Abstract
A subset of individuals exposed to Mycobacterium tuberculosis (Mtb) that we refer to as ‘resisters’ (RSTR) show evidence of IFN-γ− T cell responses to Mtb-specific antigens despite serially negative results on clinical testing. Here we found that Mtb-specific T cells in RSTR were clonally expanded, confirming the priming of adaptive immune responses following Mtb exposure. RSTR CD4+ T cells showed enrichment of TH17 and regulatory T cell-like functional programs compared to Mtb-specific T cells from individuals with latent Mtb infection. Using public datasets, we showed that these TH17 cell-like functional programs were associated with lack of progression to active tuberculosis among South African adolescents with latent Mtb infection and with bacterial control in nonhuman primates. Our findings suggested that RSTR may successfully control Mtb following exposure and immune priming and established a set of T cell biomarkers to facilitate further study of this clinical phenotype.
Similar content being viewed by others
Main
Nearly 1.7 billion people who have been exposed to Mycobacterium tuberculosis (Mtb) are clinically asymptomatic but show positive results on tuberculin skin tests (TST) or interferon (IFN)-γ release assays (IGRA), indicating they may harbor a ‘latent’ Mtb infection (referred to here as LTBI)1. We have previously described a cohort of Ugandan household contacts who never developed a positive TST or IGRA, despite a high probability of exposure to Mtb. As these individuals did not develop active tuberculosis (TB) over a median of 9.5 years of follow-up, it was proposed that they ‘resist’ Mtb infection2 (hereafter referred to as RSTR). Highly exposed individuals with negative TST and/or IGRA were also reported among healthcare workers, miners and other household contact cohorts3, even when considering variations in timing and strength of exposure, duration of follow-up and frequency of testing4,5, but whether these individuals control Mtb infection is unknown.
Early events following Mtb infection are poorly understood, because most animal models seek to recapitulate active disease. Human TB occurs along a spectrum ranging from clinically apparent disease, asymptomatic infection, and possibly resistance to Mtb infection6. T cells probably mediate resistance to Mtb infection, as several studies have conclusively demonstrated their importance in protecting against disease in humans and animal models2,7,8. However, the specific role of T cell-derived IFN-γ has been debated. CD4+ T cells can control Mtb infection even without production of IFN-γ9,10. Although mendelian defects in IFN-γ-related pathway genes confer susceptibility to mycobacterial disease11, human and mice data suggest that mechanisms other than IFN-γ may be important for protection9,12. We identified Mtb-specific IFN-γ- CD4+ T cells in both RSTR and LTBI that express interleukin (IL)-2, tumor necrosis factor (TNF) and CD154 in the absence of IFN-γ after Mtb exposure5.
Here, we asked whether Mtb-specific CD4+ T cells from RSTR expressed unique functional programs compared to LTBI. To address this question, we first analyzed a Ugandan cohort characterized by low Mtb transmission and found that Mtb-specific IFN-γ−CD4+ T cells were not detectable in the absence of Mtb exposure. Next, we identified several functional programs, including TH17 cell-like, regulatory T cell and memory T cell phenotypes, that were selectively enriched in Mtb-specific CD4+ T cells from RSTR compared to LTBI. We also leveraged public datasets to demonstrate an association between these T cell phenotypes and South African adolescents with LTBI who control Mtb infection, as well as the protective efficacy of intravenous Bacillus Calmette–Guérin (BCG) in nonhuman primates (NHPs). Taken together, our results suggest that RSTR might control Mtb after initial exposure by priming an IFN-γ−CD4+ T cell response.
Results
Mtb-specific IFN-γ−CD4+ T cells are absent in low-exposure individuals
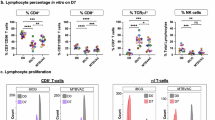
To investigate whether IFN-γ−CD4+ T cell responses were unique to household contacts, we enrolled a cohort of participants from Kampala, Uganda5 who had low exposure risk based on community TB transmission rates (hereafter low-exposure cohort; Supplementary Tables 1 and 2)13. The low-exposure cohort contained participants negative for both TST and IGRA (hereafter TST−IGRA−, n = 17, 9 males and 8 females, aged 18–26 years) (Extended Data Fig. 1a) and sex and age matched TST+IGRA+ individuals (hereafter LTBI, n = 19, 10 males and 9 females, aged 18–42 years). To study Mtb-specific responses, peripheral blood mononuclear cells (PBMC) from TST−IGRA− and LTBI participants were stimulated for 6 h with overlapping peptide pools targeting the Mtb-specific proteins ESAT6 and CFP10, which are absent in BCG vaccines (Extended Data Fig. 1b). We used intracellular cytokine staining (ICS) to identify IFN-γ+ and IFN-γ−CD4+ T cell responses and the expression of cytokines IL-2, IL-4/IL-5/IL-13, IL-17A and TNF and we also assessed the expression of the activation and memory markers CD107a and CD154 (Extended Data Fig. 1c and Supplementary Table 3)14. Four CD4+ T cell functional profiles were detected using the combinatorial polyfunctionality analysis of antigen-specific T cell subsets (referred to as COMPASS)15, three of which were IFN-γ+ (Fig. 1a,b). TST−IGRA− participants did not have IFN-γ− or polyfunctional IFN-γ+ T cell responses, while LTBI had both (Fig. 1b–e)5. Overall, IFN-γ−CD4+ T cells were absent in low-exposure individuals, indicating IFN-γ-independent T cell responses to ESAT6/CFP10 were not observed outside a high-exposure setting, such as household contacts5.
a, Representative flow cytometry showing the expression of IFN-γ and CD154 in CD4+ T cells from TST−IGRA− or LTBI in the low-exposure cohort in response to ESAT6/CFP10 stimulation. b, COMPASS-generated probability heat map of CD4+ T cell subsets including IFN-γ−IL-2+CD154+, IFN-γ+IL-2−CD154+, IFN-γ+IL-2+CD154− and IFN-γ+IL-2+CD154+ cells from TST−IGRA− or LTBI in the low-exposure cohort in response to ESAT6/CFP10. The depth of purple shading correlates to the probability of a participant-specific response above background for a given cell subset. White, no function; black/gray, presence of a function. IFN-γ+ cell subsets are noted in gray. c, The frequencies of COMPASS-identified CD4+ T cell subsets are shown, background corrected by subtracting the frequency of each subset after DMSO stimulation from the frequency after ESAT6/CFP10 stimulation as in b. d, The frequencies of polyfunctional CD4+ T cells that expressed two or more cytokines in response to ESAT6/CFP10 in CD4+ T cells as in c. e, Polyfunctionality score of CD4+ T cells specific to ESAT6/CFP10. In c−e, the statistical significance was calculated using the Wilcoxon rank-sum test. Two-sided P values are shown, while in c the reported P values were adjusted for multiple hypothesis testing using the Bonferroni method.
Mtb-specific RSTR CD4+ T cells have unique phenotypes
We next profiled the Mtb-specific CD4+ T cells in a cohort of TB household contacts (hereafter household contact cohort) in Kampala, Uganda, which contained 45 individuals who remained TST−IGRA− for a median of 9.5 years after exposure5 (RSTR; 22 males and 23 females, aged 14–67 years) and 45 individuals (23 males and 22 females, age 14–63 years) who were TST+IGRA+ at the initiation of the cohort and did not progress to disease for a median of 9.5 years after exposure5 (LTBI). Participants were matched for age, sex and exposure risk score (Supplementary Tables 4 and 5). In the discovery phase, we used PBMC collected at a median of 9.5 years after exposure5 from three RSTR and four LTBI participants (hereafter referred to as the discovery cohort) and performed index sorting and single-cell multiplex targeted transcriptomics on CD69+CD154+ and CD69+CD137+ T cells (hereafter referred to as activated T cells or antigen-specific T cells according to the stimulation antigens) after stimulation of PBMC with ESAT6/CFP10 for 6 h (Extended Data Fig. 1b). This was followed by single-cell whole transcriptomics on clonally expanded activated T cells (the method is hereafter referred to as SELECT-seq16; Fig. 2a and Extended Data Fig. 2a). For T cell phenotyping, index sorting also measured the expression of lineage (CD4 and CD8), memory (CD45RA and CD127) and additional activation (CD25, HLA-DR and CD38) markers, while targeted transcriptomics measured the clonotypic T cell receptor (TCR) ɑ and β sequences, the gene expression of canonical transcription factor usage (TBX21 (T-bet), RORC (RORγt), GATA3, FOXP3, RUNX1 and RUNX3), and characteristic cytokines (IFNG, IL2, IL17A, GZMB, PERF, IL4, IL5, IL13 and TGFB). Using FlowSOM, a tool for clustering and visualizing flow cytometry data, these markers identified 19 cell subsets of ESAT6/CFP10-specific CD4+ T cells (Fig. 2b)17. t-Distributed stochastic neighbor embedding (t-SNE) visualization indicated that some subsets, such as RORC+IL17A+ cells (cluster 6), were enriched in RSTR, while some subsets, such as RORC+IFNG+GZMB+PERF+ cells (cluster 11), were abundant in LTBI (Fig. 2c and Extended Data Fig. 2b). We also determined the T cell phenotypes in 16 RSTR (8 males and 8 females, aged 14–39 years) and 16 LTBI (9 males and 7 females, aged 15–46 years) after 12 h stimulation with whole Mtb lysate, which contains a broader set of antigens conserved across mycobacteria (Supplementary Tables 4 and 5). Index sorting and targeted transcriptomics did not identify qualitative differences in cluster enrichment in Mtb lysate-specific CD4+ T cell phenotypes between RSTR and LTBI (Extended Data Fig. 2c). Clonal expansion of ESAT6/CFP10-specific CD4+ T cells was observed in both RSTR and LTBI participants (Fig. 2d,e and Supplementary Tables 6 and 7), which confirmed the IFN-γ−CD4+ T cell recall responses reported previously5. Clonally expanded CD4+ T cells were focused within the IFNG+ TH1* subsets in LTBI (Fig. 2f), while they did not dominate in any particular T cell subsets in RSTR (Fig. 2f). These data suggested that RSTR harbored Mtb-specific T cell responses with unique functional and phenotypic profiles compared to LTBI.
a, The gating strategy is shown for the sorting of ESAT6/CFP10-specific T cells from RSTR and LTBI in the discovery household contact cohort, based first on the expression of the activation marker CD69 and then CD154 or CD137. b, A heat map showing the median marker expression of ESAT6/CFP10-specific CD4+ T cell subsets from three RSTR and four LTBI participants in the discovery household contact cohort. Clustering was performed on flow cytometry mean fluorescence intensities and binarized read counts of profiled genes. c, Dimensionality reduction (t-SNE) projection of ESAT6/CFP10-specific CD4+ T cell subsets, as in b. The arrows highlight cluster 6 and cluster 11, which were preferentially detected in RSTR or LTBI participants, respectively. d, Distribution of clonal expansion based on TCRβ chain from single-cell targeted transcriptomics in ESAT6/CFP10-specific T cells, as in b. Each dot represents a clone as defined by the TCRβ chain CDR3 sequence. The size of the dot is proportional to the frequency, which is also depicted as log2(counts) on the y axis. e, A box plot showing the median and interquartile range of frequency of TCRβ clonal expansion in ESAT6/CFP10-specific CD4+ T cells with whiskers representing minima and maxima. No statistical test was performed due to the small sample sizes. f, A histogram indicating the proportion of clonally expanded cells in ESAT6/CFP10-specific CD4+ T cell clusters (clusters 1–19), as in b, which were detected more than once in participants among the household contact cohort.
