Abstract
Kabuki syndrome type 1 is a congenital multisystem disorder caused by KMT2D mutations. While some studies suggest that KMT2D deficiency may lead to autistic-like behaviors, the role of KMT2D in social behavior remains unconfirmed due to a lack of animal model evidence. In this study, we developed a mouse knockdown model and a zebrafish knockout model to investigate the role of KMT2D in synaptic function and behavioral patterns. We also performed an RNA sequencing analysis to delve into the molecular and cellular mechanisms of KMT2D. Results showed that Kmt2d deficiency in mouse and zebrafish both exhibited autistic-like behaviors including social behaviors defects and repetitive behavior. Additionally, knockdown of Kmt2d in the mouse hippocampus decreases excitatory and increases inhibitory synaptic transmission, disrupting the excitation-inhibition balance—a hallmark of autistic-like behaviors. RNA sequencing analysis revealed that under conditions of low kmt2d expression, differentially expressed genes were associated with glutamatergic and GABAergic synapses, supporting the dysregulation of excitation-inhibition balance in the hippocampus. Taken together, our research elucidates the critical role of KMT2D in modulating animal social behavior, potentially through its impact on synaptic excitation-inhibition balance.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition typically present from early childhood, marked by difficulties in social interaction and communication, narrow interests, and repetitive, ritualistic behaviors. It affects approximately 1 in 50 children, with a male-to-female ratio of around 4:1, and has a heritability estimate ranging from 70 to 90%1.
Mulitiple single-gene forms of neurodevelopmental disorders are associated with abnormal H3K4 methylation regulation2. It is highly probable that precise control and adjustment of H3K4 methylation marks are crucial for proper brain development, as mutations leading to either a decrease (KMT2A, KMT2D) or an increase (ARX, CUL4B, KDM5A, KDM5C) in H3K4 methylation could lead to intellectual disabilities and autism, microcephaly, epilepsy, and other neurological conditions during early childhood3. Normal gene dosage of KMT2D is essential for human health, and haploinsufficiency of that is associated with developmental delay and disability4. About 75% of individuals with Kabuki syndrome type 1 (KS1) carry deleterious mutations in KMT2D5. In recent years, five patients carrying KMT2D mutations reported in the literature have described the co-occurrence of KS1 and ASD6,7,8. This evidence indicates that genetic alterations in the KMT2D gene may contribute to the development of ASD. However, current research lacks animal model data that characterize the role of KMT2D gene in the pathogenesis of ASD.
To enhance the discovery of therapeutic targets and deepen our comprehension of the pathological mechanisms behind this disorder, Kmt2d-knockout (KO) mice and zebrafish for KS were engineered9,10. The loss of KMT2D recapitulates various features of KS1 in zebrafish and mice, including hearing abnormalities, deficits in hippocampal learning and memory, skull deformities, reduced body length, and cardiac defects11,12,13,14,15. Nevertheless, Kmt2d-KO mice or kmt2d-KO zebrafish have not been documented to exhibit diminished social interest, repetitive behaviors, or impairments in social communication.
In this study, we integrated mouse and zebrafish models with electrophysiological and RNA-seq analyses to elucidate the role of KMT2D in autistic-like behaviors. We found that functional deficiency of KMT2D leads to social and behavioral impairments and disrupts the synaptic excitation-inhibition (E-I) balance, a hallmark of autistic-like behaviors. Overall, our data highlight the critical role of KMT2D in modulating social behavior and provide important insights into the underlying mechanisms of ASD.
Results
Disruption of KMT2D is associated with autism and other neurodevelopmental delay (NDD) phenotypes
We assemble genotype and phenotype data for 9 affected individuals from 9 unrelated families with predicted deleterious variants in KMT2D through literature and two web-based databases (DECIPHER and ClinVar) (Table S1). This includes 5 individuals with de novo (n = 4) and transmitted (n = 1) truncating variants, 1 individual with missense variant, and three with unknown variants (Fig. 1A).
The probands were diagnosed with autism (100%, 9/9). Intelligence disability (100%, 8/8), dysmorphic facial features (100%, 7/7), cardiac anomaly (57.14%, 4/7), Speech delay (50%, 3/6), and hypotonia (50%, 3/6) were commonly co-occurred. In addition, clinodactyly of the fifth finger (42.86%, 3/7), motor delay (33.3%, 2/6), anxiety (33.3%, 2/6), joint laxity (33.3%, 2/6), hearing loss (28.57%, 2/7), feeding difficulties (28.57%, 2/7), hyperactivity (16.67%, 1/6), coordination difficulty (16.67%, 1/6), and sleep problems (16.67%, 1/6) are also observed in subsets of the patients (Fig. 1B and Table S2). In summary, a wide range of ASD or NDD phenotypes are present in probands with KMT2D mutation.
