Abstract
Awareness of the intestinal microflora involved in insulin resistance and type 2 diabetes (T2DM) has now become more evident. However, direct mechanical insight is required to illustrate the contribution of intestinal microflora in the disease progression. In this study, we aimed to precisely assess the changes in intestinal bacteria translocation (IBT) from gut to the pancreas using combinations of FISH, 16S rRNA amplicon sequencing, and deep learning-assisted methods to track IBT in diet-induced obese (DIO) and antibiotic-induced microbiota disruption (AIMD)-DIO mouse models. Our analysis showed deep learning-assisted quantification enhanced the accuracy and objectivity of bacterial tracking. The DIO mice exhibited increased IBT, likely due to excessive intestinal lipid accumulation and compromised intestinal barrier integrity. Elevated bacterial loads in the pancreas were associated with worsened pancreatic function, indicated by higher fasting blood glucose, impaired glucose tolerance, and dysfunctional insulin secretion. In contrast, the AIMD-DIO group lowered the IBT, maintained the islet structure and improved glucose homeostasis. Comparative study between DIO and AIMD-DIO models revealed a strong correlation between number of translocated bacteria and T2DM severity. These findings provide objective evidence of bacterial migration from the intestine to the pancreas and establishes its pathological relationship with pancreatic impairment and dysfunction and underscores to utilize AI techniques more successfully in the future for evaluating IBT.
Similar content being viewed by others
Introduction
Microorganisms inhabit the human body, playing a pivotal role in shaping our physiology and significantly contributing to both health and the onset of diseases1. Recently, the emerging role of gut microbiota in the pathogenesis of metabolic disorders has been getting more attention2. Zebrafish research exposed to a high-fat diet (HFD) has revealed that the enteroendocrine cells undergo silencing and exhibit morphological changes, whereas germ-free zebrafish show resistance to diet-induced alterations3. Comparative studies between germ-free and conventional mice, as well as fecal transplantation experiments, have provided evidence that intestinal bacteria are intricately involved in diseases related to energy metabolism4.
Type 2 diabetes mellitus (T2DM) is a global chronic epidemic, characterized by an absolute or relative deficiency in insulin secretion and decreased sensitivity of target organs to insulin5. While the underlying etiology and pathogenesis of T2DM are not yet completely understood, the disease is increasingly recognized as being associated with insulin secretion deficiencies or insulin resistance (IR). Furthermore, IR is often concurrently observed with obesity6. Studies have shown that HFD can elevate serum lipopolysaccharide levels 2–3 folds in both humans and mice7,8. Researchers have reported the translocation of intestinal bacteria to extra-intestinal organs such as the lungs, liver, and pancreas9,10,11,12,13,14. This translocation has been implicated in various conditions, including insulin resistance (IR), autoimmune diseases, and malignant tumor progression9,15,16. Nevertheless, further evidence is needed to elucidate the mechanisms by which intestinal bacteria participate in the pathological process.
We hypothesize that obesity may exacerbate intestinal bacterial translocation, possibly due to increased lipid accumulation in the intestinal lumen17 and reduce mucus production, which could disrupt the mucosal barrier and increase permeability18. Such disruptions might facilitate intestinal bacterial translocation to adjacent organs, particularly the pancreas, with increased invasion of microorganisms into the pancreas potentially precipitating the onset of T2DM. To ensure the reliability of our research, we have developed a novel method using deep learning for bacterial enumeration, marking the first application of machine learning in the quantification of intestinal bacterial translocation.
Results
To investigate the potential role of intestinal bacterial translocation in the progression of obesity-related T2DM, we applied AIMD technique in DIO mice model. Following 8 weeks of HFD feeding, mice in the DIO group showed a significant increase in body weight, consistent with previous reports of HFD-induced obesity. Interestingly, however, the AIMD-DIO group, despite high food intake, showed no significant difference in body mass when compared to control group (Fig. 1a, b). This unexpected result depicts the critical role of the gut microbiota in facilitating host energy harvest, particularly in the digestion of complex polysaccharides and absorption of monosaccharides from the intestinal lumen. Correspondingly, blood glucose and serum insulin levels were significantly elevated in the DIO group, whereas the AIMD-DIO group demonstrated much lower indexes, (Fig. 1c–f). These findings suggest the depletion of gut microbiota leads to reduced net energy extraction and absorption and highlights the contribution of the gut microbiome to energy homeostasis and its potential involvement in the pathogenesis of obesity-associated metabolic disorders.
Gut microbiota contributes to obesity-related T2DM. (a) Body weight; (b) Average daily food intake; (c) Fasting blood glucose (FBG); (d) Glucose tolerance test (GTT); (e) The area under the curve (AUC) of GTT; (f) Fasting serum insulin levels. Data points represent mean ± standard deviation from independent experiments, with n = 6 mice per group. Statistical significance is denoted as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by two-way Anova with Tukey’s multiple comparisons test.
