Abstract
Haloperidol is a typical antipsychotic used to treat schizophrenia and induces dopamine D2 receptor antagonism. Long-term use of haloperidol can reduce brain size in animals and humans; however, the underlying mechanism of this effect remains unclear. Notch1 signaling regulates the development and function of the nervous system by balancing stem cell proliferation and differentiation. Therefore, we investigated the effects of long-term exposure to haloperidol on human-derived brain organoids, which served as sophisticated in vitro models of human brain development. Long-term exposure to haloperidol reduced the size of brain organoids and decreased the ventricular zone and Notch1 signaling. When propionate, which protects against haloperidol-induced toxicity, was combined with haloperidol, it rescued both the overall size of brain organoids and Notch1 expression levels. Additionally, treatment with valproic acid, a Notch1 activator, partially restored the size of brain organoids and the thickness of the ventricular layer. Taken together, these data suggest that long-term exposure to haloperidol impairs neurodevelopment via Notch1 signaling in brain organoids. These findings contribute to our understanding of antipsychotic drug safety and provide information for new neurodevelopmental toxicity assessments.
Similar content being viewed by others
Introduction
Haloperidol is a typical antipsychotic drug, widely used for the treatment of patients with schizophrenia and commonly used as a control in the development of new antipsychotic drugs1,2,3. Haloperidol antagonizes dopamine D2 receptors to reduce neurotransmission, alleviating psychotic symptoms4,5,6. However, haloperidol does not specifically inhibit dopamine D2 receptors, it also inhibits various other receptors, which can lead to a range of side effects, such as sedation, weight gain, and QT interval prolongation6,7. Haloperidol treatment of the PC12 human-derived neuroblastoma cell line resulted in neurite lesions at low concentrations and induced apoptosis at high concentrations8. In addition, haloperidol reduced the signaling of phosphorylated cAMP-responsive element-binding protein (CREB) and neuropeptide Y (NPY) in SH-SY5Y cells9. This signaling pathway is crucial for neurogenesis, including the maturation, survival, and integration of new neurons in SH-SY5Y cells. Moreover, long-term administration of haloperidol in both humans and animals has been shown to reduce brain size, suggesting that haloperidol causes brain tissue loss over time10,11. Thus, it is essential to carefully determine the dosage and treatment duration for haloperidol; however, the exact mechanisms underlying the brain size reduction associated with long-term exposure to haloperidol remain poorly understood.
Current developmental neurotoxicity (DNT) assessments rely on animal behavioral experiments, which require substantial time and financial resources12,13,14. Moreover, comparing data among different studies can be challenging because of differences in techniques and measurements used for behavioral endpoints13,15. Therefore, the Food and Drug Administration has announced the use of in vitro and in silico models as alternatives to animal models because of ethical concerns regarding animal testing16,17. Consequently, many researchers have studied the effects of chemicals on neurite outgrowth using primary rodent neuronal cells and immortalized human and rodent neuronal cell lines8,9. However, the predictive ability of neurotoxicity assessments in humans is limited by genetic modifications, and interspecific differences. Additionally, cell lines and primary cells do not accurately mimic the nervous system18,19.
We differentiated neurons derived from human induced pluripotent stem cell (iPSC). These neurons are more directly related to the human nervous system than other cell models and can provide rapid and accurate results as screening tools for drug safety evaluation20. Furthermore, we differentiated and utilized brain organoids to assess the effects of long-term exposure to haloperidol. Brain organoids offer a three-dimensional structure that mimics the complex structure and functionality of the human brain more accurately than neurons derived from stem cells in traditional two-dimensional cultures, allowing for the study of more complex biological processes, including tissue development, disease progression, and drug response21,22. Notch1 plays a crucial role in regulating the development and function of the nervous system by controlling self-renewal and differentiation of neural progenitor cells (NPCs)23,24,25,26. It is highly expressed in areas of active neurogenesis in mouse embryos, such as the diencephalon and mesencephalon24. Notch1 acts upstream of CREB and regulates CREB phosphorylation and transcription27. Recently, valproic acid, a Notch1 activator, was shown to regulate the size of brain organoids in a dose-dependent manner28,29. Therefore, the proper regulation of Notch1 signaling is crucial for proper neurodevelopment. Furthermore, Cuprizone induces a useful animal model for schizophrenia research by decreasing the expression of Notch1 signaling in the mouse forebrain30. Haloperidol also reduced Notch1 protein expression in myofibroblasts31. Taken together, these results suggest that the Notch1 signaling pathway plays a role in the development of schizophrenia.
Therefore, in the present study, we aimed to investigate the effect of haloperidol on brain organoids and identify which signal regulates the chronic application of haloperidol, possibly leading to the impairment of brain organoids. Our results will provide crucial insights into the long-term effects of haloperidol on brain development and potential strategies for mitigating its negative side effects.
