Abstract
Rheumatoid arthritis (RA) is an autoimmune disease affecting the joints and other extra-articular organs. RA has the symptoms of inflammation, joint dysfunction, and reduction in life expectancy. The main causes of RA are family history, immunogenicity, smoking, and genetic factors. Among the genetic factors, DNA damage response pathway genes are primarily involved in repairing damage caused by smoking and other carcinogens. Studies have reported an increased DNA damage frequency in RA patients. The present study is designed to illuminate the association between the DNA damage response pathway genes (PARP1, TREX1, ATM, and TP53) and RA in the Pakistani population. Methods For this purpose, 500 RA patients and 500 age/gender-matched controls were collected and DNA/RNA was extracted. The genotype frequency of selected SNPs [PARP1 (Val76Ala), ATM (Pro1054Arg), TP53 (Ala138Val), and TREX (Tyr177Tyr)] was measured using the Tetra-ARMS PCR. Expression analysis of selected genes was measured using quantitative PCR. Statistical analysis showed a significantly increased frequency of mutant allele Val76Ala (p < 0.0001), Pro1054Arg (p < 0.0001), Ala138Val (p < 0.0001), and Tyr177Tyr (p < 0.0001) in RA patients compared to controls. Linkage disequilibrium showed a strong linkage disequilibrium between selected SNPs in RA patients compared to controls. Quantitative PCR showed a significant downregulation of PARP1 (p < 0.0001), ATM (p < 0.0001), TP53 (p < 0.0001), and TREX (p < 0.0001) in RA patients. ROC curve analysis showed a good diagnostic value for selected genes in RA patients. The present study showed that increased mutant genotype frequency and expression deregulation of DNA damage response pathway genes was linked with significant increased risk of RA. This study showed that DNA damage response pathway genes can act as efficient/specific diagnostic markers for said disease. Furthermore, these findings also lay a solid foundation for further research based on targeted metabolomics/genomics, which may lead to the development of more effective treatment strategies in the future.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is one of the auto-immune disease which usually affects joints of body without any discrimination due to enduring inflammation1. RA is regarded as one of the common auto-immune disorder globally that affects 1% population with higher frequency of adult female patients2,3. In Pakistan, RA is among the devasting disease which is prevalent in more than 1% population. Risk of RA in female individuals is three times higher as compared to male individuals4. Treatment used for RA patients are based on nonpharmacological, pharmacological, and surgical treatments. Pharmacological treatment is only effective in pain reduction, improvement of quality of life with no effect on disease progression. The severity of RA disease worsen with age and surgery is only option at this stage5. Among the pharmacological treatment of RA, Biologics and methotrexate are used. Biologic agents are engineered drugs that target specific inflammatory cells, cellular interactions, and cytokines that mediate RA-related tissue damage. Such agents are designed to reduce the signs and symptoms of RA and slow disease progression. Methotrexate is disease-modifying anti-rheumatic drug (DMARD). Conventional DMARDs (e.g., methotrexate [MTX], hydroxychloroquine, and sulfasalazine) have long been the mainstay of treatment, and they are still widely used in newly diagnosed RA patients6,7. Because of its long-term effectiveness, low cost, and acceptable safety profile, oral MTX is the most widely used conventional DMARD, and it is the standard against which other DMARDs and newer RA therapies are compared. Studies have reported that two factors rheumatoid factors (RF) and cyclic citrullinated peptide (anti-CCP) are used as the screening tools for the diagnosis of RA. RF is considered as the cheapest screening modality for the RA patients especially in ophthalmic clinic with less specificity and sensitivity8. RF is not always considered as a reliable marker for RA as RF is not often detected in early RA patients. However, anti-CCP is detected in early RA patients with the 73.5% sensitivity and 100%. Specificity. On the basis of this, anti-CCP is considered as most reliable screening modality for RA patients. Anti-CCP screening should be ordered when RA suspicion is high, despite RF being normal8.
RA is a chronic inflammatory disease with unknown genetic and epigenetic pathogenic factors1. Previous studies have reported the high DNA damage in RA patients and severity of disease increased with increased frequency of adduct formation, ROS and DNA damage. This accumulated damages ultimately results in impaired repairing capacity in RA patients. This impaired repair may reflect the variations in the genes encoding the DNA repair proteins. So the investigation of DNA damage biomarkers in RA patients are urgently needed9. In present study, four vital DNA damage repair genes are selected such as poly(ADP-ribose) polymerase 1 (PARP1), three prime repair exonuclease 1 (TREX1), Ataxia-telangiectasia mutated (ATM), and tumor protein p53 (TP53). PARP1 is considered as an important protein to detect/sense the DNA damage and actively involved in the cellular response to DNA damage. During the first event of DNA damage response, PARP1 uses NAD + as a substrate and adds mono-ADP-ribose or PAR to acceptor proteins. This leads to release of repair proteins and nucleases toward the damaged site to repair10. García & Conde (2015) have reported that pharmacological inhibition and PARP1 deficiency has a protective role in inflammatory diseases like RA9. TREX1 is a DNA endonuclease that degrade the DNA fragment produced during DNA repair, cell division and apoptosis11. The loss of TREX1 gene due to mutation/variation results in accumulation of damage DNA. This accumulated DNA damage recognized by immune system as foreign particles that ultimately results in triggering of an inflammatory response12. The loss of function mutation of TREX1 is linked to autoimmune and inflammatory diseases like familial chilblain lupus (FCL) cerebral leukodystrophy (RVCL), aicardi-Goutieres syndrome (AGS), and systemic lupus erythematosus (SLE)13. In DNA damage response TREX1 interacts with PARP1 in response to genotoxic stress and contribute to repair this accumulated damage14. TP53, the third selected gene, is an important tumor suppressor gene. Yamanishi et al., (2002) have reported the low expression of TP53 in normal cells and high expression of this gene in damaged/inflamed cells15. Fourth selected DNA damage response pathway gene is ATM in present study. ATM kinase is the key molecule for activation of TP53 after DNA damage16. The ATM protein responds to DNA damage by substrate phosphorylation. The activated ATM not only phosphorylates the members but also phosphorylates a variety of proteins together to form DNA damage repair machinery16,17. Accumulation of damaged DNA is linked to the less expression of DNA damage response proteins like ATM, TREX1, PARP1, and TP53. Studies have reported the involvement of selected genes in RA with inconsistent results10,11,12,13,14,15,16. Till date no study has been reported to figure out the combined effect of these genes in RA risk and progression. Present study is designed to assess the expression variation of ATM, TREX1, PARP1, and TP53 in RA patients compared to controls. Expression pattern of selected genes was correlated with different marker of RA such as C-reactive protein (CRP), erythrocyte sedimentation (ESR) and cyclic citrullinated peptide (anti-CCP) are mainly used detection markers for RA in the laboratory.
