Abstract
Primordial germ cells (PGCs) are vital for producing sperm and eggs and are crucial for conserving chicken germplasm and creating genetically modified chickens. However, efforts to use PGCs for preserving native chicken germplasm and genetic modification via CRISPR/Cas9 are limited. Here we show that we established 289 PGC lines from eight Chinese chicken populations with an 81.6% success rate. We regenerated Piao chickens by repropagating cryopreserved PGCs and transplanting them into recipient chickens, achieving a 12.7% efficiency rate. These regenerated chickens carried mitochondrial DNA from female donor PGC and the rumplessness mutation from both male and female donors. Additionally, we created the TYRP1 (tyrosinase-related protein 1) knockout (KO) PGC lines via CRISPR/Cas9. Transplanting KO cells into male recipients and mating them with wild-type hens produced four TYRP1 KO chickens with brown plumage due to reduced eumelanin production. Our work demonstrates efficient PGC culture, cryopreservation, regeneration, and gene editing in chickens.
Similar content being viewed by others
Introduction
Domestic chickens have the widest geographic distribution among poultry. They contribute to approximately 90% of global poultry meat production and 93% of egg production1. This species was originally domesticated from the red jungle fowl2. Currently, there are more than 1600 chicken breeds worldwide, with indigenous chickens accounting for up to 80% of poultry populations in developing countries like Africa and Asia1.
These indigenous chickens typically produce lower quantities of eggs and meat than commercially improved breeds due to limited artificial selection. However, their genetic evolution and adaptation to local environments, shaped by long-term natural selection under diverse conditions, make them valuable for future breeding programs. Unfortunately, their population size and original genetic characteristics are rapidly declining due to infectious diseases and other factors3,4,5. Furthermore, the conservation of poultry genetic resources involves the management of live birds, which poses risks such as disease outbreaks, genetic complications, disasters, and management errors6,7,8.
Yunnan is a multi-ethnic province in southwest of China. It borders the provinces of Sichuan, Tibet, Guizhou and Guangxi, as well as the Southeast Asian countries of Myanmar, Laos and Vietnam. It is famous for the habitat of Red Junglefowl (Gallus gallus spadiceus) which is an ancestral subspecies of domestic chicken and other more than 20 breeds of chicken9,10. The native chicken breeds Chahua, Piao, Wuding, and Xichou black-bone are renowned for their high-quality egg and meat production. The Chahua is a small-sized, primitive breed with a phenotype similar to that of the Red Junglefowl. Contrarily, the Piao, Wuding, and Xichou black-bone breeds are medium to large-sized dual-purpose breeds. Nevertheless, a phenotypic diversity is observed within these three populations, including plumage colors, leg feathers, and black skin (fiblomelanosis, Fm)9,10. Whilst, Piao chickens exhibit a unique genetic mutation known as rumplessness (Rp), which is characterized by the absence of a tailbone11, and is also well known in Araucana chickens12. The responsible gene has not been identified in Araucana Rp mutations, only candidate regions have been reported. However, the Rp mutation in Piao has recently been identified12,13. In this study, the mutant traits including Rp and Fm as well as polydactyly (Po), and crest (Cr) mutations from recipient-crossbred chickens (PCM, Polish × Chahua derived crossbred), which being autosomal dominant, and easily phenotypically distinguishable, and thus were used as a phenotypic marker for identifying the mutations derived from donor PGCs or recipient chickens.
Chickens play a critical role in research as model organisms, representing both avian and non-avian species. Chickens serve as an important evolutionary link, bridging the gap between mammals and other vertebrates. This makes them a valuable comparative model for large-scale studies of gene function in vertebrates14. In addition, chickens are commonly used as a representative model for more than 10,000 bird species15,16. Chickens, with many naturally occurring genetic mutant lines, can provide insights into bird-specific traits as well as traits shared with other animals, aiding in gene identification and functional elucidation. Currently, over 250 Mendelian traits in chickens are listed in OMIA. However, compared to other animals, there are fewer reported mutant traits in chickens. For more than half of these traits, no causative genes or mutations have been identified. Chickens, with many naturally occurring genetic mutant lines often either endangered or already extinct, making their use challenging for academic purposes.
Currently, several different germplasm preservation methods are used to preserve poultry gametes, including semen, PGCs, somatic cells, and gonads, which are used to preserve poultry gametes17. However, the effectiveness of semen freezing is not universally guaranteed due to factors such as breed, age, and preservation techniques18,19. In addition, sperm cannot preserve the W chromosome due to female heterogametic sex and maternal mitochondrial DNA inheritance7,8,20. In addition, the presence of yolk-laden structures in eggs also limits the cryopreservation of poultry oocytes or fertilized eggs21. PGCs play a critical role as the exclusive progenitors of sperm and eggs in developing embryos. They possess unique genetic programs designed to ensure the safe transfer of genetic material to the next generation, thereby facilitating the preservation of both male and female genes through sexual reproduction22,23.
As a result, preservation of PGCs in chickens appears to be the most promising approach for germplasm conservation and production of genetically modified chickens. Therefore, in addition to in vivo conservation, PGC-based germplasm cryopreservation in poultry is highly beneficial. Successful cryobanking of PGCs will ensure the long-term conservation of poultry genetic resources, including commercial, indigenous and experimental lines. In addition, the future application of genome editing by PGCs for the creation of novel chicken mutation models and improvement of indigenous chickens is also essential.
van de Lavoir et al.24 first developed a long-term in vitro culture system for chicken germinal crescent PGCs using feeder cells to maintain their commitment to the germ cell lineage. They also successfully cryopreserved these cultured PGCs using conventional techniques. Subsequently, other researchers further improved this method by establishing a feeder-free in vitro culture system25,26. Currently, it is possible to culture PGCs from single embryonic blood or gonads, and cryopreserved PGCs can also be reintroduced into the circulatory system of recipient embryos. These technologies can be used for the conservation of genetic resources in native chickens and for genetic modification. However, there have been relatively few practical conservation initiatives using PGCs for germplasm conservation of indigenous or rare chicken breeds27,28,29. In addition, research on the production of genetically modified chickens by PGCs using CRISPR/Cas9 and TALEN remains relatively limited30,31,32,33,34,35,36.
In this study, we cryopreserved the germplasm of indigenous Chinese chickens by establishing PGC lines from blood samples obtained from single embryos and successfully regenerated rare indigenous Piao chickens derived from donor PGCs by mating only recipient chickens. In addition, we demonstrated that TYRP1 KO Chahua chickens can be generated from recipient chickens using TYRP1-KO PGCs modified with the CRISPR/Cas9 system. Our research highlights the practicality of germplasm banking with PGCs and the potential of CRISPR/Cas9-mediated gene editing in chickens. The use of PGCs for chicken germplasm conservation may enhance sustainable poultry resources and enable the production of genetically modified chickens for a wider range of biomedical research applications.
Results
Propagation of PGCs through in vitro culture and cryobanking for native chicken breeds
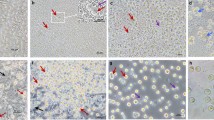
First, we conducted a preliminary experiment to evaluate the efficiency of PGC derivation from single embryonic blood samples. We made slight modifications to the culture medium, the volume of the embryonic blood sample, and the culture conditions based on the protocol of Whyte et al.25. Specifically, we adjusted the serum concentration in the medium from 0.2% to 0.4%, used a minimum of 2 μL of embryonic blood sample, and incubated at 37.8 °C, 5% CO2, and approximately 11–13% O2. Although the oxygen concentration remained unadjusted, the oxygen levels in the incubator consistently measured between 11 and 13% throughout the year, owing to the high altitude of our location. In a preliminary experiment, a total of 61 embryonic blood samples were collected at developmental stages HH13–1637 from four chicken populations: Chahua (YAU), Wuding, Xichou black bone, and PCM (crossbred, Polish × Chahua derived) (Table 1). We successfully established 45 cell lines with a derivation rate of 73.8% (45/61), including 28 males and 17 females identified by PCR sexing (Fig. 1D). Initially, blood cells dominated the culture, but by the 5th day, PGCs were observed and confirmed to proliferate. The blood cells gradually contracted and lysed, allowing them to be removed with each medium change (Fig. 1A). To reach sufficient cell numbers ( > 1.0 × 105) for cryopreservation, we expanded the cells for 15–63 days, with an average of 31.2 days to achieve the needed cell expansion (Fig. 1B). The average cell number and survival rate at cryopreservation were 3.9 × 105 cells and 86.6%, respectively (Table 1 and Fig. 1B). The mean doubling times for male PGC lines and female PGC lines, excluding outliers, were 41.5 ± 18.2 h and 43.9 ± 28.0 h, respectively. The median doubling time for males was 37.4 h and for females 30.7 h. There was no significant difference (P < 0.698) in doubling time between the male PGC line and the female PGC line (Fig. 1E–G and supplementary Data 1).
