Abstract
Metabolic associated fatty liver disease (MAFLD) represents a significant public health concern. Previous studies have shown that ACT001 has therapeutic effects on MAFLD. This study investigated the potential mechanisms by which ACT001 may treat MAFLD through an integrated approach of transcriptomics and metabolomics. MAFLD model induced by high-fat diet was established, and ACT001 was given by gavage. Histological analysis was performed, and liver enzyme and lipid levels were measured. Transcriptomic analysis was performed to identify differentially expressed genes, while metabolomic analysis was used to detect differential metabolites. Pathways enriched by genes and metabolites affected by ACT001 were also identified. The differentially expressed genes were confirmed through RT-qPCR. ACT001 reduced the levels of liver enzymes and lipids, and alleviated pathological damage such as hepatic steatosis. The integration of transcriptomic and metabolomic analyses indicated that ACT001 may alleviate high-fat diet-induced MAFLD by regulating the linoleic acid and glutathione metabolic pathways. The validation of five differentially expressed genes using RT-qPCR yielded results that were consistent with the transcriptomics data. ACT001 may exert a therapeutic effect in MAFLD mice by modulating glutathione metabolism and linoleic acid metabolism. It has the potential to be a promising treatment for MAFLD.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) has been renamed Metabolic Associated Fatty Liver Disease (MAFLD) by the International Consensus Panel1. MAFLD is rapidly emerging as one of the world’s leading causes of chronic liver disease and is now the leading cause of liver morbidity and mortality2. Currently, MAFLD is estimated to affect 38% of the general population worldwide, with much higher rates in populations that are overweight or obese3. The growing prevalence of MAFLD has led to a significant global burden on healthcare resources and economic productivity4. In consequence, there is an imperative requirement to ascertain viable and cost-efficient pharmacological objectives for MAFLD.
ACT001 (also known as dimethylamino-micheliolide, or DMAMCL) has been granted Orphan Drug Designation by the Food and Drug Administration (FDA) in the United States5. ACT001 is derived and synthesised from Parthenolide (PTL), which retains the anti-inflammatory, antioxidant and anti-tumour properties of PTL, but with improved stability, solubility, low toxicity and minimal side effects6. By reducing renal lipid deposition and inhibiting renal inflammation and oxidative stress, ACT001 improved obesity-associated kidney disease7. ACT001 was shown to improve glucose tolerance and decrease insulin resistance in mice with type II diabetes8. ACT001 decreased the levels of lipids and inflammatory factors in mice with NASH induced by a methionine-choline-deficient (MCD) diet9. Collectively, these preclinical studies indicate that ACT001 demonstrates therapeutic potential in MAFLD. Further investigation is worthy to determine its mechanism of action.
This study investigated the therapeutic benefit of ACT001 in MAFLD employing an HFD-induced MAFLD mouse model. Following treatment with ACT001, improvements were observed in liver pathology. To evaluate the efficacy of ACT001 in inducing remission in mice with MAFLD, a series of relevant biochemical parameters were measured. In order to identify potential mechanisms by which ACT001 alleviates MAFLD, we employed integrated transcriptomic and metabolomic analyses to ascertain which metabolic pathways are significantly enriched in the liver. This research indicates that ACT001 exhibits therapeutic potential for MAFLD in mice by influencing the pathways of glutathione metabolism and linoleic acid metabolism.
Method
Materials
ACT001 was provided by Accendatech Co., Ltd. (Tianjin, China; lot C10668-11). Polyphosphocholine (PPC) was commercially procured from Sanofi Pharmaceuticals Ltd. (Beijing, China; lot ABJD465B).
High-fat feed (D12492, 4075Kcal, 60% fat content) and low-fat feed (D12450J, 4075Kcal, 10% fat content) were purchased from Xiaozhi Youtai (Beijing) Biotechnology Co., LTD.
Animals
Forty C57BL/6J female mice (8 weeks old, 20 ± 2 g) were purchased from Beijing Huafukang Biotechnology Co., LTD. (Certificate No. SCXK (Beijing) 2019-0008). After the first week of adaptation period, the mice were randomly divided into four groups: ND, HFD, HFD-ACT001 (200 mg/kg/d), HFD-PPC (150 mg/kg/d). The other three groups were fed a high-fat diet, except for the control group, which was fed a low-fat diet. The control group and the high-fat group received physiological saline by gavage, while the other two groups received the drug dissolved in physiological saline by gavage. The dose was determined on the basis of the animals’ body weight. At week 21, all animals were sacrificed by cervical dislocation.
The study was conducted in accordance with the guidelines of the ARRIVE guidelines and was approved by the Ethics Committee of Nankai University (approval number: 2021-SYDWLL-000003). All methods were performed in accordance with relevant guidelines and regulations.
Liver biochemistry
Hepatic levels of alanine aminotransferase (ALT), aspartate transaminase (AST), triglycerides (TG) and total cholesterol (TC) were measured using commercially available biochemical kits.
Histopathology
The liver tissue was fixed in 10% formalin, dehydrated with gradient ethanol, transparent with xylene, and then embedded in paraffin. Sections (5 μm) were stained with hematoxylin-eosin (H&E) and Masson. All images were obtained using a pathological section scanner (C13220-01, Japan).