Already published meta-analyses and Genome-wide association studies (GWAS) have shown that the common disease-associated variants in the population may participate in a combined fashion to cause disease. These variants associated with high disease susceptibility may have diagnostic and clinical applications and may also help in better personalized medication of patients, resulting in better efficacy and success17. In second part of the study hotspot polymorphisms of PARP1 (Val76Ala), ATM (Pro1054Arg), TP53 (Ala138Val), and TREX (Tyr177Tyr) were selected and association of mutant alleles frequency with RA risk was observed.
d ().
Materials and methods
Sample collection
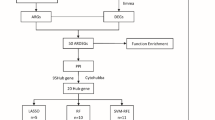
The study was conducted after ethical approval from the COMSATS University Islamabad and Combined Military Hospital. All experimental protocols were approved by the Ethical Review Committee of the Military Hospital, Rawalpindi (RTMC # RHE-2019-124−87) and complied with the Declaration of Helsinki guidelines and principles. Five hundred blood samples of RA patients and an equal number of control samples were collected. The study cohort in the present study was calculated using the G*power calculator (https://stats.oarc.ucla.edu/other/gpower/). Blood samples were collected after taking the participants’ and their legal guardians’ oral and written informed consent. The histopathological and demographic parameters of patients having RA and controls are provided in Table 1. Patients were categorized on basis of different demographic and clinicopathological parameters such as age (< 45years and ≤ 45yeras), gender (males and females), anti-cyclic citrullinated peptide status (anti-CCP negative and anti-CCP positive patients), erythrocyte sedimentation rate (ESR < 31 and ESR > 31), and C-reactive protein level (< 14 CRP and > 14 CRP). In case of treatments, patients were divided into two groups one receiving Methotrexate (10mgweek to 25mgweek) and other receiving biologics injections (etanercept 50 mg subcutaneously week), as mentioned in Table 1. SNP frequency and expression level of DNA damage response pathway genes are also compared with respect to two treatment modalities such as methotrexate and biologics used in RA patients.
DNA extraction
DNA was extracted from blood samples using the phenol-chloroform method. The blood was mixed with cell lysis buffer, it was incubated and centrifuged to separate a pellet that contained DNA. After many rounds of washing to get rid of red blood cells, the pellet was treated with Solution nuclear lysis buffer, SDS (sodium dodecyl sulfate), and proteinase K to digest it overnight. A phenol-chloroform-isoamyl alcohol mixture was used the following day to extract DNA, and Solution C, sodium acetate, and cold isopropanol were used for purification. The DNA was dried and cleaned with ethanol.
The extracted DNA was dissolved in 1x Tris-EDTA and stored at −20 °C. The spectrophotometer was used to quantify the DNA concentration.
Genetic analysis and primer designing
Four polymorphisms were selected from the SNP database for the genetic analysis of selected genes PARP1, ATM, TP53, and TREX1. SNPs were selected using the SNP selection tool (http://snpinfo.niehs.nih.gov/snpinfo/snpfunc.htm). Selection criteria for the SNPs were (i) Validated SNPs with minor allele frequency > 5% in Asian populations, (ii) SNPs selected from coding region and untranslated region of DNA damage response pathway genes; Val76Ala (coding sequence variant; PARP1), Pro1054Arg (coding sequence variant; ATM), Ala138Val (coding sequence variant; TP53), and Tyr177Tyr (3’UTR variant; TREX). Then the primers were designed for SNPs chosen, by using primer3 software as shown in Supplementary Table 1. The real-time PCR primers were designed using integrated DNA technology IDT primer software as shown in Supplementary Table 2.
PCR and mutational screening
Selected polymorphisms of these genes PARP1, ATM, TP53, and TREX1 were amplified through Tetra primers -ARMS-PCR. The tetra-primer amplification refractory mutation system PCR (ARMS-PCR) was used to amplify the targeted DNA regions. Using the mismatched strategy to generate the allele-specific response, this is a simple method for genotyping SNPs. The SNP primers were designed using Primer 1 software, the primers specificity was confirmed by Insilco PCR and primer Blast software. Further Validation and specificity of the primers was checked by running the primers at different optimizing conditions and condition specific for each primer set was selected and validate on 2% agarose gel by running the DNA ladder. PCR mixture comprised of DNA, 10 µM primers, FIREPol PCR Mix (Solisbio, Estonia) and PCR water. The primers were optimized. The PCR conditions were initial denaturation of 10 min at 95 °C, in cyclic stage 45 s of 94 °C denaturation followed by 45 s annealing and 1 min extension at 72 °C was performed. At the end 72 °C final extension was performed. For visualization of the DNA bands 2% Agarose gel electrophoresis was used for analysis of PCR results. Biodoc analyzer was used for determining the mutant, wild and heterozygous bands in each sample (Supplementary Fig. 1A-D).
