Abstract
Osteosarcoma is a prevalent primary malignant bone tumor predominantly affecting children and adolescents, characterized by a poor survival rate and prognosis. Currently, ferroptosis is a newly defined form of cell death, but the mechanism between it and osteosarcoma is unclear. To further investigate the relationship between osteosarcoma and ferroptosis, it is important to search for new biomolecular factors. We used bioinformatics to dig deeper into the ferroptosis gene PSAT1, which is closely associated with osteosarcoma. Although PSAT1 has been reported in other types of tumours and plays an important role in the development of many tumours, such as melanoma and breast cancer, little research has been done in the field of osteosarcoma. The results indicated that PSAT1 could promote the development of osteosarcoma and inhibit the ferroptosis process in osteosarcoma cells. This finding implies that PSAT1 may become a new target for the diagnosis and treatment of osteosarcoma in the future, bringing new breakthroughs to clinical practice.
Similar content being viewed by others
Introduction
Osteosarcoma is a malignant tumour commonly seen in children and adolescents1, with an annual incidence of 4.4 per million2. It commonly occurs in the long tubular epiphyses of the extremities, with the vast majority occurring around the knee joint and in the lower femur, tibia and upper fibula3. However, the pathogenesis of osteosarcoma is unknown and some studies have reported that it is thought to be associated with hereditary, viral infections, ionising radiation and chemical toxins4. The treatment of osteosarcoma usually includes chemotherapy, surgery, and radiotherapy as appropriate5. In recent years, although the survival rate of patients with osteosarcoma has improved with the use of chemotherapy, neoadjuvant chemotherapy, radiotherapy and immunotherapy, satisfactory treatment outcomes have not been achieved due to the complexity and instability of the osteosarcoma genome6. The lungs are the most common organ for osteosarcoma metastases and the vast majority of tumour patients die from complications following lung metastases7. With the development of adjuvant chemotherapy regimens, advances in surgical techniques, and improvements in radiation staging studies, 90–95% of patients with osteosarcoma can now undergo limb-salvage resection and reconstruction, and the survival rate of patients is increased8. The 10-year survival rate for patients with osteosarcoma has increased from 30% to around 50%, but the overall 10-year survival rate for patients has not changed significantly9. The prognosis for patients with osteosarcoma remains poor. Tumour cells are resistant to chemotherapy drugs. Drug resistance is a key factor in treatment failure and tumour recurrence10. Therefore, it is important to search for osteosarcoma-related gene targets and explore their associated mechanisms.
PSAT1 is a member of the transaminase family and plays a key role in purine nucleic acid metabolic pathways, especially in the synthesis of phosphoserine11. According to relevant literature, PSAT1 can also regulate the synthesis of serine in tumour cells, thus promoting the proliferation of tumour cells. However, PSAT1 has been little studied in osteosarcoma, and one paper reported that PSAT1 may act as an osteosarcoma-promoting gene in the progression of osteosarcoma12.
Dixon et al. first introduced the concept of ferroptosis13, a specific type of cell death with lipid peroxidation (LPO) aggregation and iron ion-dependent features, in 2012. In the last decade, ferroptosis has become a hot topic in tumour research mechanisms. Unlike the classical cell death previously identified, ferroptosis does not have a typical cellular necrosis or apoptotic morphology, but is characterised by reduced or absent mitochondrial cristae, concentrated mitochondrial membranes, increased density, and essentially normal cell membrane and nucleus size14. The pathogenesis of ferroptosis is complex and involves a variety of biological processes such as iron metabolism, lipid metabolism, oxidative stress, glutathione and coenzyme Q10 biosynthesis, which have important regulatory roles in osteoblasts and osteosarcoma cells15. The current hot spot regulators of ferroptosis include glutathione peroxidase 4 (GPX4)16, ferroptosis suppressor protein 1 (FSP1)17 and solute carrier family 7 member 11 (SLC7A11)18. Several related studies have shown that ferroptosis plays an important role in the progression of a variety of tumours19,20,21, and that ferroptosis plays an important role in inhibiting tumour progression by regulating cellular metabolism and promotion.
At present, the mechanism of ferroptosis and osteosarcoma has not been fully elucidated. According to relevant literature, ferroptosis not only inhibits the progression of osteosarcoma but also reduces its chemoresistance22,23. The regulatory mechanisms of ferroptosis in osteosarcoma primarily focus on the GSH-GPX4 axis24. Since the mechanism of ferroptosis and osteosarcoma action is not yet clear, it is important to study the mechanism of ferroptosis in osteosarcoma progression study and find the relevant targets for therapeutic tumour action.
Materials and methods
Database of data
Through the GEO database (https://www.ncbi.nlm.nih.gov/). The data sets GSE19276 and GSE28424 were obtained. 63 osteosarcoma tissue samples and 8 normal tissue samples were obtained and used as experimental group and control group, respectively.
