Abstract
The primary purpose was to assess the incidence and predictors for mortality in Chinese geriatric patients with perioperative transfusion following intertrochanteric fracture (IF) surgery. A total of 1260 patients who received perioperative transfusion following IF surgery between Jan. 2016 and Dec. 2018 were included. Data was performed to compare the mortality group and the survival group based on subgroup of follow-up time in univariate, and adjusted Cox regression analysis. Perioperative transfusion factors included total, pre-, intra-, and post-operation transfusion volume, and number of transfusions. In our study, the mortality rate was 0.87%, 1.6%, 2.9%, 7.0%, and 13.4% at 30-day, 3-month, 6-month, 1-year, and 2-year follow-up, respectively. Within 6-month follow-up, the adjusted Cox regression analysis revealed that a high American Society of Anesthesiologists (ASA) score, as well as an increase in volume and number of transfusions leading to rapidly increasing morbidity, were identified as risks with mortality. While advanced age and complications played important roles in mortality from 6-month follow-up. In the present study, a high ASA score, as well as the volume and number of transfusions, were identified as risk factors for mortality after IF surgery in Chinese geriatric patients within 6-month follow-up. However, age and severe complications contributed to mortality in 1- and 2-year follow-up.
Similar content being viewed by others
Introduction
Hip fracture, a common fracture in the elderly1, frequently results in hospitalization and the mortality rates ranging from 7 to 10% in 30 days2,3, which has been linked to anemia4,5. More than half of elderly patients undergoing hip fracture surgeries receive transfusions6. It is well known that transfusion not only improves the patient’s condition by mitigating the impact of blood loss due to injury and surgery but also brings in risks including increased thrombogenicity and a higher rate of infection7,8. Several observational studies9,10 have demonstrated the relationship between transfusion and increased postoperative mortality in cardiac patients.
In the field of orthopedics, Kadar11 assessed the effect of the shelf life of the allogenic blood transfusion on mortality and discovered no significant difference in mortality between ‘old’ units of blood and ‘new’ units. Furthermore, intraoperative transfusion and high-or very-high-dose transfusion were linked to an increase in mortality during short-term follow-up12,13. On the contrary, some studies14,15 suggest no close relation between transfusion and an increased rate of mortality. Although several studies have attempted to elucidate the risk factors for mortality in hip fracture cohorts, their risk factors remain debated. After reviewing related articles, no one focused on the mortality of special patients who received perioperative transfusions following intertrochanteric fracture surgery. As far as we know, this was the first article to clarify the risks of mortality in the special population based on subgroups of follow-up time.
Materials and methods
Ethics statement
The study was approved by the Institutional Review Board of Third Hospital of Hebei Medical University in compliance with the Helsinki and an exemption from the informed consent was obtained (W2021-070-1).
Patients
We included 1260 patients who received perioperative transfusion and underwent IF surgery from Jan. 2016 and Dec. 2018 in our hospital. The phone call was made at 30-day, 3-month, 6-month, 1-year, and 2-year after surgery. The inclusion criteria were: (1) patients who received perioperative leucoreduced red cells (SAGM) transfusion; (2) patients receiving IF surgery; (3) >60 years old. The exclusion criteria were: (1) patients with multiple fractures or injuries; (2) open fractures.
