Abstract
Our study aims to investigate the effect of negative pressure wound therapy (NPWT) on microRNA-155 (miR-155) in the granulation tissue of patients suffering from diabetic foot ulcers (DFUs) and its correlation with wound healing. A total of sixty patients diagnosed with DFUs were randomly assigned to either the NPWT group (n = 40) or the Non-NPWT group (n = 20) in a 2:1 ratio. After debridement, the NPWT group received NPWT treatment for one week, while the Non-NPWT group underwent routine dressing therapy. The expression of miR-155 in DFU granulation tissues was evaluated by qRT-PCR before and after treatment for one week. Following termination, wound healing rates were assessed in the NPWT group, and the correlation between variations in miR-155 expression (ΔmiR-155) and wound healing was analyzed pre and post NPWT treatment. In vitro experiments were conducted to investigate the effects of negative pressure on variations of miR-155 expression, as well as proliferation, migration, and apoptosis in normal human dermal fibroblasts (NHDFs). The NPWT group showed a decrease in miR-155 expression in wound granulation tissue compared with pre-treatment [4.12 (1.22, 14.85) vs. 6.83 (2.15, 15.72), P < 0.05]. Conversely, there was no statistically significant difference in miR-155 expression in wound granulation tissue between pre-treatment and post-treatment in the Non-NPWT group (P > 0.05). However, analysis revealed a positive correlation between ΔmiR-155 and wound healing rate after 4 weeks in the NPWT group (χ2 = 4.829, P = 0.028). The in vitro experiments showed a significant decrease in miR-155 expression in NHDFs under negative pressure measured at -125 mmHg (P < 0.05). This reduction in miR-155 expression, in turn, enhanced the proliferation and migration ability while decreasing the apoptosis rate of NHDFs by targeting the upregulation of fibroblast growth factor 7 (FGF7) gene expression (P < 0.05). It is concluded that NPWT promotes DFU healing by reducing the expression of miR-155 in granulation tissue and the efficacy of NPWT correlated with altered miR-155 expression in wound tissue.
Similar content being viewed by others
Introduction
Diabetic foot (DF) causes significant morbidity and mortality in diabetic patients, making it a major global public health concern1. Diabetic foot ulcer (DFU) is the most frequent indication of diabetic foot disease and leading cause of amputation2. Standardized management, prevention, and early detection are critical to minimizing the incidence of diabetic amputations.
Negative pressure wound therapy (NPWT) uses a device that creates controlled negative pressure to facilitate would healing. This technique has gained significant traction as a treatment for DFUs and other chronic wounds over the past few years3. Numerous studies have demonstrated that NPWT enhances local blood flow, reduces local swelling, inhibits the growth of traumatic bacteria, and promotes the growth of granulation tissue and cell proliferation. Additionally, it aids in maintaining a moist environment in trauma and peritraumatic tissues, reduces the accumulation of traumatic exudate, and alleviates post-traumatic immunosuppression4. Despite these benefits, the exact mechanism of effectiveness remains uncertain.
Wound healing is a complex process that involves local cells, the vascular system, and the extracellular matrix following skin trauma. The process comprises three sequential and overlapping phases: the inflammatory phase, the proliferative phase, and the remodeling phase5. Fibroblasts, the functional cells responsible for wound healing, play an important role. Granulation tissue formation is a crucial aspect of wound healing that encompasses the activation, migration, proliferation, and extracellular matrix secretion of fibroblasts. Notably, the proliferation and differentiation of fibroblasts represent some of the most critical cellular activities6. Research has shown that defective fibroblast function in DFU wound tissue may impede wound healing, but the exact pathogenesis is still unclear7.
MicroRNAs (miRNAs) are a type of endogenous, non-coding, small, single-stranded RNA found in eukaryotes. They measure approximately 21–23 nucleotides in length and have significant involvement in cell metabolism, proliferation, and apoptosis throughout organisms. In recent years, multiple studies have confirmed the significant involvement of miRNAs regulatory disorders in the development of chronic wounds in diabetic patients8. miR-155, an essential member of the miRNA family, plays a critical role in the development and differentiation of the body’s immune cells, inflammatory responses, immune response, and various other biological processes9,10.
It has promising potential for the treatment of skin injuries and disorders. Furthermore, it can effectively assist in the healing of skin wounds for keratinocytes11, fibroblasts12, and dermal mesenchymal stem cells13, along with other cellular functions. Studies indicate14 that miR-155 expression upregulates in diabetic murine skin wounds. It is noteworthy that miR-155, through down-regulating fibroblast growth factor 7 (FGF7) expression, hampers wound re-epithelialization, and triggers pro-inflammatory consequences. This process directly affects the migration and proliferation of keratinocytes. Suppressing miR-155 led to a significant increase in M2-like macrophages and type I collagen levels in mouse wound tissues. This increase resulted in a decrease in M1-like macrophages and inflammation. Moreover, wound repair capacity was enhanced, as documented by prior research15. In a rat skin wound model with diabetes mellitus, local injection of a miR-155 inhibitor interfered with miR-155 expression, resulting in lower numbers of inflammatory cells like T-lymphocytes, neutrophils, and macrophages in the wound tissue14,16. This decrease was associated with lower levels of IL-1β and TNF-α, leading to reduced inflammation. Furthermore, there was a significant increase in neovascularization and enhancement of collagen content in the granulation tissue with a more regular arrangement, ultimately hastening the healing of wounds.
