Abstract
Epigenetic mechanisms are crucial for neocortical development. Recent work has highlighted that ablation of Setd2, which encodes a SET domain lysine methyltransferase, from the mouse dorsal telencephalon results in deficits in reciprocal cortico-thalamic connectivity. However, analysis of broader cortical phenotypes in this model has yet to be performed. Here, we addressed this, revealing that dorsal telencephalon-specific ablation of Setd2 results in decreased structural volumes across the entire adult brain, particularly within the hippocampus. Moreover, while homozygous Setd2-deficient brains exhibited apparently normal axonal integrity within the corpus callosum, axonal connections arising from the hippocampus were significantly impacted. Furthermore, whole brain structural connectivity was significantly altered in the absence of Setd2. Finally, we revealed structural, morphological and cellular changes within the hippocampal dentate gyrus of mutant mice. Collectively, these findings demonstrate a key role for SETD2 function in the development of the adult brain.
Similar content being viewed by others
Introduction
Su(var)3–9, Enhancer of Zeste and Trithorax domain-containing protein 2 (SETD2) belongs to the nuclear receptor SET domain family of protein lysine methyltransferases1,2. Structurally, SETD2 contains several domains that are important for its catalytic activity, including the pre-SET or Associated with SET domain, SET and post-SET domains. SETD2 also contains several C-terminal domains important for protein–protein interactions, including the SETD2 hnRNP Interaction domain, Proline-rich region and Tryptophan-Tryptophan domains and the Set2 Rpb1 Interacting domain, which are important for its methyltransferase activity. SETD2 targets a number of substrates within the cell, but is most prominently associated with the deposition of the trimethylation mark on histone H3 at lysine residue 36 (H3K36me3)2. Trimethylation at H3K36 is crucial for the regulation of DNA methylation and H3K27me3 deposition, repair of DNA damage and repression of cryptic transcription2. Additional roles for SETD2 include interactions with RNA Polymerase II, methylation of actin and α-tubulin, and methylation of lysine residues on a number of different proteins including STAT1 and EZH21,2.
SETD2 is expressed both during development, and in the adult3,4,5. In humans, the importance for SETD2 activity is underscored by the fact that loss of a single allele gives rise to the rare neurodevelopmental disorder, Luscan-Lumish syndrome (LLS)6,7,8. LLS falls under the category of disorders known as overgrowth-intellectual disability disorders (OGID), which typically include macrocephaly, bodily overgrowth, intellectual disability of a varying degree, and a range of other co-morbidities, including autism spectrum disorder (ASD), language and speech acquisition delays and brain malformations6,9,10. Many of the symptoms of LLS relate to nervous system structure, such as the presence of Chiari I malformations and function, including intellectual disability and ASD6. This suggests that SETD2 is important during embryonic and postnatal nervous system development, an idea further supported by the widespread expression of this methyltransferase within the developing brain4,5.
Despite this, our understanding of how SETD2 shapes neural development is limited. While SETD2 has been shown to be critical for development of systems such as the pancreas11 and the intestinal immune system12, as well as to play a role in diseases such as clear cell renal cell carcinoma1,13,14, our understanding of how SETD2 heterozygosity culminates in LLS remains unclear. However, there have been some recent insights into the role of SETD2 in neural development. For instance, a recent study investigated how SETD2 regulates murine cortical development through the conditional ablation of this factor from the dorsal telencephalon4. Using an Emx1Cre-based approach, the authors selectively ablated Setd2 from the mouse dorsal telencephalon. This led to disrupted areal patterning of the neocortex, impaired reciprocal cortico-thalamic connectivity and aberrant behavioural responses related to social interaction, motor learning and spatial memory in homozygous conditional knockouts. Conditional heterozygous knockouts did not exhibit these phenotypes. Furthermore, transcriptomic analyses of the homozygous mutant cortices revealed alterations to the cPcdh superfamily of cadherin molecules, among others4. The authors subsequently expanded upon these findings, revealing homozygous mutant mice also exhibited deficits in lamination, migration and maturity within the hippocampal cornu ammonis (CA) 1 region15. Collectively, these studies provide an important foundation for understanding how SETD2 regulates cortical development. However, there are several important outstanding issues regarding the phenotypes observed in this model of Setd2-deficiency within the mouse forebrain. Specifically, is the size of the adult brain outside the cortex affected in mutant mice, and what are the global connectivity changes within the neocortex and other regions of the brain that retain SETD2 expression? In addition, is the hippocampal dentate gyrus, a key structure that regulates learning and memory, affected in homozygous mutant mice? Here, we addressed these gaps, using mice carrying a conditional Setd2 allele (Setd2fl/fl) crossed to mice carrying a codon-improved Cre recombinase driver under the control of the dorsal telencephalon-specific gene, Emx1 (Emx1iCre/+)16. Using these Setd2fl/fl; Emx1iCre/+ knockout (cKO) mice, we revealed reduced volume for many areas of the adult brain, and alterations to the whole-brain connectome, including changes to the hippocampal connectome. We also highlight how Setd2 ablation results in abnormal development of the adult dentate gyrus, including alterations to adult neural stem cell and immature neuronal populations. The findings shed further light on the role of SETD2 during brain development and may be informative regarding LLS.
Results
Setd2 ablation from the dorsal telencephalon results in reduced volume of the adult brain
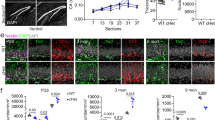
Previous work has shown that conditional ablation of Setd2 from the dorsal telencephalon culminates in a 10% smaller cortical width and hemispheric area in postnatal day (P)7 mice4. This prompted us to ask whether this phenotype was confined to the cortical hemispheres, or if the effects were more widespread. To investigate this, we used a similar model as Xu and colleagues4, namely a conditional Setd2 allele17 crossed to an Emx1iCre/+ driver18, which should ensure more efficient ablation than the Emx1IRES Cre line used previously4. In this model, Setd2 is ablated from neural stem cells within the dorsal telencephalon from embryonic (E) day 10.519. As such, most neurons and glia within the adult neocortex and hippocampus of Cre-expressing homozygous mice (barring interneurons, microglia and some oligodendrocytes) will lack SETD2 expression. To assess adult brain size, we performed volumetric magnetic resonance imaging (MRI) to compare brain volume in homozygous mice (Setd2fl/fl; Emx1iCre/+— hereafter called cKO mice) with controls (either Setd2fl/+; Emx1+/+ mice or Setd2fl/fl; Emx1+/+ mice). Analysis of these data confirmed a reduction in the size of the neocortex in cKO mice in comparison to controls, consistent with previous reports4 (Fig. 1A–J). Other areas derived from the dorsal telencephalon also exhibited reduced volume in the cKO, including the hippocampus and corpus callosum. These findings were also validated when performing haematoxylin and eosin staining of the adult brain across a range of different Bregma levels (Supp. Figure 1A–M). Critically, we also revealed that the whole adult cKO brain had a significantly reduced volume, and that areas within the diencephalon (thalamus, hypothalamus), cerebellum and brainstem (midbrain, inferior colliculi, superior colliculi) were also smaller in the cKO, despite being derived from neural stem cells that did not undergo Setd2 ablation. These data suggest that the loss of Setd2 from the dorsal telencephalon during development elicits phenotypes that extend beyond the neocortex.
Volumetric analyses reveal that Setd2 cKO brains are smaller. Volumetric model-based segmentation analyses of Setd2 Ctrl (A, D, G) and cKO (B, E, H) brains were performed using the AMBMC whole brain atlas, and representative images of Ctrl and cKO brains were overlaid to show differences in volume (C, F, I). Volume of each structure, differences in volume, and normalised structural volume are shown in J. The corpus callosum, hippocampus, neocortex and thalamus, among other sub-substructures, were significantly reduced in absolute volume, as was the entire brain. Further delineation of structures and their associated volumes are shown in Supp. File 1. All data in J were analysed using multiple unpaired t-tests with a FDR of 5%. All data are represented as mean ± SEM. All values are rounded to 2 decimal places. Differences in (normalised) volume are represented as Ctrl – cKO. Scale bar represents 1 mm. CC = corpus callosum. For all analyses, n = 10 and n = 9 were used for Ctrl and cKO brains respectively.