Possible factors were collected including: baseline factors-age, sex, time from injury to hospital, body mass index (BMI), hemoglobin (admission, discharge, minimum), type of fracture, ASA classification, time from injury to surgery, hospital stay, vein thrombosis (admission); surgical factors-transfusion (yes or no), peri-, pre-, intra- and post-operatively volume and the number of transfusion, operation time, blood loss, general anesthesia (yes or no); comorbidities-a history of electrolyte disturbance, anemia, dementia, pneumonia,, arteriosclerosis, cerebrovascular disease, hypoproteinemia, arrhythmology, valvulopathy, heart failure, myocardial infarction, diabetes, cerebral hemorrhage, cerebral infarction, coronary heart disease, hypertension; complications- heart failure, respiratory failure, cerebral infarction, stress ulcer, arrhythmology, pneumonia, delirium, anemia, vein thrombosis, electrolyte disturbance, hypoproteinemia, and hyperglycemia. Body mass index (BMI) was classified as normal with BMI < 24 kg/m2, overweight with 24 ≤ BMI < 28 kg/m2, and obesity with BMI ≥ 28 kg/m2. Operation time was grouped as ≤ 60 and >60 min. In our study, we conduct the liberal transfusion (hemoglobin<10 g/dL). We routinely administer suspended red blood cells once the hemoglobin<10 g/dL during the perioperative period. The number of units of blood transfusion depends on the patient’s hemoglobin. The peri-operative time period encompassed the entire admission period and included pre-operative, intra-operative, and post-operative transfusions that occurred during the admission period. We need to monitor the postoperative blood routine in real time. When the hemoglobin is low after operation that may caused by hidden blood loss, we need transfusion. “more than once” do the authors mean more than one transfusion episode.
Because our continuous variable did not satisfy the criteria for normality, we chose the rank sum test. Chi-square test was used for count data. Statistical significance levels were considered to be p<0.05. To identify the best predictors of mortality, univariate and adjusted Cox regression analysis were computed using SPSS, (version 26.0, Chicago, IL).
Results
From Jan. 2016 and Dec. 2018, 2708 patients with IF were evaluated for this study. 1446 patients were removed because they did not met our exclusion criteria. The total number of patients who met our inclusion and exclusion criteria were thus 1260, who were grouped as mortality group and survive group according to follow-up time.
As shown in Table 1, ASA, transfusion volumes (pre-operation, and pre-operation + post-operation), the numbers of transfusion (total, pre-operation, and pre-operation + intra-operation), hemoglobin (admission), vein thrombosis (admission), a history of pneumonia or heart failure, and complications such as heart failure, respiratory failure, and pneumonia were associated with mortality at 30-day. While adjusted Cox regression implied that ASA [p = 0.044, HR = 1.012, 95%CI (1.003, 1.418)], transfusion volume (pre-operation) [p = 0.022, HR = 3.050, 95%CI (1.900, 5.709)], the number of transfusion (total) [p = 0.040, HR = 2.033, 95%CI (1.070, 3.384)], and heart failure (complication) [p = 0.011, HR = 1.113, 95%CI (1.021, 1.608)] were risks of mortality, as presented in Fig. 1a.
At 3-month follow-up, time from injury to hospital, ASA, transfusion volume (total), the number of transfusion (total), a history of pneumonia and complications, such as heart failure and respiratory failure were found to be related to mortality. Nevertheless, as shown in Table 2. In our adjusted Cox regression, the data showed that ASA [p = 0.049, HR = 1.142, 95%CI (1.020, 1.301)], transfusion volume (total) [p = 0.022, HR = 1.122, 95%CI (1.020, 1.739)], and number of transfusions (total) [p = 0.014, HR = 1.140, 95%CI (1.011, 1.388)] were risk factors of mortality, as presented in Fig. 1b.
Age, time from injury to hospital, ASA, transfusion volume (total, pre-operation, pre-operation + intra-operation, and pre-operation + post-operation), the number of transfusions (total, pre-operation, and pre-operation + intra-operation), a history of pneumonia and heart failure, and complications such as heart failure, respiratory failure, or arrhythmia were linked with mortality at 6-month follow-up (Table 3). However, the results of adjusted Cox regression indicated that age [p = 0.002, HR = 1.129, 95%CI (1.035, 1.475)], ASA [p = 0.001, HR = 1.143, 95%CI (1.045, 1.457)], transfusion volume (pre-operation) [p = 0.001, HR = 1.080, 95%CI (1.024, 1.270)], and the number of transfusion (pre-operation + intra-operation) [p = 0.030, HR = 1.320, 95%CI (1.102, 1.998)] were identified as risks of mortality, as presented in Fig. 1c.