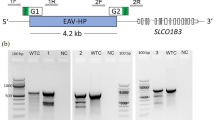
A Establishment of PGC lines from single embryo blood samples. (a) HH13–16 embryo blood samples were collected from the marginal vein near the red arrows. (b) Blood cells are predominant on days 0–5 of culture; the identification of PGCs may be theoretically possible. However, due to the predominance of blood cells, their identification is not easy. (c, d) PGCs indicated by blue and red arrows and division of PGCs were observed around day 5 of culture. PGCs and their proliferation can be observed with the contraction and lysis of blood cells until around day 5 of culture. (e, f) Predominance of embryonic blood-derived PGCs on days 12–14 of culture. Most cultured PGCs retain the morphology of typical chicken PGCs. The cultured PGCs exhibited a round shape with a diameter of 15–20 µm and contained characteristic cytoplasmic lipid vacuoles, consistent with the characteristics of PGCs well described in other reports. The scale bar represents 20 µm. B, C Distribution of culture days and expanded cell numbers. Distribution of days to reach >1.0 × 105 total PGCs in male and female PGCs for four populations (Chahua (YAU), Wuding, Xichou black bone, and PCM) in preliminary experiments. B and for two breeds (Chahua and Piao) (C). D Molecular sexing. Amplicon sizes for CHD1-Z and CHD1-W were 568 bp and 422 bp, respectively. Males have a band size of 568 bp. Females have bands at both 568 bp and 422 bp. The image shows the sexing of Piao embryos using crude DNA lysed whole embryo samples collected after blood collection for cell culture. E–G Measurement of doubling times (DTs) in PGC lines. The DTs were calculated from two cell counts taken during medium changes every other day. E DTs of 7 female PGC lines from 3 breeds: Chahua (C), Wuding (W), and Xichou black bone (X). F DTs of 25 male PGC lines from 4 breeds: Chahua (C), Wuding (W), Xichou black bone (X), and PCM (P). Median DTs for male and female PGCs are indicated by the blue horizontal line. G Non-parametric analysis using the Mann-Whitney U test with median DTs for male and female PGC lines. There was no significant difference between the DTs of male and female PGC lines (P < 0.698). The horizontal lines in the graph represent the median DTs, and the crosses represent the mean DTs (male: 41.5 ± 18.2 h, female: 43.9 ± 28.0 h).
To advance from preliminary experiments to practical implementation of PGC-based germplasm cryobanking, a total of 578 eggs were collected from heritage populations of two indigenous breeds, Chahua and Piao. This included 360 eggs from three Chahua populations (A, C, and D), and 218 eggs from one Piao population (Table 2). A total of 293 embryo blood samples (excluding unfertilized eggs, and fertilized eggs with early embryonic death) were collected from Chahua (211/360, 58.6%), and Piao (82/218, 37.6%). A total of 244 PGC lines were successfully established from these two breeds with a derivation rate of 83.3% (244/293), including 158 male and 86 female PGCs (Table 2). In the Chahua populations, the derivation efficiencies were as follows: 91.5% for population A (46 males and 19 females), 76.6% for population C (43 males and 16 females), and 82.5% for population D (36 males and 16 females). The Piao population had an efficiency of 82.9% (33 males and 35 females) (Table 2). The average culture duration was 26.3 days, with an average cell number of 2.8 × 105 and an average cell viability of 88.6% (Table 2 and Fig. 1C). The fastest growing male PGC line reached 3.0 × 105 cells in 11 days, while the slowest line took 52 days to reach 1.0 × 105 cells. Among the female PGC lines, the fastest growing lines reached 2.6 × 105 and 2.8 × 105 cells in 16 days, while the slowest line took 68 days to reach 1.0 × 105 cells (Fig. 1C).
Overall, we successfully established a sufficient number of PGC lines using a defined culture medium to enable PGC-based cryobanking for indigenous Chinese chicken breeds. In eight populations, including the preliminary experiment, the overall derivation efficiency was 81.6% (289/354). For males, the efficiency was 93.9% (186/198), while for females, it was 66.0% (103/156) (Tables 1, 2). PGC proliferation efficiency tended to be higher in both males and females when a sufficient amount ( > 2 μL) of embryonic blood collected at the appropriate developmental stage (HH14–15) was used.
Characterization and germline transmission of cultured PGCs
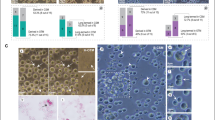
We first confirmed that cultured PGCs are round in shape, measuring 15 to 20 μm in diameter, and contain a characteristic cytoplasmic lipid vacuole, as previously reported38,39 (Fig. 2A). The immunohistochemistry results shown in Fig. 2B revealed the presence of stage-specific embryonic antigen-1 (SSEA-1), a pluripotent marker, on the surface of these cultured PGCs. In Fig. 2C, the pan-germ cell markers CVH and cDAZL were detected only in cultured PGCs by RT-PCR, and the housekeeping gene ACTB was detected in both cultured PGCs and chicken embryonic fibroblasts (CEFs), indicating that cultured PGCs are maintained as undifferentiated germ cells. To assess their ability to migrate to recipient gonads, we used transiently PKH26-labeled PGCs in a gonadal migration assay (Fig. 2D–G). We injected the PKH26-labeled PGCs into 27 recipient embryos (Chahua, YAU or PCM) and harvested whole gonads from 17 embryos at stage HH30–32. Surviving 17 recipient embryos (16/17, 94%) showed PKH26-labeled cells (Fig. 2F, G). These results suggested that cultured PGCs maintain their characteristics and efficiently colonize the gonads of recipient embryos.
A Morphologic characteristics of cultured PGCs. Cultured PGCs showed distinct features, including large nuclei and the presence of abundant lipids in their cytoplasm. Scale bars represent 20 μm. B Immunohistochemistry for pan-germ cell markers in PGC lines. (a) nuclear staining (DAPI), (b) SSEA-1, (c) merged image of SSEA-1 and DAPI. SSEA-1 was predominantly localized to the cell membrane. Scale bars represent 50 µm. C RT-PCR analysis of germ cell-related genes in cultured PGCs. RT-PCR was performed on cDNA samples from cultured PGCs to demonstrate the expression of pan-germ cell-related genes, CVH and DAZL. CEF, chicken embryonic fibroblasts; NC, negative control (H2O). D, G Gonadal migration assay of cultured PGCs into recipient gonads. Cultured PGCs (D) were transiently fluorescent labeled with PKH26 and transplanted into HH14–16 recipient embryos. Scale bars represent 50 μm. Manipulated embryos were cultured in host eggshells until day 7–8 (HH30–32), and recipient gonads, shown as yellow dashed circles, were collected (E). The female recipient embryo is on the left and the male recipient embryo is on the right. The PKH26-labeled PGCs (red cells) showed successful migration into the recipient gonads (F–G). D (left), E, F (left) and G (left) were photographed under bright light, while panels (D) (right), (F) (right) and (G) (right) were photographed under UV-light. The fluorescently labeled PGCs (red cells) were observed in the gonads of both the male and female recipient embryos. Scale bars represent 100 μm.
Generation of germline chimeras
To generate germline chimeras, we used two Piao PGC lines (male #P28 and female #P52) cryopreserved in liquid nitrogen for three months as donor PGCs. These expanded PGCs were injected into a total of 505 recipient embryos (Chahua, YAU). Of these embryos, 193 (38.2%) developed to the 18th day of incubation, and 82 (16.2%) hatched successfully. Of these 82 chicks, we raised 19 males and 19 females. Nine males and six females reached early sexual maturity and were used for mating experiments in Progeny Test I and II (Tables 3, 4). In all semen samples from nine male recipients, we detected a 558-bp amplicon specific for the fibromelanosis (Fm, black skin) mutation from donor Piao PGCs by PCR amplification (Supplementary Fig. 3A), confirming the presence of donor germ cell-derived sperm (Supplementary Fig. 3B). The same 558 bp amplicon was also found in DNA samples from the gonads of 14 recipient embryos (7 males and 7 females) that died before hatching (Supplementary Fig. 3C).
Regeneration of Piao chickens derived from donor PGCs
In Progeny Test I, using male recipients and Chahua hens, 8 out of 9 male recipients (germline chimeras) produced PGC-derived chicks (Fig. 3A). Efficiencies ranged from 0.3% to 80.2% (Table 3). These PGC-derived chicks were distinguishable from WT (recipient-derived) chicks by tailbone defects (rumplessness, Rp/rp+) and sometimes black skin (Fm/fm+) phenotypes (Fig. 4C, D).
