Abstract
Diabetic retinopathy (DR) is associated with increased cardiovascular risk, but evidence of its relationship with heart failure (HF) in people with diabetes and chronic kidney disease (CKD) is limited. Therefore, we examined the association between DR severity and concurrent HF status, as well as the risk of subsequent HF hospitalization in this population. A total of 1,503 patients with both diabetes and CKD underwent fundus photography. DR severity was classified into three groups: Group 1 (no apparent DR, mild non-proliferative DR [NPDR], moderate NPDR), group 2 (severe NPDR, very severe NPDR), and group 3 (proliferative DR [PDR], and high-risk PDR). Associations between HF status and DR severity were analyzed using ordinal regression. Cox proportional hazard models examined the association between DR severity and subsequent HF hospitalization. HF was positively associated with worse DR grade (adjusted odds ratio 2.09, 95% confidence interval [CI] 1.37–3.18). In patients without HF at baseline, a higher incidence of acute HF hospitalization over 5 years was identified in group 2 (hazard ratio 1.37, 95% CI 0.83–2.26) and group 3 (hazard ratio 1.86, 95% CI 1.22–2.83) compared to patients with less severe DR. In summary, DR severity is independently associated with both concurrent HF and risks for HF hospitalization over 5 years among those with diabetes and CKD and could aid cardiovascular risk stratification.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a significant cause of chronic kidney disease (CKD) and end-stage kidney disease1. The complications of DM have a significant impact on individuals’ health, including microvascular and macrovascular disease. Microvascular complications of DM include neuropathy and retinopathy, while macrovascular complications involve cardiovascular diseases and cerebrovascular events2,3,4. The high morbidity and mortality associated with DM are partly due to the accelerated cardiovascular disease inherent in this population5. Heart failure (HF) is one of the most feared cardiovascular complications, and its incidence is significantly increased in patients with diabetes and CKD6. The relationship between CKD and HF involves bidirectional interactions, where factors like hemodynamic stress, neurohormonal activation, oxidative stress, and pro-inflammatory cytokines contribute to the progression of both conditions7,8. The complex interactions between CKD and HF emphasize the value of multidisciplinary methods in the optimal management of both disorders and the necessity of targeted management to enhance outcomes for patients with both HF and CKD.
Diabetic retinopathy (DR) is a common microvascular complication of DM that serves as one quantifiable surrogate for systemic microvascular health9,10,11. The presence of DR lesions detectable on fundus examination shows an association with a higher likelihood of cardiovascular events and mortality in CKD12,13. Furthermore, more severe grades of DR correlating with worse retinal microangiopathy are thought to confer incrementally higher cardiovascular risk14. The pathophysiological mechanisms underlying DR, such as endothelial dysfunction, inflammation, and altered angiogenic factors, share similarities with those involved in the development and progression of HF15,16,17. However, while these relationships have been characterized for broad cardiovascular outcomes, limited evidence exists focused on the association between DR and HF, specifically among patients with diabetes and CKD. In this study, we examine the link between HF and the severity of DR ascertained through fundus examination. Our study can help physicians determine the bidirectional association between DR and HF in individuals with DM and CKD. Clarifying this relationship informs prevention and management tailored to this high-risk population.
Materials and methods
Study participants
This study was part of the Taiwan Kidney Outcome cohort (TAKO), which enrolled participants who diagnosed CKD. Taiwan’s National Health Insurance launched the Project of Integrated Care of CKD to provide multidisciplinary care. This multidisciplinary care program offers appropriate CKD education knowledge, tracks the patient’s health status, and offers comprehensive patient education and support. In this study, CKD subjects were registered in the national pre-ESRD nephrology care program at Kaohsiung Medical University Hospital (KMUH) and Kaohsiung Municipal Siaogang Hospital (KMSH) from January 2005 to December 2021. Patients older than 18 years, diagnosed as CKD having estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2 or eGFR more than 60 mL/min/1.73 m2 and presenting proteinuria for more than three months were included in the study in accordance with the criteria outlined in the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines18. Written informed consent was obtained from all subjects or their legal guardians prior to participation in the study. Patients were excluded from the study if they were ESRD receiving dialysis treatment or recipients of a kidney transplant before the index date. The index date was the day participants underwent color fundus photography examinations. We integrated the KMUH pre-ESRD program with the KMUH electronic medical records (EMRs) containing laboratory tests, medications, special procedures, and admission records. The study was approved by the Research Ethical Committee/Institutional Review Board of KMUH (KMUHIRB-E(II)−20220286). This study complies with the STROBE Statement to strengthen the Reporting of Observational Studies in Epidemiology guidelines, and the required information is presented correctly. All the methods are in compliance with relevant guidelines and regulations.