Results
Generation and characterization of brain organoids
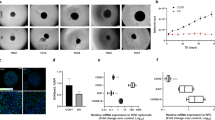
We established a 3D culture system for producing uniform brain organoids to investigate the effects of haloperidol on brain development. iPSCs were detached using collagenase, and the suspended colonies were transferred to non-adherent plates for 7 days to form spherical embryoid bodies with dual SMAD inhibitors (Fig. 1a). The cells were allowed to differentiate for 7 days and then plated onto Matrigel-coated dishes in neural induction medium and detached on day 10. NPCs were seeded in Aggrewell culture plates on day 18 and transferred to a shaker to promote the formation of brain organoids on day 19. Upon incubation in a shaker, the size of the brain organoids increased over time before they reached a consistent size (Fig. 1b, c). By day 63, the brain organoids developed into multi-layer stratified structures composed of SOX2 + NPCs (ventricular zone marker), TBR2 + intermediate progenitor cells (subventricular zone marker), and CTIP2 + neurons (cortical plate marker) (Fig. 1d). Furthermore we confirmed that positive staining for the forebrain marker FOXG1, which regions displaying forebrain, EMX1 (dorsal forebrain marker) but not NKX2.1 (ventral forebrain marker) (Supplementary Fig. 1). These results indicated self-organization and expression of classic characteristics of brain organoids, which demonstrated that the system was sufficiently optimized for use in toxicity evaluation studies.
Generation and characterization of brain organoids (a) Schematic diagram of the brain organoid protocol. (b) Light microscope images of brain organoids from 35 d – 63 d. Scale bars, 200 μm. (c) Quantification of brain organoid size from 35 d – 63 d using diameter and surface area measurements. Values represent mean ± IQR (n = 10). (d) Immunostaining for DAPI (blue), SOX2 (red), TBR2 (purple), and CTIP2 (green) of brain organoids on 63 d. Scale bars, 50 μm. ∗∗∗p < 0.0001.
Chronic exposure of haloperidol on brain organoids
A previous study reported that long-term administration of haloperidol reduced brain volume11. Therefore, we chronically exposed brain organoids to haloperidol from day 21 to day 63 to determine whether long-term haloperidol exposure had similar effects on brain organoids. We found that the size of the brain organoids reduced in a dose-dependent manner when compared with that of the control (Fig. 2a, b). Subsequently, we measured the neuronal layers and ventricular zone (VZ) to examine the detailed characteristics of the brain organoids. Brain organoids treated with haloperidol for 42 days showed a dose-dependent decrease in the VZ and neuronal layer thickness (Fig. 2c, d). Furthermore, to determine whether haloperidol affects phenotype by inducing apoptosis, we assessed cell death using cleaved-caspase-3 immunostaining. The proportion of cleaved caspase-3-positive cells did not differ significantly any haloperidol concentration compared to the control group (Supplementary Fig. 2). It suggests that long-term haloperidol exposure affects the proliferation of NPCs and cortical development without apoptosis. Sodium propionate prevents haloperidol-induced neurite lesions9. Therefore, we treated brain organoids with haloperidol and sodium propionate for 42 days to confirm whether propionate could restore the development of brain organoids treated with haloperidol. We observed that propionate prevented the haloperidol-induced reduction in the size of the brain organoids and the thickness of the VZ (Fig. 3a-d). These results suggest that sodium propionate protects against haloperidol-induced neurodevelopmental impairment.
Effect of haloperidol on human brain organoids treated with haloperidol (a) Schematic diagram of haloperidol (HAL) exposure time on brain organoids (upper). Light microscope images of brain organoids treated with HAL from 35 d – 63 d. Scale bars, 200 μm. (b) Quantification of brain organoid size with or without HAL treatment from 35 d – 63 d using surface area and diameter measurements. Values represent mean ± IQR (n ≥ 6). (c) Immunostaining for DAPI (blue), SOX2 (red) and TUJ1 (green) of brain organoids treated with HAL at 63 d. Scale bars, 50 μm. (d) Quantification of the thickness of VZ-like regions and neuronal layers of organoids treated with HAL on 63 d. Values represent mean ± IQR (n = 5 cortical structures from three organoids). ∗p < 0.05, ∗∗p < 0.005 ∗∗∗p < 0.0001.
Effect of haloperidol on human brain organoids treated with haloperidol and sodium propionate (a) Light microscope images of brain organoids treated with haloperidol (HAL) and sodium propionate (PRO) from 35 d – 63 d. Scale bars, 200 μm. (b) Quantification for brain organoid size after HAL and PRO treatment from 35 d – 63 d using surface area and diameter measurements. Values represent mean ± IQR (n ≥ 6). (c) Immunostaining for DAPI (blue), SOX2 (red) and TUJ1 (green) of brain organoids treated with HAL and PRO (300µM) on 63 d. Scale bars, 50 μm. (d) Quantification of the thickness of VZ-like regions and neuronal layers of organoids treated with HAL and PRO (300µM) on 63 d. Values represent mean ± IQR (n = 5 cortical structures from three organoids). ns; non-significant. ∗p < 0.05.
Role of Notch1 signaling in brain organoid neurodevelopment
Previous studies showed that Notch1 signaling plays a crucial role in neurodevelopment by regulating the maintenance and self-renewal of NPCs and differentiation24,26,29. Therefore, we performed immunoblotting to examine whether haloperidol can impair neurodevelopment via the regulation of Notch1. Quantitative analysis revealed that Notch1 protein levels decreased in brain organoids treated with 1 µM and 3 µM haloperidol, but Notch1 protein levels were restored in brain organoids treated with both haloperidol and sodium propionate. (Fig. 4a). These results suggest that sodium propionate may protect the neurodevelopment of brain organoids by preventing the haloperidol-induced reduction via Notch1 signaling.
