Abstract
Gallium 68 (68Ga)-labeled DOTA-conjugate ibandronic acid (DOTA-IBA) has been successfully synthesized and utilized for bone metastasis imaging. This study compares the diagnostic efficacy between 68Ga-DOTA-IBA and fluorine 18 (18F)-labeled fluorodeoxyglucose (FDG) in detecting bone metastases. This prospective study, conducted from October 2022 to September 2023, analyzed images from participants who underwent 68Ga-DOTA-IBA PET/CT and 18F-FDG PET/CT scans. Lesions were classified into five groups based on anatomical location (limbs, vertebrae, pelvis, ribs, and skull). Morphological bone changes were categorized as osteolytic, osteoblastic, or mixed. The semi-quantified radiotracer uptake, measured by the maximum standardized uptake value (SUVmax), was compared using a paired t-test. Detection rates between the two scans were analyzed using the McNemar test. A total of 46 participants (median age: 64 years [interquartile range: 53–68 years], 28 men) were evaluated. 68Ga-DOTA-IBA demonstrated higher diagnostic efficacy than 18F-FDG in detecting bone metastases in the limbs (73.2% vs. 64.1%), vertebras (78.1% vs. 67.4%), ribs (86.6% vs. 62.2%), pelvis (78.6% vs. 68.9%), and skulls (80.0% vs. 38%). For osteoblastic lesions, the detection rate for 68Ga-DOTA-IBA and 18F-FDG was 83.3% and 51.5% respectively (P < 0.001). The SUVmax of 68Ga-DOTA-IBA was 7.88 (95% CI 7.09–8.66), which was higher than that of 18F-FDG at 3.96 (95% CI 3.57–4.35) (P < 0.001). In participants with prostate cancer, the detection rate of 68Ga-DOTA-IBA and 18F-FDG was 84.7% and 55.0% respectively (P < 0.001). The SUVmax of 68Ga-DOTA-IBA was 10.44 (95% CI 8.57–12.30), which was higher than that of 18F-FDG 4.29 (95% CI 3.51–5.07) (P < 0.001). 68Ga-DOTA-IBA PET/CT demonstrates superior diagnostic performance over 18F-FDG PET/CT in detecting bone metastases, particularly in osteoblastic lesions and prostate cancer cases.
Similar content being viewed by others
Introduction
Bone metastases frequently occur in patients diagnosed with prostate and lung cancer, leading to severe complications and reduced survival rates1,2,3. Nuclear medicine plays an important role in early tumor metastasis detection, using highly sensitive agents. Fluorine 18 (18F)-labeled fluorodeoxyglucose (18F-FDG) has been proven to have a good performance in detecting bone metastases of different cancers. While 18F-FDG PET/CT is widely used for detecting bone metastases, its sensitivity in identifying osteoblastic lesions is limited4. Physiological bone marrow uptake and coexisting hematologic disorders can also affect diagnostic accuracy.
Ibandronate (Ibandronic acid, IBA) is a third-generation bisphosphonate clinically used to prevent osteoporosis in participants suffering from bone metastases5. 68Ga-DOTA-IBA has been successfully synthesized via a simple radiolabeling method in a previous study6. Pre-clinical study and biodistribution of 68Ga-DOTA-IBA have shown its high radiochemical purity, stability, and low toxicity6. Moreover, 68Ga-DOTA-IBA exhibits a high tumor-to-background ratio in bone metastases imaging, supporting its use as a novel PET tracer7,8.
This study aims to evaluate the diagnostic efficacy of 68Ga-DOTA-IBA in detecting bone metastases compared to 18F-FDG.
Participants and methods
Participants
This prospective study was conducted in our hospital. All participants were consecutively enrolled from October 2022 to September 2023. Sample size calculations are summarized in Supplementary Material (1) STARD checklist can be viewed in Supplementary Material (2) This study was approved by the ethics committee of the affiliated hospital of Southwest Medical University and registered on the Clinical Trial Registry (Clinical ethics registration number: ChiCTR2200064487). All participants had clear indications for 18F-FDG PET/CT for initial staging or restaging after therapy. Before undergoing 68Ga-DOTA-IBA, participants were asked to sign a consent agreement to receive the examination. Inclusion criteria included (a) pathologic confirmed primary disease, (b) at least one comparable bone metastatic lesion, and (c) agreement to undergo 68Ga-DOTA-IBA examination. Exclusion criteria included (a) previous treatment of bone metastases with bisphosphonate analog, (b) any treatment capable of stimulating bone marrow metabolism, and (c) any history of non-solid tumors or hematologic diseases.
