Abstract
Nutritional Programming (NP) has been studied as a means of improving dietary plant protein (PP) utilization in different fish species. This study investigated the use of enriched live feed as a vehicle for NP in larval fish. The objective of this study was to determine the effect of NP induced during the larval stage via PP-enriched live feed on: (1) growth performance; (2) expression of genes associated with inflammation and any morphological changes in the intestine; and (3) muscle free amino acid composition in largemouth bass (Micropterus salmoides) during its later life stages. Two diets were used in this study, a fish meal (FM)-based diet, and a soybean mean (SBM)-based diet, serving as the PP diet. There were 4 groups in this study. The two control groups, ( +) Control and (−) Control, were not programmed and received the FM-diet and SBM-diet, respectively throughout the whole trial after the live feed stage (27–122 days post hatch (dph). The next group, programmed, was programmed with SBM-enriched Artemia nauplii during the live feed stage (4–26 dph) and challenged with the SBM-diet during the final stage of the study (79–122 dph). The final group, non-programmed, did not receive any programming and, was challenged with the SBM-diet during the final stage of the study. The programmed group experienced a significantly higher (%) weight gain during the PP-Challenge than the non-programmed group. In addition, the live feed programming resulted in significantly longer distal villi, and a higher villi length to width ratio, compared to the non-programmed group. No significant effects on free amino acid composition and gene expression were observed between the programmed and non-programmed group, except for an increased post-prandial concentration of free proline in the programmed group. The results of this study support use of live feed as a vehicle for nutritional programming and improving the growth performance of largemouth bass fed with a SBM-based diet.
Similar content being viewed by others
Introduction
As the aquaculture industry grows, there is building pressure to find more suitable protein sources to fully replace fishmeal (FM) and other high-quality plant protein concentrates in fish diets. Different types of plant proteins (PP) have been studied extensively as additives in diets to reduce the use of FM in aquaculture1,2,3,4,5. Currently the most commonly used FM replacement is soybean meal (SBM). Its inclusion level, however, is limited. The induction of intestinal inflammation from SBM has led to significantly decreased growth performances when fed at levels exceeding 25%6,7,8,9,10. For example, in a study done on a carnivorous fish species Rainbow Trout (Oncorhynchus mykiss), the replacement of FM with SBM resulted in significantly lower weight gain and an increased FCR during the study11. Miao et al.12 also found that SBM levels over 50% in the diets of northern snakehead (Channa argus) significantly decreased weight gain and specific growth rate as soon as 21 days of feeding with the SBM diet.
One approach to mitigating the negative effects of dietary PP and improving the growth performance of fish fed PP-based diets is nutritional programming (NP). NP is a concept that an organism can be ‘programmed’ to utilize a certain dietary component through exposure during early life stages. Traditionally, NP has been induced using dry feed during the juvenile stage of the fish’s development. Previous studies have achieved positive results using this form of NP3,13,14,15. However, the digestive tract of the fish is most responsive to different environmental factors, including diet, during the larval stages, immediately after hatching. And therefore, it seems most effective to induce NP during that stage, when the digestive tract experiences a significant amount of phenotypic plasticity during its development16.
A significant limitation to larval NP however, is poor food intake and the inefficiency of dry feed utilization due to poor digestive tract development at such a young age. According to Sales17, larval fish are 2.5 times more likely to experience mortality when fed with dry diets as opposed to live feeds. Additionally, studies have found that replacing live feed with dry diets at first feeding negatively affects the growth of larval fish18,19,20,21. Larval goldfish (Carassius auratus) showed significantly reduced growth and survival on dry feeds, compared to fish fed with live food and the reduction in growth was associated with weak tryptic activity in the intestinal tracts18. In addition to reduced digestive activity, a reduction in feed intake has been observed in live feed replacement studies. Adekunle and Joyce19, reported that hybrid catfish (Herterobranchus bidorsalis x Heterobranchus longifilis) had presented lower feed intake of the dry diets compared to Artemia nauplii, and this led to reduced growth and survival of the newly-hatched catfish.
In order to avoid the negative effects of dry diets at first feeding, some researchers have utilized enriched live feed as a vehicle for nutritional components in larval fish. Past studies have found that Artemia enrichment is an effective vehicle for probiotics22, iodine23, and highly unsaturated fatty acids24. Based on these findings, we tested the use of Artemia nauplii as a vehicle for SBM to induce NP. The Artemia are filter feeders and absorb their nutrients from the water25. We hypothesized that by enriching the Artemia with SBM solution, we could program the larval largemouth bass (LMB) (Micropterus salmoides) at first feeding, while still keeping the natural feeding regime of this fish to ensure nutrient delivery and high feed intake. The objectives of this study were to determine the effect of NP with dietary PP induced during the first feeding of larval LMB using live food as a vehicle on: (1) LMB growth performance; (2) Expression of genes associated with inflammation and any morphological changes in the intestine; and (3) Muscle free amino acid (FAA) composition in LMB during its later life stages used as an indicator of dietary amino acid availability.
Materials and methods
The feeding trial was conducted in the Center for Fisheries, Aquaculture, and Aquatic Sciences at Southern Illinois University-Carbondale (SIUC), IL. All experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of SIUC. The SIUC Institutional Animal Care and Use approved all of the protocols performed (protocol# 18-051). All researchers were trained in accordance with SIUC Institutional Animal Care and Use requirements. This study was carried out in compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. During fish handling, anesthesia was performed using water bath immersion in tricaine methanesulfonate (MS222) at a recommended concentration (25 mg/l), and all efforts were made to minimize pain, stress, and discomfort in the animals. Humane endpoints were used in this study, fish were euthanized within 24 h if they showed significant signs of deteriorating quality of life. Fish were observed each day for signs of distress. No fish in this study had to be euthanized prior to the completion of the experiment. The ethical guidelines of handling and fish care follow the same protocols outlined in a previous study conducted at the same facility26.
