Abstract
Non-steroidal anti-inflammatory drugs (NSAIDs) function as antipyretic, analgesic and anti-inflammatory agents. However, its long application might result in gastrointestinal side effects. Therefore, it is imperative to hunt for novel and safer NSAIDs. Here, we report a new class of NSAIDs, M464, which was equipped with a hydrogen sulfide-releasing radical. We delved into its anti-inflammatory properties and underlying molecular mechanisms. In vitro, the relevance of the anti-inflammatory effects of M464 through the NLRP3 signaling pathway was explored by lactate dehydrogenase (LDH) release, WB (Western Blotting), ELISA assays and immunofluorescence experiments. In vivo, the influence and correlation of M464 on liver and lung tissue injury were investigated by means of histological evaluation, pathological analysis and serum index analysis. The correlation between M464 and NLRP3 signaling pathway in vivo injury model and its mechanism were investigated by Western Blotting and qPCR. In vitro results showed that M464 inactivates the assembly of the NLRP3 inflammasome, reduces the cleavage of Caspase-1 and the production of mature IL-1β, alleviates the generation of intracellular ROS, and decreases the oligomerization of the apoptosis-associated speck-like protein containing a CARD (ASC). In vivo results showed that M464 can down-regulate gene and protein expression levels of IL-1β and Caspase-1 in mice liver and lung injury models. M464 exhibits protective effects against acute lung and liver injury in mice, suggesting that M464 may be an alternative strategy to treat diseases driven by NLRP3 inflammasome activation.
Similar content being viewed by others
Introduction
Classical non-steroidal anti-inflammatory drugs (NSAIDs) play antipyretic, analgesic and anti-inflammatory roles primarily by the inhibition of PGHS-1 and PGHS-2 (Prostaglandin H Synthases-1 and -2),which participates in the biosynthesis of prostaglandin (PG)1. Currently, NSAIDs are usually prescribed for patients undergoing pain and inflammatory conditions such as long-time pain, rheumatoid arthritis, osteoarthritis, menstrual cramps, postoperative surgical conditions. While it is valid in pain and inflammation palliation, adverse effects such as gastrointestinal (GI) bleeding and ulceration have been identified, which restrict its long-term clinical use2,3.
In order to solve this problem, some new small molecules were introduced (e.g. selective or dual COX-2 inhibitors)4. However, their application was found to be involved in cardiovascular events. Recently, there is a novel category known as NO-NSAIDs5, but its safety profile has not been clearly established when compared to traditional NSAIDs. The safety of NO-NSAIDs still needs further validation through long-term clinical trials6,7. This underscores the complexity of finding new NSAIDs that are both safe and effective. In summary, despite advancements in R&D, the demand for safe and efficient NSAIDs is still an ongoing journey.
Hydrogen sulfide (H2S) was once well-known for its notorious foul smell. However, recent studies have demonstrated that H2S is a gaseous signaling molecule with significant biological functions. H2S is endogenously engendered in mammals from homocysteine and L-cysteine primarily by two pyridoxal-5′-phosphate (PLP)-dependent enzymes, i.e. CBS (cystathionine β-synthase) and CSE (cystathionine γ-lyase)8. One notable aspect of hydrogen sulfide is its involvement in cardiovascular health9. It acts as a vasodilator, promoting the relaxation of blood vessels and improving blood flow, which can help lower blood pressure and reduce the risk of cardiovascular diseases such as hypertension and atherosclerosis10,11,12. In addition to this, another beneficial role of hydrogen sulfide lies in its antioxidant and anti-inflammatory properties. Recently, findings have shown that hydrogen sulfide can inhibit the activation of inflammatory signaling pathways, such as nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs), which prevents the production of inflammatory mediators13. It has been shown to alleviate inflammation in conditions such as arthritis, colitis, and neuroinflammatory disorders14. By reducing inflammation, hydrogen sulfide may help mitigate symptoms and contribute to the overall management of these diseases.
The NLRP3 inflammasome stands as a pivotal component within the innate immune system, holding a critical role in orchestrating the regulation of inflammation. This multi-protein complex comes into formation in response to diverse danger signals, such as infection, cellular damage or stress. Comprising the NOD-like receptor protein 3 (NLRP3), the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD), and Caspase-1, this multiprotein complex collaborates intricately to initiate and modulate inflammatory responses, crucial for maintaining immune homeostasis15,16. The activated NLRP3 inflammasome promotes self-cleavage and triggers Pro-Caspase-1, leading to the secretion of the pro-inflammatory factors IL-1β/18. Caspase-1 also splits gasdermin D (GSDMD) into N-terminus GSDMD (N-GSDMD), which results in the creation of pores in the cell membrane and elicits pyroptosis, releasing inflammatory cytokines IL-1β to further potentiate the inflammatory reactions17,18,19.
Dysregulation of the NLRP3 inflammasome signaling pathway is involved in the pathogenesis of multiple human autoimmune, inflammatory, and metabolic diseases, including systemic lupus erythematosus, arthritis, and diabetes20,21. As a result, the NLRP3 inflammasome is among the most extensively investigated inflammasomes. However, no clinically approved drugs currently exist that directly target NLRP3 inflammasome activation. MCC950, a potent and selective NLRP3 inflammasome inhibitor, has exhibited robust anti-inflammatory activity across preclinical models of autoimmune diseases and metabolic disorders. While its clinical transformation faces dual challenges: (1) Gastrointestinal toxicity manifested as dose-limiting mucosal barrier disruption and dysbiosis in primate models, and (2) The effects on the central nervous system are not fully understood, particularly under prolonged exposure scenarios. Consequently, there is an urgent need to explore another safe NLRP3 inflammasome inhibitors, providing promising lead compounds for the therapeutic intervention of NLRP3-associated illnesses.