In previous studies, we found that several miRNA expression changes in the peripheral blood and wound tissue of DFU patients were closely related to the pathogenesis and prognosis of DFU17,18,19. Among them, the increase of miR-155 expression in wound tissue has a more significant adverse effect on the healing of DFU17. In addition, studies have found that NPWT can increase the expression of FGF7 in granulation tissue of DFU20. However, it remains unclear whether NPWT promotes wound healing by reducing the expression of miR-155 in DFU tissues. Therefore, the aim of our current investigation is to clarify alterations in miR-155 expression in NPWT-induced DFU tissues and their association with DFU recovery.
Materials and methods
Study subjects
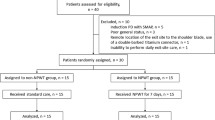
Sixty patients with DFU, hospitalized in the Department of Endocrinology at the First Affiliated Hospital of Anhui Medical University between July 2020 and December 2022, were selected for the study. The patients had foot ulcers for at least six weeks, with ulcer areas ranging from 2 to 20 cm2, Wagner grade II-III, and an ankle-brachial ratio (ABI) between 0.9 and 1.3. The study included 36 male and 24 female patients, with a mean age of (53.5 ± 10.9) years, and had diabetes mellitus for 4 to 19 years. Out of the total sample, 7 cases had type 1 diabetes mellitus, while 53 cases had type 2 diabetes mellitus. Based on clinical treatment needs and referring to randomized grouping methods in previous studies21, the patients were randomly assigned to either the NPWT treatment group (40 cases) or the Non-NPWT treatment group (20 cases) in a 2:1 ratio. All participants had no major heart, liver, or kidney problems, non-cancerous ulcers, and had not used glucocorticoids, immunosuppressants, estrogens, or external cytokines, such as epidermal growth factor, erythropoietin recombinant human granulocyte macrophage colony-stimulating factor, or recombinant human granulocyte colony-stimulating factor, in the previous 6 months. The research received approval from the Medical Ethics Committee of the First Affiliated Hospital of Anhui Medical University with the approval number LLSC20191038. Additionally, the participants provided informed consent.
Study methods
Clinical treatment protocol and retention of wound granulation tissue specimens
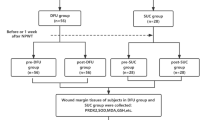
Our previous report provided the detailed protocol for clinical treatment and retention of wound tissue specimens22. Participants received conventional systemic treatment, which included anti-infective, anti-hypertensive, anti-hyperglycemic, and correction of hypoalbuminemic therapies. Debridement was performed to remove necrotic soft tissue and bone tissue in all the participants. Patients in the NPWT group received treatment using the VAC® Negative Pressure Assisted Healing Therapy System (KCI Inc., USA) after debridement. The treatment comprised continuous negative pressure suction at 125 mmHg for one week. Peripheral venous blood and wound granulation tissue were collected before VAC® treatment (on the day of debridement) and one week after VAC® treatment (on the day after removing the negative pressure device). In the Non-NPWT group, patients underwent routine dressing changes without any medical interventions or dressings with exogenous cytokines after debridement. Peripheral venous blood and wound granulation tissue on the day of debridement and one week after routine dressing change were also collected. In addition, follow-up observation was conducted on the complete wound healing of DFU after discontinuing VAC® treatment for four weeks in the NPWT group. Complete wound healing is defined as spontaneous complete closure of the wound, i.e. 100% re epithelialization.
Detection of observational indexes
Following a 10-hour fast, all participants underwent venipuncture of the median cubital vein between 8:00–8:30 am on the next morning. Blood was collected into specific anticoagulation tubes or non-anticoagulation tubes (selected according to different testing items) for measuring fasting blood glucose (FPG), glycosylated hemoglobin A1c (HbA1c), white blood cell count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), hemoglobin (Hb), and serum albumin (Alb). Digital photography was combined with ImageJ medical image analysis software (ImageJ, National Institutes of Health, USA) to measure the area of DFU. The Δ value, where Δvalue equaled post-treatment value minus pre-treatment value, was calculated based on indexes observed before and after one week of treatment.
Detection of miR-155 expression in Wound Granulation tissue before and after NPWT using qRT-PCR
The primers, which were synthesized by Sangon Biotech (Shanghai) Co., Ltd., for miR-155 consisted of an upstream sequence of 5’-CGGCGGTTAATGCTAATTGTGAT-3’ and a downstream sequence of 5’-GTGCAGGGTCCGAGGT-3’. The primers for U6 consisted of an upstream sequence of 5’-GCTTCGGCAGCAC-ATATACTAAAA-3’ and a downstream sequence of 5’-CGCTTCACGAATTTGCCTGTCAT-3’. The amplification process involved pre-denaturation at 95 °C for 5 min followed by denaturation at 95 °C for 20 s. Then, annealing occurred at 58 °C for 15 s, and extension happened at 72 °C for 10 s. To calculate the relative expression of miR-155, the 2−ΔΔCt method was used with U6 as the internal reference. This process was repeated 42 times in total. Each sample was repeated 3 times, and the final result was an average value.
