Abstract
The present vaccine against influenza virus has the inevitable risk of antigenic discordance between the vaccine and the circulating strains, which diminishes vaccine efficacy. This necessitates new approaches that provide broader protection against influenza. Here we designed a vaccine using the hypervariable receptor-binding domain (RBD) of viral hemagglutinin displayed on a nanoparticle (np) able to elicit antibody responses that neutralize H1N1 influenza viruses spanning over 90 years. Co-display of RBDs from multiple strains across time, so that the adjacent RBDs are heterotypic, provides an avidity advantage to cross-reactive B cells. Immunization with the mosaic RBD–np elicited broader antibody responses than those induced by an admixture of nanoparticles encompassing the same set of RBDs as separate homotypic arrays. Furthermore, we identified a broadly neutralizing monoclonal antibody in a mouse immunized with mosaic RBD–np. The mosaic antigen array signifies a unique approach that subverts monotypic immunodominance and allows otherwise subdominant cross-reactive B cell responses to emerge.
Similar content being viewed by others
Main
Influenza A viruses have circulated among avian and mammalian species, including humans, for over 500 years1. As a result of prolonged circulation, the viruses evolve unique antigenic traits that diversify under selective pressure2,3,4,5. Humoral immune responses to the viral hemagglutinin are the primary driving force of the selective evolution of influenza virus (that is, antigenic drift), illustrating the importance of hemagglutinin as a vulnerable target for protective antibody responses. The existence of broadly neutralizing antibodies to influenza viruses6 demonstrates the possibility of generating universal influenza immunity in humans either through natural infections7 and/or by active immunizations8,9,10,11,12. There are two structurally defined antigenic supersites on the hemagglutinin molecule that are targeted by broadly neutralizing antibodies. One is the receptor-binding site (RBS) inside the globular head region, which binds to sialic acid moieties on host cell-surface glycoproteins and glycolipids13,14,15,16. The other is a site on the hemagglutinin stem centered at the hydrophobic groove surrounding Trp21HA2 (refs. 17,18,19,20). There are other neutralization-sensitive antigenic sites on hemagglutinin that are conserved within subtypes but not between subtypes21,22,23. These sites may represent alternative vaccine targets, as the antibodies targeting those sites are often less strain dependent than are RBS-directed antibodies and have higher potency than that of stem-directed antibodies. Despite immense efforts to develop candidate universal influenza vaccines that elicit broadly neutralizing antibody responses to any of the aforementioned viral sites of vulnerability, this goal has not been achieved. Although induction of antibody-mediated heterosubtypic protective immunity to lethal challenge with influenza virus in animal models has been shown, it has not been associated with appreciable neutralizing activity24,25,26,27.
During antigen exposure from infection or vaccination, antigen-specific B cells are stimulated and undergo a process called ‘somatic hypermutation’ (SHM) to fine-tune the affinity and specificity of their B cell antigen receptors (BCRs) through germinal center reactions28. This specialization process of B cells is essential for the development of high-affinity BCRs and eventually generates highly neutralizing antibody responses. However, over-specialization of the immunodominant B cells with limited breadth against influenza viruses may impair or delay the emergence of B cells targeting conserved antigenic supersites. Since elicitation of cross-reactive B cell responses to antigenically hypervariable targets is of great interest for the development of vaccines against rapidly evolving viruses such as influenza virus, hepatitis C virus or human immunodeficiency virus type 1 (refs. 29,30), reshaping the intrinsic hierarchy of immunodominance is of critical importance for vaccine design.
Here, we developed a mosaic array by co-localizing heterotypic influenza hemagglutinin antigens on a single nanoparticle to diminish or avoid activation of strain-specific B cells and allow selective engagement of B cells that tolerate antigenic variability. This would promote cross-reactive antibody responses by adaptively targeting conserved antigenic surfaces. The heterotypic mosaic nanoparticle immunogen elicits B cell responses quantitatively and qualitatively superior to those elicited by antigenically homotypic immunogens, even when multiple specificities are admixed together. A monoclonal antibody isolated from a mouse immunized with the heterotypic mosaic nanoparticle possesses exceptional neutralization capacity directed against H1N1 viruses spanning over 90 years. Finally, the structural studies of this antibody define a site of vulnerability that should inform pan-subtypic influenza vaccine designs.
Results
Design and characterization of heterotypic influenza virus hemagglutinin mosaic RBD–np
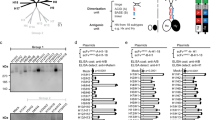
We theorized that the mosaic arrays of heterotypic antigens would reduce the likelihood of activating high-avidity B cells expressing a BCR with narrow specificity and increase opportunities for B cells expressing a cross-reactive BCR to be activated, hence altering the hierarchy of B cell response frequencies to favor the epitopes of interest. To empirically test the hypothesis, a modular self-assembling nanoparticle system based on the ferritin nanoparticle scaffold was developed25,31,32. This system allows us to manipulate homogeneity and heterogeneity of antigens displayed as an array on the assembled nanoparticle. H1N1 influenza virus hemagglutinin was chosen as a model antigen to evaluate the impact of antigenic heterogeneity on induction of cross-reactive antibodies because of the extensive genetic diversity2,33 and availability of reagents. Monomeric RBDs were expressed from a fusion construct linked to an engineered ferritin sequence31 (Fig. 1a). RBD–np expressed in transfected cells spontaneously self-assembles to form particles that are secreted into the culture supernatant. This system enables us to make homogeneously assembled RBD–np (building blocks with a single RBD sequence) as well as heterogeneously co-assembled mosaic RBD–np (building blocks with multiple RBD sequences) (Fig. 1a). To prove co-assembly of mosaic RBD–np, we used an antigenically distinct pair of hemagglutinin RBDs from two H1N1 strains, A/New Caledonia/20/99 (NC99) and A/California/04/09 (CA09), and generated three RBD–np preparations (homotypic NC99 or CA09 or co-assembled heterotypic mosaic particles) (Fig. 1b). The particle formation and antigenicity of these RBD–np preparations were confirmed by electron microscopy and immunoprecipitation using monoclonal antibodies specific to either NC99 hemagglutinin or CA09 hemagglutinin34. We found that the co-assembled mosaic RBD–np displayed a morphology analogous to that of homotypically assembled NC99 or CA09 RBD–np by negative-stain electron microscopy and possessed antigenicity of both NC99 and CA09 by immunoprecipitation, as expected (Fig. 1b,c). In addition, the co-assembled mosaic RBD–np had distinct biochemical characteristics, as evidenced by a unique profile on anion-exchange chromatography, indicating each individual particle contained both NC99 RBD components and CA09 RBD components (Supplementary Fig. 1a,b). Spacing of model antigens between 50 Å and 100 Å apart is known to be optimal for B cell activation35. Optimal distance between antigenic sites displayed in an orderly, symmetrical array strengthens the recognition of the vaccine antigen as a foreign pathogen-associated molecular pattern36. Therefore, placing antigens in a repetitive, symmetrical array with spacing of 50–100 Å was intended to maximize activation of cognate B cells via BCR cross-linking and/or BCR microclustering. To estimate the likelihood of identical antigens co-localizing adjacent to each other on a mosaic nanoparticle composed of multiple distinct specificities, we modeled the location of randomly distributed subunits graphically. A 24-meric RBD–np has 24 vertices, each of which is within 50–100 Å of five neighboring vertices (Fig. 1d,e). The RBD antigen placement on ferritin is a snub cubical geometry (Fig. 1e) with 60 edges and 38 faces and has a chromatic number of 3. Therefore, in theory, it is possible to create a perfectly heterogeneous mosaic particle with no adjacent homologous antigen pairs at the 24 vertices by co-assembling three different antigens (valency of 3) (Fig. 1f). However, the random assembly of antigens that comprise the particle allows adjacent homologous antigen pairs even at valencies of 3 or greater. Therefore, we calculated the likelihood of adjacent homologous antigen pairs forming at different valencies, making the assumption of random assortment. When two different RBDs co-assemble in a single particle, 30 ± 3.9 out of the 60 possible adjacent pairs are made of two identical RBDs (that is, A–A or B–B) and the remaining pairs are heterologous (that is, A–B) (Fig. 1g). By increasing the valency to 8, the likelihood of homologous antigen pairs is reduced to 7.5 ± 2.6 per particle, or about one eighth of RBD pairs (Fig. 1g), thus minimizing the chance for a single BCR to bivalently bind two neighboring homologous antigens or to form BCR microclusters, leading to B cell activation.
a, Design of hemagglutinin (HA) RBD–np. Alteration of residue 98 (Y98F) was made to abolish the sialic acid–binding property of hemagglutinin. Ag, antigen; SP, signal peptide; T/C, transmembrane/cytoplasmic domains. b, Negative-stain electron-microscopy images of self-assembled hemagglutinin RBD–np. RBD–np were made using either single building blocks (left and middle) or two different building blocks (right). Shown are representative images from one experiment. c, Antigenic characterization of RBD–np by immunoprecipitation (IP). The monoclonal antibodies 3u-u (anti-NC99), 2D1 (anti-CA09) and C179 (anti–hemagglutinin stem) were used to pull down NC99 RBD, CA09 RBD and hemagglutinin stem (control), respectively. Similar results were obtained from two independent experiments. d, Schematic model of ferritin-based nanoparticle. Twenty-four spatially dispersed antigens (colored individually) are displayed on the surface. Positions 2–6 are localized within 100 Å of position 1. e, Ferritin nanoparticle snub cube net with positions numbered similarly to the numbering in d. Connected lines indicate adjacent positions located within 50–100 Å. f, Twenty-four positions are colored according to the chromatic number (three) to avoid location of the a color within a 100-Å radius of the same color. g, Simulated likelihood of homologous antigen pairs made within a 100-Å radius on a single particle by using 2, 4, 6 or 8 different building blocks (valence).
