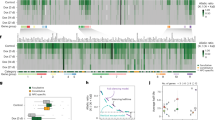
Abstract
Random X-chromosome inactivation is a hallmark of female mammalian somatic cells. This epigenetic mechanism, mediated by the long noncoding RNA Xist, occurs in the early embryo and is stably maintained throughout life, although inactivation is lost during primordial germ cell (PGC) development. Using a combination of single-cell allele-specific RNA sequencing and low-input chromatin profiling on developing mouse PGCs, we provide a detailed map of X-linked gene reactivation. Despite the absence of Xist expression, PGCs still harbor a fully silent X chromosome at embryonic day 9.5 (E9.5). Subsequently, X-linked genes undergo gradual and distinct regional reactivation. At E12.5, a substantial part of the inactive X chromosome resists reactivation, retaining an epigenetic memory of its silencing. Our findings define the orchestration of reactivation of the inactive X chromosome, a key event in female PGC reprogramming with direct implications for reproduction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All scRNA-seq, WGBS and CUT&RUN datasets were deposited to the GEO under accession numbers GSE243943 and GSE243464. The WGBS datasets of E10.5 PGCs and E12.5 PGCs were respectively extracted from the DDBJ (DRA000607)54 and the GEO (GSE76973)55. Other data are available from the corresponding author upon request. Source data are provided with this paper.
Code availability
No code was developed for this study. Details and specificities (tools, version and options) to replicate the bioinformatic pipelines are provided in the Methods.
References
Lyon, M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190, 372–373 (1961).
Loda, A., Collombet, S. & Heard, E. Gene regulation in time and space during X-chromosome inactivation. Nat. Rev. Mol. Cell Biol. 23, 231–249 (2022).
Namekawa, S. H., Payer, B., Huynh, K. D., Jaenisch, R. & Lee, J. T. Two-step imprinted X inactivation: repeat versus genic silencing in the mouse. Mol. Cell. Biol. 30, 3187–3205 (2010).
Borensztein, M. et al. Xist-dependent imprinted X inactivation and the early developmental consequences of its failure. Nat. Struct. Mol. Biol. 24, 226–233 (2017).
Marahrens, Y., Panning, B., Dausman, J., Strauss, W. & Jaenisch, R. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 11, 156–166 (1997).
Engreitz, J. M. et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science 341, 1237973 (2013).
Pandya-Jones, A. et al. A protein assembly mediates Xist localization and gene silencing. Nature 587, 145–151 (2020).
Strehle, M. & Guttman, M. Xist drives spatial compartmentalization of DNA and protein to orchestrate initiation and maintenance of X inactivation. Curr. Opin. Cell Biol. 64, 139–147 (2020).
Lyon, M. F. X-chromosome inactivation: a repeat hypothesis. Cytogenet. Cell Genet. 80, 133–137 (1998).
Chaumeil, J., Baccon, P. L., Wutz, A. & Heard, E. A novel role for Xist RNA in the formation of a repressive nuclear compartment into which genes are recruited when silenced. Genes Dev. 20, 2223–2237 (2006).
Chow, J. C. et al. LINE-1 activity in facultative heterochromatin formation during X chromosome inactivation. Cell 141, 956–969 (2010).
Żylicz, J. J. et al. The implication of early chromatin changes in X chromosome inactivation. Cell 176, 182–197(2019).
Monk, M. Methylation and the X chromosome. Bioessays 4, 204–208 (1986).
Cheng, S. et al. Single-cell RNA-seq reveals cellular heterogeneity of pluripotency transition and X chromosome dynamics during early mouse development. Cell Rep. 26, 2593–2607 (2019).
Marks, H. et al. Dynamics of gene silencing during X inactivation using allele-specific RNA-seq. Genome Biol. 16, 149 (2015).
de Andrade E Sousa, L. B. et al. Kinetics of Xist-induced gene silencing can be predicted from combinations of epigenetic and genomic features. Genome Res. 29, 1087–1099 (2019).
