Abstract
The development of a protective vaccine remains a top priority for the control of the HIV/AIDS pandemic. Here, we show that a messenger RNA (mRNA) vaccine co-expressing membrane-anchored HIV-1 envelope (Env) and simian immunodeficiency virus (SIV) Gag proteins to generate virus-like particles (VLPs) induces antibodies capable of broad neutralization and reduces the risk of infection in rhesus macaques. In mice, immunization with co-formulated env and gag mRNAs was superior to env mRNA alone in inducing neutralizing antibodies. Macaques were primed with a transmitted-founder clade-B env mRNA lacking the N276 glycan, followed by multiple booster immunizations with glycan-repaired autologous and subsequently bivalent heterologous envs (clades A and C). This regimen was highly immunogenic and elicited neutralizing antibodies against the most prevalent (tier-2) HIV-1 strains accompanied by robust anti-Env CD4+ T cell responses. Vaccinated animals had a 79% per-exposure risk reduction upon repeated low-dose mucosal challenges with heterologous tier-2 simian–human immunodeficiency virus (SHIV AD8). Thus, the multiclade env–gag VLP mRNA platform represents a promising approach for the development of an HIV-1 vaccine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The GenBank accession codes for the mRNA sequences are MZ362872, MZ362873, MZ362874, MZ362875 and MZ362876.
References
Mascola, J. R. & Haynes, B. F. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol. Rev. 254, 225–244 (2013).
Kelsoe, G. & Haynes, B. F. Host controls of HIV broadly neutralizing antibody development. Immunol. Rev. 275, 79–88 (2017).
Burton, D. R. & Mascola, J. R. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat. Immunol. 16, 571–576 (2015).
Haynes, B. F., Burton, D. R. & Mascola, J. R. Multiple roles for HIV broadly neutralizing antibodies. Sci. Transl. Med. 11, eaaz2686 (2019).
Burton, D. R. & Hangartner, L. Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu. Rev. Immunol. 34, 635–659 (2016).
Excler, J. L. & Michael, N. L. Lessons from HIV-1 vaccine efficacy trials. Curr. Opin. HIV AIDS 11, 607–613 (2016).
Hessell, A. J., Malherbe, D. C. & Haigwood, N. L. Passive and active antibody studies in primates to inform HIV vaccines. Expert Rev. Vaccines 17, 127–144 (2018).
Corey, L. et al. Immune correlates of vaccine protection against HIV-1 acquisition. Sci. Transl. Med. 7, 310rv317 (2015).
Rerks-Ngarm, S. et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361, 2209–2220 (2009).
Cohen, J. Combo of two HIV vaccines fails its big test. Science 367, 611–612 (2020).
Easterhoff, D. et al. HIV vaccine delayed boosting increases Env variable region 2-specific antibody effector functions. JCI Insight 5, e131437 (2020).
Sanders, R. W. et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 9, e1003618 (2013).
Kwon, Y. D. et al. Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat. Struct. Mol. Biol. 22, 522–531 (2015).
de Taeye, S. W. et al. Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell 163, 1702–1715 (2015).
Sullivan, J. T. et al. High-throughput protein engineering improves the antigenicity and stability of soluble HIV-1 envelope glycoprotein SOSIP trimers. J. Virol. 91, e00862-17 (2017).
Torrents de la Peña, A. et al. Improving the immunogenicity of native-like HIV-1 envelope trimers by hyperstabilization. Cell Rep. 20, 1805–1817 (2017).
Zhang, P. et al. Interdomain stabilization impairs CD4 binding and improves immunogenicity of the HIV-1 envelope trimer. Cell Host Microbe 23, 832–844 (2018).
Sanders, R. W. et al. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349, aac4223 (2015).
Klasse, P. J. et al. Sequential and simultaneous immunization of rabbits with HIV-1 envelope glycoprotein SOSIP.664 trimers from clades A, B and C. PLoS Pathog. 12, e1005864 (2016).