These volumetric data were derived using a standard mouse brain atlas, which provides analyses of 20 broad regions of interest20. While this provides a solid basis for interrogating volumetric data, these atlases often lack the resolution necessary to elucidate changes in specific sub-regions of the brain. Additionally, previous studies into Setd2-deficiency in the neocortex have shown alterations of the size and location of discrete cortical regions4, emphasising that finer resolution atlases may reveal greater insights. Therefore, we partitioned our data, and analysed hippocampal21, diencephalic22 and neocortical23 regions in more detail, in order to determine which substructures within each of these areas were affected in the cKO. Within the hippocampus, all 15 substructures were found to be significantly reduced in the cKO in comparison to the control (Supp. File 1). Similarly, of the 94 diencephalic structures analysed, 38 structures showed significant reductions in volume in the cKO; these structures largely corresponded to different thalamic nuclei (Supp. File 1). Finally, of the 74 neocortical structures analysed, almost all (71/74) showed significant reductions in volume in the cKO (Supp File 1). Using an orthogonal approach (tensor-based morphometry), we validated these findings, showing broad alterations in volume within the brains of adult cKO mice (Supp. Figure 2A–I)24,25,26. Importantly, we also validated that analyses of regional volumes did not suffer from biases resulting from arealisation defects, as we were able to confirm that the software accurately mapped S1 onto the barrel cortex in the face of areal shifts using constrained spherical deconvolution mapping (Supp. Figure 3A–L). These findings suggest that there are significant reductions in cKO brain volume across specific regions within and outside of the dorsal telencephalon, including the neocortex, hippocampus, diencephalon, brainstem and cerebellum.
Setd2 cKO brains exhibit localised connectivity differences
Xu and colleagues used anterograde AAV labelling to reveal altered reciprocal cortico-thalamic connectivity in Setd2fl/fl;Emx1IRESCre/+ mice4. To address if connectivity deficits are more widespread, we performed diffusion tensor magnetic resonance imaging (DTMRI), which allowed us to assess axon tract integrity and whole-brain connectivity. We first considered axonal integrity using DTMRI metrics, including fractional anisotropy (FA: this measures the degree of directionally dependent water diffusion in tissue), apparent diffusion coefficient (ADC: this measures the overall rate of microscopic water diffusion), axial diffusivity (AD: this measures parallel microscopic water diffusion) and radial diffusivity (RD: this measures perpendicular microscopic water diffusion)27. Interestingly, despite the reported alterations in cortico-thalamic connectivity in cKO mice4, seeding regions of interest (ROIs) within the diencephalon and basal ganglia, such as thalamic nuclei and the caudate putamen, and the primary somatosensory or motor cortices, did not reveal any changes in these DTMRI metrics (Supp. Figures 4A–P and 5A–P). These data suggest that, while axonal connectivity between the cortex and thalamus may be aberrant4, and the volume of the internal capsule was reduced in the cKO (Fig. 1J), the microstructural integrity of the axons linking these structures was not affected. Likewise, FA, ADC, AD and RD measurements of axons within the major forebrain commissures–corpus callosum, hippocampal commissure and anterior commissure–were comparable between cKO and control mice (Fig. 2A–K), apart from RD within the hippocampal commissure, which was significantly increased in the cKO (Fig. 2L). We further confirmed this through the use of an orthogonal approach (voxel-based morphometry (VBM)), whereby each voxel was analysed within the grey and white matter individually26. In doing so, we confirmed no significant alterations to FA within the major forebrain commissures but did find significant reductions in FA within the motor and somatosensory cortices of cKO brains (Fig. 2M–U). These findings may suggest changes to FA within the grey matter of affected regions (such as S1)4 which may not be seen using standard DTI assessments.
Setd2 cKO mice largely display altered DTI metrics within the grey matter, but not the white matter, of regions connected by the major forebrain commissures. Representative tractography of Setd2 Ctrl (A–D) and cKO (E–H) brains, highlighting the anterior commissure (AC; A, E), corpus callosum (CC; B, F) and hippocampal commissure (HC; C, G). All three tracts are represented as a merge panel (D, H). Diffusion tensor imaging (DTI) metrics (axial diffusivity (I), apparent diffusion coefficient (J), fractional anisotropy (K) and radial diffusivity (L)) are represented. No differences were noted in any of the major forebrain commissures, except for a significant increase in radial diffusivity within the hippocampal commissure (L). Voxel based morphometry (VBM) analyses of Setd2 Ctrl and cKO are shown in representative average-brain templates for coronal (M–R) and transverse (S–U) planes. VBM revealed decreased (Ctrl > cKO) average fractional anisotropy (blue) within both white matter and grey matter substructures. Decreased FA is represented across the rostrocaudal axis (with M being most rostral, and R being most caudal), as well as along the dorsoventral axis (with S being most dorsal and U being most ventral). No increases in FA were detected using VBM analyses, in agreeance with other findings within this article. Colours for DTI represent the following directionality: green is left–right oriented, red is dorso-ventral oriented, blue is rostro-caudal oriented; oblique fibre orientations are a combination of these colours. nd = no difference; * p < 0.05. For all analyses, n = 10 and n = 9 were used for Ctrl and cKO brains respectively.
Given that radial diffusivity was increased in the hippocampal commissure (Fig. 2L), and the hippocampus receives afferent fibres from numerous other regions (including the entorhinal cortex, prefrontal cortex and the anterior cingulate gyrus)28 which were reduced in size (Supp. File. 1), we next wanted to investigate the hippocampal formation in greater detail. To this end, we isolated ROIs of each hippocampal body (HpL and HpR) and analysed tracts passing between these bodies via the fornix (For) via tractography. Analyses of this “circuit” revealed significantly increased ADC and RD, in line with the HC increases (Fig. 2L) and significantly decreased FA (Fig. 3A–G). These data are consistent with the reduced size of the hippocampus and suggest that this part of the brain is particularly affected by loss of SETD2 activity, as has been previously shown15. Indeed, using VBM, we confirmed that there were decreases to FA within the hippocampal formation, in line with what we saw using DTI (Fig. 2M–U). Altogether, these findings are suggestive of region-specific differences in the microstructural integrity of connections within cKO brains.
Setd2 cKO mice exhibit abnormal bilateral hippocampal connectivity. Representative tractography of Setd2 Ctrl (A–C) and cKO (D–F) brains in coronal (A, D), sagittal (B, E) and transverse (C, F) planes. All tracts presented represent connectivity between the left hippocampal body (HpL) and the right hippocampal body (HpR), with only tracts passing through the fornix (For) being shown. Diffusion tensor imaging (DTI) metrics (axial diffusivity (AD), apparent diffusion coefficient (ADC), fractional anisotropy (FA) and radial diffusivity (RD)) are represented (G). All metrics but AD were significantly altered, with significant increases being seen in ADC and RD and significant decreases in FA. Colours for DTI represent the following directionality: green is left–right oriented, red is dorso-ventral oriented, blue is rostro-caudal oriented; oblique fibre orientations are a combination of these colours. * p < 0.05. For all analyses, n = 10 and n = 9 were used for Ctrl and cKO brains respectively.