Knockout of zebrafish kmt2d leads to developmental delay and autism-like behaviors
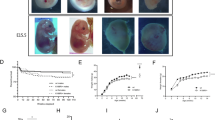
To examine whether KMT2D plays an evolutionarily conserved role, we utilized zebrafish models. We targeted the gene using the CRISPR/Cas9 system and generated a -92 + 6 bp deletion allele for kmt2d, which introduces frame shifts and early truncations in the corresponding proteins (Fig. 2A). Additionally, we conducted sanger sequencing, agarose gel electrophoresis and RT-qPCR to confirm the knockout effect of kmt2d mutant (Fig. 2B, C; Figs. S1, S4). Specifically, we conducted heterozygous-by-heterozygous crosses for the −92 + 6 bp deletion alleles and screened for phenotypes, with particular emphasis on the organs and systems affected in patients with KS. The kmt2d−/− embryos exhibiting morphological abnormalities, including tail bending, yolk malformation, pericardial edema (Fig. 2D), and reduced body length at different developmental stages (Fig. 2F). RT-qPCR analysis confirmed that kmt2d mRNA expression was significantly reduced at three points in embryos with KS phenotypes (Fig. 2E). Our results indicated that the zebrafish kmt2d knockout line has been successfully established and can be utilized for further experiments.
A–C Diagram of zebrafish kmt2d gene and mutation induced by CRISPR/Cas9, gray boxes indicate exons. A target site was on Exon 28 resulting in a 92-base deletion and 6-base insertion. B Sanger sequence. C results of agarose gel electrophoresis. D kmt2d−/− embryos exhibiting morphological abnormalities at the age of 24 h, 36 h and 72 h. E The expression levels of the Kmt2d gene decreased at three developmental point for embryos exhibiting KS phenotypes (Fifteen embryos per biological replicate were collected for zebrafish RT-qPCR, n = 3). F Body length was reduced in the kmt2d knockout zebrafish (n = 9). G Schematic diagram of the social preference behaviours experiment and motion trajectory chart for zebrafish. H Sniffing time exploring social region (n = 6 for wt, n = 7 for kmt2d-/+). I The distance traveled in the mating tank, which suggested that the kmt2d-/+ zebrafish had normal motor skill (n = 6 for wt, n = 7 for kmt2d−/+). Error bars represent as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ns: P > 0.05, Mann-Whitney U test.
To investigate whether kmt2d haploinsufficiency leads to autistic-like behaviors in zebrafish as it does in human, we monitored the social behavior of kmt2d+/− male adult zebrafish (12 weeks of age), in which a single kmt2d allele is inactivated. Zebrafish are gregarious animals with a strong inclination toward social activity. Their social preference behavior served as indicators of their social tendencies. In our research, we examined how kmt2d+/− zebrafish interacted socially. In the social preference test, the kmt2d+/+ zebrafish consistently moved through the designated social zone throughout the trial, while the kmt2d+/− zebrafish exhibited aimless swimming patterns. The kmt2d+/− zebrafish exhibited notable differences in behavior compared to their kmt2d+/+ counterparts. Specifically, they spent significantly less time in the social zone (Fig. 2G, H). Despite maintaining typical motor skills (Fig. 2I), these kmt2d+/− zebrafish displayed a propensity for large circular movements, a repetitive behavior commonly observed in zebrafish and similar to behaviors seen in individuals with autism (Fig. 2G).
Kmt2d deficiency mice showed defects in social behaviors and had repetitive behavior
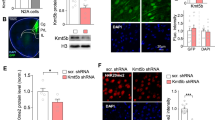
Using published single-cell RNA sequencing datasets16 (CNP0003837), we found that Kmt2d is widely expressed in different subregions of the hippocampus and in various types of neurons (Fig. S2A, B), and further confirmed the expression of KMT2D in the hippocampus via immunofluorescence experiments (Fig. S2C). To elucidate the relationship between the function of KMT2D in the hippocampus and autism-like behaviors, we selectively knocked down the Kmt2d gene in the hippocampal region of mice and examined its impact on their social behavior. We firstly screened for effective RNA interference (RNAi) fragments targeting Kmt2d in mouse HT22 cells (Figs. S3A–C; S5B, C). Then, adeno-associated virus (AAV) vectors carrying Kmt2d-shRNA (AAV-RNAi) or control-shRNA (AAV-nc) were injected into the hippocampus, respectively. After a four-week post-injection period, we analyzed sections of mouse brain tissue under a fluorescence microscope and observed positive GFP expression specifically in the hippocampal region (Fig. 3A). The results of RT-qPCR demonstrated that the expression level of the kmt2d gene was significantly reduced in the AAV-RNAi group compared to the AAV-nc control group (Fig. 3B). Given that KMT2D functions as a histone methyltransferase responsible for catalyzing the formation of H3K4me3, it is not surprising that the H3K4me3 levels were also decreased in the hippocampus of mice with kmt2d knockdown (Figs. 3C, D; S5A). These results indicate that we have successfully established a mouse model with hippocampal-specific knockdown of kmt2d.
A The adeno-associated virus (AAV) vectors carrying Kmt2d-shRNA (AAV-RNAi) or control-shRNA was delivered to the hippocampal. B The results of RT-qPCR showed a significantly reduced of kmt2d in the AAV-RNAi group compared to that of AAV-nc group (n = 3). C, D The level of the H3K4me3 protein. The band density was quantified and expressed as the relative gray value (compared with the control) by arbitrarily setting the control value as 1 (n = 3). E Schematic diagram of the three-box social experiment. F The distance traveled in different areas within the open field for the mice (n = 6). G Motion trajectory chart for the control (AAV-nc) and knockdown (AAV-RNAi) mice in an Open Field Assay. H Number of marbles buried (n = 6). I, J During a social interaction session, Kmt2d deficiency mice didn’t have social preferences. I sniffing time exploring S1 and E (n = 6). J motion trajectory chart for the control (AAV-nc) and knockdown (AAV-RNAi) mice. K, L The same as (I, J) but for social novelty test. K sniffing time exploring S1 and S2. (n = 6). L motion trajectory chart. Error bars represent as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ns: P > 0.05, Mann-Whitney U test. A Created with BioRender.com, released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
We assessed social behaviors in adult mice using the three-chamber sociability and social novelty tests (Fig. 3E). Control mice (AAV-nc) typically preferred social stimuli and spent more time with novel mice. In contrast, Kmt2d deficiency mice (AAV-RNAi) showed no preference for social stimuli or novel mice (Fig. 3I–L). Motor skills were normal in Kmt2d deficiency mice (Fig. 3F, G). Additionally, Kmt2d deficiency mice exhibited increased repetitive behaviors, burying more marbles than controls in the Marble Burying Test (Fig. 3H). Thus, Kmt2d deficiency mice displayed abnormal social behaviors and increased repetitive behaviors.