Hu, J. K. et al. Murine antibody responses to cleaved soluble HIV-1 envelope trimers are highly restricted in specificity. J. Virol. 89, 10383–10398 (2015).
Zhou, T. et al. Quantification of the impact of the HIV-1-glycan shield on antibody elicitation. Cell Rep. 19, 719–732 (2017).
Pauthner, M. et al. Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 46, 1073–1088 (2017).
McCoy, L. E. et al. Holes in the glycan shield of the native HIV envelope are a target of trimer-elicited neutralizing antibodies. Cell Rep. 16, 2327–2338 (2016).
Kwong, P. & Mascola, J. P. HIV-1 vaccines based on antibody identification, B cell ontogeny and epitope structure. Immunity 48, 855–871 (2018).
Kwong, P. D., DeKosky, B. J. & Ulmer, J. B. Antibody-guided structure-based vaccines. Semin. Immunol. 50, 101428 (2020).
Xu, K. et al. Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1. Nat. Med. 24, 857–867 (2018).
Kong, R. et al. Antibody lineages with vaccine-induced antigen-binding hotspots develop broad HIV neutralization. Cell 178, 567–584 (2019).
Steichen, J. M. et al. A generalized HIV vaccine design strategy for priming of broadly neutralizing antibody responses. Science 366, eaax4380 (2019).
Saunders, K. O. et al. Targeted selection of HIV-specific antibody mutations by engineering B cell maturation. Science 366, eaay7199 (2019).
Chen, X. et al. Vaccination induces maturation in a mouse model of diverse unmutated VRC01-class precursors to HIV-neutralizing antibodies with >50% breadth. Immunity 54, 324–339 (2021).
Bradley, T. et al. Pentavalent HIV-1 vaccine protects against simian-human immunodeficiency virus challenge. Nat. Commun. 8, 15711 (2017).
Barouch, D. H. et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 392, 232–243 (2018).
Felber, B. K. et al. Co-immunization of DNA and protein in the same anatomical sites induces superior protective immune responses against SHIV challenge. Cell Rep. 31, 107624 (2020).
Om, K. et al. Adjuvanted HIV-1 vaccine promotes antibody-dependent phagocytic responses and protects against heterologous SHIV challenge. PLoS Pathog. 16, e1008764 (2020).
Arunachalam, P. S. et al. T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat. Med. 26, 932–940 (2020).
Pardi, N. et al. Characterization of HIV-1 nucleoside-modified mRNA vaccines in rabbits and rhesus macaques. Mol. Ther. Nucleic Acids 15, 36–47 (2019).
Moyo, N. et al. Efficient induction of T cells against conserved HIV-1 regions by mosaic vaccines delivered as self-amplifying mRNA. Mol. Ther. Methods Clin. Dev. 12, 32–46 (2018).
Saunders, K. O. et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. NPJ Vaccines 6, 50 (2021).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines: a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
Alameh, M.-G., Weissman, D. & Pardi, N. Messenger RNA-based vaccines against infectious diseases. Curr. Top. Microbiol. Immunol. https://doi.org/10.1007/82_2020_202 (2020).
Corbett, K. S. et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 383, 1544–1555 (2020).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Manolova, V. et al. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 38, 1404–1413 (2008).
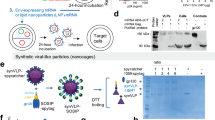
Chen, C. W., Saubi, N. & Joseph-Munne, J. Design concepts of virus-like particle-based HIV-1 vaccines. Front. Immunol. 11, 573157 (2020).
Andersson, A. C., Schwerdtfeger, M. & Holst, P. J. Virus-like-vaccines against HIV. Vaccines 6, 10 (2018).
Effio, C. L. & Hubbuch, J. Next generation vaccines and vectors: designing downstream processes for recombinant protein-based virus-like particles. Biotechnol. J. 10, 715–727 (2015).