Immune induction with heterotypic co-assembled mosaic RBD–np
To assess the impact of per-particle antigen heterogeneity on its immunogenicity, we created RBD–np from a selection of H1N1 strains with a range of valencies (Supplementary Fig. 1c–f). Mice were immunized with RBD–np presenting between one and eight different RBDs derived from various H1N1 strains either individually (that is, an admixture of up to eight homotypic particles) or together as a single mosaic particle (co-assembly using up to eight different RBDs) (Fig. 2a). The immunization dose was kept constant across different groups, thus decreasing the amount of each component as the valency increased. After two immunizations with adjuvant, hemagglutination inhibition (HAI) and neutralizing activity against homologous H1N1 NC99 virus were detected in nearly all mice, as NC99 RBD was a component of all immunogens (Fig. 2a). As expected, responses to NC99 progressively diminished as the NC99 antigen content was reduced in the admixed nanoparticle groups (Fig. 2a, left). The reduced antibody response could be explained by antigen dilution and/or antigenic competition between different RBDs. The effect of antigen dilution on antibody responses was not observed in groups immunized with mosaic nanoparticles regardless of antigen valency (Fig. 2a, right). Dose de-escalation of NC99 RBD in the mosaic nanoparticles did not diminish the response as observed in admixed nanoparticle–immunized groups. Although total NC99 antigen content decreases as valency increases, the number of particles containing NC99 RBDs should be relatively constant in the mosaic nanoparticle preparations. In contrast, the number of particles possessing NC99 RBDs decreases proportionally to the total antigen content in admixed preparations. The dilution effect on antibody response in mice immunized with admixed nanoparticles was also observed for other H1N1 strains (A/Brisbane/59/2007 (BR07) and CA09) (Supplementary Fig. 2a,b). We next assessed serum microneutralization titers generated in mice immunized with the Northern hemisphere formulation of 2006–2007, 2007–2008 or 2008–2009 trivalent inactivated influenza vaccine (TIV) and compared the levels to those of mice immunized with mosaic RBD–np (8-valent). All three TIV groups were able to elicit similar levels of neutralizing activity against A/Christchurch/1/2003 (CC03) virus, as the antigenic properties of this virus are similar to those of the vaccine viruses. In contrast, responses to antigenically dissimilar viruses were much lower than those of mice vaccinated with mosaic RBD–np (Supplementary Fig. 2c). These results indicate that the mosaic RBD–np is capable of eliciting qualitatively different responses with greater breadth than that of conventional influenza vaccines. To determine if prior exposure to influenza virus would affect the subsequent vaccine-induced response, mice were immunized with two distinct commercial inactivated influenza vaccines (IIVs) derived from different seasons to establish background influenza immunity and were subsequently immunized with mosaic RBD–np (8-valent). Before the mosaic RBD–np immunization, serum neutralizing antibody was detectable but had low-magnitude activity against antigenically mismatched viruses (for example, A/Wilson-Smith/1933 (WS33), A/Kawasaki/6/1986 (KW86) or A/Beijing/262/1995 (BJ95)). Neutralization activity against those viruses was substantially boosted after the mosaic RBD–np immunization, suggesting that the mosaic RBD–np was capable of eliciting broadly neutralizing antibody responses in the presence of pre-existing influenza immunity (Fig. 2b). Importantly, the enhancement was observed in differentially conditioned mice primed with different combinations of commercial vaccines, indicating that the individualized memory B cell repertoires established by prior influenza exposures did not adversely affect the subsequent response induced by the mosaic RBD–np. Collectively, the mosaic antigen array avoids antigenic competition, induces comparable immune responses with much lower antigen load and also effectively broadens the breadth of neutralizing antibody responses in mice with pre-existing immunity.
a, Antibody responses to homologous NC99 virus. Serum HAI and pseudotype neutralization (PN) IC50 titers were measured after two immunizations of indicated valences of either admixed or mosaic RBD–np with the Sigma adjuvant system. For HAI, cumulative data of three independent experiments (n = 15) are shown, except for the 8-valent admix group (two independent experiments, n = 10). For pseudotype neutralization, shown are representative data from one experiment (n = 5). Experiments were independently performed three times (two times for 8-valent admix group) with similar results. Lines indicate means. Statistical analyses were done with one-way ANOVA with Tukey’s multiple comparisons post-hoc test. Non-statistical significance between groups is not shown for clarity. b, Microneutralization (MN) IC50 titers against five different viruses in mice after priming with IIV and after boosting with mosaic RBD–np. Immune sera were collected at weeks 5 (prime) and 12 (boost). MN IC50 titers against each virus are plotted on five axes and are connected by a line (n = 10). MN IC50 across five H1N1 viruses is shown as geometric mean titer (GMT) ± geometric s.d. Statistical analyses were done with two-tailed paired t-test. Data are from one experiment. c, Schematic representation of different immunization modalities. d, Serum HAI titers against NC99 virus after two (NC99, admix and mosaic groups) or four (sequential) immunizations with adjuvant. Shown are representative data from one experiment (n = 10). Experiments were independently performed twice with similar results. Statistical analysis was done with one-way ANOVA with Tukey’s multiple comparison post-hoc test. e, MN IC50 titers against five H1N1 viruses. MN IC50 titers are plotted as in b. Shown are representative data from one experiment (n = 10). Experiments were independently performed twice with similar results. Statistical analysis was done with one-way ANOVA with Tukey’s multiple comparisons post-hoc test.
Differences in immune induction by immunization regimens
We next compared immune responses elicited by different immunization regimens: repeated immunizations (two immunizations of NC99 RBD–np alone, admixture (admix) of eight RBD–np, or mosaic nanoparticles made of eight different RBDs) or sequential immunizations (four immunizations with two unique homotypic RBD–np per immunization, equivalent to a total of eight different RBD–np) (Fig. 2c). We determined HAI antibody titers against NC99 virus after immunization and again observed that the group immunized with admixed RBD–np had significantly reduced neutralization activity in response to NC99 relative to that of the mosaic RBD–np and homogeneous NC99 RBD–np groups, which had titers comparable to each other (Fig. 2d). Microneutralization using a diverse set of H1N1 viruses (CA09, BR07, CC03, KW86 and WS33) revealed superior serum neutralization breadth and potency in animals immunized with mosaic nanoparticle compared to the responses in other groups (Fig. 2e). Neutralization geometric mean titers across five viruses were significantly higher for the mosaic nanoparticle–immunized group than for the other groups (Fig. 2e, right), indicating that the mosaic RBD–np elicited greater neutralizing antibody breadth than did immunization with regimens of repeated admixed nanoparticles or sequential immunizations of homotypic RBD–np.
Qualitative differences in hemagglutinin-specific B cells between homogeneous and heterogeneous RBD–np immunizations
We next asked whether there were qualitative differences in immune responses by assessing the cross-reactivity of hemagglutinin-specific B cells in immunized mice. Peripheral blood cells were collected after three immunizations with NC99 RBD–np alone or admix or mosaic RBD–np and were probed with two distinct H1N1 hemagglutinins (NC99 and CA09) to identify hemagglutinin-specific B cells by flow cytometry9 (Fig. 3a). The frequency of IgD− B cells reactive to NC99 hemagglutinin was the highest in mice immunized with NC99 RBD–np (0.245 ± 0.074%), among all groups, but the frequency was not significantly different from that in mice immunized with 8-valent admix or mosaic RBD–np (0.140 ± 0.049% or 0.235 ± 0.087%, respectively; Fig. 3b). We observed lower frequencies of B cells reactive to CA09 hemagglutinin in all the admixed or mosaic RBD–np groups (ranging from 0.151% to 0.221%) than in the CA09 RBD–np group (0.374 ± 0.226%; Fig. 3c). Interestingly, B cells stained with both NC99 hemagglutinins and CA09 hemagglutinins were rare but were detected in the majority of mice immunized with nanoparticles with two or more RBDs delivered as admix or as mosaic nanoparticles (Fig. 3d). However, these cells were detected more consistently and frequently in mice receiving the mosaic nanoparticles with RBD valency of 6 or 8 than in the other immunization groups (Fig. 3d). This suggests that increased valency promotes the induction of B cells that bind conserved regions on adjacent heterologous antigens (cross-reactivity to multiple hemagglutinins), leading to broad antibody responses. To determine how antigen-recognition patterns impact the maturation of hemagglutinin-specific B cells, we next characterized gene encoding the variable region of immunoglobulin heavy chain (VH) of sorted NC99 HA+ B cells from different immunization groups. The immunoglobulin genes were recovered by single-cell PCR with reverse transcription (RT-PCR)37, sequenced and analyzed. As expected, VH sequences from mice that received NC99 RBD–np had diverged from germline sequences by 4.1 ± 0.2% (n = 108) as a result of SHM. The mean mutation rate in groups immunized with admixed RBD–np was slightly higher at 5.0 ± 0.3% (n = 98). The mosaic RBD–np and sequential immunization groups yielded the highest mutation rates, of 5.5% ± 0.3% (n = 101) and 5.8 ± 0.5% (n = 36), respectively. The differences between groups that received NC99 RBD–np and mosaic RBD–np or sequential RBD–np were statistically significant (Fig. 3e), although the frequency of hemagglutinin-specific B cells and serum antibody responses in the sequential immunization group were relatively low. These data suggest hemagglutinin-specific B cells from mosaic nanoparticle and sequential immunization groups acquired more SHMs than did those from repeated homotypic nanoparticle immunization groups.
a, Gating strategy for identifying hemagglutinin-specific B cells by flow cytometry. Anti-CD3, anti-CD8, anti-CD14 and anti-F4/80 were combined and used to separate T cells, monocytes and macrophages (MΦ). A disparate pair of hemagglutinins (NC99 and CA09) was used to define the cross-reactivity of hemagglutinin-specific B cells. b–d, Frequencies of IgD− B cells specific to NC99 hemagglutinin (b) or CA09 hemagglutinin (c) or cross-reactive to NC99 and CA09 hemagglutinin (d) among PBMCs of mice immunized with different RBD–np (n = 15, cumulative data of three independent experiments, except for 8-valent admix group (n = 10, cumulative of two independent experiments). Statistical analyses were done with one-way ANOVA with Tukey’s multiple comparisons post-hoc test by using valence = 1 as a comparator. e, Mutation rate in genes encoding immunoglobulin VH of individually sorted NC99 HA+ B cells isolated from immunized mice (n = 3, NC99, admix and mosaic; n = 2, sequential). Total productive genes obtained were 108, 98, 101 and 36 for the NC99, admix, mosaic and sequential RBD–np groups, respectively. Statistical analysis was done with one-way ANOVA with Tukey’s multiple comparisons post-hoc test.
