Abstract
As an acute respiratory infectious disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Coronavirus Disease 2019 (COVID-19) exhibits remarkable contagiousness and has emerged as a critical global public health concern. In critically ill patients, virus-induced cytokine storms and resulting multi-organ failure represent significant therapeutic challenges. In our clinical observations, we identified that this hyperinflammatory state was correlated with reduced mRNA expression of both Tumor Necrosis Factor-α-Induced Protein 8-like Protein 2 (TIPE2)—an immune negative modulator—and the ferroptosis marker gene Glutathione Peroxidase 4 (GPx4) in peripheral blood mononuclear cells (PBMCs) of these patients. Given the emerging evidence that macrophage polarization and dysfunction play pivotal roles in COVID-19 progression, we established a THP-1-derived macrophage model stimulated with the SARS-CoV-2 spike (S) protein to mimic the host immune response. Our results demonstrated that TIPE2 modulates S protein-induced macrophage polarization and ferroptosis, specifically by suppressing M1 polarization associated with inflammation and encouraging M2 polarization linked to anti-inflammatory responses, thereby alleviating inflammatory responses. These findings suggest that TIPE2 mediates critical immunomodulatory effects during the progression of SARS-CoV-2 infection, positioning it as a promising therapeutic target for mitigating pathological inflammatory responses in COVID-19 patients gravely affected.
Similar content being viewed by others
Introduction
COVID-19, resulting from SARS-CoV-2, a single-stranded RNA virus, manifests primarily as an acute respiratory illness with significant systemic complications1. SARS-CoV-2 initiates infection by binding its spike protein to the angiotensin-converting enzyme 2 (ACE2) receptor expressed on host cells, thereby promoting viral entry and instigating a cascade of immune responses, notably driving macrophage activation and polarization2,3. In critically ill patients, severe pulmonary damage is a hallmark, characterized by excessive cell death and exuberant inflammatory responses4,5. These pathological changes are closely associated with macrophage dysregulation and a breakdown in systemic immune homeostasis6. The dramatic upregulation of inflammatory mediators, such as interleukin (IL)-6, -1β and tumor necrosis factor-alpha (TNF-α), reflects a hyperactivated immune state commonly referred to as a cytokine storm, thereby contributing to acute respiratory failure and potentially facilitating long-term immune dysfunction7,8,9. Therefore, early recognition of inflammatory dysregulation and implementation of precise immunomodulatory strategies are crucial in managing patients with severe COVID-19 to prevent adverse outcomes.
In recent years, programmed cell death (PCD) has attracted extensive attention due to its crucial roles in the development and progression of various diseases. In addition to the classical form of apoptosis, emerging forms of cell death such as ferroptosis and pyroptosis have also been recognized as key contributors under pathological conditions10. These three types of cell death differ markedly in their inducing mechanisms, signaling pathways, and morphological characteristics11. Among them, ferroptosis is a distinct type of cell death that depends on the accumulation of iron ions and elevated levels of lipid peroxides, with the buildup of lethal reactive oxygen species (ROS) serving as a major trigger12. This process ultimately results in irreversible damage to the plasma membrane and loss of cellular function. GPx413, as the pivotal regulator, functions by catalyzing the reduction of lipid hydroperoxides and removing membrane-associated lipid peroxides, thereby mitigating iron-dependent oxidative stress and effectively preventing ferroptotic cell death. In contrast, apoptosis is primarily mediated by the caspase family of cysteine proteases14. These enzymes activate a proteolytic cascade that cleaves a variety of substrates, inducing typical morphological changes such as nuclear condensation and DNA fragmentation, ultimately leading to an orderly and self-limiting degradation process15. Unlike the proteolytic function of caspases, GPx4 exerts its protective effect by preserving lipid balance and counteracting oxidative stress, highlighting a fundamental mechanistic distinction between ferroptosis and apoptosis. Pyroptosis is an inflammation-associated form of programmed cell death characterized by inflammasome-dependent activation of caspase-1, which cleaves gasdermin D (GSDMD) to form membrane pores, leading to the release of intracellular contents and pro-inflammatory cytokines16.
Of particular interest is the growing evidence supporting the role of ferroptosis in inflammatory diseases, especially those associated with viral infections. In the case of SARS-CoV-2 infection, virus-induced oxidative stress and inflammatory responses are frequently accompanied by disruption of intracellular iron metabolism17,18,19. These alterations can activate ferroptosis-related signaling pathways, resulting in irreversible oxidative injury and dysfunction of immune cells20.As essential components of the pulmonary innate immune system, alveolar macrophages play a pivotal role in pathogen clearance and immune surveillance21.However, ferroptosis in macrophages can impair their function, alter cytokine production, and exacerbate inflammatory responses22.Clinical observations have shown that iron homeostasis is often dysregulated in COVID-19 patients, characterized by reduced systemic iron levels, lower transferrin concentrations, and elevated ferritin levels19,23. These changes are closely associated with the cytokine storm observed in severe cases of COVID-1924. Collectively, these findings suggest that ferroptosis may serve as a critical mechanistic link between iron dysregulation, macrophage dysfunction, and excessive immune activation.
TIPE2, a constituent of the Tumor Necrosis Factor-α-Induced Protein 8 (TNFAIP8) family, functions as a suppressor of innate immune signaling and is crucial for orchestrating macrophage activation and preserving inflammatory equilibrium25,26,27. By modulating macrophage polarization, TIPE2 inhibits pro-inflammatory M1 phenotypes and promotes anti-inflammatory M2 polarization, thereby preserving immune homeostasis and limiting tissue injury28,29,30. In infectious disease contexts, TIPE2-mediated regulation of the immune microenvironment has been shown to suppress excessive inflammation and improve host resilience31,32,33. Beyond its immunoregulatory role, TIPE2 also contributes to cellular homeostasis through metabolic and signaling regulation. At the metabolic level, TIPE2 downregulates cholesterol and fatty acid synthesis–related genes (such as Fatty Acid Desaturase 2 (Fads2)) and inhibits mitochondrial respiration and ATP production, thereby reducing oxidative stress34. At the signaling level, TIPE2 activates the Nrf2/HO-1 antioxidant pathway30 and suppresses NF-κB signaling32—two pathways critically involved in ferroptosis inhibition and promotion, respectively35,36. Although current studies suggest that TIPE2 may influence ferroptosis through these mechanisms, its specific role in the context of SARS-CoV-2 infection remains unclear. To address this, we established a spike protein–stimulated THP-1 macrophage model to mimic post-infection immune responses and found that TIPE2 attenuates ferroptosis and restores polarization balance, indicating a potential anti-inflammatory and protective role in COVID-19.