Transcriptomic analysis of liver
Total RNA was extracted from the liver tissues of ND, HFD, HFD-ACT001 and HFD-PPC (n = 3) using Trizol reagent (Invitrogen, Carlsbad, CA, USA). The concentration and purity of the RNA were assessed using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA, USA), while RNA integrity was evaluated with the Agilent 2100 Bioanalyzer.
Multiplexed DNA libraries were homogenized and mixed in equal volume. The mixed libraries were diluted and quantified step by step, followed by sequencing in PE150 mode using Illumina sequencers. The filtered sequencing data was compared with the reference genome using HISAT2 (v2.1.0). Gene expression was quantified with HTSeq (v0.9.1), and differential expression analysis was conducted using DESeq (v1.38.3). Significant different expression genes were screened under the following conditions: p value < 0.05, |log2FoldChange| > 1. Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation (http://www.genome.jp/kegg/) was conducted to identify key biochemical metabolic and signal transduction pathways10,11,12. KEGG pathway enrichment analysis was performed using the clusterProfiler (v4.6.0) software. Additionally, a portion of the transcriptome analysis was conducted using the Parson’s Gene Cloud platform (https://www.genescloud.cn).
Non-targeted metabolomics analysis of liver
Liver specimens were randomly collected from six mice in each group for metabolomic analysis. Liver samples (50 mg) were combined with 1000 µL of tissue extract (75% methanol: chloroform, 9:1; 25% H₂O) and grinding beads, then homogenized in a tissue grinder for 2 min at − 10 °C and 50 Hz. The ground samples were subjected to ultrasonic extraction at 5 °C for 30 min, followed by centrifugation at 12,000 rpm for 10 min at 4 °C. The resulting supernatants were used for non-targeted metabolome analysis.
Liquid chromatography (LC) was conducted using a Vanquish UHPLC System (Thermo Fisher Scientific, USA), with separation achieved on an ACQUITY UPLC® HSS T3 (2.1 × 100 mm, 1.8 μm) column (Waters, Milford, MA, USA). Mass spectrometric detection of metabolites was performed using a Q Exactive mass spectrometer (Thermo Fisher Scientific, USA) equipped with an ESI ion source. Additionally, a portion of the metabolomics analysis was conducted using the Parson’s Gene Cloud platform (https://www.genescloud.cn).
Real-time fluorescence quantitative PCR analysis
Total RNA was extracted from liver tissue using an RNA extraction kit. The extracted RNA was then reverse-transcribed into cDNA using a reverse transcription kit. The resulting cDNA was added to the PCR amplification reaction mix. The PCR procedure was set as follows: initial denaturation at 95 °C for 30 s, followed by denaturation at 95 °C for 5 s, and annealing at 60 °C for 30 s. The 2−ΔΔCT method was employed for analysis. The primer sequences used for PCR amplification are listed in Supplementary Table S1.
Statistical analysis
SPSS26.0 and GraphPad Prism 9.5 are used for all statistical analysis and graph processing. Continuous variables with normal distribution were expressed as mean ± standard deviation. Independent samples t-test was used for two-group comparisons, and one-way analysis of variance (ANOVA) for multi-group comparisons. Continuous variables that did not follow a normal distribution were expressed as median (25th-75th percentiles). The Mann-Whitney U test was employed for comparisons between two groups, and the Kruskal-Wallis H test was utilized for comparisons among multiple groups. Statistical significance was determined by two-tailed P-values < 0.05.
Results
ACT001 improved hepatic pathology
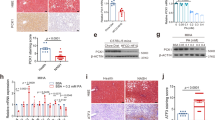
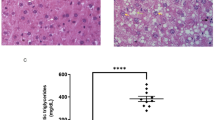
Previous research has shown that mice on a long-term HFD diet have a higher body weight than mice on a ND diet. After treatment with ACT001 or PPC, the weight of the mice decreased. Under the HE staining microscope, the liver cells in the ND group mice appeared intact, with the hepatic sinuses arranged radially in an orderly fashion. There were no noticeable abnormalities observed in the areas surrounding the portal vein and central vein. In the HFD group, there was significant bulla steatosis and balloon-like degeneration present. Many cells appeared markedly swollen, with their nuclei displaced to one side. The cytoplasm was loaded with numerous fat vacuoles, which often coalesced into large fat droplets, accompanied by signs of inflammatory infiltration. Compared to the HFD group, mice in both the HFD-ACT001 and HFD-PPC groups exhibited a reduction in liver damage and hepatocyte swelling as observed under the microscope (Fig. 1A).
Masson staining revealed collagen deposition in the hepatic sinuses of HFD mice, accompanied by a large number of lipid droplets. In contrast, the liver tissue structure of mice in the HFD-ACT001 and HFD-PPC groups showed notable improvement, with a reduction in both collagen deposition and the number of fat vacuoles compared to the HFD group (Fig. 1B).
