 (2000).
Delchier, N. et al. Thermal degradation of folates under varying oxygen conditions. Food Chem. 165, 85–91 (2014).
Zheng, Y. & Cantley, L. C. Toward a better understanding of folate metabolism in health and disease. J. Exp. Med. 216, 253–266 (2019).
Indrawati et al. Comparative study on pressure and temperature stability of 5-methyltetrahydrofolic acid in model systems and in food products. J. Agric. Food Chem. 52, 485–492 (2004).
Indrawati et al. Implications of β-mercaptoethanol in relation to folate stability and to determination of folate degradation kinetics during processing: a case study on [6 S]-5-methyltetrahydrofolic acid. J. Agric. Food Chem. 52, 8247–8254 (2004).
Servent, A., Cazals, G., Perfetto, C. & Achir, N. Kinetic modeling of four folates in a model solution at different temperatures and pH to mimic their behavior in foods during processing. J. Food Process Eng. 46, e14288 (2023).
De Brouwer, V. et al. pH stability of individual folates during critical sample preparation steps in prevision of the analysis of plant folates. Phytochem. Anal. 18, 496–508 (2007).
Nguyen, M., Oey, I., Hendrickx, M. & Van Loey, A. Kinetics of (6 R, S) 5-formyltetrahydrofolic acid isobaric–isothermal degradation in a model system. Eur. Food Res. Technol. 223, 325–331 (2006).
Nguyen, M. T. Study on folate stability during thermal processing. Can Tho University J. Sci. 4, 87–94. https://ctujs.ctu.edu.vn/index.php/ctujs/article/view/208 (2016).
Nguyen, M. T. The kinetics study on 5-formyltetrahydrofolic acid degradation and 5, 10-methenyltetrahydrofolic acid formation during thermal and combined high pressure thermal treatments. Can Tho University J. Sci. 5, 132–140 (2017).
Kariluoto, S. et al. Effect of baking method and fermentation on folate content of rye and wheat breads. Cereal Chem. 81, 134–139 (2004).
Shrestha, A. K., Arcot, J. & Yuliani, S. Susceptibility of 5-methyltetrahydrofolic acid to heat and microencapsulation to enhance its stability during extrusion processing. Food Chem. 130, 291–298 (2012).
Nguyen, M. T., Indrawati & Hendrickx, M. Model studies on the stability of folic acid and 5-methyltetrahydrofolic acid degradation during thermal treatment in combination with high hydrostatic pressure. J. Agric. Food Chem. 51, 3352–3357 (2003).
Ministry of Education Sports Science and Technology-Japan. Standard tables of food composition in Japan, 7th Revised ed., https://www.mext.go.jp/en/policy/science_technology/policy/title01/detail01/1374030.htm (2015).
Public Health England. McCance and Widdowson’s composition of foods integrated dataset, https://assets.publishing.service.gov.uk/media/60538e66d3bf7f03249bac58/McCance_and_Widdowsons_Composition_of_Foods_integrated_dataset_2021.pdf (2021).
Roe, M., Church, S., Pinchen, H. & Finglas, P. Nutrient analysis of fruit and vegetables: analytical report. 17–76 (Institute of Food Research, 2013).
Roe, M., Church, S., Pinchen, H. & Finglas, P. Nutrient analysis of fish and fish products: analytical report. 14–70 (Institute of Food Research, 2013).
Delchier, N., Reich, M. & Renard, C. M. Impact of cooking methods on folates, ascorbic acid and lutein in green beans (Phaseolus vulgaris) and spinach (Spinacea oleracea). LWT 49, 197–201 (2012).
Bognár, A. Tables on Weight Yield of Food and Retention Factors of Food Constituents for the Calculation of Nutrient Composition of Cooked Foods (Dishes) (Bundesforschungsanstalt für Ernährung, 2002).
USDA. USDA table of nutrient retention factors, Release 6, https://data.nal.usda.gov/dataset/usda-table-nutrient-retention-factors-release-6-2007 (2007).
Öhrvik, V., Carlsen, M. H., Källman, A. & Martinsen, T. A. Improving Food Composition Data by Standardizing Calculation Methods (Nordic Council of Ministers, 2015).
Bell, S. et al. Report on nutrient losses and gains factors used in European food composition databases (European Food Information Resource Network, 2006).
McKillop, D. J. et al. The effect of different cooking methods on folate retention in various foods that are amongst the major contributors to folate intake in the UK diet. Br. J. Nutr. 88, 681–688 (2002).
Stea, T. H., Johansson, M., Jägerstad, M. & Frølich, W. Retention of folates in cooked, stored and reheated peas, broccoli and potatoes for use in modern large-scale service systems. Food Chem 101, 1095–1107 (2007).
Holasova, M., Fiedlerova, V. & Vavreinova, S. Determination of folates in vegetables and their retention during boiling. Czech J. Food Sci. 26, 31–37 (2008).
Maharaj, P. P., Prasad, S., Devi, R. & Gopalan, R. Folate content and retention in commonly consumed vegetables in the South Pacific. Food Chem. 182, 327–332 (2015).
Hong, J. et al. Folate content of Korean vegetable dishes prepared outside the home: comparison between analyzed and calculated values. J. Food Compos. Anal. 103, 104088 (2021).
Renard, C. M. et al. Relative role of leaching and chemical degradation in the loss of water-soluble vitamins C and B9 from frozen vegetables cooked in water. LWT 180, 114694 (2023).
Macova, E. & Krkoskova, B. Effect of heat processing on folic acid and biotin content in food of plant origin. Agriculture 49, 349–356 (2003).
Czarnowska-Kujawska, M., Draszanowska, A. & Starowicz, M. Effect of different cooking methods on the folate content, organoleptic and functional properties of broccoli and spinach. LWT 167, 113825 (2022).
Della Lucia, C. M. et al. Folates retention in brassica vegetables consumed in Brazil after different cooking methods. Arch. Latinoam. Nutr. 64, 59–68 (2014).
Bureau, S. et al. Are folates, carotenoids and vitamin C affected by cooking? Four domestic procedures are compared on a large diversity of frozen vegetables. LWT 64, 735–741 (2015).
Melse-Boonstra, A. et al. Influence of processing on total, monoglutamate and polyglutamate folate contents of leeks, cauliflower, and green beans. J. Agric. Food Chem. 50, 3473–3478 (2002).
Puupponen‐Pimiä, R. et al. Blanching and long‐term freezing affect various bioactive compounds of vegetables in different ways. J. Sci. Food Agric. 83, 1389–1402 (2003).
Czarnowska, M. & Gujska, E. Effect of freezing technology and storage conditions on folate content in selected vegetables. Plant Foods Hum. Nutr. 67, 401–406 (2012).
Delchier, N. et al. Effects of industrial processing on folate content in green vegetables. Food Chem. 139, 815–824 (2013).
Islam, M. S. et al. Folate content in fresh corn: effects of harvest time, storage and cooking methods. J. Food Compos. Anal. 103, 104123 (2021).
Wawire, M. et al. Effect of harvest age and thermal processing on poly-γ-glutamate folates and minerals in African cowpea leaves (Vigna unguiculata). J. Food Compos. Anal. 25, 160–165 (2012).
Rumm-Kreuter, D. & Demmel, I. Comparison of vitamin losses in vegetables due to various cooking methods. J. Nutr. Sci. Vitaminol. 36, S7–S15 (1990).
Johansson, M., Furuhagen, C., Frølich, W. & Jägerstad, M. Folate content in frozen vegetarian ready meals and folate retention after different reheating methods. LWT 41, 528–536 (2008).
Gutzeit, D. et al. Folate content in sea buckthorn berries and related products (Hippophae rhamnoides L. ssp. rhamnoides): LC-MS/MS determination of folate vitamer stability influenced by processing and storage assessed by stable isotope dilution assay. Anal. Bioanal. Chem. 391, 211–219 (2008).
Wang, C., Riedl, K. M. & Schwartz, S. J. Fate of folates during vegetable juice processing—Deglutamylation and interconversion. Food Res. Int. 53, 440–448 (2013).
Strålsjö, L., Alklint, C., Olsson, M. E. & Sjöholm, I. Total folate content and retention in rosehips (Rosa ssp.) after drying. J. Agric. Food Chem. 51, 4291–4295 (2003).
Akissoé, L. et al. Impact of traditional processing on proximate composition, folate, mineral, phytate, and alpha-galacto-oligosaccharide contents of two West African cowpea (Vigna unguiculata L. Walp) based doughnuts. J. Food Compos. Anal. 96, 103753 (2021).
Ferawati, F., Hefni, M. & Witthöft, C. Flours from Swedish pulses: effects of treatment on functional properties and nutrient content. Food Sci. Nutr. 7, 4116–4126 (2019).
Coffigniez, F. & Briffaz, A. Modelling of the nutritional behaviour of cowpea seeds during soaking, germination and cooking process. Food Chem. 401, 134177 (2023).
Miftakhussolikhah et al. Folate content of mung bean flour prepared by various heat-treatments. Procedia Food Sci. 3, 69–73 (2015).
Dang, J., Arcot, J. & Shrestha, A. Folate retention in selected processed legumes. Food Chem. 68, 295–298 (2000).
Xue, S. et al. Degradation kinetics of folate (5-methyltetrahydrofolate) in navy beans under various processing conditions. LWT 44, 231–238 (2011).
Liang, Q. et al. Investigation of folate composition and influence of processing on folate stability in pulse accessions developed in China. J. Food Compos. Anal. 114, 104785 (2022).
Hefni, M. & Witthöft, C. M. Folate content in processed legume foods commonly consumed in Egypt. LWT 57, 337–343 (2014).
Coffigniez, F. et al. Localization and modeling of reaction and diffusion to explain folate behavior during soaking of cowpea. J. Food Eng. 253, 49–58 (2019).
Arcot, J., Wong, S. & Shrestha, A. K. Comparison of folate losses in soybean during the preparation of tempeh and soymilk. J. Sci. Food Agric. 82, 1365–1368 (2002).
Ginting, E., Arcot, J. & Chox, J. M. Determination of folate retention during tofu preparation using trienzyme treatment and microbiological assay. Indones. J. Agric. Sci. 4, 12–17 (2003).
Mo, H. et al. Effect of soybean processing on content and bioaccessibility of folate, vitamin B12 and isoflavones in tofu and tempe. Food Chem. 141, 2418–2425 (2013).
Ginting, E. & Arcot, J. High-performance liquid chromatographic determination of naturally occurring folates during tempe preparation. J. Agric. Food Chem. 52, 7752–7758 (2004).
Ktenioudaki, A., Alvarez-Jubete, L. & Gallagher, E. A review of the process-induced changes in the phytochemical content of cereal grains: The breadmaking process. Crit. Rev. Food Sci. Nutr. 55, 611–619 (2015).
Arcot, J. et al. Folate levels in twelve Australian wheats and changes during processing into bread. Food Aust. 54, 18–20 (2002).
Osseyi, E. S., Wehling, R. L. & Albrecht, J. A. HPLC determination of stability and distribution of added folic acid and some endogenous folates during breadmaking. Cereal Chem. 78, 375–378 (2001).
Hefni, M. & Witthöft, C. M. Increasing the folate content in Egyptian baladi bread using germinated wheat flour. LWT 44, 706–712 (2011).
Kariluoto, S. et al. Effects of yeasts and bacteria on the levels of folates in rye sourdoughs. Int. J. Food Microbiol. 106, 137–143 (2006).
Katina, K. et al. Fermentation-induced changes in the nutritional value of native or germinated rye. J. Cereal Sci. 46, 348–355 (2007).
Gujska, E., Michalak, J. & Klepacka, J. Folates stability in two types of rye breads during processing and frozen storage. Plant Foods Hum. Nutr. 64, 129–134 (2009).
Helou, C. et al. The impact of raw materials and baking conditions on Maillard reaction products, thiamine, folate, phytic acid and minerals in white bread. Food Funct. 7, 2498–2507 (2016).
Omar, R. M. et al. Effect of processing on folic acid fortified Baladi bread and its possible effect on the prevention of colon cancer. Food Chem. Toxicol. 47, 1626–1635 (2009).
Gujska, E. & Majewska, K. Effect of baking process on added folic acid and endogenous folates stability in wheat and rye breads. Plant Foods Hum. Nutr. 60, 37–42 (2005).
Anderson, W. A., Slaughter, D., Laffey, C. & Lardner, C. Reduction of folic acid during baking and implications for mandatory fortification of bread. Int. J. Food Sci. Technol. 45, 1104–1110 (2010).
Johansson, M., Witthöft, C. M., Bruce, Å. & Jägerstad, M. Study of wheat breakfast rolls fortified with folic acid: the effect on folate status in women during a 3-month intervention. Eur. J. Nutr. 41, 279–286 (2002).
Tomiuk, S. et al. Studies on the retention of microencapsulated L-5-methyltetrahydrofolic acid in baked bread using skim milk powder. Food Chem. 133, 249–255 (2012).
Liu, Y., Green, T. J., Wong, P. & Kitts, D. D. Microencapsulation of L-5-methyltetrahydrofolic acid with ascorbate improves stability in baked bread products. J. Agric. Food Chem. 61, 247–254 (2013).
López-Nicolás, R. et al. Folate fortification of white and whole-grain bread by adding Swiss chard and spinach. Acceptability by consumers. LWT 59, 263–269 (2014).
Tamene, A., Kariluoto, S., Baye, K. & Humblot, C. Quantification of folate in the main steps of traditional processing of tef injera, a cereal based fermented staple food. J. Cereal Sci. 87, 225–230 (2019).
Ekıncı, R. The effect of fermentation and drying on the water-soluble vitamin content of tarhana, a traditional Turkish cereal food. Food Chem. 90, 127–132 (2005).
Chapman, J. et al. Stability of native folate and added folic acid in micronutrient‐fortified corn masa and tortillas. Cereal Chem. 87, 434–438 (2010).
Burton, K. et al. Effect of micronutrient fortification on nutritional and other properties of nixtamal tortillas. Cereal Chem. 85, 70–75 (2008).
Dunn, M. L., Serna‐Saldivar, S. O., Sanchez‐Hernandez, D. & Griffin, R. W. Commercial evaluation of a continuous micronutrient fortification process for nixtamal tortillas. Cereal Chem. 85, 746–752 (2008).
Adolphson, S. J. et al. Evaluation of bacterial effects on folic acid loss in fortified, nixtamalized corn masa flour. Cereal Chem. 93, 508–512 (2016).
Phillips, R., Pike, O. A., Eggett, D. L. & Dunn, M. L. Folate stability in folic acid enriched corn masa flour, tortillas, and tortilla chips over the expected shelf life. Cereal Chem. 94, 917–921 (2017).
Koren, D., Hegyesné Vecseri, B. & Kun-Farkas, G. Evolution of folate content during barley malt production. Acta Aliment 50, 238–246 (2021).
Kariluoto, S. et al. Effect of germination and thermal treatments on folates in rye. J. Agric. Food Chem. 54, 9522–9528 (2006).
Yoo, J. et al. Rice-shaped extruded kernels: physical, sensory, and nutritional properties. Int. J. Food Prop. 16, 301–321 (2013).
Soongsongkiat, M. et al. Testing of folate conjugase from chicken pancreas vs. commercial enzyme and studying the effect of cooking on folate retention in Thai foods. J. Food Compos. Anal. 23, 681–688 (2010).
Gray, P. J., Conklin, S. D., Todorov, T. I. & Kasko, S. M. Cooking rice in excess water reduces both arsenic and enriched vitamins in the cooked grain. Food Addit. Contam. Part A 33, 78–85 (2016).
Silveira, C. M. M. et al. Effect of cooking methods on the stability of thiamin and folic acid in fortified rice. Int. J. Food Sci. Nutr. 68, 179–187 (2017).
Porasuphatana, S. et al. Production and shelf stability of multiple‐fortified quick‐cooking rice as a complementary food. J. Food Sci. 73, S359–S366 (2008).
Shrestha, A. K., Arcot, J. & Paterson, J. L. Edible coating materials—their properties and use in the fortification of rice with folic acid. Food Res. Int. 36, 921–928 (2003).
de Ambrosis, A. et al. Relative bioavailability of 13C5-folic acid in pectin-coated folate fortified rice in humans using stable isotope techniques. Eur. J. Clin. Nutr. 71, 103–106 (2017).
Wieringa, F. T. et al. Stability and retention of micronutrients in fortified rice prepared using different cooking methods. Ann. N. Y. Acad. Sci. 1324, 40–47 (2014).
Thiruselvam, N. et al. Micronutrients fortification of rice by parboiling: lab scale and pilot scale studies. J. Nutr. Food Sci. 4, 1–7 (2014).
Kam, K., Arcot, J. & Ward, R. Fortification of rice with folic acid using parboiling technique: Effect of parboiling conditions on nutrient uptake and physical characteristics of milled rice. J. Cereal Sci. 56, 587–594 (2012).
Tiozon, R. N. Jr et al. Efficient fortification of folic acid in rice through ultrasonic treatment and absorption. Food Chem. 335, 127629 (2021).
Bui, L. T. & Small, D. M. Folates in Asian noodles: II. a comparison of commercial samples and the impact of cooking. J. Food Sci. 72, C283–C287 (2007).
Bui, L. T. & Small, D. M. Folates in Asian noodles: III. fortification, impact of processing, and enhancement of folate intakes. J. Food Sci. 72, C288–C293 (2007).
Cheung, R. H. F., Hughes, J. G., Marriott, P. J. & Small, D. M. Investigation of folic acid stability in fortified instant Asian noodles by use of capillary electrophoresis. Food Chem. 112, 507–514 (2009).
Cheung, R. H. F., Morrison, P. D., Small, D. M. & Marriott, P. J. Investigation of folic acid stability in fortified instant noodles by use of capillary electrophoresis and reversed-phase high performance liquid chromatography. J. Chromatogr. A 1213, 93–99 (2008).
Liu, Y., Green, T. J. & Kitts, D. D. Stability of microencapsulated L-5-methyltetrahydrofolate in fortified noodles. Food Chem. 171, 206–211 (2015).
Ruggeri, S. et al. Design of cereal products naturally enriched in folate from barley pearling by-products. Nutrients 14, 3729 (2022).
Malahayati, N., Muhammad, K., Bakar, J. & Karim, R. The effect of processing method on fortified rice noodle quality and fortificant retention. Int. J. Food Sci. Nutr. 4, 30–37 (2017).
Malahayati, N., Muhammad, K., Bakar, J. & Karim, R. Quality and fortificant retention of rice noodles as affected by flour particle size. Cereal Chem. 92, 211–217 (2015).
Muehlhoff, E., Bennett, A. & McMahon, D. Milk and Dairy Products in Human Nutrition 43–64 (Food and Agriculture Organization of the United Nations, 2013).
Shetty, S. A., Young, M. F., Taneja, S. & Rangiah, K. Quantification of B-vitamins from different fresh milk samples using ultra-high performance liquid chromatography mass spectrometry/selected reaction monitoring methods. J. Chromatogr. A 1609, 460452 (2020).
Oamen, E., Hansen, A. & Swartzel, K. Effect of ultra-high temperature steam injection processing and aseptic storage on labile water-soluble vitamins in milk. J. Dairy Sci. 72, 614–619 (1989).
Van Heerden, S., Schönfeldt, H., Smith, M. & van Rensburg, D. J. Nutrient content of South African chickens. J. Food Compos. Anal. 15, 47–64 (2002).
Czarnowska-Kujawska, M., Draszanowska, A. & Gujska, E. Effect of different cooking methods on folate content in chicken liver. Foods 9, 1431 (2020).
Cáceres, E., García, M. & Selgas, M. Conventional and reduced-fat cooked meat sausages enriched with folic acid. Fleischwirtschaft 23, 58–60 (2008).
Jiratanan, T. & Liu, R. H. Antioxidant activity of processed table beets (Beta vulgaris var, conditiva) and green beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 52, 2659–2670 (2004).
Hefni, M. E., Shalaby, M. T. & Witthöft, C. M. Folate content in faba beans (Vicia faba L.)—effects of cultivar, maturity stage, industrial processing, and bioprocessing. Food Sci. Nutr. 3, 65–73 (2015).
Jägerstad, M., Jastrebova, J. & Svensson, U. Folates in fermented vegetables—a pilot study. LWT 37, 603–611 (2004).
Lester, G. E., Hallman, G. J. & Pérez, J. A. γ-Irradiation dose: effects on baby-leaf spinach ascorbic acid, carotenoids, folate, α-tocopherol, and phylloquinone concentrations. J. Agric. Food Chem. 58, 4901–4906 (2010).
Galán, I., García, M. & Selgas, M. Irradiation is useful for manufacturing ready-to-eat cooked meat products enriched with folic acid. Meat Sci. 87, 330–335 (2011).
Galán, I., García, M. & Selgas, M. Effects of irradiation on hamburgers enriched with folic acid. Meat Sci. 84, 437–443 (2010).
Luo, S., Duan, H., Zou, Y. & Wang, C. High pressure processing and post-high pressure storage induce the change of polyglutamyl folate and total folate from different legumes. J. Food Sci. Technol. 54, 3521–3531 (2017).