Knockdown of Kmt2d decreased excitatory and increased inhibitory synaptic transmission
To elucidate the neural mechanisms underlying the observed phenotypes, we further examined how the Kmt2d gene in the rat hippocampus influences the excitatory-inhibitory (E/I) balance—a key pathophysiological mechanism implicated in autism17. Rat hippocampal slice cultures are widely used in neurophysiological studies due to their larger hippocampal structure compared to mice, which facilitates higher success rates for slice culture and easier acquisition of stable synaptic activity signals. This allows for more accurate assessment of the impact of Kmt2d knockdown on synaptic function. For these experiments we used slice cultures from 8-to 10-day-old rats. We had screened for effective RNA interference fragments targeting Kmt2d in rat B104 cells (Fig. S3D, E) first. Then, Kmt2d knockdown was performed by an AAV vector containing Kmt2d-shRNA-eGFP infusion.
Based on published single-cell data, we found that the expression level of kmt2d do not significantly differ among different subregions of the hippocampus (Fig. S2A). Therefore, we hypothesize that knockdown of Kmt2d may alter synaptic function in both the CA1 and DG regions. We performed paired recordings from a transfected cells and a neighboring control cell in CA1 and DG, with synaptic responses evoked by electrical stimulation of the Schaffer collateral pathway (Fig. 4A). Our results revealed the following: First, analysis of IPSCs in kmt2d knockdown neurons from the CA1 region revealed a significant increase in amplitude compared to control neurons (Fig. 4C). Second, knockdown of kmt2d decreased the amplitude of AMPAR -mediated EPSCs in CA1 neurons but did not significantly affect NMDAR-mediated EPSCs (Fig. 4E). Finally, we observed similar changes in IPSCs and EPSCs in the DG region, consistent with the findings in the CA1 region (Fig. 4D, F). These data indicate that knockdown of KMT2D leads to functional defects in synaptic transmission, resulting in an imbalance between excitation and inhibition.
A Schematic diagram of hippocamcal cultured slice and dual whole-cell patch clamp. B Paired recordings from Kmt2d-shRNA-eGFP infection (bright cell) and control cell. C, D The amplitude of IPSCs in infected cells is increased compared to control cells in CA1 and DG regions, respectively (n = 10 for CA1 region; n = 9 for DG region). E, G The amplitude of AMPA receptor-mediated EPSCs in infected cells is decreased compared to control cells in CA1 and DG regions, respectively (n = 10 for CA1 region; n = 11 for DG region). F, H The amplitude of NMDA receptor-mediated EPSCs in infected cells remained unchanged compared to control cells in CA1 and DG regions, respectively (n = 9 for CA1 region; n = 11 for DG region). Error bars represent as mean ± SD. *P < 0.05, **P < 0.01, Mann-Whitney U test. A Created with BioRender.com, released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
KMT2D regulates downstream target genes related to synaptic response
To further explore the molecular mechanisms by which KMT2D regulates autistic-like behaviors, we used RNA-seq to compare the gene expression profiles from whole 3-dpf zebrafish exhibiting KS phenotypes (Fig. 5A). A volcano plot graphically illustrates the distribution of differentially expressed genes (DEGs), revealing that 892 genes were downregulated and 617 genes were upregulated transcriptionally in the mutant zebrafish model (Fig. 5B; Supplementary Data 2). Analyses of Gene Ontology (GO) analysis showed that the DEGs were significantly enriched in glutamatergic and GABAergic pathways (Fig. 5C; Supplementary Data 2). To delve deeper into the regulatory mechanisms of KMT2D on synaptic function, we observed that multiple genes (shank2, cnksr1, csmd2 and srgap3) in the glutamatergic signaling pathway were downregulated, whereas genes (camk4, gabarabp, gabarapl2 and npas4a) in the GABAergic signaling pathway were upregulated (Fig. 5D). These findings were further corroborated by subsequent qPCR validation (Fig. 5E, F).