Comorbidity, laboratory, and clinical variables
Sociodemographic data (age, gender), medical history, and biochemical data were obtained through EMRs. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters. BMI and Blood pressure were recorded using the data closest to the index date. All diagnoses are recorded according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) or International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM). Drug dispensing data included the type of prescriber, the name, date, amount, and prescribed dose regimen of the dispensed drug, and the length of the prescription (drug treatment period). The physicians’ clinical diagnosis of DM and hypertension were defined based on ICD-9/ICD-10 codes and/or an HbA1C ≥ 6.5% for diabetes and/or the use of blood pressure- and glucose-lowering agents. The serum creatinine level at index date was used to define the baseline eGFR, which was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, corresponding CKD stages by the following cut-off values of > 90, 60–89.9, 30–59.9, 15–29.9 and < 15 mL/min/1.73 m2.
Fundus image acquisition and diabetic retinopathy severity evaluation
Color fundus photography was obtained using a digital non-mydriatic retinal camera (Canon CRDGi with a 20 Diopter SLR backing, Canon, Japan). DR was considered present if characteristic lesions as defined by the American Academy of Ophthalmology (AAO) Preferred Practice Pattern (PPP)-updated 2019 guidelines were found on retinal photographs19. To evaluate the DR severity upon CKD progression, retinopathy was classified into seven severity grades: no apparent, mild non-proliferative DR (NPDR), moderate NPDR, severe NPDR, very severe NPDR, proliferative DR (PDR), and high-risk PDR. When only one eye’s grading was available, the severity level of that eye was used. The severity level of the eye with the more advanced retinopathy served as the participant’s level. The current study classified the DR severity into three ordered categories (no apparent, mild NPDR and moderate NPDR as group 1, severe NPDR and very severe NPDR as group 2, and PDR and high-risk PDR as group 3). The grading of DR was determined through the consensus of three independent image graders. To ensure the robustness of our DR severity evaluation, we conducted an inter-grader reliability analysis using a randomly selected subset of images. These images were graded independently by two experienced experts who were blinded to each other’s assessments. The agreement between the graders was assessed using Cohen’s kappa coefficient (κ), which is widely used to evaluate inter-rater reliability for categorical data. The inter-grader agreement was substantial, with a high level of consistency between the two graders, suggesting that the classification of DR severity in our study is reliable and reproducible.
Assessment of heart failure hospitalization
HF hospitalization was defined as having an inpatient diagnosis of HF according to the Framingham Heart Failure Diagnostic Criteria by physician with relevant treatments during the hospitalization. The hospital discharge diagnosis code of HF was 428 in International Classification of Diseases, 9 th Revision, Clinical Modification [ICD-9-CM] code and I50 in ICD-10-CM code. When a patient had multiple AHF episodes, the first episode of AHF hospitalization was recorded.
Statistical analysis
Baseline characteristics were compared between those with and without HF using chi-squared tests for categorical variables and t-tests for continuous variables. Ordinal logistic regression was used to evaluate the association between the presence of HF (independent variable) and DR severity grade (dependent variable). To ensure the appropriateness of the ordinal logistic regression model, we conducted the parallel line test, which confirmed the proportional odds assumption with a non-significant p-value. This result suggests that the ordinal logistic regression model was a suitable choice for analyzing the ordered, categorical nature of the diabetic retinopathy severity groups. Three models were created with progressive adjustment for potential confounders, including Model 1 as demographics (age, gender, smoking, BMI); Model 2 as Model 1 factors plus cardiovascular risk factors (blood pressure, hypertension, statin use); Model 3 as Model 2 factors plus laboratory measurements (HbA1c, lipid parameters, eGFR). An additional sensitivity analysis was performed using diuretic medication as a proxy indicator for HF status, analyzed with ordinal regression. Among those without baseline HF, the association between DR severity and risk of acute HF hospitalization was analyzed using Cox proportional hazards regression. The same three models used for ordinal regression were analyzed, including adjusted for demographics, cardiovascular risk factors, and laboratory measurements in hierarchical models. The cumulative probability of acute HF hospitalization over 5 years was also compared between DR severity groups using Kaplan-Meier curves, with statistical significance determined by the log-rank test. Consistency of the primary results was explored in subgroup analysis by stratified ordinal and Cox proportional hazards regression analyses. All analyses used SAS 9.4 (SAS Institute Inc., Cary, NC, USA; www.sas.com) and Jamovi 2.4.14 version (https://www.jamovi.org/). The results were presented as odds ratios (OR) with 95% confidence intervals (CIs) for the ordinal regression model, hazard ratios (HR) with 95% Cis for the Cox regression model, and a two-tailed p < 0.05 was considered statistically significant.