Pasque, V. et al. X chromosome reactivation dynamics reveal stages of reprogramming to pluripotency. Cell 159, 1681–1697 (2014).
Janiszewski, A. et al. Dynamic reversal of random X-chromosome inactivation during iPSC reprogramming. Genome Res. 29, 1659–1672 (2019).
Mak, W. et al. Reactivation of the paternal X chromosome in early mouse embryos. Science 303, 666–669 (2004).
Borensztein, M. et al. Contribution of epigenetic landscapes and transcription factors to X-chromosome reactivation in the inner cell mass. Nat. Commun. 8, 1–14 (2017).
Sugimoto, M. & Abe, K. X chromosome reactivation initiates in nascent primordial germ cells in mice. PLoS Genet. 3, e116 (2007).
Chuva de Sousa Lopes, S. M. et al. X chromosome activity in mouse XX primordial germ cells. PLoS Genet. 4, e30 (2008).
Leitch, H. G., Tang, W. W. C. & Surani, M. A. Primordial germ-cell development and epigenetic reprogramming in mammals. Curr. Top. Dev. Biol. 104, 149–187 (2013).
Tang, W. W. C., Kobayashi, T., Irie, N., Dietmann, S. & Surani, M. A. Specification and epigenetic programming of the human germ line. Nat. Rev. Genet. 17, 585–600 (2016).
Kurimoto, K. & Saitou, M. Germ cell reprogramming. Curr. Top. Dev. Biol. 135, 91–125 (2019).
Kurimoto, K. & Saitou, M. Epigenome regulation during germ cell specification and development from pluripotent stem cells. Curr. Opin. Genet. Dev. 52, 57–64 (2018).
Hajkova, P. et al. Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 452, 877–881 (2008).
Mallol, A., Guirola, M. & Payer, B. PRDM14 controls X‑chromosomal and global epigenetic reprogramming of H3K27me3 in migrating mouse primordial germ cells. Epigenetics Chromatin 12, 1–10 (2019).
Zhao, Z.-H. et al. Single-cell RNA sequencing reveals the landscape of early female germ cell development. FASEB J. 34, 12634–12645 (2020).
Sangrithi, M. N. et al. Non-canonical and sexually dimorphic X dosage compensation states in the mouse and human germline. Dev. Cell 40, 1–13 (2017).
Naik, H. C. et al. Lineage-specific dynamics of loss of X upregulation during inactive-X reactivation. Stem Cell Rep. 19, 1564–1582 (2024).
Severino, J. et al. Controlled X-chromosome dynamics defines meiotic potential of female mouse in vitro germ cells. EMBO J. 41, e109457 (2022).
Heard, E. & Turner, J. Function of the sex chromosomes in mammalian fertility. Cold Spring Harb. Perspect. Biol. 3, a002675 (2011).
de Napoles, M., Nesterova, T. & Brockdorff, N. Early loss of Xist RNA expression and inactive X chromosome associated chromatin modification in developing primordial germ cells. PLoS ONE 2, e860 (2007).
Navarro, P. et al. Molecular coupling of Xist regulation and pluripotency. Science 321, 1693–1695 (2008).
Payer, B. et al. Tsix RNA and the germline factor, PRDM14, link X reactivation and stem cell reprogramming. Mol. Cell 52, 1–14 (2013).
Keane, T. M. et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477, 289–294 (2011).
Frazer, K. A. et al. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature 448, 1050–1053 (2007).
Yeom, Y. I. et al. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 122, 881–894 (1996).
Borensztein, M., Syx, L., Servant, N. & Heard, E. Transcriptome profiling of single mouse oocytes. Methods Mol. Biol. 1818, 51–65 (2018).
Tang, F. et al. mRNA-seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Mayère, C. et al. Single-cell transcriptomics reveal temporal dynamics of critical regulators of germ cell fate during mouse sex determination. FASEB J. 35, e21452 (2021).
Yamaji, M. et al. DND1 maintains germline stem cells via recruitment of the CCR4–NOT complex to target mRNAs. Nature 543, 568–572 (2017).
