Abstract
Patients with colorectal cancer (CRC) carrying KRAS mutations face a challenging prognosis, especially due to their reduced response to EGFR inhibitor therapies. Despite the use of drugs targeting the KRASG12C mutation, the KRASG12V mutation is more common in CRC, and unfortunately, there are currently no effective targeted treatments for it. Our study shows that patients harboring KRASG12V mutation often have larger tumors, increased lymph node metastasis, elevated EGFR expression, and a tendency for right-sided colon tumors. This indicates distinct clinical and pathological traits in CRC patients with KRASG12V. Cellular studies reveal increased proliferation and decreased cell apoptosis in KRASG12V CRC cells. Bioinformatics analysis revealed a notable decrease of aquaporin 9 (AQP9) in KRASG12V CRC, confirmed by immunohistochemistry and Western blot tests. These tests showed a consistent AQP9 decrease in tissue and cell samples, linked to an increased risk of lymph node metastasis in patients with low AQP9. Importantly, AQP9 overexpression was found to hinder CRC cell proliferation and encourage apoptosis, thereby implying a potential therapeutic role for AQP9 modulation. Our study finds a link between ZHX2 and AQP9 in CRC cells, confirmed by histopathological and in vitro evidence. Increased ZHX2 expression elevates AQP9 levels, reduces CRC cell growth, and boosts apoptosis. CO-IP experiments further prove the interaction between ZHX2 and AQP9 proteins. Molecular docking studies reveal that ZHX2 can form stable complexes with AQP9, involving multiple residues. This research enhances our understanding of the molecular mechanisms regulating the growth and death of KRASG12V CRC cells, paving the way for new therapeutic strategies.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is a prevalent malignancy globally, ranking third in incidence and second in mortality among all cancers, with both rates showing a rising trend annually1. Alterations in the KRAS oncogene, specifically mutations, represent a significant genetic modification in human CRC, with an incidence rate of approximately 40% in all diagnosed cases2. Currently, aside from a minority of patients harboring KRASG12C mutation benefiting from small-molecule targeted drugs like sotorasib and adagrasib3,4, there are no effective targeted therapies available for CRC patients with other KRAS mutation types. Thus, investigating tumor-associated signaling changes and mechanisms of disease progression related to other KRAS mutation sites remains imperative.
Mutations in KRAS are strongly associated with disease progression in CRC patients5,6,7. KRAS plays a pivotal regulatory role in cellular signaling pathways, including the RAS-PI3K-AKT and RAS-RAF-MAPK pathways involved in cell proliferation8, as well as the RAS-GEF pathway linked to cytokine production9. KRAS gene consists of four coding exons and one non-coding exon, with the second exon having the highest mutation frequency, directly correlating with adverse prognosis and drug resistance10. The most frequent point mutations in KRAS gene occur at glycine 12 (G12), glycine 13 (G13), and glutamine 61 (Q61)11.
KRASG12C occurs in approximately 12% of non-small cell lung cancers. Mutation of glycine replaced by cysteine at the 12th amino acid in exon 2 promotes the active state of the KRAS protein, leading to predominance of the GTP-bound form of KRAS cancer protein, enhancing tumor cell proliferation and survival capabilities. The mutated cysteine is located near the pocket (P2) of the switch II region, which is only accessible in the inactive GDP-bound conformation of KRAS, and has been targeted for designing covalent inhibitors of KRASG12C 4. Numerous clinical studies have demonstrated the efficacy of the small molecule targeted drug Sotorasib in KRASG12C patients4, which has been FDA-approved for subsequent therapy post first-line chemotherapy progression. Recent research indicates that MRTX1133, a novel non-covalent inhibitor, shows promising results against KRASG12D pancreatic cancer in both in vitro and in vivo experiments12. Furthermore, studies have shown that KRASG60G nonsense mutations can eliminate exon splicing donor sites, generating functional KRASQ61K and affecting cellular biological functions. Researchers have designed antisense oligonucleotides to counteract aberrant splicing caused by nonsense mutations, achieving significant results13.
Our preliminary research reveals that KRASG12V occur in approximately 12–17% of CRC cases, significantly surpassing KRASG12C, which constitutes about 2–5%14,15. Therefore, investigating novel targets associated with KRASG12V in CRC patients is of paramount importance. Unlike KRASG12C, which features a cysteine mutation near the P2 pocket facilitating strong binding with covalent inhibitors, KRASG12V lacks this amino acid, resulting in markedly reduced binding affinity with such inhibitors. The valine side chain of KRASG12V extends directly from the P-loop to the γ-phosphate binding pocket, resembling KRASG12C’s cysteine side chain but with altered spatial conformation. Compared to KRASG12C, KRASG12V exhibits lower intrinsic GTPase activity, RAF kinase RBD affinity, slower hydrolysis rate, and reduced sensitivity to MEK inhibitors16. In contrast to KRASQ61L, KRASG12V cell lines demonstrate lower RAF-dependent MEK phosphorylation levels17. Studies indicate that CRC patients harboring KRASG12V show poorer responses to immunotherapy18.
In this study, we initially conducted clinical pathological analysis, revealing that KRASG12V CRC tissue samples exhibit larger tumor sizes, a higher incidence of lymph node metastasis, and a predilection for right-sided colon involvement compared to wild-type KRAS(KRASWT) and other KRAS mutation types(KRASOTHER). Subsequently, following the application of bioinformatics, we identified Aquaporin 9 (AQP9) as a crucial protein linked to KRASG12V, indicating interactions with a number of downregulated proteins. Further KEGG analysis highlighted significant enrichment of pathways involving AQP9. We validated the mRNA and protein levels of AQP9 in clinical CRC tissue samples, finding lower AQP9 expression levels in KRASG12V samples compared to KRASWT and KRASOTHER. Using CRISPR-U™-mediated genetic engineering, we constructed KRASG12V mutant cell line, which exhibited increased cell proliferation, reduced apoptosis, and decreased AQP9 protein expression compared to KRASWT cell line. Overexpression of AQP9 attenuated proliferation and promoted apoptosis in KRASG12V CRC cells. Utilizing GeneMANIA (http://genemania.org/), we predicted key proteins involved in AQP9 downregulation in CRC, identifying zinc finger and homeobox protein ZHX2 and sex-determining region Y-box 4 (SOX4) as potential candidates. RT-PCR and IHC analyses of tissue and cellular samples indicated a positive correlation between ZHX2 and AQP9 at both mRNA and protein levels, whereas SOX4 expression showed no such correlation with AQP9. We inferred that ZHX2 likely participates in the regulation of AQP9 in KRASG12V CRC. Finally, we validated the correlation between ZHX2 and AQP9 expression levels, investigated ZHX2’s impact on CRC cell functions by over-expressing ZHX2. We also confirmed the interaction between ZHX2 and AQP9 proteins through co-immunoprecipitation and molecular docking experiments. Our findings suggest that KRASG12V may modulate colorectal cancer cell proliferation and apoptosis through the ZHX2/AQP9 axis.
