Abstract
Accumulating evidence indicates that AJUBA acts as a potential target for new therapeutics to treat cancers. Nevertheless, the role of AJUBA in non-small cell lung cancer (NSCLC) remains unclear. In the current study, immunohistochemistry (IHC) showed that expression of AJUBA was upregulated in 67.55% of NSCLC tumor samples and was associated with tumor size, lymph node metastasis, advanced tumor stage, poor differentiation and poor prognosis. Loss-of-function assays of AJUBA produced by silencing RNA (siAJUBA) significantly inhibited the proliferation, invasion and migration of H1299 and A549 cell lines. Mechanistically, inhibition of extracellular signal-regulated kinases (ERKs) blocked the AJUBA-induced proliferation, invasion and migration of NSCLC cells, and decreased the expression of proteins related to the endothelial-mesenchymal transition (EMT). Silencing of AJUBA repressed tumor growth and led to a decrease in p-ERK, β-catenin and N-cadherin in vivo. In conclusion,, overexpression of AJUBA facilitates the proliferation and motility of NSCLC cells via the ERK and Wnt/β-catenin pathways. AJUBA may be useful as a prognostic marker which may provide a promising approach for the treatment of NSCLC.
Similar content being viewed by others
Introduction
According to global cancer statistics, there are more than 2 million newly diagnosed lung cancer patients annually1. Despite intensive efforts to treat non-small cell lung cancer (NSCLC) by developing novel therapeutic strategies, lung cancer has the highest mortality rate among all types of cancer globally2. Therefore, it will be valuable to explore molecular carcinogenesis and facilitate the development of novel therapeutic targets for NSCLC which can prolong the survival of patients. In mammals, the AJUBA family includes three proteins: AJUBA, Wilms tumor 1 interacting protein, and LIM domain-containing protein 1 (LIMD1). AJUBA, also known as JUB, is located on human chromosome 14 and encodes a protein with a molecular weight of 58 kDa. AJUBA protein is characterized by the presence of three LIM domains at its carboxy terminus, which can assist in its nuclear localization. LIM domains are rich in cysteine and were first discovered in many proteins that regulate cell development. The presence of multiple LIM domains in many proteins implies that AJUBA may bind to them and play a significant role3. AJUBA is present in fetal components of the developing placenta and has been shown to be involved in the process of epidermal development4. A growing body of research has demonstrated that many proteins which play a key role in development, such as the Fibulin family, 14-3-3β and β-catenin, are also indispensable in a variety of tumors. Therefore, in-depth study of the functions of proteins that play an important role in biological development and their mechanisms in tumors can help develop new strategies for tumor treatment.
Recently, numerous researchers have shown that AJUBA is upregulated in various human tumors5. AJUBA promotes colorectal cancer (CRC) growth by inhibiting apoptosis6,7.AJUBA has also been shown to stimulate breast cancer cell growth, invasion, chemoresistance and glucose uptake8,9. AJUBA is upregulated in human gastric cancers and regulates glucose uptake and mitochondrial function to increase cell growth and chemoresistance10. AJUBA is frequently overexpressed in esophageal squamous cell carcinoma tissues and promotes the tumorigenicity, motility and chemoresistance of this cancer11. AJUBA is upregulated in hepatocellular carcinoma (HCC), where it promotes proliferation, motility and endothelial-mesenchymal transition (EMT)12. AJUBA has been confirmed to be a target gene of certain microRNAs that have tumor-suppressing effects13. However, the biological functions of AJUBA in lung cancer have not yet been revealed.
The Wnt/β-catenin pathway is one of the most important molecular pathways in mammals and is overactivated in many tumors, including lung cancer14. Previous studies have shown that AJUBA is essential for modulating of the Wnt/β-catenin pathway15. Therefore, we hypothesized that AJUBA may facilitate NSCLC progression by the β-catenin pathway.
We analyzed the expression of AJUBA in NSCLC tissues and its clinical significance. The biological roles of AJUBA in NSCLC were also explored. Finally, we found that AJUBA regulates cell proliferation and motility and induces EMT through the ERK and Wnt/β-catenin pathway.
Results
AJUBA was upregulated in NSCLC tumor samples and correlated with poor prognosis
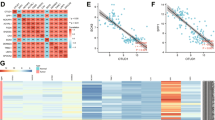
We investigated AJUBA expression status in 188 sets of cancerous and corresponding paracancerous tissues. AJUBA was expressed at higher levels in tumor tissues (67.55%; 127/188) compared to corresponding normal lung tissues (15.96%; 30/188). AJUBA was negative or weakly positive in most normal alveolar cells and bronchial epithelial cells. Only some bronchi near cancer tissues were AJUBA-positive (Fig. 1, Supplementary Fig. 1). AJUBA was highly expressed in tumor cells in 73.47% (72/98) of adenocarcinomas and in 61.11% (55/90) of squamous cell carcinomas (Fig. 1B). Next, we found no significant link between the expression of AJUBA and factors such as age (p = 0.747), gender (p = 0.175), and histological type (p = 0.710). In contrast, we found that high AJUBA expression was positively associated with tumor size (p = 0.004), lymph node metastasis (p = 0.011), advanced tumor stage (p < 0.001) and poor differentiation (p = 0.018; χ2 test, Table 1).