Buonaguro, L. et al. High efficient production of Pr55(gag) virus-like particles expressing multiple HIV-1 epitopes, including a gp120 protein derived from an Ugandan HIV-1 isolate of subtype A. Antiviral Res. 49, 35–47 (2001).
Gheysen, D. et al. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 59, 103–112 (1989).
Haffar, O. et al. Human immunodeficiency virus-like, nonreplicating, gag-env particles assemble in a recombinant vaccinia virus expression system. J. Virol. 64, 2653–2659 (1990).
Zhu, P. et al. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441, 847–852 (2006).
Keele, B. F. et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl Acad. Sci. USA 105, 7552–7557 (2008).
Voss, J. E. et al. Elicitation of neutralizing antibodies targeting the V2 apex of the HIV envelope trimer in a wild-type animal model. Cell Rep. 21, 222–235 (2017).
Kothe, D. L. et al. Antigenicity and immunogenicity of HIV-1 consensus subtype B envelope glycoproteins. Virology 360, 218–234 (2007).
Li, H. et al. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc. Natl Acad. Sci. USA 113, 3413–3422 (2016).
deCamp, A. et al. Global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 88, 2489–2507 (2014).
Han, Q. et al. Difficult-to-neutralize global HIV-1 isolates are neutralized by antibodies targeting open envelope conformations. Nat. Commun. 10, 2898 (2019).
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Gautam, R. et al. Pathogenicity and mucosal transmissibility of the R5-tropic simian/human immunodeficiency virus SHIV(AD8) in rhesus macaques: implications for use in vaccine studies. J. Virol. 86, 8516–8526 (2012).
Gautam, R. et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533, 105–109 (2016).
Gautam, R. et al. A single injection of crystallizable fragment domain-modified antibodies elicits durable protection from SHIV infection. Nat. Med. 24, 610–616 (2018).
Cottrell, C. A. et al. Mapping the immunogenic landscape of near-native HIV-1 envelope trimers in non-human primates. PLoS Pathog. 16, e1008753 (2020).
van Schooten, J. et al. Antibody responses induced by SHIV infection are more focused than those induced by soluble native HIV-1 envelope trimers in non-human primates. PLoS Pathog. 17, e1009736 (2021).
Nogal, B. et al. Mapping polyclonal antibody responses in non-human primates vaccinated with HIV Env trimer subunit vaccines. Cell Rep. 30, 3755–3765 (2020).
Haynes, B. F. et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366, 1275–1286 (2012).
Jardine, J. G. et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 351, 1458–1463 (2016).
Duan, H. et al. Glycan masking focuses immune responses to the HIV-1 CD4-binding site and enhances elicitation of VRC01-class precursor antibodies. Immunity 49, 301–311 (2018).
Prevost, J. et al. Incomplete downregulation of CD4 expression affects HIV-1 Env conformation and antibody-dependent cellular cytotoxicity responses. J. Virol. 92, e00484-18 (2018).
Richard, J. et al. Uninfected bystander cells impact the measurement of HIV-specific antibody-dependent cellular cytotoxicity responses. mBio 9, e00358-18 (2018).
Pardi, N. et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 215, 1571–1588 (2018).
Garrido, C. et al. SARS-CoV-2 vaccines elicit durable immune responses in infant rhesus macaques. Sci. Immunol. 6, eabj3684 (2021).
Painter, M. M. et al. Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 54, 2133–2142 (2021).
Locci, M. et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39, 758–769 (2013).
Moldt, B. et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl Acad. Sci. USA 109, 18921–18925 (2012).
Montefiori, D. C. & Mascola, J. R. Neutralizing antibodies against HIV-1: can we elicit them with vaccines and how much do we need? Curr. Opin. HIV AIDS 4, 347–351 (2009).
Parren, P. W. et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75, 8340–8347 (2001).
Shingai, M. et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J. Exp. Med. 211, 2061–2074 (2014).