ACT001 improved liver damage in HFD-fed mice
The HFD group demonstrated significantly elevated levels of liver ALT (p = 0.0173), AST (p < 0.0001), TC (p = 0.0002), and TG (p = 0.0029) compared to the ND group (Fig. 2A.B.C.D.). In contrast, the HFD-ACT001 group exhibited significantly reduced levels of liver ALT (p = 0.0455), AST (p = 0.0028), TC (p = 0.0477), and TG (p = 0.0121) compared to the HFD group. Similarly, the HFD-PPC group also demonstrated significantly lower levels of liver ALT (p = 0.0077), AST (p = 0.0003), TC (p = 0.0049), and TG (p = 0.0306) relative to the HFD group. The results showed that ACT001 could improve liver cell injury and elevated blood lipid induced by high fat diet in mice.
ACT001 extensively modulated hepatic metabolism
We conducted a non-targeted metabolomics analysis of liver samples from four groups of mice to examine the effects of ACT001 on metabolite profiles. Metabolome sequencing followed by principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) was conducted on mouse liver samples using both positive and negative ion modes. The results, shown in Fig. 3A–F, demonstrated clear separation among all groups. Twenty different metabolites could be attributed to a single high-fat diet factor in the positive and negative ion model. Eight differential metabolites were identified as pathogenic metabolites induced by the high-fat diet, with no improvement observed following treatment with the two drugs (Fig. 3G). These metabolites include styrene oxide, 4-pyridoxic acid, gluconic acid, palmitic acid, inosine, sumatriptan, palmitoylethanolamide, and glutathione. In both the positive and negative ion modes, the HFD group exhibited 10 up-regulated and 31 down-regulated differential metabolites compared to the ND group. In contrast, the HFD-ACT001 group displayed an up-regulation of 26 differential metabolites and a down-regulation of 60 metabolites when compared to the HFD group. Similarly, the HFD-PPC group showed 36 up-regulated and 86 down-regulated differential metabolites relative to the HFD group (Fig. 3H, I).
Metabolomics analysis of liver in each group of mice. PCA plots: (A) (ND VS HFD), (B) (HFD VS HFD-ACT001), (C) (HFD VS FD-PPC).PLS-DA plots: (D) (ND VS HFD), (E) (HFD VS HFD-ACT001), (F) (HFD VS FD-PPC). The abscissa is the first principal component and the ordinate is the second principal component. Venn diagram (G), UPSET diagram (H) and bar diagram (I) of the comparison of differential metabolites in the positive and negative ion mode.
To accurately identify differential metabolites, the following criteria were established: p-value < 0.05 and |log2FoldChange|>1, based on the relative expression levels of the metabolites. In the positive ion mode, the volcano plot of differential metabolites in the HFD group compared to the ND group is presented in Fig. 4A. Notable metabolites include Palmitoyl-L-carnitine and Palmitic acid, which are associated with fatty acid degradation. Additionally, Lithocholic acid and Choline play roles in bile secretion. Furthermore, 12-KETE and 16(R)-HETE are linked to arachidonic acid metabolism. In the negative ion mode, Fig. 4B illustrates the contrast volcano plot of differential metabolites between the HFD and ND groups. It emphasizes Homogentisate, linked to tyrosine metabolism, and also points out Arachidic acid, which plays a role in the biosynthesis of unsaturated fatty acids. Finally, Fig. 4C displays the random forest plot of the top 20 differential metabolites identified in both the positive and negative combined ion modes. The red labels indicated the metabolites mentioned above. Overall, these findings highlighted distinct metabolic alterations between the HFD and ND groups, emphasizing key pathways such as fatty acid degradation, bile secretion, and arachidonic acid metabolism.
In the positive ion mode, the volcano plot comparing the HFD group with the HFD-ACT001 group reveals several differential metabolites, as illustrated in Fig. 4D. Notably, 13-L-Hydroperoxylinoleic acid, Linoleic acid, Oleic acid, and Arachidonic acid are all implicated in linoleic acid metabolism, while Cysteinylglycine plays a role in glutathione metabolism. In the negative ion mode, the volcano plot presented in Fig. 4E highlights the differential metabolites of HFD-ACT001 in comparison to the HFD group. Here, Bovinic acid is also linked to linoleic acid metabolism. Figure 4F displays the random forest plot of the top 20 differential metabolites identified in both the positive and negative combined ion modes. These results demonstrated that ACT001 intervention significantly modulates metabolic pathways, particularly linoleic acid metabolism and glutathione metabolism, in the HFD group.
In positive ion mode, the volcano plot (Fig. 4G) of differential metabolites in the HFD-PPC group was compared to the HFD group. It revealed significant involvement of linoleic acid and 13-L-hydroperoxylinoleic acid in linoleic acid metabolism. Moreover, 2-aminobenzoic acid and L-arogenate were implicated in the biosynthesis of amino acids. In negative ion mode, the volcano plot (Fig. 4H) displayed that L-tyrosine was highlighted for its role in tyrosine metabolism, while bovinic acid also contributed to linoleic acid metabolism. Furthermore, L-glutamine, N-acetylglutamic acid, and L-tyrosine were involved in the biosynthesis of amino acids. Figure 4I illustrates the random forest plot showcasing the top 20 differential metabolites in both positive and negative combined ion modes. These results highlighted the considerable effects of PPC intervention on metabolic pathways, especially regarding linoleic acid metabolism, glutathione metabolism, and amino acid biosynthesis, within the HFD group.