The experiment was carried out using a semi-recirculated aquaculture system with two mechanical (sand) filters (Pentair, Minneapolis, MN) and two bio-filters. The water in the system was exchanged at a daily rate of 20% during the larval stage (4–26 dph) and 50% for the rest of the study. The system consisted of 40 (100 L) light blue tanks. The average water temperature was 23.8 °C (± 1.3), the pH was 7.70 (± 0.29), and the salinity was kept between 1 and 3 ppt during the live feeding stage to prolong the viability of the live food27. The photoperiod consisted of 14 h of darkness and 10 h of light, with the overhead lights on 8:00–18:00.
Feed preparation and formulation
Prior to mixing, the dry components of the diet were ground to a fine particle size (~ 0.5 mm) using a centrifugal mill (Retsch 2 M 100, Haan, Germany). Once the dry components were ground, all ingredients in the diet were mixed (HCM450 Vertical Cutter Mixer, Hobart, Troy, OH) to achieve uniform dispersion. To produce small pellet sizes, half of the mixture was extruded (Caleva Extruder 20, Sturminster Newton Dorset, England) and spheronized (Caleva Multibowl Spheronizer, Sturminster Newton Dorset, England). The other half of the mixed diets were run through a food chopper (General Slicing SD-50, General Inc., Weston, FL), to obtain a variety of larger pellet sizes. The pellets were dried (Harvest Saver Tray Dryer, Commercial Dehydration Systems Inc., Eugene, OR) to remove moisture from the diets. After drying, all pellets were separated by size using a vibratory sieve shaker (Retsch AS 200 Basic, Haan, Germany).
The diets were formulated to be isolipidic and isonitrogenous, with crude protein and lipid levels of 48.9% and 10%, respectively. The SBM diet had a full fishmeal replacement with soybean meal (46.3%) and soy protein isolate (15.4%). Soy protein isolate was utilized to adjust dietary crude protein level while also leaving room for other ingredients in the formulation, including a minimum level of starch to allow expansion of the diets. The formulations for each diet are listed in Table 1. The amino acid compositions of the diets are laid out in Table 2. In this study, the SBM diet serves at the PP-based diet.
Diet analysis
Proximate composition of diets included quantification of the following: crude protein, crude lipid, moisture, and ash. Briefly, samples were analyzed for ash by combustion (550 °C for 5 h) in a muffle furnace (Lindberg Blue M, MA); crude protein (N × 6.25) using a Leco nitrogen analyser (Model FP-628, Leco Corporation, St. Joseph, MO); and crude lipid was extracted with chloroform–methanol (2:1, v/v), as described by Folch et al.28. The amino acid profile of each feed was analyzed utilizing the Association of Official Analytical Chemists, International (AOAC) Official Method 999.13. All dietary samples were analyzed in triplicates.
Experimental design
At 4 days post hatch (dph), larval LMB (~ 0.0034 g) were randomly distributed into 12 (100 L) tanks with an initial average density of 867 (± 99) per tank. At 58 dph, the densities in each tank were reduced to 100 fish, and further reduced to 30 fish at 81 dph. The reduction in densities was done to ensure that the growth of the fish was not restricted by tank densities and that other measured parameters were not impacted by varying densities tanks. The experimental tanks started at a volume of 50 L and were increased to 100 L on 25 dph. There were 4 experimental groups utilized in this study, with 3 replicate tanks for each group. The first group was our programmed group. The programmed group was programmed with SBM through enriched Artemia nauplii (Brine Shrimp Direct, Ogden, UT) during 4–26 dph. After the programming, this group was fed with FM diet during 27–78 dph, and then exposed to the SBM diet during the “plant protein challenge” (PP-Challenge) (79–122 dph). The second group was our non-programmed group. This group underwent the same feeding regime as the programmed group, however it was not programmed with SBM during the live feed stage and, was fed spirulina enriched Artemia during 4–26 dph. The last two groups represent our control groups. The ( +) Control group and the (−) Control group both received regular spirulina enriched Artemia during 4–26 dph, and then were fed with the FM diet and SBM diet, respectively for the duration of the study (Fig. 1).
To ensure high feed intake, larval bass were fed to satiation up until 78 dph. Beginning at 79 dph, the fish were fed with a restricted feeding rate. The feeding rate was originally set by measuring the observed feed intake for each tank and setting the feeding level to the tank with the lowest feed intake. This ensured a consistent feeding rate across all tanks and ensured all food added to the tanks was consumed. Most importantly, this eliminated any potential bias that would have been caused by palatability or preference differences between the two diets that could have impacted the feed intake among groups. This helped ensure that any differences in growth performance would not be a function of varying feed intake levels. In addition, the feeding rate was adjusted daily, using an assumed FCR of 1, and also readjusted through observations of feed intake at each feeding. Also, a bi-weekly weighing was conducted during the restricted feeding period, in order to determine the actual biomass in each tank and, readjust the feeding rate accordingly. The feeding rates were 9% for 79–85 dph, 7% for 86–89 dph, 5% for 90–93 dph, 3% for 94–109 dph, and 2.5% for 110–122 dph.