Building upon the findings presented in the aforementioned studies, our laboratory synthesized a novel non-steroidal anti-inflammatory compound, M464, capable of releasing hydrogen sulfide. This paper aims to investigate its potential anti-inflammatory effects and explore its specific mechanisms of action. Our investigation revealed that M464 effectively suppressed the activation of the NLRP3 inflammasome, primarily by diminishing the generation of reactive oxygen species (ROS) and impeding the formation of ASC oligomers. Concomitantly, in vivo experiments demonstrated that M464 exhibited robust protective effects against NLRP3-driven acute lung injury and acute liver injury. As a result, M464 emerges as a promising inhibitor of the NLRP3 inflammasome, offering a potential lead compound for addressing related inflammatory conditions.
Materials and methods
Chemicals and antibodies
The synthesis methods of M464 was explored and found by Huanhuan Qiu,with its purity surpassing 99% as determined by high-performance liquid chromatography (HPLC). Lipopolysaccharide (LPS, L2880, sigma, Shanghai, China), Adenosine 5′-triphosphate disodium salt (ATP, A6363, Macklin, Shanghai, China), Phorbol 12-myristate 13-acetate (PMA, P849986, Macklin, Shanghai, China), Reactive Oxygen Species Assay Kit (ROS, S0033S, Beyotime, Shanghai, China), ELISA Kit (RK00006, ABclonal), Lactate dehydrogenase cytotoxicity assay kit (LDH, C0016, Beyotime, Shanghai, China), Propidium iodide solution (PI, C0080, Solarbio, Wuhan, China), Disuccinimidyl Suberate (DSS, S75322, Yuanye, Shanghai, China), Monosodium urate (MSU, S30775, Yuanye, Shanghai, China), D-galactosamine Hydrochloride (D-GalN, HY-42682, MCE, Shanghai, China), Malondialdehyde assay kit (MDA, A003-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China), Superoxide Dismutase assay kit (SOD, A001-3-2, Nanjing Jiancheng Bioengineering Institute, Nanjing, China), Fetal bovine serum (FBS, ShuangRu Biotech, Suzhou, China), IL-1β antibody (A16288, ABclonal), Caspase-1 antibody (P79884-2R, Abmart), ASC antibody (A1170, ABclonal and 67,824, CST), Caspase-1/P20 antibody (22,915-1-AP, Proteintech), β-actin antibody (P30002, Abmart).
Cell culture and experimental conditions
The J774A.1 murine macrophage cell line and THP-1 human monocyte cell line were purchased from the China Center for Type Culture Collection (CCTCC) under the Chinese Academy of Sciences. J774A.1 cells were cultured in DMEM high glucose medium supplemented with antibiotics (100 μg/mL streptomycin and 100 μg/mL penicillin) and 10% FBS and THP-1 cells were cultured in RPMI-1640 medium supplemented with double antibody and 10% FBS. When the cells grow to 90% in a petri dish, they can be cryopreserved or subcultured. All cells were cultured at 37 °C in moist atmosphere of 5% CO2. J774A.1 mice monocytic macrophages and THP-1 human monocytes were purchased from the Cell Bank of the Chinese Academy of Sciences.
After stimulating J774A.1 cells with 2 μg/mL LPS for 3 h, M464 was added at concentrations of 3, 6, and 12 μM for 30 min. Subsequently, the J774A.1 cells were incubated with 3 mM ATP for 30 min or 400 μg/mL MSU for 12 h to activate the NLRP3 inflammasome. The suspended THP-1 cells were first pre-treated with 50 ng/mL PMA to induce adherence, and subsequently stimulated in the same way as with J774A.1 cells.
Cell pyroptosis detection
After stimulating J774A.1 cells with 2 μg/mL LPS for 3 h, M464 was added at concentrations of 3, 6, and 12 μM for 30 min, and then added with 3 mM ATP to continue to incubate 30 min. Following this, propidium iodide (PI) reagent was added to the cell supernatant as per the test kit instructions. The dishes were then wrapped in tinfoil and kept in the dark at room temperature for 10 min. To observe cell pyroptosis using the EVOS FL AUTO fluorescence microscope, five random fields were selected from each group for photography22.
After completing the ATP stimulation, 120 μL of supernatant from each cell group was combined with 60 μL of LDH reagent and incubated in the dark for 30 min. Absorbance was then measured at 490 nm to assess cell viability.
Western blotting analysis
Protein was isolated using RIPA lysate, and the concentration of protein in each group was quantified using the BCA kit. The concentrations were then standardized across all samples before being loaded and separated by SDS-PAGE. Subsequently, the membranes were transferred and then blocked with skim milk powder for two hours each. The membranes were incubated overnight with primary antibodies against ASC, IL-1β, Caspase-1, and β-actin, followed by HRP-labeled secondary antibodies. Finally, the protein bands were visualized using an enhanced chemiluminescence (ECL) kit.
