Abstract
Diffusion-weighted magnetic resonance imaging (DW-MRI) performed before target temperature management, within 6 h of return of spontaneous circulation (ROSC), is defined as ultra-early DW-MRI. In previous studies, high-signal intensity (HSI) on ultra-early DW-MRI can predict poor neurological outcomes (Cerebral Performance Category 3–5 at 6-months post-ROSC). We aimed to assess the optimal-timing for ultra-early DW-MRI to avoid false-negative outcomes post out-of-hospital cardiac arrest, considering cardiopulmonary resuscitation (CPR) factors. The primary outcomes were HSI in the cerebral cortex or deep gray matter on ultra-early DW-MRI. The impact of CPR factors and ROSC to DW-MRI scan-interval on HSI-presence was assessed. Of 206 included patients, 108 exhibited HSI-presence, exclusively associated with poor neurological outcomes. In multivariate regression analysis, ROSC to DW-MRI scan-interval (adjusted odds ratio [aOR], 1.509; 95% confidence interval (CI): 1.113–2.046; P = 0.008), low-flow time (aOR, 1.176; 95%CI: 1.121–1.233; P < 0.001), and non-shockable rhythm (aOR, 9.974; 95%CI: 3.363–29.578; P < 0.001) were independently associated with HSI-presence. ROSC to DW-MRI scan-interval cutoff of ≥ 2.2 h was particularly significant in low-flow time ≤ 21 min or shockable rhythm group. In conclusion, short low-flow time and shockable rhythm require a longer ROSC to DW-MRI scan-interval. Prolonged low-flow time and non-shockable rhythm reduce the need to consider scan-interval.
Similar content being viewed by others
Introduction
Advancements in cardiopulmonary resuscitation (CPR) and critical care medicine have increased the survival rate of patients with out-of-hospital cardiac arrest (OHCA)1,2,3,4. However, several OHCA patients develop hypoxic–ischemic brain injury (HIBI), resulting in comatose conditions for extended periods5,6,7,8. Therefore, early determination of the degree of HIBI is crucial for providing appropriate post-resuscitation therapy and counseling to family members6,7,8,9. International guidelines advocate a 72-h delay in neurological prognostication after the return of spontaneous circulation (ROSC)10. An early and accurate prediction of neurological outcomes is important to allocate medical resources appropriately and avoid premature withdrawal of life-sustaining treatment (WLST) for OHCA patients who have the potential for neurological recovery11,12,13.
In a previous study, diffusion-weighted magnetic resonance imaging (DW-MRI), performed before target temperature management (TTM), within 6 h of ROSC, was defined as ultra-early DW-MRI14,15. A high-signal intensity (HSI) (“restricted diffusion”) in the cerebral cortex or deep gray matter on ultra-early DW-MRI indicates irreversible progression to severe HIBI, regardless of its location and extent14,15,16,17. HIBI may occur at the time of the insult; however, damage may also continue after ROSC and oxygenation are re-established18. However, HSIs on ultra-early DW-MRI is not consistently observed in patients with poor neurological outcomes due to its time-dependent nature14,15,16,17. Furthermore, HSIs on ultra-early DW-MRI is influenced by factors including the severity, duration, and progression of oxygen deprivation, as well as the absence of blood supply18,19,20,21,22,23,24,25.
We hypothesized that certain clinical factors associated with HIBI might influence HSI-presence on ultra-early DW-MRI, thereby affecting the determination of the optimal timing for scanning. Consequently, this study aimed to determine the optimal timing for ultra-early DW-MRI by analyzing the relationship between factors influencing HSI-presence and the ROSC to DW-MRI scan-interval.
Methods
Standard protocol approval, registration, and patient consent
This study was approved by the institutional review boards of each hospital (CNUSH 2023-03-019-001, CNUH 2023-04-003, and CBNUH 2023-03-038) and was conducted according to the guidelines of the Declaration of Helsinki. The extracted data included clinical data only; it did not include any personally identifiable information. Therefore, the need for obtaining informed patient consent was waived.
Study design and setting
This multicenter retrospective cohort study included data from adult (aged ≥ 18 years) comatose OHCA patients treated with TTM at university-affiliated hospitals (Chungnam National University Sejong Hospital [CNUSH], Chungnam National University Hospital [CNUH], and Chungbuk National University Hospital [CBNUH]) between March 2013 and July 2022.
Participants and clinical outcomes
The inclusion criterion was adult (age ≥ 18 years) OHCA patients who underwent DW-MRI prior to TTM. Ultra-early DW-MRI was performed before initiating TTM in OHCA patients to identify strokes and other brain lesions (e.g., brain tumors, encephalitis, and abscesses) for accurate diagnosis.
Neurologically related cardiac arrests were excluded (e.g., status epilepticus, stroke, subarachnoid hemorrhage, and encephalitis). The exclusion criteria were patients: (1) Who had brain sequelae due to prior injury; (2) whose cause of HSI on DW-MRI scans was not due to HIBI (e.g., cerebral infarction); (3) who experienced a traumatic cardiac arrest(CA); (4) whose MRI scanning time exceeded 6 h after ROSC; (3) who was not performed DW-MRI scan (received extracorporeal membrane oxygenation, cerebral hemorrhage, refusal to MRI, hemodynamic instability).
