Abstract
Diabetic kidney disease (DKD) is a major disease characterized by early albuminuria and heightened risk of renal deterioration. Increased reactive oxygen species (ROS) production, especially in glomeruli, plays an important role in the progression of DKD. ROS also cause activation of Apoptosis signal-regulating kinase 1 (ASK-1), which is implicated in various organ injuries. However, the detailed mechanisms remain unclear. This study investigates ASK-1 activation in advanced DKD and its underlying mechanisms using GS442172, an ASK-1 inhibitor. In the DKD mouse model, activation of ASK-1 was observed. Although inhibition of ASK-1 activation improved hyperpermeability in glomerular endothelial cells. ASK-1 inhibition significantly reduced glomerular injury and albuminuria, while also attenuating tubular damage and interstitial fibrosis. RNA-seq analysis revealed an aging phenotype associated with ASK-1 activation in DKD. In vitro experiments demonstrated ASK-1 activation-induced cellular senescence in tubular cells via redox signaling. These results suggested that the critical role of ASK-1 activation in DKD pathogenesis, implicating glomerular injury, tubular damage, and cellular senescence. ASK-1 inhibitors are promising therapeutic strategies to mitigate the progression of DKD.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a global public health burden, with a high prevalence and high risk of progression to end-stage renal disease (ESRD), cardiovascular disease (CVD), and premature death. In particular, DKD is an important disease for which there is still no adequate treatment to control its progression. DKD is characterized by the presence of albuminuria from an early stage. The albuminuria has been shown to be a strong risk factor for progression to renal failure1.
In DKD, elevated reactive oxygen species (ROS) production in the glomerulus occurs early in disease. ROS production sources include increased NADPH oxidase activity2 and free-radical species derived from dysfunctional mitochondria3. Normal cellular function is maintained by appropriate ROS production4. However, excessive ROS production has been shown to cause progressive renal dysfunction5. Increased NADPH oxidase activity in glomerular endothelial cells promotes the onset of albuminuria6. Thus, ROS increases play an important role in the onset and progression of DKD.
Apoptosis signal-regulating kinase 1 (ASK-1) is a ubiquitously-expressed, apical MAPK that is activated by increased oxidative stress7. ASK-1 is normally bound and repressed by thiol-containing antioxidant proteins, including thioredoxins in the cytosol and mitochondria7. In settings of elevated oxidative stress, thioredoxin undergoes oxidation and dissociation from ASK-1, leading to autophosphorylation of ASK-1 at threonine 845 (ASK-T845) and resulting in ASK-1 activity7. ASK-1 activation has been reported to be involved in the progression of organ injury by promoting apoptosis, inflammation, and fibrosis8,9. ROS-mediated ASK-1 activation has been shown to be important in the transition to heart failure after myocardial infarction10. In glomeruli of DKD also, ASK-1 is known to be activated by ROS11. It has also been shown that ER stress in glomerular epithelial cells activates ASK-1, which is involved in the disruption of the coagulation wall, and it has been reported that ASK-1 has a certain role in glomerular pathogenesis11. These preclinical data indicate that ASK-1 activation is responsible for the development of renal damage in diabetic kidney disease. However, the detailed mechanism is unknown.
On the other hand, the importance of tubular and interstitial lesions as well as glomerular lesions in the development of renal damage has been reported. Excess albumin filtered from the glomerulus is reabsorbed in the tubules. Free fatty acid bound to albumin has been shown to induce mitochondrial stress due to excessive β-oxidation during tubular metabolism. This also results in an increase in mitochondria-derived ROS, leading to the secretion of inflammatory cytokines and increased inflammation12,13. This is an important pathway for renal injury. Thus, increased reactive oxygen species have been shown to be an important driver in tubular injury as well. However, the significance of ASK-1 activation in tubular injury is unknown.
Therefore, it is possible that the damaging consequences of oxidative stress in diabetic kidney disease progresses occurs as a result of ASK-1 activation. In this study, we evaluated the significance of ASK-1 activation in advanced diabetic kidney disease and provide insight into the mechanism of renal damage progression using GS-444217, an ASK-1 inhibitor, which has been shown previously to protect against kidney fibrosis and dysfunction in rodent models of kidney disease14,15.
