Abstract
Detecting glioblastoma infiltration in the brain is challenging due to limited MRI contrast beyond the enhancing tumour core. This study aims to investigate the potential of superparamagnetic iron oxide nanoparticles (SPIONs) as contrast agents for improved detection of diffuse brain cancer. We examine the distribution and pharmacokinetics of SPIONs in glioblastoma models with intact and disrupted blood-brain barriers. Using MRI, we imaged RN1-luc and U87MG mice injected with Gadovist and SPIONs, observing differences in blood-brain barrier permeability. Peripheral imaging showed strong uptake of nanoparticles in the liver and spleen, while vascular and renal signals were transient. Susceptibility gradient mapping enabled positive nanoparticle contrast within tumours and provided additional information on tumour angiogenesis. This approach offers a novel method for detecting diffuse brain cancer. Our findings demonstrate that SPIONs enhance glioblastoma detection beyond conventional MRI, providing insights into tumour angiogenesis and opening new avenues for early diagnosis and targeted treatment strategies.
Similar content being viewed by others
Introduction
Glioblastoma is the most common type of malignant brain cancer and its prognosis is poor1. A significant challenge to the treatment of glioblastoma is the heterogenous and invasive nature of the tumour, which leads to inconsistent disruption of the blood brain barrier (BBB). While the BBB is typically disrupted within the tumour core, in regions of tumour infiltration, the BBB remains intact despite the presence of clinically significant tumour burden2. As most diagnostic contrast agents and cancer therapeutics do not cross an intact BBB, regions of infiltrating tumour are extremely difficult to both identify and treat3. In particular, while magnetic resonance imaging (MRI) of gadolinium-based contrast agents (GBCAs) is part of the standard of care for glioblastoma diagnosis, GBCAs such as Gadovist only highlight tumour in regions of significant BBB disruption4,5.
Conventional imaging of glioblastoma consists of T1-weighted (T1w) and T2-weighted (T2w) scans in addition to a T1-contrast enhanced (T1-CE) scan acquired after intravenous administration of a GBCA (Fig. 1A). While T1-CE scans boost signal in the core tumour mass, regions of hyperintensity in T2w scans beyond this core mass are a mix of infiltrative tumour lying behind an intact BBB and oedema of normal tissue6. Distinguishing between tumour and normal tissue is key to surgical and radiotherapy treatment planning. Hence, essential to the improved diagnosis and treatment of glioblastoma is the development of diagnostic tools that can provide information on tumour growth despite the presence of an intact BBB7.
(A) Clinical example of mismatch between glioblastoma T2 hyperintensity and areas of T1 gadolinium contrast enhancement. (B) To replicate the heterogeneously disrupted blood brain barrier (BBB) in clinical glioblastoma (GBM), we used a diffuse, infiltrative patient-derived xenograft, RN1, to compare PEG-SPION to Gadovist in glioblastoma, as well as in non-diffuse glioblastoma U87. We also tested the effect of PEGylation on the SPION biodistribution and generated positive SPION contrast using susceptibility gradient mapping. Human data was adapted from Claes, et al.5, with open access permission from Springer (all rights reserved).
MRI contrast agents based on nanoparticles are of increasing interest for their ability to probe additional factors within the tumour environment and could help to differentiate infiltrative tumour and healthy tissues in MRI scans. In particular, superparamagnetic iron oxide nanoparticles (SPIONs), which are nanoparticles with a 3–25 nm core of iron oxide (typically magnetite Fe3O4 or maghemite Fe2O3), are of interest for their significant MRI contrast potential and biocompatibility8,9,10. SPIONs enhance MRI contrast by shortening transverse relaxation times of nearby protons (T2 contrast) and by creating strong nanoscale magnetic inhomogeneities (T2* contrast)11,12,13. As a highly sensitive contrast agent, with long blood circulation times, there is significant potential for SPIONs to be a complementary contrast method that distinguishes regions of infiltrative tumour using MRI.
Additionally, there are growing concerns over the accumulation of GBCAs in central-nervous-system tissue, even in patients with normal renal function, which may constrain the use of GBCAs into the future14,15,16,17,18,19,20,21,22. Due to their larger size, SPIONs bypass renal deposition, and are metabolised into bioavailable haemoglobin23 rather than being deposited enduringly in tissue. Despite the improved safety profile of SPIONs, T2 relaxivity mechanisms lead to negative MRI contrast (reduced signal). Negative contrast is typically disliked by radiologists24 and there have been limited efforts to substitute SPIONs for GBCAs, which give positive contrast in T1w MRI.
Here, we preclinically evaluate the use of methoxypolyethylene-glycol (PEG) coated SPIONs (PEG-SPIONs) for identification of diffuse glioblastoma with MRI. First, we recapitulate glioblastoma in a mouse model (Fig. 1B) using cell lines known to grow aggressive tumours with a highly disrupted BBB (U87MG, GBM6) and a cell line that grows infiltrative tumours with an intact BBB (RN1)25,26. Second, we compare conventional MRI approaches to imaging glioblastoma to MRI scans acquired with PEG-SPIONs, showing that we can image angiogenesis within infiltrative tumour that is not detected with GBCAs. Third, we implement a postprocessing method called susceptibility gradient mapping (SGM)27 that generates positive contrast rather than negative MRI contrast from T2*w scans with PEG-SPIONs, overcoming one of the main barriers to the clinical use of SPIONs. We conclude by evaluating the biodistribution of PEG-SPIONs, showing that nanoparticle clearance predominantly occurs via the reticuloendothelial system. Our results show the potential of PEG-SPIONs to enhance the imaging of glioblastoma with MRI and will inform the development of new approaches to imaging and treating this disease in the clinic.
Methods
Materials
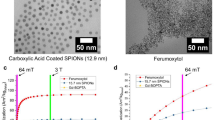
The nanoparticles evaluated in this study are novel magnetite(Fe3O4)-based, methoxy-PEG-coated SPIONs28. PEG-SPIONs (mPEG PrecisionMRX) were sourced commercially and received in aqueous solutions with elemental iron concentrations ranging from 89 to 374 mM (~ 5 to 20.9 mg/mL). PEG-SPIONs have a hydrodynamic diameter of 58.3 nm (measured by dynamic light scattering), core diameter of 24.2 nm ± 1.3 nm (measured by Rigaku SmartLab small angle X-ray scattering and JEOL 1200EX transmission electron microscopy) and polydispersity index of 0.06. Originally designed for magnetic relaxometry29, which operates at mT field strengths, these mPEG-SPIONs were shown to generate in vivo contrast in ultra-low field MRI (6.5 mT)30,31.
In addition to brain tumour imaging with PEG-SPIONs, we also evaluated the biodistribution of PEG-SPIONs and compare this to Ferumoxytol. Ferumoxytol is a SPION composed of a magnetite core of 3.25 nm diameter, which is coated with polyglucose sorbitol carboxymethylether, yielding nanoparticles with a hydrodynamic diameter of 26.3 nm. Both samples were diluted in either deoxygenated MilliQ water for relaxometric studies30 or in sterile saline for animal studies. All cell culturing reagents were supplied by Thermo Fisher Scientific unless otherwise specified.
