Abstract
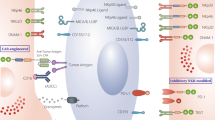
T cells and natural killer (NK) cells collaborate to maintain immune homeostasis. Current cancer immunotherapies predominantly rely on the individual application of these cells. Here we use bicistronic vectors to co-express chimeric antigen receptors (CARs) and secreted immune cell engagers (ICEs), leveraging the combined therapeutic potential of both effector cell types. After in vitro validation of immune cell engager secretion and function, various combinatorial approaches are systematically compared in mouse models, identifying a highly effective combination of bispecific killer cell engager (BiKE)-secreting CAR-T cells and NK cells. Beyond a simple combination of conventional CAR-T cells and NK cells, this strategy demonstrates superior efficacy in CD19+ B cell leukaemia and lymphoma and EGFR+ solid tumour models while reducing the dosage dependence on CAR-T cells. Moreover, CAR-T cells and BiKEs targeting distinct antigens exhibit suppression of tumour cells with heterogeneous antigen expression. These findings indicate that combining BiKE-secreting CAR-T cells and NK cells offers a promising strategy to combat tumour antigen heterogeneity and immune evasion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the Article and its Supplementary Information. The raw and analysed datasets generated during the study are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Jhunjhunwala, S., Hammer, C. & Delamarre, L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 21, 298–312 (2021).
Seliger, B. & Koehl, U. Underlying mechanisms of evasion from NK cells as rationale for improvement of NK cell-based immunotherapies. Front. Immunol. 13, 910595 (2022).
Pan, K. et al. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J. Exp. Clin. Cancer Res. 41, 119 (2022).
Krishnamurthy, A. & Jimeno, A. Bispecific antibodies for cancer therapy: a review. Pharmacol. Ther. 185, 122–134 (2018).
Tapia-Galisteo, A., Compte, M., Álvarez-Vallina, L. & Sanz, L. When three is not a crowd: trispecific antibodies for enhanced cancer immunotherapy. Theranostics 13, 1028–1041 (2023).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Raje, N. et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 380, 1726–1737 (2019).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022).
Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016).
Shah, N. N. & Fry, T. J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 16, 372–385 (2019).
Prager, I. & Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukocyte Biol. 105, 1319–1329 (2019).
Laskowski, T. J., Biederstädt, A. & Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 22, 557–575 (2022).
Myers, J. A. & Miller, J. S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 18, 85–100 (2021).
Dolstra, H. et al. Successful transfer of umbilical cord blood CD34+ hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin. Cancer Res. 23, 4107–4118 (2017).
Bachanova, V. et al. Safety and efficacy of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma. Blood 138, 823–823 (2021).
Romee, R. et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 8, 357 (2016).
Tang, X. et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer. Res. 8, 1083–1089 (2018).
Zhang, Y. et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology 121, 258–265 (2007).
Jin, S. et al. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct. Target. Ther. 7, 39 (2022).
Li, G. et al. CD3 engagement as a new strategy for allogeneic ‘off-the-shelf’ T cell therapy. Mol. Ther. Oncolytics 24, 887–896 (2022).
Goebeler, M.-E. & Bargou, R. C. T cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol. 17, 418–434 (2020).
Gleason, M. K. et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood 123, 3016–3026 (2014).
El-Khoueiry, A. et al. Abstract CT149: A phase 1/2a first-in-human study of AFM24, a CD16A/epidermal growth factor (EGFR) bispecific Innate Cell Engager (ICE®), in patients with locally advanced or metastatic EGFR-expressing solid tumors: preliminary findings from the dose-escalation phase. Cancer Res. 82, CT149–CT149 (2022).
Bordoloi, D. et al. Siglec-7 glyco-immune binding mAbs or NK cell engager biologics induce potent antitumor immunity against ovarian cancers. Sci. Adv. 9, eadh4379 (2023).
Coënon, L. & Villalba, M. From CD16a biology to antibody-dependent cell-mediated cytotoxicity improvement. Front. Immunol. 13, 913215 (2022).
Phung, S. K., Miller, J. S. & Felices, M. Bi-specific and tri-specific NK cell engagers: the new avenue of targeted NK cell immunotherapy. Mol. Diagn. Ther. 25, 577–592 (2021).