Materials and methods
Study population
Ethical approval for this investigation was obtained from the Institutional Research Ethics Committee of the Second Hospital of Jilin University (Approval No. 2023(005)). Because the study retrospectively analyzed anonymized human blood samples, informed consent was exempted. All methods were performed in accordance with the relevant guidelines and regulations. Forty-four individuals diagnosed with COVID-19 by laboratory testing were recruited from the Second Hospital of Jilin University (Changchun, China) between December 2022 and January 2023. Based on the 10th edition of the Chinese National Guidelines for the Diagnosis and Treatment of COVID-19, patients were stratified into moderate-to-severe (n = 22) and critical (n = 22) categories37. All enrolled patients had completed COVID-19 vaccination in accordance with China’s epidemic prevention policies at the time and were confirmed as first-time SARS-CoV-2 infections with no prior infection history. During the study period, the predominant circulating strain in China was Omicron38. To minimize the impact of therapeutic interventions on immune parameters, peripheral blood samples were collected within 24 h of hospital admission, prior to the administration of systemic immunomodulatory treatments (including corticosteroids). Patients with co-infections (bacterial or viral), advanced hematologic malignancies, autoimmune diseases, long-term use of immunosuppressants or corticosteroids, death or treatment withdrawal within 24 h of admission, or incomplete clinical data were excluded from the study. An additional 11 healthy individuals, confirmed to have no history or signs of SARS-CoV-2 infection, were recruited from a health examination center as controls.
Laboratory examination of blood samples
Peripheral blood samples were acquired within 24 h post-admission. To minimize the influence of confounding variables including treatment interventions and disease course, sampling time was standardized, ensuring reliable comparisons among cohorts of differing disease severity. Approximately 3 mL of venous blood was transferred into EDTA-containing tubes. Centrifugation at 3000 rpm for 10 min was used to separate plasma, which was subsequently stored at − 80 °C. Ficoll-Hypaque density gradient centrifugation was employed to obtain PBMCs, which were preserved in TRIzol (Thermo Fisher Scientific, USA) at − 80 °C.
Data collection
Demographic and clinical data, including age, gender, and symptoms (fever, sore throat, cough, sputum production, dyspnea), were collected. Laboratory data included absolute counts of neutrophils, lymphocytes, monocytes and eosinophils. Chest radiography (Siemens, Germany) was performed within 24 h of admission. Radiographs were evaluated by three independent radiologists (each with > 10 years of experience), blinded to clinical data. A semi-quantitative lung severity score was assigned by dividing each lung into three zones (upper, middle, lower) and scoring each from 0 to 3 based on the extent of radiographic opacities:
-
0 points, normal lung parenchyma;
-
1 point, interstitial involvement only;
-
2 points, visible radiopaque lung parenchyma less than 50%;
-
3 points, 50% or more visible radiopaque lung parenchyma39.
Cell culture and treatment
THP-1 cells (human monocytic lineage; Cell Bank, Chinese Academy of Sciences) were cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Procell, China). Differentiation of cells into the M0 macrophage phenotype was induced using phorbol 12-myristate 13-acetate (PMA, 100 ng/mL; AbMole, USA; Cat# M4647) for 48 h.
Cell transfection
THP-1 cells were genetically modified via lentiviral vectors delivering TIPE2-targeting shRNA, OE-TIPE2 constructs, or empty vector controls (Mijia Biotech Co. Ltd., Beijing, China). Cells were selected 72 h post-infection using the infinite dilution (single clone) method. Quantitative Real-Time PCR (qRT-PCR) and Western blotting were employed to verify TIPE2 expression.
TIPE2 shRNA sequence was: CCATGACGGCACTTAGCTTTG.
SARS-CoV-2 S glycoprotein
The recombinant SARS-CoV-2 Spike S1 + S2 ECD-His protein (Sino Biological, China; Cat# 40589-V08B1) was reconstituted and used following the manufacturer’s guidelines. The recombinant spike protein used in this study had an endotoxin level of < 1 EU/µg protein, meeting industry standards.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated using TRIzol reagent (Invitrogen, USA), and complementary DNA was synthesized utilizing the StarScript II First-Strand cDNA Synthesis Mix (GenStar, China). GAPDH and β-actin were selected as endogenous reference genes. The sequences of primers used are summarized in Table 1. All primers used in this study were designed and synthesized by Yijin Biotech Co., Ltd. (Shanghai, China).
Enzyme-linked immunosorbent assay (ELISA)
Plasma levels of IL-1β, -6, -10, TGF-β, GPx4, and ferritin were detected using ELISA kits (Bioswamp, China). TIPE2 levels were assessed using a human TNFAIP8L2 ELISA kit (SAB, USA). Intracellular coenzyme (Co) Q10 levels were determined using a human CoQ10 ELISA kit (CUSABIO, China).
Western blotting
Total cellular proteins were obtained with RIPA buffer (Solarbio, China), separated by 8–12% SDS-PAGE, and transferred to PVDF membranes (0.45 μm). Membranes were probed with primary antibodies against TIPE2 (CST, USA; Cat#53842 ), GPx4 (Proteintech, China; Cat# 67763-1-lg), SLC7A11 (CST, USA; Cat# 12691), FSP1 (Abcam, UK; Cat# ab302673), ACSL4 (Abcam, UK; Cat# ab155282), GAPDH (SAB, USA; Cat#40493 ), and α-Tubulin (SAB, USA; Cat# 37981). Detection was performed using enhanced chemiluminescence (ECL, Biosharp, China).
Cell viability assay
Cell viability analysis was performed utilizing the Cell Counting Kit-8 (CCK-8; AbMole, USA), based on the protocol provided by the manufacturer.
Transmission electron microscopy (TEM)
THP-1 macrophages were immobilized in 2.5% glutaraldehyde at 4 °C overnight, followed by postfixation in 2% osmium tetroxide, dehydrated, embedded in Epon812 resin (Merck, Germany), and sectioned into 60 nm slices. After staining with uranyl acetate and lead citrate, the sections were observed by TEM (Ftmicro, Japan).
Flow cytometry
THP-1 macrophages were stained with antibodies against CD14, CD11b, CD86, and CD206 (Biolegend, USA) for 30 min at 4 °C, washed, and analyzed using flow cytometer (BD, USA).
Measurement of iron, oxidative stress, and lipid peroxidation markers
Iron Levels: Plasma total iron and intracellular ferrous iron were quantified using colorimetric assay kits (Elabscience, China). Malondialdehyde (MDA): Intracellular MDA was measured using a detection kit (Beyotime, China; Cat#S0131S) and normalized to protein content. Reduced glutathione/ oxidized glutathione (GSH /GSSG) Ratio: Intracellular glutathione levels were assessed with a GSH/GSSG Assay Kit (Beyotime, China; Cat#S0053). NADP⁺/NADPH Ratio: Evaluated using the NADP⁺/NADPH Quantification Kit (WST-8) (Beyotime, China; Cat#S0179). Lipid ROS Detection: Lipid peroxidation was visualized by staining with BODIPY™ 581/591 C11 (Thermo Fisher, USA) under fluorescence microscopy (Olympus, Japan).