Oey, I., Van der Plancken, I., Van Loey, A. & Hendrickx, M. Does high pressure processing influence nutritional aspects of plant based food systems? Trends Food Sci. Technol. 19, 300–308 (2008).
Verlinde, P., Oey, I., Hendrickx, M. & Van Loey, A. High-pressure treatments induce folate polyglutamate profile changes in intact broccoli (Brassica oleraceae L. cv. Italica) tissue. Food Chem. 111, 220–229 (2008).
Ravichandran, C. et al. Influence of high pressure pasteurization on nutritional, functional and rheological characteristics of fruit and vegetable juices and purees-an updated review. Food Control 146, 109516 (2023).
Ramos-Parra, P. A., Hernández-Brenes, C. & Díaz de la Garza, R. I. High hydrostatic pressure modulates the folate and ascorbic acid accumulation in Papaya (Carica papaya cv. Maradol) fruit. Food Eng. Rev. 13, 613–621 (2021).
Indrawati, Van Loey, A. & Hendrickx, M. Pressure and temperature stability of 5-methyltetrahydrofolic acid: a kinetic study. J. Agric. Food Chem. 53, 3081–3087 (2005).
Butz, P. et al. Influence of high‐pressure treatment at 25°C and 80 °C on folates in orange juice and model media. J. Food Sci. 69, SNQ117–SNQ121 (2004).
Wang, C. et al. Influence of high-pressure processing on the profile of polyglutamyl 5-methyltetrahydrofolate in selected vegetables. J. Agric. Food Chem. 59, 8709–8717 (2011).
Verlinde, P. H. et al. Mechanism and related kinetics of 5-methyltetrahydrofolic acid degradation during combined high hydrostatic pressure− thermal treatments. J. Agric. Food Chem. 57, 6803–6814 (2009).
Pandrangi, S. & LaBorde, L. Retention of folate, carotenoids, and other quality characteristics in commercially packaged fresh spinach. J. Food Sci. 69, C702–C707 (2004).
O’Hare, T. et al. Impact of low temperature storage on active and storage forms of folate in choy sum (Brassica rapa subsp. parachinensis). Postharvest Biol. Technol. 74, 85–90 (2012).
Striegel, L., Chebib, S., Netzel, M. E. & Rychlik, M. Improved stable isotope dilution assay for dietary folates using LC-MS/MS and its application to strawberries. Front. Chem. 6, 11 (2018).
Phillips, K. M. et al. Stability of 5-methyltetrahydrofolate in frozen fresh fruits and vegetables. Food Chem. 92, 587–595 (2005).
Iniesta, M. D. et al. Folate content in tomato (Lycopersicon esculentum). Influence of cultivar, ripeness, year of harvest, and pasteurization and storage temperatures. J. Agric. Food Chem. 57, 4739–4745 (2009).
Frommherz, L. et al. Degradation of folic acid in fortified vitamin juices during long term storage. Food Chem. 159, 122–127 (2014).
Öhrvik, V. & Witthöft, C. Orange juice is a good folate source in respect to folate content and stability during storage and simulated digestion. Eur. J. Nutr. 47, 92–98 (2008).
Hemery, Y. M. et al. Influence of storage conditions and packaging of fortified wheat flour on microbial load and stability of folate and vitamin B12. Food Chem. X 5, 100076 (2020).
Li, Y. O., Diosady, L. L. & Jankowski, S. Folic acid stability in the presence of various formulation components including iron compounds in fortified extruded Ultra Rice® over prolonged storage at 40 °C and 60% relative humidity (RH). Int. J. Food Sci. Technol. 46, 379–385 (2011).
Zwart, S. et al. Assessment of nutrient stability in foods from the space food system after long‐duration spaceflight on the ISS. J. Food Sci. 74, H209–H217 (2009).
House, J. et al. The enrichment of eggs with folic acid through supplementation of the laying hen diet. Poult. Sci. 81, 1332–1337 (2002).
Altic, L. et al. Validation of folate-enriched eggs as a functional food for improving folate intake in consumers. Nutrients 8, 777 (2016).
Ford, J. E., Hurrell, R. F. & Finot, P. A. Storage of milk powders under adverse conditions. 2. Influence on the content of water-soluble vitamins. Br. J. Nutr. 49, 355–364 (1983).
Chitisankul, W. T., Murakami, M., Tsukamoto, C. & Shimada, K. Effects of long-term soaking on nutraceutical and taste characteristic components in Thai soybeans. LWT 115, 108432 (2019).
Coffigniez, F. et al. Modelling folates reaction kinetics during cowpea seed germination in comparison with soaking. Food Chem. 340, 127960 (2021).
Koehler, P., Hartmann, G., Wieser, H. & Rychlik, M. Changes of folates, dietary fiber, and proteins in wheat as affected by germination. J. Agric. Food Chem. 55, 4678–4683 (2007).
Hübner, F. & Arendt, E. K. Germination of cereal grains as a way to improve the nutritional value: a review. Crit. Rev. Food Sci. Nutr. 53, 853–861 (2013).
Sallam, S. M., Shawky, E. & El Sohafy, S. M. Determination of the effect of germination on the folate content of the seeds of some legumes using HPTLC-mass spectrometry-multivariate image analysis. Food Chem. 362, 130206 (2021).
Shohag, M., Wei, Y. & Yang, X. Changes of folate and other potential health-promoting phytochemicals in legume seeds as affected by germination. J. Agric. Food Chem. 60, 9137–9143 (2012).
Zhang, H. et al. Validated B vitamin quantification from lentils by selected reaction monitoring mass spectrometry. Food Chem. 359, 129810 (2021).
Ma, M. et al. Response of nutritional and functional composition, anti-nutritional factors and antioxidant activity in germinated soybean under UV-B radiation. LWT 118, 108709 (2020).
Hefni, M. & Witthöft, C. M. Effect of germination and subsequent oven-drying on folate content in different wheat and rye cultivars. J. Cereal Sci. 56, 374–378 (2012).
Chang, J. et al. Red light enhances folate accumulation in wheat seedlings. J. Zhejiang Univ. Sci. B 22, 906–916 (2021).
Liu, F. et al. The manipulation of gene expression and the biosynthesis of Vitamin C, E and folate in light-and dark-germination of sweet corn seeds. Sci. Rep. 7, 7484 (2017).
Wan, X. et al. Simultaneous extraction and determination of mono-/polyglutamyl folates using high-performance liquid chromatography-tandem mass spectrometry and its applications in starchy crops. Anal. Bioanal. Chem. 411, 2891–2904 (2019).
Romanini, E. et al. Pyridoxine and folates during small and large scale brewing. J. Inst. Brew. 127, 135–139 (2021).
Bertuzzi, T. et al. Targeted healthy compounds in small and large-scale brewed beers. Food Chem. 310, 125935 (2020).
Koren, D. et al. Folic acid content and antioxidant activity of different types of beers available in Hungarian retail. Int. J. Food Sci. Technol. 54, 1158–1167 (2017).
Koren, D., Vecseri, B. H. & Kun-Farkas, G. Evolution of folate content during wort production. Acta Aliment 49, 433–440 (2020).
Owens, J. E., Clifford, A. J. & Bamforth, C. W. Folate in beer. J. Inst. Brew. 113, 243–248 (2007).
Póo‐Prieto, R., Alonso‐Aperte, E. & Varela‐Moreiras, G. Analysis of folate form distribution in Spanish beers using combined affinity and Ion‐pair chromatography. J. Inst. Brew. 117, 188–194 (2011).
Mayer, O. Jr, Šimon, J. & Rosolova, H. A population study of the influence of beer consumption on folate and homocysteine concentrations. Eur. J. Clin. Nutr. 55, 605–609 (2001).
Walker, C., Amblar, S. & Patel, D. Die Auswirkungen des Brauprozesses auf den Folsaure-(Vitamin B9)-Gehalt im Bier. Brauwelt 142, 350–355 (2002).
Pietercelie, A., Allardin, D. & Van Nedervelde, L. Effect of fermentation conditions of brewing yeasts on folate production. Cerevisia 36, 41–45 (2011).
Walkey, C. J., Kitts, D. D., Liu, Y. & van Vuuren, H. J. Bioengineering yeast to enhance folate levels in wine. Process Biochem. 50, 205–210 (2015).
Crittenden, R., Martinez, N. & Playne, M. Synthesis and utilisation of folate by yoghurt starter cultures and probiotic bacteria. Int. J. Food Microbiol. 80, 217–222 (2003).
LeBlanc, J. G. et al. Folate production by lactic acid bacteria and other food-grade microorganisms. In: Communicating Current Research and Educational Topics and Trends in Applied Microbiology 1 (ed. Méndez Vilas, A.) 329–339 (2007).
de Giori, G. S. & LeBlanc, J. G. Folate production by lactic acid bacteria. In: Polyphenols: Prevention and Treatment of Human Disease (eds Watson, R. R., Preedy, V. R. & Zibadi, S.) Ch. 2, 15–29 (Academic Press, 2018).
Wouters, J. T., Ayad, E. H., Hugenholtz, J. & Smit, G. Microbes from raw milk for fermented dairy products. Int. Dairy J. 12, 91–109 (2002).
Xie, C. et al. Effect of amino acids on folates accumulation in wheat seedlings during germination under red light radiation. Molecules 27, 6868 (2022).
Długosz-Grochowska, O., Kołton, A. & Wojciechowska, R. Modifying folate and polyphenol concentrations in Lamb’s lettuce by the use of LED supplemental lighting during cultivation in greenhouses. J. Funct. Foods 26, 228–237 (2016).
Puthusseri, B., Divya, P., Lokesh, V. & Neelwarne, B. Enhancement of folate content and its stability using food grade elicitors in coriander (Coriandrum sativum L.). Plant Foods Hum. Nutr. 67, 162–170 (2012).
Puthusseri, B., Divya, P., Lokesh, V. & Neelwarne, B. Salicylic acid-induced elicitation of folates in coriander (Coriandrum sativum L.) improves bioaccessibility and reduces pro-oxidant status. Food Chem. 136, 569–575 (2013).
Watanabe, S. et al. Folate biofortification in hydroponically cultivated spinach by the addition of phenylalanine. J. Agric. Food Chem. 65, 4605–4610 (2017).
Hou, S. et al. Role of miRNAs in regulation of SA-mediated upregulation of genes involved in folate and methionine metabolism in foxtail millet. Front. Plant Sci. 13, 1023764 (2022).
Hu, X. et al. Biosynthesis and accumulation of multi‐vitamins in black sweet corn (Zea mays L.) during kernel development. J. Sci. Food Agric. 100, 5230–5238 (2020).
Shan, Q.-J. et al. Comprehensive evaluation of biosynthesis, accumulation, regulation of folate and vitamin C in waxy maize (Zea mays L. var. ceratina) with kernel development. J. Cereal Sci. 87, 215–224 (2019).
Luo, S. et al. Quantification of total folate, folate species and polyglutamyl folate distribution in winged beans (Psophocarus tetragonolobus (L) DC) from different cultivars and growth stages by ultra-high performance liquid chromatography tandem mass spectrometry. J. Nutr. Sci. Vitaminol. 63, 69–80 (2017).
Van Daele, J. et al. Folate profiling in potato (Solanum tuberosum) tubers by ultrahigh-performance liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 62, 3092–3100 (2014).
García-Salinas, C., Ramos-Parra, P. A. & de la Garza, R. I. D. Ethylene treatment induces changes in folate profiles in climacteric fruit during postharvest ripening. Postharvest Biol. Technol. 118, 43–50 (2016).
Waller, J. C. et al. Developmental and feedforward control of the expression of folate biosynthesis genes in tomato fruit. Mol. Plant 3, 66–77 (2010).
Tyagi, K. et al. Reduced γ-glutamyl hydrolase activity likely contributes to high folate levels in Periyakulam-1 tomato. Hortic. Res. 10, uhac235 (2023).
Tyagi, K. et al. High performance liquid chromatography coupled to mass spectrometry for profiling and quantitative analysis of folate monoglutamates in tomato. Food Chem. 179, 76–84 (2015).
Fyfe, S. et al. Folate vitamers in the Australian green plum: through growth and ripening and across locations. Front. Nutr. 9, 1006393 (2022).
Hoey, L. et al. Laying hens can convert high doses of folic acid added to the feed into natural folates in eggs providing a novel source of food folate. Br. J. Nutr. 101, 206–212 (2008).
Dickson, T. et al. Optimization of folate deposition in eggs through dietary supplementation of folic acid over the entire production cycle of Hy-Line W36, Hy-Line W98, and CV20 laying hens. J. Appl. Poult. Res. 19, 80–91 (2010).
Bagheri, S. et al. Laying hen performance, egg quality improved and yolk 5-methyltetrahydrofolate content increased by dietary supplementation of folic acid. Anim. Nutr. 5, 130–133 (2019).
Hebert, K., House, J. & Guenter, W. Effect of dietary folic acid supplementation on egg folate content and the performance and folate status of two strains of laying hens. Poult. Sci. 84, 1533–1538 (2005).
Hebert, K. et al. The effect of cereal type and exogenous enzyme use on total folate content of eggs from laying hens consuming diets supplemented with folic acid. J. Appl. Poult. Res. 20, 303–312 (2011).
Tactacan, G. et al. Characterization of folate-dependent enzymes and indices of folate status in laying hens supplemented with folic acid or 5-methyltetrahydrofolate. Poult. Sci. 89, 688–696 (2010).
Sun, D. et al. Modified EMR-lipid method combined with HPLC-MS/MS to determine folates in egg yolks from laying hens supplemented with different amounts of folic acid. Food Chem. 337, 127767 (2021).
Sheehy, T. & Sharma, S. Use of FAO food balance sheets to estimate the potential ability of novel folate-enriched eggs to increase the folate supply in European Union countries. Public Health Nutr. 14, 551–556 (2011).
Liu, F., Kariluoto, S., Edelmann, M. & Piironen, V. Bioaccessibility of folate in faba bean, oat, rye and wheat matrices. Food Chem 350, 129259 (2021).
Liu, Y. et al. Thermal oxidation studies on reduced folate, L‐5‐methyltetrahydrofolic acid (L‐5‐MTHF) and strategies for stabilization using food matrices. J. Food Sci. 77, C236–C243 (2012).
Ruiz-Rico, M. et al. Protection of folic acid through encapsulation in mesoporous silica particles included in fruit juices. Food Chem. 218, 471–478 (2017).
Oey, I., Verlinde, P., Hendrickx, M. & Van Loey, A. Temperature and pressure stability of L-ascorbic acid and/or [6s] 5-methyltetrahydrofolic acid: a kinetic study. Eur. Food Res. Technol. 223, 71–77 (2006).
Rozoy, E. et al. The use of cyclic voltammetry to study the oxidation of L-5-methyltetrahydrofolate and its preservation by ascorbic acid. Food Chem. 132, 1429–1435 (2012).
Ng, X., Lucock, M. & Veysey, M. Physicochemical effect of pH and antioxidants on mono-and triglutamate forms of 5-methyltetrahydrofolate, and evaluation of vitamin stability in human gastric juice: implications for folate bioavailability. Food Chem. 106, 200–210 (2008).
Wang, Y., Yan, B., Abbaspourrad, A. & Cheng, Y. Improved photostability of folic acid by the radical-scavenging effect of tannic acid. LWT 142, 111050 (2021).
Wusigale et al. Protection of resveratrol against the photodecomposition of folic acid and photodecomposition-induced structural change of beta-lactoglobulin. Food Res. Int. 102, 435–444 (2017).
Kadota, K. et al. Inhibition of photodegradation of highly dispersed folic acid nanoparticles by the antioxidant effect of transglycosylated rutin. J. Agric. Food Chem. 64, 3062–3069 (2016).
Wusigale et al. Mechanism for inhibition of folic acid photodecomposition by various antioxidants. J. Agric. Food Chem. 68, 340–350 (2020).
Rozoy, E. et al. Redox properties of catechins and enriched green tea extracts effectively preserve l-5-methyltetrahydrofolate: Assessment using cyclic voltammetry analysis. Food Chem. 138, 1982–1991 (2013).
Schneider, M. et al. Reaction of folic acid with reducing sugars and sugar degradation products. J. Agric. Food Chem. 50, 1647–1651 (2002).
Rychlik, M. & Mayr, A. Quantitation of N 2-[1-(1-carboxy) ethyl] folic acid, a nonenzymatic glycation product of folic acid, in fortified foods and model cookies by a stable isotope dilution assay. J. Agric. Food Chem. 53, 5116–5124 (2005).
Verlinde, P. H. et al. Influence of reducing carbohydrates on (6 S)-5-methyltetrahydrofolic acid degradation during thermal treatments. J. Agric. Food Chem. 58, 6190–6199 (2010).
Jones, M. L. & Nixon, P. F. Tetrahydrofolates are greatly stabilized by binding to bovine milk folate-binding protein. J. Nutr. 132, 2690–2694 (2002).
de Jong, R. J. et al. Bioavailability of folic acid from fortified pasteurised and UHT-treated milk in humans. Eur. J. Clin. Nutr. 59, 906–913 (2005).
Puthusseri, B. et al. Evaluation of folate-binding proteins and stability of folates in plant foliages. Food Chem. 242, 555–559 (2018).
Madziva, H., Kailasapathy, K. & Phillips, M. Alginate–pectin microcapsules as a potential for folic acid delivery in foods. J. Microencapsul. 22, 343–351 (2005).
Alborzi, S., Lim, L.-T. & Kakuda, Y. Encapsulation of folic acid and its stability in sodium alginate-pectin-poly (ethylene oxide) electrospun fibres. J. Microencapsul. 30, 64–71 (2013).
Pérez-Esteve, É. et al. Encapsulation of folic acid in different silica porous supports: a comparative study. Food Chem. 196, 66–75 (2016).
Perez-Esteve, E. et al. Enrichment of stirred yogurts with folic acid encapsulated in pH-responsive mesoporous silica particles: Bioaccessibility modulation and physico-chemical characterization. LWT 72, 351–360 (2016).
Aceituno-Medina, M., Mendoza, S., Lagaron, J. M. & López-Rubio, A. Photoprotection of folic acid upon encapsulation in food-grade amaranth (Amaranthus hypochondriacus L.) protein isolate – Pullulan electrospun fibers. LWT 62, 970–975 (2015).
Ariyarathna, I. R. & Karunaratne, D. N. Use of chickpea protein for encapsulation of folate to enhance nutritional potency and stability. Food Bioprod. Process. 95, 76–82 (2015).
do Evangelho, J. A. et al. Thermal and irradiation resistance of folic acid encapsulated in zein ultrafine fibers or nanocapsules produced by electrospinning and electrospraying. Food Res. Int. 124, 137–146 (2019).
Acevedo-Fani, A., Soliva-Fortuny, R. & Martín-Belloso, O. Photo-protection and controlled release of folic acid using edible alginate/chitosan nanolaminates. J. Food Eng. 229, 72–82 (2018).
Pérez-Masiá, R. et al. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chem. 168, 124–133 (2015).
Chapeau, A.-L. et al. Scale-up production of vitamin loaded heteroprotein coacervates and their protective property. J. Food Eng. 206, 67–76 (2017).
Chapeau, A.-L. et al. Spontaneous co-assembly of lactoferrin and β-lactoglobulin as a promising biocarrier for vitamin B9. Food Hydrocoll 57, 280–290 (2016).
Zhang, J. et al. The folic acid/β-casein complex: characteristics and physicochemical implications. Food Res. Int. 57, 162–167 (2014).
Liang, L. & Subirade, M. β-Lactoglobulin/folic acid complexes: formation, characterization, and biological implication. J. Phys. Chem. B 114, 6707–6712 (2010).
Tavares, G. M. et al. Binding of folic acid induces specific self-aggregation of lactoferrin: thermodynamic characterization. Langmuir 31, 12481–12488 (2015).
Bourassa, P., Hasni, I. & Tajmir-Riahi, H. Folic acid complexes with human and bovine serum albumins. Food Chem. 129, 1148–1155 (2011).
Fu, X., Cheng, H., Fang, Z. & Liang, L. Mechanism for improved protection of whey protein isolate against the photodecomposition of folic acid. Food Hydrocoll 79, 439–449 (2018).
Liang, L., Zhang, J., Zhou, P. & Subirade, M. Protective effect of ligand-binding proteins against folic acid loss due to photodecomposition. Food Chem. 141, 754–761 (2013).
Tofzikovskaya, Z., O’Connor, C. & McNamara, M. Synthesis, characterisation and photo-stability of a folate-modified β-cyclodextrin as a functional food additive. J. Incl. Phenom. Macrocycl. Chem. 74, 437–445 (2012).
Cho, S., Johnson, G. & Song, W. O. Folate content of foods: comparison between databases compiled before and after new FDA fortification requirements. J. Food Compos. Anal. 15, 293–307 (2002).
Zappacosta, B. et al. Homocysteine lowering by folate-rich diet or pharmacological supplementations in subjects with moderate hyperhomocysteinemia. Nutrients 5, 1531–1543 (2013).
Noam, A. et al. Folate and neural tube defects: the role of supplements and food fortification. Paediatr. Child Health 21, 145–149 (2016).