RSTR and LTBI Mtb-specific CD4+ T cells have a stem memory T (TSCM) cell phenotype
Next, we applied SELECT-seq16 to deeply profile the clonally expanded ESAT6/CFP10-specific CD4+ T cells. After sequencing quality control filtering, we analyzed 524 CD4+ T cells (Extended Data Fig. 3a) and found that the major transcriptional variance was between the RSTR and LTBI group (Extended Data Fig. 3b). Differential gene expression analysis identified 582 genes upregulated in RSTR compared to LTBI and 156 genes upregulated in LTBI compared to RSTR (Methods, Fig. 3a and Supplementary Table 8). The top upregulated genes in LTBI were proinflammatory (IFNG, TNF and IL2) and cytotoxic (GZMB and PERF) genes, aligning with an inflammatory response and IFN-γ production identified by Gene Ontology (GO) analysis (Supplementary Tables 9–11). The top upregulated genes in RSTR were linked with T cell activation (SASH3, TNFRSF4 and IKZF1) and cell membrane receptor (CCR7 and ITGAL) (Fig. 3a). GO enrichment in RSTR identified translation, cell–cell adhesion, cell movement, mitochondrial electron transport (Fig. 3b and Supplementary Tables 9–11), a process associated with oxidative phosphorylation and early T cell differentiation18, as well as T cell activation. Aligning with these, ESAT6/CFP10-specific CD4+ T cells from RSTR had higher expression of TCF7, FOXP1 and CCR7, suggesting a less differentiated and naive-like phenotype, higher ITGAL (encodes LFA1) and ITGA1 (VLA1) expression, suggesting higher trafficking mobility and homing to lymph nodes and airways19,20, and lacked expression of cytokines such as IFNG, IL2 and TNF compared to LTBI (Fig. 3c,d). The enrichment of T cell activation genes and the lack of cytokine production after short-term ESAT6/CFP10 stimulation suggested that RSTR ESAT6/CFP10-specific CD4+ T cells were likely to be TSCM cells, rather than naive T cells. The TSCM cell phenotype was further supported by the expression of the transcription factor TCF7 and an enrichment in genes belonging to the Wnt signaling pathway (Fig. 3b,d)21. Overall, SELECT-seq indicated that RSTR CD4+ T cells were less differentiated, had less inflammatory cytokine activities and more cell trafficking mobility and cell–cell adhesions compared to LTBI, suggesting a CD4+ TSCM cell phenotype.
a, A volcano plot depicting DEGs of clonally expanded activated T cells between RSTR (cell number, 231) and LTBI (cell number, 293) from the discovery household contact cohort in response to ESAT6/CFP10 based on SELECT-seq. Red, genes with |log2(FC)| >0.5 and P value <0.05. b, GO Biological Process terms related to metabolic and basic activities based on upregulated DEGs from RSTR, as in a. False discovery rate (FDR) was calculated by a modified Fisher’s exact test with FDR correction. c, A heat map displaying the mean expression of genes involved in migration, adhesion or cytokine production in each RSTR and LTBI participant in the discovery household contact cohort. The mean expression level was calculated as the mean of the scaled log-normalized gene counts. d, Violin plots depicting the expression of stem memory like T cell genes (CCR7, FOXP1 and TCF7) in RSTR and LTBI, as in c. Statistical significance was calculated using two-sided Wilcoxon rank-sum tests with the Bonferroni method. *Adjusted P value <0.05, **adjusted P value <0.005, ***adjusted P value <0.001. e, Gating strategy for naive-like CD45RA+CCR7+ T cells in ESAT6/CFP10-specific CD4+ T cells from RSTR (n = 17) and LTBI participants (n = 20) in the validation household contact cohort. f, A box plot showing the median and interquartile range of frequencies of naive-like CD45RA+CCR7+CD4+ T cells in ESAT6/CFP10-specific CD4+ T cells, as in e, with whiskers representing minima and maxima. Statistical significance was determined by the two-sided Wilcoxon rank-sum test, and the unadjusted P value is shown.
To validate the CD4+ TSCM cell phenotype, we assessed 18 additional RSTR (8 males and 10 females, aged 15–39 years) and 20 LTBI (10 males and 10 females, aged 15–63 years) from the household contact cohort who were not included in the discovery cohort (hereafter referred to as the validation cohort; Supplementary Tables 4 and 5). Flow cytometry (17 RSTR and 20 LTBI) on PBMC from the validation cohort stimulated with ESAT6/CFP10 for 12 h or whole Mtb lysate for 12 h (Fig. 3e and Extended Data Fig. 4a) indicated a trend toward a higher proportion of naive-like CD45RA+CCR7+CD4+ T cells in RSTR ESAT6/CFP10-specific CD4+ T cells compared to LTBI (16.85% versus 13.21%, P = 0.069) (Fig. 3f), while in the same participants, Mtb lysate-specific CD4+ T cells had similar frequencies of naIve-like CD45RA+CCR7+CD4+ T cells in RSTR (23.11%) versus LTBI (24.01%) (Extended Data Fig. 4b).
To explore whether the CD45+CCR7+CD4+ T cells in RSTR had a naïve or TSCM cell phenotype, PBMC from RSTR (n = 12) and LTBI (n = 12) from the validation cohort were stained with carboxyfluorescein succinimidyl ester (CFSE) and stimulated with ESAT6/CFP10 or whole Mtb lysate overnight and activated naive-like CD69+CD154+CCR7+CD45RA+CD45RO− lymphocytes were sorted by flow cytometry and cultured for 7 days to assess antigen-specific expansion22 (Extended Data Fig. 5a–c and Supplementary Table 3). At the time of sorting, we observed a significantly higher proportion of naive-like CCR7+CD45RA+CD45RO− T cells coexpressing the TSCM cell marker CD95 in LTBI compared to RSTR participants, regardless of stimulation (Extended Data Fig. 5d). The frequency of naive-like CD95+ T cells (hereafter TSCM cells)22 that proliferated in response to whole Mtb lysate or ESAT6/CFP10 was similar in RSTR and LTBI (Extended Data Fig. 5c,e). However, the Mtb lysate-specific TSCM cells in both RSTR and LTBI participants preferentially differentiated into CCR7+CD45RA− central memory T cells, while ESAT6/CFP10-stimulated TSCM cells resulted in a higher frequency of CCR7−CD45RA− effector memory T cells (Extended Data Fig. 5f,g). Taken together, Mtb-specific TSCM cells were found at similar frequencies in RSTR and LTBI, and proliferated and differentiated into various memory phenotypes after antigen re-encounter.
Mtb-specific CD4+ T cells in RSTR have a Treg phenotype
Next, we investigated the activation phenotypes of the clonally expanded ESAT6/CFP10-specific CD4+ T cells identified by SELECT-seq. GO analysis on these T cells identified T cell activation pathways, including MAPK signaling, TCR signaling, TNF signaling and noncanonical NF-κB signaling, that were enriched in RSTR compared to LTBI in the household contact cohort (Fig. 4a and Supplementary Tables 9–11). Genes encoding costimulatory molecules such as IL2RB, FAS (CD95), TNFRSF4 (OX40), ICOS, CTLA4 and IKZF1 were significantly upregulated in clonally expanded ESAT6/CFP10-specific CD4+ T cells in RSTR compared to LTBI (Fig. 4b). To further explore the CD4 signaling interactions in these RSTR cells, we conducted a protein–protein interaction network analysis on 119 immune-related genes that were upregulated in RSTR compared to LTBI. Three network clusters were identified from the genes upregulated in RSTR: a CD4 activation network centered on CTLA4, which encodes for a T cell checkpoint inhibitor, an innate immunity network, and a lymphocyte trafficking network (Supplementary Tables 12–14). The CD4 activation network identified molecules with extensive interactions with other molecules, including CTLA4, IKZF1, CD5, CCR7, IL2RB and BATF, which were defined as key signaling hubs (Fig. 4c and Supplementary Tables 15 and 16). Based on the identification of CTLA4 as a key signaling hub in RSTR, we investigated whether the SELECT-seq clonally expanded ESAT6/CFP10-specific CD4+ T cells exhibited a regulatory T (Treg) cell phenotype. We found higher expression of several Treg cell-associated genes, including IKZF2 (Helios) and TNFRSF18 (GITR), in clonally expanded ESAT6/CFP10-specific CD4+ T cells from RSTR compared to LTBI (Fig. 4d)23. Targeted transcriptomics also suggested a higher frequency of ESAT6/CFP10-specific FOXP3+ CD25+ CD4+ T cells among RSTR compared to LTBI (Fig. 4e).
a, GO terms related to T cell activation in clonally expanded ESAT6/CFP10-specific CD4+ T cells among RSTR (cell number, 231) compared to LTBI (cell number, 293) from the discovery household contact based on SELECT-seq. b, Expressions of activation and costimulation genes in RSTR and LTBI, as in a. Statistical significance was calculated by two-sided Wilcoxon rank-sum tests with the Bonferroni method. **Adjusted P value <0.005, ***adjusted P value <0.001. c, Interaction network of DEGs related to T cell activation upregulated in RSTR compared to LTBI, as in a. d, Mean expression of Treg cell-associated genes within each RSTR and LTBI, as in a. e, A box plot showing the median and interquartile range of frequencies of FOXP3+CD25+ Treg cells in ESAT6/CFP10-specific CD4+ T cells from the same RSTR (n = 3) and LTBI (n = 4) participants as in a using index sorting and targeted transcriptomics. The whiskers represent minima and maxima. No statistical test was performed due to small sample sizes. f, Gating strategy for CD25+ T cells and Foxp3+CD25+ Treg cells in ESAT6/CFP10-specific CD4+ T cells from RSTR (n = 17) and LTBI participants (n = 20) in the validation household contact cohort. g, Frequencies of CD25+CD4+ T cells in ESAT6/CFP10-specific CD4+ T cells are shown as in f. h, Frequencies of Foxp3+CD25+ Treg and IL-10+ CD4+ T cells in ESAT6/CFP10-specific CD4+ T cells are shown as in f. i, MFIs of IL-10 in supernatants after 48 h ESAT6/CFP10 or 12 h Mtb lysate stimulation based on multiplex cytokine analysis. Significance was determined by two-sided Student’s t-test. Significance in g and h was determined by two-sided Wilcoxon rank-sum tests.