Statistical analysis
All clinical data were complete, with no missing values; therefore, no imputation or exclusion was necessary. Data were analyzed using SPSS 24.0 and GraphPad Prism 9.0. Data with normal distribution were presented as mean ± SD and analyzed by Student’s t-test; non-normal data were expressed as median (Q1–Q3) and tested using the Kruskal–Wallis or Mann–Whitney U test with Bonferroni adjustment. Chi-square testing was conducted for categorical data, with p-values < 0.05 indicating statistical significance. Correlation analysis was conducted using Spearman’s rank correlation coefficient. To correct for multiple comparisons, P-values were adjusted using the false discovery rate (FDR) method of Benjamini and Hochberg. An FDR-adjusted P < 0.05 was considered statistically significant.
Results
Peripheral blood inflammatory cells in COVID-19 patients
44 patients with confirmed COVID-19 diagnosis through laboratory testing were enrolled in this investigation, including 22 with moderate-to-severe disease and 22 with critical illness. An additional control group comprised 11 healthy individuals with no history or current evidence of SARS-CoV-2 infection, providing a baseline for comparison. Age and gender were comparably distributed among the three groups, with no significant variation observed. The most common symptom among COVID-19 patients was fever (90.9% within the critically affected group vs. 100% within the moderately to severely affected group), followed by cough (72.7% vs. 86.4%), sputum production (54.5% vs. 77.3%), and dyspnea (72.7% vs. 50%). Only one critically ill patient reported a sore throat. Analysis revealed no significant disparity in the duration from symptom onset to blood sampling between the two groups. Chest X-rays were obtained for all patients, and pulmonary parenchymal abnormalities were quantified using a standardized scoring system. The median Chest x-ray (CXR) severity score at admission was significantly higher in critically ill patients (16, IQR 12–17) than in those with moderate-to-severe disease (11.5, IQR 10–14; p = 0.002) (Table 2).
Critically ill COVID-19 patients exhibited significantly elevated neutrophil counts compared to controls (critical: 7.18 [5.50–14.12] ×10⁹/L; moderate-severe: 5.86 [2.78–9.60] ×10⁹/L; control: 4.04 [3.29–5.02] ×10⁹/L; P = 0.013). The proportion of individuals with neutrophil levels > 6.3 × 10⁹/L was markedly higher in the critical (63.6%) and moderate-severe (50.0%) groups, whereas all controls were within the normal range. In contrast, lymphocyte counts were significantly reduced in COVID-19 patients (critical: 0.30 [0.20–0.55] ×10⁹/L; moderate-severe: 0.65 [0.38–1.13] ×10⁹/L; control: 2.49 [1.78–2.82] ×10⁹/L; P < 0.001). All critically ill patients and 72.7% of moderate-severe patients had lymphocyte levels below 1.0 × 10⁹/L, while all control subjects had normal values. The neutrophil-to-lymphocyte ratio (NLR) was significantly elevated in both COVID-19 subgroups, especially among critical cases (critical: 28.38 [15.12–49.13]; moderate-severe: 9.19 [4.30–18.65]; control: 1.96 [1.55–2.22]; P < 0.001). Monocyte counts did not exhibit significant difference across the groups (critical: 0.30 [0.20–0.48] ×10⁹/L; moderate-severe: 0.40 [0.28–0.53] ×10⁹/L; control: 0.40 [0.20–0.50] ×10⁹/L; P = 0.40). Eosinophil counts were significantly decreased in COVID-19 patients (critical: 0 [0–0] ×10⁹/L; moderate-severe: 0.01 [0–0.03] ×10⁹/L; control: 0.32 [0.18–0.48] ×10⁹/L; P < 0.001). Notably, 77.3% of critical and 45.5% of moderate-severe patients had undetectable eosinophil counts, whereas all control participants had normal eosinophil levels (Table 3).These alterations in peripheral blood cell counts profiles—particularly increased neutrophil counts, decreased lymphocytes and eosinophils, and elevated NLR—may reflect underlying immune dysregulation and macrophage polarization shifts during severe COVID-19.
Plasma cytokines and iron metabolism in COVID-19 patients
Circulating levels of inflammatory cytokines and iron-related markers were compared among patients with critical or moderate-to-severe COVID-19 and healthy individuals. Patients with critical illness had markedly higher IL-6 levels than moderate-to-severe cases (P < 0.01) and healthy controls (P < 0.001). Significant elevation of IL-1β occurred only in the critically ill group relative to controls (P < 0.05), whereas moderate-to-severe cases exhibited no statistical difference. Significant elevations in IL-10 were detected in both critical and moderate-to-severe COVID-19 patients compared with controls (P < 0.001 and P < 0.05, respectively), with the critical group presenting higher levels than the moderate-to-severe cohort (P < 0.05). Similarly, TGF-β levels were markedly elevated in critically ill patients compared to both moderate-severe patients (P < 0.001) and controls (P < 0.001) (Fig. 1a).
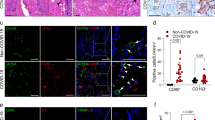
Critical COVID−19 Patients Exhibit Defects in TIPE2 and GPx4 Expression, Accompanied by Iron Dysregulation and Elevated Inflammation. a, b Plasma concentrations of inflammatory cytokines (IL-6, -1β, -10 and TGF-β) and iron metabolism markers (ferritin and total iron) in COVID-19 patients and controls. c Plasma concentrations and PBMC transcript levels of TIPE2 and GPx4 among the three groups. d Correlations between TIPE2 and clinical disease severity, GPx4, TGF-β and ferritin. P values were adjusted for multiple comparisons using the FDR method. Data are presented as mean ± SD or correlation coefficients. Statistical significance was determined using one-way ANOVA with Tukey’s multiple comparisons test for group comparisons, and Spearman’s correlation for association analyses.(*P < 0.05, **P < 0.01, ***P < 0.001.)
Figure 1b illustrates that critically ill patients exhibited substantially elevated plasma ferritin levels compared with moderate-to-severe cases (P < 0.05) and controls (P < 0.001). Plasma total iron levels were significantly lower in critically ill COVID-19 patients compared with those in patients with moderate disease (P < 0.001) and healthy controls (P < 0.001). No significant difference was detected between patients with moderate disease and healthy individuals. These results indicated that systemic inflammatory activation and iron metabolism disruption were strongly linked to disease severity in patients with COVID-19.
TIPE2 and GPx4 expression in relation to inflammation and iron dysregulation
Critically ill COVID-19 patients demonstrated significantly lower plasma protein and PBMC mRNA levels of TIPE2 and GPx4 relative to healthy participants. Plasma TIPE2 concentrations were significantly diminished in critically ill patients versus healthy controls (P < 0.01), while no meaningful differences were found between the critical and moderate-to-severe groups, or between moderate-to-severe cases and controls. Plasma GPx4 levels were markedly decreased in critically ill patients relative to both moderate-to-severe cases (P < 0.05) and healthy controls (P < 0.001). Transcriptional analysis revealed a significant reduction in PBMC TIPE2 mRNA levels in critically ill patients compared with moderate-to-severe cases and healthy controls (P < 0.001). Similarly, GPx4 expression was markedly decreased in the critical group relative to the other groups (P < 0.001 and P < 0.01, respectively) (Fig. 1c).