Detection of IL-1β in cell supernatants and lysates
IL-1β secreted into the cell supernatants was quantified by Enzyme-linked immunoabsorbent assay (ELISA) according to the manufacturer’s instruction and Western blotting analysis. The absorbance at 450 nm wavelength was finally measured using a PerkinElmer (EnSight) microplate reader in the ELISA assay. For intracellular quantification of IL-1β, cell lysates were quantified by Western blotting analysis.
Detection of intracellular ROS
The DCFH-DA fluorescent assay was used to measure the presence of intracellular reactive oxygen species (ROS). Briefly, equal numbers of cells (5 × 105 cells/mL) were seeded onto 6-well CultureSlides (LABSELECT™) and 10µM DCFH-DA in medium was added to the cells 30 min before the end of the treatments. The medium containing the fluorescent probes was removed, and the cells were rinsed with PBS, and observed in an EVOS FL Auto Cell Imaging System.
Mice
Male KM mice, approximately 20 g in weight and 42–56 days old, were purchased from Huachuang Sino Technology. They were maintained in an environment with 40–60% humidity and a temperature of 22 ± 2 °C, with a 12-h light/dark cycle. After the experiments, mice were anesthetized with 4% isoflurane for induction and 2% for maintenance, and then mice were killed by cervical dislocation. All animal experiments were carried out following the guidelines set by the Animal Experiment Ethics Committee of Nanjing Normal University (approval no: IACUC-20200504). The study was conducted in compliance with the ARRIVE guidelines and all methods were conducted in accordance with relevant guidelines and regulations.
Establishment of acute liver/lung injury model in mice
Mice were subjected to acute lung injury by intraperitoneal injection of 10 mg/kg LPS reagent. Acute liver injury was induced in mice by intraperitoneal injection of a combination of 50 μg/kg LPS and 800 mg/kg D-GalN reagent. Mice were orally administered M464 at doses of 15, 30, and 60 mg/kg one hour before LPS stimulation23. Euthanasia was performed on the mice after this period to obtain lung/liver tissues and serum. A positive control of 10mg/kg dexamethasone was employed, and the treatment protocol mirrored the same procedure24.
The ratio of lung tissue wet weight to dry weight (W/D).
First, dissect the mice to obtain the left lung lobe and weigh it as the wet weight (W) of lung tissue. Then, place the left lung lobe in a 55 °C oven and bake it for 48 h until completely dry, then weigh it again as the dry weight (D) of lung tissue, and calculate the W/D ratio25,26.
Pathological assessment of lung and liver tissues
The animal tissue was preserved in a 4% paraformaldehyde solution for over 24 h, followed by gradual dehydration and embedding in paraffin. Paraffin sections of 5 μm thickness were prepared using a slicing mechanism, and subsequently stained with hematoxylin and eosin. Imaging was performed using microscope, with 5 random visual fields chosen from each section.
Following the approach outlined by Mikawa et al27, lung injury severity in each specimen was evaluated under an optical microscope based on the degree of lung tissue congestion, changes in alveolar septal thickness, and infiltration of inflammatory cells. The degree of tissue damage was scored as follows: 0 for no injury, 1 for mild injury, 2 for moderate damage, 3 for severe injury, and 4 for extremely severe injury. A semi-quantitative assessment was carried out based on the severity of each criterion.
The Knodell Histological Activity Index scoring system was used in this study28. The scoring criteria were (1) peripheral fragmentation necrosis with or without bridging necrosis in the confluent area (0–10 points) (2) hepatocellular degeneration and focal necrosis in the lobules (0–4 points), (3) inflammation in the confluent area (0–4 points), (4) fibrosis (0–4 points), and the sum of scores of the four indexes was taken as the total score, ranging from 0 to 22.
Biochemical assay
Six hours following intraperitoneal injection of LPS/D-GalN, mice liver tissues and serum were obtained. Measure aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels according to kit instructions. Liver tissue protein was extracted and the protein concentration in each group was determined. Superoxide dismutase (SOD) and malondialdehyde (MDA) levels were also assessed based on kit protocols.
Immunohistochemistry
Mice liver tissue paraffin sections were subjected to antigen retrieval using EDTA buffer (pH 8.0). They were then treated with 3% hydrogen peroxide to suppress endogenous peroxidase activity. After blocking with a 3% bovine serum albumin (BSA) solution, the sections were incubated with the corresponding ASC primary antibody and secondary antibody, followed by color development using the DAB staining solution. Subsequently, counterstaining was performed with hematoxylin solution, and observation was conducted under microscope.
Statistical analyses
The data are expressed as the mean ± standard deviation (SD). The analyses were performed using GraphPad Prism version 8.0.2 for Windows (GraphPad Software, San Diego, California USA, www.graphpad.com). One-way analysis of variance followed by Tukey’s post hoc test and unpaired Student’s t-test were used to analyze the statistical significance among multiple groups and between two groups, respectively. *P < 0.05 was considered statistically significant.
Results
M464 suppresses NLRP3 inflammasome–mediated pyroptosis in J774A.1 and THP-1 cells
Activation of NLRP3 by microbes and danger signals leads to the release of the pro-inflammatory cytokines IL-1β and GSDMD inflammasome-mediated pyroptosis, a pro-inflammatory type of cell death. During pyroptosis, pores can form in the cell membrane, leading to the release of cellular contents and positive staining of dead cells, which can be detected by LDH release assay and propidium iodide [PI] staining, respectively.