Live feed enrichment
Finely ground particles (< 0.25 mm) of SBM were mixed with deionized water to form a solution. The formulation for the solution was 1-part dry powder to 19 parts deionized water. The solution was thoroughly homogenized and filtered through 150 µm to ensure it was able to be absorbed (filtered) by the Artemia naupli. The Artemia were hatched and after 8 h (the time after which the nauplii start the filtering process25) were enriched for at least 3 h prior to being fed to the fish. Photographs of enriched Artemia are shown in Fig. 2. Water in the enrichment jars was changed twice a day to remove waste, and the enrichment solution was continuously added throughout the day to ensure a constant supply of enrichment solution in the water (Suppl. information).
Sampling and measuring
At the conclusion of the study (122 dph), three fish from each tank were randomly sampled, euthanized with MS-222, and their intestines dissected and stored in a 10% formalin solution for histology analyses. In addition, 3 fish from each tank were euthanized with MS-222 and their intestines harvested and stored in RNALater (Sigma-Aldrich, St. Louis, MO, USA) for future analysis of gene expression. For free amino acid analysis, three fish were sampled from each tank at 3 and 24 h after feeding at the conclusion of the study and, stored at − 80 C until analysis.
Fish were weighed on a bi-weekly basis beginning at 79 dph in order to measure biomass and adjust feeding rates. The average weight per fish of each group was measured at the end of the study, as was the percent weight gain of each group during the study. Average weight was calculated for each tank by dividing the final biomass by the number of fish in the tank. Growth parameters in this study were calculated using the following formulas:
Gene expression analysis
Intestinal samples were processed using TRIzol Reagent (Ambion, Foster City, CA, USA) and RNA was extracted and purified using the On-Column PureLink DNase Treatment (PureLink RNA Mini Kit and PureLink DNase, Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Once purified, the ng/µl of each RNA sample was obtained using a spectrophotometer (Nanodrop 2000c, Thermo Fisher Scientific, Waltham, MA, USA). From this point, 2 µg of RNA from each sample was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) to obtain a 20 µl cDNA solution. The cDNA solutions were then added to a tube with 380 µl of water, to produce the cDNA sample for each tank. Gene expression of each cDNA sample was measured using a Bio-Rad (Hercules, CA, USA) CFX96 Real-Time PCR System. Each qPCR reaction mixture (20 µl) contained 9 µl of cDNA sample, 10 µl of PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, Waltham, MA, USA), and 0.5 µl of 800 nM each of forward and reverse primers. Primers (Table 3) were synthesized by Integrated DNA Technologies (Coralville, IA, USA). Each qPCR reaction was run in technical duplicates. The qPCR cycle consisted of 95 °C for 10 min, followed by 40 cycles of 95 °C for 20 s and 60 °C for 35 s, followed by a melting curve to ensure the amplification of only a single product in each well. Relative gene expression was calculated using the 2−\(\Delta \Delta\)Ct method, normalizing the target gene expression to the expression of β-actin (reference gene)29. The expression of genes in in the programmed and non-programmed groups were then compared to that of the (-) Control group to observe how the expression varied between the three groups that received the PP-based diet.
Histological analysis
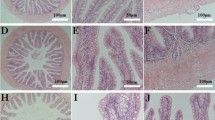
The histological analysis followed the method described in Molinari et al.26. Histological slides were prepared by Saffron Scientific Histology Services (Carbondale, IL). Intestines previously fixed in 10% neutral buffered formalin were processed to paraffin using a Sakura enclosed automated tissue processor (Netherlands). The three representative areas of LMB intestines were orientated for cross sections embedded together in the same block. Five-micrometer serial sections were cut with a Leica manual microtome (Buffalo Grover, IL) and placed on water bath at 44 °C. Sections were placed on positive charged slides. After drying, slides were stained with hematoxylin and eosin and cover-slipped using acrylic mounting media. The histological analysis focused on the hind-gut portions of fish digestive tract. Pictures of each slide at 50X magnification were obtained using a microscope (Leica DMI 300B) and camera (Leica DMC 290) combination, with the software LAS V4.4 (Leica Camera, Wetzler, Germany, https://www.leica-microsystems.com). Pictures were taken of 3 samples from each tank, with 9 pictures being analyzed for each group. From these pictures, individual lengths and widths were taken of intact villi using ImageJ (NIH, Betheseda, MD, USA). Length and width data were measured in the distal portion of the intestine. Villus length was measured from the tip of the villus to the luminal surface, and villi width was measured across the base of the villus at the luminal surface. The length-to-width ratio of each villus was determined by dividing the length by the width.
Free amino acid analysis
Muscle samples obtained from the frozen fish (− 80 ºC) were used for the analysis. Those muscle samples of three fish from each tank were combined and homogenized together with 0.1 M HCl in 1:9 (w/v) and spun at 12,000g (4 ºC, 15 min). Supernatants were collected, filtered (Milipore, 10 kDa cutoff at 15,000g, 4 ºC, 30 min) and later diluted with 0.1 M HCl (1:19 v/v) containing norvaline and sarcosine (40 µM) as internal standards. Blanks (0.1 M HCl + 40 μM norvaline and sarcosine) and external standards (Sigma acid/neutral and basic amino acids) were prepared along with the sample preparation. The same concentration of glutamine in 0.1 M HCl as an external standard was prepared and added to the basic amino acids standard. Free amino acids were quantified using Shimadzu Prominence Nexera—i LC-2040C Plus (Shimadzu, Japan) according to the Shimadzu protocol No. L529 with modifications30. Free amino acid concentrations (expressed as µmol/kg wet body weight) were calculated in LabSolutions software version 5.92 (Shimadzu, Japan, https://shimadzu.com.au/labsolutions) using internal and external standards.