A Classification of chicks from Progeny Test I (recipient Chahua♂ × Chahua WT♀). Male Chahua recipients (germline chimeras) carried sperm derived from a donor Piao PGC (#P28♂) with Rp/Rp Fm/fm+ e+/eb genotypes for three mutation traits, while the recipient Chahua males and WT Chahua hens had WT genotypes (rp+/rp+ fm+/fm+ e+/e+) for three traits. Thus, the hatched chicks in the Progeny Test I can be classified as either ♂recipient-derived chicks (Chahua♂ × Chahua♀) or ♂PGC-derived chicks (Piao♂#P28 × Chahua♀) based on the mutation phenotypes derived from the donor Piao PGC (♂#P28). In particular, the donor Piao PGCs possess homozygous genotypes for the autosomal dominant rumplessness (Rp) mutation, which results in tailbone defects. This is illustrated in Fig. 3D below. Thus, the donor Piao ♂ PGC-derived chicks (Rp/rp+) exhibit a tailbone defect phenotype. This can be identified based on the observation of the phenotype and palpation. In addition, some Piao ♂ PGC-derived chicks exhibit black skin (Fm/fm+), which results in hyperpigmentation of the skin due to the presence of the autosomal dominant fibromelanosis (Fm) mutation in a heterozygous state. This is illustrated in Fig. 3C below. Furthermore, plumage colors such as extended black (E locus, MC1R) were occasionally used for phenotyping and genotyping purposes, as the donor ♂PGCs carrying an eb allele as heterozygous genotypes (e+/eb). However, the eb allele is almost recessive for the WT e+ allele. B Classification of chicks from Progeny Test II (recipient Chahua♂ × recipient Chahua♀). As mentioned above, the six recipient Chahua hens used for mating also produce oocytes from donor Piao females PGC (#P28♀) with the Rp/Rp Fm/Fm eb/eb genotypes for the three mutation traits, similar to the recipient Chahua males. However, the male and female Chahua recipients themselves have the three WT genotypes (rp+/rp+ fm+/fm+ e+/e+). Thus, the chicks hatched in Progeny Test II can be theoretically divided into four subgroups. These include recipient-derived chicks (Chahua♂ × Chahua♀), ♂PGC-derived chicks (Piao♂#P28 × Chahua♀), ♀PGC-derived chicks (Chahua♂ × Piao♀#P52), and ♂♀PGC-derived chicks (Piao♂#P28 × Piao♀#P52). However, since the Rp and Fm traits are autosomal dominant traits, hatched chicks can be roughly classified into either ♂♀recipient-derived chicks or donor PGC-derived chicks based on the phenotypes of the three mutant traits (Rp, Fm, and eb). However, some chicks (Piao chicks) derived from male and female donor PGCs (Piao♂#P28 × Piao♀#P52) exhibit dark brown chick down coloration. These chicks are homozygous (eb/eb) for the eb allele derived from male and female donor Piao PGCs. This coloration may also be influenced by other feather color loci, but it can be distinguished from the other three subgroups. However, subsequent classification into three donor PGC-derived subgroups (♂PGC-derived, ♀PGC-derived, and ♂♀PGC-derived chicks) was finally conducted based on genetic diagnosis of mtDNA and Rp mutations (see Supplementary Table 4, Fig. 4). C WT chicks (fm+/fm+) derived from ♂♀ Chahua recipients (left) and donor Piao PGC-derived chicks (Fm/-) with the Fm mutation (right). It was assumed that the donor PGCs were heterozygous (Fm/fm+) for the Fm mutation in the male donor PGC (#P28) and homozygous (Fm/Fm) in the female donor PGC (#P52). However, the recipient Chahua was WT (fm + /fm+ ) in both sexes. It can thus be observed that a significant proportion of the donor Piao PGC-derived chicks in both Progeny Tests exhibited black skin due to excessive melanin deposition, as indicated by the yellow dashed circles. In Progeny Test I, the donor male PGC (#P28)-derived chicks were segregated into two phenotypic groups: one with a normal skin color (fm+/fm+) and one with a black skin color (Fm/fm+). In contrast, all of the donor ♀ PGC (Chahua♂ × Piao♀#P52) and ♂♀PGC (Piao♂#P28 × Piao♀#P52) derived chicks in the Progeny Test II exhibited blackish or black skin coloration, indicating the presence of either the Fm/Fm or the Fm/ fm+ genotypes. Individual differences in melanin deposition were observed in the Fm chicks, which may be attributed to the influence of Fm genotypes. D WT chicks (rp+/rp+) derived from ♂♀Chahua recipients (left) and donor Piao PGC-derived chicks (Rp/-) with the Rp mutation (right). The male (♂#P28) and female (♀#P52) donor Piao PGCs were homozygous for the Rp mutation (Rp/Rp). In contrast, both the male and female Chahua recipients exhibited WT genotypes (rp+/rp+). All donor Piao PGC-derived chicks (Rp/Rp or Rp/rp+) exhibited a tailbone defect in Progeny Test I and II, as indicated by a red dashed circle.
A Schematic representation of the genomic regions containing the IRX1 and IRX2 genes located on chromosome 2, candidate or causal mutation regions for Rp mutations in two different breeds (Araucana and Piao), and markers generated within the two regions. Three sequencing markers, indicated by the red or black circles, developed within the candidate region (125,146 bp) of the Araucana Rp mutation reported by refs. 11,12 were used to compare the sequences of donor Piao PGCs and Chahua recipients. Donor Piao PGCs (△TGAC/△TGAC) can be distinguished from recipient Chahua chickens (TGAC/TGAC) by the TGAC deletion at position 86,575,188–86,575,191 within RpSQ5. The causative mutation (4186 bp deletion) of the Piao Rp mutation reported by ref. 12 was detected by a PCR-based genotyping marker. The amplicon size of the multiplexed PCR using three primers was 548 bp in the Piao Rp allele and 431 bp in the recipient WT (rp+) allele. B Detection of the Piao Rp mutation for 25 PGC-derived chicks with mtDNA haplotype B from Progeny Test II. The two donors Piao PGCs (#P28, #P52) were homozygous (Rp/Rp) genotype (548 bp/548 bp). The recipient chickens were WT (rp+/rp+) genotype (431 bp/431 bp). The 25 chicks were divided into two groups: 17 homozygous (left, Rp/Rp, 548 bp/548 bp) and 8 heterozygous (right, Rp/rp+, 548 bp/431 bp). The 17 homozygous chicks were pure Piao chicks, resulting from the combination of male and female donor Piao PGCs. C, D Regenerated pure Piao chickens and their second generation. C The first generation (G1) of pure Piao chickens derived from donor Piao PGCs, regenerated by mating between recipient parents, consisting of four adult females (top picture) and four adult males (bottom picture). D The second generation (G2) of regenerated Piao chickens, with newly hatched chicks on the left and 2-month-old individuals on the right. These chickens were produced by mating the G1 parents.
We then conducted Progeny Test II by mating the male and female recipients to regenerate PGC-derived Piao chickens (Supplementary Fig. 3D). Two high-performing recipient (germline chimeric) males (#2610 and #2616) were mated with six recipient (putative germline chimeric) females (Table 4). A total of 134 chicks hatched, of which 94 were expected to belong to the donor male PGC-derived, the donor female PGC-derived, or both male and female donor PGCs-derived chicks. This was due to the presence of tailbone defects, and occasional black skin mutant trait (Fig. 3B and Table 4). For further categorization, we compared the mitochondrial D-loop haplotypes of the 94 chicks, donor PGCs, and recipient chickens (Supplementary Table 4). The 94 chicks could be divided into two groups: 69 with the recipient hen haplotypes (C, D, or E) and 25 with haplotype B from the donor female PGCs (♀#P52), with no haplotype A from the donor male PGCs (♂#P28) detected (Supplementary Fig. 3D and Supplementary Table 4).
We further categorized the 25 chicks with haplotype B by sequencing three markers in a candidate region (125146 bp) of the Rp locus on chromosome 2 (Fig. 4A). Only one marker (RpSQ5) differed between the two donor PGCs and eight recipients. The genotypes were TGAC/TGAC for the eight recipients and 4 bp-del/4 bp-del for the two donor PGCs. Of these 25 chicks, 17 had homozygous genotypes (△4 bp/△4 bp), indicating that they were Piao chicks derived from donor PGCs. The remaining eight chicks had heterozygous genotypes (TGAC/△4 bp), indicating that they were the offspring of a male recipient and a female donor PGC. Subsequently, Zhang et al. 13 reported that the causal mutation of Rp in Piao chickens resulted from a 4.1 kb deletion occurring upstream of the IRX1 gene on chromosome 2. Therefore, we regenotyped 25 chicks carrying haplotype B using a genotyping marker to identify this mutation (Fig. 4A, B). As a result, we confirmed that 17 chicks had a homozygous genotype (548 bp/548 bp, △4.1 kb/△4.1 kb), while 8 chicks had a heterozygous genotype (548 bp/431 bp, △4.1 kb/WT) (Fig. 4B). Furthermore, MC1R sequencing also confirmed that dark brown chicks are homozygous (eb/eb) for the eb allele from donor Piao PGCs at the extended black (E, MC1R) locus (Fig. 3B and Supplementary Table 5). In summary, we successfully regenerated full Piao chickens derived from donor PGCs with an efficiency of 12.7% (17/134) (Table 4).
The seventeen PGC-derived Piao chicks initially appeared healthy at hatching. However, approximately one week later, some experienced problems with defecation obstruction. Six of 17 (35.3%) chicks with severe defecation problems became weak and died within next few weeks, possibly due to morphological abnormalities in the periphaaca of the cloaca caused by the Rp mutation in Piao. In addition, three young chicks died within the first two months. The remaining eight reached sexual maturity and exhibited rumplessness, black or blackish skin (Fm/Fm or Fm/fm+), and plumage color similar to the original Piao chickens (Fig. 4C). These G1 hens produced twelve G2 chicks (Fig. 4D).
Generation of CRISPR/Cas9-induced TYRP1 KO chickens
A pool of Chahua PGCs (#CA10), comprising a mixture of 10 primary cultured PGC lines, was transfected with three sets of pX459 plasmid vectors (pX459-TYRP1-sgRNA#1/-#2, -#1/-#3, and -#1/-#4) (Fig. 5A). TYRP1-modified PGC lines were established by puromycin selection and proliferation for 2–4 weeks. Mutation induction efficiency was assessed by T7EI assay and Sanger sequencing. The PCR results for #1/#3 and #1/#4 were 371 bp in size, while those for the #1/#2 set were mostly approximately 300 bp (Fig. 5B). The T7E1 assay showed cleavage efficiencies of 16.8% for #1/#3, 74.7% for #1/#4, and 99.4% for #1/#2, including short products (Fig. 5B). In addition, analysis of 20 TA clones from #1/#2 revealed 73 bp deletions in 14 clones and 74 bp deletions in 6 clones (Fig. 5C). The TYRP1 KO PGC line (#CA10-TYRP1-KO) established with sgRNA#1/#2 plasmid vectors was used to generate TYRP1 KO chickens.