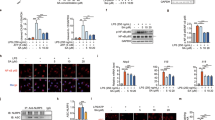
We initially observed the effect of M464 in pyroptotic cell injury. As displayed in Fig. 1A,B, compared with Con (Control group) and LPS group, the fluorescence intensity of propidium iodide increased substantially, however the activation could be reversed by M464. The reversal effect was dose-dependent, with a significant effect in the high-dose group. The lactate dehydrogenase (LDH) concentration was also detected to analyze J774A.1 and THP-1 cell death (Fig. 1C,D). The results were consistent with the PI staining analysis, which indicated the M464 inhibited pyroptosis in macrophages triggered by the NLRP3 inflammasome stimulator.
M464 inhibits cellular pyroptosis induced by NLRP3 inflammasome activation in in both J774A.1 and THP-1 cells. (A, B) Pyroptosis of PI-stained cells in J774A.1 and THP-1 cells was observed using fluorescence microscopy. Scale bar = 400 μm. (C, D) The level of LDH in the supernatant of J774A.1 and THP-1 cells were determined. The experiment was conducted independently three times, and the results were presented as the mean ± standard deviation (SD), *P < 0.05, **P < 0.01.
M464 inhibits the activation of NLRP3 inflammasome and in J774A.1 and THP-1 cells
In this experimental design, NLRP3 inflammasome is activated in two phases: the first phase of priming step is that LPS primes NLRP3 expression, and the second phase of activation step is stimulated by ATP or monosodium urate to activate the NLRP3 inflammasome. With the combined induction of LPS and ATP, Caspase-1 is generated by self-shear activation of Pro-Caspase-1, IL-1β and generation of reactive oxygen species.
We verified that the combined induction of LPS plus ATP or MSU increased the expression of Caspase-1 and mature IL-1β (17KD) by Western blotting. Furthermore, there was a significant decrease in the expression of mature IL-1β and Caspase-1 under the administration of M464 in the 6 μM and 12 μM dose groups (Fig. 2A–D). We also observed that the expression levels of Pro-IL-1β and Pro-Caspase-1 remained largely unchanged. Similarly, the same stimulation model was established in PMA-stimulated THP-1 macrophage, which also indicated that M464 could inhibit NLRP3 inflammasome activation (Fig. 2E–H). Furthermore, ELISA was utilized to quantify the level of IL-1β in the cell supernatant of each group. The findings demonstrated that M464 dose-dependently inhibited the release of IL-1β in the supernatant of J774A.1 macrophages stimulated with LPS plus ATP, consistent with the results obtained from Western Blotting (Fig. 2I). We conclude that M464 can dose-dependently prevent the activation of NLRP3, thereby improving the cellular status.
M464 inhibits the expression of marker proteins associated with NLRP3 inflammasome activation in cells. Western Blotting was used to evaluate the expression of IL-1β and Caspase-1 in J774A.1 (A–D) and THP-1 (E–H) cells’ supernatants and lysates. ImageJ was used to quantitatively analyze the levels of mature IL-1β and Caspase-1 protein in the cell supernatant. (I) The impact of M464 on IL-1β secretion activated by the NLRP3 inflammasome in J774A.1 cell supernatant was evaluated. The experiment was conducted independently three times, and the results were presented as the mean ± SD. *P < 0.05, **P < 0.01.
M464 retraining the production of intracellular ROS the formation of ASC oligomers
In addition, the change in ROS levels can also be seen as a manifestation of the change in the active state of the cells. We used cellular immunofluorescence experiments to show that the level of ROS increased dramatically in the LPS-induced group compared to the Con group, with green fluorescence occupying most of the field of view under the microscope. However, after M464 processing, a significant decrease in ROS levels was observed in terms of fluorescence level, and only a few portions of cells in the 6 μM and 12 μM groups showed green fluorescence in the field of view under the microscope (Fig. 3A–D). The above results suggest that M464 reduces ROS levels during NLRP3 inflammasome activation in macrophages.
M464 restrains the formation of ASC oligomerization and the production of intracellular ROS. M464 also hampers the expression of NLRP3 inflammasome activation marker proteins in THP-1 cells. (A–D) Green fluorescence images of ROS generation were taken by fluorescence microscopy. The fluorescence intensity of ROS production in different cell groups was measured using a microplate reader. Scale bar = 400 μm. (E) The production of ASC oligomers was detected in cell lysate precipitation. (F) Immunofluorescence detection of ASC spot formation in LPS-induced THP-1 cells treated with different doses of M464. Arrowheads indicate ASC specks. Scale bar = 10 μm. The experiment was conducted independently three times, and the results were presented as the mean ± SD, with *P < 0.05 and **P < 0.01.
Using J774A1 macrophages, we crosslinked ASC using disuccinimidyl suberate (DSS) and detected ASC oligomerization using immunoblotting. To investigate the effect of M464 on ASC oligomerisation and its own expression. First, we used LPS in combination with ATP to induce NLRP3 activation in J774A1, and then determined the changes in ASC oligomerisation after M464 administration by DSS cross-linking assay. 3 μM of the M464 had basically no effect on ASC oligomerisation, and 12 μM of the M464 was able to significantly inhibit the formation (Fig. 3E).
Using the results of the WB test, we found that with the combined stimulation of LPS and ATP, ASC oligomerisation occurred and NLRP3 inflammasome were activated. With the administration of the M464, ASC oligomerisation was alleviated. We verified this phenomenon by cellular immunofluorescence experiments. Using a laser confocal microscope, we took 100-fold images and found the changing form of the ASC protein forming spots in the cells by staining with AF488 and DAPI. In the LPS-induced group, with the activation of NLRP3 inflammasome, more ASC spots were formed. With the administration of M464, ASC green spots were significantly reduced in a concentration-dependent manner under laser confocal (Fig. 3F). These results show that M464 inhibits NLRP3 inflammasome activation at least in part by preventing ASC oligomerization and decreasing ROS production.
