Abstract
Continuous attractors are an emergent property of neural population dynamics that have been hypothesized to encode continuous variables such as head direction and eye position1,2,3,4. In mammals, direct evidence of neural implementation of a continuous attractor has been hindered by the challenge of targeting perturbations to specific neurons within contributing ensembles2,3. Dynamical systems modelling has revealed that neurons in the hypothalamus exhibit approximate line-attractor dynamics in male mice during aggressive encounters5. We have previously hypothesized that these dynamics may encode the variable intensity and persistence of an aggressive internal state. Here we report that these neurons also showed line-attractor dynamics in head-fixed mice observing aggression6. This allowed us to identify and manipulate line-attractor-contributing neurons using two-photon calcium imaging and holographic optogenetic perturbations. On-manifold perturbations yielded integration of optogenetic stimulation pulses and persistent activity that drove the system along the line attractor, while transient off-manifold perturbations were followed by rapid relaxation back into the attractor. Furthermore, single-cell stimulation and imaging revealed selective functional connectivity among attractor-contributing neurons. Notably, individual differences among mice in line-attractor stability were correlated with the degree of functional connectivity among attractor-contributing neurons. Mechanistic recurrent neural network modelling indicated that dense subnetwork connectivity and slow neurotransmission7 best recapitulate our empirical findings. Our work bridges circuit and manifold levels3, providing causal evidence of continuous attractor dynamics encoding an affective internal state in the mammalian hypothalamus.
Similar content being viewed by others
Main
Neural circuit function has been studied from two vantage points. One focuses on understanding behaviourally specialized neuron types and their functional connectivity8,9,10, whereas the other investigates emergent properties of neural networks, such as attractors1,3,11. Continuous attractors of different topologies are theorized to encode a variety of continuous variables, ranging from head direction12, location in space2, reward history14 to internal states5. Recent data-driven methodologies have allowed for the identification of such attractor-mediated computations directly in neural data5,13,14,15,16. Consequently, attractor dynamics have received increasing attention as a major type of neural coding mechanism2,3,4,12,13.
Despite this progress, establishing that these attractors arise from the dynamics of the observed network remains a formidable challenge2,3,4. This calls for combining large-scale recordings with perturbations of neuronal activity in vivo. Although this has been accomplished for a point attractor that controls motor planning in cortical area anterolateral motor cortex17,18, spatial ensembles in visual cortex that encode visually guided behaviours19,20 and for a ring attractor in Drosophila21,22, there is no study reporting such perturbations for a continuous attractor in any mammalian system. While theoretical work on continuous attractors in mammals is well developed2, the lack of direct, neural-perturbation-based experimental evidence of such attractor dynamics has hindered progress towards a mechanistic circuit-level understanding of such emergent manifold-level network features3.
Oestrogen receptor type 1 (Esr1)-expressing neurons in the ventrolateral subdivision of the ventromedial hypothalamus (VMHvlEsr1) comprise a key node in the social behaviour network and have been causally implicated in aggression23,24. Calcium imaging of these neurons in freely behaving animals has revealed mixed selectivity and variable dynamics, with time-locked attack signals sparsely represented at the single-neuron level25,26. Application of dynamical system modelling27 has revealed an approximate line attractor in the VMHvl that correlates with the intensity of agonistic behaviour, suggesting a population-level encoding of a continuously varying aggressive internal state5. This raises the question of whether the observation of a line attractor in a statistical dynamical systems model fit to VMHvlEsr1 neuronal activity reflects inherited dynamics or can be instantiated locally.
This question can be addressed, in principle, using all-optical methods to observe and perturb line-attractor-relevant neural activity3,28,29,30. A challenge in applying these methods during aggression is that current technology requires head-fixed preparations, and head-fixed mice cannot fight. To overcome this challenge, we took advantage of a recent observation that VMHvl-progesterone receptor neurons (which encompass the Esr1+ subset)31,32,33 mirror observed interindividual aggression6, to instantiate the line attractor in head-fixed mice. Using this preparation, we performed model-guided, closed-loop on- and off-manifold perturbations34 of VMHvlEsr1 activity. These experiments demonstrate that the VMHvl line attractor indeed reflects causal neural dynamics in this nucleus. They also identified selective functional connectivity within attractor-weighted ensembles, suggesting a local circuit implementation of attractor dynamics. Modelling suggests that this implementation may incorporate slow neurotransmission. Collectively, our findings elucidate a circuit-level foundation for a continuous attractor in the mammalian brain.
Line attractor for observing aggression
Recent studies have demonstrated that the VMHvl contains neurons that are active during passive observation of, as well as active participation in, aggression and that reactivating the former can evoke aggressive behaviour6. However, those findings were based on a relatively small sample of VMHvl neurons, which might comprise a specific subset distinct from those contributing to the line attractor (the latter represent around 20–25% of Esr1+ neurons5). To assess whether these mirror-like responses can be observed in Esr1+ neurons that contribute to line-attractor dynamics, we performed microendoscopic imaging35 of VMHvlEsr1 neurons expressing jGCaMP7s in the same freely behaving animals during engagement in followed by observation of aggression (Extended Data Fig. 1a–e). Analysis using recurrent switching linear dynamical systems (rSLDS)27 to fit a statistical model to each dataset (Extended Data Fig. 1f) revealed an approximate line attractor under both conditions, exhibiting ramping and persistent activity aligned and maintained across both performed and observed attack sessions (Extended Data Figs. 1g–q, 2 and 3a–f). Activity during observation of aggression in the integration dimension (x1), which contributes to the line attractor, could be reliably used to decode from held-out data instances of both observation of and engagement in attack, suggesting that this dimension encodes a similar internal state variable under both conditions (Extended Data Fig. 3g,h). Moreover, the integration dimension was weighted by a consistent and aligned set of neurons under both conditions, suggesting that a highly overlapping set of neurons (70%) contributes to line-attractor dynamics during observing or engaging in attack (Extended Data Fig. 4a–d).
The dynamical systems analysis also revealed a dimension orthogonal to the integration dimension (x2) that displayed faster dynamics time locked to the entry of the intruder(s) in both conditions (Extended Data Fig. 1g–l). To examine whether the neurons contributing to the two dimensions (x1 and x2 neurons) can be separated on the basis of physiological properties, we examined their baseline activity when solitary animals were exploring their home cage before any interaction. We did not detect a difference in amplitude or decay constant (tau) between x1 and x2 neurons (Extended Data Fig. 4e–i). However, we did see a slightly but significantly higher frequency of spontaneous calcium transients in x2 neurons (Extended Data Fig. 4f,g), suggesting that x2 neurons are more spontaneously active than x1 neurons when no interaction is taking place.
While these observed attractor dynamics could be generated in the VMHvl, they might also arise from unmeasured ramping sensory input or dynamics inherited from an input brain region36. Although behavioural perturbations in previous studies have hinted at the intrinsic nature of VMHvl line-attractor dynamics5, a rigorous test requires direct neuronal perturbations34,37 targeted to cells that contribute to the attractor. Direct on-manifold perturbation of a continuous attractor has previously been performed only in the Drosophila head direction system12,21. In mammals, although a point attractor has been perturbed off-manifold using optogenetic manipulation17,18,28, direct single-cell perturbations of neurons contributing to a continuous attractor in vivo have not been reported.
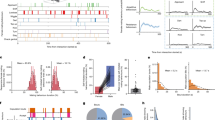
To do this, we used two-photon (2P) imaging in head-fixed mice of VMHvlEsr1 neurons expressing jGCaMP7s38 after observation of aggression and removal of the demonstrator mice (Fig. 1a–c). As described above, during observation of aggression by the head-fixed mice, rSLDS analysis identified an integration dimension with slow dynamics (x1) aligned to an approximate line attractor, and an orthogonal dimension with faster dynamics (x2) (Fig. 1d–h,k). We used the mapping between neural activity and the underlying state space to directly identify neurons contributing to each dimension (Fig. 1i,j). Neurons contributing to the integration dimension displayed more persistence than those aligned with the faster dimension (Fig. 1g,l,m). Importantly, only a small fraction of the neural activity could be explained by movements of the observer mouse (Extended Data Fig. 5a–e). Thus, a line attractor can be recapitulated in head-fixed mice observing aggression, opening the way to 2P-based perturbation experiments.
a, The experimental paradigm for 2P imaging in head-fixed mice observing aggression. b, Representative FOV through a GRIN lens in the 2P set-up (top). Bottom, fluorescence image of a coronal slice showing expression of jGCaMP7s and ChRmine. Scale bars, 100 µm. c, Neural and behavioural raster from an example mouse observing aggression in the 2P set-up (left). The arrows indicate insertion of submissive BALB/c intruders into the observation chamber for interaction with an aggressive Swiss Webster (SW) mouse. Right, example neurons from the raster to the left. d, Neural activity projected onto rSLDS dimensions obtained from models fit to 2P imaging data in one example mouse. e, rSLDS time constants across mice. n = 9 mice. Statistical analysis was performed using two-tailed Mann–Whitney U-tests. Data are mean ± s.e.m. f, The line-attractor score (Methods) across mice. n = 9 mice. Data are mean ± s.e.m. g, Behaviour-triggered average of x1 and x2 dimensions, aligned to the introduction of BALB/c mice into the resident’s cage. n = 9 mice. Data are the average activity (dark line) ± s.e.m. (shading). h, Flow fields from rSLDS model fit to 2P imaging data during observation of aggression from one example mouse. The larger blue arrows next to the neural trajectory indicate the direction flow of time. The smaller arrows represent the vector field from the rSLDS model. i, Identification of neurons contributing to x1 dimension from rSLDS model (top). The neuron’s weight is shown as an absolute (abs) value. Bottom, activity heat map of five neurons contributing most strongly to the x1 dimension. Right, neural traces of the same neurons and an indication of when the system enters the line attractor. j, As in i but for the x2 dimension. k, Dynamic velocity landscape from 2P imaging data during observation of aggression from one example mouse. Blue, stable area in the landscape; red, unstable area in the landscape. The black line shows the trajectory of neuronal activity. l, The cumulative distributions of the autocorrelation half width (ACHW) of neurons contributing to the x1 (green) and x2 (red) dimensions. n = 9 mice, 45 neurons each for the x1 and x2 distributions. m, The mean autocorrelation half width (HW) across mice for neurons contributing to the x1 and x2 dimensions. n = 9 mice. Statistical analysis was performed using a two-tailed Mann–Whitney U-test; **P = 0.0078. Data are mean ± s.e.m. ****P < 0.0001, **P < 0.01.