Detection of FGF7 expression in Wound Granulation tissue pre- and post-treatment via western blot
The wound granulation tissue (100 mg) underwent homogenization and centrifugation at 12,000 r.min− 1 at 4 °C for 10 min. The protein concentration was measured using the bacterial artificial chromosome (BAC) method. The extracted protein was denatured at 100 °C for 5 min, and subsequently sampled, electrophoresed, membrane-transferred, and closed. The FGF7 protein primary antibody (1:400, Sigma) was added and incubated overnight. Following this, the secondary antibody (1:1000, Sigma) was added and incubated for 2 h. Enhanced chemiluminescence (ECL) was then used to develop resulting bands, which were subsequently scanned and converted into images. The gray values of target bands were analyzed using an image processing system. The ratio of FGF7 content to its own glyceraldehyde-3-phosphate dehydrogenase (GAPDH) within the granulation tissue was utilized to express relative expression.
Inflammatory factor expression in Granulation tissue before and after treatment by ELISA Method
The granulation tissue of the wound was retained and prepared into homogenates both before and after treatment in both groups. After centrifuging at 4 °C 3000 r.min− 1 for 15 min, the supernatants were separated. The concentrations of IL-1β, IL-6, IL-4, and IL-10 in the supernatant were determined through ELISA method in accordance with the kit instructions from Biovision Inc., USA.
Cell culture
Primary normal human dermal fibroblasts (NHDFs) were procured from the Cell Resource Center at the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. To passage cells, NHDFs were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and dual antibodies. Incubation was carried out at 37℃ and 5% CO2. The culture medium was replenished every 2–3 days, with cells from the sixth generation employed in subsequent experiments. Prior to the experiment, the cells underwent 24-hour serum starvation to synchronize. The intervention involved the addition of D-glucose, after which the NHDFs were collected and randomly divided into four groups. A normal control group (NG group, D-glucose 5 mmol/L), a high-glucose intervention group (HG group, D-glucose 30 mmol/L), a normal control group with silenced FGF7 expression (NG + si-FGF7 group, si-FGF7 transfected cells of normal control group), and a high-glucose intervention group with silenced FGF7 expression (HG + si-FGF7 group, si-FGF7 transfected cells exposed to high levels of glucose). The NHDFs underwent cell transfection by following the procedures outlined in the Lipofectamine 2000 reagent instruction manual and then cultured for 48 h. According to the literature report18, the NG group, HG group, NG + si-FGF7 group, and HG + si-FGF7 group were further divided into normal atmospheric pressure group (negative pressure 0 mmHg) and negative pressure model group (at -125 mmHg), respectively, namely NG-0 group, NG-125 group, HG-0 group, HG-125 group, NG + si-FGF7-0 group, NG + si-FGF7-125 group, HG + si-FGF7-0 group, and HG + si-FGF7-125 group. The incubator developed by our team generated negative pressure for the in vitro study of vacuum-assisted containment22. Negative pressure was maintained at -125 mmHg for 12 h. qRT-PCR and Western blot methods were utilized to evaluate miR-155 and FGF7 protein expression in NHDF cells before and after exposure to normal atmospheric pressure and negative pressure in the NG, HG, NG + si-FGF7, and HG + si-FGF7 groups, respectively.
Dual-luciferase reporter assay
Based on prior studies and anticipated outcomes from the TargetScan website, we utilized a dual luciferase reporter gene assay to validate FGF7 as a miR-155-regulated target gene in NHDF cells. We introduced a fragment of FGF7’s 3’ untranslated region (3’UTR) with binding and mutation sites into the PGL3 luciferase reporter gene vector, resulting in the construction of both wild-type vector WT-FGF7 and mutant vector MUT-FGF7. WT-FGF7 and MUT-FGF7 were both co-transfected into NHDF cells alongside miR-155 mimics and miR-con, respectively. After being cultured for 48 h in an incubator at 37 °C, the cells were harvested and analyzed for luciferase activity using the Luciferase Activity Assay Kit (Promega, USA) following the manufacturer’s instructions.
Determination of NHDFs viability under Vacuum-assisted Closure
Cell viability was evaluated using the CCK-8 kit (Biosharp, China) before and after negative pressure treatment. After proper digestion in each group, cells were resuspended in medium at a density of 1 × 105 cells per 100 µL and added to 96-well plates. The cell culture plates were incubated overnight in a 5% CO2 incubator at 37℃. After 48 h, each well was supplemented with 10 µL of CCK8 and incubated. Finally, the absorbance value of each well was measured using the ELISA detector at optical density (OD) 450 nm.