M464 mitigates LPS-induced acute lung injury in mice by inhibiting NLRP3 inflammasome activation
Further investigate the anti-inflammatory effects of M464 and its impact on the activation of NLRP3 inflammasome in an LPS-induced acute lung injury mice model. To evaluate the protective efficacy of M464 on lung injury, the pathological changes in lung tissues were examined using H&E staining. In the LPS-induced group, significant lung congestion, collapse of alveolar structure, thickening of alveolar walls, and infiltration of inflammatory cells were observed. M464 dose-dependently reduced the pathological scores of lung injury, significantly improving the lung tissue damage in mice (Fig. 4A). The lung histopathological scores were consistent with the H&E experimental results (Fig. 4B). Additionally, pulmonary edema is a significant feature of acute lung injury in mice, so the lung wet/dry (W/D) weight ratio was measured to evaluate lung tissue edema. The lung wet/dry (W/D) weight ratio in the LPS-induced group was visibly elevated compared to the Con group, but both M464 and DXM inhibited this ratio and significantly improved pulmonary edema in mice (Fig. 4C). Furthermore, to explore whether M464 achieves its anti-inflammatory effect through NLRP3 pathway body signaling support, the levels of IL-1β and Caspase-1 in mice tissues were detected by WB, and the IL-1β in mice serum was evaluated by ELISA. WB results demonstrated that compared to the Con group, the levels of mature IL-1β and Caspase-1 in the LPS-induced group were markedly increased, while both M464 and DXM significantly reduced their expression (Fig. 4D,E). ELISA results suggested that M464 dose-dependently reduced the secretion of IL-1β (Fig. 4F). These findings indicate that M464 can protect mice against LPS-induced injury by suppressing the activation of the NLRP3 inflammasome.
M464 mitigates LPS-induced acute lung injury in mice by inhibiting NLRP3 inflammasome activation. Following intragastric administration of M464 (15, 30, and 60 mg/kg) for 1 h, mice were euthanized 12 h after induction by intraperitoneal injection of LPS (10 mg/kg). The groups included Con (control), Mod (LPS injection), DXM (Dexamethasone treatment at 10 mg/kg), and M464 at different doses (15, 30, 60 mg/kg). (A) Representative images of H&E staining were analyzed to assess pathological changes in mice lung tissues. Red arrow: Inflammatory cell infiltration; Black arrows: Areas of lung structure damage. Scale bars = 200 μm. (B) Pulmonary edema was evaluated by the lung wet/dry weight ratio. (C) Lung tissue damage scores were determined. (D) The level of IL-1β secreted in mice serum was measured by ELISA. (E, F) The impact of M464 on NLRP3 inflammasome activation-associated protein expression in mice with LPS-induced acute lung injury was assessed. Experimental results were expressed as the mean ± SD; n = 6, with *P < 0.05 and **P < 0.01.
M464 alleviates LPS/D-GalN-induced acute liver injury in mice by blocking NLRP3 inflammasome activation
To further confirm M464’s regulatory effect on the NLRP3 inflammasome, we employed an in vivo mice model of acute liver injury induced by LPS and D-GalN. Liver damage was evaluated through gross examination, including the liver-to-body weight ratio. The livers of mice in the LPS/D-GalN-induced group exhibited notable congestion and severe injury, whereas both M464 and DXM significantly ameliorated the liver injury (Fig. 5A). There was a significant increase in the ratio in LPS/D-GalN-induced group, while the positive drug DXM and the high dose group were able to return the ratio to normal (Fig. 5B). HE staining revealed that the liver tissues of mice in the LPS/D-GalN-induced group exhibited evident structural disorder, liver tissue hemorrhage, extensive hepatocyte necrosis, and infiltration of inflammatory cells (Fig. 5C,D). However, administration of M464 or DXM markedly attenuated LPS/D-GalN-induced liver injury in mice. Furthermore, serum levels of AST and ALT were assessed, indicating a significant elevation in the LPS/D-GalN-induced group compared to the Con group. M464 exhibited dose-dependent suppression of these liver enzymes (Fig. 5E,F).
M464 mitigates LPS/D-GalN-induced acute liver injury in mice. Following intragastric administration of M464 (15, 30, and 60 mg/kg) for 1 h, Mice were intraperitoneally injected with LPS at a dose of 50 μg/kg and D-GalN at 800 mg/kg and then sacrificed after 6 h of stimulation. The groups included Con (control), Mod (LPS injection), DXM (Dexamethasone treatment at 10 mg/kg), and M464 at different doses (15, 30, 60 mg/kg). (A) Gross examination of mice liver. (B) Liver-to-body weight ratio of mice. (C) HE staining to analyze histopathological changes in the liver of mice. Red arrows: Areas of hepatocyte hemorrhage; Black arrows: Areas of hepatocyte necrosis. Scale bars = 200 μm. (D) Histological scoring of the liver. (E, F) Serum levels of ALT and AST in mice. (G, H) Detection of SOD and MDA in serum, indicative of oxidative stress. (I) Detection of liver injury indicator IL-1β in mice serum. (J, K) Detection of mature IL-1β and Caspase-1 protein levels in liver tissues using Western Blotting. (L, M) Measurement of CASP1 and IL1B mRNA expression levels in mice liver tissue by RT-qPCR. (N) Analysis of ASC protein expression in liver tissues through immunohistochemical staining. Scale bars = 400 μm. Experimental results were expressed as the mean ± SD; n = 5, with *P < 0.05 and **P < 0.01.