Screening for differentially expressed genes
The Limma package of R software (version: 3.40.2) was used for data correction and quantile normalisation of the expression matrices of the samples (control and experimental groups), followed by advanced t-test analysis, and p values were corrected according to hypothesis testing to screen for corrected p values < 0.05 and |logFC|< 1; the screened genes were used as differentially expressed genes in osteosarcoma. The volcano plot was constructed and the heat map was constructed by the heat map package of R software.
Ferroptosis-related genes in ferroptosis
Access to the FerrDb database (http://zhounan.org/ferrdb/) allows for the retrieval of iron death-related genes25. The differentially expressed genes identified through differential analysis were compared with ferroptosis-related genes, and R software was utilized to find the intersection, resulting in a Venn diagram. The genes found at the intersection are identified as osteosarcoma ferroptosis-related genes.
Gene ontology (GO) and kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis
The filtering conditions were set: p value Filter = 0.05, Q value Filter = 0.05, performed GO and KEGG enrichment analysis of the intersection genes through the R software “clusterProfiler” package, and then visualized the significant enrichment results separately through the ggplot2 package26.
Protein–protein interaction network construction and module analysis
The protein–protein interaction network of the intersection genes was constructed online through the STRING (https://string-db.org/) database27, and the screening threshold was set to be 0.4. The isolated nodes were eliminated and the network was optimized to obtain the protein–protein interaction data of the intersection genes. Protein–protein interaction data were visualized using the Cytoscape software (version 3.8.2; www.cytoscape.org)28.
Cell origin and culture
Human normal osteoblast hFOB1.19 and human osteosarcoma cell lines 143B, U2OS, Saos-2, HOS, and MG-63 were purchased from the Research in Basic Medicine, Chinese Academy of Medical Sciences Institute Cell Resource Center. U2OS, HOS, MG-63 and hFOB1.19 cells were cultured in DMEM medium, and 143B cells were cultured in RPMI-1640 medium. Both DMEM medium and RPM-1640 medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin and streptomycin were cultured in a cell incubator at 37 °C and 5% CO2.
Real-time immunofluorescence detection
Cell samples were collected, sample RNA was extracted with reference to the kit, and stored at − 80 °C for later use. Purified RNA was reverse transcribed using a reverse transcription kit. Amplification by PCR instrument was performed at 37 °C for 15 min followed by 85 °C for 5 s. Take 2 µL of cell cDNA, add 12.5 µL of 2 × premix Taqin turn, 1 µL of upstream and downstream primers, add ddH2O to the total volume of 20 µL, PCR instrument amplification, expansion conditions are as follows: Predenaturation at 94 °C for 1 min was followed by denaturation at 94 °C for 30 s, annealing at 57 °C for 15 s, extension at 72 °C for 15 s, and extension at 72 °C for 10 min after 40 cycles. For the mRNA Samples, GAPDH was used as an internal control (see Table 1 for primer sequences).
Western blot analysis
RIPA lysate (150 mmol/L NaCl, 1 mmol/L EDTA, 50 mmol/L Tris HCl pH 7.4, 1% Triton X-100, 0.5% deoxycholic acid, 0. The concentration of protein lysate was determined by BCA protein quantitation kit. Thirty µg of total proteins were subjected to 10% SDS-PAGE and 120 V constant pressure electrophoresis for about 2 h, followed by membrane transfer, blocking, antibody incubation at 4 °C overnight, and second antibody incubation for 1 h. ECL reagent was added and the images were visualized on X-ray film. The protein expression levels of each sample were detected.
Construction of the PSAT1 shRNA recombinant plasmid
The sh plasmids was derived from Tsingke Biotechnology, Inc. The PSAT1-shRNA and NC-shRNA oligonucleotides were dissolved in sterile water and used as templates for PCR amplification. The PCR amplification products were ligated to the carrier by T4 DNA ligase GV-298, the ligation product was transformed into competent E. coli DH5α and incubated at 37 °C overnight. Positive clones were selected into 5 mL LB medium. After amplification, identification and verification were performed. The correctly identified recombinant plasmid was named PSAT1-shRNA.
Construction of overexpression plasmids and siRNA plasmids
Take out the corresponding receptor cells from − 80 °C refrigerator, thaw them on the ice box at room temperature, label them; add 2 µL of the corresponding plasmid into the receptor cells. Plasmids were purchased from miaolingbio and ribobio. placed in the refrigerator at 4 °C in an ice bath for 30 min; heat-stimulate the plasmid in the water bath at 42 °C for 1 min, and then put it on the ice box for 2 min immediately; add 900 µL of LB medium into each tube, shaking at 37 °C, 150 rpm × 45 min; take 100 µL of transformation solution into the corresponding resistant plate and pour in appropriate amount of glass beads to smear the liquid well; put the plate upside down in the constant temperature incubator at 37 °C to culture overnight. 150 rpm × 45 min; take 100 µL of transformation solution and add it into the corresponding resistant plate, pour in appropriate amount of glass beads and spread the liquid well; place the plate upside down in a 37 °C constant temperature incubator and incubate it overnight. siRNAs were designed and synthesized by ribobio to downregulate the expression of target genes, and siRNA-targeted none was used as the control (si-NC group). Extract the corresponding plasmid using the Plasmid Central Extraction Kit.
