Abstract
Blueberry metabolite-derived phenolic acids are thought to suppress bone resorption via interactions with the G protein-coupled receptor 109A (GPR109A). Previously, global GPR109A knockout (GPR109A−/−) mice exhibited increased bone mass and a diminished bone-protective response to phenolic acids. While GPR109A is highly expressed in osteoclast precursor macrophages, its role in bone development remains unclear. To address this, we generated a myeloid cell-specific GPR109A knockout (GPR109Aflox/flox/LysM-Cre⁺; CKO) mouse model and assessed bone phenotypes in male and female mice at 35 days, 3 months, 6 months, and 12 months using µCT. At 35 days, CKO males showed significantly increased trabecular bone in both tibia and vertebrae when compared to control genotypes (f/f, Cre⁺). However, at later time points (6 and 12 months), Cre⁺ males exhibited similar trabecular bone phenotypes compared to CKO mice. In contrast, female CKO mice displayed significantly increased trabecular bone at 6 and 12 months. Using three-point bending analysis it was found that only 12-month-old Cre⁺ and CKO male mice exhibited altered bone mechanical properties when compared to f/f mice, while for females no significant changes in bone mechanical properties were observed. These findings suggest that GPR109A regulates bone turnover pathways in a sex-specific manner.
Similar content being viewed by others
Introduction
GPR109A (HCAR2/HM74A/PUMA-G) is Gi/Go heterotrimeric G protein coupled receptor that has been reported to interact with a variety of biomolecules including short-chain fatty acids (SCFA) such as D-β-hydroxybutyric acid and β-hydroxybutyrate, butyric acid and butyrate1. In addition, GPR109A is also known to function as a receptor for niacin, mediating it’s anti-lipolytic and anti-inflammatory effects2,3,4,5. Upon binding with an agonist, GPR109A and other members of the Gi/Go family function inhibit adenylate cyclase, decreasing concentration of the secondary messenger cyclic adenosine monophosphate (cAMP) available, decreasing protein kinase A (PKA) activity leading to the expression or repression of a number of genes6,7. Blueberry metabolite phenolic acids were significantly produced by gut microbiota in mice following 5% blueberry supplemented diet, specifically, hippuric acid (HA) and 3-(3-hydroxyphenyl) propionic acid (3–3-PPA) have been shown to interact with GPR109A, likely due to their structural similarities to niacin3,4,8,9. HA and 3–3-PPA are known to promote increased bone formation and this effect is thought to be mediated at least partially through interactions with GPR109A1,10,11,12. However, how GPR109A may play a role in regulating bone cell function and bone homeostasis is currently unknown.
Previously using a whole body GPR109A gene deletion mouse model, it was discovered that bone mass and strength were significantly increased in GPR109A-/- mice when compared to wild-type control mice at both 4-weeks and 6-months of age. In addition, bone resorption markers (TNFα, TRAP, Cathepsin K) were decreased and levels of bone formation marker P1NP were increased for GPR109A-/- mice compared to wild-type controls. In addition, in GPR109A-/- mice fed an HA supplemented diet, no significant changes were found in µCT parameters and bone resorption markers compared to GPR109A-/- mice fed a control diet1. However, 5% blueberry diet was still shown to have an effect on µCT parameters and bone turnover markers in GPR109A-/- mice, suggesting additional factors from blueberry dietary supplementation than just HA and 3–3-PPA having bone protective qualities. GPR109A-/- was also found to ameliorate sex steroid deficiency induced bone loss in both male and female mice that had undergone ovari/orchiectomy. Bone resorption and formation markers were found to be significantly increased in sex steroid deficient wild-type mice but not in GPR109A-/- mice that had undergone ovari/orchiectomy13.
GPR109A is expressed at high levels in osteoclast precursor macrophage cells, however the specific role GPR109A may play in bone cell homeostasis remains poorly understood4,14. Using streptozotocin, mice with whole body gene deletion of GPR109A were found to be more susceptible to type I diabetes and had increased M1 macrophage polarization15. An additional study found that treatment of wild-type and whole body GPR109A gene deletion mouse models with nicotinic acid revealed significantly suppressed levels of inflammatory cytokines (TNF-α, IL-6, IL-12p40 and IL-1β) in wild-type bone marrow derived macrophages but did not affect inflammatory cytokine levels in macrophages isolated from GPR109A-/- mice suggesting GPR109A may serve as a negative regulator of macrophage activation. In the context of bone tissue, negative regulation of inflammatory cytokines may suppress osteoclastogenesis, further suggesting a role for GPR109A in regulating osteoclast differentiation16,17,18,19.
To investigate if GPR109A plays an important role in regulating bone turnover, via regulating differentiation of osteoclast precursor macrophages into mature osteoclasts, we have generated for the first time to our knowledge a myeloid cell (osteoclast precursor)-specific GPR109A CKO mouse model GPR109Aflox/flox/LysM-Cre+ CKO (GPR109Aflox/flox/LysM-Cre+). In this report, using µCT and three-point bending analysis we investigated change in bone phenotype in CKO mice compared to control mice (GPR109Aflox/flox, LysM-Cre+) in both tibia and vertebrae from both sexes at different time points (35 days, 3 months, 6 months, 12 months). It is our hope that results from this study will enhance our understanding of GPR109A plays a role in regulating bone turnover sexual dimorphism and ultimately guide strategies for optimizing bone development.
Results
GPR109A myeloid cell specific deletion in mice
For myeloid cell specific deletion of GPR109A, targeting vector for incorporation of floxed GPR109A into mouse genome was used as previously described (Fig. 1A)4,20. Male GPR109Aflox/+/LysM-Cre+ and female GPR109Aflox/+/LysM-Cre- offspring were crossed to generate myeloid cell specific GPR109A conditional knockout mouse models (GPR109Aflox/flox/LysM-Cre+: CKO genotype) as well as GPR109Aflox/flox/LysM-Cre- (f/f genotype) and GPR109A+/+/LysM-Cre+ (Cre+ genotype) (Fig. 1B). To confirm mouse genotypes tail DNA extraction and PCR was performed on mouse tails using primers designed to determine genotype (f/f, Cre+, CKO) (Fig. 1C). Primers used for genotyping are listed in Table 1. Agarose gel electrophoresis was performed to determine PCR product size (1.5% agarose gel, Ladder: DirectLoad Wide Range DNA Marker; D7058, Sigma).