Oxidative stress triggers NLRP3 inflammasome activation and the release of mature IL-1β. Analysis of SOD and MDA levels indicated that, relative to the Con group, the LPS/D-GalN-induced group showed a substantial reduction in SOD content and a marked increase in MDA level. These alterations were effectively reversed by M464. (Fig. 5G,H). These results demonstrated the potent inhibitory impact of M464 on oxidative stress. By Western Blotting and ELISA experiment, it was observed that mature IL-1β release and Caspase-1 production were notably enhanced following LPS plus D-GalN stimulation. However, M464 effectively suppressed these responses in a dose-dependent manner (Fig. 5I–K). Moreover, We also performed RNA extraction and reverse transcription experiments on each group and analysed IL-1β and Caspase-1 at the DNA level by RT-PCR (Fig. 5L,M). We found M464 administration can decrease the elevated level of the LPS/D-GalN-induced group, which was coincided with the WB results. According to the immunohistochemical results, there were obvious staining spots in the LPS/D-GalN-induced group, but the positive DXM group and the high-dose M464 group were exactly the opposite, which was just enough to support the results of cellular experiments (Fig. 5N). These findings indicate that M464 has the potential to inhibit NLRP3 inflammasome activation by mitigating oxidative stress, thereby exerting a protective effect in LPS/D-GalN-induced conditions.
Discussion
Novel non-steroidal anti-inflammatory drugs, designed to release hydrogen sulfide, offer a gradual release of the gas signaling molecule H2S within the body. This innovative approach addresses the adverse effects on the gastrointestinal and cardiovascular systems associated with prolonged or extensive use of conventional non-steroidal anti-inflammatory drugs29. Numerous studies have highlighted that these drugs not only mitigate these side effects but also exhibit comparable or enhanced pharmacological activities compared to their parent compounds30,31,32. The activation of NLRP3 inflammasome is intricately associated with various diseases, yet drugs for treating NLRP3 inflammasome-related conditions are scarce in clinical practice. Therefore, it is crucial to investigate lead compounds that target the NLRP3 inflammasome. Our study unveils the potential of M464, a hydrogen sulfide-releasing nonsteroidal anti-inflammatory compound synthesized by us, in exerting anti-inflammatory effects by inhibiting the NLRP3 inflammasome. In vitro experiments demonstrated that M464 effectively curtailed NLRP3 inflammasome-dependent pyroptosis. Moreover, it dose-dependently decreased the expression of IL-1β and Caspase-1 in cell supernatants, signifying a significant inhibition of NLRP3 inflammasome activation. Furthermore, our investigation into the mechanism revealed that M464 inhibited the formation of ASC oligomers. Previous research has implicated reactive oxygen species (ROS) as upstream stimulators promoting NLRP3 inflammasome activation, and our results show that M464 dose-dependently inhibits ROS production. Thus, M464 emerges as a compound capable of suppressing NLRP3 inflammasome activation by targeting ASC oligomer formation and intracellular ROS production. Additionally, in LPS/D-GalN-induced acute liver injury in mice, we found that in liver tissues, caspase-1 and IL-1β mRNA were reduced by M464. This effect might be related to H2S released from M464. Peng and his colleague33 have shown that hydrogen H₂S can protect retinal pigment epithelial (RPE) cells from high-glucose-induced damage by regulating the mRNA levels of IL-1β and caspase-1. Similarly, in a lipopolysaccharide (LPS)-induced acute lung injury model, H₂S significantly reduced the mRNA expression of IL-1β and other cytokines and had a protective effect on acute lung injury34. Although H₂S can regulate inflammatory diseases through multiple ways directly or indirectly, our results demonstrated that M464 exerts its anti-inflammatory effect, at least in part, through H₂S -mediated IL-1β and caspase-1 mRNA levels. The regulatory efficacy of M464 on such cytokines was also observed with other hydrogen-releasing agents35.
Several studies have shown that LPS stimulates the activation of NLRP3 inflammasome in macrophage36, thereby promoting the production of inflammatory factor IL-1β, leading to the occurrence of inflammatory response, which is involved in the pathological mechanism of acute liver and lung injury. The acute inflammatory response is generally a short-term process lasting from a few minutes to a few days, with clinical signs of tissue swelling, redness, pain and loss of function. Acute lung injury is a severe and diffuse lung inflammation, which triggers massive infiltration of lung inflammatory cells, thickening of lung interstitial, collapse of alveolar wall and pulmonary edema37,38. In the lung injury model of this study, M464 could effectively reduce the wet-to-dry weight ratio of the lungs, improve the infiltration of inflammatory cells as well as alveolar structural disorders, and significantly alleviate the pathological injury of the lungs. At the same time, M464 can reduce the release of activated Caspase-1 and IL-1β, and effectively inhibit the activation of NLRP3 inflammasome. Acute liver injury results in excessive inflammation leading to hyperemia of the liver tissue, massive necrosis of liver cells and impairment of liver function39,40,41. In the liver injury model of this study, M464 can effectively reduce the organ ratio of liver, improve liver bleeding, liver cell disorder and inflammatory cell infiltration. Lower ALT and AST levels and restore liver function to some extent. In tissues, M464 reduces the level of Caspase-1 and IL-1β protein or gene expression, thereby inhibiting the activation of the NLRP3 inflammasome. In this study, it was found that M464 can increase SOD content and decrease MDA level in acute liver injury model, and effectively relieve oxidative stress. In conclusion, M464 can effectively alleviate LPS-induced acute lung injury in mice and LPS combined with D-GalN induced acute liver injury in mice by regulating NLRP3 inflammasome signaling pathway and inhibiting tissue oxidative stress, thus playing a protective role in liver and lung.