Holographic activation shows integration
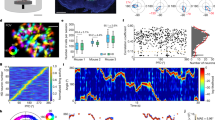
Next, to determine whether VMHvlEsr1 line-attractor dynamics are intrinsic to this hypothalamic nucleus, after removing the demonstrator mice, we performed holographic re-activation of a subset of neurons contributing to the integration dimension (x1) using soma-tagged ChRmine39, which was co-expressed with jGCaMP7s (Fig. 1b (bottom)). These neurons were identified in real-time using rSLDS fitting of data recorded during observation of aggression (in a manual closed loop), followed by 2P single-cell-targeted optogenetic reactivation of those neurons (Fig. 2a). In each field of view (FOV), we concurrently targeted five neurons, chosen on the basis of the criteria that they (1) contributed most strongly to a given dimension (x1 or x2); and (2) could be reliably reactivated by photostimulation (Fig. 2a). Repeated pulses of optogenetic stimulation (2 s, 20 Hz, 5 mW) were delivered with a 20 s interstimulus interval (ISI) (Fig. 2b–d). Under these conditions, we observed minimal off-target effects (Extended Data Fig. 6a–h) and did not observe spatial clustering of x1 or x2 neurons (Methods and Extended Data Figs. 6i–k and 7a,b).
a, The experimental paradigm for 2P perturbation in head-fixed mice. b, FOV of five x1 neurons selected for 2P activation in example mouse 1. Scale bar, 100 µm. c, Neural activity projected onto the x1 dimension after holographic activation of five x1 neurons in example mouse 1. The pink vertical lines show the time of activation. d, The average activity projected onto the x1 dimension from activation of 5 x1 neurons (left). Data are average (dark green) ± s.e.m. (shaded green area). n = 8 mice. Right, the average z-scored activity of the projected x1 dimension during the baseline or ISIs. n = 8 mice. Statistical analysis was performed using Kruskal–Wallis tests with Dunn’s correction; *P = 0.0363, **P = 0.0013 (bottom), **P = 0.0067 (top). Data are mean ± s.e.m. e, Schematic of quantifying perturbation along line attractor in neural state space. f, Flow fields from example mouse 1, showing perturbations along the line attractor after activation of 5 x1 neurons. The larger blue arrows next to the neural trajectory indicate the direction flow of time. The smaller arrows indicate the vector field from the rSLDS model. g, The Euclidian distance between time points tinitial and tstim-end across mice. n = 8 mice. Statistical analysis was performed using Kruskal–Wallis tests with Dunn’s correction; not significant (NS), P = 0.061; *P = 0.029, **P = 0.0018 (bottom), **P = 0.0059 (top). Data are mean ± s.e.m. h, As in g but for timepoints tinitial and tpost-stim. n = 8 mice. Statistical analysis was performed using Kruskal–Wallis tests with Dunn’s correction; NS, P = 0.1965; **P = 0.0082, ***P = 0.0004, *P = 0.016. Data are mean ± s.e.m. i, FOV of five x2 neurons selected for activation in example mouse 1. Scale bar, 100 µm. j, Neural activity projected onto the x2 dimension after holographic activation of x2 neurons in example mouse 1. k, The average activity projected onto the x2 dimension from activation of x2 neurons (left). Data are average (dark red) ± s.e.m. (shaded red area). n = 7 mice. Right, the average z-scored activity of the projected x2 dimension during the baseline or ISIs. n = 7 mice. Statistical analysis was performed using Kruskal–Wallis tests with Dunn’s correction; P > 0.99. Data are mean ± s.e.m. l, As in e but for x2 activation. m, Flow fields from example mouse 1, showing x2 activation. The red arrows indicate the direction of the flow of time. n, As in g but for x2 activation. n = 7 mice. Statistical analysis was performed using Kruskal–Wallis tests with Dunn’s correction; NS, P = 0.1554; *P = 0.042 (bottom), *P = 0.029 (middle), *P = 0.029 (top). Data are mean ± s.e.m. o, As in h but for x2 activation. n = 7 mice. Statistical analysis was performed using Kruskal–Wallis tests with Dunn’s correction; NS, P > 0.05 (bottom), P = 0.508 (middle), P = 0.0508 (top); *P = 0.0383. Data are mean ± s.e.m. NS, P > 0.05; *P < 0.05, ***P < 0.001, ****P < 0.0001.
In this paradigm, optogenetically induced activity along the x1 (but not the x2) dimension is predicted to exhibit integration across successive photostimulation pulses, based on the time constants of these dimensions extracted from the fit rSLDS model (Fig. 1e). Consistent with this expectation, optogenetic reactivation of cohorts of five individual x1 neurons yielded robust integration of activity in the entire x1 dimension-weighted population, as evidenced by progressively increasing peak activity during the 20 s ISI after each consecutive pulse (Fig. 2c,d; n = 8 mice). Activity decayed slowly after each peak but did not return to pre-stimulus baseline. Activated x1 neurons exhibited activity levels comparable to their response during observation of aggression (Extended Data Fig. 7d–f). Similar results were obtained using an 8 s ISI (Extended Data Fig. 8a). This activity also scaled with different laser powers (Extended Data Fig. 8e,f). Providing the same (digital optogenetic) input to the fit rSLDS model also resulted in integration by the model along the x1 dimension, similar to that observed in the data (Extended Data Fig. 8c). Importantly, x1 stimulation did not evoke appreciable activity in x2 dimension neurons (Extended Data Fig. 8g–i).
To visualize in neural-state space the effect of reactivating x1 neurons in the absence of demonstrator mice, we projected the data into a 2D flow-field based on the dynamics matrix fit to data acquired during the observation of aggression. Activation pulses transiently moved the population activity vector (PAV) ‘up’ the line attractor, followed by relaxation back down the attractor to a point that was higher than the initial position of the system (Fig. 2e,f). To quantify this effect, we calculated the Euclidean distance in state space between the initial timepoint during the baseline period (tinitial) and the timepoint at the end of stimulation or at the end of the ISI after each pulse (tstim-end and tpost-stim, respectively) (Fig. 2e–h). This revealed that the x1 perturbations resulted in progressive, stable on-manifold movement along the attractor with each consecutive stimulation, as measured by the increase in both metrics (Fig. 2g,h). However, we found that integration of optogenetic stimulation pulses saturated in the x1 dimension after the third pulse, suggesting that the line attractor occupies a finite portion of the neural state space (Extended Data Fig. 9a–d).
Importantly, activation of x2 neurons did not lead to integration (Fig. 2i–k) as predicted by the time constant derived from the fit rSLDS model (Fig. 1e (red bar)). Instead, after each pulse, we observed stimulus-locked transient activity in the x2 dimension followed by a decay back to the baseline during the ISI period, across stimulation paradigms (Fig. 2k and Extended Data Fig. 8b), with little to no effect on x1 neurons (Extended Data Fig. 8j–l). In 2D neural-state space, we observed that x2 neuron activation caused transient off-manifold movements of the PAV orthogonal to the attractor axis during each pulse (Fig. 2l–o). After each stimulus, the PAV relaxed back into the attractor, near to the initial location that it occupied before the stimulus.
To examine further the stability of different points along the line attractor, we performed photostimulation of x2 neurons after first moving activity in neural-state space further along the attractor using photostimulation of x1 neurons (Extended Data Fig. 9e, f). This x2 perturbation also resulted in transient off-manifold movements of the PAV orthogonal to the line attractor, followed by relaxation to the position occupied after the previous x1 stimulation (but before the x2 stimulation), rather than simply relaxing back to the baseline (Extended Data Fig. 9g–i). This experiment confirms the attractive nature of different points along the line. Lastly, activation of randomly selected neurons that were not weighted by either dimension did not produce activity along either the x1 or x2 dimension, emphasizing the specificity of our on- and off-manifold holographic activation (Extended Data Fig. 9j–n). Activation of either ensemble did not result in overt changes in the behaviour of the head-fixed mouse (Extended Data Fig. 5f–j). Together, these findings demonstrate that a subset of VMHvlEsr1 neurons (x1) can integrate direct optogenetic stimulation, moving the PAV along the line attractor, while a different subset (x2) pushes the PAV out of the attractor.
Line-attractor neurons form ensembles
The integration observed in the abovementioned experiments could reflect a cell-intrinsic mechanism, or it could emerge from recurrent interactions within a network40. To determine whether the latter mechanism contributes to the line attractor, we first examined whether putative x1 follower cells (that is, non-targeted neurons that were photoactivated by stimulation of targeted x1 neurons) exhibited integration. Indeed, even after excluding the targeted x1 neurons themselves as well as potentially off-target neurons located within a 50 µm radius of the targeted cell (Extended Data Figs. 6a–h and 10j–n), we observed integration in the remaining x1 neurons (Extended Data Fig. 10a–c). Moreover, optogenetically evoked integrated activity in targeted x1 neurons could be reliably decoded from the activity of their follower x1 neurons (Extended Data Fig. 10d–f). This decoding was significantly better than that obtained using the activity of non-targeted x2 neurons; furthermore, the x2 activity-based decoder performance was slightly worse than decoders trained on neurons chosen randomly (Extended Data Fig. 10g). These analyses suggest that selective functional connectivity between integration dimension-weighted x1 neurons contributes to line-attractor dynamics in the VMHvl.
To assess more precisely the extent of functional connectivity among VMHvlEsr1 neurons, we activated unitary x1 or x2 neurons and performed imaging of non-targeted neurons (Fig. 3a). These experiments revealed a slowly decaying elevation of activity during the ISI period in non-targeted x1 neurons after each pulse of activation (Fig. 3b,d) that was mostly positive (Extended Data Fig. 10h,i). Notably, the strength of functional connectivity was not positively correlated with the distance from the targeted photostimulated cell (Extended Data Fig. 10j–n) and was still observed even after excluding neurons in a 50 µm zone surrounding the targeted neuron to eliminate potential off-target effects due to ‘spillover’ photostimulation (Extended Data Fig. 10o,p). Comparing the activity of non-targeted photoactivated x1 neurons during unitary x1 neuron photoactivation versus during targeted activation of the five-x1 neuron cohorts revealed that the response strength of the non-targeted x1 neurons scaled with the number of targeted x1 neurons (Extended Data Fig. 10q,r). Importantly, the observed functional coupling between x1 neurons could not be explained by local clustering of non-targeted x1 neurons near the targeted cell (Extended Data Figs. 6i–k and 10k–l).
a, Left, the paradigm for examining activity in non-targeted x1 and x2 neurons after activation of unitary x1 neurons. Right, the average z-scored activity of the perturbed (targeted) x1 neurons (25 single neurons from n = 8 mice). Data are the average trace (dark green) ± s.e.m. (shaded green area). b, The average z-scored activity of non-targeted x1 neurons after targeting unitary x1 neurons. n = 8 mice. Data are average trace (dark green) ± s.e.m. (shaded green area). c, The average z-scored activity of non-targeted x2 neurons after targeting of unitary x1 neurons. n = 8 mice. Data are average (trace in dark red) ± s.e.m. (shaded red area). d, Quantification of activity in non-targeted x1 neurons after targeting of single x1 neurons. NS, P = 0.16; **P = 0.0037 (bottom), ***P = 0.0005, **P = 0.0016 (top). n = 8 mice. Data are mean ± s.e.m. e, Quantification of the activity in non-targeted x2 neurons after targeting of single x1 neurons (NS; n = 8 mice). Data are mean ± s.e.m. f, The paradigm for examining the activity in non-targeted x1 and x2 neurons after activation of single x2 neurons (left). Right, the average z-scored activity of targeted x2 neurons (18 single neurons from n = 7 mice). Data are the average trace (dark red) ± s.e.m. (shaded red area). g, The average z-scored activity of non-targeted x2 neurons after targeting of single x2 neurons. n = 7 mice. Data are the average trace (dark red) ± s.e.m. (shaded red area). h, The average z-scored activity of non-targeted x1 neurons after targeting of single x2 neurons. n = 7 mice. Data are the average trace (dark green) ± s.e.m. (shaded green area). i, Quantification of activity in non-targeted x1 neurons after targeting of single x2 neurons. NS, from bottom to top, P = 0.999, P = 0.31, P = 0.09; *P = 0.0316. n = 7 mice. Data are mean ± s.e.m. j, Quantification of activity in non-targeted x2 neurons after targeting of single x2 neurons (NS). n = 7 mice. Data are mean ± s.e.m.