Preparation of 18F-FDG and 68Ga-DOTA-IBA
Following a standard protocol, 18F-FDG was synthesized using an FDG-N synthesis module (PET Science & Technology, China). 68Ga was eluted from a 68Ge/68Ga generator (ITG 101, Eckert & Ziegler, Germany). DOTA-IBA was synthesized in our laboratory and provided by Shanghai New Drug Development Company, with detection performed using PET/CT (United Imaging 780, Shanghai United Imaging Medical Technology Co., Ltd.). All other equipment, software, and reagents were obtained from commercial sources.
68Ga-DOTA-IBA was prepared as previously described. Briefly, a mixture of 10 µg (10 µL) DOTA-IBA, 1 mL of sodium acetate, and 4 mL of 68GaCl3 was incubated at 95 °C for 15 min after the pH was adjusted to 4–5. The solution was then cooled and sterilized through filtration.
Radiochemical quality control was conducted via thin-layer chromatography, using a 0.1 M sodium citrate solution as the solvent. The radiochemical purity of 68Ga-DOTA-IBA was confirmed to be > 97%.
PET/CT imaging acquisition
Participants fasted for at least 6 h before imaging to ensure normal plasma glucose levels (< 8.3 mmol/L). After intravenous injection of 18F-FDG (0.1 mCi/kg), with residual activity in the empty syringe kept below 0.5 mCi, participants were instructed to drink water and rest silently. Imaging was performed 45–60 min post-injection. Whole-body CT scans (head to mid-thigh) were acquired using tube voltage: 120 kV; current: 100 mAs; slice thickness: 5.0 mm. Paired 68Ga-DOTA-IBA scans were conducted within 7 days of the 18F-FDG scans. The 68Ga-DOTA-IBA dose was calculated as 0.1 mCi/kg, with residual activity in the empty syringe below 0.5 mCi. A total acquisition of 5–6 beds at 3 min/position with a subset number 33, an iteration number 3, and a 512 \(\:\times\:\) 512 matrix were used to reconstruct PET images using CT images for attenuation correction.
Imaging review
Initially, three board-certified nuclear medicine physicians (T.X. and D.P.) with more than 5 years of experience reached a consensus on bone metastases based on CT or MRI findings. Subsequently, T.X. and D.P., blinded to each other’s assessments, independently reviewed 18F-FDG and 68Ga-DOTA-IBA images. Any discrepancies were resolved through consensus with a third nuclear medicine expert (Y.C.). Follow-up imaging 3 months later served as the reference standard for lesion confirmation. Lesions were categorized into five groups based on anatomical location: limbs, vertebras, pelvis, ribs, and skulls. Lesions were classified as positive if activity was significantly increased and markedly higher than the adjacent background; or negative: if activity was diffusely mild or indistinguishable from surrounding normal tissues.
Maximum standardized uptake values (SUVmax) for 18F-FDG and 68Ga-DOTA-IBA were automatically calculated by the software after manually placing a volume of interest (VOI) over the targeted lesion. If discrepancies arose between the two primary reviewers (T.X. and D.P.), a consensus was reached after a third review by Y.C. If there were multiple bone metastases at one site, the average value was used, which was defined as averaging the SUVmax of all lesions (no more than 5 lesions at one location) or averaging the SUVmax of 5 biggest lesions ranked by their longer length (more than 5 lesions at one location). The length of each selected lesion for SUVmax comparison was recorded. Besides, the morphological bone changes of each selected were also recorded as osteolytic, osteoblastic, and mixed.
Statistical analysis
Radiotracer uptake, semi-quantified by SUVmax, was compared using the paired t or Mann-Whitney U tests. Detection rates between the two scans were compared using the McNemar test. Two-tailed P < 0.05 was considered statistically significant. All statistical analysis and graph building were conducted using SPSS software (version 26.0; IBM) and GraphPad (version 9.5.0; PRISM).
Results
Participants
A total of 46 participants (28 men and 18 women; median age: 64 years [interquartile range: 53–68 years]) were consecutively enrolled in our hospital between October 2022 and September 2023. All participants had a confirmed diagnosis through biopsy or surgery. The characteristics of the participants are summarized in Table 1, and the study flow diagram is presented in Fig. 1. The interval between the two imaging examinations was 3.1 ± 1.2 days. Among these participants, 22 of them had lung cancer and 12 of them had prostate cancer. The number of participants suffered from nasopharyngeal, intestinal, and neuroendocrine cancer was 4, 3 and 3 respectively. Others included 1 renal carcinoma and 1 spleen hemangiosarcoma. Among all participants, 23 had osteoblastic bone lesions as major metastatic type, 13 had an osteolytic type, and 10 had a mixed type.
