Abstract
Hippocampal plasticity has an undisputed role in learning and memory. Despite decades of research focusing on the neurobiological basis of synaptic plasticity, relatively little is known about the metabolic dynamics leading to hippocampal plasticity at the single-cell level. Here we used single-cell mass spectrometry to dissect metabolomic changes of excitatory pyramidal neurons (PNs), inhibitory interneurons and astrocytes in hippocampus during long-term potentiation (LTP) and learning-related behaviors in mice. We identified an enhancement of the γ-aminobutyric acid (GABA) biosynthetic pathway in CA1 PNs during LTP. This LTP-sensitive GABA metabolic pathway was mediated through an aldehyde dehydrogenase 2 (ALDH2)-dependent mechanism. Selective deletion of PN ALDH2 blocked the LTP-related GABA enhancement and impaired learning adaptability. Thus, profiling of the single-cell metabolome is established to characterize an activity-dependent GABA pathway and its impact on hippocampal plasticity and learning. This GABA signaling pathway identified in glutamatergic neurons represents a novel target for learning and memory.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The metabolomic data generated in this study were deposited in the MassIVE database under accession code MSV000097917. Metabolites were identified by matching the accurate mass with data from the HMDB (https://hmdb.ca/). The metabolomic data are available under restricted access as the data are still being investigated. Access can be obtained by sending a request explaining the aim and use of the data to the corresponding author by email. Figure parts created with BioRender.com were made publicly available under a publication license and can be accessed using the following links: https://BioRender.com/tb0d1gy (Graphical Abstract), https://BioRender.com/xrqt8py (Fig. 1a,b), https://BioRender.com/giyunq9 (Fig. 3g), https://BioRender.com/0qfivgm (Fig. 5a,g), https://BioRender.com/2y90zzc (Fig. 6a,g), https://BioRender.com/4x7jowj (Extended Data Fig. 4e) and https://BioRender.com/6vff3e9 (Extended Data Fig. 7e). Source data are provided with this paper.
Code availability
The custom codes used in this study were uploaded to GitHub (https://github.com/qiustc/PUBLIC). Codes are being used in the further development of analytic methods in single-cell metabolomics. Therefore, the codes are under restricted access. The codes can be provided by the corresponding author upon request explaining the aim and use of the codes.
References
Kim, J. J. & Diamond, D. M. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 3, 453–462 (2002).
Lee, Y. S. & Silva, A. J. The molecular and cellular biology of enhanced cognition. Nat. Rev. Neurosci. 10, 126–140 (2009).
Malenka, R. C. The long-term potential of LTP. Nat. Rev. Neurosci. 4, 923–926 (2003).
Nicoll, R. A. A brief history of long-term potentiation. Neuron 93, 281–290 (2017).
Fuchsberger, T. & Paulsen, O. Modulation of hippocampal plasticity in learning and memory. Curr. Opin. Neurobiol. 75, 102558 (2022).
Henley, J. M. & Wilkinson, K. A. Synaptic AMPA receptor composition in development, plasticity and disease. Nat. Rev. Neurosci. 17, 337–350 (2016).
Adams, J. P. & Dudek, S. M. Late-phase long-term potentiation: getting to the nucleus. Nat. Rev. Neurosci. 6, 737–743 (2005).
Sweatt, J. D. Toward a molecular explanation for long-term potentiation. Learn Mem. 6, 399–416 (1999).
Granger, A. J. & Nicoll, R. A. Expression mechanisms underlying long-term potentiation: a postsynaptic view, 10 years on. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130136 (2014).
Baltaci, S. B., Mogulkoc, R. & Baltaci, A. K. Molecular mechanisms of early and late LTP. Neurochem. Res. 44, 281–296 (2019).
Koh, W., Kwak, H., Cheong, E. & Lee, C. J. GABA tone regulation and its cognitive functions in the brain. Nat. Rev. Neurosci. 24, 523–539 (2023).
Topolnik, L. & Tamboli, S. The role of inhibitory circuits in hippocampal memory processing. Nat. Rev. Neurosci. 23, 476–492 (2022).
Ma, H. et al. Excitation–transcription coupling, neuronal gene expression and synaptic plasticity. Nat. Rev. Neurosci. 24, 672–692 (2023).