Statistical analysis
Results are presented as means (± standard deviation). One-way ANOVA was used to test the data, and a Tukey test was run to test the differences between groups. Due to the high variability of FAA concentrations, an LSD test was run to detect differences between groups. Differences between groups are considered significant at p values < 0.05. Statistical analysis was run using R version 3.5.2 (Boston, MA).
Results
Growth performance
At the conclusion of the study (at the end of the PP Challenge), the (−) Control group had a significantly lower average weight compared to the other three groups (p = 0.0001) (Table 4). The (%) weight gains presented in the tables were calculated to show the weight gain of each group from the start (79 dph) to the end of the PP-Challenge (122 dph). The programmed group had a significantly higher weight gain than the non-programmed group (p = 0.005). The programmed group’s (%) weight gain was also not significantly different from the ( +) Control group, which was still being fed FM-diet during the PP-Challenge. In addition, the programmed group had a significantly higher FE (p = 0.033) compared to the non-programmed group. The (−) Control group did have the highest (%) weight gain (593.22 ± 27.96) during the challenge, due to poor performance and significantly smaller average weight than the other groups at the start of the challenge caused by continued SBM diet feeding throughout the study.
No significant differences were observed in survival between groups during the larval programming stage (4–26 dph), with an overall survival of 90.29 (± 2.64). During the middle stage of the study (27–28 dph), after the programming stage and before the PP-Challenge, the (−) Control group (47.19 ± 4.97) had a significantly lower survival than both the ( +) Control group (69.87 ± 7.50, p = 0.0194) and the programmed group (79.10 ± 7.79, p = 0.0026). The survival of the non-programmed group (65.26 ± 7.88) during this stage was not significantly different from any of the other groups. No significant differences in survival were observed during the PP-Challenge, with only the (−) Control group (92.22 ± 6.93) exhibiting any more than 1 mortality per tank throughout the whole period.
Intestinal health
There were no significant differences in the expression of the pro-inflammatory cytokines across the different groups (Fig. 3).
Relative gene expression. Relative gene expression of the group is represented as a fold change relative to the negative control group (fold change = 1). Values provided are mean fold change + S.E.M (standard error of the mean). Results of One-way ANOVA and Tukey test are shown on graphs when significant. No significant differences were detected.
Looking at the histology data (Table 5), the ( +) Control and programmed groups both had significantly longer distal villi than the (−) Control and non-programmed groups (p = 0.0001). In addition, the ( +) Control had significantly wider villi than the (−) Control (p = 0.005), but the programmed and non-programmed groups were not significantly different from either group. Using the length and width values, the length-to-width ratio of the villi was calculated to get a representation of its surface area. The ( +) Control group had a significantly higher length-to-width ratio than the non-programmed group (p = 0.002). The (−) Control group showed a numerically lower length-to-width ratio, although it was not significantly different from any of the other groups. Between the two non-control groups, the programmed group had a significantly higher length-to-width ratio of their distal villi, compared to the non-programmed group (p = 0.0001). Figure 4 presents examples of histology samples analyzed from each group. The raw data from the intestinal health analyses are provided in Supplementary Information 1.
Free amino acid composition
The results for muscle FAA postprandial levels 3-h after feeding are presented in Table 6. All data from the FAA analysis is provided in Supplementary Information 2. Among the 10 indispensable amino acids (IDAA) analyzed, half showed significant differences between groups. The concentration of isoleucine was significantly higher in the (−) Control (p = 0.046) and FM-PP (p = 0.022) groups, compared to the ( +) Control. The programmed group had a significantly higher concentration of leucine than the ( +) Control group (p = 0.036). The ( +) Control group had a significantly lower level of both lysine (p = 0.0001) and histidine (p = 0.0008) than all of the other groups. The level of histidine was also significantly higher in the (−) Control group than in the non-programmed group (p = 0.037). The (−) Control group also had a significantly higher level of arginine, compared to the other groups (p = 0.036).
Among the 9 dispensable amino acids (DAA) analyzed, 8 showed significant differences among groups. The level of aspartic acid was significantly higher in the (−) Control group, compared to the ( +) Control (p = 0.002) and programmed group (p = 0.039). Asparagine had the highest concentration in the (−) Control group and the lowest in the ( +) Control group, with both groups significantly different from the non-programmed and programmed groups (p = 0.0004). The same results were observed in the alanine levels, except that the programmed group was not significantly different from the (−) Control group. The ( +) Control group had a significantly higher concentration of tyrosine (p = 0.022), and a lower concentration of glutamine (p = 0.009), compared to the (−) Control group. The glycine concentration was significantly lower in the programmed group, compared to the ( +) and (−) Control groups (p = 0.011, p = 0.026). The ( +) Control group had a significantly higher level of serine, compared to all other groups (p = 0.039).
A main focus of these results was to look at differences between the programmed and non-programmed groups. There were no significant differences between these two groups for any FAA tested, except for proline, which was significantly higher in the programmed group (p = 0.023). The concentration of IDAA was significantly higher in all groups compared to the ( +) Control (p = 0.0002). In addition, the (−) Control group had a significantly higher IDAA concentration than the non-programmed group (p = 0.012). There was no significance in the concentration of dispensable amino acids (DAA) among groups, but for total free amino acids (TFAA), the (−) Control (p = 0.008) and programmed (p = 0.034) groups had significantly higher levels than the ( +) Control group.