In contrast to the observed x1-to-x1 functional connectivity, we observed little activity in non-targeted x2 neurons after activation of unitary x1 or x2 neurons (Fig. 3c,e,g,j), suggesting that functional x1–x1 connectivity is selective. While there was a trend to a gradual increase in activity in non-targeted x1 neurons after repeated activation of unitary x2 neurons (Fig. 3f–h), that increase was not statistically significant (Fig. 3i,j).
The functional connectivity that we observed could arise either from a population of sparsely but strongly interconnected neurons, or from a population with denser connections of intermediate strength41 (Fig. 4a (left)). To assess this, we calculated the distribution of pairwise influence scores in our unitary neuron stimulation experiments, defined as the average evoked z-scored activity in each non-targeted photoactivated x1 neuron after photostimulation of a single targeted cell. To estimate the amount of functional coupling within the x1 network, we considered the percentage of x1→x1 pairs that had influence scores higher than the highest x1→x2 pair, which had a z score of around 0.6 (Fig. 4a (right, vertical line)). The fraction of x1→x1 pairs above this threshold was around 36% (Fig. 4a (right)). These data suggest that VMHvlEsr1 neurons that contribute to the line attractor form relatively dense functional ensembles, consistent with theory-based predictions40.
a, Diagram of strong but sparse connectivity among x1 neurons (1), or dense interconnectivity within subnetwork (2) (left). Right, the empirical distribution of the strength of pairwise functional connectivity between x1 neurons (green) and from x1 to x2 neurons (red). n = 99 pairs, n = 7 mice. b, Cartoon illustrating different elements of an excitatory network that can determine network-level persistent activity. c, Model simulation result showing the network time constant (τn) by varying the subnetwork connectivity (σ) in the range of 0 to 20% density values and τs in the range of 0 to 20 s. Blue portions show configurations that result in unstable networks with runaway excitation. d, Magnified version of c (the region left of the dashed line) showing glutamatergic networks with a synaptic conductance time constant (τs) in range of 0.01 to 0.6 s. e, Network time constant (τn) against density of integration subnetwork for slow neurotransmitter (τs: 10, 15 and 20 s). τn varies monotonically with density for large values of τs. f, As in e but for glutamatergic networks (τs: 0.01, 0.1, 0.2 and 0.3 s). g, Cartoon showing the modified VMHvl circuit with fast feedback inhibition incorporated. h, Plot of network time constant (τn) against density of integration subnetwork for a slow neurotransmitter network with τs = 20s, for different values of strength of inhibition (inhibitory gain, ginh: 1.25, 5 and 10) (left). Right, as on the left but for a glutamatergic network with ts = 0.1 s. i, Model simulation of a slow neurotransmitter network with fast feedback inhibition (ts: 20 s, 36% density of subnetwork connectivity). Top, the input (20 s ISI) provided to the model, Bottom, spiking activity in the network. The first 200 neurons (20%) comprise the interconnected integration subnetwork. j, Ca2+ activity convolved from firing rate (Methods) of the integration subnetwork (top) and the remaining neurons (bottom). k, As in i but for a fast transmitter network (ts: 0.1 s, 36% density of subnetwork connectivity). l, As in j but for a fast transmitter network (ts: 0.1 s, 36% density of subnetwork connectivity). a.u., arbitrary units.
We next used computational approaches to investigate the kinetics of the observed functional connectivity within x1 ensembles. Such connectivity could reflect either fast, glutamatergic synapses, as typically assumed for most attractor networks40; or they could be slow neuromodulator-based connections that use GPCR-mediated second messenger pathways to sustain long-time-scale changes in synaptic conductance. To investigate systematically the density and synaptic kinetics of networks capable of generating line attractorlike dynamics with the measured integration-dimension (x1) network time constants, we turned to mechanistic modelling using an excitatory integrate and fire network7 (Fig. 4b). As VMHvl is >80% glutamatergic42, we used excitatory networks and analytically calculated the network time constant using an eigen-decomposition of the connectivity matrix40 (Extended Data Fig. 11a). By varying the synaptic conductance time constant (τs) and the density of the integration subnetwork connectivity, we found that only artificial networks based on relatively sparse connectivity (around 8–12%) and slow synaptic time constants (around 20 s) could yield network time constants (τn) in the experimentally observed range (~50–200 s; Fig. 4c,e (red shading)). By contrast, networks with fast glutamatergic connectivity failed to do so over the same range of connection densities (Fig. 4d,f).
In these purely excitatory network models, the density of connections that yielded network time constants in the observed range was much lower than the experimentally measured value (36%). To match more accurately the empirically observed connection density, we incorporated excitation-recruited fast-feedback inhibition into our integrate-and-fire network7, as VMHvl is known to receive dense GABAergic innervation from surrounding areas43,44. The addition of global strong feedback inhibition allowed networks to match the observed connection density (36%) but, importantly, maintained the slow nature of the functional connectivity (20 s; Fig. 4g and 4h (left)). Indeed, networks simulated with a long τs (20s) and dense σ (36%) could integrate digital optogenetic stimulation in a manner like that observed experimentally (Fig. 4i,j). By contrast, purely glutamatergic networks (τs = 100 ms) were unable to integrate at the observed timescales given the measured connectivity density (Fig. 4h (right) and 4k–l). Together, these results suggest an implementation of the VMHvlEsr1 line attractor that combines slow neurotransmission and relatively dense41 subnetwork interconnectivity within an attractor-creating ensemble.
Attractor stability ties to connectivity
The observed dynamics along the integration dimension exhibits two important characteristics that can reflect the stability of the line attractor, ramping activity up; and slow decay down the integrator (Fig. 5a). Both of these characteristics might either be intrinsic or be driven by external inputs to the line attractor5,40. Previously, we observed that individual differences in aggressiveness among mice were positively correlated with the stability and decay of the VMHvl line attractor during aggression5. We therefore investigated whether individual differences in line-attractor ramping or rate of decay might also be correlated with the strength of functional connectivity within the x1 ensemble (Fig. 5b–d). We plotted either the x1 decay time constants, or the rate of ramp up along the x1 dimension (obtained from rSLDS models fit to each mouse using data recorded during attack observation), against different quantitative metrics of functional connectivity between targeted x1 or x2 neurons and their non-targeted putative follower cells (obtained from the same animals by single-cell optogenetic stimulation and imaging after removal of the demonstrator intruder mice) (Fig. 5d,e and Extended Data Fig. 12a).
a, Example neural activity projected onto the x1 (integration) dimension (dimen.) of one mouse observing aggression, demonstrating a ramp when the BALB/c intruder enters the demonstrator cage containing an aggressive SW mouse (that is, movement up the line attractor) and decay after removal of the BALB/c intruder from the demonstrator cage (that is, movement down the line attractor). b, Dynamics of the integration dimension aligned to the entry of the BALB/c intruder for three example mice. Note the different rates of ramping in different mice. Norm. act., normalized activity. c, As in b, aligned to the removal of the BALB/c intruder, showing different rates of decay. d, z-scored activity of non-targeted x1 neurons after activation of individual x1 neurons in mice from b and c. The pink vertical lines show photostimulation pulses. e, Illustration of different quantitative metrics of the change in activity of non-targeted x1 neurons from d as either the average z-scored activity, or the area under the curve (AUC). The pink vertical lines show the photostimulation pulses. f, Correlation between the rate of ramping of the integration dimension from the rSLDS model fit to data obtained from observation of aggression, and the AUC of non-targeted x1 neurons measured using AUC after the third stimulus (r2 = 0.01; NS). n = 8 mice. g, The correlation between the rSLDS time constant (decay rate of the integration dimension) obtained from observation of aggression, and the AUC of non-targeted x1 neurons measured after the third stimulus (R2 = 0.87; ***P < 0.001). n = 8 mice. h, Summary of the results illustrating causal evidence of a hypothalamic line attractor. i, Diagram of the implementation of a hypothalamic line attractor encoding a behavioural internal state.
Notably, there was a strong correlation across mice between the time constant (decay) of the line attractor measured during the observation of aggression, and the strength of functional connectivity among integration-dimension (x1) neurons measured by post-observation optogenetic stimulation (Extended Data Fig. 12c,d). The strength of this correlation was higher after the third stimulus (r2 = 0.87) compared with after the first stimulus (r2 = 0.59) (Fig. 5g and Extended Data Fig. 12b), indicating that individual differences in the attractor time constant become more apparent once the system has already integrated several inputs, thereby taking longer to decay. By contrast, there was no correlation between functional connectivity and the rate of ramp-up, suggesting that the latter might be driven by extrinsic inputs to the VMHvl (Fig. 5f and Extended Data Fig. 12b–d). Importantly, the correlation between attractor stability and functional connectivity was specific to neurons in the integration (x1) subnetwork, and did not hold when rSLDS time constants were compared with the influence strength of targeted x1 neurons on x2 cells (Extended Data Fig. 12e–h). Thus, individual differences among mice in the stability of the line attractor during the observation of aggression are correlated with individual differences in the functional connection strength among attractor-contributing neurons.