Cell transfected
Cells were seeded at 1 × 105 cells/well in 6-well plates 24 h before transfection, and after 50–70% confluence of cells, Constructed corresponding plasmids and Lipofectamine 3000 were used according to Lipofectamine 3000 instructions. Then, 250 µL Opti-MEM culture medium was added and diluted, left at room temperature for 5 min, and gently mixed. Then, 400 µL mixture was added to 6-well plates by drop and transfected Subsequent experiments were performed 48 h later.
Cell proliferative activity was measured by CCK-8
Cells in the logarithmic growth phase were seeded at 2 × 103 in 96-well plates and cultured at 37 °C in a 5% CO2 conditioned incubator. At 24, 48, 72, and 96 h of cell culture, 10 µL CCK-8 reagent was added to each well, respectively, and the absorbance value at a wavelength of 450 nm was read by a microplate reader, and the cell growth curve was drawn by the absorbance value. Five complex holes were set up in parallel in each group, and the experiment was repeated three times.
Treswell cell method assay
Cells were diluted in serum-free medium, counted, and prepared into cell suspensions. 2 × 105 cells were dissolved 100 µL DMEM culture medium was placed in the upper Transwell chamber, and 700 µL conditioned medium containing 10% FBS was added to the lower chamber. The cells were incubated at 37 °C for 24 h, the upper chamber was removed, washed twice with PBS, and fixed with 4% paraformaldehyde at room temperature. 0.1% crystal violet was added and stained at room temperature for 10 min. The cells were washed twice with PBS and the upper surface cells were wiped off with a cotton ball. The results were observed and counted under a microscope.
Cell cycle assay
Flow cytometry was used to detect the effect of PSAT1 on cell cycle of 143B and MG63 cells. OS cells were seeded into 6-well culture plates. When the cells were cultured to about 60–70%, shRNA was added into the experimental group and the control group. When the cells were cultured between 48 and 72 h, the cells were collected, and the cell cycle was determined by flow cytometry according to the instructions of the cycle reagent.
Cell clone formation assay
143B and MG63 cells in the logarithmic growth phase were selected, digested into single cells, and counted. 1000 cells/well were seeded in 6-well plate medium, and the culture was continued until the number of most monoclonal cells was more than 50. They were washed with PBS, fixed with 4% paraformaldehyde for 15 min, stained with 1% crystal violet staining solution for 15 min, washed several times with PBS, dried, photographed, and counted.
Intracellular lipid ROS assay
The cells in the logarithmic growth phase were seeded and cultured in 6-well plates, and the corresponding treatment was completed according to the experimental group. DCFH-DA staining solution was added and incubated in the incubator for 1 h. The culture medium in each well was discarded, digested and collected, and then resuspended in 250 uL PBS.
The contents of MDA, GSH and ferrous ion were detected
The cells of different experimental groups were treated with ferroptosis inhibitor, digested and collected. The contents of MDA, GSH and Fe2+ in cells of each experimental group were detected at 532 nm, 405 nm and 593 nm according to the instructions.
C11-BODIPY for lipid peroxidation assay
To assess lipid peroxidation or cellular GSH/GSSG in OS cells, cells were designated to be treated for 48 h. For confocal imaging, cellular lipid peroxidation was detected by staining with probe (2.5 µM) with fluorescent dye C11-BODIPY 581/591 for 30 min at 37 °C.
Luciferase reporter gene analysis
The gene plasmid vector was constructed by inserting the PSAT1 fragment into the luciferase-expressed reporter gene vector. The cells were co-transfected with the reporter gene plasmid and phRL-TK (internal reference). Luciferase activity was determined by adding 1× PLB cell lysate and lysing the cells at room temperature for 15 min. Stop&Glo, a substrate for Renilla luciferase, was formulated to be able to terminate the reaction of LAR II. Determination of fluorescence values. Add 10 µL of cell lysate to 40 µL of LAR II, blow and mix, and detect the reading, which is the value of Firefly luciferase. Add 40 uL Stop&Glo and read again for the value of Renilla luciferase. First calculate the ratio of Firefly luciferase/Renillaluciferase for each tube, and then use the ratio for the control group as unit 1 to get the relative luciferase activity of the different treatment groups, that is, the regulatory activity of gene transcription in that treatment group.
Xenografts in mice
Five-week-old male BALB/c nude mice were used for tumor growth studies in vivo. Briefly, OS cells were subcutaneously injected into the dorsal side of mice blindly and randomly (n = 5 per group). Tumor volume was measured twice a week and calculated as follows: V = W2 × L/2. The nude mice in this experiment were euthanized at the end of the experiment. After inhalation of isoflurane anesthesia for 2–3 min, the experimenter grabbed the root of the rat tail with his right hand and lifted it, placed it on the cage cover, pressed the head and neck of the rat downward with his left thumb and index finger, and grabbed the root of the rat tail and pulled it backward and upward with his right hand, resulting in cervical dislocation, and the spinal cord and brain stem were severed. All animal experiments were performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the ethics committee of the First Affiliated Hospital of Nanchang University.