Effect of haloperidol on human brain organoids treated with haloperidol and sodium propionate or valproic acid (a) Immunoblots illustrating the protein expression levels of Notch1 and quantification of Notch1 expression normalized to β-actin in brain organoids treated with and without sodium propionate (PRO) and haloperidol (HAL) on 63 d. Values represent mean ± IQR (n = 5). (b) Immunoblots illustrating the protein expression levels of Notch1 and quantification of Notch1 expression normalized to β-actin in brain organoids treated with valproic acid (VPA) at 63 d. Values represent mean ± IQR (n = 5). (c) Light micrographs of brain organoids treated with VPA from 35 d – 63 d. Scale bars, 200 μm. (d) Quantification of brain organoid size after treatment with VPA from 35 d – 63 d using surface area and diameter measurements. Values represent mean ± IQR (n ≥ 6). (e) Immunostaining for DAPI (blue), SOX2 (red) and TUJ1 (green) of brain organoids treated with VPA at 63 d. Scale bars, 50 μm. (f) Quantification of the thickness of VZ-like regions and neuronal layers of organoids treated with VPA at 63 d. Values represent mean ± IQR (n = 5 cortical structures from three organoids). ns; non-significant. ∗∗p < 0.005 ∗∗∗p < 0.0001.
Conversely, a recent study reported that the treatment of brain organoids with valproic acid (VPA), a Notch1 activator, increased the mRNA expression levels of Sox2 and altered neuronal differentiation and maturation28. This result suggests that Notch1 signaling is involved in the self-renewal of neural progenitor cells by inhibiting neural differentiation. Therefore, we treated brain organoids with 100 µM and 300 µM VPA alone to determine whether Notch1 signaling regulates neurodevelopment in brain organoids. As a result, we observed that the Notch1 protein level increased in brain organoids treated with 300 µM VPA compared to the control group (Fig. 4b). Additionally, while 300 µM VPA treatment reduced the size of brain organoids, it significantly increased the thickness of the VZ thickness (Fig. 4c-f). Collectively, these results suggest that haloperidol impairs the neurodevelopment of brain organoids by inhibiting Notch1 signaling, which regulates the self-renewal and differentiation of NPC.
Interaction between haloperidol and valproic acid in brain organoids
We evaluated whether the neurodevelopmental impairment caused by haloperidol through the reduction of Notch1 signaling in brain organoids could be restored by treatment with VPA. We found that Notch1 expression was restored to the vehicle level with 1 µM or 3 µM haloperidol and 300 µM VPA combination treatment (Fig. 5a). Although the size of brain organoids treated with 1 µM haloperidol and 300 µM VPA showed no difference compared to those treated with 1 µM haloperidol alone (Fig. 5b, c), the size of brain organoids treated with 3 µM haloperidol and 300 µM VPA was significantly restored compared to those treated with 3 µM haloperidol alone (***, p < 0.0001) (Fig. 5b, d). Additionally, compared to the control group, the brain organoids co-treated with VPA exhibited a significantly increased size (**, p = 0.0021) compared to those treated with haloperidol alone (***, p < 0.0001). The immunohistological analysis also showed that the combined treatment of 1 µM haloperidol and 300 µM VPA caused no changes in the thickness of the VZ or the neuronal layer compared to the 1 µM haloperidol treatment alone (Fig. 5e, f). After treatment with 3 µM haloperidol and 300 µM VPA, the thickness of the VZ was significantly restored compared to that of brain organoids treated with 3 µM haloperidol alone, but it did not affect the thickness of the neuronal layer (Fig. 5e, g). These results showed that while VPA treatment restored the haloperidol-induced reduction in Notch1 signaling, the neurodevelopmental impairment caused by haloperidol was only partially recovered in the ventricular zone, suggesting that haloperidol inhibits neurodevelopment through pathways other than Notch1 signaling. Furthermore, effective combination treatment with VPA indicates that the neurodevelopmental impairment caused by haloperidol can be partially mitigated.
Effect of neurodevelopment on human brain organoids treated with haloperidol and valproic acid (a) Protein expression levels of Notch1 were visualized and quantified using immunoblotting. Notch1 expression levels were normalized to β-actin in brain organoids treated with HAL and VPA at 63 d. Values represent mean ± IQR (n = 5). (b) Light micrographs of brain organoids treated with HAL and VPA from 35 d – 63 d. Scale bars, 200 μm. (c) The size for brain organoids treated with 1 µM HAL and 300 µM VPA from 35 d – 63 d was quantified using surface area and diameter measurements. Values represent mean ± IQR (n > = 6). (d) The size for brain organoids treated with 3 µM HAL and 300 µM VPA from 35 d – 63 d was quantified using surface area and diameter measurements. Values represent mean ± IQR (n ≥ 6). (e) Immunostaining for DAPI (blue), SOX2 (red) and TUJ1 (green) of brain organoids treated with HAL and VPA at 63 d. Scale bars, 50 μm. (f) Quantification of the thickness of VZ-like regions and neuronal layers of organoids treated with 1 µM HAL and 300 µM VPA on 63 d. Values represent mean ± IQR (n = 5 cortical structures from three organoids). (g) Quantification of the thickness of VZ-like regions and neuronal layers of organoids treated with 3 µM HAL and 300 µM VPA on 63 d. Values represent mean ± IQR (n = 5 cortical structures from three organoids). ns; non-significant. ∗p < 0.05, ∗∗p < 0.005 ∗∗∗p < 0.0001.