A RNA-seq analysis of zebrafish and mice samples. B Volcano plot showing differentially expressed genes (DEGs) in the km2d+/+ and kmt2d−/− zebrafish. C GO analysis of DEGs in the km2d+/+ and kmt2d−/− zebrafish. D–F Differentially expressed genes associated with glutamatergic and GABAergic synapse in the km2d+/+ and kmt2d−/− zebrafish. D Heatmap of 4 upregulated genes associated with GABAergic synapse, and 6 downregulated genes associated glutamatergic synapse. C RT-qPCR of shank2b (n = 3); D RT-qPCR of gabarapb (n = 3). G Volcano plot showing DEGs in the Km2d-KD and control mice hippocampus. H GO analysis of DEGs in the Km2d-KD and control mice hippocampus. I–K Differentially expressed genes associated with glutamatergic and GABAergic synapse in the Km2d-KD and control mice. I Heatmap of 6 upregulated genes associated with GABAergic synapse, and 6 downregulated genes associated glutamatergic synapse. C RT-qPCR of Cnksr1 (n = 3); D RT-qPCR of Fgf7 (n = 3). Error bars represent as mean ± SD. *P < 0.05, **P < 0.01, Mann-Whitney U test. Mice of Fig. 5, created with BioRender.com, released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
When we compared the gene expression profiles of kmt2d+/+ and kmt2d−/− zebrafish, we made consistent observations of those made in hippocampus of Kmt2d-KD mice. A total of 996 DEGs were identified, including 469 downregulated genes and 527 upregulated genes (Fig. 5G; Supplementary Data 4). DEGs are significantly enriched in pathways related to glutamatergic and GABAergic function (Fig. 5H; Supplementary Data 5). In Kmt2d-KD mice, multiple genes associated with the glutamatergic signaling pathway, such as shank2, cnksr1, csmd2 and srgap3 are significantly downregulated, while several genes related to the GABAergic signaling pathway, including fgf7, gata3, syt1 and plcl1 are significantly upregulated (Fig. 5I–K). Interestingly, several genes in the glutamatergic signaling pathway, such as shank2, cnksr1, csmd2, srgap3, rap1gab and sipal1l, showed consistent changes in both Kmt2d-KD mice and kmt2d-KO zebrafish models (Fig. 5D, I). This finding suggests that the change of glutamatergic synapse is conserved across these two distinct species models. Totally, RNA-seq analysis revealed that under conditions of low kmt2d expression, differentially expressed genes were associated with glutamatergic and GABAergic synapses, supporting the dysregulation of E-I balance in the hippocampus.
The KMT2D network links the glutamatergic and GABAergic synaptic responses in ASD
A better understanding of the connections between KMT2D downstream genes and ASD-associated genes will help to more effectively link KMT2D to autism-like behaviors. By surveying 996 genes that were differentially expressed in the hypothalamus of Kmt2d-KD and control mice, we found that 61 genes were associated with ASD, 223 were related to glutamatergic synaptic responses, and 47 were related to GABAergic synaptic responses (Fig. 6A, B; Supplementary Data 6). As shown in Fig. 6C, the disrupted glutamatergic synaptic responses was one of the major causes of Kmt2d-deficiency-associated ASD (Fig. 6C; Supplementary Data 6).
A Venn-diagram showing the overlap genes between four different gene sets: DEGs: the differentially expressed genes detected in Kmt2d-KD mice RNA-seq. Glutamatergic genes: the DEGs associated with glutamatergic synaptic response. GABAergic genes: the DEGs associated with GABAergic synaptic response. ASD genes: the genes related with ASD based on SFARI. B The ratio of DEGs associated with ASD, glutamatergic and GABAergic synaptic signaling pathway. C Conduct visualization of DEGs related to the glutamatergic and GABAergic signaling pathways using the cluGO software. D The network representing protein interaction relationship between DEGs in ASD, glutamatergic and GABAergic signaling pathways based on Kmt2d-KD mice RNA-seq.
To further illustrate the connection among KMT2D-regulated genes, we studied a network of 118 DEGs that were involved in ASD, glutamatergic and GABAergic synaptic response, using the STRING database (Fig. 6D; Supplementary Data 7). The resulting network suggested that KMT2D regulated GABAergic synaptic response via ZFPM1, whose abnormal expressions and/or mutations have been associated with ASD and other neurological disorders18,19. We found that 16 genes, including Shank2, Syt1, Ar, Tek, P2rx7, Styk1, and Rheb were ASD genes that were involved in the glutamatergic synaptic response. The network also illustrated 4 ASD genes that were associated with the GABAergic synaptic response, including Mink1, Cacna1i, Adora2a and Hivep3. Specifically, Mink1, Cacna1i, Adora2a was found to be a common gene shared by the glutamatergic synaptic response. Overall, our network further illustrates that Kmt2d deficiency increases the risk of ASD by disrupting the glutamatergic and GABAergic synaptic responses.
Disscusion
Identifying new risk factors is an urgent priority in ASD research, as it can enhance our understanding of the underlying pathogenic mechanisms and potentially pave the way for the development of personalized therapies for this highly heterogeneous condition. In this study, we collected nine cases of KS1 patients with ASD through literature review and two web-based databases, all of whom carried pathogenic/likely pathogenic variants of KMT2D gene. Supportively, we showed that deficiency of kmt2d in zebrafish and mice led to autistic-like behaviors, closely resembling those observed in ASD patients with KMT2D loss-of-function mutations.
Furthermore, we showed that Kmt2d deficiency disrupted E/I imbalance, which account for the neurodevelopmental abnormalities including social deficits and repetitive behaviors. Notably, E/I imbalance has been proposed to be associated many neuropsychiatric disorders, including ASD, ID, seizures, and schizophrenia17. Previous studies have indicated that Kmt2d deficiency leads to a reduction in the number of neurons in the DG subregion of the mouse hippocampus, resulting in impaired hippocampus-dependent memory15. However, it remains unclear which specific subregion of the hippocampus is primarily affected by Kmt2d deficiency. Through analysis of published single-cell data, we found no significant differences in Kmt2d expression across the CA1 to CA3 and DG subregions of the hippocampus. Our results further demonstrated that Kmt2d deficiency induces an imbalance in the E/I ratio in both the CA1 and DG subregions. In summary, our study revealed that Kmt2d deficiency weakens synaptic excitation in hippocampal neurons and reduces the E/I ratio, which underlies the behavioral deficits observed.