Portell, C., Wenzell & Advani, A. Clinical and pharmacologic aspects of blinatumomab in the treatment of B-cell acute lymphoblastic leukemia. Clin. Pharmacol. 5, 5–11 (2013).
Lorenczewski, G. et al. Generation of a half-life extended Anti-CD19 BiTE® antibody construct compatible with once-weekly dosing for treatment of CD19-positive malignancies. Blood 130, 2815–2815 (2017).
Goyos, A. et al. Generation of half-life extended anti-BCMA Bite® antibody construct compatible with once-weekly dosing for treatment of multiple myeloma (MM). Blood 130, 5389–5389 (2017).
Al-Haideri, M. et al. CAR-T cell combination therapy: the next revolution in cancer treatment. Cancer. Cell. Int. 22, 365 (2022).
Szymczak-Workman, A. L., Vignali, K. M. & Vignali, D. A. A. Design and construction of 2A peptide-linked multicistronic vectors. Cold. Spring. Harb. Protoc. 2, 199–204 (2012).
Rafiq, S. et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 33, 847–856 (2018).
Allen, G. M. et al. Synthetic cytokine circuits that drive T cells into immune-excluded tumors. Science 378, eaba1624 (2022).
Choi, B. D. et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 37, 1049–1058 (2019).
Brentjens, R. J. et al. CD19-Targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, (2013).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, (2014).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Feucht, J. et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 25, 82–88 (2019).
Choi, B. D. et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N. Engl. J. Med. 390, 1290–1298 (2024).
Yun, J. et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR–MET bispecific antibody, in diverse models of EGFR exon 20 insertion–driven NSCLC. Cancer Discov. 10, 1194–1209 (2020).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Kagoya, Y. et al. Genetic ablation of HLA class I, class II, and the T-cell receptor enables allogeneic T cells to be used for adoptive T-cell therapy. Cancer Immunol. Res. 8, 926–936 (2020).
Jo, S. et al. Endowing universal CAR T-cell with immune-evasive properties using TALEN-gene editing. Nat. Commun. 13, 3453 (2022).
Terrén, I. et al. Cytokine-induced memory-like NK cells: from the basics to clinical applications. Front. Immunol. 13, 884648 (2022).
Wei, J., Yang, Y., Wang, G. & Liu, M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front. Immunol. 13, 1035276 (2022).
Rotte, A. et al. Dose–response correlation for CAR-T cells: a systematic review of clinical studies. J. Immunother. Cancer. 10, e005678 (2022).
Vinay, D. S. et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35, S185–S198 (2015).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Aldoss, I. et al. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am. J. Hematol. 92, 858–865 (2017).
Majzner, R. G. & Mackall, C. L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 8, 1219–1226 (2018).
Zhou, Y., Cheng, L., Liu, L. & Li, X. NK cells are never alone: crosstalk and communication in tumour microenvironments. Mol. Cancer. 22, 34 (2023).
Badrinath, S. et al. A vaccine targeting resistant tumours by dual T cell plus NK cell attack. Nature 606, 992–998 (2022).
Acknowledgements
This research was funded by The First Affiliated Hospital, Zhejiang University School of Medicine B2022010-8 (Y.F.), the National Natural Science Foundation of China grant 82161138028 (J.S.) and the National Key R&D Program of China 2021YFA0909900 (J.S.). We thank the support of Zhejiang Provincial Key Laboratory of Immunity and Inflammatory Diseases and thank Y. Xing, C. Guo and Y. Huang from the core facilities of Zhejiang University School of Medicine for their technical support.