The primary outcome was HSI-presence on ultra-early DW-MRI, while the secondary outcome was poor neurological outcomes. Six months after ROSC, an emergency physician or neurologist evaluated the neurological outcomes using the Cerebral Performance Category (CPC) via face-to-face or telephonic interviews. The CPC classifies patients into five categories: CPC 1 (good performance), CPC 2 (moderate disability), CPC 3 (severe disability), CPC 4 (vegetative state), and CPC 5 (brain death or death)26. CPC 3–5 were characterized as poor neurological outcomes. In all patients who received TTM, the evaluation of CPC was conducted as a standard protocol after 6 months.
Post-cardiac arrest care
All patients included in the study received a post-cardiac arrest care bundle, including TTM. TTM was performed using cooling devices (Arctic Sun 5000, BD, Franklin Lakes, NJ, USA) with a target temperature of 33–36 °C maintained for 24 h. The attending physician determined the target temperature (33 °C vs. 36 °C) based on the patient’s hemodynamic status or OHCA characteristics. Antiepileptic medications were administered if evidence indicated abnormal electrical brain activity or if seizures were clinically diagnosed. All patients received standard intensive care based on institutional intensive care unit protocols. In South Korea, WLST was not permitted prior to February 2018; however, 46.1% of patients died due to death by neurologic criteria or cardiopulmonary death despite medical treatment. In this study, WLST did not occur during TTM, although some patients were pronounced dead according to the circulatory or neurological criteria despite receiving maximal support. Brain death is not a legally equivalent to death in South Korea. Under South Korean medical law, even in cases of brain death (CPC 5), the patient is considered alive owing to the presence of a functioning heart. Since February 2018, WLST must be performed according to legal procedures.
Neuroimaging
DW-MRI was performed using 3-T (Ingenia Elition 3.0-T X, Philips Healthcare, Amsterdam, The Netherlands; Achieva 3.0-T, Philips Healthcare, Amsterdam, The Netherlands) and 1.5-T (Achieva 1.5T, Philips Healthcare) scanners. For 3-T DW-MRI, the scan took approximately 8–10 min, while for 1.5-T scans, it took approximately 10–12 min. During the DW-MRI imaging and transport, a ventilator (HAMILTON-MR1, Hamilton Medical AG, Switzerland, Via Crush 8, 7402 Bonaduz) and monitoring equipment available in the MRI room (MR Patient Care Portal 5000, Philips Healthcare, Amsterdam, The Netherlands) were used. The emergency MRI room is located in close proximity to the emergency department (See Supplementary Fig. S1 and Supplementary Video S1 online). HSI limited to the cerebral cortex or deep gray matter was defined as HSI on DW-MRI with corresponding hypoattenuation on the apparent diffusion coefficient map. HSI-presence on DW-MRI was confirmed if restricted diffusion was observed in the cerebral cortex or deep gray matter, irrespective of whether it extended in a gyriform pattern or was extensive (See Fig. 1). An HSI-presence pattern of multi-lobar, diffuse, and cortical involvement was defined as “diffuse and extensive anoxic injury“10. Patients with non-gyriform restricted diffusion as a single lesion or multiple HSIs limited to a specific vascular territory on DW-MRI were excluded, because they were not considered to have HIBI. The assessment was performed by neuroradiologists (I.H.L. at CNUH, K.S.L. at CBNUH, and the Korea Tele-Radiology Reading Center, Seoul, Republic of Korea, at CNUSH) who were completely blinded to the clinical information.
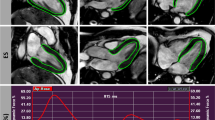
Various findings of ultra-early DW-MRI scan and ADC in OHCA patients. (a) Regional involvement. Gyriform HSI is observed in only a portion of the occipital lobe (white arrow). The patient presented with a witnessed arrest with bystander CPR. The first monitored rhythm was a non-shockable rhythm and serum lactic acid level was 11.0 mmol/L. The ROSC to DW-MRI scan-interval remained at 3.1 h. The low-flow time was 22 min. (b) Multi-regional involvement. HSIs are observed in both the occipital lobes and basal ganglia. The patient presented with an unwitnessed arrest with bystander CPR. The first monitored rhythm was a non-shockable rhythm, and the serum lactic acid level was 3.8 mmol/L. The ROSC to DW-MRI scan-interval was 2.7 h. The low-flow time was 38 min. (c) Multi-focal pattern. HSI findings, resembling emboli, are observed in the occipital and temporal lobes except white matter. The patient presented with a witnessed arrest with bystander CPR. The first monitored rhythm was shockable, and the serum lactic acid level was 13.3 mmol/L. The ROSC to DW-MRI scan-interval was 1.7 h. The low-flow time was 47 min. (d) Global involvement. HSI is observed in the occipital and temporal lobes, indicating severe HIBI. The patient presented with an unwitnessed arrest with no-bystander CPR. The first monitored rhythm was non-shockable rhythm and the serum lactic acid was 8.7 mmol/L. The ROSC to DW-MRI scan-interval of 2.9 h. The low-flow time was 7 min. OHCA, out-of-hospital cardiac arrest; DW-MRI, diffusion-weighted magnetic resonance imaging; ADC, apparent diffusion coefficient; ROSC, return of spontaneous circulation; HSI, high-signal intensity; CPR, cardiopulmonary resuscitation.
