Abstract
Pseudomonas aeruginosa is an opportunistic Gram-negative pathogen that causes severe nosocomial infections in susceptible individuals due to the emergence of multidrug-resistant strains. There are no approved vaccines against P. aeruginosa infections nor candidates in active clinical development, highlighting the need for novel candidates and strategies. Using a cell-blot proteomic approach, we reproducibly identified 49 proteins involved in interactions with human lung epithelial cells across four P. aeruginosa strains. Among these were cell division protein FtsZ and outer membrane protein OpmH. Escherichia coli BL21 cells overexpressing recombinant FtsZ or rOpmH showed a 66- and 15-fold increased ability to attach to 16HBE14o− cells, further supporting their involvement in host cell attachment. Both antigens led to proliferation of NK and CD8+ cytotoxic T cells, significant increases in the production of IFN-γ, IL-17A, TNF and IL-4 in immunised mice and elicited strong antigen-specific serological IgG1 and IgG2c responses. Immunisation with FtsZ significantly reduced bacterial burden in the lungs by 1.9-log CFU and dissemination to spleen by 1.8-log CFU. The protective antigen candidate, FtsZ, would not have been identified by traditional approaches relying on either virulence mechanisms or sequence-based predictions, opening new avenues in the development of an anti-P. aeruginosa vaccine.
Similar content being viewed by others
Introduction
Pseudomonas aeruginosa (P. aeruginosa) is a ubiquitous Gram-negative bacterium that is a significant cause of nosocomial infections in individuals admitted to intensive care units, burn and wound victims, those with compromised immune systems, or respiratory disorders such as cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), non-CF bronchiectasis (nCFBE), or ventilator-associated pneumonia1,2. In 2019, P. aeruginosa was associated with more than 500,000 deaths globally, positioning it among the five bacterial pathogens responsible for more than half of all global bacterial deaths3. Worryingly, more than 40% of P. aeruginosa-associated deaths are due to infections in the lower respiratory tract3,4, and around 20% of all pneumonia cases are caused by P. aeruginosa, usually ranking it as the first or second most common aetiologic cause5,6,7. Additionally, P. aeruginosa colonises the respiratory tract of people with CF8, COPD and nCFBE and eventually establishes chronic recalcitrant infections that are associated with increased exacerbations and mortality9,10,11.
P. aeruginosa displays a vast intrinsic and acquired resistome which often renders antibiotic therapy ineffective, increasing mortality rates and leading to poorer outcomes9,12. Indeed, drug-resistant P. aeruginosa strains caused almost 44,000 deaths in the World Health Organisation (WHO) European region and more than 300,000 worldwide in 201913,14. In 2017, the WHO included carbapenem-resistant P. aeruginosa in the list of antibiotic-resistant priority pathogens for which new antibiotics are urgently needed15, and the Centres for Diseases Control and Prevention declared it a serious threat16. Therapeutic options for P. aeruginosa infections are limited, and prophylactic vaccination of susceptible individuals may potentially present a more effective intervention to prevent infection. Despite extensive efforts towards the development of an anti-P. aeruginosa effective vaccine, only three candidates were evaluated in phase III human trials to date, namely a OprF-OprI hybrid (IC43, recently renamed as VC43)17, a bivalent flagellin preparation18, and an octavalent O-polysaccharide-endotoxin A conjugate (Aerugen®)19. Unfortunately, these failed to achieve the expected clinical benefit, and to date, there are no approved vaccines against P. aeruginosa infections, nor candidates in active clinical development20, strengthening the need for searching and evaluating novel candidates and strategies.
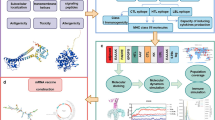
Bacterial attachment to the host epithelium is crucial for the successful initiation of infection, facilitating persistence in the host long enough to produce the necessary virulence factors to enable tissue invasion and damage21,22,23. P. aeruginosa colonisation relies on bacterial adherence to host cells and surface sensing21,23,24,25, highlighting that bacterial proteins involved in host attachment may have potential as vaccine antigens. Indeed, over half of the experimentally proven protective bacterial antigens have adhesive properties26. The use of bacterial proteins involved in host cell adhesion as antigens has already shown promise in preclinical and clinical studies of vaccines against Streptococcus pneumoniae27, Helicobacter pylori28 or Vibrio harveyi (V. harveyi)29. Moreover, at least two licensed vaccines contain adhesins as antigens. The 4CMenB vaccine against Neisseria meningitidis includes the three proteins involved in adhesion (NHBA, fHbp and NadA)30, while the Bordetella pertussis acellular vaccine comprises four adhesins (FHA, pertactin and types 2 and 3 fimbriae)31. Given the importance of bacterial proteins involved in host cell attachment as potential vaccine antigens, McClean et al.32 developed a proteomic two-dimensional cell-blot method to identify potential vaccine candidates by screening for bacterial proteins that bind to host epithelial cells. This platform was previously used to successfully identify novel and efficacious vaccine antigens in Burkholderia spp.32,33 and verocytotoxigenic Escherichia coli (E. coli)34. Here, we applied the cell-blot approach and identified a novel, immunogenic and protective vaccine antigen against P. aeruginosa, the cell division protein FtsZ.
Results
FtsZ and OpmH are involved in P. aeruginosa attachment to HBE cells
In order to identify P. aeruginosa antigens that are expressed in multiple bacterial strains, we screened six well-characterised P. aeruginosa strains from the international panel35,36 for their ability to attach to human epithelial cells (Fig. 1A). Attachment to human bronchial epithelial (HBE) cells varied considerably between strains (p < 0.05; Fig. 1B). The CF transmissible strain LES 431 showed the lowest attachment (0.58%), followed by the burn isolate Mi 162 (6.87%). Two early colonising CF strains (AA2 and ATM 0060-3) and one COPD strain (57P31PA) showed 20–30% of cells adhered, while the community-acquired pneumonia (CAP) strain (A5803) showed the highest attachment (54.91%). Confocal microscopy (multiplicity of infection (MOI) = 10) confirmed the overall trend, with LES 431 showing the lowest level of attachment and A5803 and AA2 strains showing much higher attachment (Fig. 1A, B). Single flagella were observed for strains Mi 162, ATM 0060-3 and AA2, which measured up to 10 µm (Supplementary Fig. 1). The higher level of attachment of early CF isolates AA2, ATM 0060-3 and the CAP strain A5803 is suggestive of adaptation to the infectious environment, however, overall it is apparent that P. aeruginosa host cell attachment is likely to be strain-dependent.
A Representative confocal images showing attachment of P. aeruginosa strains to HBE cells, obtained on a Opera Phenix™ High Content Screening automated confocal microscope. Bacteria are shown in green (anti-P. aeruginosa antibody + secondary antibody-Alexa Fluor®488), and HBE cells in red (membranes, CellMask™ Deep Red) and blue (nuclei, DAPI). B Attachment of P. aeruginosa to HBE cells assessed by colony counting and confocal microscopy. Bars represent the mean ± SEM, with each dot representing one independent experiment. Statistically significant differences (p value < 0.05) were calculated using one-way ANOVA and are indicated by letter-based representation of pairwise comparison. The strains were assigned a letter from “a” to “f” from left to right. The letters on top of each bar indicate the strains to which they are compared and showed a statistically significant difference.
We selected four P. aeruginosa strains with different levels of host cell attachment (LES 431, AA2, 57P31A and A5803) and isolated membrane-enriched preparations to identify novel potential adhesins. When these were separated on individual 2D gels, blotted and probed with HBE cells, 6–11 positive spots on individual cell-blots were successfully matched to bacterial proteins corresponding to the respective 2D gel (Fig. 2 and Supplementary Figs. 2–5). In the sample blot shown which was prepared from P. aeruginosa strain A5803, nine spots on the cell-blot were matched to bacterial proteins in the PageBlue-stained gel (Fig. 2A, B). The intensities of the spots on the blot are a reflection of the affinity of the bacterial protein to HBE cells, rather than bacterial protein quantity. There were also several spots on the blots that did not have a discernable spot to match with on the PageBlue-stained gel, possibly as they were weakly expressed proteins that were not detectable on staining. Negative control blots in which the HBE cells were omitted and antibodies only were applied were clear and confirm that the detected spots in the cell-blots corresponded to bacterial proteins involved in attachment to HBE cells rather than being due to nonspecific antibody binding (Fig. 2C).