Results
Baseline characteristics and stratified by diabetic retinopathy status
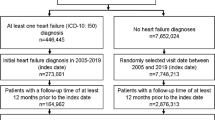
The study design is presented in Fig. 1. The study included 1,503 patients with diabetes and CKD who were stratified into three groups based on the severity of diabetic retinopathy (DR): Group 1 (no apparent, mild, or moderate NPDR, n = 1,031), Group 2 (severe or very severe NPDR, n = 174), and Group 3 (PDR or high-risk PDR, n = 298). Baseline characteristics of the study population are presented in Table 1. Patients in Group 3 were younger and had higher systolic and diastolic blood pressures, higher prevalence of HF, and lower eGFR compared to those in Groups 1 and 2 (all p < 0.001).
Association between heart failure and severity of diabetic retinopathy
Ordinal logistic regression further analyzed the association between HF and DR severity. In models adjusted for sociodemographic, comorbidities, and medication use, HF was associated with 2-fold higher odds of worse DR (OR 2.23, 95% CI 1.45–3.39, p < 0.001). This relationship persisted after the final adjustment for glycemic status, lipid parameters, and kidney function as well (OR 2.09, 95% CI 1.37–3.18, p < 0.001) (Table 2). Consistent associations were found between HF and DR severity across all examined subgroups. The 2-fold increased odds were consistent regardless of age, sex, CKD stage, or hyperlipidemia status (Fig. 2). Diuretics as a proxy for HF also showed a positive association with the severity of DR (OR 1.54, 95% CI 1.11–2.12, p = 0.009) (Supplementary Table1).
Subgroup analysis of the association between heart failure and severity of diabetic retinopathy stratified by baseline characteristics. The consistent approximately 2-fold increased odds of worse DR with HF across all subgroups demonstrates the robustness of this relationship regardless of age, sex, CKD stage, or hyperlipidemia status.
Association between severity of diabetic retinopathy and risk of heart failure hospitalization
The cumulative incidence curves for developing acute HF over 5 years of follow-up based on the DR severity category were presented (Fig. 3). Patients with advanced DR exhibited the highest risk of acute HF, reaching 35% at 5 years compared to 15% for those with no DR (log-rank p < 0.0001). In patients without baseline HF, the more severe DR grading demonstrated a higher likelihood of developing incident acute HF over time in Cox regression models (HR 1.86, 95% CI 1.22–2.83, p = 0.004 in Proliferative/high-risk PDR), confirming DR severity may predict subclinical HF progression (Table 3). Nevertheless, although patients in group 2 showed an increased risk of HF, there was no significant difference after adjusting for all variables. After adjusting for laboratory parameters (HbA1c, HDL-C, LDL-C, eGFR), the association was not statistically significant. This may reflect the substantial impact of diabetes duration and kidney function (eGFR) on both retinopathy progression and cardiovascular outcomes. Potential biases due to differences in baseline characteristics across groups should be interpreted cautiously. Positive associations of DR severity with acute HF risk were consistent across subgroup analyses, including age, gender, smoking, BMI, blood pressure control, diabetes control, eGFR, and statin use (Fig. 4).
Kaplan-Meier curve of severity of diabetic retinopathy and risk of acute heart failure hospitalization in DM and CKD patients without diagnosis of heart failure. Group 2 (Severe NPDR + Very severe NPDR) and Group 3 (PDR + High-risk PDR) demonstrate significantly higher risk of acute heart failure hospitalization compared to Group 1 (No apparent, Mild NPDR and Moderate NPDR), with Group 3 showing the highest risk (log-rank p < 0.0001).
Subgroup analysis of the association between severity of diabetic retinopathy and risk of acute heart failure hospitalization among DM and CKD patients without diagnosis of heart failure stratified by baseline characteristics. Results demonstrate consistent positive associations between DR severity and acute HF risk across all examined subgroups, supporting the robustness of this relationship.
Discussion
In this study of 1,503 patients with diabetes and CKD, the presence of HF was associated with approximately 2-fold increased odds of more severe DR after adjustment for potential confounders in the ordinal regression model. These associations were consistent regardless of patient age, sex, control of diabetes and blood pressure, kidney function, and medication use. The use of diuretics as a proxy for HF status also showed a significant association with increasing severity of DR. In patients without baseline HF, those with advanced DR stages, including severe/very severe NPDR disease and PDR/high-risk PDR, had 1.4- to 2.4-fold HRs of acute HF hospitalization over 5 years of follow-up in fully adjusted models. Overall, these results demonstrated a linkage between HF status and the presence of microvascular diabetic complications, as well as an association between advanced DR and risk for incident HF events in high-risk patients with diabetes and CKD.
