Abstract
Elucidating the complex network of communication between tumour cells is central to understanding cell fate decisions and progression of pancreatic ductal adenocarcinoma (PDAC)1,2. We previously showed that constant suppression of BMP activity by the BMP antagonist GREM1 secreted by mesenchymal PDAC cells is essential for maintaining the fate of epithelial PDAC cells3. Here we identify SPP1 (also known as osteopontin)4 as a key regulator of mesenchymal cell fate in pancreatic cancer. Proteomic analysis of plasma from patients with PDAC showed that SPP1 is substantially upregulated in late-stage disease. Inactivation of Spp1 led to a delay in tumorigenesis in mouse PDAC models and abolished metastasis formation. Spp1 was expressed in epithelial PDAC cells, and Spp1 inactivation resulted in a conversion of mesenchymal to epithelial PDAC cells. Mechanistically, SPP1 bound the CD61 receptor on mesenchymal PDAC cells to induce Bmp2 and Grem1 expression, and GREM1 inhibition of BMP signalling was required for Spp1 expression in epithelial cells, thereby forming an intercellular regulatory loop. Concomitant inactivation of Grem1 reverted the epithelial phenotype of Spp1 knockout to fully mesenchymal PDAC. Conversely, Grem1 heterozygosity combined with Spp1 knockout resulted in wild-type PDAC histology, a result that confirmed the direct antagonistic functions of these factors. Hence, mesenchymal and epithelial PDAC cell fates are determined by the reciprocal paracrine regulation of the soluble factors GREM1 and SPP1.
Similar content being viewed by others
Main
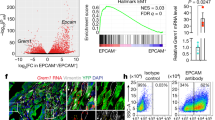
PDAC is one of the leading causes of cancer-related mortality worldwide, with a 5-year relative survival rate of only 13%5. To identify factors that control the progression of pancreatic cancer, we compared protein levels in the plasma of patients with early-stage (stage I or II) or late-stage (stage III or IV) PDAC (Fig. 1a and Supplementary Table 1). The abundance of SPP1 was significantly increased at late stages (Fig. 1b). SPP1 plasma levels were also increased in Pdx1-cre;KrasLSL-G12D/+;Trp53fl/fl;Rosa26LSL-YFP (KPCY) mice, a commonly used mouse model of PDAC6, compared with wild-type (WT) animals (Fig. 1c).
a, Schematic showing a comparison of the proteomic profiles of plasma proteins of patients with stage I or II PDAC and patients with stage III or IV PDAC. LC–MS, liquid chromatography–mass spectrometry. b, Volcano plot showing differential protein expression between plasma samples from patients with early-stage pancreatic cancer (stage I or II) or late-stage pancreatic cancer (stage III or IV). Blue dots indicate proteins with increased expression in early-stage pancreatic cancer (stage I and II), and red dots indicate proteins with increased expression in late-stage pancreatic cancer (stage III and IV). Statistical analysis was performed using two-sided t-tests. c, ELISA of plasma SPP1 levels in KPCY and WT mice (n = 3 mice). d, Immunofluorescence analysis of VIM (red), YFP (grey) and SPP1 (green) in KPCY tumours. Scale bar, 50 μm. e, Bright-field and fluorescence images of VIM–GFP in Spp1WT/WT KPFV and Spp1Δ/Δ KPFV organoids. Arrowheads indicate VIM–GFP+ cells. Scale bar, 50 μm. f, RT–qPCR analysis of expression of the epithelial markers Krt19, Cdh1 and Epcam and the mesenchymal markers Fn1, S100a4 and Vim between Spp1WT/WT KPFV and Spp1Δ/Δ KPFV organoids (n = 3 biological replicates), normalized using Spp1WT/WT KPFV values. g, Images of subcutaneous tumours formed from Spp1WT/WT KPF and Spp1Δ/Δ KPF organoids. Scale bar, 0.5 cm. h, Sizes of subcutaneous tumours formed from Spp1WT/WT KPF and Spp1Δ/Δ KPF organoids (n = 7 mice). Data are the mean ± s.e.m. (c,f,h). P values were calculated using two-sided t-tests. The diagram in a was created in BioRender. Ruiz, J. (2025) (https://biorender.com/32og8hu).
To explore the role of SPP1 in the development and progression of pancreatic cancer, we first analysed single-cell sequencing data from public databases. Cancer cell populations with high SPP1 expression had significantly higher levels of genes involved in the epithelial–mesenchymal transition (EMT) signature7,8 than populations with low SPP1 expression (Extended Data Fig. 1a–c). Moreover, by analysing publicly available transcriptome data of 644 patients with pancreatic cancer, we observed that patients with high SPP1 expression exhibited significant enrichment of the hallmark EMT gene set8. Accordingly, these patients showed worse prognosis compared with those with low SPP1 expression (Extended Data Fig. 1d–f). Together, these results suggest that SPP1 is a regulator of EMT in pancreatic cancer.
Epithelial PDAC cells secrete SPP1 to maintain mesenchymal PDAC cells
Immunofluorescence staining of KPCY tumours revealed that SPP1 was predominantly expressed in epithelial PDAC cells (EPCs) and not in vimentin (VIM)-positive mesenchymal PDAC cells (MPCs) (Fig. 1d). To understand SPP1 function, we established organoids from tumours of Pdx1-Flp;KrasFSF-G12D/+;Trp53frt/frt;Vim-GFP (KPFV) mice9, which enabled the differentiation between EPCs and MPCs on the basis of GFP fluorescence (Extended Data Fig. 1g). We performed Spp1 knockout in KPFV organoids using CRISPR–Cas9 technology (Extended Data Fig. 1h). The VIM–GFP+ MPC population was abolished after Spp1 knockout (Fig. 1e), which suggested that Spp1 is required for MPC fate. Quantitative PCR with reverse transcription (RT–qPCR) analysis showed a marked decrease in expression of the mesenchymal markers Vim, Fn1 and S100a4, concomitant with a notable increase in the epithelial markers Krt19, Cdh1 and Epcam10 (Fig. 1f). In line with this result, there were fewer allograft tumours generated from Pdx1-Flp;KrasFSF-G12D/+;Trp53frt/frt (KPF) organoids deficient in SPP1 (Spp1Δ/Δ KPF), and the tumours were small and consisted almost exclusively of EPCs. By contrast, tumours formed from control KPF organoids contained both EPC and MPC populations (Fig. 1g,h and Extended Data Fig. 1i,j). Furthermore, Spp1 overexpression in organoids from LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPR172H/+C) mice induced mesenchymal differentiation of PDAC cells (Extended Data Fig. 2a–d). Notably, these phenotypes were also observed in human PDAC organoids and xenografts with SPP1 knocked out or overexpressed (Extended Data Fig. 3a–o). Thus, SPP1 produced by EPCs is required for the maintenance of MPCs in both mouse and human PDAC.
EPC-secreted SPP1 binds to the receptor CD61 on MPCs to maintain their fate
Several receptors have been identified as receptors for SPP1, including the integrin CD61 (encoded by Itgb3)11,12,13. Immunofluorescence staining of KPCY tumours showed a markedly complementary expression pattern of SPP1 and CD61, and CD61 was predominantly expressed in MPCs (Fig. 2a). To further investigate the relationship between SPP1 and CD61, we performed CRISPR–Cas9-mediated knockout of Itgb3 in KPFV organoids (Extended Data Fig. 4a). VIM–GFP+ cells were not observed in Itgb3-deficient KPFV organoids, which also had a significant reduction in mesenchymal marker expression (Fig. 2b,c and Extended Data Fig. 4b). Furthermore, allograft formation was significantly reduced (Fig. 2d,e), and the proportion of MPCs was decreased in Itgb3-deficient allografts (Fig. 2f,g). Thus, Itgb3 knockout phenocopies Spp1 knockout (Fig. 1e–g), a result that strongly suggests that SPP1 functions through CD61.
a, Immunofluorescence analysis of CD61 (red), YFP (grey) and SPP1 (green) expression in KPCY orthotopic tumours (n = 3 mice). Scale bar, 50 μm. b, Comparative RT–qPCR analysis of the epithelial markers Krt19, Cdh1 and Epcam and the mesenchymal markers Fn1, S100a4 and Vim in Itgb3WT/WT KPFV and Itgb3Δ/Δ KPFV organoids (n = 3 biological replicates). Values are normalized to Itgb3WT/WT KPFV values. c, Comparative analysis of VIM–GFP expression between Itgb3WT/WT KPFV and Itgb3Δ/Δ KPFV organoids. Arrowheads indicate VIM–GFP+ cells. Scale bar, 50 μm. d, Images of subcutaneous tumours formed from Itgb3WT/WT KPF and Itgb3Δ/Δ KPF organoids. Scale bar, 0.5 cm. e, Sizes of subcutaneous tumours formed from Itgb3WT/WT KPF and Itgb3Δ/Δ KPF organoids (n = 4 mice). f, Immunofluorescence staining for GFP (grey), KRT19 (green) and VIM (red) of Itgb3WT/WT KPF and Itgb3Δ/Δ KPF subcutaneous tumours. Arrowheads indicate VIM+ cancer cells. Scale bar, 50 μm. g, Comparison of the percentages of different cancer cell subpopulations between subcutaneous tumours formed from Itgb3WT/WT KPF and Itgb3Δ/Δ KPF organoids (n = 3 mice). KRT19+VIM– marks epithelial cancer cells, whereas KRT19+VIM+ marks EMT hybrid cancer cells, and KRT19–VIM+ indicates mesenchymal cancer cells. Data are the mean ± s.e.m. (b,e,g). P values were calculated using two-sided t-tests.