Pauthner, M. G. et al. Vaccine-induced protection from homologous tier 2 SHIV challenge in nonhuman primates depends on serum-neutralizing antibody titers. Immunity 50, 241–252 (2019).
Nelson, J. et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. 6, eaaz6893 (2020).
Hassett, K. J. et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11 (2019).
Bahl, K. et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol. Ther. 25, 1316–1327 (2017).
Roederer, M., Nozzi, J. L. & Nason, M. C. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 79, 167–174 (2011).
Vassena, L. et al. Treatment with IL-7 prevents the decline of circulating CD4+ T cells during the acute phase of SIV infection in rhesus macaques. PLoS Pathog. 8, e1002636 (2012).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Acknowledgements
The authors thank the animal care personnel at Bioqual Inc. and at the Poolesville NIH Animal Facility. The authors also thank J. Moore (Weill-Cornell Medicine, New York) for the BG505 SOSIP.664 plasmid, J. Van Schooten (University of Amsterdam, The Netherlands) for plasmids to produce monoclonal antibodies RM20A3, RM20C, RM20E1, RM20F, RM20G, RM20J, RM15E and RM54B1, and the National Institutes of Health (NIH) AIDS Reagent Program for the reagents listed in the Methods section. This study was supported by the Intramural Research Program of the Division of Intramural Research and the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, NIH, by a grant from the NIH Office of AIDS Research, by the Bill and Melinda Gates Foundation, and by federal funds from the Frederick National Laboratory for Cancer Research, NIH, under contract HHSN261200800001 (to Y.T.).
Author information
Authors and Affiliations
Contributions
P.Z. and P.L. conceived and supervised the project; P.Z., E.N., S.D., K.B., Y.L., Q.L., H.M., H.S., D.R., S.F., S.M.E., V.P., K.B., M.P., X.C., E.K.S. and D.R.A. performed the experiments; D.W., J.M., J.S. and R.H. provided veterinary care and performed the immunizations and viral challenges; R.G. and M.A.M. provided critical reagents and expertise for the NHP challenge study; Y.T. performed the NSEM analysis; Z.H. and D.F. performed the statistical analysis; A.F., G.C., S.H., G.S.-J., A.C., A.M.D., R.A.K., J.R.M. and A.S.F. participated in the study design; P.Z. and P.L. wrote the original draft of the paper; all of the authors contributed to the final review of the paper and the editing.
Corresponding author
Ethics declarations
Competing interests
P.L., P.Z., and E.N. are named on a HIV RNA Vaccine patent (PCT/US2020/022710). E.N., S.F., S.M.E., V.P., K.B., S.H., G.S.-J. and A.C. are employees of Moderna Inc. and hold equities from the company. All other authors have no competing interests.
Additional information
Peer review information Nature Medicine thanks Joseph Jardine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Alison Farrell was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Utilization of macaque CD4 by wild-type and mutated HIV-1 Envs.
Ability of three wild-type (WT) and corresponding aa. 375-mutated HIV-1 strains to utilize rhesus macaque CD4 (rhCD4) for infection. rhCD4 transfection and pseudovirus infection are described in the Methods section.
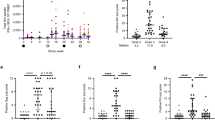
Extended Data Fig. 2 Induction of trimer-binding antibodies in the two subgroups of immunized macaques over time.
Subgroup 1 received 5 mRNA and 5 protein immunizations; subgroup 2 received 8 mRNA and 2 protein immunizations. a. Serum titers of antibodies binding to the autologous trimer as assayed by ELISA using the WITO SOSIP.664 trimer captured on lectin-coated plates. b. Serum titers of antibodies binding to the autologous V3 loop peptide (WITO pV3) as assayed using the linear WITO V3 peptide directly coated on the plate surface. c. Serum titers of antibodies binding to a heterologous trimer (AD8) as assayed by ELISA using the AD8 IP trimer captured on lectin-coated plates. Statistical differences were calculated using a paired two-tailed t-test. The sequence and timing of immunizations are indicated by color-coded triangles on the top.
