Abstract
Recurrent respiratory papillomatosis (RRP) is a pathology characterized by the presence of neoplasm in the epithelium of the airways, where the larynx is the main affected organ. The cause of this disease is the low-risk Human Papillomavirus (HPV), where subtypes 6 and 11 are the most frequent. The only proven effective treatment is surgical resection of the lesions, and although there are adjunctive treatments to try to reduce the recurrence of neoplasms, it has not been proven that there is an effective alternative that achieves this effect. A proposal as adjuvant therapy is the HPV vaccine; although used in adults to treat the disease, the effect it has on immunological changes in a pediatric population with PRR has not been studied, so the objective of this work is to assess the efficacy of vaccination as an adjuvant treatment, by observing the immunological changes that are generated after vaccination, measuring the antibody titer as well as the cytokine profile present in the patients and relating it to the impact at the clinical in pediatric population. For this, blood samples and biopsies obtained by laryngeal surgery from 10 patients before and 6 months after completing the HPV vaccination scheme were compared. Viral load of the HPV6/11 serotypes was measured by qPCR, observing a decrease in copy number of both subtypes after vaccination. The antibody titer in the serum of the same patients was measured by ELISA, observing an increase in the amount of IgG antibodies without finding modifications in the IgM antibodies against HPV L1 protein. In addition, HPV vaccination was associated with a decrease in the immunosuppressive cytokine IL-10 in papilloma tissue and a significant increase in the pro-inflammatory cytokine TNFα in serum, suggesting a shift towards a Th1-mediated immune response. All these changes had an impact on the number of surgeries that were performed to remove the neoplasms, where there was a decrease in these during a six-month follow-up after vaccination. In conclusion, the vaccination as adjuvant therapy increased the number of IgG antibodies against the virus. It increased the amount of pro-inflammatory cytokines at the systemic level, reducing the viral load and the number of recurrences of papillomas.
Similar content being viewed by others
Introduction
Human papillomavirus (HPV) is primarily transmitted through sexual contact, although it can also be spread through skin-to-skin contact or perinatal vertical transmission of HPV from infected mothers1,2,3,4. HPV is associated with several conditions, such as cervical and anal cancer, genital warts, and laryngeal or respiratory papillomatosis. There are over 200 types of HPV, each associated with different diseases. HPV types 6 and 11 account for over 90% of cases of recurrent respiratory papillomatosis (RRP), a rare condition with an estimated incidence of 1–2 cases per 100,000 people per year, where the virus infects the epithelial cells lining the larynx, leading to the formation of benign neoplasms, or papillomas, that can block the airways5,6,7.
Children and young adults are most affected by RRP, with the peak incidence occurring between the ages of 2 and 5 years8, and cases whose onset occurred before 12 years old tend to be more aggressive and recurring6,9. It is believed that this is due to the immaturity of the immune system in young children, making them more susceptible to HPV infection. HPV infection can cause the growth of papillomas in the epithelium of the air passages as the larynx, trachea, vocal cords, and bronchi10,11. RRP symptoms vary from mild to severe depending on the number, size, and location of the papillomas12. They include dysphonia, stridor, difficulty breathing, chronic cough, recurrent pneumonia, dysphagia, acute respiratory distress, and frequent respiratory infections13,14. A more aggressive disease requires tracheostomy and an increase in the annual and total number of surgical operations required12,15.
Elective RRP treatment is the surgical removal of the papillomas by CO2 laser surgery, microdebrider excision, or electrocautery; however, recurrences are common, and multiple surgeries might be needed over time16,17,18,19. There is no definitive curative treatment, and current approaches are limited to treating the symptoms and increasing patients’ quality of life15,16. In Mexico, the quality of life in children with RRP is lower than in children with acute otitis media, unilateral vocal cord paralysis, or vocal cord nodules20,21,22. Several adjuvant therapies have also been explored, including antivirals23, immune response modifiers24,25, and HPV vaccines26,27,28,29,30.
The immune response to HPV infection is complex and involves both innate and adaptive immune mechanisms. The innate immune system triggers the production of cytokines and chemokines. The adaptive immune system is mediated by neutralizing antibodies and T cells. In people with weakened immune systems, the virus can persist. Women with genital HPV infection have delayed humoral responses, which leads to almost no antibody responses. However, when vaccinated, respond robustly and produce high antibody titers against HPV31. HPV vaccination is a safe and effective way to prevent HPV infection, and HPV vaccination as a treatment is a relatively recent approach. Studies in adults using a quadrivalent HPV (types 6/11/16/18) vaccine show a decrease in the number and frequency of surgeries post-vaccination, with up to 85% no papilloma recurrence during a one-year follow-up period26,27,28,29,30,32. Recent studies in children have shown similar results33,34.