SPP1 is important for PDAC development and metastasis
We next investigated the potential function of SPP1 in PDAC progression. We combined PDAC induced by the Flp/Frt recombination system (KPF mice)14 with a floxed Spp1 (Spp1fl) allele, a Flp-inducible Rosa26FSF-creER allele14 that allows Spp1 deletion in the pancreatic parenchyma during tumour development and a Tomato/GFP-Cre reporter allele15 to generate compound Pdx1-Flp;KrasFSF-G12D/+;Trp53frt/frt;R26FSF-creER;R26mTmG;Spp1fl/fl (Spp1fl/fl KPFCT) mice (Fig. 3a).
a, Schematic showing how the Spp1fl/fl KPFCT mouse model was generated. b, Haematoxylin and eosin (H&E) staining of Spp1WT/WT KPFCT and Spp1fl/fl KPFCT tumours (n = 3 mice). Scale bar, 1.0 mm. c, Immunofluorescence staining for GFP (grey), KRT19 (green) and VIM (red) in Spp1WT/WT KPFCT and Spp1fl/fl KPFCT tumours. Scale bar, 200 μm. d, Comparison of the proportions of different cancer cell subpopulations between Spp1WT/WT KPFCT and Spp1fl/fl KPFCT tumours (n = 5 mice). e, Kaplan–Meir survival curves of Spp1WT/WT KPFCT (n = 15) and Spp1fl/fl KPFCT (n = 16) mice. The P value was calculated using the log-rank test. f, Kaplan–Meir survival curves of Spp1WT/WT KPCY (n = 14) and Spp1fl/fl KPCY (n = 14) mice. The P value was calculated using the log-rank test. g, Images of liver and lung metastases in Spp1WT/WT KPhetFCT and Spp1fl/fl KPhetFCT mice. Arrowheads indicate metastases. h, Percentage of mice with liver metastases (left) and quantification of the number of liver metastases (right) in Spp1WT/WT KPhetFCT (n = 15) and Spp1fl/fl KPhetFCT (n = 20) mice. i, Percentage of mice with lung metastases (left) and quantification of the number of lung metastases (right) in Spp1WT/WT KPhetFCT (n = 15) and Spp1fl/fl KPhetFCT (n = 20) mice. Data are the mean ± s.e.m. (d,h (right), i (right)). P values were calculated using two-sided Fisher-exact tests (h (left) and i (left)) or two-sided t-tests (d,h (right), i (right)).
Tamoxifen was administered to 25-day-old animals to inactivate Spp1 in PDAC tumours. Histology of Spp1-deficent tumours showed a notable increase in epithelial structures (Fig. 3b), and immunofluorescence analysis revealed that PDAC tumours contained a marked increase in EPCs (KRT19+VIM–) and a substantial decrease in MPCs (KRT19–VIM+) compared with control KPFCT tumours (Fig. 3c,d). Notably, Spp1fl/fl KPFCT tumours exhibited a considerable reduction in cancer-associated fibroblasts (Extended Data Fig. 4c–f). However, despite the reduction in cancer-associated fibroblasts, the degree of immune infiltration was unchanged (Extended Data Fig. 4g–n). An increase in MPCs is known as a crucial factor that leads to poor prognosis in patients with PDAC16,17. Accordingly, Spp1fl/fl KPFCT mice with tumours exhibited significantly increased survival compared with control KPFCT mice (Fig. 3e). Moreover, the absence of Spp1 also substantially reduced PDAC development in the KPCY model, with a substantial percentage of Spp1-deficient animals surviving more than 400 days (Fig. 3f).
EMT is as an important mechanism of tumour metastasis formation18. To investigate the impact of Spp1 on PDAC metastasis, we used (Pdx1-Flp;KrasFSF-G12D/+;Trp53WT/frt;R26FSF-creER;R26mTmG) KPhetFCT mice, because the heterozygosity of Trp53 means that there is a longer latency period in the formation of the tumour, which enables metastases to form and disseminate3. Ultrasound imaging showed that most mice developed tumours at around 100 days of age. Therefore, tamoxifen was administered at this point, and tumour progression and metastasis formation were monitored (Fig. 3g). Consistent with Spp1fl/fl KPFCT mice, Spp1fl/fl KPhetFCT mice (which have Spp1 knocked out) had tumours that predominantly comprised EPCs, whereas KPhetFCT mice (with WT Spp1) formed tumours with both EPCs and MPCs (Extended Data Fig. 5a,b). In KPhetFCT mice, liver metastases were detected in 23% of animals and 30% had lung metastases. By contrast, Spp1fl/fl KPhetFCT mice had no metastases in the liver or lung (Fig. 3g–i and Extended Data Fig. 5c,d). Furthermore, orthotopic transplantation of Spp1-overexpressing KPR172H/+C organoids significantly promoted liver and lung metastases (Extended Data Fig. 6a–f). By contrast, in vivo administration of a SPP1-blocking antibody reduced MPC cell fate and suppressed liver and lung metastases (Extended Data Fig. 7a–j). Taken together, these data demonstrate that SPP1 is essential for maintaining the fate of MPCs and promoting metastasis formation.
SPP1 induces BMP2 through CD61 to maintain MPC fate
We next sought to investigate the molecular mechanism of SPP1 function. We previously identified Grem1 as a BMP2 target gene to control epithelial cell identity3. RNA sequencing (RNA-seq) analysis of Spp1Δ/Δ KPFV and control organoids revealed that SPP1 function is required for Bmp2 and Grem1 expression (Fig. 4a), a result that was confirmed by RT–qPCR (Fig. 4b). Similarly, Itgb3 knockout significantly attenuated Bmp2 and Grem1 expression (Fig. 4c). Conversely, overexpressing Itgb3 increased Bmp2 and Grem1 expression (Extended Data Fig. 8a). This result suggests that the signalling axis SPP1–CD61–BMP2–GREM1 operates between EPC and MPC PDAC subpopulations.
a, Volcano plot showing gene expression profiles between Spp1WT/WT KPFV and Spp1Δ/Δ KPFV organoids. Blue dots indicate genes with higher expression in Spp1Δ/Δ KPFV organoids, and red dots indicate genes with higher expression in Spp1WT/WT KPFV organoids. P values were calculated using edgeR (two-sided). b, RT–qPCR analysis of Grem1 and Bmp2 expression in Spp1WT/WT KPFV and Spp1Δ/Δ KPFV organoids (n = 3 biological replicates). Values are normalized to Spp1WT/WT KPFV values. c, RT–qPCR analysis of BMP2 target genes Id1, Id2, Id3 and Grem1 in Itgb3WT/WT KPFV and Itgb3Δ/Δ KPFV organoids (n = 3 biological replicates), normalized to Itgb3WT/WT KPFV values. d, RT–qPCR analysis of Spp1, Id1, Id2, Id3, Bmp2 and Grem1 levels in KPF organoids treated with recombinant BMP2 protein or vehicle (n = 3 biological replicates), normalized to vehicle values. e, Immunofluorescence staining of GFP (grey), KRT19 (green) and VIM (red) in WT control (Spp1WT/WTGrem1WT/WT KPFCT), Spp1 homozygous knockout (Spp1fl/flGrem1WT/WT KPFCT), Grem1 homozygous knockout (Spp1WT/WTGrem1fl/fl KPFCT), Grem1 heterozygous knockout (Spp1WT/WTGrem1fl/WT KPFCT), Grem1 heterozygous knockout combined with Spp1 homozygous knockout (Spp1fl/flGrem1fl/WT KPFCT) and Grem1 and Spp1 double-homozygous knockout mice (Spp1fl/flGrem1fl/fl KPFCT) (n = 5 mice). Scale bar, 100 μm. f, Quantification of the proportion of KRT19+VIM– and KRT19–VIM+ cancer cells in e. Data are the mean ± s.e.m. (b–d,f). P values were calculated using two-sided t-tests.
RNA-seq analysis revealed that Itgb3 knockout organoids showed impaired NF-κB signalling (Extended Data Fig. 8b). RT–qPCR confirmed downregulation of the NF-κB target genes Ccl2 (ref. 19), Clap2 (ref. 20) and Tnf19, whereas their expression increased when Itgb3 was overexpressed (Extended Data Fig. 8c,d). Next, using the JASPAR database21, we identified two putative NF-κB-binding sites in the Bmp2 promoter, and chromatin immunoprecipitation assays for RelA confirmed binding to these two sites in the Bmp2 promoter (Extended Data Fig. 8e,f). The TAK1 kinase inhibitor 5Z-7-oxozeaenol22 significantly inhibited the binding of RelA to the site at +1597 and reduced Bmp2 mRNA levels (Extended Data Fig. 8g,h). Thus, SPP1 induces Bmp2 expression, and indirectly the expression of the BMP2 target gene Grem1, by activating the NF-κB signalling pathway.
BMP2 treatment increased Bmp2 expression, which indicated the presence of an autoregulatory feedback loop23,24, and also upregulated Grem1 but decreased Spp1 expression (Fig. 4d). BMP2 treatment led to a rapid induction of the BMP target genes Id1, Id2 and Id3, but Spp1 downregulation occurred only after 24 h and coincided with an increase in Vim expression. By contrast, treatment with the BMP inhibitor LDN193189 led to the opposite effect (Extended Data Fig. 8i–k), which indicated that Spp1 is expressed as a consequence of epithelial identity. Thus, BMP2, SPP1 and GREM1 are components of a complex regulatory loop that determines PDAC cell fate.
To better understand the mechanism of PDAC cell fate determination, we generated Spp1 and Grem1 compound double-knockout models (Extended Data Fig. 9a,b). Concomitant inactivation of Grem1 reverted the epithelial phenotype of Spp1 knockout to fully mesenchymal PDAC. Moreover, Grem1 heterozygosity combined with Spp1 knockout resulted in WT PDAC histology (Fig. 4e,f and Extended Data Fig. 9c), a result that highlighted their directly opposing functions. Furthermore, subsequent Grem1 knockout reversed the effect of Spp1 knockout and produced a mesenchymal phenotype. Conversely, Grem1 overexpression reversed the effects of Spp1 overexpression and induced an epithelia phenotype (Extended Data Figs. 10a–h and 11a–h). These results further emphasize the antagonistic relationship between SPP1 and GREM1.