We investigated the correlations between peripheral blood TIPE2 levels and disease-related parameters in patients with COVID-19. The results showed that TIPE2 mRNA expression in PBMCs was significantly positively correlated with both plasma GPx4 levels and PBMC GPx4 mRNA expression (r = 0.462, P < 0.01; r = 0.614, P < 0.001). Conversely, TIPE2 mRNA levels were significantly negatively correlated with disease severity (r = -0.820, P < 0.001) and plasma TGF-β levels (r = -0.378, P < 0.05). In addition, both plasma TIPE2 protein levels and PBMC TIPE2 mRNA expression were inversely associated with plasma ferritin concentrations (r = -0.489, P < 0.05; r = -0.368, P < 0.05) (Fig. 1d).
These findings suggest that impaired TIPE2 expression in critically ill COVID-19 patients may promote inflammatory responses, exacerbate lung injury, and accelerate disease progression. Downregulation of TIPE2 may lead to reduced GPx4 expression, impaired clearance of lipid peroxides, enhanced oxidative stress and membrane lipid damage, thereby triggering ferroptosis and creating a vicious cycle of inflammation and tissue injury. Its negative correlation with plasma ferritin further indicates that TIPE2 deficiency may disrupt iron homeostasis, increase iron overload, activate ferroptosis-related pathways, and aggravate disease severity. These results provide important insights for further investigation of the role of TIPE2 in the pathogenesis of COVID-19 and its potential as a therapeutic target.
SARS-CoV-2 spike protein induces TIPE2 and GPx4 downregulation and macrophages ferroptosis
A macrophage stimulation model was established using THP-1-derived cells treated with recombinant SARS-CoV-2 spike protein at a concentration of 20 ng/mL for 24 h. This stimulation condition was determined based on preliminary dose- and time-gradient experiments (concentrations: 0, 10, 20, 50, and 100 ng/mL; time points: 12 h and 24 h) (Fig. 2a). In addition, the selected concentration was referenced from commonly used doses in previous in vitro studies (mostly ≥ 10 µg/mL, approximately 50 nM), and was further informed by extrapolated estimates of viral protein concentrations in the body fluids of critically ill COVID-19 patients40,41,42. Under the condition of 20 ng/mL for 24 h, we observed that recombinant spike protein significantly suppressed the expression of TIPE2 and GPx4 in THP-1 macrophages at both the mRNA and protein levels (Fig. 2a, b). Moreover, prolonged exposure to the spike protein affected macrophage polarization dynamics. At 12 h, the proportions of both M1 and M2 macrophages increased. However, by 24 h, the proportion of M1 macrophages continued to rise, while that of M2 macrophages declined. The secretion levels of cytokines associated with M1 polarization (e.g., IL-6 and IL-1β) and M2 polarization (e.g., IL-10 and TGF-β) were significantly elevated (Fig. 3b, c).
SARS-CoV-2 spike protein suppresses TIPE2 and GPx4 expression and induces mitochondrial morphological changes in THP-1 macrophages. a TIPE2 and GPx4 mRNA expression levels in THP-1 macrophages were altered following treatment with different concentrations (0, 10, 20, 50, 100 ng/ml) of the SARS-CoV-2 spike protein for 12–24 h. b Protein expression levels of TIPE2 and GPx4 in THP-1 macrophages were evaluated by Western blotting after 24 h of stimulation with 20 ng/mL S protein. Cropped regions from the same blot are shown. The full-length blot, including molecular weight markers and uncropped lanes, is presented in Supplementary Fig. S1. c The mitochondrial morphology of phorbol-12-myristate-13-acetate (PMA) -induced THP-1 macrophages under transmission electron microscopy, both untreated and treated with the spike protein. Black arrows denote normal mitochondria, whereas red arrows indicate pathological changes characterized by elevated membrane density, membrane rupture, and the reduction or disappearance of mitochondrial cristae (scale bar = 500 nm). Student’s t-test evaluated differences between two groups, and one-way ANOVA with post-hoc testing (LSD or Tamhane’s T2) was used for comparisons among three groups.(*P < 0.05, **P < 0.01, ***P < 0.001).
TIPE2 alleviates SARS-CoV-2 spike protein-induced macrophage polarization imbalance and cytokine hypersecretion. a Lentiviral vectors carrying either TIPE2 shRNA or TIPE2 overexpression sequences were used to transduce THP-1 cells. The expression levels of TIPE2 were quantified by qRT-PCR and Western blotting. The blot shown was cropped to improve clarity. Molecular weight markers and uncropped original data are provided in Supplementary Fig. S2. b Flow cytometric evaluation of M1 (CD86+) and M2 (CD206+) macrophage subsets following S protein stimulation, with or without enforced TIPE2 expression. c Levels of IL-6, -1β, -10, and TGF-β in the culture supernatants of THP-1 macrophages were measured by ELISA. Data are presented as mean ± SD from at least three independent experiments. Student’s t-test evaluated differences between two groups, and one-way ANOVA with post-hoc testing (LSD or Tamhane’s T2) was used for comparisons among three groups. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P<0.0001 )
Furthermore, S protein-treated THP-1 macrophages exhibited classical ferroptotic features under electron microscopy, including reduced mitochondrial volume and disrupted cristae (Fig. 2c). Markers of ferroptosis, such as decreased GPx4, ferroptosis suppressor protein 1 (FSP1), solute carrier family 7 member 11 (SLC7A11), and elevated lipid peroxidation products (MDA, lipid ROS), increased ferrous iron levels, impaired glutathione (GSH) metabolism, as well as disruption of the CoQ10/ NADPH- dependent antioxidant system, were observed (Fig. 4a, c–i).
TIPE2 attenuates S protein-Induced inflammation and ferroptosis in macrophages
Overexpression of TIPE2 in THP-1 macrophages counteracted the polarization imbalance by reducing M1 macrophage proportions, promoting M2 macrophage restoration and decreasing cytokine(IL-6, -1β, -10 and TGF-β) secretion (Fig. 3a-c). TIPE2 overexpression significantly restored GPx4 and FSP1 expression, reduced lipid peroxidation, restored GSH/GSSG balance, enhanced the CoQ10-dependent antioxidant system, and improved cell viability following S protein stimulation (Fig. 4a, c-i). Notably, TIPE2 exhibited a more pronounced inhibitory effect on ferroptosis compared to Ferrostatin-1 (Fer-1), a known ferroptosis inhibitor43 (Fig. 4). Collectively, these results suggest that TIPE2 protects macrophages against SARS-CoV-2-induced ferroptosis and hyperinflammatory responses, highlighting its potential as a molecular target for COVID- 19 therapy.