Isolation and characterization of broadly neutralizing H1N1 antibody 441D6
As a proof of concept, we sought to isolate monoclonal antibodies to determine whether examples of cross-reactive B cells induced by mosaic nanoparticles contributing to broad serum neutralization could be detected. We sorted B cells co-stained by NC99 and CA09 hemagglutinins and cloned immunoglobulin genes recovered from individual cells into expression vectors containing constant regions of the corresponding immunoglobulin heavy and light chains. Out of 17 paired immunoglobulin genes, we identified four recombinant monoclonal antibodies that bound to NC99 hemagglutinin as well as other H1N1 hemagglutinins. Three out of four monoclonal antibodies also bound to H5N1 (A/Indonesia/05/2005) hemagglutinin (Supplementary Fig. 3a). The remaining monoclonal antibody, 441D6, is specific to H1N1, yet it had the highest neutralization potency of the four antibodies to homologous NC99 and a heterologous H1N1 virus, BJ95 (Supplementary Fig. 3b). None of the monoclonal antibodies had detectable HAI activity. We next assessed the kinetics of the binding of the antigen-binding fragment (Fab) of 441D6 to various H1N1 hemagglutinins by biolayer interferometry (Fig. 4a,b and Supplementary Fig. 4). Affinities of 441D6 Fab to hemagglutinins from a diverse set of 12 H1N1 viruses varied from sub-nanomolar to micromolar interactions, with no obvious association between apparent affinity (KD) and collection year of the viruses (Fig. 4b). To better understand how 441D6 targets diverse hemagglutinins, we solved the structure of unliganded 441D6 Fab by X-ray crystallography to 2.0 Å and determined the structure of the HA–Fab complex by cryo-electron microscopy at a resolution of 8.0 Å (Fig. 4c,d, Supplementary Table 1 and Supplementary Fig. 5). Stoichiometry of hemagglutinin-binding by 441D6 was three Fabs per trimer, resulting in a propeller-like topology when viewed along the longitudinal axis of hemagglutinin trimer (Fig. 4d). Atypical for hemagglutinin head–directed antibodies, 441D6 Fab approaches hemagglutinin nearly perpendicular to the hemagglutinin trimer vertical axis (79° relative to the hemagglutinin longitudinal axis; Fig. 4d). Coordinates corresponding to A/New York/653/1996 (H1N1) hemagglutinin and 441D6 Fab fit well into the cryo-electron microscopy density map, with only 260 Fab atoms (8% total, 3% backbone) and 994 hemagglutinin atoms (9% total, 2% backbone) outside the cryo-electron microscopy density (Fig. 4d). The epitope of 441D6 on hemagglutinin is located on the head proximal and opposite to the sialic acid–binding pocket (RBS) and distinct from the known classical antigenic sites of H1N1 hemagglutinin38,39 (Fig. 4e–g). The hemagglutinin footprint on the 441D6 Fab clusters mostly on the complementarity-determining region (CDR) loops and overlaps residues that had undergone SHM (Fig. 4c,h). Despite the high variability of the hemagglutinin head region even within subtype, the epitope is centered on a relatively conserved patch shared by H1N1 subtype viruses (Fig. 4f,g,i). To determine whether the 441D6 epitope on mosaic RBD–np is accessible for antibody and/or B cell recognition, we carried out electron microscopy to visualize the nanoparticle–Fab complex. Unlike the mosaic RBD–np alone, which yields homogeneous spherical particles with protruding short spikes with an average radius of 13.0 nm, the mosaic RBD–np in complex with 441D6 Fab resulted in larger particles with conformationally heterogeneous densities surrounding the mosaic RBD–np structure (average radius of 15.5 nm; Supplementary Fig. 6), indicating that the 441D6 Fab was able to recognize the RBD molecules displayed on the mosaic RBD–np. These results demonstrate that the 441D6-binding sites on the mosaic RBD–np are readily accessible for immune recognition.
a, Binding kinetics of 441D6 Fab to NY96 hemagglutinin, determined by biolayer interferometry. Measured sensorgram and calculated curve fit (1:1 binding model) are shown in red and black, respectively. Experiments were independently performed twice with similar results. b, Summary of binding affinities (KD) of 441D6 Fab to a diverse set of 12 H1N1 hemagglutinins. Each HA–Fab interaction was plotted and color-coded on the basis of year of identification of the virus. Binding kinetics details are found in Supplementary Fig. 4. c, 2.0-Å crystal structure of unliganded 441D6 Fab. Somatic mutations of 441D6 Fab (right). Residues that underwent SHM are colored blue. Amino acid sequence of CDRH1–3 and CDRL1–3 loops are shown with SHM residues in blue. HC, heavy chain; LC, light chain. d, Cryo-electron microscopy structure of NY96 hemagglutinin trimer in complex with 441D6 Fab. Side view along the longitudinal axis of an hemagglutinin trimer (top) and top view looking down on the three-fold axis of an hemagglutinin trimer from the membrane distal end (bottom) are shown. Superimposition of NY96 hemagglutinin (homology model, gray) and 441D6 Fab (orange-red) coordinates into the cryo-electron microscopy density map (right). White asterisks indicate sialic acid–binding pocket on each hemagglutinin protomer. e, Updated antigenic sites of H1N1 hemagglutinin. Known antigenic sites Sa, Sb, Ca1, Ca2 and Cb are shown, along with the site recognized by 441D6. f, Surface conservation of 1,368 non-overlapping H1N1 hemagglutinins. Conservation scores were calculated by the ConSurf server (http://consurf.tau.ac.il) and were colored on one NY96 hemagglutinin protomer with dark blue equating to highest conservation. g,h, Predicted 441D6 epitope (colored) mapped on NY96 hemagglutinin (g) and paratope of 441D6 (h). Each paratope residue is colored according to buried surface area (BSA) contribution. i, Amino acid sequence of HA1 subunit of NY96 hemagglutinin. 441D6 epitope residues are indicated by conservation score and BSA.
Neutralization profile of 441D6 and immune sera elicited by mosaic RBD–np
To determine the breadth of neutralization of 441D6, we used an extended panel of 17 H1N1 pseudoviruses (full list in Methods). Monoclonal antibody 441D6 neutralized all the H1N1 viruses tested in the assay, which represent about 90 years of antigenic evolution (Fig. 5a), and all except A/Iowa/1943 (IA43) were potently neutralized. This neutralization breadth of 441D6 is noteworthy, as it exceeds the breadth of well-characterized broadly neutralizing antibodies directed against the RBS and vicinity (5J8, CH65 and C05)13,15,40, and 441D6 requires ~0.1 µg ml−1 to achieve half-maximal neutralizing activity against most viruses (Fig. 5a,b). Importantly, 441D6 also neutralized viruses in microneutralization assays, as demonstrated by the use of six authentic influenza viruses (WS33, KW86, CC03, A/Solomon Islands/3/2006 (SI06), BR07 and CA09), with half-maximal inhibitory concentration (IC50) values between 0.2 μg ml–1 and 2.8 µg ml−1 (Fig. 5c), and provided protection against lethal H1N1 infections (both CA09 and A/Puerto Rico/8/1934) in murine models when given therapeutically at 24 h after infection (Supplementary Fig. 7a,b). To determine the basis for the virus neutralization of 441D6, we tested its HAI activity against multiple different H1N1 viruses. As we predicted from HA–Fab structure, there was no HAI activity against any virus, with the exception of CA09, for which we detected weak activity (Fig. 5d). Additionally, we assessed the pH-dependent hemolysis-inhibition activity41 of 441D6 and found no inhibitory activity (Fig. 5e). These results suggest that 441D6 neutralizes viruses without competing with receptor binding or inhibiting the viral fusion machinery. We next tested the virus neutralization of 441D6 in its Fab form and observed that 441D6 Fab completely lost virus-neutralizing activity, compared with that of its IgG form (Fig. 5f). Therefore, we propose that the basis for 441D6’s virus neutralization is viral aggregation, which effectively reduces inoculum size. Alternatively, cross-linking of neighboring hemagglutinin trimers with two Fab arms of IgG or binding of hemagglutinin perpendicular to its longitudinal axis could disrupt the geometric arrangement of hemagglutinin trimers on the virion and interfere with the formation of fusion pores on the host endosomal membrane (Fig. 5g).
a, Neutralization profile of broadly neutralizing antibodies, determined by a panel of 17 H1N1 pseudoviruses. Potency was displayed as a heatmap along with associated hemagglutinin phylogenetic tree (maximum-likelihood method with full-length hemagglutinin sequences). Antibodies 5J8 and CH65 (pan-H1N1 broadly neutralizing antibodies targeting RBS) and C05 (cross-subtypic broadly neutralizing antibody targeting RBS) were used as controls. Experiments were independently performed twice with similar results. b, Neutralization breadth–potency plot generated from data displayed in a. Breadth denotes the neutralization coverage of a panel of 17 H1N1 strains representing >90 years of antigenic evolution. c,d, MN IC50 (c) and HAI (d) of 441D6 against six different H1N1 viruses. Experiments were independently performed twice with similar results. e, Hemolysis-inhibition activity of 441D6. Hemolysis-inhibition assay was performed using PR34 virus. The hemolysis was calculated with the following formula: hemolysis (%) = [(Absexperimental − AbsRBC-only) / (Absno antibody − AbsRBC−only)] × 100. Experiments were independently performed twice. f, Microneutralization activity of 441D6 in its IgG and Fab forms. MN IC50 concentrations of CH65, 441D6 and CR6261 were determined by microneutralization assays with two H1N1 viruses. Experiments were independently performed twice with similar result. g, Proposed model for neutralization by 441D6, CH65 and CR6261.
We next asked if immunization with the mosaic RBD–np elicits cross-neutralizing antibody responses to diverse H1N1 viruses on the basis of 441D6-like antibodies. The breadth of serum HAI activity was measured using a panel of 14 H1N1 viruses representing about 30 years of antigenic drift. We observed variation in HAI titers to different viruses, and the breadth of response ranged between 36% and 86% in a group of five animals (Fig. 6a). Strikingly, when immune sera were tested for neutralizing activity using a panel of 13 H1N1 pseudoviruses (~90 years of coverage), we observed broad neutralizing activity against 85–100% (97.0 ± 6.7%, n = 5) of the strains, with geometric mean reciprocal IC50 titers of 4,643 across all viruses (Fig. 6b). The neutralization profile of immune sera resembles the breadth of 441D6 and is independent of HAI activity. These data suggest that co-displaying heterotypic hemagglutinin RBDs on nanoparticles can elicit potent and broadly neutralizing antibodies that are not necessarily focused on the hypervariable sequences around the sialic acid–binding pocket that tend to be immunodominant and strain specific.
a, Serum HAI titers against a panel of 14 H1N1 viruses (>30 years of coverage). HAI titers of immune sera elicited by mosaic RBD–np (8-valent) are plotted against the year of isolation of the virus (top) or are plotted individually with breadth (bottom). b, Serum pseudotype neutralization IC50 titers against a panel of 13 H1N1 pseudoviruses (~90 years of coverage). Data are presented as in a. Shown are representative data from one experiment (n = 5). Experiments were independently performed twice with similar results.