To verify the translocation of gut bacteria to other organs preferentially pancreas, we conducted a comparative analysis of pancreatic morphology and function between DIO and AIMD-DIO mice. DIO pancreas shows pronounced fatty infiltration and islets morphological disruption whereas AIMD-DIO shows improved morphology and less fat accumulation suggesting protective effect of microbiota depletion (Fig. 2a). Also, the immunofluorescence staining of Insulin and Glucagon in DIO group shows distorted positive beta cell areas indicating beta cell dysfunction which is typically observed in insulin resistant models. In contrast, the AIMD-DIO group showed much improved beta cell insulin expression and morphology (Fig. 2a, b). Our findings provide insights into the role of intestinal bacterial translocation in altering endocrine cell function within the Langerhans islets, which are central to the development of IR and T2DM.
Pancreatic dysfunction in DIO mice is rescued by AIMD. (a) Paraffin sections of the pancreas were stained with hematoxylin and eosin (H&E) (Scale bar: 50 μm) and immunofluorescence (Scale bar: 50 μm). Green: insulin, Red: Glucagon. (b) The area of the islet within the pancreas; (c, d) Quantification of insulin and glucagon secretion in pancreatic β-cell and α-cell. Data points represent mean ± standard deviation from independent experiments, with n = 6 mice per group. Statistical significance is denoted as *P < 0.05, **P < 0.01, ***P < 0.001 by two-way Anova with Tukey’s multiple comparisons test.
We then utilized 16S rRNA amplicon sequencing to evaluate the impact of altered bacterial composition in the jejunum and pancreatic tissue. The jejunum microbiota responded markedly to the antibiotic cocktail, decreasing relative abundance of Firmicutes and Bacteriodetes which are dominant in the gut microbiome, concurrent with the emergence of Proteobacteria as the predominant phylum in AIMD-DIO group indicating antibiotic-induced dysbiosis. In contrast, the DIO group showed imbalance and increase in Firmicutes/Bacteriodetes ratio when compared with control group indicating a hallmark of diet induced obesity (Fig. 3a, c). In the pancreas where Proteobacteria were abundant in control, their abundance was highly reduced in DIO group. Interestingly, AIMD-DIO group partially rescued the Proteobacteria phylum with reduction in Firmicutes (Fig. 3b, d). Further analysis at the genus level revealed a considerable increase in Prevotella and Bacteroides in the pancreatic microbiota of DIO mice (Fig. 3d). Henceforth, despite antibiotic treatment, the pancreatic microbiota in DIO and AIMD-DIO mostly remained similar indicating that antibiotics had limited impact on the pancreatic bacteria. Thus, we propose that the bacterial composition is primarily influenced by dietary factors, followed by the effects of antibiotics.
DIO significantly shapes the pancreatic bacterial composition. Analysis of bacterial composition in the jejunum (a, c) and pancreas (b, d) using 16S rRNA amplicon sequencing, depicted through PCoA plots and hierarchical taxonomic representation at phylum (left panel) and genus (right panel) levels. n = 3 mice per group.
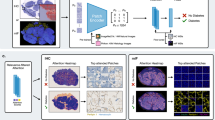
To ascertain whether DIO augments intestinal bacterial translocation, we administered fluorescently labeled bioengineered bacteria (DsRed-E. coli) in mice, thereby tracking bacterial translocation while minimizing the risk of external bacterial contamination. Following gastric administration of DsRed-E. coli in the DIO model mice, we detected translocated tracers in the pancreas of both Ctr and DIO mice (Fig. 4a). To ensure precise quantification, we trained a deep-learning model on DsRed-E. coli pancreatic tissue slides. This deep learning approach, based on fluorescent signals, was designed to enhance the quality of intestinal bacterial translocation analysis (Fig. 4b). In this study, we integrated a convolutional neural network (CNN), a standard tool for image recognition and classification, to bolster our analytical capabilities (Fig. 4c). The performance of our proposed U-Net model was rigorously evaluated using a 5-fold Cross-Validation, with results detailed in Table 1. The optimal outcomes were achieved after 200 epochs of each run, as determined by the performance on the validation set. We thoroughly assessed the model’s Dice similarity coefficient (DSC), precision, recall, and F1 scores, comparing our U-Net model with established methods such as U-Net-base, Deeplabv3, and Seg-Net, as presented in Table 2. Our U-Net model surpassed other techniques, offering enhanced objectivity, efficiency, and accuracy in bacterial enumeration compared to manual counting methods. The pancreas was segmented into the splenic lobe (SL), gastric lobe (GL), and duodenal lobe (DL) based on the dendritic distribution pattern of the pancreas along the mouse mesentery19. Utilizing deep learning-based counting, our results indicated a significant increase in tracers in the DL of DIO mice (Fig. 4d).