Cell culture
All cell cultures were incubated at 37 °C in 5% CO2. RN1-luciferase (RN1-luc)25 and GBM632 cells were grown for a maximum of five passages in KnockOut™ DMEM/F-12 basal media (Gibco) supplemented with epidermal growth factor (20 ng/mL), basic fibroblast growth factor (20 ng/mL), 2% StemPro™ neural supplement, GlutaMAX™ supplement (2 mM), 100 U/mL penicillin and 100 µg/mL streptomycin on T75 flasks coated with 0.15% Matrigel (Sigma-Aldrich) in phosphate buffered saline (PBS). RN1 is a patient-derived xenograft isolated from a 56 year old male with a primary isocitrate dehydrogenase 1 (IDH1) wild-type, O-6-methylguanine-DNA methyltransferase (MGMT) unmethylated promoter glioblastoma located in the left temporal lobe28. RN1-luc cells were selected for using 1 µg/mL blasticidin S hydrochloride (Cayman Chemical). Accutase was used for cell passaging. U87MG cells were grown in DMEM supplemented with 10% foetal calf serum, 100 U/mL penicillin and 100 µg/mL streptomycin.
Animals and glioblastoma engraftment surgery
Nineteen female mice were included in this study, and were five weeks of age at the time of surgery. Mice were sourced from Ozgene Pty Ltd. Nonobese diabetic PrkdcSCID (NOD-SCID) mice were used for the brain cancer imaging component of the study. Due to a supply shortage of NOD-SCID mice, GBM6 tumours were grown in NODRag1null IL2rγnull (NRG) mice, which is a similar strain. Nanoparticle biodistribution was evaluated in an immunocompetent mouse strain (C57BL/6).
All experimental protocols and procedures were approved by the University of Sydney Animal Ethics Committee, under protocols 1316 (nanoparticle biodistribution) and 2170 (brain cancer). All methods are reported in accordance with the ARRIVE guidelines. All methods were carried out in accordance with local guidelines and regulations. Mice were monitored for weight changes and symptoms following the mouse grimace scale33 at least twice per week throughout the study period. Mice were euthanized via transcardial perfusion under deep isoflurane anaesthesia at the conclusion of final imaging timepoints for each study.
For tumour engraftment, passaged cell pellets were resuspended in 10 ng/mL laminin from mouse Engelbreth-Holm-Swarm sarcoma (Merck) in PBS to give 100-150k cells per µL and they were kept on ice during the surgeries. Surgical tools and cotton bud tips were autoclaved prior to use. Each animal was assessed as per the mouse grimace scale for pain evaluation and monitored and weighed prior to surgery and intensively observed for the following three days. Mice were anaesthetised with 3% isoflurane and maintained under 2% isoflurane in a stereotaxic frame (Kopf Instruments) with ear bars, and breaths per minute were monitored by an assistant. Once deep anaesthesia was confirmed with a toe pinch, the scalp was shaved and washed with pHisohex (Viatris), and an approximate 10–15 mm long incision was made along the skin above the sagittal suture. A hole was drilled gently 0.1 mm posterior to bregma and 2 mm laterally, and 200k (U87MG), 250k (GBM6) or 300k (RN1-luc) cells were loaded into a 2 µL 25 G bevelled Hamilton syringe. The needle was inserted 3 mm into the brain slowly over a 2 min period, then retracted 0.5 mm and left to sit for 2 min. The cells were injected slowly at ~ 0.7 µL/min. At the conclusion of the injection, the needle was left in place for 2 min allowing the cells to settle, and retracted slowly. The burr hole was sealed with a small amount of bone wax (Ethicon) and the skin was held closed with forceps and glued using 3 M Vetbond. An analgesic subcutaneous injection of 0.1 mg/kg buprenorphine (Buprelieve®, Jurox) and an anti-inflammatory second subcutaneous injection of 5 mg/kg meloxicam (Apex) were given before turning off the isoflurane and placing the mouse in a recovery cage with body temperature warming and post-operative monitoring.
In vivo bioluminescence imaging
RN1-luc PDX progression was monitored fortnightly to weekly using either 7 T MRI with T2 weighted fast spin echo sequences (described below) or by bioluminescence using a Perkin Elmer In Vivo Imaging System (IVIS). Mice were anaesthetised using isoflurane, had scalps shaved and were injected intraperitoneally with 150 mg/kg VivoGlo™ D-luciferin (Promega). Each mouse was imaged 5 min postinjection using standard bioluminescence filters as per the Living Image software.
Magnetic resonance imaging
All MR imaging experiments were performed on MR Solutions preclinical MRI scanners. While the collection of data on a 7 T preclinical system was our preferred plan, due to unavoidable system outages, some data were collected on a 3 T preclinical system. For biodistribution experiments, three mice were imaged at 3 T and three at 7 T. For glioblastoma brain imaging, four RN1-luc, two U87MG and three GBM6 mice were imaged at 7 T, and one U87MG mouse was imaged at 3 T. Three mice which did not develop brain tumours were brain imaged at 7 T post-injection of 2, 5 or 10 mg/kg PEG-SPION to determine a desirable PEG-SPION dose for imaging.
Brain cancer imaging
Mice were transferred to our preclinical imaging facility after allowing 3 days for recovery from tumour inoculation. They were subsequently allowed 1 week acclimatization in the new facility before imaging could take place. Mice brain xenografts were monitored by MRI from two weeks post-engraftment for U87MG and GBM6, and from approximately 35 days post-engraftment for RN1-luc, onwards. MRI acquisitions to monitor tumour growth were performed which included T2-weighted fast spin echo sequences (repetition time (TR) = 4000 ms, echo time (TE) = 45 ms, flip angle (FA) = 90°, field of view (FOV) = 25 × 25 mm, number of averages (NA) = 3, and slice thickness (ST) = 0.5 mm) in the coronal plane as well as the sagittal and horizontal planes when necessary, using a mouse head coil in a 7 T MR Solutions scanner. Once tumours appeared sufficiently large (> 2–3 mm diameter) on the T2-weighted scans, or IVIS, mice proceeded to the Gadovist and PEG-SPION injection imaging stage of the study. Mice were intravenously injected with 0.1 mmol/kg Gadovist, followed by 50 µL heparin sodium (50 injection units/mL, Hospira) in saline via cannula.