Statistical analysis
All experiments were conducted a minimum of three times, and the data are presented as mean ± standard deviation. Significant differences between the experimental and control values were assessed using Student’s t-test or one-way ANOVA. Statistical analyses were carried out using GraphPad software, with a p value of less than 0.05 considered statistically significant.
Results
Screening for ferroptosis-related genes in osteosarcoma
The two databases, GSE19276 and GSE28424, were normalised to obtain 44 osteosarcoma samples and 4 normal tissue samples for GSE19276; and 19 osteosarcoma samples and 4 normal tissue samples for GSE28424 (Fig. 1). As shown, the 151 genes in the osteosarcoma samples were tagged as differentially expressed genes, including both up-regulated and down-regulated genes, compared to normal tissue samples (Fig. 2A).
Identification of DEGs in OS. (A,B) Gene expression profiles of OS tumors and normal tissues from both GSE datasets are shown with volcano plots. Black symbols represent normally expressed genes. Red and blue symbols represent the aberrantly expressed genes with |log2FC|> 1 and adjusted p < 0.05. (C,D) Heat map shows from GSE19276 and GSE28424 dataset difference expression in normal tissue and tumor tissue. DEGs were defined with |log2FC|> 1 and adjusted p < 0.05.
Acquisition of FGRs. (A) Common differentially expressed genes in GSE19276 and GSE28424 datasets. (B) The differentially expressed genes were intersected with the ferroptosis-related gene database to obtain 7 FGRs. (C) Ferroptosis related genes with significant differences of protein and protein interaction (PPI) diagram. (D,E) GO enrichment analysis and KEGG pathway enrichment analysis of genes with significant difference in ferroptosis.
Acquisition of FRGs
The differentially expressed genes in osteosarcoma obtained from the two osteosarcoma databases were compared with the ferroptosis related gene database, 19 FRGs were obtained in GSE19276 and 26 FRGs were obtained in GSE28424, as shown in the figure (Fig. 2B). The above two groups of genes were intermixed, and 7 FRGs were obtained. These are CYBB, ALOX5, CBS, PSAT1, HBA1, TRIB3 and ASNS (Fig. 2B).
PPI network of differential genes in ferroptosis-related genes osteosarcoma
The protein–protein interaction network of ferroptosis-related genes in osteosarcoma was constructed online using STRING database 16. The screening threshold was set to 0.4 to eliminate discrete nodes and optimize the network. Cytoscape software 17 was used to visualize the protein–protein interaction data (Fig. 2C).
GO and KEGG enrichment analysis of ferroptosis-related genes
GO and KEGG functional enrichment were performed for FGRs. As shown in the figure, GO enrichment showed that in biological processes, differential genes were mainly concentrated in organic acid biosynthesis process, carboxylic acid biosynthesis process and α-amino acid biosynthesis process; in cellular composition, genes were mainly concentrated in intracellular vesicles and hemoglobin complex; in molecular functions, genes were mainly concentrated in tetrapyrrole binding, heme binding and oxygen binding (Fig. 2D). KEGG enrichment showed that differential genes were mainly enriched in signaling pathways such as amino acid biosynthesis, metabolism of cysteine and methionine, and metabolism of glycine, serine and threonine (Fig. 2E).
Targeted drug prediction
We used the DSigDB database to predict potential target drugs associated with key genes for ferroptosis in osteosarcoma that may treat osteosarcoma by modulating ferroptosis. The prediction of target drugs was performed and the top 10 target drugs predicted based on the composite score are shown in Table 2.
Real-time fluorescence quantitative detection of gene expression results
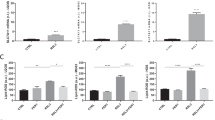
The results of real-time fluorescence quantitative PCR showed that PSAT1, CBS, TRBI3, CYBB and ALOX5 were significantly different between osteosarcoma cells and osteoblasts (p < 0.05) (Fig. 3A). We previously used bioinformatics methods to predict the obtained seven FRGs, among which CBS, PSAT1, TRIB3 and ASNS were up-regulated genes, while CYBB, ALOX5 and HBA1 were down-regulated genes. Combining the qPCR results and reviewing the literature on related FGRs, PSAT1 was selected as the subject of this study.
PSAT1 was highly expressed in osteosarcoma cells
The effect of PSAT1 on osteosarcoma was verified by in vitro cellular assays. First, the expression of PSAT1 mRNA in different osteosarcoma cell lines was detected by RT-PCR, and 143B and MG-63 cells were selected for the next step of the experiment based on the results (Fig. 3B). It was then verified using protein blotting that PSAT1 protein levels were higher in 143B and MG-63 cells than in hFOB1.19 cells (Fig. 3C).