Discussion
Cellular heterogeneity is a prominent feature used for the classification of pancreatic cancer25,26,27. We showed here that epithelial and mesenchymal PDAC subpopulations are required for the maintenance of each other through a reciprocal, paracrine signalling network of three diffusible molecules: SPP1, BMP2 and GREM1 (Extended Data Fig. 12). We speculate that the mutual dependency of epithelial and mesenchymal subpopulations may underlie the obligatory cellular heterogeneity seen in PDAC. Moreover, our data suggested that cellular heterogeneity is required for efficient pancreatic tumour formation, as decreasing the mesenchymal PDAC subpopulation through Spp1 inactivation led to a substantial extension of lifespan in the aggressive KPF and KPC PDAC models (Fig. 3e,f). Hence therapeutic strategies that aim to reduce cellular heterogeneity could be considered for PDAC treatment.
Methods
Blood samples from patients with pancreatic cancer
Peripheral venous blood samples were collected from 26 patients with pancreatic cancer at the Seventh Affiliated Hospital of Sun Yat-sen University. Plasma was isolated from the collected blood samples, and plasma proteomic analysis was subsequently performed. Informed consent was obtained from all participants, and approvals were secured from the ethics board of the Seventh Affiliated Hospital of Sun Yat-sen University for the use of these specimens in research. All clinical samples were collected with informed consent, and all experiments adhered to the regulations and approvals of the internal review and ethics board at the Seventh Affiliated Hospital of Sun Yat-sen University. The Institutional Review Board approval number is KY-2024-249-01. Clinical information on the patients, including age, sex, TNM stage, histology and differentiation, was obtained from medical and follow-up records (Supplementary Table 1).
Generation of patient-derived organoids
Human pancreatic tissue samples were obtained from patients at the Seventh Affiliated Hospital of Sun Yat-sen University who underwent surgery for pancreatic neoplasia. Informed consent was obtained from all participants, and approvals were secured from the ethics board of the Seventh Affiliated Hospital of Sun Yat-sen University for the use of these specimens in research. The tissue samples were collected in Advanced DMEM/F12 base medium with 100 µg ml–1 Primocin and minced into 1 mm2 pieces with a scissor. Minced tissue was then washed in the base medium and resuspended in 10 ml digestion medium containing Advanced DMEM/F12, 5 mg ml–1 collagenase XI, 2 mg ml–1 dispase II and 10 µg ml–1 DNase I. Tumour fragments were digested for 2–4 h on a Thermo Scientific tube rotator at 37 °C at speed 25. Digestion was stopped by adding the base medium. Tissue was then washed and centrifuged twice with the base medium and digested for 10 min with TrypLE Express (Gibco) supplemented with Y-27632 (10 µM). Tissue was then filtered through a 100 µm cell filter (Falcon), washed and centrifuged twice in the base medium. The pellet was resuspended in 5 ml red cell lysis buffer (Corning) for 5 min. Cells were then washed and centrifuged a further 2 times in the base medium, resuspended in 50 µl Matrigel per well (Corning) and plated onto pre-warmed 24-well plates. Next, 500 µl medium per well was added 15–30 min later once the Matrigel had solidified. The medium contained 1× B27 supplement, 10 mM nicotinamide, 1.25mM N-acetylcysteine, 10 nM gastrin, 100 ng ml–1 human FGF10, 100 ng ml–1 mouse Noggin, 500 nM A83-01, 100 µg ml–1 Primocin, 50 ng ml–1 EGF, 10 µM Y-27632, 10% (v/v) R-Spondin 1 conditioned medium and 50% (v/v) WNT3A conditioned medium.
Plasma proteomics
For protein extraction, cellular debris from the plasma samples was removed through centrifugation at 12,000g for 10 min at 4 °C. The supernatant was then carefully transferred to a fresh centrifuge tube, and the protein was quantified using a BCA kit (Bio-Rad) according to the manufacturer’s protocol.
For trypsin digestion, high-abundant proteins were first removed from the plasma samples using a kit (A36369, Thermo), and the solution was then treated with 5 mM dithiothreitol for 30 min at 56 °C, followed by alkylation with 11 mM iodoacetamide for 15 min at room temperature in the dark. Next, 70 μl enzyme digestion buffer was added to the beads and incubated at 95 °C for 10 min, followed by allowing the samples to return to room temperature. Trypsin was then added to a final concentration of 20 ng μl–1 for overnight digestion. The resulting peptides were desalted using C18 ZipTips (Millipore) according to the manufacturer’s instructions and then dried for subsequent mass spectrometry (MS) analysis.
For liquid chromatography–MS/MS analysis, the tryptic peptides were resuspended in solvent A and directly loaded onto a self-fabricated reversed-phase analytical column (15 cm in length, 100 μm internal diameter). The mobile phase was composed of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid and 80% acetonitrile in water). Peptides were separated using the following gradient: 0–1.6 min, 4–22.5% B; 1.6–2.0 min, 22.5–35% B; 2.0–2.6 min, 35–55% B; 2.6–2.7 min, 55–99% B; 2.7–6.8 min, 99% B; 6.8–7.6 min, 99% B, all at a constant flow rate of 300 nl min–1 on a Vanquish Neo UPLC system (ThermoFisher Scientific).
The separated peptides were analysed using an Orbitrap Astral mass spectrometer equipped with a nano-electrospray ion source. The electrospray voltage was set to 1,900 V. Precursors were analysed in the Orbitrap detector, whereas fragments were analysed in the Astral detector. The full MS scan resolution was set to 240,000 with a scan range of 380–980 m/z. The MS/MS scan was fixed with a first mass of 150.0 m/z at a resolution of 80,000. Higher-energy collisional dissociation fragmentation was performed at a normalized collision energy of 25%. The automatic gain control target was set at 500%, with a maximum injection time of 3 ms.
For database searches, the data-independent acquisition (DIA) data were processed using the DIA-NN search engine (v.1.8). Tandem mass spectra were searched against the Homo_sapiens_9606_SP_20231220.fasta database (20,429 entries) concatenated with a reversed decoy database. Trypsin/P was specified as the cleavage enzyme, allowing up to one missed cleavage. Excision on the amino-terminal Met and carbamidomethylation on Cys were specified as fixed modifications. The false discovery rate (FDR) was adjusted to be less than 1%.
Missing value imputation
To address missing values in our dataset, we used the K-nearest neighbours imputation method using the KNNImputer function from the scikit-learn library in Python28.
Genetically engineered mouse models
The KrasLSL-G12D (ref. 29), Trp53flox (ref. 30), Trp53R172H (ref. 31), Pdx1-cre (ref. 32), Pdx1-Flp, KRasFSF-G12D, Trp53frt, Rosa26FSF-creER (ref. 14), Rosa26LSL-YFP (ref. 33), Rosa26mT/mG (ref. 15) and Grem1fl (ref. 34) mouse lines have been previously described. The VIM–GFP transgenic line developed by the Gene Expression Nervous System Atlas (GENSAT) Project was obtained from the Mutant Mouse Resource & Research Centers9. Spp1fl mice (NM-CKO-210205) were purchased from the Shanghai Model Organisms Center. These lines were intercrossed to generate the desired genotypes of both sexes on a C57BL/6 background in Charles River Laboratories. Mouse genotypes were determined using real-time PCR with specific probes designed for each gene (Transnetyx).
To activate CreERT recombinase after tumour onset, Pdx1-Flp;KrasFSF-G12D;Trp53frt/frt;Rosa26FSF-creER;Rosa26mT/mG;Spp1fl/fl (Spp1fl/fl KPFCT), Spp1WT/WT KPFCT, Grem1fl/fl KPFCT, Grem1fl/WT KPFCT, Spp1fl/flGrem1fl/WT KPFCT and Spp1fl/fGrem1fl/fl KPFCT mice were given tamoxifen through intraperitoneal injections at a dose of 100 mg kg−1 body weight, once a day for 5 days at 25 days of age. Pdx1-Flp;KrasFSF-G12D;Trp53WT/frt;Rosa26FSF-creER;Rosa26mT/mG;Spp1fl/fl (Spp1fl/fl KPhetFCT) and Spp1WT/WT KPhetFCT mice were given tamoxifen at the same doses at 100 days of age. Sample sizes were determined on the basis of the results of our preliminary experiments. Mice from autochthonous tumour models were selected according to their correct genotypes.
Mouse blood samples and ELISA
Plasma was isolated from the collected blood samples from mice after humane euthanasia. The samples were centrifugated at 12,000g for 10 min at 4 °C, and the supernatant was carefully transferred to a fresh centrifuge tube. Protein levels were quantified using a BCA kit according to the manufacturer’s protocol. ELISA was performed according to the Mouse & Rat Osteopontin (OPN) ELISA Kit–Quantikine protocol (R&D System). The plasma SPP1 concentration was calculated on the basis of the Mouse/Rat OPN standard.
Subcutaneous transplantation with mouse pancreatic cancer organoids
The immunocompromised Nu/Nu mice used have been previously described35, and adults (aged 8–12 weeks) obtained from Charles River Laboratories were used for transplantation.
