Abstract
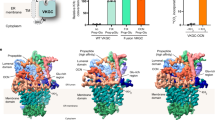
γ-Glutamyl carboxylase (GGCX) is the sole identified enzyme that uses vitamin K (VK) as a cofactor in humans. This protein catalyses the oxidation of VK hydroquinone to convert specific glutamate residues to γ-carboxyglutamate residues in VK-dependent proteins (VDPs), which are involved in various essential biological processes and diseases1,2,3. However, the working mechanism of GGCX remains unclear. Here we report three cryogenic electron microscopy structures of human GGCX: in the apo state, bound to osteocalcin (a VDP) and bound to VK. The propeptide of the VDP binds to the lumenal domain of GGCX, which stabilizes transmembrane helices 6 and 7 of GGCX to create the VK-binding pocket. After binding of VK, residue Lys218 in GGCX mediates the oxidation of VK hydroxyquinone, which leads to the deprotonation of glutamate residues and the construction of γ-carboxyglutamate residues. Our structural observations and results from binding and cell biological assays and molecular dynamics simulations show that a cholesterol molecule interacts with the transmembrane helices of GGCX to regulate its protein levels in cells. Together, these results establish a link between cholesterol metabolism and VK-dependent pathways.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The 3D cryo-EM maps for GGCX, GGCX–BGP and GGCX–BGP–MK-4 have been deposited into the Electron Microscopy Data Bank with accession numbers EMD-44912, EMD-44917 and EMD-44924, respectively. The atomic coordinates for the atomic models have been deposited into the Protein Data Bank under accession codes 9BUM, 9BUR and 9BUX. The raw cryo-EM data for GGCX, GGCX–BGP and GGCX–BGP–MK-4 have been deposited into the Electron Microscopy Public Image Archive under accession numbers EMPIAR-12453, EMPIAR-12451 and EMPIAR-12452, respectively. The MD simulation data have been deposited into Zenodo (https://doi.org/10.5281/zenodo.14150943)69. Source data are provided with this paper.
References
Wu, S. M., Cheung, W. F., Frazier, D. & Stafford, D. W. Cloning and expression of the cDNA for human γ-glutamyl carboxylase. Science 254, 1634–1636 (1991).
Furie, B., Bouchard, B. A. & Furie, B. C. Vitamin K-dependent biosynthesis of γ-carboxyglutamic acid. Blood 93, 1798–1808 (1999).
Berkner, K. L. Vitamin K-dependent carboxylation. Vitam. Horm. 78, 131–156 (2008).
Mladenka, P. et al. Vitamin K—sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 80, 677–698 (2022).
Stafford, D. W. The vitamin K cycle. J. Thromb. Haemost. 3, 1873–1878 (2005).
Shearer, M. J. & Okano, T. Key pathways and regulators of vitamin K function and intermediary metabolism. Annu. Rev. Nutr. 38, 127–151 (2018).
Rishavy, M. A. & Berkner, K. L. Vitamin K oxygenation, glutamate carboxylation, and processivity: defining the three critical facets of catalysis by the vitamin K-dependent carboxylase. Adv. Nutr. 3, 135–148 (2012).
Li, T. et al. Identification of the gene for vitamin K epoxide reductase. Nature 427, 541–544 (2004).
Rost, S. et al. Mutations in cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature 427, 537–541 (2004).
Furie, B. & Furie, B. C. The molecular basis of blood coagulation. Cell 53, 505–518 (1988).
Poser, J. W., Esch, F. S., Ling, N. C. & Price, P. A. Isolation and sequence of the vitamin K-dependent protein from human bone. Undercarboxylation of the first glutamic acid residue. J. Biol. Chem. 255, 8685–8691 (1980).
Karsenty, G. & Olson, E. N. Bone and muscle endocrine functions: unexpected paradigms of inter-organ communication. Cell 164, 1248–1256 (2016).
Shearer, M. J. Vitamin K deficiency bleeding (VKDB) in early infancy. Blood Rev. 23, 49–59 (2009).
Stock, M. & Schett, G. Vitamin K-dependent proteins in skeletal development and disease. Int. J. Mol. Sci. 22, 9328 (2021).
Wen, L. P., Chen, J. P., Duan, L. L. & Li, S. Z. Vitamin K-dependent proteins involved in bone and cardiovascular health. Mol. Med. Rep. 18, 3–15 (2018).