Discussion
Using model-guided closed-loop all-optical experiments, we provide causal evidence of line attractor-like dynamics in a mammalian system (Fig. 5h,i). Our data and modelling also provide insights into the implementation of the approximate line attractor5. We found evidence of relatively dense, selective connectivity among a physiologically distinct subset of Esr1+ neurons. Whether this subset corresponds to one of the transcriptomically distinct subtypes of Esr1+ neurons remains to be determined31. Our models confirm the importance of rapid feedback inhibition7, consistent with studies of the Drosophila ring attractor21,45. However they differ from most continuous attractor models3,40 by invoking slow neuromodulatory transmission rather than fast glutamatergic excitation. Numerous theoretical studies have posited that continuous attractors relying on recurrent glutamatergic connectivity require precise tuning of synaptic weights to sustain stable attractor dynamics40,46,47. By contrast, the inclusion of slow neurotransmission in our mechanistic models yielded network time constants in the observed range across a wide range of connectivity densities. This slow neurotransmission may have evolved not only to ensure attractor robustness, but also to implement the relatively long time scales of internal affective or motive states. These slow dynamics could be implemented by GPCR-mediated signalling triggered by biogenic amines or neuropeptides48. Consistent with this prediction, we have recently found that VMHvl line-attractor dynamics and aggression are dependent on signalling through oxytocin and/or vasopressin neuropeptide receptors expressed in Esr1+ neurons49. However, that does not exclude a contribution from recurrent glutamatergic excitation in the ventromedial hypothalamus, as in line attractors that mediate cognitive functions on shorter time scales14,50.
Lastly, our observations indicate a pronounced correlation between individual differences in the functional strength of integration subnetwork connectivity and differences in the measured stability of the line attractor, perhaps reflecting a leaky integrator. We previously found that, in freely behaving animals, individual differences in attractor stability were correlated with individual differences in aggressiveness5. By transitivity, this suggests that individual differences in the strength of functional connectivity within the attractor network might underlie individual differences in aggressiveness. As these differences are observed among genetically identical inbred mice, these observations suggest that attributes of the attractor, such as its connectivity density or strength, may be modifiable (either by epigenetic mechanisms and/or experience26). Deciphering the underlying mechanisms that afford this attractor its apparent flexibility while maintaining its robustness represents a promising avenue for future research.
Methods
Mice
All of the experimental procedures involving the use of live mice, or their tissues were carried out in accordance with NIH guidelines and were approved by the Institute Animal Care and Use Committee and the Institute Biosafety Committee at the California Institute of Technology (Caltech). All C57BL/6N mice used in this study, including wild-type and transgenic mice, were bred at Caltech. Swiss Webster (SW) male residents and BALB/c male intruder mice were bred at Caltech. Experimental C57BL/6N mice and resident SW mice were used at the age of 8–20 weeks. Intruder BALB/c mice were used at the age of 6–12 weeks and were maintained with three to five cage mates to reduce their aggression. Esr1cre/+ knock-in mice (Jackson Laboratory, 017911) were back-crossed into the C57BL/6N background and bred at Caltech. Heterozygous Esr1cre/+ mice were used for cell-specific targeting experiments and were genotyped by PCR analysis using genomic DNA from ear tissue. All mice were housed in ventilated microisolator cages in a temperature-controlled environment (median temperature, 23 °C, humidity, 60%), under a reversed 11 h–13 h dark–light cycle, with ad libitum access to food and water. Mouse cages were changed weekly.
Viruses
The following adeno-associated viruses (AAVs), along with the supplier, injection titres and injection volumes, were used in this study: AAV1-syn-FLEX-jGCaMP7s-WPRE (Addgene, 104492, around 2 × 1012 viral genomes per ml, 200 nl per injection), AAVdj-Ef1a-DIO-ChRmine-mScarlet-Kv2.1-WPRE (Janelia Vector Core, around 2 × 1012 viral genomes per ml, 200 nl per injection).
Histology
After completion of 2P/miniscope experiments, histological verification of virus expression and implant placement were performed on all of the mice. Mice lacking virus expression or correct implant placement were excluded from the analysis. Mice were perfused transcardially with 0.9% saline at room temperature, followed by 4% paraformaldehyde in 1× PBS. Brains were extracted and post-fixed in 4% paraformaldehyde overnight at 4 °C, followed by 24 h in 30% sucrose/PBS at 4 °C. The brains were embedded in OCT mounting medium, frozen on dry ice and stored at −80 °C for subsequent sectioning. Brains were sectioned at a thickness of 80 μm on a cryostat (Leica Biosystems). The sections were washed with 1× PBS and mounted onto Superfrost slides, then incubated for 30 min at room temperature in DAPI/PBS (0.5 μg ml−1) for counterstaining, washed again and a cover slip was added. The sections were imaged with epifluorescence microscope (Olympus, VS120).
Stereotaxic surgeries
Surgeries were performed on sexually experienced adult male Esr1cre/+ mice aged 6–12 weeks. Virus injection and implantation were performed as described previously25,51. In brief, mice were anaesthetized with isoflurane (5% for induction and 1.5% for maintenance) and placed onto a stereotaxic frame (David Kopf Instruments). Virus was injected into the target area using a pulled-glass capillary (World Precision Instruments) and a pressure injector (Micro4 controller, World Precision Instruments), at a flow rate of 50 nl min−1. The glass capillary was left in place for 5 min after injection before withdrawal. Stereotaxic injection coordinates were based on the Paxinos and Franklin atlas52. Virus injection: VMHvl, anteroposterior (AP), −1.5; mediolateral (ML), ±0.75; dorsoventral (DV), −5.75. For 2P experiments GRIN lenses (0.6 × 7.3 mm, Inscopix) were slowly lowered into the brain and fixed to the skull with dental cement (Metabond, Parkell). Coordinates for GRIN lens implantation: VMHvl, AP, −1.5; ML, −0.75; DV, −5.55). A permanent head bar was attached to the skull with Secure Resin cement (Parkell). For microendoscopy experiments, an additional baseplate was attached to the skull (Inscopix).
Housing conditions for behavioural experiments
All male C57BL/6N mice used in this study were socially and sexually experienced. Mice aged 8–12 weeks were initially co-housed with a female C57BL/6N female mouse for 1 day and were then screened for attack behaviours. Mice that showed attack towards males during a 10 min resident intruder assay were selected for surgery and subsequent behaviour experiments. From this point forward, these male mice were always co-housed with a female.
Behaviour annotations
Behaviour videos were manually annotated using a custom MATLAB-based behaviour annotation interface53,54. A ‘baseline’ period of 5 min when the animal was alone in its home cage was recorded at the start of every recording session. Two behaviours during the resident intruder assays were annotated: sniff (face, body, genital-directed sniffing) towards male intruders, and attack (bite, lunge).
Behavioural assays
An observation arena was built from a transparent acrylic (18 × 12.5 × 18 cm, length × width × height), and a perforated part was put in front of the mice observing aggression. Perforations were 1.27 cm diameter and spread evenly throughout the bottom third of the panel. Before initiation of the assay, the observation arena was scattered with soiled bedding from the cage of the aggressive SW demonstrator. For observation of aggression in freely behaving animals (miniscope experiments), an observer was first habituated for 15 min. A singly housed SW male demonstrator was then introduced into the observation arena, followed 1 min later with the insertion of a socially housed stimulus male (BALB/c) mouse into the same compartment. The observation of aggressive encounters persisted for around 1 min, then, after 2 min, a different intruder was introduced for another minute. Observation assays were conducted under white-light illumination. For experiments in engaging aggression, the resident mouse was first habituated 15 min then a BALB/c intruder mouse was introduced twice for 1–2 min. For the experiments comparing neural activity of mice observing aggression and mice engaging aggression, we randomly changed the order of sessions. For mice observing aggression in the 2P set-up, the approach was similar, except that the observer mouse was head-fixed and on a treadmill instead of freely behaving in his home cage.
Microendoscopy imaging
On the day of imaging, the mice were habituated for at least 15 min after installation of the miniscope in their home cage before the start of the behaviour tests. Imaging data were acquired at 30 Hz with 2× spatial downsampling; light-emitting diode power (0.1–0.5) and gain (1–7×) were adjusted depending on the brightness of GCaMP expression as determined by the image histogram according to the user manual. A transistor–transistor logic pulse from the Sync port of the data acquisition box (Inscopix) was used for synchronous triggering of StreamPix7 (Norpix) for video recording.
2P imaging and holographic optogenetics
Two to three weeks after surgery, the mice were habituated to the experimenter’s hand by handling for 15 min a day for three consecutive days. Once the mice were habituated to the experimenter’s hand, they were manually scooped and gently placed onto the treadmill. Mice were head-fixed for 3 consecutive days for habituation. Head fixation was achieved by securing the head bar into a metal clamp attached to a custom head stage. During habituation, the mice were placed underneath the objective for 15 min and given access to random presentations of chocolate milk. After habituation, combined 2P imaging and behaviour sessions were conducted. jGCaMP7s imaging was acquired using an Ultima 2P Plus and the Prairie View Software (Bruker Fluorescence Microscopy). Individual frames were acquired at 10 Hz using a galvo-resonant scanner with a resolution of 1,024 px × 1,024 px. We used a long-working-distance ×20 air objective designed for infrared wavelengths (Olympus, LCPLN20XIR, 0.45 NA, 8.3 mm working distance) combined with a femtosecond-pulsed laser beam (Chameleon Discovery, Coherent). GCaMP was excited using a 920 nm wavelength. For targeted photostimulation, the same microscope and acquisition system (Bruker) was used with a second laser path consisting of a 1,035 nm high power femtosecond pulsed laser (Monaco 1035-40-40, Coherent), spatial light modulator (512 × 512 px density) to generate multipoint stimulation montages (NeuraLight 3D, Bruker). During photostimulation, the mice were head-fixed in complete darkness on a rotating cylinder that enabled them to run. Neurons were selected for targeted photostimulation based on two criteria: (1) their weights from the rSLDS model and (2) if they responded to photostimulation. In case a neuron did not show a significant increase in activity in response to photostimulation, a new neuron was chosen until a total of five photosensitive neurons was targeted for each grouped stimulation experiment (Fig. 2). During the photostimulation session, a 128-frame average image was generated to clearly highlight all neurons. To reduce off-target effects during photostimulation, we used small targets (10 µm diameter) that were manually restricted to GCaMP-expressing neurons. Moreover, the laser power was adjusted to be a maximum of 5 mW per target. We used Prairie software to elicit holographic photostimulation (10 Hz, 2 s, 10 ms pulse width). Photostimulations were done between frames to avoid laser artefacts. Importantly, to reduce cross activation of the ChRmine from the 920 nm laser, we kept laser power for imaging to be less than 30 mW.
To extract regions of interest, data from mice observing aggression was uploaded to ImageJ. Videos were then motion corrected using the moco plugin55. Motion-corrected videos were averaged, and additional contrast and brightness adjustments were made to clearly highlight all neurons in the FOV. Cells were then manually extracted and an rSLDS model was used to identify x1 and x2 dimension neurons. Neurons were then identified on the FOV using the Prairie view software and were targeted for photostimulation. While rSLDS models was running (15–20 min, see below), control experiments were conducted.
Microendoscopy data extraction
Preprocessing
Miniscope data were acquired using the Inscopix Data Acquisition Software as 2× downsampled .isxd files. Preprocessing and motion correction were performed using Inscopix Data Processing Software. In brief, raw imaging data were cropped, 2× downsampled, median filtered and motion corrected. A spatial band-pass filter was then applied to remove out-of-focus background. Filtered imaging data were temporally downsampled to 10 Hz and exported as a .tiff image stack.
