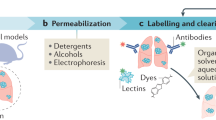
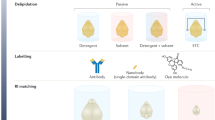
Abstract
Advances in tissue labeling and clearing methods include improvement of tissue transparency, better preservation of fluorescence signal, compatibility with immunostaining and large sample volumes. However, as existing methods share the common limitation that they can only be applied to human tissue slices, rendering intact human organs transparent remains a challenge. Here, we describe experimental details of the small-micelle-mediated human organ efficient clearing and labeling (SHANEL) pipeline, which can be applied for cellular mapping of intact human organs. We have successfully cleared multiple human organs, including kidney, pancreas, heart, lung, spleen and brain, as well as hard tissue like skull. We also describe an advanced volumetric imaging system using a commercial light-sheet fluorescence microscope that can accommodate most human organs and a pipeline for whole-organ imaging and visualization. The complete experimental process of labeling and clearing whole human organs takes months and the analysis process takes several weeks, depending on the organ types and sizes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
References
Snyder, M. P. et al. The human body at cellular resolution: the NIH Human Biomolecular Atlas Program. Nature 574, 187–192 (2019).
Rood, J. E. et al. Toward a common coordinate framework for the human body. Cell 179, 1455–1467 (2019).
Rozenblatt-Rosen, O., Stubbington, M. J. T., Regev, A. & Teichmann, S. A. The Human Cell Atlas: from vision to reality. Nature 550, 451–453 (2017).
Srivastava, S., Ghosh, S., Kagan, J. & Mazurchuk, R. The PreCancer Atlas (PCA). Trends Cancer 4, 513–514 (2018).
Ardini-Poleske, M. E. et al. LungMAP: The Molecular Atlas of Lung Development Program. Am. J. Physiol. Lung Cell Mol. Physiol. 313, L733–L740 (2017).
Amunts, K. et al. BigBrain: an ultrahigh-resolution 3D human brain model. Science 340, 1472–1475 (2013).
Deverman, B. E. et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209 (2016).
Erturk, A. et al. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat. Protoc. 7, 1983–1995 (2012).
Cai, R. et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull–meninges connections. Nat. Neurosci. 22, 317–327 (2019).
Pan, C. et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat. Methods 13, 859–867 (2016).
Park, Y.-G. et al. Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat. Biotechnol. 37, 73–83 (2019).
Renier, N. et al. Mapping of brain activity by automated volume analysis of immediate early genes. Cell 165, 1789–1802 (2016).
Belle, M. et al. Tridimensional visualization and analysis of early human development. Cell 169, 161–173 e112 (2017).
Pan, C. et al. Deep learning reveals cancer metastasis and therapeutic antibody targeting in the entire body. Cell 179, 1661–1676.e19 (2019).
Kubota, S. I. et al. Whole-body profiling of cancer metastasis with single-cell resolution. Cell Rep. 20, 236–250 (2017).
Lai, H. M. et al. Next generation histology methods for three-dimensional imaging of fresh and archival human brain tissues. Nat. Commun. 9, 1066 (2018).
Morawski, M. et al. Developing 3D microscopy with CLARITY on human brain tissue: Towards a tool for informing and validating MRI-based histology. Neuroimage 182, 417–428 (2018).
Hildebrand, S., Schueth, A., Herrler, A., Galuske, R. & Roebroeck, A. Scalable labeling for cytoarchitectonic characterization of large optically cleared human neocortex samples. Sci. Rep. 9, 10880 (2019).
Ku, T. et al. Elasticizing tissues for reversible shape transformation and accelerated molecular labeling. Nat. Methods 17, 609–613 (2020).
Zhao, S. et al. Cellular and molecular probing of intact human organs. Cell 180, 796–812 e719 (2020).
Murakami, T. C. et al. A three-dimensional single-cell-resolution whole-brain atlas using CUBIC-X expansion microscopy and tissue clearing. Nat. Neurosci. 21, 625–637 (2018).
Matsumoto, K. et al. Advanced CUBIC tissue clearing for whole-organ cell profiling. Nat. Protoc. 14, 3506–3537 (2019).
Susaki, E. A. et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157, 726–739 (2014).
Tainaka, K. et al. Whole-body imaging with single-cell resolution by tissue decolorization. Cell 159, 911–924 (2014).
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013).
Ku, T. et al. Multiplexed and scalable super-resolution imaging of three-dimensional protein localization in size-adjustable tissues. Nat. Biotechnol. 34, 973–981 (2016).
Treweek, J. B. et al. Whole-body tissue stabilization and selective extractions via tissue-hydrogel hybrids for high-resolution intact circuit mapping and phenotyping. Nat. Protoc. 10, 1860–1896 (2015).
Tainaka, K. et al. Chemical landscape for tissue clearing based on hydrophilic reagents. Cell Rep. 24, 2196–2210 e2199 (2018).
Lai, H. M., Ng, W. L., Gentleman, S. M. & Wu, W. Chemical probes for visualizing intact animal and human brain tissue. Cell Chem. Biol. 24, 659–672 (2017).