Shakur, Y. A., Garriguet, D., Corey, P. & O’Connor, D. L. Folic acid fortification above mandated levels results in a low prevalence of folate inadequacy among Canadians. Am. J. Clin. Nutr. 92, 818–825 (2010).
Shakur, Y. A. et al. How much folate is in Canadian fortified products 10 years after mandated fortification? Can. J. Public Health 100, 281–284 (2009).
Ha, A. V. V. et al. Low prevalence of folic acid supplementation during pregnancy: a multicenter study in Vietnam. Nutrients 11, 2347 (2019).
Crider, K. S. et al. Modeling the impact of folic acid fortification and supplementation on red blood cell folate concentrations and predicted neural tube defect risk in the United States: have we reached optimal prevention? Am. J. Clin. Nutr. 107, 1027–1034 (2018).
Fischer, M., Stronati, M. & Lanari, M. Mediterranean diet, folic acid, and neural tube defects. Ital. J. Pediatr. 43, 1–8 (2017).
Flynn, A. et al. Intake of selected nutrients from foods, from fortification and from supplements in various European countries. Food Nutr. Res. 53, 2038 (2009).
Czarnowska-Kujawska, M., Klepacka, J., Zielińska, O. & Samaniego-Vaesken, Md. L. Characteristics of dietary supplements with folic acid available on the Polish market. Nutrients 14, 3500 (2022).
Bailey, R. L. et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am. J. Clin. Nutr. 91, 231–237 (2010).
Bailey, R. L. et al. Total folate and folic acid intakes from foods and dietary supplements of US children aged 1–13 y. Am. J. Clin. Nutr. 92, 353–358 (2010).
Kondo, A. et al. Dietary folate intakes and effects of folic acid supplementation on folate concentrations among Japanese pregnant women. J. Obstet. Gynaecol. Re. 37, 331–336 (2011).
Anderson, C. A. et al. Effects of folic acid supplementation on serum folate and plasma homocysteine concentrations in older adults: a dose-response trial. Am. J. Epidemiol. 172, 932–941 (2010).
French, M. R., Barr, S. I. & Levy-Milne, R. Folate intakes and awareness of folate to prevent neural tube defects: a survey of women living in Vancouver, Canada. J. Am. Diet. Assoc. 103, 181–185 (2003).
Johnston, K. E., Lofgren, P. A. & Tamura, T. Folate concentrations of fast foods measured by trienzyme extraction method. Food Res. Int. 35, 565–569 (2002).
Johnston, K. E. & Tamura, T. Folate content in commercial white and whole wheat sandwich breads. J. Agric. Food Chem. 52, 6338–6340 (2004).
Czeizel, A. E., Dudás, I., Vereczkey, A. & Bánhidy, F. Folate deficiency and folic acid supplementation: the prevention of neural-tube defects and congenital heart defects. Nutrients 5, 4760–4775 (2013).
Donovan, S. et al. Folic acid from fortified foods and/or supplements during pregnancy and lactation and health outcomes: a systematic review (USDA Nutrition Evidence Systematic Review, 2020).
Bibbins-Domingo, K. et al. Folic acid supplementation for the prevention of neural tube defects: US preventive services task force recommendation statement. JAMA 317, 183–189 (2017).
Eichholzer, M., Tönz, O. & Zimmermann, R. Folic acid: a public-health challenge. Lancet 367, 1352–1361 (2006).
Ledowsky, C., Mahimbo, A., Scarf, V. & Steel, A. Women taking a folic acid supplement in countries with mandatory food fortification programs may be exceeding the upper tolerable limit of folic acid: A systematic review. Nutrients 14, 2715 (2022).
Liu, J. et al. Periconceptional folic acid supplementation and sex difference in prevention of neural tube defects and their subtypes in China: results from a large prospective cohort study. Nutr. J. 17, 1–7 (2018).
Toivonen, K. et al. Folic acid supplementation during the preconception period: a systematic review and meta-analysis. Prev. Med. 114, 1–17 (2018).
Viswanathan, M. et al. Folic Acid Supplementation: An Evidence Review for the U.S. Preventive Services Task Force (Agency for Healthcare Research and Quality, 2017).
Viswanathan, M. et al. Folic acid supplementation for the prevention of neural tube defects: an updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 317, 190–203 (2017).
De‐Regil, L. M., Peña‐Rosas, J. P., Fernández‐Gaxiola, A. C. & Rayco‐Solon, P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 12, CD007950 (2015).
Valentin, M. et al. Acid folic and pregnancy: A mandatory supplementation. Ann. Endocrinol. 79, 91–94 (2018).
Krawinkel, M. B. et al. Revised DA-CH intake recommendations for folate: how much is needed? Eur. J. Clin. Nutr. 68, 719–723 (2014).
Wolff, T., Witkop, C. T., Miller, T. & Syed, S. B. Folic acid supplementation for the prevention of neural tube defects: an update of the evidence for the US Preventive Services Task Force. Ann. Intern. Med. 150, 632–639 (2009).
Wilson, R. & O’Connor, D. Maternal folic acid and multivitamin supplementation: International clinical evidence with considerations for the prevention of folate-sensitive birth defects. Prev. Med. Rep. 24, 101617 (2021).
WHO. Guideline: Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects (World Health Organization, 2015).
EFSA Panel on Dietetic Products Nutrition and Allergies. Scientific Opinion on the substantiation of a health claim related to increasing maternal folate status by supplemental folate intake and reduced risk of neural tube defects pursuant to article 14 of regulation (EC) No 1924/2006. EFSA J. 11, 3328 (2013).
Hopkins, S. M. et al. Impact of voluntary fortification and supplement use on dietary intakes and biomarker status of folate and vitamin B-12 in Irish adults. Am. J. Clin. Nutr. 101, 1163–1172 (2015).
Kupka, R. The role of folate in malaria–implications for home fortification programmes among children aged 6–59 months. Matern. Child Nutr. 11, 1–15 (2015).
EFSA. ESCO report on analysis of risks and benefits of fortification of food with folic acid. EFSA Support. Publ. 6, 3E (2009).
Shea, B. et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst. Rev. 5, CD000951 (2013).
Visser, K. et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann. Rheum. Dis. 68, 1086–1093 (2009).
Bomba-Opoń, D., Hirnle, L., Kalinka, J. & Seremak-Mrozikiewicz, A. Folate supplementation during the preconception period, pregnancy and puerperium. Polish Society of Gynecologists and Obstetricians Guidelines. Ginekol. Polska 88, 633–636 (2017).
Wolak, N. et al. Vitamins B1, B2, B3 and B9–occurrence, biosynthesis pathways and functions in human nutrition. Mini Rev. Med. Chem. 17, 1075–1111 (2017).
Wehrli, C. Method for the production of folic acid United States patent, US5410056A (1995).
Wehrli, C. Verfahren zur Herstellung von Folsäure, EP0608693A2 (1994).
Zhang, G. et al. A kind of environment-friendly type preparation method of synthesis folic acid, China patent, CN106496231A (2016).
Qui, Y., Wang, Q., Ju, L. & Li, X. Simple and convenient folic acid environment-friendly production method, China patent, CN103896945B (2014).
Wang, G., Zhang, J. & Wang, S. Folic acid synthesis method, China patent, CN106046005A (2016).
Zhang, J., Yang, K. & Wang, X. A kind of synthetic method that folic acid is new, China patent, CN108558884A (2018).
EFSA Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Calcium L‐Methylfolate. EFSA J. 2, 135 (2004).
EFSA Panel on Food Additives and Nutrient Sources added to Food Scientific Opinion on (6S)‐5‐methyltetrahydrofolic acid, glucosamine salt as a source of folate added for nutritional purposes to food supplements. EFSA J. 11, 3358 (2013).
EFSA Panel on Nutrition Novel Foods and Food Allergens. Calcium l‐methylfolate as a source of folate added for nutritional purposes to infant and follow‐on formula, baby food and processed cereal‐based food. EFSA J. 18, e05947 (2020).
El-Sheekh, M. M., Abd-Elsalam, I. S., Shabana, S. & Zaki, A. Production of vitamin B12 and folic acid from agricultural wastes using new bacterial isolates. Afr. J. Microbiol. Res. 7, 966–973 (2013).
Mastella, L., Senatore, V., Beltrani, T. & Branduardi, P. Scheffersomyces stipitis ability to valorize different residual biomasses for vitamin B9 production. Microb. Biotechnol. 16, 392–403 (2023).
Mastella, L. et al. First report on vitamin B9 production including quantitative analysis of its vitamers in the yeast Scheffersomyces stipitis. Biotechnol. Biofuels Bioprod. 15, 98 (2022).
Revuelta, J. L., Serrano-Amatriain, C., Ledesma-Amaro, R. & Jiménez, A. Formation of folates by microorganisms: towards the biotechnological production of this vitamin. Appl. Microbiol. Biotechnol. 102, 8613–8620 (2018).
Serrano-Amatriain, C. et al. Folic acid production by engineered Ashbya gossypii. Metab. Eng. 38, 473–482 (2016).
Zhu, T., Koepsel, R., Domach, M. & Ataai, M. Metabolic engineering of folic acid production. In Fermentation Biotechnology (ed. Saha, B. C.) Ch. 13, 207-219 (American Chemical Society, 2003).
Zhu, T. et al. Engineering of Bacillus subtilis for enhanced total synthesis of folic acid. Appl. Environ. Microbiol. 71, 7122–7129 (2005).
Acevedo-Rocha, C. G. et al. Microbial cell factories for the sustainable manufacturing of B vitamins. Curr. Opin. Biotechnol. 56, 18–29 (2019).
WHO. Recommendations on wheat and maize flour fortification meeting report: Interim consensus statement (World Health Organization, 2009).
WHO. Guideline: Fortification of wheat flour with vitamins and minerals as a public health strategy (World Health Organization, 2022).
Madhari, R. S. et al. High dietary micronutrient inadequacy in peri‐urban school children from a district in South India: Potential for staple food fortification and nutrient supplementation. Matern. Child Nutr. 16, e13065 (2020).
de Benoist, B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr. Bull. 29, S238–S244 (2008).
Winkels, R. M. et al. Bread cofortified with folic acid and vitamin B-12 improves the folate and vitamin B-12 status of healthy older people: a randomized controlled trial. Am. J. Clin. Nutr. 88, 348–355 (2008).
Tucker, K. L. et al. Breakfast cereal fortified with folic acid, vitamin B-6, and vitamin B-12 increases vitamin concentrations and reduces homocysteine concentrations: a randomized trial. Am. J. Clin. Nutr. 79, 805–811 (2004).
Bird, J. K., Barron, R., Pigat, S. & Bruins, M. J. Contribution of base diet, voluntary fortified foods and supplements to micronutrient intakes in the UK. J. Nutr. Sci. 11, e51 (2022).
Hannon, E. M., Kiely, M. & Flynn, A. The impact of voluntary fortification of foods on micronutrient intakes in Irish adults. Br. J. Nutr. 97, 1177–1186 (2007).
Hennessy, A., Walsh, E., Walton, J. & Flynn, A. The contribution of fortified foods to micronutrient intake in Irish adults aged 18–64 years. Proc. Nutr. Soc. 70, E112 (2011).
Swanepoel, E. et al. Contribution of commercial infant products and fortified staple foods to nutrient intake at ages 6, 12, and 18 months in a cohort of children from a low socio‐economic community in South Africa. Matern. Child Nutr. 15, e12674 (2019).
Fulgoni, V. L. III, Keast, D. R., Bailey, R. L. & Dwyer, J. Foods, fortificants, and supplements: where do Americans get their nutrients? J. Nutr. 141, 1847–1854 (2011).
WHO/FAO. Guidelines on Food Fortification with Micronutrients (World Health Organization, 2006).
Newman, J. C., Malek, A. M., Hunt, K. J. & Marriott, B. P. Nutrients in the US diet: naturally occurring or enriched/fortified food and beverage sources, plus dietary supplements: NHANES 2009–2012. J. Nutr. 149, 1404–1412 (2019).
Berner, L. A., Keast, D. R., Bailey, R. L. & Dwyer, J. T. Fortified foods are major contributors to nutrient intakes in diets of US children and adolescents. J. Acad. Nutr. Diet. 114, 1009–1022.e1008 (2014).
Dietrich, M., Brown, C. J. & Block, G. The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States. J. Am. Coll. Nutr. 24, 266–274 (2005).
Hussain, N. M. & Sharma, S. C. Flour fortification with folate to reduce risk of Spina Bifida. Br. Student Doctor J. 4, 45–49 (2020).
Chandra-Hioe, M. V., Bucknall, M. P. & Arcot, J. Folate analysis in foods by UPLC-MS/MS: development and validation of a novel, high throughput quantitative assay; folate levels determined in Australian fortified breads. Anal. Bioanal. Chem. 401, 1035–1042 (2011).
Dwyer, J. T. et al. Fortification: new findings and implications. Nutr. Rev. 72, 127–141 (2014).
Serra-Majem, L. Vitamin and mineral intakes in European children. Is food fortification needed? Public Health Nutr. 4, 101–107 (2001).
Palchetti, C. Z. et al. Prevalence of inadequate intake of folate after mandatory fortification: results from the first National Dietary Survey in Brazil. Eur. J. Nutr. 59, 2793–2803 (2020).
Palchetti, C. Z. et al. Folate and vitamin B12 status: temporal evaluation after mandatory fortification in Brazil. Eur. J. Clin. Nutr. 76, 1266–1272 (2022).
Keats, E. C. et al. Improved micronutrient status and health outcomes in low-and middle-income countries following large-scale fortification: evidence from a systematic review and meta-analysis. Am. J. Clin. Nutr. 109, 1696–1708 (2019).
Noor, R. A. et al. Large–scale wheat flour folic acid fortification program increases plasma folate levels among women of reproductive age in urban Tanzania. PLoS One 12, e0182099 (2017).
Chan, Y.-M., MacFarlane, A. J. & O’Connor, D. L. Modeling demonstrates that folic acid fortification of whole-wheat flour could reduce the prevalence of folate inadequacy in Canadian whole-wheat consumers. J. Nutr. 145, 2622–2629 (2015).
Martiniak, Y., Heuer, T. & Hoffmann, I. Intake of dietary folate and folic acid in Germany based on different scenarios for food fortification with folic acid. Eur. J. Nutr. 54, 1045–1054 (2015).
Tablante, E. C., Pachón, H., Guetterman, H. M. & Finkelstein, J. L. Fortification of wheat and maize flour with folic acid for population health outcomes. Cochrane Database Syst. Rev. 7, CD012150 (2019).
Black, A. P. et al. High folate levels in Aboriginal children after subsidised fruit and vegetables and mandatory folic acid fortification. Aust. N. Z. J. Public Health 38, 241–246 (2014).
Cordero, J. F., Do, A. & Berry, R. Review of interventions for the prevention and control of folate and vitamin B12 deficiencies. Food Nutr. Bull. 29, S188–S195 (2008).
Dwyer, J. T. et al. Fortification and health: challenges and opportunities. Adv. Nutr. 6, 124–131 (2015).
Crider, K. S. et al. Systematic review and Bayesian meta-analysis of the dose-response relationship between folic acid intake and changes in blood folate concentrations. Nutrients 11, 71 (2019).
Powers, H. J., Stephens, M., Russell, J. & Hill, M. H. Fortified breakfast cereal consumed daily for 12 wk leads to a significant improvement in micronutrient intake and micronutrient status in adolescent girls: a randomised controlled trial. Nutr. J. 15, 1–13 (2016).
Dhonukshe-Rutten, R. et al. Dietary intake and status of folate and vitamin B12 and their association with homocysteine and cardiovascular disease in European populations. Eur. J. Clin. Nutr. 63, 18–30 (2009).
Della Lucia, C. M. et al. Impact of rice fortified with iron, zinc, thiamine and folic acid on laboratory measurements of nutritional status of preschool children. Cien. Saude Colet. 22, 583–592 (2017).
Choumenkovitch, S. F. et al. Folic acid intake from fortification in United States exceeds predictions. J. Nutr. 132, 2792–2798 (2002).
Bailey, L. B. et al. Biomarkers of nutrition for development—folate review. J. Nutr. 145, 1636S–1680S (2015).
Food Safety Authority of Ireland. Report of the scientific committee of the food safety authority of Ireland, update report on folic acid and the prevention of birth defects in Ireland (FSAI Dublin, 2016).
Mudryj, A. N., de Groh, M., Aukema, H. M. & Yu, N. Folate intakes from diet and supplements may place certain Canadians at risk for folic acid toxicity. Br. J. Nutr. 116, 1236–1245 (2016).
Chakraborty, H. et al. Folic acid fortification and women’s folate levels in selected communities in Brazil—a first look. Int. J. Vitam. Nutr. Res. 84, 286–294 (2014).
Saldanha, L. G. et al. Perspective: time to resolve confusion on folate amounts, units, and forms in prenatal supplements. Adv. Nutr. 11, 753–759 (2020).
Pfeiffer, C. M. et al. Folate status in the US population 20 y after the introduction of folic acid fortification. Am. J. Clin. Nutr. 110, 1088–1097 (2019).
Slagman, A. et al. Folic acid deficiency declined substantially after introduction of the mandatory fortification programme in Queensland, Australia: a secondary health data analysis. Public Health Nutr. 22, 3426–3434 (2019).
Obeid, R. & Herrmann, W. The emerging role of unmetabolized folic acid in human diseases: myth or reality? Curr. Drug Metab. 13, 1184–1195 (2012).
Mills, J. L., Molloy, A. M. & Reynolds, E. H. Do the benefits of folic acid fortification outweigh the risk of masking vitamin B12 deficiency? BMJ 360, k724 (2018).
Caffrey, A. et al. Maternal folate nutrition and offspring health: evidence and current controversies. Proc. Nutr. Soc. 78, 208–220 (2019).
Crider, K. S. et al. Folic acid and the prevention of birth defects: 30 years of opportunity and controversies. Annu. Rev. Nutr. 42, 423–452 (2022).
Leyvraz, M. et al. An assessment of the potential impact of fortification of staples and condiments on micronutrient intake of young children and women of reproductive age in Bangladesh. Nutrients 8, 541 (2016).
Luo, H. et al. Review of existing models to predict reductions in neural tube defects due to folic acid fortification and model results using data from Cameroon. Adv. Nutr. 12, 2401–2414 (2021).
Cuskelly, G. J., Mooney, K. M. & Young, I. S. Folate and vitamin B12: friendly or enemy nutrients for the elderly: symposium on ‘Micronutrients through the life cycle’. Proc. Nutr. Soc. 66, 548–558 (2007).
Garrett, G. S. & Bailey, L. B. A public health approach for preventing neural tube defects: folic acid fortification and beyond. Ann. N. Y. Acad. Sci. 1414, 47–58 (2018).
Haywood, P. et al. The effectiveness and cost-effectiveness of mandatory folic acid and iodine fortification (Australian Health Ministers’ Advisory Council, 2017).
Engle-Stone, R. et al. Iron, zinc, folate, and vitamin B-12 status increased among women and children in Yaounde and Douala, Cameroon, 1 year after introducing fortified wheat flour. J. Nutr. 147, 1426–1436 (2017).
New Zealand Food Safety. NZFS2019-08 Folic acid fortification: Increasing folic acid availability in food; Discussion Paper No: 2019/08 (Ministry for Primary Industries, 2019).
Patel, K. R. & Sobczyńska-Malefora, A. The adverse effects of an excessive folic acid intake. Eur. J. Clin. Nutr. 71, 159–163 (2017).
New Zealand Food Dafety. Folic acid fortification, Technical supporting document, technical paper No: 2019/04 (Ministry for Primary Industries, 2019).
Wald, N. J., Morris, J. K. & Blakemore, C. Public health failure in the prevention of neural tube defects: time to abandon the tolerable upper intake level of folate. Public Health Rev. 39, 1–11 (2018).
Low, F., Beaglehole, R. & Gluckman, P. The health benefits and risks of folic acid fortification of food (The Office of the Prime Minister’s Chief Science Advisor and the Royal Society Te Apārangi, 2018).
Sirohi, A., Pundhir, A. & Ghosh, S. Food fortification: a nutritional management strategy in India. Innov. J. Food Sci. 6, 1–8 (2018).
Haggarty, P. UK introduces folic acid fortification of flour to prevent neural tube defects. Lancet 398, 1199–1201 (2021).
Samaniego-Vaesken, Md. L., Alonso-Aperte, E. & Varela-Moreiras, G. Vitamin food fortification today. Food Nutr. Res. 56, 5459 (2012).
Samaniego-Vaesken, Md. L., Alonso-Aperte, E. & Varela-Moreiras, G. Voluntary fortification with folic acid in Spain: an updated food composition database. Food Chem. 193, 148–153 (2016).
Verkaik-Kloosterman, J. et al. Evaluation of the Dutch general exemption level for voluntary fortification with folic acid. Food Nutr. Res. 56, 5443 (2012).
Egan, E., Kelly, F. & Sweeney, M. R. Voluntary folic acid fortification levels of food staples in Ireland continue to decline: further implications for passive folic acid intakes? J. Public Health 43, 281–286 (2021).