RNA extraction and cDNA synthesis
The RNA was extracted using the Trizol method and this RNA was stored at −20 °C. Sections of tumor and control tissue from formalin-fixed paraffin-embedded blocks were deparaffinized with ethanol and xylene before being allowed to air dry. The tissues were digested using Trizol and Proteinase K, and then they were separated using chloroform. After being separated from the aqueous phase, RNA was precipitated at −20 °C using isopropanol and subsequently cleaned with ethanol. The RNA pellet was prepared for use downstream after drying and was stored at −20 °C.
RNA concentration was quantified by spectrophotometer and cDNA was synthesized using the commercially available kit (RevertAid First Strand cDNA Synthesis Kit, Thermo Scientific USA).
Expression analysis
The quantitative real-time PCR was used for the selected genes expression analysis. The mixture was prepared in reaction tubes containing cDNA, primers, SYBR green (Syber Green qPCR master mix, Thermo Scientific USA), and RNase-free water. PCR was run according to optimized conditions. The ACTB and GAPDH were used as references. The reference (usually a housekeeping gene) is used to normalize the target gene values, using internal reference genes helps keep things consistent across samples, so the data we get is more reliable18. The amplification efficiency of primer was calculated by running the melt curve analysis (Supplementary Fig. 2 A) and by providing the amplification plot (Supplementary Fig. 2B-E).
Statistical analysis
The GraphPad Prism and SPSS software were used for suitable statistical analysis. Logistic regression analysis was performed to measure the association of mutant genotypes of DNA damage response pathway genes SNP with the risk of RA pathogenesis (p < 0.05). The association of genes with other parameters i.e. age and gender, were performed by applying the student t-test. The diagnostic value was estimated using the receiver operating characteristic (ROC) curve analysis, ROC curve plots a diagnostic test’s sensitivity against its 1 − specificity. Student t-test was used to compare the expression level of DNA damage response pathway genes in RA patients receiving methotrexate and patients receiving biologics. Spearman correlation was performed for gene-to-gene correlation. P-values were get from the t-test with significance level p < 0.05.
Results
Expression analysis of selected genes in RA patients and controls
The relative expression of PARP1, ATM, TP53, and TREX1 genes was determined in RA patient and control samples using q-PCR. Results are shown in Figs. 1, 2, 3 and 4.
Expression analysis of PARP1 gene in RA patients. (A) Significant downregulation of PARP1 gene was observed in RA compared to controls. (B) Non significant association of PARP1 gene with different gender (males vs. females) and age group (< 45years vs. > 45years) of RA patients. (C) Significant deregulation of PARP1 gene in different clinicopathological parameters of RA patients such as Anti-CCP, ESR, and CRP. (D) Significant downregulation of PARP1 gene was observed in RA patients using the different treatment modalities such as methotrexate and biologics. Level of significance p < 0.05.
PARP1 expression analysis
The expression level of PARP1 was determined in RA patients compared to controls and significantly downregulated (p < 0.0001) expression was observed in RA patients compared to controls, as shown in Fig. 1A. Expression level of PARP1 gene was also compared with demographic and clinicopathological parameters of RA patients In the case of gender and age groups, a non-significant upregulation was observed in case of gender (males vs. females, p = 0.54) and different age groups of RA patients (< 45 vs. ≥ 45 years, p = 0.13) as shown in Fig. 1B. In case of clinicopathological parameters of RA patients, upregulated expression level of PARP1 was found in patients with different anti-cyclic citrullinated peptide status (anti-CCP negative vs. anti-CCP positive patients (p < 0.0001), different erythrocyte sedimentation rate (ESR < 31 vs. ESR > 31 (p < 0.0001), and different C-reactive protein level (< 14 CRP vs. > 14 CRP; p < 0.0006). The results are shown in Fig. 1C. Based on treatments strategies used for RA, patients were divided into two groups, one receiving the methotrexate, and other receiving biologics group. In association with these treatment strategies, significant upregulated expression of PARP1 gene was observed in the biologics group compared to the methotrexate (< 0.0001), as shown in Fig. 1D.
ATM expression analysis
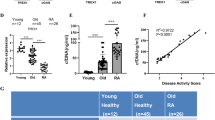
The expression pattern of the ATM gene was assessed in RA patients and controls, as shown in Fig. 2A. Significant downregulation of the ATM gene was observed in RA patients compared to the controls (p < 0.0001), as shown in Fig. 2A. In the present study, RA patients were further categorized into different groups on the basis of demographic and clinicopathological parameters. Non-significant upregulation of ATM was observed in gender groups (males vs. females; p = 0.46) and different age groups (< 45 years and ≥ 45 years; p = 0.98), as shown in Fig. 2B. In case of clinicopathological parameters, the ATM was found significantly elevated in patients with different group of anti-CCP (anti-CCP negative vs. to anti-CCP positive; p < 0.0001), different ESR group, (ESR < 31 vs. ESR > 31; (p < 0.0001), different CRP levels (< 14 CRP vs. ≥ 14; p < 0.0006). The results are shown in Fig. 2C. In case of treatment group, the ATM gene was found significantly upregulated in patients receiving biologics compared to patients receiving the methotrexate group (p < 0.0001), as shown in Fig. 2D.
Expression analysis of ATM gene in RA patients. (A) Significant downregulation of ATM gene was observed in RA patients compared to controls. (B) Non-significant association of ATM gene with different gender (males vs. females) and age group (< 45years vs. > 45years) of RA patients. (C) Significant deregulation of the ATM gene was observed in different clinicopathological parameters of RA patients such as Anti-CCP, ESR, and CRP. (D) Significant downregulation of ATM gene was observed in RA patients using the methotrexate compared to patients using biologics. Level of significance p < 0.05.