TIPE2 regulates ferroptosis-related pathways in THP-1 macrophages following SARS-CoV-2 spike protein stimulation. a, b Expression of ferroptosis-related genes in THP−1 macrophages by qRT-PCR and Western blotting. the ferroptosis-related proteins including GPx4, SLC7A11,FSP1 and ACSL4. Cropped blot is shown for clarity, retaining relevant bands. The uncropped, full-length blot with visible edges is included in Supplementary Figure S3, 4. c Cell viability was evaluated by CCK8 assay in TIPE2-transfected or control THP-1 macrophages treated with S protein alone or in combination with Fer-1 (2µM). d-h Quantification of intracellular levels of ferroptosis-associated metabolic indicators, including ferrous iron (Fe²+), Coenzyme Q10 (CoQ10), NADP⁺/NADPH ratio, malondialdehyde (MDA), and GSH/GSSG ratio in THP-1 macrophages under the indicated treatments. i Representative fluorescence microscopy images showing lipid ROS accumulation in THP-1 macrophages labeled with specific probes. Nuclei are stained blue (Hoechst), lipid ROS appear in green (FITC channel), and merged images are presented. Data are representative of at least three independent experiments. Student’s t-test evaluated differences between two groups, and one-way ANOVA with post-hoc testing (LSD or Tamhane’s T2) was used for comparisons among three groups. (*P < 0.05, **P < 0.01, ***P < 0.001)
Discussion
This study collected data primarily between December 2022 and January 2023, during which the SARS-CoV-2 Omicron variant was widely circulating in China38. Characterized by high transmissibility and notable immune evasion capacity44Omicron led to a nationwide surge in COVID-19 cases, placing substantial strain on healthcare systems. Therefore, research into the clinical features and potential therapeutic strategies during this critical period is of considerable practical significance. In this study, we systematically characterized alterations in peripheral blood immune cell profiles, inflammatory cytokine production, and iron metabolism, across different severities of COVID-19. Critically ill patients exhibited marked neutrophilia, lymphopenia, eosinopenia, and elevated NLR, reflecting a hyperinflammatory state. These observations were consistent with previous reports45including Xie et al.46who demonstrated that eosinophil depletion correlates with COVID-19 severity and may serve as a potential prognostic indicator. Mechanistically, enhanced secretion of inflammatory factors, notably IL-6 and IL-8, can impair bone marrow hematopoiesis and suppress eosinophil chemotaxis through the downregulation of chemokines such as C-C Motif Chemokine Ligand 11(CCL11)47. This disruption contributes to impaired M2 macrophage polarization, as eosinophils are a key source of IL-448. Concurrently, excessive neutrophil activation promotes reactive oxygen species (ROS) production, exacerbating tissue injury and further skewing macrophage polarization towards the pro-inflammatory M1 phenotype49,50. The resulting imbalance amplifies cytokine storms through increased M1-derived IL-6 and IL-1β secretion and diminished M2-mediated immune regulation, ultimately aggravating disease progression. Therefore, restoring macrophage polarization homeostasis may represent a promising therapeutic strategy in patients with life-threatening COVID-19 require Intensive Care Unit (ICU) care.
We evaluated plasma ferritin and total iron levels in patients with COVID-19 to preliminarily investigate infection-related disturbances in iron metabolism and their potential association with ferroptosis. Ferritin, the major intracellular iron storage protein, also functions as a sensitive acute-phase reactant51. Its elevation reflects not only increased iron burden but also systemic inflammation, and has been closely linked to ferroptotic cell death52. In contrast, plasma total iron indicates the level of bioavailable iron in circulation and is associated with iron homeostasis and oxidative stress53. Our results demonstrated that patients with more severe disease exhibited markedly elevated ferritin levels and significantly reduced plasma total iron concentrations, indicating a strong correlation between iron dysregulation and disease severity. The concurrent increase in ferritin and decrease in plasma iron may suggest abnormal iron accumulation, enhanced oxidative stress, and ferroptosis activation, which could exacerbate tissue injury and contribute to disease progression54,55,56. These outcomes underscore the possible involvement of ferroptosis in the pathogenesis of severe COVID-19.
As a critical determinant of infectivity, the spike (S) protein of SARS-CoV-2 mediates viral entry by binding to the host ACE2 receptor, a process facilitated by activation of the transmembrane protease, serine 2 (TMPRSS2)57,58. Beyond infecting epithelial cells, the S protein can directly act on alveolar macrophages that express ACE2, triggering pronounced immune activation. Studies have demonstrated that the S protein upregulates ACE2 and TMPRSS2 expression in macrophages and activates the Toll-like receptor (TLR) 2–myeloid differentiation factor 88 (MyD88)–NF-κB signaling pathway, leading to robust secretion of proinflammatory cytokines including IL-6, IL-1β, and TNF-α59,60. This promotes M1 polarization of macrophages and contributes to a highly inflammatory microenvironment. Additionally, the S protein can downregulate IL-1R-associated kinase (IRAK)-M expression via the ACE2 signaling pathway, rendering macrophages hypersensitive to TLR ligands and enhancing the production of proinflammatory cytokines such as IL-661. Collectively, these mechanisms contribute to macrophage dysfunction and sustained proinflammatory responses, playing a central role in mediating the uncontrolled inflammatory cascade in severe SARS-CoV-2 infections.
We further demonstrate that the S protein can induce ferroptosis in THP-1-derived macrophages, as evidenced by mitochondrial shrinkage, cristae disruption, increased lipid peroxidation, intracellular Fe²⁺ accumulation, and downregulation of key ferroptosis-inhibitory proteins including GPx4, FSP1, and SLC7A11, accompanied by impaired glutathione metabolism. Ferroptosis not only leads to macrophage dysfunction but may also exacerbate immune polarization and persistent inflammation by selectively depleting M2-type macrophages. Notably, we observed that S protein stimulation markedly upregulated both proinflammatory cytokines (e.g., IL-6 and IL-1β) and anti-inflammatory cytokines (e.g., IL-10 and TGF-β) in THP-1 macrophages. This suggests that the immune response triggered by the S protein is not solely proinflammatory, but rather represents a complex, dynamically regulated state characterized by the coexistence of both pro- and anti-inflammatory signals. The upregulation of IL-10 and TGF-β may reflect a feedback immunosuppressive mechanism activated by macrophages in response to intense inflammatory stimuli, aiming to mitigate host tissue damage. This phenomenon closely mirrors the immune status observed in critically ill COVID-19 patients: various reports have reported simultaneous elevation of proinflammatory (e.g., IL-6, TNF-α) and anti-inflammatory cytokines (e.g., IL-10, TGF-β), indicating a dysregulated immune state characterized by concurrent inflammatory activation and immune exhaustion8,62,63. The elevated expression of IL-10 and other anti-inflammatory cytokines in critically ill COVID-19 patients may reflect a compensatory immune regulatory response aimed at limiting inflammation-induced tissue damage under persistent immune activation. However, this response often fails to effectively suppress ongoing antigen stimulation and inflammatory signaling. As demonstrated by Aichele et al.64 in models of primary hemophagocytic lymphohistiocytosis and chronic viral hepatitis, impaired CD8⁺ T cell function leads to antigen persistence, which drives chronic inflammation and immunopathological responses. In this context, increased anti-inflammatory cytokines may not only represent a feedback mechanism against excessive inflammation but also contribute to weakened antigen clearance, promoting immune escape and exacerbating tissue injury65,66. These findings suggest that immunological disruption in severe COVID-19 is not solely due to hyperactivation, but rather arises from a complex imbalance between impaired immune clearance and compensatory immunosuppression driven by sustained antigen exposure. Therefore, S protein–induced ferroptosis may be essential to the immunopathogenesis of COVID-19 and represents a strategic therapeutic focus for modulating immune imbalance and reducing inflammatory tissue injury.