Thus, ample evidence supports the effectiveness of HPV vaccination as adjuvant therapy in RRP. However, the effect it has on the immune status in children has not been investigated. Here we explored the effects of HPV vaccination on the humoral immune response in children with RPP in a tertiary hospital.
Results
Recurrent respiratory papillomatosis (RRP) caused by human papillomavirus (HPV) types 6 and 11 significantly impacts the quality of life of affected children, often necessitating multiple surgical interventions annually to maintain airway patency. This study investigated the influence of HPV vaccination on the overall immune response and health outcomes in a pediatric cohort diagnosed with RRP. The average age was 3.4y equally affecting males and females (Table 1).
Vaccination differentiates immune profiles
Following a 3-dose HPV vaccination schedule (Fig. 1a), significant positive changes were observed in the pediatric cohort. Dimensionality reduction techniques were performed on all variables collected. t-distributed Stochastic Neighbor Embedding (tSNE; Fig. 1b) and Uniform Manifold Approximation and Projection (UMAP; Fig. 1c) revealed a distinct clustering pattern, clearly separating vaccinated children (vax) from their unvaccinated counterparts (unvax). This segregation suggests a modification of the immune landscape induced by the vaccination.
Study Design and High-Dimensional Analysis of Patient Samples Following HPV Vaccination. (a) Schematic of the study design. Patients with respiratory papillomatosis received the quadrivalent Gardasil vaccine at 0, 4, and 12 weeks. Biological samples were collected during a 6-month follow-up period after the final dose. (b, c) High-dimensional analysis of patient samples comparing fully vaccinated (Vax) and unvaccinated (Unvax) cohorts. (b) t-Distributed Stochastic Neighbor Embedding (t-SNE) plot and (c) Uniform Manifold Approximation and Projection (UMAP) plot, visualizing a clear separation between the patient groups based on their global clinical profiles.
Vaccination correlates with reduced surgical interventions and viral load
Six months following the administration of the final vaccine dose, a significant decrease in the frequency of required surgical procedures was observed (Fig. 2a). This positive development was found to be correlated with a reduction in the extent of affected areas (Fig. 2b) and a prolongation of the intervals between necessary surgical interventions (Fig. 2c). These findings suggest a potential association between vaccination and a diminished need for surgical treatment (Fig. 2d). Interestingly, a single dose of HPV vaccine was insufficient to induce an improvement in the surgery rate and highlights the importance of completing a full vaccination schedule to achieve a therapeutic effect in respiratory papillomatosis.
HPV vaccination is associated with improved clinical outcomes. Comparison of clinical parameters between unvaccinated (Unvax), single-dose (Vax-1), and fully vaccinated (Vax, 3 doses) patient groups. Data are presented for (a) the number of distinct anatomical zones affected by papilloma, (b) the mean number of surgeries required per year, (c) the mean time elapsed between surgical interventions, and (d) the overall surgery rate. Data are presented as mean ± SEM. Statistical significance was determined by unpaired t-test. *p < 0.05, ***p < 0.001.
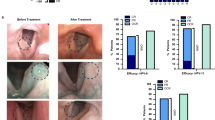
Further corroborating this link, a substantial increase in HPV-specific IgG antibodies in serum (Fig. 3a) coincided with a marked reduction in HPV 6/11 viral load in papilloma tissue (Fig. 3b, c). A strong negative correlation between antibody production and viral load was also identified (r=-0.81, p < 0.001; Fig. 3d). No significant differences were detected in production of IgM antibody against HPV L1 protein (data not shown).
Vaccination induces HPV-specific IgG antibodies that negatively correlate with viral load. (a) Comparison of HPV-specific IgG antibody titers in fully vaccinated (Vax) versus unvaccinated (Unvax) patients. Viral load of (b) HPV6 and (c) HPV11 in lesional tissue from both patient cohorts. (d) Correlation analysis in vaccinated patients demonstrating a significant negative relationship between HPV-specific IgG titers and total HPV viral load (HPV6 + HPV11 copies/ng). The line represents the best linear fit. Pearson correlation coefficient (r) and p-value are indicated. Data in (a-c) are presented as mean ± SEM. Statistical significance was determined by unpaired t-test. *p < 0.05.