To assess whether Mtb-specific CD4+ Treg cells were enriched in RSTR compared to LTBI, we performed flow cytometry and multiplex cytokine analysis (ProcartaPlex) on the validation cohort to determine the concentrations of 28 secreted cytokines in conditioned supernatants from cultured PBMC poststimulation with ESAT6/CFP10 or Mtb lysate for 6, 12, 24 and 48 h (Extended Data Figs. 7 and 8). The frequency of CD25+ cells in total CD4+ T cells and ESAT6/CFP10-specific CD4+ T cells was higher in RSTR than in LTBI (total, 17.26% versus 14.94%, P = 0.028; ESAT6/CFP10-specific, 66.83% versus 63.54%, P = 0.065) (Fig. 4f,g and Extended Data Fig. 6a). ESAT6/CFP10-specific CD4+ T cells coexpressing protein markers Foxp3 and CD25 were detectable in both RSTR and LTBI at equal frequencies (25.89% versus 24.00%, P = 0.424) (Fig. 4f,h). However, in the same participants, we also observed a higher frequency of both CD25+CD4+ T cells and FoxP3+CD25+CD4+ T cells in RSTR compared to LTBI (CD25+, 62.98% versus 57.16%, P = 0.052; FoxP3+CD25+, 19.44% versus 16.13%, P = 0.013) after Mtb lysate stimulation (Fig. 4g,h). While the frequency of IL-10+ cells in ESAT6/CFP10-specific CD4+ T cells was comparable between RSTR and LTBI using flow cytometry (0.37% versus 0.33%, P = 0.532), the concentration of IL-10 in conditioned supernatants post-ESAT6/CFP10 stimulation was higher in LTBI compared to RSTR (Fig. 4h,i). Within the same participants, IL-10 concentrations in supernatants after Mtb lysate stimulation trended higher in RSTR than LTBI (Fig. 4i). The concentration of TGF-β in supernatants post-ESAT6/CFP10 stimulation was similar between RSTR and LTBI (Extended Data Fig. 6b). Finally, the ratio of anti-inflammatory FoxP3+CD25+ Treg cell fraction to proinflammatory T cell fraction, which included RORγt+T-bet+ TH1* cells, RORγt+T-bet− TH17 cells and RORγt−T-bet+ TH1 cells, was significantly higher in RSTR than LTBI (Extended Data Fig. 6c). Together, these data indicated that RSTR Mtb-specific T cells exhibited a distinct activation profile, enriched for a Treg cell phenotype, compared to LTBI.
Mtb-specific RSTR CD4+ T cells have a TH17 cell-like phenotype
Next, we investigated the polarization of ESAT6/CFP10-specific CD4+ T cell functions in PBMC from RSTR from the discovery household contact cohort. Targeted transcriptomics revealed that RORC+TBX21+ TH1* or RORC−TBX21+ TH1 CD4+ T cells were more abundant in ESAT6/CFP10-specific CD4+ T cells among LTBI than RSTR (Fig. 5a), which is consistent with known LTBI phenotypes24, while RORC+TBX21− TH17 CD4+ T cells were enriched in RSTR (Fig. 5a and Extended Data Fig. 3c). SELECT-seq showed increased expression of several TH17 cell-associated genes, including BATF, RORA and STAT3, in clonally expanded ESAT6/CFP10-specific CD4+ T cells from RSTR compared to LTBI (Fig. 5b,c; GO:0072539 from MSigDB database)25,26, while the expression of TH1 and TH1* cell-associated genes IL2, TBX21 and CXCR3 was increased in LTBI (Fig. 5b,c).
a, Box plots showing the median and interquartile range of frequencies of RORC+TBX21+, RORC+TBX21−, RORC−TBX21+ and RORC−TBX21− cells in ESAT6/CFP10-specific CD4+ T cells in RSTR (n = 3) and LTBI (n = 4) in the discovery household contact cohort using targeted transcriptomics. The whiskers represent minima and maxima. Statistical testing was not performed due to small sample sizes. b, SELECT-seq showing the mean expression of genes associated with TH1 or TH17 phenotypes from GO:0072539 in the MSigDB database in clonally expanded ESAT6/CFP10-specific CD4+ T cells within both RSTR and LTBI in the household contact cohort as in a. c, Expressions of TH1 or TH17 cell-associated genes in RSTR and LTBI in the household contact cohort, as shown in a. Significance was determined using two-sided Wilcoxon rank-sum tests with the Bonferroni method. ***Adjusted P value <0.001. d, Representative flow cytometry showing the expression of RORγt and T-bet in ESAT6/CFP10-specific CD4+ T cells from RSTR (n = 17) and LTBI participants (n = 20) in the validation household contact cohort. e, Frequencies of RORγ+T-bet+, RORγt+T-bet−, RORγt−T-bet+ and RORγt−T-bet−cells in ESAT6/CFP10-specific CD4+ T cells or Mtb lysate-specific CD4+ T cells from RSTRs and LTBI, as in d. f, Frequencies of IL-17A+ cells in ESAT6/CFP10-specific CD4+ T cells or Mtb lysate-specific CD4+ T cells from RSTRs and LTBI as in d. g, MFIs of IL-17A or IL-23 in supernatants of PBMC are shown after 24 h or 6 h stimulation, respectively, with Mtb lysate. Significance was determined by two-sided Student’s t-test. Significance in e and f was determined by two-sided Wilcoxon rank-sum tests.
To test whether Mtb-specific TH17-like cells were enriched in RSTR compared to LTBI, we performed flow cytometry and multiplex cytokine analysis on the validation cohort. After ESAT6/CFP10 stimulation, we found an increased frequency of RORγt+T-bet+ or CXCR3+CCR6+ TH1* and RORγt−T-bet+ TH1 cells in LTBI Mtb-specific CD4+ T cells compared to RSTR (RORγt+T-bet+, 15.6% versus 12.9%, P = 0.020; CXCR3+CCR6+, 16.7% versus 12.3%, P < 0.001; and RORγt−T-bet+, 41.9% versus 30.22%, P < 0.001) compared to RSTR (Fig. 5d,e and Extended Data Fig. 9a,b)24. Conversely, we detected an increased frequency of RORγt+T-bet− TH17 T cells in RSTR compared to LTBI (5.86% versus 2.36%, P < 0.001) (Fig. 5e). Similarly, after Mtb lysate stimulation, the frequency of RORγt+T-bet+ or CXCR3+CCR6+ TH1* CD4+ T cells was higher in LTBI (RORγt+T-bet+, 20.3% versus 15.3%, P = 0.039 and CXCR3+CCR6+, 20.2% versus 13.5%, P = 0.003), while the frequency of RORγt+T-bet− TH17 CD4+ T cells was higher in RSTR (2.66% versus 1.72%, P = 0.011) (Fig. 5e and Extended Data Fig. 9b). ICS and multiplex cytokine analysis found higher production of IFN-γ in PBMC from LTBI than RSTR after both stimulations (Extended Data Fig. 7, 8 and 9b). IL-17A+ cells trended higher in RSTR Mtb-specific CD4+ T cells compared to LTBI after both stimulations, although this was not statistically significant (ESAT6/CFP10,1.24% versus 0.95%, P = 0.104 and Mtb lysate, 1.39% versus 1.20%, P = 0.209) (Fig. 5f). Multiplex cytokine analysis showed that expression of IL-17A increased in both RSTR and LTBI after ESAT6/CFP10 and Mtb lysate stimulations (Fig. 5g and Extended Data Fig. 7). We also noted higher amounts of the TH17 cell-promoting cytokine IL-23 in RSTR conditioned supernatants post-Mtb lysate stimulation than LTBI (Fig. 5g). Thus, RSTR Mtb-specific T cells were biased toward a TH17 cell-like functional program, rather than the TH1* cell program observed in LTBI.
Mtb-specific RSTR CD4+ T cell phenotypes associate with bacterial control
To further contextualize our findings, we analyzed public data from cohorts with clinically relevant endpoints. We leveraged data from the Adolescent Cohort Study (ACS), a longitudinal study of LTBI adolescents in South Africa, in which blood was collected every 6 months over 2 years to identify blood correlates of TB risk27,28. To determine whether the T cell transcriptional programs identified in RSTR were associated with a lack of progression to active TB disease, we studied 144 ACS participants who had whole-blood transcriptomic data, among which 43 participants progressed to microbiologically confirmed TB (progressors) and 101 did not develop TB (nonprogressors), and calculated gene module scores across all time points as the mean expression level of functional associated genes. A ‘TN_TSCM module score’ composed of seven genes related to the naive-like (TN) or TSCM cell phenotype we describe here, including CCR7 and TCF7 (Methods)29, was higher in nonprogressors than progressors up to 180 days before the onset of TB disease (Fig. 6a). There was a trend toward a higher score in nonprogressors compared to progressors as far back as 2 years before disease onset (Fig. 6a). A ‘T cell activation module score’, composed of 24 genes identified through network analysis30 in clonally expanded ESAT6/CFP10-specific CD4+ T cells among RSTR (Methods, Supplementary Tables 15 and 16 and Supplementary Fig. 4c), including CTLA4, IL2RB and IKZF1, was enriched in nonprogressors compared to progressors up to 180 days before disease onset (Fig. 6b). This association was also significant up to 2 years before disease onset (Fig. 6b). Thus, we found an enrichment of naive-like and T cell activation transcriptional programs among a subset of South African adolescents with LTBI who did not progress to active TB disease compared to those who did progress.
a,b, Box plots showing the median and interquartile range of RSTR-associated TN_TSCM cell module (a) or T cell activation module scores (b) from whole-blood bulk transcriptomics at serial time points before TB diagnosis among nonprogressors (n = 101) and progressors (n = 43) in the ACS (GSE79362) (ref. 28). The whiskers represent minima and maxima. Scores were computed as geometric mean of gene expressions. Significance was determined by two-sided Student’s t-test. c, Frequencies of Mtb-specific CD4+ T cells expressing RORC and/or TBX21 or FOXP3 among TB nonprogressors (n = 35) and progressors (n = 35) in ACS31 using single-cell targeted transcriptomics. Significance was determined by two-sided Wilcoxon rank-sum tests. d,e, Heat maps showing the mean expression of the top 15 enriched genes in a stem-like T cell subset (d) and a T1–T17 cell subset (e) identified in granulomas from cynomolgus macaques in clonally expanded ESAT6/CFP10-specific CD4+ T cells in RSTR (cell number, 231) and LTBI (cell number, 293) in the household contact cohort. f, Stem-like T cell- and T1–T17 T cell-associated gene module scores as in d. g, RSTR-associated TH17 cell gene module score from whole-blood bulk transcriptomics in rhesus macaques at day 0 (W0), day 2 (D2), week 2 (W2), week 4 (W4) and week 12 (W12), postintravenous vaccination with BCG. h, Flow cytometry showing cell count and frequency of IFNγ−IL-2−IL-17+TNF− TH17 CD4+ cells in the bronchoalveolar lavage of BCG-vaccinated rhesus macaques as in g. For c and f–h, significance was calculated using the two-sided Wilcoxon rank-sum tests.
As the associations within whole-blood transcriptomes did not necessarily implicate T cells directly, we analyzed the frequency of CD4+ T cell subsets using publicly available targeted transcriptomic data31 from the ACS cohort in response to stimulation with whole Mtb lysate (35 progressors and 35 nonprogressors, with all time points collapsed). The frequency of RORC+TBX21+ TH1* or RORC−TBX21+ TH1 cells in Mtb-specific CD4+ T cells was similar between progressors and nonprogressors (Fig. 6c). However, the frequencies of RORC+TBX21− TH17 and FOXP3+ Treg were higher in nonprogressors than progressors (TH17, 16.0% versus 13.3%, P = 0.01 and Treg, 3.01% versus 2.40%, P = 0.07) (Fig. 6c), indicating that RSTR-defined Treg cell-like and TH17 cell-like phenotypes were enriched in South African adolescents with LTBI who did not progress to active TB disease.