Data collection
The following data were extracted from each institution: age, sex, and Charlson Comorbidity Index (CCI) as basic demographic characteristics; witness collapse, bystander CPR, first monitored rhythm (shockable rhythm vs. non-shockable rhythm), cardiac etiology, and low-flow time as CPR factors; and serum lactic acid levels post-ROSC. Low-flow time was defined as the time from CPR to ROSC. The ROSC to DW-MRI scan-interval was defined as the time from ROSC to the start of MRI scanning. Serum lactic acid level was measured in arterial blood sampled immediately after ROSC.
Statistical analysis
Categorical variables are described as frequencies with percentiles, and continuous variables as median values with interquartile ranges, since all continuous variables had a non-normal distribution. Categorical variables were compared between the groups using χ2 (with continuity correction in 2 × 2 tables) or Fisher’s exact tests, as appropriate. The Mann–Whitney U test was used to compare continuous variables between groups with good and poor neurological outcomes and with HSI-absence and -presence groups.
All variables with P < 0.1 in the univariate analyses were included in the multivariable logistic regression model. The backward selection method was used to develop the final model. Logistic regression analysis results are reported as odds ratios (ORs) with 95% confidence intervals (CIs).
Optimal cut-off values were determined using the Youden index (sensitivity + specificity − 1) for the area under the receiver operating characteristic curve (AUROC). The diagnostic accuracy of DW-MRI, according to factors influencing HIS-presence, is expressed in terms of sensitivity, specificity, positive predictive value, and negative predictive value along with their respective 95% confidence intervals (95% CI). The data were analyzed using IBM SPSS Statistics for Windows (version 27.0; IBM Corp., Armonk, NY, USA). The AUROC was calculated using MedCalc version 15.2.2 (MedCalc Software, Mariakerke, Belgium). P < 0.05 was considered statistically significant.
Results
Baseline characteristics of the study cohort
Of the 261 OHCA patients undergoing TTM, 55 were excluded, leaving 206 patients for analysis. Among these, 98 (47.6%) were assigned to the HSI-absence group and 108 (52.4%) to the HSI-presence group (See Fig. 2). In the HSI-absence group, 74 (78.5%) patients experienced good neurological outcomes, whereas 24 (24.5%) had poor neurological outcomes. Conversely, in the HSI-presence group, the 108 patients (100%) all exhibited poor neurological outcomes (See Table 1).
The demographic and OHCA characteristics stratified according to neurological outcomes and HSI-presence/absence are presented in Table 1. The median time (h) from ROSC to DW-MRI scan-interval was 2.7 h (range: 1.8–3.8 h). The good neurological outcome and HSI-absence group had a higher proportion of patients with witnessed events, bystander CPR, shockable rhythms, cardiac etiology, shorter low-flow time, and lower serum lactic acid level. No significant differences in age, sex, CCI, and ROSC to DW-MRI scan-interval were observed between the good/poor neurological outcome and HSI-absence/-presence groups. Eight patients received WLST at a median (interquartile range) of 4 (4–7) days (See Supplementary Table S1). WLST was performed according to Korean legal WLST procedures.
Factors influencing HSI presence in ultra-early DW-MRI
In the univariate analyses, variables including unwitnessed arrest (OR: 3.095; 95%CI: 1.722–5.563; P < 0.001), no-bystander CPR (OR: 2.614; 95%CI: 1.374–4.975; P = 0.003), non-cardiac etiology (OR: 5.940; 95%CI: 2.987–11.812; P < 0.001), non-shockable rhythm (OR: 7.077; 95%CI: 3.447–14.530; P < 0.001), serum lactic acid level (OR: 1.134; 95%CI: 1.065–1.208; P < 0.001), low-flow time (OR: 1.166; 95%CI: 1.119–1.215; P < 0.001), and ROSC to DW-MRI scan-interval (OR: 1.189; 95%CI: 0.984–1.438; P = 0.07) were selected as covariates for the multivariable logistic regression model (See Table 2). The final adjusted multivariate analysis model indicated that low-flow time (adjusted odds ratio [aOR]: 1.176; 95%CI: 1.121–1.233; P < 0.001), ROSC to DW-MRI scan-intervals (aOR: 1.509; 95%CI: 1.113–2.046; P = 0.008), and non-shockable rhythm (aOR: 9.974; 95%CI: 3.363–29.578; P < 0.001) were significantly associated with HSI-presence (See Table 2).