Detecting bone metastases from different sites
Morphological imaging identified 1103 bone lesions among the 46 participants. Tables 2 and 3 show the diagnostic sensitivity and radiotracer uptake comparison between 18F-FDG and 68Ga-DOTA-IBA.
Limb lesions
-
153 bone metastases were detected in 26 participants.
-
Detection rates 18F-FDG vs. 68Ga-DOTA-IBA): 64.1% (98/153) vs. 73.2% (112/153) (P = 0.024).
-
SUVmax 18F-FDG vs. 68Ga-DOTA-IBA): 4.1 (1.8–15.2) vs. 8.5 (2.7–29.2) (P = 0.004).
Vertebral lesions
-
485 vertebral metastases were found in 37 participants.
-
Detection rates 18F-FDG vs. 68Ga-DOTA-IBA): 67.4% (327/485) vs. 78.1% (379/485) (P < 0.001).
-
SUVmax 18F-FDG vs. 68Ga-DOTA-IBA): 5.2 (2.3–14.9) vs. 9.8 (3.7–31.4) (P < 0.001).
Rib lesions
-
209 metastatic rib lesions were identified in 31 participants.
-
Detection rates 18F-FDG vs. 68Ga-DOTA-IBA): 62.2% (130/209) vs. 86.6% (181/209) (P < 0.001).
-
SUVmax 18F-FDG vs. 68Ga-DOTA-IBA): 2.6 (1.2–8.9) vs. 5.8 (2.5–21.0) (P < 0.001).
Pelvic lesions
-
206 pelvic metastases were found in 30 participants.
-
Detection rates 18F-FDG vs. 68Ga-DOTA-IBA): 68.9% (142/206) vs. 78.6% (162/206) (P= 0.019).
-
SUVmax 18F-FDG vs. 68Ga-DOTA-IBA): 5.0 (1.6–12.8) vs. 8.4 (3.5–23.0) (P= 0.001).
Skull lesions
-
50 skull metastases were detected in 19 participants.
-
Detection rates 18F-FDG vs. 68Ga-DOTA-IBA): 38.0% (19/50) vs. 80.0% (40/50) (P < 0.001).
-
SUVmax 18F-FDG vs. 68Ga-DOTA-IBA): 3.1 (1.2–8.9) vs. 5.8 (2.5–21.0) (P = 0.005).
Detection of bone metastases by different types
Among all participants involved, 23(50%) of them had osteoblastic bone lesions as major metastatic type, 13(28%) for osteolytic type and 10(22%) for mixed type. The diagnostic performance for different lesion types is summarized in Table 4, while the comparison of radiotracer uptake (SUVmax) in osteoblastic and osteolytic lesions is presented in Fig. 2. Representative imaging is shown in Fig. 3.
Comparison of radiotracer uptake of lesions with different types. *Paired samples t test (both P < 0.001), **Mann–Whitney U test (P < 0.001). The number of osteoblastic and osteolytic bone metastases to compare was 249 and 164, respectively. SUVmax was displayed with mean and 95% CI. 68Ga-DOTA-IBA, gallium 68 (68Ga)-labeled DOTA-conjugate ibandronic acid; 18F-FDG, fluorine 18 (18F)-labeled fluorodeoxyglucose; SUVmax, maximum standardized uptake value.
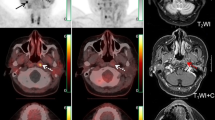
Images of a 69-year-old man diagnosed with prostate carcinoma who had a history of endocrine therapy for 2 years. (a) The maximum intensity projection (MIP) image of 18F-FDG showed moderate radiotracer uptake in a thoracic vertebra. (e) The maximum intensity projection (MIP) image of 68Ga-DOTA-IBA demonstrated more lesions in the cervical vertebra and pelvis. (b) An osteoblastic lesion in the C3 vertebra right attachment (curved arrows) was negative on 18F-FDG (SUVmax=2.0) and positive on 68Ga-DOTA-IBA (SUVmax=8.7). (c) Another osteoblastic lesion in the T10 vertebra left side (arrowheads) was both positive on 18F-FDG (SUVmax=5.2) and positive on 68Ga-DOTA-IBA (SUVmax=35.0). (d) Osteoblastic lesions in bilateral ilium (dotted arrows and arrows) were negative on 18F-FDG (left: SUVmax=1.4, right: SUVmax=1.6) and positive on 68Ga-DOTA-IBA (left: SUVmax=7.6, right: SUVmax=8.7). Follow-up imaging 3 months later confirmed the presence of these bone metastases. 68Ga-DOTA-IBA, gallium 68 (68Ga)-labeled DOTA-conjugate ibandronic acid; 18F-FDG, fluorine 18 (18F)-labeled fluorodeoxyglucose.