Calcium data extraction
After preprocessing, calcium traces were extracted and deconvolved using the CNMF-E56 large data pipeline with the following parameters: patch_dims = [4], gSig = 3, gSiz = 13, ring_radius = 17, min_corr = 0.7, min_pnr = 8. The spatial and temporal components of every extracted unit were carefully inspected manually (SNR, PNR, size, motion artefacts, decay kinetics and so on) and outliers (obvious deviations from the normal distribution) were discarded.
Terminology
We use the following terminology to refer to the design and results of our experiments:
-
(1)
x1 or x2 neurons: cells that were identified by rSLDS modelling as contributing to dimensions x1 or x2, respectively, during observation of aggression.
-
(2)
Targeted neurons: rSLDS-identified cells that were purposely photostimulated.
-
(3)
Photoactivated neurons: cells that were empirically found to increase their ∆F/F in response to photostimulation of one or more targeted neurons, that is, photoresponsive neurons. This category includes both the purposely stimulated (targeted) and not purposely stimulated neurons. The latter may include both off-target neurons and putative follower cells.
-
(4)
Off-target neurons: photoactivated neurons that were not purposely photostimulated, but which responded to photostimulation of a selected target cell(s) with an increased ∆F/F because they were close enough (within 15 µm) to be inadvertently activated by light spillover from the targeted neuron (Extended Data Fig. 6a–h).
-
(5)
Putative follower cells: neurons that responded to photostimulation and that were outside a 50 µm radius around the targeted cell (to conservatively exclude off-target neurons; Extended Data Figs. 6h and 10k–n); they are putative targets (direct or indirect) of the targeted cell.
Dynamical system models of neural data
rSLDS models16,29 were fit to neural data as previously described15. In brief, rSLDS is a generative state-space model that decomposes nonlinear timeseries data into a set of discrete states, each with simple linear dynamics. The model describes three sets of variables: a set of discrete states (z), a set of latent factors (x) that captures the low-dimensional nature of neural activity and the activity of recorded neurons (y). While the model can also allow for the incorporation of external inputs based on behaviour features, such external inputs were not included in our first analysis.
The model is formulated as follows: at each timepoint, there is a discrete state zt ∈ {1, …, K} that depends recurrently on the continuous latent factors (x) as follows:
where \({R}_{k}\in {{\mathbb{R}}}^{K\times K}\) and \({r}_{k}\in {{\mathbb{R}}}^{K}\) parameterize a map from the previous discrete state and continuous state to a distribution over the next discrete states using a softmax link function. The discrete state zt determines the linear dynamical system used to generate the latent factors at any time t:
where \({A}_{k}\in {{\mathbb{R}}}^{d\times d}\) is a dynamics matrix and \({b}_{k}\in {{\mathbb{R}}}^{D}\) is a bias vector, where D is the dimensionality of the latent space and \({{\epsilon }}_{t}\, \sim {N}(0,{Q}_{{z}_{t}})\) is a Gaussian-distributed noise (also known as innovation) term.
Lastly, we can recover the activity of recorded neurons by modelling activity as a linear noisy Gaussian observation \({y}_{t}\,\in {{\mathbb{R}}}^{N}\) where N is the number of recorded neurons:
For \(C\in {{\mathbb{R}}}^{N\times D}\) and \({\delta }_{t} \sim N(0,S)\), a Gaussian noise term. Overall, the system parameters that rSLDS needs to learn consists of the state transition dynamics, library of linear dynamical system matrices and neuron-specific emission parameters, which we write as:
We evaluate model performance using both the evidence lower bound and the forward simulation accuracy (Fig. 3a) described previously5,15, as well as by calculating the variance explained by the model on data.
We used two-dimensional models, selecting the optimal number of states through fivefold cross-validation. To ascertain which neurons contributed to each of the two model dimensions (x1 and x2), we initially confirmed the orthogonality of these dimensions by computing the subspace angle between them (88.1 ± 0.87°, n = 9 mice). Given this near orthogonality, we then used the columns of the emission matrix C to identify neurons that contributed to the two separate dimensions of the model.
The contribution of neurons to each latent dimension is defined based on their weights from the emission matrix C, which is initialized by factor analysis and then optimized by rSLDS. In the matrix C, the rows define the weights that create the latent dimensions and the columns defined the different latent dimensions (x1 and x2) in the model. The model performance is reported both as the evidence lower bound, which is equivalent to the Kullback–Leibler divergence between the approximate and true posterior as well as the variance (cross-validated R2 (cvR2)) explained. We cross-validated the model using fivefold cross-validation, for which we trained the data on four arbitrary portions of the data and tested on a left out fifth portion. In all of the experiments, the model must achieve at least 70% cvR2 before it is used for downstream analysis such as identification of x1 and x2 neurons. Models fit to miniscope data during engagement of aggression obtained a cvR2 = 84.7 ± 0.03%, while the same model explains 67.2 ± 0.02% of variance in data obtained from observation of aggression. Flow fields obtained from head-fixed animals observing aggression where fit with input terms representing the presence of the BALB/c intruder.
Estimation of time constants
We estimated the time constant of each dimension of linear dynamical systems using eigenvalues λa of the dynamics matrix of that system, derived previously as57:
The intrinsic leak rate is defined based on the time constant of the integration dimension across the whole session. The activity observed by the model takes into account both decays (that is, the decays after the first and second time the intruder is removed), and therefore gives high prediction to the holographic perturbation experiments (cVR2, ~85%; Fig. 2f,p). Note also that the dynamics captured by the perturbation experiments more closely resembles the second intruder interaction rather than the first. Furthermore, the SW mouse is still in the observation chamber between BALB/c intruders, but is removed after the second intruder. For this reason, the observed dynamics is mostly consistent and across mice the second decay seems faster.
Calculation of line attractor score
To provide a quantitative measure of the presence of line-attractor dynamics, we devised a line attractor score as defined previously5 as:
where tn is the largest time constant of the dynamics matrix of a dynamical system and \({t}_{n-1}\) is the second largest time constant.
Calculation of autocorrelation half-width
We computed autocorrelation halfwidths by calculating the autocorrelation function for each neuron timeseries data (yt) for a set of lags as described previously12. In brief, for a time series (yt), the autocorrelation for lag k is:
where ck is defined as:
and c0 is the sample variance of the data.
Mechanistic modelling
We constructed a model population of n = 1,000 standard current-based leaky integrate-and-fire neurons as previously performed7. We first modelled a purely excitatory spiking network in which each neuron has membrane potential xi characterized by dynamics:
where τm = 20 ms is the membrane time constant, W is the synaptic weight matrix, s is an input term representing external inputs and p represents recurrent inputs. To model spiking, we set a threshold (θ = 0.1), such that when the membrane potential xi(t) > θ, xi(t) is set to zero and the instantaneous spiking rate \({r}_{i}\left(t\right)\) is set to 1.
Spiking-evoked input was modelled as a synaptic current with dynamics:
where τs is the synaptic conductance time constant. In excitatory networks, the network time constant τn was derived as \(\frac{{\tau }_{{\rm{s}}}}{\left|1-{\lambda }_{\max }\right|}\), where \({\lambda }_{\max }\) is the largest eigenvalue of the synaptic weight matrix W (ref. 40).
We designed the synaptic connectivity matrix to include a subnetwork of 200 neurons (20% of the network), designated the integration subnetwork as suggested by empirical measurements, with varying densities of random connectivity as highlighted in Fig. 3. Weights of the overall network were sampled from a uniform distribution: \({W}_{{ij}} \sim {U}(\mathrm{0,1}/\sqrt{N})\), while weights of the subnetwork were sampled as \({W}_{{ij}} \sim {U}(\mathrm{0,1}/\sqrt{{N}_{p}})\), where Np = 200.
External input was provided to the network as a smoothened step function consisting of four pulses at 20 second ISI as provided in vivo. This stimulus drove a random 25% of neurons in the network.
To account for finite-size effects and runaway excitation in networks, we also simulated models with fast feedback inhibition. This was modelled as recurrent inhibition from a single graded input Iinh representing an inhibitory population that receives equal input from and provides equal input to, all excitatory units. The dynamics of Iinh evolves as:
where τI = 50 ms is the decay time constant for inhibitory currents. In this model, outside spiking events, the membrane potential evolved as:
Model dynamics were simulated in discrete time using Euler’s method with a timestep of 1 ms and a small Gaussian noise term \({\eta }_{i} \sim N(\mathrm{0,1})/5\) was added at each time step. We used g = 1 and varied ginh = 1,5,10 as suggested by measurements of inhibitory input to VMHvl43.
Spatial cluster decoder
To examine whether x1 and x2 neurons are spatially clustered in a FOV, we used a linear support vector machine decoder trained to separate cell positions of x1 and x2 neurons on each FOV. Shuffled decoder data were generated by randomly assigning neuronal identity. Shuffling was repeated 20 times for each FOV and the performance is reported as the average accuracy of each fit decoder.
Decoding behaviour from integration dimension
We trained a frame-wise decoder to discriminate bouts of attack during engaging in aggression from integration dimension activity during observation of attack. We first created ‘trials’ from bouts of attack during observation and engaging in aggression by merging all bouts that were separated by less than 5 s and balancing the data. We then trained a support vector machine to identify a decoding threshold that maximally separates the values of our normalized ‘integration dimension’ signal on frames during observation of aggression versus all other frames and tested the accuracy of the trained decoder on held-out frames. ‘Shuffled’ decoder data were generated by setting the decoding threshold on the same ‘trial’, but with the behaviour annotations randomly assigned to each behaviour bout. We repeated shuffling 20 times. We then tested the decoder trained on data from observation on frames during attack while the animals were engaging in aggression. We report performances of actual and shuffled 1D-threshold ‘decoders’ as the average accuracy score of the fit decoder, on data from all other trials for each mouse. For significance testing, the mean accuracy of the decoder trained on shuffled data was computed across mice, with shuffling repeated 1,000 times for each mouse.
Examining the effect of motion on neural encoding during observation of aggression in head-fixed mice
We used an analysis designed to detect motion from video recordings of head-fixed mice58. To detect motion this method uses singular value decomposition (SVD) to extract groups of pixels showing high differences in luminance or contrast between consecutive frames. We extracted 500 SVDs from our video recordings that reflect different sources of motion including movements of the limbs, whiskers, nose, ears and more. To predict neural activity from behaviour, we trained generalized linear models to predict the activity of each neuron k as a weighted linear combination of the first ten principal components of the 500 SVDs (reflecting over 90% of the SVDs variance) as follows:
Here, \({y}_{k}(t)\) is the calcium activity of neuron k at time t, \(\mathop{x}\limits^{\rightharpoonup }(t)\) is a feature vector of 10 binary reduced SVD dimensions at time lags ranging from t − D to t where D = 10s. \(\mathop{\beta }\limits^{\rightharpoonup }\) is a behaviour-filter that described how a neuron integrates stimulus over a 10 s period (example filters are shown in Extended Data Fig. 5c). φ is an error term. The model was fit using tenfold cross-validation with ridge regularization and model performance is reported as cvR2.
