Abstract
Olive cultivation in Rajasthan, India, has recently gained significant attention. To understand the genetic diversity among the cultivars of olive (Olea europaea L.) grown in Rajasthan, India, a cumulative approach using Random Amplified Polymorphic DNA (RAPD) markers was employed. This approach was based on morphological, biochemical, nutritional, and associated markers like antimicrobial and antioxidant potential linked to the functional activity of the primary and secondary metabolites. Eight primers (OPA-1, OPA2, OPA3, OPA4; OPB1, OPB2, OPB3, and OPB4) were used to differentiate the seven cultivars of olive. The results indicated 51 reproducible bands showing 45 polymorphism bands (88.23%). The disparity among the cultivars was evident, encompassing their morphological characteristics, biochemical features (protein, fat, carbohydrate, total phenolics, flavonoid, DPPH IC50, radical scavenging activity), and their inhibitory responses against different microorganisms. The genetic analysis using an unweighted pair group method with arithmetic averaging revealed diversity, and the dendrogram formed four distinct groups. These results demonstrate the molecular variance allowing the genetic diversity between the seven exotic cultivars imported from Israel. Considering the aforementioned phylogenetic study, this innovative method can potentially aid in understanding the future crop breeding initiatives in India and the improvement of olives.
Similar content being viewed by others
Introduction
Olive trees are recognized as subtropical trees with long lifespans of a few decades. The evergreen olive (Olea europaea L.) tree has been domesticated for over 7000 years. These trees are one of the most valued agricultural resources for good health1. Olives are widely cultivated for their significant nutritional, antioxidant, and secondary metabolite content. Many olive varieties are grown all over the world. Still, around 98% of the world’s olive trees, which are grown on more than eight million acres, are restricted to the Mediterranean region, which has the most significant number of olive-growing regions2,3. They are widely cultivated in Spain, Greece, Türkiye, Syria, Jordan, Tunisia, Morocco, Algeria, Libya, Egypt, the United States, Australia, Argentina, and Chile4. The vast gene pool of the wild olive and other olive subspecies represents plantations in traditional and emerging olive-growing nations. Cross-breeding between olive cultivars and wild olive with certain traits may be a successful plant breeding strategy and for biodiversity conservation5.Different markers are used to analyze the genetic pool of olive-cultivated varieties. Molecular markers offer trustworthy measurements that go beyond diversity6,7. Genetic diversity in the olive collection has been identified using different molecular markers such as Random amplified polymorphic DNA (RAPD), amplified fragment length polymorphisms (AFLP), single-nucleotide polymorphism (SNP), restriction fragment length polymorphism (RFLP), single-nucleotide polymorphism8, simple sequence repeats5 and chloroplast DNA (Table S1).
RAPD markers are frequently utilized as a promising marker system9,10. RAPDs have been used to establish genetic links among cultivars, differentiate among olive cultivars, examine inter- or intra-cultivar gene variation, and study genetic variations in the olive tree11. Genetic diversity using these markers for germplasm characterization in olive has been reported in olive cultivars growing in Israel12, Tunisia10, Spain13, Portugal14, Türkiye15, Greece16, and Italy17. The olive trees are not indigenous to India. It has been acclimatized and now cultivated. The cultivars grown in India were primarily imported from Israel and were planted in different climatic zones in Rajasthan, India, in 2006, in collaboration with Israeli partners. Arbequina, Barnea, Cortina, Frantoio, Koroneiki, Picholine, and Picual are the cultivars currently flowering in different climatic zones of Rajasthan. They were selected for their robust morphology, resistance to diseases, and resistance to the damaging effects of high and low temperatures and high fruit production. Detailed research on the olive plant grown in Rajasthan can help understand the genetic diversity of the cultivars. Considering the above research9,10,11,12,13,14,15,16,17, the current study analyses olive cultivars’ genetic diversity and phylogenetic relationships using the RAPD markers. Olive diversity is usually characterized by assessments of morphological trait analysis18,19,20, biochemical markers21,22, proximate composition23,24. Some studies have also considered the antioxidant activity21,25, and the antimicrobial activity25,26 to assess the genetic diversity among the cultivars of olive. Phenolic compounds and flavonoids are well-known for their antioxidant activity. These compounds also differ based on the solvents in which they are extracted. The amount of phenolics, flavonoids, and antioxidants are potential markers for distinguishing different cultivars of olive that differ among themselves and act as biochemical markers. The diversity among the cultivars based on the antioxidant activity can be related to their geographic origin. As all the cultivars grown in Barore farm had diverse geographic origins, it was interesting to understand their diversity and interrelationship among themselves. Thus, to understand this genetic diversity and phylogenetic relationship of all the olive cultivars growing in Barore, Rajasthan, India, a cumulative approach was studied using RAPD markers, considering the morphological, biochemical, proximate analysis, and their antioxidant and antimicrobial properties of the cultivars (Fig. 1). This is the first time that the genetic behavior of Israeli origin cultivars under Indian climatic conditions has been analyzed comprehensively. These cultivars are now climate resilient species that are growing luxuriantly under the concept of sustainable agriculture to provide food and oil for all.
Materials and methods
Plant material
The olive plant (Olea europaea L.) herbarium was identified and authenticated by the Department of Botany, University of Rajasthan27, and assigned the voucher accession number RUBL211669, dated March 6, 2018.
Morphological traits analysis of different cultivars of olives
Seven morphologically diverse olive cultivars from Israel were introduced to Indian soil in 2007 and planted in different locations in Rajasthan. Barore farms have shown luxuriant growth in olive trees, flowering, fruiting, and oil yield since 2012. This study was focused on this farm situated in Ganganagar (29.903839′′N latitude, 73.877190′′E longitude), Rajasthan, in northwest India. The cultivars were healthy, in complete flowering stage, and between 10 and 12 years old.
A survey on these olive cultivars was conducted with special emphasis on the leaves and fruits to get a general impression of the variability among the olive cultivars grown on the Barore farm. All the olive cultivars were tested for six morphological traits vis-à-vis. habit, height of plant (PH), number of primary branches per plant, leaf [shape of leaf (LS), length of leaf (LL), width of leaf (LW), area of leaf (LA)], flowering, fruit [shape of fruit (FS), length of fruit (FL), and width of fruit (FW), area if fruit (FA); and fruit production per plant (FP)]. A visual assessment of the data was followed by random sampling to measure the plants and their corresponding plant parts. Sorting was done among the samples collected, and only intact plant parts were taken. Data considered was in triplicate for all the morphological characteristics under investigation.
Assessment of bioactive compounds encompassing antioxidant and antimicrobial properties of the Olive leaf extract from different cultivars
The biochemical parameters and morphological parameters were also considered to understand the diversity among the different cultivars grown in Barore, Rajasthan. The shade-dried leaves from olive cultivars were ground to a fine powder (diameter < 250 μm) and stored at 37 °C. To make the olive leaf extract, 50 g of crushed olive leaves were combined with 1000 mL Milli-Q water, heated for 6 h at 60 ℃, and filtered to get the olive leaf extract28. Care was taken so that there was no intermixing of the leaves.
Phenolics content
The total amount of phenolic compounds of Olive leaf extract of seven different cultivars was quantified in both polar (ethanol, methanol, water) and non-polar (hexane, and chloroform) solvents using the Folin-Ciocalteu assay29 method. The results were expressed in milligrams of GAE per gram of dry matter. The comparison of the cultivars was also performed for the phenolic compounds using the Fourier transform infra-red (FTIR) spectroscopy in an FTIR analyser (Bruker Alpha, Germany), ranging from 4000 cm− 1 to 400 cm− 130.
Flavonoids content
Methanolic extracts were filtered through a 0.45 μm sterile syringe filter, and with the filtrate, HPTLC analysis was performed using quercetin (0.1 mg/mL) as a standard. The spots were applied using a CAMAG Linomat-5 TLC Sampler 4 (ATS 4) with nitrogen flow and a 150 nl/s delivery speed from the application syringe31. The flavonoid concentration in the methanol extract of Olive was measured using a slightly modified version of the aluminium trichloride (AlCl3) procedure32. A comparison of the cultivars was also performed with the flavonoid compounds using FTIR spectroscopy30.
Antioxidant analysis
The total antioxidant capacity of the different cultivars of olive leaf extract was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity method33. During the current research, the antioxidant activity of Olive leaf extracts of seven different cultivars in five different solvents, viz. ethanolic, methanolic, aqueous, hexane, and chloroform, was performed as per our earlier report34. The DPPH radical scavenging activity was calculated as percentage inhibition by the formula given below:
The IC50 values (mg/mL) were calculated using the concentration-response curves35.