Ju, Y. H. et al. Astrocytic urea cycle detoxifies Aβ-derived ammonia while impairing memory in Alzheimer’s disease. Cell Metab. 34, 1104–1120 e1108 (2022).
Park, J. H. et al. Newly developed reversible MAO-B inhibitor circumvents the shortcomings of irreversible inhibitors in Alzheimer’s disease. Sci. Adv. 5, eaav0316 (2019).
Dembitskaya, Y., Wu, Y. W. & Semyanov, A. Tonic GABAA conductance favors spike-timing-dependent over theta-burst-induced long-term potentiation in the hippocampus. J. Neurosci. 40, 4266–4276 (2020).
Groen, M. R. et al. Development of dendritic tonic GABAergic inhibition regulates excitability and plasticity in CA1 pyramidal neurons. J. Neurophysiol. 112, 287–299 (2014).
Jo, S. et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 20, 886–896 (2014).
Erlander, M. G., Tillakaratne, N. J., Feldblum, S., Patel, N. & Tobin, A. J. Two genes encode distinct glutamate decarboxylases. Neuron 7, 91–100 (1991).
Grone, B. P. & Maruska, K. P. Three distinct glutamate decarboxylase genes in vertebrates. Sci. Rep. 6, 30507 (2016).
Kim, J. I. et al. Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science 350, 102–106 (2015).
Wu, J. et al. Distinct connectivity and functionality of aldehyde dehydrogenase 1a1-positive nigrostriatal dopaminergic neurons in motor learning. Cell Rep. 28, 1167–1181 (2019).
Li, X. et al. Synaptic dysfunction of Aldh1a1 neurons in the ventral tegmental area causes impulsive behaviors. Mol. Neurodegener. 16, 73 (2021).
Jin, S. et al. Brain ethanol metabolism by astrocytic ALDH2 drives the behavioural effects of ethanol intoxication. Nat. Metab. 3, 337–351 (2021).
Kwak, H. et al. Astrocytes control sensory acuity via tonic inhibition in the thalamus. Neuron 108, 691–706 (2020).
Yoon, B. E. et al. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J. Physiol. 592, 4951–4968 (2014).
Le Douce, J. et al. Impairment of glycolysis-derived l-serine production in astrocytes contributes to cognitive deficits in Alzheimer’s disease. Cell Metab. 31, 503–517 (2020).
Owen, L. & Sunram-Lea, S. I. Metabolic agents that enhance ATP can improve cognitive functioning: a review of the evidence for glucose, oxygen, pyruvate, creatine, and l-carnitine. Nutrients 3, 735–755 (2011).
Izumi, Y., Izumi, M., Matsukawa, M., Funatsu, M. & Zorumski, C. F. Ammonia-mediated LTP inhibition: effects of NMDA receptor antagonists and l-carnitine. Neurobiol. Dis. 20, 615–624 (2005).
Sartini, S. et al. Maternal creatine supplementation affects the morpho-functional development of hippocampal neurons in rat offspring. Neuroscience 312, 120–129 (2016).
Zeisel, A. et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015).
Zhong, S. et al. Decoding the development of the human hippocampus. Nature 577, 531–536 (2020).
Joglekar, A. et al. A spatially resolved brain region- and cell type-specific isoform atlas of the postnatal mouse brain. Nat. Commun. 12, 463 (2021).
Chen, P. B. et al. Mapping gene expression in excitatory neurons during hippocampal late-phase long-term potentiation. Front. Mol. Neurosci. 10, 39 (2017).
Bliim, N. et al. Early transcriptome changes in response to chemical long-term potentiation induced via activation of synaptic NMDA receptors in mouse hippocampal neurons. Genomics 111, 1676–1686 (2019).
Maag, J. L. et al. Dynamic expression of long noncoding RNAs and repeat elements in synaptic plasticity. Front. Neurosci. 9, 351 (2015).
Zhu, H. et al. Single-neuron identification of chemical constituents, physiological changes, and metabolism using mass spectrometry. Proc. Natl Acad. Sci. USA 114, 2586–2591 (2017).
Zhu, H. et al. Moderate UV exposure enhances learning and memory by promoting a novel glutamate biosynthetic pathway in the brain. Cell 173, 1716–1727 (2018).
Zhu, H. et al. Metabolomic profiling of single enlarged lysosomes. Nat. Methods 18, 788–798 (2021).