Targeting vector and breeding strategy for generation of myeloid cell specific GPR109A CKO (GPR109Aflox/flox/LysM-Cre+) mouse model. (A) GPR109A CDS was cloned into pcDNA3.1 vector whose cloning site is flanked by LoxP regions. Floxed GPR109A region was incorporated into mouse embryotic stem cell allele via electroporation and homologous recombination. Embryotic stem cells carrying floxed GPR109A CDS were injected into C57BL/6 embryos which were carried to term. Adult mice carrying floxed GPR109A CDS were backcrossed to develop a stable mouse line carrying a conditional GPR109A CKO allele. (B) Female GPR109Aflox/flox mice were crossed with male LysM-Cre+ mice to give offspring with either GPR109Aflox/+/LysM-Cre+ or GPR109Aflox/+/LysM-Cre- genotypes. Male GPR109Aflox/+/LysM-Cre+ and female GPR109Aflox/+/LysM-Cre- offspring were crossed to give Cre+, f/f and CKO genotypes as well as others needed for this study. (C) Genotyping was performed on mouse tails using PCR followed by agarose gel electrophoresis (1.5%). Primers were designed to detect the presence (350 bp) or absence (400 bp) of floxed regions flanking GPR109A CDS as well as presence of LysM-Cre (750 bp) or Cre (350 bp) in DNA isolated from mouse tail samples.
Immunohistochemistry was used to confirm deletion of GPR109A in bone tissue from 6-month-old mice. In f/f mice (both male and female) GPR109A protein (in red) is present in tibia alongside LysM (in green) with DAPI staining (in blue) used as control. For myeloid cell specific GPR109A CKO mice in tibia from both male and female mice, GPR109A protein levels are significantly depleted while LysM and DAPI staining appears unaffected compared to f/f mice. Best representative images of immunohistological results from f/f and myeloid cell specific GPR109A CKO tibia are presented in Fig. 2A and 2B.
Best representative images from immunohistochemistry confirm deletion of GPR109A in tibia of both male and female CKO mice. (A, B) Immunohistochemistry was performed on tibia isolated from male and female f/f and CKO mice at 6 months of age. Loss of GPR109A protein expression (in red) was confirmed for CKO mice is present in tibia as well as the presence of LysM-Cre (in green). LysM and DAPI staining (in blue) are unaffected for CKO mice compared to f/f mice confirming myeloid cell deletion of GPR109A did not affect tibia at the cell level.
GPR109Aflox/flox/LysM-Cre+ genotype has sex dependent effect on tibia at different time points
To show the impact of myeloid cell specific deletion of GPR109A in long bone over time, µCT was performed to analyze trabecular bone in tibia isolated from male and female mice (f/f, Cre+, CKO) at each timepoint (35 days, 3 months, 6 months, 12 months). For males, at 35 days, myeloid cell deletion of GPR109A effect on bone appears limited as images for each genotype appear identical to one another (Supplemental Fig. 1A). For 35-day-old males, analysis of μCT parameters reveals BMD is significantly increased for CKO mice compared to both f/f and Cre+ controls (35 days: male f/f N = 10, Cre+ N = 10, CKO N = 10) (Fig. 3A). For 3-month-old males, tibia scans show no change in trabecular bone between f/f, Cre+ or CKO mice (Supplemental Fig. 1B). However, for 3-month-old males, BMD for Cre+ mice was significantly increased compared to f/f but not CKO mice genotypes (3 months: male f/f N = 10, Cre+ N = 10, CKO N = 9) (Fig. 3A). For 6-month-old males, µCT scans for tibia appears to show increased trabecular bone for CKO mice compared to f/f and Cre+ controls (Supplemental Fig. 1C). For 6-month-old males, Tb Th was significantly increased for CKO mice compared to both f/f and Cre+ controls, while BS/TV was increased for CKO mice compared to f/f but not Cre+ controls (6 month: male f/f N = 12, Cre+ N = 12, CKO N = 12) (Fig. 3A). For 12-month-old males, µCT collected images for show increased trabecular bone for both Cre+ and CKO mice compared to f/f controls (Supplemental Fig. 1D). At 12 months BV/TV, Tb N and BS/TV were significantly increased while Tb Sp was significantly decreased for both Cre+ and CKO mice compared to f/f. In addition, BV was also increased for 12-month-old male Cre+ and CKO mice compared to f/f controls. However, only the increase between f/f and CKO mice was found to be significant (12 months: male f/f N = 11, Cre+ N = 9, CKO N = 8) (Fig. 3A). BMD was plotted for each genotype over all time points. At 35 days, BMD for CKO mice is significantly increased compared to f/f and Cre+ mice but stays at or near this level for the remaining time points. BMD for f/f mice peaks at 6 months followed by a sharp decrease back to near 35-day levels for 12-month-old mice. For Cre+ male mice, BMD peaks at 3 months and decreases back to near 35-day levels over the next 9 months (Fig. 3B).
µCT analysis of male tibia reveals early life improvements in trabecular bone for CKO mice compared to f/f and Cre+ controls. (A) µCT parameters for male tibia (f/f, Cre+ and CKO) at different time points (35 days, 3 months, 6 months, 12 months). BMD was significantly increased at 35 days for CKO mice compared to f/f and Cre+ controls. Yet at 12 months, Cre recombinase effect is observed as BV/TV, Tb N and BS/TV are significantly increased while Tb Sp is significantly decreased for both Cre+ and CKO mice compared to f/f controls. (B) Line graph of tibia BMD over all time points shows that early life BMD increase for CKO mice compared to other genotypes is not sustained throughout life. For One-way ANOVA, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, **** P ≤ 0.0001.