Vaccination modulates cytokine and chemokine production
To elucidate the underlying mechanisms of HPV 6/11 impact and the observed vaccine efficacy, we analyzed cytokine and chemokine production in papilloma tissues and blood samples from both unvaccinated and vaccinated children. Our findings indicate a significant shift in the cytokine profile post-vaccination (Fig. 4a). Unvaccinated individuals exhibited elevated levels of the immunosuppressive cytokine IL-10 in papilloma tissue (p = 0.0380; Fig. 4b). Conversely, vaccinated children displayed increased production of TNFα in serum (p = 0.0054; Fig. 4c) - cytokines associated with potent antiviral and Th1-mediated immune responses. These contrasting cytokine profiles highlight a shift towards a Th1-mediated immune response and the vaccine’s ability to stimulate a robust and targeted immune response against HPV infection.
Vaccination promotes a pro-inflammatory cytokine environment in papilloma tissue. (a) Heatmap illustrating relative concentrations of key inflammatory cytokines measured in lesional tissue (local) and serum (systemic) from vaccinated (Vax) and unvaccinated (Unvax) patients. Purple indicates higher concentration, and white indicates lower concentration. (b-c) Quantification of Interleukin-10 (IL-10) and Tumor Necrosis Factor-alpha (TNF-α) levels in (b) lesional tissue and (c) serum, comparing the vaccinated and unvaccinated groups. Data are presented as mean ± SEM. Statistical significance was determined by unpaired t-test. *p < 0.05.
Our study provides compelling evidence for the beneficial effects of HPV vaccination in children with RRP. The observed reduction in surgical interventions, coupled with decreased viral load and positive shifts in cytokine profiles, suggests a promising avenue for improving the quality of life for these children. Further research is crucial to definitively establish a causal relationship between HPV vaccination and the observed clinical improvements. Investigating the long-term durability of these benefits and exploring potential personalized vaccination strategies will be essential in optimizing treatment protocols for RRP. Additionally, a more comprehensive analysis of the complex interplay between HPV-specific immune responses and overall health will contribute significantly to our understanding and management of this debilitating disease.
Discussion
This study provides compelling evidence that HPV vaccination, when administered as adjuvant therapy, can significantly improve clinical outcomes in children diagnosed with RRP. The HPV vaccine is safe and effective in improving the overall health and quality of life of children with recurrent respiratory papillomatosis. The vaccine was found to be well-tolerated, with no serious adverse events reported. Six months after the last vaccine dose, there was a significant reduction in the number of surgeries required to keep the airway clear. This was consistent with clinical improvement, as measured by the surgery index and clinical score.
The observed findings strongly suggest that HPV vaccination holds significant potential in improving the quality of life for children suffering from RRP. Our data demonstrates a multifaceted positive impact of vaccination on these young patients. Reduced surgical interventions due to a notable decrease in the frequency of surgical procedures needed to maintain a clear airway were observed post-vaccination, highlighting a potential reduction in disease burden.
Children reported improvements in various health indicators following vaccination, coinciding with a decrease in HPV 6/11 viral load. This correlation suggests a direct link between viral activity and the negative health impacts of respiratory papillomatosis. The reduction in both the frequency of surgical interventions and HPV viral load underscores the potential of the vaccine to modify the disease course positively.
Further, vaccination enhanced immune responses and led to a shift in the cytokine and chemokine profile, with increased production of IFNγ, IL-2, and GM-CSF, indicating a more robust and effective immune response against HPV 6/11.
The observed increase in IgG antibodies against HPV L1 protein, alongside a shift toward a pro-inflammatory cytokine profile, suggests that the vaccine effectively enhances the immune system’s ability to target and control HPV infection in these children. No significant differences in IgM levels were observed, which is consistent with the 6-month post-vaccination timepoint. By this stage, the primary humoral immune response has matured, characterized by class-switch recombination from transient IgM to a durable, long-lived IgG response.
While the exact mechanisms underlying these beneficial effects need further investigation, the findings strongly support the continued exploration of HPV vaccination as a safe and effective management strategy for RRP in the pediatric population.
