Abstract
Cisplatin is the primary chemotherapeutic agent for osteosarcoma. However, a significant proportion of patients develop resistance post-treatment, leading to disease recurrence and presenting profound clinical challenges. To understand the mechanisms underlying osteosarcoma recurrence and cisplatin resistance, particularly from the tumor microenvironment perspective, we consolidated numerous single-cell RNA sequencing datasets, offering an encompassing insight into the osteosarcoma microenvironment. When juxtaposing scRNA-seq with bulk RNA-seq data, we observed a strong correlation between high DCUN1D5 expression in osteosarcoma and patient survival. This gene amplifies osteosarcoma’s anti-apoptotic, invasive, stem-cell-like traits and PI3K/AKT/GSK3β pathway phosphorylation and fosters cisplatin resistance. Subsequent research revealed that cisplatin-resistant osteosarcoma cells excrete DCUN1D5-rich exosomes, facilitating the maturation of osteoclast precursors. Excessive osteoclast activity is a pivotal contributor to osteosarcoma recurrence and resistance. Given these insights, DCUN1D5 is a promising therapeutic target for osteosarcoma recurrence and drug resistance.

Similar content being viewed by others
Introduction
Osteosarcoma, a prevalent bone tumor, exhibits pronounced heterogeneity in clinical characteristics and therapeutic outcomes across patients. The remodeling of the immune microenvironment in osteosarcoma is the primary cause of the aforementioned consequences1. Previous studies have shown that immune cells and specific immune factors play important roles in the process mentioned above. For example, immunosuppressive cells (Tregs, MDSCs, and TAMs) secrete various immune factors (TGF-β, IL-10) to promote the formation of an immunosuppressive tumor microenvironment, thereby limiting the antitumor activity of the immune system2,3,4,5,6,7. Recent studies increasingly show that non-immune cells (such as CAFs, endothelial cells, and osteoclasts) also play an important role in disease heterogeneity. For example, a vicious cycle is formed between osteoclasts and tumor cells. Tumor cells release factors that activate osteoclasts, and osteoclasts secrete factors that promote tumor cell proliferation, migration, and exacerbate bone resorption. This cycle drives tumor expansion, recurrence, and resistance, which profoundly affects patient survival and quality of life8,9. Notably, recurrence, frequently a primary determinant of osteosarcoma prognosis, remains a grave concern. The emerging challenge in osteosarcoma management is drug resistance, which has garnered increasing attention in medical research. Numerous studies have substantiated the tight association between osteoclast overactivation, drug resistance, and recurrence10,11,12,13. Osteoclasts hold a pivotal role within the tumor immune microenvironment; any escalation in their activity markedly alters the therapeutic response to tumors.
Understanding the mechanisms underlying the occurrence and development of tumors is crucial. The ubiquitination process is closely intertwined with apoptosis, proliferation, stem cell characteristics, and tumor microenvironment remodeling14,15,16. E3s(E3) play a pivotal role in this process, and abnormalities in their function can directly contribute to tumor occurrence and development17. Therefore, E3 represents a promising therapeutic target that is of great significance. In this study, we identified a highly expressed E3, DCUN1D5, in osteosarcoma, which provides prognostic information through single-cell and bulk data analysis. Multiple studies have shown that DCUN1D5 plays an important role in cancer. In triple-negative breast cancer (TNBC), DCUN1D5 enhances its expression by interacting with the transcription factor YY1, thereby activating the FN1/PI3K/AKT pathway, promoting tumor cell proliferation and invasion18. This suggests that DCUN1D5 may become a new target for TNBC therapy and a prognostic marker. In lung adenocarcinoma, the expression of DCUN1D5 is significantly higher than in normal tissues and is associated with tumor staging and clinical prognosis. Studies have shown that high expression of DCUN1D5 is closely related to poor prognosis, potentially influencing the glycolysis process and immune cell infiltration, further exacerbating the malignancy of the tumor19. Meanwhile, as part of the E3 complex, DCUN1D5 promotes the ubiquitin-like modification of Cullin proteins. DCUN1D5 affects key biological processes such as the cell cycle and DNA repair, thereby facilitating tumor initiation and progression20. Although studies have confirmed that DCUN1D5 plays an important role in tumor initiation and progression, its role in osteosarcoma has not been fully explored. However, this study found that DCUN1D5 plays a role in regulating apoptosis, proliferation, and stem cell characteristics and in the remodeling of the tumor microenvironment in osteosarcoma. It is also closely associated with cisplatin resistance.
The standard treatment for osteosarcoma typically includes multimodal therapeutic strategies, mainly involving surgical resection of the tumor, conventional chemotherapy, and, in some instances, radiotherapy. With the advancement of scientific research, the treatment methods for osteosarcoma are continuously evolving, incorporating various biological therapies such as immunotherapy, novel chemotherapy approaches, gene therapy, targeted therapy, stem cell research, and biophysical treatments, all of which offer new hope for osteosarcoma patients. For example, immunotherapy targets tumor cells and activates and modulates the immune system to enhance the efficiency of combating tumors21,22. The novel chemotherapy approaches (copper (II) hydrazide complexes and platinum, palladium chelates) demonstrate highly effective and selective anti-tumor activity against human osteosarcoma23,24. Among numerous treatment regimens, cisplatin still plays a crucial role in treating osteosarcoma. Cisplatin is a chemotherapy drug widely used for treating various cancers. However, resistance to cisplatin has been a long-standing challenge in osteosarcoma25,26. The current research suggests that the classical mechanisms of cisplatin resistance mainly include: alterations in DNA repair mechanisms, activation of platinum efflux pumps, changes in cisplatin cellular uptake, inhibition of apoptotic pathways, alterations in metal ion metabolism, and epigenetic changes27,28,29,30,31,32. However, these studies often fail to clearly reveal the specific role of the tumor microenvironment in resistance. This study addresses this research gap by focusing on the role of DCUN1D5 in the immune microenvironment. Our research indicates that osteosarcoma cells with drug resistance exhibit elevated levels of DCUN1D5 expression. Furthermore, these cells release exosomes rich in DCUN1D5, which play a role in accelerating osteoclast maturation. These findings not only elucidate the mechanism of resistance to cisplatin in these cells but also suggest potential therapeutic avenues. This study aims to reveal the role of DCUN1D5 in the osteosarcoma immune microenvironment, particularly its potential mechanisms in cisplatin resistance and osteoclast maturation. This finding not only expands our understanding of the therapeutic response in osteosarcoma but also provides a scientific basis for the development of new targeted therapeutic strategies.
Result
Overview of the osteosarcoma tumor microenvironment at single-cell resolution
This study utilized published single-cell datasets (GSE152048, GSE162454, and GSE198896). Initially, cells exhibiting elevated mitochondrial and erythrocyte genes were excluded. Subsequently, dimensionality reduction clustering was executed using HVG and PCA analyses (Fig. S1A, B). We assessed the influence of cell cycle genes on cell clustering (Fig. S1C) and analyzed the correlations between mitochondrial-related genes, erythrocyte-related genes, nfeature, and ncount (Fig. S1D–F). Additionally, we provided a detailed account of the nfeature and ncount for each patient, alongside their cell cycle-related scores (Fig. S1G–J). Following rigorous quality control and the elimination of batch effects, we cataloged 160,065 cells. With the aid of clustree, we demonstrated the impact of varying clustering resolutions on cell grouping, presented in a dendrogram (Fig. 1A). A review of the t-SNE and UMAP plots post-batch effect mitigation revealed a uniform cell distribution (Fig. 1B). Leveraging previously curated marker genes, we discerned ten predominant cell clusters (Fig. 1C). Notably, the proportion of cell clusters varied across lesions, highlighting tumor heterogeneity and lesion consistency (Fig. 1D). Dot plots were employed to juxtapose the proportions of cells expressing cluster-specific markers against their relative expression levels, after normalization (Fig. 1E).
A Dendrogram showcasing cell clustering across varying resolutions, highlighting the stability of cluster formation and the impact of resolution choice on cell grouping. This analysis was conducted using clustree to evaluate the robustness of the clustering algorithm. B t-SNE and UMAP representations of single-cell transcriptomes from 17 osteosarcoma samples and six osteosarcoma tumor-infiltrating lymphocyte (TIL) samples. These plots demonstrate the homogeneous distribution of cells across samples, indicating the successful normalization of the data. C t-SNE and UMAP visualization of the ten primary cell types identified within osteosarcoma samples based on the expression of previously curated marker genes. This panel illustrates the cellular diversity within the tumor microenvironment and the distinct transcriptional profiles of each cell type. D Bar graphs depicting the proportional representation of cell clusters in the 17 osteosarcoma samples and six osteosarcoma-TIL samples. This panel highlights the heterogeneity of cell populations across different lesions and the consistency of specific cell types within the tumor microenvironment. E Dot plot detailing the expression of 23 feature genes across the ten identified cell clusters. The size of each dot corresponds to the proportion of cells within a cluster expressing a specific marker gene. At the same time, the color gradient represents the average expression level of that marker. This plot provides insights into the marker gene expression patterns that define each cell cluster and their relative abundance.
Malignant osteosarcoma cells display marked heterogeneity
Clinically, osteoblasts and chondrocytes represent the two predominant osteosarcoma (OS) types. Through UMAP analysis of malignant OS cells and marker gene identification (Fig. S2A), we discerned five subgroups: four aligned with the osteoblastic lineage and one with the chondrocyte lineage (Fig. S2B). These subgroups of malignant osteosarcoma cells exhibit distinct transcriptomic profiles and pathway enrichments. Osteoblastic.OS1 is enriched in the extracellular matrix, oxidative phosphorylation, and co-translational protein targeting the membrane, suggesting its potential role in tumor invasion and metastasis through extracellular matrix remodeling. Osteoblastic.OS2 shows enrichment in protein generation, pointing to its possible contribution to tumor growth via abnormal protein synthesis. Chondroblastic.OS displays enrichment in protein synthesis and localization processes, abnormalities of which may be intimately associated with tumor cell proliferation, metastasis, and other pathophysiological events. Osteoblastic.OS3’s enrichment centers around cell division and chromosome-associated biological processes, which could relate to the proliferative tendencies of tumor cells. Osteoblastic.OS4, enriched in neutrophil activation and immune responses, indicates a probable interaction between this osteosarcoma subtype and neutrophils (Fig. S2C). In comparison to primary osteosarcoma cells, recurrent osteosarcoma cells manifest distinct expression patterns (Fig. S2D). The varying proportions of cell clusters among lesions underscore the pronounced inter-tumoral heterogeneity (Fig. S2E).
Heterogeneity of myeloid cells in the immune microenvironment
In the immune microenvironment of osteosarcomas, myeloid cells serve multifaceted roles that contribute to the pathogenesis of osteosarcoma. Not only do they potentially accelerate tumor growth, invasion, and metastasis, but they also modulate immune and therapeutic responses33. Our analysis of clustering impacts at diverse resolutions led us to select an optimal resolution (Fig. S3A). Using the UMAP plot for myeloid cell visualization, and we discerned that the two-dimensional cell distribution does not exhibit a discernible relationship across samples. Our investigation identified six distinct subgroups: monocytes, M2_TAM, M1_TAM, M0_TAM, DCs, and neutrophils, with the chosen resolution illustrated during subgroup determination. Predominantly, myeloid cells reside within primary osteosarcomas; however, a fraction is evident in recurrent and metastatic osteosarcomas (Fig. S3B). Ridgeplots and Dotplots, coupled with the display of previously identified marker genes, suggest the efficacious clustering capability of these marker genes (Fig. S3C/F). Notably, the prevalent infiltrating myeloid cells in the tumor microenvironment are M2_TAM, monocytes, and DCs—a finding congruent with prior research. Despite this, substantial variations exist among the infiltrating cells across samples, emphasizing the tumor’s heterogeneity and consistency (Fig. S3D). Mirroring the characteristics of malignant tumor cells, myeloid cells from initial and recurrent lesions demonstrate marked differences in transcriptomic expression (Fig. S3E).