Dynamic changes in MRI in a subset with repeat MRI between 72 and 96 h
Overall, 168 patients underwent 72–96 h DW-MRI. Among those with HSI-absence on ultra-early DW-MRI, 19 (19.4%) showed progression to diffuse and extensive anoxic injury on 72–96 h DW-MRI. In cases of HSI presence on ultra-early DW-MRI, 80 (74.1%) already exhibited diffuse and extensive anoxic injury. In 72–96 h DW-MRI, none of the patients with diffuse and extensive anoxic injury on ultra-early DW-MRI showed improvement. All patients who exhibited HSI-presence in ultra-early DW-MRI demonstrated changes to diffuse and extensive anoxic injury in the 72–96 h DW-MRI. Although 28 patients initially had HSI presence but no diffuse and extensive anoxic injury, all eventually progressed to diffuse and extensive anoxic injury. In the good neurological outcome group, 71 individuals underwent 72–96 h DW-MRI, and all showed an absence of diffuse and extensive anoxic injury.
Cut-off values of factors influencing HSI-presence in ultra-early DW-MRI
Optimal cut-off values for the ROSC to DW-MRI scan-interval, low-flow time, and serum lactic acid level were found to be ≥ 2.2 h (sensitivity: 72% [95%CI: 63–80]; specificity: 50% [95%CI: 52–69]), more than 21 min (sensitivity: 86% [95%CI: 78–92]; specificity: 76% [95%CI: 66–84]), and > 10 mmol/L (sensitivity: 64% [95%CI: 54–73]; specificity: 73% [95%CI: 63–81]), respectively (See Table 3; Fig. 4).
In the poor neurological outcome group, when low-flow time exceeded 38 min or when the ROSC to DW-MRI scan-intervals surpassed 4 h, all patients exhibited HSI-presence (See Fig. 3). Among patients with HSI on DW-MRI, one patient had the shortest ROSC-scan interval time of 0.4 h (24 min), with a low-flow time of 38 min, non-shockable rhythm, and serum lactate level of 16 mmol/L (Fig. 4).
Distribution of HSI-absence and HSI-presence based on first monitored rhythm, low-flow time, and ROSC to DW-MRI scan-interval within the poor neurological outcome group. Green cells indicate HSI-absence and red cells indicate HSI-presence. Small cells denote shockable rhythm, whereas large cells represent non-shockable rhythm. HSI-presence escalates with extended low-flow time, an increased ROSC to DW-MRI scan-interval, and a non-shockable rhythm. ROSC, return of spontaneous circulation; DW-MRI, diffusion-weighted magnetic resonance imaging; HSI, high-signal intensity.
Statistical analysis of the area under the receiver operating characteristic curve for HSI-presence in ultra-early DW-MRI. (a) Low-flow time. (b) ROSC to DW-MRI scan-interval. (c) First monitored rhythm. ROSC, return of spontaneous circulation; DW-MRI, diffusion-weighted magnetic resonance imaging; HSI, high-signal intensity; AUROC, area under the receiver operating characteristic curve.
Predictive performance of ultra-early DW-MRI for poor neurological outcomes based on ROSC to DW-MRI scan-interval
Table 4 shows the predictive performance of ROSC to DW-MRI scan-interval (≥ 2.2 h and < 2.2 h), low-flow time (> 21 min and ≤ 21 min), and first monitored rhythm for poor neurological outcomes in each group. The optimal cut-off value was set using a specificity of 100% (i.e., false-positive rate [FPR] of 0).
For all patients, DW-MRI demonstrated a sensitivity of 82% (95%CI: 74–88) for predicting poor neurological outcomes, with an FPR of 0%. Sensitivities for DW-MRI scans-intervals of ≥ 2.2 h and < 2.2 h were 70 (95%CI: 54–83) and 88 (95%CI: 79–94), respectively. In all groups, DW-MRI scan intervals of ≥ 2.2 h showed improved sensitivities at a 0% FPR as compared to those with intervals < 2.2 h. Among these groups, the ROSC to DW-MRI scan-interval ≥ 2.2 h was more significant than the interval < 2.2 h in the low-flow time ≤ 21 min and shockable rhythm group.
Discussion
This retrospective multicenter study showed that, while all patients in the HSI-presence group had poor neurological outcomes, 24.5% of those in the HSI-absence group also had false-negative findings with poor neurological outcomes. In our cohort, prolonged low-flow time, longer ROSC to DW-MRI scan-interval, and non-shockable rhythm were independently associated with HSI-presence. Optimal cut-off values for predicting HSI-presence accurately were a ROSC to DW-MRI scan-interval ≥ 2.2 h and a low-flow time > 21 min. In particular, when predicting poor neurological outcomes using clinical factors related to OHCA based on the ROSC to DW-MRI scan-interval, the predictive performance was higher when the interval was ≥ 2.2 h than when it was < 2.2 h, in all groups.