A Schematic representation of the TYRP1 gene structure on chromosome Z and the nucleotide sequence of the exon 2 target region, which contains four target sites (sgRNA#1–#4). The four sgRNA sequences and the protospacer adjacent motif (PAM) sequences are underlined in red and light green, respectively. The two black underlined primers were used to amplify the target region (371 bp). B PCR products for the exon 2 target region using each bulk DNA sample from TYRP1 modified PGC lines using three sets of sgRNAs (#1/#2, #1/#3, and #1/#4) (left), along with cleavage efficiency assessed by the T7E1 assay (right). C Sanger sequencing analysis of TA clones of the target region for the TYRP1 modified PGC line (#CA10_TYRP1-KO) using one sgRNA set (#1/#2). Sequence chromatograms and partial sequences show two representative CRISPR/Cas9-induced mutations: 73 bp deletion (frameshift mutation) (left) and 74 bp deletion (frameshift mutation) (right) in the target region. The sequences of the sgRNAs are underlined in red and the PAM sites are underlined in light green. Red arrows indicate induced mutations.
To generate TYRP1 KO Chahua chickens, KO PGCs were transplanted into PCM recipient embryos originating from a crossbreed (Polish and Chahua), two male recipients were obtained which were homozygous for the polydactyly (Po) mutation, and heterozygous for the crest (Cr) mutation (Fig. 6A and Supplementary Table 6). Mating these PCM males (#2588 and #2595) to Chahua (YAU) hens resulted in 166 chicks (Table 3). All 65 chicks from male recipient #2588 exhibited polydactyly (Po/po+) and occasional crest (Cr/cr+) phenotypes, not from donor KO PGCs (Fig. 6A and Table 3). Male recipient #2595 had 101 chicks, including four without Po and Cr mutations, with wild-type (WT) or brown chick-down color, confirming the donor TYRP1 KO Chahua PGC origin (Fig. 6A and Table 3). These four chicks had a 73 bp deletion in exon 2 of the TYRP1 gene (Fig. 6C, D). TYRP1 is located on the Z chromosome; males (Z/Z) with heterozygous bands (371 bp/298 bp) had a WT color, while hemizygous females (Z/W) with one band (298 bp/-) had a light brown color (Fig. 6A). The 73 bp deletion from c.81 to c.153 (genomic location 30,833,037–30,832,965) created a premature TGA stop codon at c.200–202 of the TYRP1 mRNA. This resulted in a truncated 129 bp mRNA, shorter than the 1611 bp WT mRNA (Fig. 6D). The mutant mRNA shared the first 27 amino acids of WT TYRP1 (NP990376) and encoded 15 different amino acids. Since frameshift mutations are usually null alleles, this 73 bp deletion was expected to result in loss of TYRP1 function (Fig. 6E).
A Classification of chicks from mating of male recipients (PCM) and WT Chahua (YAU) hens. Chicks derived from male PCM recipients show a polydactyly (Po/po+) and occasionally a crested (Cr/cr+) phenotype, as indicated by the white dashed circles and red arrows, whereas chicks derived from TYRP1 KO Chahua PGCs do not carry either mutation. Heterozygous KO males (Z+/ZTYRP1-73bp) show WT chick down coloration (left), and hemizygous KO females (ZTYRP1-73bp/W) show brown chick down coloration with reduced eumelanin production (right). B WT and TYRP1-KO Chahua chickens in the G2 generation. Adult homozygous KO males and hemizygous KO females with a 73 bp deletion (c, d in lower panels) exhibit a brown plumage color with reduced eumelanin, whereas WT chickens (a, b in upper panels) lacking the 73 bp deletion or heterozygous males with the deletion exhibit the WT plumage color. Individual feathers from KO chickens (right) show a significant reduction in the production of eumelanin compared to WT chickens (left). The black pigmented areas change to a reddish-gray color. Pheomelanin remains largely unaffected. C PCR-based genotyping of the 73 bp deletion in exon 2 of TYRP1. The exon 2 target region was amplified using primers FD and RV. WT chickens show a single 371 bp band in females and either a 371 bp or 371/298 bp heterozygous band in males. In contrast, individuals with brown plumage show a single band of 298 bp in both sexes (right panel). D Sequences of the exon 2 target region between WT and KO alleles. The sequence chromatogram shows the WT allele (top panel) and the KO allele with a 73 bp deletion (bottom panel). The two sgRNA sequences (sgRNA#1 and sgRNA#2) are underlined in red and the PAM sequences are underlined in green. The disrupted positions marked by the two red arrows in the WT sequence result in a KO allele with a 73 bp deletion mutation in the TYRP1 target region, leading to a TGA stop codon (highlighted by the red square) at c.200–202, resulting in a shorter mRNA of 129 bp compared to the WT mRNA of 1611 bp. E Predicted amino acid sequences of TYRP1 for WT and KO individuals. The WT TYRP1 protein consists of 536 residues. The predicted truncated TYRP1 protein from the KO allele with a 73 bp deletion consists of 42 residues.
In the next generation (G2), mating one G1 male (WT/△73 bp) to two G1 females (△73 bp/-) produced 40 chicks. Of these, 17 chicks had a WT plumage color, including 10 males (△73 bp/WT) and 7 females (WT/-) (Fig. 6B). The remaining 23 chicks had a brown chick-down color, including 13 males (△73 bp/△73 bp) and 10 females (△73 bp/-) (Fig. 6B). Progeny testing confirmed complete concordance between the 73 bp deletion mutation and the brown plumage color phenotype in all 40 chickens. The TYRP1 KO mutation was recessive to the WT allele. In conclusion, we successfully generated TYRP1-KO Chahua chickens with brown plumage color using CRISPR/Cas9 technology.
Off-target analysis
Four target sequences (sgRNA#1–#4) in exon 2 of TYRP1 showed no off-target candidates for the 12-mer or 20-mer + PAM motif (NGG) regions when analyzed using CRISPRdirect (http://crispr.dbcls.jp/) based on the chicken genome (ICGSC Gallus_gallus-4.0/galGal4) (Supplementary Table 2). We also confirmed the absence of potential off-target sequences for these four sequences using updated genome databases, including Cas-OFFinder (http://www.rgenome.net/cas-offinder/) (galGal5) and the Ensembl database (http://www.ensembl.org/index.html) (galGal6). However, even with these updates, we did not find any candidate sequences that matched potential off-targets. Nevertheless, for TYRP1-sgRNA#1 and TYRP1-sgRNA#2, we selected five potential off-target candidates each for the 8-mer + PAM motif from CRISPRdirect (Supplementary Table 3). Sanger sequence analysis of the 8 TA clones for each of these candidates revealed no mutations in the off-target regions (Supplementary Table 3).
Discussion
In this study, we demonstrated that PGC lines can be efficiently established and cryopreserved from single embryonic blood samples of indigenous Chinese chicken breeds by making minor adjustments in the amount of starter blood samples used for culture, medium composition, and culture conditions. The efficiency of PGC derivation, including both sexes, reached 81.6% (289/354), exceeding previous reports of 40–67% efficiency28,29,39,40. Specifically, the male PGC derivation rate reached a high efficiency of 93.9% (186/198), while the female PGC derivation rate reached 66.0% (103/156). The occurrence of cell clustering was minimal regardless of sex, with no significant difference in doubling time observed between males and females. However, as noted in previous reports28,29,39,40, a sex difference in derivation rate eventually emerged. We used significantly more blood samples from embryos at stages HH13–16 for primary culture compared to previous research. The total number of circulating PGCs in blood at stages HH13–16 varies and is typically estimated to be between 200 and 400, depending on breeds, individuals, sex, and developmental stage22,41,42,43,44,45. Samples with developmental delay (HH13) or advanced development (HH16+) tended to require longer cultivation times due to the lower blood volume and potentially lower density of PGCs, thus increasing the risk of culture failure. Primary cultures of female PGCs were more efficient at the optimal blood collection stage around HH14–15. Therefore, blood volume and blood collection stage are critical factors in PGC primary culture. Increasing the amount of blood used as a starter can shorten the PGC culture period and thus improve efficiency. In addition, we slightly increased the serum concentration from 0.2% to 0.4% in the medium composition. Woodcock et al.28 reported that replacing chicken serum with OT (ovotransferrin) significantly improved the derivation efficiency of female PGCs in primary culture, suggesting that serum components may negatively affect derivation rates. However, since serum components contain cytokines and growth factors essential for cell proliferation, a slight increase in serum, as indicated by our results, may be more effective.