Discussion
Developmental neurotoxicity (DNT) can be caused by commercially available biochemicals to which humans are exposed, including industrial chemicals and drugs33,34,35. Current DNT tests are primarily conducted in animals and are only performed when there are specific concerns regarding neurotoxicity. However, these animal-based methods pose ethical concerns, are time-consuming, and incur high costs36,37,38. Among alternative models to overcome these problems, human induced pluripotent stem cell (iPSC)-derived neurons are a useful alternative animal model to animal cells or cell lines because they better reflect the human nervous system18,19,40. However, in vitro models using human iPSC-derived neurons in 2D culture are limited in their ability to reflect the complexity of the human central nervous system39,41. Therefore, current researchers are focusing on utilizing 3D organoids, which more accurately and comprehensively replicate the complexity of the human nervous system compared to 2D neural models, to establish precise platforms for neurotoxicity assessment21,22. In this study, we used human iPSC-derived brain organoids, representing a relevant model for examining human brain development. We differentiated brain organoids from iPSCs and demonstrated that these organoids exhibited molecular characteristics reminiscent of the developing human brain (Fig. 1c, d). Haloperidol is an antipsychotic medication used to treat schizophrenia and is often prescribed over prolonged periods to patients of various age groups42. We decided to treat brain organoids with haloperidol between days 21 and 63 to align with their critical neurodevelopmental timeline. During this period, neural progenitor cells (NPCs) actively proliferate, differentiate, and establish major cortical structures, making this time frame ideal for investigating the developmental impact of haloperidol56. Moreover, haloperidol can cross the placental barrier and potentially affect fetal neurodevelopment, including withdrawal symptoms and other adverse events43,44,57,58. By selecting this period, we aimed to capture the essential phases of NPC proliferation and differentiation when the developing brain is particularly vulnerable to damage. Our intervention studies support the key role of Notch1 in neurodevelopment. In the present study, we found that chronic exposure to haloperidol significantly impaired neurodevelopment in human stem cell-derived brain organoids (Fig. 2b). This is consistent with previous studies indicating that haloperidol reduces brain size in animals and humans through mechanisms that are not yet fully understood10,11. Notch1 signaling plays a critical role in regulating the balance between NPC proliferation and differentiation, which is crucial for proper neurodevelopment45,46. In 2D models, DAPT, a known Notch1 inhibitor, is commonly used to suppress the self-renewal ability of NPCs and promote neural differentiation53,54. In contrast, activation of Notch1 signaling in neural progenitor cells (NPCs) induces self-renewal and inhibits neurogenesis55. However, the human brain forms complex structures, including the ventricular zone and cortical areas, making the maintenance of these regions critical56. In E10.5 mouse embryos, Notch1 is highly expressed in regions where neurogenesis is actively occurring, such as the diencephalon and mesencephalon, whereas neuronal expression (TuJ1+) is lower in these areas24. We observed that long-term haloperidol exposure reduced brain organoid size and significantly decreased the ventricular zone and Notch1 signaling (Figs. 2c and 4a). Also, as shown in Fig. 4f, while the size of brain organoids treated with Valproic acid (VPA), a known Notch1 activator, decreases, the VZ thickness increases, suggesting that this thickening is due to the overexpression of Notch1 signaling, which inhibits asymmetric division during neurodevelopment. These findings suggest that this pathway is involved in haloperidol-induced neurodevelopmental toxicity. These results indicate that the Notch1 pathway is involved in haloperidol-induced neurodevelopmental toxicity, and Notch1 signaling likely regulates the maintenance of NPC populations, potentially leading to impaired neurogenesis and a reduction in brain organoid size. Our intervention studies with sodium propionate, and VPA provides promising insights into potential therapeutic strategies for mitigating the adverse effects of haloperidol. Sodium propionate protected against haloperidol-induced neuronal lesions and effectively rescued both the overall size of the brain organoids (Fig. 3b) and Notch1 expression (Fig. 4a). This finding suggests sodium propionate may counteract haloperidol’s inhibitory effect on Notch1 signaling. Additionally, we observed that the size and ventricular zone (VZ) of brain organoids treated with haloperidol and VPA improved compared to those treated with haloperidol alone. This indicates that VPA mitigates haloperidol-induced neurodevelopmental impairments by restoring Notch1 signaling, which increases the VZ size reduced by haloperidol (Figs. 5d, g). Dopamine D2 receptor expression is limited during the early stage, which we are conducting, as dopamine signaling pathways become more prominent in later stages of brain maturation51,52. While we have not specifically quantified D2 receptor expression in our organoids, Previous studies have shown that haloperidol can cause off-target effects on other signaling pathways by inhibiting Notch1 signaling in myofibroblasts31. These findings indicate that the effects of haloperidol in our model are likely mediated through pathways beyond D2 receptor activity and demonstrate the possibility that haloperidol induces deleterious effects not only by inhibiting dopamine D2 receptors but also by disrupting other signaling cascades or receptors. Also, it suggests that the significance of Notch1 signaling in maintaining the structural integrity of brain regions. It indicates that brain organoids, as a robust model for studying neurodevelopment, provide valuable insights into this process. Moreover, a previous randomized placebo-controlled study investigating the adjunctive use of divalproex, a form of valproic