Mice were subsequently injected with 10 mg/kg PEG-SPIONs after all Gadovist had cleared from the brain (at least 20 min afterwards). GBCAs are known to rapidly clear from mouse circulation with a half-life of 5–6 min34. Anatomical scans including the above mentioned T2-weighted scans and also T1-weighted fast spin echo scans (TR = 1000 ms, TR = 11 ms, FA = 90°, FOV = 25 × 25 mm, NA = 4, SL = 0.5 mm) and variable flip angle T1 mapping sequences (data not shown) were acquired pre- and post-Gadovist injection. For SPIONs imaging, T2*-weighted fast low angle shot (FLASH) gradient recalled echo (GRE) sequences (TR = 400 ms, TE = 9 ms, FA = 40°, FOV = 25 × 25 mm, NA = 4, SL = 0.5 mm) and T2*-weighted multi-gradient echo sequences (TR = 500 ms, TE = 4, 8.48, 12.96, 17.44, 21.92 and 26.4 ms, FA = 50°, FOV = 25 × 25 mm, NA = 4, ST = 0.5 mm) were acquired pre- and post- injection. T2*-weighted images were acquired immediately after PEG-SPION injection and were repeated up to 1 h post PEG-SPION injection. Repeat imaging sessions were conducted at later timepoints of 2, 3, 5 and 24 h.
We note that there was no need for randomisation or blinding in our study design as all mice received Gadovist and SPION injections. All animals were included in the study and subsequent data analysis unless a brain tumour failed to develop after inoculation with a glioblastoma cell line. Due to the aggressive nature of the tumour model, the study was designed to minimise the number of mice used. A minimum sample size of n = 3 was studied for each tumour type, yielding an effect size sufficient for distinguishing between tumour types and contrast effects.
Biodistribution imaging
C57BL/6 mice were used for the pharmacokinetics component of the study to evaluate biodistribution in an immunocompetent setting, using 3 T MRI. Mice were sedated with isoflurane and injected slowly over 1 min into the lateral tail vein via a cannula with 0.2 ml of saline containing 0.2–1.25 mg Fe/mL PEG-SPION or ferumoxytol, resulting in 2, 5 or 10 mg Fe/kg dose injected intravenously. For mice imaged at 7 T and 3 T for PEG-SPION pharmacokinetics, 5 and 10 mg/kg was injected respectively, whereas for ferumoxytol at 3 T, 2 mg/kg was injected. Mice were anaesthetised using 3% isoflurane and maintained with 1–2% isoflurane for the duration of the imaging period. For 3 T pharmacokinetics nanoparticle injections, mice were imaged at pre-injection (N), and 1, 6, 24, 48, 72 and 96 h post-injection using fast spin echo T1-weighted (TR = 350 ms, TE = 11ms, ST = 1 mm, NA = 10, number of slices = 12), T2-weighted sequences (TR = 2600ms, TE = 72 ms, ST = 1 mm, NOA = 6, number of slices = 16), and GRE FLASH T2* weighted sequences (TR = 143 ms for PEG-SPION and TR = 240 ms for ferumoxytol, TE = 9 ms, NA = 10, ST = 1 mm, number of slices = 8), using a mouse body coil. In a latter separate study, NOD/SCID mice were injected with 5 mg/kg PEG-SPION and imaged on a 7T MR Scanner (MR Solutions) with similar sequences. The 7 T sequences included a T1-weighted fast spin echo (TR = 1000 ms, TE = 11 ms, NA = 3, ST = 1 mm, FOV = 45 × 60 mm) and a T2*-weighted GRE FLASH (TR = 400 ms, TE = 9 ms, FA = 40°, number of averages = 4, slice thickness = 1 mm, field of view = 60 × 45 mm).
Histology and microscopy
Mice were transcardially perfused with 25 mL saline followed by 25 mL 10% neutral buffered formalin using a 25 G butterfly needle (Terumo) while under deep 3% isoflurane anaesthesia. Fixed tissue was transferred to 80% ethanol after 24–48 h immersion in 10% neutral buffered formalin (4 °C) and then dehydrated and paraffin embedded. Ten-micron sections were acquired onto Superfrost Plus slides using an Epredia rotary microtome with Feather S35 microtome blades including the brain, liver, spleen, heart, kidneys, and lung. Sections were stained with Perls’ Prussian blue using 2% potassium ferrocyanide mixed 1:1 with 2% hydrochloric acid immediately before use for 15 min, followed by 5 min 0.1% acetic safranin solution (0.1% glacial acetic acid, 0.1% safranin O) counterstain and cover-slipped under dibutylphthalate polystyrene xylene (DPX) mountant. Brightfield images were acquired using a Zeiss AxioScan Z1 slide scanner equipped with a Plan-Apochromat 20x objective and Hitachi HV-F202SCL camera, and visualised with ZEN v3.1 software.
Image processing and analysis
SPION biodistribution analyses were performed by comparing the mean grey values (MGV) of regions of interest (ROIs) across MRI slices of key organs (brain, liver, spleen, kidneys, lung, stomach, leg and back muscle, and bladder) at timepoints from pre-injection to 1, 6, 24, 48, 72 and 96 h post-injection. All T1, T2 and T2* weighted images were analysed. DICOMs produced by the MR Solutions preclinical MRI scanner are auto-scaled to the maximum signal intensity. Hence, for quantitative analyses, scaling-invariant images were reconstructed from raw k-space data using an inverse fast Fourier transform operation in MATLAB. For data analysis, DICOM figures were exported from MATLAB and ROIs were drawn using MicroDICOM v2024.2 software.
Positive contrast post-processing using susceptibility gradient mapping
SGM is a positive contrast method that is not reliant on longitudinal relaxation like conventional T1 positive contrast agents, but rather makes use of the fact that T2* contrast agents create local susceptibility gradients that dephase precessing magnetic moments. SGM leverages the complex k-space data obtained from a conventional GRE sequence, calculates local magnetic field gradients associated with variations in magnetic susceptibility and visualizes them in a parametric map. The following Equation 27 defines the magnitude of the susceptibility gradient map in one dimension:
Here, \(\:{\tau\:}_{x}\) is the sampling rate, \(\:{G}_{x}^{imaging}\) is the imaging gradient and \(\:{G}_{x}^{sus}\) is the susceptibility gradient that causes an unbalanced timing of the gradient echo at \(\:TE\). SGMs27 were produced from the raw k-space T2* gradient recalled echo data by performing short-term 1D Fourier transforms on each voxel and its two adjacent voxels following the procedure first described in Dahnke, et al.27. A fit was performed to calculate the magnitude of the susceptibility gradient vector. This was performed in both the x and y directions, with the two results then being combined to produce an overall SGM. This process was applied to skull stripped images of the mouse brain so as to minimize the visual contribution of susceptibility artefacts at air-tissue interfaces, which are amplified by the SGM fitting procedure.
Statistical analysis
All data is presented as mean + standard error of the mean (SEM). Pre versus post-injection brain MGVs were compared using a two-way analysis of variance (ANOVA) with a Šídák’s multiple comparison test in GraphPad Prism 10.3 Software.