Statistically, a single dose of HPV vaccine was insufficient to induce an improvement in the surgery rate. However, it is noteworthy that a positive trend in patient outcomes was observed following the administration of the initial vaccine dose. This was partially responsible for our high patient dropout. Out of an initial cohort of 50 patients, 11 (22%) completed the entirety of the study. The remaining patients dropped from the study, citing overall improvement in their condition and limiting our data for single-dose efficacy analysis.
The high dropout rate suggests that while the study may have demonstrated efficacy for a subset of participants, a significant proportion with less severe symptoms experience sufficient improvement after just a vaccine dose to warrant discontinued participation. Further investigation is required to elucidate the factors contributing to the high effectiveness rate and to determine if this is a long-lasting protection.
This study aimed to assess the efficacy of a single dose of the HPV vaccine in improving the surgery rate for patients with RRP. Our primary endpoint analysis, conducted on the cohort that completed the full 6-month follow-up, did demonstrate a statistically significant reduction in the need for surgery. However, the most significant observation was a high rate of study attrition, paradoxically driven by patient-reported clinical improvement after the initial dose. This finding must be interpreted with extreme caution due to profound methodological limitations that may obscure the vaccine’s true therapeutic effect.
The primary limitation of this study is the severe attrition bias resulting from a 78% dropout rate. The fact that patients overwhelmingly cited “overall improvement in their condition” as the reason for discontinuing participation introduces a critical survivorship bias. The final cohort of 11 patients, upon which the primary efficacy analysis was based, likely represents a subpopulation of patients with more recalcitrant disease who did not experience sufficient benefit to exit the trial. Consequently, the vaccine’s overall effectiveness is insufficiency, and the high dropout rate due to perceived benefit could, in fact, be interpreted as a strong signal of therapeutic efficacy for a majority of the initial cohort.
With such a small sample (n = 11), and the unfeasibility a randomized controlled trial in our clinical setting, the study had limited statistical power and was underpowered to detect anything but an exceptionally large effect. Furthermore, the dropout and the observed patient-reported improvement, suggests that the chosen endpoint may not have been the most sensitive or patient-relevant measure for assessing single-dose efficacy, particularly in a short-term context.
The study’s external validity is further constrained by its single-center design. As a tertiary hospital, our institution likely treats a patient population with more severe or complex disease profiles than would be seen in a general community setting. This selection bias limits the generalizability of our findings to a broader patient population, who might exhibit different response rates. Without a parallel placebo or standard-of-care control group, it is also impossible to definitively attribute the reported improvements to the vaccine, as placebo effects, regression to the mean, or the impact of concurrent treatments cannot be ruled out.
Despite these limitations, this study provides a crucial, albeit unintentional, insight. The powerful signal of patient-perceived benefit after a single dose should be considered a primary finding in itself, warranting rigorous future investigation. Future research must be specifically designed to overcome the limitations encountered here. A prospective, multi-center, randomized controlled trial with a larger sample size and an appropriate control arm is necessary. Such a trial should employ co-primary endpoints that include both objective clinical markers (e.g., surgery rate, lesion size) and validated patient-reported outcome measures (PROMs) to capture a more complete picture of the vaccine’s effectiveness and to determine the longevity of this protection.
While this study failed to evaluate the effect of a single vaccine dose to reduce surgery rates, our results with a 3-dose vaccination schedule show a promising picture for the use of HPV vaccination as a management strategy for respiratory papillomatosis; by mitigating disease severity and improving overall health, vaccination could drastically improve the quality of life for affected children and in managing RRP. Further research is crucial to elucidate the precise mechanisms by which HPV vaccination modulates the immune response and leads to the observed clinical improvements. Establishing a definitive causal relationship between HPV vaccination and the reduction in respiratory papillomatosis severity will allow to explore the long-term effects of vaccination on respiratory papillomatosis progression and recurrence. Investigating the specific immunological mechanisms by which vaccination mediates its beneficial effects will be crucial for optimizing treatment strategies and potentially developing novel therapeutic interventions for this challenging disease. Furthermore, investigating vaccine-induced memory T cell responses would provide deeper insight into the mechanisms underlying long-term protection and the potential for durable remission in RRP.
Future prospective studies should include larger cohorts and longer-term follow-up (+ 2 years) to assess the durability of the clinical and immunological effects of vaccination. Employing high-throughput transcriptomic or proteomic profiling of immune responses could help identify predictive biomarkers of vaccine efficacy, potentially allowing for the personalization of adjuvant therapy in RRP patients.