Extended Data Fig. 3 Induction of autologous neutralizing antibodies in the two subgroups of immunized macaques.
Subgroup 1 received 5 mRNA and 5 protein immunizations; subgroup 2 received 8 mRNA and 2 protein immunizations. a. Serum titers of neutralizing antibodies against the autologous Env (WITO4160.27), as assayed using pseudoviruses on TZM-bl cells. The sequence and timing of immunizations are indicated by color-coded triangles on the top. b. Effect of the autologous linear V3-loop peptide (WITO pV3) on autologous neutralization at two time points (week 29 and 37). The assays were performed by incubating the linear V3 peptide with macaque sera prior to addition to the pseudovirus stock. The asterisk denotes a p value <0.05, as assessed by two-tailed paired t-test.
Extended Data Fig. 4 Induction of heterologous neutralizing antibodies in immunized macaques.
a. Serum titers of neutralizing antibodies against a heterologous tier-1a Env (SF162). b. Serum titers of neutralizing antibodies against a heterologous tier-1b Env (BaL). c. Neutralization of SF162 in the two vaccine subgroups. d. Neutralization of BaL in the two vaccine subgroups. The asterisk denotes a p value <0.05, as assessed by two-tailed unpaired t-test. e. Neutralization of the tier-2 Env JR-FL in the two vaccine subgroups. f. Neutralization of the tier-2 Env AD8 in the two vaccine subgroups. g. Serum titers of neutralizing antibodies against a panel of heterologous tier-2 Envs at week 58 in the two vaccine subgroups. Neutralization assays were performed using pseudoviruses on TZM-bl cells. The sequence and timing of immunizations are indicated by color-coded triangles on the top. Subgroup 1 received 5 mRNA and 5 protein immunizations; subgroup 2 received 8 mRNA and 2 protein immunizations.
Extended Data Fig. 5 SIV Gag-specific T-cell responses.
Induction of SIV Gag-specific CD4+ and CD8+ T-cell responses in all 7 vaccinated macaques at weeks 27 and 60 of immunization. a. Gag-specific CD4+ T-cell responses calculated as fractions of memory CD4+ T cells expressing CD154 and the indicated intracellular cytokines or CD69. b. Gag-specific CD8+ T-cell responses calculated as fractions of memory CD8+ T cells expressing CD69 and the indicated intracellular cytokines or CD107. c. Correlation between a subset of Env-specific T-follicular helper cells (CXCR5+CXCR3-PD-1+) and protection in vaccinated macaques. The correlation was statistically significant after exclusion of a single outlier (macaque V7, highlighted in red). The correlation coefficient and P value with the inclusion of the outlier are indicated in red.
Extended Data Fig. 6 Vaccine-induced protection in the two subgroups of immunized macaques challenged with SHIV AD8.
Subgroup 1 received 5 mRNA and 5 protein immunizations; subgroup 2 received 8 mRNA and 2 protein immunizations. a. Kaplan-Meier analysis of virus-free survival in the course of 13 weekly intrarectal inoculation of 10 TCID50 of SHIV AD8 (red arrows) in the two subgroups of immunized macaques. Infection was evaluated by the appearance of plasma viremia on two subsequent tests using a sensitive real-time PCR method. Significance was calculated by the Wilcoxon exact test. b. Statistical analysis of hazard ratio and per-exposure risk in the two subgroups of immunized macaques, as assessed by 1 minus the hazard ratio, estimated from a Cox proportional hazards regression via the exact partial likelihood with group (vaccinated versus control) as the regressor. c. Mean levels of viremia (± SD) in macaques in the two vaccine subgroups synchronized by peak of viremia. Levels of viremia were evaluated by quantitative real-time PCR. The courses of viremia in different animals were aligned by setting the peak of viremia at day 21 for each animal.