Finally, we sought to examine whether RSTR CD4+ T cell phenotypes were associated with bacterial control by analyzing published NHP studies in which the Mtb burden was quantified after natural infection or experimental vaccination. We first leveraged a study of 26 granulomas from four cynomolgus macaques assessed at 10 weeks after low-dose infection with Mtb (<10 colony forming units (c.f.u.)) using single-cell RNA sequencing (RNA-seq)32 in which several T cell clusters, including GZMB+S100A10+ cytotoxic, MKI67+ proliferating, CCR7+TCF7+ stem-like and TXNIP+CCR6+ T1–T17 cell populations, were associated with reduced bacterial burden32. We found higher expression of the top genes in the macaque stem-like cell subset (such as CCR7 and TCF7; Fig. 6d) and T1–T17 population 1 subset genes (such as CCR6 and TXNIP; Fig. 6e) in clonally expanded activated CD4+ T cells in RSTR compared to LTBI. The associated gene module scores composed of the top 15 genes from each subset were significantly higher in RSTR than LTBI (Methods and Fig. 6f). We also examined the blood transcriptomes and T cell phenotypes in 34 rhesus macaques that were vaccinated intravenously with BCG, challenged with Mtb 24 weeks postvaccination, and assessed for Mtb burden by necropsy at 36 weeks postvaccination or upon development of humane endpoint clinical signs (c.f.u. <100 designated as protected and c.f.u. >100 nonprotected) to identify correlates of protective immunity33,34. Expression of a TH17 cell gene module based on genes enriched in clonally expanded activated CD4+ T cells from RSTR in Fig. 5b (CCR6, RORA, CCR4 and BATF) was higher in protected macaques than nonprotected ones (Methods and Fig. 6g). Similarly, the absolute number, but not the frequency of purified protein derivative (PPD)-specific IFN-γ−IL-2−TNF−IL-17+ CD4+ T cells in bronchoalveolar lavage was higher in protected macaques than nonprotected ones (Fig. 6h). As such, TH17 cell-like functional programs that were enriched in Mtb-specific T cells in RSTR were also associated with the control of Mtb infection in NHP models.
Discussion
Here, we showed that Mtb-specific IFN-γ− T cell responses to the Mtb antigens ESAT6/CFP10 were not detectable in a low-exposure cohort in Uganda, but were clonally expanded in individuals in a household contact cohort who ‘resist’ Mtb infection, indicating the IFN-γ− T cell functional profile may be a reliable measure of Mtb exposure and adaptive immune priming. RSTR Mtb-specific T cells expressed Treg cell and TH17 cell-like phenotypes compared to LTBI, who were characterized by TH1 and TH1* phenotypes. TH17-like and Treg cell transcriptional programs were also observed in a publicly available cohort of adolescent LTBI nonprogressors, suggesting an association with protection along the clinical spectrum of human TB. Finally, The TH17 cell-like functional programs enriched in RSTRs were also associated with early Mtb control in natural and experimental infections in NHP models. Our findings suggest that RSTR may control Mtb after initial exposure and define a set of T cell biomarkers that could be used to identify RSTR in other populations after high-intensity exposure to Mtb.
Our data support an important role for Mtb-specific T cells expressing IL-17 in the absence of IFN-γ. Studies in mice that received an ESAT6 subunit vaccine indicated a role for IL-17A-producing CD4+ T cells in recruiting TH1 cells, which directly restricted bacterial growth35. Consistent with this, RORC loss-of-function mutations in humans result in impaired IFN-γ response to mycobacteria36. A longitudinal study of Mtb-exposed household contacts in Peru found a reduction in TH17-like effector cells in individuals who previously developed active TB disease compared to those who did not37. In a repeated limiting-dose challenge model in NHPs, mucosal TH17 cells correlated with protective immunity38. Group 3 innate lymphoid cells mediated protection against Mtb through IL-17 and IL-22 induction of CXCL13 and the formation of lymphoid follicles within granulomas39. Here, we found an expansion of TH17 cell-like transcriptional program in LTBI adolescents who did not to progress to active TB over 2 years of observation compared to progressors in a South African cohort. These data support the notion that LTBI is heterogeneous and consists of individuals at risk for and individuals protected from Mtb disease. Future work may help replace the terms RSTR and LTBI, which are defined by IFN-γ+ immunity, with more clinically informative definitions.
Our results are not inconsistent with a model in which Mtb is recognized and eliminated without the assistance of T cells at the earliest stages of exposure. Alveolar macrophages are among the first airway immune cells to encounter Mtb and are generally permissive to Mtb growth40. Studies of blood-derived myeloid cells have revealed differences in the transcriptional response between RSTR and LTBIs in household contacts in Uganda41,42 and a cohort of miners in South Africa43. A study of Mtb-exposed household contacts in Indonesia reported higher IL-6 concentrations after in vitro stimulation with Escherichia coli among those who remained persistently IGRA− compared to those who became IGRA+44. Notably, IL-6 and TGF-β are required to prime TH17 responses45. These data support a model whereby differences in the early innate immune response to Mtb infection might result in differences in T cell priming. Antibodies also contribute to bacterial control through a variety of mechanisms, including activating antibacterial programs within macrophages46. Monoclonal antibodies derived from TST− Mtb-exposed healthcare workers confer protection against Mtb challenge in mice47. Thus, non-T cell mechanisms may act in concert to reduce the bacterial load and tune the inflammatory environment to prime the T cell phenotypes that we observed.
Mtb-specific IFN-γ+ CD4 T cells may be a reliable proxy for established infection with Mtb. The concentration of IFN-γ in IGRA supernatants is associated with progression to active TB48,49. The frequency of Mtb-specific IFN-γ+ CD4 T cells increases along the spectrum of IGRA nonconverters (persistently IGRA−), reverters (previously IGRA+, but now IGRA−) and persistently IGRA+ South African adolescents50. Our study characterized the phenotypes of T cells targeting ESAT6/CFP10, which are specific for Mtb and incorporated into IGRA and whole Mtb lysates, which contain many more antigens that are shared across many mycobacterial species. However, we did not identify the additional antigens present in Mtb lysate driving this T cell response. In a study of Mtb-infected mice and BCG-vaccinated humans, Mtb infection drove ESAT6-specific T cells to become more differentiated than T cells specific for Ag85B, consistent with the observation that Mtb restricts expression of Ag85B, but not ESAT6, during chronic infection51,52,53. The LTBI nonprogressors that we studied here were shown to preferentially target PE13 and CFP10 when compared to progressors31. Similar efforts may define antigens present in Mtb lysate that are preferentially targeted by RSTR compared to LTBI.
Methods
Low-exposure cohort
The low-exposure cohort was enrolled from a low TB incidence district that was identified by the Kampala Capital City Authority based on low TB transmission rates13. Subjects were screened and enrolled for health assessment and blood draws between 2017 and 2018 in Uganda (Extended Data Fig. 1). All healthy, nonpregnant participants were eligible. A total of 247 individuals were approached in this district, of which 230 consented and were screened for previous TB treatment, pregnancy, medications and serious illnesses. A total of 220 healthy, nonpregnant individuals were enrolled, and 211 of the enrolled participants were found to be noninfected with human immundeficiency virus (HIV). All participants reported no known contact with a TB case. Based on TST and IGRA (QuantiFERON-TB (QFT) Gold) concordance, we were able to classify 196 HIV-noninfected individuals as either TST+IGRA+ concordant, TST+IGRA− discordant, TST−IGRA− concordant or TST−IGRA+ discordant. For the present study, we selected 17 TST−IGRA− subjects (mean age of 21.59 years; 9 males and 8 females) and 19 TST+IGRA+ (LTBI) subjects (mean age of 24.47 years; 10 males and 9 females) after matching for age and sex.
Household contact cohort
The full details of this cohort have been previously published54. Initially, household contacts of sputum culture-positive cases of pulmonary TB were enrolled between 2002 and 2012 as part of the Kawempe Community Health Study. At baseline, individuals who had no active Mtb infection determined by sputum culture and radiology were enrolled. Upon enrollment, individuals were longitudinally screened during a 2-year follow-up period by TST (Mantoux method, 0.1 ml of 5 tuberculin units of PPD, Tubersol; Connaught Laboratories), in which a positive TST was defined as an induration of >10 mm for individuals noninfected with HIV and >5 mm for individuals infected with HIV. In this initial study, a total of 2,585 individuals were enrolled in the household contact cohort. Of these individuals, 198 (10.7%) remained persistently TST negative over the 2-year follow-up period upon their enrollment.
Between 2014 and 2017, 691 household contacts from the initial study were identified as eligible for retracing according to the epidemiologic risk score criteria previously published54. Of these individuals, 441 (63.8%) were enrolled in a subsequent longitudinal follow-up retracing study. The mean time between enrollment in the initial study and completion of the retracing study was 9.5 years. During the retracing study, individuals completed three QFT assays over 2 years. On their final visit, individuals also underwent the TST (positive TST defined above). RSTR were classified as such if all TST assays (five from the initial study and one at the end of the retracing study) and the three QFTs from the retracing study were concordantly negative, while LTBI participants were classified as such if all TST and QFT assays were positive. PBMC were isolated from whole blood collected during the retracing study by Ficoll–Hypaque density centrifugation and cryopreserved until use.
For the present study, we selected PBMC from a subset of 45 LTBI (mean age of 23.9 years; 23 males and 22 females) and 45 RSTR (mean age of 22.8 years; 22 males and 23 females) subjects from the retracing study after matching for age, sex, exposure risk score and documented lack of HIV co-infection (Supplementary Tables 4 and 5). No power calculations were performed to predetermine sample sizes, but our sample sizes are similar to those reported in our published studies of this cohort5,41. All study subjects gave written, informed consent, approved by the National AIDS Research Committee, the Uganda National Council for Science and Technology and the institutional review board at University Hospitals Cleveland Medical Center.
ACS
The ACS is a published cohort that followed healthy adolescents (n = 6,363; aged 12–18 years) from South Africa and monitored them for progression from latent TB infection to active disease over 2 years28. We obtained publicly available bulk RNA-seq data measured in whole-blood samples every 6 months for 2 years from 144 individuals (aged 12–18 years; 48 males and 96 females)28,31. At baseline, adolescents were diagnosed with LTBI by a positive QFT assay (>0.35 IU ml−1) and/or a positive TST (>10 mm). Progression to active TB was determined by evidence of intrathoracic disease (two positive sputum smears or one positive Mycobacteria Growth Indicator Tube liquid culture). Of these 144 individuals, 43 progressed to active TB (referred to here as progressors), while the remaining 101 did not develop active TB during the study period (referred to here as nonprogressors). Progressors only include individuals who developed active TB over 6 months after enrollment (or the first positive TST or positive QFT assay) to exclude early asymptomatic disease. From the same study, we also obtained publicly available data on single-cell targeted transcriptomics performed on sorted CD4+ T cells of PBMC after stimulation with Mtb lysate obtained at the same time points mentioned above, in which there were 70 participants with 35 progressors and 35 nonprogressors31. All individuals were HIV-uninfected.