Results
ASK-1-p38 pathway activation in DKD model mice
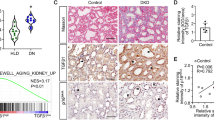
Physiological characteristics at sacrifice are shown in Table 1. Trends in body weight and blood glucose are also shown in the Supplementary Table. We first evaluated ASK-1 activation in a DKD model by western blotting of phosphorylation of p38, a downstream marker of ASK-1 activity. Compared to db/misty as control mice, eNOS-KO db/db mice showed increased phosphorylation of p38 (Fig. 1-A,B), which was abolished by the ASK-1 inhibitor GS-444217. There is a report that increased staining intensity was observed in p-p38 immunostaining in eNOS-KO/dbdb glomeruli15. In addition, immunohistochemistry revealed that ASK-1 was expressed in tubular cells (Supplementary Fig. 1). These results indicate that ASK-1 activation in the kidney occurs in diabetic kidney disease and is inhibited by GS-444217.
Expression levels of ASK-1-related genes increased in DKD model mice. These data were obtained from whole kidney tissue from 5 mice in each group. (A) Representative western blotting experiments and analysis data for phospho-p38/MAPK (Thr180/Tyr182), total-p38/MAPK and GAPDH. Original blots are presented in Supplementary Fig. 8-A,B. (B) phospho-p38/MAPK protein level expressed as fold change. Data are mean ± SEM. *P < 0.05 vs versus db/misty control. †P < 0.05 vs versus eNOS-KO/db/db control.
ASK-1 inhibition reduced glomerular injury in DKD model mice
The tissues of mice aged 10 weeks before ASK-1 inhibitor administration, the eNOS-KO db/db mice showed marked glomerular sclerosis compared to the db/misty mice (Supplementary Fig. 2A–C). PAS staining after 8 weeks of drug administration are also shown in Fig. 2-A,B and Supplementary Fig. 3. eNOS-KO db/db showed prominent glomerulosclerosis, which were significantly suppressed by ASK-1 inhibition. At 8 weeks on administration, there was a marked increase in albuminuria in the eNOS-KO db/db group, but a significant decrease in the ASK-1 inhibitor group (Fig. 2-C). For further detail morphological evaluation, we observed the capillary wall by Transmission Electron Microscope (TEM). eNOS-KO db/db mice exhibited a disappearance of endothelial cell fenestrae, indicating morphological damage to the endothelial cells (Fig. 2-D, Supplementary Fig. 4). Foot process effacement was observed in the glomerular epithelial cells. In the group treated with ASK-1 inhibitor, some foot process fusion remained, but endothelial cell swelling was markedly improved. These results indicate that ASK-1 is involved in glomerular capillary wall injury, especially endothelial injury, in DKD.
ASK-1 inhibition reduced renal function and glomerular damage. (A) PAS staining of glomeruli (bar = 20μm). (B) Percentage of Glomerulosclerosis (GS) score. data are mean ± SEM. *P < 0.05 versus db/misty control. †P < 0.05 versus eNOS-KO db/db control. (C) Measurement of Urinary albumin excretion per day (n = 6–13). Data are expressed as means ± SEM. *P < 0.05 vs versus db/misty control. (D) The cross-sectional transmission electron micrograph of glomerular capillary. Black arrow indicates endothelial fenestrae. Black arrowhead indicates the partial occlusion and disappearance of fenestrae. White arrowhead indicates swollen epithelial cells. Ep epithelial cells, Ed endothelial cells, GBM glomerular basement membrane.
Expression of glycocalyx and VE-cadherin on capillary wall via ASK-1 activation
Glycocalyx and VE-cadherin, which are important for permeability control, were evaluated. VE-cadherin in endothelial cells was decreased in eNOS-KO db/db and was preserved in the ASK-1 inhibitor treated group (Fig. 3-A,C). Endothelial glycocalyx, which is important in regulating glomerular capillary wall permeability, was evaluated by lectin staining. Normal glomeruli show smooth lectin staining along the capillary wall, whereas eNOS-KO db/db shows a discontinuity of the lectin staining (Fig. 3-B,D) and WGA staining (Supplementary Fig. 5). To further investigate the molecular mechanism, we performed in vitro experiments using glomerular endothelial cells. Phosphorylation of VE-cadherin was observed after stimulation with H2O2, but was inhibited by ASK-1 inhibitors (Fig. 3-E,F). These results indicate that increased ROS in glomerular endothelial cells promote VE-cadherin degradation via ASK-1 activation (Fig. 3-G).