Cellular classification of Tumor-Infiltrating Lymphocytes (TILs)
Tumor-infiltrating lymphocytes (TILs) are immune cells in the tumor microenvironment, often associated with immune surveillance and prognostic assessments. Several studies have established TILs as potential predictive markers for immunotherapy34,35,36. Our analysis discerned eight distinct cell subgroups: CD4/8-T, CD8 + T, CD4 + T, Proliferating.T, NK, Treg, B cell, and NKT, as shown in Fig. S4A. Using umap heat maps and dot plots to illustrate marker genes, we observed that the markers, as proposed by Zhou et al., effectively distinguish between cell types (Fig. S4B/C)37. Distinct TILs exhibit unique high-expression genes and serve various biological functions. For instance, CD4/8-T cells majorly concentrate on pathways like oxidative phosphorylation and ATP metabolism, hinting at increased energy metabolism and mitochondrial adjustments. CD8 + T cells primarily focus on T-cell activation and nuclear mRNA degradation, suggesting a regulation in gene expression to prompt differentiation. CD4 + T cells are predominantly associated with neutrophil pathways and antigen processing, indicating potential interactions with neutrophils. Proliferating.T cells, enriched in chromosome separation and nuclear division pathways, are likely in a heightened proliferative state. In osteosarcomas, NK cell pathways, which are rich in NK cell-related processes, suggest active NK cell-mediated immune responses and a focus on enhancing their antitumor activity. Treg cells primarily modulate T-cell activation, lymphocyte differentiation, and B-cell activation. B cells seem to play roles in their own activation and receptor signaling (Fig. S4D). Lastly, NKT cells potentially contribute to antiviral defenses and manage symbiotic relationships. Notably, while all patients express TILs, the representation of cell subgroups varies, underscoring TIL heterogeneity (Fig. S4E).
Robust cellular interactions characterize the osteosarcoma tumor microenvironment (TME)
The tumor microenvironment’s cellular communication is crucial for tumor development. Numerous studies highlight the central role of intercellular communication in modulating tumor growth, invasion, metastasis, immune evasion, and drug resistance38,39,40,41. In the context of osteosarcoma, our findings indicate pronounced communication among cellular subpopulations (Fig. S5A, Fig. S6, Fig. S7). Within a 2D framework, we identified the predominant sender (source) and receiver (target) cells. Predominant senders encompass malignant osteosarcoma cells, fibroblasts, myocytes, and mesenchymal stem cells. At the same time, primary receivers include macrophages, neutrophils, dendritic cells, and osteoclasts (Fig. S5B). Concurrently, we determined that osteosarcomas can modulate various cellular subgroups via multiple signaling pathways (Fig. S8). A deeper investigation into the signal distribution revealed MIF, SPP1, and MK as the predominant incoming and outgoing signals (Fig. S5C, D).
Unveiling global communication dynamics within the osteosarcoma tumor microenvironment
The challenge of coordinating the functions of various cell populations and signaling pathways emerges when studying the complex communication events between different cell subpopulations and their related signaling pathways. We utilized CellChat for pattern recognition to dissect the overall communication dynamics. Using the selectK method, we determined the number of output and input patterns, categorizing the cell subpopulations and communication patterns into different groups (output pattern number n = 4, input pattern number n = 2) (Fig. 2A, E). Our analysis focused on the intercellular communication in terms of outgoing signaling and incoming signaling. In the analysis of outgoing signaling, the intercellular communication patterns were classified into four main patterns. Pattern 1 mainly includes immune cell types such as M1_TAM, M2_TAM, M3_TAM, Monocytes, and DCs, which interact through various molecules such as ANNEXIN, OSM, GALECTIN, VISFATIN, BAFF, CCL, IGF, TNF, FCXCL, COMPLEMENT, TWEAK, GASP, and PP1. These molecules suggest that immune cells play a crucial role in immune regulation, immune evasion, and inflammatory responses in the osteosarcoma microenvironment through different signaling pathways. Pattern 2 primarily involves Proliferating.TCD4/CD8 − T cells, Endothelial cells, Pericytes, and Fibroblasts, which play important roles in tumor proliferation and angiogenesis. The associated communication patterns include PERIOSTIN, CSF, FGF, CHEMERIN, MK, ANGPT, and GAPT, which may promote tumor cell proliferation, angiogenesis, and migration. Pattern 3 includes Treg, Osteoclasts, B cells, and Chondroblastic.OS cells, which are closely related to immune tolerance and bone metastasis. Communication patterns such as BAG, VEGF, ncWNT, ANGPT, LCAL, and CRIL16 may facilitate bone metastasis and angiogenesis, influencing tumor malignancy. Pattern 4 consists of CD8 + T, NKT, and NK cells, which interact through molecules such as ANGPT, PARs, and RESISTIN, and may play important roles in tumor immune surveillance and immune evasion (Fig. 2B). In the analysis of incoming signaling, the intercellular communication patterns were divided into two main patterns. In Pattern 1, the cell types cover a broad range of immune cell types, including Neutrophils, M1_TAM, Monocytes, NK, B cells, CD4 + T, CD8 + T, TM2_TAM, Treg, DCs, and NKTM3_TAM. These cells interact through various signaling molecules such as GAS, GALECTIN, CHEMERIN, ANNEXIN, MIF, COMPLEMENT, CXCL, BAG, BAFF, IL16, TGFb, TNF, CCL, CSF, RESISTIN, and SPP1. These molecules may play a crucial role in immune evasion, immune suppression, and the migration and dissemination of tumor cells. In Pattern 2, the cell types include Proliferating.T, Myoblasts, Osteoclasts, Fibroblasts, Pericytes, MSCs, Endothelial cells, Chondroblastic.OS, Osteoblastic.OS, and CD4/CD8 − T, which participate in tumor proliferation, tissue repair, and angiogenesis. Communication molecules such as MK, PTN, IGF, PERIOSTIN, ANGPT, PARs, ANGPTL, TWEAK, PDGF, VISFATIN, ncWNT, FGF, CALCR, VEGF, and OSM are involved in shaping the tumor microenvironment, immune regulation, and tumor angiogenesis (Fig. 2F). We further clarified the potential output and input patterns and their association with cell populations and signaling pathways using Sankey diagrams (Fig. 2C, G). Moreover, the pattern recognition analysis revealed subtle changes in the output (outgoing) and input (incoming) signals of specific key signaling pathways in cells (Fig. 2D, H). Notably, malignant osteosarcoma cells, tumor-associated macrophages (TAMs), and osteoclasts play crucial roles in the overall communication dynamics.
A The number of output patterns is estimated using the Cophenetic and Silhouette indices to identify different communication patterns between cell subgroups. Based on the indices, the output pattern number is selected as 4. B Cell subtypes and their associated signaling pathways are identified, and the composition of the output patterns is analyzed to reveal the specific roles of different cell populations in regulating the communication network. C Outgoing communication patterns are shown in a Sankey diagram, providing a clearer reflection of the correspondence between specific cell types, patterns, and signaling. D A dot plot of signaling pathways displays the specific outgoing signals from various cell types as output cells. E The number of input patterns is estimated using the Cophenetic and Silhouette indices to identify different communication patterns between cell subgroups. Based on the indices, the output pattern number is selected as 2. F Cell subtypes and their associated signaling pathways are identified, and the composition of the input patterns is analyzed to reveal the specific roles of different cell populations in regulating the communication network. G Incoming communication patterns are shown in a Sankey diagram, providing a clearer reflection of the correspondence between specific cell types, patterns, and signaling. H A dot plot of signaling pathways displays the specific incoming signals to various cell types as input cells.
Patients with recurrent osteosarcoma demonstrate unique cellular communication characteristics
Recurrence of osteosarcoma typically leads to poorer prognosis. Studies have shown that intercellular communication plays an important role in disease phenotypes42,43. Therefore, a comparative analysis of the cellular communication patterns in primary and recurrent osteosarcoma can help uncover potential mechanisms of recurrence. The analysis revealed rich intercellular communication in both groups (Fig. 3A). However, there were significant differences in the total interaction and intensity of the communication networks between recurrent and primary osteosarcoma (Fig. 3B), with the overall interaction index of recurrent osteosarcoma being higher than that of primary osteosarcoma (Fig. 3C). We further analyzed the communication changes between cell subgroups using heatmaps and two-dimensional schematic diagrams. The results showed that malignant osteosarcoma cells, osteoclasts, TAMs, and fibroblasts were the main communicators in both types of osteosarcoma (Fig. 3D, E). To investigate the recurrence mechanisms of osteosarcoma, we analyzed the differential signaling pathways in different cell subgroups and found that signaling pathways such as MIF, FGF, PTN, RESISTIN, ANGPTL, ncWNT, UGRP1, IL16, OSM, and BAG were significantly overexpressed in recurrent osteosarcoma (Fig. 3F). Previous studies have shown that the high expression of these signaling pathways is related to osteoclast activity and ubiquitination and pseudo-ubiquitination modifications, further suggesting that osteoclast activity and ubiquitination and pseudo-ubiquitination modifications may play an important role in osteosarcoma recurrencee44,45,46,47,48,49. In addition, we compared the signaling pathways controlling communication probability and ligand-receptor pairs, and found obvious differences in signaling dynamics and communication likelihood between primary and recurrent osteosarcoma (Fig. S9). Compared with primary osteosarcoma, the input signals of Chondroblastic.OS and CD4 + T were significantly enhanced in recurrent osteosarcoma (Fig. 3G), while the output signals of Chondroblastic.OS and CD4/CD8 − T were also significantly enhanced (Fig. 3H). Overall signals of Chondroblastic.OS, CD4 + T, and CD4/CD8 − T were significantly increased (Fig. 3I). Further analysis of signaling pathways revealed the most prominent signal changes, including SPP1, MK, MIF, PTN, and GALECTIN. The changes in these pathways in recurrent osteosarcoma may be closely related to tumor immune evasion, cell migration, and drug resistance. Specifically, SPP1 (osteopontin) is associated with tumor cell adhesion and migration, MK (matrix metalloproteinase) is closely related to the remodeling of the tumor stroma, MIF (macrophage migration inhibitory factor) and PTN (pleiotrophin) may play a key role in the tumor immune evasion process, while GALECTIN (galectin) is known to regulate immune cell function and may influence immune evasion mechanisms by altering the tumor microenvironment.