Okamura, E. et al. Esrrb function is required for proper primordial germ cell development in presomite stage mouse embryos. Dev. Biol. 455, 382–392 (2019).
Yu, L. et al. Loss of ESRP1 blocks mouse oocyte development and leads to female infertility. Development 148, dev196931 (2021).
Cattanach, B. M. & Williams, C. E. Evidence of non-random X chromosome activity in the mouse. Genet. Res. 19, 229–240 (1972).
Calaway, J. D. et al. Genetic architecture of skewed X inactivation in the laboratory mouse. PLoS Genet. 9, e1003853 (2013).
Berletch, J. B. et al. Escape from X inactivation varies in mouse tissues. PLoS Genet. 11, e1005079 (2015).
Liu, Y. et al. Single cell RNA-seq reveals protracted germ line X chromosome reactivation dynamics directed by a PRC2 dependent mechanism. Preprint at bioRxiv https://doi.org/10.1101/2023.11.06.565813 (2023).
Yang, F., Babak, T., Shendure, J. & Disteche, C. M. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 20, 614–622 (2010).
Bauer, M. et al. Chromosome compartments on the inactive X guide TAD formation independently of transcription during X-reactivation. Nat. Commun. 12, 3499 (2021).
Pandya-Jones, A. & Plath, K. The ‘lnc’ between 3D chromatin structure and X chromosome inactivation. Semin. Cell Dev. Biol. 56, 35–47 (2016).
Simon, M. D. et al. High-resolution Xist binding maps reveal two-step spreading during X-chromosome inactivation. Nature 504, 465–469 (2013).
Kobayashi, H. et al. High-resolution DNA methylome analysis of primordial germ cells identifies gender-specific reprogramming in mice. Genome Res. 23, 616–627 (2013).
Hill, P. W. S. et al. Epigenetic reprogramming enables the primordial germ cell-to-gonocyte transition Europe PMC Funders Group. Nature 555, 392–396 (2018).
Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 16, 6–21 (2002).
Seisenberger, S. et al. Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20110330 (2013).
Monk, M. & McLaren, A. X-chromosome activity in foetal germ cells of the mouse. J. Embryol. Exp. Morphol. 63, 75–84 (1981).
Tam, P. P. L., Zhou, S. X. & Tan, S.-S. X-chromosome activity of the mouse primordial germ cells revealed by the expression of an X-linked lacZ transgene. Development 120, 2925–2932 (1994).
Panda, A., Zylicz, J. J. & Pasque, V. New insights into X-chromosome reactivation during reprogramming to pluripotency. Cells 9, 2706 (2020).
Haramoto, Y., Sakata, M. & Kobayashi, S. Visualization of X chromosome reactivation in mouse primordial germ cells in vivo. Biol. Open 10, bio058602 (2021).
Richart, L. et al. Xist loss impairs mammary stem cell differentiation and increases tumorigenicity through Mediator hyperactivation. Cell 185, 2164–2183(2022).
Generoso, S. F. et al. Cohesin controls X chromosome structure remodeling and X-reactivation during mouse iPSC-reprogramming. Proc. Natl Acad. Sci. USA 120, e2213810120 (2023).
Turner, H. H. A syndrome of infantilism, congenital webbed neck, and cubitus valgus. Endocrinology 23, 566–574 (1938).
Burgoyne, P. S. & Baker, T. G. Perinatal oocyte loss in XO mice and its implications for the aetiology of gonadal dysgenesis in XO women. J. Reprod. Fertil. 75, 633–45 (1985).
Lentini, A. et al. Elastic dosage compensation by X-chromosome upregulation. Nat. Commun. 13, 1854 (2022).
Huang, T.-C. et al. Sex-specific chromatin remodelling safeguards transcription in germ cells. Nature 600, 737–742 (2021).
Lan, Z. J., Xu, X. & Cooney, A. J. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol. Reprod. 71, 1469–1474 (2004).