Silencing PSAT1 may inhibit the proliferation, migration and invasion of osteosarcoma cells
143B and MG63 cells were transfected with sh-nc, sh-PSAT1-1 and sh-PSAT1-2 respectively. RT-PCR and WB confirmed that the expression level of PSAT1 was reduced in cells transfected with sh-1 and sh-2 compared to the control group (Fig. 4A,B). The OD values of MG63 cells and 143B cells in the sh group were significantly lower at 72 and 96 h compared to the sh-nc group (Fig. 4C). In the results of colony formation, the sh group formed fewer cell colonies. Therefore, we can conclude that PSAT1 can promote the proliferation of osteosarcoma cells (Fig. 4D). Transwell assays showed that the number of cells in the PSAT1 knockdown group was reduced, indicating that PSAT1 promoted the migration and invasion of osteosarcoma cells (Fig. 4E,F). Flow cytometry examined the effect of PSAT1 on the cell cycle of osteosarcoma cells. We found that the proportion of G1-phase cells in the sh group was lower than that in the control group, suggesting that PSAT1 may promote the proliferation of osteosarcoma cells by promoting the cellular transition of G1-S phase cells (Fig. 4G).
PSAT1 silencing inhibited the proliferation, migration and invasion of osteosarcoma cells. (A) RT-qPCR was used to detect the expression of PSAT1 mRNA in 143B and MG-63 cells. (B) Western blot was used to detect the expression of PSAT1 protein in 143B and MG-63 cells. (C) CCK8 assay was used to detect the effect of PSAT1 on the proliferation of 143B and MG-63 cells. (D) Cell clone formation method for 143B and MG-63 cell proliferation ability. (E,F) The effect of PSAT1 on the migration and invasion ability of 143B and MG-63 cells was detected by Transwell chamber assay (× 20 times). (G) The effect of PSAT1 on the cell cycle of 143B and MG-63 cells was detected by flow cytometry. sh-PSAT1: silencing PSAT1 group; sh-NC: control group. *p < 0.05; **p < 0.01; ***p < 0.001.
PSAT1 overexpression may promote OS cell migration, invasion and proliferation
We transfected with pCMV-PSAT1 to increase the expression of PSAT1 in osteosarcoma cells, and used pCMV-CMS as a control. Application of qRT—PCR and WB confirmed that PSAT1 expression was significantly increased in 143B and MG-63 cells transfected with pCMV-PSAT1 (Fig. 5A,B). The cell scratch results showed that the pCMV-PSAT1 group significantly promoted the growth and migration of 143B and MG-63 cells compared with the control group (Fig. 5C). In addition, to assess the effects of proliferation and invasion behaviours of U2OS and MG63 cells, we performed Transwell migration assay and invasion assay and cell cycle assay. We could observe that the proliferation and invasion ability of pCMV-PSAT1 cells were significantly higher compared with control cells (Fig. 5D,E). Flow cytometry was used to detect cell cycle changes in the overexpressing PSAT1 group and the control group. We found that the proportion of G1 phase cells in the overexpression group was higher than that in the control group (Fig. 5F,G). These results suggest that PSAT1 can promote the proliferation, migration and invasion of osteosarcoma cells, and the reduction of PSAT1 may be related to the progression of human osteosarcoma.
Overexpression of PSAT1 promoted tumor proliferation, migration and invasion. (A) PSAT1 mRNA content after transferring into PSAT1 overexpression plasmid. (B) PSAT1 protein after transferring into PSAT1 overexpression plasmid. (C) The effect of overexpression of PSAT1 on the migratory capacity of 143B and MG-63 cells was verified by the cell scratch method. (D,E) The effect of overexpression of PSAT1 on the invasive and migratory capacity of 143B and MG-63 cells was verified by the Treswell chamber method. (F,G) Effect of PSAT1 overexpression on the cycle of 143B and MG-63 cells. pCMV-PSAT1: overexpression PSAT1 group; pCMV-MCS: control group. *p < 0.05; **p < 0.01; ***p < 0.001.
Silencing PSAT1 induced ferroptosis in osteosarcoma cells
According to the WB results, the level of GPX4 in the sh group was significantly lower than that in the control group and increased after the use of ferrostatin-1 (Fig. 6A). In the cell viability assay, the sh group showed reduced OD values in 143B and MG63 cells compared to the control group, suggesting a decrease in viability, and an increase in cell viability after the addition of frostatin-1 (Fig. 6B), indicating that silencing PSAT1 can induce ferroptosis in osteosarcoma cells. The results showed that the MDA and ferrous ion contents were significantly higher in 143B and MG63 cells in the sh group compared to the control group, while the GSH content was significantly lower. Administration of ferrostatin-1 resulted in a decrease in MDA and ferrous ion content and an increase in GSH content (Fig. 6C). By flow cytometry analysis, ROS levels were increased in 143B and MG63 cells in the sh group compared to the control group, while ROS levels were significantly increased in the sh group after administration of ferrostatin-1 (Fig. 6D). These results suggest that silencing PSAT1 induces ferroptosis in osteosarcoma cells. Accumulation of lipid peroxidation was observed in OS cells using C11-BODIPY assay. The results showed that silencing PSAT1 elevated the lipid peroxidation level of OS cells by about 15%. Meanwhile, ferrostatin-1 rescued lipid peroxidation and led to a shift from oxidised to non-oxidised state (Fig. 6E).