Osteoblastic lesions
-
635 lesions were identified.
-
Detection rates 18F-FDG vs. 68Ga-DOTA-IBA): 51.5% (327/635) vs. 83.3% (529/635) (P < 0.001).
-
SUVmax 18F-FDG vs. 68Ga-DOTA-IBA): 3.96 (95% CI 3.57–4.35) vs. 7.88 (95% CI 7.09–8.66) (P < 0.001).
Osteolytic lesions
-
226 lesions were identified.
-
Detection rates 18F-FDG vs. 68Ga-DOTA-IBA): 79.6% (180/226) vs. 81.9% (185/226) (P = 0.533).
-
SUVmax 18F-FDG vs. 68Ga-DOTA-IBA): 6.28 (95% CI 5.71–6.85) vs. 8.40 (95% CI 7.48–9.32) (P < 0.001).
Additionally, the radiotracer uptake of 18F-FDG by osteoblastic lesions 3.96 (95% CI 3.57–4.35) was lower than that of 18F-FDG uptake by osteolytic lesions 6.28 (95% CI 5.71–6.85) (P < 0.001, Mann–Whitney U test).
Detection of bone metastases in lung and prostate cancer
Among the involved 46 participants, 22 participants were diagnosed with lung cancer and 12 participants with prostate cancer. Comparisons of diagnostic performance detecting lesions of lung or prostate cancer were summarized in Table 5. The comparison of radiotracer uptake using SUVmax of 50 lesions from lung cancer participants and 60 lesions from prostate cancer participants was shown in Fig. 4. Images of two representative participants are shown in Figs. 5 and 6.
Comparison of radiotracer uptake of bone metastases from different diseases.*Paired samples t test (Both P < 0.001), **Mann–Whitney U test (P = 0.024). The number of bone lesions from lung cancer and prostate cancer to compare SUVmax was 50 and 60, respectively. SUVmax was expressed with mean and 95% CI. 68Ga-DOTA-IBA, gallium 68 (68Ga)-labeled DOTA-conjugate ibandronic acid; 18F-FDG, fluorine 18 (18F)-labeled fluorodeoxyglucose; SUVmax, maximum standardized uptake value.
Images of a 46-year-old woman diagnosed with lung carcinoma who underwent 18F-FDG examinations for initial staging. (a) The maximum intensity projection (MIP) image of 18F-FDG showed moderate-low radiotracer uptake in the right pelvis (arrows, SUVmax=3.9) and left head of the femur (arrowheads, SUVmax=2.4). (b) The maximum intensity projection (MIP) image of 68Ga-DOTA-IBA demonstrated obvious radiotracer uptake in the right pelvis (arrows, SUVmax=13.6) and left head of the femur (arrowheads, SUVmax=9.2). (c,d) Axial fusion images of a mixed type of lesion showed that it is negative on 18F-FDG and positive on 68Ga-DOTA-IBA (curved arrow, SUVmax=2.5 vs. 8.0). CT showed osteogenic bone changes at the corresponding sites. Follow-up imaging 3 months later confirmed the presence of these bone metastases. 68Ga-DOTA-IBA, gallium 68 (68Ga)-labeled DOTA-conjugate ibandronic acid; 18F-FDG, fluorine 18 (18F)-labeled fluorodeoxyglucose.