Analysis employing deep learning techniques reveals that DIO is associated with an increased translocation of fluorescently labeled bioengineered bacteria to the pancreas. (a) Visualization of DsRed-E. coli translocation in mice following gastric administration, as detected by fluorescence microscopy on DAPI-counterstained pancreatic cryosections (Scale bar: 20 μm); (b) Schematic overview of the deep learning protocol for bacterial enumeration, including data processing, training, and counting. Green circles highlight the presence of DsRed-E. coli; (c) Depiction of the U-Net architecture, where annotations above the hierarchy indicate the number of feature maps, and numbers to the left denote the size of feature maps; (d) Quantitative assessment of DsRed-E. coli in the pancreas utilizing deep learning. Data points represent mean ± standard deviation from independent experiments, with n = 6 mice per group. Statistical significance is denoted as ##P < 0.01 by Student’s t-test. ns: not significant.
Then we conducted a comparative analysis of the indigenous bacterial populations between DIO and AIMD mice utilizing a combination of fluorescence in situ hybridization (FISH) and deep learning-assisted counting methods, as shown in Fig. 5a. We observed a reduction in jejunal bacteria due to AIMD that corresponded with a decrease in the number of bacteria in the pancreas (Fig. 5b, c). This correlation suggests that bacteria in the pancreas originates from the gut. Significantly, revealed that DIO mice harbored a higher quantity of indigenous bacteria in the pancreas compared to Ctr mice probably due to gut barrier disruption as illustrated in Fig. 5c.
DIO enhances intestinal bacterial translocation to the pancreas as assessed by FISH and deep learning-assisted quantification. (a) Detection of indigenous bacteria within the jejunum and pancreas was achieved using eubacterial FISH probe labeling (Scale bar: 50 μm); (b, c) Quantitative analysis of indigenous bacterial abundance in the jejunum and pancreas, with visualization by FISH and enumeration facilitated by deep learning-assisted image analysis. Data points represent mean ± standard deviation from independent experiments, with n = 6 mice per group. Statistical significance is denoted as *P < 0.05, **P < 0.01, ***P < 0.001 by two-way Anova with Tukey’s multiple comparisons test.
Henceforth, to support the idea of DIO mice suffering from pronounced gut leakiness, we conducted further analyses to evaluate lipid accumulation within the jejunum. Our observations revealed that DIO mice exhibited higher lipid deposition on intestinal villi and mucosal surfaces and so as AIMD-DIO group indicating the lipid storage and absorption were still active irrespective of microbiota depletion (Fig. 6a–d). This concludes that dietary fat exposure alone is sufficient to drive intestinal lipid accumulation, independent of systemic weight gain or microbiota-derived metabolic contributions. Furthermore, we examined the integrity of the intestinal barrier by quantifying the mRNA levels of genes, including claudin 3, claudin 5, and mucin 2. Our findings revealed a significant increase in intestinal permeability among DIO mice (Fig. 6e–g). This downregulation was further substantiated at the protein level, with a reduction in claudin 3 expression specifically in the villus region of DIO mice (Fig. 6h). Consequently, we suppose that the accumulation of lipids within the intestinal lumen of DIO mice severely impairs the intestinal barrier and facilitates bacterial translocation from gut to distal organs such as pancreas. This impairment is associated with a significant enhancement in the translocation of intestinal bacteria to the pancreas, which may contribute to the observed alterations in endocrine cell function within the Langerhans islets of these mice.
Accumulation of lipids in the intestine significantly compromised mucosal permeability in DIO mice. (a, b) Lipid droplets in the intestinal villi stained with oil red O (ORO) (scale bar: 50 μm) and its quantification; (c, d) Lipid deposition ORO staining (scale bar: 1 mm) and quantification on the small intestinal mucosal surfaces; (e–g) Real-time quantitative reverse transcription PCR (RT-qPCR) analysis of jejunum mRNA expression of the claudin 3, claudin 5, and mucin 2 genes; (h) Secretion of claudin 3 in the jejunum by immunofluorescence staining (Scale bar: 10 μm). Blue: DAPI, Green: claudin 3. Data points represent mean ± standard deviation from independent experiments, with n = 6 mice per group. Statistical significance is denoted as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by two-way Anova with Tukey’s multiple comparisons test.