We next identified the two residues on hemagglutinin that were critically involved in and made an hemagglutinin variant possessing two point mutations that specifically knock out binding of 441D6 (Supplementary Fig. 8a). By using this hemagglutinin variant (441D6KO), we detected a subtle but statistically significant difference between binding of serum antibody to wild-type hemagglutinin and to the 441D6KO hemagglutinin in mice immunized with the mosaic RBD–np and with the admix RBD–np, while the difference was not observed in mice immunized with NC99 RBD–np or sequential RBD–np (Supplementary Fig. 8b). The results align well with the cross-reactive B cell data and suggest that the mosaic RBD–np and the admix RBD–np elicit detectable serum antibody responses specific to the site recognized by 441D6. Furthermore, to see if humans are capable of generating 441D6-like antibodies through infection and/or vaccination, we tested 26 serum samples collected from healthy volunteers. Significant differences were detected between antibody binding to wild-type hemagglutinin and its binding to the 441D6KO hemagglutinin (Supplementary Fig. 8c), suggesting that serum antibodies directed against the 441D6-binding site can also be induced in humans through natural exposure to influenza virus and/or conventional vaccines. Indeed, a family of human antibodies has been identified42 (for example, Ab6649) that target the overlapping site of vulnerability on hemagglutinin that we have determined to be the 441D6 epitope (Figs. 4e and 7a). This lineage of antibodies broadly neutralizes H1N1 virus isolates from 1977 to 2009, suggesting that humans are capable of targeting the 441D6 epitope and can generate broadly neutralizing antibody responses targeting this site. To directly prove the human anamnestic B cell repertoire to the site, we sorted human peripheral blood mononuclear cells (PBMCs) with NC99 hemagglutinin probe labeled with one fluorochrome and NC99 hemagglutinin probe in complex with 441D6 Fab labeled with another fluorochrome to selectively enrich B cells with specificity similar to 441D6 (Fig. 7b). More than one third of B cells binding to NC99 hemagglutinin but not to NC99 HA–Fab also bound to CA09 hemagglutinin. By this targeted sorting strategy, we identified two lineages of antibodies and confirmed that the representative antibody from each lineage was capable of neutralizing 14 out of 17 (82%) H1N1 subtype viruses spanning over 90 years of antigenic evolution (Fig. 7c). Interestingly, the neutralization activity was severely impaired when these antibodies were used as Fabs, suggesting that these antibodies use a neutralization mechanism similar to that of 441D6 (Fig. 7d). Taken together, both this work and previously published work42 support the existence of antibodies with 441D6-like specificity exist in the human B cell repertoire. On the basis of our results in mice, we foresee these B cells with this specificity would represent cross-reactive lineages preferentially induced by the mosaic RBD–np.
a, Antibody cross-competition profile. Antibody binding to NC99 hemagglutinin (left) and CA09 hemagglutinin (right) was measured by biolayer interferometry in the presence or absence of competing (1°) antibody. Antibody competition was calculated by the following formula: competition (%) = 100 − [(Bmaxexperimental / Bmaxnon-competed) × 100]. Experiments were independently performed twice with similar results. Test antibodies are listed as 2º. b, Hemagglutinin staining of human B cells. CD19+IgG+ B cells were probed with NC99 hemagglutinin with two different fluorochromes (left) or probed with NC99 HA PE and NC99 HA–441D6 Fab complex APC (middle). CD19+IgG+ B cells stained with NC99 HA but not stained with HA–Fab complex (middle, green gate) were collected by single-cell sorting. Reactivity of NC99 HA+ HA–Fab complex– cells to CA09 HA BV785 and H3 A/Texas/50/2012 (TX12) HA AF488 is shown (right). Experiments were independently performed twice with similar results. c, Neutralization breadth–potency plot of two representative 441D6-like human antibodies, 33-1F04 and 33-1G06. Neutralization breadth and potency were determined by pseudotype-neutralization assays using the same 17-virus panel as that in Fig. 5. Experiments were independently performed twice with similar results. d, Microneutralization activity of Ab6649, 310-33-1F04 and 310-33-1G06 in IgG and Fab forms. MN IC50 concentrations of Ab6649, 310-33-1F04 and 310-33-1G06 were determined by microneutralization assays with two H1N1 viruses. Experiments were independently performed twice with similar results.
Model of immune induction by heterotypic mosaic antigen arrays
When the immune system encounters particulate substances that are not homotypic antigens, the outcome is less certain, since single-particle heterotypic arrays of the same protein do not exist in nature. In this scenario, B cells with cross-reactive BCRs would be favored for bivalent binding to neighboring heterologous antigen pairs, providing an avidity advantage over strain-specific BCR interactions that are incapable of facilitating bivalent binding (Fig. 8a). Even though the initial affinity may be lower than that for strain-specific BCRs, there would be less competition with high-affinity BCRs, allowing the expansion of broadly cross-reactive B cells (Fig. 8b).
a, B cell activation by homotypic or heterotypic antigen arrays. B cells possessing BCRs specific to ‘gray’ antigen or cross-reactive to multiple antigenic variants are colored gray or orange, respectively. Situations with particulate stimuli (for example, virus, vaccine, etc.) made of homotypic (left) or heterotypic (right) antigens are shown. b, Predicted immune outcome induced by homotypic (top) and heterotypic (bottom) antigen arrays.
Discussion
The ultimate goal of universal influenza immunity is to achieve broad and durable protection against future drifted and pandemic strains of influenza viruses by active immunization43. However, antigenic variability of influenza viruses poses the greatest challenge for the achievement of effective vaccine-induced immunity beyond a single influenza season. Finding ways to induce broadly neutralizing antibodies could provide an avenue for the development of broadly protective vaccines that would be useful for multiple seasons. We envision three major approaches to achieve these aims. One would be to target B cell recognition to conserved antigenic supersites such as the RBS or the Trp21HA2 groove in the hemagglutinin stem (immunofocusing approach). Another approach would be to accumulate breadth by eliciting an ensemble of neutralizing antibodies to multiple sites on multiple strains, including seasonal H1N1, H3N2 and influenza B viruses, and supplemented by the inclusion of antigens from exotic hemagglutinin subtypes, such as H5 and H7 (additive approach). This approach is inherently limited because it is dependent on and influenced by prior antigenic exposures and tends to protect ‘looking backward’ rather than forward. Immunological imprinting from prior influenza infection and vaccination results in B cell repertoires that prevent recognition or induction of antibody specificities that would be more cross-reactive with newly emerging strains. Immunodominance of strain-specific regions of hemagglutinin is a key obstacle to the achievement of broad cross-reactivity in the setting of an antigenically diverse and evolving target and is addressed by what we have termed an ‘immunosubversion’ approach. Allowing cross-reactive B cells of lower affinity from the naïve repertoire to emerge by subverting the existing strain-specific responses could potentially provide protection against future drifted seasonal strains as well as pandemic strains and may eliminate the need for annual reformulation and manufacturing that creates a substantial bottleneck in the present influenza vaccine approach44. In the antigen-naïve population (that is, infants), the mosaic RBD–np would preferentially stimulate cross-reactive B cells by providing an avidity advantage when the antigen-binding sites of the BCR can recognize more than one hemagglutinin specificity. Importantly, antigen-experienced humans appear to mount memory B cells specific to the 441D6 epitope through natural influenza exposures and/or vaccinations. These pre-existing memory B cells might be selectively stimulated and differentiated into antibody-producing cells after receiving the mosaic RBD–np.
There are a few other vaccine approaches that might elicit cross-reactive responses, including chimeric hemagglutinins, which use engineered hemagglutinin with an exotic hemagglutinin head paired with an invariant stem to promote elicitation of stem-focused immune response in individuals with pre-existing immune repertoires27,45. Another approach is the COBRA hemagglutinin, which uses computationally optimized consensus hemagglutinin sequences to help broaden the B cell reactivity46,47. Both the chimeric and COBRA hemagglutinin approaches have shown promise in preclinical models. However, there is no constraint on how the immune system responds to these immunogens — that is, providing an equal opportunity to all antigen-specific B cells regardless of specificity. One unique feature of our heterotypic mosaic antigen array is that it gives an avidity advantage to cross-reactive B cells over strain-specific B cells and hence adds an immunological constraint on the immune system. This avidity advantage may compensate for the inherently low-affinity of cross-reactive BCRs and allow cross-reactive B cells to emerge.
Conventional influenza vaccines ‘chase’ circulating viruses from year to year. This is effective as long as the antigenicity of vaccine strains matches the circulating viruses, because the present vaccines provide protection with a relatively narrow range. Using the additive approach by either serial vaccination or combined vaccination with multiple antigenic types might accumulate responses with non-overlapping specificities. Successful examples of this strategy include pneumococcal conjugate vaccines with 13 different antigens or human papillomavirus vaccines with up to nine different antigens in a single vaccine. Although these multivalent vaccines are highly efficacious in humans, the admixture could result in antigenic competition and eventually might lead to loss of efficacy for one or more components in the influenza vaccine. The accumulation of breadth by serial immunization with conventional vaccine has not been effective for the generation of broad immunity48. Importantly, for influenza virus, there is substantial antigenic variation even within subtypes, so even a subtype-specific vaccine will need to induce considerable breadth to avoid escape through antigenic drift or reassortment from zoonotic reservoirs, which leads to new viruses with pandemic potential. In contrast, the immunofocusing approach uses engineered immunogens presenting a domain or a subdomain of a protein, attempting to make only the selected antigenic sites accessible to B cells so that a response is made to a surface that is immunologically subdominant or silent in its native configuration. An example of immunofocusing for influenza would be the ‘head-less’ hemagglutinin that focuses antibody responses on the conserved stem supersite and has shown some promise24,25. Our immunosubversion approach described here adds an immunological constraint (single-particle antigenic variability) to avoid dominant strain-specific antibody responses and create an opportunity for cross-reactive antibody responses to conserved sites with shared surfaces between diverse hemagglutinin antigens. Together, our results have shown that the mosaic antigen array of hemagglutinins from diverse influenza strains allows antibody responses to unique sites that are generally not targeted otherwise. This provides a proof of concept for vaccine-induced immunosubversion with a heterotypic mosaic antigen array to give a selective avidity advantage to cross-reactive B cells.
The viral site of vulnerability defined by 441D6 is an attractive pan-subtype vaccine target. Determining the structural and molecular mechanism for virus neutralization by 441D6 informs immunogen designs that could target this site of vulnerability. Although the precise modes of virus neutralization are different, other pan-subtypic sites of vulnerability have been discovered through the isolation and characterization of monoclonal antibodies, including F005-126 for H3N2 (ref. 21), FLD194 for H5N1 (ref. 23), and CR8033, CR8071, 46B8 and C12G6 for influenza B virus hemagglutinins17,49,50. These pan-subtypic monoclonal antibodies, except for C12G6, which targets RBS, target the vestigial esterase domain of hemagglutinin that partially overlaps the 441D6 epitope. It is probable that presentation of heterotypic mosaic antigen arrays would preferentially stimulate B cells that tolerate antigenic variability for other hemagglutinin subtypes and influenza B virus lineages and allow the emergence of B cells targeting conserved antigenic sites.