Result 1
Clinical Pathological Characteristics of KRASG12V Colorectal Cancer (CRC) Patients.
Among 377 CRC patients, KRASG12V group comprised 37 cases, KRASWT group 185 cases, and KRASOTHER group 155 cases. KRASG12V accounted for approximately 10.0%, ranking second to KRASG12D (14.3%, 54/377), and stands as the predominant KRAS mutation type in CRC. The KRASG12V group exhibited significant associations with tumor maximum diameter (P = 0.023), lymph node metastasis (P = 0.014), EGFR expression (P = 0.023), and tumor location (P = 0.006) compared to the KRASWT and KRASOTHER groups. Tumors in the KRASG12V group tended to be larger, more susceptible to lymph node metastasis, and predominantly located in the right colon, often demonstrating low EGFR expression levels (Table 1).
Result 2
Bioinformatics Analysis Identifies Aquaporin 9 (AQP9) as a Central Protein Linked to KRASG12V CRC.
We conducted an investigation into the KRAS-PI3K-AKT and KRAS-BRAF-MAPK signaling pathways, both of which are implicated in KRAS-associated cell proliferation. However, our analysis did not reveal a direct correlation between AQP9 expression and the proteins related to these signaling pathways, the inconclusive results are shown in supplementary Fig. 1. As a result, we are currently exploring alternative pathways. We analyzed gene expression profiles from 511 colorectal cancer cases across two datasets (Colon and Rectal) in The Cancer Genome Atlas (TCGA) database (gene count: 15,255). This included 50 cases of KRASG12V CRC patients, 162 cases of KRASOTHER CRC patients, and 299 cases of KRASWT CRC patients. Analysis showed significant downregulation of multiple genes in the KRASG12V group compared to the other two groups (see Fig. 1A-B). Unsupervised gene set clustering analysis further confirmed differential gene expression in KRASG12V colorectal cancer compared to other groups. (see Fig. 1C-D). The Venn diagram analysis revealed 116 genes that were commonly downregulated in the KRASG12V group compared to the other two groups. (Fig. 1E).
GO enrichment analysis was performed to investigate the molecular functions, cellular components, and biological processes of variously expressed genes. As shown in Fig. 1F, these genes were significantly enriched in GO terms related to “neuroactive ligand-receptor interaction,” “cytokine-cytokine receptor interaction,” and “chemokine signaling pathway,” suggesting their involvement in cell membrane localization and cell migration. String protein interaction analysis identified AQP9 as a key protein linked with KRASG12V mutation, interacting with numerous downregulated proteins (Fig. 1G). Previous studies have indicated that AQP9, located on the cell membrane, participates in invasion and migration of various cancer cells through different signaling pathways19,20,21,22. Additionally, AQP9 has been implicated in immune response, inflammation, and cytokine-cytokine receptor interactions23. Expression levels of AQP9 in the three colorectal cancer groups were validated using the TCGA expression profile data, as depicted in Fig. 1H and I.
Result 3
AQP9 expression, linked to CRC function, is low in KRASG12V CRC tissues and cells.
We utilized immunohistochemistry to assess AQP9 protein expression levels in 107 CRC tissue samples. The findings showed significantly reduced AQP9 expression in KRASG12V CRC tissues compared to KRASOTHER and KRASWT CRC tissues (P < 0.001, Fig. 2A-B). Consistent findings were obtained from western blot experiments across the three CRC tissue groups, confirming reduced AQP9 expression in KRASG12V CRC tissues (Fig. 2C).
To elucidate the clinical-pathological correlations of AQP9 in CRC tissues, we analyzed clinical-pathological data from 107 colorectal cancer patients. The analysis revealed that among these patients, 25 exhibited low AQP9 expression, 53 exhibited moderate expression, and 29 exhibited high expression. Low AQP9 expression correlated significantly with younger age (P = 0.003), tumor maximum diameter (P = 0.033) and KRASG12V (P = 0.001), but showed no significant correlation with other clinical-pathological indicators, as shown in Table 2.
We successfully created KRASG12V CRC cell lines using CRISPR-U™-mediated genome engineering technology (Fig. 2D), and confirmed the purity of the KRASG12V mutant cell strain through Sanger sequencing (Fig. 2E). We used qRT-PCR and Western blot analysis to evaluate AQP9 expression in KRASG12V and KRASWT CRC cells (Fig. 2F-H). The results indicated lower AQP9 mRNA and protein levels in KRASG12V cells than in KRASWT cells.
To evaluate the biological functions of KRASG12V CRC cells, we compared their proliferation, invasion, migration, and apoptosis with KRASWT CRC cells. We found that KRASG12V colorectal cancer cells exhibited increased proliferation and reduced apoptosis compared to KRASWT cells, while invasion and migration capabilities showed no significant differences (Fig. 2I-L). Finally, we investigated the alterations in intracellular glycerol concentrations in CRC cells following the administration of exogenous glycerol, utilizing the Amplex Red Glycerol Assay Kit to evaluate the role of AQP9 in facilitating glycerol transport. After administering exogenous glycerol, KRASG12V CRC cells showed significantly lower glycerol concentrations at 30 and 60 min compared to KRASWT CRC cells (Fig. 2M), possibly due to differing AQP9 expression levels.
Result 4
AQP9 overexpression reduces proliferation and boosts apoptosis, while its knockdown increases proliferation and reduces apoptosis in KRASG12V CRC cells.
To investigate the impact of AQP9 protein on the biological functions of KRASG12V CRC cells, we constructed a eukaryotic expression vector for AQP9 overexpression (Fig. 3A). This vector was transfected into KRASG12V colorectal cancer cells to observe its effects on cellular functions. Following transfection, there was a significant increase in AQP9 protein expression levels (Fig. 3B & C). Compared to cells transfected with the control vector, KRASG12V CRC cells transfected with the AQP9 overexpression vector showed reduced proliferation (Fig. 3D) and increased levels of apoptosis (Fig. 3E).