Materials and methods
Study design and participants
The study was approved by the Institutional Review Board of Mexico Children’s Hospital (protocol number HIM/2017/112/SSA/1435). Written informed consent was obtained from the parents or legal guardians of all participants, and assent was obtained from children old enough to understand the study. All patient data were anonymized prior to analysis.
Informed consent forms were provided and explained to parents or Legally Authorized Representatives (LAR) in the presence of a witness. Assent from minors were obtained from all participants to ensure the process was voluntary. Ascent forms were read and explained to the participants in a comprehensible and ethically sound form, in the presence of the parents or legal guardians in accordance with institutional and international guidelines. Ascent forms language and concepts were tailored to their developmental stage. Following informed consent acquisition from parents or legal guardians and assent from pediatric participants, a longitudinal, non-blinded study was conducted.
All procedures were approved by the Mexico Children’s Hospital Research Committee, Ethics Committee, and Biosafety Committee (approval HIM/2017/112/SSA/1435), and adhered to the established guidelines and standards set forth by the Mexico Children’s Hospital Research Committee and study method was followed in accordance with the principles outlined in the Declaration of Helsinki and its subsequent amendments.
Enrollment criteria
Patients aged 0 to 18 years diagnosed with severe recurrent respiratory papillomatosis (defined as undergoing more than three surgical interventions per year) who were under the clinical care of the Pediatric Otorhinolaryngology service at Mexico Children’s Hospital were included. Participants were monitored for a minimum of 6 months following the completion of their designated vaccination regimen.
Vaccination
Tetravalent Gardasil vaccine (HPV 6, 11, 16, 18 strains) was from MSD. Intramuscular doses of Gardasil vaccine were administered at 0, 4, and 12 weeks and each patient was followed up for 6 months after the last vaccination (Fig. 1a).
Surgery
Larynx papillomas were excised using a solid-state CO2 laser.
DNA isolation
Total DNA was isolated from a 3 mm papilloma biopsy using the DNA Mini Kit (Qiagen, Düsseldorf, Germany) and quantified using a NanoDrop 2000 (Thermo, Waltham, Massachusetts).
Viral load
L1 from HPV 6/11 was amplified from patient biopsies and cloned into the pBR322 vector. The plasmid was quantified, and the copy number was calculated using the formula:
This construction was used to generate a qPCR standard curve (103–109) to determine the sample’s viral load. qPCR was performed in a Bio-Rad thermal cycler using Qiagen SYBR green master mix.
Antibody production
Specific HPV IgM (Cusabio, Houston, Texas) and IgG (Creative Diagnostics, Shirley, NY) antibodies were quantified by ELISA. Briefly, 100l serum or culture supernatant were incubated 2 h at 37 °C on pre-coated plates with recombinant VLP’s derived from HPV Type 6, 11, 16, 18. Wells were washed 5 times with wash buffer and then incubated 1 h at 37 °C with an HRP-conjugated anti-human IgG antibody. After washing again a chromogen/substrate (TMB solution) was added and developed for 20 min. The plate was read at 450 nm and the concentration of specific antibodies in the samples was determined from a standard curve.
Cytokine production
Tissue and serum samples were determined by xMAP Technology using the Cytokine Human Ultrasensitive Magnetic 10-Plex Panel (Luminex, Austin, Texas). 50 μl serum or culture supernatant were incubated with 25 μl magnetic beads for 2 h at room temperature protected from light, following the manufacturer’s instructions and analyzed in a Luminex 200 System.
Clinical data collection
Each patient was given an anatomic and clinical description of the affected areas (supraglottic, glottis, subglottic, trachea). Epidemiological data, such as sex, type of delivery, age, age of symptoms onset, age at diagnosis, time between diagnosis and surgery, number of resections, frequency of surgery, degree of airway obstruction, maternal lesion, vaccine doses, time from diagnosis to vaccination, time from vaccination to surgery, pre-vaccination surgeries, post-vaccination surgeries, and number of annual surgical interventions were also recorded. Then, the surgeries per time period rate was calculated. To ensure participant confidentiality, all collected data was de-identified. Each participant was assigned a unique, randomly generated identification number (ID), and their names were not stored with the research data. The key linking participant names to their IDs was accessible only to the participant and their supervising physician. The Principal Investigator had access solely to the de-identified dataset for analysis.