A Quantification of cell interactions in primary and recurrent osteosarcoma, showing the total number of communication events in both conditions. Both primary and recurrent osteosarcomas exhibit complex communication. B Visualization of changes in interaction counts or intensity during recurrence, with red representing enhanced expression and blue representing reduced expression. Recurrent osteosarcoma displays distinct communication quantities and intensities. C Comparison of interaction frequency and intensity between primary and recurrent osteosarcoma, with recurrent cases showing higher communication activity. D Heatmap illustrating the differences in interaction quantity or intensity, providing a visual comparison of cell communication features between primary and recurrent osteosarcoma. E Two-dimensional spatial juxtaposition analysis showing the distribution and directionality of interactions between signal sources and targets in primary and recurrent osteosarcoma patients. In recurrent osteosarcoma, osteosarcoma cells and osteoclasts occupy significant positions. F Detailed flow analysis of signaling pathways, revealing the complexity and diversity of the cell communication network. Signaling pathways such as MIF, FGF, PTN, RESISTIN, ANGPTL, ncWNT, UGRP1, IL16, OSM, and BAG are significantly upregulated in recurrent osteosarcoma. G Comparison of egress signals from different cell populations, highlighting changes in outward signaling between different cell groups. H Analysis of ingress signals in each cell population, reflecting differences in how different cell groups receive signals. I Overall signaling network overview, revealing the signaling patterns of various cell populations and their roles in cell communication.
The regulatory significance of E3 DCUN1D5 in osteosarcoma
Ubiquitination and ubiquitin-like modification has diverse roles in osteosarcoma, impacting the tumor immune microenvironment, cell cycle regulation, signaling pathway modulation, protein degradation, and therapeutic targeting50,51,52,53. E3 is essential in this process. Investigating the relationship between E3 and osteosarcoma offers insights and potential targets for therapeutic strategies54,55.
Among the 2142 E3s sourced from IUUCD and the 727 differentially expressed genes in osteogenic osteosarcoma cells, 21 OS-E3s were identified (Fig. 4A). The prognostic potential of these OS-E3s was validated using the public GEO osteosarcoma database, revealing DCUN1D5’s superior prognostic prediction capabilities (Fig. 4B, Fig. S10, Fig. S11).
A Venn diagram depicting the overlap between E3 and genes overexpressed in OS, highlighting the 21 OS-E3s identified from 2142 E3 and 727 differentially expressed genes in osteogenic osteosarcoma cells. B The Kaplan-Meier survival curves show the correlation between DCUN1D5 and overall survival (OS) in the TARGET-OS and GSE21257 databases. C Uniform Manifold Approximation and Projection (UMAP) representation of DCUN1D5 expression patterns, categorizing osteogenic osteosarcoma cells into high and low expression groups based on DCUN1D5’s median expression. D Volcano plot of genes differentially expressed based on DCUN1D5 levels, illustrating the significant upregulation and downregulation of genes in high DCUN1D5-expressing cells. E Gene Ontology (GO) analysis of the differentially expressed genes revealed associations with the extracellular matrix, immune system regulation, protease activity, and cellular components like ribosomes and organelle cavities. F Gene Set Variation Analysis (GSVA) of the differentially expressed genes, showing upregulation of metabolic and functional pathways related to ubiquitin-mediated proteolysis, vesicle transport, and energy metabolism in cells with high DCUN1D5 expression. G Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of the differentially expressed genes, indicating enrichment in ribosome pathways, cancer-related proteoglycans, and various oncogenic processes. H Gene Set Enrichment Analysis (GSEA) of the differentially expressed genes, demonstrating elevated metabolism, oxidative phosphorylation, and reactive oxygen species in cells with high DCUN1D5 expression.
To elucidate DCUN1D5’s impact on osteogenic osteosarcoma cells, we categorized the cells into high and low-expression groups according to DCUN1D5’s median expression (Fig. 4C). A volcano plot illustrated the analysis of differentially expressed genes (Fig. 4D). The differential expressed genes underwent GO analysis, revealing that BP was predominantly associated with the extracellular matrix and structural tissues, negative immune system regulation, protease activity, and fluid level control. This implies DCUN1D5’s potential involvement in extracellular matrix restructuring, immune system regulation, and protein synthesis. CC primarily pertains to ribosome components and organelle cavities, such as the endoplasmic reticulum and various vesicle cavities, suggesting DCUN1D5’s association with cellular metabolism, protein synthesis, and vesicle secretion. Meanwhile, MF concentrates on extracellular matrix and ribosomal components, molecular binding, and ubiquitin-like protein ligase binding (Fig. 4E).
GSVA analysis indicated the upregulation of several metabolic and functional pathways in cells expressing high levels of DCUN1D5, encompassing protein degradation via ubiquitination, vesicle transport, energy metabolism, and essential biological processes (Fig. 4F). KEGG-enriched pathways also correlated with ribosomes, cancer-related proteoglycans, and various oncogenic pathways (Fig. 4G). GSEA analysis showed elevated metabolism, oxidative phosphorylation, and reactive oxygen species in high-expression DCUN1D5 cells (Fig. 4H).
These findings highlight DCUN1D5’s potential regulatory influence on several facets of osteosarcoma, from extracellular matrix composition to vesicle transport. Numerous studies underscore the involvement of these processes in reshaping the osteosarcoma immune microenvironment, emphasizing DCUN1D5’s potential value in osteosarcoma diagnosis and treatment.
DCUN1D5 modulates the osteosarcoma Tumor Microenvironment (TME)
To elucidate the association between DCUN1D5 and the osteosarcoma TME, we utilized cibersortX to assess cellular infiltration within the TARGET-OS microenvironment, drawing from annotated scRNA data. Our analysis of immune infiltration across all TARGET-OS patients revealed pronounced tumor heterogeneity in osteosarcoma (Fig. 5A). Predominant cellular components within the TME encompass malignant cells from osteogenic osteosarcoma, fibroblasts, and osteoclasts (Fig. 5B). Aligning with prior research, our findings suggest that osteosarcoma cells secrete a range of cytokines and signaling molecules, modulating osteoclast and fibroblast behaviors, thus accelerating disease progression12,56,57. Notably, we identified a robust correlation between DCUN1D5 and most cells infiltrating the osteosarcoma TME(Fig. 5C), indicating that DCUN1D5 could potentially influence the TME’s architecture and contribute to an adverse prognosis in osteosarcoma.
A Immune Infiltration Analysis Across Osteosarcoma Samples. This panel displays the variation in immune cell infiltration across different osteosarcoma patient samples, highlighting the heterogeneity in tumor immune landscapes. B Proportional Representation of Cell Types in the Osteosarcoma Tumor Microenvironment. This panel illustrates the relative abundance of different cell types, including malignant osteogenic cells, fibroblasts, and osteoclasts, within the tumor microenvironment of osteosarcoma patients. C Correlation Analysis of DCUN1D5 Expression with Microenvironmental Cells in Osteosarcoma. This panel shows the strength and direction of the association between DCUN1D5 expression levels and the presence of various cell types within the osteosarcoma tumor microenvironment, indicating potential interactions and the influence of DCUN1D5 on the tumor’s cellular composition.
DCUN1D5 is implicated in the progression of osteosarcoma through its potential role in osteoclast maturation
Our findings, corroborating both bulk and single-cell data, demonstrate elevated expression levels of DCUN1D5 in osteoclasts (Fig. S12A). Osteoclasts, specialized cells vital for bone tissue absorption and remodeling, might have their functions anomalously modulated in osteosarcoma, thereby accelerating its development58,59. Through a meticulous process of osteoclast extraction, dimensionality reduction, clustering, and batch effect elimination, we analyzed the influence of varied resolutions on cell clustering (Fig. S12B). We discerned the optimal resolution and categorized osteoclasts into four predominant types: precursor, immature, mature, and non-functional (Fig. S12C, D). Notably, precursor osteoclasts predominantly express bone marrow markers CD74 and CD14. In contrast, elevated TOP2A levels in immature osteoclasts hint at osteogenic stimulation. In contrast, mature osteoclasts, with pronounced levels of CTSK, ACP5, and MMP9, are indicative of active bone resorption—the non-functional osteoclasts, however, present with subdued expression across all marker genes (Fig. S12E). Expectedly, diverse osteoclast states correspond to distinct expression patterns (Fig. S12F).
Further, we employed Monocle 2 and Monocle 3 algorithms for osteoclast trajectory analysis to elucidate their maturation pathways within OS lesions. Utilizing Monocle 2, we organized cells based on trajectory, showcasing an array of cell clusters, types, and differentiation timelines within a bi-dimensional scope (Fig. 6A–C). It became evident that DCUN1D5 is predominantly expressed in immature osteoclasts (Fig. 6D). Monocle 3 outcomes provide a more precise depiction of the comprehensive differentiation journey, spanning from osteoclast precursors to their non-functional counterparts (Fig. 6E). Remarkably, the transition from osteoclast precursors to their immature form coincides with a surge in DCUN1D5 expression (Fig. 6F). Analyzing the gene dynamics during osteoclast differentiation reveals an initial increase in DCUN1D5 expression, which subsequently declines. This peak in DCUN1D5 expression aligns with a concentration of immature osteoclasts (Fig. 6G). The emergence of branches within the single-cell trajectory is attributed to cells engaging in diverse gene expression trajectories. Intriguingly, at branch point 4, osteoclasts diverge into two distinct lineages. While one differentiates into immature and mature osteoclasts, the other progresses toward mature and non-functional osteoclasts. A pronounced rise in DCUN1D5 expression is evident during the transition to green and mature osteoclasts (Fig. 6H, I). To conclude, DCUN1D5 fosters the transition of osteoclast precursors towards their adult stages, inducing heightened activity and expediting osteosarcoma progression”.
A Monocle 2 trajectory analysis illustrates osteoclast subpopulations’ distribution and clustering, highlighting distinct developmental stages. B Detailed subgroup classification within the osteoclast lineage, emphasizing the heterogeneity and complexity of cell types. C Temporal progression of osteoclast differentiation, depicting the dynamic changes in cell states over time. D The Spatial representation of DCUN1D5 expression levels across different osteoclast subclusters showcases its predominant expression in specific developmental phases. E Monocle 3 trajectory visualization, offering a comprehensive view of osteoclast subtype dynamics, from precursors to mature and non-functional states. F Patterns of DCUN1D5 expression throughout the osteoclast maturation process, indicating its crucial role in the transition between cell stages. G Heatmap with hierarchical clustering, presenting the developmental timelines of osteoclast subtypes and their associated marker genes, elucidating the molecular signatures defining each stage. H Differential clustering heatmap highlights the distinct developmental pathways emerging from branch point 4, focusing on the differentiation trajectories leading to immature, mature, and non-functional osteoclasts. I Schematic representation of DCUN1D5’s pivotal role in directing the differentiation of precursor osteoclasts towards their active and functional mature forms, thereby influencing osteosarcoma progression through abnormal osteoclast activation.