LC–MS analysis of matched spots reproducibly identified 49 proteins across nine independent cell-blot experiments, in at least two out of four strains (Table 1). Interestingly, no protein was identified in all four strains examined, highlighting the diversity of P. aeruginosa. Among the identified proteins were previously identified antigens, OprF and FliC, both of which have already been examined as vaccine candidates and thus validate our approach. Literature searches and bioinformatic predictions (Tables S1–4) were used to shortlist the 49 identified proteins. UniProt and MoonProt databases predicted 15 were membrane proteins in P. aeruginosa, while 6 were predicted as moonlighting proteins in other bacterial species (GroEL, FtsZ, EF-Tu, GlnA, LpdG and DnaK; Fig. 3A), meaning that they can be found either in the cytoplasm or the membrane performing distinct functions at different locations. A BLASTp-based search37 showed that 34 proteins shared minimal homology with human, mouse and/or E. coli proteins (Fig. 3A). The two bioinformatic tools used to predict antigenic probability yielded very different outputs. While Vaxign2 (http://www.violinet.org/vaxign2) assigned high antigen scores to 48 proteins, only 14 were predicted as antigens by VaxiJen 3.0 (https://www.ddg-pharmfac.net/vaxijen3/). Considering all these parameters, we assigned a cut-off score of 9 to shortlist the 12 most promising candidates (Fig. 3B). Within these 12, outer membrane protein OpmH, and cell division protein FtsZ, were prioritised because they were predicted to be membrane-associated or have moonlighting capabilities; were novel antigens in P. aeruginosa; were predicted to be immunogenic and involved in adhesion and/or virulence; and did not show high similarities with human, mouse and E. coli proteins.
A Heat map representing whether the proteins fulfilled (green, score = 1) the desirable criteria for effective vaccine antigens (columns’ names) or not (red, score = 0), or inconclusive (yellow, score = 0.5). Full information is available in the Supplementary Material (Tables S2–5) B Final scores of the potential candidates, arranged from higher (green) to lower (red) scores. The higher the score, the greater the likelihood of being a promising antigen. The numbers indicate the score given to each protein.
The two antigen candidates, FtsZ and OpmH, were further characterised using additional bioinformatic tools. FtsZ was not predicted to bear a signal peptide in its amino acid sequence and is predicted to reside in the cytosol (Table 2). In contrast, OpmH resides in the outer membrane and possesses a signal peptide (Table 2). Previous studies38 showed that both proteins were expressed during PAO1 acute lung infection in mice and were highly conserved (≥99%) across six representative strains from different infection types (Table 2). A comparison of predicted epitopes indicated that OpmH showed more predicted B and T cell epitopes than FtsZ (Fig. 4).
Recombinant E. coli BL21 cells overexpressing FtsZ or OpmH confirm their involvement in attachment to HBE cells
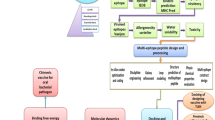
To confirm that FtsZ and OpmH were involved in host cell attachment, the attachment of IPTG-induced recombinant BL21 E. coli cultures expressing FtsZ or OpmH (Supplementary Fig. 6) to HBE cells was examined by confocal microscopy. A dramatic increase in attachment was observed in BL21 cells overexpressing either FtsZ (66-fold) or OpmH (15-fold) (Fig. 5A, B), confirming their role in host attachment. The expression of the recombinant proteins in both induced cultures was confirmed by SDS–PAGE and western blot (Fig. 5C).
A Representative images taken with the automated Opera Phenix™ confocal HSC microscope, showing the increased attachment of the induced cultures. Bacteria are shown in green (anti-E. coli antibody-FITC), and HBE cells in red (membranes, CellMask™ Deep Red) and blue (nuclei, DAPI). B Number of bacteria attached per 100 HBE cells, counted automatically by the Harmony®4.8 software In99 fields. Bars represent the mean and SD of the duplicates. C Confirmation by 12% SDS–PAGE (upper) and western blot (lower) of protein expression (red arrows) only in the induced cultures (+).
FtsZ significantly reduced bacterial burden and dissemination in an acute pneumonia challenge model
Both antigens were purified using Ni-NTA affinity columns followed by dialysis (Supplementary Fig. 7A) and their purity was visualised in an SDS–PAGE gel (Supplementary Fig. 7B, C). Their identities were confirmed by LC–MS (Supplementary Fig. 7D), and the endotoxin levels were determined to be 0.18 and 1.11 EU/mouse for FtsZ and OpmH, respectively (Supplementary Fig. 7E). FtsZ proved to be the most effective antigen, showing a 1.9-log10 reduction in lung colonisation by the heterologous strain KK1 (p = 0.0226), and a concomitant 1.8-log10 reduction in bacterial dissemination to the spleen (p = 0.0043) in immunised mice (Fig. 6B, C). Immunisation with rOpmH + SAS also reduced bacterial burden in both lungs and spleens by 0.8-log, although this was not statistically significant (p = 0.148, p = 0.6283, respectively) (Fig. 6C). The reductions in bacterial load were reflected in a significant improvement in the severity scores in both immunised groups (Fig. 6D, p < 0.05). Immunisation of mice with either antigen elicited robust humoral responses as indicated by the production of total antigen-specific serum IgG, compared to mice that received SAS alone (Fig. 6E), with reciprocal endpoint antibody titres of 7.8 × 106 in response to either antigen. IgG1 and IgG2c subtypes were also expressed (Fig. 6F) suggesting that the antigens may have stimulated mixed Th1/Th2 immune responses. The anti-rFtsZ and anti-rOpmH IgG2c/IgG1 ratios were 2 and 5, respectively, suggesting an immune response skewed towards Th1 (Fig. 6F). Opsonophagocytic killing assays performed on sera from immunised and control mice indicated that the IgG antibodies raised against both FtsZ and OpmH showed opsonophagocytic potential; however, they were inconclusive regarding their role in the protective response of FtsZ (Supplementary Fig. 8). Overall, these results indicate that while both antigens stimulated strong antibody responses, only rFtsZ was protective.
A Timeline of the immunisation schedule, indicating days of immunisation, blood collection, challenge, and organ harvest. Mice were immunised three times subcutaneously with 50 µg rFtsZ or rOpmH plus SAS, and then challenged with P. aeruginosa KK1 strain (6.3 × 106–1.8 × 107 CFU/mouse). B Bacterial burden in the lungs and spleens of mice 24 h post-challenge. C Severity score of mice 24 h after challenge. These graphs show data pooled from two independent experiments. Data are represented as mean ± SEM, and each point represents one mouse. Statistically significant differences were calculated using the non-parametric Kruskal–Wallis test or One-way ANOVA test (p value < 0.05). D Serological analysis of antigen-specific total IgG, IgG1 and IgG2c production by immunised mice. Each point represents the mean ± SD of the mice in the group. The grey, dash lines show the cut-off (2 SD + mean) for antibody titration. Significant differences between control and immunised groups were analysed via two-way ANOVA Šidák’s multiple comparison test (p value < 0.05) (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001).
Antigen-specific recall cellular immune responses show activation of T cells and NK cells and are comparable for both antigens
The immune correlates of protection required for P. aeruginosa are not well understood but increasingly a role of vaccine-induced cellular immunity is emerging39,40. Splenocytes from mice immunised with a single antigen dose were restimulated ex vivo and the cytokine-secreting cells enumerated by ELISpot. Stimulation with either antigen significantly upregulated IFN-γ secreting cells in splenocytes derived from the respective FtsZ and OpmH-immunised groups by 4.8-fold and 6.28-fold relative to adjuvant only immunised animals (Supplementary Fig. 9, p = 0.00021 and <0.00001, respectively). These results encouraged us to further explore the recall responses produced by the antigens to develop an immunoprofile of the cellular responses.
The recall responses to the antigens were immunophenotyped in ex vivo restimulated splenocytes by flow cytometry. Both rFtsZ and rOpmH significantly promoted the proliferation of CD3-expressing T cells (p < 0.0001 and p = 0.0017, respectively; Fig. 7a) and natural killer (NK) cells (p < 0.0001 and p = 0.0142, respectively; Fig. 7e), but no significant changes in proliferation of regulatory T (Treg), natural killer T (NK T), or γδ T cells (Fig. 7b–d) were observed. Strikingly, we observed a statistically significant decrease in the percentage of helper T (Th) cells in response to both rFtsZ and rOpmH (p = 0.0142 and p < 0.0001, respectively; Fig. 7f), but an increase in cytotoxic T (Tc) cells (p = 0.0023 and p < 0.0001, respectively; Fig. 7k). Both antigens stimulated significant increases in naïve (p = 0.0002 and p = 0.0056, respectively), activated (p = 0.0064 and p < 0.0001, respectively), and memory (p < 0.0001) Tc cells (Fig. 7l–n), but not effector Tc cells (Fig. 7o). By contrast, both antigens significantly decreased the percentage of effector, active and memory Th cells (Figs. 6I, J and 7g) while naïve Th cells were significantly increased (p < 0.0001; Fig. 7h). Importantly, both rFtsZ and rOpmH significantly induced IFN-γ (p < 0.0001), TNF (p < 0.0001), IL-4 (p = 0.0039, p < 0.0001), IL-17A (p = 0.0062, p = 0.0020) and IL-22 (p < 0.0001, p = 0.0003) production in non-T CD3− cells (Fig. 8a–e).
Splenocytes from mice immunised (I) with either rFtsZ (green triangles), rOpmH (red squares) or adjuvant only controls (C, blue triangles and squares) were stimulated ex vivo with rFtsZ (triangles) or OpmH (squares) 14 days post immunisation and analysed by flow cytometry. Data represent percentage of cells in response to ex vivo stimulation with rFtsZ or rOpmH adjuvanted with SAS: a total T cells; b regulatory T cells; c γδ T cells; d NK T cells; e NK cells; f–j total, activated, naïve, memory or effector helper T cells; k–o total, activated, naïve, memory or effector cytotoxic T cells. The graphs represent the mean ± SD data pooled from two independent experiments and each dot represents one mouse. Statistically significant differences (p < 0.05) were evaluated using paired Student’s t-test between antigen and its respective adjuvant only control (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001).