Determination of NHDFs migration capacity under vacuum-assisted closure
Transwell chambers were used to assess the alteration in cell migration ability following negative pressure treatment. Cells from each group were suspended in culture medium and adjusted to a concentration of 1 × 105 cells/mL. 100 µL of cell suspension was added to the upper chamber of the Transwell, and 600 µL of endothelial cell growth medium-2 (EGM-2) medium containing 50 µg/L of vascular endothelial growth factor (VEGF) was added to the lower chamber. The cells were incubated at 37℃ for 12 h before being removed from the Transwell and discarding the culture medium. After rinsing them twice with phosphate buffered saline (PBS), the cells were fixed for 15 min using 4% paraformaldehyde. Subsequently, they were air-dried at room temperature and stained with 0.1% crystal violet for 30 min. The upper layer of non-migrated cells was gently wiped using a cotton swab, and the sample was repeatedly rinsed three times with PBS solution. Finally, the number of migrating cells in five randomly selected fields was counted under the microscope.
NHDFs apoptosis assay under vacuum-assisted closure
Apoptosis was assessed pre- and post-treatment with negative pressure utilizing the V-FITC/PI Apoptosis Detection Kit (Solarbio, China). Briefly, NHDFs were suspended in 100 µL of Binding Buffer, then incubated with 5 µL of V-FITC for 15 min at 4 °C, while protected from light. This was followed by incubation with 5 µL of PI for 5 min at 4 °C. The resulting samples were promptly examined using flow cytometry.
Statistical analysis
Statistical analyses were conducted utilizing SPSS 19.0 software. The measurement data was presented as mean ± standard deviation (x̅ ± s). Paired t-tests were utilized to compare before-and-after treatment results within each group. Independent samples t-tests were used to compare the results between the two groups. One-way ANOVA was carried out to compare the results among various groups, and LSD-t analysis was used for further comparison between two groups. Non-normally distributed measurements were presented as the median and interquartile range [M (P25, P75)]. The rank sum test was used. A kappa test examined wound healing rate at four weeks between distinct groups. A Spearman correlation analysis was executed in order to evaluate the correlation between ΔmiR-155 and changes in other indices in the NPWT and Non-NPWT groups. The difference was regarded statistically significant at P < 0.05.
Results
Baseline data comparison between NPWT and Non-NPWT groups
There were no statistically significant differences foundbetween the NPWT and Non-NPWT groups in terms of age, gender composition, duration of diabetes and DFU, Wagner grade, DFU area, FPG, HbA1c, ABI, CRP, WBC, ESR, Hb, Alb, as well as the expression of T-IL-1β, T-IL-6, T-IL-4, T-IL-10, T-miR-155, and T-FGF7 proteins in the wound granulation tissues (P > 0.05). Please refer to Table 1 for details.
Comparison of observational indicators between NPWT and Non-NPWT groups
Both the NPWT and Non-NPWT groups exhibited significantly decreased FPG, CRP, WBC, T-IL-1β, and T-IL-6 (P < 0.05) after treatment compared to pretreatment. In contrast, the protein expression levels of T-IL-4, T-IL-10, and T-FGF7 were significantly increased (P < 0.05). Additionally, T-miR-155 expression was significantly reduced after treatment in the NPWT group compared to before treatment (P < 0.05), while there was no statistical difference in the Non-NPWT group. Refer to Table 2 for details.
Comparison of Δ values of observed indices before and after treatment between NPWT and Non-NPWT groups
The study results, displayed in Table 3, indicate a marked increase in T-IL-4 (ΔT-IL-4), T-IL-10 (ΔT-IL-10), and T-FGF7 (ΔT-FGF7), along with a decrease in ULA (ΔULA), CRP (ΔCRP), WBC (ΔWBC), T-IL-1β (ΔIL-1β), T-IL-6 (ΔIL-6), and T-miR-155 (ΔT-miR-155), all significantly higher than those in the Non-NPWT group (P < 0. 05). The differences in ΔFPG, ΔABI, ΔESR, ΔHb, and ΔAlb between the two groups were not statistically significant (P > 0.05).
Spearman’s correlation analysis
Table 4 showed that ΔT-miR-155 was positively correlated with ΔULA, ΔCRP, ΔWBC, ΔT-IL-1β, ΔT-IL-6 in the NPWT group, and was negatively correlated with ΔT-IL-4, ΔT-IL-10, and ΔT-FGF7 (P < 0.05). However, ΔT-miR-155 in the Non-NPWT group exhibited no correlation with the changes in other clinical indicators (P > 0.05).
Relationship between ΔmiR-155 and clinical characteristics of DFU in the NPWT group
To further validate the clinical significance of miR-155 expression alteration in wound granulation tissue following NPWT treatment, we defined a low alteration of miR-155 group (LCG group) as values below the median of ΔT-miR-155 in the NPWT group, and a high alteration of miR-155 group (HCG group) as values equal to or above the median. A comparison of the clinically relevant features of DFUs showed that the LCG cohort demonstrated a significantly greater rate of DFU recovery compared to the HCG cohort after four weeks (χ2 = 4.829, P = 0.028). There were no disparities in other DFU clinical traits between the LCG and HCG groups. Kindly refer to Table 5 for details.