DCUN1D5 overexpression may result in multidrug resistance in osteosarcoma
Patients experiencing osteosarcoma recurrence confront significant challenges due to the associated decline in prognosis. One pivotal factor driving this recurrence is drug resistance60,61. Numerous studies have underscored the role of E3 in this resistance, with DCUN1D5 emerging as a possible key player in osteosarcoma drug resistance62,63. To elucidate the effects of DCUN1D5 on drug sensitivity, we employed the OncoPredict algorithm, translating gene expression data from TARGET and GSE21257 OS tissue samples into drug sensitivity matrices. Our findings revealed that elevated DCUN1D5 expression can elevate the IC50 values for several drugs (Fig. 7A, B), suggesting its role in osteosarcoma multidrug resistance. Through molecular docking, we found that DCUN1D5 may interact with frontline drugs for osteosarcoma such as methotrexate, doxorubicin, cisplatin, and cyclophosphamide (with binding energies of −1.23 kcal/mol, −1.12 kcal/mol, −4.27 kcal/mol, and −1.37 kcal/mol, respectively), with the most vital interaction observed with cisplatin (Fig. 7C). To validate the molecular docking results of cisplatin with DCUN1D5, we further conducted molecular simulations, and the RMSF and RMSD results indicated good docking results (Fig. S12G, H). These results highlight the potential of DCUN1D5 as a therapeutic target for osteosarcoma.
A Correlation between DCUN1D5 expression and the half-maximal inhibitory concentration (IC50) values of various drugs in osteosarcoma tissue samples from the TARGET database. Each point represents a different drug, with the x-axis indicating DCUN1D5 expression levels and the y-axis showing the corresponding IC50 values. A positive correlation suggests that higher DCUN1D5 expression is associated with increased drug resistance. B Correlation between DCUN1D5 expression and the IC50 values of drugs in osteosarcoma tissue samples from the GSE21257 database. Like panel A, this graph demonstrates the relationship between DCUN1D5 expression and drug resistance, further supporting the hypothesis that DCUN1D5 plays a role in multidrug resistance in osteosarcoma. C Molecular interaction analysis between DCUN1D5 and primary osteosarcoma treatments, including methotrexate, doxorubicin, cisplatin, and Cyclophosphamide. The binding affinity values represent the interaction strength, with stronger interactions indicating a higher potential for DCUN1D5 to influence drug efficacy. This analysis highlights the potential of DCUN1D5 as a therapeutic target for overcoming drug resistance in osteosarcoma.
In vitro studies elucidate the role of DCUN1D5 in modulating drug resistance, stemness, apoptosis, and proliferation in osteosarcoma cells
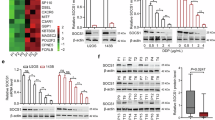
Employing a gradient increment approach, we established cisplatin-resistant osteosarcoma cell lines MG63/CDDP and U-2OS/CDDP (Fig. S13A)64. We assessed their sensitivity to cisplatin using CCK-8. Data revealed that the IC50 values for MG63/CDDP and U2OS/CDDP were 40.43 μM and 75.56 μM, respectively, while those for the parent cells MG63 and U-2OS stood at 6.13 μM and 12.35 μM, respectively. These findings confirm the successful derivation of cisplatin-resistant osteosarcoma cell lines (Fig. S13B). The drug-resistant variants demonstrated enhanced growth rates at 72 and 96 h relative to their parent cells (Fig. 8A). Both qRT-PCR and WB assays indicated a marked elevation in DCUN1D5 expression in MG63 and U-2OS close to the regular osteoblast cell line, hFOB. This increase was more pronounced in MG63 cells and even more so in their drug-resistant counterparts, MG63/CDDP (Fig. 8B, C, S13C). Immunofluorescence assays pinpointed a predominant nuclear localization for DCUN1D5, with augmented expression in the drug-resistant phenotype (Fig. 8D, S13G). The immunohistochemical results showed that the expression level of DCUN1D5 was significantly higher in osteosarcoma tissues compared to adjacent normal tissues (Fig. S13K, L).
A Growth quantification of both sensitive and drug-resistant osteosarcoma cell lines using CCK-8. *P < 0.05. B qRT-PCR assessment of DCUN1D5 levels in osteosarcoma and cisplatin-resistant cell lines. C Western blot analysis of protein expression. D Confocal imaging of DCUN1D5 in both sensitive and resistant osteosarcoma cells. E Observation of tumor spheroid formation post-DCUN1D5 knockdown in resistant cells. F 3D culture analysis post-DCUN1D5 knockdown in resistant cells. G Flow cytometric analysis of osteosarcoma cell apoptosis at 48 h. H Quantitative apoptosis assessment in osteosarcoma cells. ***P < 0.001 vs. SiRNA-NC. I a Si-NC, b Si-DCUN1D5 were co-cultured with U-2OS/CDDP cells for 24 h, c Si-NC, d Si-DCUN1D5 were co-cultured with MG63/CDDP cells for 24 h. J a Oe-NC, b Oe-DCUN1D5 were co-cultured with U-2OS/CDDP cells for 24 h, c Oe-NC, d Oe-DCUN1D5 were co-cultured with MG63/CDDP cells for 24 h. protein expression of PI3K/AKT/GSK3β signal path biomarkers were identified via immunoblotting. The quantitation data from the immunoblotting analysis were analyzed via the ImageJ program, which was expressed as mean ± SD.
To delve deeper into DCUN1D5’s impact on osteosarcoma, we synthesized two distinct siRNAs against DCUN1D5. Si-DCUN1D5-1 and Si-DCUN1D5-2 effectively curtailed DCUN1D5 expression in osteosarcoma cells, with the latter showing superior efficiency (Fig. S13D). Diverse osteosarcoma cell subsets were then exposed to graded cisplatin doses, revealing a notable reduction in cell viability and IC50 values in the Si-DCUN1D5 group as opposed to the Si-NC group (Fig. S13E, S13F). The scratch assay showed that the knockdown of DCUN1D5 reduced the closure rate of OS cells (Fig. S13M).
Tumor stemness is instrumental in dictating chemotherapy outcomes and potential tumor relapse65,66. To discern the nexus between DCUN1D5, tumor stemness, and chemotherapy resistance, we executed tumor spheroid formation assays. Post SiRNA-DCUN1D5 exposure, a significant diminution in the size and count of tumor spheroids from cisplatin-resistant osteosarcoma cells was observed, underscoring DCUN1D5’s role in amplifying tumor stemness (Fig. 8E). Concurrently, 3D cultures revealed a marked decline in the proliferative capacity of SiRNA-DCUN1D5 treated, cisplatin-resistant osteosarcoma cells over 48 h (Fig. 8F). Tumor cell apoptosis is pivotal in therapeutic interventions and in overturning drug resistance. As illustrated in Fig. 8G, H, apoptosis rates of cisplatin-resistant osteosarcoma cells post 48 h SiRNA-NC treatment were 7.28 ± 0.6% and 6.52 ± 0.8%. However, SiRNA-DCUN1D5 treatment for the same duration resulted in a pronounced spike in apoptosis (rates being 18.34 ± 1.1% and 19.5 ± 1.5%). Bioinformatics analysis using the KEGG database revealed a significant enrichment of the PI3K/AKT/GSK3β signaling pathway, prompting us to select it for further study. We subsequently investigated the influence of DCUN1D5 on the PI3K/AKT/GSK3β signaling pathway in U-2OS/CDDP and MG63/CDDP cell lines. Western blotting demonstrated that Si-DCUN1D5 substantially reduced the phosphorylation levels of PI3K, AKT, and GSK3β in osteosarcoma cells, in contrast to Oe-DCUN1D5, which enhanced these levels.
In vivo experiments evaluated the impact of DCUN1D5 on drug resistance, apoptosis, and proliferation in osteosarcoma cells
Earlier in vitro studies confirmed the critical function of DCUN1D5 in regulating phenotypes such as drug resistance, quiescence, apoptosis, and proliferation in osteosarcoma. Based on this, we constructed a subcutaneous tumor model in NSG mice to further investigate the role of DCUN1D5. Compared to the Si-NC group, the Si-DCUN1D5 group exhibited a notable decrease in tumor mass and volume (Fig. 9A–C). Moreover, in vivo, tumor sections revealed pronounced downregulation of DCUN1D5 expression in the Si-DCUN1D5 group (Fig. 9D, S13H). H&E staining results indicated a compromised structural integrity of tumor cells in the Si-DCUN1D5 group (Fig. 9E). Using TUNEL staining to ascertain the pro-apoptotic role of DCUN1D5 in vivo, we found that apoptotic cells in the SiRNA-DCUN1D5 group significantly outnumbered those in the SiRNA-NC group (Fig. 9F, S13I). These findings align with the in vitro results of SiRNA-DCUN1D5-induced cell apoptosis. Compared with the Si-NC group, no significant difference in mouse body weight was observed in the Si-DCUN1D5-treated group (Fig. S13J), suggesting that the SiRNA-mediated intervention has a lower level of toxicity.
A Representative images of subcutaneous tumors in NSG mice from different treatment groups, illustrating the impact of DCUN1D5 silencing on tumor size. B Quantitative analysis of tumor volumes in NSG mice over time. Tumor volume (V) was calculated using the formula V = (a x b^2) / 2, where ‘a’ is the longest diameter and ‘b’ is the shortest diameter of the tumor. C Comparison of tumor weights between different groups of NSG mice, highlighting the effect of DCUN1D5 knockdown on tumor growth. D Immunofluorescence staining for DCUN1D5 in dissected tumor tissues, demonstrating the downregulation of DCUN1D5 expression in the Si-DCUN1D5 group compared to the Si-NC group. E Hematoxylin and eosin (H&E) staining of tumor sections, revealing the histological changes and reduced structural integrity of tumor cells in the Si-DCUN1D5 group. F TUNEL assay and Ki-67 immunofluorescence staining in tumor sections to assess apoptosis and proliferation, respectively. The increased number of TUNEL-positive apoptotic cells and the reduced Ki-67 expression in the SiRNA-DCUN1D5 group compared to the SiRNA-NC group indicate the pro-apoptotic and anti-proliferative effects of DCUN1D5 silencing in vivo. Statistical significance is denoted by ***P < 0.005 vs. SiRNA-DCUN1D5.