TP53 expression analysis
The expression level of TP53, the third gene selected, was also estimated in RA patients compared to controls, as shown in Fig. 3. Significant downregulated expression of TP53 was observed in RA patients compared to the control samples (p < 0.0001), as shown in Fig. 3A. Expression level of TP53 was assessed with respect to different demographic and clinicopathological parameters of RA patients. No significant difference in expression level of TP53 was observed in different gender group (males vs. female; p = 0.50) and age group (< 45 years and ≥ 45 years; p = 0.72) of RA patients as shown in Fig. 3B. In case of clinicopathological parameters, significant upregulated expression of TP53 was observed in patients with different anti-CCP status (anti-CCP negative compared to anti-CCP positive; p < 0.001), with different ESR levels (ESR < 31 vs. ≥ 31; p < 0.006), and with different CRP levels (CRP < 14 CRP vs. ≥ 14 CRP samples; p < 0.0001). The results are shown in Fig. 3C. TP53 expression level was also associated with different treatment modalities for RA patients such as methotrexate and biologics. Significant upregulated expression of TP53 was observed in patients receiving the biologics compared to the patients receiving methotrexate group (p < 0.0001), as shown in Fig. 3D.
Expression analysis of TP53 gene in RA patients. (A) Significant downregulation of TP53 gene was observed in RA compared to controls. (B) Non-significant downregulation TP53 gene in different gender (males vs. females) and age group (< 45years vs. > 45years) of RA patients. (C) Significant downregulated expression of the TP53 gene was observed in different clinicopathological parameters of RA patients such as Anti-CCP, ESR, and CRP. (D) Significant downregulated expression level of TP53 gene was observed in patients using the methotrexate compared to patients using biologics. Level of significance p < 0.05.
Expression analysis of TREX1 gene in RA patients. (A) Significant downregulation of TREX1 gene was observed in RA compared to controls. (B) Non-significant difference in the expression level of the TREX1 gene was observed in different gender (males vs. females) and age group (< 45years vs. > 45years) of RA patients. (C) Significant downregulated expression level of the TREX1 gene was observed in different clinicopathological parameters of RA patients such as Anti-CCP, ESR, and CRP. (D) Significant downregulated expression level of the TREX1 gene was observed in patients using the methotrexate compared to patients using biologics. Level of significance p < 0.05.
TREX1 expression analysis
The expression level of fourth selected gene, TREX1 was observed in RA patients and controls. Expression pattern of TREX1 was observed significantly downregulated in RA patients compared to controls, as shown in Fig. 4A. Expression level of TREX1 was assessed with respect to different demographic and clinicopathological parameters of RA patients. RA patients was categorized into two groups on the basis of age and gender. Non-significant downregulation of TREX1 was observed in different gender group (males vs. females; p < 0.39) and age group (< 45 years vs. ≥ 45 years; p < 0.04), as shown in Fig. 4B. In case of clinicopathological parameters, significant increased level of TREX1 was observed in patients with different anti-CCP status (anti-CCP negative vs. anti-CCP; p < 0.001), different ESR levels (ESR ≥ 31 vs. ESR < 31; p < 0.001), and different CRP levels (< 14 CRP vs. ≥ 14 CRP; p < 0.02). The results are shown in Fig. 4C. In case of different treatment modalities give to RA patients, the TREX1 gene was found significantly upregulated in patients receiving biologics compared to the patients receiving methotrexate group (p < 0.04), as shown in Fig. 4D.
Spearman correlation of selected genes
The expression levels of PARP1, ATM, TP53. and TREX1 genes were correlated in RA patients using Spearman correlation analysis (Table 2). A significant positive correlation was observed between PARP1 versus ATM (r = 0.49 p = 0.0004), PARP1 versus TP53 (r = 0.51 p = 0.0002), PARP1 versus TREX1 (r = 0.35 p = 0.01), ATM versus TP53 (r = 0.604 p < 0.0001), ATM versus TREX1 (r = 0.55 p < 0.0001) and TP53 versus TREX1 (r = 0.47 p = 0.0007). The results are shown in Table 2.
Diagnostic significance of selected genes in RA patients
The diagnostic value of selected genes PARP1, ATM, TP53, and TREX1 was also assessed using receiver operating characteristic (ROC) curve analysis. The ROC curve compares the performance of two or more diagnostic tests and evaluates a test’s overall diagnostic performance. It is also employed to choose the best cut-off value for identifying if a disease is present or not. ROC curve analysis showed the 100% sensitivity and specificity were observed for PARP1, ATM, and TP53 in RA patients, as shown in Fig. 5A-C.
Measurement of diagnostic value of selected genes in RA patients using the ROC curve analysis. Area under the curve (AUC) was generated to measure the diagnostic value of (A) PARP1 (specificity 100% and sensitivity 100%), (B) ATM (specificity 100% and sensitivity 100%), (C) TP53 PARP1 (specificity 100% and sensitivity 100.
SNP analysis of selected polymorphisms in study cohort
In second part of present study, four SNPs were selected for the PARP1 (Val76Ala), ATM (Pro1054Arg), TP53 (Ala138Val), and TREX1 (Tyr177Tyr). These selected SNPs were screened in 500 RA patients and healthy controls. Frequency of wild and mutant allele was calculated in patients and controls. Statistical analysis was performed using the logistic regression analysis and shown in Table 3. Statistical analysis results showed that the frequency of the mutant genotype of PARP1 (Val762Ala; CC, OR 3.89; 95%CI 2.77–5.45; p < 0.0001), ATM (Pro1054Arg; GG, OR 8.87; 95%CI 5.55–14.18; p < 0.0001), TP53 (Ala138Val; TT, OR 12.22; 95%CI 6.79–21.98; p < 0.0001), and TREX1 (Tyr177Tyr; GG, OR 3.88; 95%CI 2.59–5.80; p < 0.0001) was observed significantly higher in RA patients compared to the controls. The results are shown in Table 3.