Larson, J. & Lynch, G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science 232, 985–988 (1986).
Larson, J. & Lynch, G. Role of N-methyl-d-aspartate receptors in the induction of synaptic potentiation by burst stimulation patterned after the hippocampal θ-rhythm. Brain Res. 441, 111–118 (1988).
Larson, J. & Munkacsy, E. Theta-burst LTP. Brain Res. 1621, 38–50 (2015).
Yao, H. et al. Label-free mass cytometry for unveiling cellular metabolic heterogeneity. Anal. Chem. 91, 9777–9783 (2019).
Lisman, J., Yasuda, R. & Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13, 169–182 (2012).
Nicoll, R. A. & Malenka, R. C. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann. N. Y. Acad. Sci. 868, 515–525 (1999).
Fenalti, G. et al. GABA production by glutamic acid decarboxylase is regulated by a dynamic catalytic loop. Nat. Struct. Mol. Biol. 14, 280–286 (2007).
Lee, M., Schwab, C. & Mcgeer, P. L. Astrocytes are GABAergic cells that modulate microglial activity. Glia 59, 152–165 (2011).
Bourgognon, J. M. et al. Alterations in neuronal metabolism contribute to the pathogenesis of prion disease. Cell Death Differ. 25, 1408–1425 (2018).
Sugahara, M. 4-Aminobutyraldehyde as a precursor convertible to γ-aminobutyric acid in vivo. J. Biochem. 93, 1337–1342 (1983).
Vullhorst, D. et al. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J. Neurosci. 29, 12255–12264 (2009).
Ruan, Z. et al. Alzheimer’s disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain 144, 288–309 (2021).
Fan, Y. et al. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in Ih. Nat. Neurosci. 8, 1542–1551 (2005).
Narayanan, R. & Johnston, D. Long-term potentiation in rat hippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron 56, 1061–1075 (2007).
Brager, D. H., Akhavan, A. R. & Johnston, D. Impaired dendritic expression and plasticity of h-channels in the fmr1−/y mouse model of fragile X syndrome. Cell Rep. 1, 225–233 (2012).
Shin, D. S. & Carlen, P. L. Enhanced Ih depresses rat entopeduncular nucleus neuronal activity from high-frequency stimulation or raised Ke+. J. Neurophysiol. 99, 2203–2219 (2008).
Morgan, P. J., Bourboulou, R., Filippi, C., Koenig-Gambini, J. & Epsztein, J.Kv1.1 contributes to a rapid homeostatic plasticity of intrinsic excitability in CA1 pyramidal neurons in vivo. eLife 8, e49915 (2019).
Campanac, E., Daoudal, G., Ankri, N. & Debanne, D. Downregulation of dendritic Ih in CA1 pyramidal neurons after LTP. J. Neurosci. 28, 8635–8643 (2008).
Rudy, J. W., Huff, N. C. & Matus-Amat, P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci. Biobehav. Rev. 28, 675–685 (2004).
Hsu, Y. T., Li, J., Wu, D., Sudhof, T. C. & Chen, L. Synaptic retinoic acid receptor signaling mediates mTOR-dependent metaplasticity that controls hippocampal learning. Proc. Natl Acad. Sci. USA 116, 7113–7122 (2019).
Mariottini, C. et al. Wilm’s tumor 1 promotes memory flexibility. Nat. Commun. 10, 3756 (2019).
Yang, K. et al. SENP1 in the retrosplenial agranular cortex regulates core autistic-like symptoms in mice. Cell Rep. 37, 109939 (2021).
Tzilivaki, A. et al. Hippocampal GABAergic interneurons and memory. Neuron 111, 3154–3175 (2023).
Wang, P. et al. Neuronal γ-aminobutyric acid (GABA) type A receptors undergo cognate ligand chaperoning in the endoplasmic reticulum by endogenous GABA. Front. Cell Neurosci. 9, 188 (2015).
Eshaq, R. S. et al. GABA acts as a ligand chaperone in the early secretory pathway to promote cell surface expression of GABAA receptors. Brain Res. 1346, 1–13 (2010).
Brosnan, J. T. & Brosnan, M. E. Glutamate: a truly functional amino acid. Amino Acids 45, 413–418 (2013).
Walker, M. C. & van der Donk, W. A. The many roles of glutamate in metabolism. J. Ind. Microbiol. Biotechnol. 43, 419–430 (2016).