Statistical analysis
Data were processed and analysed using Python, MATLAB and GraphPad (GraphPad PRISM 9). All data were analysed using two-tailed nonparametric tests. Mann–Whitney U-tests were used for binary paired samples. Friedman tests were used for non-binary paired samples. Kolmogorov–Smirnov tests were used for non-paired samples. Multiple comparisons were corrected using Dunn’s multiple-comparison correction.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Source data for this Article have been deposited in the DANDI repository with the accession code 001037.
Code availability
Code for fitting models is available at GitHub (https://github.com/lindermanlab/ssm).
Change history
15 September 2025
This paper was originally published under Creative Commons Attribution 4.0 International license, CC-BY-NC-ND, © The Author(s). It is now available under a Creative Commons Attribution 4.0 International license, CC-BY, © The Author(s). The error has been corrected in the online version of the article.
References
Vyas, S., Golub, M. D., Sussillo, D. & Shenoy, K. V. Computation through neural population dynamics. Annu. Rev. Neurosci. 43, 249–275 (2020).
Khona, M. & Fiete, I. R. Attractor and integrator networks in the brain. Nat. Rev. Neurosci. 23, 744–766 (2022).
Langdon, C., Genkin, M. & Engel, T. A. A unifying perspective on neural manifolds and circuits for cognition. Nat. Rev. Neurosci. 24, 363–377 (2023).
Inagaki, H. K. et al. Neural algorithms and circuits for motor planning. Annu. Rev. Neurosci. 45, 249–271 (2022).
Nair, A. et al. An approximate line attractor in the hypothalamus encodes an aggressive state. Cell 186, 178–193 (2023).
Yang, T. et al. Hypothalamic neurons that mirror aggression. Cell 186, 1195–1211 (2023).
Kennedy, A. et al. Stimulus-specific hypothalamic encoding of a persistent defensive state. Nature 586, 730–734 (2020).
Zeng, H. What is a cell type and how to define it? Cell 185, 2739–2755 (2022).
Tye, K. M. & Uchida, N. Editorial overview: neurobiology of behavior. Curr. Opin. Neurobiol. 49, iv–ix (2018).
Luo, L. Architectures of neuronal circuits. Science 373, eabg7285 (2021).
Barack, D. L. & Krakauer, J. W. Two views on the cognitive brain. Nat. Rev. Neurosci. 22, 359–371 (2021).
Hulse, B. K. & Jayaraman, V. Mechanisms underlying the neural computation of head direction. Annu. Rev. Neurosci. 43, 31–54 (2020).
Durstewitz, D., Koppe, G. & Thurm, M. I. Reconstructing computational system dynamics from neural data with recurrent neural networks. Nat. Rev. Neurosci. 24, 693–710 (2023).
Sylwestrak, E. L. et al. Cell-type-specific population dynamics of diverse reward computations. Cell 185, 3568–3587 (2022).
Rajan, K., Harvey, C. D. & Tank, D. W. Recurrent network models of sequence generation and memory. Neuron 90, 128–142 (2016).
Liu, M., Nair, A., Coria, N., Linderman, S. W. & Anderson, D. J. Encoding of female mating dynamics by a hypothalamic line attractor. Nature https://doi.org/10.1038/s41586-024-07916-w (2024).
Inagaki, H. K., Fontolan, L., Romani, S. & Svoboda, K. Discrete attractor dynamics underlies persistent activity in the frontal cortex. Nature 566, 212–217 (2019).
Daie, K., Svoboda, K. & Druckmann, S. Targeted photostimulation uncovers circuit motifs supporting short-term memory. Nat. Neurosci. 24, 259–265 (2021).
Carrillo-Reid, L., Han, S., Yang, W., Akrouh, A. & Yuste, R. Controlling Visually guided behavior by holographic recalling of cortical ensembles. Cell 178, 447–457 (2019).
Carrillo-Reid, L., Yang, W., Bando, Y., Peterka, D. S. & Yuste, R. Imprinting and recalling cortical ensembles. Science 353, 691–694 (2016).
Kim, S. S., Rouault, H., Druckmann, S. & Jayaraman, V. Ring attractor dynamics in the Drosophila central brain. Science 356, 849–853 (2017).
Green, J., Vijayan, V., Mussells Pires, P., Adachi, A. & Maimon, G. A neural heading estimate is compared with an internal goal to guide oriented navigation. Nat. Neurosci. 22, 1460–1468 (2019).
Mei, L., Osakada, T. & Lin, D. Hypothalamic control of innate social behaviors. Science 382, 399–404 (2023).
Lee, H. et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632 (2014).
Karigo, T. et al. Distinct hypothalamic control of same- and opposite-sex mounting behaviour in mice. Nature 589, 258–263 (2021).
Remedios, R. et al. Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature 550, 388–392 (2017).
Linderman, S. W. et al. Bayesian learning and inference in recurrent switching linear dynamical systems. Pr. Mach. Learn. Res. 54, 914–922 (2017).
Carrillo-Reid, L. & Yuste, R. Playing the piano with the cortex: role of neuronal ensembles and pattern completion in perception and behavior. Curr. Opin. Neurobiol. 64, 89–95 (2020).
Emiliani, V. et al. Optogenetics for light control of biological systems. Nat. Rev. Methods Primers 2, 55 (2022).
Russell, L. E. et al. All-optical interrogation of neural circuits in behaving mice. Nat. Protoc. 17, 1579–1620 (2022).
Kim, D. W. et al. Multimodal analysis of cell types in a hypothalamic node controlling social behavior. Cell 179, 713–728 (2019).
Knoedler, J. R. et al. A functional cellular framework for sex and estrous cycle-dependent gene expression and behavior. Cell 185, 654–671 (2022).
Yang, C. F. et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909 (2013).
Jazayeri, M. & Afraz, A. Navigating the neural space in search of the neural code. Neuron 93, 1003–1014 (2017).
Ghosh, K. K. et al. Miniaturized integration of a fluorescence microscope. Nat. Methods 8, 871–878 (2011).
Lo, L. et al. Connectional architecture of a mouse hypothalamic circuit node controlling social behavior. Proc. Natl Acad. Sci. USA 116, 7503–7512 (2019).
Sadtler, P. T. et al. Neural constraints on learning. Nature 512, 423–426 (2014).
Dana, H. et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 16, 649–657 (2019).
Marshel, J. H. et al. Cortical layer-specific critical dynamics triggering perception. Science 365, eaaw5202 (2019).
Goldman, M. S., Compte, A. & Wang, X. J. in Encyclopedia of Neuroscience (ed. Squire, L. R.) 165–178 (Academic, 2009).
Brunel, N. Is cortical connectivity optimized for storing information? Nat. Neurosci. 19, 749–755 (2016).
Hashikawa, Y., Hashikawa, K., Falkner, A. L. & Lin, D. Ventromedial hypothalamus and the generation of aggression. Front. Syst. Neurosci. 11, 94 (2017).
Yamamoto, R., Ahmed, N., Ito, T., Gungor, N. Z. & Pare, D. Optogenetic study of anterior BNST and basomedial amygdala projections to the ventromedial hypothalamus. eNeuro https://doi.org/10.1523/ENEURO.0204-18.2018 (2018).
Minakuchi, T. et al. Independent inhibitory control mechanisms for aggressive motivation and action. Nat. Neurosci. 27, 702–715 (2024).
Franconville, R., Beron, C. & Jayaraman, V. Building a functional connectome of the Drosophila central complex. eLife 7, e37017 (2018).
Sebastian Seung, H. Continuous attractors and oculomotor control. Neural Netw. 11, 1253–1258 (1998).
Seung, H. S. How the brain keeps the eyes still. Proc. Natl Acad. Sci. USA 93, 13339–13344 (1996).
Robinson, D. A. Integrating with neurons. Annu. Rev. Neurosci. 12, 33–45 (1989).
Mountoufaris, G. et al. A line attractor encoding a persistent internal state requires neuropeptide signaling. Cell 187, 1–18 (2024).
Mante, V., Sussillo, D., Shenoy, K. V. & Newsome, W. T. Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature 503, 78–84 (2013).
Yang, B., Karigo, T. & Anderson, D. J. Transformations of neural representations in a social behaviour network. Nature 608, 741–749 (2022).
Paxinos, G. & Franklin, K. B. Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates (Academic, 2019).
Lin, D. et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226 (2011).
Segalin, C. et al. The Mouse Action Recognition System (MARS) software pipeline for automated analysis of social behaviors in mice. eLife 10, e63720 (2021).
Dubbs, A., Guevara, J. & Yuste, R. moco: fast motion correction for calcium imaging. Front. Neuroinform. https://doi.org/10.3389/fninf.2016.00006 (2016).
Zhou, P. et al. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. eLife 7, e28728 (2018).
Maheswaranathan, N., Williams, A., Golub, M., Ganguli, S. & Sussillo, D. Universality and individuality in neural dynamics across large populations of recurrent networks. Adv. Neural Inf. Process Syst. 2019, 15629–15641 (2019).
Syeda, A. et al. Facemap: a framework for modeling neural activity based on orofacial tracking. Nat. Neurosci. 27, 187–195 (2024).