Altered expression of glycans and VE-cadherin on capillary walls via ASK-1 activation. (A) immunofluorescence staining of VE-cadherin in kidney tissue. Staining (bar = 20μm). (B) Glomerular ESL was evaluated by tomato lectin staining (bar = 20μm). (C) Expression levels of VE-cadherin. (D) Expression levels of Tomato lectin. (E) Representative western blotting experiments and analysis data for phospho-VE-cadherin (Tyr658), total-VE-cadherin, and GAPDH. Original blots are presented in Supplementary Fig. 9-A–C. (F) phosphorylated-VE-cadherin protein level expressed as fold change. Data are expressed as means ± SEM. *P < 0.05 versus H2O2. (G) Scheme showing ASK-1-mediated regulation of glomerular permeability.
ASK-1 activation induces tubular damage and interstitial fibrosis in DKD
Masson staining was used to evaluate interstitial fibrosis (Fig. 4-A). In eNOS-KO db/db, tubular cell collapse and fibrosis were observed, whereas in the ASK-1 inhibitor-treated group, the fibrosis was improved. The expression of fibrosis-related genes (αSMA, Collagen1, Fibronectin1 and TGFβ) was determined by quantitative PCR (Fig. 4-B). eNOS-KO db/db showed significantly elevated expression of these genes. ASK-1 inhibitor does not affect αSMA gene expression compared with eNOS-KO db/db group. However, the expression of other fibrosis-related genes was significantly decreased with ASK-1 inhibitors. In addition, KIM-1 staining and urinary KIM-1 excretion were evaluated as markers of tubular damage (Fig. 4-C,D). The eNOS-KO db/db group showed a marked increase in the number of KIM-1 positive tubular cells and urinary KIM-1 excretion levels. ASK-1 inhibitor treatment suppressed these proximal tubular cell damage.
ASK-1 activation induce tubular damage and interstitial fibrosis in DKD. (A) Masson trichrome staining (bar = 100 μm). (B) Expression of fibrogenic gene (alpha smooth muscle actin [α-SMA], Collagen type I [Col I], Fibronectin 1 [FN 1] and Transforming growth factor-β [TGF-β]) were assessed with quantitative reverse transcription PCR (qRT-PCR). (C) Immunohistochemical staining for KIM-1 (bar = 100μm). (D) ELISA quantifying kidney expression of KIM-1. Data are expressed as means ± SEM. *P < 0.05 versus db/misty; †P < 0.05 versus eNOS-KO db/db. db/misty control; db/misty ASK-1 inhibitor; eNOS-KO db/db control; eNOS-KO db/db ASK-1 inhibitor. The number of mice n = 6–11.
RNA-seq data revealed ASK-1 activation leads to aging phenotype in DKD
We focused on clusters that are upregulated in eNOS-KO db/db compared to db/misty and are further downregulated by ASK-1 inhibitor (Fig. 5-A cluster 2). The results of the GO analysis are presented in the Supplementary Fig. 6. In particular, Fig. 5-B shows the results of Go analysis of cluster2, where most of the significant changes in molecular function were genes related to cytoskeleton and fibrosis. On the other hand, aging was observed in the GO term of the biological process. These results suggest that cellular senescence might be caused by ASK-1 activation depending on the pathology in DKD.
RNA-seq data revealed ASK-1 activation leads to aging phenotype in DKD. (A) Z-scores of TPM values were calculated and heat maps were created. (B) GO analysis for cluster 2 was shown in bar graphs. Horizontal axis: number of genes. Vertical axis: GO term. Color: adjusted p-value. GO analysis for all clusters is shown in the Supplementary Fig. 6. (B) GO analysis for cluster 2 was shown in bar graphs. Horizontal axis: number of genes. Vertical axis: GO term. Color: adjusted p-value. GO analysis for all clusters is shown in the Supplementary Fig. 6.