Table 4 showed that age, BMI, time from injury to hospital, a history of pneumonia, and complications such as heart failure, were correlated with mortality at 1-year follow-up. Furthermore, Fig. 1d suggested that in adjusted Cox regression analysis, age [p = 0.046, HR = 1.428, 95%CI (1.198, 1.858)] and heart failure (complication) [p = 0.031, HR = 1.526, 95%CI (1.294, 1.942)] were considered as increased mortality.
Table 5 presented that age, BMI, time from injury to surgery, and complications such as delirium were linked with mortality at 2-year follow-up. What’s more, Fig. 1e demonstrated that age [p = 0.001, HR = 1.037, 95%CI (1.016, 1.060)], and delirium (complication) [p = 0.008, HR = 1.064, 95%CI (1.017, 1.114)] were considered as increased mortality in adjusted Cox regression.
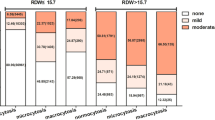
In Fig. 2, relations between the overall morbidity and transfusion volume and number of transfusions were performed, and our findings showed that overall morbidity increased with the increase in transfusion volume and number of transfusions perioperatively, preoperatively, intraoperatively, and postoperatively.
Discussion
In this present study, the mortality rate was 0.87%, 1.6%, 2.9%, 7.0% and 13.4% at 30-day, 3-month, 6-month, 1-year, and 2-year follow-up, respectively. The high ASA score was an independent risk factor for mortality within the 6-month follow-up. Preoperative transfusion volume and number of perioperative transfusions at 30-day, perioperative transfusion volume and number of transfusion at 3-month, and preoperative transfusion volume and number of pre- and intra-operative transfusions at 6-month were discovered to be risk factors for mortality. However, advanced age and serious complications became risk factors for mortality from 6-month follow-up.
Previous studies3,16 reported a 4.0-10% 30-day mortality and a 19-36% one-year mortality, which was significantly higher than our results of 0.87% mortality at 30-day and 7.0% mortality at 1-year. Two possible reasons might account for the difference in mortality between this study and previous studies. First, it might be related to different demographics, including age, distribution of gender, and comorbidity in each study. Second, our institution has specialist geriatric wards, based on a collaborative multidisciplinary team care model, in the management of older trauma and orthopedic patients who are over 75 years old. Physicians provide professional treatment for internal medical diseases of the elderly before and after surgery to minimize postoperative complications and allow the elderly to recover more quickly, which has greatly contributed to lower in-hospital and post-discharge mortality.
A growing number of articles studied the effect of transfusions on mortality, yet it remained debated. Johnston DJ14 believed that perioperative transfusion was not associated with an increased risk of mortality in patients with hip fracture. In contrast, increased transfusion volumes have been demonstrated to be associated with increased morbidity and mortality in other studies4,16. A retrospective study including 971,455 patients from Turan A4 focused on lower transfusion doses ranging from 0 to 10 units and suggested that patients who received 5 or greater units had a significantly increased 30-day mortality than those who received 1–4 units or without transfusion following non-cardiac surgery. While Johnson P13 evaluated the relationship between high or very high dose and morbidity and mortality in 3,523 patients who received greater than 10 units, he found that a linear relationship between high dose and mortality indicated a 10% increase with every 10 units. Besides, they also discovered overall morbidity increased in a dose-related curvilinear manner. Even though Turan A4 and Johnson P13 provide innovative points for us, they did not perform a medium- or long-term follow-up and just paid attention to the overall period of transfusion. Gupta P12 conducted a further study and stratified perioperative transfusions into four periods (peri-operative, pre-operative, intraoperative, or post-operative periods) to better understand the risk and association with mortality and mentioned that transfusions preoperatively may cause future systemic implications. But only 2-month follow-up was performed in Gupta P’s study.