Acknowledgements
We thank B. Yang for help in miniscope imaging and initial construct of the ChRmine plasmid and for his feedback; I. Landau and H. Inagaki for feedback; T. Karigo for help in behavioural experiments; J.-S. Kim for help in stereotaxic surgeries and histology; X. Da for help in histology; Y. Huang for help with genotyping and maxi-prep; the members of the Techlab at Caltech for help with 3D printing; D. Wagenaar for help with hardware; Y. Jung for help securing the funds for the 2P-SLM set-up; staff at Bruker, and especially S. Trier, T. Fothergill and E. Cho, for their help in establishing and maintaining the 2P-SLM set-up; the former Anderson laboratory members B. Weissbourd and A. Kennedy for initial feedback on this work; the current Anderson laboratory members for continuous feedback; the Caltech OLAR staff and especially K. Lee for animal care; and C. Chiu, G. Mancuso and L. Chavarria for laboratory management and administrative assistance. D.J.A. is an investigator of the Howard Hughes Medical Institute. This work was supported by grants from the NIH (RO1MH112593, RO1MH123612 and RO1NS123916) and by the Simons Collaboration on the Global Brain. A.N. is supported by a National Science Scholarship from the Agency of Science, Technology and Research, Singapore; and A.V. by a fellowship from the Human Frontiers Science Program and is a postdoctoral fellow at the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
A.V., A.N. and D.J.A. designed the study and wrote the manuscript, with critical input from S.W.L. A.N. and A.V. performed data analysis and data collection with help from J.H.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Michael Hausser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Shared line attractor dynamics in freely behaving mice engaging in or observing aggression.
a. Implantation of miniscope, field of view (top) and fluorescence image showing histology (bottom) with jGCaMP7s expression in VMHvl. N = 5 mice. b. Experimental paradigm to record VMHvlEsr1 activity in mice engaging in aggression. c. Left: neural & behavioural raster of example mouse 1 when engaging in aggression. Right: example neurons. d. Experimental paradigm to record VMHvlEsr1 activity in same mice in Extended Data Fig. 1c during observation of aggression. e. Left: neural & behavioural raster of example mouse 1 during observation of aggression. Right: example neurons. f. Overview of rSLDS analysis. g. Left: rSLDS time constants in example mouse 1. Right: Normalized neural activity projected onto two dimensions (x1 and x2) of dynamical system. h. Behaviour triggered average of normalized x1 and x2 dimensions, aligned to introduction of male intruder (n = 5 mice, average trace in dark red and black ± sem in shaded area). i. Behaviour triggered average of x1 dimensions, aligned to first attack onset (n = 5 mice, average trace in dark red ± sem in shaded red area). j. Left: rSLDS time constants in example mouse 1 during observation of aggression. Right: Neural activity projected onto two dimensions (x1 and x2) of dynamical system. k. Behaviour triggered average of normalized x1 and x2 dimensions from observation of aggression, aligned to introduction of BALB/c into resident’s cage (n = 5 mice, average trace in dark purple and black ± sem in shaded area). l. Behaviour triggered average of x1 dimensions from observation of aggression, aligned to first bout of observing attack (n = 5 mice, average trace in dark purple ± sem in shaded purple area). m. Average activity in the x1 dimension during sniffing of the SW mouse, vs observing the SW mouse a BALB\c intruder (n = 4 mice, *p = 0.0286, Two-tailed Mann Whitney U-test, error bars - sem). n. rSLDS time constants across mice engaging in aggression (n = 5 mice, *p = 0.0079, Two-tailed Mann Whitney U-test, error bars - sem). o. Line attractor score across mice engaging in aggression (n = 5 mice, error bars - sem). p. rSLDS time constants across mice during observation of aggression (n = 5 mice, *p = 0.0079,Two-tailed Mann Whitney U-test, error bars - sem). q. Line attractor score across mice during observation of aggression (n = 5 mice, error bars - sem).
Extended Data Fig. 2 Flow fields from miniscope experiments during engagement and observation of aggression.
Flow fields from all mice showing neural trajectories aligned to removal of the intruder or demonstrator mouse in either observation or engagement of aggression. Dashed lines highlight region of slow points (line attractor).
Extended Data Fig. 3 Comparing neuronal activity of x1 neurons during engaging vs. observing aggression.
a. Normalized neuronal activity of all x1 neurons from example mouse 1 when engaging in aggression (left) and observing aggression (right). Bottom: Raster plots of the activity of all neurons from x1 dimension in mouse 1. b. Same as in panel a but for example mouse 2. c. comparing the activity of x1 neurons between observing and engaging in aggression. Left: Average activity across mice (n = 5 mice, shaded area is sem). Right: comparison of the activity during observing attack bouts and engaging in attack (n = 5 mice, p = 0.42, Two-tailed Mann-Whitney U-test, error bars - sem). d. Activity of x1 neurons aligned to removal of last intruder during observation and engaging in aggression (n = 5 mice, shaded area is sem). e. Quantification of autocorrelation half-width for x1 neurons in both conditions during the full interaction (mean achw during observation: 25 ± 0.8s, mean achw during engagement: 20 ± 1.7s, n=5 mice, p = 0.125, Two-tailed Mann-Whitney U-test, error bars - sem). f. Quantification of achw for x1 neurons in both conditions aligned to removal of last intruder (mean achw during observation: 14 ± 1s, mean achw during engagement: 11 ± 1.6s, n = 5 mice, p = 0.187, Two-tailed Mann-Whitney U-test, error bars - sem). g. Decoding bouts of attack during engaging in aggression from integration dimension activity during observation of attack. Left: Decoder strategy. A SVM decoder was trained on data from integration dimension activity to separate bouts of observing attack from non attack bouts. Right: Quantification of the decoder accuracy performance (n = 5 mice, p = 0.0079, Two-tailed Mann-Whitney U-test, error bars - sem). h. Left: Strategy for testing the decoder. The SVM decoder that was trained on observation of attack is tested with data from engaging in attack. Right: Quantification of the performance of the decoder on engaging vs shuffled data (n = 5 mice, p = 0.0079, Two-tailed Mann-Whitney U-test, error bars - sem).
Extended Data Fig. 4 Single cell comparison of integration neurons across conditions.
a. Single cell contribution of x1 dimension (rSLDS weights) from engagement of aggression in example mouse. b. Single cell contribution of x1 dimension (rSLDS weights) from observation of aggression in example mouse. c. Overlap in neurons contributing to line attractor x1 & x2 dimension from rSLDS performing independently in engaging versus observing aggression. Left: Example mouse, Right: Average across 5 mice, error bars - sem. d. Dot product of x1 neural weight vectors during observation vs. engagement in aggression. rSLDS weights of the x1 dimension during observation were compared to model weights of the x1 and x2 dimensions during engagement using a dot product of the two weight vectors. (n = 5 mice, ***p = 0.0079, Two-tailed Mann-Whitney U-test, error bars - sem). e. Example raster of baseline activity from one mouse freely behaving while solitary in its home cage. f. Example single-cell traces from raster in Ex. Data Fig.e. Top - x1 neurons, bottom - x2 neurons. g. Comparison of frequency of Ca+2 transients (above 1.5σ in z-score activity) during baseline recordings across mice (mean frequency x1: 1.6 ± 0.2 events, mean frequency x2: 2.3 ± 0.2 events, n = 5 mice, *p = 0.012, Two-tailed Mann-Whitney U-test, error bars - sem). h. Comparison of the mean amplitude of Ca+2 transients in x1 vs. x2 neurons during baseline recordings, averaged across mice (mean amplitude x1: 0.58 ± 0.04 z-score, mean amplitude x2: 0.71 ± 0.08 z-score, n = 5 mice, p = 0.188, Two-tailed Mann-Whitney U-test, error bars - sem). i. Comparison of the decay time of Ca+2 events during baseline recordings across mice (mean tau x1: 1.7 ± 0.6s, mean tau x2: 2.3 ± 0.4s, n = 5 mice, p = 0.34, Two-tailed Mann-Whitney U-test, error bars - sem).
Extended Data Fig. 5 Readouts of behaviour and motion in head-fixed mice.
a. Top: Experimental paradigm for 2-photon imaging in head-fixed mice observing aggression. A 920nm 2-photon laser was used to monitor activity of Esr1+ neurons in VMHvl. Middle: One frame from a video recorded during observation of aggression. Bottom: An example of one motion SVD. b. Top: Neural activity raster during observation of aggression. Bottom: examples of SVD outputs over time during observation of aggression. c. Top: Predicted neuronal activity of single neurons and their variance explained by a generalized linear model (GLM) from SVDs readout over time. Bottom: Two example cells with different levels of variance explained. d. Estimated cumulative distribution of variance explained by the GLM of either x1 or x2 neurons across all mice. e. Statistical comparison of variance explained by GLM of x1 activity or x2 activity neurons per mouse (n = 7 mice, p = 0.8125, Two-tailed Mann-Whitney U-test, error bars - sem). f. Top: One frame from a video recorded during group photo-activation of x1 neurons. Middle: An example of one motion SVD. Bottom: Time-evolving activity of top 3 SVDs aligned to x1 activation (vertical red bars = photoactivation pulses). g. Projection of top 5 motion SVDs and stimulus triggered average of each SVD aligned to the start of x1 activation. h. Average response in top 5 SVDs during pre-stimulus and stimulus periods (n = 8 mice, p >0.05, Two-tailed Mann-Whitney U-test, error bars - sem). i. Same as g, but for activation of x2 neurons. j. Same as h, but for activation of x2 neurons.
Extended Data Fig. 6 Controls for off-target effects of 2P photoactivation.
a. GRIN lens changes the spatial resolution based on the axial depth. Top: imaging a calibration slide with 40 μm fluorescent squares at different axial distances below the GRIN lens. Bottom: imaging in-vivo jGCaMP7s expressing Esr1+ neurons in the VMHvl at different axial distances below the GRIN lens. b. Magnification ratio at different imaging depths calculated from the fluorescent calibration slide. c. Quantification of the relationship between imaging depth and magnification error. Linear regression is used to estimate the degree of aberration caused by the GRIN lens. d. Example field of view illustrating the experimental procedure for mapping the spatial resolution of 2P targeted photo-stimulation through the GRIN lens. Reference neurons were targeted first centred on their somata, and then again stepwise at different distances from the soma centre along each of the four cardinal directions, using 10 µm diameter stimulation spirals. N = 17 cells. e. Average response of all tested neurons to stimulation at each location from the soma. Shaded area represents standard error of the mean. The red-boxed trace indicates the response observed when the stimulation is centred on the reference cell (0 µm). f. Estimated cumulative distribution of the reference cell responses at different distances from soma. Lighter shades of red represent responses at distances progressively further from the soma. n = 17 neurons. g. Raster of neural activity of all 17 reference neurons tested using the procedure in Ex. Data Fig. e. Note that at 15 µm the average response in the reference cells is close to zero. h. Normalized average activity of all neurons at different distances from soma. Each row is a different experiment on a different reference cell. i. Representative examples of field of views from two mice. Green - all x1 neurons, Red – all x2 neurons, black - non x1 or x2 neurons. Fov - field of view. j. Example illustrating how distances are calculated for estimating the spatial clustering of x1 and x2 neurons. k. Quantification of average distance within x1 and x2 neurons and between x1 and x2 neurons, across mice (n = 8 mice, p > 0.05: Kruskal-Wallis test with Dunn’s correction for multiple comparison, error bars - sem).
Extended Data Fig. 7 Spatial clustering of neurons and activity comparison.
a. Support vector machine decoder trained to separate cell positions of x1 versus x2 neurons. Scenario 1 shows a cartoon where cells are perfectly separated by the SVM decoder and scenario 2 shows a cartoon where cells are inseparable based on their spatial location and shows low classifier accuracy. b. Accuracy of SVM decoder trained on data versus shuffled control (n=10 mice, p=0.156: Two-tailed Mann-Whitney U-test, error bars - sem). c. Classification width of SVM decoder trained on data versus shuffled control (n=10 mice, p=0.578: Two-tailed Mann-Whitney U-test). d. Neural activity of five x1 neurons selected for grouped optogenetic targeting during observation of aggression. e. Neural activity of the same five x1 neurons in panel d during grouped optogenetic activation. f. Comparison of peak z-score of x1 neurons selected for grouped optogenetic activation during observation of aggression and during optogenetic activation (n = 8 mice, p > 0.05: Two-tailed Mann-Whitney U-test, error bars - sem).