Setd2 cKO brains exhibit altered whole-brain structural connectivity
In addition to assessing axonal integrity, DTMRI can also be used to investigate global structural connectivity within the brain. Previous studies investigating ASD in patients have utilised network-based statistic (NBS) approaches to model how patients may present with altered functional connectivity29. The authors found altered connectivity using resting state functional MRI and showed alterations to regions involved in memory and learning (hippocampus), as well as socialisation and emotional responses (the limbic system). Based upon these findings, we theorised that the use of a similar NBS approach would allow us to determine whether there were alterations to whole-brain connectivity in cKO mice. To do this, we utilised the NBS toolbox30, allowing us to visualise structural connectivity, and perform connection-wise comparisons between “nodes” (regions) of the brain31. Using a 106-node template, we constructed the structural connectome to determine which nodes showed altered (increased or decreased) connectivity in cKO brains compared to controls (Fig. 4A–D). NBS analyses of control and cKO brains revealed increased connectivity primarily within areas associated with sensory processing, such as the somatosensory and visual cortices (Fig. 4E), and decreased connectivity primarily within areas associated with the hippocampus i.e., dorsal hippocampal fissures, as well as projections and connectivity i.e., CC and cingulum (Fig. 4F; see Supp. File 1 for all findings). LLS, which is caused by heterozygous loss of function of SETD2, is often comorbid with ASD and language acquisition delays. In line with this, we observed alterations across the brain in regions and “networks” typically associated with ASD aetiology using a structural NBS approach29, suggesting that ablation of Setd2 from the neocortex results in altered structural connectivity within the adult mouse brain.
Structural connectome mapping reveals abnormal inter-nodal connectivity within Setd2 cKO brains. Connection-wise comparisons between Setd2 controls compared to cKO brains was performed using Network Based Statistic analysis. The intensity component (total number of connections) and primary threshold (t = 2.8, p = 0.0064) results were used for all representations above. Brain nodes are represented as circles scaled in size according to the degree of change in a node, which corresponds to the number of different connections made with that node. Abnormal nodes are shown in sagittal (A, B) and axial (C, D) views. Nodes that had increased connectivity in Setd2 cKO mice compared to controls are depicted in red, while nodes with decreased connectivity are depicted in blue. (A, C) Compared to control brains, Setd2 cKO brains predominantly show a significantly increased degree of inter-nodal connections within primary integration areas, as represented in the bar graph provided (E). However, Setd2 cKO brains primarily show a significantly decreased degree of inter-nodal connectivity within hippocampal structures, as well as structures for long range projections, as represented in the bar graph provided (F). For all analyses, n = 10 and n = 9 were used for Ctrl and cKO brains respectively. A2-L, Secondary auditory cortex, Left; Ac-L, Anterior cingulate, Left; Ac-R, Anterior cingulate, Right; An-L, Accumbens nucleus, Left; An-R, Accumbens nucleus, Right; Ant_com, Anterior commissure; CC-im, Corpus callosum (inside midline); CCec-L, Corpus callosum/external capsule, Left; CCec-R, Corpus callosum/external capsule, Right; Cl-L, Claustrum, Left; Cng-L, Cingulum, Left; Cng-R, Cingulum, Right; Dhf-in, Ventral hippocampal commissure inclusion; Dhf-L, Dorsal hippocampal fissure, Left; Dhf-R, Dorsal hippocampal fissure, Right; Dof-R, Dorsal fornix, Right; Dor_for, Dorsal Fornix; Ect-R, Ectorhinal cortex, Right; End-L, Endopiriform nucleus, Left; Ent-L, Entorhinal cortex, Left; Ent-R, Entorhinal cortex, Right; Fmcc-L, Forceps minor of the corpus callosum, Left; Fmcc-R, Forceps minor of the corpus callosum, Right; For, Fornix; Hind-R, Hindbrain, Right; Hp-L, Hippocampus, Left; Hp-R, Hippocampus, Right; Hyp-R, Hypothalamus, Right; Ic-R, Inferior colliculus, Right; In-L, Insular cortex, Left; In-R, Insular cortex, Right; Inc-L, Internal capsule, Left; Inc-R, Internal capsule, Right; Laot-R, Lateral olfactory tract, Right; Lgp-L, Lateral globus pallidus, Left; Lgp-R, Lateral globus pallidus, Right; Lo-L, Lateral orbital cortex, Left; M2-R, Secondary motor cortex, Right; Midli-wocc, Midline (except corpus callosum); Nsb-L, Nosebulb, Left; Pa-L, Parietal cortex, Left; Pir-L, Piriform cortex, Left; Pir-R, Piriform cortex, Right; Pr-L, Perirhinal cortex, Left; Pr-R, Perirhinal cortex, Right; Rs-L, Retrosplenial cortex, Left; Rs-R, Retrosplenial cortex, Right; S1-L, Primary somatosensory cortex, Left; S1-R, Primary somatosensory cortex, Right; S2-L, Secondary somatosensory cortex, Left; S2-R, Secondary somatosensory cortex, Right; Sc-L, Superior colliculus, Left; Sc-R, Superior colliculus, Right; Sep-L, Septum, Left; Sub-R, Subiculum, Right; Ta-L, Temporal association area, Left; Ta-R, Temporal association area, Right; Thal-L, Thalamus, Left; Thal-R, Thalamus, Right; V1-L, Primary visual cortex, Left; V1-R, Primary visual cortex, Right; V2l-L, Secondary visual cortex, lateral part, Left; V2l-L, Secondary visual cortex, lateral part, Right; V2ml-L, Secondary visual cortex, mediolateral part, Left; V2ml-R, Secondary visual cortex, mediolateral part, Right; Vhipf-in, Ventral hippocampal commissure inclusion; Vmo-L, Ventromedial orbital cortex, Left; Vmo-R, Ventromedial orbital cortex, Right.
Setd2 ablation results in abnormal cellular composition of the adult hippocampus
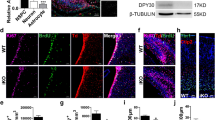
Previous studies in cKO mice have shown arealisation defects in the cortex, albeit with typical cortical lamination4, as well as lamination and migratory deficits within CA1 of the hippocampus15. Additionally, Xu and colleagues identified abnormal behavioural and learning responses4. Because of the important role played by the dentate gyrus (DG) in these processes, we next turned our attention to analysing the DG. We first looked at gross morphology of the adult control and cKO brain using haematoxylin and eosin staining. The area of the hippocampus was significantly reduced, and the dentate gyrus blades were smaller in area, thickness and length (Fig. 5A–J), consistent with our MRI analyses (Fig. 1J). Both excessive cell death and reduced proliferation can contribute to changes in structure size, however, using the expression of the apoptotic marker CC332, we did not observe any significant changes in apoptosis within the DG of cKO mice in comparison to controls (Supp. Figure 6A–E). The adult mouse hippocampus is one of the few regions of the forebrain in which neurogenesis continues throughout life, with quiescent and proliferative adult neural stem cells (aNSCs) present within the subgranular zone (SGZ) of the dentate gyrus33. Could impaired adult neurogenesis contribute to the reduction in hippocampal volume? To address this, we assessed the expression of SOX2 (a marker for aNSCs and astrocytes more broadly)34 and GFAP (an astrocytic/aNSC marker)35. Cell counts revealed fewer SOX2+ cells within the dentate gyrus of the cKO (Fig. 6A–F), and, importantly, a reduced number of SOX2-expressing cells within the SGZ of the mutant (Fig. 6G), suggestive of reduced aNSCs in the cKO. Despite this, the number of radially orientated GFAP-expressing fibres within the granular zone of the cKO was not significantly different in the cKO compared to controls (Fig. 6H), which may point to differences in the numbers of quiescent versus active aNSCs within the SGZ36.