Cells dissociated from organoids were subcutaneously injected in 50 μl cold growth-factor-reduced Matrigel (Corning). For Spp1 knockout organoid transplantation, a total 10,000 Spp1Δ/Δ KPF and Spp1WT/WT cells were injected into contralateral flanks of Nu/Nu mice. For Itgb3 knockout organoid transplantation, a total 100,000 Itgb3Δ/Δ KPF and Itgb3WT/WT cells were injected into contralateral flanks of Nu/Nu mice. For the inducible Grem1 deletion after Spp1 deletion analysis, we used CRISPR–Cas9-mediated knockout of Spp1 in GFP-labelled Grem1fl/fl KPFC organoids, and a total 100,000 cells dissociated from GFP-labelled Grem1fl/flSpp1Δ/Δ KPFC organoids were injected into the flanks of Nu/Nu mice. After tumours reached a size of 100 mm3, tamoxifen or peanut oil (vehicle) was intraperitoneally injected at a dose of 100 mg kg−1 body weight once a day for 5 days. For the inducible Grem1 overexpression after Spp1 overexpression analysis, we used Plv-SPP1–GFP and pINDUCER-GREM1 overexpression lentiviral vectors in KPFC organoids, and a total of 100,000 cells dissociated from Plv-SPP1–GFP and pINDUCER-GREM1 KPF organoids were injected into the flanks of Nu/Nu mice. After tumours reached a size of 100 mm3, doxycycline or saline (vehicle) was intraperitoneally injected at a dose of 2 mg kg–1 body weight twice weekly.
Tumour growth was monitored twice a week and mice were humanely euthanized when the tumour size reached a mean diameter of 1.2 cm. The tumours were weighed and fixed in 10% neutral buffered formalin (Cellstor) for histological analyses.
All animal experiments were approved by the Institute of Cancer Research, London’s Animal Welfare and Ethical Review Body and conformed to UK Home Office regulations under the Animals (Scientific Procedures) Act 1986, including Amendment Regulations 2012. The mice were housed in individually ventilated cages at constant temperature and humidity (23 ± 2 °C, 50–60%) in a pathogen-free controlled environment, with a standard 12 h light–12 h dark cycle and unlimited access water and food. For subcutaneous transplantation experiments, the maximum tumour size did not exceed the mean diameter of 1.2 cm, in compliance with project licence PP9490916.
Ultrasound-guided pancreas orthotopic transplantation
The injection technique was adapted from published literature36,37. Under anaesthesia, mice were placed in a supine position on a heated (37 °C) ultrasound platform. The platform was rotated 180° anticlockwise to position the injection mount towards the left upper quadrant of the abdomen. Ultrasound gel was applied to the upper part of the abdomen and the pancreas was located by using the left kidney and spleen as a reference. Once a clear image of the pancreas was located, a syringe was mounted into the holder and the needle was brought towards the field of view using the x axis micromanipulator. With an appropriate angle, the needle was pierced through the skin and pushed forward until the tip was in the pancreas. KPR172H/+C Spp1 overexpression (n = 10,000) cells and KPR172H/+C control (n = 10,000 cells) cells, suspended in 25 μl of 90% cold growth-factor-reduced Matrigel (Corning), were slowly injected into the pancreas using a 1 ml syringe with a 29 gauge needle. Successful injection was confirmed by the presence of hyperechogenic contrast from the Matrigel inside the pancreas. The needle was slowly withdrawn after 15 s of delay to prevent leakage.
Ultrasound imaging of pancreatic tumours
The precise acquisition of ultrasound images of pancreatic tumours was performed using a Microscan transducer MS-550D (VEVO 2100; Visualsonics). Mice were gently anaesthetized with 1.5–2.5% isoflurane in oxygen at a flow rate of 1 l min−1 and placed in a supine position on a preheated (37 °C) ultrasound platform. Pancreatic tumours were located and labelled using the 2D B-mode setting. For tumour volume measurement, consecutive 2D-mode images were acquired by scanning across the entire tumour at intervals of 0.2 mm using the 3D stage control system, and then reconstructed into a 3D volume. The heart rate, respiration rate and body temperature were monitored throughout the scanning process to ensure stable vital signs of the mice. The 3D tumour volume was calculated using the 3D analysis tool Vevo Lab (v.3.2.0).
Patient-derived organoid pancreatic cancer xenografts
Organoids were grown to 80–90% confluence before injection. Organoids were dissociated using TrypLE Express supplemented with Y-27632 (10 µM) into single cells and small clusters, and then counted using an automated cell counter. Cells were then resuspended to the indicated concentration in a 50% Matrigel and 50% organoid culturing medium. Next, 50 µl of the cell mixture was injected into the flank of male NSG mice aged 6 weeks. The tumour size was monitored twice weekly with callipers, and tumours were allowed to grow for about 2 months. Tumour volumes were calculated using the formula volume = length × width2/2, as previously described3. Mice were killed at either the study end point or when the tumour reached the maximum allowable volume (tumour volume = 1.5 cm2), and tissues were collected for downstream analyses. The animal use protocol listed was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), Sun Yat-Sen University (approval no. SYSU-IACUC-2025-B0073).
SPP1 neutralization experiments
KPR172H/+CY mice (100 days old) were intraperitoneally injected 3 times a week for 3 months with mouse SPP1 antibody (BioxCell, clone MPIIIB10; 200 μg per mouse per injection) or control antibody (mouse IgG isotype control, Cell Sciences; 200 μg per mouse per injection) in independent experiments.
Mouse tumour cell isolation and organoid cultures
After humane euthanasia, mice were disinfected with 70% ethanol and pancreatic tumours were dissected and placed into ice-cold DMEM–F-12 containing 50 U ml–1 penicillin–streptomycin (Thermo Fisher Scientific). The tissue samples were minced on ice using sterile tissue scissors and then incubated in DMEM containing 1 mg ml–1 collagenase V (Sigma-Aldrich) in a shaking incubator for 1 h. After digestion, the process was halted by adding ice-cold G solution, and the cells were centrifuged at 300g for 5 min at 4 °C. Subsequently, the cells underwent further digestion with TrypLE (Thermo Fisher Scientific) for 10 min at 37 °C, followed by stopping the reaction with ice-cold 2% FCS in PBS and centrifugation. The cells were resuspended, filtered through a 40 μm nylon mesh, centrifuged again and then plated in Matrigel. Organoid medium was used as previously described38. To ensure the selection of cancer cells in the culture, for the first five passages, they were cultured in medium without EGF. After the fifth passage, complete organoid medium was used for culturing and experimentation.
Organoid staining
Organoids were grown in chamber slides (Ibidi) and washed twice with ice-cold PBS, fixed for 15 min in 4% paraformaldehyde in PBS, permeabilized in 0.5% Triton X-100 in PBS for 20 min and blocked in PBS containing 10% FCS (Gibco), 1% BSA (Sigma-Aldrich) and 0.2% Triton X-100 (Sigma-Aldrich). Primary antibodies were incubated in blocking buffer at 4 °C overnight. Fluorophore-conjugated secondary antibodies together with 3 μM DAPI were incubated in blocking buffer for 1 h at room temperature. The antibodies used are listed in Supplementary Table 2. Stained organoids were imaged on a Leica SP8 confocal microscope and analysed with Imaris (v.9.5.1).
Immunohistochemical staining
After standard dissection of mice, tumour tissue samples were collected and fixed in 10% neutral buffered formalin for 24 h. The tumours were then transferred to 70% ethanol for storage, followed by gradual dehydration in ethanol and embedding in paraffin. The paraffin-embedded tissues were sectioned into 4-μm-thick slices. H&E staining and DAB immunohistochemistry were conducted by the Histopathology Core Facility Breast Cancer Research at The Institute of Cancer Research using standard procedures. All staining was carried out using a Dako Autostainer Link48 (Agilent Technologies) immunostaining platform with epitope retrieval and simultaneous dewaxing using the Dako PT Link module (Agilent Technologies) according to the manufacturer’s instructions. Formalin-fixed paraffin-embedded sections were cut at 4 µm onto APEX adhesive slides (Leica Biosystems), dried at 60 °C for 60 min and subject to epitope retrieval at 97 °C for 20 min using either Dako Target Retrieval Solution pH 9 (Agilent Technologies, K800421-2) or pH 6 (Agilent Technologies, K800521-2) as described below. Primary antibodies were diluted in Dako FLEX antibody diluent (Agilent, K800621-2) and endogenous peroxidases were blocked using Dako REAL peroxidase block (Agilent, S202386-2). Antibody binding reactions were visualized using Dako DAB+ (Agilent, K346811-2) and sections were counterstained using Dako FLEX Haematoxylin (Agilent, K800821-2). The following antibodies were used for the indicated experiments: CK19 DSHB rat monoclonal TROMA III (primary dilution 1:200), epitope retrieval = pH 9, detection system = anti-rat N-histofine polymer-HRP (Nichirei Biosciences, 414311F); vimentin, Cell Signaling Technology rabbit monoclonal D21H3, 5741 (primary dilution 1:200), epitope retrieval = pH 9, detection system = Dako rabbit EnVision polymer-HRP (Agilent, K400311-2). Stained slides were imaged on a NanoZoomer (Hamamatsu Photonics) slide scanner and analysed with QuPath (v.0.4.2)39. Analyses and grading of PDACs were performed by a certified pathologist based on H&E staining. For liver and lung metastases, H&E-stained sections of paraffin-embedded tissues were analysed, and the number of metastases per section was quantified using ImageJ.
Immunofluorescence staining
Paraffin sections from tumour tissues were prepared as described above, and antigen retrieval was performed by heating in 10 mM sodium citrate (Sigma-Aldrich) buffer (adjust pH 6.0 by using hydrochloric acid). The sections were blocked using blocking buffer (PBS containing 10% FCS and 1% BSA from Sigma-Aldrich) at room temperature for 1 h, followed by overnight incubation with primary antibodies prepared in blocking buffer at 4 °C in a cold room. On the next day, fluorophore-conjugated secondary antibodies (1:200) along with 3 μM DAPI (Sigma-Aldrich) in blocking buffer were applied in the dark at room temperature for 1 h. A list of the antibodies used is provided in Supplementary Table 2. Before mounting, slides were incubated in 0.1% (w/v) Sudan Black B (Sigma-Aldrich) in fresh 70% ethanol to reduce background signal. Stained slides were imaged on a Leica confocal microscope or a NanoZoomer (Hamamatsu Photonics) slide scanner and analysed using Imaris.