acid, with haloperidol demonstrated significantly greater improvements in baseline measures of Clinical Global Impression (CGI), Brief Psychiatric Rating Scale (BPRS) and Scale for the Assessment of Negative Symptoms (SANS) compared to haloperidol monotherapy47. Similarly, in clinical trials, the group treated with divalproex in combination with atypical antipsychotics such as olanzapine or risperidone showed an improvement in the total Positive and Negative Syndrome Scale (PANSS) score over the 28-day treatment period59. Furthermore, a recent study indicated that quetiapine, an antipsychotic drug, also relieved schizophrenia via its effects on Notch1 signaling. Quetiapine has been shown to rescue the cuprizone-induced reduction in the expression of Notch1 in a mouse model of schizophrenia, thereby improving schizophrenia-like behaviors30. Based on these findings, we propose that VPA or sodium propionate may alleviate the side effects of haloperidol. Furthermore, the consistency of our results with clinical observations strengthens the case for brain organoids as a preclinical model for neurodevelopmental studies. Thus, combining haloperidol with VPA or sodium propionate may not only confer clinical benefits but also reduce the risk of antipsychotic-induced neurodevelopmental toxicity. However, in the rescue experiment, when VPA was co-administered with haloperidol, the restoration of Notch1 expression led to recovery of the VZ compared to haloperidol treatment alone. Nevertheless, the VZ was still not fully restored compared to the control group (Fig. 5a and d). This finding suggests that haloperidol may impair neurodevelopment through an additional off-target mechanism beyond the Notch1 pathway, also requiring further molecular studies to elucidate the precise pathways affected by haloperidol and the interactions of sodium propionate or VPA with these pathways. In addition, a limitation of the present study is the use of a single dermal-fibroblast-derived iPSC line. Previous reports show that line-specific genetic and epigenetic backgrounds can modulate organoid cell-type composition and even alter drug sensitivity60,61. Moreover, although the dorsal forebrain model we generated is useful for evaluating haloperidol’s off-target effects beyond its dopamine D2 action, the drug should also be tested in midbrain or ventral forebrain models, where D2 receptors are more abundant62. To better capture inter-individual variability and enhance clinical relevance, we plan to employ multiple, genetically diverse iPSC lines and to differentiate additional midbrain or ventral forebrain organoid models in future studies. Specifically, brain organoids treated with a combination of 1 µM haloperidol and VPA showed no changes in organoid size, ventricular zone, or neuronal layer compared to brain organoids treated with haloperidol alone (Fig. 5c, f). This suggests that investigating other signaling pathways related to neuronal differentiation and neurogenesis is crucial for fully understanding the complete spectrum of haloperidol-induced neurodevelopmental toxicity. To develop safer therapeutic strategies for managing schizophrenia and other neuropsychiatric disorders, toxicity assessments using midbrain organoids through dopamine D2 receptors, the primary targets of haloperidol, are necessary in conjunction with in vivo studies. In conclusion, our findings highlight the critical role of Notch1 signaling in neurodevelopment and emphasize its vulnerability to pharmacological disruption by haloperidol. We demonstrated that long-term haloperidol exposure reduces the size of human iPSC-derived brain organoids and diminishes the VZ, accompanied by decreased Notch1 signaling. Sodium propionate successfully restored both organoid size and Notch1 expression, and VPA partially alleviated the impairment of neurodevelopmental processes. Taken together, these results indicate that Notch1 is pivotal for maintaining normal neurogenesis and suggest that targeting Notch1 may help mitigate the adverse effects of long-term antipsychotic use. Moreover, these findings also imply that alterations in Notch1 signaling could underlie neurodevelopmental toxicity triggered by various exogenous agents. Collectively, these findings suggest a preliminary mechanistic framework for haloperidol-induced neurodevelopmental toxicity and offer an initial basis for future studies aimed at developing strategies to safeguard early brain development during long-term antipsychotic therapy.
Materials and methods
HiPSC culture and generation of NPCs
hFSiPS3-1, a human stem cell line derived from dermal fibroblast was obtained from the Korea Stem Cell Bank (National Institute of Health of Korea) and cultured using TeSR-E8 culture medium (Stem cell Technologies) on vitronectin (Stem cell Technologies) coated culture dishes and passaged every 4–5 days. To generation of neural progenitor cell, we followed the protocol described48. Briefly, hFSiPS3-1 was treated with collagenase type IV (Stem cell Technologies) for 30 min and suspended to induce cell neuroectoderm from the embryoid body. The suspended stem cells were cultured in the neural induction medium (DMEM/F12 (Gibco) supplemented with 20% knockout serum replacement (Thermo Fisher Scientific), 55 µM/ml β-mercaptoethanol (Sigma-Aldrich), 1X MEM-NEAA (Thermo Fisher Scientific), 10 µM/ml SB431542 (Tocris), 5 µM/ml dorsomorphin (Tocris), and 1% penicillin/streptomycin (Thermo Fisher Scientific). Cells were grown in Petri dishes (SPL) for 7 days (days 0–7). On the 7th day, embryoid bodies induced to differentiate into neuroectoderm were attached to a Matrigel-coated culture dish and incubated in rosette culture medium (DMEM/F12 with 1X N2 supplement (Thermo Fisher Scientific), 20 ng bFGF (Peprotech)) for 3 days. On days 7–10, 2.5 µl/ml insulin (Thermo Fisher Scientific) and 1% penicillin/streptomycin were added. On the 10th day, the central part was separated using a glass rod and placed in a Matrigel-coated culture dish containing NPC medium (DMEM/F12 with 1X N2 supplement, 1X B27 supplement (Thermo Fisher Scientific) with bFGF, 20 ng/ml EGF (Peprotech), and 1% penicillin/streptomycin and was passaged every 2–3 days (days 10–18).