We used shRNA knockdown to study AQP9’s role in CRC cells. Lentiviral vectors with AQP9-specific shRNAs were introduced into KRASG12V CRC cells, and stable cells were selected with puromycin. Three distinct shRNAs, “sh AQP9-S1,” “sh AQP9-S2,” and “sh AQP9-S3,” were used to avoid sequence overlap. This method significantly reduced AQP9 protein levels by up to 70% in 2 out of 3 cases, as shown in Fig. 3F&G. Silencing AQP9 with targeted shRNAs in KRASG12V CRC cells resulted in more proliferation and less apoptosis compared to control (Fig. 3H&I).
Result 5
KRASG12V CRC Cells Regulate AQP9 via ZHX2, Affecting CRC Cell Functions.
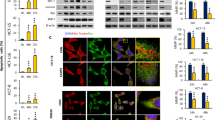
To further explore the specific mechanism by which KRASG12V regulates AQP9, we utilized the GeneMANIA database (http://genemania.org/) to predict their interaction relationships. We identified zinc finger and homeobox protein ZHX2 and sex-determining region Y-box 4 (SOX4) as potentially significant proteins (Fig. 4A). RT-PCR and IHC analysis of tissue samples revealed decreased levels of ZHX2 mRNA and protein in the KRASG12V group (Fig. 4B-D), consistent with its expression in KRASG12V cell lines (Fig. 4E-G). Conversely, no differences expression of SOX4 were observed between KRASG12V and KRASWT (data not shown), suggesting that ZHX2 likely participates in KRASG12V-mediated regulation of AQP9.
ZHX2 functions as a transcriptional repressor involved in gene regulation at the transcriptional level. It interacts with nuclear factor Y subunit A (NF-YA) to inhibit downstream target gene expression and can form homodimers or heterodimers with family members like ZHX1/ZHX3 to exert transcriptional repression24. Studies have shown that ZHX2 regulates tumor-related genes to inhibit cell proliferation, migration, and drug resistance, ultimately suppressing tumor progression25,26. Clinical-pathological analysis of ZHX2 revealed that patients with high ZHX2 expression had larger tumor diameters, consistent with the clinical-pathological features of KRASG12V colorectal cancer and AQP9 (Table 3).
To investigate the regulatory role of ZHX2 on AQP9 in the context of KRASG12V, we engineered a plasmid for the overexpression of ZHX2 (Fig. 4H). Subsequent to the overexpression of ZHX2 in KRASG12V CRC cells, we noted an augmentation in AQP9 expression (Fig. 4I-J). Additionally, the overexpression of ZHX2 resulted in the inhibition of tumor cell proliferation (Fig. 4K) and the promotion of apoptosis (Fig. 4L).
We conducted AQP9 knockdown experiments to determine whether the knockdown of AQP9 could counteract the effects of ZHX2 overexpression. As anticipated, our findings revealed that in the group with ZHX2 overexpression, AQP9 knockdown was able to replicate the enhanced proliferative capacity, reduced apoptosis and reduced glycerol transport observed in CRC cells (Fig. 5A-D).
To further validate the relationship between ZHX2 and AQP9 proteins, we uploaded ZHX2 (PDB ID: 3NAU, crystal structure of ZHX2, homeodomain 2) and AQP9 (PDB file was generated in SWISS-MODEL(https://swissmodel.expasy.org ) using AQP9 amino acid sequence ) to ZDOCK for rigid-body docking to predict the receptor-ligand complex model. The ZDOCK Score values and their best pose interaction were calculated, The Z-score of ZHX2 and AQP9 was 1813.361(shown in Supplementary Table 3 ). ZDOCK score, calculated by ZDOCK program, measures shape complementarity between proteins, factoring in potential energy, spatial complementarity, and electric field force. A higher score suggests better docking. The score threshold varies based on docking results and indicates the likelihood of approaching the true binding state rather than providing an absolute measure27. Visualization analysis revealed that ZHX2 interacts with residues on AQP9 through hydrogen bonds, ensuring stable binding (Fig. 5E). Utilizing the PDBePISA database (https://www.ebi.ac.uk/pdbe/pisa/), we predicted the pertinent amino acid residues potentially involved in the interaction between ZHX2 and AQP9. The predicted amino acid residues, which may engage in hydrogen bonding, such as AQP9 Glu468- ZHX2 SER457, AQP9 Glu468-ZHX2 GLN460, are depicted in Fig. 5F, with additional details provided in the supplementary Fig. 2. Comprehensive analysis revealed that proteins AQP9 and ZHX2 formed a stable protein docking model. Furthermore, we verified the ZHX2 and AQP9 interaction using co-immunoprecipitation (Fig. 5G). This implies that KRASG12V CRC cells control AQP9 via ZHX2, affecting CRC cell functions (Fig. 5H).
Discussion
KRAS is a common and significant oncogene in human colorectal cancer(CRC)28. The status of KRAS is closely associated with the standardized diagnosis and treatment of patients29,30. Multiple countries’ diagnostic and treatment guidelines have clearly stated that patients with wild-type KRAS, NRAS, and BRAF may benefit from the monoclonal antibodies cetuximab and panitumumab29. Small molecule targeted drugs such as sotorasib and adagrasib have been approved for the treatment of non-small cell lung cancer (NSCLC) with KRASG12C, while targeted drugs for other mutations of KRAS remain under investigation. In CRC, KRASG12V accounts for approximately 12–17%, representing a common type of KRAS mutation in CRC. However, there are no specific drugs targeting KRASG12V, underscoring the need for in-depth exploration of this mutation in CRC.