Cardiac arrest occurs in 2.5–4% of patients with acute ischemic stroke. Ultra-early DW-MRI in OHCA patients helps identify strokes and other brain lesions, such as brain tumors and infections, ensuring accurate diagnosis and treatment27. Brain tumors, especially those in the brainstem, can compress vital structures, disrupting cardiac and respiratory regulation. Infections such as encephalitis or meningitis increase intracranial pressure and affect brain regions controlling heart rate28. Findings from early DW-MRI have significantly guided specific interventions without affecting the implementation of TTM or other post-cardiac arrest care protocols. This methodology enables tailored care while adhering to established post-cardiac arrest treatment protocols.
DW-MRI serves as the initial imaging sequence to depict changes in HIBI, typically revealing HSIs in areas such as the cerebral cortex (particularly the perirolandic and visual cortices), deep gray matter (basal ganglia and thalami), and hippocampus29,30,31. HSIs on ultra-early DW-MRI scans are likely to indicate an irreversible progression to severe HIBI, regardless of their location or extent14,15,16,17,18. This interpretation differs from that of a post-TTM DW-MRI, where the neurological outcome varies depending on the location and extent of HSI10,14,15,16,17,18,31,32,33. The results of our study revealed that all patients in the HSI-presence group exhibited changes to diffuse and extensive anoxic injury in the 72–96 h DW-MRI. These results are consistent with our previous findings. Furthermore, unlike previous single-center studies, this study was a multicenter study. Notably, 74.1% of patients in ultra-early DW-MRI HSI-presence group had already shown diffuse and extensive anoxic injury. While our study indicates that those with HSI on ultra-early DW-MRI had diffuse findings of anoxic injury on subsequent MRI and definitively poor outcome, prior studies have shown that patients who received WLST owing to perceived poor neurologic prognosis had no pathologic evidence of HIBI34. Our study suggests that ultra-early MRI may be a reliable prognostic marker; however, further research is needed to understand the pathologic correlates of these findings. Our previous studies, and the present one, demonstrated that the presence of HSI in ultra-early DW-MRI predicts diffuse and extensive anoxic injury on DW-MRI after 72 h of ROSC. This progression to diffuse and extensive anoxic injury is attributable to the time-dependent development of brain edema following post-CA brain injury14,15,16. Therefore, the necessity of indirectly confirming HIBI through ultra-early DW-MRI can be further emphasized to prevent inappropriate WLST decisions before TTM.
The optimal timing for ultra-early DW-MRI in OHCA patients remains unknown. An ischemic attack-to-DW-MRI scan-interval ≥ 2 h reduces false-negatives in transient ischemic attacks and cerebral infarction33,35,36. In this study, the cut-off value for the ROSC to DW-MRI scan-interval was 2.2 h. This finding was similar to that related to ischemic attacks. The DW-MRI changes post-cardiac arrest are expected to show time-dependent patterns.
Ultra-early DW-MRI signal alterations can differ based on the severity of hypoxia during cardiac arrest and the scanning time14,15,37. Unlike cerebral infarction, ultra-early DW-MRI is performed after the blood flow is restored throughout the brain following cardiac arrest-induced HIBI. Cytotoxic edema, hemodynamic changes, and altered substance diffusion speed, which progress throughout the entire brain, are critical factors in the early stages of HIBI5,18. Cytotoxic edema involves increased cell membrane permeability, leading to temporary reduction in diffusion and potentially smaller or absent lesions on DW-MRI scans33,35. Hemodynamic changes can disrupt the blood flow, further impacting substance diffusion around the injury site38,39. Moreover, slower substance diffusion rates in the whole brain may also contribute to the DW-MRI presentation of a smaller HSI35,36. However, the potential occurrence of false-negative findings on ultra-early DW-MRI due to these factors can be overcome by adjusting the scan timing38,39. In this study, these factors showed a clear pattern in which false-negative findings decreased as the severity of hypoxia increased (e.g., longer low-flow time and non-shockable rhythm). Additionally, these false negative findings were reduced as the ROSC to DW-MRI scan-interval increased. Therefore, these factors and DW-MRI scan timing might collectively play a significant role in false-negative findings on ultra-early DW-MRI.
In this study, the cut-off value for the low-flow time of HSI-presence was > 21 min, supporting the findings of previous studies reporting poor neurological outcomes for low-flow durations of ≥ 20 min or > 30 min19,20,21,32,40,41,42. Particularly, in the patients with low-flow time > 30 min in the poor neurological outcome group, all but one patient exhibited HSI-presence. However, favorable outcomes were observed in some cases with prolonged low-flow time of > 30 min. HSI was absent in all of them. Moreover, non-shockable rhythm indicated poor neurological outcomes24,32,40,41,42. However, favorable outcomes have also been observed in a significant number of non-shockable cases. Therefore, for OHCA patients with low-flow time > 21 min or a non-shockable rhythm, the significance of ultra-early DW-MRI becomes more evident. The presence or absence of HSI provides adequate data for predicting neurological outcomes. Thus, in cases with a low-flow time of > 21 min and a non-shockable rhythm, the integration of ultra-early DW-MRI into objective neurological outcome predictions seems to be warranted.