Our results showed that among patients without HF at baseline, those with DR had a greater risk of acute HF hospitalization for 5 years than individuals without any discernible retinopathy. Consistent with our findings, a 13-year countrywide cohort research discovered a 3.482 hazard ratio linking DR to an increased risk of HF20. A population-based study included 1,021 type 2 diabetic persons with normal renal function and found that the presence of DR, particularly more severe grade, was linked to a higher risk of HF21. Furthermore, retinopathy was found to be an independent predictor of HF in a prospective 7-year cohort study, even in individuals without preexisting hypertension, diabetes, or coronary heart disease22. As demonstrated in our study, DR is associated with an increased risk of HF, independent of other known risk factors. The presence of DR may be an independent predictor of HF in CKD patients.
Few studies have explored regarding DR and HF relationships, specifically among patients with established CKD. A study assessed the association of DR and diabetic kidney disease with all-cause and CVD mortality, including acute myocardial infarction, coronary heart disease, hypertensive and other heart disease, HF, and stroke. In that study, DR and diabetic kidney disease were independently associated with an increased risk of all-cause and CVD mortality in people with diabetes. Moreover, risks of all-cause and CVD mortality were significantly higher in those with diabetic kidney disease and DR23. Another study examined the association of albuminuria and retinopathy with cardiovascular events, renal events, and mortality in Chinese patients with type 2 diabetes, finding that the coexistence of DR and albuminuria associated with heightened risks for cardiovascular events (HF or angina, myocardial infarction, lower limb amputation, revascularization procedures, and stroke), renal failure progression, and mortality24. One cohort study also reported that retinopathy concomitant with microalbuminuria posed a higher CVD-related mortality risk than was observed with retinopathy alone25. Our analysis added robust data showing stepped increases in HF risks conferring to worsening DR grade, suggesting concordant microvascular disease burden predicting HF development in patients with diabetes and CKD. Further mechanistic and outcomes studies are still needed in this population.
The potential mechanistic links between DR and HF shared microvascular dysfunctions and systemic consequences of diabetes, involving both macrovascular and microvascular pathological processes26. Macrovascular disease primarily manifests as ischemic coronary heart disease, characterized by atherosclerotic plaque formation, vessel stenosis, and acute coronary events27. Conversely, microvascular disease presents as diabetic cardiomyopathy, involving structural and functional cardiac changes such as myocardial hypertrophy, fibrosis, and impaired cardiac muscle function26,28. Despite their apparent differences, both macrovascular and microvascular pathways share a common underlying mechanism: oxidative stress29. A pivotal molecular mechanism underlying diabetic complications involves glycation end-products (AGEs) activating their receptor on vascular and immune cells, triggering oxidative stress, nitric oxide depletion, and inflammation that drive myocardial remodeling, diabetic cardiomyopathy and cardiovascular deterioration26,30. In the ocular microenvironment, hyperglycemia induces oxidative stress, inflammation, vascular endothelial growth factor accumulation, and glycation product formation, leading to vascular permeability, leukocyte adhesion, and endothelial dysfunction. Serine racemase overexpression further promotes neurodegeneration via N-methyl-D-aspartate receptor activation, collectively driving vascular remodeling, hypoxia, and progression of diabetic retinopathy26. Several underlying mechanisms appeared to contribute to the link between DR and HF, which is evident in clinical observations. Primary among these were endothelial dysfunction and impaired endothelium-dependent vasodilation. In both DR and HF, there was a loss of vasodilator and antithrombotic effects of nitric oxide or increased release of vasoconstrictors, resulting in reduced blood flow and tissue oxygenation31,32,33. The persistent hyperglycemia associated with diabetes also promotes cellular damage through increased production of free radicals coupled with decreased antioxidant capacity, known as oxidative stress34,35,36,37. These changes produce vascular endothelial damage in both DR and HF. Additional mechanisms further exacerbated vascular complications. Platelet dysfunction leads to abnormal platelet activation and aggregation, which could worsen microvascular occlusion in DR38,39. Myocardial damage might also result from such changes in HF as platelets accumulate in the myocardium40. Nonenzymatic glycation of proteins and macromolecules gave rise to AGEs, causing structural and functional changes that promote injury in both micro- and macro-vessels. Studies have demonstrated significantly higher serum levels of AGEs in patients with PDR compared to non-diabetic controls, as well as an association between higher AGE levels and increased risk of prevalent HF in the general population41,42,43. Beyond these vascular effects, there was evidence that low-grade systemic inflammation played a key role in DR and HF44. Proinflammatory cytokines could directly inflict damage or modulate other pathways, leading to tissue injury45,46,47. Chronic kidney disease, a prevalent comorbidity in diabetes, accelerates vascular injury through early calcium deposition and apoptosis of vascular smooth muscle cells, promoting calcification and arterial stiffness48. This vascular remodeling exacerbates ischemic and hypoxic insults in the retina and myocardium, amplifying the risks and severity of both DR and HF. To sum up, the relationship between HF and DR was multifaceted. These conditions were often interlinked, and their coexistence might be attributed to shared pathological mechanisms.