As with male mice, µCT analysis was performed on tibia isolated from female mice. At 35 days, µCT collected images show no change in trabecular bone between f/f, Cre+ and CKO female mice (Supplemental Fig. 1A). In addition, no significant changes in µCT parameters were detected between genotypes (35 days: female f/f N = 9, Cre+ N = 10, CKO N = 9) (Fig. 4A). For 3-month-old females, µCT scans show increased trabecular bone for CKO mice compared to f/f and Cre+ control mice (Supplemental Fig. 1B). In 3-month-old female mice, BV/TV and BMD were significantly increased while Tb Sp was significantly decreased for both Cre+ and CKO genotypes compared to f/f. BV, Tb N and BS/TV were increased for both Cre+ and CKO 3-month-old female mice compared to f/f mice. However, only the increase between CKO and f/f mice was found to be significant (3 months: female f/f N = 10, Cre+ N = 5, CKO N = 10) (Fig. 4A). For 6-month-old female mice, µCT collected images show an increase in trabecular bone for CKO mice compared to both f/f and Cre+ control mice (Supplemental Fig. 1C). For 6-month-old females; BMD was significantly increased for CKO mice compared to both f/f and Cre+ controls, while a Cre recombinase effect was also observed as BS/TV was significantly increased and Tb Sp was significantly decreased for both Cre+ and CKO mice (6 month: female f/f N = 12, Cre+ N = 10, CKO N = 12) (Fig. 4A). For 12-month-old female mice, images from μCT scans show that trabecular bone is increased for CKO mice compared to both f/f and Cre+ controls (Supplemental Fig. 1D). For 12-month-old CKO female mice; BV, BV/TV, Tb N, BS/TV and BMD were significantly increased compared to both f/f and Cre+ controls. Tb Sp was decreased for both Cre+ and CKO mice compared to f/f however, only the decrease from f/f to CKO was found to be significant (12 months: female f/f N = 10, Cre+ N = 7, CKO N = 9) (Fig. 4A). As with male mice, female BMD was plotted for each genotype over each time point. At 35 days, BMD for CKO mice is statistically similar to BMD for f/f and Cre+ mice and reaches a maximum at 6 months before decreasing back to 35-day levels for 12-month-old mice. For Cre+ female mice, BMD is significantly increased at 3 months, before being decreased to below CKO levels at 6 months and below 35-day-old Cre+ levels at 12 months. For f/f female mice, BMD is statistically unchanged from 35 days to 6 months before being decreased to below baseline levels at 12 months (Fig. 4B).
µCT analysis of female tibia reveals later in life improvements in trabecular bone for CKO mice compared to f/f and Cre+ controls. (A) µCT parameters for female tibia (f/f, Cre + and CKO) at different time points (35 days, 3 months, 6 months, 12 months). For female mice, impact of GPR109A myeloid cell deletion is primarily observed at 12 months as evident by significant increases in BV, BV/TV, Tb N, BS/TV and BMD for CKO mice compared to both f/f and Cre+ mice. For CKO mice, BMD is also significantly increased at 6 months when compared to both f/f and Cre+ controls. (B) Line graph of female mice tibia BMD over all time points showing peak BMD occurs at 6 months for CKO mice. For One-way ANOVA, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, **** P ≤ 0.0001.
GPR109A/LysM CKO has sex dependent effect on L3-L5 vertebrae at different time points
In addition to tibia, the effect of myeloid cell specific deletion of GPR109A on vertebrae was also investigated by performing µCT on L5 vertebrae isolate from f/f, Cre+, and CKO mice from both sexes at each timepoint (35 days, 3 months, 6 months, 12 months). Based on images collected during µCT scanning of 35-day-old male L5 vertebrae, trabecular bone appears to be increased in CKO mice compared to both f/f and Cre+ control mice (Supplemental Fig. 2A). For 35-day old males; BV/TV, Tb N and BS/TV were significantly increased while Tb Sp was significantly decreased for CKO mice compared to both f/f and Cre+ controls (35 days: male f/f N = 10, Cre+ N = 10, CKO N = 10) (Fig. 5A). For 3-month-old male mice, based on μCT collected images there does not appear to be any changes in trabecular bone for CKO mice compared to either f/f or Cre+ controls (Supplemental Fig. 2B). Interestingly, for 3-month-old male mice Tb N and BS/TV for L5 vertebrae were decreased for CKO mice compared to both f/f and Cre+ controls. However, for both Tb N and BS/TV only the decrease from Cre+ to CKO was found to be significant (3 months: male f/f N = 10, Cre+ N = 11, CKO N = 10) (Fig. 5A). For 6-month-old male mice, µCT imaging of L5 vertebrae shows increased trabecular bone for Cre+ and CKO mice compared to f/f controls (Supplemental Fig. 2C). A Cre recombinase effect was observed for 6-month-old male L5 vertebrae, as BS/TV was significantly increased for both Cre+ and CKO mice compared to f/f controls. In addition, Tb Th was significantly increased for CKO mice compared to f/f while the difference for Tb Th between Cre+ and CKO genotypes was not found to be significant; while BMD was increased for both Cre+ and CKO mice compared to f/f with only the increase from f/f to CKO found to be significant (6 months: male f/f N = 10, Cre+ N = 10, CKO N = 10) (Fig. 5A). For 12-month-old male mice, images collected during µCT analysis of L5 vertebrae show increased trabecular bone for both Cre+ and CKO mice when compared to f/f controls (Supplemental Fig. 2D). For 12-month-old males, Tb Sp was significantly decreased for CKO mice compared to f/f and Cre+ control mice. However, a Cre recombinase effect was observed as Tb N was significantly increased for both Cre+ and CKO genotypes compared to f/f. In addition, BS/TV was increased for both Cre+ and CKO mice compared to f/f, however, only the increase from f/f to CKO was found to be significant (12 months: male f/f N = 11, Cre+ N = 9) (Fig. 5A). As with tibia, for male mice BMD for L5 vertebrae was plotted for each genotype over each time point. BMD is similar for f/f, Cre+ and CKO mice at 35 days and is significantly increased to similar levels for all three genotypes at 3 months. From 3 to 6 months BMD is increased to nearly identical levels for Cre+ and CKO mice compared to f/f controls. Finally, from 6 to 12 months, BMD for Cre+ and CKO male mice is slightly decreased but still greater than that for f/f mice (Fig. 5B).
µCT analysis of male mice L5 vertebrae reveal early life improvements in CKO trabecular bone compared to f/f and Cre+ controls. (A) µCT parameters for male L5 vertebrae (f/f, Cre+ and CKO) at different time points (35 days, 3 months, 6 months, 12 months). BV/TV, Tb N and BS/TV are significantly increased while Tb Sp is significantly decreased for 35-day-old CKO mice compared to f/f and Cre+ controls. As with tibia, a Cre recombinase effect was observed at latter time points as BS/TV is significantly increased for 6-month-old Cre+ and CKO mice compared to f/f and Tb N is significantly increased for Cre+ and CKO mice compared to f/f at 12 months. In addition, at both 6 and 12 months, BMD for Cre+ and CKO are similar, and both are increased compared to f/f mice but not to a significant extent. (B) Line graph of male L5 vertebrae BMD over all time points shows no change between genotypes at early time points but similar increases for both Cre+ and CKO mice compared to f/f at 6 and 12 months. For One-way ANOVA, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, **** P ≤ 0.0001.