NHPs
We obtained publicly available gene sets from single-cell sequencing data of granuloma homogenate from cynomolgus macaques (n = 4; mean age of 7 years, 3 males and 1 female)32. The cynomolgus macaques were infected with low-dose Mtb via bronchoscopic instillation (<10 c.f.u., Erdman strain). Infection was confirmed at 4 weeks by positron emission tomography computed tomography and monitored until necropsy 10-weeks postinfection by clinical and radiographic examinations. We utilized gene sets from single-cell sequencing data obtained from 26 granulomas sampled 10-weeks postinfection at necropsy.
Additionally, we obtained whole-blood transcriptome data and flow cytometry data of bronchoalveolar lavage fluid from rhesus macaques (n = 34; median age of 4.4 years, 16 males and 18 females)33,34. The rhesus macaques were randomized into six vaccine groups (based on birth colony, gender and prevaccination CD4 T cell responses to Mtb PPD) and received intravenous BCG vaccination of varying doses (4.5–7.5 log10 c.f.u. in half-log increments). The rhesus macaques received Mtb challenge via bronchoscope (average 12 c.f.u., Erdman strain) 24 weeks postvaccination. We analyzed bulk RNA-seq data from whole blood collected at baseline, 2 days, 2 weeks, 4 weeks and 12 weeks postvaccination. We analyzed flow cytometry data from bronchoalveolar lavage fluid collected at baseline, 2 weeks, 4 weeks, 8 weeks and 12 weeks postvaccination. The rhesus macaques were euthanized either 36 weeks postvaccination or when they developed clinical signs of humane endpoints, and total Mtb c.f.u. was measured upon necropsy.
Antigens
Overlapping peptide pools targeting ESAT6 and CFP10 were used to stimulate T cells for these studies (BEI Resources). Peptides were 15 or 16 mers with 11 or 12 amino acid overlaps for ESAT6 protein and 11 amino acid overlaps for CFP10. Mtb whole-cell lysate from H37Rv was also used to stimulate T cells (BEI Resources). Dimethyl sulfoxide (DMSO) (Sigma-Aldrich) was used as a negative control. Staphylococcus enterotoxin B (List Biological Laboratories) was used as a positive control for the low-exposure cohort.
ICS
ICS was performed on samples from the low-exposure cohort as we have previously described5. The same ICS assay and flow cytometry acquisition method was performed on samples from the household contacts with minor modifications. Before staining, samples from the household contacts were divided in half to be analyzed using two multiparameter flow cytometry panels, one for the analysis of Treg subsets and one for TH subsets (panel details in Supplementary Table 3). Cells were permeabilized and underwent intracellular staining using the eBioscience Foxp3/Transcription Factor Staining Buffer Set (eBioscience) according to the manufacturer’s directions to allow for the analysis of transcription factors. In the household contacts data, PBMC from two RSTR subjects were excluded from data acquisition and analysis due to bacterial contamination of the samples after overnight rest. The investigators were blinded to group allocation during acquisition of flow cytometry data in the validation household contact cohort.
SELECT-seq on Mtb-reactive T cells
We conducted single-cell whole transcriptomics using the SELECT-seq protocol, which includes stimulation and sorting procedures16,31. In brief, PBMC from RSTR and LTBI samples were stimulated as described in the ICS methods above. Notably, before the stimulation, the cells were cultured in 1 μg ml−1 anti-CD154 antibody for 30 min to prevent CD154 downregulation. After stimulation, cells were first stained (Supplementary Table 3) and index sorted on live CD3+/TCRαβ+ cells positive for CD69 and CD154 and/or CD137 on a BD FACSAria Fusion. These antigen-specific T cells were fluorescence-activated cell sorted (FACS) into individual wells of a 96-well PCR plate with lysis buffers. To assess the technical variability, we mixed the lysis buffers with the external RNA control—ERCC RNA Spike-In (a final estimate of ~5,000 molecules per well, Thermo Fisher Scientific). We used the modified Smart-seq2 protocol (Clontech Laboratories) to generate the complementary DNA library. From the library, we took a small aliquot (1 μl) for the nested PCR to amplify and sequence 15 targeted RNAs (TBX21, RORC, GATA3, FOXP3, RUNX1, RUNX3, IFNG, IL2, IL17A, GZMB, PERF, IL4, IL5, IL13 and TGFB) and the CDR3 regions of both TCRα and TCRβ chains. Based on the TCR sequences, the Smart-seq2-generated complementary DNAs of the clonally expanded activated T cells were manually selected only on the ESAT6/CFP10-reactive cells for the high-coverage in-depth single-cell full transcriptomic sequencing. We applied the tagmentation and indexing protocol as in the Smart-seq2 protocol and amplified the tagmented DNA. The final pooled library was prepared using the Nextera XT library prep kit (96 index primers; Illumina) protocol and was sequenced on an Illumina HiSeq 2500.
T cell proliferation assay
PBMC from RSTR (n = 12) and LTBI (n = 12) were thawed and enumerated as described above. After resting the samples for 2 h, the cells were washed with cold CFSE buffer (PBS supplemented with 5% FBS) and adjusted to 5 × 106 cells ml−1. The samples were then stained with 5 µM CFSE (BioTracker 488 Green CSFE Cell Proliferation Kit; Sigma-Aldrich) for 5 min at room temperature (RT), followed by three successive washes using CFSE buffer. After the third wash, the cells were reconstituted in media and incubated with anti-CD154 (1 µg ml−1) (Miltenyi) for 30 min at 37 °C to prevent internalization of surface CD154. The cells were then stimulated overnight with DMSO, the whole Mtb lysate (100 µg ml−1) or ESAT6/CFP10 peptide pool (1 µg ml−1). Following stimulation, the cells were stained with LIVE/DEAD Fixable Aqua (Thermo Fisher Scientific) for 15 min at RT. Next, the cells were washed with FACS buffer and stained with CCR7 (phycoerythrin (PE)) for 30 min at 37 °C. The cells were centrifuged and washed with FACS buffer and stained with CD154 (BV711), CD69 (BV450), CD95 (PE–Dazzle 594), CD45RA (PE–Cyanine7) and CD45RO (PerCP-Cyanine5.5) (panel details in Supplementary Table 3) for 30 min at 4 °C. The live CD154+/CD69+CCR7+CD45RA+CD45RO− populations were sorted into 5-ml FACS tubes using a BD FACSAria III Cell Sorter. These cells were then transferred to a 96-well U-bottom plate (Corning) and cultured in sterile-filtered RPMI 1640 (Gibco) supplemented with 10% FBS (HyClone) and 50 units ml−1 recombinant human IL-2 (Prometheus Pharmaceuticals through UWMC Clinical Pharmacy) at 37 °C. After 7 days in culture, the cells were stained with LIVE/DEAD Fixable Aqua (Thermo Fisher Scientific) for 15 min at RT. Next, the cells were washed and stained with CCR7 (PE) for 30 min at 37 °C. The cells were then washed and stained with additional phenotypic markers (Supplementary Table 3) for 30 min at 4 °C. Finally, the cells were fixed using 1% paraformaldehyde (Electron Microscopy Sciences) for 15 min at 4 °C and acquired using a BD LSRFortessa Cell Analyzer. Data were analyzed using FlowJo version 10.10.00 (BD Biosciences).
Multiplex cytokine analysis and ELISA
Cytokine profiles were assessed for 18 RSTR and 20 LTBI participants from the household contact cohort using custom 27-plex ProcartaPlex Panel kits (Invitrogen) and enzyme-linked immunosorbent assy (ELISA). Briefly, 100 μl of supernatant from PBMC stimulated with DMSO or ESAT6/CFP10 peptide pool for 6, 12, 24 and 48 h was collected and stored at −80 °C for downstream analyses. For assessment via multiplex cytokine analysis, 50 μl of undiluted culture supernatant was incubated with magnetic capture beads conjugated to analyte-specific antibodies in 96-well plates. The plates were then washed, and the wells containing samples and beads were incubated with detection antibodies followed by streptavidin conjugated to phycoerythrin. Cytokine secretion data (Granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-α, IFN-γ, IL-1α, IL-1β, IL-10, IL-12p70, IL-13, IL-15, IL-17A, IL-18, IL-1RA, IL-2, IL-21, IL-22, IL-23, IL-27, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IP-10, TNF-α and TNF-β) were then acquired using the Bio-Plex 200 suspension array system (Bio-Rad). Only samples with bead counts >50 were considered. Due to the concentration of several analytes falling outside the linear range of their respective standard curve, the mean fluorescence intensity (MFI) value for each cytokine per sample was extracted and analyzed in R.
Due to incompatibility with ProcartaPlex chemistry, TGFβ-1 was detected separately via ELISA. Briefly, 96-well high-binding ELISA plates (Millipore) were coated overnight with mouse anti-human TGFβ-1 IgG (BioLegend) in carbonate coating buffer at 4 °C. Then, the plates were washed and blocked using 2% BSA in PBS for 3 h at 37 °C. After blocking, the plates were washed, 50 μl of the experimental sample or TGFβ-1 standard was added to each well, and the plates were incubated overnight at 4 °C. The supernatant was collected, the plates were washed and 50 μl of biotinylated mouse anti-human TGFβ-1 IgG (BioLegend) was added to each well, and the plates were incubated at 37 °C for 3 h. Next, the plates were washed, and 50 μl of streptavidin conjugated to horseradish peroxidase (BD Biosciences) was added to each well. The plates were incubated at room temperature for 30 min and then washed, and 50 μl of substrate buffer (o-phenylenediamine dihydrochloride (Thermo Fisher Scientific) and Pierce Stable Peroxide Buffer) was added to each well. The plates were developed for 10–15 min at RT before plate absorbance was read at 450 nm using a CLARIOstar Plus Microplate Reader (BMG LabTech).
Computation/statistics
Flow cytometry
ICS data were compensated and gated using FlowJo (v9.9.6) (BD Biosciences). Representative gating trees of the low-exposure controls and the household contacts are shown in Extended Data Fig. 2. The data were then processed using the OpenCyto framework (V2.16.1) in the R programming environment (V4.1.2)55. With the data from the low-exposure controls COMPASS (V1.19.4) was used to achieve a comprehensive and unbiased analysis of the activation profiles of antigen-specific T cells as previously described5,15. For a given subject, COMPASS was also used to compute a polyfunctionality score that summarizes the entire functionality profile into a single number in which greater weight is given to subsets with more than one function. COMPASS was performed on data from the antigen stimulations for CD4+ T cells to assess T cell subsets expressing IFN-γ, IL-17A, IL-4/5/13, CD107a, TNF, IL-2 and CD154. Poor-quality samples were identified by low CD3 (<10,000 cells) or CD4 (<3,000 cells) counts and were excluded from downstream analysis. The R package ComplexHeatmap (V1.15.1) was used to visualize COMPASS posterior probabilities of response56. For all flow cytometry data, magnitudes of T cell responses were calculated as the proportion of gated events. We used Wilcoxon rank-sum tests to compare magnitudes of T cell responses between sample groups.