Haplotype analysis of selected SNPs
Haplotype analysis for selected SNPs was performed using haploview 4.2. The haplotypes were generated for selected polymorphisms of PARP1 (Val76Ala), ATM (Pro1054Arg), TP53 (Ala138Val), and TREX1 (Tyr177Tyr) among patients and controls. A total of nine haplotypes were generated and shown in Table 4. Among the nine haplotypes, CCTG (p = 7.34e-014) haplotype was associated with an increased risk of RA. In contrast, CGTG and TCCA (p = 8.88e-016) haplotypes were associated with a decreased risk of RA in patients. The results are summarized in Table 4.
Linkage disequilibrium was generated using the haploview 4. Software and shown in Fig. 6. Results showed that all the SNPs are in strong linkage disequilibrium in RA patients compared with controls as shown in Fig. 6.
Discussion
Rheumatoid arthritis (RA), a chronic inflammatory disease affecting synovial joints, is caused due to inadequate immune response19. RA pathogenesis is caused by different genetic, epigenetic and environmental factor variations whose details are still needs to be explore20. Among genetic factors, DNA damage pathway genes play an important role in the pathogenesis of RA21,22,23. Genetic abnormalities in DNA damage pathway genes, including polymorphisms/variations and expression deregulation, lead to an impaired DNA damage response and generation of oxidative stress. Consequently, environmental, and genetic stress has been converted to RA-related immune dysfunction through the premature apoptosis of T cells24.
Previous studies have reported the involvement of different DNA repair pathway genes in pathogenesis of RA, but results are inconsistent. Furthermore, most of the published studies on RA has focused on immune system malfunction and joint damage9 resulting in lack of knowledge regarding the involvement of DNA damage pathway genes as a biomarker for this commonly occurring disease. The scarcity of data concerning DNA damage pathway genes has created a gap in the knowledge about the effective treatment and individual susceptibility to RA.
Present study is designed to assess the expression deregulation and genetic variation of DNA repair pathway genes (PARP1, ATM, TP53, and TREX1) in RA patients. In the first phase of the present study, we examined the association of single nucleotide polymorphisms (SNPs) for DNA damage pathway genes, including PARP1 (Val762Ala, coding sequence variant), ATM (Pro1054Arg, coding sequence variant), TP53 (Ala138Val, coding sequence variant), and TREX1 (Tyr177Tyr, 3’UTR variant) in RA patients. We found a strong association between these SNPs and susceptibility to RA, and a strong linkage disequilibrium was observed between selected SNPs.
The Val762Ala/rs1136410 (PARP1) polymorphism has been reported to affect the PARP1 gene and results in functional impairment25. In present study mutant allele frequency of the PARP1 (Val76Ala) gene was observed significantly higher in RA patients compared to controls. The PARP1 (Val762Ala) polymorphism has been studied in many diseases25,26 and found associated with an increased risk of gastric and thyroid cancers, particularly in the Asian population25,26,27. Another study has reported the association of Val762Ala with an increased risk of different inflammatory conditions such as coronary artery disease28,. Few studies have reported the association of Val76Ala with RA, with conflicting results across various ethnic groups. Hur et al., (2006) have reported that Val76Ala is associated with the risk of RA in the Korean population29. Another study by Onaran et al., (2009) has found no association of this polymorphism with the Turkish population30. Our study suggested the involvement of Val762Ala in RA increased risk and susceptibility. One possible mechanism of its involvement in RA is the location of Val762Ala is in the catalytic domain of PARP1 and its influences on the enzyme kinetic activity of said gene31. This SNP may affect the activation and binding mechanisms of the PARP1 gene, leading to expression deregulation of inflammatory genes, which can result in an inflammatory response manifesting as RA32.
ATM selected SNP (Pro1054Arg), located in exon splicing sites and can alter gene functioning by disrupting alternative splicing33. Our present study observed the significant higher frequency of mutant allele of (Pro1054Arg in RA patients compared to controls. ATM SNP Pro1054Arg, has been extensively studied in various cancers, including breast cancer, renal carcinoma, and thyroid cancer. It has been reported that this SNP has an association with cancer susceptibility34,35,36,37. Various SNPs of ATM have been reported to affect inflammatory diseases. However, a few studies have reported an association between ATM SNPs and RA susceptibility24. Till date no studies have been reported on the association of ATM SNP Pro1054Arg with RA susceptibility. Present study suggests an association between the mutant allele frequency of Pro1054Arg and RA susceptibility. One possible mechanism behind this could be that the downregulation of the ATM protein due to SNPs present in the exon splicing region leads to unrepaired damaged DNA in T-cells, causing premature apoptosis of T-cells and, consequently, inflammation leading to RA susceptibility24. Moreover, bone density is also lost due to decreased expression of ATM in B cells, contributing to the pathophysiology of RA38.
Ala138Val located in catalytic domain of TP53 and increased mutant allele frequency of Ala138Val contributes to reduced gene activity and increased disease pathogenesis39. Our present study observed significant increased mutant allele frequency of Ala138Val () in RA patients compared to controls. Ala138Val has been comprehensively studied in various diseases including Li-Fraumeni syndrome (LFS), glioma cancer, and breast cancer40,41,42,47,50. However, no study has been reported on its association with any inflammatory disease. The present study suggests the association of increased mutant allele frequencyof Ala138Val with RA susceptibility. Mousavi et al., (2023) have reported that TP53 mutations contribute to the pathogenicity of RA43. TP53 and NF-κB antagonize each other and these mutations can lead to over-activated NF-κB which causes chronic inflammation and increased risk of RA pathogenesis44.