This large observational study of over 1,500 patients with diabetes and CKD has several research strengths. Color fundus photography allowed for objective, graded measurements of DR severity based on characteristic lesions. The sample size provided ample statistical power to detect associations between DR and the risk of incident HF. EMRs enabled reliable ascertainment of comorbid conditions using diagnosis codes and clinical documentation. Multiple statistical methods, including adjusted regression modelling, substantiated a relationship wherein more severe diabetic retinal disease conferred higher odds of concurrent HF and more significant hazards for acute HF hospitalization over follow-up. The findings carry important clinical implications for patients with diabetes and CKD. The observed association between diabetic retinopathy severity and heart failure risk suggests the need for comprehensive cardiovascular screening in patients with advanced microvascular complications. Clinicians should consider retinopathy severity as a potential marker for cardiovascular risk stratification, warranting more intensive cardiac monitoring for patients in higher retinopathy severity groups. From a public health perspective, these results emphasize the importance of multidisciplinary management approaches that integrate ophthalmologic and cardiovascular assessments for high-risk diabetic populations.
However, limitations in the observational study design preclude determining causal mechanisms in the association between microvascular disease and macrovascular outcomes. Although our study was conducted in a Taiwanese population, certain findings may potentially apply to other ethnic groups. However, it is imperative to acknowledge the inherent limitations regarding generalizability. Extant literature demonstrates significant ethnic variations in genetic susceptibility to DR, with evidence suggesting that specific polymorphisms may confer differential risk profiles between Eastern and Western populations49,50. To address these considerations, future studies in diverse populations are warranted to validate our observations and explore potential ethnic or systemic healthcare disparities. Outcomes based on clinical documentation can be prone to misclassification biases if diagnoses are incorrect or overlooked. In our study, HF hospitalizations were identified using ICD codes and Framingham criteria, without differentiating between HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF). These subtypes may exhibit distinct associations with diabetic microvascular disease. Notably, diabetes is more strongly linked to an increased risk of HFpEF, potentially due to shared pathophysiological mechanisms such as endothelial dysfunction and chronic inflammation. Therefore, future prospective studies incorporating systematic echocardiographic assessment are warranted to determine whether the association between diabetic retinopathy severity and HF risk differs by HF subtype. An important limitation of our study is the lack of data on diabetes duration, which could be a confounder since both diabetic retinopathy severity and cardiovascular complications typically correlate with longer duration of diabetes51. Consequently, the strength of the observed association between DR severity and HF risk may be partly attributable to unmeasured differences in diabetes duration across our study groups. Without controlling for this variable, we cannot fully discern whether the observed relationship represents a direct pathophysiological link between microvascular and macrovascular disease or simply reflects the parallel progression of both complications with increasing diabetes chronicity. Future studies should prioritize collecting comprehensive data on diabetes duration, ideally from time of diagnosis, to better delineate the independent contribution of DR severity to HF risk after controlling for this crucial time-dependent variable. Information on statin medication adherence was also not available, which plausibly contributes to complications risk. Poor adherence to statin therapy has been consistently associated with an increased risk of cardiovascular events, including heart failure52,53. Although we documented statin prescriptions in our cohort (with rates of 53.0%, 47.7%, and 59.4% across our three retinopathy severity groups), we were unable to measure actual adherence to these medications. This represents a notable limitation as adherence patterns may vary substantially across different retinopathy severity groups. Nonetheless, this study provides evidence that the severity of DR may mirror and predict subclinical macrovascular disease progression in the form of incident HF among diabetes patients with coexisting CKD.
Conclusion
Among over 1,500 adults with diabetes and CKD, the severity of DR assessed through photographic retinal grading served as an independent indicator of both prevalent and incident HF risk. Quantifying microvascular health through diabetic retinal lesions may enable risk stratification, guide cardiovascular monitoring, and tailor preventive care for populations with dual diabetes and CKD. Clinicians should consider integrating DR severity assessments into comprehensive cardiovascular risk evaluation protocols, potentially using retinal imaging as an early screening tool for identifying patients at higher risk of heart failure progression.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Jung, C. Y. & Yoo, T. H. Novel biomarkers for diabetic kidney disease. Kidney Res. Clin. Pract. 41, S46–S62. https://doi.org/10.23876/j.krcp.22.084 (2022).