Exosomes secreted by osteosarcoma drug-resistant cell lines enhance osteoclast differentiation and bone resorption
In investigating the role of DCUN1D5 in osteosarcoma drug resistance and recurrence, we assessed DCUN1D5 expression in the conditioned medium (CM) from drug-sensitive and drug-resistant osteosarcoma cells. We determined that drug-resistant osteosarcoma cells had a comparatively elevated DCUN1D5 expression in their CM compared to the drug-sensitive cells (Fig. 10A). Interestingly, In contrast, the DCUN1D5 levels in osteosarcoma CM remained constant post-RNase A treatment, they markedly declined following a combined treatment of RNase A and Triton X-100 (Fig. 10B). This suggests that extracellular DCUN1D5 is membrane-encapsulated rather than being directly secreted. Exosomes were extracted from the supernatants of MG63 and MG63/CDDP cultures and subsequently characterized using transmission electron microscopy, protein immunoblotting, and Nanosight. The identified exosomes predominantly exhibited a round or oval vesicular structure consistent with typical exosomal morphology (Fig. 10C). Nanoparticle tracking analysis (NTA) revealed an exosomal diameter ranging between 30 nm and 200 nm, peaking around 100 nm (Fig. 10D). Western blot assays indicated a pronounced presence of specific exosomal surface proteins CD63 and CD9, but an absence of the endoplasmic reticulum protein calnexin control (Fig. 10E). These findings validate the successful isolation of osteosarcoma-derived exosomes. To discern if DCUN1D5 chiefly originates from these exosomes, we employed GW4869 to inhibit their secretion. We observed a significantly enhanced DCUN1D5 expression in the osteosarcoma-derived exosomes compared to those from GW4869-treated cells (Fig. 10F). Additionally, qPCR evaluations of total CM, exosome-depleted CM (via ultracentrifugation), and exosomes showed elevated DCUN1D5 expression in both total CM and exosomes compared to the depleted CM (Fig. 10G). This indicates the primary encapsulation of DCUN1D5 within osteosarcoma-derived exosomes. Comparative analysis of DCUN1D5 expression between exosomes from regular and cisplatin-resistant osteosarcoma cells revealed significantly higher levels in the latter (Fig.10H, I). After a 6-hour incubation, PKH26-tagged MG63/CDDP-Exo, co-cultured with RAW264.7 cells, displayed cytoplasmic red fluorescence under a confocal microscope, suggesting RAW264.7 cell internalization of MG63/CDDP-Exo (Fig. 10J). Concurrently, MG63/CDDP-Exo exposure enhanced RAW264.7, MMP9, and CTSK expressions (Fig. 10K, L), indicative of activated osteolytic activity. In vivo experiments demonstrated that although there was no significant difference between the exosome group and the control group in the overall three-dimensional images of the tibia (Fig. 10M, P), radiographic analysis of femur and tibia cross-sections showed that the exosome-treated group had significantly reduced bone mass compared to the control group (Fig. 10N, O, Q, R). Compared with the control group, the MG63/CDDP-exo group showed significant reductions in bone mineral density (BMD), bone surface area (BS), bone volume/tissue volume ratio (BV/TV), trabecular thickness (Tb.Th), and trabecular number (Tb.N) in the cancellous bone at the distal metaphysis of the femur, while trabecular spacing (Tb.Sp) significantly increased. In the cortical bone of the mid-femur, the MG63/CDDP-exo group exhibited significant increases in total cross-sectional area (Tt.Ar), cortical area (Ct.Ar), cortical area/total cross-sectional area ratio (Ct.Ar/Tt.Ar), and cortical thickness (Ct.Th) (Fig. S14).
A DCUN1D5 expression in the culture medium (CM) of osteosarcoma parental and drug-resistant cell lines analyzed using qPCR. B DCUN1D5 expression in MG63 and MG63/CDDP cell lines after treatment with control medium, RNase A (2 mg/mL), or a combination with Triton X-100 (0.1%) for 0.5 h, assessed by qPCR. C Transmission microscopy-based phenotypic analysis of exosomes derived from MG63 cells, and (D) MG63/CDDP nanoparticle tracking with Nano Sight. E Detection of exosome biomarkers (TSG101, CD9, ALIX) in osteosarcoma cell lines using Western blotting. F, G qPCR assessment of DCUN1D5 expression in CM of osteosarcoma cells after exosome depletion using GW4869 (F) or ultracentrifugation (G), compared to MG63 and MG63/CDDP CM. H, I Comparative analysis of DCUN1D5 expression in osteosarcoma-derived exosomes and those from cisplatin-resistant cells using qRT-PCR (H) and Western blotting (I). J Uptake of PKH26 (red)-labeled exosomes by CFSE (green)-labeled RAW264.7 cells, visualized using confocal microscopy. K, L MMP9 and CTSK expression were analyzed using QRT-PCR and Western blotting. *P < 0.05, **P < 0.01, ***P < 0.001. M, P are three-dimensional gross images of the femur in the exosome treatment group and the control group, respectively. (N, Q) are images of various important sections of the femur in the exosome treatment group and the control group, respectively. O, R are images of various important sections and three-dimensional gross images of the tibia in the exosome treatment group and the control group, respectively.
Discussion
Osteosarcoma displays marked heterogeneity. Upon recurrence, patients face a substantially worsened prognosis67. Unraveling the mechanisms underpinning osteosarcoma recurrence is paramount for its scientific significance and implications on treatment selection. Previous studies have shown that Groenlandicine significantly enhances the sensitivity of cisplatin-resistant osteosarcoma cells to cisplatin by regulating the BAX/Bcl-2/Caspase-9/Caspase-3 signaling pathway68. Another study demonstrated that the GEMDOX chemotherapy regimen achieved good efficacy in patients with recurrent osteosarcoma69. These findings suggest that the development of new therapeutic strategies for cisplatin-resistant and recurrent osteosarcoma patients is an important direction for future research. Crucially, tumor cells don’t operate in isolation; they interact intimately with cells within the tumor microenvironment. Many studies have shown that tumor cells can remodel the tumor microenvironment, a process that leads to significant heterogeneity and immune suppression in the osteosarcoma immune microenvironment, which may result in suboptimal outcomes from conventional chemotherapy or immunotherapy70. This is the main driving factor of osteosarcoma recurrence. Recently, interventions targeting the tumor microenvironment have emerged as promising contenders in staving off drug resistance71. Consequently, probing osteosarcoma’s drug resistance pathways through its microenvironment lens could pave the way for innovative breakthroughs. In our investigation, we harnessed multiple scRNA-seq and bulk-seq datasets to delve into the cellular heterogeneity of osteosarcoma, contrasting primary with recurrent lesions. A meticulous cell atlas assessment of malignant entities and the microenvironment components further shed light on the tumor’s intricacy, suggesting potential therapeutic targets.
Ubiquitination and ubiquitin-like modification, as key post-translational modifications, involve the attachment of ubiquitin and ubiquitin-like molecules to specific proteins72,73. Such alterations can incite a spectrum of cellular functional shifts, encompassing protein degradation, relocation, and activity modulation. Ample evidence posits that ubiquitination aberrations are inextricably intertwined with cancer recurrence and heightened drug resistance63. Central to the ubiquitination and ubiquitin-like modification cascade, the variety and selectivity of E3 are determinative, dictating the proteins subjected to ubiquitin governance, thus spotlighting E3 as the linchpin enzyme74,75. By synergizing scRNA-seq with Bulk-seq datasets, we pinpointed an E3 enzyme, DCUN1D5, prominently expressed in osteosarcoma, with links to survival outcomes. Leveraging analytic tools such as GO, KEGG, GSVA, and GSEA, we charted the prospective roles of DCUN1D5 in osteosarcoma, spanning facets from extracellular matrix regulation to its seminal role in osteosarcoma’s immune milieu. Recent studies have demonstrated that the PI3K/AKT/GSK3β signaling pathway is instrumental in the pathogenesis and progression of multiple tumors, including breast, lung, and prostate cancers and leukemia. Aberrant activation of the PI3K/AKT/GSK3β pathway can promote epithelial-mesenchymal transition (EMT) and engage osteosarcoma cancer stem cells (CSC) in metastasis and recurrence. Western blotting was employed in this investigation to evaluate the expression of PI3K, p-AKT, and GSK3β proteins in drug-resistant osteosarcoma cell lines treated with Si-DCUN1D5. The findings revealed a significant reduction in PI3K, p-AKT, and GSK3β phosphorylation. Subsequent reversal experiments showed that Oe-DCUN1D5 could enhance the phosphorylation of these proteins. These results suggest that DCUN1D5 might modulate the onset, drug resistance, and relapse of osteosarcoma through the activation of the PI3K/AKT/GSK3β signaling pathway. Of significance, analyses on chemotherapy drug sensitivity and affinity unraveled that DCUN1D5 not only escalates the IC50 of several drugs but also exhibits pronounced affinity to frontline chemotherapy agents. In alignment, our in vitro and in vivo experimental endeavors corroborate our bioinformatic findings, revealing that DCUN1D5 possesses attributes from impeding apoptosis to amplifying osteosarcoma’s cisplatin resistance.
Cellular communication networks are pivotal in cancer drug resistance and recurrence76. Through comprehensive analysis of cell communication patterns, we identified a sophisticated communication network within osteosarcoma’s immune microenvironment. Here, osteosarcoma cells communicate with non-tumor cells using various mechanisms. Exosomes transport substances, including DNA, RNA, and proteins, acting as carriers, thereby serving as a vital conduit for cellular communication77. Current research suggests that osteosarcoma-derived exosomes profoundly influence drug resistance and recurrence. For a deeper insight into the specific cell communication leading to recurrence, we juxtaposed the communication features of primary and recurrent cancer cells. It is salient that pathways such as RESISTIN, ANGPTL, ncWNT, UGRP1, IL16, OSM, and BAG are markedly upregulated in recurrent cases. Substantial evidence shows that these pathways correlate with ubiquitylation, cancer drug resistance and osteoclast hyperactivity78,79,80,81. Notably, exosomes from cisplatin-resistant osteosarcoma cells, unlike those from sensitive cells, are enriched in DCUN1D5. Utilizing combined scRNA-seq and bulk-seq analyses, we observed a high expression of DCUN1D5 in osteoclasts. This suggests that the exosomes from drug-resistant osteosarcoma may target osteoclasts directly. Besides being integral to the osteosarcoma immune microenvironment, osteoclasts are crucial to recurrence and drug resistance in osteosarcoma. Pseudo-time analysis indicated that DCUN1D5 accelerates the maturation of osteoclast precursors. In vitro assays confirmed that DCUN1D5-rich exosomes activate osteoclasts, potentially driving osteosarcoma recurrence.
Our data underscore that DCUN1D5 enhances osteosarcoma cell stemness, proliferation, and resistance to apoptosis, intensifying resistance to cisplatin. Exosomes from cisplatin-resistant osteosarcoma cells can release DCUN1D5, likely inducing osteoclast hyperactivation—a potential key to recurrence. Thus, DCUN1D5 has a dual role in cancer drug resistance and osteoclast overactivation, contributing to osteosarcoma recurrence. Consequently, future interventions targeting DCUN1D5 might augment osteosarcoma’s sensitivity to cisplatin, offering novel therapeutic approaches for osteosarcoma patients.
This study still has some limitations. First, the mechanism by which DCUN1D5 exerts its E3 ligase function has not been fully elucidated. Additionally, the specific target proteins of DCUN1D5, its binding domains, and binding sites require further investigation. The role of DCUN1D5 in osteosarcoma, as an important biomarker, has been shown to alleviate cisplatin resistance. However, how to develop potential therapeutic strategies targeting DCUN1D5 remains to be explored in depth. Existing literature suggests that plant-derived natural compounds have significant efficacy in cancer treatment. Future research plans will focus on screening and developing plant natural compounds that target and inhibit DCUN1D5, hoping to achieve a synergistic effect when combined with cisplatin, thereby enhancing the cytotoxic effect on tumor cells. The zebrafish model and the chorioallantoic membrane (CAM) model, due to their high-throughput screening capacity, visual observation of tumor development, and relatively low experimental cost, have become important tools in tumor biology and drug screening82. This study only employed a mouse model, and future research plans will extend to the zebrafish model and CAM model to further validate the potential application of DCUN1D5 in cancer therapy.
This study provides a comprehensive analysis of the tumor microenvironment in osteosarcoma and explores potential mechanisms related to recurrence and resistance. Through screening, the E3 ligase DCUN1D5 was identified as a key factor significantly overexpressed in osteosarcoma, with a significant impact on patient prognosis. Our experimental results demonstrate that DCUN1D5 plays a crucial role in promoting osteosarcoma cell stemness, cisplatin resistance, proliferation, apoptosis inhibition, phosphorylation of the PI3K/p-AKT/GSK3β signaling pathway, and modulation of the tumor immune microenvironment. Therefore, DCUN1D5 is considered a promising therapeutic target. Future research will further investigate the specific mechanisms of action of DCUN1D5 as an E3 ligase, develop drugs targeting DCUN1D5, particularly relevant therapeutic strategies involving natural compounds, and validate the function of DCUN1D5 in osteosarcoma through multi-model approaches. It is hoped that these studies will provide new theoretical foundations and therapeutic strategies for the clinical treatment of osteosarcoma.