TIPE2, a TNFAIP8 family protein, has emerged as a central modulator of immune balance and inflammation-associated signaling, highlighting its immunoregulatory importance67,68. In Pseudomonas aeruginosa–induced keratitis, Wang et al.32 demonstrated that TIPE2 protects corneal tissue from inflammatory injury by inhibiting the (Transforming growth factor-β-activated kinase 1) TAK1/NF-κB pathway, thereby reducing the expression of IL-6, IL-1β, and chemokines, as well as limiting neutrophil infiltration. In parallel, TIPE2 has been shown to restrain M1 macrophage activation and promote M2 polarization, contributing to innate immune homeostasis69. In a model of LPS-induced acute lung injury, TIPE2 ameliorates inflammation by simultaneously suppressing c-Jun N-terminal kinase (JNK) and NF-κB signaling pathways, triggering decreased pulmonary cell apoptosis and reduced neutrophil accumulation27. Moreover, TIPE2 expression is consistently downregulated in viral infections such as hepatitis B and C, suggesting its broader involvement in antiviral immune regulation70,71. Building on this, we further found that TIPE2 expression exhibited a significant decline in peripheral blood mononuclear cells (PBMCs) from patients with criticall ill COVID-19. Notably, TIPE2 levels positively correlated with GPx4, a key enzyme counteracting ferroptosis, and inversely correlated with TGF-β and ferritin levels. These findings indicate that TIPE2 may not only participate in virus-induced immune dysregulation but also act as a molecular link between ferroptosis and inflammation, providing new insights into its functional role in COVID-19 and other viral diseases.
Based on the above findings, we focused on the key regulatory role of TIPE2 in S protein-mediated ferroptosis and immune activation. Experimental results showed that TIPE2 overexpression significantly alleviated the ferroptotic phenotype induced by the S protein, reduced inflammatory responses, and maintained the balance of M1/M2 macrophage polarization. Mechanistically, it is speculated that TIPE2 may sustain glutathione metabolic homeostasis to support the function and expression of GPx4, thereby inhibiting the ferroptosis process. In addition, its upregulation of FSP1 may enhance the reductive capacity of coenzyme Q10, synergistically suppressing the lipid peroxidation chain reaction72. However, direct evidence is still lacking to determine whether TIPE2 is involved in the transcriptional regulation of GPx4 or whether it exerts its effects through the glutathione metabolic pathway. The potential involvement of pathways such as NRF2 and NF-κB also requires further investigation35,73. Notably, under conditions of TIPE2 overexpression, we observed that it effectively inhibited M1 polarization and pro-inflammatory cytokine expression, thereby attenuating the inflammatory response. Meanwhile, as the inflammatory environment was alleviated, M2 macrophages no longer maintained compensatory overexpression of anti-inflammatory factors, and the levels of IL-10 and TGF-β subsequently declined. This change does not indicate a weakening of the anti-inflammatory response, but rather may reflect the gradual restoration of immune homeostasis. These findings suggest that TIPE2 may exert a bidirectional regulatory function in modulating macrophage activity by coordinating both pro-inflammatory and anti-inflammatory pathways, playing a critical role in suppressing excessive inflammation and maintaining immune balance. This regulatory capacity is particularly important in the context of immune exhaustion associated with severe COVID-1974, where TIPE2 may help terminate the positive feedback loop of inflammation and prevent the progression of immune dysregulation.
It is important to acknowledge several limitations. First, this single-center retrospective study with a limited sample size may constrain statistical power and generalizability. Despite efforts to minimize bias, results should be interpreted cautiously. The restricted study period may also limit applicability across different viral variants or healthcare contexts. Future multicenter, prospective studies with larger cohorts and longer follow-up are warranted to validate and extend these findings. Second, due to clinical and ethical constraints, bronchoalveolar lavage fluid (BALF) samples could not be obtained from COVID-19 patients. Consequently, our conclusions regarding macrophage ferroptosis and polarization imbalance are primarily based on peripheral blood analyses and in vitro experiments, which may not fully reflect the pulmonary microenvironment where key immunopathological processes occur. Third, the in vitro model has inherent limitations. THP-1 cells were used for standardization, but differ from primary or tissue-resident macrophages, potentially affecting physiological relevance. The use of recombinant SARS-CoV-2 spike protein allows targeted investigation but does not fully mimic viral replication, RNA sensing, or multi-protein host interactions. Future studies will incorporate additional viral components and classical stimuli to better assess the specificity and broader immunopathology of SARS-CoV-2.
Conclusion
Overall, our study revealed that critically ill COVID-19 patients exhibited significant immune dysregulation, characterized by altered macrophage profiles, elevated inflammatory cytokines, and disrupted iron metabolism. Mechanistic experiments results indicated that SARS-CoV-2 spike protein induced macrophage ferroptosis and polarization imbalance, while TIPE2 restoration alleviateed ferroptosis and mitigates inflammation. These results offer novel perspectives on the immunopathogenesis of severe COVID-19 and imply that TIPE2 could be a potential therapeutic candidate.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Coronavirus 2
- COVID-19:
-
Coronavirus Disease 2019
- TIPE2:
-
Tumor Necrosis Factor-α-Induced Protein 8-like Protein 2
- GPx4:
-
Glutathione Peroxidase 4
- PBMCs:
-
Peripheral blood mononuclear cells
- ACE2:
-
Angiotensin-converting enzyme 2
- TNF-α:
-
Tumor necrosis factor-alpha
- PCD:
-
Programmed cell death
- GSDMD:
-
Gasdermin D
- TNFAIP8:
-
Tumor Necrosis Factor-α-Induced Protein 8
- Fads2:
-
Fatty Acid Desaturase 2
- CXR:
-
Chest x-ray
- FDR:
-
False discovery rate
- MDA:
-
Malondialdehyde
- GSH /GSSG:
-
Reduced glutathione/ oxidized glutathione
- Co Q10:
-
Coenzyme Q10
- NLR:
-
Neutrophil-to-lymphocyte ratio
- FSP1:
-
Ferroptosis suppressor protein 1
- SLC7A11:
-
Solute carrier family 7 member 11
- ACSL4:
-
Acyl-CoA synthetase long chain family menmber 4
- Fer-1:
-
Ferrostatin-1
- CCL11:
-
C-C Motif Chemokine Ligand 11
- ICU:
-
Intensive Care Unit
- TMPRSS2:
-
Transmembrane protease, serine 2
- LPS:
-
Lipopolysaccharide
- JNK:
-
c-Jun N-terminal kinase
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- TLR:
-
Toll-like receptor
- MyD88:
-
Myeloid differentiation factor 88
- IRAK:
-
IL-1R-associated kinase
- TAK1:
-
Transforming growth factor-β-activated kinase 1
- BALF:
-
Bronchoalveolar lavage fluid
References
Yang, H. & Rao, Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 19, 685–700 (2021).