AJUBA expression was upregulated in NSCLC tumor samples and correlated with poor prognosis in NSCLC patients. (A) IHC analysis of AJUBA in NSCLC tissue samples and non-tumor tissues (200X). The tissue samples were obtained from three different patients. (B) Expression of AJUBA in adenocarcinoma and squamous cell carcinoma (200X). (C) Survival rates based on AJUBA were analyzed by the Kaplan-Meier survival method in NSCLC patients.
Subsequently, Kaplan–Meier analysis revealed that among 188 patients, patients with low expression of AJUBA experienced better overall survival (81.445 ± 5.794 months, p < 0.001; Fig. 1C), which indicated that AJUBA was correlated with a worse prognosis for NSCLC (51.710 ± 3.893 months). Furthermore, multivariate Cox regression analysis uncovered that advanced TNM stage (p = 0.042) and high expression of AJUBA (p = 0.002) were independent variables associated with decreased survival rates (Cox proportional risk regression model, Table 2). Collectively, we concluded that AJUBA is overexpressed in NSCLC and is closely related to tumorigenesis and progression.
Downregulation of AJUBA inhibited the growth of NSCLC cells
We next measured the protein levels of AJUBA in four different NSCLC cell lines compared to immortalized human normal HBE cells. Consistent with the in vivo findings, we found the AJUBA was also overexpressed in NSCLC cell lines (Fig. 2A, Supplementary Fig. 2 A). We next silenced AJUBA in H1299 and A549 cells in loss-of-function assays and used Trilencer-27 Universal Scrambled siRNA as a negative control(NC) (Fig. 2B and C, Supplementary Fig. 2B). CCK-8 assays showed that the proliferation of NSCLC cells after siAJUBA treatment decreased in a time-dependent manner (Fig. 2D). The colony-formation capacity of the siAJUBA group was also inhibited compared with the control group (Fig. 2E).
Downregulation of AJUBA attenuated the proliferation of HCC cells. (A) Protein expression levels of AJUBA in normal lung epithelial cells (HBE) and NSCLC cell lines (H1299, A549, H460 and H1650). One-way ANOVA was used to analyze the differences in grayscale among different groups. (B) The mRNA levels of AJUBA in AJUBA-knockdown H1299 and A549 cells. Two-tailed Student’s t-test was used to analyze the differences between the two groups. NC, negative control. ***P < 0.001. (C) The protein levels of AJUBA in AJUBA-knockdown H1299 and A549 cells. One-way ANOVA was used to analyze the differences in grayscale among different groups. (D) Cell viability was detected in AJUBA-knockdown H1299 and A549 cells. Effect of AJUBA knockdown on cell growth of H1299 and A549 cells was analyzed by colony formation assay. Two-tailed Student’s t-test was used to analyze the differences between the two groups,. **P < 0 01, ***P < 0.001. (E) Effect of AJUBA knockdown on cell growth of H1299 and A549 cells was analyzed by colony formation assay. Two-tailed Student’s t-test was used to analyze the differences between the two groups. **P < 0.01.
Downregulation of AJUBA inhibited the invasion and migration of NSCLC cells
To explore the effect of AJUBA on the motility of lung cancer cells, we conducted migration and invasion assays in Transwell plates. After transfection with siAJUBA to downregulate its expression, the migratory capacity of the two cell lines was significantly reduced compared to the control groups (Fig. 3A). In the invasion assay using Matrigel to simulate the in vivo environment, we found that downregulating AJUBA significantly reduced the number of cells penetrating the lower membrane. After statistical analysis, the number of cells that penetrated the membrane in the two siAJUBA groups was significantly lower than in the control groups (Fig. 3B).
The expression of AJUBA modulated the migration and invasion of NSCLC cells in vitro. (A) H1299 and A549 cells were transfected with or without siAJUBA and a wound healing assay was undertaken (100X). Two-tailed Student’s t-test was used to analyze the differences between the two groups. **P < 0.01, ***P < 0.001. (B) Transwell assay analysis of AJUBA knockdown in H1299 and A549 cells (200X). Two-tailed Student’s t-test was used to analyze the differences between the two groups. ***P < 0.001.
