Abstract
Staphylococcus aureus has been posing a significant global health threat, underscoring an urgent need for innovative preventive strategies, notably vaccines. This study presents an evaluation of a multi-target mRNA vaccine against S. aureus, engineered to target five pivotal virulence factors: the manganese transporter MntC, enterotoxin SEB, exotoxin HLA, adhesion factor FnBPA, and iron surface binding protein IsdB. In a parallel control setting, mice were immunized with either monovalent mRNA-LNPs, a multivalent mRNA-LNP cocktail, or a protein cocktail, and were subsequently assessed for humoral and cellular immune responses as well as the vaccines’ protective efficacy. The findings demonstrated that the multivalent mRNA-LNP cocktail vaccine induced a robust and sustained humoral immune response, along with a stronger cellular immune response compared to both monovalent mRNA vaccines and the protein cocktail vaccine. This was characterized by increased secretion of IFN-γ, IL-2, IL-4, and IL-17A, suggesting a potent Th1/Th2/Th17 mixed immune response. Moreover, the cocktail vaccine demonstrated improved survival rates and a reduction in bacterial loads and organ damage. These results underscore the promise of a multi-target mRNA vaccine strategy in combating antibiotic-resistant Staphylococcus aureus.
Similar content being viewed by others

Introduction
Staphylococcus aureus is a formidable bacterial pathogen capable of causing a spectrum of infections, ranging from superficial skin infections to severe, life-threatening conditions such as pneumonia, sepsis, and endocarditis1,2. The rise of antibiotic-resistant strains, notably methicillin-resistant S. aureus (MRSA) and vancomycin-resistant S. aureus (VRSA), has compromised the efficacy of traditional antibiotic treatments, precipitating a global health crisis and highlighting the urgent need for novel prophylactic and therapeutic interventions3,4. Vaccines, leveraging the multifaceted human immune system, are considered a promising alternative due to their potential to prevent infections and reduce the development of antibiotic resistance5.
The historical development of S. aureus vaccines dates back to 1902, with Dr. Wright’s use of a heat-killed S. aureus vaccine to treat recurrent furunculosis6. However, despite advancements, clinical trials of several S. aureus vaccines have ended in failure, with no approved vaccine candidates to date. Notable examples include StaphVAX, which targeted capsular polysaccharides type 5 and 8 (CP5 and CP8), and failed to meet the primary endpoint for reducing S. aureus bacteremia (NCT00130260)7,8,9. Pfizer’s SA4Ag, composed of recombinant MntC, ClfA, and CP5 and CP8 conjugated to a detoxified form of diphtheria toxin, showed high immunogenicity in Phase I trials but failed to demonstrate efficacy in a subsequent Phase IIb trial (NCT02388165)10,11,12. Merck’s V710 vaccine, including the iron surface determinant factor B (IsdB), minimally reduced surgical site infections in a Phase III clinical trial, with an alarming increase in mortality among patients with postoperative S. aureus infections (NCT00518687)13,14. These setbacks have underscored the multifaceted pathogenesis and immune evasion mechanisms of S. aureus15,16,17, driving vaccine development towards multivalent formulations, such as GSK’s SA-5Ag vaccine18, Olymvax’s rFSAV19 and Integrated Biotherapeutics’ IBT-V0220, which target multiple antigens.
The success of COVID-19 mRNA vaccines has accelerated the adoption of mRNA vaccine technology, offering a promising avenue for combating bacterial infections21,22,23,24. mRNA vaccines instruct host cells to produce antigens through synthetic mRNA, eliciting an immune response. This approach offers rapid development, scalability, and the ability to encode multiple antigens, which is particularly beneficial for multivalent vaccine development25. Moreover, mRNA vaccines can induce both humoral and robust cellular immune responses, crucial for clearing intracellular pathogens like S. aureus. To our knowledge, only Guo et al. have reported an mRNA vaccine targeting Staphylococcal enterotoxin B (SEB) to date26, and more in-depth studies are still required to advance this emerging field.
In this study, we present a multi-target mRNA vaccine strategy against S. aureus, hypothesizing that targeting multiple virulence factors would elicit a more comprehensive immune response compared to single-target vaccines. We conducted systemic immunological evaluations in murine models, assessing antibody responses, T-cell activation, cytokine production, and protection against bacterial challenge. Through this approach, we aim to determine the efficacy of mRNA vaccines in preventing bacterial infections and contribute to the development of next-generation vaccines capable of overcoming the growing threat of antibiotic resistance.
Results
Rationale for the selection of vaccine antigens
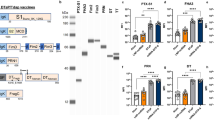
The failure of a series of S. aureus vaccine candidates has highlighted the insufficiency of single-target vaccines to address the extensive antigenic variability characteristic of S. aureus18,27,28. Therefore, we have opted to develop a multivalent vaccine strategy targeting multiple antigens of S. aureus. This approach is designed to include a range of bacterial toxin molecules, outer membrane proteins, and proteins vital to bacterial growth and metabolism, which have previously shown to be immunologic and protective1. In this study, we have selected five conserved antigens to address this challenge, including the manganese transporter MntC29,30, a detoxified variant of the enterotoxin SEB with mutations at L45R, Y89A, and Y94A31,32, an inactivated exotoxin Hla mutant (H35L)16,33,34, the immunodominant region (110-263) of the adhesion factor FnBPA35, and the N2 domain (348-465) of the iron surface binding protein IsdB15,36. Vaccines based on these antigens are intended to neutralize or block key virulence factors of S. aureus. To assess the conservation of our vaccine targets across various S. aureus strains, we performed a comparative genomic analysis. This involved aligning the amino acid sequences of our target antigens with those from 99 S. aureus strains retrieved from NCBI. Our analysis demonstrated that the proteins MntC, HLA, and IsdB exhibit a high degree of conservation, with average amino acid sequence similarities exceeding 98%. Additionally, FnBPA shows significant conservation, with an average amino acid sequence similarity of 87.27%, and SEB displays a moderate level of conservation, with an average amino acid sequence similarity of 59.69% (Fig. 1A). Together, this conservation profile underscores its potential as a robust candidate for developing a broadly protective vaccine, positioning it to overcome challenges posed by strain variability and enhance the translational viability of vaccine development.