To our knowledge, a few studies have broken down the transfusion period to assess the impact of transfusions on mortality at long-term follow-up. The highlight of our study lies in exploring the mortality based on follow-up time (30-day, 3-month, 6-month, 1-year, and 2 year) in relation to volume and number of transfusions at different times (peri-, pre-, intra, post-, pre- + intra-perative, pre- + post-perative, and intra-+ post-operative). A crude result indicated an increase in volume and number of transfusions associated with an high risk of mortality within 6-month follow-up. A hypothesis that the effect of transfusion diminishes over time may account for the result. It is well known that transfusion-related immunomodulation leads to a reduced host response to pathologic organisms, which increases the risk of prosthetic infection and transfusion-associated circulatory overload such as serious complications or even causes death17,18. More transfusion volume and number of transfusions mean a higher rate of mortality. Meanwhile, our own immune system adjusts the body to gradually reduce the impact of transfusion reactions.
Moreover, a further and meticulous analysis was conducted to find out which period in volume and number of transfusions affected mortality in short-term follow-up. Surprisingly, our findings in adjusted model indicated that preoperative transfusion volume and the number of perioperative transfusion at 30-day, perioperative transfusion volume and the number of transfusion at 3-month and preoperative transfusion volume and number of pre- + intra-operative transfusion at 6-month were related with increased risk of mortality. In addition, an irregular linear relationship was observed between overall morbidity with volume and number of transfusions during different periods, as shown in Fig. 2. Obviously, no matter which period, the irregular linear relationship was able to be divided into three stages, including the first-rising stage, the plateau stage, and the second-rising stage. Regardless of which period, overall morbidity turned into a plateau stage since receiving the first transfusion or 2 units of blood product, while it was not clear when it changed into the second-rising stage.
In terms of transfusion volume, per- with intra-operatively had a markedly higher overall morbidity in comparison with other periods. However, its real reasons were unclear. We inferred that four continuous strikes (injury, pre-operative transfusion, surgery, and intra-operative transfusion) against the body of the geriatric patients made them more prone to serious complications. Regarding the number of transfusions, it was significantly related to highly increased morbidity and mortality in the risk-adjusted model, which was consistent with Johnson P13. Although transfusion was not found to be associated with mortality at 1- and 2-year follow-up in the adjusted model.
In the current study, higher ASA classification and advanced age were risk factors for postoperative mortality, which had been demonstrated in previous studies19,20. Differently, at short-term follow-up ranging from 30-day to 6-month, higher ASA classification was closely linked with mortality, while varying from 6-month to 2-year, age has become a crucial role in mortality, companied with the generally disappearing role of transfusion.
We has some limitations. First, As we know, little research has focused on transfusion-stratified to assess risks of mortality, but we need a multi-center, randomized controlled study was needed. Second, another factor such as a history of smoking that might influence mortality was not fully included due to retrospective studies’ limitations. Third, although eight doctors conducted the operations that may affect the accuracy, they were had good surgical skills and experience.
In conclusion, the lower mortality rate was discovered in our study because of the geriatric orthopedics wards with collaborative multidisciplinary teams in our institution. Furthermore, the increase in transfusion volume and the number of transfusions were regarded as independent risk factors for mortality after IF surgery in Chinese geriatric patients with a short follow-up. Since the 6-month follow-up, mortality has been primarily attributed to age and severe complications. This article aimed to share some experience with the surgeons and suggested a reduction in transfusion volume and the number of transfusions, especially for preoperative and intraoperative use, may be an effective measure to lower mortality after IF surgery.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Change history
26 June 2025
The original online version of this Article was revised: The original version of this Article omitted an affiliation for Zhiyong Hou. The article has been corrected.
Abbreviations
- IF:
-
Intertrochanteric Fracture
- BMI:
-
body mass index
- ASA:
-
American Society of Anesthesiologists
References
Johnell, O. & Kanis, J. A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 17 (12), 1726–1733 (2006).