AJUBA was co-expressed with β-catenin in NSCLC tumor samples and its Silencing inhibited the expression of β-catenin and EMT-associated proteins in NSCLC cell lines
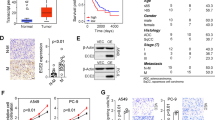
Previous studies have shown that AJUBA can directly bind to β-catenin in hepatocellular carcinoma16, and β-catenin also plays a crucial regulatory role in the proliferation and migration of NSCLC. To identify the mechanism of AJUBA-mediated tumor promotion, we used IHC to examine the relationship between AJUBA and β-catenin expression in NSCLC cells of different histology. In Fig. 4A, the expression of AJUBA and β-catenin was positively correlated in both adenocarcinomas and squamous cell carcinomas (χ2 test, Table 3, Supplementary Fig. 3 A). In addition to β-catenin, which mainly acts through the Wnt signaling pathway, other important molecules in this pathway were examined to explore the biological function of AJUBA in NSCLC cell lines. After knockdown of AJUBA, β-catenin, N-cadherin and Vimentin were decreased. Furthermore, Cyclin D1, MMP-9 and p-ERK expression levels were also decreased in both AJUBA-knockdown NSCLC cell lines. These have all been confirmed to be downstream genes in the Wnt pathway. In summary, then, these data indicated that AJUBA positively regulates the Wnt/β-catenin pathway (Fig. 4B, Supplementary Fig. 3B).
Elevated AJUBA expression was positively associated with β-catenin expression in NSCLC and regulated the expression of EMT-related proteins. (A) Correlation of the protein level of AJUBA and β-catenin in NSCLC tumor tissues by IHC (200X). (B) Western blotting analysis for AJUBA, Cyclin D1, MMP-9, β-catenin, N-cadherin and Vimentin in H1299 and A549 cells after transfection with or without siAJUBA, GAPDH served as an internal control. One-way ANOVA was used to analyze the differences in grayscale among different groups. (C) Western blotting analysis was employed to detect the protein level of AJUBA, p-ERK, β-catenin, N-cadherin and Vimentin in H1299 and A549 cells with AJUBA overexpression and/or PD98059 treatment. One-way ANOVA was used to analyze the differences in grayscale among different groups.
AJUBA upregulated β-catenin via the ERK signaling pathway and regulated EMT progression in NSCLC cells
A previous study showed that the activation of ERK signaling molecules is associated with EMT17, ERK1/2 phosphorylates the Ser9 residue of GSK3β, leading to β-catenin upregulation18. Therefore, we explored whether AJUBA contributed to EMT via ERK activation. After we upregulated the expression of AJUBA, the expression of β-catenin, N-cadherin and Vimentin were significantly increased; meanwhile the expression level of EMT markers was decreased by the ERK inhibitor PD98059 (Fig. 4C, Supplementary Fig. 3 C). Therefore, ERK inhibition can reverse the AJUBA-induced upregulation of β-catenin and other key proteins in EMT. We next investigated whether AJUBA promotes proliferation and motility via ERK activation. CCK-8, colony formation (Fig. 5A and B), wound healing and cell migration assays (Fig. 5C and D) demonstrated that phosphorylation of ERK inhibitor PD98059 reversed the effects of AJUBA on growth and motility of human lung cancer cells.
ERK inhibitor PD98059 reversed the proliferation, migration and invasion effects of AJUBA plasmid in lung cancer cells. (A) The growth curves of H1299 and A549 cells transfected with AJUBA plasmid and/or PD98059. Two-tailed Student’s t-test was used to analyze the differences between the two groups. **P < 0.01, ***P < 0.001. (B) The colony formation capacity of H1299 and A549 cells transfected with AJUBA plasmid and/or PD98059. Two-tailed Student’s t-test was used to analyze the differences between the two groups. *P < 0.05; **P < 0.01. (C) The migration of H1299 and A549 cells transfected with AJUBA plasmid and/or PD98059 (100X). Two-tailed Student’s t-test was used to analyze the differences between the two groups. *P < 0.05, ***P < 0.001. (D) The invasion of H1299 and A549 cells transfected with AJUBA plasmid and/or PD98059 (200X). Two-tailed Student’s t-test was used to analyze the differences the between the two groups. **P < 0.01, ***P < 0 001.
Silencing of AJUBA repressed tumor growth and led to a decrease in p-ERK, β-catenin and N-cadherin in vivo
The effect of AJUBA deficiency on NSCLC growth was measured in a mouse model. Mice treated with shAJUBA showed lower tumor growth volumes compared with control mice (Fig. 6A and B). These mice exhibited significantly lower tumor weights than the control group (Fig. 6C). Furthermore, the expression of p-ERK, β-catenin, Ncadherin and Vimentin decreased in the shAJUBA group (Fig. 6D). Taken together, these data indicate that AJUBA promoted tumor growth via regulation of the ERK and Wnt/β-catenin pathway in NSCLC.