Szabo, P. E., Hübner, K., Schöler, H. & Mann, J. R. Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech. Dev. 115, 157–160 (2002).
Skene, P. J. & Henikoff, S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife 6, 1–35 (2017).
Dura, M. et al. DNMT3A-dependent DNA methylation is required for spermatogonial stem cells to commit to spermatogenesis. Nat. Genet. 54, 469–480 (2022).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Shen, W., Le, S., Li, Y. & Hu, F. SeqKit: a cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS ONE 11, e0163962 (2016).
Krueger, F., James, F., Ewels, P., Afyounian, E. & Schuster-Boeckler, B. FelixKrueger/TrimGalore: v0.6.7. Zenodo https://doi.org/10.5281/zenodo.5127899 (2021).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Krueger, F. & Andrews, S. R. SNPsplit: allele-specific splitting of alignments between genomes with known SNP genotypes. F1000Res. 5, 1479 (2016).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. Gigascience 10, giab008 (2021).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Zhang, Y. et al. Model-based Analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Smallwood, S. A. et al. Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat. Methods 11, 817–820 (2014).
Walter, M., Teissandier, A., Pérez-Palacios, R. & Bourc’his, D. An epigenetic switch ensures transposon repression upon dynamic loss of DNA methylation in embryonic stem cells. eLife 5, e11418 (2016).
Krueger, F. & Andrews, S. R. Bismark: a flexible aligner and methylation caller for Bisulfite-seq applications. Bioinformatics 27, 1571–1572 (2011).
Gel, B. et al. regioneR: an R/Bioconductor package for the association analysis of genomic regions based on permutation tests. Bioinformatics 32, 289–291 (2016).
Acknowledgements
We acknowledge the M.A.S. and D.B. laboratories for insightful discussions. We thank K. Harnish, Cambridge Stem Cell Institute Genomics Facility and the MGX-Montpellier GenomiX platform for advice and deep sequencing of the libraries. We thank the pathogen-free barrier animal facility of Gurdon Institute and the PCEA and ZEFI of IGMM, UMR5535, in Montpellier. We are grateful to J. Barau, D. Helmlinger and L. Bonneville for their advice and reagents related to the low-input CUT&RUN. We thank Y. Moyano-Rodríguez, T. Forné and E. J. Kremer for proofreading the manuscript. This work was supported by a Centre National de la Recherche Scientifique et Institut National de la Santé et de la Recherche Médicale (CNRS-INSERM) ATIP-Avenir grant, the Amorçage Jeune Equipe from Fondation Recherche Médicale (FRM AJE202005011598) and Agence National de la Recherche under the ‘Investissements d’avenir’ program (ANR-16-IDEX-0006) to M.B., by the Wellcome Trust (096738 and 092096) and Cancer Research UK program (C6946/A14492) to M.A.S., by a PhD fellowship from La Ligue Nationale Contre le Cancer to C.R. and by FRM (SPE20150331826) and Marie Sklodowska-Curie Individual (H2020-MSCA-IF-2015, no. 706144) fellowships to M.B. We acknowledge financial support to MGX from the France Génomique National infrastructure, funded as part of the ‘Investissement d’Avenir’ program managed by Agence Nationale pour la Recherche (contract ANR-10-INBS-09).
Author information
Authors and Affiliations
Contributions
M.B. conceptualized the study with M.A.S. and performed the scRNA-seq experiments. C.R. performed the chromatin analysis, handled the mouse colonies and collected the embryos with the help and supervision of K.C. L.S., E.B., P.R. and D.Z. performed the bioinformatics analysis, supervised by N.S. and M.B. A.T. performed the repeats and WGBS bioinformatics analysis. C.L. contributed to the animal husbandry and sample collection. M.W. and D.B. conducted the WGBS experiments. M.B., M.A.S. and D.B. secured the funding. M.B. wrote the original draft. C.R. and L.S. reviewed and edited the manuscript, with input from coauthors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editors: Carolina Perdigoto and George Inglis, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Differential gene expression upon female PGC development and key markers of soma and PGC.