Discussion
Previous studies have found that NP using dry feed improves the utilization of dietary PP3,13,14,15. Kwasek et al.13 also tested the use of live feed for NP and found that, although non-significant, the live feed-programmed fish achieved numerically higher weight gain on a PP-based diet than non-programmed fish. The authors argued, however, that prolonged feeding duration could have made the result significant but the model choice (zebrafish Danio rerio) and its sexual dimorphism forced investigators to terminate the study earlier. This present experiment focuses on aquaculture species, LMB, and found that NP through live feed enrichment significantly improved the growth performance of LMB fed an SBM-diet during pre-adult stages. The programmed fish achieved an average (%) weight gain of 357% compared to a (%) weight gain of 278% for the non-programmed group. More significantly, the programmed group had close to the same (%) weight gain and FE feeding on a PP-diet as the ( +) Control group which was fed a diet based 100% on marine animal protein sources. The high weight gain observed in the (−) Control group is misleading due to its significantly lower initial weight at the start of the PP-Challenge. The average initial weight of the (−) Control group (2.18 g (± 0.11)) was more than 50% smaller than any of the other groups at that stage. Over the 43-day span of the PP-Challenge the (−) Control had a (%) weight gain of 593.22 (± 27.96). When the other three groups were of a comparable initial size (~ 2 g), their (%) weight gain over a shorter period (37-day span; 58–95 dph) similarly amounted 545.68 (± 96.17) compared to the lower (%) weight gain of 335.70 (± 48.73) achieved during PP-Challenge when the average initial fish size was 5.86 g (± 0.69). Thus, based on the growth trends for the fish in the other groups in this study, it can be assumed that the increased (%) weight gain in the (−) Control group can be attributed to its smaller size (and hence higher growth rate) at the start of the PP-Challenge. In addition, because of the low survival of the (−) Control group before PP Challenge (~ 47%), the growth response presented by this group fed SBM diet during the last 43 days of the study was likely a result of genetic selection of individuals characterized by higher resistance to the negative effects of SBM.
Other carnivorous species have been found to be negatively impacted by the inclusion of SBM into their diets. In spotted rose snapper (Lutjanus guttatus), a FM-replacement level of 60% with SBM significantly reduced the growth rate of the fish8. The same effects were observed at an even lower inclusion level of 30% SBM in Korean rockfish (Sebastes schlegeli)10. Similar to the other carnivorous species, LMB are also known to be sensitive to high SBM inclusion levels in their diet. A previous study found that the inclusion of dietary SBM for LMB led to a significant decrease in both growth and digestibility, even at a level of 50% FM replacement31. This study provides support that, with the use of NP with SBM through live feed, a carnivorous aquaculture species like LMB can grow as efficiently on a ~ 60% soy-based diet, as they do on a typical FM-based diet.
The mechanism behind NP is believed to occur through epigenetic modifications in the DNA. In a review by Lillycrop and Burdge32, it was determined that nutritional exposure in early life stages alters the epigenome and consequently, affects the regulation of certain genes in later life stages. This possible epigenetic effect of early exposure to PP has been observed to affect the expression of genes associated with digestive hormones13 and also appetite regulation33. Kwasek et al.13 found that NP in zebrafish had a significant impact on ghrelin, cholecystokinin, and neuropeptide Y expression. Additionally, NP has been observed to impact the expression of intestinal peptide transporter PepT126. The same epigenetic effect that NP has had on appetite and digestion related genes, has also been observed in intestinal inflammation-related genes. In this study, we looked at the expression of genes associated with intestinal inflammation in LMB and their response to re-exposure to SBM in the later life stages, similar to Perera and Yufera34. More specifically, this study analyzed the expression of proinflammatory cytokines IL-1 (Interleukin-1), IL-8 (Interleukin-8), tnf- α (tumor necrosis factor alpha), and IL-12p40 (Interleukin 12 subunit p40). Hedrera et al.35 found that both IL-1 and IL-8 were significantly up-regulated in the intestines of zebrafish that were exposed to SBM. In addition, the up-regulation of tnf-α has been found to be a result of SBM induced intestinal inflammation36. Wang et al.37 observed that the genes IL-1, tnf- α, and IL-12p40 all showed an increased expression with increases in SBM levels in the diet. This finding is consistent with another programming study that found that NP of zebrafish significantly downregulated expression of pro-inflammatory cytokines when the fish were reintroduced to SBM later in life34. The findings of these studies may signify that the intestines of the programmed fish have adapted to the SBM leading to reduced level of inflammation induced by the SBM diets. This study however, did not find a significant effect of NP on the intestinal inflammatory cytokines in LMB.
SBM inclusion in diets has been found to trigger an inflammatory response that negatively affects the morphology of the intestines of fish. The SBM induced inflammation (enteritis) is characterized by shorter villi and a decreased capacity for absorption in the epithelial enterocytes6,38,39,40,41. Zhang et al.40 found that 75% replacement of FM with SBM resulted in significantly shorter intestinal villi and, correlated with increased enteritis and a significantly reduced growth rate in Japanese sea bass (Lateolabrax japonicus). Similar results were observed in beluga sturgeon (Huso huso), where SBM inclusion resulted in shorter and wider distal villi41. In addition, Wang et al.6 observed that the complete replacement of FM with SBM in diets reduced the length of the intestinal villi and also the surface area of the villi. The negative impact on the intestinal morphology led to a significantly reduced growth rate and feeding efficiency for the group fed a 100% SBM-based diet6. Previously, NP has been found to increase the surface area for absorption in the intestine, represented by longer villi and a higher length-to-width ratio of the villi13. This study found that the programmed group had longer distal villi and also a higher length-to-width ratio of the distal villi, compared to the non-programmed group. It is possible that the programming may have made the intestines of the bass more resistant to the inflammatory properties in SBM, leading to increased surface area for absorption in the intestine and ultimately improved nutrient absorption capacity reflected by higher weight gain during the PP-Challenge.