Walls, A. C. et al. Structure, function, and antigenicity of the SARS-CoV-2 Spike glycoprotein. Cell 181, 281–292e6 (2020).
Chiok, K., Hutchison, K., Miller, L. G., Bose, S. & Miura, T. A. Proinflammatory responses in SARS-CoV-2 and soluble Spike glycoprotein S1 subunit activated human macrophages. Viruses 15, 754 (2023).
Pfortmueller, C. A., Spinetti, T., Urman, R. D., Luedi, M. M. & Schefold, J. C. COVID-19-associated acute respiratory distress syndrome (CARDS): current knowledge on pathophysiology and ICU treatment—a narrative review. Best Pract. Res. Clin. Anaesthesiol. 35, 351–368 (2021).
Sun, C. et al. Regulated necrosis in COVID-19: a double-edged sword. Front. Immunol. 13, 917141 (2022).
Liao, M. et al. Single-cell landscape of Bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 26, 842–844 (2020).
Del Valle, D. M. et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 26, 1636–1643 (2020).
Han, H. et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infections. 9, 1123–1130 (2020).
Ghaffarpour, S. et al. Cytokine profiles dynamics in COVID-19 patients: a longitudinal analysis of disease severity and outcomes. Sci. Rep. 15, 14209 (2025).
Ai, Y., Meng, Y., Yan, B., Zhou, Q. & Wang, X. The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol. Cell. 84, 170–179 (2024).
Lee, E., Song, C. H., Bae, S. J., Ha, K. T. & Karki, R. Regulated cell death pathways and their roles in homeostasis, infection, inflammation, and tumorigenesis. Exp. Mol. Med. 55, 1632–1643 (2023).
Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421 (2022).
Ma, T. et al. GPx4-independent ferroptosis—a new strategy in disease’s therapy. Cell. Death Discov. 8, 434 (2022).
Sahoo, G., Samal, D., Khandayataray, P. & Murthy, M. K. A review on caspases: key regulators of biological activities and apoptosis. Mol. Neurobiol. 60, 5805–5837 (2023).
Vitale, I. et al. Apoptotic cell death in disease—current understanding of the NCCD 2023. Cell. Death Differ. 30, 1097–1154 (2023).
Broz, P. Pyroptosis: molecular mechanisms and roles in disease. Cell. Res. 35, 334–344 (2025).
Bauer, M. E. & Fuente, M. D. L. The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mechanisms of Ageing and Development 158, 27–37 (2016).
Dev, S. & Babitt, J. L. Overview of iron metabolism in health and disease. Hemodialysis Int. 21, S6–S20 (2017).
Habib, H. M., Ibrahim, S., Zaim, A. & Ibrahim, W. H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother. 136, 111228 (2021).
Huang, R., Wu, J., Ma, Y. & Kang, K. Molecular mechanisms of ferroptosis and its role in viral pathogenesis. Viruses 15, 2373 (2023).
Kopf, M., Schneider, C. & Nobs, S. P. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 16, 36–44 (2015).
Yang, Y. et al. Interaction between macrophages and ferroptosis. Cell. Death Dis. 13, 355 (2022).
Chakurkar, V. et al. Increased serum catalytic iron may mediate tissue injury and death in patients with COVID-19. Sci. Rep. 11, 19618 (2021).
Peleman, C. et al. Ferroptosis and pyroptosis signatures in critical COVID-19 patients. Cell. Death Differ. 30, 2066–2077 (2023).
Sun, H. et al. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 133, 415–426 (2008).
Goldsmith, J. R. Regulation of inflammation and tumorigenesis by the TIPE family of phospholipid transfer proteins. Mol. Immunol. 14, 482–487 (2017).
Wu, X. et al. TIPE2 ameliorates lipopolysaccharide-induced apoptosis and inflammation in acute lung injury. Inflamm. Res. 68, 981–992 (2019).
Liu, R. et al. Negative immune regulator TIPE2 promotes M2 macrophage differentiation through the activation of PI3K-AKT signaling pathway. PLoS ONE. 12, e0170666 (2017).
Jiang, Y. et al. TIPE2 governs macrophage polarization via negative regulation of mTORC1. Mol. Med. Rep. 17, 952–960 (2018).
Shi, B. et al. TIPE2 May target the Nrf2/HO-1 pathway to inhibit M1 Macrophage–Related neutrophilic inflammation in asthma. Front. Immunol. 13, 883885 (2022).
Xi, W. et al. Roles of TIPE2 in hepatitis B virus-induced hepatic inflammation in humans and mice. Mol. Immunol. 48, 1203–1208 (2011).
Wang, Q. et al. TIPE2 suppresses Pseudomonas aeruginosa keratitis by inhibiting NF-κB signaling and the infiltration of inflammatory cells. J. Infect. Dis. 220, 1008–1018 (2019).
Du, Y. et al. TIPE2 regulates periodontal inflammation by inhibiting NF-κB p65 phosphorylation. J. Appl. Oral Sci. 31, e20230162 (2023).
Li, T. et al. Genome-wide analysis reveals TNFAIP8L2 as an immune checkpoint regulator of inflammation and metabolism. Mol. Immunol. 99, 154–162 (2018).
Li, S. et al. RSL3 Drives Ferroptosis through NF- κ B Pathway Activation and GPx4 Depletion in Glioblastoma. Oxidative Medicine and Cellular Longevity 2915019 (2021). (2021).
Chen, Y., Jiang, Z. & Li, X. New insights into crosstalk between Nrf2 pathway and ferroptosis in lung disease. Cell. Death Dis. 15, 841 (2024).
Released by National Health Commission of People’s Republic of China & National Administration of Traditional Chinese Medicine on January 5. Diagnosis and treatment protocol for COVID-19 patients (Tentative 10th Version). Health Care Science 2, 10–24 (2023).
Pan, Y. et al. Characterisation of SARS-CoV-2 variants in Beijing during 2022: an epidemiological and phylogenetic analysis. Lancet 401, 664–672 (2023).
Monaco, C. G. et al. Chest x-ray severity score in COVID-19 patients on emergency department admission: a two-centre study. Eur. Radiol. Exp. 4, 68 (2020).
George, S. et al. Evidence for SARS-CoV-2 Spike protein in the urine of COVID-19 patients. Kidney360 2, 924–936 (2021).