(A) PCA of the 30 most differentially expressed genes (DEGs) that contributed to lineage segregation. The different stages are denoted by different colours. Orange arrows represent genes contributing to PGC lineage, and blue arrows represent genes contributing to soma lineages. (B-D) Volcano plots represent differentially expressed genes (DEGs) between the two developmental stages of female PGCs. Differential expression was measured by TMM method (trimmed mean of M values, see Method section), followed by Benjamini–Hochberg correction. Genes with an adjusted P value < 0.05 were considered significant. A few transcriptional changes have been observed in migratory PGCs. Changes arise once PGCs colonize the gonads. Some examples of DEGs are highlighted. Red dots represent upregulated genes, and blue dots represent downregulated genes. X-linked genes are shown in orange.
Extended Data Fig. 2 Clustering of PGCs by sex.
(A) Pseudotime representation of the scRNA-seq data based on the first principal component for XX females (pink), XO females (red), and XY males (blue). (B) Level of Xist expression and degree of reactivation in each single cell. Each dot represents a single cell. Most female soma cells exhibit high Xist expression and a low number of biallelically expressed genes. Genes with RPRT expression < 4 were considered to be unexpressed.
Extended Data Fig. 3 Contribution to the kinetics of X-chromosome reactivation.
(A) Representation of the Gene Ontology analysis of Biological process performed on the best correlated genes with X-linked gene reactivation (adj.p-value ≤ 0.05) (Supplementary Data 3). Correlation and anti-correlation between gene expression levels (autosomes and X chromosomes) and the percentage of X-linked gene reactivation (allelic ratio >0.15 and <0.85 for X-linked genes) were measured using two-sided Pearson’s correlation and the Benjamini–Hochberg correction. (B) Comparison of reactivation timing for 7 X-linked genes between our study (129 x Mus musculus castaneus PGCs) and published scRT-PCR digested by restriction enzyme (Mus musculus domesticus x Mus musculus mollossinus PGCs) 21. In the extracted data from Sugimoto and Abe, an arbitrary threshold of ≥ 50% of biallelic cells has been applied to consider the gene reactivated. (C) Distance to escapee genes. Distribution of genomic distances to escapees (Mb) for each X-linked gene reactivation class. The transcription start site (TSS) of each gene was used to measure the distance from the closest escaping gene. No significant differences were found between reactivation classes by the KW test (p-value = 0.13), despite very late-reactivated genes being statistically further to escapees than early-reactivated genes by MW test (p-value = 0.02). Boxplots represent the medians with lower and upper quartiles. (D) Reactivation classes in female PGCs compared to the in vitro PGC-like cell system 32. (E) Xist RNA entry sites are regions of the X chromosome showing early accumulation of Xist RNA upon initiation of X-chromosome inactivation and are thought to be the closest to the Xist locus in 3D spatial proximity. Allelic expression of X-linked genes classified on the basis of their relative position to Xist RNA entry sites (as identified during XCI induction in ESC 6): inside (TSS located inside a Xist RNA site, 17 informative genes), next to (TSS located less than 100 kb away from an entry site, 17 informative genes) and outside (over 100 kb from an entry site, 163 informative genes). Box plots represent medians (centre lines) with lower and upper quartiles (box limits). Whiskers represent 1.5× the interquartile range. Outliers are represented by dots.
Extended Data Fig. 4 Validation of H3K27me3 low-input CUT&RUN in enriched regions from both chromosomes.
Integrative Genomics Viewer tracks for total H3K27me3 normalized reads (dark blue) and allele-specific reads (light blue: CAST; red: B6) at the HoxC locus (A) and Sox9 locus (B) in Count Per Million mapped reads (CPM, bin 10). Orange blocks are statistically-called H3K27me3 broad domains by MACS2, compared to IgG (top track). Each replicate is shown at E11.5 and E12.5. Location is given in mm10 genome, with gene isoforms extracted from Integrative Genome viewer and UCSC.