Redox status induced cellular senescence via ASK-1 activation in tubular cells
To investigate the direct involvement of ASK-1 activation and cellular senescence, we performed in vitro experiments using proximal tubular cells. SA-β-GAL (senescence-associated beta-galactosidase) staining was used to evaluate cellular senescence. The SA-β-GAL activity in tubular cells were increased by incubation with BSA + FFA (Fig. 6-A). This increase in activity was suppressed by administration of ASK-1 inhibitors. The p21 signaling pathway, a marker of aging, was evaluated by western blotting. Expression of p21 in Primary human proximal tubule epithelial cells (hPTECs; Lonza, Basel, Switzerland) were upregulated by the addition of BSA + FFA, but was suppressed by ASK-1 inhibitor treatment (P = 0.0983 H2O2 versus H2O2 + ASK-1inhibitor) (Fig. 6-B,C). In vivo, eNOS-KO db/db mice showed higher p53 protein expression than db/misty mice, which was suppressed by ASK-1inhibitor treatment. Moreover, p21 protein levels, a transcriptional target of p53, was suppressed after treatment with ASK-1inhibitor (Fig. 6-D, Supplementary Fig. 7). The expression of genes associated with SASP (Senescence-associated secretory phenotype) (IL-1β, IL-18 and F4/80) was measured by quantitative PCR (Fig. 6-E). The lipopolysaccharide-mediated signaling pathway of RNA-seq bioprocess exhibited the same trend (Supplementary Fig. 6). These results indicate that elevated ROS in proximal tubules promotes cellular senescence via ASK-1 activation.
Redox status induced cellular senescence via ASK-1 activation in tubular cells. (A) Representative images of SA–β-gal–stained hPTECs. (B) Immunoblot analysis quantifying cell expression of p21 and GAPDH. Original blots are presented in Supplementary Fig. 10-A,B. (C) p21 protein level expressed as fold change. Data are expressed as means ± SEM. *P < 0.05 versus control. (D) In vivo, representative western blotting experiments for p53, p21 and GAPDH. These marker protein level expressed as fold change in Supplementary Fig. 7. Original blots are presented in Supplementary Fig. 10-C. (E) Expression of SASP related gene (interleukin-1β[IL-1β], interleukin-18[IL-18], F4/80) were assessed with quantitative reverse transcription PCR (qRT-PCR). Data are expressed as means ± SEM. *P < 0.05 versus eNOS-KO db/db control.
The results of this study show that in DKD, ASK-1 activation in glomerular endothelial cells causes increased permeability due to accelerated VE-cadherin degradation, resulting in increased albuminuria and tubular damage. ROS produced during this process also promote cellular senescence via ASK-1 activation and are implicated in the formation of tubulointerstitial lesions.
Discussion
This study investigated the role of ASK-1 activation in the eNOS-KO db/db mouse model of DKD, and build on previous research15. Herein, we demonstrate that ASK-1 activation was observed in eNOS-KO db/db mice compared to db/misty mice as assessed by phospho-p38, a downstream marker of ASK-1 activity. Inhibition of ASK-1 with the ASK-1-selective inhibitor, GS-444217, significantly reduced p-p38 and reduced glomerular injury, as evidenced by decreased glomerulosclerosis and albuminuria. ASK-1 inhibition also reduced endothelial injury in glomerular capillary walls and reduced disruption of glycocalyx and VE-cadherin expression. Moreover, ASK-1 inhibition reduced tubular damage and interstitial fibrosis, as shown by histological and molecular markers. Bulk kidney RNA-seq data indicated that the eNOS-KO db/db mouse model results in a senescence gene signature, which was reversed by ASK-1 inhibition. Mechanistic studies performed in vitro demonstrated that ASK-1 activation induced cellular senescence in tubular cells via redox signaling. Overall, these findings suggest that ASK-1 activation plays a crucial role in DKD pathogenesis by promoting glomerular injury, tubular damage, and cellular senescence.