µCT analysis was also performed on L5 vertebrae isolated from female mice of each genotype (f/f, Cre+, CKO). For 35-day-old female mice, images collected during scanning of L5 vertebrae show increased trabecular bone for CKO mice compared to both f/f and Cre+ control mice (Supplemental Fig. 2A). For female mice at 35 days; BV/TV, Tb N and BS/TV were significantly increased for CKO mice compared to f/f but not Cre+ mice while for BMD, significant increases were observed for both Cre+ and CKO mice compared to f/f. and BMD were significantly increased for CKO mice compared to f/f but not Cre+ mice(35 days: female f/f N = 10, Cre+ N = 10, CKO N = 10) (Fig. 6A). For 3-month-old female mice, µCT collected images of L5 vertebrae do not show any changes in trabecular bone between f/f, Cre+ and CKO genotypes (Supplemental Fig. 2B). For 3-month females, Tb N was significantly increased for both Cre+ and CKO mice compared to f/f control mice. Interestingly, for L5 vertebrae BV/TV and BS/TV were found to be significantly increased for 3-month-old female Cre+ mice compared to f/f but not CKO mice (3 months: female f/f N = 11, Cre+ N = 10, CKO N = 10) (Fig. 6A). For 6-month-old female L5 vertebrae, µCT imaging appears to show increased trabecular bone for CKO and Cre+ mice when compared to f/f control mice (Supplemental Fig. 2C). For 6-month-old females, BV/TV, Tb N and BMD for L5 vertebrae were significantly increased while Tb Sp was significantly decreased for CKO mice compared to both f/f and Cre+ controls. However, a Cre recombinase effect was observed for BV and BS/TV as both were significantly increased for Cre+ and CKO mice compared to f/f controls (6 months: female f/f N = 10, Cre+ N = 10, CKO N = 11) (Fig. 6A). For 12-month-old females, µCT collected images of L5 vertebrae show increased trabecular bone for CKO mice compared to both f/f and Cre+ control mice (Supplemental Fig. 2D). For 12-month-old female mice; BV, BV/TV and Tb N were found to be significantly increased while Tb Sp was significantly decreased for CKO mice compared to both f/f and Cre+ controls. mice. Interestingly, BS/TV was significantly increased for CKO mice compared to Cre+ but not f/f controls (12 months: female f/f N = 10, Cre+ N = 7, CKO N = 10) (Fig. 6A). As with males, L5 vertebrae BMD from female mice of each genotype was plotted over all time points. For females, at 35 days BMD for Cre+ and CKO mice is greater than BMD for f/f control mice. From 35 days to 3 months, BMD is increased for all genotypes however there were no significant differences in BMD detected between the genotypes at 3 months. From 3 to 6 months, BMD for female CKO peaks and is significantly greater than BMD for both f/f and Cre+ mice. Finally, at 12 months BMD CKO mice decreases back to f/f and Cre+ levels, consistent with 3 and 6 months (Fig. 6B).
µCT analysis of female L5 vertebrae reveal later in life improvements in CKO trabecular bone compared to both f/f and Cre+ controls. (A) µCT parameters for L5 vertebrae from female mice (f/f, Cre+ and CKO) at different time points (35 days, 3 months, 6 months, 12 months). Impact of GPR109A myeloid cell deletion is primarily observed at later time points as observed by significant increases in BV, BV/TV and Tb N as well as a significant decrease in Tb Sp at both 6 and 12 months for CKO mice compared to both f/f and Cre+ controls. In addition, BS/TV was significantly increased for CKO mice at 12 months when compared to Cre+ but not f/f mice. Finally, at 6 months BMD for CKO mice was found to be significantly increased compared to f/f and Cre+ controls. (B) Line graph of female L5 vertebrae BMD over all time points showing peak BMD occurs at 6 months for CKO mice. For One-way ANOVA, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, **** P ≤ 0.0001.
LysM-Cre+ male specific effect on femur biomechanical properties from 12-month-old mice
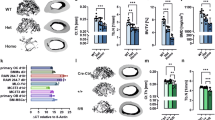
Three-point bending analysis of isolated femurs from f/f, Cre+ and CKO mice; at 12 months, in both sexes; was performed to analyze the impact of myeloid cell specific GPR109A on bone biomechanical properties. For males, when compared to f/f control mice Yield Stress, Ultimate Stress and Elastic Modulus were significantly increased for both Cre+ and CKO mice, with their being no significant difference in the presented three-point bending parameters between Cre+ and CKO genotypes (Fig. 7A). However, for females, there is no significant difference detected for Yield Stress, Ultimate Stress or Elastic Modulus between ff, Cre+ or CKO mice (Fig. 7B) (male: f/f N = 10, Cre+ N = 8, CKO N = 8; female f/f N = 10, Cre+ N = 7, CKO N = 10).
Cre recombinase male specific effect is also observed in 3-point bending analysis results. (A) For males, 3-point bending analysis of femur from 12-month old mice reveals significant increases in Yield Stress, Ultimate Stress and Elastic Modulus for both Cre+ and CKO mice compared to f/f mice. (B) For females, 3-point bending analysis of 12-month old femur shows no significant changes in Yield Stress, Ultimate Stress and Elastic Modulus between f/f, Cre+ or CKO mice. For One-way ANOVA, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, **** P ≤ 0.0001.
Discussion
Previously using a global GPR109A gene knockout mouse model, a significant increase in bone mass was observed for GPR109A-/- mice compared to wildtype controls. In addition, for GPR109A-/- mice protein and mRNA levels for osteoclastogenesis factors; including MMP9, NFATc1 and Cathepsin K were unchanged following 5% blueberry or HA dietary supplementation, suggesting a role for GPR109A in mediating the osteoclast suppressing effects of blueberry diet derived metabolites such as HA and 3–3-PPA1,10,11,12. µCT results from this study seem to hint that myeloid cell specific GPR109A plays a role in regulating osteoclastogenesis, as deletion of GPR109A from these osteoclast precursor cells resulted in increased trabecular bone formation (tibia and vertebrae) in both male and female mice depending on the timepoint measured1. It must be reiterated that these results only describe the bone phenotype, and that further research will be required to understand the mechanisms by which myeloid cell specific GPR109A regulates osteoclastogenesis21.