Ju, Y. H. et al. Astrocytic urea cycle detoxifies Aβ-derived ammonia while impairing memory in Alzheimer’s disease. Cell Metab. 34, 1104–1120 (2022).
Xie, N. et al. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target Ther. 5, 227 (2020).
Nguyen, T. et al. Uncovering the role of N-acetyl-aspartyl-glutamate as a glutamate reservoir in cancer. Cell Rep. 27, 491–501 (2019).
Yang, C. et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol. Cell 56, 414–424 (2014).
Gisabella, B., Scammell, T., Bandaru, S. S. & Saper, C. B. Regulation of hippocampal dendritic spines following sleep deprivation. J. Comp. Neurol. 528, 380–388 (2020).
Wozny, C. et al. VGLUT2 functions as a differential marker for hippocampal output neurons. Front. Cell Neurosci. 12, 337 (2018).
Billwiller, F. et al. GABA–glutamate supramammillary neurons control θ and γ oscillations in the dentate gyrus during paradoxical (REM) sleep. Brain Struct. Funct. 225, 2643–2668 (2020).
Yin, L. et al. Selective activation of cholinergic neurotransmission from the medial septal nucleus to hippocampal pyramidal neurones improves sepsis-induced cognitive deficits in mice. Br. J. Anaesth. 130, 573–584 (2023).
Goenawan, I. H., Bryan, K. & Lynn, D. J. DyNet: visualization and analysis of dynamic molecular interaction networks. Bioinformatics 32, 2713–2715 (2016).
Acknowledgements
We express our gratitude to T. Pan for synthesizing [15N]ABAL. We acknowledge support from National Key R&D Program of China (2021YFA0804900 to W.X. and H.Z. and 2020YFA0112203 to W.X.), National Natural Science Foundation of China (32430045, 32225020, 92049304 and 32121002 to W.X.; 32322033 and 32471080 to H.Z.), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB39050000 to W.X.), Key Research Program of Frontier Science (ZDBS-LY-SM002 to W.X.), Chinese Academy of Sciences (CAS) Interdisciplinary Innovation Team (JCTD-2018-20 to W.X.), Youth Innovation Promotion Association CAS, Fundamental Research Funds for the Central Universities, USTC Research Funds of the Double First-Class Initiative (YD9100002001 to W.X. and YD9100002005 to H.Z.), CAS Project for Young Scientists in Basic Research (YSBR-013 to W.X.), CAS Collaborative Innovation Program of Hefei Science Center (2021HSC-CIP003 to W.X.), University Synergy Innovation Program of Anhui Province (GXXT-2022-033 to H.Z.), Research Funds of Center for Advanced Interdisciplinary Science and Biomedicine of IHM (QYZD20230011 to W.X.) and Major Science and Technology Special Projects, Anhui Provincial Department of Science and Technology (202303a07020005 to W.X.).
Author information
Authors and Affiliations
Contributions
W.X. initialized, designed and supervised the study with the assistance of H.Z. Q.C. and H.Z. conducted the single-cell MS with assistance from M.C., L.Y. and S.G. Q.C. and C.M. conducted the electrophysiological recordings and immunohistochemistry, with assistance from S.W. in the latter. Q.C. and Y.S. conducted the stereotaxic surgery and virus injection. Q.C. and W.Q. conducted the animal behavioral tests. Q.C., W.L., T.L., M.Y. and H.Z. analyzed the data. Q.C., L.Z. and W.X. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Hui-Quan Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Single-cell metabolomics analysis of metabolites during LTP in single hippocampal cells.