The muscle FAA results in this study reveal a significant difference between the utilization of dietary FM and SBM. Previous studies have found that FM replacement with PP leads to a significant delay in the peak of FAA in the body, which means that the timing of post-prandial FAA peak in fish tissues is significantly affected by the dietary protein source42. Gomez-Requeni et al.43, observed that the post-prandial level of FAA in the muscle of gilthead sea bream (Sparus aurata) was significantly higher in fish that were fed with a PP-based diet, compared to a FM-based diet. The authors determined that this increase in muscle TFAA signified a lower utilization rate of the dietary protein and, was correlated with a reduced growth rate in the PP-fed group43. The results of this study might support these findings. The levels of IDAA and TFAA were significantly higher in the PP-fed (−) Control group, compared to the FM-fed ( +) Control group. The same trend can be observed in many of the individual free amino acids like asparagine, histidine, alanine, and lysine, which were all significantly lower in the ( +) Control group compared to the three PP-fed groups. Similar to the study done by Gomez-Requeni et al.43 the high TFAA and IDAA levels in the (−) Control group were also associated with a significant reduction in growth rate for the PP-fed fish. We can assume that the significant differences among FAA concentrations in the study between the ( +) Control group and the three PP-fed groups are attributed to the different dietary protein source. The effect of the difference in digestive efficiency between the two dietary protein sources could be further amplified by the fact that LMB is a warm-water, carnivorous species. One study done on rainbow trout, a cold-water, carnivorous species, found that the peak FAA levels in the plasma occurred ~ 12 h after feeding with FM and ~ 21 h after feeding with SBM44. Another study done on rainbow trout observed a similar delay, with FAA peaking in the plasma at 6–8 h after feeding with FM and 12 h after feeding with PP42. While those studies were done on cold-water fish, it has been found that there is a temperature effect on amino acid absorption, with absorption rates increasing with rising temperatures45. In a study done on yellowtail (Seriola quinqueradiata), a warm-water carnivore, the peaks of FAA appeared just 2–4 h after feeding with raw fish46. Based on these studies, it is apparent that in carnivorous species, amino acids are absorbed much more rapidly from FM-based diets compared to PP-based diets, and that amino acid absorption and utilization is quicker in warm-water species, compared to cold-water species. Thus, it is feasible that at 3 h after feeding, more of the FAA in the ( +) Control group may have been absorbed and utilized prior to sampling, while the delayed digestion of the SBM-diet in the other groups may have caused the FAA to just reach the muscle pool at the time of sampling.
However, the results of the FAA analysis for this study showed minimal difference between the programmed and non-programmed groups. The lack of differences between the two groups may be linked to the relatively low dynasticity of the muscle FAA pool. Mente et al.47 found that the white muscle FAA concentrations of Atlantic salmon (Salmo salar) remained undifferentiated for at least 12 h after being fed with diets containing varying levels of maize gluten as a protein source. The same study determined that the white muscle FAA pool may be resistant to significant post-prandial changes in FAA concentrations immediately after feeding47, whereas FAA pools in other areas of the fish body, including blood plasma, have been shown to be more responsive to FAA changes after feeding48. Among the muscle FAA measured, the only significant difference between the programmed and non-programmed groups was the concentration of free proline. Proline is a DAA that plays a key role in protein synthesis, including the synthesis of collagen49. l-proline as a dietary supplement has been found to increase growth performance in pigs and, improve the functionality of the gastrointestinal tract50. While it is unlikely that the higher concentration of proline had a significant effect on the increased growth performance of the programmed fish, it is still worth noting due to its functional role in tissue protein accretion.
Conclusion
The results from this study support the use of live feed as a vehicle of SBM for NP in larval LMB. The programmed bass experienced significantly higher weight gain (%) during the PP-Challenge, compared to the non-programmed fish and achieved a weight gain similar to that of the bass that were fed FM. The mechanism behind the programming seemed to lie in the intestine. While no significant effect was observed on the expression on inflammatory cytokines, the significantly higher length-to-width ratio of the distal villi signifies an increased surface area for nutrient absorption in the intestine as a result of the NP. The programming did not have a significant effect on FAA concentrations, except that free proline had a higher concentration in the programmed group.
By using enriched live feed to program at first feeding, we are able to begin introduction of PP to the larval fish at the earliest possible point (first feeding) and, prevent the programming process from having a negative effect on the fish development in its early life stages. Further research would be beneficial into determining the viability of live feed NP across a wider variety of aquaculture species, and also providing a deeper look into the physiological mechanisms behind NP. The findings from this study are important for the industry of aquaculture allowing for NP at first feeding, without disrupting the natural feeding regime of the fish ultimately leading to increased utilization of feeds containing SBM and hence reduction in costs of commercial PP-based dietary formulations.
Data availability
All data is available in the supplementary information files.
References
de Francesco, M. et al. Effect of long-term feeding with a plant protein mixture based diet on growth and body/fillet quality traits of large rainbow trout (Oncorhynchus mykiss). Aquaculture 236(1–4), 413–429 (2004).
Rimoldi, S. et al. Intestinal B 0 AT1 (SLC6A19) and PEPT1 (SLC15A1) mRNA levels in European sea bass (Dicentrarchus labrax) reared in fresh water and fed fish and plant protein sources. J. Nutr. Sci. 4 (2015).
Geurden, I. et al. The positive impact of the early-feeding of a plant-based diet on its future acceptance and utilisation in rainbow trout. PLoS One. 8(12), e83162 (2013).