This study described the clinical and pathological characteristics of CRC harboring KRASG12V (shown in Table 1). Compared with CRC patients harboring KRASWT or KRASOTHER, these with KRASG12V typically presents with larger tumor diameters, a higher propensity for lymph node metastasis, elevated levels of epidermal growth factor receptor (EGFR), and a predilection for tumor occurrence in the right colon. Collectively, these observations underscore the distinctive clinical and pathological phenotypes associated with KRASG12V in CRC (shown in Table 1). The uniqueness of KRASG12V CRC is also manifested in its unique molecular characteristics. Utilizing bioinformatics analysis, we discerned a downregulation in the expression of aquaporin-9 (AQP9) in CRC harboring KRASG12V. This downregulation was further validated in KRASG12V CRC tissues and cell lines. AQP9 is a member of the transmembrane water channel protein family, playing a crucial role in transepithelial and transcellular water movement31. It facilitates not only water permeation but also the transport of glycerol and other small solutes32. Existing research provides evidence that AQP9 may play a role in the migration and invasion of prostate cancer cells by engaging in the extracellular signal-regulated kinase (ERK) signaling pathway21. In hepatocellular carcinoma, AQP9 overexpression has been shown to inhibit epithelial-mesenchymal transition, thereby suppressing cancer cell invasion20. Additionally, AQP9 has been implicated in the inhibition of liver cancer cell growth and metastasis through the Wnt/β-catenin signaling pathway19. In this study, we observed that AQP9 is downregulated in KRASG12V CRC tissues and cells, which correlates with enhanced proliferation and reduced apoptosis in CRC cells. Overexpression of AQP9 was found to suppress proliferation and promote apoptosis of CRC cells. These findings are congruent with Huang’s results, which demonstrated that AQP9 can enhance the sensitivity of CRC cells to chemotherapeutic agents, suggesting that elevated AQP9 levels may inhibit the growth of CRC cells33. However, unlike KRASWT cells with normal AQP9 expression, KRASG12V cells with low AQP9 levels showed no changes in migration and invasion in CRC cells; Likewise, AQP9 overexpression did not affect CRC cell migration or invasion. This result may highlight the unique role of AQP9 expression in KRASG12V CRC cells. We also examined changes in intracellular glycerol levels in CRC cells after adding external glycerol to assess AQP9’s role in glycerol transport. KRASG12V CRC cells had notably lower glycerol concentrations at 30 and 60 min than KRASWT cells, likely due to varying AQP9 expression. The downregulation of AQP9 is more prevalent in younger patients and those with larger tumor volumes, aligning with the clinical characteristics of CRC patients harboring KRASG12V(shown in Table 2). We employed shRNA-mediated knockdown to investigate the role of AQP9 in CRC cells. Our findings reveal that silencing AQP9 using specific shRNAs in KRASG12V CRC cells led to increased cell proliferation and reduced apoptosis in comparison to the control group. This finding aligns with the results reported by Li, which demonstrated that the knockdown of AQP9 leads to an increased proliferation of laryngeal cancer cells34.
Utilizing the GeneMANIA database, we discovered that the expression of zinc finger-containing homolog ZHX2 may exhibit a correlation with AQP9 in colorectal cancer. ZHX2, as a nuclear-localized transcriptional repressor, possesses the capability to recognize and augment promoter activity35. It can interact with the nuclear factor YA (NF-YA) to repress the expression of downstream target genes and is known to form homodimers or heterodimers with family members ZHX1/ZHX3 to exert transcriptional repression functions24. ZHX2 exhibits overexpression in both triple-negative breast cancer cell lines and patients, where it plays a pivotal role in promoting the oncogenic HIF1α signaling pathway25. In thyroid cancer, knockdown of ZHX2 is associated with enhanced metastatic potential, and it can impede thyroid cancer progression by disrupting tumor metastasis mediated by the S100 calcium-binding protein A14 (S100A14)26. Similarly, ZHX2 expression is diminished in lung cancer cell lines, where it can inhibit cell proliferation and promote apoptosis by suppressing the p38MAPK signaling pathway36. In renal clear cell carcinoma tissues, the overexpression of ZHX2 correlates with an unfavorable prognosis for patients. ZHX2 can stimulate the proliferation and migration of renal cancer cells by activating the MEK/ERK signaling cascade and can induce resistance to sunitinib through the modulation of autophagy in renal clear cell carcinoma cells37. In hepatocellular carcinoma, diminished expression of ZHX2 is associated with adverse patient outcomes. The downregulation of ZHX2 is implicated in the advancement of liver tumors and the dissemination of liver cancer stem cells (CSCs). ZHX2 also suppresses the re-emergence of liver cancer CSCs’ initiation, self-renewal, and resistance to sorafenib38.Upon overexpression of ZHX2 in CRC cell lines, we detected an elevation in AQP9 levels, concurrent with a reduction of proliferation and an increase of apoptosis in CRC cells. We conducted further experiments to see if reducing AQP9 could counter ZHX2 overexpression effects. As expected, AQP9 knockdown in the ZHX2 overexpression group replicated increased proliferation and reduced apoptosis in CRC cells, knockdown of AQP9 also effectively restores the diminished glycerol transport function. Co-immunoprecipitation assays substantiated the presence of an interaction between ZHX2 and AQP9 proteins. Molecular docking studies elucidated that ZHX2 can engage in stable binding with AQP9 through the formation of hydrogen bonds and van der Waals interactions with multiple residues. These findings indicate that KRASG12V colorectal cancer cells may exert regulatory control over AQP9 via ZHX2, thus influencing the biological functions of CRC cells.
This study acknowledges several limitations: (1) The exact molecular process of how KRASG12V affects growth and cell death in colorectal cancer cells through AQP9, a key aquaporin for water and small solute transport, is unclear and requires further study; (2) The exact interaction between ZHX2 and AQP9 is still unclear. More research is needed on ZHX2, a nuclear-localized transcriptional repressor, to understand how it regulates AQP9 expression; (3) This study focused mainly on CRC tissues and cell lines, and the results need to be confirmed through animal model studies.
Previous genetic research has primarily focused on the comparative analysis of mutant and wild-type alleles of specific genes. For instance, patients with wild-type RAS and RAF genes have been shown to derive clinical benefit from cetuximab and panitumumab, whereas those with mutations in EGFR gene may respond to EGFR tyrosine kinase inhibitors. The progression of research has unveiled those mutations at different loci within the same gene, or distinct mutational types at the same locus, are associated with the disease status and treatment outcomes of patients. For instance, a study has stratified EGFR genes into four distinct categories: classical-like mutations that were distant from the ATP-binding pocket, T790M-like mutations in the hydrophobic core, insertions in the loop at the C-terminal end of the αC-helix in exon 20, and mutations on the interior surface of the ATP-binding pocket or C-terminal end of the αC-helix (PVR mutations), which exhibit a profound correlation with the personalized therapeutic strategies and their corresponding efficacies in patient care39. In the therapeutic landscape of CRC, clinical trials are underway for drugs targeting the KRASG12C. The combination of Adagrasib with cetuximab has demonstrated an impressive objective response rate of 46% in patients with advanced CRC harboring KRASG12C40. However, there is little research on KRASG12V in CRC. In addition to the direct development of small molecule drugs targeting the KRASG12V protein, the exploration of the upstream and downstream molecular alterations associated with KRASG12V is also of significant importance. This study has initiated an exploration of the clinical and pathological features, as well as the molecular characteristics, of KRASG12V in CRC and has begun to elucidate the role of AQP9 in cells harboring KRASG12V, with the understanding that further research is imperative.