Antimicrobial assay
The nonpathogenic bacterial isolates, Staphylococcus aureus (MTCC No. 9542), Escherichia coli (ATCC 25923), Bacillus subtilis (MZ683213.1), and Pseudomonas aeruginosa (MZ348930) were administered to the antimicrobial assay. Antimicrobial assay of crude plant extract fractions was performed using nutrient agar media through the agar-well diffusion method34 using olive leaf extract of Arbequina, Barnea, Coratina, Frantoio, Koroneiki, Picual, Picholine cultivars, and Amikacin as positive control. They were allowed to diffuse for one hour at room temperature and later left undisturbed in an incubator at room temperature for the whole night36. The zone of inhibition (mm) was measured after every 24 h for 3 days.
Proximate analysis in the Olive leaf extract of different cultivars
Olive leaf extract was analyzed using the Standard Procedure (AOAC, 1995) to evaluate its moisture, crude fiber, protein, ash, and fat levels37,38. The comparison of the cultivars was also performed for the primary metabolites related to proximate analysis using the FTIR spectroscopy30.
Genomic DNA extraction and PCR amplification
This study thoroughly rinsed olive leaves with running water and gently washed them with sterile distilled water. The genomic DNA from the olive leaves of the different cultivars was extracted using the modified CTAB process39. This genomic DNA was screened using 8 decamer primers (Table 1). The RAPD primer sequences used in the present study were obtained from The Operon Technologies, USA (publicly available), and the primers were synthesized by M/s Bangalore Genei Pvt. Ltd., India.
The PCR amplification was performed using double-distilled water, 0.5 mM dNTP mix, 2.5 mM MgCl2, 1 U of Taq polymerase, 8 times reaction buffer, 1.0 µM primer, and 25 ng of genomic DNA. Using a BioRad C1000 Touch thermal cycler (Bio-Rad Laboratories), the amplification was carried out under reaction conditions consisting of a 3-minute pre-denaturation step at 94 °C for 3 min, 45 cycles of denaturation at 94 °C for 30 s, 1 min of annealing at 45 °C, and 1.5 min of extension at 72 °C. The final extension occurred at 72 °C for 7 min, with a hot temperature of 4 °C. Electrophoresis was performed on 0.8% agarose gels stained with ethidium bromide (1 µg/ml). The voltage of the gel was maintained at 90 V/cm until the loading dye/bromophenol blue reached the other end of the gel.The transparent gel was photographed using a gel documentation system.
Data analysis
Amplified bands generated from RAPD amplification were scored based on the presence (1) or absence (0) of bands in primer and were used to calculate a SM similarity matrix among seven cultivars, triple readings for each of the variables using NTSYS-pc version 2.140. Cluster analysis was based on morphological, biochemical, antimicrobial, and molecular data. Similarity matrices were created for all pairings of accessions using SM similarity coefficients. Jaccard’s similarity coefficient was used to calculate the similarity coefficient between seven olive cultivars. Cluster analysis was performed using the Unweighted Pair-Group Method with Arithmetic Mean (UPGMA) analysis, and Dendrograms were also created. Principal component analysis (PCA) was also used with the NTSYS-pc software to discover any genetic relationship between the genotypes. The matrix measuring similarity and variation was made using the simple matching (SM) similarity coefficient, which was calculated using NTSYS-pc software (Numerical Taxonomy & Multivariate Analysis System; Version 2.1, Exeter Software, Applied Biostatistics Inc., New York), and the dendrogram was created using this matrix40.
Statistical analysis
The morphological and biochemical data were subjected to mean values and relative standard deviations. One-way analysis of variance (ANOVA) was carried out for the statistical evaluation of the results using Microsoft Excel’s Data Analysis tool, with a significance level (p ≤ 0.05). The F-test was used to validate the quantitative attributes displaying substantial variation among the populations. All morphological and biochemical data were analyzed to determine their mean values and relative standard deviations. The analytical results were used for statistical comparisons, and all assays were performed in triplicate.
Results and discussion
Morphological markers of the different Olive cultivars
The different cultivars showed variations in habit (canopy) of the olive tree and trunk pattern, as shown in Fig. 2. It is believed that evolution gave rise to the tree’s mechanically efficient shape. Arbequina (origin Spain) is one of the most productive olive cultivars, and it is cultivated in Spain and Argentina. The Arbequina tree showed maximum plant height, while the Coratina and Picholine had the lowest height (Table 2). The evergreen tree of Arbequina (origin Spain) showed an upright spreading habit of growth with medium-to-dense branching structure (Fig. 2A-B), and less refined foliage. The Barnea (origin Israel) cultivar is a vigorously growing tree cultivated in Israel. It showed an erect habit with less branching and produced thin, narrow gray-green leaves (Fig. 2C-D). Coratina (origin Italy) tree is a quick-growing Italian olive cultivar with a generally dense and erect growth habit. As with most trees, regular pruning promotes the formation of an even thicker canopy (Fig. 2E-F). The fruit has dark green foliage and small, white blossoms with a vast root system. The cultivar Frantoio (origin Italy) tree grows slowly, with medium vigour and less branching (Fig. 2G-H). The Koroneiki, Greece’s most common oil cultivar, showcases moderate growth and an open, expansive habit, a bushy tree with a delicate canopy (Fig. 2I-J). Picholine (origin France) cultivar has a medium vigour, spreading semi-dense canopy, and an open habit (Fig. 2K-L). The tree of Picual (origin Spain) cultivar of olive is recognized for its upright growth pattern, healthy foliage with long, thin, and dark green leaves (Fig. 2M-N).
The number of primary branches per plant was lowest in Barnea and Coratina (˂ 7 no) and the greatest in Picholine and Picual (˂ 9 no), respectively (Fig. 2I-J). Olive fruits and leaves despite the challenging environmental circumstances in which they develop showed diversity considering the various morphological factors, height of the plant, leaf shape, length, width, shape of the fruit, length, width, number of primary branches per plant, and fruit yield per plant (Table 2; Figure S1). The leaves of Coratina and Barnea fruits were the largest among all cultivars studied (Figure S1). Arbequina and Barnea types had elliptical leaves; Picual had long leaves; and Coratina, Frantoio, Koroneiki, and Picholine had elliptical-lanceolate leaves. The length of the leaves shows significant differences. They ranged from 4.6 to 10.1 cm, while the width of the leaves ranged from 0.6 to 1.6 cm. Coratina (16.1 cm) had the highest average leaf size, while Koroneiki (2.7 cm) had the lowest (Table 2).
The reproductive structures of olive trees were the axillary buds found on the leaves of the previous year’s shoots. In olive cultivars grown in Rajasthan, India, inflorescence development (Fig. 3A-U) was observed from mid-February, when the trees are subjected to sufficiently cold temperatures for a prolonged period. Their floral buds finally emerge from hibernation, and after bud break occurs. The Arbequina olive tree produces small, white flowers with four petals, measuring 1–2 cm in length (Fig. 3A-C). These delicate, feathery blooms appear in late spring or early summer, growing in clusters and emitting a fragrant scent. Arbequina is partially self-fertile, capable of producing fruit without cross-pollination41. However, having a pollinator variety nearby, such as ‘Koroneiki’ or ‘Frantoio’, can enhance fruit set and yield by 10–20%42. Despite this, Arbequina remains a productive variety even without cross-pollination. The fruit of the Arbequina olive is distinctive, with a spherical shape that sets it apart from other cultivars. Fruit sizes range from 1.4 to 2.5 cm in length and 0.6 to 1.0 cm in width. The area of fruit sizes ranged from 0.9 to 2.25 cm (Table 2). Within cultivars, olive fruit production per plant also varies. The annual production of Arbequina fruit was 20–25 kg, whereas the plant’s annual production of Barnea fruit was nearly 55 kg/ plant per year. Similarly, the yearly fruit yield per plant was Coratina 30 kg, Frantoio 5 kg, Koroneiki 10–12 kg, Picholine 5 kg, and Picual, respectively. The morphological characteristics, specifically the fruits and leaves of four olive cultivars grown in Greece43 and 28 different olive cultivars from Turkey44, were investigated and found to vary in shape and size. In another study from Iran45, fruit was also a good marker for distinguishing 243 different cultivars of olive. In addition to leaf and fruit, the bark of 25 Italian olive cultivars was also reported46,47 as a morphological marker.