Altered expression of miR-155 and FGF7 in NHDFs under Vacuum-assisted Closure
Under normal atmospheric pressure conditions, the NHDFs in the HG group exhibited a noteworthy increase in miR-155 expression and a significant decrease in FGF7 protein expression in comparison to the NG group (P < 0.05). When subjected to -125 mmHg atmospheric pressure compared to normal conditions, both the NG and HG groups presented notable reductions in miR-155 expression and significant increases in FGF7 protein expression (P < 0.05). For visual representation, please refer to Fig. 1A and B, and Fig. 1C. The original version of Fig. 1B can be found in the Supplementary Information file.
Expression of miR-155 and FGF7 protein in the NHDFs under negative pressure at 0 mmHg and − 125 mmHg. (A) The expression of miR-155 was detected by qRT-PCR. (B) Representative images of FGF7 expression detected by Western blotting analysis in the NHDFs. (C) The protein expression of FGF7 was detected by Western blot and quantified by the image processing system. *P < 0.05 vs. NG-0 group, #P < 0.05 vs. HG-0 group; △P < 0.05 vs. NG + si-FGF7-0 group, ▲P < 0.05 vs. HG + si-FGF7-0 group. n = 3 in each group. NHDFs, human dermal fibroblasts; FGF7, fibroblast growth factor 7; NG-0: normal control (5 mmol/L D-glucose) at negative pressure 0 mmHg group; NG-125: normal control (5 mmol/L D-glucose) at negative pressure − 125 mmHg group; HG-0: high-glucose intervention (30 mmol/L D-glucose) at negative pressure 0 mmHg group; HG-125: high-glucose intervention (30 mmol/L D-glucose) at negative pressure − 125. mmHg group; NG + si-FGF7-0: normal control (5 mmol/L D-glucose) transfected with si-FGF7 at negative pressure 0 mmHg group; NG + si-FGF7-125: normal control (5 mmol/L Dglucose) transfected with si-FGF7 at negative pressure − 125 mmHg group; HG + si-FGF7-0: high-glucose intervention (30 mmol/L D-glucose) transfected with si-FGF7 at negative pressure 0 mmHg group; HG + si-FGF7-125: high-glucose intervention (30 mmol/L D-glucose) transfected with si-FGF7 at negative pressure − 125 mmHg group.
Targeted regulation of FGF7 expression by miR-155
Targeted regulation of FGF7 expression was predicted by miR-155’s TargetScan, which revealed the existence of complementary nucleotide sequences between miR-155 and FGF7. This indicates that FGF7 expression is being targeted, as seen in Fig. 2A. The dual luciferase reporter gene assay demonstrated that miR-155 co-transfection effectively suppressed the relative luciferase activity of the 3’UTR reporter gene of wild-type FGF7 (WT-FGF7) (P < 0.05). In contrast, miR-155 had no significant effect on the luciferase activity of the mutant FGF7 3’UTR reporter gene (MUT-FGF7) (Fig. 2B). Transfection with miR-155 resulted in a significant inhibition of FGF7 protein expression, while transfection with anti-miR-155 significantly increased FGF7 protein levels (P < 0.05) (shown in Fig. 2C and Fig. 2D). To view the original version of Fig. 2C, please refer to the Supplementary Information file.
Dual-luciferase reporter gene assay. (A) The miR-155 and FGF7 had complementary nucleotide sequences. (B) The luciferase activity of miR-155 group and miR-con group after transfection of the FGF7-3’UTR WT and MUT vector plasmid. (C) and (D) The protein expression of FGF7 was detected in the NHDFs transfected with miR-155 or anti-miR-155. *P <. 0.05 vs. miR-con group; #P < 0.05 vs. anti-miR-con group; n = 3 in each group. NHDFs, human dermal fibroblasts; FGF7, fibroblast growth factor 7.
Changes in proliferation, migration capacity and apoptosis of NHDFs under vacuum-assisted closure
FGF7 protein in the NG + si-FGF7 group was significantly lower (P < 0.05) compared to the NG and HG groups, indicating successful transfection, as depicted in Fig. 1C. Compared to the NG group, human NHDFs in the HG group experienced significant reductions in both their proliferative and migratory abilities under normal atmospheric pressure, as well as a significantly increased rate of apoptosis (P < 0.05). At an atmospheric pressure of -125 mmHg, the NHDFs in both the NG and HG groups exhibited significant increases in proliferation and migration abilities, along with significant decreases in the apoptosis rate (P < 0.05). When FGF7 protein expression was silenced, there was no effect observed on the proliferation ability, migration ability, or apoptosis rate of NHDFs under negative pressure (P < 0.05). Refer to Fig. 3A and B, and 3C for visual representation.
Effects of negative pressure on proliferation and migration of NHDFs. (A) Cell proliferation was monitored by CCK-8 assay. (B) Cell migration was tested. using Transwell inserts. (C) Apoptosis rate of NHDFs was determined by flow. cytometry. *P < 0.05 vs. NG-0 group, #P < 0.05 vs. HG-0 group. n = 3 in each group. NHDFs, human dermal fibroblasts; FGF7, fibroblast growth factor 7; NG-0: normal. control (5 mmol/L D-glucose) at negative pressure 0 mmHg group; NG-125: normal control (5 mmol/L D-glucose) at negative pressure − 125 mmHg group; HG-0: high-glucose intervention (30 mmol/L D-glucose) at negative pressure 0 mmHg group; HG-125: high-glucose intervention (30 mmol/L D-glucose) at negative pressure − 125. mmHg group; NG + si-FGF7-0: normal control (5 mmol/L D-glucose) transfected with si-FGF7 at negative pressure 0 mmHg group; NG + si-FGF7-125: normal control (5 mmol/L Dglucose) transfected with si-FGF7 at negative pressure − 125 mmHg group; HG + si-FGF7-0: high-glucose intervention (30 mmol/L D-glucose) transfected with si-FGF7 at negative pressure 0 mmHg group; HG + si-FGF7-125: high-glucose intervention (30 mmol/L D-glucose) transfected with si-FGF7 at negative pressure − 125 mmHg group.