In summary, this research has uncovered that AQP9 exhibits reduced expression in CRC tissues and cells harboring KRASG12V, with this diminished expression being associated with enhanced proliferation and attenuated apoptosis in CRC cell lines. AQP9 overexpression was found to ameliorate the proliferative and apoptotic profiles in CRC cell. Furthermore, a positive correlation between ZHX2 and AQP9 expression levels was identified within CRC tissues and cell lines, with an upregulation of AQP9 occurring subsequent to ZHX2 overexpression. Co-immunoprecipitation and molecular docking studies have provided evidence of an interactive relationship between ZHX2 and AQP9. Nonetheless, the underlying mechanisms of their functional interplay warrant further investigation.
Bioinformatics Analysis Identifies Aquaporin 9 (AQP9) as a Central Protein Linked to KRASG12V colorectal cancer (CRC). (A, B) Volcano plot showing differential gene expression between KRASG12V/KRASWT, KRASG12V/KRASOTHER in CRC. (C, D) Heat map of differentially expressed genes between KRASG12V/KRASWT, KRASG12V/KRASOTHER in CRC. (E) Venn diagram showing commonly differentially expressed genes in KRASG12V/KRASWT and KRASG12V/KRASOTHER of CRC. (F) Gene ontology analyses of commonly differentially expressed genes. (G) String protein-protein interaction analysis to identify AQP9. (H, I) AQP9 expression is significantly downregulated in CRC with KRASG12V compared to KRASWT and KRASOTHER.
AQP9 expression, linked to CRC cell function, is low in KRASG12V CRC tissues and cells. A&B: Expression of AQP9 in KRASWT, KRASOTHER, and KRASG12V CRC tissues (Immunohistochemistry). (A) Microscopic results, (B) Quantitative results. (C) Expression of AQP9 in KRASWT, KRASOTHER, and KRASG12V CRC tissues (Western blot). (D) Construction of KRASG12V colorectal cancer cell lines using CRISPR/Cas9 RNP technology. (E) Sanger sequencing validation of KRASG12V CRC cell line. (F) Relative mRNA quantification of AQP9 in KRASWT and KRASG12V CRC cell. (G, H) Relative protein quantification of AQP9 in KRASWT and KRASG12V CRC cell. (G) Western blot, (H) Quantitative results. (I) Proliferation levels of KRASWT and KRASG12V CRC cell (CCK8 assay). (J) Invasion levels of KRASWT and KRASG12V CRC cell (Transwell assay). (K) Migration levels of KRASWT and KRASG12V CRC cell (Scratch assay). (L) Apoptosis levels of KRASWT and KRASG12V CRC cell (FITC-Annexin V/PI flow cytometry assay). (M) Alterations in glycerol concentration in the two cell types at various time points following the administration of exogenous glycerol. (B) n = 6, variance analysis; (F, H-M) n = 3, two independent sample t-tests.
AQP9 overexpression reduces proliferation and boosts apoptosis, while its knockdown increases proliferation and reduces apoptosis in KRASG12V CRC cells. (A) Schematic representation of the construction of the AQP9 overexpression plasmid. (B, C) Protein quantification of AQP9 following overexpression in KRASG12V CRC cells. (B) Western blot, (C) Quantitative results. (D) Proliferation levels of KRASG12V CRC cell following AQP9 overexpression. (E) Apoptosis levels of KRASG12V CRC cell following AQP9 overexpression. (F, G) AQP9 knockdown protein levels in KRASG12V CRC cells (F Western blot, G Quantitative analysis). (H) KRASG12V CRC cell proliferation post-AQP9 knockdown. (I) KRASG12V CRC cell apoptosis post-AQP9 knockdown.
ZHX2 expression in KRASG12V CRC tissues and cells positively correlates with AQP9. (A) GeneMANIA database prediction of the regulatory interaction between KRASG12V and AQP9. (B) mRNA expression levels of ZHX2 in KRASWT, KRASOTHER, and KRASG12V CRC tissues. (C, D) Protein expression levels of ZHX2 in KRASWT, KRASOTHER, and KRASG12V CRC tissues (immunohistochemistry). (C) Microscopic observations, (D) Quantitative analysis. (E) mRNA expression levels of ZHX2 in KRASWT and KRASG12V CRC cell. (F, G) Protein expression levels of ZHX2 in KRASWT and KRASG12V CRC cell (Western blot). (F) Western blot, (G) Quantitative analysis. (H) Schematic diagram of the construction of the ZHX2 overexpression plasmid. (I, J) ZHX2 overexpression upregulated the expression of AQP9 (I Western blot, J Quantitative analysis). (K) ZHX2 overexpression reduced proliferation of KRASG12V CRC cell (CCK8 assay). (L) ZHX2 overexpression promoted apoptosis of KRASG12V CRC cell (FITC-Annexin V/PI flow cytometry assay).
KRASG12V CRC Cells Regulate AQP9 via ZHX2, Affecting CRC Cell Functions. (A) Knockdown of AQP9 in ZHX2 overexpression KRASG12V CRC cell. (B) Proliferative capacity & (C) Apoptosis of KRASG12V CRC cell with AQP9 knockdown and ZHX2 overexpression. (D) Alterations in glycerol concentration in KRASG12V CRC cell with AQP9 knockdown and ZHX2 overexpression following the administration of exogenous glycerol. (E) Molecular docking simulation revealed that ZHX2 can stably bind with AQP9 (visual representations of ZHX2 and AQP9 interaction). (F) The specific residues involved in these interactions by hydrogen bonds by the PDBePISA database (https://www.ebi.ac.uk/pdbe/pisa/). G CO-IP confirmed the binding of ZHX2 to AQP9. H The schematic diagram of ZHX2/AQP9 interaction in KRASG12V CRC cell (By Figdraw, https://www.home-for-researchers.com/).