We propose that the juxtaposition of heterotypic influenza virus hemagglutinin antigens in a mosaic array will represent a novel immunological pattern that preferentially engages B cells with cross-reactive potential and changes the hierarchy of immunodominance. This immunosubversion approach may be applicable to other pathogens with extreme antigenic variability, especially in cases in which strain-specific B cells dominate the response and prevent the emergence of cross-reactive B cells through competition or other forms of immunodominance. Advances in protein engineering and manufacturing technologies make this approach achievable and provide a new tool with which to combat pathogens that exploit genetic plasticity and antigenic variation as a primary immunoevasion mechanism.
Methods
Study design
The aim of this study was to elucidate and characterize the immune response elicited by the non-natural mosaic arrays of antigenically heterotypic influenza virus antigens in mice. The experimental design involved in vivo and in vitro experiments, including design, purification, biochemical, biophysical and antigenic characterizations of nanoparticle immunogens, mathematical simulation, enzyme-linked immunosorbant assay (ELISA), virus neutralization, fluorescence-activated cell sorting (FACS), single-cell RT-PCR, immunogenetics analysis, recombinant antibody production, biolayer interferometry, X-ray crystallography and cryo-electron microscopy. The animal experiments were not randomized. The investigators were not blinded to the allocation during experiments and analyses unless otherwise indicated. Experimental replication is indicated in the figure legends. Animal experiments were carried out in accordance with all federal regulations and NIH guidelines and were approved by the Institutional Animal Care and Use Committee. All human serum samples for this study were collected with informed consent of volunteers, and approval for this study was obtained under protocol number VRC 700 (Clinicaltrials.gov NCT01262079)51. All volunteers were at least 6 years of age, were healthy, and ranged in age from 7 years to 93 years. Human PBMC samples were obtained from VRC 310, a single-site, phase 1, open-label, randomized clinical trial conducted at the National Institutes of Health (NIH) Clinical Center by the NIAID VRC (Clinicaltrial.gov NCT01086657)52. These studies were approved by the NIAID Intramural Institutional Review Board. US Department of Health and Human Services guidelines for conducting clinical research were followed.
Gene synthesis and vector construction
All genes used in the study were human codon optimized (GenScript). The gene encoding ferritin (Helicobacter pylori–bullfrog hybrid) was designed and characterized previously31. Briefly, the ferritin was composed of residues 2–9 of bullfrog (Rana catesbeiana) ferritin lower subunit (UniProt: P07797 with a point mutation at residue 8 (N8Q) to abolish a potential N-glycosylation site) to H. pylori non-heme ferritin (UniProt accession code Q9ZLI1; residues 3–167) with mutations at residues 7 (I7E) and 19 (N19Q) to maintain the salt bridge found in bullfrog ferritin and to abolish a potential N-glycosylation site, respectively. All the hemagglutinin (HA) RBD sequences used in the study correspond to residues 56–264 (H3 numbering) and possess a mutation that knocks out the sialic acid–binding property (Y98F)9 and are derived from various H1N1 strains listed below. To construct RBD–np, a modified bovine prolactin signal sequence31 was attached upstream of the RBD and was fused to the ferritin with a Ser-Gly linker. All genes were then cloned into the CMV/R (VRC 8400) or CMV/R 8κb (VRC 8405) mammalian expression vector for protein production.
Biosynthesis of recombinant proteins and purification
The expression vectors were transiently transfected (1.0 mg l−1 of 2.5 × 106 cells) into Expi293F cells (Thermo Fisher Scientific) using ExpiFectamine 293 transfection reagents (Thermo Fisher Scientific). For co-assembly, cells were co-transfected with equal amount of multiple RBD–np plasmids (total of 1.0 mg l−1 of 2.5 × 106 cells). Four days after transfection, culture supernatants were collected and cleared. The RBD–np were purified by ion-exchange chromatography using HiTrap Q HP columns at pH 8.0 (GE Healthcare), followed by size-exclusion chromatography with a Superose 6 PG XK 16/70 column (GE Healthcare) in PBS. Recombinant hemagglutinin ectodomain trimers constructed with T4 fibritin foldon and hexa-histidine tag were produced and purified as described elsewhere32,53. Recombinant hemagglutinin probes (Y98F) for FACS were made as previously described9,54.
Immunization
Female 8- to 10-week-old BALB/cAnNTac mice (Jackson Laboratories) were immunized (n = 5–10) intramuscularly with 2 μg of purified nanoparticle in 100 μl of 50% (v/v) mixture of SAS adjuvant (Sigma) in PBS. For the experiments shown in Fig. 2a, mice were immunized at weeks 0 and 4. For the experiments shown in Fig. 2b, mice were immunized with 1.5 µg of TIV from indicated season at weeks 0 and 3 to generate influenza immunity and then were immunized with 2 µg of mosaic RBD–np (8-valent) formulated with SAS and given at weeks 6 and 10. For the experiments shown in Figs. 2d,e and 3, mice were immunized at weeks 0, 4 and 12 (NC99, admix and mosaic) or weeks 0, 4, 8 and 12 (sequential). For the experiments shown in Fig. 6a,b, mice were immunized at weeks 0, 4 and 8. Sera were collected before the first dose and periodically 2–3 weeks after each immunization.
H1N1 viruses and recombinant hemagglutinins
Influenza virus strains used for microneutralization and HAI assays are: A/USSR/90/1977; A/Kawasaki/6/1986; A/Chile/4795/2000; A/Texas/36/1991; A/Shanghai/8/1996; A/Auckland/65/2001; A/Ostrava/801/1998; A/Johannesburg/159/1997; A/Beijing/262/1995; A/Brisbane/59/2007; A/Christchurch/1/2003; A/New Caledonia/20/1999; A/California/07/2009; A/Singapore/6/1986; and A/WSN/1933 (made by reverse genetics). Influenza virus strains used for pseudotype neutralization assays are: A/South Carolina/1/1918 (GenBank AF117241, SC18); A/Puerto Rico/8/1934 (GenBank CY009444, PR34); A/New Jersey/1976 (GenBank CY021957, NJ76); A/Singapore/6/1986 (GenBank CY020477, SG86); A/Beijing/262/1995 (GenBank CY033614, BJ95); A/New Caledonia/20/1999 (GenBank CY033622, NC99); A/Solomon Islands/3/2006 (GenBank CY031340, SI06); A/Brisbane/59/2007 (GenBank CY058487, BR07); A/California/04/2009 (GenBank GQ117044, CA09); A/Albany/4835/1948 (GenBank CY019947, AB48); A/Baylor/4052/1981 (GenBank CY021029, BA81); A/Memphis/39/1983 (GenBank ABO37988, ME83); A/New York/146/2000 (GenBank AAX56530, NY00); A/New York/653/1996 (GenBank ABF47649, NY96); A/New York/8/2006 (GenBank ABK79959, NY06); A/Texas/36/1991 (GenBank CY033655, TX91); A/Hong Kong/117/1977 (GenBank ABD60944, HK77); A/Iowa/1943 (GenBank ABO38373, IA43); and A/Fort Monmouth/1/1947 (GenBank CY009612, FM47). Recombinant hemagglutinin proteins were made by fusing hemagglutinin ectodomain with T4 fibritin trimerization motif (foldon) and purification tag as described previously53 for NY96, FM47, SI06, NC99, BJ95, BR07, SG86, SC18, NY00, CA09, IA43 and PR34.
Hemagglutination inhibition assay
Seed stocks of the influenza viruses were obtained from the Division of Viral Products, US Food and Drug Administration (FDA) and the viruses were propagated in Madin–Darby canine kidney (MDCK) cells. Immune sera were pretreated with receptor-destroying enzyme (RDE II; Denka Seiken), and HAI assays were performed using four hemagglutinating units per well and 0.5% turkey red blood cells (Lampire Biological Laboratories). Viruses used in the assays are listed above.
Flow cytometry
Mouse peripheral white blood cells were prepared from heparinized whole blood by lysing red blood cells with ACK lysing buffer (Thermo Fisher Scientific). After hemolysis, white blood cells were washed and stained with fluorochrome-conjugated monoclonal antibodies to mouse CD3-Cy5PE (BD Bioshiences, 553065, clone 145-2C11, 1:300 dilution), CD4-Cy5PE (BD Biosciences, 553654, clone H129.19, 1:300 dilution), CD8-Cy5PE (BD Biosciences, 553034, clone 53-6.7, 1:300 dilution), F4/80-Cy5PE (Biolegend, 1231111, clone BM8, 1:300 dilution), CD19-PE-CF594 (BD Biosciences, 562329, clone 1D3, 1:300 dilution), IgD-BV421 (BD Biosciences, 744291, clone 11-26 c.2a, 1:600 dilution). PE- and APC-labeled hemagglutinin probes were prepared as described previously9. AQUA dead cell stain was added for live or dead discrimination (Thermo Fisher Scientific). Samples were analyzed on an LSR II (BD Biosciences), and data analysis was done in FlowJo 9 (TreeStar). For single-cell sorting, hemagglutinin-specific B cells were stained as above, and HA+ IgD− B cells were sorted as single cells into 96-well plates using a FACSAria II (BD Biosciences). The addition of two hemagglutinin probes and index sorting was used to determine the binding of each sorted B cell to hemagglutinin of two strains simultaneously. Human PBMCs were stained with fluorochrome-conjugated monoclonal antibodies to human CD3-BV510 (BioLegend, 317332); CD56-BV510 (Biolegend, 318340); CD14-BV510 (Biolegend, 301842); CD27-BV605 (Biolegend, 302830); CD38-AF700 (Biolegend, 303524); CD19-ECD (Beckman Coulter, A07770); IgG-BV421 (BD Biosciences, 562581); IgM-PerCP-Cy5.5 (BD Biosciences, 561285); NC99 HA-PE or -APC; NC99 HA+441D6 Fab-APC; H3 TX12 HA-AF488; CA09 HA-BV785; and CA09 HA stem-BV510. All CA09 HA stem–binding B cells were excluded before gating with NC99 HA.