Furie, B. C. et al. The γ-carboxylation recognition site is sufficient to direct vitamin K-dependent carboxylation on all adjacent glutamate-rich region of thrombin in a propeptide–thrombin chimera. J. Biol. Chem. 272, 28258–28262 (1997).
Jorgensen, M. J. et al. Recognition site directing vitamin K-dependent γ-carboxylation resides on the propeptide of factor-IX. Cell 48, 185–191 (1987).
Freedman, S. J., Furie, B. C., Furie, B. & Baleja, J. D. Structure of the calcium ion-bound γ-carboxyglutamic acid-rich domain of factor-IX. Biochemistry 34, 12126–12137 (1995).
Spyropoulos, A. C., Hayth, K. A. & Jenkins, P. Anticoagulation with anisindione in a patient with a warfarin-induced skin eruption. Pharmacotherapy 23, 533–536 (2003).
Watzka, M. et al. Bleeding and non-bleeding phenotypes in patients with gene mutations. Thromb. Res. 134, 856–865 (2014).
Tie, J., Wu, S. M., Jin, D. Y., Nicchitta, C. V. & Stafford, D. W. A topological study of the human γ-glutamyl carboxylase. Blood 96, 973–978 (2000).
Tie, J. K. et al. Characterization of vitamin K-dependent carboxylase mutations that cause bleeding and nonbleeding disorders. Blood 127, 1847–1855 (2016).
Tie, J. K. et al. Determination of disulfide bond assignment of human vitamin K-dependent γ-glutamyl carboxylase by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Biol. Chem. 278, 45468–45475 (2003).
Holm, L., Laiho, A., Törönen, P. & Salgado, M. DALI shines a light on remote homologs: one hundred discoveries. Protein Sci. 32, e4519 (2023).
Wu, S. M., Mutucumarana, V. P., Geromanos, S. & Stafford, D. W. The propeptide binding site of the bovine γ-glutamyl carboxylase. J. Biol. Chem. 272, 11718–11722 (1997).
Hoang, Q. Q., Sicheri, F., Howard, A. J. & Yang, D. S. C. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature 425, 977–980 (2003).
Lin, P. J. et al. The putative vitamin K-dependent γ-glutamyl carboxylase internal propeptide appears to be the propeptide binding site. J. Biol. Chem. 277, 28584–28591 (2002).
Hao, Z. Y. et al. γ-Glutamyl carboxylase mutations differentially affect the biological function of vitamin K dependent proteins. Blood 137, 533–543 (2021).
Parker, C. H. et al. A conformational investigation of propeptide binding to the integral membrane protein γ-glutamyl carboxylase using nanodisc hydrogen exchange mass spectrometry. Biochemistry 53, 1511–1520 (2014).
Mutucumarana, V. P., Acher, F., Straight, D. L., Jin, D. Y. & Stafford, D. W. A conserved region of human vitamin K-dependent carboxylase between residues 393 and 404 is important for its interaction with the glutamate substrate. J. Biol. Chem. 278, 46488–46493 (2003).
Rishavy, M. A. & Berkner, K. L. Insight into the coupling mechanism of the vitamin K-dependent carboxylase: mutation of histidine 160 disrupts glutamic acid carbanion formation and efficient coupling of vitamin K epoxidation to glutamic acid carboxylation. Biochemistry 47, 9836–9846 (2008).
Rishavy, M. A. et al. Bronsted analysis reveals Lys218 as the carboxylase active site base that deprotonates vitamin K hydroquinone to initiate vitamin K-dependent protein carboxylation. Biochemistry 45, 13239–13248 (2006).
Rishavy, M. A. et al. A new model for vitamin K-dependent carboxylation: the catalytic base that deprotonates vitamin K hydroquinone is not Cys but an activated amine. Proc. Natl Acad. Sci. USA 101, 13732–13737 (2004).
Mosley, S. T., Brown, M. S., Anderson, R. G. W. & Goldstein, J. L. Mutant clone of Chinese hamster ovary cells lacking 3-hydroxy-3-methylglutaryl coenzyme A reductase. J. Biol. Chem. 258, 3875–3881 (1983).
Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. Nature 343, 425–430 (1990).
Metherall, J. E., Goldstein, J. L., Luskey, K. L. & Brown, M. S. Loss of transcriptional repression of three sterol-regulated genes in mutant hamster cells. J. Biol. Chem. 264, 15634–15641 (1989).