The Tyr177Tyr variant is a common synonymous SNP of TREX1 which can influence the normal exonuclease activity of said gene Tyr177Tyr45. Loss of function of TREX causes interferopathy in humans and mice46. Our present study found that the mutant allele frequency of TREX SNP (Tyr177Tyr) was found significantly higher in RA patients compared to controls. Studies have reported the association of mutant allele frequency of Tyr177Tyr with different autoimmune disease and cancer such as Primary Sjogren’s syndrome, breast and ovarian cancer45,47. No study has reported the association of this SNP with the susceptibility of RA. However, the present study suggests a possible role of TREX Tyr177Tyr in the increased risk of RA. TREX Tyr177Tyr is involved in inflammations and immune responsesand has been reported to contribute to increased production of type 1 interferon (IFN)48 which ultimately resultin pathogenesis of RA49.
In the second phase of the present study, we assessed the expression levels of PARP1, ATM, TP53, and TREX1 genes and significant downregulated expression level was observed. Observed expression deregulations was correlated with different treatment modalities such as methotrexate and biologics. All genes were upregulated in the group treated with biologics compared to those treated with methotrexate. Additionally, gene-to-gene interactions between PARP1, ATM, TP53, and TREX1 were observed, and the diagnostic specificity of these genes was also analyzed.
PARP1, ATM, TP53, and TREX1 play multifaceted roles by influencing DNA repair, inflammation, cell cycle regulation, oxidative stress response, and immune system function and contribute in various diseases, including cancer and inflammatory diseases23,51. PARP1 has been upregulated in different cancers and inflammatory diseases. Previous studies have reported that upregulated PARP1 activates the hypoxia by expression variations of HIF1α and HIF2α and increase the aggressiveness of the disease52,53. Li et al., (2016) have reported the upregulation of the PARP1 gene in RA patients by modulation of NF-κB and pro-inflammatory gene. This ultimately results in increased expression of inflammatory cytokines, and may contribute to chronic inflammation in the joints54. However, the results of the expression deregulation analysis from the present study showed downregulation of mRNA expression of PARP1 in RA patients compared to healthy controls. One possible reason for downregulation of PARP1 gene in present study is increased frequency of mutant allele of Val762Ala(rs1136410) in RA patients.
Rondeau et al. (2015) have reported the downregulation of ATM in breast cancer and suggested it as a good prognostic marker for breast cancer55. However, a few other studies have reported no significant difference in the expression level of ATM in colorectal and gastric cancer patients56,57. Reason behind this downregulation is still needs to explore.Shao et al., (2015) have reported that SNPs in the important domain of ATM gene may contribute the downregulated expression of said gene57. Studies have reported the that downregulation of ATM was linked with earlier apoptosis of T cells and loss of bone density in RA patients and can act as the poor prognostic factor35,36.
In present study, TP53is third selected gene which plays a vital role in the cell cycle, apoptosis, and maintaining genomic stability. In present study significant downregulated expression of TP53 was observed in RA patients compared to controls. Studies have reported the downregulated of TP53 in various diseases, such as t cancers and inflammatory diseases58. Peng et al. (2022) have found upregulated expression of TP53 in the synovial joints of RA patients59. Previous studies have reported that upregulated expression of TP53 can potentially modulate the inflammatory response by reducing the production of pro-inflammatory cytokines and dampening chronic inflammation59,60.
In the present study, significant downregulated expression of TREX1 was observed in the present study. Similar expression of TREX1 gene has been reported in many diseases, including cancer, autoimmune diseases, and RA47,50. Downregulation of TREX1 can disrupt various important functions, including immune response and DNA damage repair46. This downregulation could be due to transcriptional inactivation or mutations such as SNPs in the gene50. In the present study, the selected SNPs could be a potential reason for the downregulation of gene expressions; however, the detailed mechanism still needs to be explored.
Pro1054ArgTyr177TyrPresent study showed that mutant frequency of selected SNP of PARP1 (Val76Ala), ATM (Pro1054Arg), TP53 (Ala138Val), and TREX1 (Tyr177Tyr) was found associated with increased risk of RA. Furthermore, significant deregulation of selected DNA damage repair pathway gene was also observed in present study.
Conclusion
In conclusion, this study highlights the significant role of DNA damage pathway genesin the susceptibility to rheumatoid arthritis (RA). The study sheds light on the mechanisms by which these genetic variations contribute to RA. The PARP1 Val762Ala polymorphism, known for impairing PARP1 functionality, may influence the expression of inflammatory genes, leading to RA-related inflammation. Similarly, the ATM Pro1054Arg SNP may disrupt alternative splicing, resulting in impaired DNA damage response and premature T-cell apoptosis, contributing to RA susceptibility. The TP53 Ala138Val SNP, associated with reduced TP53 gene activity, and the TREX Tyr177Tyr variant, linked to increased type 1 interferon production, further underlines the complex genetic interplay in RA development. Moreover, our study reveals that these genes are downregulated in RA patients, with expression levels significantly higher in those treated with biologics compared to methotrexate. This suggests that biological treatments may mitigate some of the genetic and epigenetic disruptions seen in RA. ROC curve analysis showed that deregulation of PARP1, ATM, TP53 and TREX1 can act as the good diagnostic markers. One of study limitation in finding the diagnostic value of selected genes is difficult to differentiate the effects of treatment from those of the disease itself.
The identification and understanding of these genetic factors provide valuable insights into pathogenesis of RA and offer potential targets for therapeutic interventions. Future research should continue to explore detailed mechanisms of various treatments on these pathways to develop more effective strategies for managing RA.
Data availability
All data generated or analysed during this study are included in this published article.
References
Mun, S. et al. Proteomics approach for the discovery of rheumatoid arthritis biomarkers using mass spectrometry. Int. J. Mol. Sci. 20 (18), 4368 (2019).
Radu, A. F. & Bungau, S. G. Management of rheumatoid arthritis: an overview. Cells 10 (11), 2857 (2021).
Padyukov, L. Genetics of rheumatoid arthritis. In Seminars in immunopathology. 44, 47–62 (2022).
Mukhtar, M. et al. Vitamin D Receptor Gene Polymorphism: an Important Predictor of Arthritis Development (BioMed research international, 2019).