Formation of brain organoids
To generation of brain organoids, we followd the protocol with slight modifications, as originally described by Kim49. AggreWell™800 (Stem cell Technologies) plates containing 300 microwells were used to obtain uniformly-sized brain organoids. Approximately 3 × 106 single NPC cells (Day 18) were added per AggreWell™800 well in neural differentiation medium (mixed 1: 1 DMEM/F12 and Neurobasal (Gibco) with 1X N2 supplement, 1X B27 supplement and BDNF, 20 ng/ml GDNF (Peprotech), 200 nM/ml ascorbic acid (Sigma-Aldrich), 100 µM/ml dibutyryl cyclic adenosine monophosphate (Sigma-Aldrich), 1% penicillin/streptomycin), centrifuged at 100 x g for 3 min to capture the cells in the microwells and incubated at 37 ºC with 5% CO2. After 24 h, organoids from each microwell were harvested using a serological pipette and transferred into 100 mm plastic petri dishes on a 60 rpm orbital shaker. The brain organoids were cultured at approximately 15–20 per dish and medium changed every 2–3 days.
Exposure of brain organoids to chemicals
The brain organoids were treated with various concentrations of haloperidol (0.1, 1, 3 µM) (Sigma-Aldrich) with or without propionate (300 µM) (Sigma-Aldrich) or different concentrations of valproic acid (100, 300 µM) (Sigma-Aldrich). In particular, we differentiated brain organoids in two batches to minimize variability across organoid cultures and generated all the brain organoids during drug testing at the same time. We treated them in parallel under different conditions. All chemicals were administered to the brain organoids for 42 days, from day 21 to day 63. All chemical quantitative analyses were conducted on randomly picked cortical structures. The thickness of the VZ and neuronal layer of the brain organoids were measured as reported previously50 at 0, 45, and 90 degrees. All parameters were measured using ImageJ software.
Immunoblotting
Cellular lysates were obtained using RIPA buffer (Thermo Fisher Scientific). The lysates were centrifuged at 10,000 g for 15 min at 4 °C, and the supernatant fractions were collected. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. The proteins were electro transferred using the iBlot 2 Gel Transfer Device (Thermo Fisher Scientific). The membranes were incubated in blocking buffer (0.05% Tween 20 with 5% bovine serum albumin in TBS) for 1 h. After washing three times with TBST, the membranes were incubated with primary antibody overnight. Detection of specific proteins was carried out with an ECL Western blotting kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Signal intensity was analyzed using the iBright Analysis Software (Thermo Fisher Scientific).
Cryosection and Immunofluorescence
The brain organoids were retrieved from the dish on day 63 to precisely identify the detailed characterizations of brain organoids exposed to chemicals by immunohistochemical staining. Brain organoids were fixed in 4% paraformaldehyde (PFA) and incubated at 4 °C overnight. The fixed organoids were washed three times in PBS, transferred to a 30% sucrose solution, and incubated at 4 °C overnight. After dehydration, the tissues were embedded in O.C.T. compound (SAKURA) and stored at − 80 °C. For immunofluorescence staining, 10 μm thick sections were obtained using a cryostat (Leica, Germany). Cryosections on adhesive slides were washed in PBS to remove excess O.C.T. and permeabilized with 0.5% Triton X-100 in PBS for 10 min at room temperature. The sections were then blocked with 5% bovine serum albumin for 1 h. Subsequently, the sections were incubated overnight at 4 °C with a primary antibody diluent. The following primary antibodies were used for immunohistochemistry: TUJ1 antibody-conjugated to Alexa Fluor® 488 (1:200, Santa Cruz, USA), SOX2 antibody-conjugated to Alexa Fluor® 647(1:200, Cell signaling, USA), TBR2 antibody-conjugated to Alexa Fluor® 568 (1:200, Abcam), CTIP2 antibody-conjugated to FITC (1:200 Abcam) and cleaved-caspse3 (1:200, Cell signaling, USA). The samples were washed three times with PBS to remove excess antibodies. Nuclei were counterstained with Mounting Medium containing DAPI (Abcam) for 15 min. All images were captured using a Zeiss LSM700 confocal microscope using ZEN software (Zeiss).
Statistical analysis
All data analysis was performed using GraphPad Prism (GraphPad Software). The results are expressed as medians ± interquartile range (IQR). A two-tailed Mann–Whitney U test was used for statistical significance, and the P values are reported in the figure legends.
Data availability
All the raw data supporting this study’s results are available from the corresponding author upon request.
References
Huang, X. F. & Song, X. Effects of antipsychotic drugs on neurites relevant to schizophrenia treatment. Med. Res. Rev. 39, 386–403 (2019).
Orzelska-Górka, J., Mikulska, J., Wiszniewska, A. & Biała, G. New atypical antipsychotics in the treatment of schizophrenia and depression. Int J. Mol. Sci 23, 10624 (2022).
Pahwa, M., Sleem, A., Elsayed, O. H., Good, M. E. & El-Mallakh, R. S. New antipsychotic medications in the last decade. Curr. Psychiatry Rep. 23, 87 (2021).
Ginovart, N. & Kapur, S. Role of dopamine D(2) receptors for antipsychotic activity. Handb. Exp. Pharmacol. 212, 27–52 (2012).