Molecular analysis of polymorphism among different cultivars of Olive growing in Barore forms in Rajasthan
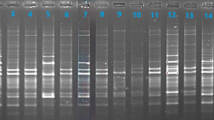
Establishing a fingerprint for each species is necessary to identify polymorphic DNA using RAPD markers. The presence or absence of bands resulting from the multiplication of specific plant genome segments was used to analyze the genetic relationship in the study’s results. The molecular weights of those bands depended on the quantity and locations of the prefix sequences on the DNA template, and very light bands were ignored. Divergence based on molecular marker (RAPD) profiling was performed. Based on the eight primers selected for RAPD analysis, based on their reproducibility and banding patterns, a total of 51 bands were generated from 8 RAPD primers. Forty-five bands were polymorphic (56.25%), averaging 6.37 polymorphic bands per primer. The OPA-2 and OPB-2 primers amplified the highest number of amplicons (7) with 100% polymorphism, whereas the OPB-1 primer generated the lowest number of amplicons (2). A dendrogram has been generated using NTSYS-pc version 2.148, considering morphological (leaf and fruit) parameters using UPGMA cluster analysis based on Jaccard’s similarity coefficient value, which revealed that the average similarity percentage was 62% (Fig. 4A-H).
RAPD Fingerprinting of Olive Cultivars. Agarose gel electrophoresis of RAPD fragments amplified using different primers: [A–D]: OPA primers (OPA_1 to OPA_4; [E–H]: OPB primers (OPB_1 to OPB_4); Lane Details: Lane 1: Molecular Marker (10 kb ladder with fragments ranging from 1000 to 50 kb); Lanes 1–7: Olive cultivar samples.
Diversity analysis of Olive using RAPD markers and clustering (Fig. 5) revealed two significant clusters with 59% similarities. The genetic similarity values ranged from 0.52 to 0.73 based on morphological and RAPD markers. Based on these data, this similarity coefficient amongst the seven olive cultivars showed an average of 0.62. All seven cultivars were differentiated and clustered using a dendrogram based on RAPD data into two major clusters and two subclusters Fig. 5A-B. First cluster A was the smallest and comprised only two cultivars consist, Picholine and Frantoio, while second cluster B was the biggest and comprised five olive cultivars, Arbequina, Barnea, Coratina, Picual, and Koroneiki. Subclusters B-I and B-II were created from Cluster B. Subcluster B-I comprised Koroneiki, Picual, and Coratina cultivars. In contrast, subcluster B-II was made up of Arbequina and Barnea. Picual and Coratina’s cultivars had the highest similarity coefficient (0.73%), and Koroneiki had a 0.65% similarity coefficient in subcluster B1. Cultivar Picholine and Frantoio had cluster A’s lowest similarity coefficient (0.57%). The second subcluster B2 included two distinct cultivars, Arbequina and Barnea, with a similarity coefficient of 0.68%, and Koroneiki with a similarity coefficient of 0.65. This study demonstrated the ability of RAPD indicators to differentiate between seven olive cultivars on the Barore farm in Rajasthan olive cultivars under investigation, India, and identify the genetic diversity and degree of proximity between them. The correlation coefficient (r) was calculated using Mantel Z-statistics49 and estimated at 0.73. This number was regarded as a good fit for the data by the UPGMA cluster pattern. The observed morphological variations among the cultivars could be attributed to genetic differences, environmental influences, or a combination. For instance, plant height and leaf shape differences might be related to genetic variations in growth habit and leaf morphology. Similarly, the fruit size and shape variations could be influenced by environmental factors such as climate, soil, and water availability.
Using RAPD markers13 used the primers, OPK16, OPA19, OPF06, OPX09, and OPZ11, to discriminated 103 olive Spanish cultivars. Later, they found that a combination of the primers, OPK-16, OPA-19, and OPX-09, was most favourable and consistent in distinguishing most cultivars. Inter simple sequence repeats (ISSRs) and start codon targeted (SCoT) markers have recently been used to evaluate the genetic polymorphism among the olive cultivars Arbequina, Rosana, Aski, Coratina, Frantoio, Koroneiki, Manzanillo, and Picual from Saudi Arabia50. Earlier investigations51 from Portugal compared 17 ISSR and 20 RAPD markers to identify genetic variation and distances across wild olives and reported 73 to 100% polymorphism. RAPD, ISSR, and SSR molecular markers depicted a 95% polymorphism among the 101 olive Greek cultivars16. RAPD and ISSR marker was used to evaluate the relationship between polymorphic markers and olive oil properties, of nineteen Tunisian olive cultivars and noted 80% polymorphic bands using RAPD markers52. According to the study made by Abood and his colleagues53, 95.9% polmorphism was reported among the olive cultivars, where a cluster study based on RAPD revealed that Arbequina, Picual, and Arbosana were aggregating and forming one cluster based on their geographical origin. Still, the wild accession and Koroneiki cultivar failed to create a cluster and were individually separated. Spanish olive cultivars from the Extremadura region were documented and identified using three RAPD primers (OPF-6, OPA-8, and OPK-16) and morphological traits54. The findings showed a relationship between cultivars in terms of geographical origin. The results of our RAPD analysis motivated us to analyze the diversity considering a combination of markers related to its quantitative assessment of different primary and secondary metabolites and their related antioxidant and antimicrobial characteristics.
Biochemical markers as a toolkit for deciphering the genetic diversity among the different cultivars of Olive
Qualitative assessment of the primary and secondary metabolites in different cultivars of Olive
The qualitative analysis of the phenolic components present in the olive leaves from different cultivars was distinguished using FTIR spectroscopy (Fig. 6A-G). The present study noted the presence of a broad absorption band aligning with the functional group, primary amine (N-H), at 3310 cm− 1 in all seven olive cultivars. O-H stretching bands at 2929 cm− 1 and 2936 cm− 1, indicating the occurrence of the aromatic ring and alkyl group. In Picholine and Coratina cultivars, the 2929 and 2936 cm− 1 bands were absent. Peaks at 681, 519, 525, 539, and 573 cm− 1 may indicate C-I or C-Br stretching, suggesting the presence of halogenated compounds. Our FTIR results align with previous studies, associated with phenolic compounds in various reports for different olive cultivars55,56,57,58,59,60, specifically for the cultivars , Arbequina, Arbosana (Chile), Kalamata, Koroneiki, Picual (Spain), Koroneiki (Greece), Picholine (Morocco), at 3310 cm− 1, 1612.02 cm− 1, 1386.23 cm− 1 and 1230 cm− 1, respectively.
Qualitative and quantitative analysis of secondary metabolites in leaf extract of different Olive cultivars [A–G], FTIR analysis of [A] Arbequina [B] Barnea [C] Coratina, [D] Frantoio [E] Koroneiki [F] Picholine [G] Picual; [H] Total phenolics content present in the leaves of different olive cultivars extracted using different solvents.
The qualitative analysis of the flavonoid component present in the olive leaves from different cultivars was distinguished using FTIR spectroscopy (Figs. 6A-G). In the current research, the absorption peak for the flavonoid (C = C) functional group at 1636 cm− 1 was present in all the cultivars except in Coratina. C = C stretching, bands at 1636 cm− 1, indicates the presence of amide and alkene group occurrence. According to a report from Chile, the spectrum areas between 1600 –1500 cm− 1 indicate the detection of flavonoid compounds in Arbequina and Arbosana Olive cultivars58. Previous research61 reported that the peak between 1400 and 1650 cm− 1 is attributed to the stretching vibration of C = C groups from aromatic compounds in olive Picual and Koroneiki olive leaves harvested from Spain and Greece61 and in leaves of the Neb jmel olive from Tunisia62.
Using HPTLC, the derivatized samples showed the presence of the flavonoids as light greenish-blue luminous zones under UV 366 nm 30. To find the spectral peak, samples of olive leaf extract from all seven kinds were scanned at 254 and 366 nm wavelengths. The spectral peak indicating the presence of flavonoids in olive methanolic extracts was detected at 254 nm (Fig. 7A) and 366 nm. The Rf value of quercetin was determined at a value of 0.30. Coratina, Frantoio, Picholine, and Koroneiki show a Quercetin peak at 0.03 Rf value, and Arbequina, Barnea, and Picual show a quercetin peak at 0.02 Rf at 254 nm. While Coratina shows Quercetin peak at 0.02 and 0.03 Rf, Frantoio at 0.18 Rf, Picholine 0.02 and 0.18 Rf, Picual 0.18 Rf, Arbequina and Koroneiki 0.03 Rf, and Barnea shows zero peak at 366 nm. The present investigation exhibits more peaks and area in Picholine and Coratina cultivars detected at 366 nm as compared to 254 nm (Fig. 7B-D). Alvarez and Gutierrez reported58 that the phenolic substances in olives are linked to high antioxidant and anti-inflammatory capabilities. The presence of the phenolic compound and flavonoid can be directly correlated with the antioxidant property of the different cultivars of the olives63,64,65,66,67.