Nordheim, E. & Geir Jenssen, T. Chronic kidney disease in patients with diabetes mellitus. Endocr. Connect. 10, R151–R159. https://doi.org/10.1530/EC-21-0097 (2021).
Lim, A. Diabetic nephropathy - complications and treatment. Int. J. Nephrol. Renovasc Dis. 7, 361–381. https://doi.org/10.2147/IJNRD.S40172 (2014).
de Boer, I. H. et al. Diabetes management in chronic kidney disease: A consensus report by the American diabetes association (ADA) and kidney disease: improving global outcomes (KDIGO). Diabetes Care. 45, 3075–3090. https://doi.org/10.2337/dci22-0027 (2022).
Evans, M. et al. A narrative review of chronic kidney disease in clinical practice: current challenges and future perspectives. Adv. Ther. 39, 33–43. https://doi.org/10.1007/s12325-021-01927-z (2022).
Vijay, K., Neuen, B. L. & Lerma, E. V. Heart failure in patients with diabetes and chronic kidney disease: challenges and opportunities. Cardiorenal Med. 12, 1–10. https://doi.org/10.1159/000520909 (2022).
Romero-González, G. et al. Burden and challenges of heart failure in patients with chronic kidney disease. A call to action. Nefrologia (Engl Ed). 40, 223–236. https://doi.org/10.1016/j.nefro.2019.10.005 (2020).
Paterson, E. N. et al. Association of reduced inner retinal thicknesses with chronic kidney disease. BMC Nephrol. 21 https://doi.org/10.1186/s12882-019-1679-1 (2020).
Siemianowicz, K., Francuz, T., Gminski, J., Telega, A. & Syzdól, M. Endothelium dysfunction markers in patients with diabetic retinopathy. Int. J. Mol. Med. 15, 459–462 (2005).
Cheung, N. & Wong, T. Y. Diabetic retinopathy and systemic vascular complications. Prog Retin Eye Res. 27, 161–176. https://doi.org/10.1016/j.preteyeres.2007.12.001 (2008).
Sorrentino, F. S., Matteini, S., Bonifazzi, C., Sebastiani, A. & Parmeggiani, F. Diabetic retinopathy and endothelin system: microangiopathy versus endothelial dysfunction. Eye (Lond). 32, 1157–1163. https://doi.org/10.1038/s41433-018-0032-4 (2018).
Xu, X. H. et al. Diabetic retinopathy predicts cardiovascular mortality in diabetes: a meta-analysis. BMC Cardiovasc. Disord. 20, 478. https://doi.org/10.1186/s12872-020-01763-z (2020).
Guo, V. Y., Cao, B., Wu, X., Lee, J. J. W. & Zee, B. C. Prospective association between diabetic retinopathy and cardiovascular Disease-A systematic review and Meta-analysis of cohort studies. J. Stroke Cerebrovasc. Dis. 25, 1688–1695. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.03.009 (2016).
Pongrac Barlovic, D. et al. The association of severe diabetic retinopathy with cardiovascular outcomes in Long-standing type 1 diabetes: A longitudinal Follow-up. Diabetes Care. 41, 2487–2494. https://doi.org/10.2337/dc18-0476 (2018).
Drera, A., Rodella, L., Brangi, E., Riccardi, M. & Vizzardi, E. Endothelial dysfunction in heart failure: what is its role?? J. Clin. Med. 13 https://doi.org/10.3390/jcm13092534 (2024).
Wei, L. et al. The pathophysiological mechanisms underlying diabetic retinopathy. Front. Cell. Dev. Biol. 10, 963615. https://doi.org/10.3389/fcell.2022.963615 (2022).
Pandey, A. K. et al. Mechanisms of VEGF (Vascular endothelial growth Factor) Inhibitor-Associated hypertension and vascular disease. Hypertension 71, e1–e8. https://doi.org/10.1161/hypertensionaha.117.10271 (2018).
Rossing, P. et al. Executive summary of the KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease: an update based on rapidly emerging new evidence. Kidney Int. 102, 990–999. https://doi.org/10.1016/j.kint.2022.06.013 (2022).
Mitsch, C. et al. eHealth 2015 special issue: impact of electronic health records on the completeness of clinical Documentation generated during diabetic retinopathy consultations. Appl. Clin. Inf. 6, 478–487. https://doi.org/10.4338/ACI-2014-11-RA-0104 (2015).
Hsu, C. Y. et al. The association of diabetic retinopathy and cardiovascular disease: A 13-Year nationwide Population-Based cohort study. Int. J. Environ. Res. Public. Health. 18 https://doi.org/10.3390/ijerph18158106 (2021).
Cheung, N. et al. Diabetic retinopathy and risk of heart failure. J. Am. Coll. Cardiol. 51, 1573–1578. https://doi.org/10.1016/j.jacc.2007.11.076 (2008).