Silencing PSAT1 may promote ferroptosis in OS cells. (A) The ferroptosis-related proteins in each group were determined by Western blot. (B) After silencing PSAT1 or adding ferrostatin-1, the relative cell viability of each group was measured by CCK8 assay. (C) After silencing PSAT1 and adding ferrostatin-1, the relative contents of MDA, ferrous ion and GSH in each group were measured by kits. (D) Flow cytometry was used to measure the relative content of ROS in each group after silencing PSAT1 or adding ferrostatin-1. (E) The accumulation of lipid peroxidation was measured by C11-BODIPY staining after silencing PSAT1 or adding ferrostatin-1. sh-PSAT1: silencing PSAT1 group; sh-NC: control group, fer-1 ferrostatin-1. *p < 0.05; **p < 0.01; ***p < 0.001.
The inhibition of PSAT1 suppresses tumorigenesis in vivo
The stable knockdown of PSAT1 resulted in a suppression of tumor growth and weight, as well as a decrease in PSAT1 expression in xenografts within OS cell-engineered athymic nude mice (Fig. 7). This finding suggests that the inhibition of PSAT1 effectively suppresses tumorigenesis in vivo. Collectively, these results indicate that PSAT1 regulates ferroptosis in osteosarcoma by modulating GSH and GPX4 proteins (Fig. 8).
PSAT1 inhibits tumorigenesis in vivo. (A) Representative images in nude mice established by the subcutaneous injection of OS cells stably transfected with sh-NC, sh-PSAT1. (B) Tumor volume curves of nude mice. (C) Tumor weight of nude mice. (D) RT-qPCR to determine the expressions of PSAT1 in tumors. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Ferroptosis is an iron-dependent and oxidation-damaged cell death process with the following changes in cell morphology: mitochondrial membrane condensation, mitochondrial cristae reduction, and mitochondrial membrane29. As a new mode of cell death, ferroptosis plays a crucial role in tumor biology30. Osteosarcoma is the most common malignant tumor of the whole skeletal system. It is derived from mesenchymal cells with osteogenic potential, also known as osteosarcoma. It is highly malignant and prone to lung metastasis in the early stage31. Osteosarcoma is a highly malignant tumor with poor prognosis. Therefore, it is necessary to study the new mechanism of osteosarcoma progression and improve the prognosis of patients. In recent years, ferroptosis has become a hot topic, and the number of studies on the role of ferroptosis in malignant tumors has been increasing. Multiple drugs and molecules have been shown to be effective in inhibiting osteosarcoma progression by inducing ferroptosis. Liu et al. found that synthetic analogues of curcumin induced ferroptosis by increasing MDA levels, ROS levels, and intracellular iron ion levels32. Wen et al. found that baicalin induced ferroptosis in osteosarcoma through a novel Nrf2/xCT/GPX4 regulatory axis33. Zoledronic acid induces ferroptosis by upregulating POR in osteosarcoma34. However, the mechanism of ferroptosis in osteosarcoma has not been fully studied. Therefore, it is necessary to systematically study the mechanism of FRGs in osteosarcoma, so as to better understand the mechanism of osteosarcoma progression and find a new target for improving the prognosis of patients with osteosarcoma.
In this study, we began by screening for differentially expressed genes associated with ferroptosis in osteosarcoma. Ultimately, we identified seven ferroptosis-related genes (FRGs) using a bioinformatics approach: CBS, PSAT1, TRIB3, and ASNS were found to be up-regulated, while CYBB, ALOX5, and HBA1 were down-regulated. Verification through real-time PCR confirmed that the genes consistent with the bioinformatics predictions included ALOX5, CBS, PSAT1, TRIB3, and HBA1. Notably, ALOX5 and CBS have been documented in the literature concerning osteosarcoma. Consequently, after a comprehensive evaluation of the bioinformatics predictions, real-time PCR results, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses, as well as relevant literature, PSAT1 was selected as the primary focus of this research project. PSAT1 is one of the key enzymes in the serine synthesis pathway, which catalyzes the conversion of 3-phosphohydroxypyruvate to phosphoserine, and also generates 3-phosphopyruvate by glycolysis of 3-phosphoglycerate. It participates in the complex metabolic network regulation, provides material basis and energy for tumor cell proliferation, and maintains intracellular REDOX balance35. PSAT1 may be an oncogene that plays an important role in various cancer types36. It has been reported that PSAT1 is highly expressed in various malignant tumors, promotes the proliferation of tumor cells, and is associated with poor prognosis of tumors37,38,39. PSAT1 has been reported to be associated with ferroptosis in amyotrophic lateral sclerosis and diffuse large B-cell lymphoma40,41. Based on this, we hypothesized that PSAT1 might be involved in the regulation of ferroptosis in osteosarcoma. To test this hypothesis, the expression of PSAT1 in osteosarcoma cells and normal osteoblasts was assessed using real-time immunofluorescence. The results indicated that PSAT1 was significantly overexpressed in osteosarcoma cells. Further analysis revealed that silencing PSAT1 inhibited both the proliferation and invasion of osteosarcoma cells while promoting the progression of ferroptosis. Collectively, these findings suggest that PSAT1 may play a regulatory role in the progression of osteosarcoma through the modulation of ferroptosis.