Splenocytes from mice immunised (I) with either rFtsZ (green triangles), rOpmH (red squares) or adjuvant only controls (C, blue triangles and squares) were stimulated ex vivo with rFtsZ (triangles) or OpmH (squares) 14 days post immunisation and analysed by flow cytometry. Data represent percentage of cells producing IFN-γ, TNF, IL-4, IL-17A or IL-22 in response to immunisation with rFtsZ + SAS or rOpmH + SAS. a–e non-T cells; f–j T cells; k–o helper T cells; p–r NK cells; s, t cytotoxic T cells. C control, I immunised. The graphs show data pooled from two independent experiments, which is represented as the average ± SD, and each dot represents one mouse. Statistically significant differences (p < 0.05) were evaluated using paired Student’s t-test (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001).
In the context of immune correlates of protection, there were no distinct cellular differences between FtsZ and OpmH-immunised mice, and only a marginal difference was observed in TNF-producing NK cells which significantly proliferated in response to rFtsZ but not rOpmH (p = 0.0437, Fig. 8q), while no significant increase was found in IFN-γ- or IL-17A-producing NK cells (Fig. 8p, r). Cytokine-producing CD3+ T cells did not proliferate in response to either antigen (Fig. 8f–j). No significant changes were observed in the Th cell produced cytokines in response to either candidate (Fig. 8k–o), apart from marginal decreases in production IL-4 (p = 0.0109) and IL-22 (p = 0.0283) production in response to rOpmH (Fig. 8m, o). Despite the observed increase in the percentage of Tc cells, there was no increase in cytokine production by these cells in response to either antigen (Fig. 8s, t). Overall, it is surprising that the recall responses between the two antigens were so comparable when one offered almost 2-log CFU protection and one did not show a statistically significant reduction in bacterial burden.
Discussion
P. aeruginosa is a notorious opportunistic pathogen worldwide41,42,43, and treatment options are often limited due to the high percentage of multidrug-resistant isolates4,44,45. Prophylactic vaccination of susceptible individuals (e.g., immunocompromised patients; people undergoing extended-term hospital stays; people with CF, COPD and nCFBE; or groups at high risk of suffering from wounds and burns) has the potential to reduce the mortality and economic burden associated with these infections20,46. The failure of previous clinical trials highlights the urgent need to advance the evaluation of new vaccinology approaches20,47,48. Therefore, we aimed to identify novel proteins involved in the attachment of P. aeruginosa to lung epithelial cells with potential as vaccine antigens. We observed high inter-strain variability in the attachment of P. aeruginosa to HBE cells, likely because the adaptation of the strains to different infection niches led to the loss of well-described virulence traits (i.e., pilin, flagella), overproduction of polysaccharides, or changes in LPS structures, all of which participate in adhesion to host cells35,49,50,51. Indeed, the observed variability in the presence of flagella in these strains further suggests the adaptation to infectious environments. Nevertheless, multiple bacterial proteins are expressed, and preserved despite adaptation, which seem to be involved in the binding of P. aeruginosa.
Using a 2D cell-blot approach32 we reproducibly identified 49 proteins that are involved in host cell attachment, and in combination with bioinformatic predictions and experimental evidence from the literature, 12 were ranked as the most promising candidates. Among these, three were recognised P. aeruginosa adhesins (OprF, FliC and OprQ)1,52,53, two of which have progressed to clinical trials (OprF and FliC)17,18, giving us confidence in our approach. Moreover, homologues of OpmH, BamB, LptH and GroEL are involved in the attachment of other pathogens, including Salmonella typhimurium, Klebsiella pneumoniae and Neisseria meningitis29,54,55,56,57,58. Consequently, FtsZ and OpmH were both identified in three independent P. aeruginosa strains, and were further evaluated for their potential as vaccine antigens. FtsZ is involved in P. aeruginosa cell division by determining the division site and initiating the assembly of the divisome machinery59. Given its importance for bacterial growth, it has recently gained interest as a drug target for CF pathogens60. This is the first report of its involvement in P. aeruginosa host cell attachment, and the exact mechanism requires further investigation. Bioinformatic predictions showed that FtsZ does not contain a signal peptide and should reside in the cytoplasm, playing a role in cell division61. Therefore, it may act as a moonlighting protein to enhance bacterial adhesion to host cells62. We identified two other known P. aeruginosa moonlighting proteins (EF-Tu and LpdG) in the cell-blots. Lysine trimethylation of EF-Tu enhances P. aeruginosa attachment to airway respiratory cells63, and Lpd is a dihydrolipoamide dehydrogenase when in the cytoplasm but a vitronectin- and clusterin-binding protein when located in the surface of P. aeruginosa64. The E. coli cell division protein YftB normally resides in the cytoplasm and no signal sequence was computationally predicted; however, it was recently shown to also be associated with the outer membrane, where it plays a role in adhesion to human kidney cells65. A moonlighting function was also recently proposed for other two cell division proteins from Neisseria gonorrhoeae (N. gonorrhoeae) (FtsN and NGO0265)66. Moreover, Group A Streptococcus SpyAD is involved in cell division and adhesion to host cells, and also conferred protection in mice67. Interestingly, in Streptococcus pyogenes, SpyAD interacts with FtsZ and both proteins were exposed on the surface, again supporting the notion that P. aeruginosa FtsZ is a moonlighting protein. It was also identified within the proteome of P. aeruginosa OMVs produced via budding, along with other nucleoid-localised proteins, including FtsK, which is also part of the cell divisome68.
Recombinant FtsZ proved to be the most protective antigen. Previously, mice immunised with rFtsZ from Streptococcus suis serotype 2 were protected against lethal challenge (60% survival over 10 days)69, and mice immunised with cell division proteins FtsN and NGO0265 from N. gonorrhoeae reduced vaginal colonisation by multidrug-resistant strains66. These results indicate the potential of bacterial cell division proteins as promising vaccine antigen candidates. Although mouse survival was not examined in consideration of refinement (3Rs), the observed near 2-log reductions in both lung bacterial load and dissemination to the spleen are substantial in terms of mouse survival. Other P. aeruginosa vaccine studies reporting ≤1.5-log CFU reductions translated into lung pathology improvement or 60–100% survival70,71,72. Moreover, in the development of the acute pneumonia model, we observed that increases in bacterial dose of 1-log could lead to up to 2-fold increases in severity scores and humane endpoints being reached (in press)73.
For its part, OpmH is the constitutively expressed outer membrane channel of the TriABC efflux pump that is specifically involved in the resistance to triclosan74, facilitating direct interaction with host cell receptors, similar to other outer membrane proteins such as OprF or OprI75,76. Surprisingly, rOpmH was not statistically protective against P. aeruginosa challenge, in contrast to homologues in other bacteria (TolC). For example, 80% of TolC immunised fish survived over 14 days after V. harveyi lethal challenge and 50% of immunised mice survived over 7 days after challenge with Haemophilus parasuis serovar 529,77. Mice immunised with rOpmH showed lower severity and lower bacterial loads in both lungs and spleen compared to the control group. Given the robust cellular recall responses elicited by this antigen together with the lower severity scores, it may have potential to contribute to protection in a future multivalent vaccine.
Given that the immune correlates of protection are not well established, we rigorously evaluated the immune responses to both antigens to identify key markers that FtsZ elicited but that OpmH did not. Both antigens elicited comparable antigen-specific humoral responses, indicating that IgG antibody responses alone are not sufficiently protective, consistent with other studies of P. aeruginosa vaccines78,79,80. Indeed, antibody affinity and avidity rather than quantity may be more relevant for protection81. Both antigens also elicited comparable recall responses which was surprising, including the proliferation of NK cells. The only discernable difference between the antigens was increased TNF production in NK cells in response to rFtsZ only, suggesting that this may be one of the main contributors to protection. NK cells were previously demonstrated to be important for P. aeruginosa clearance82,83. Strikingly, we observed an increase in Tc cells in both antigen-immunised groups. Subunit vaccines typically fail to elicit CD8+ T cell proliferation as internalised antigens are presented on MHC-II molecules. However adjuvant-induced cross presentation is widely described whereby internalised antigens can also be presented by antigen presenting cells on MHC-I molecules84,85,86, and may explain the observed proliferation of cytotoxic cells following immunisation with the subunit vaccines. In previous studies, we also observed the proliferation of Tc cells after immunisation with the Burkholderia pseudomallei (B. pseudomallei) OmpW subunit antigen adjuvanted with SAS87 and with CAF0188. CD8+ T cells have not been widely investigated in the context of P. aeruginosa infection, but the protection conferred against secondary P. aeruginosa pneumonia after administration of human rIL-7 to mice was correlated with increased levels of CD8+ Tc cells89, and Tc cells proliferated in response to a protective P. aeruginosa X-Pa vaccine90. Growing evidence suggests that Th cell responses are required to confer protection, as depletion of CD4+ T cells abrogated protection levels achieved with several vaccine candidates91,92. However, consistent with our data, Ma et al. did not observe an increase in total CD4+ T cells after vaccination with the protective X-Pa, which led to an increase in naïve CD4+ T cells and a decrease in effector T cells, most likely because these migrate to non-lymphoid organs90. NK T cells did not proliferate in response to either antigen, suggesting that they are unlikely to have a role in protection against P. aeruginosa lung infection in mice. This is consistent with Kinjo et al.93, but contrasts with several studies that observe a beneficial role of these cells in fighting against P. aeruginosa infection94,95,96. However, the role of NK T cells against P. aeruginosa infections may depend on the mouse strain, as these cells showed a beneficial role in BALB/c mice but not in C56BL/6 mice95, which may explain the observed irresponsiveness of NK T cells to rFtsZ or rOpmH. In the case of γδ T cells, no differences were observed between the antigens, consistent with other reports that failed to observe a beneficial role of γδ T cells in immunised97. Overall, despite extensive analysis of systemic responses, the immune responses involved in vaccine-induced protection against P. aeruginosa are complex and not well understood, and induction of multi-pronged immunity is likely to be required40.