Preparation and characterization of mRNA-LNPs and antigen proteins
To evaluate the vaccine potential of the five selected antigens—MntC, SEB, Hla, FnBPA, and IsdB, we synthesized their corresponding mRNAs through T7 polymerase-mediated in vitro transcription, with uridine (U) replaced by N1-methyl-pseudouridine nucleoside to enhance mRNA stability and reduce mRNA immunogenicity, the synthesized mRNA molecule primarily includes a 5′ cap, 5′ and 3′ untranslated regions (UTRs), an open reading frame (ORF), and a 3′ poly(A) tail. (Fig. 1B). The integrity of the synthesized mRNA molecules was confirmed as high by both capillary electrophoresis (Fig. 1C) and agarose gel electrophoresis (Supplementary Fig. 1). For in vivo delivery, the mRNA was encapsulated into lipid nanoparticles (LNPs) using microfluidic technology. The resulting LNPs had an average size of ~100 nm (ranging from 80 to 115 nm) as determined by dynamic light scattering, and a polydispersity index (PDI) below 0.2 (Fig. 1D), indicating a uniform particle size distribution. This homogeneity was further confirmed by cryo-electron microscopy (cryo-EM) analysis (Supplementary Fig. 2). The encapsulation efficiency of mRNA within the LNPs post-encapsulation exceeded 90%, as quantified using the Quant-iTTM RiboGreenTM RNA kit (Fig. 1E). Transfection of HEK293T cells and macrophages J774A.1 with each mRNA-LNPs resulted in high expression levels of the target proteins, as detected by western blot (Fig. 1F). The original blots are given in Supplementary Fig. 3. Notably, WB analysis revealed multiple electrophoretic bands for both FnBPA and IsdB, and a single band for the Hla protein, which was larger than its predicted molecular weight, suggesting the presence of post-translational modifications such as glycosylation (Supplementary Fig. 4). Moreover, cell viability remained above 90% following transfection with high doses of mRNA-LNPs, demonstrating the low toxicity of our mRNA-LNP formulations (Fig. 1G). In addition, the mRNA-LNPs exhibited no hemolytic activity against mouse erythrocytes (Supplementary Fig. 5). Following intramuscular or intravenous injection of 50 μg multivalent mRNA-LNPs, serum levels of alanine transaminase (ALT), aspartate transaminase (AST), creatinine and urea displayed slight elevation at 24 h post-injection. These transient increases may be attributed to mild immune activation induced by antigen expression, which triggered a localized inflammatory response. By 72 h, all parameters had returned to baseline levels, indicating that the LNPs do not cause persistent tissue damage (Supplementary Fig. 6). Subsequently, the five target proteins were expressed using an Escherichia coli expression system and purified to high homogeneity, as confirmed by SDS-PAGE analysis (Fig. 1H).
A The maximum likelihood phylogenetic tree illustrating the amino acid sequence identity of the antigens (MntC, SEB, Hla, FnBPA, and IsdB) encoded by the mRNA constructs, compared to those from 99 S. aureus strains, was created using Parsnp. B Schematic of five mRNA constructs used in this study. C Capillary electrophoresis image displaying the quality and purity of in vitro synthesized mRNAs. D Particle size distribution of mRNA-LNPs as detected by Malvern particle size analyzer, data presented as average particle diameter. PDI Polydispersity Index. E Average encapsulation efficiency of mRNA-LNPs. F Western Blot analysis showing protein expression in 293T and J774A.1 cells 48 h post-transfection with mRNA-LNPs. NT, no-treatment. G CCK-8 assay evaluating the viability of HEK293T cells 72 h post-transfection with mRNA-LNPs. H SDS-PAGE analysis showing the purity of purified target proteins. Data in (D, E, G) were presented as mean ± SD, n = 3.
Humoral immune response evaluation of monovalent and multivalent mRNA-LNP/protein vaccines
Subsequently, we immunized mice to evaluate the humoral immune responses induced by monovalent, multivalent mRNA-LNP and protein vaccines. Mice were divided to eight treatment groups: one group received blank LNPs (without payload) as a negative control (NC), five groups received individual mRNA-LNPs (10 μg per component), one group received a five-component mRNA-LNPs cocktail (10 μg per component, total 50 μg), and one group received a cocktail of five corresponding proteins (10 ug per component, total 50 μg). Vaccinations were administered intramuscularly in two doses, with a 2-week interval between doses. Serum samples were collected on specified days for the detection of antigen-specific antibodies (Fig. 2A). ELISA analysis revealed that both individual and cocktail vaccines, whether mRNA or protein-based, induced modest antigen-specific antibody titles after the initial immunization (D14), with a slightly increase following the booster immunization (D21, D28) (Fig. 2B-F). Consistent with prior observations in multivalent influenza vaccine37, both monovalent and multivalent mRNA-LNP vaccines maintained robust antibody titers against their antigens, regardless of valency. Direct comparison of the mRNA cocktail vaccine and protein cocktail vaccine revealed antigen-specific disparities: the mRNA vaccine elicited significantly higher anti-MntC (Fig. 2B) and anti-SEB titers (Fig. 2C) than its protein counterpart, whereas anti-Hla (Fig. 2D) and anti-FnBPA (Fig. 2E) responses were significantly lower. Anti-IsdB antibody levels were comparable between the two platforms (Fig. 2F). These differential responses may reflect antigen competition or a shift in immune response dominance, or they may be due to the uncertainty in mRNA translation efficiency within the body, making it difficult to directly compare equivalent doses of mRNA and protein. Additionally, antigen post-translational modifications may contribute to observed differences. Antibody isotyping analysis revealed rapid IgM production (Fig. 2G) followed by a mixed IgG1/IgG2 response, with IgG1 levels modestly exceeding IgG2 (Fig. 2H). This profile suggests a balanced Th1/Th2 polarization with a slight Th1 bias. Collectively, these data underscore the antigen-specific immunogenicity of mRNA-LNP cocktails and highlight their potential for tailoring immune responses.