Index sort and targeted RNA-seq
The index sort flow cytometry MFI and targeted RNA-seq count dataset were analyzed in R using the packages FlowSOM (V2.1.11)17 and CATALYST (V1.14.1)57. First, all MFI values were shifted by a minimal constant to ensure they were above zero. The non-negative MFI values helped prevent the data overspill. Next, the MFIs underwent an arcsinh5 transformation. The RNA-seq count data were binarized with a cutoff of 5 due to its binary property58. The combined transformed data matrix was standardized to have a mean of 0 and a standard deviation of 1 within each marker across all cells. The CATALYST workflow conducted two series of clustering for the computational efficiency with many cells. The cells were clustered by FlowSOM into 100 groups with a grid size of 15. These 100 groups were meta-clustered by the agglomerative hierarchical clustering to a preset cluster number 40 to identify rare subsets, such as Treg and TH1*. We visualized these cell phenotypes in the t-SNE plot calculated in CATALYST. Next, we manually merged and annotated these cell phenotype clusters. We used Wilcoxon rank-sum tests to compare the cell phenotype subset composition between the two groups and corrected for multiple hypothesis testing using the Bonferroni method.
Full transcriptomic scRNA-seq
Python (V3.9) was used for preprocessing of raw sequencing data. To convert the sequencing read fastq files into single-cell count tables, we used STAR59 to align with the reference GRCh38.genome.ERCC.fa and an index length of 150 bp, and featureCounts60 to calculate the count tables. The single-cell count tables were imported as a Seurat object using the Seurat package (V4) in R (V4.1.2) for downstream analysis. We filtered cells with unique featureCounts over 4,000, total counts below 5 × 105 and mitochondrial counts >5% for doublets, dropouts and dying cells (Extended Data Fig. 4a). CD8+ T cells were removed due to a low cell number. We gated CD4+ T cells based on their CD4 and CD8 flow MFIs. The principal component analysis reduced the expressed genes into 30 components. To study cell phenotypic heterogeneity, these CD4+ T cells were clustered using the graphical-based clustering method and visualized in the Uniform Manifold Approximation and Projection plot. To identify significantly differentially expressed genes (DEGs) between the two groups, we used the Wilcoxon rank-sum test with cutoffs of 0.05 in the Benjamini–Hochberg-adjusted P values and 0.5 in the absolute log2 of fold change (FC) of the average expression between the two groups. The significantly upregulated genes in RSTR were used to run the GO analysis using DAVID GO61,62. For the network analysis, we screened the immune-related genes (GO0002376: immune_system_process) and ran the STRINGdb (V2.16.4) network clustering algorithm in R30. The gene regulatory network inference and motif discovery were conducted using SCENIC (V1.1.2)63.
Mutiplex cytokine analysis and ELISA
The multiplex cytokine and TGFβ ELISA data were exported as comma-separated value (CSV) files from the Bio-Plex 200 CLARIOstar Plus Microplate Reader, respectively. All the cytokine data were merged, cleaned and analyzed in R. Due to the low concentration of cytokines detectable by the Procartaplex kit, the MFI data for each cytokine were extracted and assessed. Background-corrected signals were computed by subtracting the signal from the DMSO condition from the antigen stimulation condition. To choose the precise time points with which to compare protein secretion (measured by ProcartaPlex and ELISA) between the RSTR and LTBI groups, we identified samples exhibiting the most favorable signal-to-noise ratio in response to stimulation (ESAT6/CFP10 or Mtb lysate) versus DMSO. This selection was based on applying the t-statistic at each time point assessed. Subsequently, statistical testing between the two groups was performed on a single time point for each analyte using the Student’s t-test (Extended Data Figs. 7 and 8). P values less than 0.05 were considered significant. Data distribution was assumed to be normal but this was not formally tested.
ACS cohort analysis
We summarized a 7-gene differentiation module (CCR7, SELL, LEF1, TCF7, FOXP1, IL7R and CD27) and the 24-gene activation module (CTLA4, IKZF1, CD5, IL2RB, BATF, TNFRSF4, CXCR4, ICOS, JAK3, TNFRSF18, FAS, JAK1, UBASH3A, S1PR4, NFKB2, PIK3CD, PRKCQ, ETS1, AHR, RASGRP1, SASH3, POU2F2, TTC7A and RFTN1) as the geometric mean of the module gene expression level, and we evaluated whether these modules were differentially expressed between progressors and nonprogressors at all time points before sputum conversion28. The phenotypic CD4+ T cell fractions were calculated from the non-mucosal-associated invariant T (MAIT) CD4+ T cells previously published31. The targeted transcriptional profiling count data were binarized with a cutoff of 5 as we have done previously36 to study cells expressing RORC, TBX21 and FOXP3.
NHP cohort analysis
We derived the gene expression patterns identified in 26 granulomas from 4 cynomolgus macaques obtained 10 weeks after low-dose Mtb infection and analyzed using single-cell RNA-seq32. We selected the top 15 enriched genes in each of the stem-like cell subset and T1–T17 population 1 cell subset and calculated their mean expression using scaled log-normalized gene counts in RSTR and LTBI subjects using the SELECT-seq dataset. Genes that were not expressed or not found were excluded from the analysis. We used the AddModuleScore function from Seurat to calculate the associated gene module scores.
We examined the expression of the TH17 gene module in the whole-blood transcriptome of 34 rhesus macaques from a dose-ranging study of intravenous BCG vaccination followed by Mtb challenge. Data collection and preprocessing have been described in detail previously33. We calculated the RSTR-associated TH17 module score using the geometric mean of genes identified in Fig. 5b that could be mapped to the rhesus macaque genome (CCR6, CCR4, BATF and RORA). We then compared this module score across all time points between macaques that were protected (n = 18) versus not protected (n = 16) against Mtb challenge, as determined by total Mtb c.f.u. upon necropsy, using the two-sided Wilcoxon rank-sum test. Flow cytometry was performed on freshly collected bronchoalveolar lavage fluid obtained from these same macaques34. From these data, we extracted the IL-17-monofunctional T cell phenotype measured by IFNγ−IL-2−IL-17+TNF− among CD4+ T cells after PPD stimulation.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the validation flow cytometry data are available for download via ImmPort at https://www.immport.org under study accession number SDY2277 and via Fairdomhub at https://fairdomhub.org/studies/1179. The processed Seurat object generated from the SELECT-seq data is available via Zenodo at https://zenodo.org/records/7946277 (ref. 64). The raw and processed SELECT-seq data is available at Gene Expression Omnibus (GEO; accession number GSE267774). The gene sets GO:0072539 and GO:0002376 from MSigDB were used to analyze SELECT-seq data. From the ACS cohort, whole-blood bulk transcriptomics data is available at GEO (accession number GSE79362)28 and single-cell targeted transcriptomics data can be found in supplementary materials in the published study from Musvosvi et al.31.
Code availability
Code to complete multiplex cytokine and flow cytometry analyses can be found via GitHub at https://github.com/seshadrilab/cd4-phenotypes-sun-2024-procartaplex and https://github.com/seshadrilab/cd4-phenotypes-sun-2024-flow. Code to complete SELECT-seq analysis and analyses of ACS and NHP cohort data can be found via GitHub at https://github.com/ttsunmeng/TB_RSTR_ESAT6CFP10_SelectSeq_analysis_pipeline.
Change history
19 August 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41590-024-01959-x
References
Houben, R. M. & Dodd, P. J. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 13, e1002152 (2016).
Lin, P. L. et al. CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res Hum. Retroviruses 28, 1693–1702 (2012).
Gutierrez, J., Kroon, E. E., Möller, M. & Stein, C. M. Phenotype definition for ‘resisters’ to Mycobacterium tuberculosis infection in the literature-a review and recommendations. Front. Immunol. 12, 619988 (2021).
Verrall, A. J., Netea, M. G., Alisjahbana, B., Hill, P. C. & van Crevel, R. Early clearance of Mycobacterium tuberculosis: a new frontier in prevention. Immunology 141, 506–513 (2014).
Lu, L. L. et al. IFN-γ-independent immune markers of Mycobacterium tuberculosis exposure. Nat. Med. 25, 977–987 (2019).
Simmons, J. D. et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat. Rev. Immunol. 18, 575–589 (2018).
Zumla, A., Raviglione, M., Hafner, R. & von Reyn, C. F. Tuberculosis. N. Engl. J. Med. 368, 745–755 (2013).
Mogues, T., Goodrich, M. E., Ryan, L., LaCourse, R. & North, R. J. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193, 271–280 (2001).
Gallegos, A. M. et al. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 7, e1002052 (2011).
Sallin, M. A. et al. Host resistance to pulmonary Mycobacterium tuberculosis infection requires CD153 expression. Nat. Microbiol 3, 1198–1205 (2018).
Jouanguy, E. et al. Interferon–gamma-receptor deficiency in an infant with fatal bacille Calmette–Guérin infection. N. Engl. J. Med. 335, 1956–1961 (1996).
Bustamante, J., Boisson-Dupuis, S., Abel, L. & Casanova, J. L. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin. Immunol. 26, 454–470 (2014).
Wobudeya, E., Sekadde-Kasirye, M., Kimuli, D., Mugabe, F. & Lukoye, D. Trend and outcome of notified children with tuberculosis during 2011–2015 in Kampala, Uganda. BMC Public Health 17, 963 (2017).
De Rosa, S. C., Carter, D. K. & McElrath, M. J. OMIP-014: validated multifunctional characterization of antigen-specific human T cells by intracellular cytokine staining. Cytom. A 81, 1019–1021 (2012).
Lin, L. et al. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat. Biotechnol. 33, 610–616 (2015).
Huang, H. et al. Select sequencing of clonally expanded CD8+ T cells reveals limits to clonal expansion. Proc. Natl Acad. Sci. USA 116, 8995–9001 (2019).
Van Gassen, S. et al. FlowSOM: using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A 87, 636–645 (2015).
Araki, K. et al. Translation is actively regulated during the differentiation of CD8. Nat. Immunol. 18, 1046–1057 (2017).
Zens, K. D. & Farber, D. L. Memory CD4 T cells in influenza. Curr. Top. Microbiol Immunol. 386, 399–421 (2015).
Eckert, I. N. et al. VLA-1 binding to collagen IV controls effector T cell suppression by myeloid-derived suppressor cells in the splenic red pulp. Front. Immunol. 11, 616531 (2020).
Gattinoni, L. et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 15, 808–813 (2009).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297 (2011).
Zemmour, D. et al. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nat. Immunol. 19, 291–301 (2018).
Lindestam Arlehamn, C. S. et al. Memory T cells in latent Mycobacterium tuberculosis infection are directed against three antigenic islands and largely contained in a CXCR3+CCR6+ Th1 subset. PLoS Pathog. 9, e1003130 (2013).
Sopel, N., Graser, A., Mousset, S. & Finotto, S. The transcription factor BATF modulates cytokine-mediated responses in T cells. Cytokine Growth Factor Rev. 30, 39–45 (2016).
Arlehamn, C. L. et al. Transcriptional profile of tuberculosis antigen-specific T cells reveals novel multifunctional features. J. Immunol. 193, 2931–2940 (2014).
Mahomed, H. et al. TB incidence in an adolescent cohort in South Africa. PLoS ONE 8, e59652 (2013).
Zak, D. E. et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 387, 2312–2322 (2016).
Galletti, G. et al. Two subsets of stem-like CD8+ memory T cell progenitors with distinct fate commitments in humans. Nat. Immunol. 21, 1552–1562 (2020).
Szklarczyk, D. et al. The STRING database in 2023: protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–D646 (2023).