Setd2 cKO brains show reductions in size across the adult hippocampus. (A) Coronal sections taken at the level of the dentate gyrus of the hippocampus from Setd2 control (A, left) and cKO (B, right) brains, stained using haematoxylin and eosin. Higher magnification zooms of the dentate gyrus are shown below (C, D). Quantification of (E) average total hippocampal area, (F) average hippocampal CA region thickness, (G) average angle between blades of the DG (at the hilus), (H) average DG blade width, (I) average DG blade length and (G) average DG blade area. nd = no difference, ns = not significant, * p < 0.05; ** p < 0.01; *** p < 0.001. Scale bar in D represents 1 mm for A & B, and 250 μm for C & D. CA = cornu ammonis, DG = dentate gyrus, IB = inferior blade, SB = superior blade. For all analyses, an n = 5 was used for each genotype.
Setd2 cKO brains exhibit alterations in cellular populations within the hippocampus. Coronal sections taken at the level of the dentate gyrus of the hippocampus from Setd2 control (A–B'") and cKO (F–D'") brains, revealing the expression of SOX2 (red), GFAP (green) and DAPI (white). Quantification of (E) the average number of SOX2+ cells within the DG, (F) density of those cells in the DG, (G) average number of SOX2+ cells within the SGZ and H) average number of radially oriented GFAP+ fibres. nd = no difference, ns = not significant; ** p < 0.01. Scale bar in D'"H represents 25 µm for all large panels, and 5 µm for all insets. For all analyses, an n = 5 was used for each genotype.
To probe this further, we next aimed to determine if there was a reduction in the number of proliferating aNSCs. To assess proliferation within the hippocampal dentate gyrus directly, we performed immunofluorescence staining using antibodies against SOX2 and KI-67, a marker of cells progressing through the cell cycle. Cell counts revealed a significant reduction in the number and density of KI-67-expressing cells within the dentate gyrus of the cKO, as well as a small, though non-significant reduction in the number of cells within the SGZ expressing both SOX2 and KI-67 (proliferating aNSCs; p = 0.0622; Fig. 7A–H). Expression of PROX1 (a marker for mature granule neurons) revealed a reduction in the granular zone of the superior and inferior blades of the dentate gyrus (Fig. 8A–E), which was also accompanied by reduced number, density and blade length occupied by immature neurons (Doublecortin (DCX)-positive cells; Fig. 8G–H). Taken together, these findings suggest that Setd2 ablation results in a reduction in the number of active aNSCs, accompanied by a reduction in newborn neurons in the adult brain.
Proliferation within the SGZ is affected in Setd2 cKO brains. Coronal sections taken at the level of the dentate gyrus of the hippocampus from Setd2 control (A–B’”) and cKO (C–D’”) brains, revealing the expression of SOX2 (red), Ki67 (green) and DAPI (white). Quantifications of (E) the average number of KI-67+ cells in the SGZ, (F) the average density of KI-67+ cells in the SGZ, G) the average number of SOX2+/KI-67- cells in the SGZ and (H) the average number of SOX2+/KI-67+ cells in the SGZ. Scale bar in D’” represents 25 µm for all large panels, and 5 µm for all insets. ns = not significant, * = p < 0.05, ** = p < 0.01. For all analyses, an n = 5 was used for each genotype.
Setd2 cKO brains show alterations in hippocampal neuronal populations. Coronal sections taken at the level of the dentate gyrus of the hippocampus from Setd2 control (A–B’”) and cKO (C–D’”) brains, revealing the expression of PROX1 (red), Dcx (green) and DAPI (white). Quantification of (E) the PROX1+ area of each blade of the dentate gyrus, (F) the density of PROX1 cells within the DG, (G) the number of Dcx+ neuroblasts and (H) the length of each blade of the cKO DG occupied by Dcx+ neuroblasts as a percentage of control brains. ns = not significant; * p < 0.05; ** p < 0.01; *** p < 0.001. Scale bar in D’” represents 100 µm for all large panels, and 20 µm for all insets. For all analyses, an n = 5 was used for each genotype.
Finally, as previous studies did not consider how ablation of Setd2 from the dorsal telencephalon affects glial populations4,15, we investigated this feature within the hippocampus and neocortex. As outlined above, we identified a decrease in the total number of SOX2-expressing cells within the entire dentate gyrus (Fig. 6E), suggestive of a reduction in astrocyte numbers within the hilar region of the dentate gyrus. Similarly, analysis of OLIG2 expression revealed a significant reduction in the total number of cells of the oligodendrocyte lineage in the cKO hippocampus (Supp. Figure 7A–C). However, we did not observe any difference in the number or density of SOX2- or OLIG2-expressing cells within the motor cortex (Supp. Figure 8A–P).
Discussion
In the current study, we have uncovered previously unappreciated roles for SETD2 in regulating cortical development. We reveal that SETD2 regulates brain volume and whole-brain structural connectivity, while at the circuit level Setd2 ablation results in deficits within hippocampal tract integrity. We highlight how Setd2 ablation results in reduced proliferation of aNSCs within the subgranular zone of the dentate gyrus, resulting in reduced neurogenesis as well as alterations to several different cellular populations within the adult hippocampus. Altogether, these findings expand upon previously established phenotypes shown to exist within the cKO brain4,15, and highlight a key role for SETD2 in neural development.
Within this study, we have highlighted reductions in brain size and volume in Setd2 cKO mice. Previous studies into mouse models of other epigenetic genes have shown brain size changes depending on gene dosage. These include models of Dnmt3a deficiency, heterozygosity of which causes the human overgrowth disorder Tatton-Brown-Rahman Syndrome, which manifest with tibial/femoral length increase, adult onset obesity and brain size alterations37,38. Studies of three different models of Dnmt3a deficiency revealed that despite craniofacial morphometry being largely unchanged and no change in skull size being noted37,38, there was a late-adulthood onset of reduced brain volume, not noted at 8 weeks of age, which was worsened by mutation severity (R878H vs P900L mutations)37. Yet another study into Chd8, a DNA binding protein implicated in chromatin remodelling, autism spectrum disorder and CHD8-related overgrowth disorder39,40,41,42, revealed how gene dosage drives distinct outcomes for brain growth43. While haploinsufficiency for Chd8 resulted in brain overgrowth, severe reductions in gene expression (~ 91% reduction at protein level) resulted in brain undergrowth43. Studies of Dnmt3a and Chd8 show similarities to the findings we and others4,15 have observed in Setd2 cKO mice, and suggest that total ablation of Setd2 may result in different outcomes as opposed to heterozygous ablation—what then is driving these brain size reductions in our Setd2-deficient mice?
There are likely numerous distinct mechanisms at work which are driving the reductions in brain size. Previous studies into regulators of hippocampal volume have revealed that reduced neurogenesis, as well as stress, drives CA3/DG-specific reductions in volume44. Chronic stress reduced volume across the entire hippocampus, while inhibition of neurogenesis reduced volume only within the dentate gyrus (after 4 weeks), and then the CA3 region (after 16 weeks)44. Because Setd2 cKO mice have previously been demonstrated to exhibit anxiety-like phenotypes4, and we have shown here that Setd2 ablation results in reduced neurogenesis, we hypothesise that hippocampal volume reductions result from a combination of reduced neurogenesis and an increased anxiolytic effect. As for volume within the cortex, one possibility for reduced neocortical volume may lie in the connectivity deficits we and others have characterised4. Reduced volume and connectivity, as well as aberrant targeting of connections4, have previously been linked to reduced brain volumes18,31—indeed, a previous study18 where Emx1 was used to ablate the gene-of-interest revealed reduced volumes in regions which do not derive from Emx1-expressing progenitors, similar to our findings herein. Against this backdrop, we posit that the volumetric reductions in the hippocampus derive from neurogenesis-related mechanisms, and neocortical reductions derive from abnormal connectivity.