A Schematic overview of the experimental timeline for vaccination, blood collection, challenge, and cellular immunity assessment drawn with FIGDRAW. B–F The antigen-specific antibody titers detected by ELISA. Serum samples were collected on day 14 post-primary immunization, and on days 21 (7 days post-booster) and 28 (14 days post-booster) following the booster immunization. Antigen-specific IgG titers against MntC (B), SEB (C), Hla (D), FnBPA (E), and IsdB (F) were analyzed by ELISA. G Specific IgM titers induced on day 7 post-immunization with monovalent mRNA-LNPs. H Antibody titers of IgG1 and IgG2a induced on day 28 by five monovalent mRNA-LNP vaccines. Data are represented as mean ± SD and analyzed by one-way ANOVA with Tukey’s multiple comparisons test. n = 5. ns represents no significant, p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****).
The evaluation of cellular immune responses induced by monovalent and multivalent mRNA-LNP/protein vaccines
To comprehensively evaluate the cellular immune responses in above eight groups of mice following vaccination, splenocytes were harvested on day 21 for ELISPOT analysis to assess the secretion of cytokines in response to antigen stimulation. The ELISPOT results demonstrated a robust cellular immune response, particularly in mice vaccinated with the multivalent mRNA-LNP cocktail, which exhibited significantly higher levels of IFN-γ (Fig. 3A), IL-2 (Fig. 3B), IL-4 (Fig. 3C), and IL-17A (Fig. 3D) compared to other groups. These results suggested a potent Th1/Th2/Th17 mixed immune response, reflecting broad and effective immune activation. Additionally, the mRNA-FnBPA vaccine also induced a stronger cytokine response than other monovalent mRNA vaccine (Fig. 3), suggesting its potential as one of the key antigens in vaccine formulations. Furthermore, the flow cytometry data also demonstrated the multivalent mRNA-LNP cocktail vaccine induced a higher proportion of CD4+ T cells and CD8 + T cells secreting IFN-γ, IL-2, IL-4, IL-17A compared to the protein cocktail vaccine (Fig. 4, Supplementary Fig. 7).
Elispot assay quantifying IFN-γ (A), IL-2 (B), IL-4 (C), and IL-17A (D) secretion from mice splenocytes collected 21 days post-immunization and stimulated with recombinant proteins. Data are represented as mean ± SD and analyzed by one-way ANOVA with Dunnett’s post-hoc test. n = 3. ns represents no significant, p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****).
CD8+ T cells and CD4+ T cells in splenic lymphocytes were assayed for IFN-γ+ (A, B), IL-2+ (C, D), IL-4+ (E, F), and IL-17A+ (G, H) expression by flow cytometry after restimulation with corresponding proteins. Data are represented as mean ± SD and analyzed by one-way ANOVA with Tukey’s multiple comparisons test. n = 5. ns represents no significant. p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****).
Protective efficacy of monovalent and multivalent mRNA-LNP/protein vaccines against Staphylococcus aureus infection in murine models
Building on the immune responses characterized above, we next assessed the functional activity and protective efficacy of the vaccine candidates. The opsonophagocytic killing (OPK) assay was then used to evaluated the bactericidal activity of vaccine-candidate-raised antibodies. The results indicate that, the sera from mice immunized with vaccines exhibited higher opsonophagocytic activity against S. aureus (Fig. 5A). Quantification of intracellular bacterial loads further confirmed enhanced macrophage phagocytosis of S. aureus in vaccine serum-treated cultures, demonstrating the functional potency of the humoral response (Supplementary Fig. 8). Subsequently, the protective efficacy of monovalent and multivalent mRNA-LNP and protein vaccines against Staphylococcus aureus infection was assessed by challenging mice with MRSA strain (MRSA252) 2 weeks following the booster immunization. We initially employed a systemic infection model to assess the protective efficacy of the vaccines. The model was established by administering an intravenous (IV) injection of a lethal dose of Staphylococcus aureus (1 × 109 CFU). Three independent parallel experiments demonstrated that the multivalent mRNA vaccine provided the best protection efficiency, followed by the protein vaccine, which outperformed all monovalent vaccines. Among five monovalent mRNA vaccines, Hla mRNA-LNP vaccine exhibited the highest protective efficacy, followed by MntC and SEB mRNA vaccines, while FnBPA and IsdB mRNA vaccines provided minimal efficacy. These trends were supported by bacterial burden analyses in blood, lung, and kidneys, which showed the lowest bacterial loads in mice vaccinated with the multivalent mRNA, multivalent protein, and Hla monovalent vaccines (Fig. 5D–F). Additionally, histological analysis of lung tissues revealed markedly improved pulmonary conditions in vaccinated mice, particularly those receiving the mRNA cocktail vaccine, as compared to the control group that received blank LNPs. These improvements included enhanced alveolar integrity, diminished inflammatory cell infiltration, and reduced severity of hemorrhage and tissue damage (Fig. 5G, H). Similar improvements were observed in liver, spleen, and kidney tissues (Supplementary Fig. 9).
A Comparative analysis of the opsonophagocytic killing activity of immune serum against Staphylococcus aureus. B Survival rate of mice over 7 days after challenge with a lethal dose of the MRSA252. C Average survival rate of three parallel experiments (n = 7). D–F Bacterial load per milliliter of blood, per gram of lung and kidney tissue 24 h after challenge with a sublethal dose of the MRSA252 (5 × 108). n = 5. Asterisks beneath each experimental group denote statistical significance versus the control group. G Hematoxylin and eosin (H&E) stained histopathological images of mice lung tissues 48 h after challenge with a lethal dose of the MRSA252. H Severity scores of lungs (n = 5) from mice 48 h post infection were shown. Data are represented as mean ± SD and analyzed by one-way ANOVA with Dunnett’s post-hoc test (C, H) and Tukey’s multiple comparisons test (D, E, F). ns represents no significant. p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****).