Volcano plot and random forest plot for pairwise comparison of differential metabolites in the four groups of mice. Volcano plot of the comparison of differential metabolites in ND VS HFD in positive (A) and negative (B) ion mode. Volcano plot of the comparison of differential metabolites of HFD VS HFD-ACT001 in the positive ion mode (D) and negative ion mode (E). Volcano plot of the HFD VS HFD-PPC differential metabolites in the positive ion mode (G) and negative ion mode (H). Random forest plots of differential metabolites in the positive and negative ion modes of ND VS HFD (C), HFD VS HFD-ACT001 (F), and HFD VS HFD-PPC (I).
The KEGG analysis was employed to identify the regulatory signaling pathways through which ACT001 improved MAFLD. In both positive and negative ion modes, the top 10 KEGG-enriched pathways were analyzed for the ND and HFD groups, revealing key pathways such as tyrosine metabolism, bile secretion, and fatty acid degradation (Fig. 5A, B). In comparing the HFD and HFD-ACT001 groups, the enriched pathways included linoleic acid metabolism, glutathione metabolism, and biosynthesis of amino acids (Fig. 5C, D). Furthermore, the comparison between the HFD group and the HFD-PPC group highlighted enriched pathways related to protein digestion and absorption, biosynthesis of amino acids, and metabolic pathways (Fig. 5E, F). In summary, these findings revealed unique alterations in metabolic pathways triggered by HFD. They also emphasized the regulatory roles of ACT001 and PPC interventions on lipid, amino acid, and glutathione metabolism.
KEGG enrichment analysis of metabolic substances in MAFLD mice across each group. Bar graph illustrates the KEGG enrichment analysis of differential metabolites in positive and negative ion modes for the following comparisons: ND vs. HFD (A), HFD vs. HFD-ACT001 (C), and HFD vs. HFD-PPC (E). Similarly, bubble plots demonstrate KEGG pathways between groups: ND vs. HFD (B), HFD vs. HFD-ACT001 (D), and HFD vs. HFD-PPC (F).
ACT001 extensively modulated hepatic transcription
In the transcriptome sequencing analysis of mouse liver samples, we observed that the ND group distinctly separated from the HFD group in the overall PCA map. Furthermore, the drug treatment group exhibited a closer proximity to the normal group, as shown in Fig. 6A. This suggests drug therapy may be working. In a Venn diagram, 258 differentially expressed genes (DEGs) were found to be exclusively linked to high fat diets. Of these, twenty-nine DEGs are identified as disease-causing genes that were induced by the high-fat diet and showed no improvement with the two drugs, as shown in Fig. 6B. Compared to the ND group, the HFD group showed 193 up-regulated and 149 down-regulated DEGs. Compared to the HFD group, the HFD-ACT001 group exhibited 102 up-regulated and 91 down-regulated DEGs. Similarly, the HFD-PPC group presented 131 up-regulated and 144 down-regulated DEGs compared to the HFD group (Fig. 6C). The transcriptome analysis reveals clear differences in gene expression patterns between the ND and HFD groups. Drug treatments, including ACT001 and PPC, were observed to partially reverse these changes, indicating their potential therapeutic effects.
Volcano plots was used to identify DEGs in comparisons between groups. Compared to the ND group, several DEGs were found to be upregulated in the HFD group (Fig. 6D). Genes implicated in glutathione metabolism, including Gsta1, Gstm1, and Gstp1, were elevated, alongside Col1a1 (PI3K-Akt signaling), Plin4 (PPAR pathway), and Hspa1b (MAPK signaling). Critical metabolic pathways were linked to several downregulated DEGs in the HFD group. Lepr (implicated in non-alcoholic fatty liver disease and adipocytokine/JAK-STAT/AMPK signaling), Cyp2c54 and Cyp3a16 (involved in linoleic acid and steroid hormone metabolism), along with Cyp4a31 and Cyp4a14 (participating in PPAR signaling and arachidonic acid metabolism), were suppressed. Additionally, Socs2 (associated with insulin and JAK-STAT signaling) showed decreased expression. This pattern underscored a systemic dysregulation of lipid metabolism, oxidative stress responses, and inflammatory signaling in the HFD group, highlighting potential mechanistic drivers of diet-induced metabolic dysfunction.
Comparative transcriptomic analysis between the HFD and HFD-ACT001 groups identified significant differential gene expression patterns (Fig. 6E). Upregulation was observed in genes implicated in lipid metabolism and signaling pathways, including Cyp3a44 (linoleic acid metabolism/steroid hormone biosynthesis), Map3k13 (MAPK signaling), Chac1 (glutathione/linoleic acid metabolism), Cyp2c54 (linoleic/arachidonic acid metabolism/steroid biosynthesis), Egfr (MAPK signaling), Cyp2b10 (arachidonic acid metabolism/steroid biosynthesis), and Hgf (PI3K-Akt signaling). In contrast, downregulation was detected in Hspa1b (MAPK signaling) and Hsp90aa1 (PI3K-Akt signaling). These findings suggested that ACT001 intervention modulates lipid metabolism and stress-related signaling pathways, potentially counteracting HFD-induced dysregulation through coordinated gene expression changes.