Noteworthy, single-cell transcriptomic analysis revealed elevated expression of KMT2D in oligodendrocytes, suggesting its potential role in indirectly regulating synaptic function through neuron-glia interaction networks. However, the conventional knockdown model employed in this study presents inherent limitations that preclude definitive discrimination between neuron-autonomous effects and oligodendrocyte-mediated indirect influences. Therefore, the results of this expression analysis may affect the interpretation of the electrophysiological analysis results in Fig. 4. Future investigations should employ cell-type-specific conditional knockout models coupled with neuron-glia coculture platforms to systematically dissect the relative contributions of cell-autonomous neuronal defects versus non-cell-autonomous glial effects in KMT2D-associated neurodevelopmental pathogenesis.
Neural circuits are primarily composed of glutamatergic and GABAergic neurons, which communicate through synaptic connections to form the E-I balance. Theoretically, the reduced synaptic excitation caused by Kmt2d deficiency may stem from the downregulation of glutamatergic-related genes, while the increased synaptic inhibition could be associated with the upregulation of GABAergic-related genes. As a histone methyltransferase, KMT2D promotes gene transcription by generating the H3K4me3 modification20. Therefore, KMT2D deficiency may directly lead to the downregulation of glutamatergic-related genes, thereby impeding excitatory synaptic transmission. RNA-seq results showed that over 20% of the DEGs were related to the glutamatergic synaptic signaling pathway, and the downregulated DEGs were significantly enriched in this pathway, supporting this hypothesis. In contrast, the regulation of GABAergic-related genes by KMT2D may involve more complex mechanisms to maintain the modulation of inhibitory synaptic transmission. Through analysis on the STRING website, we mapped the interaction networks of DEGs related to glutamatergic and GABAergic genes. Results demonstrate that KMT2D is linked to the GABAergic signaling pathway via ZFPM1, a cofactor for the GATA family of genes21. Deficiency in ZFPM1 can cause upregulation of GATA3, which has been implicated in autism22. Overall, this study uncover the crucial role of KMT2D in synaptic function, expanding our understanding of its physiological functions. It also provides insights and potential therapeutic targets for KMT2D-related autism and other neurodevelopmental disorders.
We attempted to inject AAV at 4-6 weeks of age in mice during our preliminary experiments. However, we found that this timing led to a significant increase in mortality in both the experimental and control groups. Therefore, we adjusted our strategy and chose to inject AAV at 8 weeks of age in mice to ensure their survival rate and the feasibility of the experiment. In future studies, we will construct a conditional Kmt2d knockout mouse model to further investigate the specific roles of Kmt2d in behavioral performance and neurodevelopment during the early developmental stages of mice.
In this study, the combination of mouse and zebrafish models provides a more comprehensive perspective for understanding the role of KMT2D in social behavior. Although most ASD studies rely on rodent models, the zebrafish model, with its unique advantages, has become a powerful complementary tool for investigating the complexity and variability of ASD. For example, by 2 to 3 dpf, zebrafish undergo primary neuron replacement and major brain formation and differentiation, a stage analogous to embryonic day E12.5 to E13.5 in mice23. We compared the gene expression profiles of 3-dpf zebrafish and the hippocampus of 12-week-old mice, revealing significant enrichment of KMT2D target genes in both glutamatergic and GABAergic signaling pathways. This finding underscores the value of the zebrafish model in complementing mouse studies and deepening our understanding of the role of KMT2D in neurodevelopment.
Zebrafish can be used to identify simple phenotypic effects of genetically regulated autistic-like pathology24. The SFARI foundation has recently identified 12 ASD risk genes using validated zebrafish mutant models (https://www.sfari.org/resource/zebrafish-models/). In kmt2d KO zebrafish, a decrease in larval body length is associated with a general delay in development that mirrors the condition observed in KS patients. Importantly, the zebrafish model utilized in our research effectively mirrors the autistic-like behaviors of individuals carrying loss-of-function mutations in KMT2D. These findings in zebrafish extend the observations in mice, providing an another zebrafish model for ASD.
In summary, our study elucidates the critical role of KMT2D in modulating animal social behavior. It provides a mechanistic framework for understanding how KMT2D, synaptic function, and autistic-like behaviors may be potentially interrelated.
Methods
Mice and zebrafish
The mice were housed under specific pathogen-free conditions with a 12 h light/dark cycle and provided ad libitum access to water and a normal diet. Adult the zebrafish were maintained under standard laboratory conditions (28.5 °C and 14 h/10 h light-dark cycle). In both zebrafish and mouse experiments, study groups (experimental and control) were established using a littermate-controlled design to ensure genetic and environmental consistency. With n = 6 per group (both experimental and control), this sample size adheres to standard animal research protocols. Preliminary data demonstrating low variability confirmed that n = 6 yields adequate statistical power for our experimental design. Behavioral tests used counterbalanced sequences to control for order effects. Experiments were approved by the Fujian Medical University (Project No. FJMU-2024-Y-1633). All efforts were made to minimize animal suffering and the number of animals used.