Concurrent with the systemic infection model, we established a pneumonia infection model to further evaluate the vaccines’ protective efficacy. This model was induced by intranasal administration of a sub-lethal dose of Staphylococcus aureus (5 × 108). Compared to the control group, the vaccinated group exhibited a significant reduction in neutrophil number (Fig. 6A) and pulmonary bacterial loads (Fig. 6B), although no significant differences were observed among the vaccinated groups. Histological examination of lung tissues in the pneumonia model, similar to the systemic infection model, revealed marked improvements in the vaccinated groups (Fig. 6C, D).
A Evaluation of neutrophil percentage in blood of infected mice. B Bacterial load per gram of lung tissue in a pneumonia infection model following a sublethal dose of MRSA252 at 24 h post-infection. C Severity scores of lungs (n = 5) from mice 48 h post infection were shown. D Hematoxylin and eosin (H&E) stained histopathological images of mice lung tissues.
Discussion
The development of an effective vaccine against Staphylococcus aureus (S. aureus) has been a significant challenge in the field of infectious disease research. Over the past two decades, multiple attempts have been made, yet the success of these endeavors has been limited by several factors, including the pathogen’s adaptability, antigenic diversity, and the complexity of the immune response required for effective immunity18. The present study evaluates a multi-target mRNA vaccine strategy against S. aureus, targeting five key virulence factors: MntC, SEB, HLA, FnBPA, and IsdB. This approach was designed to elicit a robust humoral and cellular immune response, which is crucial for combating this pathogen.
The rationale for selecting these antigens is based on their roles in the pathogenesis of S. aureus, including bacterial toxins, immune evasion factors, and proteins associated with bacterial growth and metabolism. The multivalent vaccine strategy aims to provide broad coverage against the diverse antigenic landscape of S. aureus, thereby enhancing the likelihood of vaccine success.
This study has presented a comprehensive evaluation of both humoral and cellular immune responses elicited by monovalent, multivalent mRNA-LNPs and multivalent protein vaccines targeting S. aureus. The mRNA-LNP platform induced robust antigen-specific antibody titers, with notable differences across targets. The multivalent cocktail elicited significantly higher IgG titers against MntC (Fig. 2B) and SEB (Fig. 2C) compared to the protein vaccine, likely due to native-like antigen folding enabled by endogenous expression. In contrast, lower anti-Hla and anti-FnBPA titers in the multivalent mRNA group (Fig. 2D, E) may reflect antigenic competition or epitope masking caused by post-translational modifications (e.g., glycosylation of FnBPA, Fig. S3). The IgG1/IgG2a balance (Fig. 2H) indicates a mixed Th1/Th2 polarization, with IgG1 (Th2-associated) slightly predominating. This balanced response is advantageous: IgG1 enhances opsonophagocytic via Fcγ receptors on macrophages, while IgG2a (Th1-associated) promotes complement activation and neutrophil-mediated killing38. Functional validation via OPK assays confirmed that vaccine-induced antibodies enhanced bacterial clearance (Fig. 5A), correlating with reduced bacterial loads in systemic and pulmonary challenge models (Figs. 5D–F, 6B). The multivalent mRNA-LNP vaccine outperformed protein counterparts in activating T-cell responses. ELISPOT data revealed elevated IFN-γ (Th1), IL-2 (Th1), IL-4 (Th2), and IL-17A (Th17) secretion (Fig. 3A–D), suggesting coordinated activation of multiple T-helper subsets. IFN-γ primes macrophages for intracellular pathogen killing39, critical for eliminating S. aureus persisting in phagosomes, and IL-17A recruits neutrophils and strengthens mucosal barriers, explaining the reduced lung pathology in vaccinated mice40,41. Moreover, the activated CD8 + T cells may target S. aureus-infected host cells, a mechanism rarely exploited by traditional subunit vaccines42.
The protective efficacy observed in murine models demonstrates that the multivalent mRNA-LNP cocktail vaccine outperformed both monovalent mRNA vaccines and protein-based cocktail vaccines in terms of survival rates and bacterial load reduction post-challenge. This superior performance may be attributed to two key factors. First, endogenous antigen expression via mRNA enables native-like post-translational modifications, critical for conformational epitope presentation43. Second, LNPs act as adjuvants through TLR7/8 activation, synergizing with antigen-specific responses44,45.
While this study demonstrates the feasibility of multi-target mRNA vaccines against S. aureus, several limitations warrant attention. First, protective efficacy was tested against a single MRSA strain (MRSA252), leaving coverage of diverse clinical strains unaddressed. Second, the durability of long-term memory cell responses and sustained protection beyond 28 days remain uncharacterized. Third, the potential for antigen competition or immunodominance shifts in multivalent formulations is not systematically evaluated. Fourth, due to the uncertainty of mRNA translation efficiency in the body, it is necessary to explore the impact of dose and immune regimen on the protective effect. Finally, the study does not model the effects of pre-existing immunity to vaccine antigens, a critical factor in clinical populations. Addressing these gaps—including cross-strain efficacy testing, long-term immune monitoring, antigen formulation optimization, and pre-existing immunity assessments—will be essential to strengthen the clinical translation potential of this vaccine strategy. Addressing these gaps in future studies will strengthen clinical translation potential.
In conclusion, our study demonstrates that a multi-target mRNA vaccine against S. aureus is feasible and effective, offering a promising approach to combat antibiotic-resistant infections.