Discussion
The intestinal microbiota plays a pivotal role in the etiology and progression of T2DM20. However, the precise mechanisms by which the gut microbiota contribute to the onset and development of T2DM remains to be elucidated. Numerous reports have documented the translocation of gut microbiota to other distal organs and tissues, even in previously thought sterile positions, such as the uterus9,10,11,12,13,21,22,−23. However, translocation of intact bacteria into the pancreas, which is in close spatial proximity to the gut, has received less attention24. The anatomical positioning is well distinct in humans and mice. Pancreas in humans is a solitary organ whereas in mice, it is diffused in the mesentery of proximal duodenum. Therefore, mice pancreas is more pronounced to gut derived microbiota than in humans and this anatomical difference might be translational challenge in our findings. However, despite this, both species have well preserved exocrine and endocrine functions and both contain alpha, beta and delta cells responding to rise in blood glucose by secreting insulin. Therefore, studying and corelating small animals preferably mice remain translatable. Moreover, previous clinical studies by Li et al.25 and BJ Ammori et al.26 supported the idea of increased bacterial translocation and gut permeability in acute pancreatitis patients. In both species, the gut-pancreas axis plays a vital role in nutrient sensing and immune response. Furthermore, the disruption mechanism of intestinal barrier, translocation of bacteria and its impact on endocrinal functions of pancreas remains fundamentally elusive. Therefore, this study using DIO and AIMD-DIO mice proves to be a robust system for investigating microbe translocation from gut to pancreas offering insight to metabolic disease progression. Our assessment of pancreatic function in DIO mice revealed a pronounced dysfunction, characterized by significantly impaired glucose and insulin secretion. In contrast, the AIMD-DIO group displayed well-functioning islets with significantly stable glucose and insulin secretion. These findings underscore the significant impact of translocated bacteria on the endocrine function of the Langerhans islets in the pancreas. The bacteria translocated exhibited a clear diet-dependent pattern. The lipid accumulation in the small intestine aggravated the bacterial migration to the pancreas, connecting obesity and T2DM from a novel perspective.
16S rRNA amplicon sequencing results indicate the pancreatic bacterial composition aligns with diet-dependent patterns rather than AIMD-driven alterations. At the genus level, our analysis revealed a notable increase in Prevotella and Bacteroides within the pancreatic microbiota of DIO mice. This result aligns with research establishing a causal relationship between specific bacterial species and obesity, such as Prevotella sp. enhancing clinical and inflammatory traits27and the obesity-associated prevalence of Bacteroides sp. in Indian populations28. Notably, Prevotella copri and Bacteroides vulgatus have been implicated as key drivers in the exacerbation of IR in T2DM29. Furthermore, Cerf et al. highlighted the early islet cell dysfunction is more critical in T2DM development than early IR in pathogenesis30.
To display and count translocated bacteria, development of an objective quantification method for assessing intestinal bacterial translocation was deemed essential. We therefore employed a deep learning-assisted approach for bacterial enumeration, which represents, to our knowledge, a novel application within the domain of intestinal bacterial translocation research. Our deep learning method for bacterial counting outperforms manual methods by enhancing accuracy, efficiency, and objectivity. It scales well for high-throughput analysis, ensuring consistent and repeatable results. Our research has uncovered a significant increase in tracer bacteria within the duodenal lobe of the pancreas in DIO mice. This finding demonstrates that obesity significantly enhances intestinal bacterial translocation to the pancreas. Utilizing a combination of FISH and deep learning-assisted counting methods, we detected an increased indigenous bacterial population in the pancreas of DIO mice. This observation is consistent with our results using bioengineered bacteria, providing further validation that obesity enhances the translocation of intestinal bacteria to the pancreas. However, further investigations involving bacterial culture and viability assays will be crucial to determine the metabolically active bacterial colonization in the pancreas.
Meanwhile, we found that intestinal integrity disruption contributes to the systematic translocation of gut bacteria. We observed a pronounced accumulation of lipids on the intestinal mucosal surfaces and villi in DIO mice compared to the Ctr. This is in alignment with prior research that has documented increased mucosal triglyceride levels in DIO mice, linking this lipid accumulation to the downregulation of genes essential for lipolysis and fatty acid oxidation17,31. Moreover, a related study has established a correlation between increased visceral adiposity and elevated intestinal permeability in a sample of healthy women32. We found the tight junction and mucin related genes of the small intestine, one of the structures sustaining intestinal integrity, was disrupted due to the HFD in the present study which supports the ideal hallmarks of enhanced gut permeability. Dysbiosis, an imbalance in the gut microbiota, contributes to the breakdown of tight junctions, predominantly affecting the small intestines33. We have identified a decrease in mucin2 mRNA in the DIO model, suggesting reduced mucus could diminish the spatial separation between microorganisms and the endothelium, promoting bacterial translocation to adjacent organs such as the pancreas. Typically, gut microorganisms are compartmentalized to interact with the host, but dysbiosis can disrupt this segregation. Moreover, HFD-induced dysbiosis can trigger inflammation and eosinophil depletion, both of which are known to increase intestinal permeability34. These observations collectively suggest a correlation between intestinal permeability and lipid accumulation within the intestine. The association between bacterial translocation and increased intestinal permeability35,36implies a potential mechanism for bacterial translocation to the pancreas. Therefore, we addressed the mechanism of this pathogenesis is associated with the accumulation of lipids in the intestine of DIO mice, along with their deposition on the intestinal wall, leading to significant impairment to the intestinal barrier, and subsequently resulting in increased intestinal bacterial translocation.