Method
Data collection
We used the following keywords to search for osteosarcoma data: (((osteosarcoma) AND single cell*) AND “Homo sapiens”[porgn:__txid9606]), and manually filtered the relevant studies. Ultimately, we selected the entire content of the GSE152048 and GSE162454 datasets, as well as the osteosarcoma-related data from the GSE198896 dataset (GSM5959132, GSM5959134, GSM5959136, and GSM5959138) as the subjects for our study37,83,84. Additionally, clinical data for these patients were extracted from the supplementary data provided in these studies. Clinical samples of bulk RNA-seq denoted as TARGET-OS and GSE21257 were obtained from TARGET and GEO databases (https://www.ncbi.nlm.nih.gov/).
scRNA-seq data analysis
The scRNA-seq data were primarily processed using the Seurat software (version 4.3.0). Low-quality single cells were filtered based on the criteria nFeature_RNA < 200, 12,000 < nFeature_RNA, nCount_RNA < 500, nCount_RNA < 200,000, and 20 < percent.mt. The RunHarmony function from the HARMONY package was used to integrate patient data, and batch correction was performed on the first 50 principal components (PCA components) to eliminate batch effects between patients. The data matrix was subsequently normalized with Seurat’s NormalizeData function. Major cell subtypes were clustered unsupervisedly using Seurat’s FindClusters function and represented using 2D UMAP or t-distributed stochastic neighbor embedding (tSNE). Cell cluster markers were then identified and annotated using Seurat’s FindAllMarkers function. The relationships between clusters at varying resolutions were visualized using Clustree. For heatmap presentations and enrichment analysis of differentially expressed genes across subtypes, we utilized ClusterGVis. Visualization of Marker genes was facilitated by the Nebulosa package, scCustomize package, and the Ridgeplot function85. Lastly, the transcriptional trajectories of osteoclasts were analyzed using Monocle2 and Monocle386,87.
Intercellular communication
We conducted an intercellular communication analysis using CellChat-1.6.1, available in the R package88. Initially, we established a CellChat object, utilizing the “createCellChat” function based on RNA expression matrices and pertinent cell information. We then proceeded with downstream analyses using ligand-receptor interaction databases such as “Secreted Signaling”, “ECM-Receptor”, and “Cell-Cell Contact”. The “computeCommunProb” function was employed to gauge communication probabilities. The “selectK” function was applied to discern global communication patterns, setting nPatterns at 2 and 4 for inbound and outbound communication patterns, respectively.
Survival analysis of E3 Ubiquitin and Ubiquitin-like Ligase-related Genes
We consolidated 2407 ubiquitin-associated genes derived from the integrated annotations of the Ubiquitin and Ubiquitin-like Conjugation Database (IUUCD) (http://iuucd.biocuckoo.org/). For survival analyses of these individual genes, refer to our prior studies.
Pathway analysis
We identified DEGs between clusters using Seurat’s FindMarkers function, applying a cutoff threshold of Padj. P-value < 0.05 and log2FC > 0.25. Subsequent analyses on the DEGs, encompassing both GO and KEGG, were executed using clusterProfiler. For GSEA and GSEV analyses, we adopted the GSEA and GSVA R packages, respectively.
Correlation with bulk-RNA seq in clinical cohorts
We utilized CibersortX and the mean expression of signature genes to interpret infiltration scores for each cell subtype in the TARGET clinical cohort89. The association between DCUN1D5 and cell clusters was determined via Spearman correlation.
OncoPredict for drug sensitivity analysis
Maeser and team developed the OncoPredict R package to forecast in vivo drug responses in cancer patients90. The software matches tissue gene expression profiles with the IC50 of drugs and gene expression from the Genomics of Drug Sensitivity in Cancer (GDSC; https://www.cancerrxgene.org/). This alignment fits the cancer cell line profile in the Broad Institute’s Cancer Cell Line Encyclopedia (CCLE; https://portals.broadinstitute.org/ccle_legacy/home). We computed a total of 198 drugs and examined the correlation between their IC50 and the DCUN1D5 gene using Spearman analysis.
Molecular docking analysis and molecular dynamics
The critical target protein’s crystal structure was procured from the SWISS-MODEL protein database (https://swissmodel.expasy.org/) and stored in “PDB” format. Drug structures in sdf format were sourced from PubChem (https://pubchem.ncbi.nlm.nih.gov/) and converted to mol2 format via the Open Babel GUI software. Using PyMOL and AutoDock4, protein conformation adjustments were made, including the removal of water molecules, the addition of non-polar hydrogen atoms, computation of protein charge, identification of rotatable bonds in ligands, and exportation in PDBQT format. Docking parameters were conFigured based on the receptor and ligand dimensions. Molecular docking was executed using AutoDock4, with results visualized in PyMOL. The selection threshold was set at a binding energy below -2.0 kcal/mol. We use the “Desmond” module of Schrodinger 2019 software for operations. We will simulate water molecules using the predefined SPC water model and OPLS2005 force field. To neutralize the system charge, an appropriate amount of chloride/sodium ions will be added to balance the system charge and randomly placed in the solvated system. After constructing the solvated system, energy minimization will be performed using the default protocol integrated with the Desmond module (using OPLS 2005 force field parameters). Nose-Hoover temperature coupling and isotropic scaling will be employed to maintain the temperature and pressure at 300 K and 1 atm; following this, NPT simulations will be run, with trajectory saving at intervals of 100 ps.
Establishment of drug-resistant osteosarcoma cell line
The human osteosarcoma cell lines MG63 and U2OS were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). In our previous study, we successfully established a drug-resistant cell line(MG63/CDDP and U-2OS/CDDP) using a stepwise increase in drug concentration induction method91. The drug-resistant cell line was created by inducing the osteosarcoma cell line with cisplatin over an appropriate period, thereby reducing its sensitivity to the drug.
Real-time fluorescent quantitative PCR
RNA, extracted with TRIzol (Invitrogen, USA), was reverse transcribed using the PrimeScript RT kit (TaKaRa, Japan). qPCR operations were conducted on the Applied Biosystems 7300 system employing SYBR Premix ExTaq (TaKaRa, Japan). mRNA primer sequences can be referenced in Table S1.
RNA interference
Invitrogen synthesized two siRNA pairs targeting human DCUN1D5, designated as Gene-SiRNA1 and Gene-SiRNA2. Primer sequences are elaborated in Supplementary Table S2. Osteosarcoma cells were seeded at a density of (Rosen, 1985; Ritter and Bielack, 2010; Li et al., 2011; Goguet-Surmenian et al. 2013; Smeland et al. 2019) × 10^5 per well in a 512-well plate. As cells reached 80% confluency, siRNA transfection was administered using Lipofectamine™ 3000, in alignment with manufacturer guidelines. Total RNA was isolated using TRIzol and reverse transcribed into cDNA, and mRNA levels were ascertained via RT-qPCR. The most potent siRNA was pinpointed through these trials, with the entire procedure being replicated thrice.
CCK-8 detection
Cells are cultured in a transparent 96-well plate and treated as described in the Figure legend. Following the manufacturer’s guidelines, cell proliferation is assessed using the CCK-8 assay kit (Sigma-Aldrich, St. Louis, MO). Absorbance at 450 nm is measured and corrected by subtracting the reference wavelength. The data presented in the Figure represents the relative optical density (OD).
Protein western blotting
Cells are lysed using the radioimmunoprecipitation assay buffer (RIPA, Beyotime, People’s Republic of China), and proteins are extracted and subsequently quantified through BCA analysis (Beyotime). The proteins are separated via 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Sigma-Aldrich, USA). The membrane is incubated with primary antibodies: anti-DCUN1D5 (1:1000, proteintech), anti-MMP9 (1:500, proteintech), anti-CTSK (1:500, proteintech), anti-AKT (1:1000; Cell Signaling Technology, USA), anti-PI3K (1:1000; Cell Signaling Technology), anti-GSK3β (1:1000; Cell Signaling Technology), anti-p-Akt (Ser-473; 1:1000; Cell Signaling Technology), anti-p-PI3K (Tyr458; 1:1000; Cell Signaling Technology), anti-p- GSK3β (Ser-9; 1:1000; Cell Signaling Technology), anti-p-PI3K (Tyr458; 1:1000; Cell Signaling Technology) and anti-GAPDH (1:1000, abcam), followed by secondary antibodies (1:5000, Cell Signaling Technology). Post-washing, the signals are detected using a chemiluminescence system (Bio-Rad, USA) and analyzed via the Image Lab software (Bio-Rad).
Immunofluorescence
In vivo immunofluorescence staining techniques detect apoptosis (TUNEL staining) and identify essential genes (DCUN1D5). These procedures adhere to the manufacturer’s instructions. Cell nuclei are stained with DAPI and visualized under a laser confocal microscope.
Immunohistochemistry
The osteosarcoma tissue sections are first subjected to EDTA or citrate-based decalcification until the tissue softens. The section thickness is 4-5 μm, and after dewaxing, dehydration is carried out using an alcohol gradient, followed by washing with PBS. Antigen retrieval is performed by incubating the tissue sections in a citrate buffer at 95–100 °C for 10–20 min. After removing endogenous peroxidase activity, the sections are incubated overnight with a primary antibody at a 1:1000 dilution (e.g., DCUN1D5). The sections are then incubated with a secondary antibody for 30–60 min, followed by treatment with ABC solution for 30 min. Finally, the sections are stained with DAB for 2–5 min. After staining, dehydration is performed, and the slides are mounted for microscopic observation.
Scratch assays
Osteosarcoma cells from different treatment groups were seeded into 6-well plates and cultured until 70% confluence. A sterile pipette tip made uniform vertical scratches in each well. PBS was used to wash away the detached cells, and a fresh medium was added to continue the culture. The scratch area was observed and imaged under a microscope, and the image was recorded at 0 h. Subsequently, the cells were incubated in a cell incubator, and the repair process was monitored by regularly capturing images at 12-hour intervals.
Tumor sphere formation assay
Cells (1000 per well) were seeded into ultra-low attachment 24-well plates (Corning, Corning, NY) and cultured in Dulbecco’s Modified Eagle Medium (DMEM)/F12 supplemented with human epidermal growth factor (EGF, ten ng/ml, PeproTech), human basic fibroblast growth factor (10 ng/ml, PeproTech), and N-2 supplement (1×, Gibco, Waltham, MA). Cultures were maintained for 14 days at 37 °C, 5% CO2. Cells were fed every three days by adding 100 μl of fresh culture medium to each well. Sphere diameter and number were recorded using an inverted microscope, and the dryness of tumor cells was assessed by comparing the average diameter and number of spheres under different conditions92.
Flow Cytometry Detection of Cell Apoptosis
Following the described transfection, the supernatant is harvested after a 48-hour incubation. The cells are treated with trypsin, omitting EDTA and yielding single-cell suspensions. After resuspension in cold PBS and centrifugation, cells are washed with binding buffer and stained using annexin V-FITC and propidium iodide. Apoptosis analysis is promptly conducted via flow cytometry.