Kelly, F. et al. Folic acid levels in some food staples in Ireland are on the decline: implications for passive folic acid intakes? J. Public Health 38, 265–269 (2016).
Laird, E. J. et al. Voluntary fortification is ineffective to maintain the vitamin B12 and folate status of older Irish adults: evidence from the Irish Longitudinal Study on Ageing (TILDA). Br. J. Nutr. 120, 111–120 (2018).
Wang, A. et al. Impact of voluntary folic acid fortification of corn masa flour on RBC folate concentrations in the US (NHANES 2011–2018). Nutrients 13, 1325 (2021).
Hennessy, Á., Walton, J. & Flynn, A. The impact of voluntary food fortification on micronutrient intakes and status in European countries: a review. Proc. Nutr. Soc. 72, 433–440 (2013).
Bundesinstitut für Risikobewertung. Nutzen-Risiko-Abwägung einer flächendeckenden Anreicherung von Mehl mit Folsäure: Stellungnahme Nr. 027/2017 des BfR vom 13. September 2017 https://doi.org/10.17590/20170913-100236 (2017).
Bundesinstitut für Risikobewertung. Updated recommended maximum levels for the addition of vitamins and miner als to food supplements and conventional foods, https://www.bfr.bund.de/en/press_information/2021/11/maximum_levels_for_vitamins_and_minerals_in_food_supplements_and_fortified_foods-270796.html (2021).
Czeizel, A. E. & Kökény, M. Bread is fortified with folic acid in Hungary. BMJ 325, 391 (2002).
Hamner, H. C. & Tinker, S. C. Fortification of corn masa flour with folic acid in the United States: an overview of the evidence. Ann. N. Y. Acad. Sci. 1312, 8–14 (2014).
Fleischman, A. R. & Oinuma, M. Fortification of corn masa flour with folic acid in the United States. Am. J. Public Health 101, 1360–1364 (2011).
Flores, A. L. et al. Adding folic acid to corn Masa flour: partnering to improve pregnancy outcomes and reduce health disparities. Prev. Med. 106, 26–30 (2018).
Redpath, B., Kancherla, V. & Oakley, G. P. Availability of corn masa flour and tortillas fortified with folic acid in Atlanta after national regulations allowing voluntary fortification. JAMA 320, 1600–1601 (2018).
Khalid, S. I. et al. The impact of voluntary folate fortification of corn masa flour on US pregnancies complicated by neural tube defects. Childs Nerv. Syst. 39, 1813–1819 (2023).
Jentink, J., van de Vrie-Hoekstra, N. W., de Jong-van den Berg, L. T. & Postma, M. J. Economic evaluation of folic acid food fortification in the Netherlands. Eur. J. Public Health 18, 270–274 (2008).
WHO. Guideline: Fortification of maize flour and corn meal with vitamins and minerals (World Health Organization, 2016).
FSSAI. Food Safety and Standards (Fortification of Foods) Regulations, https://www.fssai.gov.in/upload/uploadfiles/files/Compendium_Food_Fortification_Regulations_30_09_2021.pdf (2018).
Alavi, S. et al. Rice fortification in developing countries: a critical review of the technical and economic feasibility, https://www.semanticscholar.org/paper/Rice-Fortification-in-Developing-Countries-%3A-A-of/e63515126eeda28d442f25615fb6cbf9aeecfcac (2008).
Kam, K., Arcot, J. & Adesina, A. A. Folic acid fortification of parboiled rice: Multifactorial analysis and kinetic investigation. J. Food Eng. 108, 238–243 (2012).
Kam, K., Murray, J. M., Arcot, J. & Ward, R. Fortification of parboiled rice with folic acid: Consumer acceptance and sensory evaluation. Food Res. Int. 49, 354–363 (2012).
Waller, A. W. et al. Stakeholder’s perceptions of Mexico’s federal corn flour fortification program: a qualitative study. Nutrients 12, 433 (2020).
Orjuela, M. A. et al. Fortification of bakery and corn masa–based foods in Mexico and dietary intake of folic acid and folate in Mexican national survey data. Am. J. Clin. Nutr. 110, 1434–1448 (2019).
Barkley, J. S., Wheeler, K. S. & Pachón, H. Anaemia prevalence may be reduced among countries that fortify flour. Br. J. Nutr. 114, 265–273 (2015).
Murphy, M. E. & Westmark, C. J. Folic acid fortification and neural tube defect risk: analysis of the food fortification initiative dataset. Nutrients 12, 247 (2020).
Biemi, F. D. & Ganji, V. Temporal relation between double fortification of wheat flour with iron and folic acid, and markers and prevalence of anemia in children. Nutrients 13, 2013 (2021).
Southern African Development Community (SADC). English SADC Fortification Minimum Standards, https://www.sadc.int/document/english-sadc-fortification-minimum-standards (2020).
Muthayya, S. et al. Rice fortification: an emerging opportunity to contribute to the elimination of vitamin and mineral deficiency worldwide. Food Nutr. Bull. 33, 296–307 (2012).
Saha, S. & Roy, A. Whole grain rice fortification as a solution to micronutrient deficiency: Technologies and need for more viable alternatives. Food Chem. 326, 127049 (2020).
Cardoso, R. V. et al. Flour fortification for nutritional and health improvement: a review. Food Res. Int. 125, 108576 (2019).
Thurston, L., Borman, B. & Bower, C. Mandatory fortification with folic acid for the prevention of neural tube defects: a case study of Australia and New Zealand. Childs Nerv. Syst., 39, 1737–1741 (2023).
Barboza-Argüello, M. D. L. P. et al. Neural tube defects in Costa Rica, 1987–2012: origins and development of birth defect surveillance and folic acid fortification. Matern. Child Health J. 19, 583–590 (2015).
Lu, B. et al. Simultaneous determination of four water-soluble vitamins in fortified infant foods by ultra-performance liquid chromatography coupled with triple quadrupole mass spectrometry. J. Chromatogr. Sci. 46, 225–232 (2008).
Liberato, S. C. & Pinheiro-Sant’Ana, H. M. Fortification of industrialized foods with vitamins. Rev. Nutr. 19, 215–231 (2006).
European Commission. Commission directive 2006/125/EC on processed cereal-based foods and baby foods for infants and young children. OJEU, 16–35 (European Commission, 2006).
European Commission. Commission directive 2006/141/EC of 22 December 2006 on infant formulae and follow-on formulae and amending Directive 1999/21/EC. OJEU 49, 1–33 (2006).
Samaniego-Vaesken, M. D. L., Alonso-Aperte, E. & Varela-Moreiras, G. Analysis and evaluation of voluntary folic acid fortification of breakfast cereals in the Spanish market. J. Food Compos. Anal. 23, 419–423 (2010).
Samaniego-Vaesken, M. D. L., Alonso-Aperte, E. & Varela-Moreiras, G. Voluntary folic acid fortification levels and nutrient composition of food products from the Spanish market: A 2011–2015 update. Nutrients 9, 234 (2017).
Kuriyan, R. et al. The effects of regular consumption of a multiple micronutrient fortified milk beverage on the micronutrient status of school children and on their mental and physical performance. Clin. Nutr. 35, 190–198 (2016).
Pentieva, K. et al. Acute absorption of folic acid from a fortified low-fat spread. Eur. J. Clin. Nutr. 57, 1235–1241 (2003).
van den Boom, A. et al. The contribution of ready-to-eat cereals to daily nutrient intake and breakfast quality in a Mediterranean setting. J. Am. Coll. Nutr. 25, 135–143 (2006).
Parliament, E. U. Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. OJEU 50, 26–38 (2006).
McGee, E. J. T., Sangakkara, A. R. & Diosady, L. L. Double fortification of salt with folic acid and iodine. J. Food Eng. 198, 72–80 (2017).
Li, Y. O., Diosady, L. L. & Wesley, A. S. Folic acid fortification through existing fortified foods: iodized salt and vitamin A—fortified sugar. Food Nutr. Bull. 32, 35–41 (2011).
Vinodkumar, M. & Rajagopalan, S. Multiple micronutrient fortification of salt. Eur. J. Clin. Nutr. 63, 437–445 (2009).
Modupe, O. & Diosady, L. L. Quadruple fortification of salt for the delivery of iron, iodine, folic acid, and vitamin B12 to vulnerable populations. J. Food Eng. 300, 110525 (2021).
Modupe, O., Krishnaswamy, K. & Diosady, L. L. Technology for triple fortification of salt with folic acid, iron, and iodine. J. Food Sci. 84, 2499–2506 (2019).
Modupe, O., Siddiqui, J., Jonnalagadda, A. & Diosady, L. L. Folic acid fortification of double fortified salt. Sci. Rep. 11, 14561 (2021).
Kancherla, V. et al. Modeling shows high potential of folic acid‐fortified salt to accelerate global prevention of major neural tube defects. Birth Defects Res. 112, 1461–1474 (2020).
Vora, R. M. et al. Potential for elimination of folate and vitamin B12 deficiency in India using vitamin-fortified tea: a preliminary study. BMJ Nutr. Prev. Health. 4, 293–306 (2021).
Vora, R. M. & Antony, A. C. The unresolved tragedy of neural-tube defects in India: The case for folate-and vitamin-B12-fortified tea for prevention. J. Indian Assoc. Pediatr. Surg. 27, 1–8 (2022).
Tapola, N., Karvonen, H., Niskanen, L. & Sarkkinen, E. Mineral water fortified with folic acid, vitamins B6, B12, D and calcium improves folate status and decreases plasma homocysteine concentration in men and women. Eur. J. Clin. Nutr. 58, 376–385 (2004).
Järvenpää, J. et al. Fortified mineral water improves folate status and decreases plasma homocysteine concentration in pregnant women. J. Perinat. Med. 35, 108–114 (2007).
Engle-Stone, R. et al. Analyses using national survey data from Cameroon, Haiti, and Ghana indicate the potential for bouillon fortification to help fill dietary gaps for 5 nutrients. Curr. Dev. Nutr. 5, 640 (2021).
Engle‐Stone, R. et al. Weighing the risks of high intakes of selected micronutrients compared with the risks of deficiencies. Ann. N. Y. Acad. Sci. 1446, 81–101 (2019).
De Steur, H., Feng, S., Xiaoping, S. & Gellynck, X. Consumer preferences for micronutrient strategies in China. A comparison between folic acid supplementation and folate biofortification. Public Health Nutr. 17, 1410–1420 (2014).
De Steur, H. et al. Potential impact and cost-effectiveness of multi-biofortified rice in China. N. Biotechnol. 29, 432–442 (2012).
Strobbe, S. & Van Der Straeten, D. Folate biofortification in food crops. Curr. Opin. Biotechnol. 44, 202–211 (2017).
De Steur, H. et al. Health impact in China of folate-biofortified rice. Nat. Biotechnol. 28, 554–556 (2010).
De Lepeleire, J. et al. Folate biofortification of potato by tuber-specific expression of four folate biosynthesis genes. Mol. Plant 11, 175–188 (2018).
McLean, E., de Benoist, B. & Allen, L. H. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr. Bull. 29, S38–S51 (2008).
Strobbe, S. & Van Der Straeten, D. Toward eradication of B-vitamin deficiencies: considerations for crop biofortification. Front. Plant Sci. 9, 443 (2018).
Yu, S. & Tian, L. Breeding major cereal grains through the lens of nutrition sensitivity. Mol. Plant 11, 23–30 (2018).
Kumar, J. et al. Current knowledge on genetic biofortification in lentil. J. Agric. Food Chem. 64, 6383–6396 (2016).
Piironen, V., Edelmann, M., Kariluoto, S. & Bedo, Z. Folate in wheat genotypes in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 56, 9726–9731 (2008).
Riaz, B. et al. Folate content analysis of wheat cultivars developed in the North China Plain. Food Chem. 289, 377–383 (2019).
Shewry, P. R. et al. Natural variation in grain composition of wheat and related cereals. J. Agric. Food Chem. 61, 8295–8303 (2013).
Hefni, M. E., Schaller, F. & Witthöft, C. M. Betaine, choline and folate content in different cereal genotypes. J. Cereal Sci. 80, 72–79 (2018).
Kariluoto, S., Edelmann, M. & Piironen, V. Effects of environment and genotype on folate contents in wheat in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 58, 9324–9331 (2010).
Zheng, J. et al. Folate (vitamin B9) content analysis in bread wheat (Triticum aestivum L.). Front. Nutr. 9, 933358 (2022).
Ashokkumar, K. et al. Genomics-integrated breeding for carotenoids and folates in staple cereal grains to reduce malnutrition. Front. Genet. 11, 414 (2020).
Shahid, M. et al. Folate monoglutamate in cereal grains: Evaluation of extraction techniques and determination by LC-MS/MS. J. Food Compos. Anal. 91, 103510 (2020).
Andersson, A. A. et al. Phytochemical and dietary fiber components in barley varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 56, 9767–9776 (2008).
Kariluoto, M. S., Vahteristo, L. T. & Piironen, V. I. Applicability of microbiological assay and affinity chromatography purification followed by high‐performance liquid chromatography (HPLC) in studying folate contents in rye. J. Sci. Food Agric. 81, 938–942 (2001).
Shewry, P. R. et al. Effects of genotype and environment on the content and composition of phytochemicals and dietary fiber components in rye in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 58, 9372–9383 (2010).
Shewry, P. R. et al. Phytochemical and fiber components in oat varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 56, 9777–9784 (2008).
De Brouwer, V. et al. Ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) for the sensitive determination of folates in rice. J. Chromatogr. B 878, 509–513 (2010).
Ashokkumar, K., Sivakumar, P. & Saradhadevi, M. Identification and determination of naturally occurring folates in grains of rice (Oryza sativa L.) by UPLC-MS/MS analysis. Nat. Prod. Res. 32, 1733–1737 (2018).
Dong, W. et al. Identification of QTLs underlying folate content in milled rice. J. Integr. Agric. 13, 1827–1834 (2014).
Xiao, Y. et al. A genome-wide association study of folates in sweet corn kernels. Front. Plant Sci. 13, 1004455 (2022).
Lian, T. et al. Comparative transcriptome analysis reveals mechanisms of folate accumulation in maize grains. Int. J. Mol. Sci. 23, 1708 (2022).
Hou, S. et al. Folate metabolic profiling and expression of folate metabolism‐related genes during panicle development in foxtail millet (Setaria italica (L.) P. Beauv). J. Sci. Food Agric. 102, 268–279 (2022).
Wang, Y. et al. Foxtail millet [Setaria italica (L.) P. Beauv.] grown under nitrogen deficiency exhibits a lower folate contents. Front. Nutr. 10, 1035739 (2023).
Goyer, A. & Sweek, K. Genetic diversity of thiamin and folate in primitive cultivated and wild potato (Solanum) species. J. Agric. Food Chem. 59, 13072–13080 (2011).
Robinson, B. R., Sathuvalli, V., Bamberg, J. & Goyer, A. Exploring folate diversity in wild and primitive potatoes for modern crop improvement. Genes 6, 1300–1314 (2015).
Singh, J. et al. Genetic variability for vitamin B9 and total dietary fiber in lentil (Lens culinaris L.) cultivars. Int. J. Food Prop. 19, 936–943 (2016).
Jha, A. B. et al. Genetic diversity of folate profiles in seeds of common bean, lentil, chickpea and pea. J. Food Compos. Anal. 42, 134–140 (2015).
Agyenim-Boateng, K. G. et al. Profiling of naturally occurring folates in a diverse soybean germplasm by HPLC-MS/MS. Food Chem. 384, 132520 (2022).
Agyenim-Boateng, K. G. et al. Identification of quantitative trait loci and candidate genes for seed folate content in soybean. Theor. Appl. Genet. 136, 149 (2023).
Agyenim-Boateng, K. G. et al. Folate biofortification in soybean: challenges and prospects. Agron 13, 241 (2023).
Marshall, J. et al. Targeted quantification of B vitamins using ultra-performance liquid chromatography-selected reaction monitoring mass spectrometry in faba bean seeds. J. Food Compos. Anal. 95, 103687 (2021).
Goyer, A., Navarre, D. A. & Miklas, P. N. Folate content in select dry bean genotoypes. 132–133 (Annual Report-Bean Improvement Cooperative, 2008).
Martin, C. J., Torkamaneh, D., Arif, M. & Pauls, K. P. Genome-wide association study of seed folate content in common bean. Front. Plant Sci. 12, 696423 (2021).
Khanal, S. et al. Quantitative trait loci analysis of folate content in dry beans, Phaseolus vulgaris L. Int. J. Agron 2013, 983641 (2013).
Jha, A. B. et al. Folate profile diversity and associated SNPs using genome wide association study in pea. Euphytica 216, 1–16 (2020).
Shohag, M. et al. Genetic and physiological regulation of folate in pak choi (Brassica rapa subsp. Chinensis) germplasm. J. Exp. Bot. 71, 4914–4929 (2020).
Blancquaert, D., De Steur, H., Gellynck, X. & Van Der Straeten, D. Present and future of folate biofortification of crop plants. J. Exp. Bot. 65, 895–906 (2014).
Blancquaert, D. et al. Enhancing pterin and para-aminobenzoate content is not sufficient to successfully biofortify potato tubers and Arabidopsis thaliana plants with folate. J. Exp. Bot. 64, 3899–3909 (2013).
Blancquaert, D. et al. Rice folate enhancement through metabolic engineering has an impact on rice seed metabolism, but does not affect the expression of the endogenous folate biosynthesis genes. Plant Mol. Biol. 83, 329–349 (2013).
De Steur, H. et al. Genetically modified rice with health benefits as a means to reduce micronutrient malnutrition: global status, consumer preferences, and potential health impacts of rice biofortification. In Wheat and Rice in Disease Prevention and Health (eds Watson, R. R., Preedy, R. V. & Zibadi, S.) Ch. 21, 283–299 (Academic Press, 2014).
Sanahuja, G. et al. A question of balance: achieving appropriate nutrient levels in biofortified staple crops. Nutr. Res. Rev. 26, 235–245 (2013).
Bekaert, S. et al. Folate biofortification in food plants. Trends Plant Sci. 13, 28–35 (2008).
Díaz de la Garza, R. et al. Folate biofortification in tomatoes by engineering the pteridine branch of folate synthesis. Proc. Natl. Acad. Sci. 101, 13720–13725 (2004).
Storozhenko, S. et al. Folate enhancement in staple crops by metabolic engineering. Trends Food Sci. Technol. 16, 271–281 (2005).
Rébeillé, F. et al. Roles of vitamins B5, B8, B9, B12 and molybdenum cofactor at cellular and organismal levels. Nat. Prod. Rep. 24, 949–962 (2007).
Tyagi, K. et al. Seeing the unseen: a trifoliate (MYB117) mutant allele fortifies folate and carotenoids in tomato fruits. Plant J. 112, 38–54 (2022).
Song, L. et al. Weighted gene co-expression network analysis unveils gene networks regulating folate biosynthesis in maize endosperm. 3 Biotech 11, 1–16 (2021).
Basset, G. J., Quinlivan, E. P., Gregory, J. F. III & Hanson, A. D. Folate synthesis and metabolism in plants and prospects for biofortification. Crop. Sci. 45, 449–453 (2005).
Blancquaert, D. et al. Improving folate (vitamin B9) stability in biofortified rice through metabolic engineering. Nat. Biotechnol. 33, 1076–1078 (2015).
Hossain, T. et al. Enhancement of folates in plants through metabolic engineering. Proc. Natl. Acad. Sci. 101, 5158–5163 (2004).
Nunes, A. C., Kalkmann, D. C. & Aragao, F. J. Folate biofortification of lettuce by expression of a codon optimized chicken GTP cyclohydrolase I gene. Transgenic Res. 18, 661–667 (2009).
Díaz de la Garza, R. I., Gregory, J. F. III & Hanson, A. D. Folate biofortification of tomato fruit. Proc. Natl. Acad. Sci. 104, 4218–4222 (2007).
Zanga, D. et al. Freedom‐to‐operate analysis of a transgenic multivitamin corn variety. Plant Biotechnol. J. 14, 1225–1240 (2016).
Liang, Q. et al. Improved folate accumulation in genetically modified maize and wheat. J. Exp. Bot. 70, 1539–1551 (2019).
Ramírez Rivera, N. G., García‐Salinas, C., Aragão, F. J. & Díaz de la Garza, R. I. Metabolic engineering of folate and its precursors in Mexican common bean (Phaseolus vulgaris L.). Plant Biotechnol. J. 14, 2021–2032 (2016).
Dong, W. et al. Overexpression of folate biosynthesis genes in rice (Oryza sativa L.) and evaluation of their impact on seed folate content. Plant Foods Hum. Nutr. 69, 379–385 (2014).
Naqvi, S. et al. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc. Natl. Acad. Sci. 106, 7762–7767 (2009).
Storozhenko, S. et al. Folate fortification of rice by metabolic engineering. Nat. Biotechnol. 25, 1277–1279 (2007).
Capozzi, V. et al. Lactic acid bacteria producing B-group vitamins: a great potential for functional cereals products. Appl. Microbiol. Biotechnol. 96, 1383–1394 (2012).
LeBlanc, J. et al. B‐Group vitamin production by lactic acid bacteria–current knowledge and potential applications. J. Appl. Microbiol. 111, 1297–1309 (2011).