Toledano, E. et al. A meta-analysis of mortality in rheumatic diseases. Reumatologia Clin. 8 (6), 334–341 (2012).
Curtis, J. R. & Singh, J. A. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin. Ther. 33 (6), 679–707. https://doi.org/10.1016/j.clinthera.2011.05.044 (2011).
Zhang, J. et al. Impact of biologic agents with and without concomitant methotrexate and at reduced doses in older rheumatoid arthritis patients. Arthritis Care Res. (Hoboken). 67 (5), 624–632. https://doi.org/10.1002/acr.22510 (2015).
Singh, P., Kumar, A. & Chandra, P. Rheumatoid factor versus anti - cyclic citrullinated peptide antibody as screening tool for rheumatoid arthritis in an ophthalmic clinic. Indian J. Ophthalmol. 68 (1), 236–238. https://doi.org/10.4103/ijo.IJO_526_19 (2020).
Hassan Sr, W. M. & Oxidative, D. N. A. Damage and zinc status in patients with rheumatoid arthritis in Duhok Iraq. Cureus 16(1), 1–7 (2024).
Beck, C., Robert, I., Reina-San-Martin, B., Schreiber, V. & Dantzer, F. Poly (ADP-ribose) polymerases in double-strand break repair: focus on PARP1, PARP2 and PARP3. Exp. Cell. Res. 329 (1), 18–25 (2014).
García, S. & Conde, C. The role of Poly (ADP-ribose) Polymerase-1 in rheumatoid arthritis. Mediators Inflamm. 2015 (1), 837250 (2015).
Ghodke-Puranik, Y. & Niewold, T. B. Immunogenetics of systemic lupus erythematosus: A comprehensive review. J. Autoimmun. 64, 125–136. https://doi.org/10.1016/j.jaut.2015.08.004 (2015).
Crow, Y. J. & Rehwinkel, J. Aicardi-Goutieres syndrome and related phenotypes: linking nucleic acid metabolism with autoimmunity. Hum. Mol. Genet. 18, 130–136 (2009).
Wang, Y., Luo, W. & Wang, Y. PARP-1 and its associated nucleases in DNA damage response. DNA Repair. 81, 102651 (2019).
Yamanishi, Y. et al. Regulation of joint destruction and inflammation by p53 in collagen-induced arthritis. Am. J. Pathol. 160 (1), 123–130 (2002).
Cheng, Q. & Chen, J. Mechanism of p53 stabilization by ATM after DNA damage. Cell. Cycle. 9 (3), 472–478 (2010).
Dedmon, L. E. The genetics of rheumatoid arthritis. J. Rheumatol. 59 (10), 2661–2670 (2020).
Ling, D., Salvaterra, P. M. & Robust RT-qPCR data normalization: validation and selection of internal reference genes during post-experimental data analysis. PloS one, 6(3), e17762. (2011).
Debreova, M. et al. Rheumatoid arthritis: from synovium biology to cell-based therapy. Cytotherapy 24 (4), 365–375 (2022).
Souliotis, V. L., Vlachogiannis, N. I., Pappa, M., Argyriou, A. & Sfikakis, P. P. DNA damage accumulation, defective chromatin organization and deficient DNA repair capacity in patients with rheumatoid arthritis. Clin. Immunol. 203, 28–36 (2019).
Arya, R. & Bassing, C. H. V (D) J recombination exploits DNA damage responses to promote immunity. Trends Genet. 33 (7), 479–489 (2017).
Fang, Q., Zhou, C. & Nandakumar, K. S. Molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediators Inflamm. 2020 (1) 3830212 (2020).
Taghadosi, M., Adib, M., Jamshidi, A., Mahmoudi, M. & Farhadi, E. The p53 status in rheumatoid arthritis with focus on fibroblast-like synoviocytes. Immunol. Res. 69 (3), 225–238 (2021).
Shao, L. DNA damage response signals transduce stress from rheumatoid arthritis risk factors into T cell dysfunction. Front. Immunol. 9, 415589 (2018).
Hua, R. et al. Association between the PARP1 Val762Ala polymorphism and cancer risk: evidence from 43 studies. PLoS One 9(1), e87057 (2014).
Bashir, K., Sarwar, R., Saeed, S., Mahjabeen, I. & Kayani, M. A. Interaction among susceptibility genotypes of PARP1 SNPs in thyroid carcinoma. PLoS One 13(9), e0199007 (2018).
Zhang, Q. et al. PARP-1 Val762Ala polymorphism, CagA + H. Pylori infection and risk for gastric cancer in Han Chinese population. Mol. Biol. Rep. 36, 1461–1467 (2009).
Wang, X. B. et al. PARP-1 variant Rs1136410 confers protection against coronary artery disease in a Chinese Han population: a two-stage case-control study involving 5643 subjects. Front. Physiol. 8, 916 (2017).
Hur, J. W. et al. Poly (ADP-ribose) polymerase (PARP) polymorphisms associated with nephritis and arthritis in systemic lupus erythematosus. Rheumatol 45 (6), 711–717 (2006).
Onaran, İ. et al. The Val762Ala Polymorphism in the Poly (ADP-ribose) Polymerase-1 gene is not associated with susceptibility in Turkish rheumatoid arthritis patients. Rheumatol. Int. 29, 797–800 (2009).
Kauppinen, T. M. Multiple roles for Poly (ADP-ribose) Polymerase-1 in neurological disease. Neurochem Int. 50 (7–8), 954–958 (2007).
Koc, A. et al. Association of three SNPs in the PARP-1 gene with hashimoto’s thyroiditis. Hum. Genome Var. 1 (1), 1–6 (2014).
Qian, D. et al. A pleiotropic ATM variant (rs1800057 C > G) is associated with risk of multiple cancers. Carcinogenesis 43 (1), 60–66 (2022).