Gomes, F. V. & Grace, A. A. Beyond dopamine receptor antagonism: new targets for schizophrenia treatment and prevention. Int J. Mol. Sci 22, 4467 (2021).
Jafari, S., Fernandez-Enright, F. & Huang, X. F. Structural contributions of antipsychotic drugs to their therapeutic profiles and metabolic side effects. J. Neurochem. 120, 371–384 (2012).
Hatta, K. et al. The association between intravenous haloperidol and prolonged QT interval. J. Clin. Psychopharmacol. 21, 257–261 (2001).
Dai, Y. et al. Haloperidol induces the nuclear translocation of phosphatidylinositol 3′-kinase to disrupt Akt phosphorylation in PC12 cells. J. Psychiatry Neurosci. 32, 323–330 (2007).
Hu, M. et al. Propionate protects haloperidol-induced neurite lesions mediated by neuropeptide Y. Front. Neurosci. 12, 743 (2018).
Dorph-Petersen, K. A. et al. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology 30, 1649–1661 (2005).
Ho, B. C., Andreasen, N. C., Ziebell, S., Pierson, R. & Magnotta, V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry. 68, 128–137 (2011).
Bal-Price, A. et al. Strategies to improve the regulatory assessment of developmental neurotoxicity (DNT) using in vitro methods. Toxicol. Appl. Pharmacol. 354, 7–18 (2018b).
Crofton, K. M. et al. Undertaking positive control studies as part of developmental neurotoxicity testing: a report from the ILSI research foundation/risk science Institute expert working group on neurodevelopmental endpoints. Neurotoxicol Teratol. 30, 266–287 (2008).
Tsuji, R. & Crofton, K. M. Developmental neurotoxicity guideline study: issues with methodology, evaluation and regulation. Congenit Anom. 52, 122–128 (2012).
Crofton, K. M., Makris, S. L., Sette, W. F., Mendez, E. & Raffaele, K. C. A qualitative retrospective analysis of positive control data in developmental neurotoxicity studies. Neurotoxicol Teratol. 26, 345–352 (2004).
Han, J. J. FDA Modernization Act 2.0 Allows for Alternatives To Animal Testing (Wiley Online Library, 2023).
Uesawa, Y. Efficiency of pharmaceutical toxicity prediction in computational toxicology. Toxicol 40, 1–9 (2024).
Hunsberger, J. G. et al. Induced pluripotent stem cell models to enable in vitro models for screening in the central nervous system. Stem Cells Dev. 24, 1852–1864 (2015).
Ryan, K. R. et al. Neurite outgrowth in human induced pluripotent stem cell-derived neurons as a high-throughput screen for developmental neurotoxicity or neurotoxicity. Neurotoxicology 53, 271–281 (2016).
Cerneckis, J., Cai, H. & Shi, Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Signal. Transduct. Target. Ther. 9, 112 (2024).
Kim, J., Sullivan, G. J. & Park, I. H. How well do brain organoids capture your brain? iScience 24, 102063 (2021).
Kim, S. H. & Chang, M. Y. Application of human brain organoids—opportunities and challenges in modeling human brain development and neurodevelopmental diseases. Int. J. Mol. Sci. 24, 12528 (2023).
Christensen, J. et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309, 1696–1703 (2013).
Hatakeyama, J. & Kageyama, R. Notch1 expression is spatiotemporally correlated with neurogenesis and negatively regulated by Notch1-independent Hes genes in the developing nervous system. Cereb. Cortex 16(suppl 1)(suppl_1):i132-i137, i132–i137 (2006).
Sachan, N., Mutsuddi, M. & Mukherjee, A. Notch signaling: from neurogenesis to neurodegeneration. Insights Hum. Neurodegener Lessons Learnt Drosophila, 7, 185–221 (2019).
Zhang, R., Engler, A. & Taylor, V. Notch: an interactive player in neurogenesis and disease. Cell. Tissue Res. 371, 73–89 (2018).
Brai, E. et al. Notch1 regulates hippocampal plasticity through interaction with the reelin pathway, glutamatergic transmission and CREB signaling. Front. Cell. Neurosci. 9, 447 (2015).
Cui, K. et al. Neurodevelopmental impairment induced by prenatal valproic acid exposure shown with the human cortical organoid-on-a-chip model. Microsyst. Nanoeng. 6, 49 (2020).
Zang, Z. et al. Valproic acid exposure decreases neurogenic potential of outer radial glia in human brain organoids. Front. Mol. Neurosci. 15, 1023765 (2022).
Wang, H. N. et al. Quetiapine ameliorates schizophrenia-like behaviors and protects Myelin integrity in Cuprizone intoxicated mice: the involvement of Notch signaling pathway. Int. J. Neuropsychopharmacol. 19, pyv088 (2015).
Rehman, M. et al. High-throughput screening discovers antifibrotic properties of haloperidol by hindering myofibroblast activation. JCI Insight 4, 8 (2019).
Chauhan, P., Garg, S., Tikka, S. K. & Khattri, S. Efficacy of intensive cerebellar intermittent theta burst stimulation (iCiTBS) in treatment-resistant schizophrenia: a randomized placebo-controlled study. Cerebellum 20, 116–123 (2021).
Harrill, J. A. et al. Testing for developmental neurotoxicity using a battery of in vitro assays for key cellular events in neurodevelopment. Toxicol. Appl. Pharmacol. 354, 24–39 (2018).