It is worth noting that we only used female mice to test vaccine efficacy in this study, as it has been widely described that females are more susceptible to P. aeruginosa infections and undergo significantly poorer outcomes98,99,100. Consequently, biological sex has not been evaluated as a variable and this limitation will be addressed in future studies which will include cohorts with mixed genders. Another limitation of the present study was the lack of analysis of mucosal immunity as we anticipated that mucosal responses would be sub-optimal following subcutaneous vaccination. Thus far, only a handful of studies have investigated mucosal vaccination against P. aeruginosa in preclinical models39, and only one mucosal formulation using the OprF-OprI hybrid protein antigen was evaluated in clinical trials101. However, because mucosal surfaces are a common site for P. aeruginosa infection, the importance of mucosal immunity has been recently highlighted47,102. Therefore, an immunisation strategy with systemic priming followed by mucosal boosting (e.g., intranasal delivery) is critical and may potentially enhance the protective efficacy of these antigens. Moreover, the evaluation of lung cellular responses and IgA production should help elucidate the correlates of protection. These studies are currently ongoing.
In addition to the immunisation route, the choice of adjuvant also impacts vaccine efficacy and the immune response. The assessment of adjuvant’s contribution to vaccine formulation has been largely neglected, restricting our knowledge about adjuvants in P. aeruginosa vaccines39. Adjuvants mounting a Th2-biased response appear sub-optimal to prevent P. aeruginosa infections103,104, while a more balanced Th1/Th2 response may be more beneficial39. Our previous work utilising the TLR-4 agonist SAS as adjuvant stimulated Th1 responses in combination with B. pseudomallei OmpW87, conferring high levels of protection (75% survival for 81 days)33. These promising results rationalised the choice of SAS; however, more studies are needed to inform adjuvant selection. Given the recently highlighted importance of Th17 responses against P. aeruginosa40, the evaluation of the novel antigens with other adjuvants that stimulate Th1/Th17 responses will be pursued.
In summary, the use of the 2D cell-blot approach allowed the identification FtsZ, which protected mice against P. aeruginosa acute lung infection in mice. Moreover, it is highly conserved in six representative clinical isolates from different sources of infections and was identified in three independent strains originally isolated from CF, COPD and CAP infections underlining its potential breadth of coverage. FtsZ was not predicted to be involved in host cell interactions and appears to act as a moonlighting protein, which highlights the limitations of screening vaccine antigens using approaches that rely on traditional virulence mechanisms or sequence-based prediction for vaccine antigen design and opens new venues to advance in the development of vaccination strategies against P. aeruginosa infections.
Methods
Bacterial strains and culture
Seven well-characterised P. aeruginosa strains originating from different infection sources obtained from the P. aeruginosa International Reference Panel36 were used (LES 431, AA2, ATM 0060-3, Mi 162, 57P31PA, KK1 and A5803). Bacterial cultures were stored at −80 °C in 60% glycerol and LB broth (1:1), after being cultured to mid-logarithmic phase (OD600 = 0.6). Unless otherwise stated, the strains were routinely grown in LB broth at 37 °C with orbital shaking (200 rpm).
Mammalian cell culture
The HBE cell line 16HBE14o− was routinely grown in Eagle’s Minimum Essential Medium (MEM) supplemented with 10% foetal bovine serum, 1% penicillin/streptomycin and 1% l-glutamine, at 37 °C in a humidified incubator at 5% CO2. The cells were routinely cultured on collagen/fibronectin-coated T75 flasks.
Bacterial attachment to HBE cells
To quantitate the attachment of P. aeruginosa strains to lung epithelial cells, HBE cells were seeded at a density of 4 × 105 cells/mL in coated 24-well plates, incubated for 24 h, and equilibrated once with MEM alone before incubating with the P. aeruginosa strains. Bacterial inocula were applied to cells at a MOI of 5:1, plates were centrifuged at 700 × g for 5 min, and cells were incubated with bacteria for 30 min at 37 °C in 5% CO2. Cells were then washed twice with sterile phosphate-buffered saline (PBS), and adherent bacteria were detached with 0.5% Triton X-100 in PBS for 10 min incubation at room temperature (RT) and removed by scraping with a pipette tip. The lysates were serially diluted in PBS, plated onto LB agar plates in duplicate and incubated at 37 °C for 24 h, and resulting CFU were counted. The CFU/mL were calculated, and the attachment to host cells was expressed as the percentage of CFU attached relative to the theoretical starting inoculum (4 × 106 CFU/mL). At least three technical replicates and three independent experiments per strain were performed.
Alternatively, bacterial attachment was determined by confocal microscopy as previously described105 with the following modifications. P. aeruginosa cultures were applied to seeded HBE cells at an MOI 5:1 or 10:1 as described above, and a rabbit anti-P. aeruginosa polyclonal antibody (Invitrogen, USA) (1:8,000 dilution in PBS + 1% BSA) and an Alexa Fluor®488-conjugated goat anti-rabbit antibody (Invitrogen) (1:1,000 dilution in PBS + 1% BSA) were used to stain bacteria. Images were taken on an automated Opera Phenix™ High Content Screening confocal microscope and the number of epithelial cells and bacteria attached were automatically counted using a specific pipeline with the Harmony®4.8 High Content Imaging and Analysis software (Supplementary Fig. 10). Bacterial attachment was measured by counting the number of bacteria attached per 100 cells. At least two technical replicates and three independent experiments per strain were performed.
The attachment of recombinant E. coli BL21 clones to HBE cells was also analysed by confocal microscopy with the following modifications. IPTG-induced and uninduced cultures of recombinant E. coli BL21 were adjusted to OD600 = 0.1 and applied to seeded HBE cells (4 × 105 cells/mL). Inocula were serially diluted, plated onto selective LB agar, and incubated for 24 h at 37 °C to count the CFU and calculate the actual inocula applied. Aliquots from the inocula were also taken to confirm recombinant protein expression by SDS–PAGE and Western blot analysis with an anti-6xHis antibody (Invitrogen, 1:1,000 dilution). Bacterial cells were labelled by a single overnight incubation at 4 °C with an anti-E. coli serotype O/K polyclonal antibody conjugated to fluorescein isothiocyanate (FITC; Thermo Fisher). This experiment was performed once in duplicate. The number of bacteria per 100 cells were compared between induced recombinant E. coli BL21 clones and uninduced counterparts by calculating the fold change (FC; induced/uninduced).
Preparation of bacterial membrane-enriched proteins
Membrane protein-enriched preparations from four P. aeruginosa strains with different attachment levels (LES 431, AA2, 57P31PA and A5803) were obtained as described32 with modification. Stationary phase bacterial cultures were pelleted by centrifugation at 5000 × g and 4 °C for 10 min, and pellets were lysed in ice-cold PBS with 5% CHAPS (3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate hydrate) and 1X cOmplete™ mini EDTA-free protease inhibitor cocktail (Roche, Switzerland). Cell debris was removed by centrifugation at 7000 × g and 4 °C for 15 min. Supernatants were retained and ultracentrifuged at 30,000 × g and 4 °C for 30 min. Then, supernatants were discarded and the pellets containing the proteins were resuspended in 2 mM MgCl2, 50 mM Tris base (pH = 8) and protease inhibitor cocktail, and ultracentrifuged in the same conditions. The supernatants were removed and the pellets were resuspended in 2% Triton X-100, 50 mM Tris base (pH = 8) and protease inhibitor cocktail, incubated for 30 min at 40 °C with gentle shaking, and ultracentrifuged as before. The supernatants were discarded, and pellets were resuspended in 50 mM Tris base (pH = 8). A final ultracentrifugation step was performed, supernatants were discarded, and pelleted proteins were resuspended in rehydration buffer [8 M urea, 2 M thiourea, 4% CHAPS, 1% Triton X-100, 10 mM Tris base, 65 mM dithiothreitol and 0.8% immobilised pH gradient]. The protein concentration of the samples was determined by a bicinchoninic acid (BCA) assay using the Pierce™ BCA Protein Assay Kit (Thermo Fisher), following manufacturer’s instructions.