Statistical analysis
For dimension reduction, data transformation on a raw dataset was performed to calculate the nearest neighbors and distances between them. The resulting matrix associates each data point to a fixed number of nearest neighbours and the distances between them.
For all our analyses, we performed t-SNE, UMAP, Pearson correlation, and Student´s unpaired t-test in R 4.3.2 and GraphPad Prism 10.3.0 (436).
Data availability
All data are available in the main text. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Puranen, M. H., Yliskoski, M. H., Saarikoski, S. V., Syrjänen, K. J. & Syrjänen, S. M. Exposure of an infant to cervical human papillomavirus infection of the mother is common. Am. J. Obstet. Gynecol. 176, 1039–1045 (1997).
Medeiros, L. R. et al. Vertical transmission of the human papillomavirus: a systematic quantitative review. Cad Saúde Pública. 21, 1006–1015 (2005).
Rombaldi, R. L., Serafini, E. P., Mandelli, J., Zimmermann, E. & Losquiavo, K. P. Perinatal transmission of human papilomavirus DNA. Virol. J. 6, 83 (2009).
Koskimaa, H. M. et al. Human papillomavirus genotypes present in the oral mucosa of newborns and their concordance with maternal cervical human papillomavirus genotypes. J. Pediatr. 160, 837–843 (2012).
Rimell, F. L. et al. Pediatric respiratory papillomatosis: prognostic role of viral typing and cofactors. Laryngoscope 107, 915–918 (1997).
Donne, A. J., Hampson, L., Homer, J. J. & Hampson, I. N. The role of HPV type in recurrent respiratory papillomatosis. Int. J. Pediatr. Otorhinolaryngol. 74, 7–14 (2010).
Sanchez, G. I. et al. Human papillomavirus genotype detection in recurrent respiratory papillomatosis (RRP) in Colombia. Head Neck. 35, 229–234 (2012).
Shah, K. V., STERN, W. F., SHAH, F. K., BISHAI, D. & Kashima, H. K. Risk factors for juvenile onset recurrent respiratory papillomatosis. Pediatr. Infect. Dis. J. 17, 372–376 (1998).
Gi, R. E. A. T. P. et al. Clinical course of recurrent respiratory papillomatosis: comparison between aggressiveness of human papillomavirus-6 and human papillomavirus-11. Head Neck. 37, 1625–1632 (2014).
Katsenos, S. & Becker, H. D. Recurrent respiratory papillomatosis: a rare chronic disease, difficult to treat, with potential to lung cancer transformation: apropos of two cases and a brief literature review. Case Rep. Oncol. 4, 162–171 (2011).
Marchiori, E. et al. Papilomatose laringotraqueobrônquica: aspectos Em Tomografia computadorizada de tórax. J. Bras. Pneumol. 34, 1084–1089 (2008).
Omland, T. et al. Risk factors for aggressive recurrent respiratory papillomatosis in adults and juveniles. PLoS ONE. 9, e113584 (2014).
Derkay, C. S. & Wiatrak, B. Recurrent respiratory papillomatosis: a review. Laryngoscope 118, 1236–1247 (2008).
Fortes, H. R. et al. Recurrent respiratory papillomatosis: a state-of-the-art review. Respir Med. 126, 116–121 (2017).
Wiatrak, B. J., Wiatrak, D. W., Broker, T. R. & Lewis, L. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope 114, 1–23 (2004).
Derkay, C. S. Task force on recurrent respiratory papillomas: a preliminary report. Arch. Otolaryngol. Head Neck Surg. 121, 1386–1391 (1995).
Pasquale, K., Wiatrak, B., Woolley, A. & Lewis, L. Microdebrider versus CO2 laser removal of recurrent respiratory papillomas: a prospective analysis. Laryngoscope 113, 139–143 (2003).
Ivancic, R., Iqbal, H., deSilva, B., Pan, Q. & Matrka, L. Current and future management of recurrent respiratory papillomatosis. Laryngoscope Investig. Otolaryngol. 3, 22–34 (2018).
Liu, S., Wang, J. & Shao, J. Safety of different surgical modalities for recurrent respiratory papillomatosis resection: a systematic review and meta-analysis. Clin. Otolaryngol. 48, 403–413 (2023).