Single-cell immunoglobulin amplification and cloning
Reverse transcription was performed on sorted cells, and multiplexed PCR was used to amplify genes encoding immunoglobulin heavy and light chains as described elsewhere37. PCR products were sequenced by Beckman Coulter or ACGT and were analyzed using IMGT55. Heavy- and light-chain sequences were synthesized and cloned by Genscript into murine IgG2a and kappa expression vectors. To produce antibodies recombinantly, 293 F cells were transfected with plasmids encoding immunoglobulin heavy and light chain pairs with 293fectin (Thermo Fisher Scientific). Monoclonal antibodies were purified from the cell supernatant using Protein A Sepharose Fast Flow (GE Healthcare).
Virus-neutralization assay
All influenza viruses except A/WSN/1933 were obtained from Division of Viral Products, FDA. A/WSN/1933 virus was made by reverse genetics with eight plasmids encoding each influenza A virus segment under the control of biscistronic polymerase I/polymerase II promoters obtained from St Jude Children’s Research Hospital. All viruses were propagated in MDCK cells in the presence of TPCK-treated trypsin (1 µg ml−1, Sigma) and were titrated in MDCK cells as described elsewhere (http://www.who.int/influenza/gisrs_laboratory/2010_12_06_serological_diagnosis_of_influenza_by_microneutralization_assay.pdf). Briefly, DMEM–TPCK (DMEM with 1 µg ml−1 TPCK-trypsin and penicillin/streptomycin) was used to make four-fold serial dilutions of RDE-treated sera or monoclonal antibodies and to dilute influenza viruses to a final concentration of 100 TCID50 per well. In a 96-well plate, serum or antibody and virus were mixed and incubated 1 h at 37 °C before adding to substrate cells. Control wells of virus alone and diluent alone were included on each plate. Cells were seeded at 1.5 × 104 cells per well 24 h before the assays and were washed once with PBS before use. Antibody–virus mixture (50 µl) was then added to wells of pre-washed cells in duplicate, and the plates were incubated for 18 h at 37 °C and 5% CO2 humidified atmosphere. The cells were then fixed with 80% cold acetone and were allowed to air dry. The presence of viral nucleoprotein was detected by ELISA with biotin-conjugated antibodies to influenza virus nucleoprotein (MAB8257B and MAB8258B, EMD Millipore) and was visualized with HRP-conjugated streptavidin and SureBlue TMB Microwell Peroxidase Substrate (KPL). Absorbance was read at 450 nm (A450) and 650 nm (A650) with the SpectraMax Paradigm microplate reader (Molecular Devices). The A650 was used to subtract plate background. The percentage neutralization was calculated by constraining the virus-alone control as 0% and the diluent alone control as 100% and was plotted against serum or antibody concentration. A curve fit was generated by a four-parameter nonlinear fit model in Prism 7 (GraphPad). The IC50 was obtained from the curve fit for each serum sample or monoclonal antibody, respectively. The pseudotype neutralization assay was performed as previously described56,57. Briefly, pseudovirus was produced by transfection of 293 T cells of hemagglutinin and corresponding neuraminidase, along with the lentiviral packaging and reporter plasmids. A plasmid encoding the human type II transmembrane serine protease TMPRSS2 was also co-transfected for efficient proteolytic activation of hemagglutinin. At 48 h after transfection, supernatants were collected, filtered and frozen until use.
Electron microscopy
For negative-stain electron microscopy analysis of mosaic RBD–np alone and in complex with 441D6 Fab, 50 µg ml−1 of samples were adsorbed to freshly glow-discharged carbon-coated grids, then were rinsed with distilled water and stained with 2% ammonium molybdate, 0.7% uranyl formate or phosphotungstic acid (PTA). Images were recorded on a T12 microscope operating at 100 kV (FEI) with a OneView camera (Gatan) using SerialEM for semi-automated data collection58. RELION was used to make 2D class averages; rotationally averaged images were created from RELION class averages using EMAN2 image processors. Rotationally averaged images were analyzed with FIJI59 to yield a profile of image intensity as a function of radius.
Immunoprecipitation
Antibodies (5 µg) specific to NC99 (3u-u; ref. 53) and CA09 (2D1; refs. 34,60) or hemagglutinin stem (C179; ref. 19) were incubated with purified nanoparticles (5 µg) at ambient temperature for 30 min. Protein G Dynabeads (Thermo Fisher Scientific) were then added and incubated for another 30 min. Immune complexes were separated by magnetic force, washed and eluted in Laemmli buffer containing reducing agent. Half of each reaction was analyzed by SDS polyacrylamide gel electrophoresis (SDS–PAGE) followed by InstantBlue staining (Expedeon).
Simulation of particle assembly with multiple antigens
The ferritin particle was represented as a snub cube graph with 24 vertices and 60 edges. For k different antigens (colors), graph coloring was generated by assigning one of the k possible colors to each of the 24 vertices in the graph. A total of 106 random graphs were generated for each k. In each of the 106 graphs, the number of homologous edges (where an edge exists between two vertices of the same color) was counted and the fraction of 106 graphs with 0, 1, 2, …, 60 homologous edges was computed. For a given k, the number of homologous edges was reported as mean ± s.d.
Biolayer interferometry
All biosensors were hydrated in PBS before use. Recombinant hemagglutinin proteins were immobilized either on SA or HIS1K biosensors through conjugated biotin or hexa-histidine tag, respectively. After briefly dipping in assay buffer (1% BSA in PBS), the biosensors were dipped in a two-fold dilution series of Fab for 5 min. Biosensors were then dipped in assay buffer to allow Fab to dissociate from hemagglutinin for 10 min. All assay steps were performed at 30°C with agitation set at 1,000 r.p.m. in the Octet HTX instrument (fortéBio). Correction to subtract non-specific baseline drift was carried out by subtracting the measurements recorded for a sensor loaded with Epstein–Barr virus gp350 incubated with the Fabs as previously described61. Data analysis and curve fitting were carried out using Octet analysis software (version 9.0). Experimental data were fitted with the binding equations describing a 1:1 interaction. Global analyses of the complete data sets assuming binding was reversible (full dissociation) were carried out using nonlinear least-squares fitting allowing a single set of binding parameters to be obtained simultaneously for all concentrations used in each experiment.
X-ray crystallography
The 441D6 antigen-binding fragment (Fab) was generated by digestion of the engineered antibody, which has a HRV 3C cleavage site introduced in the hinge region, using HRV 3C protease (EMD Millipore) at 10 units mg−1 of Fab for 3–4 h at ambient temperature. The digestion reaction was then passed over a Protein A affinity column (GE Healthcare) to remove the crystallizable (Fc) fragments. The flow-through and PBS wash were pooled and concentrated to about 8 mg ml−1, and 768 crystallization conditions were screened in 192-well plates (Corning) using a Mosquito crystallization robot. Initial crystal conditions were observed in a condition containing 12% PEG 3350, 0.1 M HEPES, pH 7.5, 5 mM cobalt chloride, 5 mM calcium chloride, 5 mM magnesium chloride and 5 mM nickel chloride, with the final optimized crystals grown in 12% PEG 3350, 0.1 M HEPES, pH 7.5, 5 mM magnesium chloride and 5 mM nickel chloride. Crystals were cryoprotected using 15% 2R, 3R and butanediol, and diffraction data were collected at the Advanced Photon Source (Argonne National Laboratory) SER-CAT ID-22 beamline, at wavelength 1.00 Å and temperature 100 K, and were processed with HKL2000 (ref. 62). Iterative cycles of model building and refinement were carried out using COOT63 and PHENIX64 software packages, respectively, with 5% of the data acting as an R-free cross validation test set. All structural images were generated in UCSF Chimera65.
Cryo-electron microscopy
Samples of complexes of hemagglutinin NY96 and 441D6 Fab purified by gel-filtration chromatography were applied to glow-discharged Quantifoil R2/2 300 mesh grids (Quantifoil) at a concentration of 1 mg ml−1 and were plunge frozen in a Vitrobot Mark IV plunge freezer (FEI) under 100% humidity. The sample was blotted for 4 s after a blot delay of 1 s, and then the grid was rapidly plunged into liquid ethane to form vitreous ice. Plunge-frozen grids were imaged using a Titan Krios electron microscope (FEI) operating at 300 kV at a magnification of ×59,000 using a Falcon 2 direct electron detector (FEI). The detector was operated in movie mode to collect multiple frames per micrograph at a pixel size of 1.38 Å. Exposures were 1 s total, dose fractionated into seven frames. The accumulated dose per micrograph was 34 e−/Å2. A total of 464 micrographs were collected, with defocus values ranging between −2 µm and −7 µm. Micrographs were motion-corrected by frame alignment using MotionCorr66. Defocus values were determined using CTFFIND4 (ref. 67). A total 1,800 particles were manually picked and these particles were used to create 2D class averages with RELION (ref. 68). The best resolved of these 2D class averages were used as templates for auto-picking with RELION69 from all micrographs, which yielded 225,000 particles. Batches of particles were subjected to 2D classification using EMAN2 (ref. 70) to separate out overlapping particles and false-positive auto-picked particles, finding 195,314 particles judged to be HA–Fab complexes. Particles were transitioned back to RELION for further 2D classification. Three-fold symmetry was readily observable in the 2D class averages; thus, we created an initial model from 2D class averages using EMAN2. and this 3D model was used for refinement and additional 3D classification in RELION. The project was transitioned to RELION2 (ref. 71), and original micrographs were motion corrected using MotionCor2 (ref. 72), before micrograph coordinates corresponding to the curated working set of particles from RELION were used to re-extract particles from dose-weighted motion-corrected micrographs. A final set of 75,233 particles was refined using the ‘gold-standard’ protocol as implemented in RELION2 and was post-processed with an ad hoc B-factor of −300, then the map resolution was determined as 8.0 Å using an FSC cutoff of 0.143. Molecular modeling of the HA–Fab complex and epitope footprint was performed by rigid docking using UCSF Chimera65. The coordinates of hemagglutinin and the Fab were independently fitted into the cryo-electron microscopy map of the complex, and the position of both molecules was well determined by the map. Images rendered with surfaces were created using vertices exported from UCSF Chimera and were rendered with Blender (Blender Foundation, www.blender.org), an open-source 3D rendering program.
Virus challenge in mice
All virus challenge studies were conducted in an ABSL2 facility at Bioqual in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. Groups of ten BALB/cAnNHsd mice (5–9 weeks old, female, Envigo) were anesthetized and infected intranasally with 10 LD50 A/California/07/2009 (H1N1) or A/Puerto Rico/8/1934 (H1N1) and were given a purified monoclonal IgG antibody via intraperitoneal route 24 h later. The animals were monitored twice daily for development of clinical signs and were weighed daily for 14 days. Any animals that had lost 20% or more of their initial body weight were humanely euthanized.