Flavonoid analysis of leaf extract of Olive cultivars using qualitative and quantitative analysis [A] HPTLC fingerprinting at [a-b] 254nm, [c and d], 366 nm. [B] HPTLC densitogram of [a, aʹ] Quercetin, [b, bʹ] (Coratina), [c, cʹ] Frantoio, [d, dʹ] Barnea, [e, eʹ] Picholine, [f, fʹ] Picual, [g, g’] Arbequina, [h, hʹ] Koroneiki, at 254 nm at 366 nm; [C, D] Densitometry analysis compared to quercetin at [C] 254 nm and [D] 366 nm [E]; Quantitative analysis using AlCl₃ method.
The qualitative analysis of the primary metabolites related to the nutritional content in the olive leaves from different cultivars was distinguished using FTIR spectroscopy (Fig. 6A-G). The prominent peak between 1030 and 1070 cm− 1 was attributed to polysaccharides, aromatic ethers, and carbohydrates68,69. In a current report, the broad absorption band for the protein with the functional group (C = C) at 1636 cm− 1 was present in all the cultivars except Coratina. C-O stretching, bands at 1055 and 1061 cm− 1 were denoted for the presence ofcarbohydrate. Leaves have a small amount of fats, so fatty acid peaks are absent in all seven cultivars of olive.
Quantitative assessment of the primary and secondary metabolites in different cultivars of Olive
The significant components of food, such as moisture, ash, crude fiber, total carbohydrate, protein, fat, and calories, are known as proximate analysis, as shown in Figure S2. In the present study, a proximate analysis of the leaves of olive cultivars showed ash content varying between 0.87 and 10.93%, and moisture content between 0.86 and 6.99%, respectively. Coratina cultivars have a higher moisture and ash content. Koroneiki is 6.99% and 15.49%, respectively, and Koroneiki cultivars have the least moisture and ash content, 0.86%, respectively. Among the primary metabolites, carbohydrate content in all cultivars varied between 76.19% and 97.85%, and total energy ranged from 300 to 400 kcal. Therefore, it can be said that the concentration of different nutritional parameters related to proximate analysis of the olive leaves differs among cultivars. Eventually, in 100 g of olive leaf extract, 0.86 g to 6.99 g of moisture, 0.15 g to 0.84 g of fat, and 0.17 g to 2.35 g of protein was noted. On analysis of one-way ANOVA, there was a significant difference (p = 0.0002) among the cultivars considering these characteristics. Carbohydrate content was found to range from 88 to 91%, although crude fiber was absent. According to a study from Portugal, the nutritional assessment of the leaves of olive cultivars, Madural, Verdeal, and Cobrançosa, was noted in the following trend: total carbohydrate > moisture > total protein > ash > total fat70. The nutritional content of Koroneiki olive leaves from Brazil showed protein ranging from 10.5 to 13.1 g/100 whereas the total carbohydrate ranged from 8.74 to 32.63%71. The nutritional composition of Egyptian olive leaves also reported that the protein ranged from 10.6 to 10.7 g/100 and total carbohydrate content was 74.5 to 74.7%, respectively72. This type of result was also observed in a study from Portugal37,73 where the protein was 8.02 g/100 dry leaves, and carbohydrate content was 37.65 g/100 dry leaves.
In the present study , the total amount of total phenolic content in olive leaves [extracted using different solvents, aqueous, ethanolic, methanolic, hexane, and chloroform] ranged from 31.6 to 93.23 GAE mg/g, respectively (Fig. 6H). The total phenolic content in different cultivars of olive leaves extracted using methanol was highest and ranged from 44.27 GAE mg/g to 90.05 GAE mg/g, respectively. The results were found to be significant (p-value 0.0036) according to one-way ANOVA analysis. The significant difference in total phenolic content among the cultivars suggests that some olive leaf cultivars may have higher antioxidant potential than others. The chloroform leaf extract of the Picual cultivar had the least total phenolic content in comparison to other cultivars. The methanolic extract of the leaves of all the cultivars showed high phenolic content (49 to 95 mg GAE/g DE) as evaluated using leaf extracts from different cultivars. In a previous study74, the high phenolic content of the methanolic olive leaf extract (131.7 ± 9.4 mg GAE/g dw) was noted from olive leaves growing in Brazil. A recent study75 reported that the ethanolic leaf extract of Tunisian, Sigoise, and Rougette olive cultivars had the highest phenolic concentration of 161.54 ± 0.99 and 160.53 ± 1.17 mg GAE/g DE, respectively. In contrast, the hexanolic leaf extract from olive cultivars75, Octoubri, Verdal, and Sofiana, showed low phenolic values (17.12 ± 0.77 mg GAE/g DE, 18.48 ± 0.50 mg GAE/g DE, and 18.53 ± 1.41 mg GAE/g DE. The effect of using different polar and non-polar solvents was also reported65,76 on Kalamon, Koroneiki and Megaritiki olive cultivars from Greece in different solvents.
Earlier study77 found that the leaves of 17 cultivars of olive viz. Manzanilla, Conservolea, Arbequina, Mishen, Coratina, Roghani, Kalamon, Amphissis, Yellow, Amigdalifolia, Mary, Leccino, Shenge, Gordal, Sevillenca, Fishomi, and Beleidi, growing in the farms of Iran showed that the methanolic extract of cultivars, Kalamon, Gordal, and Coratina had the highest concentration of total phenolic compounds ranging from 155.91 ± 0.06 mg to 190.65 ± 0.03 GAE/g extract, respectively. It was found that the methanolic extract of the Koroneiki cultivar of olive showed the highest total phenolic content, and the Kalamon cultivar showed the least phenolic content. On examination of the total phenolics in the methanolic extract of the Moroccan Meknès region78, cultivars viz. Arbequina, Hojiblanca, Frantoio, Koroneiki, Lechín, Luque, Manzanilla, Picholine de Languedoc, Picholine Marocaine, Picual, and Verdal, it was found that the olive cultivar Picholine showed the highest total phenolic content (44 g/kg dry weight). Picual showed the lowest amount at 11 g/kg dry weight. In the present study, the methanolic extract showing high total phenolic content can be considered a potential biochemical marker for the analysis of the genetic diversity.
According to the findings, the flavonoid content in different cultivars of olive leaves extracted using methanol ranged from 10.58 to 20.16 mg QE/g, respectively, as shown in Fig. 7E. The least flavonoid content was 10.58 mg QE/g in the Frantoio cultivar, and the highest flavonoid content was observed at 20.16 mg QE/g in the Picholine cultivar. The highest total flavonoid concentration of the olive cultivar growing in Spain37 was found to be 20.45 mg QE/g dried extract. In a recent report from Egypt33, Picual showed the highest total flavonoid content of 59.00 ± 1.00 µg CE/g, and Shamlali had the lowest 42.00 ± 0.60 µg CE/g of total flavonoids, respectively. According to research done on five olive leaf cultivars (Zarrazi, Limouni, Chemlali Tataouine, Dhokkar, and Toffehi) from Tunisia, the Tofffehi methanolic extracts had the highest flavonoid content of 42.754 ± 0.674 mg RE/g of dry extract79. The flavonoid concentration of Brazilian olive leaves was 19.4 ± 1.3 mg QE/g DW75. The flavonoid concentration of a methanolic extract of the Chemlali variety from Tunisia70, at two distinct harvesting stages, October and January, was between 98.4 and 377.06 mg CE/100 g DE.
Antioxidant analysis among different cultivated cultivars of Olive growing in Barore forms in Rajasthan
Their phenolic component content is the primary factor for olive leaves’ antioxidant potential. To find the activity of scavenging free radicals or other reactive species is measured by its antioxidant activity. Presuming that the antioxidant potential also differs among the different cultivars as phenolic content, this potential of the Olive was also considered a biochemical marker. Using varying ascorbic acid concentrations as standard as discussed in the earlier study34, the values of DPPH were estimated. The result displayed the ability of ethanol, methanol, aqueous, hexane, and chloroform leaf extracts of olive to scavenge this radical. In the current study, there was a high inhibition activity found in the ethanolic Arbequina leaf extracts at 94%. The lowest inhibition activity was represented by chloroform Picholine leaf extract and hexanoic Koroneiki at 89% in Fig. 8A. In all types of solvent extracts, the activity was not variable for all the cultivars, ranging from 89%to 94%. IC50 values of olive leaf extract were determined to be 0.38 to 0.41 mg/ml at different concentrations (0.2 to 0.6 mg/ml) respectively in Fig. 8B. The observed significant differences in the IC50 values (p = 0.46) among all the cultivars of olive in our present study can be related to the biochemical profiles and structural composition of the extracts. For instance, Barnea chloroform, and Frantoio ethanolic extracts exhibited the lowest IC50 values (IC50 = 0.38 mg mL− 1), can be related to less amount of the phenolic compounds.