Rossi, M., Amaretti, A. & Raimondi, S. Folate production by probiotic bacteria. Nutrients 3, 118–134 (2011).
Levit, R., Savoy de Giori, G., De Moreno De Leblanc, A. & LeBlanc, J. G. Recent update on lactic acid bacteria producing riboflavin and folates: application for food fortification and treatment of intestinal inflammation. J. Appl. Microbiol. 130, 1412–1424 (2021).
Mahara, F. A., Nuraida, L. & Lioe, H. N. Fermentation of milk using folate-producing lactic acid bacteria to increase natural folate content: a review. Appl. Biotechnol. Rep. 6, 129–136 (2019).
Mahara, F. A., Nuraida, L. & Lioe, H. N. Folate in milk fermented by lactic acid bacteria from different food sources. Prev. Nutr. Food Sci. 26, 230–240 (2021).
Meucci, A. et al. Folates biosynthesis by Streptococcus thermophilus during growth in milk. Food Microbiol. 69, 116–122 (2018).
Laiño, J. E. et al. Folate production and fol genes expression by the dairy starter culture Streptococcus thermophilus CRL803 in free and controlled pH batch fermentations. LWT 85, 146–150 (2017).
Tomar, S., Srivatsa, N., Ramya, I. & Rameshwar, S. Estimation of folate production by Streptococcus thermophilus using modified microbiological assay. Milchwissenschaft 64, 260–263 (2009).
Sybesma, W. et al. Effects of cultivation conditions on folate production by lactic acid bacteria. Appl. Environ. Microbiol. 69, 4542–4548 (2003).
Ayad, E. H. Starter culture development for improving safety and quality of Domiati cheese. Food Microbiol. 26, 533–541 (2009).
Padalino, M. et al. Effect of fructooligosaccharides and galactooligosaccharides on the folate production of some folate-producing bacteria in media cultures or milk. Int. Dairy J. 27, 27–33 (2012).
Albano, C., Silvetti, T. & Brasca, M. Screening of lactic acid bacteria producing folate and their potential use as adjunct cultures for cheese bio-enrichment. FEMS Microbiol. Lett. 367, fnaa059 (2020).
Laiño, J. E., del Valle, M. J., de Giori, G. S. & LeBlanc, J. G. J. Development of a high folate concentration yogurt naturally bio-enriched using selected lactic acid bacteria. LWT 54, 1–5 (2013).
Laiño, J. E., del Valle, M. J., de Giori, G. S. & LeBlanc, J. G. J. Applicability of a Lactobacillus amylovorus strain as co-culture for natural folate bio-enrichment of fermented milk. Int. J. Food Microbiol. 191, 10–16 (2014).
Rad, A. H., Khosroushahi, A. Y., Khalili, M. & Jafarzadeh, S. Folate bio-fortification of yoghurt and fermented milk: a review. Dairy Sci. Technol. 96, 427–441 (2016).
Olanbiwoninu, A. et al. Microbial-based biofortification to mitigate African micronutrients deficiency: A focus on plant-based fermentation as source of B-group vitamins. Food Biosci 55, 102996 (2023).
Mahara, F. A., Nuraida, L., Lioe, H. N. & Nurjanah, S. The occurrence of folate biosynthesis genes in lactic acid bacteria from different sources. Food Technol. Biotechnol. 61, 226–237 (2023).
Patel, A., Shah, N. & Prajapati, J. Biosynthesis of vitamins and enzymes in fermented foods by lactic acid bacteria and related genera-A promising approach. Croat. J. Food Sci. Technol. 5, 85–91 (2013).
Mosso, A. L. et al. Increasing the folate content of tuber based foods using potentially probiotic lactic acid bacteria. Food Res. Int. 109, 168–174 (2018).
Mosso, A. L. et al. Effect of fermentation in nutritional, textural and sensorial parameters of vegan-spread products using a probiotic folate-producing Lactobacillus sakei strain. LWT 127, 109339 (2020).
Carrizo, S. L. et al. Ancestral Andean grain quinoa as source of lactic acid bacteria capable to degrade phytate and produce B-group vitamins. Food Res. Int. 89, 488–494 (2016).
Kariluoto, S. et al. In situ enrichment of folate by microorganisms in beta-glucan rich oat and barley matrices. Int. J. Food Microbiol. 176, 38–48 (2014).
Thompson, H. O. et al. Fermentation of cauliflower and white beans with Lactobacillus plantarum–impact on levels of riboflavin, folate, vitamin B 12, and amino acid composition. Plant Foods Hum. Nutr. 75, 236–242 (2020).
Da Silva, F. F. P., Biscola, V., LeBlanc, J. G. & de Melo Franco, B. D. G. Effect of indigenous lactic acid bacteria isolated from goat milk and cheeses on folate and riboflavin content of fermented goat milk. LWT 71, 155–161 (2016).
Tamene, A., Baye, K. & Humblot, C. Folate content of a staple food increased by fermentation of a cereal using selected folate-producing microorganisms. Heliyon 8, e09526 (2022).
Divya, J. B. & Nampoothiri, K. M. Folate fortification of skim milk by a probiotic Lactococcus lactis CM28 and evaluation of its stability in fermented milk on cold storage. Int. J. Food Sci. Technol. 52, 3513–3519 (2015).
Panda, S. et al. Characterization of novel folate producing Lactobacillus rhamnosus and its appliance in fortification of ragi (Eleusine coracana) gruel. Food Biosci. 21, 100–106 (2018).
Okoroafor, I., Banwo, K., Olanbiwoninu, A. A. & Odunfa, S. A. Folate enrichment of Ogi (a fermented cereal gruel) using folate producing starter cultures. Adv. Microbiol. 9, 177 (2019).
Tamene, A., Mekuriyaw, T. & Baye, K. Effectiveness of Lactiplantibacillus plantarum in enhancing the folate content of injera made with different cereals. Food Sci. Nutr. 11, 6213–6222 (2023).
Greppi, A. et al. Ability of lactobacilli isolated from traditional cereal-based fermented food to produce folate in culture media under different growth conditions. LWT 86, 277–284 (2017).
Pompei, A. et al. Folate production by bifidobacteria as a potential probiotic property. Appl. Environ. Microbiol. 73, 179–185 (2007).
Celik, O. F. & O’Sullivan, D. J. Correlation of gene content in selected bifidobacteria with folate supplier or scavenger status during growth in laboratory media. Food Biosci. 51, 102324 (2023).
Czarnowska-Kujawska, M. & Paszczyk, B. Changes in the folate content and fatty acid profile in fermented milk produced with different starter cultures during storage. Molecules 26, 6063 (2021).
Hugenschmidt, S., Schwenninger, S. M., Gnehm, N. & Lacroix, C. Screening of a natural biodiversity of lactic and propionic acid bacteria for folate and vitamin B12 production in supplemented whey permeate. Int. Dairy J. 20, 852–857 (2010).
Hugenschmidt, S., Schwenninger, S. M. & Lacroix, C. Concurrent high production of natural folate and vitamin B12 using a co-culture process with Lactobacillus plantarum SM39 and Propionibacterium freudenreichii DF13. Process Biochem. 46, 1063–1070 (2011).
Hugenholtz, J., Hunik, J., Santos, H. & Smid, E. Nutraceutical production by propionibacteria. Le Lait 82, 103–112 (2002).
Poonam et al. Multifaceted attributes of dairy propionibacteria: a review. World J. Microbiol. Biotechnol. 28, 3081–3095 (2012).
Van Wyk, J. & Britz, T. J. A rapid high-performance liquid chromatography (HPLC) method for the extraction and quantification of folates in dairy products and cultures of Propionibacterium freudenreichii. Afr. J. Biotechnol. 11, 2087–2098 (2012).
Van Wyk, J., Witthuhn, R. C. & Britz, T. J. Optimisation of vitamin B12 and folate production by Propionibacterium freudenreichii strains in kefir. Int. Dairy J. 21, 69–74 (2011).
Korhola, M. et al. Production of folate in oat bran fermentation by yeasts isolated from barley and diverse foods. J. Appl. Microbiol. 117, 679–689 (2014).
Hjortmo, S., Patring, J. & Andlid, T. Growth rate and medium composition strongly affect folate content in Saccharomyces cerevisiae. Int. J. Food Microbiol. 123, 93–100 (2008).
Hjortmo, S. B., Hellström, A. M. & Andlid, T. A. Production of folates by yeasts in Tanzanian fermented togwa. FEMS Yeast Res. 8, 781–787 (2008).
Greppi, A. et al. Potential probiotic Pichia kudriavzevii strains and their ability to enhance folate content of traditional cereal-based African fermented food. Food Microbiol. 62, 169–177 (2017).
Chandrasekar Rajendran, S. et al. Biofortification of riboflavin and folate in idli batter, based on fermented cereal and pulse, by Lactococcus lactis N8 and Saccharomyces boulardii SAA655. J. Appl. Microbiol. 122, 1663–1671 (2017).
Herranen, M. et al. Isolation and characterization of folate-producing bacteria from oat bran and rye flakes. Int. J. Food Microbiol. 142, 277–285 (2010).
Deatraksa, J. et al. Isolation of folate-producing Weissella spp. from Thai fermented fish (Plaa Som Fug). LWT 89, 388–391 (2018).
Kariluoto, S. et al. Production of folate by bacteria isolated from oat bran. Int. J. Food Microbiol. 143, 41–47 (2010).
Laiño, J. E., LeBlanc, J. G. & Savoy de Giori, G. Production of natural folates by lactic acid bacteria starter cultures isolated from artisanal Argentinean yogurts. Can. J. Microbiol. 58, 581–588 (2012).
Saubade, F., Humblot, C., Hemery, Y. M. & Guyot, J.-P. PCR screening of an African fermented pearl-millet porridge metagenome to investigate the nutritional potential of its microbiota. Int. J. Food Microbiol. 244, 103–110 (2017).
D’Aimmo, M. R. et al. Biosynthesis and cellular content of folate in bifidobacteria across host species with different diets. Anaerobe 30, 169–177 (2014).
Masuda, M. et al. Production potency of folate, vitamin B12, and thiamine by lactic acid bacteria isolated from Japanese pickles. Biosci. Biotechnol. Biochem. 76, 2061–2067 (2012).
Misci, C. et al. Fermentation as a tool for increasing food security and nutritional quality of indigenous African leafy vegetables: the case of Cucurbita sp. Food Microbiol. 99, 103820 (2021).
Hjortmo, S., Patring, J., Jastrebova, J. & Andlid, T. Biofortification of folates in white wheat bread by selection of yeast strain and process. Int. J. Food Microbiol. 127, 32–36 (2008).
Sybesma, W. et al. Multivitamin production in Lactococcus lactis using metabolic engineering. Metab. Eng. 6, 109–115 (2004).
Sybesma, W. et al. Increased production of folate by metabolic engineering of Lactococcus lactis. Appl. Environ. Microbiol. 69, 3069–3076 (2003).
Sybesma, W. et al. Controlled modulation of folate polyglutamyl tail length by metabolic engineering of Lactococcus lactis. Appl. Environ. Microbiol. 69, 7101–7107 (2003).
Wegkamp, A., van Oorschot, W., de Vos, W. M. & Smid, E. J. Characterization of the role of para-aminobenzoic acid biosynthesis in folate production by Lactococcus lactis. Appl. Environ. Microbiol. 73, 2673–2681 (2007).
Santos, F. et al. High-level folate production in fermented foods by the B12 producer Lactobacillus reuteri JCM1112. Appl. Environ. Microbiol. 74, 3291–3294 (2008).
Wegkamp, A. et al. Transformation of folate-consuming Lactobacillus gasseri into a folate producer. Appl. Environ. Microbiol. 70, 3146–3148 (2004).
Liu, Y. et al. Enhancing the natural folate level in wine using bioengineering and stabilization strategies. Food Chem. 194, 26–31 (2016).
LeBlanc, J. G. et al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 24, 160–168 (2013).
Shin, Y. S. et al. Subcellular localization of gamma-glutamyl carboxypeptidase and of folates. Biochim. Biophys. Acta 444, 794–801 (1976).
Hou, Z. & Matherly, L. H. Biology of the major facilitative folate transporters SLC19A1 and SLC46A1. Curr. Top. Membr. 73, 175–204 (2014).
Ducker, G. S. & Rabinowitz, J. D. One-carbon metabolism in health and disease. Cell Metab. 25, 27–42 (2017).
Watkins, D. & Rosenblatt, D. S. Inherited Disorders of Folate and Cobalamin Transport and Metabolism. In The Online Metabolic and Molecular Bases of Inherited Disease (eds Valle, D. L. et al.) (McGraw-Hill Education, 2019). https://doi.org/10.1036/ommbid.420.
Froese, D. S., Fowler, B. & Baumgartner, M. R. Vitamin B(12), folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 42, 673–685 (2019).
Tibbetts, A. S. & Appling, D. R. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 30, 57–81 (2010).
Zhao, R., Matherly, L. H. & Goldman, I. D. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev. Mol. Med. 11, e4 (2009).
Bosson, G. Reduced folate carrier: biochemistry and molecular biology of the normal and methotrexate-resistant cell. Br. J. Biomed. Sci. 60, 117–129 (2003).
Salazar, M. D. & Ratnam, M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 26, 141–152 (2007).
Low, P. S. & Kularatne, S. A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 13, 256–262 (2009).
Gao, W. et al. Chemotherapeutic drug delivery to cancer cells using a combination of folate targeting and tumor microenvironment-sensitive polypeptides. Biomaterials 34, 4137–4149 (2013).
Zhao, R. & Goldman, I. D. Folate and thiamine transporters mediated by facilitative carriers (SLC19A1-3 and SLC46A1) and folate receptors. Mol. Asp. Med. 34, 373–385 (2013).
Alpers, D., Taylor, B., Bier, D. & Klein, S. Manual of Nutritional Therapeutics 6th edn. (Lippincott Williams & Wilkins, 2015).
Reisenauer, A. M., Krumdieck, C. L. & Halsted, C. H. Folate conjugase: two separate activities in human jejunum. Science 198, 196–197 (1977).
Wang, T. T., Reisenauer, A. M. & Halsted, C. H. Comparison of folate conjugase activities in human, pig, rat and monkey intestine. J. Nutr. 115, 814–819 (1985).
Inoue, K. et al. Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G660–G668 (2008).
Subramanian, V. S., Marchant, J. S. & Said, H. M. Apical membrane targeting and trafficking of the human proton-coupled transporter in polarized epithelia. Am. J. Physiol. Cell Physiol. 294, C233–C240 (2008).
Greiner, P. O., Zittoun, J., Marquet, J. & Cheron, J. M. Pharmacokinetics of (-)-folinic acid after oral and intravenous administration of the racemate. Br. J. Clin. Pharmacol. 28, 289–295 (1989).
Garza-Aguilar, S. M. et al. The complexity of folate polyglutamylation in plants: postharvest ripening and ethylene modulate polyglutamylated profiles in climacteric fruits plus systematic analysis of the glutamyl tail-editing enzymes. Sci. Hortic. 273, 109588 (2020).
Habeck, L. L. et al. Substrate specificity of mammalian folylpolyglutamate synthetase for 5,10-dideazatetrahydrofolate analogs. Mol. Pharmacol. 48, 326–333 (1995).
Herbert, V. & Zalusky, R. Interrelations of vitamin B12 and folic acid metabolism: folic acid clearance studies. J. Clin. Invest. 41, 1263–1276 (1962).
Gregory, J. III et al. Kinetic model of folate metabolism in nonpregnant women consuming [2H2]folic acid: isotopic labeling of urinary folate and the catabolite para-acetamidobenzoylglutamate indicates slow, intake-dependent, turnover of folate pools. J. Nutr. 128, 1896–1906 (1998).
Steinberg, S. E., Campbell, C. L. & Hillman, R. S. Kinetics of the normal folate enterohepatic cycle. J. Clin. Invest. 64, 83–88 (1979).
Gregory, J. III Bioavailability of folate. Eur. J. Clin. Nutr. 51, S54–S59 (1997).
Gregory, J. III, Williamson, J., Bailey, L. & Toth, J. Urinary excretion of [2H4]folate by nonpregnant women following a single oral dose of [2H4]folic acid is a functional index of folate nutritional status. J. Nutr. 128, 1907–1912 (1998).
McPartlin, J. et al. The quantitative analysis of endogenous folate catabolites in human urine. Anal. Biochem. 206, 256–261 (1992).
Galivan, J. et al. Glutamyl hydrolase: pharmacological role and enzymatic characterization. Pharmacol. Ther. 85, 207–215 (2000).
Suh, J. R., Herbig, A. K. & Stover, P. J. New perspectives on folate catabolism. Annu. Rev. Nutr. 21, 255–282 (2001).
Garrow, T. A. et al. Cloning of human cDNAs encoding mitochondrial and cytosolic serine hydroxymethyltransferases and chromosomal localization. J. Biol. Chem. 268, 11910–11916 (1993).
Gregory, J. III et al. Primed, constant infusion with [2H3]serine allows in vivo kinetic measurement of serine turnover, homocysteine remethylation, and transsulfuration processes in human one-carbon metabolism. Am. J. Clin. Nutr. 72, 1535–1541 (2000).
Herbig, K. et al. Cytoplasmic serine hydroxymethyltransferase mediates competition between folate-dependent deoxyribonucleotide and S-adenosylmethionine biosyntheses. J. Biol. Chem. 277, 38381–38389 (2002).
Ducker, G. S. et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 23, 1140–1153 (2016).
Labuschagne, C. F. et al. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 7, 1248–1258 (2014).
Yamaoka, T. et al. Amidophosphoribosyltransferase limits the rate of cell growth-linked de novo purine biosynthesis in the presence of constant capacity of salvage purine biosynthesis. J. Biol. Chem. 272, 17719–17725 (1997).
Shane, B. Folate and vitamin B12 metabolism: overview and interaction with riboflavin, vitamin B6, and polymorphisms. Food Nutr. Bull. 29, S5–S16 (2008).
Lan, X., Field, M. S. & Stover, P. J. Cell cycle regulation of folate-mediated one-carbon metabolism. Wiley Interdiscip. Rev. Syst. Biol. Med. 10, e1426 (2018).
Prem Veer Reddy, G. Catalytic function of thymidylate synthase is confined to S phase due to its association with replitase. Biochem. Biophys. Res. Commun. 109, 908–915 (1982).
Benkovic, S. J. & Wagner, C. R. Dihydrofolate reductase. In Protein Design and the Development of New Therapeutics and Vaccines (eds Hook, J. B., Poste, G. & Schatz, J.) Ch. 5, 237–249 (Springer US, 1990).
Santi, D. & Danenberg, P. Folates in pyrimidine nucleotide biosynthesis. Folates Pterins 1, 345–398 (1984).
Shane, B. Folate, vitamin B12 and vitamin B6. In Biochemical, Physiological, Molecular Aspects of Human Nutrition 2nd edn (ed. Stipanuk, M. H.) 693–732 (W.B. Saunders, 2006).
Shane, B. & Stokstad, E. L. Vitamin B12-folate interrelationships. Annu. Rev. Nutr. 5, 115–141 (1985).
Fenech, M. The role of folic acid and vitamin B12 in genomic stability of human cells. Mutat. Res. Fundam. Mol. Mech. Mutagen. 475, 57–67 (2001).
Jacques, P. F. et al. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N. Engl. J. Med. 340, 1449–1454 (1999).
Refsum, H., Ueland, P. M., Nygård, O. & Vollset, S. E. Homocysteine and cardiovascular disease. Annu. Rev. Med. 49, 31–62 (1998).
Kim, J., Kim, H., Roh, H. & Kwon, Y. Causes of hyperhomocysteinemia and its pathological significance. Arch. Pharm. Res. 41, 372–383 (2018).
Meiser, J. & Vazquez, A. Give it or take it: the flux of one-carbon in cancer cells. FEBS J. 283, 3695–3704 (2016).
Field, M. S. et al. Human mutations in methylenetetrahydrofolate dehydrogenase 1 impair nuclear de novo thymidylate biosynthesis. Proc. Natl. Acad. Sci. 112, 400–405 (2015).
Stead, L. M. et al. Is it time to reevaluate methyl balance in humans? Am. J. Clin. Nutr. 83, 5–10 (2006).
Prem veer Reddy, G. & Pardee, A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc. Natl. Acad. Sci. 77, 3312–3316 (1980).
Anderson, D. D. et al. Serine hydroxymethyltransferase anchors de novo thymidylate synthesis pathway to nuclear lamina for DNA synthesis. J. Biol. Chem. 287, 7051–7062 (2012).
Blount, B. C. et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc. Natl. Acad. Sci. 94, 3290–3295 (1997).
Field, M. S., Kamynina, E., Chon, J. & Stover, P. J. Nuclear folate metabolism. Annu. Rev. Nutr. 38, 219–243 (2018).
Kapiszewska, M., Kalemba, M., Wojciech, U. & Milewicz, T. Uracil misincorporation into DNA of leukocytes of young women with positive folate balance depends on plasma vitamin B12 concentrations and methylenetetrahydrofolate reductase polymorphisms. A pilot study. J. Nutr. Biochem. 16, 467–478 (2005).