ASK-1 has been shown to be involved in the regulation of vascular permeability as well as cell death. In a stroke model, ASK-1 activation has been shown to exacerbate brain edema16. ASK-1 is also involved in regulating the expression of VE-cadherin, a tight junction that is important in the regulation of vascular permeability. It has been shown that VE-cadherin is degraded by ASK-1 activation in tumor lesions. This increases vascular permeability, which is inhibited by ASK-1 inhibitors17. Interestingly, it has recently been reported that decreased VE-cadherin expression causes decreased glycocalyx expression in diabetic kidney disease18. The results of the present study indicate that ASK-1 activation is involved in hyperpermeability in diabetic kidney disease via direct VE-cadherin degradation in glomerular endothelial cells.
Free fatty acid (FFA) that is bound to albumin has been shown to induce tubular damage. Excess FFAs induce mitochondrial damage and promote the production of mitochondria-derived reactive oxygen species (ROS)19. Improving mitochondrial damage in proximal tubular cells in DKD is one therapeutic strategy. We also demonstrated previously, that selective estrogen receptor modulators manifest nephroprotective effects through suppression of mitochondrial damage12. Mitochondrial dysfunction is known to induce apoptosis, a Caspase 3-dependent cell death20. It is now known that ASK-1 activity is downstream of mitochondrial ROS in kidney disease, resulting in apoptosis21. The results of our study also indicate that mitochondrial dysfunction and ROS in proximal tubular cells promotes interstitial lesions in DKD via ASK-1 activation.
There is a close association between ROS induction and cellular senescence. An increase in ROS through NADPH oxidase activity, for example, causes cellular senescence via DNA damage22. Interestingly, the use of NADPH oxidase inhibitors can also prevent aging23. Mitochondria-derived ROS have also been shown to be involved in cellular senescence24. The senescent cells promote organ damage by producing SASP and inducing inflammation. On the other hand, SASP-related genes have been shown to move through various pathways. CCAAT/enhancer binding protein-β (C/EBP-β), nuclear factor kappa-light chain enhancer (NF-κB) of activated B cells, H2A histone family member X (γH2AX), ATM, macroH2A1 histone mutant, Janus kinase 1/2 (JAK1/2), p38 and mitogen-activated protein kinase (MAPK) are also involved in SASP25. The present results indicate that MAPKs, especially ASK-1 activation, are involved in cellular senescence in tubular cells.
In summary, ASK-1 activation was shown to be involved in the pathogenesis of DKD. In glomerular lesions, ASK-1 activation is involved in the regulation of the permeability of the glomerular wall. In tubular damage, ASK-1 activation was found to promote aging. These are direct pathways in which ASK-1 inhibitors may potentially provide therapeutic intervention.
Methods
Animals
The experimental protocols (no. 23-069) were approved by the Animal Research Committee of Kawasaki Medical School and were based on the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996). Additionally, this study was reported in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (https://arriveguidelines.org). Experiments in eNOS-KO db/db mice were performed as described previously26. db/misty, eNOS-KO db/db were randomly divided into two groups. Each genotype was randomly divided into two groups, one continuing on a control diet from the age of 10 weeks and a feed mixed with ASK-1 inhibitor (0.3% GS-444217; provided by Gilead Sciences Inc, db/misty control, db/misty + ASK-1inhibitor, eNOS-KO db/db control and eNOS-KO db/db + ASK-1inhibitor, n = 6–11 mice in each group). The mice were housed in a temperature- and humidity-controlled room with a 14:10 h light–dark cycle and were fed standard laboratory animal chow with free access to tap water. The pentobarbital (200 mg/kg) used for euthanising the mice.
Physiological and biochemical measurement
Physiological parameters were measured just before the mice were killed at 18 weeks of age. Blood samples were obtained using a 21-gauge needle inserted into the right atrium of mice that fasted for 24 h. Body weights were recorded, and blood pressures and pulse rates were measured using the tail-cuff method with an automatic sphygmomanometer (BP98A; Softron Co., Ltd., Tokyo, Japan). Blood glucose was measured by using Glutestmint (Sanwa Chemistry, Aichi, Japan). The 24-h urine samples were collected before the feed change, 4 weeks after the feed change, and 8 weeks after the feed change before euthanasia. Urinary albumin levels were determined by ELISA using the primary monoclonal antibody of mouse albumin (A90-134A, Bethyl Laboratories, TX, USA). Urinary KIM-1 levels were determined by ELISA using the primary monoclonal antibody of mouse albumin (A90-134A, Bethyl Laboratories, TX, USA). Serum creatinine and urea nitrogen levels were measured at a central clinical laboratory (SRL, Inc., Tokyo, Japan).