CRISPR–Cas9-mediated knockout of target gene in organoids
The target gene was deleted in organoids using the CRISPR–Cas9 system. The protocol for engineering organoids was adapted and modified from a previous study40. Organoids were dissociated into single cells using TrypLE and diluted to 1 × 106 cells per electroporation. The cell pellet was then resuspended in a precise 100 μl solution containing BTXpress solution (BTX), 7.2 μg PB-CMV-MCS-EF1a-RFP-T2A-Puro (System Biosciences) or pPB[Exp]-CMV>Hygro (Vectorbuilder), 2.8 μg CMV-pHyPBase or pRP[Exp]-mCherry-CAG>hyPBase (Vectorbuilder), and 10 μg PX330 (provided by F. Zhang, Addgene, 4223062) with a target gRNA. The cell–DNA mixture was transferred to 2 mm electroporation cuvettes (Nepagene) and electroporated using a NEPA21 super electroporator (Nepagene), with settings described in our previous publication41. After electroporation, the cell suspension was transferred to complete medium containing Y-27632 (10 μM) without antibiotics. After 30 min, cells were centrifuged at 300g for 5 min at 4 °C, resuspended in Matrigel and cultured in antibiotic-free complete medium supplemented with 10 μM Y-27632. On day 5 after electroporation, organoids were selected with 2 μg ml–1 puromycin or 100 mg ml–1 hygromycin B. After 3 days of selection, organoids were flow sorted into single cells into a 96-well plate with 30 μl Matrigel per well. gDNA was extracted using a Purelink Genomic DNA Minikit per the manufacturer’s instructions (Thermo Fisher Scientific). PCR was performed using Q5 High-Fidelity 2× master mix (New England Biolabs) with pre-designed primers and gDNA. Subsequently, PCR products were amplified and cloned using a Zero Blunt PCR Cloning kit (Thermo Fisher Scientific) and sequenced by Sanger sequencing from Genewiz. A list of the gRNAs used is provided in Supplementary Table 3.
Flow cytometry
Organoids or tissues were dissociated into single cells as mentioned above. Single cells were suspended in FACS buffer (PBS containing 2% FCS). The cells were incubated with fluorophore-conjugated isotype control or experimental antibodies in FACS buffer on ice in the dark for 30 min. A list of the antibodies used is provided in Supplementary Table 2. Cells were washed 3 times with PBS, filtered through a 40 μm nylon mesh (Corning) and then incubated in FACS buffer containing 3 μM DAPI or propidium iodide. Single-colour and fluorescence minus one staining control were used as necessary. The cells were analysed using FACSymphony A5 (BD Biosciences) analyzer. Data were analysed using FlowJo 10 software. The gating strategy is shown in Supplementary Fig. 2.
RT–qPCR
RNA was extracted from organoids using either a RNAqueous-Micro Total RNA Isolation kit (Thermo Fisher Scientific) or a RNeasy Mini kit (Qiagen) following the manufacturer’s instructions. For BMP2 and LDN193189 treatment, organoids were starved in DMEM with 1% FCS overnight and treated with vehicle, 100 ng ml–1 recombinant BMP2 Protein (R&D Systems) or 100 nM LDN193189 (Tocris) for 12 h, followed by RNA extraction from the cells. cDNA was generated using an iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s instructions. The cDNA was diluted 1:10 in distilled water, and 2 μl was used per qPCR reaction. PowerUp SYBR Green master mix (Thermo Fisher Scientific) was used for qPCR on an ABI7500 or QuantStudio 6 Flex (Applied Biosystems) system. Primers were designed using the Primer-BLAST tool at NCBI and made by Sigma-Aldrich. Housekeeping gene mRNA levels (Actb or Rplp0) were used for normalization. mRNA levels were quantified using the difference in Ct values and calculated using the \({2}^{-\Delta \Delta {C}_{{\rm{t}}}}\) method42. A list of the qPCR primers used is provided in Supplementary Table 4.
Western blotting
Matrigel was removed from cells on ice using Cell Recovery solution (Corning) following the manufacturer’s instructions. The Matrigel-depleted cells were lysed in ice-cold cell lysis buffer (NEB) as previously described3. After brief sonication, lysates were centrifuged at 13,000 r.p.m. for 15 min at 4 °C. Protein quantification was performed using a Protein Assay kit (Bio-Rad), and proteins were separated by SDS–PAGE on 10% gels (Bio-Rad), then transferred onto PVDF membranes (Bio-Rad) using a Trans-Blot Turbo system (Bio-Rad) according to the manufacturer’s instructions. The membranes were blocked in TBST containing 3% BSA at room temperature for 1 h, then incubated with primary antibodies prepared in TBST with 1% BSA overnight at 4 °C. HRP-conjugated secondary antibodies were subsequently incubated at room temperature for 1 h. Chemiluminescence was detected using Amersham ECL Prime Western Blotting Detection reagent (GE Healthcare) in an Amersham ImageQuant 800 imaging system. A list of the antibodies used is provided in Supplementary Table 2.
Lentiviral production and infection
The Itgb3 overexpression, Spp1 overexpression, SPP1 overexpression, control and GFP vectors were purchased from Vectorbuilder. GFP and inducible Grem1 were cloned into pLenti6 V5-DEST (Thermo Fisher Scientific) or pInducer20-Blast (Addgene, 109334) using the BP and LR Recombination System (Thermo Fisher Scientific). Lentiviral particles were produced in HEK293T cells using psPAX2 and pMD2.G helper plasmids (Addgene) and transfected into HEK293T cells using jetPRIME transfection reagent (jetPRIME). For organoid infection, a single-cell suspension was prepared and incubated with lentiviruses supplemented with 4 μg ml–1 polybrene (Santa Cruz Biotechnology) in a rocking incubator for 6 h. Cells were then seeded in Matrigel and antibiotic selection was applied 3 days later.
Chromatin immunoprecipitation
To predict transcription factor binding motifs in the Bmp2 promoter, DNA sequences ranging from −1000 to +500 bp from the transcription start site were scanned in the JASPAR 2000 database (http://jaspar.genereg.net/) using a relative profile score threshold of 80%. The JASPAR relative score is defined as 1 for the maximum-likelihood binding site21.
Organoids were dissociated and plated on 2D culture dishes and cultured in DMEM with 5% FCS. After exponential growth, the cells were switched to DMEM containing 1% FCS and starved overnight, followed by treatment with vehicle or 5Z-7-oxozeaenol for 6 h. The cells were fixed and crosslinked chromatin was prepared as previously described3. For immunoprecipitation, 10 μg chromatin was incubated with 10 μl anti-histone H3 rabbit IgG (CST, used as a positive control), 2 μl normal rabbit IgG (CST) or 5 μl of anti-RelA antibody (CST) overnight at 4 °C. Before immunoprecipitation, 2% of the chromatin (v/v) was used as input. Protein G magnetic beads were used to capture chromatin–protein–antibody complexes, followed by reversal of crosslinking to release chromatin and purification using a SimpleChIP Enzymatic Chromatin IP kit (CST). DNA was quantified by qPCR using primers targeting predicted Rela-binding regions at the Bmp2 promoter. The DNA level was normalized to the input, and the fold enrichment was calculated by normalizing to the control. A list of the chromatin immunoprecipitation qPCR primers is provided in Supplementary Table 5. A list of the antibodies used is provided in Supplementary Table 2.
RNA-seq analysis
For the RNA-seq analysis of Spp1 and Itgb3 WT and knockout organoids, RNA was extracted from organoids using a RNeasy Mini kit (Qiagen) following the manufacturer’s instructions. mRNA capture and library preparation were performed by Genewiz. Biological replicate libraries were sequenced on an Illumina NovaSeq platform at Genewiz, which generated an average of approximately 25 million 150 bp paired-end reads per sample. Sequence reads were processed using Trimmomatic (v.0.36) to remove possible adapter sequences and nucleotides with poor quality43. Reads were aligned to the mouse reference assembly to the Mus musculus GRCm38 reference genome available on ENSEMBL using STAR aligner (v.2.5.2b)44. Normalization of raw counts and regularized log-transformation were performed as previously described3.
Gene set enrichment analysis
For the RNA-seq dataset of Spp1 and Itgb3 WT and knockout organoids, GSEA (v.4.0.3) analysis was performed using the rlog-transformed counts, with the following settings applied: number of permutations = 1000, permutation type = gene set, enrichment statistics = weighted, metric for ranking genes = Signal2Noise. Normalized expression values of PDAC samples from the datasets GSE71729, ICGCarray, ICGCseq, TCGA, GSE62452 and GSE79668 were batch corrected using ComBat algorithm. Samples were grouped into SPP1high and SPP1low based on the median expression level. For GSEA, the following settings were applied: including the number of permutations = 1000, permutation type = phenotype, enrichment statistics = weighted, metric for ranking genes = Signal2Noise. The EMT gene set of 200 genes was checked for significant enrichment with an FDR q < 0.2.
scRNA-seq analysis
For human PDAC scRNA-seq data, fastq files of 24 PDAC and 11 normal pancreas samples were downloaded from the Chinese National Genomics Data Center (Genome Sequence Archive: CRA001160) and processed as previously described45. Uniform manifold approximation and projection plots were generated using the R package Seurat (v.3.2.2) with default settings. Cells were coloured according to cluster assignments from the reference. For evaluating SPP1 expression and its association with EMT in ductal cells (type 1 and 2), raw counts were normalized to counts per million (CPM) using the edgeR package in R (v.4.0.3) and log2[CPM + 1] transformed. Cells belonging to ductal clusters 1 and 2 were categorized into SPP1high and SPP1low based on expression levels.