An, S., Kumar, R., Sheets, E. D. & Benkovic, S. J. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science 320, 103–106 (2008).
Field, M. S., Anderson, D. D. & Stover, P. J. Mthfs is an essential gene in mice and a component of the purinosome. Front. Genet. 2, 36 (2011).
Froese, D. S. et al. Restricted role for methionine synthase reductase defined by subcellular localization. Mol. Genet. Metab. 94, 68–77 (2008).
Mellman, I., Willard, H. F. & Rosenberg, L. E. Cobalamin binding and cobalamin-dependent enzyme activity in normal and mutant human fibroblasts. J. Clin. Invest. 62, 952–960 (1978).
Rassin, D. K. & Gaull, G. E. Subcellular distribution of enzymes of transmethylation and transsulphuration in rat brain. J. Neurochem. 24, 969–978 (1975).
Khan, K. M. & Jialal, I. Folic Acid Deficiency (StatPearls Publishing, 2021).
Duthie, S. J. Epigenetic modifications and human pathologies: cancer and CVD. Proc. Nutr. Soc. 70, 47–56 (2011).
Grindulis, K. A. & McConkey, B. Does sulphasalazine cause folate deficiency in rheumatoid arthritis? Scand. J. Rheumatol. 14, 265–270 (1985).
Stover, P. J. Folate biochemical pathways and their regulation. In Folate in Health and Disease 2nd edn (ed. Bailey, L. B.) 49–74 (CRC Press Boca Raton, 2009).
Chen, J., Xu, X., Liu, A. & Ulrich, C. M. Folate and cancer: epidemiological perspective. In Folate in Health and Disease 2nd edn (ed. Bailey, L. B.) 205–233 (CRC Press Boca Raton, 2009).
Berger, S. H., Pittman, D. L. & Wyatt, M. D. Uracil in DNA: consequences for carcinogenesis and chemotherapy. Biochem. Pharmacol. 76, 697–706 (2008).
Blencowe, H. et al. Estimates of global and regional prevalence of neural tube defects for 2015: a systematic analysis. Ann. N. Y. Acad. Sci. 1414, 31–46 (2018).
Khoshnood, B. et al. Long term trends in prevalence of neural tube defects in Europe: population based study. BMJ 351, h5949 (2015).
Liu, J. et al. Prevalence and trend of neural tube defects in five counties in Shanxi province of Northern China, 2000 to 2014. Birth Defects Res. A Clin. Mol. Teratol. 106, 267–274 (2016).
MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 338, 131–137 (1991).
Czeizel, A. E. & Dudás, I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 327, 1832–1835 (1992).
McNulty, H. et al. Addressing optimal folate and related B-vitamin status through the lifecycle: health impacts and challenges. Proc. Nutr. Soc. 78, 449–462 (2019).
Martinez, H. et al. Global strategies for the prevention of neural tube defects through the improvement of folate status in women of reproductive age. Childs Nerv. Syst., 39, 1719–1736 (2023).
Martinez, H., Pachón, H., Kancherla, V. & Oakley, G. P. Jr Food fortification with folic acid for prevention of spina bifida and anencephaly: the need for a paradigm shift in evidence evaluation for policy-making. Am. J. Epidemiol. 190, 1972–1976 (2021).
Benavides-Lara, A. et al. Integrated surveillance strategy to support the prevention of neural tube defects through food fortification with folic acid: the experience of Costa Rica. Childs Nerv. Syst. 39, 1743–1754 (2023).
Saing, S. et al. Real-world cost effectiveness of mandatory folic acid fortification of bread-making flour in Australia. Appl. Health Econ. Health Policy 17, 243–254 (2019).
Hertrampf, E. & Cortés, F. Folic acid fortification of wheat flour: Chile. Nutr. Rev. 62, S44–S48 (2004).
Williams, L. J. et al. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology 66, 33–39 (2002).
De Wals, P. et al. Reduction in neural-tube defects after folic acid fortification in Canada. N. Engl. J. Med. 357, 135–142 (2007).
García-Fragoso, L., García-García, I. & Rivera, C. E. The use of folic acid for the prevention of birth defects in Puerto Rico. Ethn. Dis. 18, S2–168-171 (2008).
Ray, J. G. Folic acid food fortification in Canada. Nutr. Rev. 62, S35–S39 (2004).
Oakley, G. P. & Tulchinsky, T. H. Folic acid and vitamin B12 fortification of flour: a global basic food security requirement. Public Health Rev. 32, 284–295 (2010).
Santos, L. M. P. et al. Prevention of neural tube defects by the fortification of flour with folic acid: a population-based retrospective study in Brazil. Bull. World Health Organ. 94, 22–29 (2016).
Pardo, R. et al. Neural tube defects prevalence does not increase after modification of the folic acid fortification program in Chile. Birth Defects Res. 114, 259–266 (2022).
Rosenthal, J. et al. Neural tube defects in Latin America and the impact of fortification: a literature review. Public Health Nutr. 17, 537–550 (2014).
Cordero, A. et al. CDC grand rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb. Mortal. Wkly. Rep. 59, 980–984 (2010).
Castillo-Lancellotti, C., Tur, J. A. & Uauy, R. Impact of folic acid fortification of flour on neural tube defects: a systematic review. Public Health Nutr. 16, 901–911 (2013).
Atta, C. A. et al. Global birth prevalence of spina bifida by folic acid fortification status: a systematic review and meta-analysis. Am. J. Public Health 106, e24–e34 (2016).
Ebara, S. Nutritional role of folate. Congenital Anomalies 57, 138–141 (2017).
Grosse, S. D. et al. Retrospective assessment of cost savings from prevention: folic acid fortification and spina bifida in the US. Am. J. Prev. Med. 50, S74–S80 (2016).
Grosse, S. D., Waitzman, N. J., Romano, P. S. & Mulinare, J. Reevaluating the benefits of folic acid fortification in the United States: economic analysis, regulation, and public health. Am. J. Public Health 95, 1917–1922 (2005).
Fajardo, V. & Varela-Moreiras, G. Efficacy of adding folic acid to foods. Int. J. Vitam. Nutr. Res. 82, 177 (2012).
Williams, J. et al. Updated estimates of neural tube defects prevented by mandatory folic acid fortification - United States, 1995-2011. MMWR Morb. Mortal. Wkly. Rep. 64, 1–5 (2015).
Arth, A. et al. A 2015 global update on folic acid‐preventable spina bifida and anencephaly. Birth Defects Res. A Clin. Mol. Teratol. 106, 520–529 (2016).
Liu, S. et al. A comprehensive evaluation of food fortification with folic acid for the primary prevention of neural tube defects. BMC Pregnancy Childb 4, 1–10 (2004).
Wang, H. et al. Effectiveness of folic acid fortified flour for prevention of neural tube defects in a high risk region. Nutrients 8, 152 (2016).
Rodrigues, V. B., Silva, E. N. D. & Santos, M. L. P. Cost-effectiveness of mandatory folic acid fortification of flours in prevention of neural tube defects: A systematic review. PLoS One 16, e0258488 (2021).
Hoddinott, J. The investment case for folic acid fortification in developing countries. Ann. N. Y. Acad. Sci. 1414, 72–81 (2018).
Običan, S. G. et al. Folic acid in early pregnancy: a public health success story. FASEB J. 24, 4167–4174 (2010).
Osterhues, A., Ali, N. S. & Michels, K. B. The role of folic acid fortification in neural tube defects: a review. Crit. Rev. Food Sci. Nutr. 53, 1180–1190 (2013).
Wald, N. J. Folic acid and neural tube defects: discovery, debate and the need for policy change. J. Med. Screen. 29, 138–146 (2022).
Wald, N. J. & Hoffbrand, A. V. Mandatory UK folic acid fortification. Lancet 398, 1961–1962 (2021).
Shlobin, N. A., LoPresti, M. A., Du, R. Y. & Lam, S. Folate fortification and supplementation in prevention of folate-sensitive neural tube defects: a systematic review of policy. J. Neurosurg. Pediatr. 27, 294–310 (2020).
Moore, D. & Young, M. Folic acid fortification: both society and individuals benefit (Ministry for Primary Industries, 2019).
Ricks, D. J. et al. Peru’s national folic acid fortification program and its effect on neural tube defects in Lima. Pan Am. J. Public Health 32, 391–398 (2012).
Johnston, R. B. Will increasing folic acid in fortified grain products further reduce neural tube defects without causing harm?: consideration of the evidence. Pediatr. Res. 63, 2–8 (2008).
Jägerstad, M. Folic acid fortification prevents neural tube defects and may also reduce cancer risks. Acta Paediatr. 101, 1007–1012 (2012).
Morris, J. K. et al. Prevention of neural tube defects in Europe: a public health failure. Front. Pediatr. 9, 647038 (2021).
Kancherla, V. Countries with an immediate potential for primary prevention of spina bifida and anencephaly: Mandatory fortification of wheat flour with folic acid. Birth Defects Res. 110, 956–965 (2018).
Kancherla, V. et al. Mandatory food fortification with folic acid–Authors’ reply. Lancet Glob. Health 10, e1391–e1392 (2022).
Kancherla, V. et al. Preventing birth defects, saving lives, and promoting health equity: an urgent call to action for universal mandatory food fortification with folic acid. Lancet Glob. Health 10, e1053–e1057 (2022).
Kancherla, V. et al. A global update on the status of prevention of folic acid‐preventable spina bifida and anencephaly in year 2020: 30‐Year anniversary of gaining knowledge about folic acid’s prevention potential for neural tube defects. Birth Defects Res. 114, 1392–1403 (2022).
Oakley, G. P. Jr Failure to fortify staple foods with folic acid—still public health malpractice. Childs Nerv. Syst. 39, 1699–1701 (2023).
Crider, K. S., Bailey, L. B. & Berry, R. J. Folic acid food fortification—its history, effect, concerns, and future directions. Nutrients 3, 370–384 (2011).
Wald, N. J. & Oakley, G. P. Should folic acid fortification be mandatory? Yes. BMJ 334, 1252–1252 (2007).
Scientific Advisory Committee on Nutrition. Update on folic Acid (Scientific Advisory Committee on Nutrition, 2017).
Choi, J.-H. et al. Contemporary issues surrounding folic acid fortification initiatives. Prev. Nutr. Food Sci. 19, 247–260 (2014).
Ghotme, K. A. et al. Barriers and facilitators to the implementation of mandatory folate fortification as an evidence-based policy to prevent neural tube defects. Childs Nerv. Syst. 39, 1805–1812 (2023).
Berry, R. J. et al. Fortification of flour with folic acid. Food Nutr. Bull. 31, S22–S35 (2010).
Rabovskaja, V., Parkinson, B. & Goodall, S. The cost-effectiveness of mandatory folic acid fortification in Australia. J. Nutr. 143, 59–66 (2013).
Wald, N. J. Postscript to ‘Folic acid and neural tube defects: Discovery, debate and the need for policy change. J. Med. Screen. 29, 147–147 (2022).
McNulty, H. The B-Vitamins. In Sustainable Nutrition in a Changing World (eds Biesalski, H. K. et al) Ch. 29, 371–388 (Springer International Publishing AG, 2017).
Wald, N. J., Morris, J. K. & Blakemore, C. Urgent need for folic acid fortification of flour and grains: response to the 2019 UK Government’s public consultation. Arch. Dis. Child. 105, 6–9 (2020).
McNulty, H. & Scott, J. M. Intake and status of folate and related B-vitamins: considerations and challenges in achieving optimal status. Br. J. Nutr. 99, S48–S54 (2008).
Odewole, O. A. et al. Near-elimination of folate-deficiency anemia by mandatory folic acid fortification in older US adults: reasons for geographic and racial differences in stroke study 2003–2007. Am. J. Clin. Nutr. 98, 1042–1047 (2013).
Aune, D. et al. Dietary folate intake and the risk of 11 types of cancer: a case–control study in Uruguay. Ann. Oncol. 22, 444–451 (2011).
Wang, X. et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet 369, 1876–1882 (2007).
Wang, H. et al. Association of maternal plasma folate and cardiometabolic risk factors in pregnancy with elevated blood pressure of offspring in childhood. Am. J. Hypertens. 30, 532–540 (2017).
Wang, Y. et al. The effect of folic acid in patients with cardiovascular disease: a systematic review and meta-analysis. Medicine 98, e17095 (2019).
Huo, Y. et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA 313, 1325–1335 (2015).
Li, Y. et al. Folic acid supplementation and the risk of cardiovascular diseases: a meta‐analysis of randomized controlled trials. J. Am. Heart Assoc. 5, e003768 (2016).
Qin, X. et al. Homocysteine-lowering therapy with folic acid is effective in cardiovascular disease prevention in patients with kidney disease: a meta-analysis of randomized controlled trials. Clin. Nutr. 32, 722–727 (2013).
O’Connor, D. M. et al. Low folate predicts accelerated cognitive decline: 8-year follow-up of 3140 older adults in Ireland. Eur. J. Clin. Nutr. 76, 950–957 (2022).
Mason, J. B. Folate and colon cancer: dietary habits from the distant past coming home to roost. Am. J. Clin. Nutr. 114, 1–2 (2021).
Petch, S. et al. Folic acid fortification of flour to prevent neural tube defects in Europe–A position statement by the European Board and college of obstetrics and gynaecology (EBCOG). Eur. J. Obstet. Gynecol. Reprod. Biol. 279, 109–111 (2022).
Durga, J. et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 369, 208–216 (2007).
Kawakita, D. et al. Association between dietary folate intake and clinical outcome in head and neck squamous cell carcinoma. Ann. Oncol. 23, 186–192 (2012).
Xu, J. et al. Non-linear associations of serum and red blood cell folate with risk of cardiovascular and all-cause mortality in hypertensive adults. Hypertens. Res. 46, 1504–1515 (2023).
Zhang, K. et al. Association between dietary folate intake and cognitive impairment in older US adults: National Health and Nutrition Examination Survey. Arch. Gerontol. Geriatr. 109, 104946 (2023).
Stewart, C. P. et al. Antenatal micronutrient supplementation reduces metabolic syndrome in 6-to 8-year-old children in rural Nepal. J. Nutr. 139, 1575–1581 (2009).
Otsu, Y., Ae, R. & Kuwabara, M. Folate and cardiovascular disease. Hypertens. Res. 46, 1816–1818 (2023).
Wu, K. et al. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma. Am. J. Clin. Nutr. 90, 1623–1631 (2009).
Kim, Y.-I. Current status of folic acid supplementation on colorectal cancer prevention. Curr. Pharmacol. Rep. 2, 21–33 (2016).
Obeid, R., Koletzko, B. & Pietrzik, K. Critical evaluation of lowering the recommended dietary intake of folate. Clin. Nutr. 33, 252–259 (2014).
Pieroth, R., Paver, S., Day, S. & Lammersfeld, C. Folate and its impact on cancer risk. Curr. Nutr. Rep. 7, 70–84 (2018).
Bo, Y. et al. Association between folate and health outcomes: an umbrella review of meta-analyses. Front. Public Health 8, 550753 (2020).
Naninck, E. F., Stijger, P. C. & Brouwer-Brolsma, E. M. The importance of maternal folate status for brain development and function of offspring. Adv. Nutr. 10, 502–519 (2019).
Shulpekova, Y. et al. The concept of folic acid in health and disease. Molecules 26, 3731 (2021).
Tian, T. et al. Folic acid supplementation for stroke prevention in patients with cardiovascular disease. Am. J. Med. Sci. 354, 379–387 (2017).
Ismail, S., Eljazzar, S. & Ganji, V. Intended and unintended benefits of folic acid fortification—a narrative review. Foods 12, 1612 (2023).
Wondemagegn, A. T. & Afework, M. The association between folic acid supplementation and congenital heart defects: Systematic review and meta-analysis. SAGE Open Med. 10, https://doi.org/10.1177/20503121221081069 (2022).
Mason, J. B. Folate status and colorectal cancer risk: a 2016 update. Mol. Asp. Med. 53, 73–79 (2017).
Fu, H. et al. Folate intake and risk of pancreatic cancer: a systematic review and updated meta-analysis of epidemiological studies. Dig. Dis. Sci. 66, 2368–2379 (2021).
Jiang, Z., Qu, H., Chen, K. & Gao, Z. Beneficial effects of folic acid on inflammatory markers in the patients with metabolic syndrome: meta-analysis and meta-regression of data from 511 participants in 10 randomized controlled trials. Crit. Rev. Food Sci. Nutr. 64, 5450–5461 (2022).
Jones, P. et al. Folate and inflammation–links between folate and features of inflammatory conditions. J. Nutr. Intermed. Metab. 18, 100104 (2019).
Xu, X. et al. Association of folate intake with cardiovascular-disease mortality and all-cause mortality among people at high risk of cardiovascular-disease. Clin. Nutr. 41, 246–254 (2022).
Smith, A. D., Kim, Y.-I. & Refsum, H. Is folic acid good for everyone? Am. J. Clin. Nutr. 87, 517–533 (2008).
Smith, A. D. et al. Mandatory food fortification with folic acid. Lancet Glob. Health 10, e1389 (2022).
Maruvada, P. et al. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am. J. Clin. Nutr. 112, 1390–1403 (2020).
Kim, Y. Folate: a magic bullet or a double edged sword for colorectal cancer prevention? Gut 55, 1387–1389 (2006).
Looi, M.-K. Folic acid: The case to rethink the UK’s food fortification plans. BMJ 381, p1158 (2023).
House, S. H., Nichols, J. A. & Rae, S. Folates, folic acid and preconception care–a review. JRSM Open 12, h6198 (2021).
Field, M. S. & Stover, P. J. Safety of folic acid. Ann. N. Y. Acad. Sci. 1414, 59–71 (2018).
Colapinto, C. K. et al. Systematic review of adverse health outcomes associated with high serum or red blood cell folate concentrations. J. Public Health 38, e84–e97 (2016).
Ulrich, C. M. & Potter, J. D. Folate supplementation: too much of a good thing? Cancer Epidemiol. Biomark. Prev. 15, 189–193 (2006).
Boyles, A. L., Yetley, E. A., Thayer, K. A. & Coates, P. M. Safe use of high intakes of folic acid: research challenges and paths forward. Nutr. Rev. 74, 469–474 (2016).
Scientific Committee on Food. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Folate, https://ec.europa.eu/food/fs/sc/scf/out80e_en.pdf (2000).
Alnabbat, K. I. et al. High dietary folic acid intake is associated with genomic instability in peripheral lymphocytes of healthy adults. Nutrients 14, 3944 (2022).
Allen, L. H. Pros and cons of increasing folic acid and vitamin B12 intake by fortification. In Nestle Nutrition Institute workshop series 70, 175–183 (Karger Publishers, 2012).
Quinlivan, E. P. In vitamin B12 deficiency, higher serum folate is associated with increased homocysteine and methylmalonic acid concentrations. Proc. Natl. Acad. Sci. 105, E7–E7 (2008).
EFSA Panel on Nutrition Novel Foods and Food Allergens. Scientific opinion on the tolerable upper intake level for folate. EFSA J. 21, e08353 (2023).
Fardous, A. M. & Heydari, A. R. Uncovering the hidden dangers and molecular mechanisms of excess folate: a narrative review. Nutrients 15, 4699 (2023).
Liu, M. et al. Relationship of several serum folate forms with the risk of mortality: a prospective cohort study. Clin. Nutr. 40, 4255–4262 (2021).
Cortés, F. et al. Wheat flour fortification with folic acid: changes in neural tube defects rates in Chile. Am. J. Med. Genet. A 158a, 1885–1890 (2012).
Morris, M. S., Jacques, P. F., Rosenberg, I. H. & Selhub, J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am. J. Clin. Nutr. 91, 1733–1744 (2010).
Mills, J. L. Fortification of foods with folic acid—how much is enough? N. Engl. J. Med. 342, 1442–1445 (2000).
Mills, J. L. et al. Low vitamin B-12 concentrations in patients without anemia: the effect of folic acid fortification of grain. Am. J. Clin. Nutr. 77, 1474–1477 (2003).
Hirsch, S. et al. The Chilean flour folic acid fortification program reduces serum homocysteine levels and masks vitamin B-12 deficiency in elderly people. J. Nutr. 132, 289–291 (2002).
Wyckoff, K. F. & Ganji, V. Proportion of individuals with low serum vitamin B-12 concentrations without macrocytosis is higher in the post–folic acid fortification period than in the pre–folic acid fortification period. Am. J. Clin. Nutr. 86, 1187–1192 (2007).
Qi, Y. P. et al. The prevalence of low serum vitamin B-12 status in the absence of anemia or macrocytosis did not increase among older US adults after mandatory folic acid fortification. J. Nutr. 144, 170–176 (2014).
Morris, M. S., Selhub, J. & Jacques, P. F. Vitamin B‐12 and folate status in relation to decline in scores on the Mini‐Mental State Examination in the Framingham Heart Study. J. Am. Geriatr. Soc. 60, 1457–1464 (2012).
Molloy, A. M. Adverse effects on cognition caused by combined low vitamin B-12 and high folate status—we must do better than a definite maybe! Am. J. Clin. Nutr. 112, 1422–1423 (2020).