Downregulation of AJUBA inhibits the growth of NSCLC cells in vivo. (A) Image of tumors in nude mouse tissues from the two groups. (B) Tumor volume in the shctrl and shAJUBA groups. Two-tailed Student’s t-test was used to analyze the differences between the two groups. ***P < 0.001. (C) The tumor weights in the NSCLC mouse model were measured in the shctrl and shAJUBA groups. Two-tailed Student’s t-test was used to analyze the differences between the two groups. ***P < 0.001. (D) The expression levels of AJUBA, Ki67, p-ERK, β-catenin and N-cadherin were investigated in control and AJUBA-depleted groups by IHC (200X).
Discussion
Although the diagnostic technology and treatment methods for NSCLC have been continuously improving, its five-year survival rate remains very low19. It is necessary to explore the core mechanisms of tumor progression to discover novel biomarkers. Although involvement of AJUBA in several signaling cascades has been reported20, and the function of AJUBA investigated in a series of human cancers16,21,22,23,24, there have been no reports to the best of our knowledge on the in vivo role of AJUBA in lung cancer and its impact on prognosis. In this study, we demonstrated that AJUBA was markedly increased in NSCLC while deletion of AJUBA caused a reduction in the proliferation and motility of NSCLC cells. Furthermore, knockdown of AJUBA regulates growth in both cell lines and mouse models by repressive activity on the Wnt/β-catenin pathway. Our study thus provides new evidence showing AJUBA as a tumor biomarker in NSCLC.
Previous research has found that AJUBA expression is related to poor prognosis in HCC, CRC, breast cancer and esophageal squamous cell carcinoma6,9,11,12, but the impact of AJUBA on the survival rate in lung cancer has not been reported. In this study, AJUBA overexpression was observed in 127/188 NSCLC specimens and was associated with progression of existing clinicopathological factors. Survival analysis results showed that AJUBA expression was significantly related to reduced survival. This is consistent with previous results in HCC patients12. We also found that siAJUBA treatment induced an anticancer effect on H1299 and A549 cells.
AJUBA is a scaffold or adapter protein containing multiple LIM domains. These domains might identify a shared structural characteristic of proteins, given that there isn’t a universal LIM domain recognition sequence among proteins3. Previous studies have shown that AJUBA interacts with various proteins such as Snail25, sp126and p6227to play multiple roles. β-catenin is one of these proteins, which initially aroused our interest. The Wnt/β-catenin pathway plays a significant role in lung cancer, and currently, there is no research on its regulatory relationship with AJUBA in lung cancer. The Wnt/β-catenin pathway is involved in cellular processes, tumorigenesis and EMT. In HCC, AJUBA is necessary for activation of the EMT pathway and HCC progression by increasing the levels of mesenchymal features12. Our present study demonstrated that AJUBA was positively correlated with β-catenin in adenocarcinoma (p = 0.012) and squamous cell carcinoma (p = 0.014) tissues. Down-regulation of AJUBA led to a decrease in a series of key molecules in EMT as well as downstream genes in the Wnt pathway. Thus, the impact of AJUBA on tumor prognosis may involve regulating the Wnt/β-catenin pathway.
Inappropriate activation of ERK signaling is implicated in lung cancer, and the ERK pathway is necessary to induce EMT progression28, while β-catenin is upregulated by p-ERK and contributes to lung cancer metastasis29. According to the literature, the earliest functional studies demonstrated that AJUBA binds to Grb2, resulting in activation of the ERK pathway in a RAS-dependent manner4. These findings prompted us to focus on the impact of p-ERK levels on the function of AJUBA in lung cancer. In our study, AJUBA overexpression increased malignant phenotypes of NSCLC cell lines, and EMT-related proteins were also increased in the AJUBA overexpression group. Targeting ERK with an inhibitor effectively reduced AJUBA-induced tumor progression. Moreover, ERK dephosphorylation induces downregulation of β-catenin, N-cadherin and Vimentin. The NSCLC mouse model we constructed showed that shAJUBA inhibited the xenograft growth in models using NSCLC cells, and AJUBA ablation suppressed the expression of Ki67, p-ERK, β-catenin, N-cadherin and Vimentin simultaneously in tumor tissues. Thus, AJUBA promotes proliferation, motility and EMT by targeting the ERK and Wnt/β-catenin pathways.