Author information
Authors and Affiliations
Contributions
J.S. conceived the study. J.S. and Y.F. designed the experiments. Y.F., Y.D., J.C., Y.W., K.S., J.J. and L.S. performed the experiments. Y.F. analysed the experimental data. C.Z. and M.S. revised the manuscript written by Y.F. and J.S. H.H. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Der-Yang Cho, Jeffrey Miller and Michael Mitchell for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Additionally verifying the expression of 1928ζ-CARs and CD19-BiKEs and BiTEs.
a. Surface CAR expression of HEK293T cells transfected with plasmid vectors as indicated, examined by flow cytometry. b. Coomassie Blue staining of SDS-PAGE gels to detect CD19-BiKEs (top) or CD19-BiTEs (bottom) secreted by HEK293T cells transfected with plasmid vectors as indicated. Unconc, unconcentrated, Conc, concentrated. c. Binding traits of secreted BiKEs (left) and BiTEs (right) towards T cells and NK cells respectively as negative controls, examined through flow cytometry. SN, supernatant. d. Statistics on the surface expression of CAR (represented as MFI) for 1928ζ BiKE− or BiKE+ CAR-T cells (left) and 1928ζ BiTE− or BiTE+ CAR-NK cells (right) generated from three independent donors. Paired, two-tailed Student’s t-tests were performed. e. Retroviral transduction efficiency in the production of 1928ζ BiKE− or BiKE+ CAR-T cells (left) and 1928ζ BiTE− or BiTE+ CAR-NK cells (right) from the other two donors. f. Representative contour plots showing the simultaneous expression of CAR (left) and GFP (right) for T cells transduced with 1928ζ-P2A-GFP retroviral vectors. g. Scatter plot showing the linear correlation between the expression of CAR and GFP for 1928ζ-P2A-GFP CAR-T cells generated from three independent donors, with the Pearson correlation coefficient (r) and P-value calculated.
Extended Data Fig. 2 Additionally testing the in vitro properties and functions of 1928ζ CAR-T and CAR-NK cells and redirection of NK cells.
a. Histograms for extra two biological replicates of CFSE assays showing the percentage of proliferated 1928ζ BiKE− or BiKE+ CAR-T cells (top) and 1928ζ BiTE− or BiTE+ CAR-NK cells (bottom) with or without antigen stimulation. b. Percentage of lysed NALM6 cells after 16 h coculture with primary NK cells at indicated E:T ratios in supernatant collected from 1928ζ BiKE− or BiKE+ CAR-T cells. SN, supernatant. NK cells from independent donors (n = 3) were sampled. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. P-values greater than 0.05 are not shown. c. Histograms showing the surface expression of three exhaustion markers on 1928ζ BiKE− or BiKE+ CAR-T cells of extra two biological replicates with or without consistent antigen stimulation. d. The concentration of CD19-BiKEs secreted by equal amounts of 1928ζ BiKE+ CAR-T cells with or without consistent antigen stimulation. CAR-T cells from independent donors (n = 3) were sampled. Unpaired, two-tailed Student’s t-tests were performed and data are presented as mean values ± SD.
Extended Data Fig. 3 Additionally evaluating the in vivo efficacy of BiKE-secreting CAR-T cells combined with NK cells against B-cell lymphoma.
a. Schedule of the initial subcutaneous B-cell lymphoma model. Tumour cells and effector cells (wild-type 1928ζ CAR-T cells and unmodified NK cells) were injected at the time points and doses as indicated. b. Growth curves of repeated experiments (top and bottom), showing the time-lapse subcutaneous tumour volumes for NSG mice in different groups of Supplementary Fig. 3a (n = 5). Arrows and dashed lines indicate the time points when NK cells were adoptively transferred. Two-way analysis of variance (ANOVA) and Tukey’s multiple comparisons tests were performed and data are presented as mean values ± SD. Statistical significances between the last two groups are annotated. c. Schedule of the subcutaneous B-cell lymphoma model. Tumour cells and relatively higher doses of effector cells (1928ζ-1XX CAR-T cells and unmodified NK cells) were injected at the time points as indicated. d. Growth curves of repeated experiments (top and bottom), showing the time-lapse subcutaneous tumour volumes for NSG mice in different groups of Supplementary Fig. 3c (n = 5). Arrows and dashed lines indicate the time points when NK cells were adoptively transferred. Two-way analysis of variance (ANOVA) and Tukey’s multiple comparisons tests were performed and data are presented as mean values ± SD. Statistical significances for the terminal point are annotated. e. Schedule of the subcutaneous B-cell lymphoma model. Tumour cells and effector cells (unmodified NK cells) were injected at the time points and doses as indicated. Purified BiKEs were injected for three weeks after the initial adoptive transfer of effector cells. f. Growth curves showing the time-lapse subcutaneous tumour volumes for NSG mice in different groups of Supplementary Fig. 3e (n = 5). Arrows and dashed lines indicate the time points when NK cells were adoptively transferred. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. Statistical significances for the terminal point are annotated.