The deep learning-assisted bacterial counting method utilized in this study demonstrates significant potential over traditional manual counting methods, offering marked improvements in both efficiency and accuracy. However, we recognize that the refinement of our method is an ongoing process, which will undoubtedly benefit from future advancements in artificial intelligence technology37,38,39. This iterative validation will be crucial for enhancing the precision of our approach. Moreover, our research has revealed intriguing correlations between fat accumulation, intestinal barrier damage, bacterial translocation, and the progression toward T2DM. While these findings are suggestive, we are cognizant of the complex and multifactorial molecular mechanisms, acknowledging that this area warrants further investigation.
In conclusion, the present study bridged a potential link between intestinal lipid accumulation and bacterial translocation, and this novel insight shed light on the potential pathogenesis of obesity-associated T2DM. It provides valuable concept for future studies exploring microbiota–host interactions and developing microbiome-targeted therapeutic strategies for metabolic disorders.
Methods
Animals
Seven-week-old male C57BL/6 N mice were purchased from Charles River Laboratories (Beijing) and housed in a specific pathogen-free environment. After one week of adaptation with free access to a normal chow diet (Ctr, Beijing KeAo XieLi Feed CO, China) and water, the mice were randomly assigned into four experimental groups: Ctr (n = 12), DIO (n = 12), AIMD-Ctr (n = 6), AIMD-DIO (n = 6). Body weight, fasting blood glucose (FBG), and glucose tolerance tests (GTT) were employed to evaluate the development of the DIO-T2DM model. For the AIMD mouse model, mice were administered a cocktail of antibiotics and antifungals (Aladdin company, China) in drinking water, as previously described40. The drinking water was mixed with ampicillin (1 mg/ml; A102048, Aladdin, China), vancomycin (0.5 mg/ml; V105495, Aladdin, China), neomycin (0.5 mg/ml; N109017, Aladdin, China), metronidazole (1 mg/ml; M109874, Aladdin, China), and amphotericin B (0.5 µg/ml; A105482, Aladdin, China). This mixture was freshly prepared daily and used within 24 h. AIMD treatment was initiated concurrently with the feeding and continued for 8 weeks. All mice were euthanized at 16 weeks of age, following a 5-hour fasting period. All protocols were approved and performed in accordance with Ethics Committee for Animal Research, Shenzhen Institutes of Advanced Technology, Chinese Academy of Science (SIAT-IRB-170401-YGS-RPG-A0312-01) and in compliance with institutional guidelines for the care and use of animals. We confirm that all methods were performed in accordance with the relevant guidelines and regulations and the study is reported in accordance with ARRIVE guidelines.
FBG, GTT, and fasting serum insulin measurements
As described in our previous study41blood glucose levels were measured using blood glucose strips and an Accu Check glucometer (Roche, Switzerland) from tail-tip blood obtained by tail snipping. FBG was tested after a 5-hour fasting period. GTT was conducted following a 16-hour overnight fasting period. Glucose (2 g/kg body weight; Sigma, USA) was administered intraperitoneally, and blood glucose levels were measured at 0-, 15-, 30-, 60-, 90-, and 120-minute post-glucose injection. The fasting serum insulin level was determined using the EZRMI-13 K insulin assay kit (Merck, Germany), following the manufacturer’s instructions.
16S rRNA amplicon sequencing
Bacterial amplicon sequencing was performed by TinyGene Bio-Tech Company (Shanghai, China) using an Illumina Miseq PE 300 platform (Illumina, USA). Concurrently, to rigorously assess and control potential contamination, blank controls were established and handled under identical conditions to the experimental samples. These controls were meticulously managed alongside the samples to ensure any background signals were accurately identified and subsequently subtracted from the dataset. Genomic DNA was extracted from the contents of the jejunum and pancreas using the QIAamp DNA Stool Mini Kit (QIAGEN, Germany) and purified with the AxyPrepDNA kit (Axygen, USA), following the manufacturer’s instructions. The V3-V4 region of the 16S rRNA gene was selected for amplification. DNA libraries for paired-end sequencing with single indices were constructed using the TruSeq DNA PCR Free Library Prep kit (Illumina, USA).
Raw data analysis was performed using the USEARCH pipeline40. Reads ranging from 400 to 480 base pairs were retained. Amplicon sequence variants (ASVs) were generated using the UNOISE3 algorithm. Non-bacterial ASVs were filtered out against the SILVA 99% database based on 97% identity using VSEARCH. Subsequently, ASVs were assigned to taxa using Naive Bayes classifiers pre-trained on SILVA_138 99% OTUs full-length sequences in QIIME2 (version 2021.2). The copy numbers of 16S rRNA genes were normalized using the default plugin in QIIME2.