It is worth mentioning that, unlike AJUBA, the other two members of the AJUBA family (Wilms tumor 1 interacting protein and LIMD1) seem to play different roles in lung cancer30,31. This may be attributed to their distinct impacts on the same signaling pathways in lung cancer. In NSCLC, high expression of LIMD1 can inhibit the over-activation and nuclear localization of yes-associated protein (YAP), thereby suppressing the progression of lung cancer32. In addition to the WNT pathway, previous literature documented that AJUBA could also regulate the Hippo pathway3, which is required in many processes such as cell proliferation, EMT and carcinoma formation20. In CRC, the Hippo/YAP pathway was activated significantly after AJUBA knockdown and attenuated the growth of CRC cells23. High AJUBA levels enhanced cervical cancer cell drug resistance to cisplatin by upregulating YAP and the transcriptional coactivator TAZ. Overexpression of AJUBA also regulated glucose uptake and mitochondrial potential through Hippo/YAP in human gastric cancer10. The above conclusion seems to imply that AJUBA has different regulatory effects on YAP in different types of tumors. The relationship between AJUBA and Hippo/YAP in lung cancer has not been reported until now. Because the Hippo and Wnt pathways both affect tumorigenesis, it will be essential to study the cross-talk between AJUBA, WNT and Hippo in NSCLC in future experiments.
AJUBA has functioned as an oncogene in most studies, but also has been shown to have tumor-suppressing effects in certain cancers. For example, AJUBA functions as a haploinsufficient tumor suppressor by promoting NOTCH signaling in head and neck squamous cell carcinomas33. AJUBA’s effects are context-dependent and tissue-specific in tumorigenesis, which will require further research.
This study does have some limitations. When we conducted the AJUBA upregulation experiment with the addition of an ERK inhibitor, we found that the experimental dose of the ERK inhibitor did not completely offset the protein upregulation and enhanced tumor malignancy caused by AJUBA upregulation. Since the purpose of this experiment was to verify that upregulation of AJUBA could promote p-ERK expression, we did not use a high concentration of the ERK inhibitor. Meanwhile, the experimental results suggested that AJUBA may also regulate tumor malignancy through ERK-independent pathways, which needs to be confirmed in future studies. The in vivo experiments in this study did confirm that AJUBA inhibited tumor growth and we observed a reduction in p-ERK in vivo. To further verify whether AJUBA inhibits tumor growth in vivo through the suppression of the ERK pathway, the next step should include mouse experiments with the injection of ERK inhibitors. Furthermore, in vivo experiments need to be conducted to verify whether AJUBA can be used as a gene target for the treatment of NSCLC. This should involve conducting dose-response experiments with AJUBA inhibitors, exploring AJUBA’s role in resistance to existing chemical therapies, and validating findings across a broader range of NSCLC subtypes.
In conclusion, our results suggest that AJUBA is commonly and significantly up-regulated in NSCLC, which suggests an important role in the acquisition of a poor prognostic phenotype. AJUBA promotes the growth of lung cancer cells both in vitro and in vivo. AJUBA also accelerates EMT in NSCLC by inducing the ERK and Wnt/β-catenin pathways. Our findings thus indicate that AJUBA may be a key prognostic marker in NSCLC. This new understanding of the underlying mechanisms involving the AJUBA and Wnt/β-catenin pathways will provide a potential new target for the treatment of NSCLC.
Materials and methods
Immunohistochemistry
A collection of 188 paired tumor and paracancerous paraffin tissues without any preoperative treatment were used for IHC staining and were purchased from the Shanghai Outdo Biotech Company. The study was performed in strict compliance with the Declaration of Helsinki and was approved by the Ethics Committee of the Taizhou Hospital of Zhejiang province (SHYJS-CO-1904014,1910013). Sections were subjected to dewaxing with xylene and gradient alcohol dehydration followed by antigen retrieval using heat-mediated antigen retrieval in citrate buffer. The sections were blocked with normal goat serum (Maixin) for 10 min at room temperature. Subsequently, the sections were incubated with primary anti-AJUBA rabbit polyclonal antibody (HPA006171; 1:100, Sigma), anti-β-catenin (ab32572;1:400, Abcam), anti-Ncadherin (ab76011; 1:400, Abcam), anti-p-ERK (4370; 1:400, Cell Signaling Technology), or anti-Ki67 (RMA-0542; ready-to-use, Maixin) at 4 °C. The next morning, the sections were incubated with the Elivision super Kit from Maixin (KIT-9921). DAB-2031 (Maixin) was added for 2 min to visualize the proteins. The intensity of staining of AJUBA was graded as follows: 1 (no staining), 2 (light yellow) and 3 (deep yellow). The percentage of staining was assigned as 1 (< 10%), 2 (10–50%) and 3 (51–100%). Scores for intensity and percentage of staining were assigned to each sample and then multiplied together. Resulting values ≥ 4 were considered to indicate high expression. The intensity of staining scores for β-catenin were assigned based on previous studies34. The criteria for differentiation were as follows: For adenocarcinoma, if the proportion of high-grade components (solid, micropapillary, cribriform and complex glandular structures) was less than or equal to 20%, it was considered well-differentiated. If the proportion was greater than 20%, it was considered poorly differentiated. For squamous cell carcinoma, the presence of keratin pearls and intercellular bridges in cells with incomplete keratinization indicated good differentiation, while the absence of those features indicated poor differentiation. Two independent experienced investigators were assigned to examine all tumor slides randomly.