Our research substantiates M464’s robust anti-inflammatory efficacy, potentially attributed to its modulation of the NLRP3 inflammasome pathway. Because the exploration of NLRP3-related diseases is a developing field with imperative need for the discovery of multiple lead compounds or drug candidates, our study positions M464 as a versatile depressant of the NLRP3 inflammasome, offering a promising candidate for the treatment of interconnected illnesses. This underscores its potential as a novel therapeutic option in the ongoing pursuit of addressing NLRP3-related diseases.
In conclusion, M464 effectively hinders the activation of downstream Caspase-1 expression and the release of IL-1β by impeding ASC oligomerisation and curtailing ROS production. This dual action manifests a potent anti-inflammatory impact both in vitro and in vivo. These findings underscore the potential of M464 as a novel lead compound for addressing NLRP3-related inflammatory diseases, offering promising prospects for therapeutic interventions in this domain.
Conclusions
In conclusion, M464 effectively hinders the activation of downstream Caspase-1 expression and the release of IL-1β by impeding ASC oligomerisation and curtailing ROS production. This dual action manifests a potent anti-inflammatory impact both in vitro and in vivo. These findings underscore the potential of M464 as a novel lead compound for addressing NLRP3-related inflammatory diseases, offering promising prospects for therapeutic interventions in this domain.
Data availability
All datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- NLRP3:
-
NOD-like receptor family, pyrin domain containing 3
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PGHS:
-
Prostaglandin H synthases
- PG:
-
Prostaglandin
- GI:
-
Gastrointestinal
- H2S:
-
Hydrogen sulfide
- PLP:
-
Pyridoxal-5′-phosphate
- CBS:
-
Cystathionine β-synthase
- CSE:
-
Cystathionine γ-lyase
- NF-κB:
-
Nuclear factor-kappa B
- MAPKs:
-
Mitogen-activated protein kinases
- GSDMD:
-
Gasdermin D
- ROS:
-
Reactive Oxygen Species
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- ASC:
-
Apoptosis-associated speck-like Protein containing a CARD
- ELISA:
-
Enzyme-linked immunoabsorbent assay
- D-GalN:
-
D-galactosamine
- DSS:
-
Disuccinimidyl suberate
- IL-1β:
-
Interleukin-1β
- LDH:
-
Lactate dehydrogenase
- LPS:
-
Lipopolysaccharide
- MSU:
-
Monosodium urate
References
Vane, J. R. & Botting, R. M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 47(Suppl 2), S78-87 (1998).
Lee, M. & Feldman, M. The aging stomach: Implications for NSAID gastropathy. Gut 41(4), 425–426 (1997).
García-Rayado, G., Navarro, M. & Lanas, A. NSAID induced gastrointestinal damage and designing GI-sparing NSAIDs. Expert Rev. Clin. Pharmacol. 11(10), 1031–1043 (2018).
Cui, J. & Jia, J. Natural COX-2 inhibitors as promising anti-inflammatory agents: An update. Curr. Med. Chem. 28(18), 3622–3646 (2021).
Fiorucci, S. & Antonelli, E. NO-NSAIDs: From inflammatory mediators to clinical readouts. Inflamm. Allergy Drug Targets 5(2), 121–131 (2006).
Davies, N. M. et al. NO-naproxen versus naproxen: Ulcerogenic, analgesic and anti-inflammatory effects. Aliment Pharmacol. Ther. 11(1), 69–79 (1997).
Rigas, B. Novel agents for cancer prevention based on nitric oxide. Biochem. Soc. Trans. 35(Pt 5), 1364–1368 (2007).
di Masi, A. & Ascenzi, P. H2S: A “double face” molecule in health and disease. BioFactors 39(2), 186–196 (2013).
van Goor, H. et al. Hydrogen sulfide in hypertension. Curr. Opin. Nephrol. Hypertens 25(2), 107–113 (2016).
Wallace, J. L. et al. Hydrogen sulfide-releasing therapeutics: Translation to the clinic. Antioxid Redox Signal 28(16), 1533–1540 (2018).
Henein, M. Y. et al. The role of inflammation in cardiovascular disease. Int. J. Mol. Sci. 23(21), 12906 (2022).
Gemici, B. et al. H2S-releasing drugs: Anti-inflammatory, cytoprotective and chemopreventative potential. Nitric Oxide 46, 25–31 (2015).
Chan, S. J. & Wong, P. T. Hydrogen sulfide in stroke: Protective or deleterious?. Neurochem. Int. 105, 1–10 (2017).
Mao, Y. G. et al. Hydrogen sulfide therapy: A narrative overview of current research and possible therapeutic implications in future. Med. Gas Res. 10(4), 185–188 (2020).
Fu, J. & Wu, H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu. Rev. Immunol. 41, 301–316 (2023).