Graphical abstracts
Diagrams in Fig. 1a and Extended Data Figs. 7a, 10a, 11a and 12 were created in BioRender (https://biorender.com).
Statistical analysis
All images and graphs represent the results of multiple experiments, which were conducted on different biological replicate samples and different dates. Each experiment was independently repeated. Differential gene expression analysis for RNA-seq data was performed using the edgeR package in R. All other statistical analyses were conducted using GraphPad Prism 8 for Windows. Specific statistical tests are specified in the figure legends. All t-tests tests, Fisher-exact tests and Mann–Whitney tests were two-sided.
Survival analysis
The survival plots for Spp1fl/fl KPFCT and Spp1WT/WT KPFCT mice receiving tamoxifen treatment and for Spp1fl/fl KPCY, Spp1WT/WT KPCY mice and SPP1 neutralization experiments were created and analysed using two-sided log-rank tests in GraphPad Prism 8. The following PDAC datasets were used: GSE71729, ICGCarray, ICGCseq, TCGA, GSE62452 and GSE79668. The normalized ComBat batch-corrected expression values were used for categorizing samples to SPP1high and SPP1low based on surv_cutpoint function in survminer R package. This function determines the optimal cut-off by maximally selected rank statistics.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
RNA-seq data for Spp1WT/WT KPFV and Spp1Δ/Δ KPFV organoids and for Itgb3WT/WT KPFV and Itgb3∆/∆ KPFV organoids have been deposited into the Gene Expression Omnibus under the accession codes GSE269971 and GSE269972, respectively. The MS proteomic data are available from ProteomeXchange with the identifier PXD053603. Source data are provided with this paper.
References
Yachida, S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1117 (2010).
Connor, A. A. & Gallinger, S. Pancreatic cancer evolution and heterogeneity: integrating omics and clinical data. Nat. Rev. Cancer 22, 131–142 (2022).
Lan, L. et al. GREM1 is required to maintain cellular heterogeneity in pancreatic cancer. Nature 607, 163–168 (2022).
Bill, R. et al. CXCL9:SPP1 macrophage polarity identifies a network of cellular programs that control human cancers. Science 381, 515–524 (2023).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Ferreira, R. M. M. et al. Duct- and acinar-derived pancreatic ductal adenocarcinomas show distinct tumor progression and marker expression. Cell Rep. 21, 966–978 (2017).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Heintz, N. Gene expression nervous system atlas (GENSAT). Nat. Neurosci. 7, 483 (2004).
Tonelli, C. et al. A mucus production programme promotes classical pancreatic ductal adenocarcinoma. Gut 73, 941–954 (2024).
Nallasamy, P. et al. Pancreatic tumor microenvironment factor promotes cancer stemness via SPP1–CD44 axis. Gastroenterology 161, 1998–2013 (2021).
Dai, B. et al. Blockage of osteopontin–integrin β3 signaling in infrapatellar fat pad attenuates osteoarthritis in mice. Adv. Sci. 10, e2300897 (2023).
Erikson, D. W., Burghardt, R. C., Bayless, K. J. & Johnson, G. A. Secreted phosphoprotein 1 (SPP1, osteopontin) binds to integrin alpha v beta 6 on porcine trophectoderm cells and integrin alpha v beta 3 on uterine luminal epithelial cells, and promotes trophectoderm cell adhesion and migration. Biol. Reprod. 81, 814–825 (2009).
Schönhuber, N. et al. A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nat. Med. 20, 1340–1347 (2014).
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Zheng, X. et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525–530 (2015).
Yamada, S. et al. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery 154, 946–954 (2013).
Aiello, N. M. et al. EMT subtype influences epithelial plasticity and mode of cell migration. Dev. Cell 45, 681–695 (2018).
Taniguchi, K. & Karin, M. NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 18, 309–324 (2018).
Mahoney, D. J. et al. Both cIAP1 and cIAP2 regulate TNFα-mediated NF-κB activation. Proc. Natl Acad. Sci. USA 105, 11778–11783 (2008).
Fornes, O. et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 48, D87–d92 (2020).
Ruiz, E. J. et al. LUBAC determines chemotherapy resistance in squamous cell lung cancer. J. Exp. Med. 216, 450–465 (2019).
Ghosh-Choudhury, N. et al. Autoregulation of mouse BMP-2 gene transcription is directed by the proximal promoter element. Biochem. Biophys. Res. Commun. 286, 101–108 (2001).
Ghosh-Choudhury, N., Abboud, S. L., Mahimainathan, L., Chandrasekar, B. & Choudhury, G. G. Phosphatidylinositol 3-kinase regulates bone morphogenetic protein-2 (BMP-2)-induced myocyte enhancer factor 2A-dependent transcription of BMP-2 gene in cardiomyocyte precursor cells. The. J. Biol. Chem. 278, 21998–22005 (2003).
Evan, T., Wang, V. M. & Behrens, A. The roles of intratumour heterogeneity in the biology and treatment of pancreatic ductal adenocarcinoma. Oncogene 41, 4686–4695 (2022).
Milan, M., Diaferia, G. R. & Natoli, G. Tumor cell heterogeneity and its transcriptional bases in pancreatic cancer: a tale of two cell types and their many variants. EMBO J. 40, e107206 (2021).
Espinet, E., Klein, L., Puré, E. & Singh, S. K. Mechanisms of PDAC subtype heterogeneity and therapy response. Trends Cancer 8, 1060–1071 (2022).
Zhang, Z. Introduction to machine learning: k-nearest neighbors. Ann. Transl. Med. 4, 218 (2016).
Jackson, E. L. et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 15, 3243–3248 (2001).
Marino, S., Vooijs, M., van Der Gulden, H., Jonkers, J. & Berns, A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 14, 994–1004 (2000).
Olive, K. P. et al. Mutant p53 gain of function in two mouse models of Li–Fraumeni syndrome. Cell 119, 847–860 (2004).
Hingorani, S. R. et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437–450 (2003).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Gazzerro, E. et al. Conditional deletion of gremlin causes a transient increase in bone formation and bone mass. J. Biol. Chem. 282, 31549–31557 (2007).
Flanagan, S. P. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 8, 295–309 (1966).
Huynh, A. S. et al. Development of an orthotopic human pancreatic cancer xenograft model using ultrasound guided injection of cells. PLoS ONE 6, e20330 (2011).
Hay, C. A., Sor, R., Flowers, A. J., Clendenin, C. & Byrne, K. T. Ultrasound-guided orthotopic implantation of murine pancreatic ductal adenocarcinoma. J. Vis. Exp. https://doi.org/10.3791/60497 (2019).
Boj, S. F. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338 (2015).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Fujii, M., Matano, M., Nanki, K. & Sato, T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 10, 1474–1485 (2015).
Nelson, J. K. et al. USP25 promotes pathological HIF-1-driven metabolic reprogramming and is a potential therapeutic target in pancreatic cancer. Nat. Commun. 13, 2070 (2022).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the \({2}^{-\Delta \Delta {C}_{{\rm{t}}}}\) Method. Methods 25, 402–408 (2001).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Peng, J. et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 29, 725–738 (2019).
Acknowledgements
We thank Breast Cancer Now for funding this work as part of Programme Funding to the Breast Cancer Now Toby Robins Research Centre and grants from the National Natural Science Foundation of China (no. 82473128) and the Science and Technology planning project of Guangdong province (no. 2021B1212040006). We thank staff at the Breast Cancer Now Toby Robins Research Centre Translational Research Support in Vitro Modelling Team, the Human Organoid Facility at the CRUK Convergence Science Centre (CTRQQR-2021\100009) and the Institute of Cancer Research Proteomics Core Facility for their technical support.
Author information
Authors and Affiliations
Contributions
A.B. and H.L. conceived and designed the study. H.L. participated in manuscript preparation and formal analyses and performed most of the experiments. L.L. and H.C performed proteomics on blood samples from patients with pancreatic cancer and in vivo and in vitro experiments using patient-derived organoids. M.Z.T. helped with ultrasound imaging of pancreatic tumours and pancreas orthotopic transplantation. M.Z.T. and M.A. helped with subcutaneous transplantation. H.P. and A.S. performed human PDAC subtype correlation and human PDAC scRNA-seq analyses. J.K.N., I.M.E., E.J.R., L.L., L.T. and R.C. assisted with the study design and manuscript preparation. N.G. performed immunohistochemical staining. J.M., T.Y. and C.W. collected blood samples from patients. Y.H. and S.-Z.L. provided intellectual feedback. A.B. and C.Z. supervised the study. All authors discussed the results and implications and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Dieter Saur and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The role of SPP1 in human and mouse PDAC.
a, UMAP plot of single cells from a scRNA-seq dataset for human PDACs (24 PDAC and 11 normal pancreas samples). Ductal cell type 1 and 2 represent the two main PDAC cancer cell populations. b, Cancer cells were categorized into the high SPP1 expression population (SPP1hi, n = 6980 cells) and the low SPP1 expression population (SPP1lo, n = 6981 cells) groups based on the expression of SPP1. c, EMT signature score plot for SPP1 high (n = 6980 cells) and SPP1 low (n = 6981 cells) cancer cell populations. d, Normalised RNA read counts (RSEM) for SPP1 from the PDAC dataset of GSE71729, ICGCarray, ICGCseq, TCGA, GSE62452 and GSE79668. The samples were categorised into SPP1hi (n = 321) and SPP1lo groups (n = 323) based on its expression, higher or lower than the median value. e, GSEA plot for enrichment of the hallmark EMT gene set in the transcriptome of SPP1hi PDACs. NES, normalized enrichment score; FDR, false discovery rate. f, Kaplan-Meir test for the survival analysis of SPP1 high expression patients (n = 321) and SPP1 low expression patients (n = 323). P value was calculated with the logrank test. g, KPFV pancreatic cancer mouse model schematic diagram. h, Validation of CRISPR/Cas9 Spp1 knockout by Western blot. n = 3 independent experiments. Gel source data are provided in Supplementary Fig. 1. i, H&E and immunohistochemistry for Krt19 and Vimentin in KPFV; Spp1wt/wt (n = 3 mice) and KPFV; Spp1Δ/Δ allografts (n = 2 mice). Scale bar: 2.5 mm. j, Immunofluorescence staining for DAPI (Blue), GFP (gray), Krt19 (green), and Vimentin (red) of KPF; Spp1wt/wt (n = 3 mice) and KPF; Spp1Δ/Δ subcutaneous tumours (n = 2 mice). Scale bar is 50um. For b and d data, the lines in violin plots indicate the median, the upper quartile and the lower quartile respectively. For b, c, d data, P value is from two-sided Mann-Whitney test.