Moreover, this study utilized self-prepared avian KO-DMEM as an alternative to custom-made avian KO-DMEM, following the protocol previously described by Idoko-Akoh and McGrew46. The custom-made avian KO-DMEM is devoid of calcium, as elevated calcium concentrations in the medium could impede the culture of female PGCs. However, the solution contains physiologically low levels of calcium (0.15 mM)25. Additionally, the solution is adjusted to an optimal osmolarity of 250 mOsmkg[−1 25. Conversely, while our avian KO-DMEM alternative is likely to be equivalent to the custom-made product, the precise values of osmotic pressure and calcium concentration remain unknown. Consequently, minor discrepancies in the characteristics of the commercial avian KO-DMEM and our avian KO-DMEM alternative may have some effect on the efficiency of PGC induction. Nevertheless, more detailed studies are still needed as we altered more than one parameter of the in vitro culture conditions.
Regarding the cultivation conditions, we maintained a temperature of 37.8 °C and 5% CO2 without adjusting the oxygen concentration. However, due to our high-altitude location, the oxygen concentration inside the incubator was maintained at 11–13% during PGC cultivation. This lower oxygen condition may also have contributed to the higher effects of PGC derivatives. Ezaki et al.47 achieved stable cultivation efficiency under conditions of 38 °C, 5% CO2, and 3% O2 by adding 1% serum to their custom medium. Therefore, future studies should investigate the effects of these factors on cultivation success rates and establish optimal conditions accordingly.
We successfully regenerated pure Piao chickens from male and female donor PGCs by mating only recipient parents, achieving an efficiency of 12.8% (17/134 chicks). The regenerated Piao chickens exhibited characteristics that were consistent with the original Piao chickens, such as plumage color, body shape, and the presence of a complete tailbone defect caused by the Rp mutation. We confirmed at the molecular level that the regenerated Piao chickens are homozygous for a specific 4.1 kb deletion, which is the causative mutation of the Piao Rp mutation, and that they carry the same mitochondrial D-loop haplotype as the female donor PGC. Initially, healthy Piao chicks (G1) developed fecal obstruction problems approximately one week after hatching. A proportion of them had severe defecation problems (6/17, 35.3%), which eventually led to death. Similar problems were observed in chicks from the original Piao population, suggesting an influence of the Rp mutation. Nevertheless, the surviving individuals reached sexual maturity, showed normal fertility and no abnormalities except those related to Rp. We were also able to obtain the G2 generation. However, the hatchability of the G2 generation (12/43, 27.9%) was even lower than that of the original population (9/20, 45%). This finding was consistent with the observations of Dunn and Landauer, who emphasized the significant effect of Rp homozygosity on hatchability and mortality48 Dunn and Landauer, along with Zwilling49, emphasized abnormalities in the muscles around the cloaca in fully Rp homozygous individuals, affecting defecation and resulting in mortality. We further examined the fixation of Rp mutations in the original population and found that the embryos used in PGC culture had incomplete fixation of the Rp mutation. Approximately 33% of the individuals were Rp heterozygous (13/39), and approximately 5% were WT (2/39). This incomplete fixation of the Rp mutation in the original Piao population may explain the disadvantages faced by Rp homozygous individuals in terms of hatchability and post-hatch survival. Therefore, PGC conservation is emerging as an effective approach to preserve rare breeds affected by genetic diseases, such as Piao chickens, while maintaining live bird populations.
In this study, we successfully generated TYRP1 KO Chahua chickens by genome editing of cultured PGCs using the CRISPR/Cas9 system. We were able to induce relatively large deletion mutations within the target region by using two sgRNAs, which facilitated genotyping. TYRP1 is a critical enzyme involved in melanin synthesis in vertebrates, and loss-of-function mutations in this gene result in a change from a black-based coat and feather color to brown or chocolate in vertebrates50,51,52,53. In humans, TYRP1 is known to be the causative gene for oculocutaneous albinism type 3 (OCA3)54,55. In poultry, natural mutations of TYRP1 in Japanese quail (Coturnix japonica) result in a brown plumage color in both males and females, referred to as “br” or “roux”56,57. Similarly, chocolate-colored mutant (choc) chickens transform the black (E)-based plumage color to chocolate and reduce eumelanin production as observed in our KO individuals58,59. In this study, we utilized PGCs from Chahua chickens with WT (e+) plumage color background similar to Red Junglefowl for genome editing. The TYRP1 KO Chahua birds exhibited a clear reduction in eumelanin production, resulting in brown plumage in both sexes. Additionally, the black parts of the individual feathers in both sexes also showed a reddish-gray color due to reduced eumelanin. However, pheomelanin production was largely unaffected. The KO chickens of TYRP1 located on the Z chromosome were confirmed to be recessive to the WT. The sexes of male and female chicks produced by mating KO homozygous males to WT females could be distinguished by the color of their down feathers. Female chicks (ZTYRP1-/W) have a brown color, while heterozygous males (Z/ZTYRP1-) have a down color typical of WT Chahua chicks. In addition, the survival and reproductive performance of the KO individuals were equivalent to those of the WT, suggesting the potential application to the sexing of male and female chicks, similar to the br mutant quail already used commercially, and may help to further promote the use of Chahua, which has high egg and meat quality.
Natural mutations in chickens can be valuable research models, but identifying causative genes generally requires generating reference families and genome-wide gene analysis60. CRISPR/Cas9-mediated genome editing of cultured PGCs offers a powerful tool to generate mutants for target and related genes, enabling a wide range of applications, including the production of beneficial proteins through the introduction of foreign genes and breeding improvements.
Conclusion
In conclusion, this study highlights the importance of germplasm conservation through PGC methods and CRISPR/Cas9 genome editing in biomedical research and genetic resource conservation. It is crucial to protect the genetic diversity of several indigenous chicken breeds in China through PGC conservation due to their declining population which will ensure their future use in sustainable poultry production and breeding improvement. In addition, the application of genome editing techniques facilitates the creation of genetically modified chickens, further enhancing the utility of chicken models for research purposes.
Methods
Fertilized eggs
Fertilized eggs were collected from four indigenous Chinese chicken breeds (Chahua-Yunnan Agricultural Uiversity colony (Chahua-YAU), Wuding, and Xichou black bone) and one crossbreed (PCM, Polish × Chahua derived). These fertilized eggs were used in preliminary experiments to confirm the culture conditions. In addition, four populations from two native breeds (Chahua and Piao) were used for practical conservation of genetic resources of rare indigenous Chinese chicken breeds based on PGC. Fertilized eggs were collected from three Chahua populations which were selected at the base of body weight (A, large; C, medium; and D, small), and from a Piao population in four batches from 2020 to 2021. These populations have been maintained as heritage populations ( > 1500 chickens/population) at an Indigenous Chicken Genetic Stock Farm in the Xishuangbanna Autonomous Region in Yunnan Province, China. PCM or Chahua (YAU) fertilized eggs were used as recipients for gonadal migration assays and germline chimera production. Animal care and all experimental procedures were approved by the Animal Care and Use Committee of Yunnan Agricultural University (approval code: YAUACUC01) and were performed in accordance with the Animal Experiment Regulations of Yunnan Agricultural University.
Isolation, culture and cryopreservation of PGCs
Fertilized eggs were incubated at 37.8 °C and 60–70% relative humidity for 55–60 h, resulting in embryonic development at stages HH14–1637. To isolate PGCs from blood, approximately 0.5–6 μL of embryonic blood was collected from the marginal vein of individual embryos using a fine glass micropipette (Ø 40 µm) (GD-1, Narishige Co., Tokyo, Japan) under a stereomicroscope (SMZ745; Nikon, Japan). The embryonic blood was washed with 300 µL of phosphate-buffered saline without Ca2+ and Mg2+ (PBS[-]) containing 1% penicillin-streptomycin. After centrifugation at 400 × g for 5 min, each sample was transferred to a well of a 48-well plate containing 300 µL of PGC culture medium. The PGC culture medium was prepared based on a composition previously defined by ref.25. Briefly, this medium consisted of avian KO-DMEM (equivalent to 250 mOsmkg-1 and calcium chloride free) supplemented with 1× GlutaMAX (Life Technologies, Carlsbad, CA, USA), 1× nonessential amino acids (Life Technologies), 1× EmbryoMax nucleosides (Merck Millipore, Darmstadt, Germany), 1.2 mM sodium pyruvate (Life Technologies), 0.1 mM β-mercaptoethanol (Life Technologies), 0.2% ovalbumin (Sigma-Aldrich, St. Louis, USA), 0.01% sodium heparin (Sigma-Aldrich), 4 ngmL−1 FGF2 (PeproTech, Rocky Hill, NJ, USA), and 25 ngmL−1 activin A (PeproTech). The self-made avian KO-DMEM was prepared as an alternative to the commercially available avian KO-DMEM (Life Technologies-ThermoFisher Scientific, #041-96570 M), in accordance with the protocol by Idoko-Akoh and McGrew46. Briefly, 50 ml of the avian KO-DMEM replacement were prepared by mixing the following components: 37.5 ml of calcium-free DMEM (Gibco-Life Technologies, 21068028), 11.6 ml of distilled water (Life Technologies), 0.5 ml of 50× MEM amino acids solution (Life Technologies), 0.5 ml of 100 mM sodium pyruvate (Life Technologies), and 0.5 ml of 100× MEM vitamin solution (Life technologies). To prepare the FAOTc medium, the basal medium was supplemented with 0.4% chicken serum (Sigma-Aldrich) and 10 µgml−1 conalbumin (ovotransferrin) (Sigma-Aldrich). A third of the culture medium was replaced every other day. PGCs were cultured at 37.8 °C and 5% CO2 for 2 to 10 weeks until the cell number exceeded 1 × 105 cells. The oxygen concentration was not adjusted, but the oxygen level in the incubator ranged from 11 to 13% due to the high altitude. PGCs were then cryopreserved after measuring cell viability using the trypan blue assay. The expanded PGCs were evenly divided into 1–3 polypropylene cryovials per PGC line and resuspended in freezing medium containing 5% dimethyl sulfoxide (DMSO) and 4% chicken serum. These cryovials were then frozen in a −80 °C freezer in a freezing container and stored in liquid nitrogen after freezing overnight. The established cell lines were then used as donor PGCs.