Myeloid cell specific deletion of GPR109A mouse model (GPR109Aflox/flox/LysM-Cre+, CKO) was developed through breeding of female GPR109Aflox/flox mice with male LysM-Cre+. Offspring from this first breeding pair were then bred to each other to give the genotypes used for this experiment (f/f, Cre+, CKO)22. Of note myeloid cell specific deletion of GPR109A revealed differences in bone phenotype between the different genotypes investigated for both sexes. For both tibia and L3-L5 vertebrae, at 6 and 12 months, trabecular bone was increased for female CKO mice compared to both f/f and Cre+ control mice. Yet at these timepoints for male CKO mice, trabecular bone was found to be statistically unchanged compared to Cre+ control mice. In addition to µCT analysis, three-point bending results also show a significant increase in Yield Stress, Ultimate Stress and Elastic Modulus in femur isolated from 12-month-old male Cre+ and CKO mice compared to f/f controls.
Interestingly for female CKO mice at 12 months, μCT and bone mechanical results from three-point bending analysis (Yield Stress, Ultimate Stress, Elastic Modulus) are not consistent with one another. Femur Yield Stress, Ultimate Stress and Elastic Modulus are unchanged for CKO mice when compared to controls yet, BV/TV and BMD from tibia are significantly increased compared to f/f and Cre + controls. Such discrepancies may be the result of changes in bone remodeling genes between male and female CKO mice. This will need to be confirmed in future histology and gene expression analysis experiments 23,24,25. additional factors, including sexual dimorphism, explaining the increase μCT parameters with no change bone mechanical properties for female mice may be at play as well 26. However, this will need to be investigated further in future studies.
Both µCT and three-point bending results for male mice hint at a sex specific effect for the Cre+ genotype on bone. Cre recombinase producing distinct phenotypes is a known phenomenon27,28,29,30. In addition, a review of relevant literature shows that Cre recombinase male specific effects in mice have previously been observed. For instance, in Synapsin 1 (Syn1)-Cre rat models, used for investigating neuronal function, a significant increase in human growth hormone (HGH) transcription was observed in both male and female mice compared to wild-type controls. However only male Syn1-Cre mice exhibited decreased body weight and femur length likely through decreased liver Igf1 expression31. In addition, male Myosin heavy chain, α isoform (Myh6)-Cre mice, used for investigating cardiac function, were found to have decreased ejection fraction and left atrial dilation compared to control mice32,33. While Cre male specific effects in mice have been observed, this is the first time to our knowledge that male LysM-Cre+ mice and not female mice have had an effect bone phenotype comparable to CKO mice. The potential mechanism explaining LysM-Cre+ genotype having increased bone mass in male, but not female mice may need to have further investigation.
It cannot be said for certain if the increase in trabecular bone for male CKO mice at 6 or 12 months is due to deletion of GPR109A or the presence of Cre recombinase. However, at 35 days in male CKO mice tibia BMD and vertebrae BV/TV, Tb N and Tb Sp were significantly improved compared to both f/f and Cre+ mice. These differences in µCT parameters were not nearly as apparent for female 35-day old CKO mice, suggesting a male specific effect for GPR109A on early life bone formation and development. Previous research has found that ketone bodies, including GPR109A substrate β-hydroxybutyrate, lower testosterone levels in young males34. G protein-coupled receptor family C group 6-member A (GPRC6A) has previously been shown to regulate testosterone production and energy metabolism35. It may be that GPR109A acts as a suppressor of testosterone production through unknown mechanisms, leading to suppressed bone formation and explaining how GPR109A deletion would lead to increased μCT parameters 36. Yet it is unknown if myeloid cell gene deletion of GPR109A will impact testosterone levels.
While the potential effects of GPR109A gene deletion in male mice appear to be primarily on early life bone development, in female mice, myeloid cell specific deletion of GPR109A effect on bone (tibia and L3-L5) is seen primarily at later time points (6 months, 12 months). Structural similarities between GPR109A and G-coupled protein estrogen receptor 1 (GPER1) as well as molecular docking studies, suggest that the two may share substrates including estrogens, known to play a huge role in regulating bone metabolism by inhibiting bone resorption 37,38,39,40,41,42,43. GPR109A, through inhibition of cAMP/PKA signaling is associated with increased bone resorption 44. Interactions between GPR109A and bone anabolic promoting substrates such as HA and 3–3-PPA are inhibitory, leading to increased cAMP/PKA signaling and decreased osteoclastogenesis 1,10. Due to myeloid cell gene deletion of GPR109A leading to increased bone formation in female mice it may be that GPR109A suppresses downstream effects of estrogen 10,41,42,43,45,46. While estrogen binds to and activates GPER1, it is unknown if interactions between specific G-coupled protein receptors and estrogen may suppress the downstream effects of estrogen. ERβ, whose activity can be regulated by GPER1 can act as a repressor of estrogen downstream effects in certain situations 47,48. It may be that GPR109A also regulates ERα and ERβ activities through currently unknown mechanisms to increase bone resorption.
In this study the effect of myeloid cell specific (osteoclast precursor) deletion of GPR109A on bone phenotype was investigated at different time points (35 days, 3 months, 6 months and 12 months) in both male and females using for the first time to our knowledge a GPR109A/LysM CKO mouse model, hinting towards a role for GPR109A in regulating bone turnover throughout life. In early life GPR109A myeloid cell deletion was shown to significantly improve bone formation in male but not female mice. While at later ages, female CKO mice had significantly increased trabecular bone compared to both f/f and Cre+ controls. Results suggest GPR109A and sex steroid signaling may coordinate to explain the observed sexual dimorphism in mice at different measured time points. However, we can only speculate on the mechanism explaining how GPR109A and androgen/estrogen may coordinate to explain GPR109A effects on bone phenotype for both genders. Future experiments will be performed to investigate how sex steroid signaling and GPR109A collaborate to explain sexual dimorphism present in bone. Further understanding of the osteoclastogenesis pathways regulated by GPR109A will assist in the development of novel treatments and therapeutics for alleviating high bone resorption disorders.