Kaushik, S. J., Coves, D., Dutto, G. & Blanc, D. Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 230(1–4), 391–404 (2004).
Torstensen, B. E. et al. Novel production of Atlantic salmon (Salmo salar) protein based on combined replacement of fish meal and fish oil with plant meal and vegetable oil blends. Aquaculture 285(1–4), 193–200 (2008).
Wang, Y. R., Wang, L., Zhang, C. X. & Song, K. Effects of substituting fishmeal with soybean meal on growth performance and intestinal morphology in orange-spotted grouper (Epinephelus coioides). Aquacult. Rep. 5, 52–57 (2017).
Snyder, G. S. et al. Effects of carnosine supplementation to an all-plant protein diet for rainbow trout (Oncorhynchus mykiss). Aquaculture 338, 72–81 (2012).
Silva-Carrillo, Y., Hernández, C., Hardy, R. W., González-Rodríguez, B. & Castillo-Vargasmachuca, S. The effect of substituting fish meal with soybean meal on growth, feed efficiency, body composition and blood chemistry in juvenile spotted rose snapper Lutjanus guttatus (Steindachner, 1869). Aquaculture 364, 180–185 (2012).
Wang, Y., Kong, L. J., Li, C. & Bureau, D. P. Effect of replacing fish meal with soybean meal on growth, feed utilization and carcass composition of cuneate drum (Nibea miichthioides). Aquaculture 261(4), 1307–1313 (2006).
Lim, S. R. et al. Effects of dehulled soybean meal as a fish meal replacer in diets for fingerling and growing Korean rockfish Sebastes schlegeli. Aquaculture 231(1–4), 457–468 (2004).
Kumar, V. et al. Comparative evaluation of processed soybean meal (EnzoMealTM) vs. regular soybean meal as a fishmeal replacement in diets of rainbow trout (Oncorhynchus mykiss): Effects on growth performance and growth-related genes. Aquaculture. 516, 734652 (2020).
Miao, S. et al. Effect of dietary soybean meal associated with feeding time on the growth performance and intestinal microbiota composition of northern snakehead. Aquac. Res. 50(10), 2751–2759 (2019).
Kwasek, K. et al. Nutritional programming improves dietary plant protein utilization in zebrafish Danio rerio. PloS One. 15(3), e0225917 (2020).
Kemski, M., Wick, M. & Dabrowski, K. Nutritional programming effects on growth and reproduction of broodstock and embryonic development of progeny in yellow perch (Perca flavescens) fed soybean meal-based diets. Aquaculture 497, 452–461 (2018).
Izquierdo, M. S. et al. Nutritional programming through broodstock diets to improve utilization of very low fishmeal and fish oil diets in gilthead sea bream. Aquaculture 449, 18–26 (2015).
Pittman, K. et al. Fantastically plastic: Fish larvae equipped for a new world. Rev. Aquac. 5, S224–S267 (2013).
Sales, J. First feeding of freshwater fish larvae with live feed versus compound diets: A meta-analysis. Aquacult. Int. 19(6), 1217–1228 (2011).
Abi-Ayad, A. & Kestemont, P. Comparison of the nutritional status of goldfish (Carassius auratus) larvae fed with live, mixed or dry diet. Aquaculture 128(1–2), 163–176 (1994).
Adekunle, A. I. & Joyce, A. O. Effect of partial and total replacement of live feed (artemia) with formulated diets in early stage growth of hybrid catfish (Heterobranchus bidorsalis x Heterobranchus longifilis) fry. Int. J. Fish. Aquat. Stud. 1, 143–146 (2014).
Curnow, J., King, J., Bosmans, J. & Kolkovski, S. The effect of reduced Artemia and rotifer use facilitated by a new microdiet in the rearing of barramundi Lates calcarifer (BLOCH) larvae. Aquaculture 257(1–4), 204–213 (2006).
Kwasek, K., Zhang, Y. & Dabrowski, K. Utilization of dipeptide/protein based diets in larval and juvenile Koi carp – Post-prandial free amino acid levels. J. Anim. Physiol. Anim. Nutr. 94, 35–43 (2010).
Arndt, R. E. & Wagner, E. J. Enriched Artemia and probiotic diets improve survival of Colorado River cutthroat trout larvae and fry. N. Am. J. Aquac. 69(2), 190–196 (2007).
Moren, M., Opstad, I., Van der Meeren, T. & Hamre, K. Iodine enrichment of Artemia and enhanced levels of iodine in Atlantic halibut larvae (Hippoglossus hippoglossus L.) fed the enriched Artemia. Aquacult. Nutr. 12(2), 97–102 (2006).
Furuita, H., Takeuchi, T., Toyota, M. & Watanabe, T. EPA and DHA requirements in early juvenile red sea bream using HUFA enriched Artemia nauplii. Fish. Sci. 62(2), 246–251 (1996).
Van Stappen, G. 4.1. Introduction, Biology and Ecology of Artemia (1996).
Molinari, G. S. et al. Can intestinal absorption of dietary protein be improved through early exposure to plant-based diet? Plos One 15(6), e0228758 (2020).
Dabrowski, K. & Miller, M. Contested paradigm in raising zebrafish (Danio rerio). Zebrafish 15(3), 295–309 (2018).
Folch, J., Lees, M. & Stanley, G. S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226(1), 497–509 (1957).
Byadgi, O., Chen, C. W., Wang, P. C., Tsai, M. A. & De Chen, S. C. novo transcriptome analysis of differential functional gene expression in largemouth bass (Micropterus salmoides) after challenge with Nocardia seriolae. Int. J. Mol. Sci. 17(8), 1315 (2016).