Cell culture
In this study, all NSCLC cell lines and the HBE cell line were maintained in DMEM/F12 (1:1) medium (Gibco), with 10% FBS (Biological Industries) at 37 °C under 5% CO2. For transfection, negative control siRNA and siAJUBA#1 (SR313776) were purchased from Origene. siAJUBA#2 5’-GGACCGGGA.
UUAUCACUUUTT-3 was synthesized by Gene Pharma (Shanghai). For AJUBA overexpression, NSCLC cells were transfected with Plasmid Control(PC) and pCMV6-AJUBA plasmid (RC215384, Origene). Lipofectamine 3000 (Invitrogen) was used as transfection reagent. Transfected cells were used for further experiments after 48 h.
Real-time PCR
TRIzol was used to extract total RNA from the samples. Real-time PCR was conducted using Supermix from Bio-Rad in a 20-ul reaction system, with triplicate samples analyzed on the Light Cycler®480 II. GAPDH was used as an internal reference, and relative quantification of all samples was performed using the 2−ΔΔCT method. The sequences of the primers are listed in Table S1.
Cell counting Kit-8 (CCK-8) assays
After 24 h of transfection with siAJUBA or pCMV6-AJUBA plasmids, NSCLC cells were seeded in 96-well plates (5 × 103 cells per well). CCK-8 reagent (10 µl; Beyotime) was added 4 h later and the cells were incubated for an additional 2 h at 37 °C. OD values were read at a wavelength of 450 nm using a full-wavelength microplate reader (Thermo Fisher Scientific). Growth curves were plotted using data from all observation time points, and statistical analysis was performed using data from the end point of observations.
Colony formation assay
H1299 or A549 cells (1 × 103 cells/well) were transfected with siAJUBA or pCMV6-AJUBA plasmids in 6-well plates. Crystal violet (Solarbio, Beijing, China) was used to stain the cell colonies after 14 days.
Matrigel invasion assay
Migration experiments were conducted using 24-well plates, with matrix gel (BD) diluted 1:6 in serum-free culture medium. Cells were incubated at 37 °C 16 h, then cells which had not invaded the lower layer were removed using a cotton tip. The cells that had passed through the membrane were fixed with formalin for 15 min and then stained with crystal violet for 15 min. Photos were taken and statistical analysis was performed on 10 randomly selected high-power microscopic fields for each sample.
Cell migration assay
H1299 and A549 cells were seeded into 6-well plates 48 h after transfection. Once the cells were confluent, a sterile 20-µL pipette tip was used to scratch the cell surface. The cells were observed and photographed by a digital camera at the same position at 0 h and 24 h later. The scratch width was measured and quantitatively analyzed with Image-Pro Plus 6.0 software.
Protein Immunoblotting experiment
Total protein was isolated from cells by M-PER™ (78503; Thermo Fisher Scientific) with Halt Protease and Phosphorylase Inhibitor Cocktail (78441; Thermo Fisher Scientific), then quantified using Pierce™ BCA assays (23225; Thermo Fisher Scientific). Sample buffer was added to the protein mixture to achieve a total protein concentration of 60 ug per 20 ul, and the cells were incubated at 100 ˚C for 5 min to denature the protein. After 10% SDS-PAGE, we use semi-dry transfer to move the proteins onto a PVDF membrane (Millipore) with a pore size of 0.45 mm. Next, non-specific binding was blocked with 5% milk and the membrane was incubated at 4℃ overnight with one of the following primary antibodies: AJUBA (HPA0061710; 1:1000, Sigma); Cyclin D1 (2978), Matrix Metalloproteinase-9 (MMP-9; 13667), ERK (4695) or p-ERK (4370, all 1:1000 from Cell Signaling Technology); β-catenin (ab32572), N-cadherin (ab76011) and Vimentin (ab92547), all 1:1000 from Abcam); and GAPDH (1:3000, Affinity Biosciences). The next day, after thorough washing, the membranes were incubated with the appropriate secondary antibodies at 37℃ for 90 min. Chemiluminescence reagent (Bio-Rad) was used to visualize the protein bands with the ECL detection system (Bio-Rad). Image Lab™ Software (Bio-Rad) was used to quantify the relative density of the bands. Images of full-length, original, unprocessed blot densitometric data and calculations of significance are provided in the supplementary materials.