Yang, T. et al. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell 110, 489–500 (2002).
Qi, X., Friedberg, L., De Bose-Boyd, R., Long, T. & Li, X. Sterols in an intramolecular channel of Smoothened mediate Hedgehog signaling. Nat. Chem. Biol. 16, 1368–1375 (2020).
Dowd, P., Hershline, R., Ham, S. W. & Naganathan, S. Vitamin K and energy transduction—a base strength amplification mechanism. Science 269, 1684–1691 (1995).
Berkner, K. L. & Pudota, B. N. Vitamin K-dependent carboxylation of the carboxylase. Proc. Natl Acad. Sci. USA 95, 466–471 (1998).
Hallgren, K. W., Zhang, D., Kinter, M., Willard, B. & Berkner, K. L. Methylation of γ-carboxylated Glu (Gla) allows detection by liquid chromatography–mass spectrometry and the identification of Gla residues in the γ-glutamyl carboxylase. J. Proteome Res. 12, 2365–2374 (2013).
de Boer-van den Berg, M. A., Thijssen, H. H. & Vermeer, C. The in vivo effects of acenocoumarol, phenprocoumon and warfarin on vitamin K epoxide reductase and vitamin K-dependent carboxylase in various tissues of the rat. Biochim. Biophys. Acta 884, 150–157 (1986).
Tie, J. K., Jin, D. Y., Straight, D. L. & Stafford, D. W. Functional study of the vitamin K cycle in mammalian cells. Blood 117, 2967–2974 (2011).
Di Minno, A. et al. Old and new oral anticoagulants: food, herbal medicines and drug interactions. Blood Rev. 31, 193–203 (2017).
Goldstein, J. L. & Brown, M. S. A century of cholesterol and coronaries: from plaques to genes to statins. Cell 161, 161–172 (2015).
Undas, A., Brummel-Ziedins, K. E. & Mann, K. G. Anticoagulant effects of statins and their clinical implications. Thromb. Haemost. 111, 392–400 (2014).
Jiang, S. Y. et al. Schnyder corneal dystrophy-associated UBIAD1 mutations cause corneal cholesterol accumulation by stabilizing HMG-CoA reductase. PLoS Genet. 15, e1008289 (2019).
Schumacher, M. M., Elsabrouty, R., Seemann, J., Jo, Y. & DeBose-Boyd, R. A. The prenyltransferase UBIAD1 is the target of geranylgeraniol in degradation of HMG CoA reductase. eLife 4, e05560 (2015).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
McFarlane, M. R. et al. Scap is required for sterol synthesis and crypt growth in intestinal mucosa. J. Lipid Res. 56, 1560–1571 (2015).
Li, H., Robertson, A. D. & Jensen, J. H. Very fast empirical prediction and rationalization of protein pK values. Proteins 61, 704–721 (2005).
Brooks, B. R. et al. Charmm—a program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 4, 187–217 (1983).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Lomize, M. A., Lomize, A. L., Pogozheva, I. D. & Mosberg, H. I. OPM: orientations of proteins in membranes database. Bioinformatics 22, 623–625 (2006).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
MacKerell, A. D. et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Elghobashi-Meinhardt, N. Structure and mechanism of vitamin K-dependent gamma-glutamyl carboxylase (GGCX) MD simulation data. Zenodo https://doi.org/10.5281/zenodo.14150943 (2024).
Acknowledgements
We thank M. Brown, J. Goldstein, E. Olson, B. Wang, R. DeBose-Boyd and X. Li for discussions; D. Stoddard, J. Martinez-Diaz, C. Baker, R. Welch, C. Brautigam, S. Tso, L. Esparza and Y. Qin for technical support; A. Lemoff at the UTSW Proteomics Core for mass spectrometry analysis; and P. Schmiege for editing the manuscript. Cryo-EM data were collected at the UT Southwestern Medical Center Cryo-EM Facility (funded in part by CPRIT Award RP220582). This work was supported by NIH P01HL160487 (to R.W.) and the Endowed Scholars Program in Medical Science of UT Southwestern Medical Center and CPRIT (RR230054 to X.Q.). N.E.-M. acknowledges the Irish Centre for High-End Computing (ICHEC) for the provision of computational facilities and support. X.Q. is a CPRIT Scholar and a Michael L. Rosenberg Scholar in Medical Research of UT Southwestern Medical Center.