Results
RN1-luc xenografts have a Gadovist-impermeable blood brain barrier in contrast to U87MG xenografts
U87MG orthotopic xenografts appear highly permeable towards 0.1 mmol/kg Gadovist®, as shown by the positive contrast in T1-weighted turbo spin echo sequences post-Gadovist injection (Fig. 2A). However, RN1-luc stem-like patient-derived orthotopic xenografts displayed limited contrast after Gadovist injection (Fig. 2A), likely indicating a Gadovist impermeable BBB despite T2 hyperintensity. Significant growth of the RN1-luc tumour is also evident from displacement of the ventricles, which appear as hyperintense regions in T2w scans due to the long T2 relaxation time of cerebrospinal fluid. Tumour infiltration by RN1-luc in regions that did not show tumour infiltration was later confirmed by histology (Fig. 2B). The limited BBB permeability of Gadovist observed in RN1-luc infiltrative tumour tissue was also observed to an extent in another patient-derived xenograft, GBM6 (Figure S1).
(A) Representative examples of U87MG and RN1-luc xenograft anatomy in T1 and T2 weighted scans, including post-Gadovist (0.1 mmol/kg). (B) Brain histology of U87MG and RN1-luc patient-derived orthotopic xenografts, stained with Perls’ Prussian blue and counterstained with 0.1% safranin O. RN1-luc shows a distinctly diffuse phenotype whereby it has spread laterally across hemispheres, whereas U87MG shows a demarcated non-diffuse phenotype. Scale bars = 1 mm and 400 microns respectively. Images were acquired using a 20x objective. Tumour affected areas are outlined in dashed green in accordance with T2 weighted hyperintensity and histological staining.
Glioblastoma angiogenesis was contrast enhanced via SPION T2* vascular signal
Patient-derived orthotopic xenografts RN1-luc and U87MG were evaluated for PEG-SPION uptake using T2*, T2 and T1 weighted scans. In both tumours the nanoparticles showed strong T2* contrast effects, which were predominantly located in normal neurovasculature and angiogenic tumour areas (Fig. 3). There was a statistically significant change in tumour vascular contrast in U87MG and RN1-luc compared to control regions following injection of PEG-SPION (Fig. 3C). The PEG-SPION T2* contrast was largely washed out from the vasculature after 5 h from injection, with no detectable amount of signal in the RN1-luc tumour area at 24 h. However, both U87MG and RN1-luc tumour vascular PEG-SPION T2* contrast was visible at 2 h and 3 h post injection. The lack of Perls’ Prussian blue staining in the tumour area 1 h post-perfusion (Fig. 2B) is indicative that the PEG-SPION was localised to the tumour vasculature rather than tumour cells themselves.
T1- and T2*-weighted MR images of (A) U87MG and (B) RN1-luc patient-derived xenografts pre- and two minutes post-injection of either 0.1 mmol/kg Gadovist or 10 mg/kg PEGSPIONs. (C) Quantification of change in pre- to post- injection from representative regions of interest (ROIs) in T1 and T2* weighted images. ROIs were drawn around the brighter (T1w) or darker (T2*w, e.g. yellow arrow) appearing tumour regions and quantified relative to the same ROIs in the pre-injection images, and compared to control hemisphere non-tumour area, giving mean ± SEM post/pre injection values (n = 3). One U87MG mouse (blue data point) was imaged at 3 T rather than 7 T. * denotes p < 0.05, ** p < 0.01 and *** p < 0.001, as measured by two-way ANOVA with Šídák’s multiple comparisons test.
SPION positive contrast from quantitative susceptibility gradient mapping
We found that SGM can highlight PEG-SPIONs in the U87MG and RN1-luc tumours as positive contrast, with a trade-off being a lower effective resolution (Fig. 4). In the case of RN1-luc which has a functional BBB, the borders of this tumour were not clear from T2* SGM but instead abnormal vasculature and blood pooling in the tumour area appeared bright. Abnormal vasculature was defined as that in which appears in the tumour area but not the control hemisphere. The full extent of RN1-luc spread and infiltration was most visible in T2 weighted fast spin echo sequences rather than T1 or T2* weighted scans post-contrast. Across multiple timepoints, in U87MG, the T2* signal was converted to bright contrast surrounding the tumour area, while for RN1-luc vascular signal was converted to positive contrast, though the full extent of RN1-luc spread was still not entirely visible using this method (Fig. 4, Figure S2).
Bright contrast of PEG-SPIONs generated by susceptibility gradient mapping (SGM) post-processing of T2* weighted gradient recalled echo sequences calculated from Eq. (1), shown in (A) U87MG and (B) RN1-luc. In RN1-luc, abnormal blood vessels are visible in the right hemisphere post-SPION injection (yellow arrows). Tumour affected area is outlined in dotted green, corresponding to T2 weighted hyperintensity. U87MG, which is a more permeable tumour, shows a high concentration of PEG-SPION in a highly vascularised region.
Figure S4 indicates a large susceptibility gradient at the kidneys, stomach, liver, and spleen 1 h post PEG-SPION injection, indicating interfaces between iron-rich areas and the surrounding iron-poor tissue. The insides of these organs remain dark, indicating a homogeneous distribution of nanoparticles within the organ, which in turn results in a lack of large susceptibility gradient vectors within the organ and thus little-to-no bright contrast is seen. In organs with a very high concentration of SPIONs, there is no measurable signal in these regions due to the short T2*. Hence, signal voids are produced in the image and as a result no SGM can be calculated for these regions. This serves in contrast to hypointensities in the lung, which are dark both pre- and post- injection and are not as significantly highlighted by this post-processing method – due to the presence of air and a low signal-to-noise ratio which prevents accurate calculation of the susceptibility gradient.
Biodistribution results
Quantification of the signal intensity in T2*w MR images pre-injection and ≥ 1 h post PEG-SPION injection (Fig. 5B) revealed a decrease in the MGV post-injection in the liver and spleen. A similar biodistribution profile was observed for ferumoxytol (Figure S3). The strong inhomogeneity in magnetic susceptibility caused by the SPIONs in vasculature also appeared to enhance contrast within the kidneys, with the renal vasculature being sharply defined against the surrounding renal medulla, but only at early time points (generally < 1 h) post injection (Fig. 5B, Figure S4).
(A) Biodistribution of the PEG-SPIONs (representative image, n = 3), as shown by 7T MR T1-weighted spin echo images in NOD-SCID mice. From left to right, top to bottom, lung (Lu), stomach (St), liver (Li), spleen (Sp), kidney (Ki), muscles (Mu) and bladder (Bl) are encircled in yellow. Displayed window width and window level are equal for all images. (B) Quantification of unscaled reconstructed k-space of 3 T T2*-weighted gradient recalled echo acquisitions in C57BL/6 mice over longer time points. Graph represents mean + SEM grey values (arbitrary units) from oval-shaped regions of interest in T2* weighted FLASH 3 T images (n = 2). (C) Representative histology images of 10 micron sections stained with Perls’ Prussian blue and 0.1% safranin O. Iron oxide is shown by blue colour, notably in the liver and spleen, and to some extent in the kidney at 1 h. Scale bar = 100 microns.