Soft agar colony formation
The soft agar colony formation assay is executed as follows: A foundational layer containing 0.6% low-melting agarose is established using MEM culture medium enriched with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin. Subsequently, a top layer, constituted of 0.3% agarose and 5000 specified cells, is formulated using the identical MEM culture medium. The plates undergo incubation at 37 °C, with 5% CO2, in a humidity-controlled environment for approximately two weeks. Cell colonies are stained for 20 min using a 0.5% crystal violet blend and 25% methanol. These plates are subsequently scanned and captured, with colony count determined using Quantity One v.4.0.3 software by Bio-Rad, Hercules, CA.
Exosome isolation, characterization, and uptake by RAW264.7 Cells
The in-depth exosome isolation and characterization procedures are delineated in previous research93. RAW264.7 cells, at a density of 5 × 10^4/mL, are cultured on dishes appropriate for laser confocal microscopy and are left to incubate overnight. The exosomes undergo labeling with PKH26, as per the PKH26 dye guidelines, followed by introducing 15ug/mL PKH26-Exosome to the RAW264.7 cells. After a 6-hour incubation, the RAW264.7 cells are collected with the supernatant,and the supernatant is discarded. The cells are then fixed with 4% paraformaldehyde for 10 min, rinsed thrice with PBS, overlaid with CFSE dye for 5 min, and finally stained with DAPI for 3 min. The cellular uptake of exosomes is visualized using a confocal microscope.
Animal experiments
The establishment of humanized NSG mice has been described in previous studies93. The method involves irradiating newborn NOD-scid IL2rγnull (NSG) mice followed by intravenous injection of human CD34+ hematopoietic stem cells. After 8 weeks, the proportion of human CD45+ cells is measured, with a proportion greater than 10% indicating successful modeling, making these mice suitable for subsequent experiments. Next, 1 × 10^7 U2OS/R cells were subcutaneously injected into the NSG mice to construct a drug-resistant tumor model. The successfully modeled NSG mice were randomly divided into two groups: the SiRNA-NC group (subcutaneously injected with Si-NC/MG63 cells and intraperitoneally injected with cisplatin) and the SiRNA-DCUN1D5 group (subcutaneously injected with Si-DCUN1D5/MG63 cells and intraperitoneally injected with cisplatin). Each group consisted of three mice. Tumor growth was continuously observed, and tumor volume was recorded. When the tumor volume reached 50 mm^3, the mice were assigned according to their groups and intraperitoneally injected with cisplatin (diluted with saline to 1 μg/g) twice weekly. Tumor volume and mouse weight were evaluated weekly. At the fourth week, the mice were euthanized; tumors were excised, weighed, and analyzed for DCUN1D5 and TUNEL expression, as well as HE staining.
To evaluate the impact of exosomes derived from drug-resistant cell lines on bone resorption, 100 μL of PBS solution containing 10 μg of MG63/CDDP cell-derived exosomes or regular PBS solution was injected into the tail vein of NSG mice every three days. After four weeks, the NSG mice were euthanized, and the femurs and tibias were fixed in 4% paraformaldehyde and stored in PBS at 4 °C. The specimens were scanned using high-resolution micro-CT (Skyscan 1276, Skycan, Aartselaar, Belgium) with a resolution of 20.376 μm per pixel, a voltage of 100 kV, and a current of 200 μA. To analyze trabecular bone parameters, including bone mineral density (BMD), bone surface area (BS), bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp), the region of interest (ROI) was selected to extend 0.45 mm from below the distal growth plate of the femur towards the proximal direction. Additionally, cortical bone parameters in the midshaft of the femur were evaluated, including total cross-sectional area (Tt.Ar), cortical area (Ct.Ar), cortical area ratio (Ct.Ar/Tt.Ar), and cortical thickness (Ct.Th), within a range of 0.2 mm.
Statistics and Reproducibility
Data from at least three independent biological replicates were reported as the mean ± SD. Statistical differences were determined by an unpaired Student’s t test or two-way ANOVA with the corresponding two-tailed significance determined. Statistical analysis was performed using GraphPad Prism 9 software (GraphPad Software Inc., San Diego, CA), and differences were considered significant at p < 0.05.
Data availability
The scRNA-seq reported in this paper are publicly available through GSE152048, GSE162454, and GSE198896. All the relevant data are available from the corresponding author upon reasonable request. The raw data for all the figures in the manuscript can be found as Supplementary Data 1. Uncropped Western blot images are available in the Supplementary Information.
References
Tang, N. et al. Single-Cell Spatial-Temporal Analysis of ZNF451 in Mediating Drug Resistance and CD8<SUP>+</SUP> T Cell Dysfunction. Research 7, 23 (2024).
Qiu, J. et al. Dissecting the Distinct Tumor Microenvironments of HRD and HRP Ovarian Cancer: Implications for Targeted Therapies to Overcome PARPi Resistance in HRD Tumors and Refractoriness in HRP Tumors. Adv. Sci. (Weinh.) 11, e2309755 (2024).
Yang, L. et al. Synergetic Functional Nanocomposites Enhance Immunotherapy in Solid Tumors by Remodeling the Immunoenvironment. Adv. Sci. (Weinh.) 6, 1802012 (2019).
Jin, H. et al. Tumor Ablation and Therapeutic Immunity Induction by an Injectable Peptide Hydrogel. ACS Nano 12, 3295–3310 (2018).
Low, J. T., Ho, P. C. & Matsushita, M. TAM-tastic: from resistance to resilience in cancer. Trends Pharm. Sci. 45, 953–954 (2024).
Tang, N. et al. Decoding the role of SLC25A5 in osteosarcoma drug resistance and CD8+ T cell exhaustion: The therapeutic potential of phyllanthin. Phytomedicine 136, 156291–156291 (2024).
Wu, Z. G. et al. N6-Methyladenosine-Related LncRNAs Are Potential Remodeling Indicators in the Tumor Microenvironment and Prognostic Markers in Osteosarcoma. Front. Immunol. 12, 19 (2022).
Wang, X. et al. Single-cell RNA-seq reveals the genesis and heterogeneity of tumor microenvironment in pancreatic undifferentiated carcinoma with osteoclast-like giant-cells. Mol. Cancer 21, 133 (2022).
Beider, K. et al. CXCL13 chemokine is a novel player in multiple myeloma osteolytic microenvironment, M2 macrophage polarization, and tumor progression. J. Hematol. Oncol. 15, 144 (2022).
Wu, X. et al. A Targeted Erythrocyte Membrane-Encapsulated Drug-Delivery System with Anti-osteosarcoma and Anti-osteolytic Effects. ACS Appl. Mater. Interfaces 13, 27920–27933 (2021).
Jiang, Z. et al. Versatile Polymer-Initiating Biomineralization for Tumor Blockade Therapy. Adv. Mater. 34, e2110094 (2022).
Lamora, A. et al. Overexpression of smad7 blocks primary tumor growth and lung metastasis development in osteosarcoma. Clin. Cancer Res. 20, 5097–5112 (2014).
Liu, W. B. et al. A simple, universal and multifunctional template agent for personalized treatment of bone tumors. Bioact. Mater. 12, 292–302 (2022).
Deng, L., Meng, T., Chen, L., Wei, W. & Wang, P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct. Target Ther. 5, 11 (2020).
Lim, S. O. et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell 30, 925–939 (2016).
Xiong, W. et al. USP8 inhibition reshapes an inflamed tumor microenvironment that potentiates the immunotherapy. Nat. Commun. 13, 1700 (2022).
Cruz Walma, D. A., Chen, Z., Bullock, A. N. & Yamada, K. M. Ubiquitin ligases: guardians of mammalian development. Nat. Rev. Mol. Cell Biol. 23, 350–367 (2022).
Lin, Y. et al. YY1 mediated DCUN1D5 transcriptional activation promotes triple-negative breast cancer progression by targeting FN1/PI3K/AKT pathway. Biol. Direct 19, 42 (2024).
Zhao, S. et al. DCUN1D5 is a prognostic biomarker and correlated immune infiltrates and glycolysis in lung adenocarcinoma. Sci. Rep. 15, 403 (2025).
Bommeljé, C. C. et al. Oncogenic function of SCCRO5/DCUN1D5 requires its Neddylation E3 activity and nuclear localization. Clin. Cancer Res. 20, 372–381 (2014).
Chen, C. et al. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 500, 1–10 (2021).
Wu, X., Deng, Z. & Zhao, Q. Q. Immunotherapy improves disease prognosis by affecting the tumor microenvironment: A bibliometric study. Front. Immunol. 13, 20 (2022).
Martins, A. S. et al. Cytotoxicity of Platinum and Palladium Chelates against Osteosarcoma. Chemistryselect 5, 5993–6000 (2020).
Balsa, L. M. et al. Cu(II)-acylhydrazone complex, a potent and selective antitumor agent against human osteosarcoma: Mechanism of action studies over in vitro and in vivo models. Chem.-Biol. Interact. 384, 110685 (2023).
Niu, J. et al. The COPS3-FOXO3 positive feedback loop regulates autophagy to promote cisplatin resistance in osteosarcoma. Autophagy 19, 1693–1710 (2023).
Zhou, Y. et al. miR-33a is up-regulated in chemoresistant osteosarcoma and promotes osteosarcoma cell resistance to cisplatin by down-regulating TWIST. J. Exp. Clin. Cancer Res.: CR 33, 12 (2014).
Engel, J. L. et al. The Fanconi anemia pathway induces chromothripsis and ecDNA-driven cancer drug resistance. Cell 187, 6055–6070.e6022 (2024).
Rottenberg, S. et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl Acad. Sci. USA 105, 17079–17084 (2008).
Zhu, Y. et al. Targeting gut microbial nitrogen recycling and cellular uptake of ammonium to improve bortezomib resistance in multiple myeloma. Cell Metab. 36, 159–175.e158 (2024).
Pi, M. et al. Targeting metabolism to overcome cancer drug resistance: A promising therapeutic strategy for diffuse large B cell lymphoma. Drug Resist Updat 61, 100822 (2022).
Jin, P. et al. Mitochondrial adaptation in cancer drug resistance: prevalence, mechanisms, and management. J. Hematol. Oncol. 15, 97 (2022).
Swamydas, M., Murphy, E. V., Ignatz-Hoover, J. J., Malek, E. & Driscoll, J. J. Deciphering mechanisms of immune escape to inform immunotherapeutic strategies in multiple myeloma. J. Hematol. Oncol. 15, 17 (2022).
Kansara, M. et al. Infiltrating Myeloid Cells Drive Osteosarcoma Progression via GRM4 Regulation of IL23. Cancer Discov. 9, 1511–1519 (2019).
Fu, L., Zhang, W., Zhou, X., Fu, J. & He, C. Tumor cell membrane-camouflaged responsive nanoparticles enable MRI-guided immuno-chemodynamic therapy of orthotopic osteosarcoma. Bioact. Mater. 17, 221–233 (2022).
Ge, Y. X. et al. Enhancement of anti-PD-1/PD-L1 immunotherapy for osteosarcoma using an intelligent autophagy-controlling metal organic framework. Biomaterials 282, 121407 (2022).
Ma, S. W., Wu, X., Wu, Z. G. & Zhao, Q. Q. Treatment-prognostication-adjustment a new therapeutic idea by analyzing T cell immune checkpoint in tumor microenvironment by algorithm: A bibliometric analysis. Hum. Vaccines Immunother. 19, 16 (2023).