Author information
Authors and Affiliations
Contributions
X.Q., together with R.W., conceived the project and designed the research. R.W., B.C. and X.Q. purified the proteins. R.W. and X.Q. carried out cryo-EM work and refined the structures. R.W., B.C., A.A., N.Z. and X.Q. performed the functional assays. N.E.-M. conducted the MD simulations. J.-K.T. provided the GGCX-knockout HEK293 cell line. R.W., N.E.-M. and X.Q. analysed the data. X.Q. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Doreen Matthies and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Biochemical and cryo-EM analyses of GGCX and the GGCX–BGP complex.
a, Representative gel-filtration chromatograms of GGCX purified without any compound (blue), with menaquinone-4 (MK-4) (orange), with Anisindione (teal) and with vitamin K1 epoxide (VKO1) and Warfarin (magenta). The SDS-PAGE gels of the fractions used for cryo-EM study are shown. b, Preliminary cryo-EM maps of GGCX purified without any compound, with MK-4, and with Anisindione. The cleft of the detergent micelle in each map is indicated by a dashed line. c, Summary of cryo-EM data processing procedures of GGCX purified with VKO1 and Warfarin. d, Fourier shell correlation (FSC) curves between two half maps of GGCX purified with VKO1 and Warfarin. e, Local resolution of the cryo-EM map of GGCX purified with VKO1 and Warfarin. Maps are colored according to local resolution, estimated using cryoSPARC. f, Cryo-EM maps of the major structural elements of GGCX purified with VKO1 and Warfarin, as well as the cryo-EM map of cholesterol (CLR). TM, transmembrane helix. g, GGCX structure docked into the low-resolution cryo-EM map of GGCX purified with MK-4. The cholesterol density and the surrounding residues are shown in the lower panel. h, Comparison of the gel-filtration chromatograms of GGCX–BGP complex (green) and GGCX (blue) purified without any compound. The ratio of protein used for cryo-EM study in total protein is around 3.7% in GGCX and 23.4% in GGCX–BGP samples. The SDS-PAGE gel of the fractions of the GGCX–BGP complex used for cryo-EM study is shown. i, The SDS-PAGE gel and western blot result of the GGCX–BGP complexes expressed and purified with either MK-4 (GGCX–BGP–MK-4) or Anisindione (GGCX–BGP–ANI). The BGP bands in the SDS-PAGE gel were subjected for Mass spectrometry (MS) analysis. The western blot was performed with the same samples using the antibody specifically recognizing the Gla68 of BGP. BGPGla, carboxylated BGP protein. j, Mass spectrometry results for detecting the carboxylation of BGP in GGCX–BGP–MK-4 and GGCX–BGP–ANI samples.
Extended Data Fig. 2 Cryo-EM analyses of the GGCX–BGP complex.
a, Summary of cryo-EM data processing procedures of the GGCX–BGP complex. b, Fourier shell correlation (FSC) curves between two half maps of the GGCX–BGP complex. c, Local resolution of cryo-EM map of the GGCX–BGP complex. Maps are colored according to local resolution, estimated using cryoSPARC. d, Cryo-EM map of the GGCX–BGP (unsharpened) at different contour levels. The strong cholesterol (yellow) is visible at a high contour level, while the unassigned lipid/detergent densities (cyan) are invisible. e, Cryo-EM maps of the major structural elements of GGCX and BGP, as well as the cholesterol and Phosphatidylcholine (PC) in GGCX–BGP complex.
Extended Data Fig. 3 Sequence alignment of GGCX.
Sequence alignment of GGCX proteins from Homo sapiens (NP_000812), Mus musculus (NP_062776), Parus major (XP_015504226), Xenopus tropicalis (NP_001116905), and Danio rerio (XP_003199339). The residue numbers and major structural elements are shown above the sequence. The conserved residues are colored in red.
Extended Data Fig. 4 Sequence alignment of human Gla proteins and the structures of Gla domains.