Osaka, Y. Simultaneous Analysis of Amino Acids Using Automatic Pretreatment Function of ProminenceTM-i Integrated LC System. https://www.shimadzu.com/an/sites/shimadzu.com.an/files/pim/pim_document_file/applications/application_note/10845/jpl219002.pdf (2019).
Sampaio-Oliveira, A. M. B. M. & Cyrino, J. E. P. Digestibility of plant protein-based diets by largemouth bass Micropterus salmoides. Aquac. Nutr. 14(4), 318–323 (2008).
Lillycrop, K. A. & Burdge, G. C. Epigenetic mechanisms linking early nutrition to long term health. Best Pract. Res. Clin. Endocrinol. Metab. 26(5), 667–676 (2012).
Balasubramanian, M. N. et al. Molecular pathways associated with the nutritional programming of plant-based diet acceptance in rainbow trout following an early feeding exposure. BMC Genomics 17(1), 449 (2016).
Perera, E. & Yúfera, M. Soybean meal and soy protein concentrate in early diet elicit different nutritional programming effects on juvenile zebrafish. Zebrafish 13(1), 61–69 (2016).
Hedrera, M.I. et al. Soybean meal induces intestinal inflammation in zebrafish larvae. PLoS ONE 8(7), e69983 (2013).
Urán, P. A. et al. Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 25(6), 751–760 (2008).
Wang, J. et al. The effect of partial replacement of fish meal by soy protein concentrate on growth performance, immune responses, gut morphology and intestinal inflammation for juvenile hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂). Fish Shellfish Immunol. 98, 619–631 (2020).
Stone, D. A., Bellgrove, E. J., Forder, R. E., Howarth, G. S. & Bansemer, M. S. Inducing subacute enteritis in yellowtail kingfish Seriola lalandi: The effect of dietary inclusion of soybean meal and grape seed extract on hindgut morphology and inflammation. N. Am. J. Aquacult. 80(1), 59–68 (2018).
Miao, S. et al. Dietary soybean meal affects intestinal homoeostasis by altering the microbiota, morphology and inflammatory cytokine gene expression in northern snakehead. Sci. Rep. 8(1), 1 (2018).
Zhang, C. et al. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquaculture 483, 173–182 (2018).
Sohrabnezhad, M., Sudagar, M. & Mazandarani, M. Effect of dietary soybean meal and multienzyme on intestine histology of beluga sturgeon (Huso huso). Int. Aquat. Res. 9(4), 271–280 (2017).
Larsen, B. K., Dalsgaard, J. & Pedersen, P. B. Effects of plant proteins on postprandial, free plasma amino acid concentrations in rainbow trout (Oncorhynchus mykiss). Aquaculture 326, 90–98 (2012).
Gómez-Requeni, P. et al. Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata). Aquaculture 232(1–4), 493–510 (2004).
Dabrowski, K., Poczyczynski, P., Köck, G. & Berger, B. Effect of partially or totally replacing fish meal protein by soybean meal protein on growth, food utilization and proteolytic enzyme activities in rainbow trout (Salmo gairdneri). New in vivo test for exocrine pancreatic secretion. Aquaculture 177(1), 29–49 (1989).
Conceição, L. E., Ozório, R. O., Suurd, E. A. & Verreth, J. A. Amino acid profiles and amino acid utilization in larval African catfish (Clarias gariepinus): Effects of ontogeny and temperature. Fish Physiol. Biochem. 19(1), 43–58 (1998).
Shimeno, S., Takeda, M., Takii, K. & Ono, T. Post-feeding changes of digestion and plasma constituent in young yellowtail fed with raw fish and formulated diets. Nippon Suisan Gakkaishi 59(3), 507–513 (1993).
Mente, E., Deguara, S., Santos, M. B., & Houlihan, D. White muscle free amino acid concentrations following feeding a maize gluten dietary protein in Atlantic salmon (Salmo salar L.). Aquaculture 225(1–4), 133–147 (2003).
Brezas, A. & Hardy, R. W. Improved performance of a rainbow trout selected strain is associated with protein digestion rates and synchronization of amino acid absorption. Sci. Rep. 10(1), 1–12 (2020).
Li, P. & Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50(1), 29–38 (2018).
Kang, P. et al. Effects of L-proline on the growth performance, and blood parameters in weaned lipopolysaccharide (LPS)-challenged pigs. Asian Australas. J. Anim. Sci. 27(8), 1150 (2014).
Acknowledgements
We thank Saffron Scientific Histology Services (Carbondale, IL) for providing the histological services for this study.
Author information
Authors and Affiliations
Contributions
K.K. and M.W. contributed to the funding acquisition and experimental conception and design. G.S.M. and M.W. contributed to the acquisition and analysis of data. All authors contributed to the preparation of tables and figures. M.W. and K.K. contributed to the methodology utilized in this study. G.S.M. wrote the original manuscript. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Molinari, G.S., Wojno, M. & Kwasek, K. The use of live food as a vehicle of soybean meal for nutritional programming of largemouth bass Micropterus salmoides. Sci Rep 11, 10899 (2021). https://doi.org/10.1038/s41598-021-89803-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-89803-2
This article is cited by
-
Impact of environmental endocrine disruptors on adolescent sexual development: a comprehensive review with a focus on specific chemicals
Discover Applied Sciences (2025)
-
Modulation of gut microbiota composition and predicted metabolic capacity after nutritional programming with a plant-rich diet in Atlantic salmon (Salmo salar): insights across developmental stages
Animal Microbiome (2024)