There was little overall difference between quantification of images in terms of SPIONs T2 susceptibility effects in 3 T versus 7 T scanners, nor were there any strong differences in the peripheral organ changes between T1, T2 and T2* weighted images (data not shown). Although vascular T2* signal was visible in healthy brain at doses of 2, 5 and 10 mg/kg PEG-SPION (Figure S5), pilot SGMs indicated that 10 mg/kg generated a favourable amount of T2* signal. Uptake of the nanoparticles into the liver and spleen parenchyma was confirmed using Perls’ Prussian blue staining of 10 micron paraffin-embedded slices post transcardial saline and formalin perfusion (Fig. 5C).
Discussion
Choice of orthotopic glioblastoma model is vital for evaluating nanoparticle, nanomedicine or other systemic therapeutic uptake and biodistribution26. The tumour border of U87MG is extremely clear in MRI scans as shown by both PEG-SPION and Gadovist contrast enhancement (Fig. 3). As U87MG tumours have high cellularity, they are visible even in pre-contrast T1w and T2w MRI scans. The aggressive tumour nature causes near uniform blood-brain barrier disruption and thus, gadolinium enhancement in T1w images is visible across the entire tumour region. RN1-luc tumours on the other hand, more faithfully recapitulate intratumoural BBB functionality, with the stem-like properties of the cells allowing growth as a diffuse dense mass within healthy tissue that spreads throughout the brain into healthy circuitry tissue32 over a longer time period. This infiltrative growth is difficult to clearly distinguish using MRI, though asymmetries and invasive changes in tissue density become evident in T2-weighted scans (Figs. 2 and 4). However, the true extent of the RN1-luc glioblastoma infiltration can only be visualised postmortem through histology and microscopy, which is also largely the case in human patients. Interestingly, Gadovist did not provide clear information regarding the infiltration extent of RN1-luc tumours, even in highly progressed cases (Fig. 2), whereas the PEG-SPIONs provided information on abnormal vasculature and some angiogenic areas of the tumour (Figs. 3 and 4, Figure S2).
Other radiological imaging modalities including positron emission tomography, magnetic resonance spectroscopy and diffusion tensor imaging may provide further qualitative information of glioblastoma location to that reported here. However, while the sensitivities of these modalities are favourable, the resolutions of these technologies are generally more limited than standard MRI sequences, and they have not yet been widely incorporated into clinical workflows. Two limitations for using diffuse glioblastoma patient-derived xenografts in our hands, are the long in vivo growth period of over 90 or 155 days, and the compromised immune system required. Long tumour growth periods constrained the number of animals studied, particularly for RN1-luc. While our sample size of n = 3 for each tumour type was sufficient to enable statistically significant identification of large contrast effects35 (see Fig. 3), studies with larger cohorts will be needed to improve the statistical power and identify any other subtle image contrast effects (e.g. contrast resulting from accumulation of SPIONs at low concentrations within the tumour tissue). Furthermore, any passage of patient derived xenografts as allografts to maintain tumourigenicity inevitably changes the phenotype of the cells compared to the original host tumour25,36. Use of a genetic glioblastoma models such as triple Pten, Trp53, and Rb1 mutation induction via a Cre-loxP system also encounters a long accelerating tumour growth period of approximately 100–200 days, and though these mice have a functional immune system, the growth location of each individual tumour is unpredictable37.
The PEGylated nanoparticles used in this study exhibit strong MRI vascular contrast at doses compatible with clinical use, and appear to be phagocytosed by hepatic and splenic reticuloendothelial macrophages of the mononuclear phagocytic system (Fig. 5), following a several-hour blood circulation and blood-pool T2* contrast effects. Similar to clinically used carbohydrate-coated SPIONs such as ferumoxytol (Feraheme®), PEGylated SPIONs may have applications in not only glioblastoma angiogenesis MRI, but also possibly as a general alternative to gadolinium-based contrast. Careful modification of the surface coating of SPION contrast agents with high affinity targeting ligands may offer further highlighting of aggressive tumour infiltration, though the complexity and cost of generating functionalised SPIONs may limit the large-scale clinical translatability of this approach. Future studies may explore how antibody or ligand-targeted and passive PEGylated stealth nanoparticles are clinically applicable to glioblastoma prognosis and treatment response monitoring and evaluation. Feasibility of large-scale Good Manufacturing Practice grade nanoparticle production will also be an important consideration for future clinical implementation.
SPIONs such as ferumoxytol are prescribed off-label for clinical neuroimaging purposes at concentrations of ~ 1–11 mg Fe/kg (up to a 510 mg bolus)38, corresponding well in the range of that used in this study. The optimal concentration for imaging is governed by the iron-oxide contrast agent pharmacological, metabolic, and physical properties. A key physical property is the relaxivity of the contrast agent at the field strength chosen for MR imaging. The nanoparticles used here were initially developed for use as a contrast agent in superparamagnetic relaxometry (magnetic particle imaging, MPI) and were thus tuned for very high saturation magnetization that gives high transverse relaxivity (r2 = 177 ± 9 mM− 1 s− 1 at 3 T), while the r1 of this nanoparticle on the other hand is close to 0 at clinical field strengths30. Therefore, this nanoparticle is a strong candidate for use as a MRI contrast agent, as its saturation r2 relaxivity is approximately 2.8 times greater than that of clinically used ferumoxytol39 (r2 = 62.3 ± 3.7 s− 1 mM− 1) and greater than that of ferumoxtran-10 (r2 = 127.8 s− 1 mM− 1)40. In principle this allows for the administration of a smaller quantity of PEG-SPION while maintaining still-substantive T2 dark contrast in regions of low SPION uptake, where contrast enhancement with existing clinical methods would not be possible. This passive PEG-SPION may therefore have also strong applications in functional liver MRI (Fig. 5), as the liver rapidly uptakes SPIONs with little evidence of clearance across a 72-hour period, while non-functional liver would be expected to show a lack of uptake37. Hepatic Kupffer-Browicz cells are known to be highly endocytic and readily phagocytose most nanoparticles, particularly iron oxide nanoparticles41,42. Furthermore, with the advent of affordable, portable MRIs reaching the market, such as the Hyperfine Swoop® system, future low-cost screening of early-stage cancers such as in breast, prostate, liver and brain using affordable MR is becoming increasingly viable, and in such cases low-toxicity, high-sensitivity contrast agents are paramount.