Xenograft tumor assay
Female nude mice (body weight: 18–20 g) were obtained from Liaoning Changsheng Biotechnology and were meticulously cared for in accordance with the Laboratory Animal Care protocols established by the First Hospital of China Medical University. These protocols are aligned with the recommendations from the US National Institutes of Health and adhere to the ARRIVE guidelines, which can be accessed at the following link (https://arriveguidelines.org) for further details. The mice were allowed to adapt to a specific pathogen-free environment with a temperature range of 23 to 24˚C, relative humidity levels between 30 and 50%, and a 12-h alternating light/dark cycle. During the entire experimental process, food and water were freely available. H1299 cells were transfected with lentiviral supernatant containing shRNA targeting AJUBA (TL303846, Origene) or shControl (TR30021, Origene). Stable cell lines were positively selected using 34 ug/ml Chloramphenicol. The mice were divided into the shAJUBA group and the shCtrl group. Each experimental group was implanted with 5 × 106 stably transfected H1299 cells. Measurement of tumor nodules was performed once per week. The tumor volume was calculated using the following formula: Tumor Volume = Longest Diameter × (Shortest Diameter/2)/2. All mice were euthanatized 3 weeks later, at which time the tumor tissues were dissected and weighed.
Statistical analysis
All statistical analyses were conducted using SPSS 27.0 software (IBM). The specific statistical methods used in each experiment are explained in the text and figure legends. Data are presented as means plus or minus standard deviation (SD). The levels of statistical significance are denoted by p-values.
Data availability
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request.
Abbreviations
- (si)RNA:
-
Silencing RNA
- TNM:
-
Tumor Node Metastasis stage
- ERK:
-
Extracellular Signal-Regulated kinases
- EMT:
-
Epithelial-Mesenchymal Transition
- WTIP:
-
Wilms Tumor 1Interacting Protein
- LIMD1:
-
LIM Domain-Containing Protein 1
- CRC:
-
Colorectal Cancer
- ESCC:
-
Esophageal Squamous Cell Carcinoma
- HCC:
-
Hepatocellular Carcinoma
- IHC:
-
Immunohistochemistry
- HBE:
-
Human Bronchial Epithelial
- FBS:
-
Fetal Calf Serum
- DMEM:
-
Dulbecco’s Modified Eagle’s Medium
- NC:
-
Negative Control
- PC:
-
Plasmid Ccontrol
- GAPDH:
-
Glyceraldehyde-3-Phosphate Dehydrogenase
- CCK-8:
-
Cell Counting Kit-8
- MMP-9:
-
Matrix Metalloproteinase-9
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Chen, P., Liu, Y., Wen, Y. & Zhou, C. Non-small cell lung cancer in China. Cancer Commun. (Lond). 42, 937–970 (2022).
Schimizzi, G. V. & Longmore, G. D. Ajuba proteins. Curr. Biol. 25, R445–446 (2015).
Schleicher, K. & Schramek, D. A. J. U. B. A. A regulator of epidermal homeostasis and cancer. Exp. Dermatol. 30, 546–559 (2021).
Song, N. et al. The LIM protein AJUBA is a potential oncogenic target and prognostic marker in human Cancer via Pan-Cancer analysis. Front. Cell. Dev. Biol. 10, 921897 (2022).
Dommann, N. et al. The LIM Protein Ajuba Augments Tumor Metastasis in Colon Cancer. Cancers (Basel) 12, 1913 (2020).
Jia, H. et al. The LIM protein AJUBA promotes colorectal cancer cell survival through suppression of JAK1/STAT1/IFIT2 network. Oncogene 36, 2655–2666 (2017).
Xu, B. et al. The LIM protein Ajuba recruits DBC1 and CBP/p300 to acetylate ERalpha and enhances ERalpha target gene expression in breast cancer cells. Nucleic Acids Res. 47, 2322–2335 (2019).
Li, X. et al. ,. Ajuba Overexpression Promotes Breast Cancer Chemoresistance and Glucose Uptake through TAZ-GLUT3/Survivin Pathway. Biomed Res Int 3321409 (2022). (2022).
Li, H. et al. Ajuba overexpression regulates mitochondrial potential and glucose uptake through YAP/Bcl-xL/GLUT1 in human gastric cancer. Gene 693, 16–24 (2019).
Shi, X. et al. AJUBA promotes the migration and invasion of esophageal squamous cell carcinoma cells through upregulation of MMP10 and MMP13 expression. Oncotarget 7, 36407–36418 (2016).
Zhang, C. et al. Super-enhancer-driven AJUBA is activated by TCF4 and involved in epithelial-mesenchymal transition in the progression of hepatocellular carcinoma. Theranostics 10, 9066–9082 (2020).
Choi, K. H., Shin, C. H., Lee, W. J., Ji, H. & Kim, H. H. Dual-strand tumor suppressor miR-193b-3p and – 5p inhibit malignant phenotypes of lung cancer by suppressing their common targets. Biosci. Rep. 39, BSR20190634 (2019).