At present, the study of PSAT1 is still in the initial stage, and its regulatory role and related mechanisms on tumor biological behavior are still not elucidated. In recent years, many studies have shown that ferroptosis plays a crucial role in clearing cancer cells and overcoming treatment resistance42. However, there are still many unexplored areas between ferroptosis and osteosarcoma. In this study, 7 ferroptosis-related osteosarcoma differentially expressed genes were identified based on high-throughput sequencing data. There are some limitations and lack of validation with large clinical samples, and further clinical studies are needed to demonstrate the reliability of this study. Although the effect of PSAT1 on the function of osteosarcoma cells and the specific mechanisms need to be further explored, our study provides a new idea for studying the biological mechanism of osteosarcoma and finding effective therapeutic targets.
Conclusion
In summary, we investigated the potential role of the ferroptosis-related gene PSAT1 in ferroptosis within osteosarcoma through bioinformatics research methods and cellular experimental studies. Our findings indicate that PSAT1 promotes the proliferation, migration, and invasion of osteosarcoma cells by inhibiting ferroptosis. Therefore, PSAT1 may serve as a promising target for the development of new therapeutic strategies aimed at improving the prognosis of osteosarcoma patients.
Data availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
References
Harrison, D. J., Geller, D. S., Gill, J. D., Lewis, V. O. & Gorlick, R. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 18(1), 39–50 (2018).
Deng, Y. et al. A four-methylated LncRNA signature predicts survival of osteosarcoma patients based on machine learning. Genomics 113(1 Pt 2), 785–794 (2021).
Klein, M. J. & Siegal, G. P. Osteosarcoma: Anatomic and histologic variants. Am. J. Clin. Pathol. 125(4), 555–581 (2006).
Gill, J. & Gorlick, R. Advancing therapy for osteosarcoma. Nat. Rev. Clin. Oncol. 18(10), 609–624 (2021).
Wittig, J. C. et al. Osteosarcoma: A multidisciplinary approach to diagnosis and treatment. Am. Fam. Physician 65(6), 1123–1132 (2002).
Wu, C. C. & Livingston, J. A. Genomics and the immune landscape of osteosarcoma. Adv. Exp. Med. Biol. 1258, 21–36 (2020).
Meyers, P. A. et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high dose methotrexate. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 23(9), 2004–2011 (2005).
Da, W. et al. A 10-year bibliometric analysis of osteosarcoma and cure from 2010 to 2019. BMC Cancer 21(1), 115 (2021).
Simpson, E. & Brown, H. L. Understanding osteosarcomas. JAAPA 31(8), 15–19 (2018).
Qiu, C. et al. Novel therapeutic savior for osteosarcoma: The endorsement of ferroptosis. Front. Oncol. 12, 746030 (2022).
Jiang, J. et al. Targeting PSAT1 to mitigate metastasis in tumors with p53–72Pro variant. Signal Transduct. Target Ther. 8(1), 65 (2023).
Tang, X. et al. Therapeutic potential of targeting HSPA5 through dual regulation of two candidate prognostic biomarkers ANXA1 and PSAT1 in osteosarcoma. Aging (Albany NY) 13(1), 1212–1235 (2020).
Dixon, S. J. et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149(5), 1060–1072 (2012).
Xie, Y. et al. Ferroptosis: Process and function. Cell Death Differ. 23(3), 369–379 (2016).
Lin, H. et al. EF24 induces ferroptosis in osteosarcoma cells through HMOX1. Biomed. Pharmacother. 136, 111202 (2021).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156(1–2), 317–331 (2014).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575(7784), 688–692 (2019).
Koppula, P., Zhuang, L. & Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 12(8), 599–620 (2021).
Mou, Y. et al. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 12(1), 34 (2019).
Zhao, L. et al. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. (Lond.) 42(2), 88–116 (2022).
Wang, Y., Wei, Z., Pan, K., Li, J. & Chen, Q. The function and mechanism of ferroptosis in cancer. Apoptosis 25(11–12), 786–798 (2020).