Sexing of samples
PCR-based sexing was performed using primers (CHD1-F and CHD1-R) for the CHD1 genes on chromosomes Z and W (Supplementary Table 1). Genomic DNA was prepared by lysing the whole embryos after blood collection using QuickExtract DNA Extraction Solution (Lucigen, Co., Middleton, WI, USA), or extracted from approximately 1.0 μL of blood by DNAZOL solution (Molecular Research Center, Cincinnati, USA), followed by PCR amplification in a 10 μL reaction mixture consisting of 1.0 μL template (approximately 100 ngμL−1), 0.01 μM of each primer, and 5 μL ES-Taq Master Mix (Tiangen Biotech, Co., Ltd., Beijing, China). The amplicon sizes for CHD1-Z and CHD1-W were 568 bp and 422 bp, respectively.
Immunofluorescence staining
For immunofluorescence analysis, cultured PGCs (1 × 105) were fixed with 4% paraformaldehyde (PFA) at RT for 10 min. After washing with PBS(-), the cells were permeabilized with 0.5% Triton-X100-PBS(-) solution for 5 min at RT. The fixed samples were washed with PBST (PBS(-) with 0.1% Tween 20) and incubated with 10% goat serum-PBST for 15 min at RT. The samples were then incubated overnight at 4 °C with a mouse monoclonal IgM antibody against SSEA-1 at a dilution of 1:150 (DSHB, IA, USA). The samples were then washed with PBST and incubated with a secondary antibody, Alexa Fluor 594 goat anti-mouse IgM (1:250; Invitrogen, Carlsbad, CA, USA), for 3 h at RT. The samples were then washed with PBS(-) and counterstained with DAPI (Prolong Diamond Antifade Mountant with DAPI, Invitrogen). Imaging was performed with a fluorescence microscope (Axio Vert. A1; Carl Zeiss Meditec AG, Jena, Germany).
RT-PCR
The expression of germ cell-specific genes (cDAZL, CVH) was evaluated by RT-PCR in cultured PGCs and control cells (CEFs, chicken embryonic fibroblasts). The CEFs were prepared as follows: The hindlimb of day 8 embryo was minced in PBS, and the samples were treated with 0.5% trypsin in PBS for 20 min at 38 °C. Subsequently, the samples were centrifuged at 1700 rpm for 5 min, the supernatant was drained, and cultured in medium containing 7.5% serum. The culture medium consisted of Dulbecco’s Modified Eagle Medium (DMEM; Gibco-Life Technologies, 11965092) supplemented with 1× GlutaMAX (Life Technologies), 1× non-essential amino acids (Life Technologies), 1× sodium pyruvate (Life Technologies), 1× penicillin-streptomycin (Life technologies), 1× MEM vitamins (Life technologies), 1× MEM amino acid (Life technologies), and 7.5% chicken serum (Sigma-Aldrich). The supernatant was replaced with a flesh medium every two days. At 70–80% confluency, the cells were treated with PBS supplemented with 0.25% trypsin for 5 min at 38°C. After several passages, the cells that exhibited characteristics consistent with fibroblasts were utilized as CEFs. Total RNA was extracted from the cells using TRIzol reagent (Invitrogen), and 1.0 μg of total RNA was reverse transcribed using the PrimeScript RT-PCR Kit (Takara, Otsu, Japan) with oligo dT primers. RT-PCR was performed in a 25 μL reaction containing 25 ng cDNA, 1× KOD FX buffer, 100 µM dNTPs, 0.75 μL of 10 μM forward and reverse primers (Supplementary Table 1), and 1 unit of KOD FX DNA polymerase (TOYOBO, Osaka, Japan). PCR cycles included an initial denaturation at 94 °C for 2 min, followed by 35 cycles of 98 °C for 10 s, 64 °C for 30 s, and 68 °C for 2 min, with a final extension at 68 °C for 7 min. The PCR products were electrophoresed on a 2.5% agarose gel.
Gonadal migration assay
Two Chahua male and female PGC lines, expanded after cryopreservation in liquid nitrogen, were used as donor cells to assess the migration and colonization ability of cultured PGCs within recipient gonads. The two PGC lines were mixed in equal proportions (7.5 × 105 cells per each line) to prepare a mixture of 1.5 × 106 cells. These PGCs were transiently labeled with the PKH26 Red Fluorescent Cell Linker Mini Kit (Sigma) according to the manufacturer’s instructions. Nevertheless, the volume of the reaction solution was reduced to 1/10 and a final concentration of 0.4 μM PKH26 dye was used. After counting these cells, the labeled cells were microinjected into the dorsal aorta of HH14–16 recipient embryos (Chahua, YAU, or PCM) in a final volume of 1 µL containing 1500–4500 cells. The engineered embryos were cultured to the HH30–32 stage using the surrogate eggshell culture system (System III)61. Whole gonads were then collected from the embryos, and PKH26-positive cells were identified by fluorescence microscopy.
Transplantation of donor PGCs into recipient embryos and generation of germline chimeras
Two Piao PGC lines, one male (#P28) and one female (#P52), cryopreserved for three months, were used as donors for transplantation into recipient embryos. These donor PGC lines were expanded and subcultured over a period of weeks to months during their use in transplantation experiments. The two PGC lines were mixed in approximately equal proportions, and a 1 µL volume of medium containing 5,800 to 16,000 cells was microinjected into the dorsal aorta of HH13–16 stage recipient embryos (Chahua, YAU). Most recipient embryos were used after reduction of blood containing endogenous PGCs as described in a previously reported method62. These embryos were cultured until hatching using surrogate eggshells as described above. The hatched recipient chicks were reared to sexual maturity. To determine whether the male recipients could produce donor-derived sperm, genomic DNA samples were extracted from semen and blood for detection of the Piao PGC-derived black skin (fibromelanosis, Fm) mutation using PCR. The PCR was performed using a primer pair (Fm_FD and Fm_RV) listed in Supplementary Table 1, which was specifically designed to target an inverted duplicated region near EDN3 on chromosome 20 that is unique to the Fm mutation, as described by Dorshorst et al.63 (Supplementary Fig. 3A). A 588 bp Fm-specific fragment was amplified in a 10 μL reaction volume containing 1.0 μL lysis sample, 0.01 μM of each primer, and 5 μL ES-Taq Master Mix (Tiangen Biotech).
Progeny testing
Two progeny tests, Progeny Test I using male recipients and Progeny Test II using recipients of both sexes, were performed (Fig. 3B, Supplementary Fig. 1A and Supplementary Fig. 2). In Progeny Test I, we performed mating between each male Chahua recipient and WT Chahua hens to assess whether male recipients that tested positive for Fm in sperm could actually produce offspring derived from donor PGCs (Supplementary Fig. 1A). In Progeny Test II, we mated two recipient Chahua males with high efficiency in producing donor-derived offspring with six recipient females to regenerate the original PGC-derived Piao chickens (Fig. 3B, Supplementary Fig. 1A and Supplementary Fig. 2).
In both progeny tests, we distinguished donor Piao PGC-derived chicks from recipient Chahua-derived chicks by phenotypic observations (Supplementary Table 6). Because both donor Piao PGCs were homozygous for the autosomal dominant rumplessness (Rp) mutation, which is characterized by the absence of free caudal vertebrae and uropygial glands, the donor PGC-derived chicks lacked tails (Rp/rp+), whereas recipient-derived chicks had normal tails (rp+/rp+). In addition, Piao donor PGCs had black skin (Fm/-) and a brown chick-down color (eb allele at the E locus, MC1R) mutations in at least one dose. Therefore, in some cases, we also used Fm and E mutations as phenotypic markers to identify donor PGC-derived chicks.
In Progeny Test II, donor Piao PGC-derived chicks whose phenotype was confirmed by the presence of Piao PGC-derived mutant traits, including the Rp mutation, were theoretically assumed to be the mixture of three subgroups (male Piao PGC-derived, female Piao PGC-derived, and both Piao PGC-derived). These chicks were further classified using Sanger sequencing, and PCR genotyping using genomic DNA and mitochondrial DNA (mtDNA). At first, these three subgroups were classified into two subgroups i.e. male Piao PGC-derived chicks and female Piao PGC-both Piao PGC-derived chicks at the base of sequence haplotypes of the D-loop region (1370 bp) in mtDNA. Subsequently, chicks from the female or both donor Piao PGCs were further classified by Sanger sequencing of markers strongly associated with the Rp mutation and genotyping of the Rp causative mutation. Initially, since the causative mutation of Rp had not been identified in the Piao chickens, we used a sequencing marker (RpSQ5: 863 bp) that could discriminate between donor Piao and recipient Chahua genotypes from three linkage markers designed within the Rp candidate region for Araucana chicken located between the IRX1 and IRX2 genes on chromosome 2, as reported by Freese et al.12. Since the causative mutation of Rp in Piao chickens was later identified as a 4.1 kb deletion located upstream of the IRX1 gene by Zhang et al.13, we genotyped the donor-derived chicks with newly designed PCR-based genotyping markers based on this information. The primers used for PCR amplification-sequencing are listed in Supplementary Table 1.