Methods
Animals
Specific pathogen-free female 6–8 weeks old BALB/c mice (20–30 g) were purchased from Kangde Biological (Guangzhou, China) and kept under specific pathogen-free (SPF) conditions. This study was performed under strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. This protocol was approved by the laboratory animal ethics committee of the Southern University of Science and Technology (JY202308066) and The Hong Kong Polytechnic University (NO.22-23/286-OTHERS-R-SZG). Primary vaccination was performed when the mice were 7–8 weeks old.
Bacteria
The standard S. aureus strain MRSA252 was a gift from Professor Hao Zeng of Army Medical University. Bacteria were revived and cultured on tryptic soy agar (TSA) overnight at 37 °C. The next day, one colony was transferred into tryptic soy broth (TSB) and cultured in a shaking incubator (200 rpm) overnight at 37 °C. The bacterial culture was diluted 1:100 in fresh TSB and cultured at 37 °C for 2 h at least. The concentration was measured at OD600 using a Nanodrop 2000c spectrophotometer (Thermo Fisher Scientific, US). The bacteria were centrifuged at 8000 × g and resuspended in sterile PBS before use, and stored at 4 °C until needed. Counts confirmed by CFU assay on tryptic soy agar (TSA).
Phylogenomic analyses
A maximum likelihood phylogenetic tree was constructed using Parsnp v1.7.4 for 99 Staphylococcus aureus isolates from NCBI (Supplementary Table 1). The phylogenetic tree was visualized using intermediate nodes in the interactive Tree Of Life (iTOL) v6.8 tool. Homology comparisons for five selected target proteins were performed across these 99 S. aureus strains using the BLAST tool, generating homology scores that were subsequently used for visualization. For further visualization and phylogenetic annotation, ggplot2 and ggtree were employed.
In vitro transcription of mRNA
MntC, SEB, Hla, FnBPA, and IsdB protein sequences were retrieved from Uniprot (Supplementary Note 1). The vaccine sequence was composed of a 5’ UTR, coding region, 3’ UTR, and a polyA tail. The leader sequence of tissue plasminogen activator was added to the N-terminus of this segment, and a 6×His tag was appended to the C-terminus. This sequence was cloned into the PVAXI vector for expression. The gene synthesis and cloning were performed by GeneWiz Biotech Co., Ltd. (Suzhou, China). The linearized plasmid DNA served as the template for the synthesis of optimized mRNA via an in vitro transcription reaction mediated by an optimized T7 RNA polymerase. The reaction components were added in the following order: nuclease-free water, 7.5 mM of each NTP (ATP, GTP, N1-methyl-pseudouridine triphosphate, CTP), 6 mM Clean Cap® Reagent AG (3’ OMe), 10× HY T7 Buffer, 1 µg/20 µL template DNA, 4 U/µL RNase Inhibitor, 0.005 U/µL inorganic pyrophosphatase, and finally, 5 U/µL T7 RNA Polymerase. The reaction was incubated at 37 °C for 2 h, followed by the addition of 0.5 U/µL DNase I and incubation at 37 °C for 30 min. Purify mRNA using the Monarch® Spin RNA Cleanup Kit (New England Biolabs, US).
Capillary electrophoresis and agarose gel electrophoresis
RNA integrity was evaluated by the 28S/18S ribosomal RNA ratio and an RNA Quality Number, using the Bioptic Qsep 100 Capillary Electrophoresis System (Bioptic, Taiwan, China) equipped with an R1 RNA cartridge. Samples of mRNA were denatured at 70 °C for 5 min before being transferred to microvials for loading onto the instrument. The separation conditions were set to run at 4 kV for 8 min. The separated mRNA was visualized in the electropherogram as peaks with distinct migration times. The instrument was operated according to the manufacturer’s protocols.
The denatured mRNA was simultaneously loaded into a 1% agarose gel for electrophoresis to analyze its integrity. The conditions were set to 5 V/cm, 30 min.
mRNA-LNP preparation
Lipid nanoparticle (LNP) formulations were prepared with organic phase components including ionizable lipid (SM-102), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol, and polyethylene glycol lipid (mPEG-DMG) in a molar ratio of 50:10:38.5:1.5, dissolved in ethanol to a final concentration of 10 mg/mL. The aqueous phase consisted of mRNA dissolved at 150 ng/µL in 50 mM sodium citrate buffer (pH 4). The organic and aqueous phases were mixed using a microfluidic device (Inano E, Micro & Nano, Shanghai, China) at a flow rate of 12 mL/min and a 1:3 ratio through a microfluidic chip to yield the mRNA-LNP solution. This mRNA-LNP solution was then concentrated and exchanged into PBS buffer (pH 7.4) using an Amicon® Ultra-15 centrifugal filter (cut-off = 10 kDa), filtered through a 0.22 μm pore size membrane, and stored at 4 °C for future use.
In vitro characterization of mRNA-LNP
Dynamic Light Scattering (DLS) was employed to measure the particle size of the lipid nanoparticles (LNPs). The hydrodynamic diameter was assessed using a Master sizer 3000(Malvern Panalytical, US) with measurements conducted at 25 °C and a scattering angle of 90°. The resulting data provided the average particle size and polydispersity index (PDI), indicating the size distribution and uniformity of the LNPs. The mRNA content in LNP-mRNA was quantified using the Quant-iTTM RiboGreenTM RNA Assay Kit (Invitrogen, R11490).
Western blotting
Cells were seeded in 6-well plates at densities of 1 × 106 cells/well for HEK293T and 2 × 106 cells/well for J774A.1, and cultured overnight. Subsequently, add 10 µg mRNA-LNPs to each well incubate for 48 h. Cell lysates were separated by SDS-page and subsequently transferred onto polyvinylidene difluoride (PVDF) membranes using an eBlotTM L1 Fast Wet Transfer Device (GenScript, Nanjing, China). Protein expression were probed with an anti-His-HRP conjugated antibody (HRP66005, Proteintech, USA) and a SHST Analysis chemiluminescence imaging system (SHENHUA, Hangzhou, China).