The DPPH scavenging among all these cultivars were found to be significantly different (p = 0.015) on ANOVA analysis. Figure 8B depicts a graphical representation of the DPPH% inhibition in several solvents (aqueous, ethanol, methanol, chloroform, and hexane) ranging from 89 to 94%. The ethanolic Frantoio extract exhibited the least activity (IC50 = 0.38 mg mL− 1), whereas the aqueous Arbequina extract had the highest IC50 value 0.41 mg mL− 1. According to earlier studies35, the DPPH IC50 activity differed among the cultivars Picual, Tofahi, and Shemlali cultivars from Alexandria, respectively, whereas Picual shows the highest antioxidant activity. Previous report78 showed that the DPPH radical scavenging activity of olive leaf extract for different solvents ranged from 22.72 to 69.81%. In another similar study79, DPPH radical scavenging activity of olive leaf in different cultivars were Arbequina 43.7, Arbosana 36.9, Changlot Real 46.8, Frantoio 41.2, Koroneiki 36.7, Picual 33.03, and Sikitita 45.83 mg of TE g− 1 dry weight respectively.
DPPH IC50 activity of aqueous extract of Picual leaves was reported as 48.14 ± 0.15 µg/mL63. Among the different solvents (ethanol, methanol, acetone, and water) in which olive leaf extract was extracted, the antioxidant activity of methanolic extracts (80%) of the cultivar Kalamata showed the highest antioxidant activity (28.09 µg/mL)64. While the antioxidant activity value reported in the Kalamata cultivar is notable, it is essential to recognize that activity can vary significantly depending on factors like extraction methods, solvent composition, and plant cultivar80. Further studies are needed to fully understand the range of antioxidant activity in olive leaf extracts. The methanolic extract of the following cultivars, Arbequina, Hojiblanca, Frantoio, Koroneiki, Lechín, Luque, Manzanilla, Picholine de Languedoc, Picholine Marocaine, Picual, and Verdal, reported that the antioxidant activity (DPPH IC50) ranged from 33 ± 04 TE g− 1 d.w. to 46.8 ± 0.2 mg of TE g − 1 d.w81.
Antimicrobial activity of the different cultivars of Olive leaf extracts
The antimicrobial activity of olive leaf extract against specific nonpathogenic bacteria (B. subtilis, P. aeruginosa, S. aureus, and E. coli) was obtained by the well diffusion method illustrated in Fig. 9A-D. According to the present study, it was found that the olive leaf extracts from different cultivars effectively inhibited the different test organisms. Results indicated that B. subtilis was the microorganism that was most responsive to Olive leaf extract, whereas E. coli, P. aeruginosa, and S. aureus were the least susceptible. It was also statistically verified that all the cultivars showcased significant differences (p = 0.002) of inhibition of microbial growth. Similar results were also observed by previous researchers,82,83,84,85,86. The macerated extracts of olive leaves showed potent antibacterial action against Escherichia coli, Staphylococcus aureus, Bacillus subtilis, and Listeria monocytogenes. In an interesting study82, the antimicrobial activities of olive leaf aqueous extracts against S. aureus and foodborne pathogens were also evident. According to another recent study83, the olive leaf ethanolic leaf extract demonstrated strong antibacterial activity against five pathogenic bacteria (Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Bacillus subtilis, and Listeria monocytogenes). It was reported36 that olive leaf aqueous extract can inhibit B. subtilis, P. aeruginosa, S. aureus, and E. coli.
According to our results there was an impact on the metabolic content based on the solvent used, thereby this had an profound role in contribution of the functional role in imparting their antioxidant and antimicrobial activity. The observed significant differences in the IC50 values (p = 0.46) among all the cultivars of olive in our present study can be related to the biochemical profiles and structural composition of the extracts. For instance, chloroform extract of Barnea, and ethanolic extract of Frantoio exhibited low IC50 value of 0.38 mg mL− 1. It can be related to less amount of the phenolic compounds. The methanolic extract of Frantoio showed 20.16 QE/g flavonoid content and 44.27 GAE.mg/g phenolic content, while the same methanolic extract of anther olive cultivar, Barnea, showed 20.09 mg QE/g. Flavonoid content and 76.01 GAE.mg/g phenolic content. The same methanolic extract of Frantoio and Barnea showcased an IC50 activity of 0.4 mg mL− 1 and hence conclude to have high antioxidant activity. The methanolic extract of Barnea and Frantoio exhibited good antimicrobial activity in comparison to olive leaf methanolic extracts of other cultivars, Picholine, Picual and Koroneiki. Hence, both cultivars exhibited profound biological functions (antioxidant and antimicrobial activity) due to the presence of the hydrophilic compounds like flavonoids and phenols extracted in polar solvents. When the extract was prepared in hexanolic solvent (non-polar), the antioxidant and antimicrobial activity was minimal, as both phenols and flavonoids were absent in these non-polar extracts.
Cumulative data analysis of morphological, biochemical, nutritional, antimicrobial, and molecular data using the UPGMA method
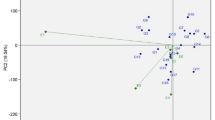
Using all the above data from the morphological, biochemical, nutritional, and antimicrobial studies, the current study predicted genetic variability among olive cultivars (genotypes) growing in the Barore farm in Rajasthan, India. The earlier morphological characterization of polymorphisms was the foundation for adaptability to various climatic situations. Morphological and biochemical assessment using several features indicated high variation in leaf, fruit, and stem properties, indicating that olive cultivars have the potential to be used in plant breeding and improvement. All these cultivars contain a reasonably high quantity of glucose, potassium, magnesium, and calcium, and hence might be used as a nutritional source. Based on morphological, biochemical, nutritional, and antimicrobial variables, the seven genotypes showed pairwise similarity ranging from 0.03 to 0.12 with an average of 0.9 (Fig. 9). The seven olive genotypes show a comparatively low degree of genetic similarity, with an average of 0.9 and pairwise similarity indices ranging from 0.03 to 0.12. This implies that the genotypes are genetically varied, which aligns with our conclusions drawn from the antimicrobial, nutritional, biochemical, and morphological traits.
All seven cultivars were differentiated and clustered using a dendrogram based on the cumulative (morphological, biochemical, nutritional, and antimicrobial) data into two major clusters, A and B, and the A cluster was divided into three subclusters. In contrast, the B cluster had only 1 subcluster. The first sub cluster included two genotypes: Arbequina, and Picholine (0.8%) similarity index. In the present study, Arbequina showed high phenolic content, and Picholine showed high flavonoid content. This can be related to their high antioxidant activity and ability to inhibit microorganisms. Moreover, as per the previous reports, Arbequina and Picholine are self-incompatible (cannot pollinate themselves) and also cross-compatible (can pollinate each other, and they were grouped in the G1 group based on single-nucleotide polymorphisms85). A genetic system probably prevents self-fertilization. This confirms the genetic relationship between Arbequina and Picholine, as evidenced by the present study.
The second sub-cluster included two germplasms, Barnea and Picual, showing a 0.8% similarity index. The flavonoid content in both cultivars was almost the same, thus confirming a similar high DPPH activity. Using RAPD and ISSR markers85. It was reported that Barnea and Picual are closely related based on the morphological traits of the fruits. The final third sub-cluster has two cultivars, Frantoio and Koroneiki. The highest similarity (0.12%) was seen in two genotypes of this cluster, Frantoio and Koroneiki. In the present study, the antioxidant activity of both cultivars was similar due to their high flavonoid and phenolic content. Similar evidence also found similarity among the olive cultivars Frantoio and Koroneiki in the context of their high phenolic content in the olive oil86,87.
Arbequina, Picholine, and Barnea, Picual had the similarity index of 8%. In comparison, Frontoio and Koroneiki had the highest (0.12%) and B cluster has one subcluster with one genotype Coratina, has the lowest similarity (0.04%). In Fig. 10, the 3-D PCA plot and the 2-D dendrogram based on molecular similarity between various genotypes showed that Coratina is the most diverse species since it has not formed any group with other genotypes. The genotypes are exotic arrivals from Israel that might have experienced genetic drift, mutation, or selection during their adaption to the Indian environment, may be the cause of the low similarity indices. The prospective use of these similarity indicators in olive breeding programs gives them biological significance88. Additionally, the low similarity indices imply that the genotypes might have unique adaptive strategies, which could help us comprehend how they react to various environmental circumstances. The variability of some olive cultivars using microsatellite markers and morphological trait was studied and found that Coratina and Frantoio were both monoclonal and similar 88,89. But in the present context the fruit morphology differed, and all the biochemical parameters were also different. Hence on RAPD analysis, this cultivar (genotype) was found to be the most diverse among the others. The average similarity across all germplasm was 0.07, showing a low level of genetic similarity between genotypes.