Compared to the HFD group, several genes were upregulated in the HFD-PPC group (Fig. 6F). Upregulation was observed in genes associated with lipid metabolism, including Cyp7a1 (cholesterol metabolism, bile secretion, PPAR signaling), Cyp2c54 (linoleic/arachidonic acid metabolism and steroid biosynthesis), and Chac1 (glutathione metabolism). Additional upregulated genes implicated in signaling processes included Map3k13 (MAPK signaling) and Slco1a1 (bile secretion). Conversely, downregulation was detected in Hspa1b (MAPK signaling), Hsp90aa1 (PI3K-Akt signaling), and Hspa8 (MAPK signaling). These transcriptional changes highlighted the regulatory effects of PPC on lipid metabolism and stress-related signaling pathways, suggesting its potential in mitigating HFD-induced metabolic dysregulation.
Transcriptome analysis of liver in MAFLD mice. Overall PCA plots of the four groups of MAFLD mice (A). The abscissa is the first principal component and the ordinate is the second principal component. Venn diagram (B) and bar graph (C) of differentially expressed genes in four groups of mice. Volcano plot of differentially expressed genes in ND VS HFD (D), HFD VS HFD-ACT001 (E) and HFD VS HFD-PPC (F).
Gene Ontology (GO) enrichment analysis revealed distinct functional pathways across groups, as detailed below. Transcriptome sequencing identified the top 20 enriched GO pathways in both the ND and HFD groups, which included lipid metabolic processes, steroid metabolic processes and steroid hydroxylase activity, among others (Fig. 7A). The HFD and HFD-ACT001 groups showed pathways related to lipid metabolic processes, steroid sulfotransferase activity, and steroid hydroxylase activity (Fig. 7C). Furthermore, both the HFD and HFD-PPC groups exhibited pathways associated with insulin-activated receptor activity, negative regulation of gluconeogenesis, the enzyme-linked receptor protein signaling pathway, and negative regulation of lipid localization (Fig. 7D). These results collectively demonstrated that HFD induced broad dysregulation in lipid and steroid metabolism, while ACT001 and PPC interventions partially restored pathway activity through distinct regulatory mechanisms.
KEGG pathway enrichment analysis revealed distinct metabolic signatures across experimental groups. The top 20 KEGG pathways enriched in transcriptome sequencing for both the ND and HFD groups included bile secretion, linoleic acid metabolism, glutathione metabolism, and arachidonic acid metabolism, among others (Fig. 7B). The HFD and HFD-ACT001 groups were found to have pathways related to arachidonic acid metabolism, linoleic acid metabolism, the MAPK signaling pathway, the PI3K-Akt signaling pathway, cholesterol metabolism, and bile secretion (Fig. 7E). Additionally, the HFD and HFD-PPC groups exhibited enrichment in linoleic acid metabolism, glutathione metabolism, and MAPK signaling pathways (Fig. 7F). These findings also underscored the central role of lipid metabolism, oxidative stress regulation, and signaling cascades in HFD-induced metabolic perturbations, with ACT001 and PPC interventions demonstrating pathway-specific modulatory effects.
Performed GO and KEGG enrichment analyses on four groups of MAFLD mice. The bubble maps for GO enrichment analysis are presented for the comparisons ND VS HFD (A), HFD VS HFD-ACT001 (C), and HFD VS HFD-PPC (E). The bubble maps for KEGG enrichment analysis are shown for the comparisons ND VS HFD (B), HFD VS HFD-ACT001 (D), and HFD VS HFD-PPC (F).
Integrated analysis of transcriptomics and metabolomics
Integrated transcriptomic and metabolomic analyses demonstrated that a high-fat diet (HFD) primarily induces MAFLD through the bile secretion pathway (Fig. 8A). In this pathway, the up-regulated differentially expressed genes (DEGs) included Slc4a4, Ugt1a5, Ugt2b37, Sult2a7, and Slco1a1, while the down-regulated DEGs included Slc10a2, Cyp7a1, Sult2a4, Sult2a5, and Sult2a1. Among the metabolites, Glutathione and Choline were up-regulated, whereas Uric acid and Lithocholic acid were down-regulated. The correlation between the differential metabolites and DEGs is presented in Fig. 8B.
Combined transcriptomic and metabolomic analyses revealed that ACT001 primarily functions through three key pathways: Linoleic acid metabolism, central carbon metabolism, and glutathione metabolism, all of which played a role in improving MAFLD in mice (Fig. 8C). We further identified significantly different metabolites (VIP > 1 and p < 0.05) and differentially expressed genes (|log2FC|≥1, p < 0.05). The Spearman algorithm was employed to analyze the correlation between these differential metabolites and genes, with the results shown in Fig. 8D. The metabolites associated with lipid metabolism included 13-L-Hydroperoxylinoleic acid, Palmitic acid, Linoleic acid, Oleic acid, Norlinolenic acid, Stearidonic acid, and Deoxycorticosterone. Cyp2c54, Cyp3a44, Linoleic acid, and 13-L-Hydroperoxylinoleic acid were involved in linoleic acid metabolism. Notably, Linoleic acid and 13-L-Hydroperoxylinoleic acid showed positive correlations with Hspalb, Hsp90aa1, and Hyou1. Ascorbate and Pyroglutamic acid were found to be involved in glutathione metabolism, with both metabolites exhibiting a negative correlation with Cyp3a44. The multi-omics findings highlighted the regulatory effects of ACT001 on lipid homeostasis, offering mechanistic insights into its therapeutic potential for the treatment of MAFLD.