Injection of adenovirus-associated vector (AAV)
To investigate the role of KMT2D in autism-like behaviors, adeno-associated virus (AAV) vectors carrying Kmt2d-shRNA or control-shRNA were utilized (Hanhen, Shanghai, China). Briefly, Kmt2d-shRNA or control-shRNA was inserted into the pHBAAV-U6-MCS-CMV-EGFP vector (AAV9, 1.0 × 10^12 TU/ml), and the constructs were verified by sequencing. The recombinant plasmids were prepared using a triple-transfection, helper-free method and then purified. The sequences for the scrambled Kmt2d-shRNA and control-shRNA were 5′-GACTGGTCTAGCCGATGTAAA-3′ and 5′-CAGCTAGAGAGGAACTTGAAGCTAA-3′, respectively. For virus infusion, animals were anesthetized with isoflurane, and 500 nl of either control-shRNA or Kmt2d-shRNA was injected into the hippocampus (−2 mm anteroposterior (AP), ±1.5 mm mediolateral (ML), −2.3 mm dorsoventral (DV)) of 8-week-old male mice at a rate of 0.1 μl/min using a 5 μl Hamilton syringe fitted with a 30-gauge needle. Subsequently, the animals were returned to their home cages and kept warm. After a 4-week recovery period, the effects of transfection in vivo were observed using fluorescence microscopy, RT-qPCR and western blot (WB), followed by the performance of behavioral tests. Mice were anesthetized by intraperitoneal injection of tribromoethanol (0.1 ml/10 g).
RT-qPCR and WB
We used the FreeZol Reagent kit (Vazyme, China) to extract the total RNA of zebrafish embryos or cells and reverse-transcribed it into cDNA following the manufacturer’s protocol. The expression level of mRNA was assessed by RT-qPCR using specific primers and ChamQ SYBR qPCR Master Mix (Vazyme). The level of mRNA was calculated by using the 2−ΔΔCt method. The quantitative PCR primers are shown in supplemental material.
The primary antibodies of rabbit monoclonal antihistone H3 (1:1000; trimethyl K4; Abcam, USA, ab8580) was used to perform WB analysis under standard protocol. Protein expression levels were quantitated by using Image J software (NIH, USA).
Immunofluorescence
The mice were anesthetized and perfused for fixation, after which brain tissue was obtained to prepare frozen sections. Antigen retrieval was performed on the sections to enhance antigen exposure. The sections were subjected to immunostaining with a primary antibody of KMT2D (1: 100; Abcam, USA; ab231239) followed by a secondary antibody conjugated with a fluorescent label (1:500; Sanying, China; RGAMOO3). Finally, the sections were mounted with a mounting medium and observed and imaged under a fluorescence microscope.
Behavioral tests in mice models
Three-chamber test
In this experimental paradigm, the test mouse was positioned in the center chamber of a three-chamber apparatus, with two empty wire baskets placed in the other two chambers (Fig. 3E). The test comprised three phases, beginning with an initial 10-minute habituation period during which the test mouse acclimated to the empty baskets. In the second phase, a social stimulus (a novel mouse) was introduced into one of the baskets, while a nonsocial stimulus was placed in the other basket. In the third phase, the familiar mouse remained in its basket, and the nonsocial stimulus was replaced with another novel mouse. The duration of time the test mouse spent investigating each stimulus was recorded.
Open-field assay
In brief, mice were placed in a 40 cm × 40 cm × 40 cm rectangular plastic enclosure, divided into peripheral and central areas. They were allowed to explore freely for 30 min, with their movement speed, total distance traveled, and time spent in the central area being tracked using a video tracking system.
Marble burying test
The marble burying test was conducted in transparent cages filled with approximately 4.5 cm of fine sawdust. 16 glass marbles were arranged in a 3 × 6 grid with equal spacing between them. Mice were introduced into the cage with the marbles and allowed a 10 min period to interact with the environment. The test concluded with the removal of the mice. A marble was considered buried if at least two-thirds of its surface was covered by the sawdust, and the number of buried marbles was recorded.
Organotypic hippocampal slice culture and viral transfection
Organotypic hippocampal slice cultures are derived from 8- to 10-day-old pups with slight modifications to the original protoco25. The procedure begins by anesthetizing the pups on ice, followed by decapitation and the extraction of the brain. The hippocampi are then harvested and sectioned into 400-micrometer slices using a vibratome. These slices are promptly transferred to a cold dissecting medium. The organotypic slices are gently placed onto a 0.4-micrometer membrane insert, which is positioned within a petri dish under a laminar flow hood. The slices are incubated at 34 °C, and the culture medium is refreshed every 48 h to maintain optimal conditions. Viral transfection of the slices is performed on the first day in vitro (DIV1) following slicing. For electrophysiological recordings, the slices are typically utilized 8–10 days post-transfection, allowing sufficient time for the virus to express its genetic payload and for the neurons to recover from the transfection process.