Tracer bacteria Gavage and enumeration
Tracer bacteria DsRed-E. coli (1.0 × 109 colony-forming units in 200 µl saline, obtained from Dr. Jie Feng’s Laboratory) were administered via gavage to both control (Ctr) and DIO mice. Three hours of post-gavage, mice were euthanized using CO2, and the pancreases were harvested under sterile conditions. The pancreases were fixed, cryosectioned, and counterstained with DAPI (AR1177, Boster, China). Subsequently, the sections were examined under a fluorescence microscope (BX53, Olympus, Japan). In a single-blind manner, 40–50 fields of view were observed for each sample. Enumeration of the tracer bacteria was performed using deep learning-assisted counting.
Deep learning-assisted counting
Architecture:
The U-Net structure employed here was symmetrical and comprised three parts: (a) a subsampled feature extraction path, (b) an upsampling feature pooling path, and ca. linear pixel classifier. A sliding clipping method with a 50% overlap was utilized for the augmentation of the training data. Following network training, a model possessing optimal network parameters (weights and deviations) and a minimized loss function were identified. The objective function was defined as:
Where W* denotes the optimal network parameters obtained from the training procedure. The Dice loss \(\:\left(L\left({Y}_{n},\:f\left({X}_{n},\:W\right)\right)\right)\) was defined as the minimization of the overlap between the predicted label and ground truth:
We set ε = 1 in this experiment to ensure numerical stability and to prevent division by zero.
Counting:
Automatic counting was performed using the label function in the skimage. measure module. The number of bacteria present in the predicted image was determined by the number of connected objects identified in the labeled connected components.
Evaluation Metric:
In addition to utilizing Dice for evaluation, quantity-based Precision and Recall were also employed as metrics for quantitative evaluation. Given the challenge of achieving high values for both metrics simultaneously, we introduced the F - measure, which is the harmonic mean of Precision and Recall.
FISH
FISH was conducted on 5 μm paraffin sections of jejunal and pancreatic tissues. The sections were deparaffinized, rehydrated, and then treated with proteinase K in a dry oven at 37 °C for 30 min. Subsequently, they were washed in DEPC-treated 1×PBS for 1 min. The sections were hybridized with a FITC-labeled universal eubacterial probe EUB338 (FBPC-10, Creative Bioarray, USA). Hybridization was carried out overnight at an optimal temperature of 40 °C in a humidified chamber. Post-hybridization, the sections were washed and counterstained with DAPI. Imaging was performed using a confocal fluorescence microscope (Nikon, Japan) with appropriate filter sets, and the deep learning method described previously was utilized for analysis.
RT-qPCR
Total RNA from the jejunum was isolated through homogenization in TRIzol reagent (1559608, ThermoFisher, USA). Reverse transcription was performed using a Reverse Transcription PCR kit (RR036, Takara, Japan). RT-qPCR was conducted using a LightCycler (Roche, Switzerland) and a SYBR quantitative real-time PCR kit (RR820, Takara, Japan). The GAPDH gene served as an endogenous control.
Histological and immunohistological stainings
H&E staining
Pancreatic tissues were fixed overnight in 4% paraformaldehyde (AR1068, Boater, China) and embedded in paraffin. Section (5 μm) were adhered to positively charged glass slides (188105, Citotest, China). Tissue morphology was observed following H&E (C0105S, Beyotime, China) staining under a microscope (Olympus, Japan). The area of the islets of Langerhans was quantified using Image J software.
ORO staining
Jejunum tissues fixed in paraformaldehyde were embedded in O.C.T. (4583, SAKURA, USA) and cryosectioned (12 μm, Leica, Germany). Lipid droplets in the tissue were stained by ORO (O-0625, Sigma, USA) and quantified using Image J. To observe lipid accumulation on the small intestinal mucosal surfaces macroscopically, the jejunum from mice was dissected. Three stained sections per group were randomly selected, and ORO eluted with 100% isopropanol was measured for optical density (OD) at a wavelength of 500 nm (Thermo, USA).
Immunostainings
Jejunum paraffin sections (5 μm) were incubated with an anti-claudin 3 antibody (34-1700, Invitrogen, USA) overnight at 4 °C, then detected with a fluorochrome-conjugated secondary antibody (Invitrogen, USA). Confocal fluorescence microscopy was performed after DAPI counterstaining.
Pancreatic paraffin sections (5 μm) were incubated with anti-insulin (ab7842, Abcam, UK) or anti-glucagon polyclonal antibody (15954, Proteintech, China), followed by a selected secondary antibody (Invitrogen, USA). Confocal fluorescence microscopy was used to visualize the stained sections. Mean fluorescence intensity (MFI) was quantified.
Statistical analysis
All data were presented as the mean ± standard deviation. Statistical significance is denoted as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by two-way Anova with Tukey’s multiple comparisons test on GraphPad Prism version 8.0.0 for Windows, San Diego, California USA.
Data availability
The datasets presented in this study can be found in NCBI with the accession number PRJNA873796.
References
Hou, K. et al. Microbiota in health and diseases. Signal. Transduct. Target. Ther. 7, 135. https://doi.org/10.1038/s41392-022-00974-4 (2022).