Validation of differentially expressed genes
To validate the differentially expressed genes, we selected Cyp2c54 (involved in linoleic acid metabolism), Hsp90aa1 (part of the PI3K-Akt pathway), Chac1 (in the glutathione metabolism pathway) as well as Map3k13 and Hspa1b (related to the MAPK pathway) for RT-qPCR analysis. The results indicated that Cyp2c54 mRNA expression was significantly up-regulated (p < 0.0001), whereas Hsp90aa1 mRNA expression was significantly down-regulated (p = 0.0056) in the ACT001 group compared to the HFD group (Fig. 9A, B). Additionally, the mRNA expression of Chac1 was increased in the ACTOO1 group relative to the HFD group, while the levels of Map3k13 and Hspa1b were decreased; however, these changes did not reach statistical significance (Fig. 9C, D, E).
Discussion
Previous studies have explored the mechanism of ACT001 improving NASH at the proteomic level9. In this study, we attempted to explore the potential mechanisms by which ACT001 improves MAFLD at the transcriptional and metabolic levels. Firstly, we established a mouse model of HFD-induced MAFLD and conducted biochemical and histopathological assessments to validate the therapeutic efficacy of ACT001 on MAFLD. The administration of ACT001 improved lipid and liver function in MAFLD mice, with pathological assessments revealing a reduction in hepatic steatosis. Secondly, transcriptomics and metabolomics were employed to investigate the potential pathways through which ACT001 exerts its effects in the treatment of MAFLD. Finally, the integration of transcriptomics and metabolomics revealed that ACT001 may improve MAFLD by modulating linoleic acid metabolism and glutathione metabolism.
The combination of transcriptome and metabolomics analyses revealed that a high-fat diet primarily induces MAFLD by modifying the bile secretion pathway. Developing MAFLD is mostly associated with accumulating lipids and lipotoxicity13. The enrichment pathway analysis for the ND group and HFD group indicates a significant relationship with lipid and steroid metabolic processes, suggesting that a high-fat diet may influence the progression of MAFLD through lipid metabolism. Bile secretion is an important function of the liver to provide bile acids that are essential for fat digestion and absorption, promote the elimination of metabolites and exogenous substances, and facilitate the intestinal absorption of lipids and fat-soluble vitamins14. The formation of bile acids (BA) from cholesterol occurs through either the classical pathway, involving 7α-hydroxylation of cholesterol via CYP7A1, or through an alternative pathway that utilizes CYP39A1 or CYP7B115. Our study also found that in the biliary secretion pathway, the CYP7A1 gene was down-regulated in the high-fat group compared to the normal group. bile acids (BA) regulate the metabolic balance of bile acids, cholesterol, lipids, and glucose by activating nuclear hormone receptors like farnesoid X receptor (FXR) and pregnane X receptor (PXR)16. The connection between bile acids (BA) and MAFLD may involve several mechanisms, including enhanced synthesis in hepatocytes, decreased intestinal conversion to secondary bile acids, and reduced enterohepatic circulation17.
Combined transcriptomic and metabolomic analysis revealed that ACT001 may alleviate HFD-induced MAFLD by regulating linoleic acid metabolic pathway and Glutathione metabolism pathway. This suggests that lipid metabolism and oxidative stress may be important in the mechanism by which ACT001 alleviates MAFLD.
By increasing the expression of Cyp2c54, which is involved in the metabolism of linoleic acid, ACT001 may improve MAFLD. Cytochromes P450 (CYPs) participate in a diverse range of reactions such as S-oxidation and hydroxylation, and they are essential for the metabolism of xenobiotics, steroids, and fatty acids18. Notably, hepatic Cyp2c54 expression was significantly suppressed in HFD-induced MAFLD mouse models, suggesting its potential role in disease pathogenesis19.Previous proteomic analyses have shown that bicyclol may improve MAFLD by increasing the expression of Cyp2c5420.
The upregulation of Cyp2c54 by ACT001 may enhance linoleic acid (LA) metabolism, thereby altering the balance of its downstream metabolites, including arachidonic acid (AA). LA can be converted into AA, a key player in the inflammatory process, as it acts as a substrate for the production of pro-inflammatory compounds that lead to the release of mediators such as tumor necrosis factor α (TNF-α) and interleukin-1 (IL-1)21. Some scholars have suggested, based on previous studies, that LA might not directly trigger the production of inflammatory cytokines. Instead, its endogenous conversion to AA appears to drive pro-inflammatory signaling22. Previous studies examining the fatty acid composition in the liver tissue of patients with MAFLD have revealed that AA levels are reduced in individuals with liver disease; Additionally, a lower ratio of AA to LA has been linked to the presence of MAFLD and NASH23. This clinical study suggested that AA deficiency may be a potential biomarker for disease progression. Intriguingly, ACT001 treatment in HFD-fed mice reversed this imbalance, elevating AA level while reducing LA accumulation. This suggests that a significant amount of LA was converted to AA, maybe contributing to anti-inflammatory effect.