Extended Data Fig. 4 Additionally evaluating the in vivo efficacy of BiKE-secreting CAR-T cells combined with NK cells against B-cell leukaemia.
a. Schedule of the initial systemic B-ALL model. Tumour cells and effector cells (1928ζ-1XX CAR-T cells and unmodified NK cells) were injected at the time points and doses as indicated. b. Grouped NSG mice of the systemic B-ALL model captured in weekly bioluminescence imaging. c. Time-lapse luminescence flux of every mouse in different groups (n = 5). Arrows and dashed lines indicate time points when NK cells were adoptively transferred. d. Survival curves of NSG mice in different groups. Kaplan-Meier survival estimates (log-rank tests) were performed. e. Gating strategy for counting human CAR-T cells and NK cells in the peripheral blood of mice injected with BiKE-secreting CAR-T cells and unmodified NK cells, from Fig. 3d–g. f. Statistics on cell numbers of CAR-T cells (top) and NK cells (bottom) in mouse peripheral blood from the “BiKE+ with NK” group in Fig. 3d–g (n = 5) on the 10th and the 20th days since the first adoptive transfer of effector cells. Paired, two-tailed Student’s t-tests were performed and data are presented as mean values ± SD.
Extended Data Fig. 5 In vivo efficacy of other ICE-based combinatorial strategies.
a. Schedule for the systemic B-ALL model. Tumor cells and effector cells (1928ζ CAR-NK cells and unmodified T cells) were injected at the time points and doses as indicated. b. Grouped NSG mice of the systemic B-ALL model captured in weekly bioluminescence imaging. c. Time-lapse luminescence flux of every mouse in different groups (n = 5). Arrows and dashed lines indicate time points when T cells were adoptively transferred. d. Survival curves of NSG mice in different groups. Kaplan-Meier survival estimates (log-rank tests) were performed. e. Gating strategy for counting human CAR-NK cells and T cells in the peripheral blood of mice injected with BiTE-secreting CAR-NK cells and unmodified T cells, from a–d. f. Statistics on cell numbers of CAR-NK cells (top) and T cells (bottom) in mouse peripheral blood from the “BiTE+ with T” group in a–c (n = 5) on the 3rd and the 17th days since the first adoptive transfer of effector cells. Paired, two-tailed Student’s t-tests were performed and data are presented as mean values ± SD. g. Schedule for the subcutaneous B-cell lymphoma model. Tumour cells and effector cells (1928ζ-1XX BiKE+ CAR-T cells combined with NK cells (left) or 1928ζ-1XX BiTE+ CAR-T cells combined with T cells (right)) were injected at the time points and doses as indicated. h. Growth curves showing the time-lapse subcutaneous tumour volumes of NSG mice in different groups (n = 5). Arrows and dashed lines indicate the time points when T cells or NK cells were adoptively transferred. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. Statistical significances for the terminal point are annotated.
Extended Data Fig. 6 Additionally verifying the expression of EGFR28ζ-CARs and EGFR-BiKEs.
a. Surface CAR expression of HEK293T cells transfected with plasmid vectors as indicated, examined by flow cytometry. b. Coomassie Blue staining of SDS-PAGE gels to detect EGFR-BiKEs secreted by HEK293T cells transfected with plasmid vectors as indicated. Unconc, unconcentrated. Conc, concentrated. c. Immunoblotting identification of EGFR-BiKEs secreted by HEK293T cells transfected with EGFR28ζ BiKE− or BiKE+ plasmid vectors. d. Binding traits of EGFR-BiKEs secreted by HEK293T cells transfected with EGFR28ζ BiKE+ plasmid vectors towards SK-OV-3 cells and NK cells, examined through flow cytometry. SN, supernatant. e. Binding traits of secreted BiKEs towards T cells as a negative control, examined through flow cytometry. SN, supernatant. f. Statistics on the surface expression of CAR (represented as MFI) for EGFR28ζ BiKE− or BiKE+ CAR-T cells generated from three independent donors. Paired, two-tailed Student’s t-tests were performed. g. Retroviral transduction efficiency in the production of EGFR28ζ BiKE− or BiKE+ CAR-T cells from two other donors.