a, Representative confocal images of PNs labeled by tdT (red) and immunostained for DAPI (blue) in the CA1 of Vglut2-Cre;Ai9 mice. Interneurons labeled by GAD67 (green) and immunostained for DAPI (blue) in the CA1 of GAD1-EGFP mice. Astrocytes labeled by SR101 (red) and immunostained for DAPI (blue) in the CA1. Scale bars, 20 µm. b, The metabolites detected in the mass spectrum of the blank sample (bottom) or PN sample (top). The expanded views were mass spectra of ABAL (I), GABA (II), Aspartate (III), Glutamine (IV), Arginine (V) and NAA (VI), which were either notably absent or markedly low. c, Heatmaps of characteristic metabolites and their relative intensities in single PNs with baseline stimulation or TBS. Color indicates z scores of metabolites in each sample (n = 4 mice). d-f, Volcano plot showing correlations between P values and relative intensities (FCs) of all detected metabolites with LTP compared to baseline stimulation (Ctrl) in vehicle-treated PNs (n = 6 mice) (d), MK801-treated PNs (e), and KN93-treated PNs (f). g, h, Heatmaps of characteristic metabolites and their relative intensities in single interneurons (g) or astrocytes (h) with baseline stimulation or TBS. Color indicates z scores of metabolites in each sample. Red, upregulated metabolites; Blue, downregulated metabolites. Unless otherwise stated, data were collected from 3 mice.
Extended Data Fig. 2 Single-cell metabolomics analysis confirms the structure of iGABA and its related metabolites in single hippocampal PNs.
a-h, MS/MS spectra for GABA (a), ABAL (b), Glutamine (c), Glutamate (d), Proline (e), Aspartate (f), Arginine (g) and NAA (h) from single hippocampal PNs (top) and standards (bottom). Higher-energy collisional dissociation (HCD) at 35% energy. i, GABA calibration curve constructed by plotting MS intensity/Total Ion Chromatography (TIC) against the concentration of the GABA standard. The area shows the range of intracellular GABA concentrations in single CA1 PNs (cyan) or interneurons (red). j, The concentrations of GABA intensity in CA1 PNs or interneurons (PNs, n = 14 neurons; Interneurons, n = 17 neurons). Neurons pooled from 3 mice. Data are represented as mean ± SEM. Statistics were calculated by unpaired two-tailed t test (j). ****P < 0.0001.
Extended Data Fig. 3 The identification of glutamate-GABA pathway in CA1 neurons.
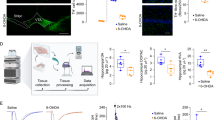
a, Representative confocal microscopy images of immunostaining for GAD65 (cyan) and DAPI (blue) fluorescence in the CA1 of Vglut2-Cre;Ai9 mice. The CA1 PNs were labeled with tdT (red). Images are representative of three independent experiments. Scale bar, 20 μm. b, c, Representative MS spectra for 15N-GABA and normalized intensities (c) of intracellular 15N-GABA with preincubation of 15N-glutamate in CA1 interneurons and PNs (Interneurons, n = 29; PNs, n = 22). Data were normalized to values from interneurons. d-f, Normalized intracellular concentrations of iGABA (d), iGlutamate (e), and iABAL (f) in single CA1 PNs under 15N-glutamate treatment (n = 29 Ctrl, 34 LTP) versus untreated controls (n = 28 Ctrl, 33 LTP). Values were normalized to their respective untreated control groups. g, h, Representative MS spectra (g) and normalized intensities (h) of iGABA in single CA1 interneurons treated with vehicle or 3-MPA (Vehicle, n = 36; 3-MPA, n = 31). Data were normalized to values from the vehicle group. Unless otherwise stated, each dot represents one neuron and all neurons measured from 3 mice. Data are represented as mean ± SEM. Statistics were calculated by unpaired two-tailed t-test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 4 The effects of inhibitor against ALDH1a1 or shRNA against ALDH2 on LTP-induced GABA increase.