Lung epithelial cell-probed blots
This was performed using the method of McClean et al.32, with some modifications. Bacterial protein preparations (60–180 μg) were rehydrated overnight and separated by isoelectric focusing on an Ettan™ IPGphor™ 3 IEF System (Cytvia, USA) following a three-step protocol (30 min at 300 V, 30 min at 1000 V, and 4 h at 5000 V) on pairs of strips run in parallel. The strips were placed on 12% SDS–PAGE gels and the proteins further separated at 120 V for 2.5 h. One gel was stained overnight with PageBlue™ Protein Staining Solution (Thermo Fisher), while the other gel was transferred onto a polyvinylidene difluoride (PVDF) membrane at 30 mA and 4 °C for 16 h. Membranes were blocked overnight at 4 °C in PBS with 5% BSA and 3% milk powder before being probed with 4 × 105 HBE cells/mL in PBS for 4 h at 4 °C. Unbound cells were washed four times with 0.5% Tween 20 in PBS, and attached cells were detected with a mouse anti-epithelial cell-specific antigen antibody (clone VD-1D9, Sigma-Aldrich; 1:1,1000 dilution) in 5 mL of PBS+5% BSA + 0.04% Tween 20 and incubated overnight at 4 °C. A rabbit polyclonal anti-mouse antibody conjugated to horseradish peroxidase (HRP; Abcam, UK; 1:36,000 dilution) was applied for 1 h at RT after the blots were washed, and chemiluminescence detection was carried out on a Vilber Fusion Fx (Vilber, France) imaging system using the Pierce™ ECL Western kit (Thermo Fisher), according to manufacturer’s instructions. The spots revealed by chemiluminescence were matched with the corresponding protein spots observed after destaining the counterpart PageBlue-stained gel, based on spot position (theoretical isoelectric point and molecular weight) and shape. At least two independent experiments per bacterial strain were performed. Reproducible identification thresholds were set to include only proteins that were identified in at least three experiments (nine independent experiments in total) and in two out of four strains.
Protein identification by LC–MS analysis
Tryptic digestion of proteins contained in the matched spots was performed following the in-gel digestion method described by Shevchenko et al.106. Tryptic peptides were redissolved in 0.5% trifluoroacetic acid (TFA) and ZipTip®Pipette tips (Millipore, USA) were wetted in 0.1% TFA in 80% acetonitrile, and equilibrated in 0.1% TFA. Then, peptides were adsorbed into the tip, washed with 0.1% TFA, eluted in 0.1% TFA in 60% acetonitrile and dried down in a vacuum. Peptides were resuspended in 0.1% formic acid and analysed in the Mass Spectrometry Core service at the Conway Institute in University College Dublin (UCD). Proteins’ spectra were obtained by liquid chromatography–mass spectrometry (LC–MS) and matched with a P. aeruginosa reference proteome from strains ATCC 15692, DSM 22644, CIP 104116, JCM 14847, LMG 12228, 1C, PRS 101 and PAO1 (UniProt accession number: UP000002438). The sequence coverage threshold was set at ≤15%, and proteins whose molecular weight and isoelectric point (obtained from Compute pI/Mw tool from Expasy, https://web.expasy.org/compute_pi/) matched that of the corresponding spot in the stained gel were selected for shortlisting.
Antigen candidate shortlisting process
To ensure reproducibility and coverage among strains, proteins that were identified in at least three out of nine experiments and in two out of four strains were selected for further shortlisting with a scoring method that takes into account desirable criteria for effective vaccine antigens (Supplementary Table 5)46. Surface or membrane association was predicted in either P. aeruginosa or other pathogens using UniProt database107 and MoonProt 2.0 database108. Literature searches were performed to evaluate the candidates’ novelty, immunogenicity and role in adhesion or virulence in P. aeruginosa or other pathogens (Supplementary Table 6). Bioinformatic predictions on their adhesin and antigen probability were performed using the Vaxign2109 and VaxiJen 3.0110 tools. Pathogen specificity was evaluated by assessing candidates’ similarity with proteins from human (Homo sapiens, taxid:9606), mouse (Mus musculus, taxid:10090), and E. coli strain K-12 (taxid:562) in the curated UniProtKB/Swiss-Prot database, using the BLASTp online tool37.
Bioinformatic predictions on the vaccine candidates
The CELLO2GO server111 was used to predict the most likely subcellular location of the antigen candidates, setting the prediction model from Gram-negative bacteria and the default threshold to limit the homology (E-value = 0.001). The presence of different types of signal peptides was analysed with the SignalP 6.0 software112 and the UniProt database. Intraspecies conservation was evaluated in six P. aeruginosa strains chosen as representative strains from different sources of infection. Their genomes are sequenced and publicly available in Pseudomonas Genome database (Supplementary Table 7). These sequences were aligned using ClustalW113, and compared to the reference sequence (PAO1 strain)114. The DualSeq Database115 was used to confirm whether the antigen candidates were expressed by P. aeruginosa during infection, following authors’ criteria. Data available on the database was obtained from a murine acute lung infection model (16 h)38.
Cloning and expression of antigen candidates
The genomic DNA from the reference strain PAO1 was extracted using the GenElute™ Bacterial Genomic DNA Kit (Sigma-Aldrich) and the ftsZ and opmH genes were amplified by PCR with the PfuUltra II Fusion High-fidelity DNA Polymerase (Agilent, USA) and specific primers (Tables S8 and S9), and gene amplification was confirmed by 1.5% agarose gel electrophoresis. The amplicons were purified using the QIAquick PCR purification kit (Qiagen, Germany), and cloned into pET-28a(+) plasmid. Constructs were transformed into the expression host One Shot™ BL21 Star™ (DE3) Chemically Competent E. coli cells (Thermo Fisher) as per manufacturer’s instructions. Correct insertion of the gene into the plasmid was assessed by colony PCR using T7 primers (Supplementary Table 4). Lack of mutations in the genes was confirmed by sequencing. Cultures (1 L) of recombinant E. coli BL21 cells were induced with 1 mM IPTG and grown in selective LB broth for 2 h (BL21_FtsZ) and 5 h (BL21_OpmH) at 37 °C. The cultures were then centrifuged at 5000 × g for 10 min, and the cell pellets were stored at −20 °C until purification was performed.
Purification of His-tagged recombinant proteins
Bacterial cell pellets were thawed and resuspended in a basic buffer containing 50 mM NaH2PO4, 300 mM NaCl and 10 mM imidazole (pH 8; 50 mL/1 L culture) supplemented with 1 mg/mL lysozyme and 1X cOmplete™ mini EDTA-free protease inhibitor cocktail, and sonicated at 20% amplitude. The crude lysates were then centrifuged for 40 min at 16,000 × g and 4 °C, and soluble fractions were separated from the insoluble pellets. Recombinant FtsZ was purified from soluble fractions, while rOpmH was resolubilised from the pellets with 8 M urea. HisPur™ Ni-NTA (nickel-nitrilotriacetic acid) Superflow Agarose resins (Thermo Fisher) were equilibrated twice with 10 mM imidazole and incubated with protein extracts for 1 h at RT with mild rotation. The mixtures were passed through gravity columns to remove unbound proteins and resins were washed twice with 10 mM and then with 20 mM imidazole. The recombinant proteins were eluted twice with 250 and 300 mM imidazole. Urea and imidazole were removed by extensive dialysis using a 10 K MWCO (molecular weight cut-off) cellulose membrane SnakeSkin™ Dialysis Tubing (Thermo Fisher), and dialysed protein fractions were concentrated by centrifugation (4000 × g, 4 °C, 1 h) in an Amicon® Ultra 15 mL centrifugal devices with Ultracel® 10 MWCO filter unit (Sigma-Aldrich). The endotoxin levels of the purified protein batches were quantified using the Pierce™ Chromogenic Endotoxin Quant Kit (Thermo Fisher), following manufacturer’s instructions116. PageBlue-stained SDS–PAGE gels were imaged and processed in the ImageJ v1.8.0 software to obtain densitograms and purity percentages. Protein concentration was measured using the Pierce™ BCA Protein Assay Kit, and protein identity was confirmed by LC–MS.
Ethical statement
All the work involving the animals was approved by the UCD Animal Research Ethics Committee (AREC-21-19), and mice were maintained according to the regulations of the Health Products Regulatory Authority (Directive 2010/63/EU and Irish Statutory Instrument 543 of 2012), under Authorisation Number AE18982/P209.