EFSA. Tolerable Upper Intake Levels for Vitamins and Minerals (Publications Office 2006).
Moore, E. M. et al. Among vitamin B12 deficient older people, high folate levels are associated with worse cognitive function: combined data from three cohorts. J. Alzheimer’s Dis. 39, 661–668 (2014).
Doets, E. L. et al. Interactions between plasma concentrations of folate and markers of vitamin B12 status with cognitive performance in elderly people not exposed to folic acid fortification: the Hordaland Health Study. Br. J. Nutr. 111, 1085–1095 (2014).
Carter, B. et al. Plasma methylmalonic acid concentration in folic acid–supplemented depressed patients with low or marginal vitamin B-12: a randomized trial. J. Nutr. 151, 3738–3745 (2021).
Smith, A. D. Folic acid fortification: the good, the bad, and the puzzle of vitamin B-12. Am. J. Clin. Nutr. 85, 3–5 (2007).
Berry, R. J., Carter, H. K. & Yang, Q. Cognitive impairment in older Americans in the age of folic acid fortification. Am. J. Clin. Nutr. 86, 265–267 (2007).
Reynolds, E. What is the safe upper intake level of folic acid for the nervous system? Implications for folic acid fortification policies. Eur. J. Clin. Nutr. 70, 537–540 (2016).
Reynolds, E. H. The risks of folic acid to the nervous system in vitamin B12 deficiency: rediscovered in the era of folic acid fortification policies. J. Neurol. Neurosurg. Psychiatry 88, 1097–1098 (2017).
Selhub, J. et al. Perspective: the high-folate–low-vitamin B-12 interaction is a novel cause of vitamin B-12 depletion with a specific etiology—a hypothesis. Adv. Nutr. 13, 16–33 (2022).
Selhub, J. & Rosenberg, I. H. Excessive folic acid intake and relation to adverse health outcome. Biochimie 126, 71–78 (2016).
Bailey, R. L. et al. High folic acid or folate combined with low vitamin B-12 status: potential but inconsistent association with cognitive function in a nationally representative cross-sectional sample of US older adults participating in the NHANES. Am. J. Clin. Nutr. 112, 1547–1557 (2020).
Clarke, R. et al. Folate and vitamin B12 status in relation to cognitive impairment and anaemia in the setting of voluntary fortification in the UK. Br. J. Nutr. 100, 1054–1059 (2008).
Ding, Z. et al. Non-linear association between folate/vitamin B12 status and cognitive function in older adults. Nutrients 14, 2443 (2022).
Deng, Y., Wang, D., Wang, K. & Kwok, T. High serum folate is associated with brain atrophy in older diabetic people with vitamin B12 deficiency. J. Nutr. Health Aging 21, 1065–1071 (2017).
Morris, M. S., Jacques, P. F., Rosenberg, I. H. & Selhub, J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am. J. Clin. Nutr. 85, 193–200 (2007).
Miller, J. W. et al. Metabolic evidence of vitamin B-12 deficiency, including high homocysteine and methylmalonic acid and low holotranscobalamin, is more pronounced in older adults with elevated plasma folate. Am. J. Clin. Nutr. 90, 1586–1592 (2009).
Lökk, J. Association of vitamin B12, folate, homocysteine and cognition in the elderly. Food Nutr. Res. 47, 132–138 (2003).
Osterhues, A., Holzgreve, W. & Michels, K. B. Shall we put the world on folate? Lancet 374, 959–961 (2009).
Kim, Y.-I. Folate and cancer: a tale of Dr. Jekyll and Mr. Hyde? Am. J. Clin. Nutr. 107, 139–142 (2018).
Mason, J. B. Folate, cancer risk, and the Greek god, Proteus: a tale of two chameleons. Nutr. Rev. 67, 206–212 (2009).
Tomita, L. Y. Folate and cancer: is there any association? J. Inborn Errors Metab. Screen. 4, https://doi.org/10.1177/2326409816661357 (2016).
Mackerras, D., Tan, J. & Larter, C. Folic acid, selected cancers and all-cause mortality: a meta-analysis. Int. Food Risk Anal. J. 4, https://doi.org/10.5772/58396 (2014).
Vollset, S. E. et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet 381, 1029–1036 (2013).
Kotsopoulos, J., Kim, Y.-I. & Narod, S. A. Folate and breast cancer: what about high-risk women? Cancer Causes Control 23, 1405–1420 (2012).
Drake, B. F. & Colditz, G. A. Assessing cancer prevention studies—a matter of time. JAMA 302, 2152–2153 (2009).
Miller, J. W. & Ulrich, C. M. Folic acid and cancer—where are we today? Lancet 381, 974–976 (2013).
Ferrazzi, E., Tiso, G. & Di Martino, D. Folic acid versus 5-methyl tetrahydrofolate supplementation in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 253, 312–319 (2020).
Datta, M. & Vitolins, M. Z. Food fortification and supplement use-are there health implications? Crit. Rev. Food Sci. Nutr. 56, 2149–2159 (2016).
Mills, J. L. & Dimopoulos, A. Folic acid fortification for Europe? BMJ 351, https://doi.org/10.1136/bmj.h6198 (2015).
Lee, J. E. & Chan, A. T. Fruit, vegetables, and folate: cultivating the evidence for cancer prevention. Gastroenterology 141, 16–20 (2011).
Ebbing, M. et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 302, 2119–2126 (2009).
Moazzen, S. et al. Staple food fortification with folic acid and iron and gastrointestinal cancers: critical appraisal of long-term national fortification. Nutr. Cancer 73, 1534–1538 (2021).
Asemi, Z. et al. Effects of long-term folate supplementation on metabolic status and regression of cervical intraepithelial neoplasia: a randomized, double-blind, placebo-controlled trial. Nutr 32, 681–686 (2016).
Tu, H. et al. Is folic acid safe for non–muscle-invasive bladder cancer patients? An evidence-based cohort study. Am. J. Clin. Nutr. 107, 208–216 (2018).
Jennings, B. A. & Willis, G. How folate metabolism affects colorectal cancer development and treatment; a story of heterogeneity and pleiotropy. Cancer Lett 356, 224–230 (2015).
Figueiredo, J. C. et al. Folate‐genetics and colorectal neoplasia: what we know and need to know next. Mol. Nutr. Food Res. 57, 607–627 (2013).
Figueiredo, J. C. et al. Folic acid and prevention of colorectal adenomas: a combined analysis of randomized clinical trials. Int. J. Cancer 129, 192–203 (2011).
Cole, B. F. et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 297, 2351–2359 (2007).
Mason, J. B. Folate consumption and cancer risk: a confirmation and some reassurance, but we’re not out of the woods quite yet. Am. J. Clin. Nutr. 94, 965–966 (2011).
Mason, J. B. et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol. Biomark. Prev. 16, 1325–1329 (2007).
Luebeck, E. G. et al. Does folic acid supplementation prevent or promote colorectal cancer? Results from model-based predictions. Cancer Epidemiol. Biomark. Prev. 17, 1360–1367 (2008).
Hubner, R. A., Houlston, R. D. & Muir, K. R. Should folic acid fortification be mandatory? No. BMJ 334, 1253–1253 (2007).
Kim, D.-H. et al. Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. Cancer Causes Control 21, 1919–1930 (2010).
Kim, Y.-I. Role of folate in colon cancer development and progression. J. Nutr. 133, 3731S–3739S (2003).
Kim, Y. I. Folate and colorectal cancer: An evidence‐based critical review. Mol. Nutr. Food Res. 51, 267–292 (2007).
Kherbek, H. et al. The relationship between folic acid and colorectal cancer; a literature review. Ann. Med. Surg. 80, 104170 (2022).
Gylling, B. et al. Low folate levels are associated with reduced risk of colorectal cancer in a population with low folate status. Cancer Epidemiol. Biomark. Prev. 23, 2136–2144 (2014).
Protiva, P. et al. Altered folate availability modifies the molecular environment of the human colorectum: implications for colorectal carcinogenesis. Cancer Prev. Res. 4, 530–543 (2011).
Haas, C. B. et al. Interactions between folate intake and genetic predictors of gene expression levels associated with colorectal cancer risk. Sci. Rep. 12, 18852 (2022).
Fife, J., Raniga, S., Hider, P. & Frizelle, F. Folic acid supplementation and colorectal cancer risk: a meta‐analysis. Colorectal Dis 13, 132–137 (2011).
Geijsen, A. J. et al. Circulating folate and folic acid concentrations: associations with colorectal cancer recurrence and survival. JNCI Cancer Spectr 4, pkaa051 (2020).
Gigic, B. et al. Cohort profile: biomarkers related to folate-dependent one-carbon metabolism in colorectal cancer recurrence and survival–the FOCUS Consortium. BMJ Open 12, e062930 (2022).
Moazzen, S. et al. Folic acid intake and folate status and colorectal cancer risk: a systematic review and meta-analysis. Clin. Nutr. 37, 1926–1934 (2018).
Stevens, V. L. et al. High levels of folate from supplements and fortification are not associated with increased risk of colorectal cancer. Gastroenterology 141, 98–105.e101 (2011).
O’Reilly, S. L. et al. Folic acid supplementation in postpolypectomy patients in a randomized controlled trial increases tissue folate concentrations and reduces aberrant DNA biomarkers in colonic tissues adjacent to the former polyp site. J. Nutr. 146, 933–939 (2016).
Keum, N. & Giovannucci, E. L. Folic acid fortification and colorectal cancer risk. Am. J. Prev. Med. 46, S65–S72 (2014).
Qin, T. et al. Folic acid supplements and colorectal cancer risk: meta-analysis of randomized controlled trials. Sci. Rep. 5, 12044 (2015).
Burr, N. E., Hull, M. A. & Subramanian, V. Folic acid supplementation may reduce colorectal cancer risk in patients with inflammatory bowel disease. J. Clin. Gastroenterol. 51, 247–253 (2017).
Lee, J. E. et al. Folate intake and risk of colorectal cancer and adenoma: modification by time. Am. J. Clin. Nutr. 93, 817–825 (2011).
Sanjoaquin, M. A. et al. Folate intake and colorectal cancer risk: a meta‐analytical approach. Int. J. Cancer 113, 825–828 (2005).
Kennedy, D. A. et al. Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis. Cancer Epidemiol. 35, 2–10 (2011).
Hubner, R. & Houlston, R. Folate and colorectal cancer prevention. Br. J. Cancer 100, 233–239 (2009).
Stolzenberg-Solomon, R. Z. et al. Folate intake, alcohol use, and postmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 83, 895–904 (2006).
Chen, P. et al. Higher dietary folate intake reduces the breast cancer risk: a systematic review and meta-analysis. Br. J. Cancer 110, 2327–2338 (2014).
Kim, S. J. et al. Folic acid supplement use and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a case–control study. Breast Cancer Res. Treat. 174, 741–748 (2019).
Ren, X. et al. Association of folate intake and plasma folate level with the risk of breast cancer: A dose-response meta-analysis of observational studies. Aging 12, 21355 (2020).
Zhang, Y.-F. et al. Folate intake and the risk of breast cancer: a dose-response meta-analysis of prospective studies. PLoS One 9, e100044 (2014).
Wien, T. N. et al. Cancer risk with folic acid supplements: a systematic review and meta-analysis. BMJ Open 2, e000653 (2012).
De Vogel, S. et al. Serum folate and vitamin B12 concentrations in relation to prostate cancer risk—a Norwegian population-based nested case–control study of 3000 cases and 3000 controls within the JANUS cohort. Int. J. Epidemiol. 42, 201–210 (2013).
Rycyna, K. J., Bacich, D. J. & O’Keefe, D. S. Opposing roles of folate in prostate cancer. Urology 82, 1197–1203 (2013).
Figueiredo, J. C. et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J. Natl. Cancer Inst. 101, 432–435 (2009).
Price, A. J. et al. Circulating folate and vitamin B12 and risk of prostate cancer: a collaborative analysis of individual participant data from six cohorts including 6875 cases and 8104 controls. Eur. Urol. 70, 941–951 (2016).
Downer, M. K., Van Blarigan, E. L., Peisch, S. F. & Stampfer, M. J. Should we fear folate? Eur. Urol. 70, 952–953 (2016).
Håberg, S. E. et al. Folic acid supplements in pregnancy and early childhood respiratory health. Arch. Dis. Child. 94, 180–184 (2009).
Parr, C. L. et al. Maternal folate intake during pregnancy and childhood asthma in a population-based cohort. Am. J. Respir. Crit. Care Med. 195, 221–228 (2017).
Alfonso, V. H., Bandoli, G., von Ehrenstein, O. & Ritz, B. Early folic acid supplement initiation and risk of adverse early childhood respiratory health: a population-based study. Matern. Child Health J. 22, 111–119 (2018).
Yang, L. et al. High dose of maternal folic acid supplementation is associated to infant asthma. Food Chem. Toxicol. 75, 88–93 (2015).
Yang, F. et al. Relationship between maternal folic acid supplementation during pregnancy and risk of childhood asthma: systematic review and dose-response meta-analysis. Front. Pediatr. 10, 1000532 (2022).
Best, K. P. et al. Maternal late-pregnancy serum unmetabolized folic acid concentrations are not associated with infant allergic disease: a prospective cohort study. J. Nutr. 151, 1553–1560 (2021).
Molloy, A. M. & Mills, J. L. Folic acid and infant allergy: Avoiding rash judgments. J. Nutr. 151, 1367–1368 (2021).
Bekkers, M. B. et al. Maternal use of folic acid supplements during pregnancy, and childhood respiratory health and atopy. Eur. Respir. J. 39, 1468–1474 (2012).
Wang, T. et al. Is folate status a risk factor for asthma or other allergic diseases? Allergy Asthma Immunol. Res. 7, 538–546 (2015).
Trivedi, M. K. et al. Folic acid in pregnancy and childhood asthma: a US cohort. Clin. Pediatr. 57, 421–427 (2018).
Whitrow, M. J., Moore, V. M., Rumbold, A. R. & Davies, M. J. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am. J. Epidemiol. 170, 1486–1493 (2009).
McGowan, E. C. et al. Association between folate metabolites and the development of food allergy in children. J. Allergy Clin. Immunol. Pract. 8, 132–140.e135 (2020).
Liu, J. et al. Periconceptional folic acid supplementation and risk of parent-reported asthma in children at 4–6 years of age. ERJ Open Res. 6; https://doi.org/10.1183/23120541.00250-2019 (2020).
Adgent, M. A. et al. Periconceptional folic acid supplementation and child asthma: a Right From the Start follow-up study. J. Matern. Fetal Neonatal Med. 35, 10232–10238 (2022).
Moser, S. S. et al. High dose folic acid during pregnancy and the risk of autism; The birth order bias: A nested case-control study. Reprod. Toxicol. 89, 173–177 (2019).
DeVilbiss, E. A., Gardner, R. M., Newschaffer, C. J. & Lee, B. K. Maternal folate status as a risk factor for autism spectrum disorders: a review of existing evidence. Br. J. Nutr. 114, 663–672 (2015).
DeVilbiss, E. A. et al. Antenatal nutritional supplementation and autism spectrum disorders in the Stockholm youth cohort: population based cohort study. BMJ 359, j4273 (2017).
Raghavan, R. et al. A prospective birth cohort study on cord blood folate subtypes and risk of autism spectrum disorder. Am. J. Clin. Nutr. 112, 1304–1317 (2020).
Levine, S. Z. et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry 75, 176–184 (2018).
Egorova, O. et al. Maternal blood folate status during early pregnancy and occurrence of autism spectrum disorder in offspring: a study of 62 serum biomarkers. Mol. Autism. 11, 1–15 (2020).
McNulty, H. et al. Effect of continued folic acid supplementation beyond the first trimester of pregnancy on cognitive performance in the child: a follow-up study from a randomized controlled trial (FASSTT Offspring Trial). BMC Med. 17, 1–11 (2019).
Caffrey, A. et al. Effects of maternal folic acid supplementation during the second and third trimesters of pregnancy on neurocognitive development in the child: an 11-year follow-up from a randomised controlled trial. BMC Med. 19, 1–13 (2021).
Valera-Gran, D. et al. Effect of maternal high dosages of folic acid supplements on neurocognitive development in children at 4–5 y of age: the prospective birth cohort Infancia y Medio Ambiente (INMA) study. Am. J. Clin. Nutr. 106, 878–887 (2017).
Murray, L. K., Smith, M. J. & Jadavji, N. M. Maternal oversupplementation with folic acid and its impact on neurodevelopment of offspring. Nutr. Rev. 76, 708–721 (2018).
Obeid, R., Holzgreve, W. & Pietrzik, K. Is 5-methyltetrahydrofolate an alternative to folic acid for the prevention of neural tube defects? J. Perinat. Med. 41, 469–483 (2013).
Kelly, D., O’Dowd, T. & Reulbach, U. Use of folic acid supplements and risk of cleft lip and palate in infants: a population-based cohort study. Br. J. Gen. Pract. 62, e466–e472 (2012).
Rozendaal, A. M. et al. Periconceptional folic acid associated with an increased risk of oral clefts relative to non-folate related malformations in the Northern Netherlands: a population based case-control study. Eur. J. Epidemiol. 28, 875–887 (2013).
Cheng, Z., Gu, R., Lian, Z. & Gu, H. F. Evaluation of the association between maternal folic acid supplementation and the risk of congenital heart disease: a systematic review and meta-analysis. Nutr. J. 21, 20 (2022).
Silva, C., Keating, E. & Pinto, E. The impact of folic acid supplementation on gestational and long term health: Critical temporal windows, benefits and risks. Porto Biomed. J. 2, 315–332 (2017).
Bailey, S. W. & Ayling, J. E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl. Acad. Sci. 106, 15424–15429 (2009).
Plumptre, L. et al. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am. J. Clin. Nutr. 102, 848–857 (2015).
Sweeney, M. R. et al. Persistent circulating unmetabolised folic acid in a setting of liberal voluntary folic acid fortification. Implications for further mandatory fortification? BMC Public Health 9, 1–7 (2009).
Obeid, R. Serum unmetabolized folic acid: the straw that broke dihydrofolate reductase’s back? J. Nutr. 145, 387–390 (2015).
Palchetti, C. Z. et al. Association between serum unmetabolized folic acid concentrations and folic acid from fortified foods. J. Am. Coll. Nutr. 36, 572–578 (2017).
Page, R. et al. Total folate and unmetabolized folic acid in the breast milk of a cross-section of Canadian women. Am. J. Clin. Nutr. 105, 1101–1109 (2017).
Cho, E. et al. Unmetabolized folic acid in prediagnostic plasma and the risk for colorectal cancer. J. Natl. Cancer Inst. 107, djv260 (2015).
Hefni, M. E., Witthöft, C. M. & Moazzami, A. A. Plasma metabolite profiles in healthy women differ after intervention with supplemental folic acid v. folate-rich foods. J. Nutr. Sci. 7, e32 (2018).
Troen, A. M. et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J. Nutr. 136, 189–194 (2006).
Hu, J., Wang, B. & Sahyoun, N. R. Application of the key events dose-response framework to folate metabolism. Crit. Rev. Food Sci. Nutr. 56, 1325–1333 (2016).
Williamson, J. M. et al. High folate, perturbed one-carbon metabolism and gestational diabetes mellitus. Nutrients 14, 3930 (2022).
Page, R., Wong, A., Arbuckle, T. E. & MacFarlane, A. J. The MTHFR 677C> T polymorphism is associated with unmetabolized folic acid in breast milk in a cohort of Canadian women. Am. J. Clin. Nutr 110, 401–409 (2019).
Berry, R. J. Lack of historical evidence to support folic acid exacerbation of the neuropathy caused by vitamin B12 deficiency. Am. J. Clin. Nutr. 110, 554–561 (2019).
Committee on toxicity of chemicals in food consumer products and the environment. Folic acid – statement on the tolerable upper level (TUL) - Lay summary (The Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment, 2019).
Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline (National Academies Press, 1998).
FDA. Food additives permitted for direct addition to food for human consumption; folic acid. Final rule. Fed. Reg. 81, 22176–22183 (2016).
National Toxicology Program. NTP monograph: identifying research needs for assessing safe use of high intakes of folic acid, https://ntp.niehs.nih.gov/sites/default/files/ntp/ohat/folicacid/final_monograph_508.pdf (2015).
Porter, K. et al. Causes, consequences and public health implications of low B-vitamin status in ageing. Nutrients 8, 725 (2016).
Kar, A. et al. Mandatory food fortification with folic acid. Lancet Glob. Health 10, e1390 (2022).
Johnson, M. A. If high folic acid aggravates vitamin B12 deficiency what should be done about it? Nutr. Rev. 65, 451–458 (2007).
Molloy, A. M. et al. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics 123, 917–923 (2009).
Wang, Z.-P., Shang, X.-X. & Zhao, Z.-T. Low maternal vitamin B12 is a risk factor for neural tube defects: a meta-analysis. J. Matern. Fetal Neonatal Med. 25, 389–394 (2012).