Parenti, I., Rabaneda, L. G., Schoen, H. & Novarino, G. Neurodevelopmental disorders: from genetics to functional pathways. Trends Neurosci. 43, 608–621 (2020).
Rice, D. & Barone, S. Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 108 (suppl 3), 511–533 (2000).
Raffaele, K. C. et al. The use of developmental neurotoxicity data in pesticide risk assessments. Neurotoxicol Teratol. 32, 563–572 (2010).
Shafer, T. J. et al. Evaluation of chemical effects on network formation in cortical neurons grown on microelectrode arrays. Toxicol. Sci. 169, 436–455 (2019).
Bal-Price, A. et al. Recommendation on test readiness criteria for new approach methods (NAM) in toxicology: exemplified for developmental neurotoxicity (DNT). ALTEX 35, 306–352 (2018a).
Schmidt, B. Z. et al. In vitro acute and developmental neurotoxicity screening: an overview of cellular platforms and high-throughput technical possibilities. Arch. Toxicol. 91, 1–33 (2017).
Aschner, M. et al. Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: example lists and criteria for their selection and use. ALTEX 34, 49–74 (2017).
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell. Stem Cell. 3, 519–532 (2008).
Hutchings, E. J., Waller, J. L. & Terry, A. V. Differential long-term effects of haloperidol and Risperidone on the acquisition and performance of tasks of Spatial working and short-term memory and sustained attention in rats. J. Pharmacol. Exp. Ther. 347, 547–556 (2013).
Gener, T. et al. Serotonin 5-HT1A, 5-HT2A and dopamine D2 receptors strongly influence prefronto-hippocampal neural networks in alert mice: contribution to the actions of Risperidone. Neuropharmacology 158, 107743 (2019).
Li, P., Snyder, G. L. & Vanover, K. E. Dopamine targeting drugs for the treatment of schizophrenia: past, present and future. Curr. Top. Med. Chem. 16, 3385–3403 (2016).
Ables, J. L. et al. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J. Neurosci. 30, 10484–10492 (2010).
Zhou, Z. D., Kumari, U., Xiao, Z. C. & Tan, E. K. Notch as a molecular switch in neural stem cells. IUBMB Life. 62, 618–623 (2010).
Wassef, A. A. et al. Randomized, placebo-controlled pilot study of divalproex sodium in the treatment of acute exacerbations of chronic schizophrenia. J. Clin. Psychopharmacol. 20, 357–361 (2000).
Kang, H. et al. Assessment of neurotoxicity on neurite outgrowth in human induced pluripotent stem cell-derived neurons using high-content screening. JSAAE 16, 43–52 (2022).
Kim, H. & Jiang, P. Generation of human pluripotent stem cell-derived fused organoids with oligodendroglia and Myelin. STAR. Protocols. 2, 100443 (2021).
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016).
Reumann, D. et al. In vitro modeling of the human dopaminergic system using spatially arranged ventral midbrain-striatum-cortex assembloids. Nat. Methods. 20, 2034–2047 (2023).
Money, K. M. & Stanwood, G. D. Developmental origins of brain disorders: roles for dopamine. Front. Cell. Neurosci. 7, 260 (2013).
Borghese, L. et al. Inhibition of Notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells. 28, 955–964 (2010).
Venkatesh, K. et al. NOTCH signaling is essential for maturation, self-renewal, and tri-differentiation of in vitro derived human neural stem cells. Cell. Reprogramming. 19, 372–383 (2017).
Kaltezioti, V. et al. Prox1 regulates the Notch1-mediated Inhibition of neurogenesis. PLoS Biol. 8, e1000565 (2010).
Lancaster, M. A. & Knoblich, J. A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 9, 2329–2340 (2014).
Gentile, S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophrenia bulletin 36, 518–544. (2010).
Akar, M. et al. Transient nephrogenic diabetes insipidus caused by fetal exposure to haloperidol. Ren. Fail. 36, 951–952 (2014).
Casey, D. E. et al. Effect of divalproex combined with olanzapine or Risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacology 28, 182–192 (2003).
Quadrato, G. et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53 (2017).
Yoon, S-J. et al. Modeling alzheimer’s disease with patient-derived brain organoids. Cell. Stem Cell. 24, 977–993 (2019).
Sebastian, R. et al. Method to generate dorsal forebrain brain organoids from human pluripotent stem cells. Bio-protocol 14, e4608 (2024).
Acknowledgements
This work was supported by a grant from the Korea Institute of Toxicology (1711195889 & 2710008763) and the Ministry of Food and Drug Safety (22213MFDS391).
Author information
Authors and Affiliations
Contributions
Conceptualization: Hyunsu Kang, Ki-Suk Kim; methodology: Hyunsu Kang; formal analysis and investigation: Hyunsu Kang; writing original draft preparation: Hyunsu Kang; writing review and editing: Hana Cho, Ki-Suk Kim; funding acquisition: Jae-Hyeok Lee, Ki-Suk Kim; supervision: Hana Cho, Ki-Suk Kim. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kang, H., Lee, JH., Cho, H. et al. Chronic haloperidol exposure impairs neurodevelopment via Notch1 signaling in human stem cell-derived brain organoids. Sci Rep 15, 25945 (2025). https://doi.org/10.1038/s41598-025-08855-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08855-w