Zhao, J., Zhao, Y., Ma, X., Zhang, B. & Feng, H. Targeting ferroptosis in osteosarcoma. J. Bone Oncol. 30, 100380 (2021).
Wang, L. & Pan, S. The regulatory effects of p53 on the typical and atypical ferroptosis in the pathogenesis of osteosarcoma: A systematic review. Front. Genet. 14, 1154299 (2023).
Liu, X., Du, S., Wang, S. & Ye, K. Ferroptosis in osteosarcoma: A promising future. Front. Oncol. 12, 1031779 (2022).
Zhou, N. & Bao, J. FerrDb: A manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database (Oxford) 2020, baaa021 (2020).
Ashburner, M. et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25(1), 25–29 (2000).
Szklarczyk, D. et al. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43(Database issue), D447–D452 (2015).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13(11), 2498–2504 (2003).
Gao, M., Monian, P., Quadri, N., Ramasamy, R. & Jiang, X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59(2), 298–308 (2015).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22(4), 266–282 (2021).
Klein, M. J. & Siegal, G. P. Osteosarcoma: Anatomic and histologic variants. J. Clin. Pathol. 125(4), 555–581 (2006).
Liu, Z., Ma, H. & Lai, Z. The role of ferroptosis and cuproptosis in curcumin against hepatocellular carcinoma. Molecules 28(4), 1623 (2023).
Wen, R. J. et al. Baicalin induces ferroptosis in osteosarcomas through a novel Nrf2/xCT/GPX4 regulatory axis. Phytomedicine 116, 154881 (2023).
Jiacong, H. et al. Zoledronic acid induces ferroptosis by upregulating POR in osteosarcoma. Med. Oncol. 40(5), 141 (2023).
DeNicola, G. M. et al. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat. Genet. 47(12), 1475–1481 (2015).
Feng, M. et al. An integrated pan-cancer analysis of PSAT1: A potential biomarker for survival and immunotherapy. Front. Genet. 13, 975381 (2022).
Gao, S. et al. PSAT1 is regulated by ATF4 and enhances cell proliferation via the GSK3β/β-catenin/cyclin D1 signaling pathway in ER-negative breast cancer. J. Exp. Clin. Cancer Res. 36(1), 179 (2017).
Li, S. et al. ADH1C inhibits progression of colorectal cancer through the ADH1C/PHGDH/PSAT1/serine metabolic pathway. Acta Pharmacol. Sin. 43(10), 2709–2722 (2022).
Zhang, Y. et al. PSAT1 regulated oxidation-reduction balance affects the growth and prognosis of epithelial ovarian cancer. OncoTargets Ther. 13, 5443–5453 (2020).
Zhang, Q. et al. The classification and prediction of ferroptosis-related genes in ALS: A pilot study. Front. Genet. 13, 919188 (2022).
Chen, H. et al. Ferroptosis-related gene signature: A new method for personalized risk assessment in patients with diffuse large b-cell lymphoma. Pharmgenom. Pers. Med. 14, 609–619 (2021).
Woo, Y., Lee, H. J., Jung, Y. M. & Jung, Y. J. Regulated necrotic cell death in alternative tumor therapeutic strategies. Cells 9(12), 2709 (2020).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (Grant Number. 82060546). The funding bodies had no role in the design of the study, data collection, analysis, and interpretation of data or in writing the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant Number: 82060546) and the Education Development Foundation of Nanchang University (Grant number: ZL005). The funding bodies had no role in the design of the study, data collection, analysis, and interpretation of data or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
PW: Conceptualization, methodology, software, formal analysis, writing-original draft; visualization; JX: Software, validation, investigation; JZ: Investigation, data curation; FY: Software, validation, investigation; ML: Investigation, data curation; TL: Investigation, data curation; HZ: Conceptualization, methodology, writing-review and editing, supervision; HL: Conceptualization, methodology, writing-review and editing, supervision, funding acquisition. This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. The study design was approved by the appropriate ethics review board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. We have read and understood your journal’s policies, and we believe that neither the manuscript nor the study violates any of these. There are no conflicts of interest to declare.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This subject study is reported in accordance with ARRIVE guidelines. All animal experiments were performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the ethics committee of the First Affiliated Hospital of Nanchang University. The nude mice in this experiment were euthanized at the end of the experiment. After inhalation of isoflurane anesthesia for 2–3 min, the experimenter grabbed the root of the rat tail with his right hand and lifted it, placed it on the cage cover, pressed the head and neck of the rat downward with his left thumb and index finger, and grabbed the root of the rat tail and pulled it backward and upward with his right hand, resulting in cervical dislocation, and the spinal cord and brain stem were severed.
Consent for publication
All authors approved the final manuscript and the submission to this journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, P., Xiao, J., Zeng, J. et al. PSAT1 inhibits ferroptosis in osteosarcoma cells by activating the Xct/GPX4 signaling axis. Sci Rep 15, 26425 (2025). https://doi.org/10.1038/s41598-025-09568-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-09568-w