Immunisation and bacterial challenge
Female C57BL/6J mice (6–8 weeks old; Charles River, UK) were randomly housed in groups of 3–4 mice/cage. Food and water were available ad libitum. Mice (n = 7) were immunised subcutaneously three times (100 μL /dose), 2 weeks apart, with 50 µg/mouse of either rOpmH or rFtsZ mixed 1:1 v/v with Sigma Adjuvant System® (SAS), or SAS alone as a control. Two weeks after the last immunisation, mice were challenged by oropharyngeal aspiration with 6.3 × 106–1.8 × 107 CFU/mouse of the heterologous strain, KK1. Briefly, mice were individually anaesthetised in a chamber with 5% isoflurane gas and 2 L/min oxygen for 1 min. Once anaesthetised, mice were placed on a backboard in a semi-recumbent supine position and suspended by the upper incisors from a thread. The nares were occluded, and the tongue was gently pulled outside the mouth using blunt tweezers to allow an aliquot of 50 µL of either a bacterial suspension or PBS to be deposited into the oral cavity, close to the entrance of the trachea. Mice were allowed to aspirate the bacterial suspension for 10 breaths after which the animals were allowed to recover (in press73). The optimal bacterial dose and challenge time were selected based on having reproducible bacterial load without impacting severely on animal welfare73. After 24 h, mice were euthanised by CO2 exposure (2 L/min) for 9 min plus a 5 min delay, confirming death by sensing the absence of heartbeat before lungs, spleens and stomachs were aseptically harvested in PBS, weighed, and homogenised in the TissueLyser II (Qiagen) for 15 min at the maximum frequency (30 Hz/s) using 3.2-mm stainless-steel beads. Organ homogenates were then serially diluted in PBS, plated onto LB agar in duplicate, and incubated overnight at 37 °C. CFU were counted and normalised to organs’ weight (CFU/mg). Two independent experiments were performed. Mice were weighed and monitored after challenge using an experiment-specifically designed welfare scoresheet (Supplementary Table 10).
Determination of antigen-specific serum antibodies by indirect ELISA
Blood samples were collected from individual mice via submandibular vein bleed at day 35. Blood samples were centrifuged at 16,000 × g and 4 °C for 30 min to isolate sera. Indirect ELISA was performed to determine the anti-FtsZ and anti-OpmH antibodies as before32, with the following changes. Plates were coated with 1 μg/mL of either antigen, and blocked with 10% FBS. The cut-off values for antibody titres were determined as the mean OD of the control sera plus 2 standard deviations.
Immunophenotyping by splenocyte ex vivo restimulation assays and flow cytometry
Female C57BL/6J mice (6–8 weeks old) were immunised once subcutaneously with 50 µg of individual antigens plus SAS (1:1 v/v) or SAS alone as a control. After 14 days, mice were humanely killed, and ex vivo splenocyte restimulation was performed as before87. The antibodies were titrated for optimal performance and Beckman Coulter Versacomp beads were used for compensation. A Beckman Coulter CytoFLEX LX (NUV full configuration) was used for the analysis of the samples with the gains for each one of the markers optimised by performing a gaintration. Blocking and cell staining for flow cytometry was performed as before87 using Viakrome 808 as viability dye and fluorochrome-labelled antibodies against CD3, CD4, CD8, CD25, CD44, CD49b, CD26L, γδ, Foxp3, IL-4, IL-17A, IL-22, IFN-γ and TNF (Supplementary Table 11). Fluorescence minus one (FMO) controls were prepared and used for every single marker (<0.5%). Gating strategies and corresponding FMOs are available in Fig. S8. Data were extracted as the percentage of parental cells. Data analysis was performed with Beckman Coulter CytExpert v. 2.4.
Statistical analysis
Statistically significant differences (p value < 0.05) in the attachment of P. aeruginosa strains to HBE cells were analysed using one-way ANOVA. Differences in antibody production between control and immunised groups were analysed via two-way ANOVA Šidák’s multiple comparison test. Data relating to organ colonisation of immunised mice was analysed with the unpaired non-parametric Kruskal–Wallis test. Data relating to the flow cytometric analysis of splenocyte restimulation was analysed with the Student’s t-test. All statistical analysis was performed with GraphPad Prism v9.4.1 and all graphs were created using this same software.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE117 partner repository with the dataset identifier PXD055823. Data for this work are available in the Zenodo repository (https://doi.org/10.5281/zenodo.13628876).
References
Morin, C. D., Déziel, E., Gauthier, J., Levesque, R. C. & Lau, G. W. An organ system-based synopsis of Pseudomonas aeruginosa virulence. Virulence 12, 1469–1507 (2021).
Wood, S. J., Kuzel, T. M. & Shafikhani, S. H. Pseudomonas aeruginosa: infections, animal modeling, and therapeutics. Cells 12, 199 (2023).
Collaborators, G. B. D. A. R. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 400, 2221–2248 (2022).
Shortridge, D. et al. Geographic and temporal patterns of antimicrobial resistance in Pseudomonas aeruginosa over 20 years from the SENTRY Antimicrobial Surveillance Program: 1997-2016. Open Forum Infect. Dis. 6, S63–S68 (2019).
Herkel, T. et al. Epidemiology of hospital-acquired pneumonia: results of a Central European multicenter, prospective, observational study compared with data from the European region. Biomed. Pap. 160, 448–455 (2016).
Weiner-Lastinger, L. M. et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015-2017. Infect. Control Hosp. Epidemiol. 41, 1–18 (2020).
Tilahun, M. et al. Bacteriology of community-acquired pneumonia, antimicrobial susceptibility pattern and associated risk factors among HIV patients, Northeast Ethiopia: cross-sectional study. SAGE Open Med. 11, 20503121221145569 (2023).
Fundation, C. F. Patient Registry: Annual Data Report 2022 (©2023 Cystic Fibrosis Foundation, Bethesda, Maryland, 2023).
Durda-Masny, M. et al. The determinants of survival among adults with cystic fibrosis-a cohort study. J. Physiol. Anthropol. 40, 19 (2021).
Kwok, W. C., Ho, J. C. M., Tam, T. C. C., Ip, M. S. M. & Lam, D. C. L. Risk factors for Pseudomonas aeruginosa colonization in non-cystic fibrosis bronchiectasis and clinical implications. Respir. Res. 22, 132 (2021).
Martínez-García, M. et al. Risk factors and relation with mortality of a new acquisition and persistence of Pseudomonas aeruginosa in COPD patients. COPD 18, 333–340 (2021).
Horcajada, J. P. et al. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin. Microbiol. Rev. 32, e00031–00019 (2019).
Collaborators, A. R. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
European Antimicrobial Resistance, C. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis. Lancet Public Health 7, e897–e913 (2022).
Tacconelli, E. et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327 (2018).
CDC, Antibiotic Resistance Threats in the United States, 2019. (Department of Health and Human Services, CDC, Atlanta, GA, USA, 2019).
Adlbrecht, C. et al. Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients-a randomized clinical trial. Crit. Care 24, 74 (2020).
Döring, G., Meisner, C., Stern, M. & Group, F.V.T.S. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 104, 11020–11025 (2007).
Döring, G. Prevention of Pseudomonas aeruginosa infection in cystic fibrosis patients. Int. J. Med. Microbiol. 300, 573–577 (2010).
Frost, I. et al. The role of bacterial vaccines in the fight against antimicrobial resistance: an analysis of the preclinical and clinical development pipeline. Lancet Microbe 4, e113–e125 (2023).
Ribet, D. & Cossart, P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 17, 173–183 (2015).
Reynolds, D. & Kollef, M. The epidemiology and pathogenesis and treatment of Pseudomonas aeruginosa infections: an update. Drugs 81, 2117–2131 (2021).
Muggeo, A., Coraux, C. & Guillard, T. Current concepts on Pseudomonas aeruginosa interaction with human airway epithelium. PLoS Pathog. 19, e1011221 (2023).
Siryaporn, A., Kuchma, S. L., O’Toole, G. A. & Gitai, Z. Surface attachment induces Pseudomonas aeruginosa virulence. Proc. Natl. Acad. Sci. USA 111, 16860–16865 (2014).
Stones, D. H. & Krachler, A. M. Against the tide: the role of bacterial adhesion in host colonization. Biochem. Soc. Trans. 44, 1571–1580 (2016).
Ong, E., Wong, M. U. & He, Y. Identification of new features from known bacterial protective vaccine antigens enhances rational vaccine design. Front. Immunol. 8, 1382 (2017).
Entwisle, C. et al. Safety and immunogenicity of a novel multiple antigen pneumococcal vaccine in adults: a phase 1 randomised clinical trial. Vaccine 35, 7181–7186 (2017).
Banga Ndzouboukou, J. L. et al. Helicobacter pylori adhesins: HpaA a potential antigen in experimental vaccines for H. pylori. Helicobacter 26, e12758 (2021).
Zhu, Z., Dong, C., Weng, S. & He, J. Identification of outer membrane protein TolC as the major adhesin and potential vaccine candidate for Vibrio harveyi in hybrid grouper, Epinephelus fuscoguttatus (♀) × E. lanceolatus (♂). Fish Shellfish Immunol. 86, 143–151 (2019).
Pizza, M., Bekkat-Berkani, R. & Rappuoli, R. Vaccines against meningococcal diseases. Microorganisms 8, 1521 (2020).
Dewan, K. K., Linz, B., DeRocco, S. E. & Harvill, E. T. Acellular pertussis vaccine components: today and tomorrow. Vaccines 8, 217 (2020).