Materials and methods
Production of GPR109A/LysM-CKO mouse model and animal care
Myeloid cell specific deletion of GPR109A in mice was accomplished using selective breeding. Briefly, female mice with loxP sites flanking GPR109A (GPR109Aflox/flox) were generated through a collaboration with Texas A&M Institute for Genomic Medicine and Emory University School of Medicine. GPR109A targeting vector was constructed by using PCR on full-length coding sequence of mouse GPR109A and cloning of amplified region (to be flanked by loxP regions) into pcDNA3.1 vector containing a gene for neomycin resistance flanked by Frt sites4,20. Targeting vector was transformed into mouse embryotic stem cells via electroporation with loxP regions from targeting vector incorporated into genomic DNA via homologous recombination. Embryotic stem cells containing floxed GPR109A region are selected for using Neomycin and injected into C57BL/6 embryos. Backcrossing of chimeric GPR109Aflox/flox mice with C57BL/6 mice was performed to develop stable GPR109Aflox/flox mouse line4. Deletion of GPR109A in myeloid cell line (monocytes, mature macrophages, osteoclasts) was accomplished through breeding of female GPR109Aflox/flox mice with male mice containing the coding sequence for Cre recombinase inserted into the first ATG codon of the lysozyme 2 gene (LysM), an antibacterial enzyme expressed in myeloid cells (LysM-Cre+), Jackson laboratory, B6.129P2-Lyz2tm1(cre)Ifo/J, Strain #:004781). From the offspring male GPR109Aflox/+/LysM-Cre+ and female GPR109Aflox/+/LysM-Cre- mice were interbred based on previously published methods to generate the following genotypes; wild-type, GPR109Aflox/+/LysM-Cre-, GPR109Aflox/flox/LysM-Cre- (f/f), GPR109A+/+/LysM-Cre+ (Cre+), GPR109Aflox/+/LysM-Cre+ and GPR109Aflox/flox/LysM-Cre+ (CKO)22. For this study only Cre+, f/f and CKO mice were used and additional breeding was performed as necessary to generate these needed genotypes.
Following generation of needed genotypes. Mice were weighed and randomized into 4 different lifespan groups (35 days, 3 months, 6 months, 12 months). Mice were housed 6 per cage in small shoe box cages and fed a purified control diet. Mice were weighed weekly and weight/mouse at the completion of lifespan are listed in Supplemental file 1. After the completion of lifespans, mice were euthanized via inhalation of CO2 followed by exsanguination. Tibia and L3-L5 vertebrae from mice were collected and stored at -80 ºC in formalin. Mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved animal facility in the Arkansas Children’s Nutrition Center Animal Studies Core at the Arkansas Children’s Research Institute, with constant humidity and lights on from 06:00–18:00 h. at 22 °C. All animal procedures were approved by the Institutional Animal Care and Use Committee at University of Arkansas for Medical Sciences (AUP#3595 UAMS, Little Rock, AR).
Mouse tail genotyping
Genotyping of offspring mice was performed on mice tails using Extract-N-Amp PCR kit (Sigma-Aldrich). Briefly, newborn mice were anesthetized using isoflurane and end of tails were snipped and collected. 50 µl of extraction solution and 12.5 µl of preparation solution were added to microcentrifuge tubes containing tails. Samples were first incubated at 55° C for 1 h. followed by incubation 95° C for 5 min. Following incubation, 50 µl of neutralization solution was added and PCR was performed for both Cre recombinase and GPR109Aflox/flox. Primers for LysM-Cre and floxed GPR109A CDS are listed in Table 1. Genotypes of offspring mice were determined based on PCR product size using Jackson Laboratory protocols.
Bone immunohistology analysis
Mouse right tibia samples were decalcified using EDTA, embedded, cut and slides were prepared by Histology Special Procedures at the Arkansas Children’s Nutrition Center Histology Core. Slides for f/f and CKO mouse genotypes were washed with 1X PBS (10–15 min., room temperature). PBS was removed and 2.5% Horse Blocking serum was added (20 min., room temperature). Blocking serum was removed via aspiration and bone tissue slides were incubated with primary antibody (GPR109A: A02511, Boster LysM: 66456-1-Ig; Proteintech) diluted 1:50 in 2.5% horse blocking serum containing 1% IGEPAL overnight at 4° C. Slides were washed with 1X PBS containing 0.05% IGEPAL (3 min., 3 times at room temperature) and secondary antibody was added (Goat anti-Rabbit IgG Secondary Antibody, Alexa Fluor 647; Goat anti-mouse IgG Secondary antibody, AlexaFluor 546) (1 h., room temperature, protected from light). Final slides were covered with DAPI-Fluoromount-G and observed using Nikon Eclipse T/2 epifluorescent microscope.
µCT scan of GPR109A/LysM-cKO tibia and L3-L5 vertebrae
Micro-computed tomography (CT) measurements of tibia and L5 vertebrae from CKO, Cre+ and f/f mice (all age groups) were evaluated using a Skyscan µCT scanner (SkyScan 1272.). Tibia and vertebrae were cleaned of muscle tissue and stored in formalin for at least 24 h. prior to scanning. For µCT scanning and analysis of both tibia and vertebrae trabecular bone, the region of interest (ROI) was selected to include the region extending 0.9 mm distally and 0.03 mm from the physis49. Images were obtained at 70 kV X-ray tube voltage and 142 μA current, from a 0.5 mm aluminum filter. Images were reconstructed using NRecon software (Skyscan). Random movement and flat field correction were turned on and beam hardening correction was set to 38%. Total bone volume (BV mm3), tissue volume (TV mm3), bone volume fraction (BV/TV %), bone surface (BS/TV mm2), trabecular thickness (Tb Th, mm), trabecular separation (Tb Sp, mm), trabecular number (Tb N, 1/mm) and bone mineral density (BMD g/cm3) were calculated using Skyscan provided software and averaged for each age group.
Three-point bending of GPR109A/LysM-cKO femur
Before mechanical testing, right femurs were thawed and re-wrapped in wet gauze then scanned using micro computed tomography (µCT: Bruker Skyscan 1272, Billerica, MA). Images were obtained at 60 kV X-ray tube voltage and 166 μA current, using a 0.5 mm aluminum filter, 1026 ms exposure time, and 25.90 μm image pixel size. For each specimen, a series of 628 projection images were obtained (a rotation step 2.0°, averaging 3 frames). Images were reconstructed to obtain images using NRecon software (Skyscan). Next, images were subjected to morphometric analysis using CTAn software (CT Analyser 1.13.5.1, Skyscan). Average minimum moment of inertia and centroid were calculated from 100 slices centered at the mid-diaphysis. Following µCT scanning, right femurs were loaded to failure in three-point bending using a Z2.5 material testing machine with an XForceP 0.2 KN load cell (Zwick/Roell, Ulm, Germany). The fixed distance between the lower supporting bars was 6.82 mm, with a displacement rate of 0.5 mm/min. The anterior mid-diaphysis was pre-loaded at 0.5 N, and the tests were analyzed using the load displacement curve created by the system’s analysis software TestXpert III (Zwick/Roell, Version 1.6). Yield was defined as the point at which the regression line that represents a 5% loss in stiffness crosses the load displacement curve.