Establishment of TYRP1 gene knockout PGC lines
We selected the TYRP1 gene, located on chromosome Z, as the target gene for the generation of genetically modified chickens using PGCs (Supplementary Fig. 1B). We specifically targeted the second exon of the TYRP1 gene and selected four highly specific target sequences for sgRNAs (sgRNA#1 to -#4) with minimal off-target effects using CRISPRdirect (http://crispr.dbcls.jp/)64 (Supplementary Table 2 and Fig. 5A). These target sequences were individually inserted into the pSpCas9(BB)-2A-Puro plasmid (pX459; Addgene plasmid #48139). TYRP1-KO PGC lines were established using a mixture (#CA10) of primary cultured PGC lines, in which 10 male Chahua embryos (A population) were cultured for 44–47 days each. Each PGC line was adjusted to a total of 1.5 × 106 cells, and a mixture was prepared by equal aliquots from the ten PGC lines. Subsequently, 3.5 × 105 PGCs were transfected with 0.25 µg of each mixture of three sets of pX459 plasmid vectors (pX459-TYRP1-sgRNA#1/-#2, pX459-TYRP1-sgRNA#1/-#3, and pX459-TYRP1-sgRNA#1/-#4) using Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific, MA, USA). PGCs were suspended in Opti-MEM I medium (Thermo Fisher Scientific) mixed with plasmid vectors and transfection reagent for 4 h, centrifuged, resuspended in FAOTcs medium, and cultured at 37.8 °C for 24 h. To enrich the transfected PGCs, cells were exposed to 1 µgml-1 puromycin for 72 h and then expanded in FAOTcs medium for 2 to 4 weeks.
Genomic DNA samples from a subset of expanded PGCs were extracted using QuickExtract DNA Extraction Solution (Lucigen) to evaluate induced mutations and targeting efficiencies at the TYRP1 locus. CRISPR/Cas9-induced mutations within the TYRP1 target region were detected by PCR product size analysis, and a T7 endonuclease I (T7EI) assay was performed by agarose gel electrophoresis. To confirm the exact mutations, we sequenced 20 clones using the pMD19-T vector cloning kit (Takara). These primers for PCR amplification of the target region are listed in Supplementary Table 1. PCR amplification was performed using KOD FX DNA polymerase (TOYOBO) according to the manufacturer’s protocol. For the T7EI assay, 100 ng of PCR product was incubated with 10 units of T7 endonuclease I (Vazyme, Nanjing, China) in T7 endonuclease I reaction buffer for 1.5 h at 37 °C according to the manufacturer’s instructions. Electrophoresis of digested PCR products was performed on a 1.5% agarose gel, and targeting efficiencies were determined based on band intensities quantified using Image Lab5.0 software (Bio-Rad Laboratories, Hercules, CA, USA). Finally, we selected a TYRP1-targeted PGC line (#CA10-TYRP1-KO) with a 73 bp deletion (frameshift mutation) or a 74 bp deletion (frameshift mutation) within the target site using a set of plasmid vectors (pX459-TYRP1-sgRNA#1 and -#2).
Transplantation of TYRP1-KO PGCs into recipients
A total of 3,500–5,000 TYRP1-KO PGC cells were microinjected into the dorsal aorta of HH13–16 crossbred (PCM) recipient embryos using the protocol described above. These recipient embryos were then transferred to the System III shell of surrogate eggshell culture systems and incubated at 37.8 °C until hatching.
Progeny testing and screening of TYRP1 KO chickens
Hatched recipient chicks that were transplanted with a donor TYRP1 KO Chahua PGC line (#CA10-TYRP1-KO) were reared to sexual maturity. The putative germline chimeric males were then mated with WT Chahua (YAU) hens. The differentiation between chicks derived from donor KO PGCs and those from recipients was based on their phenotypic characteristics. Specifically, the recipient PCM males were homozygous for the polydactyly (Po) mutation, resulting in the formation of an extra digit on the feet. In contrast, the donor Chahua PGCs and the mating Chahua females were normal, without polydactyly (po+/po+) (Supplementary Fig. 1B and Supplementary Table 6). Consequently, chicks with normal digits (po+/po+) were expected to be the offspring of donor KO PGCs, while those with an extra digit (Po/po+) were determined to be the offspring of recipient males. In addition, since TYRP1 is a sex-linked gene located on the Z chromosome, we expected that female chicks (ZTYRP1-/W) with the TYRP1 mutation would have reduced eumelanin in their chick-down color compared to that in wild-type chicks. In contrast, male chicks (Z+/ZTYRP1-) with the TYRP1 mutation were expected to have the typical Chahua (wild-type) chick-down color. These phenotypes were finally confirmed by PCR-based genotyping and sequence analysis of the target region. The PCR fragment containing the two target sites was amplified and sequenced using the primer pair listed in Supplementary Table 1.
Off-target analysis
We selected four highly specific sgRNA sequences (sgRNA#1–#4) targeting TYRP1 exon 2 using CRISPRdirect (http://crispr.dbcls.jp/) based on the genome database (ICGSC Gallus_gallus-4.0/galGal4) (Supplementary Table 2). These sequences did not have any off-target candidates in the 12-mer and 20-mer+PAM motif (NGG) regions. However, as a precaution, we also searched for potential off-target sequences for TYRP1-sgRNA#1 and -#2 using the updated genome databases Cas-OFFinder (http://www.rgenome.net/cas-offinder/) (Galgal5) and Ensembl Database (http://www.ensembl.org/index.html) (Galgal6). No off-target candidates were found in either database. Nevertheless, we selected five candidates from each of the 8-mer + PAM motif regions using CRISPRdirect (Supplementary Table 3). PCR products were amplified from the genomic DNA of KO PGCs (#CA10-TYRP1-KO) using the primers listed in Supplementary Table 1, and eight TA clones each were sequenced by the Sanger method.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
References
FAO. “FAOSTAT Database.” Poultry Production. https://www.fao.org/poultry-production-products/production/poultry-species/en/ (2020).
Wang, M. S. et al. 863 genomes reveal the origin and domestication of chicken. Cell Res. 30, 693–701 (2020).
Mtileni, B. J. et al. Genetic diversity and conservation of South African indigenous chicken populations. J. Anim. Breed. Genet. 128, 209–218 (2011).
Malomane, D. K. et al. Genetic diversity in global chicken breeds in relation to their genetic distances to wild populations. Genet. Sel. Evol. 53, 36 (2021).
Zhuang, Z. et al. Genetic diversity and breed identification of Chinese and Vietnamese local chicken breeds based on microsatellite analysis. J. Anim. Sci. 101, https://doi.org/10.1093/jas/skad182 (2023).
Petitte, J. N. Avian germplasm preservation: embryonic stem cells or primordial germ cells? Poult. Sci. 85, 237–242 (2006).
Nakamura, Y. Poultry genetic resource conservation using primordial germ cells. J. Reprod. Dev. 62, 431–437 (2016).
Santiago-Moreno, J. &E. Blesbois Animal board invited review: Germplasm technologies for use with poultry. Animal 16, 100475 (2022).
Huo, J. L. et al. Genetic diversity of local Yunnan chicken breeds and their relationships with Red Junglefowl. Genet. Mol. Res. GMR 13, 3371–3383 (2014).
Alsoufi, M. & Changrong, G. Genetic Diversity and Evolution of Yunnan Chicken Breeds of China, in Population Genetics. 2022, IntechOpen.
Song, C. et al. Detection and genetic research of rumpless trait in Piao chicken. China Poult. 37, 12/5 (2015).
Freese, N. H. et al. A novel gain-of-function mutation of the proneural IRX1 and IRX2 genes disrupts axis elongation in the Araucana rumpless chicken. PLoS One 9, e112364 (2014).
Zhang, J. et al. A ∼4.1 kb deletion in IRX1 gene upstream is completely associated with rumplessness in Piao chicken. Genomics 114, 110515 (2022).
Brown, W. R., Hubbard, S. J., Tickle, C. & Wilson, S. A. The chicken as a model for large-scale analysis of vertebrate gene function. Nat. Rev. Genet. 4, 87–98 (2003).
Andersson, L. Domestic animals as models for biomedical research. Ups. J. Med. Sci. 121, 1–11 (2016).
Flores-Santin, J. & Burggren, W. W. Beyond the Chicken: Alternative Avian Models for Developmental Physiological Research. Front. Physiol. 12, 712633 (2021).
Sun, Y. et al. Poultry genetic heritage cryopreservation and reconstruction: advancement and future challenges. J. Anim. Sci. Biotechnol. 13, 115 (2022).
Blesbois, E. Current status in avian semen cryopreservation. J. World’s Poult. Sci. 63, 213–222 (2007).
Zong, Y. et al. Chicken Sperm Cryopreservation: Review of Techniques, Freezing Damage, and Freezability Mechanisms. Agriculture 13, 445 (2023).