Moreno-Indias, I., Salgado-Somoza, A., Azzouzi, E., Murri, M., Editorial & H. & Emerging roles of the gut microbiota in the pathogenesis of metabolic disorders. Front. Endocrinol. (Lausanne). 12, 736371. https://doi.org/10.3389/fendo.2021.736371 (2021).
Ye, L. et al. High fat diet induces microbiota-dependent Silencing of enteroendocrine cells. Elife 8, e48479. https://doi.org/10.7554/eLife.48479 (2019).
Woting, A. & Blaut, M. The intestinal microbiota in metabolic disease. Nutrients 8, 202. https://doi.org/10.3390/nu8040202 (2016).
Shaw, J. E., Sicree, R. A. & Zimmet, P. Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87, 4–14. https://doi.org/10.1016/j.diabres.2009.10.007 (2010).
Item, F. & Konrad, D. Visceral fat and metabolic inflammation: The portal theory revisited. Obes. Rev. 13 (Suppl 2), 30–39. https://doi.org/10.1111/j.1467-789X.2012.01035.x (2012).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772. https://doi.org/10.2337/db06-1491 (2007).
Amar, J. et al. Energy intake is associated with endotoxemia in apparently healthy men. Am. J. Clin. Nutr. 87, 1219–1223. https://doi.org/10.1093/ajcn/87.5.1219 (2008).
Amar, J. et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 3, 559–572. https://doi.org/10.1002/emmm.201100159 (2011).
Dinakaran, V. Microbial translocation in the pathogenesis of cardiovascular diseases: A Microbiome perspective. J. Cardiol. Curr. Res. 8, 00305. https://doi.org/10.15406/jccr.2017.08.00305 (2017).
Manfredo Vieira, S. et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359, 1156–1161. https://doi.org/10.1126/science.aar7201 (2018).
Fine, R., Vieira, S. M., Ruiz, D. F. Z. & Kriegel, M. A. Gut pathobiont translocation induces lymphocyte migration to internal organs in autoimmunity. J. Immunol. 200, 102116–102116 (2018).
Teltschik, Z. et al. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology 55, 1154–1163. https://doi.org/10.1002/hep.24789 (2012).
Dickson, R. P. & Huffnagle, G. B. The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathog. 11, e1004923. https://doi.org/10.1371/journal.ppat.1004923 (2015).
Nicholson, J. K. et al. Host-gut microbiota metabolic interactions. Science 336, 1262–1267. https://doi.org/10.1126/science.1223813 (2012).
Denou, E. et al. Defective NOD2 peptidoglycan sensing promotes diet-induced inflammation, dysbiosis, and insulin resistance. EMBO Mol. Med. 7, 259–274. https://doi.org/10.15252/emmm.201404169 (2015).
Douglass, J. D. et al. Intestinal mucosal triacylglycerol accumulation secondary to decreased lipid secretion in obese and high fat fed mice. Front. Physiol. 3, 25. https://doi.org/10.3389/fphys.2012.00025 (2012).
Song, C. et al. Intestinal mucus components and secretion mechanisms: What we do and do not know. Exp. Mol. Med. 55, 681–691. https://doi.org/10.1038/s12276-023-00960-y (2023).
Dolensek, J., Rupnik, M. S. & Stozer, A. Structural similarities and differences between the human and the mouse pancreas. Islets 7, e1024405. https://doi.org/10.1080/19382014.2015.1024405 (2015).
Lai, Y., Huang, X., Sun, H., Hui, Q. & Hu, S. Research progress in the relationship between intestinal flora and diabetes mellitus. Endocr. Metab. Immune Disord. Drug Targets https://doi.org/10.2174/0118715303308965240624054156 (2024).
Baker, J. M., Chase, D. M. & Herbst-Kralovetz, M. M. Uterine microbiota: residents, tourists, or invaders? Front. Immunol. 9, 208. https://doi.org/10.3389/fimmu.2018.00208 (2018).
Nakamoto, N. et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat. Microbiol. 4, 492–503. https://doi.org/10.1038/s41564-018-0333-1 (2019).
Owyang, C. & Wu, G. D. The gut microbiome in health and disease. Gastroenterology 146, 1433–1436. https://doi.org/10.1053/j.gastro.2014.03.032 (2014).
Akshintala, V. S., Talukdar, R., Singh, V. K. & Goggins, M. The gut microbiome in pancreatic disease. Clin. Gastroenterol. Hepatol. 17, 290–295. https://doi.org/10.1016/j.cgh.2018.08.045 (2019).
Li, Q. et al. Identification and characterization of blood and neutrophil-associated microbiomes in patients with severe acute pancreatitis using next-generation sequencing. Front. Cell. Infect. Microbiol. 8, 5 (2018).
Ammori, B. J. et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J. Gastrointest. Surg. 3, 252–262 (1999).
Larsen, J. M. The immune response to prevotella bacteria in chronic inflammatory disease. Immunology 151, 363–374. https://doi.org/10.1111/imm.12760 (2017).