Wong, T. Y. et al. Retinopathy and risk of congestive heart failure. Jama 293, 63–69. https://doi.org/10.1001/jama.293.1.63 (2005).
Sabanayagam, C. et al. Association of diabetic retinopathy and diabetic kidney disease with All-Cause and cardiovascular mortality in a multiethnic Asian population. JAMA Netw. Open. 2, e191540. https://doi.org/10.1001/jamanetworkopen.2019.1540 (2019).
Tong, P. C. et al. Interactive effect of retinopathy and macroalbuminuria on all-cause mortality, cardiovascular and renal end points in Chinese patients with type 2 diabetes mellitus. Diabet. Med. 24, 741–746. https://doi.org/10.1111/j.1464-5491.2007.02145.x (2007).
Fisher, D. E. et al. Mortality in older persons with retinopathy and concomitant health conditions: the age, gene/environment Susceptibility-Reykjavik study. Ophthalmology 123, 1570–1580. https://doi.org/10.1016/j.ophtha.2016.02.045 (2016).
Li, Y. et al. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal. Transduct. Target. Ther. 8, 152. https://doi.org/10.1038/s41392-023-01400-z (2023).
Katakami, N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J. Atheroscler Thromb. 25, 27–39. https://doi.org/10.5551/jat.RV17014 (2018).
Jia, G., Hill, M. A. & Sowers, J. R. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ. Res. 122, 624–638. https://doi.org/10.1161/circresaha.117.311586 (2018).
Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 107, 1058–1070. https://doi.org/10.1161/circresaha.110.223545 (2010).
Tan, Y. et al. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat. Rev. Cardiol. 17, 585–607. https://doi.org/10.1038/s41569-020-0339-2 (2020).
Shityakov, S., Nagai, M., Ergün, S., Braunger, B. M. & Förster, C. Y. The protective effects of neurotrophins and MicroRNA in diabetic retinopathy, nephropathy and heart failure via regulating endothelial function. Biomolecules 12 https://doi.org/10.3390/biom12081113 (2022).
Gamrat, A., Surdacki, M. A., Chyrchel, B. & Surdacki, A. Endothelial dysfunction: A contributor to adverse cardiovascular remodeling and heart failure development in type 2 diabetes beyond accelerated atherogenesis. J. Clin. Med. 9 https://doi.org/10.3390/jcm9072090 (2020).
Cheung, N. et al. Diabetic retinopathy and the risk of coronary heart disease: the atherosclerosis risk in communities study. Diabetes Care. 30, 1742–1746. https://doi.org/10.2337/dc07-0264 (2007).
Kowluru, R. A. & Chan, P. S. Oxidative stress and diabetic retinopathy. Exp Diabetes Res 43603, (2007). https://doi.org/10.1155/2007/43603 (2007).
Demaison, L. Oxidative stress and Obesity- and type 2 Diabetes-Induced heart failure. Antioxid. (Basel). 9 https://doi.org/10.3390/antiox9080653 (2020).
Koniari, I. et al. Anti-Diabetic therapy, heart failure and oxidative stress: an update. J. Clin. Med. 11 https://doi.org/10.3390/jcm11164660 (2022).
Kayama, Y. et al. Diabetic cardiovascular disease induced by oxidative stress. Int. J. Mol. Sci. 16, 25234–25263. https://doi.org/10.3390/ijms161025234 (2015).
Walinjkar, R. S., Khadse, S., Kumar, S., Bawankule, S. & Acharya, S. Platelet indices as a predictor of microvascular complications in type 2 diabetes. Indian J. Endocrinol. Metab. 23, 206–210. https://doi.org/10.4103/ijem.IJEM_13_19 (2019).
Dindar, S., Cinemre, H., Sengul, E. & Annakkaya, A. N. Mean platelet volume is associated with glycaemic control and retinopathy in patients with type 2 diabetes mellitus. West. Indian Med. J. 62, 519–523. https://doi.org/10.7727/wimj.2012.284 (2013).
Kaya, H. et al. Plasma osmolality predicts mortality in patients with heart failure with reduced ejection fraction. Kardiol Pol. 75, 316–322. https://doi.org/10.5603/KP.a2016.0168 (2017).
Arshi, B., Chen, J., Ikram, M. A., Zillikens, M. C. & Kavousi, M. Advanced glycation end-products, cardiac function and heart failure in the general population: the Rotterdam study. Diabetologia 66, 472–481. https://doi.org/10.1007/s00125-022-05821-3 (2023).