Mehmood, A., Kayani, M. A., Ahmed, M. W., Nisar, A. & Mahjabeen, I. Association between single nucleotide polymorphisms of DNA damage response pathway genes and increased risk in breast cancer. Future Oncol. 16 (26), 1977–1995 (2020).
Navinchandra, S. A. et al. ATM polymorphisms and their relationship to radiation toxicity in breast Cancer patients. Braz Appl. Sci. Rev. 4 (5), 3123–3148 (2020).
Bensouilah, F. Z. et al. Association of single nucleotide polymorphisms with renal cell carcinoma in Algerian population. AFR. J. UROL. 26, 1–8 (2020).
Gielecińska, A., Kciuk, M., Kołat, D., Kruczkowska, W. & Kontek, R. Polymorphisms of DNA repair genes in thyroid Cancer. Int J. Mol. Sci 25(11), 5995 (2024).
Mensah, K. A. et al. Impaired ATM activation in B cells is associated with bone resorption in rheumatoid arthritis. Sci. Transl Med. 11 (519), 4626 (2019).
Xavier, C. B. et al. Suspected germline TP53 variants and clonal hematopoiesis of indeterminate potential: lessons learned from a molecular tumor board. Oncologist 28 (7), 624–627 (2023).
Szeliga, M., Bogacińska-Karaś, M., Kuźmicz, K., Rola, R. & Albrecht, J. Downregulation of GLS2 in glioblastoma cells is related to DNA hypermethylation but not to the p53 status. Mol. Carcinog. 55 (9), 1309–1316 (2016).
Fortuno, C. et al. A quantitative model to predict pathogenicity of missense variants in the TP53 gene. Hum. Mutat. 40 (6), 788–800 (2019).
Song, R. et al. Clinical features of Li-Fraumeni syndrome in Korea. Cancer Res. Treat. 56 (1), 334 (2024).
Mousavi, M. J. et al. Transformation of fibroblast-like synoviocytes in rheumatoid arthritis; from a friend to foe. Auto Immun. Highlights. 12, 1–13 (2021).
Cooks, T., Harris, C. C. & Oren, M. Caught in the cross fire: p53 in inflammation. Carcinogenesis 35 (8), 1680–1690 (2014).
Nezos, A. et al. TREX1 variants in sjogren’s syndrome related lymphomagenesis. Cytokine 132, 154781 (2020).
Chauvin, S. D. et al. Inherited C-terminal TREX1 variants disrupt homology-directed repair to cause senescence and DNA damage phenotypes in drosophila, mice, and humans. Nat. Commun. 15 (1), 1–23 (2024).
Namjou, B. et al. Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun. 12 (4), 270–279 (2011).
Queiroz, M. A. F. TREX1 531 C/T polymorphism and autoantibodies associated with the immune status of HIV-1-infected individuals. Int. J. Mol. Sci. 24 (11), 9660 (2023).
Castañeda-Delgado, J. E. et al. Type I interferon gene response is increased in early and established rheumatoid arthritis and correlates with autoantibody production. Front. Immunol. 8, 285 (2017).
Neidhart, M., Karouzakis, E., Schumann, G. G., Gay, R. E. & Gay, S. Trex-1 deficiency in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 62 (9), 2673–2679 (2010).
Pazzaglia, S. & Pioli, C. Multifaceted role of PARP-1 in DNA repair and inflammation: pathological and therapeutic implications in cancer and non-cancer diseases. Cells 9 (1), 41 (2019).
Gil-Kulik, P. et al. Different regulation of PARP1, PARP2, PARP3 and TRPM2 genes expression in acute myeloid leukemia cells. BMC cancer. 20, 1–9 (2020).
Kupczyk, P. et al. PARP1 as a marker of an aggressive clinical phenotype in cutaneous melanoma—a clinical and an in vitro study. Cells 10 (2), 286 (2021).
Li, G. The rheumatoid arthritis risk variant CCR6DNP regulates CCR6 via PARP-1. PLoS Genet. 12(9), e1006292 (2016).
Rondeau, S. ATM has a major role in the double-strand break repair pathway dysregulation in sporadic breast carcinomas and is an independent prognostic marker at both mRNA and protein levels. Br. J. Cancer. 112 (6), 1059–1066 (2015).
Xiong, H. & Zhang, J. Expression and clinical significance of ATM and PUMA gene in patients with colorectal cancer. Oncol. Lett. 14 (6), 7825–7828 (2017).
Pádua, J. D. B. mRNA expression and methylation of the RAD51, ATM, ATR, BRCA1, and BRCA2 genes in gastric adenocarcinoma. Biomark 19, 11772719231225206 (2024).
Shao, L. et al. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J. Exp. Med. 206 (6), 1435–1449 (2009).
Peng, C. Y., Hu, L., Wu, Z. J., Wang, J. & Cai, R. L. Effects of moxibustion on p53, SLC7A11, and GPX4 expression in synovial tissues of rats with adjuvant arthritis. J. Acupunct. Res. 47 (1), 21–26 (2022).
Malemud, C. J., Haque, A., Louis, N. A. & Wang, J. Immune response and apoptosis–introduction. J. Clin. Cell. Immunol. 3, e001 (2012).
Acknowledgements
The authors are thankful to the patients and staff of CMH Multan Institute of Medical Sciences, for their contribution to this research.
Author information
Authors and Affiliations
Contributions
All of the authors read and approved the final version of manuscript. MZH, MSH, and MHK collected and isolated the exosomes, DNA and RNA samples. RH, AM, and MFH performed the expression analysis study. IM and RS performed statistical analyses of the data and draft of the manuscript. IM supervised the project and provided critical revisions. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hussain, M.Z., Khan, M.H., Haris, M.S. et al. Association of DNA damage response pathway genes with rheumatoid arthritis risks: a case-control study. Sci Rep 15, 20937 (2025). https://doi.org/10.1038/s41598-025-06656-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06656-9