Statistical analysis
Multi-group comparisons were done by one-way analysis of variance (ANOVA) with the Tukey’s post-hoc test performed in Prism 7 (GraphPad) unless mentioned otherwise. Differences were considered significant when P values were less than 0.05. Statistical methods and exact P values can be found in Figure legends and Figures, respectively.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary information. Coordinates for 441D6 Fab structure and cryo-electron microscopy density map have been deposited in the Protein Data Bank (PDB) under accession code 5TR8 and electron microscopy Data Bank (EMDB) under accession code EMD-7021. Hemagglutinin RBD–np and antibody sequences are deposited in the GenBank under accession codes MK273069–MK273076 and MK283705–MK283710. Requests for materials should be addressed to M.K. and B.S.G.
Change history
12 April 2019
In the version of this article initially published, the labels (50 Å) above the scale bars in Fig. 1b were incorrect. The correct size is 50 nm. The error has been corrected in the HTML and PDF versions of the article.
References
Morens, D. M. & Taubenberger, J. K. Historical thoughts on influenza viral ecosystems, or behold a pale horse, dead dogs, failing fowl, and sick swine. Influenza Other Respir. Viruses 4, 327–337 (2010).
Bedford, T. et al. Integrating influenza antigenic dynamics with molecular evolution. eLife 3, e01914 (2014).
Koel, B. F. et al. Substitutions near the receptor binding site determine major antigenic change during influenza virus evolution. Science 342, 976–979 (2013).
Smith, D. J. et al. Mapping the antigenic and genetic evolution of influenza virus. Science 305, 371–376 (2004).
Webster, R. G., Laver, W. G., Air, G. M. & Schild, G. C. Molecular mechanisms of variation in influenza viruses. Nature 296, 115–121 (1982).
Air, G. M. Influenza virus antigenicity and broadly neutralizing epitopes. Curr. Opin. Virol. 11, 113–121 (2015).
Wrammert, J. et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208, 181–193 (2011).
Joyce, M. G. et al. Vaccine-induced antibodies that neutralize group 1 and group 2 influenza a viruses. Cell 166, 609–623 (2016).
Whittle, J. R. et al. Flow cytometry reveals that H5N1 vaccination elicits cross-reactive stem-directed antibodies from multiple Ig heavy-chain lineages. J. Virol. 88, 4047–4057 (2014).
Li, G. M. et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc. Natl Acad. Sci. USA 109, 9047–9052 (2012).
Schmidt, A. G. et al. Viral receptor-binding site antibodies with diverse germline origins. Cell 161, 1026–1034 (2015).
Andrews, S. F. et al. Preferential induction of cross-group influenza A hemagglutinin stem-specific memory B cells after H7N9 immunization in humans. Sci. Immunol. 2, eaan2676 (2017).
Ekiert, D. C. et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 489, 526–532 (2012).
Lee, P. S. et al. Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc. Natl Acad. Sci. USA 109, 17040–17045 (2012).
Whittle, J. R. et al. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc. Natl Acad. Sci. USA 108, 14216–14221 (2011).
Lee, P. S.et al. Receptor mimicry by antibody F045-092 facilitates universal binding to the H3 subtype of influenza virus. Nat. Commun. 5, 3614 (2014).
Dreyfus, C. et al. Highly conserved protective epitopes on influenza B viruses. Science 337, 1343–1348 (2012).
Ekiert, D. C. et al. Antibody recognition of a highly conserved influenza virus epitope. Science 324, 246–251 (2009).
Okuno, Y., Isegawa, Y., Sasao, F. & Ueda, S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 67, 2552–2558 (1993).
Corti, D. et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333, 850–856 (2011).
Iba, Y. et al. Conserved neutralizing epitope at globular head of hemagglutinin in H3N2 influenza viruses. J. Virol. 88, 7130–7144 (2014).
Krause, J. C. et al. A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of the influenza H1N1 virus hemagglutinin. J. Virol. 85, 10905–10908 (2011).
Xiong, X. et al. Structures of complexes formed by H5 influenza hemagglutinin with a potent broadly neutralizing human monoclonal antibody. Proc. Natl Acad. Sci. USA 112, 9430–9435 (2015).
Impagliazzo, A. et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 349, 1301–1306 (2015).
Yassine, H. M. et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 21, 1065–1070 (2015).
Mallajosyula, V. V. et al. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc. Natl Acad. Sci. USA 111, E2514–E2523 (2014).
Krammer, F., Pica, N., Hai, R., Margine, I. & Palese, P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 87, 6542–6550 (2013).
Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu. Rev. Immunol. 30, 429–457 (2012).
Doria-Rose, N. A. & Joyce, M. G. Strategies to guide the antibody affinity maturation process. Curr. Opin. Virol. 11, 137–147 (2015).
Lanzavecchia, A., Fruhwirth, A., Perez, L. & Corti, D. Antibody-guided vaccine design: identification of protective epitopes. Curr. Opin. Immunol. 41, 62–67 (2016).
Kanekiyo, M. et al. Rational design of an epstein-barr virus vaccine targeting the receptor-binding site. Cell 162, 1090–1100 (2015).
Kanekiyo, M. et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499, 102–106 (2013).
Zimmer, S. M. & Burke, D. S. Historical perspective--Emergence of influenza A (H1N1) viruses. N. Engl. J. Med. 361, 279–285 (2009).
Xu, R. et al. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 328, 357–360 (2010).
Dintzis, H. M., Dintzis, R. Z. & Vogelstein, B. Molecular determinants of immunogenicity: the immunon model of immune response. Proc. Natl Acad. Sci. USA 73, 3671–3675 (1976).
Bachmann, M. F. et al. The influence of antigen organization on B cell responsiveness. Science 262, 1448–1451 (1993).
Tiller, T., Busse, C. E. & Wardemann, H. Cloning and expression of murine Ig genes from single B cells. J. Immunol. Methods 350, 183–193 (2009).
Gerhard, W., Yewdell, J., Frankel, M. E. & Webster, R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature 290, 713–717 (1981).
Caton, A. J., Brownlee, G. G., Yewdell, J. W. & Gerhard, W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 31, 417–427 (1982).
Hong, M. et al. Antibody recognition of the pandemic H1N1 Influenza virus hemagglutinin receptor binding site. J. Virol. 87, 12471–12480 (2013).
Yewdell, J. W., Gerhard, W. & Bachi, T. Monoclonal anti-hemagglutinin antibodies detect irreversible antigenic alterations that coincide with the acid activation of influenza virus A/PR/834-mediated hemolysis. J. Virol. 48, 239–248 (1983).
Raymond, D. D. et al. Conserved epitope on influenza-virus hemagglutinin head defined by a vaccine-induced antibody. Proc. Natl Acad. Sci. USA 115, 168–173 (2018).
Neu, K. E., Henry Dunand, C. J. & Wilson, P. C. Heads, stalks and everything else: how can antibodies eradicate influenza as a human disease? Curr. Opin. Immunol. 42, 48–55 (2016).
Lambert, L. C. & Fauci, A. S. Influenza vaccines for the future. N. Engl. J. Med. 363, 2036–2044 (2010).
Hai, R. et al. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J. Virol. 86, 5774–5781 (2012).
Carter, D. M. et al. Elicitation of protective antibodies against a broad panel of H1N1 viruses in ferrets pre-immune to historical H1N1 influenza viruses.J. Virol. 91, e01283-17 (2017).
Carter, D. M. et al. Design and characterization of a computationally optimized broadly reactive hemagglutinin vaccine for H1N1 influenza viruses. J. Virol. 90, 4720–4734 (2016).
Belongia, E. A. et al. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev. Vaccines 16, 1–14 (2017).
Chai, N. et al. A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action. Nat. Commun. 8, 014234 (2017).
Shen, C. et al. A multimechanistic antibody targeting the receptor binding site potently cross-protects against influenza B viruses. Sci. Transl. Med. 9, eaam5752 (2017).
Ngwuta, J. O. et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med. 7, 309ra162 (2015).
Ledgerwood, J. E. et al. Prime-boost interval matters: a randomized phase 1 study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect. J. Infect. Dis. 208, 418–422 (2013).
Wei, C. J. et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 329, 1060–1064 (2010).
Wheatley, A. K. et al. H5N1 vaccine-elicited memory B cells are genetically constrained by the IGHV locus in the recognition of a neutralizing epitope in the hemagglutinin stem. J. Immunol. 195, 602–610 (2015).
Brochet, X., Lefranc, M. P. & Giudicelli, V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 36, W503–W508 (2008).
Kong, W. P. et al. Protective immunity to lethal challenge of the 1918 pandemic influenza virus by vaccination. Proc. Natl Acad. Sci. USA 103, 15987–15991 (2006).
Yang, Z. Y. et al. Immunization by avian H5 influenza hemagglutinin mutants with altered receptor binding specificity. Science 317, 825–828 (2007).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Yu, X. et al. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature 455, 532–536 (2008).
Misasi, J. et al. Structural and molecular basis for Ebola virus neutralization by protective human antibodies. Science 351, 1343–1346 (2016).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods 10, 584–590 (2013).
Rohou, A. & Grigorieff, N. CTFFIND4: fFast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Scheres, S. H. Semi-automated selection of cryo-EM particles in RELION-1.3. J. Struct. Biol. 189, 114–122 (2015).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Kimanius, D., Forsberg, B. O., Scheres, S. H. & Lindahl, E. Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. eLife 5, e18722 (2016).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Acknowledgements
We thank J. Weir (Food and Drug Administration) for providing influenza viruses; R. Webby (St Jude Children’s Research Hospital) for influenza reverse genetics plasmids; M. Dillon, K. Wuddie, G. Sarbador, C. Chiedi, A. Taylor, H. Bao and D. Scorpio (VRC) for help with animal studies; A. Kumar (VRC) for help with protein production; V. Nair and E. Fischer (NIAID) for cryo-electron microscopy data collection; M. Chen (VRC), D. Angeletti and J. Yewdell (NIAID) for help with initial epitope mapping. This work used the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov) and also the Office of Cyber Infrastructure and Computational Biology (OCICB) High Performance Computing (HPC) cluster at the NIAID, NIH. Use of insertion device 22 (SER-CAT) at the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Basic Energy Sciences (under contract W-31-109-Eng-38). Support for this work was provided by the NIAID Intramural Research Program to the VRC and Division of Intramural Research. This work was also supported in part with federal funds from the Frederick National Laboratory for Cancer Research, NIH (under contract HHSN261200800001E).