According to a recent study, several marker attributes, including polymorphism percentage, polymorphism information content, effective multiple ratio, marker index, and resolving power for RAPD markers, were utilized to evaluate the informativeness of the primer sets. The mean polymorphic information content (PIC) of 0.20 was noted10. In the present study on the olive cultivars growing on Barore farm, the RAPD study showed that primer OPB-2 had the highest PIC value (0.40), whereas primer OPB-01 had the lowest (0.24) value. EMR readings varied from 0.48 to 2.87, with a mean of 2.0. The average PIC value for RAPD, a measure of allelic diversity, was 0.34. The resolving power (Rp) of the evaluated RAPD markers ranged from 1.42 to 3.42, with an average value of 2.73 (Table 3). The highest resolving power (3.42) was found in RAPD for OPA-1 and OPA-3. Marker Index varied from 0.11 to 1.16, with an average of 0.72, and the correlation coefficient (r) was estimated as 0.67. The r value of 0.67 was considered a good fit of the UPGMA cluster pattern to the data.
In the present study, a comprehensive analysis of the genetic diversity and the relationship of RAPD molecular markers considering the morphological, biochemical, antioxidant, and antimicrobial properties in seven Indian acclimatized olive cultivars showed distinct differences between them due to diverse chemical characteristics, in addition to geographical provenance. Since RAPD is associated with a few limitations, like dominant nature, less reproducibility, etc., for further breeding programs, the SSR and SNP markers based on the advancement of genomic facilities need to be developed and validated for olive cultivars growing in India.
Conclusion
Finally, the findings show that olive cultivars show genetic diversity among seven olive cultivars from Barore farm. This study demonstrated the effectiveness of the RAPD technique in differentiating between the analyzed olive types as well as identifying the genetic distance and degree of closeness between them. Using RAPD primers, OPA1, OPA2, OPA3, OPA4, OPB1, OPB2, OPB3, and OPB4 produced distinct, reproducible bands that could be evaluated. The OPB1 gene produced the 7 amplicons and the OPA1 gene produced the fewest 2 amplicons. Olive cultivars (leaves) showed variations among nutritive value and were rich in phenolics and flavonoids showcasing high antioxidant, and antimicrobial activity. Arbequina leaf extract showed the greatest phenolic content whereas the highest flavonoid content was observed in the Picholine cultivar. Arbequina showed the highest radical scavenging activity and effectively inhibited microorganisms. A high degree of genetic variation was observed, and four distinct groups were noted. Both Picholine and Arbequina cultivar can be used for both table olives and olive oil production. They showed certain similarities that helped to group both of them in one cluster, yet they showed differences among themselves. On the basis of the morphology, Arbequina and Barnea were placed in the same cluster. Arbequina is often not a compatible pollinator for Barnea, meaning they do not effectively cross-pollinate each other; in some cases, Barnea is even considered partially self-incompatible when pollinated with Arbequina pollen. Barnea and Picual also showed similarity in secondary metabolite content, yet it is noted that Picual is primarily used for olive oil production due to its high oil content, whereas Barnea can also be used for table olives. Frantoio is one of the hardy cultivars and can resist very low temperatures. Although both Frantoio and Koroneiki show similarities in metabolite content and antioxidant activities, and they were clustered as one group. Yet in context to morphological characteristics, the fruits of Frantoio are bigger than those of Koroneiki. It was evident that the claim for phylogenetic relationship was pronounced by studying the morphological, biochemical, and antimicrobial properties, correlating them to molecular differences among the olive cultivars. This study used marker systems to better understand the performance of the olive cultivars, despite their geographically disparate origins. This information can be helpful for olive breeding programs and cultivation in India.
Data availability
Data is provided within the manuscript or supplementary information files.
Abbreviations
- pH:
-
Potential of hydrogen
- TDS:
-
Total dissolved solids
- RH:
-
Relative Humidity
- BD:
-
Bulk Density
- EVOO:
-
Extra virgin olive oil
- FAME:
-
Fatty acid methyl ester
- kg:
-
Kilogram
- g/cm3 :
-
Gram per centimeter cube
- h:
-
Hour
References
Anwar, M. A. et al. A compilation of applications for enhancing agricultural traits, disease resistance, biotic interaction, byproducts valorization, and quality control purposes of Olive. Trends Food Sci. Technol. 143, 104311 (2024). Metabolomics.
Peralbo-Molina, Á. & de Luque, M. D. Potential of residues from the mediterranean agriculture and agrifood industry. Trends Food Sci. Technol. 32, 16–24 (2013).
Torres, M. et al. Olive cultivation in the Southern hemisphere: flowering, water requirements and oil quality responses to new crop environments. Front Plant. Sci 8, (2017).
Torrecillas, C. & Martínez, C. Patterns of specialization by country and sector in Olive applications. Technol. Soc. 70, 102003 (2022).
Yadav, S., Carvalho, J., Trujillo, I. & Prado, M. Microsatellite markers in olives (Olea europaea l.): utility in the cataloging of germplasm, food authenticity and traceability studies. Foods 10, 1907 (2021).
Coskun, O. F. & Gulsen, O. Determination of markers associated with important agronomic traits of watermelon (Citrullus lanatus L). J. Agric. Sci. Technol. 26, 1359–1371 (2024).
Coşkun, Ö. F., Toprak, S. & Mavi, K. Some seed properties and molecular analysis with inter-primary binding site (iPBS) retrotranposons markers of edible-seeded watermelon genotypes. Genet. Resour. Crop Evol. 71, 3151–3162 (2024).
Zhu, C. et al. Construction of a high-density genetic linkage map and QTL mapping for growth traits in Pseudobagrus ussuriensis. Aquaculture 511, 734213 (2019).
Bandelj, D., Jak¿e, J. & Javornik, B. Characterisation of Olive (Olea europaea l.) cultivars by RAPD markers. Acta Hortic. 133–136. https://doi.org/10.17660/ActaHortic.2002.586.20 (2002).
Abdelhamid, S. et al. Application of RAPD and ISSR markers tools for commercial monovarietal Tunisian extra Virgin Olive oils (EVOO) authenticity and traceability. EuroMediterr J. Environ. Integr. https://doi.org/10.1007/s41207-024-00614-z (2024).
Besnard, G., Baradat, P., Chevalier, D., Tagmount, A. & Bervillé, A. Genetic differentiation in the Olive complex (Olea europaea) revealed by RAPDs and RFLPs in the rRNA genes. Genet. Resour. Crop Evol. 48, 165–182 (2001).
Wiesman, Z. Molecular characterization of common Olive varieties in Israel and the West bank using randomly amplified polymorphic DNA (RAPD) markers. Sci. Hortic. 123, 837–841 (1998).
Belaj, A. Optimal use of RAPD markers for identifying varieties in Olive (Olea europaea L.) germplasm collections. Hortic. Sci. 192, 266–270 (2004).
Gemas, V. J. V., Almadanim, M. C., Tenreiro, R., Martins, A. & Fevereiro, P. Genetic diversity in the Olive tree (Olea europaea L. Subsp. europaea) cultivated in Portugal revealed by RAPD and ISSR markers. Genet. Resour. Crop Evol. 51, 501–511 (2004).
Sesli, M. & Yegenoglu, E. D. Genetic relationships in wild olives (Olea europaea Ssp. oleaster) by ISSR and RAPD markers. Biotechnol. Biotechnol. Equip. 31, 897–904 (2017).
Linos, A., Nikoloudakis, N., Katsiotis, A. & Hagidimitriou, M. Genetic structure of the Greek Olive germplasm revealed by RAPD, ISSR and SSR markers. Sci. Hortic. 175, 33–43 (2014).
Muzzalupo, I. & Perri, E. Genetic diversity in Olive tree cultivars from Italy and other countries of the mediterranean basin as revealed by RAPD and SSR molecular marker. Adv. Hortic. Sci. 23, 1000–1013 (2009).
Dehghan-Seresht, M., Khadivi, A. & Tunç, Y. Morphological and Pomological characterizations of Olive (Olea europaea L.) cultivars. Genet Resour. Crop Evol Pg. 1–17. https://doi.org/10.1007/s10722-024-02303-w (2024).
Blazakis, K. N. et al. Discrimination of 14 Olive cultivars using morphological analysis and machine learning algorithms. Front Plant. Sci 15, (2024).
Al-Saif, A. M. et al. Morphological, physiological, and biochemical responses of some Olive tree cultivars to low temperature stress. Bioresources 19, 9582–9605 (2024).
Tunç, Y. et al. Characterization of Olive (Olea Europaea L.) cultivars; colour properties, biochemical contents, antioxidant activity and nutrient contents. Genet. Resour. Crop Evol. https://doi.org/10.1007/s10722-024-01991-8 (2024).