Images of a 52-year-old woman diagnosed with lung carcinoma treated by surgery who underwent 18F-FDG examinations for restaging. (a) The maximum intensity projection (MIP) image of 18F-FDG showed obvious radiotracer uptake in multiple vertebras (arrowheads, mean SUVmax=5.7) and right ribs (arrows, SUVmax=6.2). (b) The maximum intensity projection (MIP) image of 68Ga-DOTA-IBA also demonstrated obvious radiotracer uptake in multiple vertebras (arrowheads, mean SUVmax=19.4) and right ribs (arrows, SUVmax=12.0). (c,d) Axial fusion images of an osteoblastic lesion showed that it is negative on 18F-FDG and positive on 68Ga-DOTA-IBA (curved arrow, SUVmax=2.9 vs. 14.7). CT showed osteogenic bone changes at the corresponding sites. Follow-up imaging 3 months later confirmed the presence of these bone metastases. 68Ga-DOTA-IBA, gallium 68 (68Ga)-labeled DOTA-conjugate ibandronic acid; 18F-FDG, fluorine 18 (18F)-labeled fluorodeoxyglucose.
Lung cancer
-
303 metastatic lesions were identified.
-
Detection rates 18F-FDG vs. 68Ga-DOTA-IBA): 78.2% (237/303) vs. 87.1% (264/303) (P < 0.001).
-
SUVmax 18F-FDG vs. 68Ga-DOTA-IBA): 6.01 (95% CI: 4.84–7.19) vs. 9.10 (95% CI: 7.57–10.63) (P < 0.001).
Prostate cancer
-
593 metastatic lesions were identified.
-
Detection rates 18F-FDG vs. 68Ga-DOTA-IBA) 55.0% (326/593) vs. 84.7% (502/593) (P < 0.001).
-
SUVmax 18F-FDG vs. 68Ga-DOTA-IBA): 4.29 (95% CI: 3.51–5.07) vs. 10.44 (95% CI: 8.57–12.30) (P < 0.001).
Additionally, the radiotracer uptake of 18F-FDG by lesions from lung cancer 6.01 (95% CI 4.84–7.19) was higher than 18F-FDG uptake by lesions from prostate cancer 4.29 (95% CI 3.51–5.07) (P = 0.024, Mann–Whitney U test).
Discussion
Early and precise diagnosis of bone metastases is crucial for patient management. Although various bone-seeking imaging agents are available, 18F-FDG is commonly used for initial staging and restaging. Our study supports the rationale for using 68Ga-DOTA-IBA to improve the management of patients with bone metastases, considering the therapeutic efficacy of 177Lu-DOTA-IBA. (7) In addition, it has been shown that 18F-NaF is a response predictor in nasopharyngeal, breast, and prostate cancer, including radiotherapy, endocrine therapy or 223RaCl9,10,11,12,13. 68Ga-DOTA-IBA may also have prognostic value for precision therapy.
Recent studies have shown that 68Ga-DOTA-IBA and 18F-NaF have comparable diagnostic efficacy and are superior to bone scan, and they are commonly used imaging agents for targeting bone metastases14,15. 68Ga-labeled fibroblast activation protein (FAP) inhibitor (FAPI) is an emerging imaging agent that may be superior to 18F-FDG in the diagnosis of bone metastases in different tumors and may have prognostic significance irrespective of the primary lesion16,17,18,19. However, a recent meta-analysis found that there may be no significant difference between 18F-FDG and 68Ga-FAPI in diagnosing bone metastases20. This may be because some benign lesions have 68Ga-FAPI uptake21. On the other hand, there are fewer studies of 68Ga-FAPI in diagnosing bone metastases. Prostate-specific membrane antigen (PSMA) is an imaging agent targeting prostate cancer and has a high accuracy for prostate cancer with bone metastases22. A recent meta-analysis found that 68Ga-PSMA PET/CT was superior to bone scan for diagnosing bone metastases23. There is a need to investigate 68Ga-DOTA-IBA versus these emerging imaging agents in the future as necessary.
A previous study reported that bone metastasis was most frequently detected by 18F-FDG PET in vertebras (74%), followed by pelvic bones (70%), and ribs (65%)24. Our findings align with this, as 37 (80.4%) participants had lesions in the vertebrae, 31 (67%) in the ribs, and 30 (65%) in the pelvis. The lower detection rate of 18F-FDG in the skull is likely due to physiological uptake in the brain25. Our study found a 38% sensitivity in detecting skull metastases, similar to a previous report of 42.9%26.
The sensitivity of 18F-FDG PET to detect bone metastases from lung cancer is 78.2% in our study which is lower than the pooled sensitivity previously reported in two meta-analyses27,28. This discrepancy may be due to the small sample size and the heterogeneity of participants in our study.