Biering, S. B. et al. SARS-CoV-2 Spike triggers barrier dysfunction and vascular leak via integrins and TGF-β signaling. Nat. Commun. 13, 7630 (2022).
Barhoumi, T. et al. SARS-CoV-2 coronavirus Spike Protein-Induced apoptosis, inflammatory, and oxidative stress responses in THP-1-Like-Macrophages: potential role of Angiotensin-Converting enzyme inhibitor (Perindopril). Front. Immunol. 12, 728896 (2021).
Miotto, G. et al. Insight into the mechanism of ferroptosis Inhibition by ferrostatin-1. Redox Biol. 28, 101328 (2020).
Chatterjee, S., Bhattacharya, M., Nag, S., Dhama, K. & Chakraborty, C. A. Detailed overview of SARS-CoV-2 omicron: its Sub-Variants, mutations and pathophysiology, clinical characteristics, immunological landscape, immune escape, and therapies. Viruses 15, 167 (2023).
Rosenberg, H. F., Foster, P. S. & Eosinophils COVID-19: diagnosis, prognosis, and vaccination strategies. Semin Immunopathol. 43, 383–392 (2021).
Xie, G. et al. The role of peripheral blood eosinophil counts in COVID-19 patients. Allergy 76, 471–482 (2021).
Lavandoski, P. et al. Eotaxin-1/CCL11 promotes cellular senescence in human-derived fibroblasts through pro-oxidant and pro-inflammatory pathways. Front. Immunol. 14, 1243537 (2023).
Kolbinger, A. et al. Eosinophil-derived IL-4 is necessary to establish the inflammatory structure in innate inflammation. EMBO Mol. Med. 15, e16796 (2023).
Schuster, S., Ewald, J. & Kaleta, C. Modeling the energy metabolism in immune cells. Curr. Opin. Biotechnol. 68, 282–291 (2021).
Loh, W. & Vermeren, S. Anti-Inflammatory neutrophil functions in the resolution of inflammation and tissue repair. Cells 11, 4076 (2022).
Moreira, A. C., Mesquita, G. & Gomes, M. S. Ferritin: an inflammatory player keeping iron at the core of Pathogen-Host interactions. Microorganisms 8, 589 (2020).
Kotla, N. K., Dutta, P., Parimi, S. & Das, N. K. The role of ferritin in health and disease: recent advances and understandings. Metabolites 12, 609 (2022).
Ru, Q. et al. Iron homeostasis and ferroptosis in human diseases: mechanisms and therapeutic prospects. Sig Transduct. Target. Ther. 9, 271 (2024).
Yang, M. & Lai, C. L. SARS-CoV-2 infection: can ferroptosis be a potential treatment target for multiple organ involvement? Cell. Death Discov. 6, 130 (2020).
Bozkurt, F. T. et al. Can ferritin levels predict the severity of illness in patients with COVID-19? Cureus 13, e12832 (2021).
Zhao, K. et al. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: A retrospective study. Open. Forum Infect. Dis. 7, ofaa250 (2020).
Thunders, M. & Delahunt, B. Gene of the month: TMPRSS2 (transmembrane Serine protease 2). J. Clin. Pathol. 73, 773–776 (2020).
Zhang, Y., Yan, R. & Zhou, Q. ACE2, B0AT1, and SARS-CoV-2 Spike protein: structural and functional implications. Curr. Opin. Struct. Biol. 74, 102388 (2022).
Palestra, F. et al. SARS-CoV-2 Spike protein activates human lung macrophages. Int. J. Mol. Sci. 24, 3036 (2023).
Khan, S. et al. SARS-CoV-2 Spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. eLife 10, e68563 (2021).
Pantazi, I. et al. SARS-CoV-2/ACE2 interaction suppresses IRAK-M expression and promotes pro-inflammatory cytokine production in macrophages. Front. Immunol. 12, 683800 (2021).
Mazzoni, A., Salvati, L., Maggi, L., Annunziato, F. & Cosmi, L. Hallmarks of immune response in COVID-19: exploring dysregulation and exhaustion. Semin. Immunol. 55, 101508 (2021).
Li, M. et al. Elevated exhaustion levels of NK and CD8 + T cells as indicators for progression and prognosis of COVID-19 disease. Front. Immunol. 11, 580237 (2020).
Aichele, P. et al. Immunopathology caused by impaired CD8 + T-cell responses. Eur. J. Immunol. 52, 1390–1395 (2022).
Tinoco, R., Alcalde, V., Yang, Y., Sauer, K. & Zuniga, E. I. Cell-Intrinsic transforming growth factor-β signaling mediates virus-specific CD8 + T cell deletion and viral persistence in vivo. Immunity 31, 145–157 (2009).
Smith, L. K. et al. Interleukin-10 directly inhibits CD8 + T cell function by enhancing N-Glycan branching to decrease antigen sensitivity. Immunity 48, 299–312e5 (2018).
Laliberté, B. et al. TNFAIP8: a new effector for Galpha(i) coupling to reduce cell death and induce cell transformation. J. Cell. Physiol. 225, 865–874 (2010).
Gao, J., Zhang, H. & Zhang, F. Research progress of TIPE2 in immune-related diseases. Int. Immunopharmacol. 121, 110514 (2023).
Li, F., Zhu, X., Yang, Y., Huang, L. & Xu, J. TIPE2 alleviates systemic lupus erythematosus through regulating macrophage polarization. Cell. Physiol. Biochem. 38, 330–339 (2016).
Fan, Y. C., Wang, N., Sun, Y. Y., Xiao, X. Y. & Wang, K. TIPE2 mRNA level in PBMCs serves as a novel biomarker for predicting short-term mortality of acute-on-chronic hepatitis B liver failure: a prospective single-center study. Medicine 94, e1638 (2015).
Kong, L. et al. Downregulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with chronic hepatitis C. Hepatol. Int. 7, 844–849 (2013).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPx4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Koppula, P. et al. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat. Commun. 13, 2206 (2022).
Alahdal, M. & Elkord, E. Exhaustion and over-activation of immune cells in COVID-19: challenges and therapeutic opportunities. Clin. Immunol. 245, 109177 (2022).
Funding
This work was supported by the Science and Technology Project for Social Development of Jilin Province (20240304201SF) and Natural Science Foudation of Jilin Province (YDZJ202501ZYTS686).
Author information
Authors and Affiliations
Contributions
SZ: Research concept and Writing the article. WL: Data collection and analysis. YH: Data check. LZ: Research concept and design. PG: Research concept and design, Final approval of article. All authors reviewed and endorsed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Statement.
The research involving human subjects was reviewed and approved by the Ethics Committee of the Second Hospital of Jilin University (approval no.: 2023(005)). All participants provided written informed consent prior to their enrollment in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, S., Li, W., Hao, Y. et al. TIPE2 suppresses ferroptosis and pro-inflammatory polarization in macrophages triggered by SARS-CoV-2 spike protein. Sci Rep 15, 30246 (2025). https://doi.org/10.1038/s41598-025-14235-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14235-1