Musvosvi, M. et al. T cell receptor repertoires associated with control and disease progression following Mycobacterium tuberculosis infection. Nat. Med. 29, 258–269 (2023).
Gideon, H. P. et al. Multimodal profiling of lung granulomas in macaques reveals cellular correlates of tuberculosis control. Immunity 55, 827–846.e810 (2022).
Liu, Y. E. et al. Blood transcriptional correlates of BCG-induced protection against tuberculosis in rhesus macaques. Cell Rep. Med 4, 101096 (2023).
Darrah, P. A. et al. Airway T cells are a correlate of i.v. Bacille Calmette–Guerin-mediated protection against tuberculosis in rhesus macaques. Cell Host Microbe 31, 962–977.e968 (2023).
Khader, S. A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8, 369–377 (2007).
Okada, S. et al. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 349, 606–613 (2015).
Nathan, A. et al. Multimodally profiling memory T cells from a tuberculosis cohort identifies cell state associations with demographics, environment and disease. Nat. Immunol. 22, 781–793 (2021).
Dijkman, K. et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 25, 255–262 (2019).
Ardain, A. et al. Group 3 innate lymphoid cells mediate early protective immunity against tuberculosis. Nature 570, 528–532 (2019).
Cohen, S. B. et al. Alveolar macrophages provide an early Mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe 24, 439–446.e434 (2018).
Simmons, J. D. et al. Monocyte metabolic transcriptional programs associate with resistance to tuberculin skin test/interferon-γ release assay conversion. J. Clin. Invest. 131, e140073 (2021).
Seshadri, C. et al. Transcriptional networks are associated with resistance to Mycobacterium tuberculosis infection. PLoS ONE 12, e0175844 (2017).
Chihota, V. N. et al. Resistance to Mycobacterium tuberculosis infection among highly TB exposed South African gold miners. PLoS ONE 17, e0265036 (2022).
Verrall, A. J. et al. Early clearance of Mycobacterium tuberculosis is associated with increased innate immune responses. J. Infect. Dis. 221, 1342–1350 (2020).
Bhaumik, S. & Basu, R. Cellular and molecular dynamics of Th17 differentiation and its developmental plasticity in the intestinal immune response. Front. Immunol. 8, 254 (2017).
Lu, L. L. et al. A functional role for antibodies in tuberculosis. Cell 167, 433–443.e414 (2016).
Li, H. et al. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 114, 5023–5028 (2017).
Andrews, J. R. et al. The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents. Am. J. Respir. Crit. Care Med. 191, 584–591 (2015).
Andrews, J. R. et al. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir. Med. 5, 282–290 (2017).
Mpande, C. A. M. et al. Mycobacterium tuberculosis-specific T cell functional, memory, and activation profiles in QuantiFERON-reverters are consistent with controlled infection. Front Immunol. 12, 712480 (2021).
Moguche, A. O. et al. Antigen availability shapes T cell differentiation and function during tuberculosis. Cell Host Microbe 21, 695–706.e695 (2017).
Rogerson, B. J. et al. Expression levels of Mycobacterium tuberculosis antigen-encoding genes versus production levels of antigen-specific T cells during stationary level lung infection in mice. Immunology 118, 195–201 (2006).
Shi, L., North, R. & Gennaro, M. L. Effect of growth state on transcription levels of genes encoding major secreted antigens of Mycobacterium tuberculosis in the mouse lung. Infect. Immun. 72, 2420–2424 (2004).
Stein, C. M. et al. Long-term stability of resistance to latent Mycobacterium tuberculosis infection in highly exposed tuberculosis household contacts in Kampala, Uganda. Clin. Infect. Dis. 68, 1705–1712 (2019).
Finak, G. et al. OpenCyto: an open source infrastructure for scalable, robust, reproducible, and automated, end-to-end flow cytometry data analysis. PLoS Comput. Biol. 10, e1003806 (2014).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Chevrier, S. et al. Compensation of signal spillover in suspension and imaging mass cytometry. Cell Syst. 6, 612–620.e615 (2018).
Han, A., Glanville, J., Hansmann, L. & Davis, M. M. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat. Biotechnol. 32, 684–692 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Sherman, B. T. et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50, W216–W221 (2022).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Van de Sande, B. et al. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat. Protoc. 15, 2247–2276 (2020).
Sun, M. et al. Specific CD4+ T cell phenotypes associate with bacterial control in people who ‘resist’ infection with Mycobacterium tuberculosis. Zenodo https://zenodo.org/records/7946277 (2023).
Acknowledgements
We acknowledge the invaluable contribution made by the Kawempe study team’s medical officers, health visitors, laboratory and data personnel in Uganda and the USA. The present study would not have been possible without the generous participation of the Ugandan TB patients and their families. We thank D. Lauffenberger from MIT in collaborating to analyze the intravenous BCG data. We thank members of the Human Immune Monitoring Center for facilitating this work, especially A. Cheruku. Furthermore, we thank J. Wilhelmy, A. McSween, Q. Xia and C. Wang from the Davis lab for their input on assay development and data analysis. Cell sorting/flow cytometry analysis for this project was done on instruments in the Stanford Shared FACS Facility. We thank the Hi-IMPAcTB Data Management team for organizing the data associated with this project for FAIR sharing, namely C. Demurjian, E. Koo, S. Levine (MIT BioMicro Center) and D. Mugahid (Harvard School of Public Health). This work was supported by the US National Institutes of Health (R01-AI124348 to W.H.B., C.M.S. and T.R.H.; U01-AI115642 to W.H.B., T.R.H., C.M.S. and H.M.-K.; K24-AI137310 to T.R.H.; R01-AI125189 and R01-AI146072 to C.S.; and 75N93019C00071 to C.S. and W.H.B.), the Bill and Melinda Gates Foundation (OPP1151836 and OPP1109001 to W.H.B., T.R.H., C.M.S. and H.M.-K. and OPP1113682 Center for Human Systems Immunology award to M.M.D.) and the Howard Hughes Medical Institute (M.M.D.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Additionally, we acknowledge E.D. Layton for technical assistance. Finally, we would like to thank E. Nemes and K. Urdahl for providing critical feedback on the paper before submission.
Author information
Authors and Affiliations
Contributions
C.S. and M.M.D. conceived the study. M.S., J.M.P. and N.S.K. performed the experiments, analyzed the data, and generated the figures and tables. M.T.S., H.H., S.G., S.-H.C., M.G. and P.K. facilitated computational analyses. K.K.Q.Y., G.O., H.T.M., A.K., S.S., N.G., M.R. and P.A. facilitated SELECT-seq experiments at the Stanford Human Immune Monitoring Core. C.M.S., H.M.-K., T.R.H. and W.H.B. contributed to epidemiologic analyses and established the clinical cohorts. Y.E.L., C.W. and P.K. contributed the analyses in the NHP study. M.S., J.M.P., N.S.K. and C.S. wrote the paper with contributions from all authors. C.M.S. and W.H.B. facilitated access to biospecimens. C.M.S., W.H.B., T.R.H., M.M.D. and CS provided funding and oversight for the work. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Fatoumatta Darboe and Jayne Sutherland for their contribution to the peer review of this paper. Primary Handling Editor: Ioana Staicu, in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Enrollment of low exposure cohort, study schema and gating strategy.
(a) Screening and enrollment of low exposure cohort participants for health assessment and blood draw. Enrollment took place in Uganda from July 2017 to March 2018. All healthy non-pregnant participants were eligible. TB, tuberculosis; HIV, human immunodeficiency virus; TST, tuberculin skin test; IGRA, IFN-γ release assay. (b) Schematic of the low exposure cohort, containing 19 LTBI and 17 TST−IGRA− participants, and the household contact cohort, containing 45 RSTR and 45 LTBI participants. T cell responses to ESAT6/CFP10 or whole Mtb lysate were assessed using intracellular cytokine staining, targeted transcriptomics and SELECT-seq, and validated using flow cytometry and multiplex cytokine analysis on independent samples. (c) Flow cytometry gating strategy for CD4+ T cell subsets with cytokine and activation marker expressions from PBMCs in the low exposure control cohort stimulated with ESAT6/CFP10 peptide pool for 6 hours.
Extended Data Fig. 2 The composition of Mtb-specific T cell subsets.
(a) Gating strategy of ESAT6/CFP10-specific CD4+ T cells among PBMCs from the household contact cohort using index sort. T cells were identified as CD3+CD14−/CD19−. T cells positive for the TCRɑβ marker were gated on and separated into CD4+ or CD8+ subsets. Activated CD3+TCRαβ+ T cells were gated on using CD69, CD137, and CD154 markers. (b) Box plots showing the median and interquartile range of frequencies of ESAT6/CFP10-specific CD4+ T cell clusters defined in Fig. 2b among RSTR (n = 3) and LTBI (n = 4) donors in the household contact cohort using index sort and targeted transcriptomics. Whiskers represent minima and maxima. Statistical testing was not performed due to the small sample sizes. (c) Dimensionality reduction (t-SNE) projection of activated CD4+ T cell subsets sorted from PBMCs among the household contact cohort after stimulation with Mtb lysate for 12 hours.
Extended Data Fig. 3 Quality control filtering and key T cell transcriptional factor gene visualization of the SELECT-Seq data.
(a) Filtered cell number by quality control parameters, including the number of unique expressed genes, total gene counts, and percentage of mitochondrial (MT) gene expression, from the whole transcriptomic data of clonally expanded ESAT6/CFP10-specific CD4+ T cells in the household contact cohort. (b) Dimensionality reduction (UMAP) projection of these clonally expanded CD4+ T cells showing group, subject, sex, sequencing batches, and subset clusters. (c) Expression of transcriptional factor genes essential for T cell polarization on these clonally expanded CD4+ T cells. The expression levels were calculated as the log-normalized gene counts from the whole transcriptomic data.
Extended Data Fig. 4 Gating strategy for TH panel and naïve-like Mtb lysate-specific cell frequency in the validation cohort.
(a) Flow cytometry gating strategy for cells expressing memory markers, transcriptional factors and intracellular cytokines associated with TH1 and TH17 among the validation household contact cohort in response to stimulation with ESAT6/CFP10. A time gate was applied to exclude events affected by sample acquisition aberrations, followed by T cell identification by lymphocyte size, CD3, CD14, and CD19 markers. A singlet gate was then applied, followed by the identification of viable cells and a second lymphocyte gate. A secondary gate was applied to identify IL-17A+ cells due to spillover spreading. The same gating strategy was applied to samples stimulated with whole Mtb lysate (not shown). (b) Flow cytometry showing the median and interquartile range of frequencies of CD45RA+CCR7+ naïve-like cells in Mtb lysate-specific CD4+ T cells among validation household contact cohort RSTR (n = 17) and LTBI (n = 20). Whiskers represent minima and maxima. Statistical significance was determined by the two-sided Wilcoxon rank-sum test.
Extended Data Fig. 5 RSTR TSCM cells proliferate similarly to LTBI TSCM cells in response to Mtb antigen.