Histological analysis and immunohistochemistry
Right kidney tissue was fixed in 4% paraformaldehyde and embedded in paraffin for histological analysis. Paraffin-embedded tissue was sectioned at 4 µm thickness and frozen tissue at 10 µm. Tissue sections were deparaffinized and stained with PAS and Masson staining. Three nephrologists semiquantitatively analysed PAS stained sections in a blind fashion. One point was counted if the sclerosis involved the entire glomerulus. Deparaffinized kidney sections were heated in a microwave at 500 W, 15 min for antigen retrieval and then incubated overnight with antibody against KIM-1 (AF1817, R&D Systems, Minneapolis, MN, USA). The primary antibody was detected using the Histofine Simple Stain MAX PO kit (Nichirei Corporation, Tokyo, Japan) and 3,3ʹ-diaminobenzidine (Sigma-Aldrich). All images were obtained with an all-in-one fluorescence microscope (BZ-9000, KEYENCE, Osaka, Japan).
Frozen tissue sections were incubated overnight with antibody against VE-cadherin (ab33168, abcam, Cambridge, UK); secondary antibody was FITC (F0205, Dako, Glostrup, DNK).
The glomerular glycocalyx, which is a main component of endothelial surface layer (ESL), was evaluated by staining paraffin-embedded tissue with tomato lectin (L0401, SIGMA, Kanagawa, Japan).
Western blot analysis
SDS-PAGE was performed (20–30 μg protein/lane) with antibodies against phospho-p38/MAPK (Thr180/Tyr182) (9211S, Cell signaling, Danvers, USA), total-p38/MAPK (9211, Cell signaling, Danvers, USA), phospho-VE-cadherin (Tyr658) (44-1144G, Invitrogen, Waltham, USA), total-VE-cadherin (ab33168, abcam, Cambridge, UK) and glyceraldehyde phosphate dehydrogenase (1E6D9, proteintech, Rosemont, Illinois, USA). Signals were detected using Amersham ECL Western Blotting Detection Reagents (Cytiva, Marlborough, MA, USA). The relative optical densities of the bands were quantified using Image J software version 1.53t.
RNA extraction and real-time quantitative PCR
Total RNA extraction and real-time quantitative RT-PCR were performed, as previously described27. Briefly, total RNA was isolated from the kidneys using TRIzol (Invitrogen; Thermo Fisher Scientific Inc., Waltham, MA, USA), followed by digestion with DNase (Sigma-Aldrich). cDNA was synthesized from total RNA (1 µg) using Moloney murine leukemia virus reverse transcriptase (Thermo Fisher Scientific Inc.) with oligo (dT)12–18 as a primer (Thermo Fisher Scientific Inc.). Reverse transcription was performed for 50 min at 37°C according to the manufacturer’s protocol (Thermo Fisher Scientific Inc.). The primers and probes for TaqMan analysis were designed using sequence information from GenBank (National Institutes of Health)28 and the Primer3 online software (http://frodo.wi.mit.edu/primer3/; accessed July 1, 2015). The primer and probe sequences are listed in Table 2. TaKaRa Premix Ex Taq (Takara Bio Inc., Otsu, Japan), with a final reaction volume of 20 µL, was used for the TaqMan probe-based RT-PCR reaction, which was performed on an Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific Inc.). Plasmid cDNA of each gene was used to prepare absolute standards. The mRNA expression levels of each gene were normalized to those of the housekeeping 18S ribosomal RNA gene.
RNA-sequencing
RNA-sequencing was performed by using mRNA form whole kidney. Samples were processed and analyzed by Novaseq (Gene Nex, Beijing, China). Gene ontology (GO) enrichment analysis was preformed using goatoools29 and GOplot30. These data analysis was performed by Rhelixa Inc., Tokyo 104-0042, Japan.