Extended Data Fig. 7 In vitro properties and functions of EGFR28ζ CAR-T cells and redirection of NK cells.
a. Growth curve showing the total number of EGFR28ζ BiKE− or BiKE+ CAR-T cells counted at indicated time points after subculture. CAR-T cells from independent donors (n = 3) were sampled. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. b. Histograms of CFSE assays showing the percentage of proliferated EGFR28ζ BiKE− or BiKE+ CAR-T cells with or without antigen stimulation, and statistics of three biological replicates. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. c. Percentage of lysed SK-OV-3 cells after 16 h coculture with EGFR28ζ BiKE− or BiKE+ CAR-T cells at indicated E:T ratios. CAR-T cells from independent donors (n = 3) were sampled. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. d. Percentage of lysed SK-OV-3 cells after 3 h coculture with primary NK cells at indicated E:T ratios in supernatant collected from EGFR28ζ BiKE− or BiKE+ CAR-T cells. SN, supernatant. NK cells from independent donors (n = 3) were sampled. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. P-values greater than 0.05 are not shown. e. The concentration of proinflammatory cytokines released by EGFR28ζ BiKE− or BiKE+ CAR-T cells after 16 h coculture with target cells. CAR-T cells from independent donors (n = 3) were sampled. Unpaired, two-tailed Student’s t-tests were performed and data are presented as mean values ± SD. f. The concentration of proinflammatory cytokines released by primary NK cells after 3 h coculture with target cells in supernatant collected from EGFR28ζ BiKE− or BiKE+ CAR-T cells. NK cells from independent donors (n = 3) were sampled. Unpaired, two-tailed Student’s t-tests were performed and data are presented as mean values ± SD. g. Histograms showing surface expression of three exhaustion markers on EGFR28ζ BiKE− or BiKE+ CAR-T cells with or without consistent antigen stimulation, and statistics of three biological replicates. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. h. Concentration of EGFR-BiKEs secreted by equal amounts of EGFR28ζ BiKE+ CAR-T cells after or without consistent antigen stimulation. CAR-T cells from independent donors (n = 3) were sampled. Unpaired, two-tailed Student’s t-tests were performed and data are presented as mean values ± SD.
Extended Data Fig. 8 Additional in vivo data of BiKE-secreting EGFR28ζ-1XX CAR-T cells together with NK cells.
a. Schedule of the initial subcutaneous EGFR-positive solid tumour model. Tumour cells and effector cells (EGFR28ζ-1XX CAR-T cells and unmodified NK cells) were injected at the time points and doses as indicated. b. Growth curves showing the time-lapse subcutaneous tumour volumes for NSG mice in different groups of Supplementary Fig. 8a (n = 5). Arrows and dashed lines indicate the time points when NK cells were adoptively transferred. Two-way analysis of variance (ANOVA) and Tukey’s multiple comparisons tests were performed and data are presented as mean values ± SD. Statistical significances for the terminal point are annotated. c. Histograms showing the expression of CD3 (left) and CD56 (right) on CAR-T cells and NK cells before being adoptively transferred. d. Histogram showing the expression of CD56 on SK-OV-3 cells before being implanted. e. Statistics on densities of tumour-infiltrating CAR-T cells (left) and NK cells (right) from the “BiKE- with NK” and “BiKE+ with NK” group in Fig. 4g (n = 5). Unpaired, two-tailed Student’s t-tests were performed and data are presented as mean values ± SD.