Glutathione (GSH) is a widely occurring non-protein thiol found in all organisms, serving crucial roles in antioxidant defense and detoxification while maintaining cellular redox balance24. The abundance of GSH support the liver’s defense mechanisms against oxidative toxicity and are believed to play an essential protective role in several liver conditions, including non-alcoholic fatty liver disease and other liver disorders25. Our results showed that compared with HFD group, Chac1 and glutathione in HFD-ACT001 group were up-regulated, while GPX6 and Gsta1 were down-regulated. In vitro, the overexpression of Chac1, which is a proapoptotic γ-glutamyl cyclotransferase that depletes glutathione, triggered apoptosis in macrophages and was inhibited by the administration of cell-permeable glutathione26. In mammals, glutathione peroxidase family (GPX) collaborates with superoxide dismutase and catalase to create an enzymatic antioxidant system that diminishes reactive oxygen species (ROS) and mitigates their toxicity27. GSTA1 was significantly elevated in cell models of ethanol-induced acute liver injury28. A study indicated that GSTA1 may be secreted by the liver and function as an antioxidant to help prevent acute liver damage29. The connection between ACT001 and the MAFLD mouse model, as well as glutathione metabolism-related genes, requires further investigation.
In this study, ACT001 demonstrated promising efficacy in improving HFD-induced MAFLD in mice, indicating that its therapeutic effects may be linked to pathways associated with glutathione and linoleic acid metabolism. This establishes a strong basis for considering ACT001 as a potential treatment for MAFLD. However, the study has some limitations. First, the transcriptomic and metabolomic analyses were conducted with limited sample sizes (n = 3 for transcriptomics; n = 6 for metabolomics), which may reduce statistical power to detect subtle but biologically relevant changes. Small cohort sizes increase the risk of type II errors (false negatives) and variability in omics data interpretation, potentially obscuring minor pathways or metabolite-gene correlations. While the observed alterations in glutathione and linoleic acid metabolism are robust, validation in larger cohorts is essential to confirm reproducibility and generalize the conclusions. secondly, the research did not include experiments with inhibitors or agonists targeting the aforementioned pathways. Notably, the causal relationships between ACT001 intervention and glutathione/ linoleic acid metabolism metabolic reprogramming remain unverified without functional cellular studies, such as hepatocyte culture models treated with pathway-specific modulators.
Future studies should prioritize expanding omics sample sizes to enhance statistical reliability and ensure comprehensive pathway coverage. Additionally, it is important to incorporate inhibitors, such as GSH synthesis blockers, and agonists, like linoleic acid pathway activators, to establish causality between ACT001 and its proposed mechanisms. Furthermore, validating key findings in vitro using cell types such as hepatocytes or macrophages will be essential for dissecting cell-type-specific effects. Despite these limitations, the integrative multi-omics approach robustly identifies glutathione and linoleic acid metabolism as central pathways modulated by ACT001, offering an actionable framework for preclinical development and mechanistic refinement.
Data availability
The datasets presented in this research are available from online repositories. Repository names and accession numbers are given below: NCBI’s BioProject data base: accession PRJNA1201872 Metabolomics data have been deposited to the EMBL-EBI MetaboLights database with the identifier MTBLS12051.
References
Eslam, M., Sanyal, A. J. & George, J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158. https://doi.org/10.1053/j.gastro.2019.11.312 (2020).
Byrne, C. D. & Targher, G. NAFLD: A multisystem disease. J. Hepatol. 62, S47–S64. https://doi.org/10.1016/j.jhep.2014.12.012 (2015).
Wei, S., Wang, L., Evans, P. C. & Xu, S. NAFLD and NASH: Etiology, targets and emerging therapies. Drug Discov. Today 29, 103910. https://doi.org/10.1016/j.drudis.2024.103910 (2024).
Allen, A. M., Lazarus, J. V. & Younossi, Z. M. Healthcare and socioeconomic costs of NAFLD: A global framework to navigate the uncertainties. J. Hepatol. 79, 209–217. https://doi.org/10.1016/j.jhep.2023.01.026 (2023).
Cai, L. et al. ACT001 attenuates microglia-mediated neuroinflammation after traumatic brain injury via inhibiting AKT/NFκB/NLRP3 pathway. Cell. Commun. Signal. 20, 56. https://doi.org/10.1186/s12964-022-00862-y (2022).
Luo, H. et al. ACT001 ameliorates ionizing radiation-induced lung injury by inhibiting NLRP3 inflammasome pathway. Biomed. Pharmacother. 163, 114808. https://doi.org/10.1016/j.biopha.2023.114808 (2023).
Zhou, Y. et al. ACT001 alleviates chronic kidney injury induced by a high-fat diet in mice through the GPR43/AMPK pathway. Lipids Health Dis. 22, 198. https://doi.org/10.1186/s12944-023-01949-2 (2023).
Yu, Q. et al. Alleviative effects of the parthenolide derivative ACT001 on insulin resistance induced by sodium propionate combined with a high-fat diet and its potential mechanisms. Eur. J. Pharmacol. 971, 176529. https://doi.org/10.1016/j.ejphar.2024.176529 (2024).