Although previous studies have demonstrated the potential of 18F-FDG for detecting bone metastases across different cancer types29,30. It shows limited diagnostic efficacy in prostate cancer due to the low metabolism of prostate cancer cells. In our study, there are 12 participants diagnosed with prostate cancer, and 2 of them were treated by androgen deprivation therapy. Our results are consistent with previous studies. Shreve’s study demonstrated a sensitivity of 65% in 22 untreated prostate cancer participants31. Pietrzak reported a low SUVmax of bone metastases from 27 participants32. Moreover, diagnostic efficacy could be reduced to 18% for those treated with androgen deprivation therapy33.
Another reason why 18F-FDG is poor in detecting bone metastases for prostate cancer may attributed to the osteoblastic type. In our study, 18F-FDG showed lower sensitivity for osteoblastic lesions (51.5%) compared to osteolytic lesions (79.6%), with significantly lower radiotracer uptake (3.96 vs. 6.28). Cook et al. reported lower metabolic activity in osteoblastic metastases (0.95) compared to osteolytic metastases (6.77) in 23 breast cancer participants34. Nakai’s study reported a visualization rate of 55.6% vs. 100%, analyzing 18 blastic and 10 lytic sites35. Previous studies also reported similar results in other cancers36,37,38.
Bisphosphonates are the standard treatment for tumor patients after diagnosis of bone metastases. The effect of bisphosphonates on their radiolabeled analogs remains unclear. Previous studies have suggested that bisphosphonate therapy does not affect the diagnostic efficacy of bone scan39,40,41. Thus, bisphosphonates may also not affect the diagnostic efficacy of 68Ga-DOTA-IBA, but further studies are needed.
Although our study indicates that 68Ga-DOTA-IBA PET may outperform 18F-FDG in detecting bone metastases, 18F-FDG remains valuable for providing information about primary lesions. Additionally, we excluded participants who had received bisphosphonates before the examination, as it remains uncertain whether these treatments affect diagnostic efficacy. Other limitations of our study include the small sample size and the absence of breast cancer participants, despite breast cancer being a common cause of bone metastases. Moreover, limb lesion analysis was incomplete because 18F-FDG PET scans were limited to the head-to-mid-thigh region, and hand and forearm imaging was missing due to participant positioning. Lastly, we only analyzed lesions with visible morphological changes. We did not record false-positive lesions or calculate specificity, as it would be unethical to perform lesion-by-lesion biopsies or delay treatment for confirmation.
Conclusions
68Ga-DOTA-IBA PET may outperform 18F-FDG in bone metastase detection, especially for those with osteoblastic bone destruction or those from prostate cancer.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 68Ga-DOTA-IBA:
-
Gallium 68 (68Ga)-labeled DOTA-conjugate ibandronic acid
- 18F-FDG:
-
Fluorine 18 (18F)-labeled fluorodeoxyglucose
- SUVmax :
-
Maximum standardized uptake value
- CI:
-
Confidence interval
References
Coleman, R. E. Skeletal complications of malignancy. Cancer 80 (8 Suppl), 1588–1594 (1997).
Fornetti, J., Welm, A. L. & Stewart, S. A. Understanding the bone in cancer metastasis. J. Bone Min. Res. 33 (12), 2099–2113 (2018).
Sathiakumar, N. et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: A population-based analysis of US medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 14 (2), 177–183 (2011).
Nakai, T. et al. Pitfalls of FDG-PET for the diagnosis of osteoblastic bone metastases in participants with breast cancer. Eur. J. Nucl. Med. Mol. Imaging. 32 (11), 1253–1258 (2005).
Dooley, M. & Balfour, J. A. Ibandronate. Drugs 57 (1), 101–110. (1999).
Wang, Y. et al. Preparation, biological characterization and preliminary human imaging studies of 68Ga-DOTA-IBA. Front. Oncol. 12, 1027792 (2022).
Qiu, L. et al. Safety and efficacy of 68 Ga- or 177 Lu-Labeled DOTA-IBA as a novel theranostic radiopharmaceutical for bone metastases: A phase 0/I study. Clin. Nucl. Med. 48 (6), 489–496 (2023).
Yang, J. et al. Biodistribution and internal dosimetry of 68 Ga-DOTA-IBA PET imaging for participants with bone metastases. Clin. Nucl. Med. 48 (10), 847–852 (2023).
Peterson, L. M. et al. Prospective study of serial 18F-FDG PET and 18F-Fluoride PET to predict time to Skeletal-Related events, time to progression, and survival in patients with bone-dominant metastatic breast cancer. J. Nucl. Med. 59 (12), 1823–1830 (2018).