Statistical analysis
For experiments, numerical variables were expressed as mean + /- SD (Standard Deviation); n equals to the number of samples/group. Statistical analysis was performed with GraphPad Prism 9.0 (GraphPad Software, Inc., San Diego, Ca, USA). Outliers were determined using ROUT method (Q = 10%) and removed50. Differences within groups were evaluated using one-way ANOVA and corrected for multiple comparisons by Tukey post hoc test. We confirm that the study is reported in accordance with ARRIVE guidelines.
Data availability
The source of mice was used in this study. The data that support the findings of this study are available in the methods and/or supplemental material of this article.
References
Chen, J.-R. et al. GPR109A mediates the effects of hippuric acid on regulating osteoclastogenesis and bone resorption in mice. Commun. Biol. https://doi.org/10.1038/s42003-020-01564-2 (2021).
Singh, N. et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139. https://doi.org/10.1016/j.immuni.2013.12.007 (2014).
Ren, N. et al. Phenolic acids suppress adipocyte lipolysis via activation of the nicotinic acid receptor GPR109A (HM74a/PUMA-G). J. Lipid Res. 50, 908–914. https://doi.org/10.1194/jlr.M800625-JLR200 (2009).
Tunaru, S. et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9, 352–355. https://doi.org/10.1038/nm824 (2003).
Miyamoto, J. et al. Ketone body receptor GPR43 regulates lipid metabolism under ketogenic conditions. Proc. Natl. Acad. Sci. USA 116, 23813–23821. https://doi.org/10.1073/pnas.1912573116 (2019).
Meinkoth, J. L. et al. Signal transduction through the cAMP-dependent protein kinase. Mol. Cell Biochem. 127–128, 179–186. https://doi.org/10.1007/bf01076769 (1993).
Hanoune, J. & Defer, N. Regulation and role of adenylyl cyclase isoforms. Annu. Rev. Pharmacol. Toxicol. 41, 145–174. https://doi.org/10.1146/annurev.pharmtox.41.1.145 (2001).
Bhandari, D. et al. Exploring GPR109A receptor interaction with hippuric acid using MD simulations and CD spectroscopy. Int. J. Mol. Sci. https://doi.org/10.3390/ijms232314778 (2022).
Chen, J.-R. et al. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/β-catenin canonical Wnt signaling. J. Bone Miner. Res. 25, 2399–2411. https://doi.org/10.1002/jbmr.137 (2010).
Zhao, H., Lazarenko, O. P. & Chen, J. R. Hippuric acid and 3-(3-hydroxyphenyl) propionic acid inhibit murine osteoclastogenesis through RANKL-RANK independent pathway. J. Cell. Physiol. 235, 599–610. https://doi.org/10.1002/jcp.28998 (2020).
Caviness, P. C. et al. Phenolic acids prevent sex-steroid deficiency-induced bone loss and bone marrow adipogenesis in mice. J. Nutr. Biochem. 127, 109601. https://doi.org/10.1016/j.jnutbio.2024.109601 (2024).
Chen, J. R. et al. 3-(3-Hydroxyphenyl)-propionic acid (PPA) suppresses osteoblastic cell senescence to promote bone accretion in mice. JBMR Plus https://doi.org/10.1002/JBM4.10201 (2019).
Chen, J.-R., Lazarenko, O. P. & Blackburn, M. L. GPR109A gene deletion ameliorates gonadectomy-induced bone loss in mice. Bone 161, 116422. https://doi.org/10.1016/j.bone.2022.116422 (2022).
Wise, A. et al. Molecular identification of high and low affinity receptors for nicotinic acid*. J. Biol. Chem. 278, 9869–9874. https://doi.org/10.1074/jbc.M210695200 (2003).
Zhang, Z. et al. GPR109a regulates phenotypic and functional alterations in macrophages and the progression of type 1 diabetes. Mol. Nutr. Food Res. 66, e2200300. https://doi.org/10.1002/mnfr.202200300 (2022).
Zandi-Nejad, K. et al. The role of HCA2 (GPR109A) in regulating macrophage function. FASEB J. 27, 4366–4374. https://doi.org/10.1096/fj.12-223933 (2013).
Adamopoulos, I. E. Inflammation in bone physiology and pathology. Curr. Opin. Rheumatol. 30, 59–64. https://doi.org/10.1097/bor.0000000000000449 (2018).
Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2, 17023. https://doi.org/10.1038/sigtrans.2017.23 (2017).
Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1, a001651. https://doi.org/10.1101/cshperspect.a001651 (2009).
Elangovan, S. et al. The niacin/butyrate receptor GPR109A suppresses mammary tumorigenesis by inhibiting cell survival. Cancer Res. 74, 1166–1178. https://doi.org/10.1158/0008-5472.Can-13-1451 (2014).
Zhao, B. et al. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat. Med. 15, 1066–1071. https://doi.org/10.1038/nm.2007 (2009).
Caviness, P. C. et al. Decreased bone resorption in Ezh2 myeloid cell conditional knockout mouse model. FASEB J. 37, e23019. https://doi.org/10.1096/fj.202201673RR (2023).
Wang, L., You, X., Zhang, L., Zhang, C. & Zou, W. Mechanical regulation of bone remodeling. Bone Res. 10, 16. https://doi.org/10.1038/s41413-022-00190-4 (2022).
Grover, K., Lin, L., Hu, M., Muir, J. & Qin, Y. X. Spatial distribution and remodeling of elastic modulus of bone in micro-regime as prediction of early stage osteoporosis. J. Biomech. 49, 161–166. https://doi.org/10.1016/j.jbiomech.2015.11.052 (2016).
Blázquez-Carmona, P., Mora-Macías, J., Pajares, A., Mármol, Á. & Reina-Romo, E. On the influence of structural and chemical properties on the elastic modulus of woven bone under healing. Front. Bioeng. Biotechnol. 12, 1476473. https://doi.org/10.3389/fbioe.2024.1476473 (2024).
Callewaert, F., Sinnesael, M., Gielen, E., Boonen, S. & Vanderschueren, D. Skeletal sexual dimorphism: Relative contribution of sex steroids, GH-IGF1, and mechanical loading. J. Endocrinol. 207, 127–134. https://doi.org/10.1677/JOE-10-0209 (2010).