Yasuda, Y. et al. Glyceraldehyde-derived advanced glycation end-products are associated with left ventricular ejection fraction and brain natriuretic peptide in patients with diabetic adverse cardiac remodeling. Scand. Cardiovasc. J. 56, 208–216. https://doi.org/10.1080/14017431.2022.2095013 (2022).
Mengstie, M. A. et al. Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications. Front. Mol. Biosci. 9, 1002710. https://doi.org/10.3389/fmolb.2022.1002710 (2022).
Si, Y., Chen, Q., Xiong, X. & Zheng, M. The association of inflammatory biomarkers with clinical outcomes in diabetic retinopathy participants: data from NHANES 2009–2018. Diabetol. Metab. Syndr. 16, 181. https://doi.org/10.1186/s13098-024-01419-4 (2024).
Ramos, H., Hernández, C., Simó, R., Simó-Servat, O. & Inflammation The link between neural and vascular impairment in the diabetic retina and therapeutic implications. Int. J. Mol. Sci. 24 https://doi.org/10.3390/ijms24108796 (2023).
Zhang, Q., Chen, Y. E., Zhu, X. X., Wang, X. & Qu, A. J. [The role of inflammation in heart failure with preserved ejection fraction]. Sheng Li Xue Bao. 75, 390–402 (2023).
Peh, Z. H. et al. Inflammation as a therapeutic target in heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 10, 1125687. https://doi.org/10.3389/fcvm.2023.1125687 (2023).
Shroff, R. C. et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 118, 1748–1757. https://doi.org/10.1161/circulationaha.108.783738 (2008).
Hampton, B. M., Schwartz, S. G., Brantley, M. A. Jr. & Flynn, H. W. Jr. Update on genetics and diabetic retinopathy. Clin. Ophthalmol. 9, 2175–2193. https://doi.org/10.2147/opth.S94508 (2015).
Priščáková, P., Minárik, G. & Repiská, V. Candidate gene studies of diabetic retinopathy in human. Mol. Biol. Rep. 43, 1327–1345. https://doi.org/10.1007/s11033-016-4075-y (2016).
Li, F. R. et al. Diabetes duration and glycaemic control as predictors of cardiovascular disease and mortality. Diabetes Obes. Metab. 23, 1361–1370. https://doi.org/10.1111/dom.14348 (2021).
Rodriguez, F. et al. Association of Statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 4, 206–213. https://doi.org/10.1001/jamacardio.2018.4936 (2019).
De Vera, M. A., Bhole, V., Burns, L. C. & Lacaille, D. Impact of Statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br. J. Clin. Pharmacol. 78, 684–698. https://doi.org/10.1111/bcp.12339 (2014).
Acknowledgements
The funding sources did not play any role in the design or conduct of the study, collection, management, analysis, interpretation of the data, or preparation, review, or approval of the manuscript. The study was funded by grants from the Ministry of Science and Technology, Taiwan (NSTC 113-2314-B-037 -097 -MY3), Kaohsiung Medical University Hospital, Taiwan (KMUH111-1M09, KMUH112-2M08, KMUH113-3R19, KMUH-DK(B)110003-1), and Kaohsiung Medical University, Taiwan (NPUST-KMU-111-P001, NPUST-KMU-114-P001, KT112P012, KT113P006, KMUH-DK(C)112001, NHRIKMU-111-I001-3). The authors thank the help from the Division of Medical Statistics and Bioinformatics, Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung Medical University, the Center for Big Data Research (KMU-TC109B08), and Kaohsiung Medical University for providing administrative support, including the Kaohsiung Medical University Hospital Research Database (KMUHRD) (KMUTC111IFSP0).
Author information
Authors and Affiliations
Contributions
Conceptualization, Yi-Chen Huang, Feng-Ching Shen, Pei-Kang Liu, Wei-Chun Su, Ping-Hsun Wu; Study design and analysis plan, Yi-Chen Huang, Feng-Ching Shen, Pei-Kang Liu, Wei-Chun Su, Mei-Chuan Kuo, Ping-Hsun Wu; Statistical analysis, Feng-Ching Shen, Teng-Hui Huang, Ming-Yen Lin, Ping-Hsun Wu; Funding acquisition, Yi-Wen Chiu, Ping-Hsun Wu; Writing– first draft, Yi-Chen Huang, Ping-Hsun Wu; Writing – review & editing, Pei-Kang Liu, Ming-Yen Lin, Mei-Chuan Kuo, Yi-Wen Chiu, Shang-Jyh Hwang, Yi-Ting Lin.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, YC., Shen, FC., Liu, PK. et al. Dissecting the relationship between heart failure and diabetic retinopathy severity in patients with diabetes and chronic kidney disease: an observational cohort study. Sci Rep 15, 24346 (2025). https://doi.org/10.1038/s41598-025-04523-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04523-1