Holt, G., Smith, R., Duncan, K., Finlayson, D. F. & Gregori, A. Early mortality after surgical fixation of hip fractures in the elderly: an analysis of data from the Scottish hip fracture audit. J. Bone Joint Surg. Br. 90 (10), 1357–1363 (2008).
Lewiecki, E. M. et al. Hip fracture trends in the united States, 2002 to 2015. Osteoporos. Int. 29 (3), 717–722 (2018).
Turan, A. et al. Morbidity and mortality after massive transfusion in patients undergoing non-cardiac surgery. Can. J. Anaesth. 60, 761–770 (2013).
Smilowitz, N. R. et al. Association between anemia, bleeding, and transfusion with Long-term mortality following noncardiac surgery. Am. J. Med. 129 (3), 315–323 (2016). e2.
Halm, E. A. et al. Effects of blood transfusion on clinical and functional outcomes in patients with hip fracture. Transfusion 43 (10), 1358–1365 (2003).
Blajchman, M. A. Immunomodulation and blood transfusion. Am. J. Ther. 9, 389–395 (2002).
Shander, A., Javidroozi, M., Ozawa, S. & Hare, G. M. What is really dangerous: anaemia or transfusion? Br. J. Anaesth. 107 (Suppl 1), i41–59 (2011).
Koch, C. G. et al. Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. Ann. Thorac. Surg. 81, 1650–1657 (2006).
Kuduvalli, M. et al. Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coronary artery bypass surgery. Eur. J. Cardiothorac. Surg. 27, 592–598 (2005).
Kadar, A. et al. The effects of ‘old’ red blood cell transfusion on mortality and morbidity in elderly patients with hip fractures–a retrospective study. Injury 6, 747–750 (2013).
Gupta, P., Kang, K. K., Pasternack, J. B., Klein, E. & Feierman, D. E. Perioperative Transfusion Associated with Increased Morbidity and Mortality in Geriatric Patients Undergoing Hip Fracture Surgery1221514593211015118 (Geriatric orthopaedic surgery & rehabilitation, 2021).
Johnson, D. J. et al. Morbidity and mortality after High-dose transfusion. Anesthesiology 2, 387–395 (2016).
Johnston, P. et al. Is perioperative blood transfusion a risk factor for mortality or infection after hip fracture? J. Orthop. Trauma. 10, 675–679 (2006).
Saleh, A. et al. Allogenic blood transfusion following total hip arthroplasty: results from the nationwide inpatient sample, 2000 to 2009. J. Bone Joint Surg. Am. 18, e155 (2014).
Neuman, M. D. et al. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Int. Med. 174 (8), 1273–1280 (2014).
Opelz, G. & Terasaki, P. I. Improvement of kidney-graft survival with increased numbers of blood transfusions. Transplant Proc. 1973;299: 253–259. (1973).
Vamvakas, E. C. Possible mechanisms of allogeneic blood transfusion associated postoperative infection. Transfus. Med. Rev. 16, 144–160 (2002).
Meng, D. et al. Patient and perioperative factors influencing the functional outcomes and mortality in elderly hip fractures. J. Invest. Surg. 34 (3), 262–269 (2021).
Vochteloo, A. J. et al. Outcome in hip fracture patients related to anemia at admission and allogeneic blood transfusion: an analysis of 1262 surgically treated patients. BMC Musculoskelet. Disord. 12, 262 (2011).
Author information
Authors and Affiliations
Contributions
TW was responsible for study concept and writing the article. YBL and ZQW were responsible for screened the abstracts and reviewed the article. ZYH was responsible for reviewing and writing the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, T., Long, Y., Wang, Z. et al. The role of perioperative transfusion in mortality in geriatric patients with intertrochanteric fracture. Sci Rep 15, 16699 (2025). https://doi.org/10.1038/s41598-025-00881-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00881-y
Keywords
This article is cited by
-
Pre-operative Red Blood Cell Transfusion for Chronically Anemic Hip Fracture Patients is Associated with Increased Mortality
Indian Journal of Orthopaedics (2025)