Extended Data Fig. 9 Additionally verifying the expression of 1928ζ-CARs and EGFR-BiKEs.
a. Surface CAR expression of HEK293T cells transfected with plasmid vectors as indicated, examined by flow cytometry. b. Coomassie Blue staining of SDS-PAGE gels to detect EGFR-BiKEs secreted by HEK293T cells transfected with plasmid vectors as indicated. Unconc, unconcentrated. Conc, concentrated. c. Immunoblotting identification of EGFR-BiKEs secreted by HEK293T cells transfected with 1928ζ BiKE− or BiKE+ plasmid vectors. d. Binding traits of EGFR-BiKEs secreted by HEK293T cells transfected with 1928ζ BiKE+ plasmid vectors towards SK-OV-3 cells and NK cells, examined through flow cytometry. SN, supernatant. e. Retroviral transduction efficiency in the production of 1928ζ BiKE− or BiKE+ CAR-T cells from three donors. f. Statistics on the surface expression of CAR (represented as MFI) for 1928ζ BiKE− or BiKE+ CAR-T cells generated from three independent donors. Paired, two-tailed Student’s t-tests were performed. g. Detection of EGFR-BiKEs secreted by 1928ζ BiKE− or BiKE+ CAR-T cells at indicated time points after transduction. CAR-T cells from independent donors (n = 3) were sampled. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD.
Extended Data Fig. 10 Validation of 1928ζ CAR-T cells and secreted EGFR-BiKEs and the endogenous expression of EGFR and exogenous expression of CD19 and FFLuc on SK-OV-3 cells.
a. Growth curve showing the total number of 1928ζ BiKE− or BiKE+ CAR-T cells counted at indicated time points after subculture. CAR-T cells from independent donors (n = 3) were sampled. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. b. Histograms of CFSE assays showing the percentage of proliferated 1928ζ BiKE− or BiKE+ CAR-T cells with or without antigen stimulation, and statistics of three biological replicates. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. c. Percentage of lysed NALM6 cells after 16 h coculture with 1928ζ BiKE− or BiKE+ CAR-T cells at indicated E:T ratios. CAR-T cells from independent donors (n = 3) were sampled. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. d. Percentage of lysed SK-OV-3 cells after 3 h coculture with primary NK cells at indicated E:T ratios in supernatant collected from 1928ζ BiKE− or BiKE+ CAR-T cells (n = 3). SN, supernatant. NK cells from independent donors (n = 3) were sampled. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. P-values greater than 0.05 are not shown. e. The concentration of proinflammatory cytokines released by 1928ζ BiKE− or BiKE+ CAR-T cells after 16 h coculture with target cells. CAR-T cells from independent donors (n = 3) were sampled. Unpaired, two-tailed Student’s t-tests were performed and data are presented as mean values ± SD. f. The concentration of proinflammatory cytokines released by primary NK cells after 3 h coculture with target cells in supernatant collected from 1928ζ BiKE− or BiKE+ CAR-T cells. NK cells from independent donors (n = 3) were sampled. Unpaired, two-tailed Student’s t-tests were performed and data are presented as mean values ± SD. g. Histograms showing surface expression of three exhaustion markers on 1928ζ BiKE− or BiKE+ CAR-T cells with or without consistent antigen stimulation, and statistics of three biological replicates. Unpaired, two-tailed multiple t-tests were performed and data are presented as mean values ± SD. h. Concentration of EGFR-BiKEs secreted by equal amounts of 1928ζ BiKE+ CAR-T cells after or without consistent antigen stimulation. CAR-T cells from independent donors (n = 3) were sampled. Unpaired, two-tailed Student’s t-tests were performed and data are presented as mean values ± SD. i. Histograms showing the endogenous expression of EGFR (top) and exogenous expression of CD19 (bottom) on SK-OV-3 cells. WT, wild-type. j. Histogram showing the exogenous expression of eGFP on SK-OV-3 cells. k. Statistics on luminescence intensity indicating the exogenous expression of FFLuc on SK-OV-3 cells. Three biological replicates of WT or GFP-FFLuc SK-OV-3 cells were sampled. Unpaired, two-tailed Student’s t-tests were performed and data are presented as mean values ± SD.
Supplementary information
Supplementary Data 1
Coding sequences of bicistronic vectors.
Source data
Source Data Figs. 1–5, Extended Data Figs. 1–10
A single file containing all source data.
Blots and Gels Fig. 1, Extended Data Figs. 6 and 9
A single file containing all unprocessed western blots and gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, Y., Duan, Y., Chen, J. et al. Bispecific killer cell engager-secreting CAR-T cells redirect natural killer specificity to enhance antitumour responses. Nat. Biomed. Eng 10, 390–403 (2026). https://doi.org/10.1038/s41551-025-01450-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01450-4