Cequier-Sánchez, E., Rodríguez, C., Ravelo, A. G. & Zárate, R. Dichloromethane as a solvent for lipid extraction and assessment of lipid classes and fatty acids from samples of different natures. J. Agric. Food Chem. 56, 4297–4303 (2008).
Schmidt, M. M. et al. Collagen extraction process. Int. Food Res. J. 23, 913–922 (2016).
Yanagishita, M., Podyma-Inoue, K. A. & Yokoyama, M. Extraction and separation of proteoglycans. Glycoconj. J. 26, 953–959 (2009).
Todorov, M. I. et al. Machine learning analysis of whole mouse brain vasculature. Nat. Methods 17, 442–449 (2020).
Ugryumova, N., Matcher, S. J. & Attenburrow, D. P. Measurement of bone mineral density via light scattering. Phys. Med. Biol. 49, 469–483 (2004).
Greenbaum, A. et al. Bone CLARITY: clearing, imaging, and computational analysis of osteoprogenitors within intact bone marrow. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aah6518 (2017).
Grüneboom, A. et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat. Metab. 1, 236–250 (2019).
Gonzalez-Chavez, S. A., Pacheco-Tena, C., Macias-Vazquez, C. E. & Luevano-Flores, E. Assessment of different decalcifying protocols on osteopontin and osteocalcin immunostaining in whole bone specimens of arthritis rat model by confocal immunofluorescence. Int. J. Clin. Exp. Pathol. 6, 1972–1983 (2013).
Savi, F. M., Brierly, G. I., Baldwin, J., Theodoropoulos, C. & Woodruff, M. A. Comparison of different decalcification methods using rat mandibles as a model. J. Histochem. Cytochem. 65, 705–722 (2017).
Liu, A. K. et al. Bringing CLARITY to the human brain: visualization of Lewy pathology in three dimensions. Neuropathol. Appl. Neurobiol. 42, 573–587 (2016).
Murray, E. et al. Simple, scalable proteomic imaging for high-dimensional profiling of intact systems. Cell 163, 1500–1514 (2015).
Perbellini, F. et al. Free-of-Acrylamide SDS-based Tissue Clearing (FASTClear) for three dimensional visualization of myocardial tissue. Sci. Rep. 7, 5188 (2017).
Nojima, S. et al. CUBIC pathology: three-dimensional imaging for pathological diagnosis. Sci. Rep. 7, 9269 (2017).
Jensen, K. H. & Berg, R. W. CLARITY-compatible lipophilic dyes for electrode marking and neuronal tracing. Sci. Rep. 6, 32674 (2016).
Mann, D. M., Yates, P. O. & Stamp, J. E. The relationship between lipofuscin pigment and ageing in the human nervous system. J. Neurol. Sci. 37, 83–93 (1978).
Schnell, S. A., Staines, W. A. & Wessendorf, M. W. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J. Histochem. Cytochem. 47, 719–730 (1999).
Neumann, M. & Gabel, D. Simple method for reduction of autofluorescence in fluorescence microscopy. J. Histochem. Cytochem. 50, 437–439 (2002).
Yang, J. et al. Quenching autofluorescence in tissue immunofluorescence [version 1; peer review: 2 approved with reservations, 1 not approved]. Wellcome Open Res. https://doi.org/10.12688/wellcomeopenres.12251.1 (2017).
Helsby, M. A. et al. CiteAb: a searchable antibody database that ranks antibodies by the number of times they have been cited. BMC Cell Biol. 15, 6 (2014).
Voigt, F. F. et al. The mesoSPIM initiative: open-source light-sheet microscopes for imaging cleared tissue. Nat. Methods 16, 1105–1108 (2019).
Preibisch, S., Saalfeld, S. & Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25, 1463–1465 (2009).
Acknowledgements
We thank A. Ghasemi Mag for developing the Python script ‘Stitching.py’ to stitch sequences of images. We thank I. Horvath, K. Joseph, K. Biniossek and E. Süheda for revising the manuscript. We thank Miltenyi Biotec for providing the PE-conjugated antibodies. A schematic of the SHANEL pipeline (Fig. 1) was created with BioRender.com. This work was supported by the Vascular Dementia Research Foundation, Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy, ID 390857198) and DFG (SFB 1052, project A9; TR 296 project 03), H.M. and Z.R. would like to thank the China Scholarship Council (CSC) for the financial support (no. 201806780034 and no. 201806310110).
Author information
Authors and Affiliations
Contributions
A.E. conceived and led the project. S.Z. developed the original SHANEL protocol. H.M., Z.R. and S.Z. performed the experiments and wrote the manuscript. R.C. performed the antibody screening. H.S. and I.B. dissected and provided the human organs. All authors commented on the manuscript text.
Corresponding author
Ethics declarations
Competing interests
A.E. and S.Z. have filed a patent on the SHANEL technology described in this protocol.