Patil, D. P. et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J. Biosci. 37, 647–657. https://doi.org/10.1007/s12038-012-9244-0 (2012).
Pedersen, H. K. et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381. https://doi.org/10.1038/nature18646 (2016).
Cerf, M. E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. (Lausanne). 4, 37. https://doi.org/10.3389/fendo.2013.00037 (2013).
Uchida, A. et al. Reduced triglyceride secretion in response to an acute dietary fat challenge in obese compared to lean mice. Front. Physiol. 3, 26. https://doi.org/10.3389/fphys.2012.00026 (2012).
Gummesson, A. et al. Intestinal permeability is associated with visceral adiposity in healthy women. Obes. (Silver Spring). 19, 2280–2282. https://doi.org/10.1038/oby.2011.251 (2011).
Teixeira, T. F., Collado, M. C., Ferreira, C. L. & Bressan, J. Peluzio M. C. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr. Res. 32, 637–647. https://doi.org/10.1016/j.nutres.2012.07.003 (2012).
Johnson, A. M. et al. High fat diet causes depletion of intestinal eosinophils associated with intestinal permeability. PLoS One. 10, e0122195. https://doi.org/10.1371/journal.pone.0122195 (2015).
de Vos, W. M., Tilg, H., Van Hul, M. & Cani, P. D. Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032. https://doi.org/10.1136/gutjnl-2021-326789 (2022).
Tropini, C., Earle, K. A., Huang, K. C. & Sonnenburg, J. L. The gut microbiome: Connecting Spatial organization to function. Cell. Host Microbe 21, 433–442. https://doi.org/10.1016/j.chom.2017.03.010 (2017).
Ferrari, A., Lombardi, S. & Signoroni, A. Bacterial colony counting by Convolutional Neural Networks. In Annu Int Conf IEEE Eng Med Biol Soc 7458–7461, (2015). https://doi.org/10.1109/EMBC.2015.7320116 (2015).
Signoroni, A., Savardi, M., Pezzoni, M., Guerrini, F., Arrigoni, S. and Turra, G. Combining the use of CNN classification and strength-driven compression for the robust identification of bacterial species on hyperspectral culture plate images. IET Comput. Vis. 12, 941–949 (2018).
Cohen, J. P., Boucher, G., Glastonbury, C. A., Lo, H. Z. & Bengio, Y. Count-ception: counting by fully convolutional redundant counting. In 2017 IEEE Int. Conf. Comput. Vis. Workshops (Iccvw 2017). 18–26 https://doi.org/10.1109/Iccvw.2017.9 (2017).
Yu, Z., Yu, X. F., Kerem, G. & Ren, P. G. Perturbation on gut microbiota impedes the onset of obesity in high fat diet-induced mice. Front. Endocrinol. (Lausanne). 13, 795371. https://doi.org/10.3389/fendo.2022.795371 (2022).
Teng, B. et al. Newly identified peptide hormone inhibits intestinal fat absorption and improves NAFLD through its receptor GPRC6A. J. Hepatol. 73, 383–393. https://doi.org/10.1016/j.jhep.2020.02.026 (2020).
Acknowledgements
The authors thank Dr. Jie Feng’s Laboratory (Institute of Microbiology, Chinese Academy of Sciences) for DsRed-E. coli, GFP-E. coli, Luciferase-E. coli, and GFP-PA strains.
Funding
This work was supported by National Key Research and Development Program of China (grant number 2021YFA0719303); National Natural Science Foundation of China (grant number 32271166, 32100572); Guangdong Basic and Applied Basic Research Foundation (grant number 2024A1515013017); Shenzhen Science and Technology Program (grant number JCYJ20200109115441918, JCYJ20210324102013035, KCXFZ20201221173400002); High-level Key Clinical Specialty project of Guangdong Provincial Health Commission (Supporting construction funds of Shenzhen) (grant number SZGSP012); Guangdong High-level Hospital Construction Fund and Guangdong High-level Hospital Construction Fund Clinical Research Project of Shenzhen Children’s Hospital (grant number LCYJ2022071).
Author information
Authors and Affiliations
Contributions
ZS, YZ, PR. Methodology: XY, ZY, WX. Investigation: XY, ZY, WX, GK, BT. Formal analysis: XY, ZY, WX, GK. Resources: WX, BT, GK. Funding acquisition: BT, ZS, PR. Software: ZY, WX. Project administration: PR. Supervision: YZ. Validation: XY, GK, BT, ZS, YZ, PR. Writing original draft: XY, WX, YZ. Writing– review & editing: YZ, JZ, ZS, PR, CG. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, XF., Gurung, C., Yu, Z. et al. Intestinal bacteria translocation promotes β-cell dysfunction in DIO mice. Sci Rep 15, 31034 (2025). https://doi.org/10.1038/s41598-025-15244-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15244-w