Discussion
A significant decrease in miR-155 expression and an increase in FGF7 protein expression were identified in the wound granulation tissues of DFU patients who underwent 1 week of NPWT. Notably, no significant changes in miR-155 or FGF7 expression were observed in the group that received routine dressing therapy (Non-NPWT). Further analysis demonstrated a direct correlation between the degree of down-regulated expression of miR-155 in wound granulation tissue of the NPWT group, and the rate of wound healing. Hence, a greater extent of miR-155 down-regulation in this tissue resulted in higher rates of wound healing. An in vitro study revealed a significant increase in miR-155 expression in NHDFs under a high-glucose environment, while miR-155 expression decreased substantially under vacuum-assisted closure conditions. Regulating FGF7 gene expression significantly improved NHDFs’ proliferation and migration, while reducing apoptosis rates. These findings suggest that miR-155 expression suppression followed by FGF7 upregulation facilitates wound healing with NPWT. miR-155 is proposed to be a biomarker for gauging NPWT effectiveness. Our research represents the inaugural study demonstrating the role of NPWT in modulating miR-155 expression levels in DFU wound tissue and its impact on DFU healing.
Both animal experiments and clinical studies23,24 have confirmed that short-term use of NPWT leads to significant alterations in the expression of many gene profiles, including miRNAs that play a role in various physiological processes such as wound remodeling, cell adhesion, inflammation, growth factors, and signaling pathways in trabecular tissue. However, the regulatory mechanism through which NPWT affects miR-155 expression in DFU wound tissue remains unknown.
It has been suggested25,26 that trauma-induced inflammation critically governs miRNA expression within the organis. This study reports decreased wound inflammation levels in both NPWT and Non-NPWT groups after a week of treatment, manifested in a significant decrease in peripheral blood CRP and WBC levels and the expression of pro-inflammatory factors IL-1βand IL-6 in traumatic tissues, coupled with a marked increase in anti-inflammatory factors IL-4 and IL-10. But in terms of improvement, the NPWT group is superior to the Non NPWT group, consistent with our previous research results21,22. Moreover, subsequent analysis suggested a significant correlation between ΔmiR-155 and changes in inflammatory indicator values, but only in the NPWT group. Recently, a study27 demonstrated that inflammatory factors can regulate miR-155 expression through multiple mechanisms. It was hypothesized that the differences in miR-155 expression between the two groups could be attributed to changes in inflammatory status. It is worth noting that the significant improvement in wound inflammation in the NPWT group may also be partially attributed to the use of antibiotics and high-quality debridement. Therefore, when evaluating the correlation between changes in miRNA-155 expression in wound granulation tissue and changes in inflammatory factors in the NPWT group, these confounding factors need to be fully considered. Further rigorous research designs are needed to confirm this. Additionally, research has shown that NPWT can modify DNA methylation, regulate gene expression, and alter epigenetic inheritance to affect the healing process of DFUs28. Further research is required to examine the mechanisms underlying the effect of NPWT on miR-155 expression in DFU wound granulation tissue.
This study found that NPWT significantly decreased miR-155 expression in the wound granulation tissue of DFU patients, thereby effectively enhancing DFU healing. Xu M et al. found that the increase in miR-155 expression levels in peripheral blood and wound edge tissues is closely related to the poor prognosis of DFU, which supports our findings17. Nevertheless, Yang L et al. revealed that miR-155 has a promoting effect on wound healing, which may be mediated by upregulating MMP-2 levels to accelerate the migration of keratinocytes11. The above findings are inconsistent with the results of this study. Upon investigation, it is speculated that Yang L et al. explored the impact of changes in miR-155 expression on wound healing under non high glucose conditions, while this study investigated the impact of changes in miR-155 expression on wound healing under high glucose and negative pressure conditions. The specific mechanism of discrepancy needs further exploration.