Previous studies4 have shown that cKO brains show altered reciprocal corticothalamic connectivity. In the current study, we were unable to directly assess this possibility using DTI techniques but have highlighted that axons within these “circuits” do not show altered DTI metrics (i.e. changes to FA), while axons within the hippocampal “circuit” are affected in their axonal microstructural integrity. One possibility for these differences likely comes down to glial, and specifically oligodendrocyte, populations. While previous studies have failed to consider these crucial populations4,15, here we have shown a reduction in the number of hippocampal glial populations. Oligodendrocytes are crucial in myelinating axons throughout the brain, and myelination status could explain the alterations seen in DTI metrics in our study. While we were unable to directly assess myelination differences in this study, myelination has been implicated in neurodevelopmental disorders such as autism previously45, and may explain differences seen within cKO brains in relation to both DTI metrics and altered connectivity. As such, we suggest that oligodendrocyte differences may potentially contribute to hippocampal “circuit” alterations, while altered connectivity may have another underlying cause yet to be identified.
In the current work, we have focused our analyses on adult mice. However, since Setd2 is ablated from ~ E10.5 through Emx119, it is likely that these phenotypes originate much earlier in development. Indeed, Xu and colleagues identified reduced brain hemispheric area as early as P74, suggesting that the onset of brain volume reductions may be occurring earlier in development. In future studies it would be important to conduct DT-MRI analyses at earlier postnatal, and indeed embryonic ages, to map the onset of deficits. Given the early onset of reduced brain size (P7) and the fact that major aspects of mouse brain connectivity are not complete until 2–3 weeks of age, with full maturation occurring even beyond this point46,47, what may be driving this early phenotypic onset? While Xu and colleagues have shown that apoptosis is not increased at P0, this does not preclude a later onset of increased cell death, or indeed other modalities of cell death48,49, driving reduced brain volume4. Assessment of cell death using complementary methods, such as TUNEL staining, across several ages may reveal if postnatal cell death is contributing to reduced brain size. Similarly, while proliferation has been assessed through the use of BrdU, Tbr2 and KI-67 stains4,15 and has shown typical cell proliferation across most of the embryonic brain, with the exception of elevated hippocampal neuroepithelium-localised KI-67+ cells at E15.515, it is nevertheless possible that proliferation is reduced during the early postnatal period. This possibility could be assessed through the use of neurosphere assays to assess proliferation potential of neural stem cells.
Finally, we have demonstrated that Setd2 ablation results in alterations to adult neural stem cell populations within the subgranular zone (SGZ) of the hippocampus, and that these cells are incompetent at producing newborn neurons. Adult neurogenesis is crucial for several reasons, including replenishment of dead/dying cells and plasticity for memory and learning33,50,51. Neurogenesis is also acutely linked to health and disease, including Alzheimer’s, Parkinsons and typical aging50,52. We have revealed that in the absence of Setd2, there are reductions in the number of active aNSCs residing in the SGZ, as well as the number of newborn neurons of the DG, suggesting that the active aNSCs that do exist are unable to produce the same numbers of newborn neurons in the absence of Setd2. Proliferative capacity of active aNSCs could be investigated using methods such as neurosphere assays to assess proliferative competency and stemness53. In addition, the use of methods such as single nucleus multiome sequencing methods (snRNA/snATACseq)54, combined with methods such as CUT&RUN for profiling of histone modifications55, or even spatial sequencing methodologies56,57, would allow for an in-depth characterisation of how these aNSCs differ in the absence of Setd2. Indeed, these sequencing methodologies would likely allow us to elucidate specific gene pathways or networks that may altered in the absence of Setd2 and provide possible targets for future research. In closing, we have provided further characterisation of the phenotypes present in the cortices of Setd2 cKO mice, highlighting previously unappreciated roles for SETD2 in neural development. Further research should be undertaken in more clinically relevant settings, such as the use of heterozygous murine models of LLS or in vitro approaches derived from patients own tissues, to continue to advance our understanding of the breadth of roles that SETD2 plays.
Materials and methods
Animals
Animals used in this study were bred at The University of Queensland under approval from the Institutional Animal Ethics Committee (2023/AE000007). To study the role of SETD2 in cortical development, a conditional allele was used; Setd2fl/fl, which was generated from embryonic stem cells as previously described58. This conditional line was crossed with mice carrying a codon-improved Cre recombinase under control of the Emx1 promoter (Emx1iCre/+)16, which has been used successfully previously by our lab18 and others59. Use of this Emx1iCre/+ line enables the ablation of Setd2 from neural stem cells of the dorsal telencephalon from approximately E10.5. This generated Setd2fl/+; Emx1iCre/+ progeny. These animals were crossed with Setd2flfl mice to generate control (Setd2fl/fl; Emx1+/+ or Setd2fl/+; Emx1+/+; hereafter referred to as controls) or homozygous (Setd2fl/fl; Emx1iCre/+; hereafter referred to as cKO mice) animals. All experiments were performed according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and according to the ARRIVE guidelines. Pregnant females were acquired by placing male and female mice together overnight. The next day, females were inspected for the presence of a vaginal plug. The day of birth was designated as postnatal day (P) 0. Mice were housed in Optimice IVC caging, with double HEPA filter and built in ventilation. Food and water were available ad libitum. Animals at P69–P72 were used in this study and were collectively termed “adult” mice. Mice were genotyped by PCR; primers are available on request (Supp. Table 1). The experiments performed within this manuscript were performed on the same cohort of mice (i.e. the same mice used for MRI analyses were subsequently used for histological and immunohistochemical analyses).
Magnetic resonance imaging
For volumetric and diffusion MRI analyses, adult Setd2 control (male = 6, female = 4) and Setd2 cKO (male = 6, female = 3) brains were used. Mice were deeply anesthetised with Lethabarb (1:50 dilution, 0.7–0.9 mL injection; VIRBAC PTY) and euthanised via transcardial perfusion with 0.1 M phosphate-buffered saline (PBS), followed by 4% (w/v) paraformaldehyde (pH 7; Sigma-Aldrich) in PBS. Before MRI scanning, the brains were washed in 0.1 M PBS with 0.2% v/v gadopentetate dimeglumine (Magnevist, Bayer, Leverkusen) for four days60. MRI data were acquired using a 16.4 T vertical bore microimaging system (Bruker Biospin, Rheinstetten; ParaVision v6.01) equipped with a Micro2.5 imaging gradient and a 15 mm linear surface acoustic wave coil (M2M, Brisbane, Australia). Three-dimensional (3D) T1/T2*-weighted FLASH structural images were acquired using a gradient echo imaging sequence with the following parameters: repetition time (TR) = 50 ms, echo time (TE) = 12 ms, bandwidth = 50 kHz, field of view (FOV) = 19.6 × 11.4 × 8.4 mm and matrix size = 654 × 380 × 280, which results in 30 μm isotropic image resolution, with an acquisition time of 30 min. 3D diffusion-weighted images (DWI) data were acquired using a Stejskal-Tanner DWI spin-echo sequence with TR = 200 ms, TE = 23 ms, δ/Δ = 2.5/12 ms, bandwidth = 50 kHz, FOV = 19.6 × 11.4 × 8.4 mm and matrix size = 196 × 114 × 84, image resolution = 100 μm, 30 direction diffusion encoding with b-value = 5000 s/mm2, two b = 0 images, with an acquisition time of 17 h. DWI datasets were zero-filled by a factor of 1.5 in all dimensions prior to Fourier transform to improve fibre tracking61.