Toxicity assessment
HEK 293T cells were seeded in 96-well plates at densities of 3000 cells/well for HEK293T, and cultured overnight. Subsequently, 10 µg of mRNA was added to each well to assess the cytotoxicity of mRNA-LNPs. After 72 h, 10 µL of CCK-8 solution (Vazyme, Nanjing, China) was added to each well, and the plates were incubated for at least 30 min. Cell viability was quantified by measuring the optical density at 450 nm using a microplate reader.
Blood was collected from mice into anticoagulant tubes and washed three times with PBS (300 g, 4 °C, 5 min). Red blood cells were resuspended in PBS to prepare a 2% suspension. Two different doses of mRNA-LNP (1 µg, 10 µg) were added to the red blood cell suspension, with PBS or the same concentration of LNP serving as the negative control, and 1% Triton X-100 in plasma as the positive control. The mixture was incubated at 37 °C for 3 h, followed by centrifugation. The supernatant was collected and transferred to a 96-well plate. Hemolytic activity was quantified by measuring the optical density at 450 nm using a microplate reader. The hemolysis rate was calculated using the formula: Hemolysis rate = (ODLNP − ODnegative)/(ODpositive − ODnegative).
Mice were injected with 50 µg of multivalent mRNA-LNP via the tail vein or intramuscular injection, with PBS as the control group. After 24 and 72 h, ALT, AST, CR, and creatinine levels were measured to evaluate acute toxicity.
Purification of recombinant vaccine antigens
For five proteins, the DNA sequences were synthesized by GeneWiz Biotech Co., Ltd. (Suzhou, China). The SEB, FnBPA, IsdB protein was cloned into the pet-30a vector, and the MntC, Hla protein was cloned into the pGEX-6P-1 vector. Both constructs contained a C-terminal HIS tag. The constructed plasmid was transformed into BL21(DE3) pLysS competent cells. Bacteria were grown in LB medium supplemented with 100 μg/mL ampicillin until an OD600 of 0.6–0.8 was reached. Expression of the recombinant protein was induced by adding IPTG to a final concentration of 0.5 mM and incubating at 16 °C and 200 rpm overnight. Bacterial cultures were harvested and subjected to high-pressure homogenization for cell disruption. The supernatant was purified using Ni-NTA Beads 6FF or Glutathione Beads 4FF (Smart-Lifesciences, China) loaded on a protein purification system (SDL-030-F2, Sepure Instruments Inc.). The N-terminal GST tag on the MntC and Hla protein was cleaved using PreScission Protease (GE, USA) after elution. Protein concentrations were determined using the PierceTM BCA Protein Assay Kit.
Immunization regimen
The immunization protocol was designed to administer two doses of vaccine on days 0 and 14. For the mRNA-LNP vaccine group, 100 μL of the preparation diluted in PBS was administered directly, whereas for the protein vaccine group, the immunogen solution was combined with Imject Alum (#77161, Thermo Fisher Scientific, USA) at a 1:1 volume ratio, and all immunizations were subsequently performed via intramuscular injection.
Enzyme-linked immunosorbent assays
Antibody titers were determined using an indirect enzyme-linked immunosorbent assay (ELISA) and was defined by Frey method (99.5% confidence interval). Target proteins were diluted to 2 ng/µl in coating buffer (0.05 M Carbonate Buffer) and used to coat ELISA microplates (Corning, USA) overnight at 4 °C. Plates were washed three times with PBST, 2% BSA was added to each well and blocked at 37 °C for 2 h. Plates were washed three times, 100 μl of serially diluted mouse sera were added to each well, incubated 37 °C for 60 min. Plates were washed three times, 100 μl of secondary antibodies- HRP-labeled Goat Anti-Mouse IgG H&L (ab6789, Abcam, UK), HRP-conjugated Affinipure Goat Anti-Mouse IgG1 (SA00012-1, Proteintech, UK), Goat Anti-Mouse IgM (SA00012-6, Proteintech, UK) or Goat Anti-Mouse IgG 2a (SA00012-2, Proteintech, UK) was added and incubated at 37 °C for 60 min for the reaction. After washed three times with PBST, 100 μl of Enhanced TMB Chromogen Solution(P0210, Beyotime) was added and reacted at 37 °C for 30 min in the dark; ultimately, 100 μl of Stop Solution for TMB Substrate(P0215, Beyotime) was added to stop the reaction, the absorbance was measured at 450 nm using a microplate reader.
Enzyme-linked immunospot assay
ELISPOT assay was performed on splenocytes isolated from immunized mice. Splenocytes were serially diluted in RPMI containing 10% FBS from 5 × 106 cells/well into 96-well pre-coated plate (3321-4AST-2, 3441-4APW-2, 3311-4APW-2, 3521-4HPW-2, MABTECH, Sweden), Then add antigen to stimulate (10 µg of corresponding protein for monovalent groups and 10 µg of five proteins for multivalent groups) for 36 h. The primary antibody was detection antibodies conjugated with biotin and the secondary antibody was streptavidin-HRP. Afterwards, the positive cells were detached using BCIP/NBT solution. Spots were counted using an ELISpot reader.
Intracellular flow cytometry analysis
Lymphocytes were isolated as described above. Splenocytes were seeded at a density of 2 × 106 cells/ml and stimulated as above. After culturing at 37 °C for 6 h, Brefeldin A Solution (Biolegend, US) was added, and the culture continued for an additional 18 h. Cells were incubated with TruStain FcXTM (anti-mouse CD16/32) Antibody (1:100, 101320, Biolegend) to block nonspecific Fc receptor binding, followed by surface staining with BV421 anti-mouse CD3 Antibody (100227, 0.25 µg per million cells, Biolegend), FITC anti-mouse CD4 Antibody (100405, 0.25 µg per million cells, Biolegend) and BV510 anti-mouse CD8a Antibody (100751, 0.5 µg per million cells, Biolegend). Cells were then fixed and permeabilized using BD Cytofix/Cytoperm (BD Biosciences) and stained intracellularly with APC anti-mouse IL-2 Antibody (503809, 0.25 µg per 106 cells, Biolegend) and PE anti-mouse IFN-γ Antibody (163503, 0.125 µg per million cells, Biolegend) or APC anti-mouse IL-17A Antibody (506915, 0.25 µg per million cells, Biolegend) and PE anti-mouse IL-4 Antibody (504103, 0.25 µg per 106 cells, Biolegend) in 1× Cytoperm buffer. Samples were analyzed using a BD FACS Array flow cytometer (BD Biosciences, US) and FlowJo software (BD Biosciences). The gating strategy is outlined in the supplementary Fig. 7.