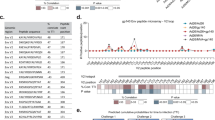
Extended Data Fig. 7 Epitope mapping of trimer-binding antibodies in serum from vaccinated macaques.
a. Competition with HIV-1 bNAbs, as assessed by ELISA on lectin-captured HIV-1 BG505 SOSIP.664 trimers. b. Competition with macaque monoclonal antibodies directed against off-target and non-bNAb-target regions (RM20A3, RM20C, RM20E1, RM20F, RM20G, RM20J, RM15E and RM54B1). TB: trimer base; GH: glycan holes; V1: V1 loop; FP: fusion peptide.
Extended Data Fig. 8 Polyclonal epitope mapping of trimer-binding antibodies in a representative protected animal by negative-staining electron microscopy (NSEM).
The analysis was performed using purified Fab fragments isolated from serum collected from macaque V3 (subgroup 1) at week 58. The arrow heads point to the trimer-bound Fab fragments. Visualization of antibodies binding to: a. A region compatible with the CD4-BS deep in the inter-protomer groove; b. A region in the inter-protomer groove more distal from the trimer axis than the CD4-BS; c. A region(s) at the trimer apex targeted by 2 Fabs simultaneously; d. A region at the trimer apex compatible with the V2-glycan supersite targeted by a single Fab; e. A region at the trimer base, presumably constituted by neo-epitopes produced by gp41 truncation.
Extended Data Fig. 9 Correlation between vaccine-induced protection and antibodies against specific epitopes, infected cells or ADCC.
a. Correlation between protection and antibodies against an autologous linear V3 peptide (WITO pV3). b. Correlation between protection and antibodies against a heterologous linear V3 peptide (JR-FL V3). c. Correlation between protection and antibodies against a V1V2 scaffolded protein (gp70 V1V2). d. Correlation between protection and antibodies binding to the surface of SHIV AD8-infected primary CD4+ T cells. The results are mean values from two experiments. The correlation becomes significant after exclusion of an outlier (macaque V7, highlighted in red). The correlation coefficient and P value with the inclusion of the outlier are indicated in red. e. Correlation between protection and antibodies mediating ADCC against SHIV AD8-infected primary CD4+ T cells. The results are mean values from two experiments. The correlation becomes significant after exclusion of an outlier (macaque V1, highlighted in red). The correlation coefficient and P value with the inclusion of the outlier are indicated in red. f. Inhibitory effect of a VRC01 Fab fragment on macaque antibody binding to SHIV AD8-infected primary CD4+ T cells. C denotes Fab-untreated controls. All correlations were determined by the Spearman test.
Supplementary information
Supplementary Information
Methods, Supplementary references, Supplementary Tables 1–8, Supplementary Fig. 1
Rights and permissions
About this article
Cite this article
Zhang, P., Narayanan, E., Liu, Q. et al. A multiclade env–gag VLP mRNA vaccine elicits tier-2 HIV-1-neutralizing antibodies and reduces the risk of heterologous SHIV infection in macaques. Nat Med 27, 2234–2245 (2021). https://doi.org/10.1038/s41591-021-01574-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-021-01574-5
This article is cited by
-
mRNA technology for the prevention and treatment of HIV-1 infection
Nature Reviews Bioengineering (2026)
-
Lipid nanoparticle–based mRNA platforms for mucosal HIV vaccines: formulation advances, immune mechanisms, and translational pathways
Archives of Microbiology (2026)
-
Research progress of mRNA vaccines for infectious diseases
European Journal of Medical Research (2025)
-
Revolutionizing immunization: a comprehensive review of mRNA vaccine technology and applications
Virology Journal (2025)
-
Recent advances and perspectives on the development of circular RNA cancer vaccines
npj Vaccines (2025)