There are well over 100 preclinical nanoparticle studies published in recent years for the magnetic resonance imaging of glioblastoma43. However, a large proportion of these rely solely on U87MG tumours26,43 or similar differentiated C6 or 9 L tumours, and in some cases flank subcutaneous xenografts which all have poorly relevant blood brain barrier properties. Ferumoxtran-10 and ferumoxytol have been imaged in U87MG and human glioblastoma cases for over 20 years, with strong uptake appearing at approximately 24 h post-injection42,43,44. However, the engulfment of these iron oxide nanoparticles appears to be largely by reactive macrophages rather than the tumour cells themselves44 − 44, which is different to what we observed regarding the wash in and wash out signal of PEG-SPION in U87MG and RN1-luc. Recent clinical studies comparing ferumoxytol to GBCAs have found differences in glioblastoma borders and dynamic susceptibility contrast, further indicating that these dextran-coated SPIONs are predominantly engulfed by tumour associated macrophages45,46, though by the lack of iron staining in brain tumour tissue post-perfusion in our study, it is doubtful that this is the case for PEG-coated SPIONs. Rather, the PEG-SPION T2* weighted signal seen in vivo in this study is indicative of tumour related-vascular abnormalities rather than acting as a direct marker of infiltrative tumour clusters. Vascular abnormalities in T2-weighted hyperintense regions of GBMs that do not show Gadolinium-based contrast-enhancement have been shown to represent areas of infiltrating tumour at early stages of angiogenesis which are associated with worse clinical outcomes and, hence might reflect more severe infiltrating tumour regions47. As these regions are normally missed from surgical resection margins, novel imaging agents that enable granular visualisation of these regions on pre-operative images, such as the PEG-SPIONs presented in our study, would enable adaptation of surgical resection margins, likely leading to improved patients survival outcomes.
It is important to note that both SPION ligand coating and size can confer potential biodistribution and toxicity effects. For instance, hyaluronic acid and chitosan coated 5 nm SPIONs show lower haemolysis at high concentrations compared to 10 nm and 30 nm polyacrylic acid coated SPIONs, as well as different complement activation, due to stability differences and avoidance of aggregation48. Interestingly, this aggregation can also lead to changes in both T1 and T2 relaxation49 and changes in the tumour microenvironment such as hypoxia can also affect SPION relaxivity50. As evidenced in this study, hydrophilic PEG coatings are generally favourable in terms of constituting low immunogenicity, low fouling and resistance to degradation51. PEG can also be chaperoned for targeting purposes by employing PEG-targeted bispecific antibodies37,52. Notably, we did not observe any signs of toxicity in mice injected with PEG-SPIONs in this study.
Our biodistribution results are consistent with those of Gobbo et al.53, who administered mice with 12–15 nm SPIONs coated with dimercaptosuccinic acid at an intravenous concentration of 10 mg Fe/kg. T2-weighted MR imaging was performed at 7 T using turbo spin echo sequences post intravenous injection. They observed a maximum change in MRI signal intensity at the 3-hour mark for both the liver and spleen, from which the signal remained relatively constant up-unto the 96-hour mark, and a blood half-life of 32 min. In our study, we found rapid uptake of the PEG-SPION by the liver, spleen and less so in the kidneys within 10 min to 1 h. For the kidneys in Gobbo et al.53, a signal peak was reached 48 h post-injection, with a gradual decline observed out until the 96-hour mark. This is indicative of a slower renal uptake and clearance of the dimercaptosuccinic acid coated 15 nm SPIONs when compared to PEG-SPION.
In another study, Pham et al.54. obtained ex vivo biodistribution and clearance profiles in mice intraperitoneally-administered novel 25 nm core, methoxypolyethylene glycol coated SPIONs. This analysis quantified the iron concentrations in various peripheral organs via atomic absorption spectroscopy measurements. The mice were administered with a much larger dose of SPIONs – 90 mg Fe/kg – and were examined up to 1-week post-injection. Iron concentrations in the liver, spleen, kidney, and bladder all peaked at 1 h post injection, with observed iron concentrations being relatively stable up to 1-week post injection, aside from a gradual reduction in iron content of the kidney by the 48-hour mark. In our in vivo imaging study, we observed a more rapid clearance of nanoparticles from both the kidney (Figs. 5) and bladder (data not shown) by the 24-hour mark, which may also be related to differences between intravenous and intraperitoneal administration. While a limited number of cytotoxic effects have been described in the literature for SPIONs, such as oxidative stress through Fenton reactions53, the frequency and severity of adverse events seen in clinic for currently used SPION ferumoxytol has been low54. No imaging or treatment-related adverse events have been reported in a thirteen patient clinical trial (IBI10103) of the PEG-SPION used in this study, suggesting that they are also low risk.
The SGM postprocessing step was performed to create image hypointensities as a result of SPION presence in the body, as regions of high SPION uptake will produce a strong susceptibility gradient at interfaces with regions of low SPION uptake. Positive contrast from iron-oxide nanoparticles can also be achieved via sequences such as balanced steady-state free precession, though this would be challenging at the field strengths used here due to banding artefacts30,31. The benefits of this SGM post-processing approach when compared to other approaches for generating bright contrast from dark T2 SPION MR contrast include a higher sensitivity to field inhomogeneity and susceptibility gradients combined with a reasonable suppression of background signal and water signals. Additionally, the signal-to-noise ratio is high when compared to other methods27,55,56. FDA approved nanoparticles such as Ferumoxytol could be a rapid route to clinical tests of the imaging techniques demonstrated here. However, we note that the SGM positive contrast technique shown would have reduced efficacy for Ferumoxytol due to the smaller size, and resultant magnetization, of the iron oxide core28,39. Likely, functionalizations other than PEG may exist that would give superior contrast when diagnosing glioblastoma57.
The sensitivity to local susceptibility that the SGM approach provides in tandem with the high magnetization of the nanoparticle may have applications in the detection of microhemorrhages or calcification within tissue, as there will be a high susceptibility gradient between SPION-rich blood and these regions of interest27,58. As shown in Fig. 4, this approach could have application in the detection of glioblastoma, where SPIONs continue to be investigated for their applicability as an alternative to gadolinium-based MR contrast agents. While Gadovist is the clinical standard for diagnosis of glioblastoma, other Gd-based contrast agents are clinically available. In future work, Gd-based contrast agents designed for long circulation times should be compared to SPION-based contrast agents for imaging glioblastoma infiltration and vascular abnormalities45. Given growing concerns over gadolinium retention in the brain and body, SPIONs may provide a safer alternative for long-term vascular imaging in glioblastoma and glioma. Future studies will be needed to evaluate the sensitivity of our SPION imaging methods in the quantification of glioblastoma response to treatment, which could include measuring changes in BBB permeability or vascularisation.
In conclusion, PEGylated SPIONs provide additional vasculature information to that obtained with conventional glioblastoma MRI. While small gadolinium-based contrast agents and ultrasmall SPIONs have shown rapid renal uptake59 and deposition throughout the body60, medium-sized SPIONs such as those used in this study appear to show a more preferential hepatobiliary macrophagic (Kupffer-Browicz cell) and splenic uptake, where it predominantly remains and is slowly metabolised. Further investigations in humans may be made using SGM approaches to test the clinical utility of positive contrast imaging of SPIONs.
Data availability
The authors declare that original data supporting the findings of this research are available within the article. Raw data is available from the corresponding author upon reasonable request.