He, Y., Hara, H. & Núñez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 41(12), 1012–1021 (2016).
Hu, Y. et al. The Gasdermin D N-terminal fragment acts as a negative feedback system to inhibit inflammasome-mediated activation of Caspase-1/11. Proc. Natl. Acad. Sci. USA 119(45), e2210809119 (2022).
Huang, Y., Xu, W. & Zhou, R. NLRP3 inflammasome activation and cell death. Cell Mol. Immunol. 18(9), 2114–2127 (2021).
Grebe, A., Hoss, F. & Latz, E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ. Res. 122(12), 1722–1740 (2018).
Esser, N. et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 105(2), 141–150 (2014).
Chen, X. et al. NLRP3 inflammasome and IL-1β pathway in type 2 diabetes and atherosclerosis: Friend or foe?. Pharmacol. Res. 173, 105885 (2021).
Guo, J. et al. Protective effect of berberine against LPS-induced endothelial cell injury via the JNK signaling pathway and autophagic mechanisms. Bioengineered 12(1), 1324–1337 (2021).
Hwang, J. S. et al. Glucosamine improves survival in a mouse model of sepsis and attenuates sepsis-induced lung injury and inflammation. J. Biol. Chem. 294(2), 608–622 (2019).
Zhang, T. et al. Oridonin alleviates d-GalN/LPS-induced acute liver injury by inhibiting NLRP3 inflammasome. Drug Dev. Res. 82(4), 575–580 (2021).
Nguyen, N. et al. ISM1 suppresses LPS-induced acute lung injury and post-injury lung fibrosis in mice. Mol. Med. 28(1), 72 (2022).
Tang, J. et al. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 91, 107272 (2021).
Shi, S. et al. Salidroside pretreatment alleviates PM(2.5) caused lung injury via inhibition of apoptosis and pyroptosis through regulating NLRP3 Inflammasome. Food Chem. Toxicol. 177, 113858 (2023).
Leung, N. Liver disease-significant improvement with lamivudine. J. Med. Virol. 61(3), 380–385 (2000).
Grosser, T., Ricciotti, E. & FitzGerald, G. A. The cardiovascular pharmacology of nonsteroidal anti-inflammatory drugs. Trends Pharmacol. Sci. 38(8), 733–748 (2017).
Yu, B. et al. Prodrugs of sulfide and persulfide species: Implications in their different pharmacological activities. Curr. Opin. Chem. Biol. 75, 102329 (2023).
Fonseca, M. D. et al. NOSH-aspirin (NBS-1120), a dual nitric oxide and hydrogen sulfide-releasing hybrid, reduces inflammatory pain. Pharmacol. Res. Perspect. 3(3), e00133 (2015).
Gugliandolo, E. et al. Anti-inflammatory effect of ATB-352, a H2S -releasing ketoprofen derivative, on lipopolysaccharide-induced periodontitis in rats. Pharmacol. Res. 132, 220–231 (2018).
Peng, W. et al. Hydrogen sulfide attenuates high glucose-induced human retinal pigment epithelial cell inflammation by inhibiting ROS formation and NLRP3 inflammasome activation. Mediat. Inflamm. 2019, 8908960 (2019).
Wang, P. et al. Effect of hydrogen sulfide on inflammatory factor in acute lung injury induced by lipopolysaccharide in rats. Chin. J. Crit. Care Med. 29(11), 973–977 (2009).
Ramos-Inza, S. et al. NSAIDs: Old acquaintance in the pipeline for cancer treatment and prevention─Structural modulation, mechanisms of action, and bright future. J. Med. Chem. 64(22), 16380–16421 (2021).
Kim, J. et al. Donepezil regulates LPS and Aβ-stimulated neuroinflammation through MAPK/NLRP3 inflammasome/STAT3 signaling. Int. J. Mol. Sci. 22(19), 10637 (2021).
Long, M. E., Mallampalli, R. K. & Horowitz, J. C. Pathogenesis of pneumonia and acute lung injury. Clin. Sci. (Lond.) 136(10), 747–769 (2022).
Mokrá, D. Acute lung injury - from pathophysiology to treatment. Physiol. Res. 69(Suppl 3), S353-s366 (2020).
Luck, J., Bell, D. & Bashir, G. Paintball-related traumatic liver injury. BMJ Case Rep., 2016, (2016).
Yuan, R. et al. Protective effect of acidic polysaccharide from Schisandra chinensis on acute ethanol-induced liver injury through reducing CYP2E1-dependent oxidative stress. Biomed. Pharmacother. 99, 537–542 (2018).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464(7285), 104–107 (2010).
Acknowledgements
This work was supported by Funding from Institute for Life and Health, Nanjing Drum Tower Hospital, Nanjing Normal University, Project 81,773,948 supported by National Natural Science Foundation of China, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), NSFC for Talents Training in Basic Science (Grant Nos. J1103507, J1210025).
Author information
Authors and Affiliations
Contributions
W. W and Z. Y.K. carried out experimental verification and writing manuscripts, L.S and W.T. visualization and data analysis, H.K.J. 、 L. W.W.、 C. C. and F. X.Y. investigated and wrote,X. G.L. carried out conceptualization, investigation, method, supervision, verification, project management and fund acquisition.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, W., Zhao, Y., Li, S. et al. M464 inhibits activation of NLRP3 inflammasome and inflammatory response in mice. Sci Rep 15, 33878 (2025). https://doi.org/10.1038/s41598-025-07834-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07834-5