When the ROSC to DW-MRI scan-interval was ≥ 2.2 h, ultra-early DW-MRI exhibited excellent performance in predicting poor neurological outcomes, notably in patients with a low-flow time of > 21 min or a non-shockable rhythm. Moreover, a ROSC to DW-MRI scan-interval of < 2.2 h still provided adequate prediction of neurological prognosis. However, for patients with a low-flow time ≤ 21 min or a shockable rhythm, a minimum ROSC to DW-MRI scan-interval of 2.2 h was essential. If the ROSC to DW-MRI scan-interval was < 2.2 h, the neurological outcome predictions were less reliable. Thus, ultra-early DW-MRI conducted beyond 2.2 h were found to be more dependable, particularly in patients with a low-flow time of ≤ 21 min or a shockable rhythm.
These results indicated that increased hypoxic severity increases the likelihood of detecting HSI on ultra-early DW-MRI, which suggests poor neurological outcomes, particularly in prolonged low-flow time and non-shockable rhythm scenarios. Moreover, in less severe hypoxic situations, such as a short low-flow time or shockable rhythm, the timing of ultra-early DW-MRI becomes more pivotal. Ultra-early DW-MRI can potentially enhance the diagnostic precision for OHCA patients by optimizing the scan timing, particularly in relation to low-flow time and first monitored rhythm. Therefore, as low-flow time and first monitored rhythm are uncontrollable factors, refining ultra-early DW-MRI timing based on these factors is crucial for a more accurate prediction of neurological outcomes.
The strengths of our study include its multicenter design and the training provided at each site. Despite advancements in this field, ultra-early DW-MRI is less commonly used in clinical settings than is post-TTM DW-MRI, partly because of the lack of established guidelines for imaging timing, and particularly concerning factors such as low-flow time and first monitored rhythm. Therefore, once validated, our data may contribute to future evidence-based guidelines for employing ultra-early DW-MRI for prognostication before TTM.
This study also had some limitations. First, as this was a retrospective study with a limited sample size, a larger prospective study is needed to ensure generalizability of the results. Second, a self-fulfilling prophecy bias might have occurred because the attending physicians had access to the DW-MRI results. However, WLST was not permitted in South Korea before February 2018 and during TTM in this study. Additionally, early WLST was not performed on any patient and all included patients received standard intensive care based on institutional intensive care unit protocols. Third, the analysis of no-flow time data was not included. In unwitnessed cases, determining the precise no-flow time was not feasible42,43. Therefore, prolonged no-flow time could potentially impact the severity and affect HSI-presence42,43. Fourth, Utstein data on cardiac arrest are lacking. Especially we were unable to include qualitative aspects of basic life support and advanced cardiovascular life support in our analysis44,45,46,47. These aspects could influence the severity of HIBI, thereby affecting HSI-presence. Fifth, images obtained with 3.0-T DW-MRI demonstrated superior tissue contrast and conspicuity compared to 1.5-T48. Thus, HSIs may be more frequently observed with 3.0-T DW-MRI compared to 1.5-T DW-MRI. Fifth, this study may have been affected by selection bias. While our protocol dictates performing DW-MRI before TTM, 261 patients had undergone TTM during the study, and 55 (21.1%) were excluded, possibly causing selection bias, and limiting the generalizability of our findings. Finally, our model is not standard of care. Currently, no data supporting the use of MRI to guide care limitation or intervention selection exist. However, our unique setup, with access to rapid sequence MRI and a higher proportion of neurogenic arrest, has made this model feasible and effective.
In conclusion, our study indicated that a longer ROSC to DW-MRI scan-interval is necessary with a shorter low-flow time or shockable rhythm. However, with a longer low-flow time or non-shockable rhythm, the urgency for the ROSC to DW-MRI scan-interval diminishes. Therefore, we recommend an optimal timing for ultra-early DW-MRI of ≥ 2.2 h, and particularly in cases with a low-flow time of ≤ 21 min or shockable rhythm. These findings warrant further validation via a prospective multicenter study to confirm their reliability and generalizability.
Data availability
Anonymized data not published in this article can be made available upon reasonable request from any qualified investigator, subject to approval from the CNUSH, CNUH, and CBNUH Institutional Review Board. The data supporting the findings of this study can be requested from the corresponding author, Jin Hong Min, at laphir@cnu.ac.kr.
References
Benjamin, E. J. et al. Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 137, e67–e492 (2018).
Hypothermia after cardiac arrest study group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 346, 549–556 (2002).
Bernard, S. A. et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 346, 557–563 (2002).
Nielsen, N. et al. Targeted temperature management at 33 ºC versus 36 ºC after cardiac arrest. N. Engl. J. Med. 369, 2197–2206 (2013).
Sekhon, M. S., Ainslie, P. N. & Griesdale, D. E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A two-hit model. Crit. Care 21, 90 (2017).
Rossetti, A. O., Rabinstein, A. A. & Oddo, M. Neurological prognostication of outcome in patients in coma after cardiac arrest. Lancet Neurol. 15, 597–609 (2016).