Opsonophagocytic killing assay
J774A.1 macrophages were centrifuged at 400 × g for 3 min, then resuspended in DMEM at a concentration of 106 cells/mL in 96-well plate. Bacteria at a multiplicity of infection (MOI) of 10 (107 CFU/mL) were added, followed by the addition of 30 µL of immune serum to the bacterial-macrophage suspension. After incubation for 2 h in 37 °C, the supernatant was serially diluted with PBS and plated on TSA agar plates. Moreover, the macrophages were washed three times with sterile cold PBS to terminate phagocytosis, followed by the addition of sterile water to lyse the cells. The lysates were then subjected to serial dilution and plated onto TSA plates. Colony counts were performed after 24 h.
Construction of murine S.aureus infection models
To establish a systemic infection model, a lethal dose (1 × 109 CFU) or sublethal dose (5 × 108 CFU) of log-phase Staphylococcus aureus was collected, washed and resuspended in PBS. 100 µL of bacterial suspension was used for intravenous (i.v.) inoculation of mice. Mice survival was monitored and recorded every 24 h, and were euthanized according to IACUC protocols at humane endpoints.
To establish pneumonia models, mice were first anesthetized, then pinch their nasal passages gently to compel oral breathing. Subsequently, the mice were intratracheally inoculated with sublethal doses of MRSA252(5 × 108 CFU), suspended in 30 μL of PBS.
Evaluation of bacterial load
Twenty-four hours post-infection with a sublethal dose, blood samples were collected into EDTA-coated tubes. The plasma was serially diluted in PBS, and bacterial counts were determined by plating on TSA plates. The mice were then euthanized using carbon dioxide, and their lungs and kidneys were harvested. These organs were homogenized in PBS, followed by serial dilution, and bacterial loads were determined by plating the dilutions on TSA plates.
Histopathological analysis
Mice with a lethal dose of systemic infection and a sublethal dose of pneumonia infection. were euthanized 48 h post-infection using carbon dioxide. Lung, liver and spleen samples were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned and stained with hematoxylin and eosin (HE). The pathological score is based on the area of damage according to its severity. The scoring is as follows: No damage = 1, 25% damage = 2, 50% damage = 3, 75% damage = 4, Diffuse damage = 5.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism 8.0. The data are presented as mean ± standard deviation (SD) and analyzed by one-way ANOVA with Dunnett’s post-hoc test (C, H) and Tukey’s multiple comparisons test. Differences were considered statistically significant when P < 0.05.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Masters, E. A. et al. Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 20, 385–400 (2022).
Linz, M. S., Mattappallil, A., Finkel, D. & Parker, D. Clinical impact of Staphylococcus aureus skin and soft tissue infections. Antibiotics 12, 557 (2023).
Ikuta, K. S. et al. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 400, 2221–2248 (2022).
Lee, A. S. et al. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 4, 18033 (2018).
Micoli, F., Bagnoli, F., Rappuoli, R. & Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 19, 287–302 (2021).
Wright, A. E. Notes on the treatment of furunculosis, sycosis, and acne by the inoculation of a staphylococcus vaccine: and generally on the treatment of localised bacterial invasions by therapeutic inoculations of the corresponding bacterial vaccines. Lancet 159, 874–884 (1902).
Fattom, A. et al. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine 23, 656–663 (2004).
Osterholm, M. T. Nabi corporation stages presentation of StaphVAX data at the interscience conference on antimicrobial agents and chemotherapy, despite lack of abstract submission. Clin. Infect. Dis. 31, i–ii (2000).
Anderson, A. S. et al. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum. Vaccines Immunother. 8, 1585–1594 (2012).
Scully, I. L. et al. Performance of a four-antigen Staphylococcus aureus vaccine in preclinical models of invasive S. aureus disease. Microorganisms 9, 177 (2021).
Frenck, R. W. et al. Safety, tolerability, and immunogenicity of a 4-antigen Staphylococcus aureus vaccine (SA4Ag): results from a first-in-human randomised, placebo-controlled phase 1/2 study. Vaccine 35, 375–384 (2017).
Creech, C. B. et al. Persistence of immune responses through 36 months in healthy adults after vaccination with a novel Staphylococcus aureus 4-antigen vaccine (SA4Ag). Open Forum Infect. Dis. 7, ofz532 (2020).
McNeely, T. B. et al. Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: an analysis of possible contributing host factors. Hum. Vaccin. Immunother. 10, 3513–3516 (2014).
Harro, C. D. et al. The immunogenicity and safety of different formulations of a novel Staphylococcus aureus vaccine (V710): results of two phase I studies. Vaccine 30, 1729–1736 (2012).
Tsai, C.-M. et al. Non-protective immune imprint underlies failure of Staphylococcus aureus IsdB vaccine. Cell Host Microbe 30, 1163–1172.e6 (2022).
Teymournejad, O., Li, Z., Beesetty, P., Yang, C. & Montgomery, C. P. Toxin expression during Staphylococcus aureus infection imprints host immunity to inhibit vaccine efficacy. npj Vaccines 8, 3 (2023).
Garzoni, C. & Kelley, W. L. Return of the Trojan horse: intracellular phenotype switching and immune evasion by Staphylococcus aureus. EMBO Mol. Med. 3, 115–117 (2011).