Author information
Authors and Affiliations
Contributions
M.K. and B.S.G. conceptualized and devised studies. M.K. designed immunogens. M.K., R.A.G., H.M.Y. and S.B.B. performed animal studies. M.G.J. crystallized and solved Fab structure. J.R.G. and A.K.H. determined cryo-electron microscopy structure. S.F.A., A.K.W., B.E.F., D.R.A. and M.S.P. performed FACS and single-cell PCR. M.K., R.A.G., S.F.A. and H.M.Y. produced and characterized proteins. A.C. made viruses by reverse genetics. R.A.G., H.M.Y., K.L., E.S.Y. and W.-P.K. performed virus neutralization assays. I.S.G. performed mathematical simulations. Y.T. and U.B. performed electron microscopy experiments. H.A. performed challenge studies. K.L.Z. and J.E.L. conducted clinical trials and provided human samples. M.K., M.G.J., R.A.G., J.R.G., S.F.A., H.M.Y., A.K.W., A.K.H., R.A.K., P.D.K., A.B.M., J.R.M. and B.S.G. analyzed data. M.K. and B.S.G. wrote paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The US National Institutes of Health has filed a patent application on the basis of the studies presented in this paper; M.K., H.M.Y. and B.S.G. are listed as inventors on the patent application.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Fig. 1 Biochemical characterization of hemagglutinin RBD–np.
Chromatograms of homogeneously assembled and heterogeneously co-assembled hemagglutinin RBD–np on anion-exchange column (a) and size exclusion column (b). NC99 and CA09 hemagglutinin RBD–np were produced in Expi293 cells by transient transfection and the culture supernatants were purified. Anion-exchange chromatography was carried out using a HiTrap Q HP column eluted with linear gradient of NaCl (a). Fractions containing RBD–np corresponding to the shaded area were collected and subjected to size exclusion chromatography (SEC). SEC was carried out using a Superose 6 matrix to confirm particle formation (b). Experiments were independently performed three times with similar results. Purified RBD–np expressing various hemagglutinin RBD from indicated H1N1 strains homogeneously (c) or heterogeneously co-assembled (mosaic) (d) were analyzed on SDS–PAGE and blue staining. For comparison, mechanical mixtures of 2, 4, 6 or 8 different RBD–np were prepared and analyzed on the same gel (admix) (d). Experiments were independently performed two times with similar results. Size exclusion chromatography profiles of hemagglutinin RBD–np shown in panel c and d (e and f, respectively). Experiments were performed two times with similar results.
Supplementary Fig. 2 Antibody responses elicited by hemagglutinin RBD–np and commercial vaccines to influenza virus.
Serum HAI and neutralization antibody titers to CA09 (a) and BR07 (b). BALB/c mice were immunized with NC99 RBD–np (valence 1) or various preparations of various RBD–np either as admixtures (admix) or as co-assembled particles (Mosaic). Represented data were generated using sera collected 2 weeks after the second immunization with adjuvant (2 µg of RBD–np per immunization). Shown results were obtained from a representative experiment (n = 5). Each dot indicates individual mouse with mean of each group indicated as lines. Animal experiments were independently performed two times with similar results. Statistical analyses are done with one-way ANOVA with Tukey’s multiple comparisons post-hoc test. Non-statistical significance between groups is not shown for clarity. (c) Microneutralization (MN) titers against WS33, KW86, and CC03 viruses of pooled immune sera. Mice (n = 5) were immunized with 2 µg of mosaic RBD–np (8-valent), or 1.5 µg of commercial TIV (from indicated season) formulated with SAS and delivered at weeks 0 and 4. Immune sera were collected at week 6, RDE-treated, heat-inactivated, and pooled for microneutralization assays. Animal experiment was performed once.
Supplementary Fig. 3 Hemagglutinin-binding and H1N1 neutralization of monoclonal antibodies.
(a) Hemagglutinin-binding of monoclonal antibodies measured by biolayer interferometry. Recombinant hemagglutinin trimer proteins were immobilized on HIS1K biosensors through hexa-histidine tag and used to assess binding of monoclonal antibodies. All assays were performed in the Octet HTX instrument (fortéBio) at 30ºC with agitation at 1,000 rpm. PBS with 1% BSA was used as assay buffer for all steps. Plotted data indicate the response value during the mAb association step at 300 s. Control monoclonal antibodies 3u-u, 2D1 and CH65 were shown as gray bars. Experiments were performed two times with similar results. (b) Neutralization capacity of monoclonal antibodies. Pseudotype neutralization assays were used to assess neutralization potency of isolated monoclonal antibodies. Homologous NC99 and heterologous BJ95 strains were selected for screening. Experiments were performed two times with similar results.
Supplementary Fig. 4 Hemagglutinin-binding kinetics of 441D6 Fab by biolayer interferometry.
Binding kinetics measurements were carried out using biolayer interferometry with Octet HTX instrument (fortéBio). Recombinant hemagglutinin trimers were immobilized either on HIS1K or SA sensors through hexa-histidine tag or conjugated biotin, respectively. Fab 441D6 was prepared by introducing HRV3C cleavage site in the heavy chain hinge and digesting with HRV3C enzyme followed by Protein A affinity purification to remove Fc and uncleaved antibodies as described elsewhere7. Dilution series of Fab 441D6 was made in assay buffer (PBS with 1% BSA). All assays were performed in the Octet HTX instrument (fortéBio) at 30ºC with agitation at 1,000 rpm. Represented data contain measured sensograms (red) and global fitting with 1:1 binding model (black) generated by Octet Analysis software (version 9.0, fortéBio). Experiments were independently performed two times with similar results.
Supplementary Fig. 5 Structure determination of HA–441D6 complex by cryo-electron microscopy.
(a) Micrographs indicated the HA–441D6 complex partitioned equally between the carbon surface and carbon holes. (b) Picked particles contributing to the determined structure are indicated by green circles, visualized on the same micrograph shown in a. (c). Model-free 2D class averages reveal 3-fold symmetry of the complex. Experiment was performed once. (d) Class occupancy from 2D classification is plotted corresponding to classes in c. (e) Fourier-shell correlation analysis of ‘gold-standard’ refinement using a cutoff of 0.143 estimates the model resolution as 8.0 Å.
Supplementary Fig. 6 electron microscopy analysis of mosaic RBD–np particle and its complex with 441D6 Fab.
(a) Raw electron microscopy images and representative 2D classes of mosaic RBD–np. Individual particles were visualized by PTA negative staining, revealing the ferritin core particle as either a filled or hollow white circle, depending on stochastic penetration of the ferritin core by the negative stain. RBD domains were seen as points immediately surrounding the ferritin core. The inset shows 4 representative class averages from 60,842 picked particles, after initial picking of 65,517 particles in 174 micrographs. (b) Mosaic RBD–np in complex with 441D6 Fab was similarly analyzed by PTA negative staining electron microscopy. The extra density surrounding the ferritin core is consistent with the presence of a coat of bound 441D6 Fab. The inset shows representative class averages of the complex, but unlike the RBD–np alone, the particles that stained hollow or filled required separate processing as they did not naturally partition into distinct classes during classification. From a total pool of 9,187 particles in 134 micrographs, final class averages of filled particles were made from 6,926 particles, and hollow particles were made from 957 particles. Experiment was performed once. (c) Rotationally averaged images of RBD–np particles (bottom) and 441D6 Fab complexes (top) were synthesized from class averages, and then juxtaposed to illustrate the bulk effect of 441D6 binding. The inner sphere represents the ferritin core, while the middle density of lesser intensity is either RBD or RBD+Fab. The outermost layer resulted from lighter staining of the electron microscopy grid carbon surface, since the negative stain accumulated in higher amounts proximal to particles. (d) Relative mean intensity of the rotational averages was plotted as a function of particle radius. High intensity corresponds to white in images in c. The outer radius of the RBD–np is 13 nm, while mosaic RBD–np–441D6 Fab complexes extend to 15.5 nm from the particle center. We interpret the first minima near 7 nm for the 441D6 complex as accumulation of negative stain between the ferritin core and Fabs that are tethered via the RBD domains at varying orientations.
Supplementary Fig. 7 Therapeutic effect of 441D6 in mouse infection model.
Therapeutic administration of control VRC01 (anti-HIV) and 441D6 monoclonal antibodies in mice infected with A/California/07/2009 (H1N1) (a) or A/Puerto Rico/8/1934 (H1N1) (b). BALB/c mice were intranasally infected with a 10 × LD50 of either H1N1 viruses 24 hours before receiving monoclonal antibodies via intraperitoneal injection. Clinical symptoms and body weight were monitored throughout the study. Lines in plots showing body weight change indicate mean of group with standard deviations including only live animals at indicated time points. Experiment was performed once (n = 10).
Supplementary Fig. 8 Generation of 441D6KO hemagglutinin and its recognition by immune serum.
(a) Generation and characterization of hemagglutinin with 441D6KO mutation. Two point mutations, E116K and K172E were introduced in the NC99 hemagglutinin (highlighted in black on the hemagglutinin molecular surface). Antigenicity of 441D6KO hemagglutinin was assessed by ELISA using 441D6, CH65, and anti-His (Covance) antibodies. Binding curves of antibodies to WT and 441D6KO hemagglutinin were depicted. Assays were independently performed two times with similar results. (b) Serum binding antibody titers to WT and 441D6KO hemagglutinin. The endpoint titers in immune sera raised by NC99 HA-, admix (8-valent)-, mosaic (8-valent)-, and sequential RBD–np immunization were measured by ELISA (n = 10, biologically independent samples). Assays were independently performed two times with similar results. (c) Serum binding antibody titers to WT and 441D6KO hemagglutinin. The endpoint titers were determined as above (n = 26, biologically independent samples). Assays were independently performed two times with similar results. Statistical analyses were done with nonparametric t-test using Wilcoxon matched-paired signed rank test (two-tailed).
Supplementary information
Supplementary Figs. 1-8
Supplementary Figs. 1-8 Supplementary Table 1
Rights and permissions
About this article
Cite this article
Kanekiyo, M., Joyce, M.G., Gillespie, R.A. et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat Immunol 20, 362–372 (2019). https://doi.org/10.1038/s41590-018-0305-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41590-018-0305-x
This article is cited by
-
Elicitation of liver-stage immunity by nanoparticle immunogens displaying P. falciparum CSP-derived antigens
npj Vaccines (2025)
-
Enhancing antibody responses by multivalent antigen display on thymus-independent DNA origami scaffolds
Nature Communications (2024)
-
Vaccine design via antigen reorientation
Nature Chemical Biology (2024)
-
Nanoparticle display of neuraminidase elicits enhanced antibody responses and protection against influenza A virus challenge
npj Vaccines (2024)
-
An attachment glycoprotein nanoparticle elicits broadly neutralizing antibodies and protects against lethal Nipah virus infection
npj Vaccines (2024)