Jung, Y. S. & Park, J. I. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond beta-catenin and the destruction complex. Exp. Mol. Med. 52, 183–191 (2020).
Haraguchi, K. et al. Ajuba negatively regulates the Wnt signaling pathway by promoting GSK-3beta-mediated phosphorylation of beta-catenin. Oncogene 27, 274–284 (2008).
Liu, M. et al. Ajuba inhibits hepatocellular carcinoma cell growth via targeting of beta-catenin and YAP signaling and is regulated by E3 ligase Hakai through neddylation. J. Exp. Clin. Cancer Res. 37, 165 (2018).
Nagarajan, D., Melo, T., Deng, Z., Almeida, C. & Zhao, W. ERK/GSK3beta/Snail signaling mediates radiation-induced alveolar epithelial-to-mesenchymal transition. Free Radic Biol. Med. 52, 983–992 (2012).
Ding, Q. et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol. Cell. 19, 159–170 (2005).
Jurisic, V. et al. ,. EGFR Polymorphism and Survival of NSCLC Patients Treated with TKIs: A Systematic Review and Meta-Analysis. J Oncol 1973241 (2020). (2020).
Lei, Q. Y. et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the Hippo pathway. Mol. Cell. Biol. 28, 2426–2436 (2008).
Du, P. et al. Comprehensive genomic analysis of oesophageal squamous cell carcinoma reveals clinical relevance. Sci. Rep. 7, 15324 (2017).
Yang, X. et al. MicroRNA-92a as a potential biomarker in diagnosis of colorectal cancer: a systematic review and meta-analysis. PLoS One. 9, e88745 (2014).
Wang, X., Chen, Y., Liu, W., Liu, T. & Sun, D. Hsa_circ_0128846 promotes tumorigenesis of colorectal cancer by sponging hsa-miR-1184 and releasing AJUBA and inactivating Hippo/YAP signalling. J. Cell. Mol. Med. 24, 9908–9924 (2020).
Yang, D. et al. Smad1 promotes colorectal cancer cell migration through Ajuba transactivation. Oncotarget 8, 110415–110425 (2017).
Marie, H. et al. The LIM protein Ajuba is recruited to cadherin-dependent cell junctions through an association with alpha-catenin. J. Biol. Chem. 278, 1220–1228 (2003).
Zhang, B. et al. The LIM protein Ajuba/SP1 complex forms a feed forward loop to induce SP1 target genes and promote pancreatic cancer cell proliferation. J. Exp. Clin. Cancer Res. 38, 205 (2019).
Ferrand, A., Chevrier, V., Chauvin, J. P. & Birnbaum, D. Ajuba: a new microtubule-associated protein that interacts with BUBR1 and Aurora B at kinetochores in metaphase. Biol. Cell. 101, 221–235 (2009).
Singh, M., Yelle, N., Venugopal, C. & Singh, S. K. EMT: mechanisms and therapeutic implications. Pharmacol. Ther. 182, 80–94 (2018).
Yang, Y. F. et al. A positive feedback loop of IL-17B-IL-17RB activates ERK/beta-catenin to promote lung cancer metastasis. Cancer Lett. 422, 44–55 (2018).
Wu, Z. et al. WT1-interacting protein inhibits cell proliferation and tumorigenicity in non-small-cell lung cancer via the AKT/FOXO1 axis. Mol. Oncol. 13, 1059–1074 (2019).
Sharp, T. V. et al. The chromosome 3p21.3-encoded gene, LIMD1, is a critical tumor suppressor involved in human lung cancer development. Proc. Natl. Acad. Sci. U S A. 105, 19932–19937 (2008).
Li, W. H. et al. PLOD3 regulates the expression of YAP1 to affect the progression of non-small cell lung cancer via the PKCdelta/CDK1/LIMD1 signaling pathway. Lab. Invest. 102, 440–451 (2022).
Loganathan, S. K. et al. Rare driver mutations in head and neck squamous cell carcinomas converge on NOTCH signaling. Science 367, 1264–1269 (2020).
Qu, L., Tian, Y., Wang, F. & Li, Z. NOVA1 promotes NSCLC proliferation and invasion by activating Wnt/beta-catenin signaling. BMC Cancer. 22, 1091 (2022).
Acknowledgements
We promise the manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal.
Author information
Authors and Affiliations
Contributions
Lianyue Qu: Methodology, Data curation, Investigation, Visualization. Fan Wang: Visualization, Resources, Writing. Yuxiang Wang: Methodology, Software, Data curation. Zixuan Li: Conceptualization, Project administration, Writing – review &editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qu, L., Wang, F., Wang, Y. et al. AJUBA promotes the proliferation, invasion and migration of NSCLC cells by activating the ERK/β-catenin pathway. Sci Rep 15, 13123 (2025). https://doi.org/10.1038/s41598-025-98156-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98156-z