Cell culture
Primary human glomerular endothelial cells (hGEC; cell systems, Seattle, USA) were used for the in vitro assays. After starvation, subconfluent cells (0.5% fetal bovine serum for 24 h) were stimulated with 200 μM H2O2 for 1 h. ASK-1 inhibitor (10μM) was added to the culture medium 30 min before stimulation with H2O2.
hPTECs were cultured in a renal epithelial basal medium (Lonza) supplemented with 5% fetal bovine serum. After starvation, subconfluent cells (0.5% fetal bovine serum for 24 h) were stimulated with 0.5% BSA (MilliporeSigma) binding 450 mM palmitic acid (BSA + FFA) for 6 h. ASK-1 inhibitor (10μM) was added to the culture medium 30 min before stimulation with BSA + FFA.
Senescence-associated β-galactosidase (SA-β-gal) staining
SA-β-gal staining in hPTECs were performed using a Senescence β-Galactosidase Staining Kit (#9860; Cell Signaling), following the manufacturer’s instructions.
Statistical analyses
Data are expressed as the mean ± standard error of the mean (SEM). Statistical analyses were performed using GraphPad Prism7 software (GraphPad Software, La Jolla, CA, USA). Statistical significance was evaluated using one-way ANOVA with Tukey–Kramer post hoc tests for comparisons among multiple groups. P values less than 0.05 were considered statistically significant.
Data availability
The datasets generated and analysed during the current study are available in the Gene Expression Omnibus (GEO) repository, GSE275379.
References
Iseki, K. et al. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 63(4), 1468–1474. https://doi.org/10.1046/j.1523-1755.2003.00868.x (2003).
Asaba, K. et al. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 67(5), 1890–1898. https://doi.org/10.1111/j.1523-1755.2005.00287.x (2005).
Gao, L. & Mann, G. E. Vascular NAD(P)H oxidase activation in diabetes: A double-edged sword in redox signalling. Cardiovasc. Res. 82(1), 9–20. https://doi.org/10.1093/cvr/cvp031 (2009).
Ozeki, M. et al. Reactive oxygen species mediate compensatory glomerular hypertrophy in rat uninephrectomized kidney. J. Physiol. Sci. 59(5), 397–404. https://doi.org/10.1007/s12576-009-0048-4 (2009).
Kishi, S. et al. Oxidative stress and the role of redox signalling in chronic kidney disease. Nat. Rev. Nephrol. 20(2), 101–119. https://doi.org/10.1038/s41581-023-00775-0 (2024).
Nagasu, H. et al. Activation of endothelial NAD(P)H oxidase accelerates early glomerular injury in diabetic mice. Lab. Invest. 96(1), 25–36. https://doi.org/10.1038/labinvest.2015.128 (2016).
Ichijo, H. et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275(5296), 90–94. https://doi.org/10.1126/science.275.5296.90 (1997).
Chang, H. Y. et al. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 281(5384), 1860–1863. https://doi.org/10.1126/science.281.5384.1860 (1998).
Tobiume, K. et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2(3), 222–228. https://doi.org/10.1093/embo-reports/kve046 (2001).
Xu, W. et al. TRAF1 exacerbates myocardial ischemia reperfusion injury via ASK1-JNK/p38 signaling. J. Am. Heart Assoc. 8(21), e012575. https://doi.org/10.1161/jaha.119.012575 (2019).
Kaufman, D. R. et al. Deletion of inositol-requiring enzyme-1α in podocytes disrupts glomerular capillary integrity and autophagy. Mol. Biol. Cell 28(12), 1636–1651. https://doi.org/10.1091/mbc.E16-12-0828 (2017).
Nishi, Y. et al. Selective estrogen receptor modulation attenuates proteinuria-induced renal tubular damage by modulating mitochondrial oxidative status. Kidney Int. 83(4), 662–673. https://doi.org/10.1038/ki.2012.475 (2013).
Zhuang, Y. et al. Mitochondrial dysfunction confers albumin-induced NLRP3 inflammasome activation and renal tubular injury. Am. J. Physiol. Renal Physiol. 308(8), F857–F866. https://doi.org/10.1152/ajprenal.00203.2014 (2015).
Tesch, G. H. et al. ASK1 inhibitor halts progression of diabetic nephropathy in Nos3-deficient mice. Diabetes 64(11), 3903–3913. https://doi.org/10.2337/db15-0384 (2015).