Electrophysiological recording
AMPAR-mediated EPSCs were measured as the peak of the EPSCs recorded at −70 mV, whereas NMDAR-mediated EPSCs were measured as the amplitude of the EPSCs 100 ms after the stimulus at +40 mV to avoid contamination by AMPAR currents26. Record EPSCs using an intracellular solution that contained Cs+ (to block various potassium channels and hyperpolarization-activated cation channels) and QX314 (to block voltage-gated sodium channels). Add picrotoxin (100 µM) to block inhibition in external solution. And to isolate AMPAR-currents and NMDAR-currents, different holding potentiation was used. When holding at −70mV, NMDA receptors are block by magnesium ion and AMPAR-EPSCs are recorded. While holding at +40 mV, NMDA receptors open with removing magnesium ion. Filtering the faster AMPA EPSCs 100 ms after the stimulus, NMDA EPSCs were analyzed. To record GABA IPSCs, the cell membrane was held at 0 mV. For evoke IPSCs, APV (50 μM) and BNQX (10 μM) were added to block excitatory currents and 2-Chloroadenosine (4 μM) was used to control epileptiform activity.
Generation of kmt2d mutant zebrafish
CRISPR/Cas9-mediated gene editing was conducted using established methods. A synthetic specific guide RNA (sgRNA) (sequence: 5’-cagtctgcagcgttgggagaagg-3’) and Cas9 mRNA (concentrations of 320 ng/μL and 800 ng/μL, respectively) were coinjected into each WT zebrafish embryo at the single-cell stage in a total volume of 1 nL. Genomic DNA was extracted, and genotyping was performed to screen for mutation frequency by comparing with WT zebrafish samples. The method of genotype identification is shown in Fig. S1. Mutant chimeric zebrafish were crossed with the WT strain to purify the genetic background and generate kmt2d+/− zebrafish. kmt2d+/+, kmt2d+/−, and kmt2d−/− littermates were obtained by crossing male and female kmt2d+/− zebrafish.
Social and repetitive behavior tests in zebrafish models
The social behaviors of 12-week-old post-fertilization male zebrafish, including individual social preference and repetitive behavior, were evaluated at 28.5 °C. In the experiment, a single zebrafish was placed in one compartment of a standard mating tank, while four WT male zebrafish were placed in the opposite compartment, separated by a transparent plastic divider to assess social preference (Fig. 2G). The area containing the single zebrafish was designated as the social zone (Region 2), and the area without any zebrafish was the nonsocial zone (Region 1). Each trial lasted for 5 min. Behavioral recording commenced after a 1–2 min acclimation period, allowing the zebrafish to adjust to the tank environment. The social behavior of the zebrafish was quantified based on their proximity to the group, measured as either distance or the regions they occupied. The proportion of time spent in the social area was used to directly gauge the social activity level of an individual zebrafish.
During our examination of the spontaneous movements of zebrafish, we noted a large circular movement, which was considered as a repetitive behavior in the kmt2d−/+ zebrafish (Fig. 2G). Large circular movement described the zebrafish swimming in a circular pattern, either counterclockwise or clockwise, along the perimeter of the tank.
RNA-seq
Total RNA was extracted from 3-day post-fertilization whole embryos using AG RNAex Pro Reagent (AG21101, China) following the manufacturer’s protocol. RNA integrity and contamination were assessed on 1% agarose gels. The RNA concentration was determined using a Qubit® 4.0 fluorometer (Thermo, USA), while RNA integrity was evaluated with an Qsep400TM (Bioptic, Taiwan, China). For RNA sample preparation, three micrograms of RNA per sample were used. Sequencing libraries were constructed for Illumina® (New England Biolabs, USA) as per the manufacturer’s guidelines, incorporating index codes to distinguish each sample. Sequencing data from the high-throughput sequencer were processed to convert image data into sequence reads containing sequence information and corresponding quality scores. Differential gene expression analysis was performed with the DESeq2 R package, which employs a negative binomial distribution model to statistically determine differential expression from digital gene expression data. The resulting P values were adjusted using the Benjamini and Hochberg method to control the false discovery rate. Genes with an P value of less than 0.05 were classified as DEGs.
Statistics and reproducibility
All experiments in this study were repeated at least three times. The experimental data presented in all figures are expressed as the mean ± standard deviation (SD). Measurements were taken from distinct samples. The Mann-Whitney U test was used for intergroup comparison. A score of P < 0.05 was considered statistically. GraphPad Prism, version 8.0 software, was used for all statistical analyses.
Availability of data and materials
The data supporting the findings of this study are available within the article and supplementary data 1. The RNA-seq data that support the findings of this study are made available (https://www.ncbi.nlm.nih.gov/sra/PRJNA1289345; PRJNA1289345).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
References
Genovese, A., Butler, M. G. The autism spectrum: behavioral, psychiatric and genetic associations[J]. Genes 14, 677 (2023).
Vallianatos, C. N. & Iwase, S. Disrupted intricacy of histone H3K4 methylation in neurodevelopmental disorders[J]. Epigenomics 7, 503–519 (2015).
Shen, E., Shulha, H., Weng, Z. & Akbarian, S. Regulation of histone H3K4 methylation in brain development and disease[J]. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1652, 369 (2014).
Froimchuk, E., Jang, Y., Ge, K. J. G. Histone H3 lysine 4 methyltransferase KMT2D[J]. Genes 627, 337-342 (2017).
Boniel, S., Szymańska, K., Śmigiel, R., Szczałuba, K. Kabuki syndrome-clinical review with molecular aspects[J]. Genes 12, 468 (2021).
Sertçelik, M., Uğur, Ç, Şahin, A. A. & Gürkan, C. A child with Kabuki syndrome and autism spectrum disorder[J]. Noro Psikiyatr Ars 53, 280–282 (2016).