Extended Data Fig. 8 Characterization of line attractor properties.
a. Average activity projected onto x1 dimension from activation of x1 neurons across mice using 8s inter stimulus interval (n = 7 mice). Shaded area – sem. Right: Quantification of average z-scored activity of projected x1 dimension during baseline or inter stimulus intervals (n = 7 mice, n.s p = 0.3, **p = 0.0012, **p = 0.0012, *p = 0.0192 Kruskal-Wallis test with Dunn’s correction for multiple comparison, error bars - sem). b. Average activity projected onto x2 dimension from activation of x2 neurons across mice using 8s inter stimulus interval (n = 7 mice). Shaded area – sem. Right: Quantification of average z-scored activity of projected x2 dimension during baseline or inter stimulus intervals (n.s p > 0.05, n = 7 mice, Kruskal-Wallis test with Dunn’s correction for multiple comparison, error bars - sem). c. Data and model prediction of applying stimulation paradigm in Fig. 2c to rSLDS model trained on observing aggression. d. Data and model prediction of applying stimulation paradigm in Fig. 2j to rSLDS model trained on observing aggression. e. x1 integration dimension activity with 1mW per neurons (blue) and 5mW per neuron (red). Shaded area – sem. n = 8 mice. f. Quantification of average z-scored activity of projected x1 dimension neurons in 1mW and 5mW per neuron during baseline or various inter stimulus intervals (n = 8 mice, *p = 0.0295, *p = 0.0186, *p = 0.045, n.s p = 0.7, Two-tailed Mann-Whitney U-test, error bars - sem). g. Paradigm for examining activity in x2 dimension upon grouped holographic activation of x1 neurons. h. Average z-score activity of neural activity projected onto x2 dimension across mice (n = 8 mice). Shaded area – sem. i. Quantification of activity in non-targeted x2 dimension upon grouped holographic activation of x1 neurons (n.s, n = 8 mice, Kruskal-Wallis test with Dunn’s correction for multiple comparison, error bars - sem). j. Paradigm for examining activity in x1 dimension upon grouped holographic activation of x2 neurons. k. Average z-score activity of neural activity projected onto x1 dimension across mice (n = 8 mice, Shaded area – sem). l. Quantification of activity in non-targeted x1 dimension upon grouped holographic activation of x2 neurons (n.s p = 0.276, n.s p = 0.276, **p = 0.0072, *p = 0.03, n = 8 mice, Kruskal-Wallis test with Dunn’s correction for multiple comparison, error bars - sem).
Extended Data Fig. 9 Examination of finite nature and stability of line attractor.
a. Top: model prediction, assuming there is a finite length of the attractor, after the system reaches a certain point along the attractor, further pulses of activity will not cause a further ramp. Bottom: If the line attractor is infinite, then each activation should push the system further along the attractor. b. Example from one mouse comparing the prediction of finite (top) and infinite (bottom) model of the line attractor. Pink lines represent time of photoactivation. Mse - mean square error between model and the data. c. Comparison of the mse of the whole trace between the data and either the finite or infinite models (n = 8 mice, **p<0.001, Two-tailed Mann-Whitney U-test, error bars - sem). d. Same as Extended Data Fig. 9c but comparing only after the third pulse. Note that the scale of the y axis in Extended Data Fig. 9d is twice as big as in Extended Data Fig. 9c (n = 8 mice, **p<0.001, Two-tailed Mann-Whitney U-test, error bars - sem). e. Testing off-manifold perturbations further along the attractor. Experimental design: first we ramp the activity mid-way along the line attractor using activation of x1 neurons, then test the population vector trajectory after targeting of x2 neurons. f. Left: stimulation paradigm. Right: Scheme of the quantification approach for the effect of off manifold targeting further along the attractor. g. State space and the activity ramp following x1 photo-activation (showing only three pulses to avoid clutter). h. Same as Extended Data Fig. 9g but for x2 photo-activation. i. Quantification of the activity distance from baseline after each photostimulation (n = 8 mice, Kruskal-Wallis test with Dunn’s correction for multiple comparison, **p = 0.0025, n.s p > 0.05, error bars - sem). j. Effect of grouped holographic activation of randomly selected neurons on activated neurons. Shaded area – sem, n = 5 mice. k. Average z-score activity of non-targeted x1 dimension upon activation of random neurons. Shaded area – sem n = 5 mice. l. Average z-score activity of non-targeted x2 dimension upon activation of random neurons. Shaded area – sem, n = 5 mice. m. Left: Quantification of activity in non-targeted x1 dimension upon grouped holographic activation of random neurons (n.s, p > 0.05, Kruskal-Wallis test with Dunn’s correction for multiple comparison, n = 5 mice, error bars - sem). Right: Comparison of grouped activation of x1 neurons (green, reproduced from Fig. 2d, right) and grouped activation of random neurons on activity of x1 dimension (black, reproduced from Extended Data Fig. 3m, left, error bars - sem). n. Quantification of activity in non-targeted x2 dimension upon grouped holographic activation of random neurons (n.s, p > 0.05, Kruskal-Wallis test with Dunn’s correction for multiple comparison, n = 5 mice, error bars - sem).
Extended Data Fig. 10 Impact of functional connectivity measurements on non-targeted neurons.
a. Experimental design. We grouped activated five x1 neurons (three are shown for illustrative purposes) and examined the activity of non-targeted photoactivated x1 neurons following exclusion of off-target neurons. b. Z-score activity of x1 dimension photoactivated neurons not targeted for photo-stimulation. N = 8 mice. Shaded area – sem. c. Quantification of average z-scored activity of a weighted average of non-targeted x1 dimension neurons during baseline or various inter- photo-stimulation intervals. (n = 8 mice, error bars - sem, ***p<0.001). d. Experimental design for decoding analysis. We examined whether the activity of non-targeted but photoactivated x1 or x2 dimension neurons can be used to decode integration of direct photo-stimulation by groups of five targeted x1 neurons (three are shown for simplicity), using a support vector machine (SVM) decoder. e. One example mouse showing the activity of targeted x1 dimension neurons (black), activity decoded from non-targeted x1 neurons (green), and activity decoded from x2 non-targeted neurons (orange). f. Same as Extended Data Fig. 9e but averaged over 8 mice. Shaded area – sem. g. Decoding from non-targeted x1 neurons can explain significantly more variance (80% versus 40%) than non-targeted x2 or randomly selected neurons (n = 8 mice, *p = 0.01, ***p = 0.0003, n.s. p >0.05, Kruskal Wallis test with Dunn’s correction for multiple comparisons, error bars - sem). h. Fraction of non-targeted neurons with either positive or negative response (defined by whether their mean response post photostimulation of targeted x1 neuron is 1.5 std above or below baseline activity). i. Averaged activity of non-targeted neurons with either a positive (left), negative (middle) or no significant response (right). Shaded area – sem. N = 8 mice. j. Cartoon illustrating how the relationship between spatial distance and response in putative “follower” x1 neurons is assessed. k. Example field of view showing z-score response in all neurons in a field of view. The filled-in black cell is the targeted x1 neuron and the shaded region around it shows a 50 µm stringent zone of exclusion. Putative follower cells are shaded according to their z-score response (see colour scale). Note that some of the most strongly activated cells are located >100 µm from the targeted cell. l. Histogram of distance between targeted x1 neuron and all putative “follower” x1 neurons (mean: 139 ± 35 µm). m. Scatter plot showing the relationship between distance and response in putative “follower” x1 neurons. Blue line shows the regression line. 11% of all assessed putative “follower” x1 neurons are within 50 µm of the targeted x1 neurons. n. Average response from scatter plot in ‘m’. Black line –mean over moving window of 15um. Shaded area – sem. o. Average response in non-targeted x1 neurons from photo-stimulation of single x1 neuron with (black trace) and without (green trace) exclusion of neurons within a 50 µm radius of the targeted neuron (pink shaded region in Extended Data Fig. 10l–n). Shaded area – sem. N = 8 mice. p. Quantification of data from Extended Data Fig. 10o at various time periods after each photo-stimulation pulse. n.s: not significant, Kruskal-Wallis test with Dunn’s correction for multiple comparisons, error bars - sem. N = 8 mice q. x1 integration dimension activity with activation of one neuron (blue) versus five neurons (red). N = 8 mice. Shaded area – sem. r. Quantification of average z-scored activity of projected x1 dimension neurons with one neuron (blue) versus five neurons (red) during baseline or various inter stimulus intervals. N = 8 mice, *p = 0.0239, **p = 0.0063, **p = 0.0074, *p = 0.0341, Kruskal-Wallis test with Dunn’s correction for multiple comparisons, error bars - sem.
Extended Data Fig. 11 Deriving network time constant for model simulations.
a. Analytical derivation of network time constant from connectivity matrix of purely excitatory recurrent neural network.
Extended Data Fig. 12 Additional quantifications of the correlation between functional connectivity and the stability of the decay and ramp.
a. Illustration of different quantification approaches to the change in activity of non-targeted x1 neurons from Fig. 5e as either the average z-score activity following different stimulus pulses, or the area under the curve (auc). Red vertical lines, photostimulation pulses. b. Left: Correlation between the rate of ramping of the integration dimension obtained from observation of aggression and average z-score of non-targeted x1 neurons measured using the average z-score post third stimulus (r2: 0.01, n.s, n = 8 mice). Right: Correlation between rSLDS time constant obtained from observation of aggression and average z-score across non-targeted x2 neurons measured using the average z-score post third stimulus (r2: 0.87, ***p < 0.001, n = 8 mice). c. Same as b) but calculated from non-targeted x1 neurons measuring the auc of activity post first stimulus. d. Same as c), calculated from non-targeted x1 neurons measuring the average z-score activity. e. Same as c) but calculated from non-targeted x2 neurons measuring the AUC of activity post third stimulus. f. Same as e) but calculated using the average z-score activity. g. Same as e) but calculated post first stimulus. h. Same as g) but calculated using the average z-score activity.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vinograd, A., Nair, A., Kim, J.H. et al. Causal evidence of a line attractor encoding an affective state. Nature 634, 910–918 (2024). https://doi.org/10.1038/s41586-024-07915-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-07915-x
This article is cited by
-
A neural manifold view of the brain
Nature Neuroscience (2025)
-
What neuroscience can tell AI about learning in continuously changing environments
Nature Machine Intelligence (2025)
-
A neural circuit for context-dependent multimodal signaling in Drosophila
Nature Communications (2025)
-
Probabilistic inference of social presence across brain scales reveals enhanced synaptic efficacy
Communications Biology (2025)
-
24-hour simultaneous mPFC’s miniature 2-photon imaging, EEG-EMG, and video recording during natural behaviors
Scientific Data (2025)