Methods
Patients and tissue specimens
Following receipt of informed written consent from the patients or their legal representatives, and subsequent approval from the Medical Ethics Committee of Affiliated Hospital of Jiangnan University (Ethical Approval Number: LS2024007), a cohort of 377 paraffin-embedded colorectal cancer (CRC) tissue specimens and a subset of surgically excised tissues cryopreserved at -80 °C were procured from Affiliated Hospital of Jiangnan University over the period of 2021 to 2022. The genetic status of KRAS, NRAS, and BRAF in these specimens was ascertained through routine clinical polymerase chain reaction (PCR) testing. All samples had been verified as CRC in histopathology with comprehensive clinical and pathological data available for review. All experiments were performed in accordance with relevant named guidelines and regulations.
Bioinformatics analysis
Differential gene expression analysis
The colon and rectal datasets utilized in this experiment were derived from The Cancer Genome Atlas Program (TCGA) database. Differential gene expression analysis was conducted using the T-test and Bayesian algorithms, with gene expression levels quantified using Transcripts Per Million (TPM). The selection criteria were: |log2Fold Change| > 1 and P < 0.05.
String Protein-Protein interaction analysis and genemania prediction
The protein interaction network diagram was obtained by inputting the gene sequences of interest into the String database (https://string-db.org/) and aligning them with protein sequences in the database. GeneMANIA (http://genemania.org/) was employed to explore gene interactions and predict functions.
GO enrichment analysis
Gene Ontology (GO) analyses were performed to elucidate the functions and pathways associated with differentially expressed genes. Fisher’s exact test was used to calculate the statistical significance of the enrichment results. The criterion for significance was P < 0.05.
Cell culture
The human colorectal cancer cell line HCT 116 was purchased from the Ubigene Biosciences. The cells were cultured in McCoy’s 5 A medium (Ubigene Biosciences) at 37 °C in a humidified incubator with 5% CO₂.
Construction and sequencing verification of KRASG12V cell
Genomic DNA was isolated from CRC HCT116 cell line, and the targeted genomic region was amplified via polymerase chain reaction (PCR). The guide RNA (gRNA) sequences are detailed in Supplementary Table 1. Following the optimization of experimental conditions, the cells were subjected to electroporation. Post-electroporation, the cells were seeded into 96-well plates for culture, and subsequent pool screening and monoclonal isolation were conducted (Fig. 2D). A specific 96-well plate was then selected for further monoclonal selection and characterization.
DNA was extracted from constructed KRASG12V cells for PCR amplification. The PCR products were examined using a 1% agarose gel electrophoresis to visualize the bands. The target PCR bands were excised, purified, and sequenced using the Sanger sequencing to verify for KRASG12V (2E and Supplementary Fig. 3).
Reagents and antibodies
The following antibodies were sourced from their respective suppliers: Anti-AQP9, anti-LaminB1, and anti-β-tubulin antibodies were purchased from Proteintech Company. Anti-ZHX2 antibodies were purchased from Santa Cruz Biotechnology. ERK, P-ERK, AKT, P38 antibodies were purchased from Beyotime Biotechnology. Additionally, horseradish peroxidase (HRP)-conjugated secondary antibodies specific for goat anti-rabbit and goat anti-mouse were procured from APExBIO Company.
Assessment of cell viability, invasion, migration, and apoptosis
CCK-8 (Cell Counting Kit-8) Proliferation Assay: Cells were plated into 96-well plates at the required densities and incubated for 24 h. CCK-8 reagent was then added, and the plates were incubated in the dark for 2 h. The absorbance was measured at 450 nm using a microplate reader, from which the proliferation rate of the cells was inferred.
Transwell Migration Assay: Cells were seeded into the upper compartment of a 24-well transwell chamber, with medium containing 20% serum in the lower compartment. After overnight incubation at 37 °C, the cells were fixed with precooled 4% paraformaldehyde and stained with crystal violet. The stained cells were photographed and counted under a microscope after the membrane was mounted.
Wound-Healing Assay: Cells were seeded in 6-well plates at appropriate densities and incubated overnight at 37 °C. Straight-edged tools were used to create wounds, with initial images taken immediately under a light microscope. After a 24-hour incubation period, the wounds were rephotographed to document the healing process.
Flow Cytometric Apoptosis Assay: Cells were placed in 6-well plates, incubated overnight at 37 °C, washed, and resuspended in Binding Buffer. Annexin V-FITC and propidium iodide were added to each flow cytometry tube, mixed, and incubated in the dark for 15 min. After another wash and resuspension in Binding Buffer, the cells were analyzed using a flow cytometer within an hour.
RNA quantification
For this investigation, primers for reverse transcription polymerase chain reaction (RT-PCR) were meticulously designed utilizing the Primer Premier 5.0 software suite and subsequently synthesized by Shanghai Bioengineering Corporation. Adhering to the protocol for quantitative real-time PCR, the reaction mixture was meticulously assembled by adding, in an orderly fashion, deionized water (ddH2O), SYBR Green Master Mix, forward and reverse primers, and cDNA template to the PCR tubes, thus preparing the PCR reaction cocktail. The specific sequences of the primers are listed in Supplementary Table 2.
Immunohistochemistry
Tissue microarrays were sectioned to a thickness of 4 μm, and the sections were deparaffinized by incubation in a 70 °C oven for 2 h. Following this, antigen retrieval and blocking were conducted. The primary antibody solution was applied to the tissue sections and incubated at 4 °C with refrigeration overnight to facilitate specific antigen-antibody binding. Once the primary antibody incubation was complete, the sections were incubated with the secondary antibody at room temperature for 30 min. The slides were then processed for chromogenic visualization, mounted, and examined microscopically.
Western blot analysis
Gel formulations for both resolving and concentrating gels were prepared. Following protein loading, the samples underwent electrophoretic separation, which was succeeded by the transfer of proteins onto a membrane. The membrane was then incubated under non-denaturing conditions for 1 h to ensure complete blocking. The primary antibody was applied and incubated in 4 °C overnight to allow for specific antigen recognition. Subsequently, the membrane was incubated with the secondary antibody at room temperature for 1 h. Ultimately, the immunoreactive bands were visualized using an Enhanced Chemiluminescence (ECL) substrate, and the emitted luminescence was documented with a chemiluminescence imaging system. As full as possible length gels and blots are included in the Supplementary Fig. 4.