McClean, S. et al. Linocin and OmpW are involved in attachment of the cystic fibrosis-associated pathogen Burkholderia cepacia complex to lung epithelial cells and protect mice against infection. Infect. Immun. 84, 1424–1437 (2016).
Casey, W. T. et al. Identification of an OmpW homologue in Burkholderia pseudomallei, a protective vaccine antigen against melioidosis. Vaccine 34, 2616–2621 (2016).
Quinn, C. et al. GlnH, a novel antigen that offers partial protection against verocytotoxigenic Escherichia coli Infection. Vaccines 11, 175 (2023).
Cullen, L. et al. Phenotypic characterization of an international Pseudomonas aeruginosa reference panel: strains of cystic fibrosis (CF) origin show less in vivo virulence than non-CF strains. Microbiology 161, 1961–1977 (2015).
De Soyza, A. et al. Developing an international Pseudomonas aeruginosa reference panel. Microbiologyopen 2, 1010–1023 (2013).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Damron, F. H., Oglesby-Sherrouse, A. G., Wilks, A. & Barbier, M. Dual-seq transcriptomics reveals the battle for iron during Pseudomonas aeruginosa acute murine pneumonia. Sci. Rep. 6, 39172 (2016).
Sainz-Mejias, M., Jurado-Martin, I. & McClean, S. Understanding Pseudomonas aeruginosa-host interactions: the ongoing quest for an efficacious vaccine. Cells 9, 2617 (2020).
Baker, S. M., McLachlan James, B. & Morici Lisa, A. Immunological considerations in the development of Pseudomonas aeruginosa vaccines. Hum. Vaccines Immunother. 16, 412–418 (2020).
ECDC in ECDC. Annual Epidemiological Report for 2019 (ECDC, Stockholm, 2023).
Diekema, D. J. et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 63, e00355–00319 (2019).
Ozlu, O. & Basaran, A. Infections in patients with major burns: a retrospective study of a burn intensive care unit. J. Burn Care Res. 43, 926–930 (2022).
Liu, Q. et al. Influence of carbapenem resistance on mortality of patients with Pseudomonas aeruginosa infection: a meta-analysis. Sci. Rep. 5, 11715 (2015).
Pérez, A. et al. High incidence of MDR and XDR Pseudomonas aeruginosa isolates obtained from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J. Antimicrob. Chemother. 74, 1244–1252 (2019).
López-Siles, M., Corral-Lugo, A. & McConnell, M. J. Vaccines for multidrug resistant gram negative bacteria: lessons from the past for guiding future success. FEMS Microbiol. Rev. 45, fuaa054 (2021).
Killough, M., Rodgers, A. M. & Ingram, R. J. Pseudomonas aeruginosa: recent advances in vaccine development. Vaccines 10, 1100 (2022).
Priebe, G. P. & Goldberg, J. B. Vaccines for Pseudomonas aeruginosa: a long and winding road. Expert Rev. Vaccines 13, 507–519 (2014).
Planet, P. J. Adaptation and evolution of pathogens in the cystic fibrosis lung. J. Pediatr. Infect. Dis. Soc. 11, S23–S31 (2022).
Freschi, L. et al. Genomic characterisation of an international Pseudomonas aeruginosa reference panel indicates that the two major groups draw upon distinct mobile gene pools. FEMS Microbiol. Lett. 365 (2018).
Patel, H. & Gajjar, D. Cell adhesion and twitching motility influence strong biofilm formation in Pseudomonas aeruginosa. Biofouling 38, 235–249 (2022).
Arhin, A. & Boucher, C. The outer membrane protein OprQ and adherence of Pseudomonas aeruginosa to human fibronectin. Microbiology 156, 1415–1423 (2010).
Paulsson, M., Su, Y. C., Ringwood, T., Uddén, F. & Riesbeck, K. Pseudomonas aeruginosa uses multiple receptors for adherence to laminin during infection of the respiratory tract and skin wounds. Sci. Rep. 9, 18168 (2019).
Buckley, A. M. et al. The AcrAB-TolC efflux system of Salmonella enterica serovar Typhimurium plays a role in pathogenesis. Cell. Microbiol. 8, 847–856 (2006).
Hsieh, P. F., Hsu, C. R., Chen, C. T., Lin, T. L. & Wang, J. T. The Klebsiella pneumoniae YfgL (BamB) lipoprotein contributes to outer membrane protein biogenesis, type-1 fimbriae expression, anti-phagocytosis, and in vivo virulence. Virulence 7, 587–601 (2016).
Takahashi, H. et al. Modification of lipooligosaccharide with phosphoethanolamine by LptA in Neisseria meningitidis enhances meningococcal adhesion to human endothelial and epithelial cells. Infect. Immun. 76, 5777–5789 (2008).
Moraes, C. T. et al. Flagellin and GroEL mediates in vitro binding of an atypical enteropathogenic Escherichia coli to cellular fibronectin. BMC Microbiol. 15, 278 (2015).
Ensgraber, M. & Loos, M. A 66-kilodalton heat shock protein of Salmonella typhimurium is responsible for binding of the bacterium to intestinal mucus. Infect. Immun. 60, 3072–3078 (1992).
Ur Rahman, M., Wang, P., Wang, N. & Chen, Y. A key bacterial cytoskeletal cell division protein FtsZ as a novel therapeutic antibacterial drug target. Bosn. J. Basic Med. Sci. 20, 310–318 (2020).
Buroni, S. et al. The cell division protein FtsZ as a cellular target to hit cystic fibrosis pathogens. Eur. J. Med. Chem. 190, 112132 (2020).
Rahman, M. U. et al. Assembly properties of bacterial tubulin homolog FtsZ regulated by the positive regulator protein ZipA and ZapA from Pseudomonas aeruginosa. Sci. Rep. 10, 21369 (2020).
Jeffery, C. Protein moonlighting: what is it, and why is it important?. Philos. Trans. R. Soc. B 373, 20160523 (2017).
Barbier, M. et al. Lysine trimethylation of EF-Tu mimics platelet-activating factor to initiate Pseudomonas aeruginosa pneumonia. mBio 4, e00207–e00213 (2013).
Hallström, T. et al. Pseudomonas aeruginosa uses dihydrolipoamide dehydrogenase (Lpd) to bind to the human terminal pathway regulators vitronectin and clusterin to inhibit terminal pathway complement attack. PLoS ONE 10, e0137630 (2015).
Bottomley, A. L. et al. The novel E. coli cell division protein, YtfB, plays a role in eukaryotic cell adhesion. Sci. Rep. 10, 6745 (2020).
Gulati, S. et al. Preclinical efficacy of a cell division protein candidate gonococcal vaccine identified by artificial intelligence. mBio 14, e0250023 (2023).
Gallotta, M. et al. SpyAD, a moonlighting protein of group A Streptococcus contributing to bacterial division and host cell adhesion. Infect. Immun. 82, 2890–2901 (2014).
Zavan, L. et al. The mechanism of Pseudomonas aeruginosa outer membrane vesicle biogenesis determines their protein composition. Proteomics 23, e2200464 (2023).
Feng, L. et al. Immunogenicity and protective capacity of EF-Tu and FtsZ of Streptococcus suis serotype 2 against lethal infection. Vaccine 36, 2581–2588 (2018).
Sheweita, S. A. et al. Bacterial ghosts of Pseudomonas aeruginosa as a promising candidate vaccine and its application in diabetic rats. Vaccines 10, 910 (2022).
Farjah, A. et al. Immunological evaluation of an alginate-based conjugate as a vaccine candidate against Pseudomonas aeruginosa. APMIS 123, 175–183 (2015).
Sokol, P. A., Kooi, C., Hodges, R. F., Cachia, P. & Woods, D. E. Immunization with a Pseudomonas aeruginosa elastase peptide reduces severity of experimental lung infections due to P. aeruginosa or Burkholderia cepacia. J. Infect. Dis. 81, 1682–1692 (2000).
Jurado-Martín, I. et al. Development of acute Pseudomonas aeruginosa and Acinetobacter baumannii lung mono-challenge models in mice using oropharyngeal instillation. Access Microbiol. 000860.v1 (2024).
Chuanchuen, R., Murata, T., Gotoh, N. & Schweizer, H. P. Substrate-dependent utilization of OprM or OpmH by the Pseudomonas aeruginosa MexJK efflux pump. Antimicrob. Agents Chemother. 49, 2133–2136 (2005).
Loots, K., Revets, H. & Goddeeris, B. M. Attachment of the outer membrane lipoprotein (OprI) of Pseudomonas aeruginosa to the mucosal surfaces of the respiratory and digestive tract of chickens. Vaccine 26, 546–551 (2008).
Cassin, E. K. & Tseng, B. S. Pushing beyond the envelope: the potential roles of OprF in Pseudomonas aeruginosa biofilm formation and pathogenicity. J. Bacteriol. 201, e00050–00019 (2019).
Li, M. et al. Evaluation of immunogenicity and protective efficacy of recombinant outer membrane proteins of Haemophilus parasuis serovar 5 in a murine model. PLoS ONE 12, e0176537 (2017).