Clegg, J. et al. Staphylococcus aureus vaccine research and development: the past, present and future, including novel therapeutic strategies. Front. Immunol. 12, 705360 (2021).
Zeng, H. et al. Rapid and broad immune efficacy of a recombinant five-antigen vaccine against Staphylococcus aureus infection in animal models. Vaccines 8, 134 (2020).
Karauzum, H. et al. IBT-V02: A multicomponent toxoid vaccine protects against primary and secondary skin infections caused by Staphylococcus aureus. Front. Immunol. 12, 624310 (2021).
Han, X. et al. Adjuvant lipidoid-substituted lipid nanoparticles augment the immunogenicity of SARS-CoV-2 mRNA vaccines. Nat. Nanotechnol. 18, 1105–1114 (2023).
Kon, E. et al. A single-dose F1-based mRNA-LNP vaccine provides protection against the lethal plague bacterium. Sci. Adv. 9, eadg1036 (2023).
Mayer, R. L. et al. Immunopeptidomics-based design of mRNA vaccine formulations against Listeria monocytogenes. Nat. Commun. 13, 6075 (2022).
Wang, X. et al. Strong immune responses and protection of PcrV and OprF-I mRNA vaccine candidates against Pseudomonas aeruginosa. npj Vaccines 8, 76 (2023).
Alameh, M.-G. et al. A multivalent mRNA-LNP vaccine protects against Clostridioides difficile infection. Science 386, 69–75 (2024).
Luo, F. et al. mRNA-based platform for preventing and treating Staphylococcus aureus by targeted staphylococcal enterotoxin B. Front. Immunol. 15, 1490044 (2024).
Chen, W. H. et al. Safety and immunogenicity of a parenterally administered, structure-based rationally modified recombinant staphylococcal enterotoxin B protein vaccine. STEBVax. Clin. Vaccin. Immunol. 23, 918–925 (2016).
Shinefield, H. et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346, 491–496 (2002).
Yu, W. et al. Protective humoral and CD4+ T cellular immune responses of Staphylococcus aureus vaccine MntC in a murine peritonitis model. Sci. Rep. 8, 3580 (2018).
Salazar, N. et al. Staphylococcus aureus manganese transport protein C (MntC) Is an extracellular matrix- and plasminogen-binding protein. PLoS One 9, e112730 (2014).
Krakauer, T. & Stiles, B. G. The staphylococcal enterotoxin (SE) family: SEB and siblings. Virulence 4, 759–773 (2013).
Choi, J. Y. et al. A novel staphylococcal enterotoxin B subunit vaccine candidate elicits protective immune response in a mouse model. Toxicon 131, 68–77 (2017).
Pivard, M., Moreau, K. & Vandenesch, F. Staphylococcus aureus arsenal to conquer the lower respiratory tract. mSphere 6, e00059–21 (2021).
Adhikari, R. P. et al. Novel structurally designed vaccine for S. aureus α-hemolysin: protection against bacteremia and pneumonia. PLOS One 7, e38567 (2012).
Gaudreau, M.-C., Lacasse, P. & Talbot, B. G. Protective immune responses to a multi-gene DNA vaccine against Staphylococcus aureus. Vaccine 25, 814–824 (2007).
Kuklin, N. A. et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect. Immun. 74, 2215–2223 (2006).
Arevalo, C. P. et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 378, 899–904 (2022).
Vidarsson, G., Dekkers, G. & Rispens, T. IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 5, 520 (2014).
Kak, G., Raza, M. & Tiwari, B. K. Interferon-gamma (IFN-γ): exploring its implications in infectious diseases. Biomol. Concepts 9, 64–79 (2018).
Gómez, M. I., Sordelli, D. O., Buzzola, F. R. & García, V. E. Induction of cell-mediated immunity to Staphylococcus aureus in the mouse mammary gland by local immunization with a live attenuated mutant. Infect. Immun. 70, 4254–4260 (2002).
Narita, K. et al. Role of interleukin-17A in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor A. Infect. Immun. 78, 4234–4242 (2010).
Friot, A. et al. Antigen specific activation of cytotoxic CD8+ T cells by Staphylococcus aureus infected dendritic cells. Front. Cell. Infect. Microbiol. 13, 1245299 (2023).
Ojha, R. & Prajapati, V. K. Cognizance of posttranslational modifications in vaccines: a way to enhanced immunogenicity. J. Cell. Physiol. 236, 8020–8034 (2021).
Lee, Y., Jeong, M., Park, J., Jung, H. & Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 55, 2085–2096 (2023).
Hald Albertsen, C. et al. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 188, 114416 (2022).
Acknowledgements
We extend our heartfelt thanks to the participants who willingly participated in the on-site experiment and every member of Peng George Wang’s team. We thank Dr. Hao Zeng at National Immuno-Biological Products Engineering Technology Research Center of Army Medical University, for kindly providing the strain MRSA252. This research was funded by Nation Key R& D Program of China (Grant No. 2023YFC3403200), the Natural Science Foundation of Guangdong Province, China (Grant No. 2024A1515011286), Shenzhen Science and Technology Innovation Com-mission, Key Project of Basic Research Special Project (Grant No. JCYJ20220818100402004), and Shenzhen Key Medical Discipline Construction Fund (Grant No. SZXK045).
Author information
Authors and Affiliations
Contributions
X.G., Y.H., and P.W. conceived the project; X.G., Y.Z., X.W., and J.J. performed experiments; X.G., Y.Z., X.W., J.J., C.L., and C.Y. contributed to methodology; Y.H. and P.W. acquired funding; X.G., Y.Z., and X.W. collected and analyzed the data; X.G. drafted the manuscript; Y.H. and P.W. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, X., Zheng, Y., Wang, X. et al. A multivalent mRNA-LNP cocktail vaccine confers superior efficacy against Staphylococcus aureus infection in murine models. npj Vaccines 10, 210 (2025). https://doi.org/10.1038/s41541-025-01244-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41541-025-01244-4