Abbreviations
- BBB:
-
blood–brain barrier
- FA:
-
flip angle
- FOV:
-
field of view
- GRE:
-
gradient recalled echo
- MGV:
-
mean grey value
- MRI:
-
magnetic resonance imaging
- NA:
-
number of averages
- PDX:
-
patient–derived xenograft
- PEG:
-
polyethylene glycol
- T1w:
-
T1–weighted
- T2w:
-
T2–weighted
- T2*w:
-
T2*–weighted
- TE:
-
echo time
- TR:
-
repetition time
- SGM:
-
susceptibility gradient mapping
- ST:
-
slice thickness
- SPION:
-
superparamagnetic iron oxide nanoparticle
References
Bae, S. et al. Radiomic MRI phenotyping of glioblastoma: improving survival prediction. Radiology 289, 797–806 (2018).
Sarkaria, J. N. et al. Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-oncology 20, 184–191 (2018).
Arvanitis, C. D., Ferraro, G. B. & Jain, R. K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer. 20, 26–41 (2020).
Wirsching, H.-G., Galanis, E., & Weller, M. Glioblastoma. Handb. Clin. Neurol. 134, 381–397 (2016).
Claes, A., Idema, A. J. & Wesseling, P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 114, 443–458 (2007).
Yamahara, T. et al. Morphological and flow cytometric analysis of cell infiltration in glioblastoma: a comparison of autopsy brain and neuroimaging. Brain Tumor Pathol. 27, 81–87 (2010).
Tang, W. et al. Emerging blood–brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 48, 2967–3014 (2019).
Daldrup-Link, H. E. Ten things you might not know about iron oxide nanoparticles. Radiology 284, 616–629 (2017).
Gholami, Y. H. et al. A radio-nano-platform for T1/T2 dual-mode PET-MR imaging. Int. J. Nanomed. 15, 1253–1266 (2020).
Liu, H. et al. Application of iron oxide nanoparticles in glioma imaging and therapy: from bench to bedside. Nanoscale 8, 7808–7826 (2016).
Gallo, J., Long, N. J. & Aboagye, E. O. Magnetic nanoparticles as contrast agents in the diagnosis and treatment of cancer. Chem. Soc. Rev. 42, 7816–7833 (2013).
Lee, N. & Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 41, 2575–2589 (2012).
Shin, T. H., Choi, Y., Kim, S. & Cheon, J. Recent advances in magnetic nanoparticle-based multi-modal imaging. Chem. Soc. Rev. 44, 4501–4516 (2015).
van der Molen, A. J., Quattrocchi, C. C., Mallio, C. A., Dekkers, I. A., European Society of Magnetic Resonance in Medicine. & B. G. R. Ten years of gadolinium retention and deposition: ESMRMB-GREC looks backward and forward. Eur. Radiol. 34, 600–611 (2024). Educational Committee.
Kanda, T., Oba, H., Toyoda, K., Kitajima, K. & Furui, S. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Japanese J. Radiol. 34, 3–9 (2016).
Rogosnitzky, M. & Branch, S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals 29, 365–376 (2016).
Hua, N. et al. Gadolinium deposition in the rat brain measured with quantitative MRI versus elemental mass spectrometry. Radiology 306, 244–251 (2023).
Splendiani, A. et al. Visible T1-hyperintensity of the dentate nucleus after multiple administrations of macrocyclic gadolinium-based contrast agents: yes or no? Insights into Imaging. 10, 1–10 (2019).
Murata, N., Murata, K., Gonzalez-Cuyar, L. F. & Maravilla, K. R. Gadolinium tissue deposition in brain and bone. Magn. Reson. Imaging. 34, 1359–1365 (2016).
Semelka, R. C. & Ramalho, M. Gadolinium deposition disease: current state of knowledge and expert opinion. Invest. Radiol. 10, 1097 (2023).
Semelka, R. C. et al. Gadolinium deposition disease: initial description of a disease that has been around for a while. Magn. Reson. Imaging. 34, 1383–1390 (2016).
Burke, L. M. et al. Self-reported gadolinium toxicity: a survey of patients with chronic symptoms. Magn. Reson. Imaging. 34, 1078–1080 (2016).
Weissleder, R. et al. Superparamagnetic iron oxide: pharmacokinetics and toxicity. Am. J. Roentgenol. 152, 167–173 (1989).
Estelrich, J., Sánchez-Martín, M. J. & Busquets, M. A. Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. Int. J. Nanomed. 10, 1727–1741 (2015).
Stringer, B. W. et al. A reference collection of patient-derived cell line and xenograft models of proneural, classical and mesenchymal glioblastoma. Sci. Rep. 9, 4902 (2019).
Brighi, C. et al. Comparative study of preclinical mouse models of high-grade glioma for nanomedicine research: the importance of reproducing blood-brain barrier heterogeneity. Theranostics 10, 6361 (2020).
Dahnke, H., Liu, W., Herzka, D., Frank, J. A. & Schaeffter, T. Susceptibility gradient mapping (SGM): a new postprocessing method for positive contrast generation applied to superparamagnetic iron oxide particle (SPIO)-labeled cells. Magn. Reson. Med. 60, 595–603 (2008).
De Haro, L. P. et al. Magnetic relaxometry as applied to sensitive cancer detection and localization. Biomedical Engineering/Biomedizinische Technik. 60, 445–455 (2015).
Adolphi, N. L. et al. Imaging of Her2-targeted magnetic nanoparticles for breast cancer detection: comparison of SQUID‐detected magnetic relaxometry and MRI. Contrast Media Mol. Imaging. 7, 308–319 (2012).
Waddington, D. E., Boele, T., Maschmeyer, R., Kuncic, Z. & Rosen, M. S. High-sensitivity in vivo contrast for ultra-low field magnetic resonance imaging using superparamagnetic iron oxide nanoparticles. Sci. Adv. 6, eabb0998 (2020).
Shen, S. et al. Enhancing organ and vascular contrast in preclinical ultra-low field MRI using superparamagnetic iron oxide nanoparticles. Commun. Biology. 7, 1197 (2024).
Chiarelli, P. A. et al. Iron oxide nanoparticle-mediated radiation delivery for glioblastoma treatment. Mater. Today. 56, 66–78 (2022).
Langford, D. J. et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods. 7, 447–449 (2010).
Tweedle, M. F., Wedeking, P. & Kumar, K. Biodistribution of radiolabeled, formulated gadopentetate, Gadoteridol, Gadoterate, and gadodiamide in mice and rats. Invest. Radiol. 30, 372–380 (1995).
Desmond, J. E. & Glover, G. H. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J. Neurosci. Methods. 118, 115–128 (2002).
Wang, J. et al. A reproducible brain tumour model established from human glioblastoma biopsies. BMC cancer. 9, 1–13 (2009).
Janowicz, P. W. et al. Understanding nanomedicine treatment in an aggressive spontaneous brain cancer model at the stage of early blood brain barrier disruption. Biomaterials 283, 121416 (2022).
Toth, G. B. et al. Current and potential imaging applications of Ferumoxytol for magnetic resonance imaging. Kidney Int. 92, 47–66 (2017).
Knobloch, G. et al. Relaxivity of Ferumoxytol at 1.5 T and 3.0 T. Invest. Radiol. 53, 257–263 (2018).