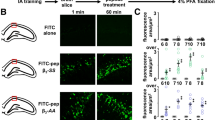
a, The ALDH-mediated GABA biosynthetic pathway and inhibitors. b, Representative confocal microscopy images of immunostaining for ALDH1a1 (cyan) and DAPI (blue) fluorescence in the CA1 of Vglut2-Cre;Ai9 mice. Images are representative of three independent experiments. Scale bar, 20 μm. c, Normalized iGABA intensities in CA1 PNs with baseline stimulation or TBS (Vehicle: n = 29 Ctrl, 29 LTP; CM37: n = 26 Ctrl, 29 LTP). The PNs were treated with vehicle or CM37 (10 µM). Data normalized to Vehicle-Ctrl group. d, The LTP induced by TBS with 10 µM CM37 absent or present in the internal solution (Vehicle, n = 5 neurons; CM37, n = 6 neurons). Top, representative electrophysiological traces at the indicated time. e, Schematic illustration depicting viral shALDH2 constructs (ALDH2* indicates an shRNA-resistant ALDH2) and experimental configuration. Panel e is created with BioRender.com. f, Quantitative analysis of ALDH2 mRNA by RT-qPCR from CA1 after injection of AAV virus (n = 4 mice for each group). g, Confocal images depicting the infection of virus (EGFP) and ALDH2 (red) in CA1. Images are representative of three independent experiments. Scale bars, 50 μm. h, Normalized iGABA intensities of in CA1 PNs with baseline stimulation or TBS. The PNs were from Vglut2-Cre mice receiving intra-CA1 injection of AAV-DIO-scramble (shScr), AAV-DIO-shALDH2 (shALDH2) or AAV-DIO-shALDH2-ALDH2* (shALDH2 Rescue) (n values from left to right = 37, 34, 30 and 30). Data were normalized to values from the shScr-Ctrl group. i, Normalized 15N-GABA intensities in CA1 PNs with baseline stimulation or TBS. The PNs were incubated with 15N-ABAL (n values from left to right = 32, 35, 33 and 26) (n values from left to right = 32, 35, 33 and 26). Data normalized to shScr-Ctrl group. Unless otherwise stated, data were collected from 3 mice. Data are represented as mean ± SEM. Statistics were calculated by two-way ANOVA (c), one-way ANOVA with Tukey’s multiple comparison post hoc test (h) or unpaired two-tailed t-test (f, i). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns, not significant.
Extended Data Fig. 5 Pharmacologically or genetically inhibition of ALDH2 on basal excitatory or inhibitory transmission.
a, eEPSCs in pyramidal neurons were evoked at holding potential of -70 mV by the paired stimulation (Ctrl: n = 9 neurons from 3 mice; Cyanamide: n = 9 neurons from 3 mice; shALDH2: n = 8 neurons from 3 mice). b, Input-output curves measured of eEPSCs in hippocampal SC-CA1 synapses (Ctrl: n = 7 neurons from 3 mice; Cyanamide: n = 6 neurons from 3 mice; shALDH2: n = 6 neurons from 3 mice). c, Trace records of the sEPSC under different conditions. d, f, The quantitative frequency (d) or amplitude (f) of sEPSCs from CA1 PNs. e, g, Cumulative probability plot of sEPSCs frequency (e) or sEPSCs amplitude (g) (Ctrl: n = 18 neurons from 3 mice; Cyanamide: n = 20 neurons from 3 mice; shALDH2: n = 18 neurons from 3 mice). Statistical significance indicated as ‘ns’ for Ctrl vs. Cyanamide, and ‘NS’ for the comparison between Ctrl and shALDH2. h, Trace records of the sIPSC under different conditions. i, k, The quantitative frequency (i) or amplitude (k) of sIPSCs from CA1 PNs. j, l, Cumulative probability plot of sIPSCs frequency (j) or sIPSCs amplitude (l) (Ctrl: n = 20 neurons from 3 mice; Cyanamide: n = 17 neurons from 3 mice; shALDH2: n = 18 neurons from 3 mice). Statistical significance indicated as ‘*’ or ‘ns’ for Ctrl vs. Cyanamide, and ‘#’ or ‘NS’ for Ctrl vs. shALDH2. Data are represented as mean ± SEM. Statistics were calculated by one-way ANOVA with Tukey’s multiple comparison post hoc test (d, f, i, k) or Kolmogorov-Smirnov test (e, g, j, l). **P < 0.01, ****P < 0.0001. ns, not significant.
Extended Data Fig. 6 Role of ALDH2-mediated increase in iGABA in modulating mIPSC of PNs.