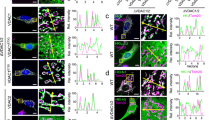
a, Sequence alignment of the propeptide and Gla domain of human Prothrombin (Factor II, F2), Factor VII (F7), Factor IX (F9), Factor X (F10), Protein C, Protein S, Protein Z, Transmembrane Gla proteins (TMGs) 1-4, Growth arrest-specific gene 6 (Gas6), Matrix Gla protein (MGP) and BGP. The propeptide and Gla domain are indicated by brown and orange lines, respectively. The conserved -16, -10 and -6 positions of the propeptide are labeled above the sequence. The disulfide bonds in Gla domains are indicated by black lines. The residues of BGP observed in the GGCX–BGP structure are indicated by a red rectangle. b and c, Crystal structures of the mature Gla domain of human F9 (PDB: 1NL0, light orange) (b) and mature porcine BGP (PDB: 1Q8H, grey) (c). The side chains of Gla residues are shown as sticks and Ca2+ ions are shown as green balls. d, Cryo-EM structure of unmature human BGP in GGCX–BGP complex. The propeptide and Gla domain are shown in brown and orange, respectively. The invisible residues are indicated by dashed lines.
Extended Data Fig. 5 Models of the propeptides from other VDPs binding to GGCX.
a, Propeptide of Prothrombin (residue 26-38) modeled to GGCX. The potential interactions are indicated by dashed lines. The residues of Prothrombin are denoted by underlining. b, Propeptide of F9 (residue 29-41) modeled to GGCX. The potential interactions are indicated by dashed lines. The residues of F9 are denoted by underlining. c, Propeptide of Protein C (residue 25-37) modeled to GGCX. The potential interactions are indicated by dashed lines. The residues of Protein C are denoted by underlining. d, Propeptide of MGP (residue 32-44) modeled to GGCX. The potential interactions are indicated by dashed lines. The residues of MGP are denoted by underlining.
Extended Data Fig. 6 Cryo-EM analyses of GGCX–BGP–MK-4.
a, Summary of cryo-EM data processing procedures of the GGCX–BGP–MK-4 complex. b, Fourier shell correlation (FSC) curves between two half maps of the GGCX–BGP–MK-4 complex. c, Local resolution of cryo-EM map of the GGCX–BGP–MK-4 complex. Maps are colored according to local resolution, estimated using cryoSPARC. d, Cryo-EM map of GGCX–BGP–MK-4 (unsharpened) at different contour levels. The strong cholesterol (yellow) is visible at a high contour level, while the unassigned lipid/detergent densities (cyan) are invisible. e, Comparison of the Gla-binding site in GGCX–BGP and GGCX–BGP–MK-4 cryo-EM maps. GGCX, the propeptide and Gla domain of BGP were colored in grey, brown, and orange, respectively. The Gla peptide observed in GGCX–BGP structure but not in GGCX–BGP–MK-4 structure was indicated by a red arrow. f, Cryo-EM maps of the major structural elements of GGCX and BGP, as well as the cholesterol and PC in GGCX–BGP–MK-4 complex.
Extended Data Fig. 7 Chemical structures of VKs and Warfarin modeled to the VK-binding pocket in GGCX.
a, Structure of VK1, VK2 family and VK3. b, Warfarin modeled into the VK-binding pocket. The head group of Warfarin was aligned to that of MKO-4. The potential steric clash between GGCX and Warfarin is indicated by a dashed oval. The chemical structure of Warfarin is shown.
Extended Data Fig. 8 Model of GGCX C-terminus self-carboxylation.
a, The flexible C-terminus of GGCX (residues 726-758) is modeled into the center cavity of GGCX. Glu733, Glu757 and Glu748 are shown and Glu748 is modeled into the reaction center. MKH2-4, MK-4 hydroquinone. b, the top view of a.
Supplementary information
Supplementary Information
This file contains Supplementary Fig. 1 (uncropped gels) and Supplementary Table 1.
Supplementary Video 1
MD simulations of GGCX without cholesterol. MD simulations were performed for 200 ns. POPC is shown as sticks.
Supplementary Video 2
MD simulations of GGCX with cholesterol. MD simulations were performed for 200 ns. POPC and cholesterol are shown as sticks.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, R., Chen, B., Elghobashi-Meinhardt, N. et al. Structure and mechanism of vitamin-K-dependent γ-glutamyl carboxylase. Nature 639, 808–815 (2025). https://doi.org/10.1038/s41586-024-08484-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08484-9
This article is cited by
-
Molecular basis of vitamin K-dependent protein γ-glutamyl carboxylation
Cell Research (2025)