Glycerol transport testing
Glycerol levels in cells were measured using the Amplex Red Glycerol Assay Kit (Beyotime Biotech Inc, Shanghai, China). Cells were collected, homogenized, and the supernatant was diluted. The diluted sample was added to a 96-well plate with 20 µl of test buffer. Then, 80 µl of glycerol detection solution was added to each well and incubated at 37 °C in the dark for 30 min. A microplate reader measured A570 absorbance values. The kit’s standards were used to create a standard curve and determine the glycerol concentration in the sample. In this experiment, 200 µL of a 100 mM glycerol solution was added to 9.8 mL of medium to assess the intracellular glycerol concentration at time intervals of 0, 30, and 60 min, with each condition being replicated three times(reference to article41).
AQP9 knockdown
Three distinct short hairpin RNAs (shRNAs) AQP9 were developed: “shAQP9-S1” (targeting GCTCCTAAATGCAGCTATTGA), “shAQP9-S2” (targeting GAGGTAGAATTGCCACTATTG), and “shAQP9-S3” (targeting GAGCTCTCAAGAAAGTATTGT). Additionally, a scrambled control shRNA (“c-sh”) was included. All constructs were cloned into the LV3(H1/GFP&Puro) vector provided by Genepharma. Lentiviral particles were produced by co-transfecting these shRNA constructs along with viral packaging plasmids into HEK-293 cells. The resulting viral particles were then utilized to infect KRASG12V CRC cells. After a 48-hour incubation period, the cells were transferred to fresh complete medium containing puromycin. Stable cell lines with silenced AQP9 expression were established after five passages, and the efficiency of AQP9 knockdown was confirmed through Western blotting assays.
Statistical analysis
The statistical analyses were performed utilizing SPSS version 29, with data represented as the mean ± standard error of the mean (SEM). Differences between two groups were evaluated using the two-tailed Student’s t-test. In instances of experiments with multiple groups, a one-way or two-way analysis of variance (ANOVA) was conducted, succeeded by Tukey’s multiple comparisons test. Graphical representations of the data were generated with GraphPad Prism version 8. The number of replicates for both biological and technical aspects of each experiment is specified in the corresponding figure legends, with statistical significance denoted by P-value < 0.05.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263. https://doi.org/10.3322/caac.21834 (2024).
Wood, L. D. et al. The genomic landscapes of human breast and colorectal cancers. Science 318, 1108–1113. https://doi.org/10.1126/science.1145720 (2007).
Jänne, P. A. et al. Adagrasib in Non-Small-Cell lung cancer harboring a KRAS(G12C) mutation. N Engl. J. Med. 387, 120–131. https://doi.org/10.1056/NEJMoa2204619 (2022).
Hong, D. S. et al. KRAS(G12C) Inhibition with Sotorasib in advanced solid tumors. N Engl. J. Med. 383, 1207–1217. https://doi.org/10.1056/NEJMoa1917239 (2020).
Pereira, A. A. et al. Association between KRAS mutation and lung metastasis in advanced colorectal cancer. Br. J. Cancer. 112, 424–428. https://doi.org/10.1038/bjc.2014.619 (2015).
Vakiani, E. et al. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J. Clin. Oncol. 30, 2956–2962. https://doi.org/10.1200/jco.2011.38.2994 (2012).
Artale, S. et al. Mutations of KRAS and BRAF in primary and matched metastatic sites of colorectal cancer. J. Clin. Oncol. 26, 4217–4219. https://doi.org/10.1200/jco.2008.18.7286 (2008).
Wang, Z. et al. Quadruple-editing of the MAPK and PI3K pathways effectively blocks the progression of KRAS-mutated colorectal cancer cells. Cancer Sci. 112, 3895–3910. https://doi.org/10.1111/cas.15049 (2021).
Chen, C. C. et al. KRAS K104 modification affects the KRAS(G12D)-GEF interaction and mediates cell growth and motility. Sci. Rep. 10, 17447. https://doi.org/10.1038/s41598-020-74463-5 (2020).
Perkins, G., Pilati, C., Blons, H. & Laurent-Puig, P. Beyond KRAS status and response to anti-EGFR therapy in metastatic colorectal cancer. Pharmacogenomics 15, 1043–1052. https://doi.org/10.2217/pgs.14.66 (2014).
Prior, I. A., Lewis, P. D. & Mattos, C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 72, 2457–2467. https://doi.org/10.1158/0008-5472.Can-11-2612 (2012).
Hallin, J. et al. Anti-tumor efficacy of a potent and selective non-covalent KRAS(G12D) inhibitor. Nat. Med. 28, 2171–2182. https://doi.org/10.1038/s41591-022-02007-7 (2022).
Kobayashi, Y. et al. Silent mutations reveal therapeutic vulnerability in RAS Q61 cancers. Nature 603, 335–342. https://doi.org/10.1038/s41586-022-04451-4 (2022).
Liu, Y. et al. Analysis of KRAS mutations in Circulating tumor DNA and colorectal cancer tissue. Biotech. Histochem. 96, 376–383. https://doi.org/10.1080/10520295.2020.1810775 (2021).
Liu, Y. et al. Value of a novel polymerase chain reaction method in evaluating the efficacy of targeted therapy in patients with colorectal cancer. Chin. J. Experimental Surg. 39, 1371–1374 (2022).
Hunter, J. C. et al. Biochemical and structural analysis of common Cancer-Associated KRAS mutations. Mol. Cancer Res. 13, 1325–1335. https://doi.org/10.1158/1541-7786.Mcr-15-0203 (2015).
Buhrman, G., Kumar, V. S., Cirit, M., Haugh, J. M. & Mattos, C. Allosteric modulation of Ras-GTP is linked to signal transduction through RAF kinase. J. Biol. Chem. 286, 3323–3331. https://doi.org/10.1074/jbc.M110.193854 (2011).
Jung, J., Park, S., Lee, J. & Kim, S. Solid tumor patients with G12V and G13D KRAS aberrations have poor survival following ICI treatment. J. Clin. Oncol. 40, e14567–e14567. https://doi.org/10.1200/JCO.2022.40.16_suppl.e14567 (2022).
Liao, S. et al. Aquaporin 9 inhibits growth and metastasis of hepatocellular carcinoma cells via Wnt/β-catenin pathway. Aging (Albany NY). 12, 1527–1544. https://doi.org/10.18632/aging.102698 (2020).
Zhang, W. G. et al. Aquaporin 9 is down-regulated in hepatocellular carcinoma and its over-expression suppresses hepatoma cell invasion through inhibiting epithelial-to-mesenchymal transition. Cancer Lett. 378, 111–119. https://doi.org/10.1016/j.canlet.2016.05.021 (2016).