Rico, E. I., de la Fuente, G. C. M., Morillas, A. O. & Ocaña, A. M. F. Physiological and biochemical study of the drought tolerance of 14 main Olive cultivars in the mediterranean basin. Photosynth Res. 159, 1–16 (2024).
Sousa, M. M. et al. Effect of different time/temperature binomials on the chemical features, antioxidant activity, and natural microbial load of Olive pomace paste. Molecules 28, 2876 (2023).
Abozid, M. & Eldmardash, M. Olive and artichoke leaves attenuate hyperglycemia and protect liver in alloxan-induced diabetic rats. Menoufia J. Agricultural Biotechnol. 10 (2), 31–41 (2025).
El-Hadary, A. A. R., Abulmakarm, A. M., Sulieman, A. M., El-Shorbagy, G. A. & Mohamed, A. S. Impact of the unconventional extraction on antioxidants and antimicrobial characteristics of hibiscus and Olive leaves extracts. Heliyon, 11(2) (2025).
Alowaiesh, B. F., Alhaithloul, H. A. S., Saad, A. M. & Hassanin A. A. green biogenic of silver nanoparticles using polyphenolic extract of Olive leaf wastes with focus on their anticancer and antimicrobial activities. Plants 12, 1410 (2023).
Majumder, D. et al. Metabolic profiling and investigations on crude extract of Olea europaea L. leaves as a potential therapeutic agent against skin cancer. J. Funct. Foods. 58, 266–274 (2019).
Sisodiya, S. & Debnath, M. Comparison of the phytochemical constituents and antimicrobial activity of zinc nanoparticle of two varieties of Olive. NanoWorld J 08, (2022).
Singleton, V. L. & Rossi, J. A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158 (1965).
Agatonovic-Kustrin, S., Gegechkori, V., Petrovich, D. S., Ilinichna, K. T. & Morton, D. W. HPTLC and FTIR fingerprinting of Olive leaves extracts and ATR-FTIR characterisation of major flavonoids and polyphenolics. Molecules 26, 6892 (2021).
Libin, K. V. et al. Bioefficacy, chromatographic profiling and drug-likeness analysis of flavonoids and terpenoids as potential inhibitors of H1N1 influenza viral proteins. Int. J. Biol. Macromol. 281, 136125 (2024).
Khlif, I. et al. Characteristics, phytochemical analysis and biological activities of extracts from Tunisian Chetoui Olea europaea variety. J Chem, 418731 (2015). (2015)(1).
Palmeri, R. et al. Olive leaves, a promising byproduct of Olive oil industry: assessment of metabolic profiles and antioxidant capacity as a function of cultivar and seasonal change. Agronomy 12 (9), 2007 (2022).
Sisodiya, S., Gautam, S., Aneja, D. & Debnath, M. Exploring the usage of Olive leaf and green synthesized Olive leaf metallic nanoparticles as soap supplement for the formulation and optimization of innovative germicidal herbal soap. Ind. Crops Prod. 219, 119012 (2024).
Baliyan, S. et al. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 27 (4), 1326 (2022).
Borjan, D., Leitgeb, M., Knez, Ž. & Hrnčič, M. K. Microbiological and antioxidant activity of phenolic compounds in Olive leaf extract. Molecules 25, 5946 (2020).
Safarzadeh Markhali, F., Teixeira, J. A. & Rocha, C. M. R. Effect of ohmic heating on the extraction yield, polyphenol content and antioxidant activity of Olive mill leaves. Clean. Technol. 4, 512–528 (2022).
Ganogpichayagrai, A. & Suksaard, C. Proximate composition, vitamin and mineral composition, antioxidant capacity, and anticancer activity of Acanthopanax trifoliatus. J. Adv. Pharm. Technol. Res. 11, 179 (2020).
Aboul-Maaty, N. A. F. & Oraby, H. A. S. Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method. Bull. Natl. Res. Cent. 43, 25 (2019).
Rohlf, F. J. NTSYS-Pc. Numerical Taxonomy and Multivariate Analysis System. (2004).
Kaur, G., Joshi, A., Jain, D., Choudhary, R. & Vyas, D. Diversity analysis of green gram (Vigna radiata (L.) Wilczek) through morphological and molecular markers. Turk. J. Agric. For. 40, 229–240 (2016).
Bashir, M. A., Bertamini, M., Gottardini, E., Grando, M. S. & Faralli, M. Olive reproductive biology: implications for yield, compatibility conundrum, and environmental constraints. J. Exp. Bot. 75, 4300–4313 (2024).
Koubouris, G. C., Breton, C. M., Metzidakis, I. T. & Vasilakakis, M. D. Self-incompatibility and pollination relationships for four Greek Olive cultivars. Sci. Hortic. 176, 91–96 (2014).
Maiuf, M. H. & Al-Mayahi, F. H. Morphological study of Olive cultivars olea europaea L. cultivated in central and Southern of Iraq. IOP Conf. Ser. Earth Environ. Sci. 1262, 042064 (2023).
Nikpeyma, Y. Determination of morphological, Pomological, and biochemical characteristics of important Turkish Olive varieties grown in the southeastern Anatolian region (Turkey) conditions. Erwerbs-Obstbau 5, 1755–1768 (2023).
Khadivi, A., Mirheidari, F., Moradi, Y. & Paryan, S. Identification of the promising Olive (Olea europaea L.) cultivars based on morphological and Pomological characters. Food Sci. Nutr. 10, 1299–1311 (2022).
Markhali, F. S. & Teixeira, J. A. Extractability of Oleuropein, Hydroxytyrosol, Tyrosol, Verbascoside and flavonoid-derivatives from Olive leaves using ohmic heating (a green process for value addition). Sustain. Food Technol. 2, 461–469 (2024).
Bianco, R., Lo, Panno, G. & Avellone, G. Characterization of Sicilian Olive genotypes by multivariate analysis of leaf and fruit chemical and morphological properties. J Agric. Science 5, (2013).
Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 27, 209–220 (1967).
Alhaithloul, H. A. S. et al. Genetic diversity, chemical constituents and anatomical analysis of eight popular Olive (Olea europaea L.) cultivars in Al-Jouf region, Saudi Arabia. Sci. Rep. 14, 14688 (2024).
Martins-Lopes, P. et al. RAPD and ISSR molecular markers in Olea europaea L.: genetic variability and molecular cultivar identification. Genet. Resour. Crop Evol. 54, 117–128 (2007).
Omri, A. et al. Genetic diversity and association of molecular markers with biochemical traits in Tunisian Olive cultivars. Genet. Resour. Crop Evol. 68, 1181–1197 (2021).
Abood, A. A. et al. Molecular and phytochemical analysis of wild type and Olive cultivars grown under Saudi Arabian environment. 3 Biotech. 7, 289 (2017).
D’Imperio, M. et al. Integration between molecular and morphological markers for the exploitation of Olive germoplasm (Olea europaea). Sci. Hortic. 130, 229–240 (2011).
Parra-Lobato, M. C., Delgado-Martinez, F. J. & Gomez-Jimenez, M. C. Morphological traits and RAPD markers for characterization and identification of minor Spanish Olive cultivars from the Extremadura region. Genet. Mol. Res. 11, 2401–2411 (2012).
Alhajri, H. M. et al. Olive leaf extracts for a green synthesis of silver-functionalized multi-walled carbon nanotubes. J. Funct. Biomater. 13, 224 (2022).
Tu, J. L. & Yuan, J. J. Thermal decomposition behavior of Hydroxytyrosol (ht) in nitrogen atmosphere based on TG-FTIR methods. Molecules 23, 404 (2018).
Alvarez, C. & Gutierrez, M. Olive residues as a source of bioactive compounds. in ECSOC 2024 68 (MDPI, Basel Switzerland, doi:https://doi.org/10.3390/ecsoc-28-20183. (2024).
Grabska-Zielińska, S., Gierszewska, M., Olewnik-Kruszkowska, E. & Bouaziz, M. Polylactide films with the addition of Olive leaf extract—physico-chemical characterization. Materials 14 (24), 7623 (2021).
Bouknana, D., Caid, S., Hammouti, H., Rmili, B., Hamdani, I. Diagnostic study of the Olive oil industry in the Eastern region of Morocco. Mater. Today Proc. 45, 7782–7788 (2021).
Magyari-Pavel, I. Z. et al. Antioxidant extracts from Greek and Spanish Olive leaves: antimicrobial, anticancer and antiangiogenic effects. Antioxidants 13, 774 (2024).
Brahmi, F., Mechri, B., Dabbou, S., Dhibi, M. & Hammami, M. The efficacy of phenolics compounds with different polarities as antioxidants from Olive leaves depending on seasonal variations. Ind. Crops Prod. 38, 146–152 (2012).