To further explore the molecular mechanism by which NPWT downregulates miR-155 expression in granulation tissue of DFU wounds and promotes wound healing, we conducted in vitro studies. In vitro experiments, we demonstrated a significant increase in miR-155 expression in NHDFs under high glucose conditions. Furthermore, we observed that high miR-155 expression causes a reduction in NHDFs’ migration and proliferation ability and increases their apoptosis rate. This suggests that the elevated miR-155 expression in diabetic patients could be one of the contributing factors for DFUs to become chronic and difficult to heal wounds. These findings support our prior research17. A negative correlation between miR-155 expression and FGF7 protein expression in the granulation tissue in the NPWT group was demonstrated through a correlation analysis. Previous studies have confirmed that miR-155 targets the FGF7 gene in keratinocytes and causes downregulation of its expression. Conversely, upregulation of FGF7 expression can enhance keratinocyte proliferation, migration, wound blood flow reconstruction, and antimicrobial capacity, thus promoting wound healing14,29. Our study also confirms that miR-155 targets the FGF7 gene in NHDFs. The in vitro experiments showed that NPWT significantly reduced miR-155 expression in NHDFs, increased the proliferation and migration capacity of NHDFs, and decreased the apoptosis rate in both low-glucose and high-glucose environments. This effect was attributed to miR-155 regulation of FGF7 expression. Additional research is needed to investigate whether miR-155 exerts its impact via alternative molecular mechanisms.
The study had limitations, as it was only conducted in a single center with a relatively small sample size, which may have resulted in selection bias. Thus, further research with larger sample size in multicencer trials is necessary to validate the findings. Second, the four-week follow-up time after NPWT was short. To better compare the long-term outcomes and recurrence rates of DFU after NPWT and non-NPWT treatments, studies with a longer follow-up time with at least half a year is required. Moreover, our findings might have been impacted by the hypoglycemic medications that were administered to our included patients. However, it should be mentioned that practically every patient in this trial was on insulin when they first entered the study. Hypoglycemic therapy is therefore unlikely to have affected the study’s findings, but we cannot rule out the possibility of other known and unknown causes. Additionally, the current research was unable to uncover the underlying mechanism of miR-155 expression change in DFU wound tissue due to NPWT treatment. Our research provides a hint that miR-155 may act as a potential factor in promoting wound healing of DFUs. More studies are imperative to investigate the effects of NPWT on miR-155 expression alteration and to assess miR-155’s potential as an indicator of NPWT’s efficacy in DFU treatment. Last but not least, further exploration of the downstream molecular effects of miR-155 regulation and FGF7 upregulation, including other relevant pathways in wound healing, could enhance the depth of the study and is warranted in the near future.
Data availability
All data generated or analysed during this study and supporting our findings are included and can be found in the manuscript. The raw data can be provided by corresponding author on reasonable request.
Abbreviations
- NPWT:
-
negative-pressure wound therapy group
- Non-NPWT:
-
without receiving NPWT group
- ULA:
-
ulcer area
- FPG:
-
fasting plasma glucose
- HbA1c:
-
glycated hemoglobin A1c
- ABI:
-
ankle brachial index
- CRP:
-
C-reactive protein
- WBC:
-
white blood cell
- ESR:
-
erythrocyte sedimentation rate
- Hb:
-
hemoglobin
- Alb:
-
albumin
- IL:
-
interleukin
- T-IL-1β:
-
concentration of IL-1β in the supernatant of granulation tissue
- T-IL-6:
-
concentration of IL-6 in the supernatant of granulation tissue
- T-IL-4:
-
concentration of IL-4 in the supernatant of granulation tissue
- T-IL-10:
-
concentration of IL-10 in the supernatant of granulation tissue
- T-miR-155:
-
miR-155 expression in wound granulation tissue
- T-FGF7:
-
FGF7 protein expression in wound granulation tissue
References
Mishra, S. C., Chhatbar, K. C., Kashikar, A. & Mehndiratta, A. Diabet. foot BMJ ; 359:j5064. (2017).
Armstrong, D. G., Tan, T. W., Boulton, A. J. M. & Bus, S. A. Diabetic foot ulcers: a review. JAMA 330 (1), 62–75 (2023).
Ji, S. et al. Consensus on the application of negative pressure wound therapy of diabetic foot wounds. Burns Trauma. 9, tkab018 (2021).
Chen, L. et al. A systematic review and meta-analysis of efficacy and safety of negative pressure wound therapy in the treatment of diabetic foot ulcer. Ann. Palliat. Med. 10 (10), 10830–10839 (2021).
Baron, J. M., Glatz, M. & Proksch, E. Optimal support of wound healing: new insights. Dermatology 236 (6), 593–600 (2020).
Boehnke, K. et al. Effects of fibroblasts and microenvironment on epidermal regeneration and tissue function in long-term skin equivalents. Eur. J. Cell. Biol. 86 (11–12), 731–746 (2007).
Liu, Y. et al. Fibroblasts: immunomodulatory factors in refractory diabetic wound healing. Front. Immunol. 13, 918223 (2022).
Goodarzi, G., Maniati, M. & Qujeq, D. The role of microRNAs in the healing of diabetic ulcers. Int. Wound J. 16 (3), 621–633 (2019).
Vigorito, E., Kohlhaas, S., Lu, D. & Leyland, R. miR-155: an ancient regulator of the immune system. Immunol. Rev. 253 (1), 146–157 (2013).
Arbore, G. et al. MicroRNA-155 is essential for the optimal proliferation and survival of plasmablast B cells. Life Sci. Alliance. 2 (3), e201800244 (2019).
Yang, L. et al. miR-155 promotes cutaneous wound healing through enhanced keratinocytes migration by MMP-2. J. Mol. Histol. 48 (2), 147–155 (2017).