Mu, X. et al. 18F-NaF uptake in skull-base bone as a predictor of treatment response in advanced nasopharyngeal carcinoma. Sci. Rep. 14 (1), 29501 (2024).
Azad, G. K. et al. Prediction of therapy response in bone-predominant metastatic breast cancer: Comparison of [18F] Fluorodeoxyglucose and [18F]-fluoride PET/CT with whole-body MRI with diffusion-weighted imaging. Eur. J. Nucl. Med. Mol. Imaging. 46 (4), 821–830 (2019).
Letellier, A. et al. Uptake of Radium-223 dichloride and early [18F]NaF PET response are driven by baseline [18F]NaF parameters: A pilot study in castration-resistant prostate cancer patients. Mol. Imaging Biol. 20 (3), 482–491 (2018).
Jadvar, H. & Colletti, P. M. 18F-NaF/223RaCl2 theranostics in metastatic prostate cancer: Treatment response assessment and prediction of outcome. Br. J. Radiol. 91 (1091), 20170948 (2018).
Deng, J., Yang, J., Wang, Y., Liu, G. & Chen, Y. Comparison of the relative diagnostic performance of 68Ga-DOTA-IBA and 18F-NaF for the detection of bone metastasis. Front. Oncol. 14, 1364311 (2024).
Xiang, F. et al. Prospective comparison of 68Ga-DOTA-ibandronate and bone scans for detecting bone metastases in breast cancer. Front. Oncol. 14, 1428498 (2024).
Wu, J. et al. Comparison of the relative diagnostic performance of [68Ga]Ga-DOTA-FAPI-04 and [18F]FDG PET/CT for the detection of bone metastasis in patients with different cancers. Front. Oncol. 11, 737827 (2021).
Arak, H., Elboga, U., Cayirli, Y. B. & Aytekin, A. Prognostic significance of 68 Ga-FAPI PET/CT in patients with bone metastases in various cancers. Ann. Nucl. Med. 38 (8), 630–638 (2024).
Tian, X. et al. Evaluating the diagnostic value of 18F-FAPI-04 PET/CT in various malignant tumors: A head-to-head comparison with 18F-FDG PET/CT. Jpn J. Radiol. https://doi.org/10.1007/s11604-024-01714-0(2024).
Sidrak, M. M. A. et al. Fibroblast activation protein inhibitor (FAPI)-based theranostics-where we are at and where we are heading: A systematic review. Int. J. Mol. Sci. 24 (4), 3863 (2023).
Wu, G. et al. Head-to-head comparison of [68Ga]Ga-FAPI PET and [18F]FDG PET in the detection of bone and lymph node metastasis in various cancers: A systematic review and meta-analysis. Eur. J. Radiol. 171, 111302 (2024).
Qin, C. et al. Increased uptake of 68Ga-DOTA-FAPI-04 in bones and joints: Metastases and beyond. Eur. J. Nucl. Med. Mol. Imaging. 49 (2), 709–720 (2022).
Sachpekidis, C. et al. 68Ga-PSMA PET/CT in the evaluation of bone metastases in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 45 (6), 904–912 (2018).
Daniels, H., Gilbert, R. & Bonin, L. The diagnostic accuracy of 68Ga-PSMA PET/CT versus 99mTc-MDP bone scintigraphy for identifying bone metastases in persons with prostate cancer: A systematic review. J. Med. Imaging Radiat. Sci. 54 (3), 545–555 (2023).
Nakamoto, Y., Osman, M. & Wahl, R. L. Prevalence and patterns of bone metastases detected with positron emission tomography using F-18 FDG. Clin. Nucl. Med. 28 (4), 302–307 (2003).
Rodrigues, M. et al. Diagnostic performance of [18F] FDG PET-CT compared to bone scintigraphy for the detection of bone metastases in lung cancer patients. Q. J. Nucl. Med. Mol. Imaging. 60 (1), 62–68 (2016).
Zhang, Y. et al. Comparison of 18F-NaF PET/CT and 18F-FDG PET/CT for detection of Skull-Base invasion and osseous metastases in nasopharyngeal carcinoma. Contrast Media Mol. Imaging. 2018, 8271313 (2018).
Liu, T. et al. Fluorine-18 Deoxyglucose positron emission tomography, magnetic resonance imaging and bone scintigraphy for the diagnosis of bone metastases in patients with lung cancer: Which one is the best?--a meta-analysis. Clin. Oncol. (R Coll. Radiol). 23 (5), 350–358 (2011).