Sandron, C., D’Arrigo, S. & Nolan, J. P. Prognostication after cardiac arrest. Crit. Care. 22, 150 (2018).
Geocadin, R. G. et al. Standards for studies of neurological prognostication in Comatose survivors of Cardiac arrest: A scientific statement from the American Heart Association. Circulation 140, e517–e542 (2019).
Dragancea, I. et al. Protocol-driven neurological prognostication and withdrawal of life-sustaining therapy after cardiac arrest and targeted temperature management. Resuscitation. 117, 50–57 (2017).
Nolan, J. P. et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 47, 369–421 (2021).
Elmer, J. et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 102, 127–135 (2016).
Grossestreuer, A. V. et al. Factors associated with post-arrest withdrawal of life-sustaining therapy. Resuscitation. 110, 114–119 (2017).
Lee, B. K., Min, J. H., Park, J. S., Kang, C. & Lee, B. K. Early identified risk factors and their predictive performance of brain death in out-of-hospital cardiac arrest survivors. Am. J. Emerg. Med. 56, 117–123 (2022).
Park, J. S. et al. Ultra-early neurologic outcome prediction of out-of-hospital cardiac arrest survivors using combined diffusion-weighted imaging findings and quantitative analysis of apparent diffusion coefficient. Resuscitation 148, 39–48 (2020).
Kang, C. S. et al. Association of ultra-early diffusion-weighted magnetic resonance imaging with neurological outcomes after out-of-hospital cardiac arrest. Crit. Care 27, 16 (2023).
Park, J. S. et al. Efficacy of diffusion-weighted magnetic resonance imaging performed before therapeutic hypothermia in predicting clinical outcome in comatose cardiopulmonary arrest survivors. Resuscitation 88, 132–137 (2015).
Jeon, C. H. et al. Comparison of brain computed tomography and diffusion-weighted magnetic resonance imaging to predict early neurologic outcome before target temperature management comatose cardiac arrest survivors. Resuscitation 118, 21–26 (2017).
Busl, K. M. & Greer, D. M. Hypoxic-ischemic brain injury: Pathophysiology, neuropathology and mechanisms. NeuroRehabilitation 26, 5–13 (2010).
Welbourn, C. & Efstathiou, N. How does the length of cardiopulmonary resuscitation affect brain damage in patients surviving cardiac arrest? A systematic review. Scand. J. Trauma. Resusc. Emerg. Med. 26, 77 (2018).
Coppler, P. J. et al. Duration of cardiopulmonary resuscitation and phenotype of post-cardiac arrest brain injury. Resuscitation 188, 109823 (2023).
Otani, T. et al. Low-flow time is associated with a favorable neurological outcome in out-of-hospital cardiac arrest patients resuscitated with extracorporeal cardiopulmonary resuscitation. J. Crit. Care 48, 15–20 (2018).
Tateishi, K. et al. Prehospital predicting factors using a decision tree model for patients with witnessed out-of-hospital cardiac arrest and an initial shockable rhythm. Sci. Rep. 13, 16180 (2023).
Lee, S. Y., Hwang, S. S., Park, J. H., Song, K. J. & Shin, S. D. Impact of awareness time interval on the effect of bystander cardiopulmonary resuscitation on out-of-hospital cardiac arrest: A nationwide study. Yonsei Med. J. 64, 327–335 (2023).
Han, K. S., Lee, S. W., Lee, E. J., Kwak, M. H. & Kim, S. J. Association between shockable rhythm conversion and outcomes in patients with out-of-hospital cardiac arrest and initial non-shockable rhythm, according to the cause of cardiac arrest. Resuscitation 142, 144–152 (2019).
Funada, A., Goto, Y., Okada, H., Maeda, T. & Takamura, M. Effects of witness status and time to cardiopulmonary resuscitation by emergency medical services on neurological outcomes in out-of-hospital cardiac arrest patients with non-shockable rhythm. Eur. Heart J. 40(Suppl_1), ehz7460075 (2019).
Rittenberger, J. C., Raina, K., Holm, M. B., Kim, Y. J. & Callaway, C. W. Association between cerebral performance category, modified Rankin scale, and discharge disposition after cardiac arrest. Resuscitation 82, 1036–1040 (2011).
Adams, R. J. et al. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: A scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association. Circulation 9, 1278–1290 (2003).
Benghanem, S. et al. Brainstem dysfunction in critically ill patients. Crit. Care 24, 5 (2020).
Pai, V., Sitoh, Y. Y. & Purohit, B. Gyriform restricted diffusion in adults: looking beyond thrombo-occlusions. Insights Imaging 11, 20 (2020).
Howard, R. S. et al. Hypoxic-ischaemic brain injury: Imaging and neurophysiology abnormalities related to outcome. QJM 105, 551–561 (2012).
Park, J. Y. et al. Association between the extent of diffusion restriction on brain diffusion-weighted imaging and neurological outcomes after an out-of-hospital cardiac arrest. Resuscitation 187, 109761 (2023).