Peer review
Peer review information
Nature Protocols thanks Etsuo A. Susaki and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Zhao, S. et al. Cell 180, 796–812 (2020): https://doi.org/10.1016/j.cell.2020.01.030
Extended data
Extended Data Fig. 1 Human brain clearing by perfusion system.
a, The dissection of human brain with CL and CR (left and right carotids, respectively), VL and VR (left and right vertebral arteries, respectively). b, The human brain after dissection and fixation. c, The setup to clear a human brain by perfusion system under the fume hood. The human brain was put in a glass container and connected with input and output tubing controlled by a peristaltic pump. There are four channels controlling the four connecting tubes as indicated in c (i). Two output tubes are connected to the carotid arteries as shown in c (ii), and two output tubes are connected to the vertebral arteries as shown in c (iii). The input tubes are protected with gauze to avoid solid impurities entering and blocking the tubing (see c (iv)). c (v) shows the decolorization effects of CHAPS/NMDEA that manifest as the blooming of dark-green color from the organ. d, The glass container was sealed with several layers of plastic wrap to prevent evaporation of the running solutions, especially EtOH, DCM and MeOH. e, Photo of human brain after SHANEL pretreatment and before clearing. Remaining blood in the vessels (b) was decolorized.
Extended Data Fig. 2 Passively stained conjugated antibody in centimeter-size human kidney and lung tissue.
a,b, Collagen IV, catalase and cytokeratin 19 antibody staining (green) in human kidney (a) and lung (b). Top panel in a and b shows immunofluorescent staining images obtained by confocal microscopy to verify antibody compatibility with SHANEL. Bottom panel shows 3D reconstruction of tissue with antibody labeling in Imaris. Scale bars, 20 µm and 500 µm, respectively.
Extended Data Fig. 3 Imaging software settings.
The user interface of LaVision Imspector software. a, Settings of the objective lens and magnification. b, Names of the devices should be listed in the correct order. c, One example for settings of the tiling scan with 4 × 7 tiles and 33% overlap.
Extended Data Fig. 4 Key steps for data stitching, renaming and compression.
a, One example for correcting information in ‘Stitching Image Grid Sequence’; a1 highlights the replacement of red letters in Step 42 about ‘file names’. b, The key information of ‘TileConfiguration’ txt file; b1 highlights the responding channel number that should be corrected and saved in the respective separate folder, and b2 highlights that the starting Z number should be ‘0000’ for all ‘TileConfiguration’ files. c, The information of ‘Stitching.py’. d, One example for settings of ‘Multi-Rename Tool. e, One example of compressing tiff files with ‘LZW TIFF’. Panel a produced with software from ref. 50, Oxford University Press.
Extended Data Fig. 5 Key steps for Arivis fusion of 3D images.
a, An example of flipping one set of volumetric dataset in X, Y or Z direction to match with another volumetric dataset. b, Overview of two volumetric datasets oriented in the same XYZ direction after flipping. c,d, Examples of identical structural markers from two volumetric datasets for fusion. e, Loading of three key markers for optimal fusion. f, 3D fused image of pancreas from two volumetric datasets.
Extended Data Fig. 6 Key steps for Imaris data loading and visualization.
a, An example of converting a stitched image sequence data to the .ims format using Imaris File Converter. b, The settings for file names with a delimiter should be changed to ‘C _ Z.tif’. c, 3D image properties including XYZ voxel size and channel color can be set in Imaris for analysis.
Extended Data Fig. 7 Overview of SHANEL workflow.
This workflow summarizes the main steps after organ collection: experimental setup (Steps 1–5), pretreatments (Steps 6–10), labeling and clearing (Steps 11–18), imaging (Steps 19–39) and data processing (Steps 40–79). We describe two ways of handling samples: active perfusion with a pump, if the blood vessels can be connected with external tubes, and passive incubation on a shaker. We also outline the differences in the procedure that are dependent on the organ components, organ size, targeted labeling and imaging equipment.
Supplementary information
Supplementary Information
Supplementary Table 1.
Supplementary Video 1
Change position of tubing to prevent breakage during active pumping.
Supplementary Video 2
Use a syringe to remove bubbles in the cleared sample.
Supplementary Video 3
Set the start point and end point in the Z direction during the light-sheet microscope imaging.
Supplementary Video 4
Human organs with vessel labeling.
Rights and permissions
About this article
Cite this article
Mai, H., Rong, Z., Zhao, S. et al. Scalable tissue labeling and clearing of intact human organs. Nat Protoc 17, 2188–2215 (2022). https://doi.org/10.1038/s41596-022-00712-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-022-00712-8
This article is cited by
-
Reverse impact of chordae tendineae structural changes on its biomechanical properties as a part of pathogenesis in canine myxomatous mitral valve disease
BMC Veterinary Research (2025)
-
A novel protein-preserving passive tissue clearing approach using sodium cholate and urea for whole-organ imaging
Experimental & Molecular Medicine (2025)
-
Spatial landmark detection and tissue registration with deep learning
Nature Methods (2024)
-
SOLID: minimizing tissue distortion for brain-wide profiling of diverse architectures
Nature Communications (2024)
-
INSIHGT: an accessible multi-scale, multi-modal 3D spatial biology platform
Nature Communications (2024)