Chen, Q. et al. Effect of AQP9 expression in Androgen-Independent prostate cancer cell PC3. Int. J. Mol. Sci. 17 https://doi.org/10.3390/ijms17050738 (2016).
Liu, X., Xu, Q., Li, Z. & Xiong, B. Integrated analysis identifies AQP9 correlates with immune infiltration and acts as a prognosticator in multiple cancers. Sci. Rep. 10, 20795. https://doi.org/10.1038/s41598-020-77657-z (2020).
Xiao, S. J. et al. Uncovering the differentially expressed genes and pathways involved in the progression of stable coronary artery disease to acute myocardial infarction using bioinformatics analysis. Eur. Rev. Med. Pharmacol. Sci. 25, 301–312. https://doi.org/10.26355/eurrev_202101_24396 (2021).
Perincheri, S., Dingle, R. W., Peterson, M. L. & Spear, B. T. Hereditary persistence of alpha-fetoprotein and H19 expression in liver of BALB/cJ mice is due to a retrovirus insertion in the Zhx2 gene. Proc. Natl. Acad. Sci. U S A. 102, 396–401. https://doi.org/10.1073/pnas.0408555102 (2005).
Fang, W. et al. ZHX2 promotes HIF1α oncogenic signaling in triple-negative breast cancer. Elife 10 https://doi.org/10.7554/eLife.70412 (2021).
Zhang, Y. et al. ZHX2 inhibits thyroid cancer metastasis through transcriptional Inhibition of S100A14. Cancer Cell. Int. 22, 76. https://doi.org/10.1186/s12935-022-02499-w (2022).
Pierce, B. G. et al. ZDOCK server: interactive Docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 30, 1771–1773. https://doi.org/10.1093/bioinformatics/btu097 (2014).
Tang, J. et al. Exploring the role of sporadic BRAF and KRAS mutations during colorectal cancer pathogenesis: A spotlight on the contribution of the endosome-lysosome system. Cancer Lett. 585, 216639. https://doi.org/10.1016/j.canlet.2024.216639 (2024).
Allegra, C. J. et al. American society of clinical oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J. Clin. Oncol. 27, 2091–2096. https://doi.org/10.1200/jco.2009.21.9170 (2009).
Ye, W., Lu, X., Qiao, Y. & Ou, W. B. Activity and resistance to KRAS(G12C) inhibitors in non-small cell lung cancer and colorectal cancer. Biochim. Biophys. Acta Rev. Cancer. 1879, 189108. https://doi.org/10.1016/j.bbcan.2024.189108 (2024).
Moon, C. S., Moon, D. & Kang, S. K. Aquaporins in cancer biology. Front. Oncol. 12, 782829. https://doi.org/10.3389/fonc.2022.782829 (2022).
Viadiu, H., Gonen, T. & Walz, T. Projection map of aquaporin-9 at 7 A resolution. J. Mol. Biol. 367, 80–88. https://doi.org/10.1016/j.jmb.2006.12.042 (2007).
Huang, D. et al. AQP9-induced cell cycle arrest is associated with RAS activation and improves chemotherapy treatment efficacy in colorectal cancer. Cell. Death Dis. 8, e2894. https://doi.org/10.1038/cddis.2017.282 (2017).
Ren, L., Li, P., Li, Z. & Chen, Q. AQP9 and ZAP70 as immune-related prognostic biomarkers suppress proliferation, migration and invasion of laryngeal cancer cells. BMC Cancer. 22, 465. https://doi.org/10.1186/s12885-022-09458-8 (2022).
Kawata, H. et al. Zinc-fingers and homeoboxes (ZHX) 2, a novel member of the ZHX family, functions as a transcriptional repressor. Biochem. J. 373, 747–757. https://doi.org/10.1042/bj20030171 (2003).
Tian, X., Wang, Y., Li, S., Yue, W. & Tian, H. ZHX2 inhibits proliferation and promotes apoptosis of human lung cancer cells through targeting p38MAPK pathway. Cancer Biomark. 27, 75–84. https://doi.org/10.3233/cbm-190514 (2020).
Zhu, L., Ding, R., Yan, H., Zhang, J. & Lin, Z. ZHX2 drives cell growth and migration via activating MEK/ERK signal and induces Sunitinib resistance by regulating the autophagy in clear cell renal cell carcinoma. Cell. Death Dis. 11, 337. https://doi.org/10.1038/s41419-020-2541-x (2020).
Lin, Q. et al. ZHX2 restricts hepatocellular carcinoma by suppressing stem cell-like traits through KDM2A-mediated H3K36 demethylation. EBioMedicine 53, 102676. https://doi.org/10.1016/j.ebiom.2020.102676 (2020).
Robichaux, J. P. et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature 597, 732–737. https://doi.org/10.1038/s41586-021-03898-1 (2021).
Yaeger, R. et al. Adagrasib with or without cetuximab in colorectal cancer with mutated KRAS G12C. N Engl. J. Med. 388, 44–54. https://doi.org/10.1056/NEJMoa2212419 (2023).
Ohgusu, Y. et al. Functional characterization of human Aquaporin 9 as a facilitative glycerol carrier. Drug Metab. Pharmacokinet. 23, 279–284. https://doi.org/10.2133/dmpk.23.279 (2008).
Acknowledgements
The authors are grateful to Doc. Yan Qin of biobank in Affiliated hospital of Jiangnan University for providing the colorectal cancer tissue and for assistance during biological sampling. Thanks are also expressed to Doc. Yin Yuan of the Wuxi Cancer Institute, Affiliated Hospital of Jiangnan University for her constructive suggestions.
Funding
This work was funded by Project of Wuxi Translational Medicine Research Institution (Grant no. LCYJ202309).
Author information
Authors and Affiliations
Contributions
Project ideation: Liu Y., D.J., T.Q., S.N. and Q.X. Biological sampling: Liu Y., D.J., T.Q., C.S. and Li Y. Molecular analyses: T.Q. and W.R. Cell Experiment: D.J., T.Q. and W.R. Histological analysis: T.Q., Li Y. and W.R. Figure preparation: Liu Y., T.Q. and S.N. Funding: S.N. and Q.X. Manuscript writing (first draft): Liu Y. and T.Q. Manuscript review: all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Dou, J., Tan, Q. et al. Aquaporin 9 downregulation in KRASG12V colorectal cancer and associated with increased proliferation and decreased apoptosis in cancer cells. Sci Rep 15, 12298 (2025). https://doi.org/10.1038/s41598-025-95513-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95513-w