Extended Data Fig. 2 Spp1 overexpression induces mesenchymal transition of PDAC.
a, Immunofluorescent analysis of Vimentin (yellow), DAPI(blue), and Krt19 (red) in KPR172H/+C; Plv-con and KPR172H/+C; Spp1 overexpression organoids. Scale bar length is 50μm. b, RT-qPCR analysis of the expression of epithelial markers Krt19, Cdh1, and Epcam, and mesenchymal markers Fn1, S100a4, and Vim between KPR172H/+C; Plv-con and KPR172H/+C; Spp1 overexpression organoids (n = 3 biological replicates), normalised using KPR172H/+C; Plv-con values. c, Immunofluorescent staining for GFP (gray), Krt19 (green), and Vimentin (red) in KPR172H/+C; Plv-con and KPR172H/+C; Spp1 overexpression allografts. n = 3 mice. Scale bar: 50um. d, H&E and Immunohistochemistry for Krt19 in KPR172H/+C; Plv-con and KPR172H/+C; Spp1 overexpression allografts. n = 3 mice. Scale bar: 1.0 mm. For b data are mean ± s.e.m. P values were calculated using two-sided t-tests.
Extended Data Fig. 3 The role of SPP1 in human PDAC development.
a, RT-qPCR analysis of the expression of epithelial markers KRT19, CDH1, and EPCAM, and mesenchymal markers FN1, S100A4, and VIM between Human PDAC; SPP1wt/wt and Human PDAC; SPP1Δ/Δ organoids (n = 3 biological replicates), normalised using SPP1wt/wt organoids values. b, Validation of CRISPR/Cas9 SPP1 knockout by Western blot. n = 3 independent experiments. Gel source data are provided in Supplementary Fig. 1. c, Image of the subcutaneous tumours formed by Human PDAC; SPP1wt/wt and Human PDAC; SPP1Δ/Δ organoids. Scale bar length is 0.5 cm. d, The sizes of subcutaneous tumours of (c) (n = 6 mice). e, Immunofluorescence staining for KRT19 (green), and VIMENTIN (red) of subcutaneous tumours from (c). Scale bar is 50um. f, Comparison of the percentages of different cancer cell subpopulations of (e) (n = 5 mice). g, h, H&E and immunohistochemistry for KRT19 of subcutaneous tumours from (c). n = 3 mice. Scale bar: 2 mm. i, RT-qPCR analysis of the expression of epithelial markers KRT19, CDH1, and EPCAM, and mesenchymal markers FN1, S100A4, and VIM between Human PDAC; Plv-con and Human PDAC; SPP1 overexpression organoids (n = 3 biological replicates), normalised using Plv-con organoids values. j, Image of the subcutaneous tumours formed by Human PDAC; Plv-con and Human PDAC; SPP1 overexpression organoids. Scale bar length is 0.5 cm. k, The sizes of subcutaneous tumours of (j) (n = 6 mice). l, Immunofluorescence staining for KRT19 (green), and VIMENTIN (red) of subcutaneous tumours from (j). Scale bar is 50um. m, Comparison of the percentages of different cancer cell subpopulations of (l) (n = 5 mice). n, o, H&E and immunohistochemistry for KRT19 of subcutaneous tumours from (j). n = 3 mice. Scale bar: 2 mm. For a, d, f, i, k, m data are mean ± s.e.m. P values were calculated using two-sided t-tests.
Extended Data Fig. 4 Spp1 to maintain identity of MPCs by Cd61 and the effect of tumor stromal composition and immune cell infiltration upon Spp1 deletion.
a, Flow cytometry for Cd61 in Cd61wt/wt and Cd61Δ/Δ PDAC organoids. b, GSEA of the hallmark EMT gene set between of KPFV; Cd61wt/wt and KPFV; Cd61Δ/Δ transcriptomes. c, Flow cytometry for Cd45−/Cd31−/GFP− in tumors from KPFCT; Spp1wt/wt and KPFCT; Spp1fl/fl mice. d, Comparison of the percentages of Cd45−/Cd31−/GFP− in tumors from KPFCT; Spp1wt/wt and KPFCT; Spp1fl/fl mice (n = 5 mice). e, Immunofluorescence staining for aSMA (red), GFP (green), and Vimentin (Pink) of KPFCT; Spp1wt/wt and KPFCT; Spp1fl/fl tumours (n = 5 mice). Scale bar is 50um. f, Comparison of the percentages of aSMA cell subpopulations with Cancer cells between tumours formed by KPCTF; Spp1wt/wt and KPFCT; Spp1 fl/fl. g-m, Comparison of the percentages of Cd4 T cells (g), Cd8 T cells (h), B220 B cells (i), NK cells(j), CD11b monocytes cells (k), F4/80 macrophages cells (l), dendritic cells (m), neutrophils cells (n) in tumors from KPFCT; Spp1wt/wt and KPFCT; Spp1 fl/fl mice (n = 5 mice). For d, f, g, h, i, j, k, l, m and n data are mean ± s.e.m. P values were calculated using two-sided t-tests.
Extended Data Fig. 5 Loss of Spp1 inhibited PDAC development and metastasis formation.
a, H&E and immunohistochemistry of Krt19 in tumors from KPhetFCT; Spp1wt/wt and KPhetFCT; Spp1fl/fl mice. n = 3 mice. Scale bar: 2.5 mm. b, Immunofluorescence staining for GFP (gray), Krt19 (green), and Vimentin (red) of KPhetFCT; Spp1wt/wt and KPhetFCT; Spp1fl/fl tumours. n = 3 mice. Scale bar: 50 μm. c, H&E and immunohistochemistry for Vimentin in livers from KPhetFCT; Spp1wt/wt (n = 15 mice) and KPhetFCT; Spp1fl/fl mice (n = 20 mice). Scale bar: 10 mm. d, H&E and immunohistochemistry for Vimentin in lungs from KPhetFCT; Spp1wt/wt (n = 15 mice) and KPhetFCT; Spp1fl/fl mice (n = 20 mice). Scale bar: 5 mm.
Extended Data Fig. 6 Spp1 overexpression promotes PDAC metastasis formation.
a, Ultrasound imaging of pancreatic tumours in Pancreas Orthotopic transplantation mice. The dashed red circles indicate the tumours in the pancreas. b, H&E immunohistochemistry in tumour from KPR172H/+C; Plv-con and KPR172H/+C; Spp1 overexpression Pancreas Orthotopic transplantation mice. n = 3 mice. Scale bar: 10 mm. c, Percentage of mice with of liver metastases (left) and quantification of number of liver metastases (right) in KPR172H/+C; Plv-con and KPR172H/+C; Spp1 overexpression Pancreas Orthotopic transplantation mice (n = 4 mice). d, Percentage of mice with of lung metastases (left) and quantification of number of lung metastases (right) in KPR172H/+C; Plv-con and KPR172H/+C; Spp1 overexpression Pancreas Orthotopic transplantation mice (n = 4 mice). e, Immunohistochemistry for Vimentin in livers from KPR172H/+C; Plv-con and KPR172H/+C; Spp1 overexpression Pancreas Orthotopic transplantation mice. n = 4 mice. Scale bar: 10 mm. f, Immunohistochemistry for Krt19 in lung from KPR172H/+C; Plv-con and KPR172H/+C; Spp1 overexpression Pancreas Orthotopic transplantation mice. n = 4 mice. Scale bar: 5 mm. For c(right) and d(right) data are mean ± s.e.m. c (left) and d (left) P values were calculated using two-sided Fisher exact test. c (right) and d (right) P values were calculated using two-sided t-tests.
Extended Data Fig. 7 Spp1 antibody treatment induces epithelial differentiation by blocking MPCs in pancreatic cancer.
a, Scheme depicting the experimental approach for Spp1 neutralization experiments in KPR172H/+CY Model. b, Kaplan-Meir test for the survival analysis of KPR172H/+CY mice treat with Spp1 antibody or IgG control. P value was calculated with the logrank test. c, Percentage of mice with of liver metastases (left) and quantification of number of liver metastases (right) in KPR172H/+CY mice treat with Spp1 antibody or IgG control (n = 12 mice). d, Percentage of mice with of lung metastases (left) and quantification of number of lung metastases (right) in KPR172H/+CY mice treat with Spp1 antibody or IgG control (n = 12 mice). e, Immunofluorescent staining for YFP (gray), Krt19 (green), and Vimentin (red) in KPR172H/+CY mice treat with Spp1 antibody or IgG control. Scale bar: 50um. f, Comparison of the proportions of different cancer cell subpopulations between KPR172H/+CY mice treat with Spp1 antibody or IgG control tumours (n = 5 mice). g, H&E immunohistochemistry in tumors from KPR172H/+CY mice treat with Spp1 antibody or IgG control. n = 3 mice. Scale bar: 2 mm. h, Immunohistochemistry of Krt19 in tumors from KPR172H/+CY mice treat with Spp1 antibody or IgG control. n = 3 mice. Scale bar: 2 mm. i, Immunohistochemistry for Krt19 in livers from KPR172H/+CY mice treat with Spp1 antibody or IgG control mice. n = 12 mice. Scale bar: 5 mm. j, Immunohistochemistry for Krt19 in lung from KPR172H/+CY mice treat with Spp1 antibody or IgG control. n = 12 mice. Scale bar: 2.5 mm. For c(right), d(right) and f data are mean ± s.e.m. c (left) and d (left) P values were calculated using two-sided Fisher exact test. c (right), d (right) and f, P values were calculated using two-sided t-tests. The diagram in a was created in BioRender. Ruiz, J. (2025) (https://biorender.com/adqe56v).