Bishai, D., Kashima, H. & Shah, K. The cost of Juvenile-Onset recurrent respiratory papillomatosis. Arch. Otolaryngol. Head Neck Surg. 126, 935–939 (2000).
Chadha, N. K. et al. The quality of life and health utility burden of recurrent respiratory papillomatosis in children. Otolaryngol. - Head Neck Surg. 143, 685–690 (2010).
Montaño-Velázquez, B. B. et al. Jáuregui-Renaud, quality of life of young patients with recurrent respiratory papillomatosis. J. Laryngol x0026 Otol. 131, 425–428 (2017).
Shehab, N., Sweet, B. V. & Hogikyan, N. D. Cidofovir for the treatment of recurrent respiratory papillomatosis: a review of the literature. Pharmacother : J. Hum. Pharmacol. Drug Ther. 25, 977–989 (2005).
Maturo, S. & Hartnick, C. J. Use of 532-nm pulsed potassium Titanyl phosphate laser and adjuvant intralesional bevacizumab for aggressive respiratory papillomatosis in children: initial experience. Arch. Otolaryngol. Head Neck Surg. 136, 561–565 (2010).
Sidell, D. R., Nassar, M., Cotton, R. T., Zeitels, S. M. & de Alarcon, A. High-Dose sublesional bevacizumab (Avastin) for pediatric recurrent respiratory papillomatosis. Ann. Otol. Rhinol. Laryngol. 123, 214–221 (2014).
Chirilă, M. & Bolboacă, S. D. Clinical efficiency of quadrivalent HPV (types 6/11/16/18) vaccine in patients with recurrent respiratory papillomatosis. Eur. Arch. Oto-Rhino-Laryngol. 271, 1135–1142 (2013).
Mauz, P. S., Schäfer, F. A., Iftner, T. & Gonser, P. HPV vaccination as preventive approach for recurrent respiratory papillomatosis - a 22-year retrospective clinical analysis. BMC Infect. Dis. 18, 343 (2018).
Milner, T. D. et al. A retrospective case-control analysis of the efficacy of Gardasil® vaccination in 28 patients with recurrent respiratory papillomatosis of the larynx. Clin. Otolaryngol. 43, 962–965 (2018).
Smahelova, J. et al. Outcomes after human papillomavirus vaccination in patients with recurrent respiratory papillomatosis. JAMA Otolaryngol. Head Neck Surg. 148, 654–661 (2022).
Matsuzaki, H. et al. Human papillomavirus vaccination as an adjuvant therapy for recurrent respiratory papillomatosis: additional case series. J. Voice. https://doi.org/10.1016/j.jvoice.2021.07.019 (2021).
Buchinsky, F. J. et al. In RRP, serologic response to HPV is frequently absent and slow to develop. PLoS ONE. 15, e0230106 (2020).
Goodman, E., Reuschenbach, M., Kaminski, A. & Ronnebaum, S. Human papillomavirus vaccine impact and effectiveness in six High-Risk populations: a systematic literature review. Vaccines 10, 1543 (2022).
Mészner, Z., Jankovics, I., Nagy, A., Gerlinger, I. & Katona, G. Recurrent laryngeal papillomatosis with oesophageal involvement in a 2 year old boy: successful treatment with the quadrivalent human papillomatosis vaccine. Int. J. Pediatr. Otorhinolaryngol. 79, 262–266 (2015).
Hermann, J. S. et al. Effectiveness of the human papillomavirus (types 6, 11, 16, and 18) vaccine in the treatment of children with recurrent respiratory papillomatosis. Int. J. Pediatr. Otorhi. 83, 94–98 (2016).
Funding
Mexico Ministry of Health grant HIM/2017/112/SSA/1435 (OMC).
Author information
Authors and Affiliations
Contributions
Conceptualization: OMCFormal analysis: MAPI, EGD, DCVSInvestigation: MAPI, EGD, DCVS, CCB, EHC, DCEVisualization: MAPI, EGD, DCVS, CCB, EHC, DCESupervision: HAN, OMCWriting—original draft: DCVS, DCE, OMCFunding acquisition: OMCMAPI and EGD contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Prieto-Islas, M.A., Godoy-Dahbura, E., Villalpando-Sánchez, D.C. et al. HPV vaccination improves immune response in children with respiratory papillomatosis. Sci Rep 15, 29885 (2025). https://doi.org/10.1038/s41598-025-14787-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14787-2