Zhang, C. et al. Comparative evaluation of the phytochemical profiles and antioxidant potentials of Olive leaves from 32 cultivars grown in China. Molecules 27 (4), 1292 (2022).
Salido, S., Pérez-Bonilla, M., Adams, R. P. & Altarejos, J. Phenolic components and antioxidant activity of wood extracts from 10 main Spanish Olive cultivars. J. Agric. Food Chem. 63 (29), 6493–6500 (2015).
Kiritsakis, K. et al. Composition and antioxidant activity of Olive leaf extracts from Greek Olive cultivars. J. Am. Oil Chem. Soc. 87, 369–376 (2010).
Salama, Z. A. et al. In vitro antioxidant, antimicrobial and anticancer activities of banana leaves (Musa acuminata) and Olive leaves (Olea europaea L.) as by-products. Res. J. Pharm. Technol. 13, 687 (2020).
Olmo-García, L. et al. Establishing the phenolic composition of Olea europaea L. leaves from cultivars grown in Morocco as a crucial step towards their subsequent exploitation. Molecules 23, 2524 (2018).
Amir, R. M. et al. Application of fourier transform infrared (FTIR) spectroscopy for the identification of wheat varieties. J. Food Sci. Technol. 50, 1018–1023 (2013).
Siregar, S., Nurhikmat, A., Amdani, R. Z., Hatmi, R. U., Kobarsih, M., Kusumaningrum,A., … Yuliyanto, P. Estimation of proximate composition in rice using ATR-FTIR spectroscopy and Chemometrics. ACS omega, 9(30), 32760–32768 (2024).
de Oliveira, N. M., Machado, J., Chéu, M. H., Lopes, L., Barroso, M. F., Silva, A.,… Grosso, C. Potential therapeutic properties of Olea europaea leaves from selected cultivars based on their mineral and organic profiles. Pharmaceuticals, 17(3), 274 (2024).
Cavalheiro, C. V. et al. Olive leaves offer more than phenolic compounds – Fatty acids and mineral composition of varieties from Southern Brazil. Ind. Crops Prod. 71, 122–127 (2015).
Ibrahim, E. H., Abdelgaleel, M. A., Salama, A. A. & Metwalli, S. M. Chemical and nutritional evaluation of Olive leaves and selection of the optimum conditions for extraction their phenolic compounds. J. Sustain. Agric. Sci. 42, 445–459 (2016).
Ferreira, D. M. et al. Updated organic composition and potential therapeutic properties of different varieties of Olive leaves from Olea europaea. Plants 12, 688 (2023).
Lins, P. G., Piccoli Pugine, M. & Scatolini, S. Melo, M. P. In vitro antioxidant activity of Olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 4, e00805 (2018).
Khelouf, I., Karoui, I. J., Lakoud, A., Hammami, M. & Abderrabba, M. Comparative chemical composition and antioxidant activity of Olive leaves Olea europaea L. of Tunisian and Algerian varieties. Heliyon 9, e22217 (2023).
Kiritsakis, K., Kiritsakis, A., Manousaki-Karacosta, E. & Genigeorgis, F. Table olives and olive oil: production, processing, composition, and nutritional qualities. in Handbook of Vegetables and Vegetable Processing 663–682Wiley, (2010). https://doi.org/10.1002/9780470958346.ch33
Ghasemi, S. et al. Investigation of phenolic compounds and antioxidant activity of leaves extracts from seventeen cultivars of Iranian Olive (Olea europaea L). J. Food Sci. Technol. 55, 4600–4607 (2018).
Bechar, S. et al. Extraction methods and geographical variability influence on phenolic content, fatty acid composition and physicochemical quality, of Moroccan Picholine Olive oils in the Fes-Meknes region. OCL 31, 20 (2024).
Sifaoui, I., López-Arencibia, A., Martín-Navarro, C. M., Chammem, N., Mejri, M., Lorenzo-Morales,J., … Piñero, J. E.Activity assessment of Tunisian olive leaf extracts against the trophozoite stage of Acanthamoeba. Parasitol res, 112, 2825–2829 (2013).
Sellami, M. K., Khemakhem, I., Mkadmini, K. & Bouzouita, N. Chemical composition, antioxidant potential and phenolic profile of oil mill waste water from Tunisian Olive varieties (Chetoui and Chemlali). Mediterr. J. Chem. 5 (6), 605–614 (2016).
El-Rahmana, S. N. A., Abubshaitb, S. A., Abubshaitc, H. A., Elsharifb, A. M. & Kamound, M. The anti-aging, anti-tuberculosis and antioxidant potential benefits of Saudi Arabia Olea-Europaea leaves extracts. Braz J. Biol 84, (2024).
Martín-García, B. et al. Comparative extraction of phenolic compounds from Olive leaves using a sonotrode and an ultrasonic bath and the evaluation of both antioxidant and antimicrobial activity. Antioxidants 11, 558 (2022).
Ghomari, O. et al. Phenolic profile (HPLC-UV) of Olive leaves according to extraction procedure and assessment of antibacterial activity. Biotechnol. Rep. 23, e00347 (2019).
Giacometti, J., Milovanović, S., Jurc\̌ić Momc\̌ilović, D. & Bubonja-S\̌onje, M. Evaluation of antioxidant activity of Olive leaf extract obtained by ultrasound‐assisted extraction and their antimicrobial activity against bacterial pathogens from food. Int. J. Food Sci. Technol. 56, 4843–4850 (2021).
de Oliveira, N. M. et al. Potential therapeutic properties of Olea europaea leaves from selected cultivars based on their mineral and organic profiles. Pharmaceuticals 17, 274 (2024).
Šimat, V. et al. Antioxidant and antimicrobial activity of hydroethanolic leaf extracts from six mediterranean Olive cultivars. Antioxidants 11, 1656 (2022).
Biton, I., Many, Y., Mazen, A. & Ben-Ari, G. Compatibility between Arbequina and Souri Olive cultivars may increase Souri fruit set. Agronomy 10, 910 (2020).
Contreras, R., Aguayo, F., Guerra, A., Tapia, F. & Porcile, V. Genetic characterization of centennial Olive trees from Northern Chile: the case of extra Virgin Olive oil from Huasco in the process of designation of origin. Chil. J. Agric. Animl Sci. 34, 126–139 (2018).
Muzzalupo, I., Chiappetta, A., Benincasa, C. & Perri, E. Intra-cultivar variability of three major Olive cultivars grown in different areas of central-southern Italy and studied using microsatellite markers. Sci. Hortic. 126 (3), 324–329 (2010).
Acknowledgements
Financial assistance to SS from Rajasthan Olive Cultivation limited (ROCL), Durgapura Campus for the research work is highly acknowledged. Sincere gratitude to Rajasthan Olive Cultivation Limited (ROCL), Barore farm, for providing olive leaves, fruits, olive oil from the olive farms for this work. Special thanks to all the managers of Barore farm, Rajasthan Olive cultivation, for providing agricultural data, soil, plant resources, and information that were relevant for completing this manuscript. The whole work was financed under the Memorandum of understanding (dated 11-06-2019) between ROCL, Manipal University Jaipur, Tripura University and Jiwaji University for development of innovative products from Olives.
Funding
Open access funding provided by Manipal University Jaipur.
Financial support to M.D. from Manipal Research Board (MRB seed grant, DoR MRB|2023 |SG-03) and to Department of Biosciences in the form of DST-FIST project (DST/2022/1012) from Govt. of India, are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
M.D. received the olive plant sample from ROCL, prepared herbaria and deposited to University of Rajasthan for identification. S.S. analyzed the morphological nature of the olive samples and their functional attributes. D.J. analyzed the samples for the molecular diversity studies; S.S. wrote the main manuscript, and M.D. revised the entire manuscript and supervised the whole work, S.S prepared the Figs. 1, 2, 3, 4, 5, 6, 7, 8 and 9 and S.S with the help of S.S.S. collected all resources like pictures of olive plant in different stages, the leaves of fresh olive and fruits for the study from the Barore olive farm. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Compliance with ethical standards
The authors affirm that they are free of any known potential conflicts of interest or close personal ties that might have looked to have affected the research presented in this study. The research did not involve human or animal as participants and every author has consented to participate in and publish this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sisodiya, S., Debnath, M., Jain, D. et al. An integrated approach using morphological, biochemical, and RAPD markers to assess the genetic diversity of Olive (Olea Europaea L.) cultivars in India. Sci Rep 15, 18664 (2025). https://doi.org/10.1038/s41598-025-02815-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02815-0
Keywords
This article is cited by
-
Multivariate analysis highlights genetic structure in Olive (Olea Europaea L.) germplasm using Resource-Efficient RAPD marker system
Molecular Biology Reports (2026)