Zhou, Y. et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 11, 6322 (2020).
Cervantes-Villagrana, R. D., Albores-García, D., Cervantes-Villagrana, A. R. & García-Acevez, S. J. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduct. Target Ther. 5, 99 (2020).
He, G. et al. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol. Cancer 21, 19 (2022).
Kalluri, R. & McAndrews, K. M. The role of extracellular vesicles in cancer. Cell 186, 1610–1626 (2023).
Zhang, S. et al. Monitoring of cell-cell communication and contact history in mammals. Science 378, eabo5503 (2022).
Bayik, D. & Lathia, J. D. Cancer stem cell-immune cell crosstalk in tumour progression. Nat. Rev. Cancer 21, 526–536 (2021).
Lai, Z. Z. et al. Single-cell transcriptome profiling of the human endometrium of patients with recurrent implantation failure. Theranostics 12, 6527–6547 (2022).
He, J. et al. Obacunone targets macrophage migration inhibitory factor (MIF) to impede osteoclastogenesis and alleviate ovariectomy-induced bone loss. J. Adv. Res. 53, 235–248 (2023).
Torres-Rodríguez, C. et al. Effects of bleaching on osteoclast activity and their modulation by osteostatin and fibroblast growth factor 2. J. Colloid Interface Sci. 461, 285–291 (2016).
Walker, E. C. et al. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J. Clin. Invest 120, 582–592 (2010).
Khoso, M. H. et al. Purification and extraction of fibroblast growth factor 21 (FGF-21) protein by sumo fusion in Escherichia coli. Pak. J. Pharm. Sci. 35, 1813–1818 (2022).
Niu, J. et al. Efficient treatment of Parkinson’s disease using ultrasonography-guided rhFGF20 proteoliposomes. Drug Deliv. 25, 1560–1569 (2018).
Pauws, E. & Stanier, P. FGF signalling and SUMO modification: new players in the aetiology of cleft lip and/or palate. Trends Genet 23, 631–640 (2007).
Shen, S. et al. CircECE1 activates energy metabolism in osteosarcoma by stabilizing c-Myc. Mol. Cancer 19, 151 (2020).
Wang, X. et al. Targeting the CK1α/CBX4 axis for metastasis in osteosarcoma. Nat. Commun. 11, 1141 (2020).
Williams, S. A. et al. USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell 146, 918–930 (2011).
Zeng, C. et al. Targeting the Lysosomal Degradation of Rab22a-NeoF1 Fusion Protein for Osteosarcoma Lung Metastasis. Adv. Sci. (Weinh.) 10, e2205483 (2023).
Cao, J. et al. Hypoxia-Induced WSB1 Promotes the Metastatic Potential of Osteosarcoma Cells. Cancer Res. 75, 4839–4851 (2015).
Zhang, T. et al. CDK7/GRP78 signaling axis contributes to tumor growth and metastasis in osteosarcoma. Oncogene 41, 4524–4536 (2022).
Zhang, Y. et al. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics 11, 1473–1492 (2021).
Lu, K. H., Lu, E. W., Lin, C. W., Yang, J. S. & Yang, S. F. New insights into molecular and cellular mechanisms of zoledronate in human osteosarcoma. Pharm. Ther. 214, 107611 (2020).
Sun, S. G., Lau, Y. S., Itonaga, I., Sabokbar, A. & Athanasou, N. A. Bone stromal cells in pagetic bone and Paget’s sarcoma express RANKL and support human osteoclast formation. J. Pathol. 209, 114–120 (2006).
Zheng, L. et al. Destabilization of macrophage migration inhibitory factor by 4-IPP reduces NF-κB/P-TEFb complex-mediated c-Myb transcription to suppress osteosarcoma tumourigenesis. Clin. Transl. Med. 12, e652 (2022).
Li, Y. et al. Amplification of LAPTM4B and YWHAZ contributes to chemotherapy resistance and recurrence of breast cancer. Nat. Med 16, 214–218 (2010).
Hackl, H., Astanina, K. & Wieser, R. Molecular and genetic alterations associated with therapy resistance and relapse of acute myeloid leukemia. J. Hematol. Oncol. 10, 51 (2017).
Yang, S. et al. The E3 ubiquitin ligase Pellino3 protects against obesity-induced inflammation and insulin resistance. Immunity 41, 973–987 (2014).
Ming, H. et al. Protein degradation: expanding the toolbox to restrain cancer drug resistance. J. Hematol. Oncol. 16, 6 (2023).
Zhan, H. et al. Exosomal CTCF Confers Cisplatin Resistance in Osteosarcoma by Promoting Autophagy via the IGF2-AS/miR-579-3p/MSH6 Axis. J. Oncol. 2022, 9390611 (2022).
Yang, Z. et al. Single-cell Sequencing Reveals Variants in ARID1A, GPRC5A and MLL2 Driving Self-renewal of Human Bladder Cancer Stem Cells. Eur. Urol. 71, 8–12 (2017).
Ohta, Y. et al. Cell-matrix interface regulates dormancy in human colon cancer stem cells. Nature 608, 784–794 (2022).
Duffaud, F. et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 20, 120–133 (2019).
Zhao, Z. et al. Groenlandicine enhances cisplatin sensitivity in cisplatin-resistant osteosarcoma cells through the BAX/Bcl-2/Caspase-9/Caspase-3 pathway. J. Bone Oncol. 48, 100631 (2024).
Low, K., Foulkes, P., Hills, F., Roberts, H. C. & Stordal, B. The efficacy of gemcitabine and docetaxel chemotherapy for the treatment of relapsed and refractory osteosarcoma: A systematic review and pre-clinical study. Cancer Med. 13, e70248 (2024).
Recine, F. et al. Clinical and translational implications of immunotherapy in sarcomas. Front Immunol. 15, 1378398 (2024).
Zhang, Z. et al. Tumor Microenvironment-Derived NRG1 Promotes Antiandrogen Resistance in Prostate Cancer. Cancer Cell 38, 279–296.e279 (2020).
Popovic, D., Vucic, D. & Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 20, 1242–1253 (2014).
Wu, X. et al. Spatiotemporal evolution of AML immune microenvironment remodeling and RNF149-driven drug resistance through single-cell multidimensional analysis. J. Transl. Med. 21, 21 (2023).
Oltion, K. et al. An E3 ligase network engages GCN1 to promote the degradation of translation factors on stalled ribosomes. Cell 186, 346–362.e317 (2023).
Pao, K. C. et al. Activity-based E3 ligase profiling uncovers an E3 ligase with esterification activity. Nature 556, 381–385 (2018).
Maacha, S. et al. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol. Cancer 18, 55 (2019).
Cully, M. Exosome-based candidates move into the clinic. Nat. Rev. Drug Discov. 20, 6–7 (2021).
Geethadevi, A. et al. Oncostatin M Receptor-Targeted Antibodies Suppress STAT3 Signaling and Inhibit Ovarian Cancer Growth. Cancer Res. 81, 5336–5352 (2021).
Skundric, D. S., Cruikshank, W. W. & Drulovic, J. Role of IL-16 in CD4+ T cell-mediated regulation of relapsing multiple sclerosis. J. Neuroinflammation 12, 78 (2015).
Alberti, S. et al. Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J. Biol. Chem. 277, 45920–45927 (2002).
Cardamone, M. D. et al. A protective strategy against hyperinflammatory responses requiring the nontranscriptional actions of GPS2. Mol. Cell 46, 91–104 (2012).
Giusti, V. et al. Xenografting Human Musculoskeletal Sarcomas in Mice, Chick Embryo, and Zebrafish: How to Boost Translational Research. Biomedicines 12, 1921 (2024).
Cillo, A. R. et al. Ewing Sarcoma and Osteosarcoma Have Distinct Immune Signatures and Intercellular Communication Networks. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 28, 4968–4982 (2022).
Rothzerg, E. et al. Single-Cell Transcriptome Analysis Reveals Paraspeckles Expression in Osteosarcoma Tissues. Cancer Inf. 21, 11769351221140101 (2022).
Alquicira-Hernandez, J. & Powell, J. E. Nebulosa recovers single-cell gene expression signals by kernel density estimation. Bioinformatics 37, 2485–2487 (2021).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Steen, C. B., Liu, C. L., Alizadeh, A. A. & Newman, A. M. Profiling Cell Type Abundance and Expression in Bulk Tissues with CIBERSORTx. Methods Mol. Biol. 2117, 135–157 (2020).
Maeser, D., Gruener, R. F. & Huang, R. S. oncoPredict: an R package for predicting in vivo or cancer patient drug response and biomarkers from cell line screening data. Brief. Bioinform 22, bbab260 (2021).
Xin, W., Tang, N., Zhao, Q. & Xiong, J. Spatiotemporal evolutionary process of osteosarcoma immune microenvironment remodeling and C1QBP-driven drug resistance deciphered through single-cell multi-dimensional analysis. Bioeng. Transl. Med. https://doi.org/10.1002/btm2.10654 (2024).
Li, H. et al. FOXP1 drives osteosarcoma development by repressing P21 and RB transcription downstream of P53. Oncogene 40, 2785–2802 (2021).
Wu, X. et al. Unveiling the PDK4-centered rituximab-resistant mechanism in DLBCL: the potential of the “Smart” exosome nanoparticle therapy. Mol. Cancer 23, 144 (2024).
Acknowledgements
I would like to thank my wife Ethna for her unwavering support of this study. This work was supported by the National Natural Science Foundation of China (No. 82260365, 82372315), the Guangxi Natural Science Foundation (No. 2025GXNSFDA069032), the Liuzhou Science and Technology Program (2021YB0104B060,2024YB0103A012), the Hunan Provincial Innovation Foundation for Postgraduate (CX20230296), The 2024 Guangxi Zhuang Autonomous Region Bagui Young Top-notch Talent Program, Document No. Gui Ke Fa [2025] No. 181, the Basic Research Program of Qinghai Province – Applied Basic Research Program (2023-ZJ-716),and the High-Level Talent Research Start-up Fund of Liuzhou People’s Hospital (LRYGCC202407).
Author information
Authors and Affiliations
Contributions
X.W. and W.D.D. conceived and supervised the study. X.W. performed the bioinformatics analysis. Q.Q.Z. and J.B.X. analyzed the data. Q.Q.Z. and J.B.X. conducted the experiments. X.W. and JBX wrote the draft. X.W., Q.Q.Z., and J.B.X. revised and validated the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. The authors declare no competing interests. Figure 6I, Figure S13A and graphic abstract, was created with BioRender software (https://biorender.com/, accessed on 3 March 2023).
Ethical approval
All animal care and experimental procedures were conducted in accordance with the guidelines and were approved by the Ethics Committee of the Department of Laboratory Animal Science, Central South University (Approval Number: CSU-2022-0664; Approval Date: October 10, 2022). We have complied with all relevant ethical regulations for animal use.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Dr. Silvia Belluti and Dr. Ophelia Bu.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, X., Deng, W., Zhao, Q. et al. Single-cell analysis links DCUN1D5 to immune remodeling and cisplatin resistance in recurrent osteosarcoma. Commun Biol 8, 1019 (2025). https://doi.org/10.1038/s42003-025-08409-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08409-w