Kawamoto, S. et al. Site-directed mutagenesis of Glu-141 and His-223 in Pseudomonas aeruginosa elastase: catalytic activity, processing, and protective activity of the elastase against Pseudomonas infection. Infect. Immun. 61, 1400–1405 (1993).
Matsumoto, T. et al. Efficacies of alkaline protease, elastase and exotoxin A toxoid vaccines against gut-derived Pseudomonas aeruginosa sepsis in mice. J. Med. Microbiol. 47, 303–308 (1998).
Kashef, N. et al. Synthesis and characterization of Pseudomonas aeruginosa alginate-tetanus toxoid conjugate. J. Med. Microbiol. 55, 1441–1446 (2006).
Zuercher, A. W. et al. Antibody responses induced by long-term vaccination with an octovalent conjugate Pseudomonas aeruginosa vaccine in children with cystic fibrosis. FEMS Immunol. Med. Microbiol. 47, 302–308 (2006).
Broquet, A. et al. Depletion of natural killer cells increases mice susceptibility in a Pseudomonas aeruginosa pneumonia model. Crit. Care Med. 42, e441–e450 (2014).
Feehan, D. D. et al. Natural killer cells kill extracellular Pseudomonas aeruginosa using contact-dependent release of granzymes B and H. PLoS Pathog. 18, e1010325 (2022).
Embgenbroich, M. & Burgdorf, S. Current concepts of antigen cross-presentation. Front. Immunol. 9, 1643 (2018).
Baljon, J. J. & Wilson, J. T. Bioinspired vaccines to enhance MHC class-I antigen cross-presentation. Curr. Opin. Immunol. 77, 102215 (2022).
Ho, N. I., Huis In ‘t Veld, L. G. M., Raaijmakers, T. K. & Adema, G. J. Adjuvants enhancing cross-presentation by dendritic cells: the key to more effective vaccines? Front. Immunol. 9, 2874 (2018).
Tomas-Cortazar, J. et al. BpOmpW antigen stimulates the necessary protective T-cell responses against melioidosis. Front. Immunol. 12, 767359 (2021).
Tomas-Cortazar, J. et al. BpOmpW antigen administered with CAF01 adjuvant stimulates comparable T cell responses to Sigma adjuvant system. Vaccine X 17, 100438 (2024).
Shindo, Y. et al. Interleukin 7 immunotherapy improves host immunity and survival in a two-hit model of Pseudomonas aeruginosa pneumonia. J. Leukoc. Biol. 101, 543–554 (2017).
Ma, C. et al. A novel inactivated whole-cell Pseudomonas aeruginosa vaccine that acts through the cGAS-STING pathway. Signal Transduct. Target. Ther. 6, 353 (2021).
Behrouz, B. et al. Immunization with bivalent flagellin protects mice against fatal Pseudomonas aeruginosa pneumonia. J. Immunol. Res. 2017, 5689709 (2017).
Staczek, J., Gilleland, L. B., van der Heyde, H. C. & Gilleland, H. E. DNA vaccines against chronic lung infections by Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 37, 147–153 (2003).
Kinjo, T. et al. NKT cells play a limited role in the neutrophilic inflammatory responses and host defense to pulmonary infection with Pseudomonas aeruginosa. Microbes Infect. 8, 2679–2685 (2006).
Smith, D. J., Hill, G. R., Bell, S. C. & Reid, D. W. Reduced mucosal associated invariant T-cells are associated with increased disease severity and Pseudomonas aeruginosa infection in cystic fibrosis. PLoS ONE 9, e109891 (2014).
Benoit, P. et al. The role of CD1d-restricted NKT cells in the clearance of Pseudomonas aeruginosa from the lung is dependent on the host genetic background. Infect. Immun. 83, 2557–2565 (2015).
Tanno, H. et al. Contribution of invariant natural killer T cells to the clearance of Pseudomonas aeruginosa from skin wounds. Int. J. Mol. Sci. 22, 3931 (2021).
Cabral, M. P. et al. A live auxotrophic vaccine confers mucosal immunity and protection against lethal pneumonia caused by Pseudomonas aeruginosa. PLoS Pathog. 16, e1008311 (2020).
Jackson, A. D. et al. Validation and use of a parametric model for projecting cystic fibrosis survivorship beyond observed data: a birth cohort analysis. Thorax 66, 674–679 (2011).
Vidaillac, C., Yong, V. F. L., Jaggi, T. K., Soh, M. M. & Chotirmall, S. H. Gender differences in bronchiectasis: a real issue? Breathe 14, 108–121 (2018).
Zhou, Y. Y. et al. Gender differences in clinical characteristics of patients with non-cystic fibrosis bronchiectasis in different age groups in northern China. Clin. Respir. J. 17, 311–319 (2023).
Baumann, U., Göcke, K., Gewecke, B., Freihorst, J. & von Specht, B. U. Assessment of pulmonary antibodies with induced sputum and bronchoalveolar lavage induced by nasal vaccination against Pseudomonas aeruginosa: a clinical phase I/II study. Respir. Res. 8, 57 (2007).
Baker, S. M., McLachlan, J. B. & Morici, L. A. Immunological considerations in the development of Pseudomonas aeruginosa vaccines. Hum. Vaccines Immunother. 16, 412–418 (2020).
Rello, J. et al. A randomized placebo-controlled phase II study of a Pseudomonas vaccine in ventilated ICU patients. Crit. Care 21, 22 (2017).
Yang, F. et al. Protective efficacy of the trivalent Pseudomonas aeruginosa vaccine candidate PcrV-OprI-Hcp1 in murine pneumonia and burn models. Sci. Rep. 7, 3957 (2017).
O’Connor, A. et al. A universal stress protein upregulated by hypoxia has a role in Burkholderia cenocepacia intramacrophage survival: implications for chronic infection in cystic fibrosis. Microbiologyopen 12, e1311 (2023).
Shevchenko, A., Tomas, H., Havlis, J., Olsen, J. V. & Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 (2006).
Consortium, U. UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Res. 51, D523–D531 (2023).
Chen, C., Zabad, S., Liu, H., Wang, W. & Jeffery, C. MoonProt 2.0: an expansion and update of the moonlighting proteins database. Nucleic Acids Res. 46, D640–D644 (2018).
Ong, E. et al. Vaxign2: the second generation of the first web-based vaccine design program using reverse vaccinology and machine learning. Nucleic Acids Res. 49, W671–W678 (2021).
Zaharieva, N., Dimitrov, I., Flower, D. R. & Doytchinova, I. VaxiJen dataset of bacterial immunogens: an update. Curr. Comput. Aided Drug Des. 15, 398–400 (2019).
Yu, C. S. et al. CELLO2GO: a web server for protein subCELlular LOcalization prediction with functional gene ontology annotation. PLoS ONE 9, e99368 (2014).
Teufel, F. et al. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 40, 1023–1025 (2022).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Winsor, G. L. et al. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 44, D646–D653 (2016).
Macho Rendón, J., Lang, B., Ramos Llorens, M., Gaetano Tartaglia, G. & Torrent Burgas, M. DualSeqDB: the host-pathogen dual RNA sequencing database for infection processes. Nucleic Acids Res. 49, D687–D693 (2021).
Malyala, P. & Singh, M. Endotoxin limits in formulations for preclinical research. J. Pharm. Sci. 97, 2041–2044 (2008).
Perez-Riverol, Y. et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47, D442–D450 (2019).
Acknowledgements
This study was funded by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska–Curie grant agreement BactiVax (anti-Bacterial Innovative Vaccines) number 860325. It was also partially funded by the Higher Education Authority, Department of Further and Higher Education, Research, Innovation and Science and the Shared Island Fund under the North-South Research Programme, Strand 2 grant agreement AIVRT. The funders played no role in the study design, data collection, analysis and interpretation of data, or the writing of this manuscript. The authors acknowledge the help from the staff of the Biomedical Facility at UCD.
Author information
Authors and Affiliations
Contributions
S.McC. conceived the study; I.J.-M. identified, characterised and prepared the vaccine antigens; I.J.-M., J.T.-C., Y.H. and M.S.-M. performed immunisation and challenge studies; I.J.-M. and J.T.-C. performed flow cytometry experiments; J.T.-C. performed ELISpot; I.J.-M., M.M.M. and J.C.S. performed confocal microscopy experiments; O.S., J.H. and F.R.-S. performed OPA experiments; J.A.B. developed and performed acute infection model. All authors participated in the data analysis. S.McC. supervised the study. I.J.-M. and S.McC. wrote and revised the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. University College Dublin has filed a patent application related to this study with I.J.-M. and S.McC. as inventors; UK IPO application number: 2405503.0. The application covers the identification of FtsZ and other P. aeruginosa antigens as protective vaccine candidates in mice.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jurado-Martín, I., Tomás-Cortázar, J., Hou, Y. et al. Proteomic approach to identify host cell attachment proteins provides protective Pseudomonas aeruginosa vaccine antigen FtsZ. npj Vaccines 9, 204 (2024). https://doi.org/10.1038/s41541-024-00994-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-024-00994-x