Simon, G. H. et al. T1 and T2 relaxivity of intracellular and extracellular USPIO at 1.5 T and 3T clinical MR scanning. Eur. Radiol. 16, 738–745 (2006).
Boey, A. & Ho, H. K. All roads lead to the liver: metal nanoparticles and their implications for liver health. Small 16, 2000153 (2020).
Hunt, N. J. et al. Rapid intestinal uptake and targeted delivery to the liver endothelium using orally administered silver sulfide quantum Dots. ACS Nano. 14, 1492–1507 (2020).
Zhuang, D., Zhang, H., Hu, G. & Guo, B. Recent development of contrast agents for magnetic resonance and multimodal imaging of glioblastoma. J. Nanobiotechnol. 20, 1–21 (2022).
Neuwelt, E. A. et al. Imaging of iron oxide nanoparticles by MR and light microscopy in patients with malignant brain tumours. Neuropathol. Appl. Neurobiol. 30, 456–471 (2004).
Barajas, R. et al. Distinguishing extravascular from intravascular Ferumoxytol pools within the brain: proof of concept in patients with treated glioblastoma. Am. J. Neuroradiol. 41, 1193–1200 (2020).
Wongsawaeng, D. et al. Comparison of dynamic susceptibility contrast (DSC) using gadolinium and iron-based contrast agents in high-grade glioma at high-field MRI. Neuroradiol. J. 37, 473–482 (2024).
Bobholz, S. A. et al. Multi-Site retrospective analysis of diffusion and perfusion MRI correlates to glioma characteristics derived from Radio-Pathomic maps. Neuro-Oncology, noaf044. https://doi.org/10.1093/neuonc/noaf044 (2025).
Liu, T. et al. The effect of size and surface ligands of iron oxide nanoparticles on blood compatibility. RSC Adv. 10, 7559–7569 (2020).
Zhou, H. et al. In vivo aggregation-induced transition between T 1 and T 2 relaxations of magnetic ultra-small iron oxide nanoparticles in tumor microenvironment. Nanoscale 9, 3040–3050 (2017).
Zhou, H. et al. Hypoxia-triggered self-assembly of ultrasmall iron oxide nanoparticles to amplify the imaging signal of a tumor. J. Am. Chem. Soc. 143, 1846–1853 (2021).
Wu, Z. et al. Advances in magnetic resonance imaging contrast agents for glioblastoma-targeting theranostics. Regenerative Biomaterials. 8, rbab062 (2021).
Lim, M. et al. Targeted hyperbranched nanoparticles for delivery of doxorubicin in breast cancer brain metastasis. Mol. Pharm. 20, 6169–6183 (2023).
Gobbo, O. L. et al. Biodistribution and Pharmacokinetic studies of SPION using particle electron paramagnetic resonance, MRI and ICP-MS. Nanomedicine 10, 1751–1760 (2015).
Pham, B. T. et al. Biodistribution and clearance of stable superparamagnetic maghemite iron oxide nanoparticles in mice following intraperitoneal administration. Int. J. Mol. Sci. 19, 205 (2018).
Çukur, T., Yamada, M., Overall, W. R., Yang, P. & Nishimura, D. G. Positive contrast with alternating repetition time SSFP (PARTS): A fast imaging technique for SPIO-labeled cells. Magn. Reson. Medicine: Official J. Int. Soc. Magn. Reson. Med. 63, 427–437 (2010).
Lin, C., Cai, S. & Feng, J. Positive contrast imaging of SPIO nanoparticles. J. Nanomaterials. 2012, 6–6 (2012).
Xie, H. et al. Lactoferrin-conjugated superparamagnetic iron oxide nanoparticles as a specific MRI contrast agent for detection of brain glioma in vivo. Biomaterials 32, 495–502 (2011).
Liu, T. et al. Cerebral microbleeds: burden assessment by using quantitative susceptibility mapping. Radiology 262, 269–278 (2012).
Li, X., Sun, Y., Ma, L., Liu, G. & Wang, Z. The renal clearable magnetic resonance imaging contrast agents: state of the Art and recent advances. Molecules 25, 5072 (2020).
Choi, J. W. & Moon, W. J. Gadolinium deposition in the brain: current updates. Korean J. Radiol. 20, 134–147 (2019).
Acknowledgements
We acknowledge the support of the Sydney Imaging Core Research Facility and the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability at the University of Sydney, including Dr Nguyen Pham, Dr Sofie Trajanovska, Dr Raj Parajuli, and Dr Xiang Feng (MR Solutions), for their assistance with the MR imaging. We also thank Professor Bryan Day and QCell for providing the RN1-luc patient-derived xenograft cells, Dr Kelly McKelvey for providing advice on animal surgeries, and the University of Sydney Laboratory Animal Services including the Kearns Facility for housing of the mice. We acknowledge the use of services provided by the Preclinical pipeline for Advanced Cancer Therapeutics (PACT) at the Kolling Institute. We thank Imagion Biosciences for providing the nanoparticles used in this study, Dr Emily Hewson and Jeremy Lim for providing critical reading of the manuscript, Dr Samson Dowland for histological advice and Dr Yingying Su for assistance with microscopy. PWJ was supported by a Cancer Institute New South Wales Translational Program Grant (TPG2165). DEJW was supported by Australian National Health and Medical Research Council (NHMRC) Investigator Grant 2017140. CB and TB were supported by NHMRC Investigator Grant 1194004.
Author information
Authors and Affiliations
Contributions
The study was conceptualized by D.E.J.W., C.B., Z.K., and P.W.J. Methodology was developed by P.W.J., D.E.J.W., R.M., Y.G., C.B., E.G.K., B.S., M.Z., S.S., T.D.T.T., F.W., and A.T. MRI investigations were conducted by P.W.J., R.M., and Y.G. Data visualization and analysis were handled by P.W.J., R.M., T.B., and D.E.J.W. Funding was secured by Z.K. and D.E.J.W. Project administration was managed by D.E.J.W. and Z.K. Supervision was provided by D.E.J.W., Z.K., and L.M. The original draft was written by P.W.J. and D.E.J.W. All authors contributed to review and editing of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
DEJW, CB and ZK have received research support from Imagion Biosystems Inc. and Patrys. MZ is an employee of Imagion Biosystems Inc. All the remaining authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Janowicz, P.W., Boele, T., Maschmeyer, R.T. et al. Enhanced detection of glioblastoma vasculature with superparamagnetic iron oxide nanoparticles and MRI. Sci Rep 15, 14283 (2025). https://doi.org/10.1038/s41598-025-97943-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97943-y
Keywords
This article is cited by
-
Brain neurovascular unit in response to intra- and extra-central nervous system cancers: implications, mechanisms, and challenges
Cancer Cell International (2025)
-
Mapping two decades of nanomaterials research in glioblastoma: trends, networks, and translational insights
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)
-
Multifunctional bio-nanostructure for cancer therapy: mini review
Discover Applied Sciences (2025)