Gibson, T. M. et al. Pre-and postfortification intake of folate and risk of colorectal cancer in a large prospective cohort study in the United States. Am. J. Clin. Nutr. 94, 1053–1062 (2011).
van der Pols, J. C., Baade, P. & Spencer, L. B. Colorectal cancer incidence in Australia before and after mandatory fortification of bread flour with folic acid. Public Health Nutr. 24, 1989–1992 (2021).
Wang, F. et al. Association of folate intake and colorectal cancer risk in the postfortification era in US women. Am. J. Clin. Nutr. 114, 49–58 (2021).
Fu, H. et al. Folate intake and risk of colorectal cancer: a systematic review and up-to-date meta-analysis of prospective studies. Eur. J. Cancer Prev. 32, 103–112 (2023).
Crider, K. S. et al. Prenatal folic acid and risk of asthma in children: a systematic review and meta-analysis. Am. J. Clin. Nutr. 98, 1272–1281 (2013).
Wang, M., Li, K., Zhao, D. & Li, L. The association between maternal use of folic acid supplements during pregnancy and risk of autism spectrum disorders in children: a meta-analysis. Mol. Autism 8, 1–4 (2017).
Liu, X. et al. Prenatal folic acid supplements and offspring’s autism spectrum disorder: a meta-analysis and meta-regression. J. Autism Dev. Disord. 52, 522–539 (2022).
Perera, N., Rudland, V. L., Simmons, D. & Price, S. A. Folate supplementation in women with pre-existing diabetes. Nutrients 15, 1879 (2023).
Sweeney, M. R., McPartlin, J. & Scott, J. Folic acid fortification and public health: report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health 7, 1–7 (2007).
Murphy, M. S. et al. Impact of high-dose folic acid supplementation in pregnancy on biomarkers of folate status and 1-carbon metabolism: An ancillary study of the Folic Acid Clinical Trial (FACT). Am. J. Clin. Nutr. 113, 1361–1371 (2021).
Koenig, K. L. et al. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate and risk of breast cancer: A nested case-control study. Eur. J. Clin. Nutr. 74, 1306–1315 (2020).
Food Standards Australia New Zealand. L-methylfolate, calcium as a permitted form of folate (Food Standards Australia New Zealand, 2008).
Patanwala, I. et al. Folic acid handling by the human gut: implications for food fortification and supplementation. Am. J. Clin. Nutr. 100, 593–599 (2014).
EFSA Panel on Nutrition Novel Foods and Food Allergens. Safety of monosodium salt of l-5-methyltetrahydrofolic acid as a novel food pursuant to Regulation (EU) 2015/2283 and the bioavailability of folate from this source in the context of Directive 2002/46/EC, Regulation (EU) No 609/2013 and Regulation (EC) No 1925/2006. EFSA J. 21, e8417 (2023).
Troesch, B. et al. Suitability and safety of L-5-methyltetrahydrofolate as a folate source in infant formula: A randomized-controlled trial. PloS One 14, e0216790 (2019).
Niederberger, K. et al. Safety evaluation of calcium L-methylfolate. Toxicol. Rep. 6, 1018–1030 (2019).
Menezo, Y., Elder, K., Clement, A. & Clement, P. Folic acid, folinic acid, 5 methyl tetrahydrofolate supplementation for mutations that affect epigenesis through the folate and one-carbon cycles. Biomolecules 12, 197 (2022).
Greenberg, J. A., Bell, S. J., Guan, Y. & Yu, Y.-H. Folic acid supplementation and pregnancy: more than just neural tube defect prevention. Rev. Obstet. Gynaecol. 4, 52–59 (2011).
Hoekstra, J. et al. Integrated risk–benefit analyses: method development with folic acid as example. Food Chem. Toxicol. 46, 893–909 (2008).
Striepen, B. et al. Gene transfer in the evolution of parasite nucleotide biosynthesis. Proc. Natl. Acad. Sci. 101, 3154–3159 (2004).
Watkins, W. M., Mberu, E. K., Winstanley, P. A. & Plowe, C. V. The efficacy of antifolate antimalarial combinations in Africa: a predictive model based on pharmacodynamic and pharmacokinetic analyses. Trends Parasitol. 13, 459–464 (1997).
Fernández-Villa, D., Aguilar, M. R. & Rojo, L. Folic acid antagonists: antimicrobial and immunomodulating mechanisms and applications. Int. J. Mol. Sci. 20, 4996 (2019).
Anderson, A. C. Targeting DHFR in parasitic protozoa. Drug Discov. Today 10, 121–128 (2005).
Al-Rashood, S. T. et al. Synthesis, biological evaluation and molecular modeling study of 2-(1, 3, 4-thiadiazolyl-thio and 4-methyl-thiazolyl-thio)-quinazolin-4-ones as a new class of DHFR inhibitors. Bioorg. Med. Chem. Lett. 24, 4557–4567 (2014).
Wang, M. et al. Synthesis and antiproliferative activity of a series of novel 6-substituted pyrido[3,2-d]pyrimidines as potential nonclassical lipophilic antifolates targeting dihydrofolate reductase. Eur. J. Med. Chem. 128, 88–97 (2017).
Matherly, L. H. & Hou, Z. Structure and function of the reduced folate carrier a paradigm of a major facilitator superfamily mammalian nutrient transporter. Vitam. Horm. 79, 145–184 (2008).
Tanyi, J. L. et al. A phase III study of pafolacianine injection (OTL38) for intraoperative imaging of folate receptor–positive ovarian cancer (Study 006). J. Clin. Oncol. 41, 276–284 (2023).
Moore, K. N. et al. Mirvetuximab soravtansine in FRα-positive, platinum-resistant ovarian cancer. N. Engl. J. Med. 389, 2162–2174 (2023).
Coleman, R. L. et al. Mirvetuximab soravtansine in folate receptor alpha (FRα)–high platinum-resistant ovarian cancer: final overall survival and post hoc sequence of therapy subgroup results from the SORAYA trial. Int. J. Gynecol. Cancer, ijgc-2024-005401; https://doi.org/10.1136/ijgc-2024-005401 (2024).
Heo, Y.-A. Mirvetuximab soravtansine: first approval. Drugs 83, 265–273 (2023).
Shimizu, T. et al. First-in-human phase 1 study of morab-202, an antibody–drug conjugate comprising farletuzumab linked to eribulin mesylate, in patients with folate receptor-Α–positive advanced solid tumors. Clin. Cancer Res. 27, 3905–3915 (2021).
Gupta, A. et al. Vaccination with folate receptor-alpha peptides in patients with ovarian cancer following response to platinum-based therapy: A randomized, multicenter clinical trial. Gynecol. Oncol. 189, 90–97 (2024).
Spicer, J. et al. Safety and anti-tumour activity of the IgE antibody MOv18 in patients with advanced solid tumours expressing folate receptor-alpha: a phase I trial. Nat. Commun. 14, 4180 (2023).
Panda, P. K. et al. Efficacy of oral folinic acid supplementation in children with autism spectrum disorder: a randomized double-blind, placebo-controlled trial. Eur. J. Pediatr. 183, 4827–4835 (2024).
Renard, E. et al. Folinic acid improves the score of Autism in the EFFET placebo-controlled randomized trial. Biochimie 173, 57–61 (2020).
Batebi, N. et al. Folinic acid as adjunctive therapy in treatment of inappropriate speech in children with autism: a double-blind and placebo-controlled randomized trial. Child Psychiatry Hum. Dev. 52, 928–938 (2021).
Bao, H. et al. Combined use of amlodipine and folic acid are significantly more efficacious than amlodipine alone in lowering plasma homocysteine and blood pressure among hypertensive patients with hyperhomocysteinemia and intolerance to ACEI: A multicenter, randomized, double‐blind, parallel‐controlled clinical trial. J. Clin. Hypertens. 25, 689–699 (2023).
Zhang, S. et al. Effects of individualized administration of folic acid on prothrombotic state and vascular endothelial function with H-type hypertension: a double-blinded, randomized clinical cohort study. Med 101, e28628 (2022).
Asadi, M. et al. Effect of folic acid on the sexual function of postmenopausal women: a triple-blind randomized controlled trial. J. Sex. Med. 20, 1180–1187 (2023).
Chen, H. et al. Effects of folic acid and vitamin B12 supplementation on cognitive impairment and inflammation in patients with Alzheimer’s disease: A randomized, single-blinded, placebo-controlled trial. J. Prev. Alzheimers Dis. 8, 249–256 (2021).
Kvestad, I. et al. Vitamin B12, folate, and cognition in 6-to 9-year-olds: a randomized controlled trial. Pediatrics 145, e20192316 (2020).
Van Soest, A. P., Van de Rest, O., Witkamp, R. F. & De Groot, L. C. Positive effects of folic acid supplementation on cognitive aging are dependent on ω-3 fatty acid status: a post hoc analysis of the FACIT trial. Am. J. Clin. Nutr. 113, 801–809 (2021).
Li, M. et al. Effect of folic acid combined with docosahexaenoic acid intervention on mild cognitive impairment in elderly: a randomized double-blind, placebo-controlled trial. Eur. J. Nutr. 60, 1795–1808 (2021).
Araghi, S. O. et al. Long-term effects of folic acid and vitamin-B12 supplementation on fracture risk and cardiovascular disease: Extended follow-up of the B-PROOF trial. Clin. Nutr. 40, 1199–1206 (2021).
Satapathy, S. et al. Folic acid and vitamin B12 supplementation in subjects with type 2 diabetes mellitus: A multi-arm randomized controlled clinical trial. Complement. Ther. Med. 53, 102526 (2020).
Schisterman, E. F. et al. Effect of folic acid and zinc supplementation in men on semen quality and live birth among couples undergoing infertility treatment: a randomized clinical trial. JAMA 323, 35–48 (2020).
Jenkins, T. et al. The impact of zinc and folic acid supplementation on sperm DNA methylation: results from the folic acid and zinc supplementation randomized clinical trial (FAZST). Fertil. Steril. 117, 75–85 (2022).
Huang, W. J., Lu, X. L., Li, J. T. & Zhang, J. M. Effects of folic acid on oligozoospermia with MTHFR polymorphisms in term of seminal parameters, DNA fragmentation, and live birth rate: a double‐blind, randomized, placebo‐controlled trial. Andrology 8, 110–116 (2020).
Türkomp. Turkish food composition database https://turkomp.gov.tr/main (2017).
Zhao, M., Lin, Y. & Chen, H. Improving nutritional quality of rice for human health. Theor. Appl. Genet. 133, 1397–1413 (2020).
Nystrom, L. et al. Phytochemicals and dietary fiber components in rye varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 56, 9758–9766 (2008).
Satyavathi, C. T., Ambawat, S., Khandelwal, V. & Srivastava, R. K. Pearl millet: a climate-resilient nutricereal for mitigating hidden hunger and provide nutritional security. Front. Plant Sci. 12, 659938 (2021).
Adebo, O. A. African sorghum-based fermented foods: past, current and future prospects. Nutrients 12, 1111 (2020).
Arya, S. S., Salve, A. R. & Chauhan, S. Peanuts as functional food: a review. J. Food Sci. Technol. 53, 31–41 (2016).
King, J. C. et al. Tree nuts and peanuts as components of a healthy diet. J. Nutr. 138, 1736S–1740S (2008).
Bonku, R. & Yu, J. Health aspects of peanuts as an outcome of its chemical composition. Food Sci. Hum. Wellness. 9, 21–30 (2020).
Sathe, S. K., Monaghan, E. K., Kshirsagar, H. H. & Venkatachalam, M. Chemical composition of edible nut seeds and its implications in human health. In Tree Nuts: Composition, Phytochemicals, and Health Effects (eds Alasalvar, C. & Shahidi, F.) Ch. 2, 11–35 (CRC Press, 2008).
Barreca, D. et al. Almonds (Prunus dulcis Mill. DA webb): a source of nutrients and health-promoting compounds. Nutrients 12, 672 (2020).
Fajardo, V., Alonso-Aperte, E. & Varela-Moreiras, G. Folate content in fresh-cut vegetable packed products by 96-well microtiter plate microbiological assay. Food Chem. 169, 283–288 (2015).
Food Standards Australia New Zealand. Nutritional impact of phytosanitary irradiation of fruits and vegetables (Food Standards Australia New Zealand, 2014).
Ratajczak, A. E. et al. Does folic acid protect patients with inflammatory bowel disease from complications? Nutrients 13, 4036 (2021).
Pandrangi, S. & LaBorde, L. F. Optimization of microbiological assay of folic acid and determination of folate content in spinach. Int. J. Food Sci. Technol. 39, 525–532 (2004).
Dreher, M. L. & Davenport, A. J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 53, 738–750 (2013).
USDA. FoodData Central, https://fdc.nal.usda.gov/ (2019).
O’Connor, A. An overview of the role of bread in the UK diet. Nutr. Bull. 37, 193–212 (2012).
Probst, Y. Nutrient Composition of Chicken Meat (Rural Industries Research and Development Corporation, 2009).
Lister, C. Nutritional analysis of mushrooms-a summary (A Plant & Food Research, 2015). https://meadowmushrooms.co.nz/storage/wysiwyg/files/final-nutritional-analysis-of-meadow-mushrooms-a-summary.pdf.
Gahruie, H. H., Eskandari, M. H., Mesbahi, G. & Hanifpour, M. A. Scientific and technical aspects of yogurt fortification: a review. Food Sci. Hum. Wellness. 4, 1–8 (2015).
Johnston, K., DiRienzo, D. & Tamura, T. Folate content of dairy products measured by microbiological assay with trienzyme treatment. J. Food Sci. 67, 817–820 (2002).
Redeuil, K. M. et al. Simultaneous quantification of 21 water soluble vitamin circulating forms in human plasma by liquid chromatography-mass spectrometry. J. Chromatogr. A 1422, 89–98 (2015).
Oosterink, J. E. et al. Accurate measurement of the essential micronutrients methionine, homocysteine, vitamins B6, B12, B9 and their metabolites in plasma, brain and maternal milk of mice using LC/MS ion trap analysis. J. Chromatogr. B 998-999, 106–113 (2015).
Redeuil, K. et al. A novel methodology for the quantification of B-vitamers in breast milk. J. Anal. Bioanal. Tech. 8, 352 (2017).
Khaksari, M. et al. Detection and quantification of vitamins in microliter volumes of biological samples by LC‐MS for clinical screening. AIChE J. 64, 3709–3718 (2018).
Zayed, A., Bustami, R., Alabsi, W. & El-Elimat, T. Development and validation of a rapid high-performance liquid chromatography–tandem mass spectrometric method for determination of folic acid in human plasma. Pharm 11, 52 (2018).
Asante, I. et al. Simultaneous quantitation of folates, flavins and B6 metabolites in human plasma by LC–MS/MS assay: Applications in colorectal cancer. J. Pharm. Biomed. Anal. 158, 66–73 (2018).
Nandania, J., Kokkonen, M., Euro, L. & Velagapudi, V. Simultaneous measurement of folate cycle intermediates in different biological matrices using liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 1092, 168–178 (2018).
Kahoun, D. et al. Development and validation of an LC-MS/MS method for determination of B vitamins and some its derivatives in whole blood. PLoS One 17, e0271444 (2022).
Gu, Y. et al. A novel automated multi-cycle magnetic solid-phase extraction coupled to LC-MS/MS to study the disorders of six functional B vitamins in patients with gastroenterology and hyperhomocysteinemia. J. Pharm. Biomed. Anal. 241, 115989 (2024).
Kang, L. et al. Rapid determination of folic acid and riboflavin in urine by polypyrrole magnetic solid-phase extractant combined ultra-performance liquid chromatography. J. Chromatogr. A 1648, 462192 (2021).
Akbari, A. et al. Determination of B vitamins by double-vortex-ultrasonic assisted dispersive liquid–liquid microextraction and evaluation of their possible roles in susceptibility to COVID− 19 infection: Hybrid Box–Behnken design and genetic algorithm. J. Chromatogr. Sci. 60, 897–906 (2022).
Xu, H. et al. Voltammetric determination of folic acid at physiological pH values by using a glassy carbon electrode modified with a multilayer composite consisting of polyoxometalate (H8P2Mo16V2O62) and reduced graphene oxide and prepared via layer-by-layer self-assembly and in-situ photoreduction. Mikrochim. Acta 184, 4295–4303 (2017).
Wang, M. et al. Nitrogen-doped carbon quantum dots as a fluorescence probe combined with magnetic solid-phase extraction purification for analysis of folic acid in human serum. Anal. Bioanal. Chem. 409, 7063–7075 (2017).
Gao, X. et al. Synthesis of graphene/ZnO nanowire arrays/graphene foam and its application for determination of folic acid. J. Electroanal. Chem. 808, 189–194 (2018).
Güney, S. Electrochemical synthesis of molecularly imprinted poly (p-aminobenzene sulphonic acid) on carbon nanodots coated pencil graphite electrode for selective determination of folic acid. J. Electroanal. Chem. 854, 113518 (2019).
Hussain, S. et al. Facile preparation of molybdenum carbide (Mo2C) nanoparticles and its effective utilization in electrochemical sensing of folic acid via imprinting. Biosens. Bioelectron. 140, 111330 (2019).
Wang, Q. et al. A fast and facile electrochemical method for the simultaneous detection of epinephrine, uric acid and folic acid based on ZrO2/ZnO nanocomposites as sensing material. Anal. Chim. Acta 1104, 69–77 (2020).
Yang, B. et al. A water-stable MOF-AgClO4-abtz as fluorescent sensor for detection of folic acid based on inner filter effect. Talanta 217, 121019 (2020).
Yang, M. et al. Visual detection of folic acid based on silica coated CdTeS quantum dots in serum samples. Mater. Res. Bull. 144, 111509 (2021).
Fereja, S. L. et al. Silver-enhanced fluorescence of bimetallic Au/Ag nanoclusters as ultrasensitive sensing probe for the detection of folic acid. Talanta 233, 122469 (2021).
Yadav, D. et al. Nanohybrid comprising gold nanoparticles–MoS2 nanosheets for electrochemical sensing of folic acid in serum samples. Electroanalysis 35, e202200286 (2023).
Sun, Y., Wang, X. & Zhang, H. Sensitive and stable electrochemical sensor for folic acid determination using a ZIF-67/AgNWs nanocomposite. Biosens 12, 382 (2022).
Li, K., Quan, X. & Yan, B. Eu (III)-functionalized iCOF hybrids by “tandem post-synthetic modifications” for fluorescent detection of folic acid and trimethoprim: a logical judgement by combination of neural networks and logic gates. Sens. Actuators B Chem. 392, 134078 (2023).
Xu, Y. et al. In situ electrodeposition of bismuth oxide nanowires@ MWNT on the carbon fiber microelectrode for the sensitively electrochemical detection of folic acid. Talanta 253, 123944 (2023).
Vegad, Y. et al. Folic acid detection using β-cyclodextrin-functionalized copper nanoclusters and vitamin B6 cofactor pyridoxal. ACS Appl. Nano Mater. https://doi.org/10.1021/acsanm.3c05697 (2024).
Kıranşan, K. D. & Topçu, E. Free‐standing and flexible MoS2/rGO paper electrode for amperometric detection of folic acid. Electroanalysis 30, 810–818 (2018).
Immundiagnostik A. G. ID-Vit® Folic acid, https://alpco-docs.s3.amazonaws.com/30/IFU-30-KIF005.pdf (2019).
Abbexa. Folic Acid/Vitamin B9 ELISA Kit, https://www.abbexa.com/documents/manual/abx150387_ifu.pdf (2023).
Cell Biolabs Inc. Folic Acid ELISA Kit, https://www.cellbiolabs.com/sites/default/files/MET-5068-folic-acid-elisa-kit.pdf (2024).
LSBio. All species Folic Acid ELISA Kit (Competitive EIA), https://www.lsbio.com/elisakits/manualpdf/ls-f4330.pdf (2024).
Raimondi, M. V. et al. DHFR inhibitors: reading the past for discovering novel anticancer agents. Molecules 24, 1140 (2019).
Acknowledgements
This open-access review paper was supported by The project New Technologies for Translational Research in Pharmaceutical Sciences /NETPHARM, project ID CZ.02.01.01/00/22_008/0004607, is co-funded by the European Union, and the Erasmus+ Programme of the European Union, Key Action 2: Strategic Partnerships, Project no. 2020-1-CZ01-KA203-078218. P.H. thanks to the Charles University (SVV 260 662). C.S., K.M., and L.K.K. thank MH-CZ-DRO (UHHK, 00179906). The graphical abstract was created with BioRender.com. [Embed link to http://biorender.com].
Author information
Authors and Affiliations
Contributions
T.S. elaborated with help of P.H. and Z.L. dietetic aspects, M.a.M., M.o.M. & P.M. were responsible for biological and pharmacological aspects while K.M., C.S. & L.K.K. for analytical and chemical aspects. P.M. prepared the concept of this paper. All authors contributed to the revision of the first version before submission as well as participated in revision based on reviewer comments. All authors have read and agreed with the final version of this paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siatka, T., Mát’uš, M., Moravcová, M. et al. Biological, dietetic and pharmacological properties of vitamin B9. npj Sci Food 9, 30 (2025). https://doi.org/10.1038/s41538-025-00396-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00396-w