Pomplun, D., Florian, S., Schulz, T., Pfeiffer, A. F. & Ristow, M. Alterations of pancreatic beta-cell mass and islet number due to Ins2-controlled expression of Cre recombinase: RIP-Cre revisited; part 2. Horm. Metab. Res 39, 336–340. https://doi.org/10.1055/s-2007-976538 (2007).
Semprini, S. et al. Cryptic loxP sites in mammalian genomes: genome-wide distribution and relevance for the efficiency of BAC/PAC recombineering techniques. Nucleic Acids Res. 35, 1402–1410. https://doi.org/10.1093/nar/gkl1108 (2007).
Loonstra, A. et al. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc. Natl. Acad. Sci. USA 98, 9209–9214. https://doi.org/10.1073/pnas.161269798 (2001).
Huh, W. J., Mysorekar, I. U. & Mills, J. C. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia, and regenerative changes in the absence of “floxed” alleles. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G368-380. https://doi.org/10.1152/ajpgi.00021.2010 (2010).
Baghdadi, M., Mesaros, A., Purrio, M. & Partridge, L. Sex-specific effects of Cre expression in Syn1Cre mice. Sci. Rep. 13, 10037. https://doi.org/10.1038/s41598-023-37029-9 (2023).
Pugach, E. K., Richmond, P. A., Azofeifa, J. G., Dowell, R. D. & Leinwand, L. A. Prolonged Cre expression driven by the α-myosin heavy chain promoter can be cardiotoxic. J. Mol. Cell Cardiol. 86, 54–61. https://doi.org/10.1016/j.yjmcc.2015.06.019 (2015).
McLean, B. A. et al. PI3Kα is essential for the recovery from Cre/tamoxifen cardiotoxicity and in myocardial insulin signalling but is not required for normal myocardial contractility in the adult heart. Cardiovasc. Res. 105, 292–303. https://doi.org/10.1093/cvr/cvv016 (2015).
Svart, M. et al. Oral 3-hydroxybuturate ingestion acutely lowers circulating testosterone concentrations in healthy young males. Scand. J. Med. Sci. Sports 33, 1976–1983. https://doi.org/10.1111/sms.14441 (2023).
Oury, F. et al. Endocrine regulation of male fertility by the skeleton. Cell 144, 796–809. https://doi.org/10.1016/j.cell.2011.02.004 (2011).
Behre, H. M., Kliesch, S., Leifke, E., Link, T. M. & Nieschlag, E. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J. Clin. Endocrinol. Metab. 82, 2386–2390. https://doi.org/10.1210/jcem.82.8.4163 (1997).
Chou, Y. S., Chuang, S. C., Chen, C. H., Ho, M. L. & Chang, J. K. G-protein-coupled estrogen receptor-1 positively regulates the growth plate chondrocyte proliferation in female pubertal mice. Front. Cell Dev. Biol. 9, 710664. https://doi.org/10.3389/fcell.2021.710664 (2021).
Chuang, S. C., Chen, C. H., Chou, Y. S., Ho, M. L. & Chang, J. K. G Protein-coupled estrogen receptor mediates cell proliferation through the cAMP/PKA/CREB pathway in murine bone marrow mesenchymal stem cells. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21186490 (2020).
Santolla, M. F. et al. Niacin activates the G protein estrogen receptor (GPER)-mediated signalling. Cell. Signal. 26, 1466–1475. https://doi.org/10.1016/j.cellsig.2014.03.011 (2014).
Grande, F. et al. Computational approaches for the discovery of GPER targeting compounds. Front. Endocrinol. 11, 517. https://doi.org/10.3389/fendo.2020.00517 (2020).
Kameda, T. et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J. Exp. Med. 186, 489. https://doi.org/10.1084/jem.186.4.489 (1997).
Shevde, N. K., Bendixen, A. C., Dienger, K. M. & Pike, J. W. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc. Natl. Acad. Sci. USA 97, 7829–7834. https://doi.org/10.1073/pnas.130200197 (2000).
Krum, S. A. et al. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 27(3), 535–545. https://doi.org/10.1038/sj.emboj.7601984 (2008).
Yang, D. C. et al. cAMP/PKA regulates osteogenesis, adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS ONE 3, e1540. https://doi.org/10.1371/journal.pone.0001540 (2008).
Wang, L. et al. Dopamine suppresses osteoclast differentiation via cAMP/PKA/CREB pathway. Cell Signal 78, 109847. https://doi.org/10.1016/j.cellsig.2020.109847 (2021).
Weivoda, M. M. et al. Wnt signaling inhibits osteoclast differentiation by activating canonical and noncanonical cAMP/PKA pathways. J. Bone Miner. Res. 31, 65–75. https://doi.org/10.1002/jbmr.2599 (2016).
Hall, J. M. & McDonnell, D. P. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140, 5566–5578. https://doi.org/10.1210/endo.140.12.7179 (1999).
Zhou, Y. & Liu, X. The role of estrogen receptor beta in breast cancer. Biomark. Res. 8, 39. https://doi.org/10.1186/s40364-020-00223-2 (2020).
Osterhoff, G. et al. Bone mechanical properties and changes with osteoporosis. Injury 47(Suppl 2), S11-20. https://doi.org/10.1016/s0020-1383(16)47003-8 (2016).
Motulsky, H. J. & Brown, R. E. Detecting outliers when fitting data with nonlinear regression: A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 7, 123. https://doi.org/10.1186/1471-2105-7-123 (2006).
Acknowledgements
Authors would like to thank Jim Sikes, Hoy Pittman and Bobby Fay for their technical assistances on animal experiments. This work was supported by sub-objective to J.R.C. by United States Department of Agriculture (USDA) / Agricultural Research Service (ARS) Project # USDA-ARS Project 6026-51000-012-06S as well National institute of health project R37 AA18282 sub-awarded to J.R.C. Authors declare that they have no competing interests. All data are available in the main text or the supplemental materials.
Funding
This study is supported by USDA-ARS Project 6026-10700-001-000D; and NIH R01 project R37 AA18282 sub-awarded to JRC.
Author information
Authors and Affiliations
Contributions
J.R.C. designed the study; P.C.C. wrote the original draft and produced figures; J.R.C., and P.C.C. revised and edited the paper; O.P.L. and M.L.B. performed ex vivo experiments; P.C.C., J.R.C., O.P.L. and M.L.B. analyzed results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Caviness, P.C., Lazarenko, O.P., Blackburn, M.L. et al. Sex dependent effects of GPR109A gene deletion in myeloid cells on bone development in mice. Sci Rep 15, 26515 (2025). https://doi.org/10.1038/s41598-025-12017-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12017-3