(a) Schematic of TSCM study where RSTR (n = 12) and LTBI (n = 12) PBMC from the household contact cohort were thawed, rested for 2 hours, stained with 5 µM CFSE, and stimulated with ESAT6/CFP10 or Mtb lysate overnight. (b) Gating strategy of activated naive-like CD69+CD154+CCR7+CD45RA+CD45RO−lymphocytes. (c) Representative flow cytometry showing sorted TSCM cells from RSTR and LTBI expressing the proliferation marker CFSE after 7-day culture. Representive CD45RA and CCR7 staining of the CFSElow population in response to ESAT6/CFP10 and Mtb lysate 7-day post-stimulation (d) Box plots showing the median and interquartile range of frequencies of TSCM cells (CD95+CCR7+CD45RA+CD45RO−) at the time of sorting from PBMC stimulated with Mtb lysate or ESAT6/CFP10. Whiskers represent minima and maxima. (e) Frequencies of CFSElowCD95+ CD4+ TSCM cells after 7-day culture. (f-g) Frequencies of proliferated cells in response to (f) Mtb lysate and (g) ESAT6/CFP10 stimulation. CM, central memory; EM, effector memory. Significance in d-g was calculated using the two-sided Wilcoxon rank-sum tests.
Extended Data Fig. 6 Gating strategy of Treg and its functional profiles.
(a) Flow cytometry gating strategy for cells expressing Treg cell- and activation- associated markers, transcriptional factors and intracellular cytokines among the validation household contact cohort in response to stimulation with ESAT6/CFP10. A time gate was applied to exclude events affected by sample acquisition aberrations, followed by T cell identification by lymphocyte size, CD3, CD14, and CD19 markers. A singlet gate was then applied, followed by the identification of viable cells and a second lymphocyte gate. The same gating strategy was applied to samples stimulated with whole Mtb lysate (not shown). (b) ELISA showing the median and interquartile range of background-corrected absorbance level of TGF-β in conditioned supernatants from PBMCs in response to ESAT6/CFP10 stimulation among RSTR (n = 18) and LTBI (n = 20) in the validation household contact cohort. Whiskers represent minima and maxima. (c) Ratio of anti-inflammatory FoxP3+CD25+ Treg cell frequency to pro-inflammatory CD4 T cell frequency, including RORγt-T-bet+ TH1, RORγt+T-bet−TH17, and RORγt+T-bet+ TH1* cells, among validation donors in response to ESAT6/CFP10 (left) or Mtb lysate (right) from flow cytometry data. Significance in b,c was determined by the two-sided Wilcoxon rank-sum tests.
Extended Data Fig. 7 Cytokine secretion from LTBI and RSTR PBMC following ESAT6/CFP10 stimulation.
(a) Heatmap showing paired t-statistic of cytokine expression levels between ESAT6/CFP10 and DMSO stimulations from supernatants among RSTR (n = 18) and LTBI (n = 20) donors at 6, 12, 24, and 48 hours after stimulation. (b) Box plots showing the median and interquartile range of cytokine mean fluorescence intensities at the timepoint that exhibited the highest signal identified as in a. Whiskers represent minima and maxima. Significance was calculated by two-sided student’s t-test. n.s. (not significant), p-value > 0.05; *, p-value < 0.05; **, p-value < 0.01; ***, p-value < 0.001.
Extended Data Fig. 8 Cytokine secretion from LTBI and RSTR PBMC following Mtb lysate stimulation.
(a) Heatmap showing paired t-statistic of cytokine expression levels between Mtb lysate and DMSO stimulations from supernatants among RSTR (n = 18) and LTBI (n = 20) donors at 6, 12, 24, and 48 hours after stimulation. (b) Box plots showing the median and interquartile range of cytokine mean fluorescence intensities at the timepoint that exhibited the highest signal identified as in a. Whiskers represent minima and maxima. Significance was calculated by two-sided student’s t-test. n.s. (not significant), p-value > 0.05; *, p-value < 0.05; **, p-value < 0.01; ***, p-value < 0.001.
Extended Data Fig. 9 Representative staining of CXCR3+CCR6+ TH1 cells and IFN-γ+ CD4+ T cell frequencies.
(a) Representative staining for CXCR3+CCR6+ TH1 cells in ESAT6/CFP10-specific CD4+ T cells from a participant in the validation household contact cohort. (b) Median and interquartile range of frequencies of CXCR3+CCR6+ TH1 and IFN-γ+ CD4+ T cells in ESAT6/CFP10-specific CD4+ T cells or Mtb lysate-specific CD4+ T cells among RSTR (n = 17) and LTBI (n = 20). Whiskers represent minima and maxima. Significance was determined by two-sided Wilcoxon rank-sum tests.
Supplementary information
Supplementary Tables
Supplementary Table 1. Summary of clinical and demographic characteristics of low exposure cohort. Data presented here include all participants of the wider study cohort. Samples used in this study are from a subset of the concordant positive (TST+IGRA+; LTBI) and concordant negative (TST−IGRA−) subjects, and all subjects included in this study were HIV-negative adults. Categorization was done based on TST and IGRA results, examining quantitative responses. Any subjects that were not clearly TST+ and/or IGRA+ based on quantitative QFT were included in the ‘unevaluable’ category. Means are presented as indicated. BCG scar indicates prior vaccination with BCG. Clinical and demographic characteristics are shown for each individual where available. *Eleven subjects had TST readings between 5 and 10 mm, two had indeterminate QFT results and two had missing TST readings. **QFT results >10 were capped at 10. HIV, human immunodeficiency virus; TST, tuberculin skin test; IGRA, IFN-γ release assay; QFT, QuantiFERON-TB Gold test; BMI, body mass index. Supplementary Table 2. Clinical and demographic characteristics of low-exposure cohort. Raw data used to generate the values shown in Supplementary Table 1 are presented here. Data presented here represent the subjects included in the ICS assay of the low exposure cohort, which are a subset of the concordant positive (TST+IGRA+; LTBI) and concordant negative (TST−IGRA−) subjects presented in Supplementary Table 1. All subjects included in this study were HIV-negative adults. Clinical and demographic characteristics are shown for each individual where available. BMI, body mass index. Supplementary Table 3. Multiparameter flow cytometry panels. The results reported in Figs. 1–5 and Extended Data Fig. 5 utilized four different multiparameter flow cytometry panels to analyze T cells following in vitro simulation. Details regarding the antibodies used for these experiments are provided here. The ICS results in Fig. 1 utilized the ‘LEC’ (low-exposure cohort) panel, the index sort results in Fig. 2 utilized the ‘Index sort’ panel, the flow cytometry results in Figs. 3 and 5 utilized the ‘Th’ panel, the flow cytometry results in Fig. 4 utilized the ‘Treg’ panel and the flow cytometry results in Extended Data Fig. 5 utilized the ‘Tscm’ panel. Supplementary Table 4. Summary of clinical and demographic characteristics of household contact cohort. Subjects presented here are demographically representative of the wider study cohort and consist of individuals included in the discovery and validation phases of this study. Means are presented as indicated. The risk score is determined as previously described54. HIV, human immunodeficiency virus. Supplementary Table 5. Clinical and demographic characteristics of household contact cohort. Raw data used to generate the values shown in Supplementary Table 4 are presented here. Data presented here represent the subjects from the household contact cohort included for SELECT-seq, flow cytometry, multiplex cytokine analysis and ELISA. Clinical and demographic characteristics are shown for each individual where available. FULLIDNO, subject identification number; RS_SUB_ACCESSION_NO, sample accession number; M0_KCVAGE, age at study baseline visit for initial 24 months follow-up; KCHCA_AGE_YR_CURRENT, age at substudy retrace visit; ADULT_RISK_TBINF, adult risk score; CURRENT_HIVSTAT, current HIV status. Supplementary Table 6. TCR sequencing of RSTR ESAT6/CFP10-specific T cells. The TCR sequencing data from three RSTR using the SELECT-seq approach was analyzed to calculate the clonally expanded T cells. Supplementary Table 7. TCR sequencing of LTBI ESAT6/CFP10-specific T cells. The TCR sequencing data from four LTBI using the SELECT-seq approach was analyzed to calculate the clonally expanded T cells. Supplementary Table 8. DEGs between RSTR and LTBI. The DEGs between RSTR and LTBI were identified from the whole transcriptomic data in the SELECT-seq. The adjusted P values were calculated using the Wilcoxon rank-sum test. The average log2 FC was calculated as the difference in mean log-normalized expression levels between RSTR and LTBI cells. A positive log2(FC) indicates higher mean expression levels in the RSTR group compared to the LTBI group. Supplementary Table 9. Pathway analyses of RSTR DEGs. GO Biological Process (BP) analysis was performed to analyze the DEGs. The false discovery rate (FDR) was calculated to quantify the significance of each GO BP term in the RSTR group. Supplementary Table 10. Pathway analyses of LTBI DEGs. GO BP analysis was performed to analyze the DEGs. The FDR was calculated to quantify the significance of each GO BP term in the LTBI group. Supplementary Table 11. Gene set enrichment analysis of DEGs between RSTR and LTBI. The DEGs found in RSTR and LTBI groups were analyzed using gene set enrichment analysis (GSEA). Supplementary Table 12. Network analysis of upregulated DEGs in RSTR using STRINGdb: cluster 1. The upregulated DEGs related to the immune system process in RSTR were analyzed using the STRINGdb network clustering algorithm. The analysis yielded three distinct clusters, where each gene was a node in a network cluster. The network properties of each gene node in cluster 1 were calculated and presented. Supplementary Table 13. Network analysis of upregulated DEGs in RSTR using STRINGdb: cluster 2. The upregulated DEGs related to the immune system process in RSTR were analyzed using the STRINGdb network clustering algorithm. The analysis yielded three distinct clusters, where each gene was a node in a network cluster. The network properties of each gene node in cluster 2 were calculated and presented. Supplementary Table 14. Network analysis of upregulated DEGs in RSTR using STRINGdb: cluster 3. The upregulated DEGs related to the immune system process in RSTR were analyzed using the STRINGdb network clustering algorithm. The analysis yielded three distinct clusters, where each gene was a node in a network cluster. The network properties of each gene node in cluster 3 were calculated and presented. Supplementary Table 15. Gene regulatory network analysis of RSTR in the household contact cohort. The whole transcriptomic data of the SELECT-seq was used to conduct the gene regulatory network analysis. The enrichment score of each transcription factor was calculated separately for the RSTR group. Supplementary Table 16. Gene regulatory network analysis of LTBI in the household contact cohort. The whole transcriptomic data of the SELECT-seq was used to conduct the gene regulatory network analysis. The enrichment score of each transcription factor was calculated separately for the LTBI group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, M., Phan, J.M., Kieswetter, N.S. et al. Specific CD4+ T cell phenotypes associate with bacterial control in people who ‘resist’ infection with Mycobacterium tuberculosis. Nat Immunol 25, 1411–1421 (2024). https://doi.org/10.1038/s41590-024-01897-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-024-01897-8
This article is cited by
-
Exploring T-cell metabolism in tuberculosis: development of a diagnostic model using metabolic genes
European Journal of Medical Research (2025)
-
Alveolar macrophage transcriptional signatures associated with resistance to TST/IGRA conversion following Mycobacterium tuberculosis exposure
BMC Genomics (2025)
-
Deep immune profiling delineates hallmarks of disease heterogeneity in extrapulmonary tuberculosis
Nature Communications (2025)
-
Recasting resistance to Mycobacterium tuberculosis
Nature Immunology (2024)