Liles, J. T. et al. ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J. Clin. Invest. 128(10), 4485–4500. https://doi.org/10.1172/jci99768 (2018).
Song, J. et al. The effect of ASK1 on vascular permeability and edema formation in cerebral ischemia. Brain Res. 1595(1), 43–55. https://doi.org/10.1016/j.brainres.2014.11.024 (2015).
Yin, M. et al. ASK1-dependent endothelial cell activation is critical in ovarian cancer growth and metastasis. JCI Insight. https://doi.org/10.1172/jci.insight.91828 (2017).
Li, L. et al. Aberrant activation of Notch1 signaling in glomerular endothelium induces albuminuria. Circ. Res. 128(5), 602–618. https://doi.org/10.1161/circresaha.120.316970 (2021).
Erkan, E., Devarajan, P. & Schwartz, G. J. Mitochondria are the major targets in albumin-induced apoptosis in proximal tubule cells. J. Am. Soc. Nephrol. 18(4), 1199–1208. https://doi.org/10.1681/asn.2006040407 (2007).
Zhou, Y. et al. Mitochondrial outer membrane protein Samm50 protects against hypoxia-induced cardiac injury by interacting with Shmt2. Cell Signal. 120, 111219. https://doi.org/10.1016/j.cellsig.2024.111219 (2024).
Jiang, W. et al. Hirsutine ameliorates myocardial ischemia-reperfusion injury through improving mitochondrial function via CaMKII pathway. Clin. Exp. Hypertens 45(1), 2192444. https://doi.org/10.1080/10641963.2023.2192444 (2023).
Marazita, M. C. et al. Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: Implications for Age-related Macular Degeneration. Redox Biol. 7, 78–87. https://doi.org/10.1016/j.redox.2015.11.011 (2016).
Kwak, J. Y. et al. Nicotinamide exerts antioxidative effects on senescent cells. Mol. Cells 38(3), 229–235. https://doi.org/10.14348/molcells.2015.2253 (2015).
Wiley, C. D. et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 23(2), 303–314. https://doi.org/10.1016/j.cmet.2015.11.011 (2016).
Freund, A., Patil, C. K. & Campisi, J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. Embo J. 30(8), 1536–1548. https://doi.org/10.1038/emboj.2011.69 (2011).
Zhao, H. J. et al. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J. Am. Soc. Nephrol. 17(10), 2664–2669. https://doi.org/10.1681/asn.2006070798 (2006).
Satoh, M. et al. Mitochondrial damage-induced impairment of angiogenesis in the aging rat kidney. Lab. Invest. 91(2), 190–202. https://doi.org/10.1038/labinvest.2010.175 (2011).
Benson, D. A. et al. GenBank. Nucleic Acids Res. 41(Database issue), D36-42. https://doi.org/10.1093/nar/gks1195 (2013).
Klopfenstein, D. V. et al. GOATOOLS: A python library for gene ontology analyses. Sci. Rep. 8(1), 10872. https://doi.org/10.1038/s41598-018-28948-z (2018).
Walter, W., Sánchez-Cabo, F. & Ricote, M. GOplot: An R package for visually combining expression data with functional analysis. Bioinformatics 31(17), 2912–2914. https://doi.org/10.1093/bioinformatics/btv300 (2015).
Acknowledgements
We thank Etsuko Yorimasa, Yuriko Katayama, and Chisa Fujiwara for providing animal care. We thank Keiko Satoh for assisting with the in vitro assays. We also thank Gilead Sciences Inc. for supporting the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Study design: E.K., H.N. N.K.; Interpretation of data and statistical analysis: E.K. M.T., R.T., A.H., I.T., R.U., Y.W.; Drafting of the manuscript: E.K., H.N., S.K.; Revision of the submitted manuscript: E.K. H.N. S.I., H.K., K.K., T.S., N.K. All authors performed the experiments in this study and designed research plan.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kajimoto, E., Nagasu, H., Takasu, M. et al. ASK-1 activation exacerbates kidney dysfunction via increment of glomerular permeability and accelerates cellular aging in diabetic kidney disease model mice. Sci Rep 14, 26438 (2024). https://doi.org/10.1038/s41598-024-77577-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77577-2