To perform volumetric analyses, the Australian Mouse Brain Mapping Consortium atlases for whole brain (https://imaging.org.au/AMBMC/Model/), hippocampus (https://imaging.org.au/AMBMC/Hippocampus/), neocortex (https://imaging.org.au/AMBMC/Cortex/) and diencephalon (https://imaging.org.au/AMBMC/Diencephalon/) were registered to the FLASH images using FMRIB Software Library’s linear registration (FLIRT, fsl.fmrib.ox.ac.uk), followed by ANTs diffeomorphic registration (https://github.com/ANTsX/ANTs)62. Model-based segmentation of each atlases brain regions was performed on each sample and their volumes were measured using ITK-SNAP (https://www.itksnap.org/pmwiki/pmwiki.php)63. For all subsequent analyses, only the whole brain model atlas was used unless specified. To analyse the diffusion MRI data, image intensity were first bias corrected using ANTs N4BiasFieldCorrection and processed using MRtrix3 software (https://www.mrtrix.org/)64. Fiber orientation distribution (FOD) was reconstructed using constrained spherical deconvolution (CSD) method, and probabilistic tractography was performed using iFOD2 algorithm. Tractography was performed for specific major white matter tracts and ROIs, and for the whole brain for the structural connectome analyses. Firstly, the seeding regions of interest (ROIs) were manually drawn in the midsagittal and coronal sections of the colour vector map, and fibre tracks were generated for the corpus callosum, hippocampal commissure, and anterior commissure at 100 seeds per voxel. From these structures, parametric maps, including tract density imaging (TDI, which measures number of tracts within a voxel unit)65 and diffusion-tensor MRI (DTMRI) metrics, including fractional anisotropy (which measures the microstructural integrity of axonal tracts), and mean, radial and axial diffusivities (which measures the overall water motility, and in the perpendicular and parallel orientations to axonal bundles, respectively)66 were calculated for each structure and compared between control and Setd2 cKO brains. For non-forebrain commissure tractographic analyses (hippocampus and M1/S1 to thalamus), ROIs taken from the 106 node Centre for Advanced Imaging (CAI)-John Hopkins MRI atlas67 were overlaid on brains and used to guide tractography. Voxel based morphometry was performed by firstly creating a multivariate template image using the ANTs program and followed by FSL Randomise with 1000 permutations and threshold-free cluster enhancement (FWE-corrected, p < 0.05). To perform structural connectome analyses, whole-brain probabilistic tractography was generated using 10 seeds per voxel. The modified Centre for Advanced Imaging (CAI)-John Hopkins MRI atlas67 was used to segment the whole brain tractography into a connectivity matrix comprising of 106 nodes. The degree of a node in a network corresponds to the number of connections a node has with other nodes. The Network Based Statistic (NBS) toolbox30 was used to detect changes in the brain connectivity network between control and Setd2 cKO mouse brains. NBS results were examined using a range of primary thresholds (t = 2.5 to 3.5) to avoid false positive and bias from using a single threshold67. The final brain connectivity changes were presented using the results calculated at t = 2.8 based on the highest statistical significance (the lowest family wise error rate (FWER)-corrected p-value of any components). No differences in the volumetric and tractography analyses were found between sexes within each genotype in our dataset, hence results shown include combined genders.
Immunohistochemistry
Adult mice were transcardially perfused (0.1 M phosphate buffered saline, followed by 4% paraformaldehyde (PFA)) and postfixed in 4% PFA at 4 °C as described above. Brains were removed from the skull, embedded coronally in 3% agar and sectioned at 50 µm (Leica VT1000S Vibratome). Sections at bregma level − 1.955 mm were mounted on Superfrost + slides and dried in a 37 °C oven before heat-mediated antigen retrieval (NxGen Decloaker) was performed in 10 mM sodium citrate solution (90 °C for 10 min). Fluorescence immunohistochemistry (IF) was then performed as previously described68. Briefly, sections were incubated overnight with primary antibodies against target proteins (Supp. Table 2). The following day, sections were rinsed in phosphate buffered saline (PBS) and then incubated with the relevant secondary antibodies for 3 h at room temperature. A list of all antibodies used is listed in Supp. Table 2. Sections were rinsed in 0.9% saline and counterstained with 4’, 6-diamidino-2-phenylindole (DAPI) and mounted in fluorescence mounting media (DAKO).
Histological staining
Adult mice were transcardially perfused as described above. Sections at bregma levels 0.745 mm, 0.145 mm, − 0.755 mm, and − 1.955 m were stained with Haematoxylin and Eosin (H&E) using standard protocols69. For all H&E-stained images, sections were imaged using a brightfield slide scanner (Aperio XT Brightfield) at 40 × magnification (NA 0.75). Images were captured using ScanScope XT, and post-processed using Aperio ImageScope × 64. Images were exported as tiffs and quantified in blinded conditions (quantifications performed described in Supp. Figure 9A–D).
Imaging and cell counts
To capture higher resolution representative images, images were acquired on a Zeiss LSM900 Airyscan 2 confocal microscope at 20x (0.8 NA) magnification. Images were acquired as WF confocal images acquired as 25 μm tiled z-stacks (0.32 μm z-step), which were stitched and rendered as maximum intensity projections using ZEN Blue software. To quantify cell number and morphology within the whole cortex, 8 μm tiled z-stacks (0.49 μm z-step) of control and cKO brains were captured at 20 × magnification (0.8 NA) using a Zeiss Axioscan Z1 slide scanner with a Colibri 7-LED light source, pentaband emission filter set (112 MBP) and Hamamatsu Orca Flash 4 sCMOS camera. The tiled z-stacks were stitched and rendered as maximum intensity projections using ZEN Blue software. All fluorophores used are provided in Supp. Table 2.
For all IF analyses except DCX, PROX1, CC3 and proliferative marker quantifications, the StarDist plugin for ImageJ70 was used to automate the cell count process and minimise bias. Default settings (available from StarDist’s documentation) were used for all cell counts, except for the number of tiles—this was set to 100 to accommodate whole section counts for the largest sections to be performed. Counts were performed using StarDist as previously described71. For DCX counts, the DG was traced and isolated from all images (encompassing dendritic area for completeness). These files were then blinded and manually quantified for soma number and occupied length. For PROX1 cell counts, the DG was traced and isolated using DAPI to guide trace thickness. These files were then blinded and manually quantified for soma number and occupied area. Cells were counted in 1000 μm2 grids, with 2 adjacent grids (atop each other) being quantified at 3 different positions for each blade of the DG. For CC3 cell counts, images were blinded, and both hippocampi were manually counted and quantified in Zeiss Zen Blue. For KI-67, files were blinded and manually quantified using the Cell Counter functionality within ImageJ. For co-localised proliferative cell counts, multiple channels were stacked in ImageJ (Stack functionality) and then quantified using the Cell Counter functionality. For DG- or cortex-localised SOX2+ cells, the DG (using DAPI as a guide) or the cortex were traced and isolated. All cells contained within the blades of the DG (as well as the hilus), or the cortex (M1) were then quantified using StarDist as described above. For hippocampal and cortically located Olig2 cells, either the hippocampus (using CA and DG as landmarks for tracing) or the cortex (M1) were traced and isolated and were then quantified using StarDist as described above.
For all histological analyses, .tiffs were blinded and quantified manually in Fiji. A description of all software used is provided in Supp. Table 3, and descriptions of all analyses completed is provided in Supp. Table 4. All analyses were completed on an n = 5 for both genotypes unless stated otherwise.
Statistical analyses
All DTI data was validated using multiple unpaired t-tests—for all analyses which were analysing the same metric across different regions/structures, multiple comparisons were performed using a 5% false discovery rate (FDR), while analyses comparing different metrics across the same region/structure did not have multiple comparisons. Analyses of sex effects were performed using a 2-way ANOVA, with mice split by sex and genotype (data not shown). As no significant differences between sexes were found, sexes were pooled for all analyses. All IF and histological data were analysed using either multiple unpaired t-tests with Holm-Sidak correction, or Welch’s t-tests—multiple unpaired t-tests were used for all instances of multiple regions being analysed for the same measure within a graph (i.e. Fig. 5F), and Welch’s t-tests were used for all instances of a single measure on a single region (i.e. Fig. 5G). All data are presented as mean ± SEM. For all DTI analyses, 10 Ctrl and 9 cKO brains were used unless specified. For all histological and IF analyses, an n = 5 for both genotypes were used unless specified.
Data availability
Please contact the senior author (Michael Piper) for all data requests.