Extended Data Fig. 5 CUT&RUN tracks at escapee and early-reactivated X-linked locus.
Integrative Genomics Viewer tracks for total H3K27me3 normalized reads (dark blue) and allele-specific reads (light blue: Xi/CAST; red: Xa/B6) at the Kdm5c region (very-late reactivated gene) (A) and Smc1a region (early-reactivated gene) (B) in Count Per Million mapped reads (CPM, bin 10). Orange blocks are statistically-called H3K27me3 broad domains by MACS2, compared to IgG. Each replicate is shown at E11.5 and E12.5. Location is given in mm10 genome, with gene isoforms extracted from Integrative Genome viewer and UCSC.
Extended Data Fig. 6 CUT&RUN tracks at candidate X-linked gene locus.
(A) Integrative Genomics Viewer tracks for total H3K27me3 normalized reads (dark blue) and allele-specific reads (light blue: Xi/CAST; red: Xa/B6) at the Gjb1 (silent gene), Zmym3 (very-late reactivated gene) and Nono (early-reactivated gene) locus. Each replicate is shown at E11.5 and E12.5. (B-D) Integrative Genomics Viewer tracks for total H3K27me3 normalized reads (dark blue) and allele-specific reads (light blue: Xi/CAST; red: Xa/B6) at (B) the Med14 early reactivated gene, (C) the Kif4 early-reactivated gene, and (D) the Cacna1f silent gene in Count Per Million mapped reads (CPM, bin 10). Orange blocks are statistically-called H3K27me3 broad domains by MACS2, compared to IgG. Location is given in mm10 genome, with gene isoforms extracted from Integrative Genome viewer and UCSC.
Extended Data Fig. 7 H3K27me3 occupancy at active and inactive X chromosomes.
(A) Heatmaps of H3K27me3 occupancy in E11.5 and E12.5 female PGCs. Profile plots and their corresponding heatmaps centred on the transcription start sites (TSS) +/- 3 kb of all X-linked genes for H3K27me3 repressive histone marks. The purple-to-yellow gradient indicates low to high sum score of H3K27me3 in the corresponding regions clustered by k-mean =5 on total H3K27me3 at E11.5. Genome B6: Xa and Genome Cast: Xi. (B) Repartition of reactivation classes and silent genes in the different clusters, calculated by k-mean, based on their H3K27me3 occupancy at E11.5. Silent gene class has been characterised as genes silenced from both X chromosomes in all female PGC scRNA-seq. Numbers in pie charts are TSS.
Supplementary information
Supplementary Figures
Supplementary Figs. 1–4.
Supplementary Data 1
Information on the scRNA-seq datasets.
Supplementary Data 2
Allelic ratio of informative X-linked genes and escapees from Fig. 3 heatmaps and their reactivation classes.
Supplementary Data 3
Correlation between gene expression and percentage of X-linked gene reactivation (XX soma and PGC cells).
Supplementary Data 4
DEGs at E12.5 PGCs. Differential expression was measured by the trimmed mean of M values (Methods), followed by Benjamini–Hochberg correction. Genes with an adjusted P value < 0.05 were considered significant.
Supplementary Data 5
Primers for allele-specific DNA methylation assays.
Source data
Source Data Figs. 1–6 and Extended Data Figs. 1–3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roidor, C., Syx, L., Beyne, E. et al. Temporal and regional X-linked gene reactivation in the mouse germline reveals site-specific retention of epigenetic silencing. Nat Struct Mol Biol 32, 926–939 (2025). https://doi.org/10.1038/s41594-024-01469-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41594-024-01469-2
This article is cited by
-
Single nuclei and spatial profiling of sacrococcygeal teratomas reveals cellular composition and X inactivation heterogeneity
npj Precision Oncology (2026)
-
Single-cell long-read Hi-C, scNanoHi-C2, details 3D genome reorganization in embryonic-stage germ cells
Nature Structural & Molecular Biology (2025)
-
Slow awakening of the silent X chromosome in female primordial germ cells
Nature Structural & Molecular Biology (2025)