Parisi, L., Filippo, T. & Roccella, M. Autism spectrum disorder in Kabuki syndrome: clinical, diagnostic and rehabilitative aspects assessed through the presentation of three cases[J]. Minerva Pediatr. 67, 369–375 (2015).
Luo, J., Wang, Q., Cheng, S., Chen, A. X. & Yuan, H. M. Analysis of clinical manifestation and a mosaic frameshift variant of the KMT2D gene in a Chinese patient with Kabuki syndrome[J]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 38, 861–864 (2021).
Lee, J. E. et al. H3K4 mono- and di-methyltransferase MLL4 is required for enhancer activation during cell differentiation[J]. Elife 2, e01503 (2013).
Serrano, M., Demarest, B. L., Tone-Pah-Hote, T., Tristani-Firouzi, M., Yost, H. J. Inhibition of Notch signaling rescues cardiovascular development in Kabuki Syndrome[J]. PLoS Biol. 17, e3000087 (2017).
Fahrner, J. A. et al. Precocious chondrocyte differentiation disrupts skeletal growth in Kabuki syndrome mice[J]. JCI Insight 4, e129380 (2019).
Shpargel, K. B., Mangini, C. L., Xie, G., Ge, K. & Magnuson, T. The KMT2D Kabuki syndrome histone methylase controls neural crest cell differentiation and facial morphology[J]. Development 147, dev187997 (2020).
Laarhoven, P. et al. Kabuki syndrome genes KMT2D and KDM6A: functional analyses demonstrate critical roles in craniofacial, heart and brain development[J]. Hum. Mol. Genet. 24, 4443–4445 (2015).
Kalinousky, A. J. et al. KMT2D deficiency causes sensorineural hearing loss in mice and humans[J]. Genes 15, 48 (2023).
Bjornsson, H. T. et al. Histone deacetylase inhibition rescues structural and functional brain deficits in a mouse model of Kabuki syndrome[J]. Sci. Transl. Med. 6, 256ra135 (2014).
Lei, H. et al. Single-cell spatial transcriptomic atlas of the whole mouse brain. Neuron 113, 0 (2025).
Sohal, V. S. & Rubenstein, J. L. R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders[J]. Mol. Psychiatry 24, 1248–1257 (2019).
Novara, F. et al. Haploinsufficiency for ANKRD11-flanking genes makes the difference between KBG and 16q24.3 microdeletion syndromes: 12 new cases[J]. Eur. J. Hum. Genet. 25, 694–701 (2017).
Tikker, L. et al. Inactivation of the GATA cofactor ZFPM1 results in abnormal development of dorsal raphe serotonergic neuron subtypes and increased anxiety-like behavior[J]. J. Neurosci. 40, 8669–8682 (2020).
Froimchuk, E., Jang, Y. & Ge, K. Histone H3 lysine 4 methyltransferase KMT2D[J]. Gene 627, 337–342 (2017).
Morceau, F. et al. GATA-1: friends, brothers, and coworkers[J]. Ann. N. Y Acad. Sci. 1030, 537–554 (2004).
Rout, U. K. & Clausen, P. Common increase of GATA-3 level in PC-12 cells by three teratogens causing autism spectrum disorders[J]. Neurosci. Res. 64, 162–169 (2009).
Mario, F. & Wullimann Secondary neurogenesis and telencephalic organization in zebrafish and mice: a brief review.[J]. Integr. Zool. 4, 0 (2009).
Washbourne, P. Can we model autism using zebrafish?[J]. Dev. Growth Differ. 65, 453–458 (2023).
Stoppini, L., Buchs, P. A. & Muller, D. A simple method for organotypic cultures of nervous tissue[J]. J. Neurosci. Methods 37, 173–182 (1991).
Chen, X. et al. CaMKII autophosphorylation is the only enzymatic event required for synaptic memory[J]. Proc. Natl. Acad. Sci. USA 121, e2402783121 (2024).
Acknowledgements
We express our gratitude to the funding of National Natural Science Foundation of China (No. 82471436), Major Program of National Science Foundation of Fujian Province, China (No. 22SCZZX007), Natural Science Foundation of Fujian Province, China (No. 2022J02029) to W.T., the China Postdoctoral Science Foundation (No. 2024M750469) and the Startup Fund for Scientific Research, Fujian Medical University (No. 2023QH1003) to H.S., and the Key Clinical Special Discipline Construction Program of Fujian Province (NO. 20230103) to R.C. We appreciate the guidance in zebrafish behavioral testing provided by Yuqing Lei from the Medical Research Center, Fujian Maternity and Child Health Hospital for experimental assistance. We also thank BioRender.com for providing the tools to create scientific figures.
Author information
Authors and Affiliations
Contributions
H.S. and X.W. performed the experiment, conducted the data analysis and interpretation, and wrote the manuscript. J.g.H. help perform electrophysiological recording and data analysis. J.H. help prepare brain slices and viral infections. Z.C. and X.C. helped in conducting the RT-qPCR and genotyping experiments. J.Y. helped to conducted IF and WB. J.Z., B.Y., and R.Y. help conduct behavioral experiments in mice models. R.C. and W.T. contributed to the research design, data analysis, and writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Masayuki Itoh and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Christoph Anacker and Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shangguan, H., Huang, J., Wei, X. et al. Deficiency of KMT2D causes autistic-like behavior in mice and zebrafish. Commun Biol 8, 1311 (2025). https://doi.org/10.1038/s42003-025-08635-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-08635-2