References
Michail, C., Rodrigues Lima, F., Viguier, M. & Deshayes, F. Structure and function of the lysine methyltransferase SETD2 in cancer: From histones to cytoskeleton. Neoplasia 59, 101090. https://doi.org/10.1016/j.neo.2024.101090 (2025).
Mitchell, B., Thor, S. & Piper, M. Cellular and molecular functions of SETD2 in the central nervous system. J. Cell Sci. https://doi.org/10.1242/jcs.261406 (2023).
Science, A. I. f. B. (Allen Institute, 2004). Allen Mouse Brain Atlas [69529053]. Available from mouse.brain-map.org/experiment/show/69529053
Xu, L. et al. Abnormal neocortex arealization and Sotos-like syndrome-associated behavior in setd2 mutant mice. Sci. Adv. https://doi.org/10.1126/sciadv.aba1180 (2021).
Roadmap Epigenomics, C. et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330. https://doi.org/10.1038/nature14248 (2015).
Pappas J, Rabin R. SETD2 Neurodevelopmental Disorders. 2021 Dec 30 [Updated 2022 Sep 22]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK575927/
Luscan, A. et al. Mutations in SETD2 cause a novel overgrowth condition. J. Med. Genet. 51, 512–517. https://doi.org/10.1136/jmedgenet-2014-102402 (2014).
Lumish, H. S., Wynn, J., Devinsky, O. & Chung, W. K. Brief Report: SETD2 mutation in a child with autism, intellectual disabilities and epilepsy. J. Autism. Dev. Disord. 45, 3764–3770. https://doi.org/10.1007/s10803-015-2484-8 (2015).
Parra, A. et al. Clinical heterogeneity and different phenotypes in patients with SETD2 variants: 18 new patients and review of the literature. Genes https://doi.org/10.3390/genes14061179 (2023).
Atterton, C. et al. Overgrowth-intellectual disability disorders: Progress in biology, patient advocacy and innovative therapies. Dis. Models Mech. https://doi.org/10.1242/dmm.052300 (2025).
Lu, P. et al. Spatiotemporal role of SETD2-H3K36me3 in murine pancreatic organogenesis. Cell Rep. 43, 113703. https://doi.org/10.1016/j.celrep.2024.113703 (2024).
Chang, J. et al. Setd2 determines distinct properties of intestinal ILC3 subsets to regulate intestinal immunity. Cell Rep. 38, 110530. https://doi.org/10.1016/j.celrep.2022.110530 (2022).
Fahey, C. C. & Davis, I. J. SETting the stage for cancer development: SETD2 and the consequences of lost methylation. Cold Spring Harb. Perspect. Med. https://doi.org/10.1101/cshperspect.a026468 (2017).
Carvalho, S. et al. SETD2 is required for DNA double-strand break repair and activation of the p53-mediated checkpoint. Elife 3, e02482. https://doi.org/10.7554/eLife.02482 (2014).
Hu, G. et al. Histone methyltransferase SETD2 is required for proper hippocampal lamination and neuronal maturation. Mol. Biol. Cell 35, ar54. https://doi.org/10.1091/mbc.E23-12-0492 (2024).
Kessaris, N. et al. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9, 173–179. https://doi.org/10.1038/nn1620 (2006).
Park, I. Y. et al. Dual chromatin and cytoskeletal remodeling by SETD2. Cell 166, 950–962. https://doi.org/10.1016/j.cell.2016.07.005 (2016).
Kasherman, M. A. et al. Abnormal behavior and cortical connectivity deficits in mice lacking Usp9x. Cereb. Cortex 31, 1763–1775. https://doi.org/10.1093/cercor/bhaa324 (2021).
Gorski, J. A. et al. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 22, 6309–6314. https://doi.org/10.1523/JNEUROSCI.22-15-06309.2002 (2002).
Janke, A. L. & Ullmann, J. F. Robust methods to create ex vivo minimum deformation atlases for brain mapping. Methods 73, 18–26. https://doi.org/10.1016/j.ymeth.2015.01.005 (2015).
Richards, K. et al. Segmentation of the mouse hippocampal formation in magnetic resonance images. Neuroimage 58, 732–740. https://doi.org/10.1016/j.neuroimage.2011.06.025 (2011).
Watson, C. et al. An ontologically consistent MRI-based atlas of the mouse diencephalon. Neuroimage 157, 275–287. https://doi.org/10.1016/j.neuroimage.2017.05.057 (2017).
Ullmann, J. F., Watson, C., Janke, A. L., Kurniawan, N. D. & Reutens, D. C. A segmentation protocol and MRI atlas of the C57BL/6J mouse neocortex. Neuroimage 78, 196–203. https://doi.org/10.1016/j.neuroimage.2013.04.008 (2013).
Avants B., Tustison N., Song G. "Advanced Normalization Tools: V1.0". The Insight Journal. 2009 Jul. http://hdl.handle.net/10380/3113
Kim, J. et al. Structural consequences of diffuse traumatic brain injury: A large deformation tensor-based morphometry study. Neuroimage 39, 1014–1026. https://doi.org/10.1016/j.neuroimage.2007.10.005 (2008).
Nguyen, V. T. et al. Magnetic resonance imaging and micro-computed tomography reveal brain morphological abnormalities in a mouse model of early moderate prenatal ethanol exposure. Neurotoxicol. Teratol. 77, 106849. https://doi.org/10.1016/j.ntt.2019.106849 (2020).
Leong, D. et al. Correlation of diffusion tensor imaging parameters in the canine brain. Neuroradiol. J. 28, 12–18. https://doi.org/10.15274/NRJ-2014-10110 (2015).
Anand, K. S. & Dhikav, V. Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 15, 239–246. https://doi.org/10.4103/0972-2327.104323 (2012).
Lin, Q. et al. Functional brain network alterations in the co-occurrence of autism spectrum disorder and attention deficit hyperactivity disorder. Eur. Child Adolesc. Psychiatry 33, 369–380. https://doi.org/10.1007/s00787-023-02165-0 (2024).
Zalesky, A., Fornito, A. & Bullmore, E. T. Network-based statistic: Identifying differences in brain networks. Neuroimage 53, 1197–1207. https://doi.org/10.1016/j.neuroimage.2010.06.041 (2010).
Oishi, S. et al. Heterozygosity for nuclear factor one X in mice models features of malan syndrome. EBioMedicine 39, 388–400. https://doi.org/10.1016/j.ebiom.2018.11.044 (2019).
Ogane, N. et al. Cleaved caspase-3 expression is a potential prognostic factor for endometrial cancer with positive peritoneal cytology. Cytopathology 29, 254–261. https://doi.org/10.1111/cyt.12550 (2018).
Abbott, L. C. & Nigussie, F. Adult neurogenesis in the mammalian dentate gyrus. Anat. Histol. Embryol. 49, 3–16. https://doi.org/10.1111/ahe.12496 (2020).
Favaro, R. et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat. Neurosci. 12, 1248–1256. https://doi.org/10.1038/nn.2397 (2009).
Yang, Z. & Wang, K. K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 38, 364–374. https://doi.org/10.1016/j.tins.2015.04.003 (2015).
Meng, H. et al. Quiescent adult neural stem cells: Developmental origin and regulatory mechanisms. Neurosci. Bull. 40, 1353–1363. https://doi.org/10.1007/s12264-024-01206-1 (2024).
Beard, D. C. et al. Distinct disease mutations in DNMT3A result in a spectrum of behavioral, epigenetic, and transcriptional deficits. Cell Rep. 42, 113411. https://doi.org/10.1016/j.celrep.2023.113411 (2023).
Christian, D. L. et al. DNMT3A haploinsufficiency results in behavioral deficits and global epigenomic dysregulation shared across neurodevelopmental disorders. Cell Rep. 33, 108416. https://doi.org/10.1016/j.celrep.2020.108416 (2020).