Extended Data Fig. 8 Molecular analysis of Bmp2 regulation by Spp1 and Bmp2 indirectly modulates Spp1 expression by regulating mesenchymal cell fate.
a, RT-qPCR analysis of the expression of Id1, Id2, Id3 and Grem1 in control and Cd61 overexpressing KPFV organoids. Values are normalised to control. b, GSEA of the TNFα NF-κB hallmark gene set between KPFV; Cd61wt/wt and KPFV; Cd61Δ/Δ organoids. c, RT-qPCR analysis of the NF-κB signaling pathway target genes Ccl2, Clap2, and Tnf in KPFV; Cd61wt/wt and KPFV; Cd61Δ/Δ organoids. Values are normalised to the KPFV; Cd61wt/wt values. d, RT-qPCR analysis of Ccl2, Clap2, and Tnf expression in control and Cd61 overexpressing KPFV organoids. Values are normalised to the control. e, Potential binding sites of RelA in the Bmp2 promoter were predicted using JASPAR. f, Schematic diagram of the binding sites of RelA to the Bmp2 promoter. g, ChIP analysis for RelA binding to the Bmp2 promoter and Tnf promoter in KPFV organoids. ChIP was conducted using IgG control or RelA antibody, with or without treatment with TAK1 inhibitor (5Z-7), followed by qPCR analysis. Values are normalised to the input. h, RT-qPCR analysis of the expression of Bmp2 in Vehicle and TAK1 inhibitor (5Z-7) treated organoids. Values are normalised to vehicle treatment. i, RT-qPCR analysis of the expression of Spp1, Id1, Id2, Id3, Bmp2 and Grem1 in KPF organoids treated with the Bmp2 inhibitor LDN193189 and vehicle. Values are normalised to the vehicle. 5Z-7: 5Z-7-oxozeaenol. j, Comparative RT-qPCR analysis of Id1, Id2, Id3, Spp1, Krt19 and Vim, in KPF organoids treated with Bmp2 for 0, 3, 6, 9 and 24 h. Values are normalised to Bmp2 0 h treatment. k, Comparative RT-qPCR analysis of Id1, Id2, Id3, Spp1, Krt19 and Vim, in KPF organoids treated with LDN193189 for 0, 3, 6, 9 and 24 h. Values are normalised to the LDN193189 0 h treatment. For a, c, d, g, h, i, j, k data are mean ± s.e.m and n = 3 biological replicates. P values were calculated using two-sided t-tests.
Extended Data Fig. 9 Histological analysis of Spp1 and Grem1 compound mutant mice.
a, Schematic diagram showing the mouse model KPFCT; Grem1fl/fl. b, Schematic diagram showing the KPFCT; Spp1fl/fl; Grem1fl/fl mouse model. c, Immunohistochemical staining of Krt19 in control (KPFCT; Spp1wt/wt; Grem1wt/wt), homozygous Spp1 knock out (KPFCT; Spp1fl/fl; Grem1wt/wt), homozygous Grem1 knock out (KPFCT; Spp1wt/wt; Grem1fl/fl), heterozygous Grem1 knock out (KPFCT; Spp1wt/wt; Grem1fl/wt), heterozygous Grem1 knock out combined with homozygous Spp1 knock out (KPFCT; Spp1fl/fl; Grem1fl/wt) and homozygous double knock out mice (KPFCT; Spp1fl/fl; Grem1fl/fl). n = 3 mice. The scale bar: 500 μm.
Extended Data Fig. 10 Grem1 deletion reverses the epithelial cell state induced by Spp1 knock out.
a, Scheme depicting the experimental approach for establishing tumours from Spp1 CRISPR/Cas9 knockout and Tamoxifen-inducible Grem1 knockout organoids. b, RT-qPCR analysis of the expression of epithelial markers Krt19, Cdh1, and Epcam, and mesenchymal markers Fn1, S100a4, and Vim in organoids from either KPFC; Spp1 wt/wt; Grem1wt/wt, KPFC; Spp1Δ/Δ; Grem1fl/fl organoids and subsequently treated with either vehicle or with 4-OH Tamoxifen as noted in the figure (n = 3 biological replicates); all data was normalised against the KPFC; Spp1 wt/wt; Grem1wt/wt group. c, Image of the subcutaneous tumours generated by the implantation of KPFC; Spp1Δ/Δ; Grem1fl/fl organoids into nude mice and subsequently treated with either vehicle or with Tamoxifen as noted in the figure. Scale bar length is 1 cm. d, Quantification of the subcutaneous tumours detailed in (c) (n = 5 mice). e, Immunofluorescence staining for GFP (gray), Krt19 (green), and Vimentin (red) of KPFC; Spp1Δ/Δ; Grem1fl/fl & GFP (treated with vehicle) and KPFC; Spp1Δ/Δ; Grem1fl/fl & GFP (treated with Tamoxifen) subcutaneous tumours. Scale bar is 1 mm. f, Comparison of the proportions of epithelial cancer cell subpopulations between the tumours detailed in (e) (n = 5 mice). g, Comparison of the proportions of mesenchymal cancer cell subpopulations the tumours detailed in (e). h, Immunohistochemistry for H&E from subcutaneous tumours formed by KPFC; Spp1Δ/Δ; Grem1fl/fl &GFP treated with vehicle or Tamoxifen. n = 3 mice. Scale bar: 1 mm. For b, d, f and g data are mean ± s.e.m. P values were calculated using two-sided t-tests. The diagram in a was created in BioRender. Ruiz, J. (2025) (https://biorender.com/2uaw5xd).
Extended Data Fig. 11 Grem1 overexpression reverses the mesenchymal cell state established by initial Spp1 overexpression.
a, Scheme depicting the experimental approach for establishing tumours from Spp1 overexpression and doxycycline-inducible Grem1 overexpression organoids. b, RT-qPCR analysis of the expression of epithelial markers Krt19, Cdh1, and Epcam, and mesenchymal markers Fn1, S100a4, and Vim in organoids from either KPF; Plv-GFP&pINDUCER-con or KPF; Plv-Spp1-GFP&pINDUCER-Grem1 mice treated with either vehicle or doxycycline as noted in the figure (n = 3 biological replicates). c, Image of the subcutaneous tumours formed by KPF; Plv-Spp1-GFP&pINDUCER-Grem1 organoids treated with either vehicle or Doxycycline as indicated. Scale bar length is 1 cm. d, Quantification of the subcutaneous tumours detailed in (c). e, Immunofluorescence staining for GFP (gray), Krt19 (green), and Vimentin (red) of the subcutaneous tumours formed by KPF; Plv-Spp1-GFP&pINDUCER-Grem1 organoids treated with either vehicle or Doxycycline as indicated (n = 5 mice). Scale bar is 1 mm. f, Comparison of the proportions of epithelial cancer cell subpopulations between of the subcutaneous tumours detailed in (e) (n = 5 mice). g, Comparison of the proportions of mesenchymal cancer cell subpopulations of the subcutaneous tumours detailed in (e) (n = 5 mice). h, Immunohistochemistry for H&E from subcutaneous tumours formed by KPF; Plv-Spp1-GFP&pINDUCER-Grem1 treated with vehicle or Doxycycline. n = 3 mice. Scale bar: 1 mm. For b, d, f and g data are mean ± s.e.m. P values were calculated using two-sided t-tests. The diagram in a was created in BioRender. Ruiz, J. (2025) (https://biorender.com/2uaw5xd).
Extended Data Fig. 12 Model for the mechanism of Spp1, Bmp2 and Grem1 in maintaining cellular heterogeneity in pancreatic cancer.
Schematic cartoon depicting the mechanism for the paracrine interaction between epithelial and mesenchymal cancer cells. This diagram was created in BioRender. Ruiz, J. (2025) (https://biorender.com/e3c38ka).
Supplementary information
Supplementary Figures
Supplementary Fig. 1. Uncropped raw images for western blots. For Extended Data Fig. 1h, anti-SPP1 and β-actin were run on the same gel. For Extended Data Fig. 3b, anti-SPP1 and β-actin were run on the same gel. Supplementary Fig. 2. Gating strategies for flow cytometry analysis. Gating strategies for flow cytometry analysis of Extended Data Fig. 4a,c,g–n.
Supplementary Table 1
Clinicopathological characteristics of patients with pancreatic cancer. Information includes the patient’s sex, age, histology, differentiation, TNM stage and AJCC (8th edn) staging.
Supplementary Table 2
A detailed list of antibodies used in the study. Included are details of the primary antibodies, the fluorophore-conjugated primary antibodies and secondary antibodies used.
Supplementary Table 3
Sequences of gRNAs used in the study. Included are the sequences of sgSpp1-1, sgSpp1-2, sgSPP1 -1, sgSPP1-2, sgCd61-1 and sgCd61-2.
Supplementary Table 4
Sequences of RT–qPCR primers used in the study. Included are the target sequences of forward (5′ to 3′) and reverse (5′ to 3′) primers.
Supplementary Table 5
Sequences of ChIP–qPCR primers used in the study. Included are the target sequences of forward (5′ to 3′) and reverse (5′ to 3′) primer.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Lan, L., Chen, H. et al. SPP1 is required for maintaining mesenchymal cell fate in pancreatic cancer. Nature (2025). https://doi.org/10.1038/s41586-025-09574-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-025-09574-y