Oren, N. C., Chang, E., Yang, C. W. & Lee, S. K. Brain diffusion imaging findings may predict clinical outcome after cardiac arrest. J. Neuroimaging 29, 540–547 (2019).
Els, T., Kassubek, J., Kubalek, R. & Klisch, J. Diffusion-weighted MRI during early global cerebral hypoxia: A predictor for clinical outcome?. Acta Neurol. Scand. 110, 361–367 (2004).
Endisch, C. et al. Hypoxic-ischemic encephalopathy evaluated by brain autopsy and neuroprognostication after cardiac arrest. JAMA Neurol. 77, 1430–1439 (2020).
Shono, K. et al. Optimal timing of diffusion-weighted imaging to avoid false-negative findings in patients with transient ischemic attack. Stroke 48, 1990–1992 (2017).
Ahlhelm, F., Schneider, G., Backens, M., Reith, W. & Hagen, T. Time course of the apparent diffusion coefficient after cerebral infarction. Eur. Radiol. 12, 2322–2329 (2002).
Soul, J. S., Robertson, R. L., Tzika, A. A., Plessis, A. J. & du Volpe, J. J. Time course of changes in diffusion-weighted magnetic resonance imaging in a case of neonatal encephalopathy with defined onset and duration of hypoxic-ischemic insult. Pediatrics 108, 1211–1214 (2001).
Jin, O. K. & Soo, J. L. Early false-negative diffusion-weighted image in acute ischemic stroke presenting sudden isolated vertigo. J. Neurosonol. Neuroimag 10, 169–171 (2018).
Schönfeld, M. H. et al. Improved detectability of acute and subacute brainstem infarctions by combining standard axial and thin-sliced sagittal DWI. PloS One 13, e0200092 (2018).
Park, S. et al. Optimal cardiopulmonary resuscitation duration for favorable neurological outcomes after out-of-hospital cardiac arrest. Scand. J. Trauma. Resusc. Emerg. Med. 30, 5 (2022).
Goto, Y., Funada, A., Maeda, T. & Goto, Y. Termination-of-resuscitation rule in the emergency department for patients with refractory out-of-hospital cardiac arrest: A nationwide, population-based observational study. Crit. Care 26, 137 (2022).
Okada, Y. et al. Association between low pH and unfavorable neurological outcome among out-of-hospital cardiac arrest patients treated by extracorporeal CPR: A prospective observational cohort study in Japan. J. Intensive Care 8, 34 (2020).
Mutter, E. L. & Abella, B. S. Duration of cardiac arrest resuscitation: Deciding when to call the code. Circulation 133, 1338–1340 (2016).
Matsuyama, T., Ohta, B., Kiyohara, K. & Kitamura, T. Cardiopulmonary resuscitation duration and favorable neurological outcome after out-of-hospital cardiac arrest: A nationwide multicenter observational study in Japan (the JAAM-OHCA registry). Crit. Care 26, 120 (2022).
Krammel, M. et al. The impact of a high-quality basic life support police-based first responder system on outcome after out-of-hospital cardiac arrest. PloS One 15, e0233966 (2020).
Honarmand, K., Mepham, C., Ainsworth, C. & Khalid, Z. Adherence to advanced cardiovascular life support (ACLS) guidelines during in-hospital cardiac arrest is associated with improved outcomes. Resuscitation 129, 76–81 (2018).
Simmons, K. M., McIsaac, S. M. & Ohle, R. Impact of community-based interventions on out-of-hospital cardiac arrest outcomes: A systematic review and meta-analysis. Sci. Rep. 13, 10231 (2023).
Wood, R., Bassett, K. 5th, Spry, C. & Tong, L. 1.5 tesla magnetic resonance imaging scanners compared with 3.0 tesla magnetic resonance imaging scanners: Systematic review of clinical effectiveness. CADTH Technol Overv. 2, e2201 (2012).
Funding
This work was supported by the research fund by the National Research Foundation of Korea grant funded by the government (MIST) (No. 2023R1A2C1006309).
Author information
Authors and Affiliations
Contributions
JSP, CSK, and JHM contributed to the study of conception, design analyzed the data, and wrote the paper. YHY, WJJ, HJA, and YNI contributed to data acquisition. YMK and SKO contributed to data analysis and interpretation. SYJ, IHL, HSJ, and BKL contributed to the statistical analysis. All authors made relevant intellectual contributions to the manuscript, and all authors approved the final version for journal submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the institutional review boards of each hospital (CNUSH 2023-03-019-001; approval date: April 27, 2023, CNUH 2023-04-003; approval date: April 28, 2023, CBNUH 2023-03-038; approval date: April 19, 2023). The extracted data included clinical data only, and no personally identifiable information. Therefore, the need for obtaining informed consent from the patients was waived.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 4
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, J.S., Kang, C., Min, J.H. et al. Optimal timing of ultra-early diffusion-weighted MRI in out-of-hospital cardiac arrest patients based on a retrospective multicenter cohort study. Sci Rep 14, 25284 (2024). https://doi.org/10.1038/s41598-024-76418-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76418-6