Mitchel MW, Myers SM, Heidlebaugh AR, et al. CHD8-Related Neurodevelopmental Disorder with Overgrowth. 2022 Oct 27. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK585456/
Alotaibi, M. & Ramzan, K. A de novo variant of CHD8 in a patient with autism spectrum disorder. Discoveries 8, 107. https://doi.org/10.15190/d.2020.4 (2020).
Bernier, R. et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 158, 263–276. https://doi.org/10.1016/j.cell.2014.06.017 (2014).
Katayama, Y. et al. CHD8 haploinsufficiency results in autistic-like phenotypes in mice. Nature 537, 675–679. https://doi.org/10.1038/nature19357 (2016).
Hurley, S. et al. Distinct, dosage-sensitive requirements for the autism-associated factor CHD8 during cortical development. Mol. Autism. 12, 16. https://doi.org/10.1186/s13229-020-00409-3 (2021).
Schoenfeld, T. J., McCausland, H. C., Morris, H. D., Padmanaban, V. & Cameron, H. A. Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biol. Psychiatry 82, 914–923. https://doi.org/10.1016/j.biopsych.2017.05.013 (2017).
Khelfaoui, H., Ibaceta-Gonzalez, C. & Angulo, M. C. Functional myelin in cognition and neurodevelopmental disorders. Cell Mol. Life Sci. 81, 181. https://doi.org/10.1007/s00018-024-05222-2 (2024).
Hammelrath, L. et al. Morphological maturation of the mouse brain: An in vivo MRI and histology investigation. Neuroimage 125, 144–152. https://doi.org/10.1016/j.neuroimage.2015.10.009 (2016).
Rahn, R. M. et al. Functional connectivity of the developing mouse cortex. Cereb. Cortex 32, 1755–1768. https://doi.org/10.1093/cercor/bhab312 (2022).
Shen, S., Shao, Y. & Li, C. Different types of cell death and their shift in shaping disease. Cell Death Discov. 9, 284. https://doi.org/10.1038/s41420-023-01581-0 (2023).
Wong, F. K. & Marin, O. Developmental cell death in the cerebral cortex. Annu. Rev. Cell Dev. Biol. 35, 523–542. https://doi.org/10.1146/annurev-cellbio-100818-125204 (2019).
Toda, T., Parylak, S. L., Linker, S. B. & Gage, F. H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 24, 67–87. https://doi.org/10.1038/s41380-018-0036-2 (2019).
Kempermann, G., Song, H. & Gage, F. H. Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol. 7, a018812. https://doi.org/10.1101/cshperspect.a018812 (2015).
Bond, A. M., Ming, G. L. & Song, H. What Is the relationship between hippocampal neurogenesis across different stages of the lifespan?. Front. Neurosci. 16, 891713. https://doi.org/10.3389/fnins.2022.891713 (2022).
Heng, Y. H. et al. NFIX regulates proliferation and migration within the murine svz neurogenic niche. Cereb. Cortex 25, 3758–3778. https://doi.org/10.1093/cercor/bhu253 (2015).
Chen, Z. et al. Single-nucleus chromatin accessibility and RNA sequencing reveal impaired brain development in prenatally e-cigarette exposed neonatal rats. iScience 25, 104686. https://doi.org/10.1016/j.isci.2022.104686 (2022).
Matsui, Y. & Peng, J. C. CUT & RUN to profile chromatin-bound proteins in primary mouse neural progenitor cells. Methods Mol. Biol. 2599, 99–111. https://doi.org/10.1007/978-1-0716-2847-8_8 (2023).
Zhang, D. et al. Spatial epigenome-transcriptome co-profiling of mammalian tissues. Nature 616, 113–122. https://doi.org/10.1038/s41586-023-05795-1 (2023).
Deng, Y. et al. Spatial-CUT&Tag: Spatially resolved chromatin modification profiling at the cellular level. Science 375, 681–686. https://doi.org/10.1126/science.abg7216 (2022).
Yuan, H. et al. Histone methyltransferase SETD2 modulates alternative splicing to inhibit intestinal tumorigenesis. J. Clin. Invest. 127, 3375–3391. https://doi.org/10.1172/JCI94292 (2017).
Stegeman, S. et al. Loss of Usp9x disrupts cortical architecture, hippocampal development and TGFbeta-mediated axonogenesis. PLoS ONE 8, e68287. https://doi.org/10.1371/journal.pone.0068287 (2013).
Kurniawan, N. D. MRI in the study of animal models of neurodegenerative diseases. Methods Mol. Biol. 1718, 347–375. https://doi.org/10.1007/978-1-4939-7531-0_21 (2018).
Tournier, J.-D., Calamante, F. & Connelly, A. MRtrix: Diffusion tractography in crossing fiber regions. Int. J. Imaging Syst. Technol. https://doi.org/10.1002/ima.22005 (2012).
Avants, B. B. et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044. https://doi.org/10.1016/j.neuroimage.2010.09.025 (2011).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31, 1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015 (2006).
Tournier, J. D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202, 116137. https://doi.org/10.1016/j.neuroimage.2019.116137 (2019).
Calamante, F. et al. Super-resolution track-density imaging studies of mouse brain: Comparison to histology. Neuroimage 59, 286–296. https://doi.org/10.1016/j.neuroimage.2011.07.014 (2012).
Alexander, A. L. et al. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. 1, 423–446. https://doi.org/10.1089/brain.2011.0071 (2011).
Liu, C. et al. Altered structural connectome in adolescent socially isolated mice. Neuroimage 139, 259–270. https://doi.org/10.1016/j.neuroimage.2016.06.037 (2016).
Piper, M. et al. NFIA controls telencephalic progenitor cell differentiation through repression of the Notch effector Hes1. J. Neurosci. 30, 9127–9139. https://doi.org/10.1523/JNEUROSCI.6167-09.2010 (2010).
Piper, M. et al. Nuclear factor one X regulates the development of multiple cellular populations in the postnatal cerebellum. J. Comp. Neurol. 519, 3532–3548. https://doi.org/10.1002/cne.22721 (2011).
Rueden, C. T. et al. Image J2: ImageJ for the next generation of scientific image data. BMC Bioinform. 18, 529. https://doi.org/10.1186/s12859-017-1934-z (2017).
Atterton, C. et al. The Hippo effector TEAD1 regulates postnatal murine cerebellar development. Brain Struct. Funct. 230, 42. https://doi.org/10.1007/s00429-025-02903-x (2025).
Acknowledgements
The work was funded by grants from the Simons Foundation Autism Research Initiative (Pilot award to MP and ST), the Australian Research Council (DP220100985 and DP230101750) to MP and ST, and a grant from the National Health and Medical Research Council of Australia to ST (2019372). CA and BM were supported by Research Training Program stipends from the Australian Government. We acknowledge the supports from the Queensland NMR Network and the National Imaging Facility (a National Collaborative Research Infrastructure Strategy capability) for the operation of 16.4 T MRI at the Centre for Advanced Imaging. We thank Matthew Lorincz and Cheryl Walker for the provision of the Setd2 conditional strain. The authors acknowledge the School of Biomedical Sciences Histology Facility and the School of Biomedical Sciences Microscopy and Analytics Facility for providing the excellent research environment and core facilities that enabled this research. Finally, we thank the animal team from UQ Biological Resources (QBI) for their exceptional care and housing of our animals.
Author information
Authors and Affiliations
Contributions
Study conception and design; CA, MP and ST. All authors contributed to material preparation, data collection and analysis. The first draft of the manuscript was written by CA, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript prior to publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Atterton, C., Naumann, H., Mitchell, B. et al. The histone methyltransferase SETD2 regulates adult brain structure, connectivity and neurogenesis. Sci Rep 15, 33411 (2025). https://doi.org/10.1038/s41598-025-18780-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18780-7