Fulton, J. Avian genetic stock preservation: an industry perspective. Poult. Sci. 85, 227–231 (2006).
Long, J. Avian semen cryopreservation: what are the biological challenges? Poult. Sci. 85, 232–236 (2006).
Nakamura, Y. et al. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult. Sci. 86, 2182–2193 (2007).
Szczerba, A., Kuwana, T. & Paradowska, M. &M. Bednarczyk In Vitro Culture of Chicken Circulating and Gonadal Primordial Germ Cells on a Somatic Feeder Layer of Avian Origin. Animals 10, 1769 (2020).
van de Lavoir, M. C. et al. Germline transmission of genetically modified primordial germ cells. Nature 441, 766–769 (2006).
Whyte, J. et al. FGF, insulin, and SMAD signaling cooperate for avian primordial germ cell self-renewal. Stem Cell Rep. 5, 1171–1182 (2015).
Taylor, L. et al. Efficient TALEN-mediated gene targeting of chicken primordial germ cells. Development 144, 928–934 (2017).
Nakamura, Y. et al. Efficient system for preservation and regeneration of genetic resources in chicken: concurrent storage of primordial germ cells and live animals from early embryos of a rare indigenous fowl (Gifujidori). Reprod. Fert. Dev. 22, 1237–1246 (2010).
Woodcock, M. E. et al. Reviving rare chicken breeds using genetically engineered sterility in surrogate host birds. Proc. Natl Acad. Sci. USA 116, 20930–20937 (2019).
Lázár, B. et al. Successful cryopreservation and regeneration of a partridge colored Hungarian native chicken breed using primordial germ cells. Poult. Sci. 100, 101207 (2021).
Park, T. S. et al. Targeted gene knockout in chickens mediated by TALENs. Proc. Natl Acad. Sci. USA. 111, 12716–12721 (2014).
Oishi, I. et al. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci. Rep. 6, 23980 (2016).
Oishi, I., Yoshii, K., Miyahara, D. & Tagami, T. Efficient production of human interferon beta in the white of eggs from ovalbumin gene-targeted hens. Sci. Rep. 8, 10203 (2018).
Lee, H. J. et al. Targeted gene insertion into Z chromosome of chicken primordial germ cells for avian sexing model development. Faseb J. 33, 8519–8529 (2019).
Kim, G. D. et al. Generation of myostatin-knockout chickens mediated by D10A-Cas9 nickase. Faseb J. 34, 5688–5696 (2020).
Ballantyne, M. et al. Direct allele introgression into pure chicken breeds using Sire Dam Surrogate (SDS) mating. Nat. Commun. 12, 659 (2021).
Ezaki, R. et al. Transcription activator-like effector nuclease-mediated deletion safely eliminates the major egg allergen ovomucoid in chickens. Food Chem. Toxicol. 175, 113703 (2023).
Hamburger, V., & Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 (1951).
Macdonald, J. et al. Characterisation and germline transmission of cultured avian primordial germ cells. PLoS One 5, e15518 (2010).
Altgilbers, S. et al. Cultivation and characterization of primordial germ cells from blue layer hybrids (Araucana crossbreeds) and generation of germline chimeric chickens. Sci. Rep. 11, 12923 (2021).
Nandi, S. et al. Cryopreservation of specialized chicken lines using cultured primordial germ cells. Poult. Sci. 95, 1905–1911 (2016).
Fujimoto, T., Ukeshima, A. & Kiyofuji, R. The origin, migration and morphology of the primordial germ cells in the chick embryo. Anat. Rec. 185, 139–145 (1976).
Nakamura, M. et al. Ectopic colonization of primordial germ cells in the chick embryo lacking the gonads. Anat. Rec. 229, 109–115 (1991).
Tajima, A. et al. Study on the concentration of circulating primordial germ cells (cPGCs) in early chick embryos. J. Exp. Zool. 284, 759–764 (1999).
Motono, M., Ohashi, T., Nishijima, K. & Iijima, S. Analysis of chicken primordial germ cells. Cytotechnology 57, 199–205 (2008).
De Melo Bernardo, A. et al. Chicken primordial germ cells use the anterior vitelline veins to enter the embryonic circulation. Biol. Open 1, 1146–1152 (2012).
Idoko-Akoh, A. & McGrew, M. J. Generation of Genome-Edited Chicken Through Targeting of Primordial Germ Cells. Methods Mol. Biol. 2631, 419–441 (2023).
Ezaki, R., Ichikawa, K., Matsuzaki, M. & Horiuchi, H. Targeted Knock-in of a Fluorescent Protein Gene into the Chicken Vasa Homolog Locus of Chicken Primordial Germ Cells using CRIS-PITCh Method. J. Poult. Sci. 59, 182–190 (2022).
Dunn, L. C., & Landauer, W. The genetics of the rumpless fowl with evidence of a case of changing dominance. J. Genet. 29, 217–243 (1934).
Zwilling, E. The Development of Dominant Rumplessness in Chick Embryos. Genetics 27, 641–656 (1942).
Adalsteinsson, S., Bjarnadottir, S., Vage, D. I. & Jonmundsson, J. V. Brown coat color in Icelandic cattle produced by the loci Extension and Agouti. J. Hered. 86, 395–398 (1995).
Schmutz, S. M., Berryere, T. G. & Goldfinch, A. D. TYRP1 and MC1R genotypes and their effects on coat color in dogs. Mamm. Genome 13, 380–387 (2002).
Lyons, L. A., Foe, I. T., Rah, H. C. & Grahn, R. A. Chocolate coated cats: TYRP1 mutations for brown color in domestic cats. Mamm. Genome 16, 356–366 (2005).
Ren, J. et al. A 6-bp deletion in the TYRP1 gene causes the brown colouration phenotype in Chinese indigenous pigs. Heredity 106, 862–868 (2011).
Manga, P. et al. Rufous oculocutaneous albinism in southern African Blacks is caused by mutations in the TYRP1 gene. Am. J. Hum. Genet. 61, 1095–1101 (1997).
Rooryck, C. et al. Oculocutaneous albinism with TYRP1 gene mutations in a Caucasian patient. Pigment Cell Res. 19, 239–242 (2006).
Wakasugi, N. & Kondo, K. Breeding methods for maintenance of mutant genes and establishment of strains in the Japanese quail. Jikken Dobutsu 22, 151–159 (1973).
Nadeau, N. J., Mundy, N. I., Gourichon, D. & Minvielle, F. Association of a single-nucleotide substitution in TYRP1 with roux in Japanese quail (Coturnix japonica). Anim. Genet. 38, 609–613 (2007).
Carefoot, W. C. Chocolate: a sex-linked recessive plumage colour mutant of the domestic fowl. Br. Poult. Sci. 37, 867–868 (1996).
Li, J. et al. A missense mutation in TYRP1 causes the chocolate plumage color in chicken and alters melanosome structure. Pigment Cell Melanoma Res. 32, 381–390 (2019).
Kinoshita, K. et al. Combined deletions of IHH and NHEJ1 cause chondrodystrophy and embryonic lethality in the Creeper chicken. Commun. Biol. 3, 144 (2020).
Perry, M. M. A complete culture system for the chick embryo. Nature 331, 70–72 (1988).
Naito, M., Tajima, A., Yasuda, Y. & Kuwana, T. Production of germline chimeric chickens, with high transmission rate of donor-derived gametes, produced by transfer of primordial germ cells. Mol. Reprod. Dev. 39, 153–161 (1994).
Dorshorst, B. et al. A complex genomic rearrangement involving the endothelin 3 locus causes dermal hyperpigmentation in the chicken. PLoS Genet. 7, e1002412 (2011).
Naito, Y., Hino, K., Bono, H. & Ui-Tei, K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31, 1120–1123 (2015).
Acknowledgements
We are grateful to Professor George McDonald Church, Harvard Medical School for guidance and suggestion for study. We extend our thanks to the Major Science and Technology Project of Yunnan Province (Grant# 202102AA100054) and Introduction of Poultry Genetic Resources Cryopreservation Technology by National Animal Husbandry Station (Grant#13200398) for funding. We are also thankful to Professor Changrong Ge (Wuding Chicken), Xinglong Breeding Professional Cooperative in Xichou County, Wenshan Prefecture, Yunnan Province, China (Xichou Black Bone Chicken) and Yunling Chahua Chicken Industry Development Co., Ltd, Xishuangbanna Prefecture, Yunnan Province, China (Piao and Chahua Chicken) for providing animal materials.
Author information
Authors and Affiliations
Contributions
K.K., K.T., H-Y.Z., and H.-J.W. designed research; K.K., and K.T. performed research; K.K., K.T., Y.N., K.-I.N., T.S., Y.O., S.M., M.-S.W., S.U.K, T.W., H.Z., S.F., G.L., F.Z., and Y.S. contributed reagents/materials/analysis tools/technical support; K.K., and K.T., analyzed data; K.K., K.X., M.A.J., and H-J. W. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Mike (J) McGrew and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editors: Dr Simona Chera and Dr Ophelia Bu.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kinoshita, K., Tanabe, K., Nakamura, Y. et al. PGC-based cryobanking, regeneration through germline chimera mating, and CRISPR/Cas9-mediated TYRP1 modification in indigenous Chinese chickens. Commun Biol 7, 1127 (2024). https://doi.org/10.1038/s42003-024-06775-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-06775-5