Shojania, H. R., Momeni-Moghaddam, M., Hossini, S. E., Armin, M. & Omrani Bidi, J. MicroRNA 155 downregulation by vitamin C-Loaded human serum Albumin nanoparticles during Cutaneous Wound Healing in mice. Int. J. Low Extrem Wounds. 18 (2), 143–152 (2019).
Hou, R. X. et al. Increased mir-155-5p expression in dermal mesenchymal stem cells of psoriatic patients: comparing the microRNA expression profile by microarray. Genet. Mol. Res. 15(3). https://doi.org/10.4238/gmr.15038631 (2016).
Moura, J. et al. microRNA-155 inhibition restores fibroblast growth factor 7 expression in diabetic skin and decreases wound inflammation. Sci. Rep. 9 (1), 5836 (2019).
van Solingen, C., Araldi, E., Chamorro-Jorganes, A., Fernández-Hernando, C. & Suárez, Y. Improved repair of dermal wounds in mice lacking microRNA-155. J. Cell. Mol. Med. 18 (6), 1104–1112 (2014).
Ye, J. et al. MicroRNA-155 inhibition promoted wound healing in diabetic rats. Int. J. Low Extrem Wounds. 16 (2), 74–84 (2017).
Xu, M. et al. Increased expression of miR-155 in peripheral blood and wound margin tissue of type 2 diabetes mellitus patients associated with diabetic foot ulcer. Diabetes Metab. Syndr. Obes. 15, 3415–3428 (2022).
Wu, T. et al. Enhanced expression of miR-34c in peripheral plasma associated with diabetic foot ulcer in type 2 diabetes patients. Diabetes Metab. Syndr. Obes. 14, 4263–4273 (2021).
Li, X., Tang, Y., Jia, Z., Zhao, X. & Chen, M. Decreased expression of miR-24 in peripheral plasma of type 2 diabetes mellitus patients associated with diabetic foot ulcer. Wound Repair. Regen. 28 (6), 728–738 (2020).
Jia, Z. et al. Proteomics changes after negative pressure wound therapy in diabetic foot ulcers. Mol. Med. Rep. 24 (6), 834 (2021).
Mu, S. et al. Effect of negative-pressure wound therapy on the circulating number of peripheral endothelial progenitor cells in diabetic patients with mild to moderate degrees of ischaemic foot ulcer. Vascular 27 (4), 381–389 (2019).
Liu, L. et al. Downregulation of hsa-miR-203 in peripheral blood and wound margin tissue by negative pressure wound therapy contributes to wound healing of diabetic foot ulcers. Microvasc Res. 139, 104275 (2022).
Borys, S. et al. Negative pressure wound therapy in the treatment of diabetic foot ulcers may be mediated through differential gene expression. Acta Diabetol. 56 (1), 115–120 (2019).
Derrick, K. L., Norbury, K., Kieswetter, K., Skaf, J. & McNulty, A. K. Comparative analysis of global gene expression profiles between diabetic rat wounds treated with vacuum-assisted closure therapy, moist wound healing or gauze under suction. Int. Wound J. 5 (5), 615–624 (2008).
Kapusta, P. et al. Negative pressure wound therapy affects circulating plasma microRNAs in patients with diabetic foot ulceration. Diabetes Res. Clin. Pract. 165, 108251 (2020).
Leffler, M. et al. Changes of anabolic processes at the cellular and molecular level in chronic wounds under topical negative pressure can be revealed by transcriptome analysis. J. Cell. Mol. Med. 15 (7), 1564–1571 (2011).
Chen, M., Wang, F., Xia, H. & Yao, S. MicroRNA-155: regulation of immune cells in sepsis. Mediators Inflamm, 2021:8874854. (2021).
Ludwig-Slomczynska, A. H. et al. DNA methylation analysis of negative pressure therapy effect in diabetic foot ulcers. Endocr. Connect. 8 (11), 1474–1482 (2019).
Peng, C. et al. Lack of FGF-7 further delays cutaneous wound healing in diabetic mice. Plast. Reconstr. Surg. 128 (6), 673e–684e (2011).
Acknowledgements
We are grateful to the all patients for participating in the study. We thank the participants of this study including the doctors, nurses, and researchers from the Department of Endocrinology in the First Affiliated Hospital of Anhui Medical University.
Funding
This study was supported by the Natural Science Foundation of Anhui Province in China (2108085MH269), the Natural Science Research Project of Colleges and Universities in Anhui Province (KJ2021A0274). The funding body had no role in the design of the study, or the collection, analysis, and interpretation of data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
YH was involved in the overall study, designed the analysis plan, analyzed the data, and wrote the manuscript. YY, MX and XZ recruited and collected clinical samples. XZ, LL and YT collected the data and analyzed the data. MC and DT contributed to the discussion and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the medical ethics committee of the First Affiliated Hospital of Anhui Medical University as LLSC20191038, and informed consent was obtained from the subjects.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, Y., Yu, Z., Xu, M. et al. Negative pressure wound therapy promotes wound healing by down-regulating miR-155 expression in granulation tissue of diabetic foot ulcers. Sci Rep 15, 6733 (2025). https://doi.org/10.1038/s41598-025-90643-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-90643-7