Zhou, H. et al. iTRAQ-based quantitative proteomics analysis of the effect of ACT001 on non-alcoholic steatohepatitis in mice. Sci. Rep. 13, 11336. https://doi.org/10.1038/s41598-023-38448-4 (2023).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–D677. https://doi.org/10.1093/nar/gkae909 (2025).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Guo, X., Yin, X., Liu, Z. & Wang, J. Non-Alcoholic fatty liver disease (NAFLD) pathogenesis and natural products for prevention and treatment. Int. J. Mol. Sci. 23. https://doi.org/10.3390/ijms232415489 (2022).
Cai, J. et al. The metabolic pathway of bile secretion is vulnerable to Salmonella enterica exposure in Porcine intestinal epithelial cells. Animals 14. https://doi.org/10.3390/ani14050789 (2024).
Smelt, A. H. M. Triglycerides and gallstone formation. Clin. Chim. Acta 411, 1625–1631. https://doi.org/10.1016/j.cca.2010.08.003 (2010).
Gottlieb, A. & Canbay, A. Why bile acids are so important in Non-Alcoholic fatty liver disease (NAFLD) progression. Cells 8. https://doi.org/10.3390/cells8111358 (2019).
Arab, J. P., Karpen, S. J., Dawson, P. A., Arrese, M. & Trauner, M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 65, 350–362. https://doi.org/10.1002/hep.28709 (2017).
Abass, K., Reponen, P., Anyanwu, B. & Pelkonen, O. Inter-species differences between humans and other mammals in the in vitro metabolism of Carbofuran and the role of human CYP enzymes. Environ. Toxicol. Pharmacol. 102, 104243. https://doi.org/10.1016/j.etap.2023.104243 (2023).
Wang, C., Tao, Q., Wang, X., Wang, X. & Zhang, X. Impact of high-fat diet on liver genes expression profiles in mice model of nonalcoholic fatty liver disease. Environ. Toxicol. Pharmacol. 45, 52–62. https://doi.org/10.1016/j.etap.2016.05.014 (2016).
Wu, J. et al. Bicyclol attenuates high fat diet-induced non-alcoholic fatty liver disease/non-alcoholic steatohepatitis through modulating multiple pathways in mice. Front. Pharmacol. 14, 1157200. https://doi.org/10.3389/fphar.2023.1157200 (2023).
Fritsche, K. L. Too much Linoleic acid promotes inflammation-doesn’t it? Prostaglandins Leukot. Essent. Fat. Acids 79, 173–175. https://doi.org/10.1016/j.plefa.2008.09.019 (2008).
Burns, J. L., Nakamura, M. T. & Ma, D. W. L. Differentiating the biological effects of Linoleic acid from arachidonic acid in health and disease. Prostaglandins Leukot. Essent. Fat. Acids 135, 1–4. https://doi.org/10.1016/j.plefa.2018.05.004 (2018).
Puri, P. et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46, 1081–1090 (2007).
Aquilano, K., Baldelli, S. & Ciriolo, M. R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 5, 196. https://doi.org/10.3389/fphar.2014.00196 (2014).
Hong, S. H., Yu, X., Zhu, Y. & Chen, Y. Liver epigenomic signature associated with chronic oxidative stress in a mouse model of glutathione deficiency. Chem. Biol. Interact. 398, 111093. https://doi.org/10.1016/j.cbi.2024.111093 (2024).
Allawzi, A. et al. Redistribution of EC-SOD resolves bleomycin-induced inflammation via increased apoptosis of recruited alveolar macrophages. FASEB J. 33, 13465–13475. https://doi.org/10.1096/fj.201901038RR (2019).
Pei, J., Pan, X., Wei, G. & Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 14, 1147414. https://doi.org/10.3389/fphar.2023.1147414 (2023).
Chang, Y. C. et al. Glutathione S-transferase A1—a sensitive marker of alcoholic injury on primary hepatocytes. Hum. Exp. Toxicol. 36, 386–394. https://doi.org/10.1177/0960327116650013 (2017).
Ma, X. et al. Expression of glutathione S-transferase A1, a phase II drug-metabolizing enzyme in acute hepatic injury on mice. Exp. Ther. Med. 14, 3798–3804. https://doi.org/10.3892/etm.2017.4957 (2017).
Acknowledgements
We would like to thank the Institute of Liver Medicine, Tianjin Second People’s Hospital for their support of this project. We would also like to acknowledge the help and support we received from Shangde Pharmaceutical Technology Co., LTD. (Tianjin, China).
Funding
This research was funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-059B).
Author information
Authors and Affiliations
Contributions
J.L., Y.Z. and P.L. conceived and designed the experiments. J.L., Y.Z., Y.L., Z.C., X.J., J.H. and Q.W. performed the experiments. J.L. and Y.Z. analyzed the data. J.L. wrote the manuscript. Y.M. and P.L. supervised the study. All authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was conducted in accordance with the ARRIVE guidelines and was approved by the Ethics Committee of Nankai University (Approval No. 2021-SYDWLL-000003).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, J., Zhou, Y., Li, Y. et al. Integrative transcriptomic and metabolomic analysis explores the mechanisms by which ACT001 treats MAFLD in mice. Sci Rep 15, 12494 (2025). https://doi.org/10.1038/s41598-025-97312-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97312-9