Qu, X., Huang, X., Yan, W., Wu, L. & Dai, K. A meta-analysis of 18FDG-PET-CT, 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with lung cancer. Eur. J. Radiol. 81 (5), 1007–1015 (2012).
Rong, J. et al. Comparison of 18 FDG PET-CT and bone scintigraphy for detection of bone metastases in breast cancer patients. A meta-analysis. Surg. Oncol. 22 (2), 86–91 (2013).
Kato, H. et al. Comparison between whole-body positron emission tomography and bone scintigraphy in evaluating bony metastases of esophageal carcinomas. Anticancer Res. 25 (6 C), 4439–4444 (2005).
Shreve, P. D., Grossman, H. B., Gross, M. D. & Wahl, R. L. Metastatic prostate cancer: Initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology 199 (3), 751–756 (1996).
Pietrzak, A. K., Czepczynski, R., Wierzchoslawska, E. & Cholewinski, W. Detection of the prostate Cancer bone metastases: Is it feasible to compare 18F-fluorocholine PET/CT, 18F-fluorodeoxyglucose PET/CT and 99mTc-methyl diphosphonate bone scintigraphy?? Urol. J. 15 (5), 242–247 (2018).
Yeh, S. D. J. et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl. Med. Biol. 23 (6), 693–697 (1996).
Cook, G. J., Houston, S., Rubens, R., Maisey, M. N. & Fogelman, I. Detection of bone metastases in breast cancer by 18FDG PET: Differing metabolic activity in osteoblastic and osteolytic lesions. J. Clin. Oncol. 16 (10), 3375–3379 (1998).
Nakai, T. et al. Pitfalls of FDG-PET for the diagnosis of osteoblastic bone metastases in patients with breast cancer. Eur. J. Nucl. Med. Mol. Imaging. 32 (11), 1253–1258 (2005).
Liu, N. et al. Bone metastasis in patients with non-small cell lung cancer: The diagnostic role of F-18 FDG PET/CT. Eur. J. Radiol. 74, 231 (2010).
Nakamoto, Y., Cohade, C., Tatsumi, M., Hammoud, D. & Wahl, R. L. CT appearance of bone metastases detected with FDG PET as part of the same PET/CT examination. Radiology 237 (2), 627–634 (2005).
Özülker, T., Küçüköz Uzun, A., Özülker, F. & Özpaçac, T. Comparison of 18F-FDG-PET/CT with 99mTc-MDP bone scintigraphy for the detection of bone metastases in cancer patients. Nucl. Med. Commun. 31 (6), 597–603 (2010).
Roudier, M. P. et al. Bone histology at autopsy and matched bone scintigraphy findings in patients with hormone refractory prostate cancer: The effect of bisphosphonate therapy on bone scintigraphy results. Clin. Exp. Metastasis. 20 (2), 171–180 (2003).
Pecherstorfer, M. et al. Effect of clodronate treatment on bone scintigraphy in metastatic breast cancer. J. Nucl. Med. 34 (7), 1039–1044 (1993).
Morris, P. G. et al. Intravenous bisphosphonate therapy does not acutely alter nuclear bone scan results. Clin. Breast. Cancer. 10 (1), 33–39 (2010).
Funding
This work was supported by the Luzhou science and technology plan projects (2022-JYJ-118, 2021LZXNYD-C02, and 2021LZXNYD-P03) and major science and technology project in Gansu Province (23ZDFA014).
Author information
Authors and Affiliations
Contributions
All authors contributed to the original research. Linwei Li completed the first draft of the manuscript. Lingzhi Chen contributed to data collection and statistical analysis. Jian Yang commented on previous versions of the manuscript. Tingting Xu and Dengsai Peng contributed to imaging review. Yue Chen gave some meaningful suggestions and theoretical support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the ethics committee of the affiliated hospital of southwest medical university and registered on Clinical Trial Registry (Clinical ethics registration number: ChiCTR2200064487). All procedures performed in studies in involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all subjects and/or their legal guardian(s).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, L., Chen, L., Yang, J. et al. Comparison of 18F-FDG and 68Ga-DOTA-IBA in detecting bone metastases: a lesion-basis study. Sci Rep 15, 12766 (2025). https://doi.org/10.1038/s41598-025-97920-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97920-5
Keywords
This article is cited by
-
The radiological response of patients with advanced bone metastases to lutetium-177-labeled DOTA-ibandronic acid assessed by metabolic tumor volume
Journal of Cancer Research and Clinical Oncology (2025)