a, The quantitative frequency of GABA mIPSCs from CA1 PNs. b, c, Cumulative probability plot of mIPSCs frequency from CA1 PNs in Vglut2-Cre mice with intra-CA1 injection of AAV-DIO-scramble (shScr) (b) or AAV-DIO-shALDH2 (shALDH2) (c) (shScr: n = 24, 25 and 23; shALDH2: n = 23, 25 and 22). Statistical significance indicated as ‘*’ for Ctrl vs. GABA intra-pipette delivery, and ‘#’ for Ctrl vs. LTP. Each dot represents one neuron and all neurons measured from 4 mice. d, The quantitative amplitude of GABA mIPSCs from CA1 PNs in the presence of NNC-711 (Ctrl: n = 18 neurons from 3 mice; n = 17 neurons from 4 mice for other groups). e, Cumulative probability plot of mIPSCs amplitude from CA1 PNs in the presence of NNC-711. Statistical significance indicated in blue for the comparison between the Ctrl and GABA intra-pipette comparison, and red for the comparison between Ctrl and LTP comparisons. f, The quantitative AP threshold with different treatment (shScr: n = 17, 20 and 20; shALDH2: n = 21, 18 and 21). Each dot represents one neuron and all neurons measured from 3 mice. g, h, The trace (g) and spike number (h) of the CA1 PNs with different treatment in the presence of 20 µM Bicuculline (Ctrl, n = 20 neurons; GABA intra-pipette, n = 21 neurons; LTP, n = 21 neurons). All neurons measured from 3 mice. Data are represented as mean ± SEM. Statistics were calculated by two-way (a, f), Kolmogorov-Smirnov test (b, c, e), one-way ANOVA (d, h) with Tukey’s multiple comparison post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns, not significant.
Extended Data Fig. 7 GABA-related KEGG pathways and effects of ALDH2 knockdown on the learning-related behaviors.
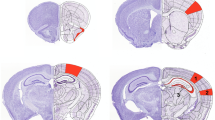
a, b, The top 10 metabolic pathways related to upregulated metabolites enriched by KEGG. The pathways directly involving GABA biosynthesis were marked in red. Metabolites in b marked in color represent those upregulated after CFC, while those marked with asterisk (*) indicate metabolites that showed no significant change, others were not detected or SNR < 3. c, Representative low-magnification coronal brain sections depicting viral coverage in both sides of CA1 for each behavioral test. Scale bar, 500 μm. d, The freezing time of shScr or shALDH2 group of mice in the first knowledge, CFC test (shScr, n = 9 mice; shALDH2, n = 8 mice). e, Schematic illustration of the novel object location (NOL) task. Panel e is created with BioRender.com. f, Exploration behavior of shScr or shALDH2 group mice towards objects in familiar and novel locations during the NOL test 2-hour post-training (shScr, n = 8 mice; shALDH2, n = 8 mice). g, Exploration behavior of shScr or shALDH2 group mice towards objects in familiar and novel locations during the NOL test 24-hour post-training (shScr, n = 8 mice; shALDH2, n = 10 mice). Data are represented as mean ± SEM. Statistics were calculated by two-sided Student’s t-tests. *P < 0.05, **P < 0.01. ns, not significant.
Extended Data Fig. 8 Effects of ALDH2 knockdown on the Barnes maze.
a, The top 10 metabolic pathways related to upregulated metabolites enriched by KEGG. The pathways directly involving GABA biosynthesis were marked in red. b, The learning curves during the 8 + 8 sessions of learning trials and reversal trials in Barnes maze (shScr, n = 17 mice; shALDH2, n = 17 mice). c-e, The latency time to locate the target hole (c) and time spent in the Target quadrant (T), Left quadrant (L), Opposite quadrant (O), Right quadrant (R) during the BM probe test (d) or during the BM reversal test (e) (shScr, n = 17 mice; shALDH2, n = 17 mice). Data are represented as mean ± SEM. Statistics were calculated by two-way ANOVA with Šídák’s multiple comparison post hoc test (d, e) or unpaired two-tailed t-test (c). ****P < 0.0001. ns, not significant.
Extended Data Fig. 9 Comparative analysis of enriched KEGG pathways during LTP and behavioral learning paradigms.
a, The metabolic pathways associated with upregulated metabolites enriched by KEGG in LTP and behavioral learning paradigms, with the pathways directly involved in GABA biosynthesis highlighted in red. b, A Venn diagram illustrating the overlap of KEGG pathways identified during LTP, BM, and CFC training. c, Shared KEGG pathways between LTP and learning processes. d, Differential KEGG pathways between LTP and CFC. e, Differential KEGG pathways between LTP and BM.
Supplementary information
Supplementary Information
Supplementary Figs 1–3 and Supplementary Tables 1–3.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Q., Miao, C., Ge, S. et al. ALDH2-mediated GABA biosynthesis regulates hippocampal LTP and learning adaptability. Nat Chem Biol (2025). https://doi.org/10.1038/s41589-025-01984-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41589-025-01984-3