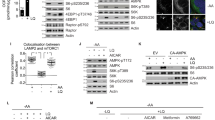
Abstract
Cultured cancer cells frequently rely on the consumption of glutamine and its subsequent hydrolysis by glutaminase (GLS). However, this metabolic addiction can be lost in the tumour microenvironment, rendering GLS inhibitors ineffective in the clinic. Here we show that glutamine-addicted breast cancer cells adapt to chronic glutamine starvation, or GLS inhibition, via AMPK-mediated upregulation of the serine synthesis pathway (SSP). In this context, the key product of the SSP is not serine, but α-ketoglutarate (α-KG). Mechanistically, we find that phosphoserine aminotransferase 1 (PSAT1) has a unique capacity for sustained α-KG production when glutamate is depleted. Breast cancer cells with resistance to glutamine starvation or GLS inhibition are highly dependent on SSP-supplied α-KG. Accordingly, inhibition of the SSP prevents adaptation to glutamine blockade, resulting in a potent drug synergism that suppresses breast tumour growth. These findings highlight how metabolic redundancy can be context dependent, with the catalytic properties of different metabolic enzymes that act on the same substrate determining which pathways can support tumour growth in a particular nutrient environment. This, in turn, has practical consequences for therapies targeting cancer metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA-seq data are deposited in the Gene Expression Omnibus (GEO) database under accession number GSE263696. Source data are provided with this paper.
References
Curthoys, N. P. & Watford, M. Regulation of glutaminase activity and glutamine metabolism. Annu. Rev. Nutr. 15, 133–159 (1995).
Cluntun, A. A., Lukey, M. J., Cerione, R. A. & Locasale, J. W. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 3, 169–180 (2017).
Altman, B. J., Stine, Z. E. & Dang, C. V. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 619–634 (2016).
Wang et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 18, 207–219 (2010).
Gross, M. I. et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther. 13, 890–901 (2014).
Yang, W. H., Qiu, Y., Stamatatos, O., Janowitz, T. & Lukey, M. J. Enhancing the efficacy of glutamine metabolism inhibitors in cancer therapy. Trends Cancer 7, 790–804 (2021).
DeBerardinis, R. J. & Cheng, T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29, 313–324 (2010).
Cheng, T. et al. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc. Natl Acad. Sci. USA 108, 8674–8679 (2011).
Dos Reis, L. M. et al. Dual inhibition of glutaminase and carnitine palmitoyltransferase decreases growth and migration of glutaminase inhibition-resistant triple-negative breast cancer cells. J. Biol. Chem. 294, 9342–9357 (2019).
Daemen, A. et al. Pan-cancer metabolic signature predicts co-dependency on glutaminase and de novo glutathione synthesis linked to a high-mesenchymal cell state. Cell Metab. 28, 383–399 (2018).
Timmerman, L. et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 24, 450–465 (2013).
Muir, A. et al. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. Elife 6, e27713 (2017).
Shin, C. S. et al. The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nat. Commun. 8, 15074 (2017).
Lukey, M. J., Greene, K. S., Erickson, J. W., Wilson, K. F. & Cerione, R. A. The oncogenic transcription factor c-Jun regulates glutaminase expression and sensitizes cells to glutaminase-targeted therapy. Nat. Commun. 7, 11321 (2016).
Pan, M. et al. Regional glutamine deficiency in tumours promotes dedifferentiation through inhibition of histone demethylation. Nat. Cell Biol. 18, 1090–1101 (2016).
Kamphorst, J. J. et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75, 544–553 (2015).
Edwards, D. N. et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J. Clin. Invest. 131, e140100 (2021).
Pavlova, N. N. et al. As extracellular glutamine levels decline, asparagine becomes an essential amino acid. Cell Metab. 27, 428–438 (2018).
Pacold, M. E. et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat. Chem. Biol. 12, 452–458 (2016).
Weinstabl, H. et al. Intracellular trapping of the selective phosphoglycerate dehydrogenase (PHGDH) inhibitor BI-4924 disrupts serine biosynthesis. J. Med. Chem. 62, 7976–7997 (2019).
Possemato, R. et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 (2011).
Fell, D. A. & Snell, K. Control analysis of mammalian serine biosynthesis. Feedback inhibition on the final step. Biochem. J. 256, 97–101 (1988).
Koper, K., Han, S. W., Pastor, D. C., Yoshikuni, Y. & Maeda, H. A. Evolutionary origin and functional diversification of aminotransferases. J. Biol. Chem. 298, 102122 (2022).
Ouzounis, C. & Sander, C. Homology of the NifS family of proteins to a new class of pyridoxal phosphate-dependent enzymes. FEBS Lett. 322, 159–164 (1993).
Chen, W. W., Freinkman, E., Wang, T., Birsoy, K. & Sabatini, D. M. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 166, 1324–1337 (2016).
Bailey, J., Bell, E. T. & Bell, J. E. Regulation of bovine glutamate dehydrogenase. The effects of pH and ADP. J. Biol. Chem. 257, 5579–5583 (1982).
Glinghammar, B. et al. Detection of the mitochondrial and catalytically active alanine aminotransferase in human tissues and plasma. Int. J. Mol. Med. 23, 621–631 (2009).
Huynh, Q. K., Sakakibara, R., Watanabe, T. & Wada, H. Glutamic oxaloacetic transaminase isozymes from rat liver. J. Biochem. 88, 231–239 (1980).
Davoodi, J. et al. Overexpression and characterization of the human mitochondrial and cytosolic branched-chain aminotransferases. J. Biol. Chem. 273, 4982–4989 (1998).
Matsuzawa, T. Characteristics of the inhibition of ornithine-δ-aminotransferase by branched-chain amino acids. J. Biochem. 75, 601–609 (1974).
Chou, T. C. Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res. 70, 440–446 (2010).
Fazzari, J. & Singh, G. Effect of glutaminase inhibition on cancer-induced bone pain. Breast Cancer Targets Ther. 11, 273–282 (2019).
Pakos-Zebrucka, K. et al. The integrated stress response. EMBO Rep. 17, 1374–1395 (2016).
Garcia, D. & Shaw, R. J. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 66, 789–800 (2017).
Selvarajah, B. et al. mTORC1 amplifies the ATF4-dependent de novo serine-glycine pathway to supply glycine during TGF-β1-induced collagen biosynthesis. Sci. Signal 12, 3048 (2019).
Myers, R. W. et al. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science 357, 507–511 (2017).
Yun, H. J. et al. AMPK-HIF-1α signaling enhances glucose-derived de novo serine biosynthesis to promote glioblastoma growth. J. Exp. Clin. Cancer Res. 42, 340 (2023).
DeNicola, G. M. et al. NRF2 regulates serine biosynthesis in non–small cell lung cancer. Nat. Genet. 47, 1475–1481 (2015).
Joo, M. S. et al. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol. Cell. Biol. 36, 1931–1942 (2016).
Lukey, M. J., Katt, W. P. & Cerione, R. A. Targeting amino acid metabolism for cancer therapy. Drug Discov. Today 22, 796–804 (2017).
Baixauli, F. et al. An LKB1–mitochondria axis controls TH17 effector function. Nature 610, 555–561 (2022).
Kottakis, F. et al. LKB1 loss links serine metabolism to DNA methylation and tumorigenesis. Nature 539, 390–395 (2016).
Galan-Cobo, A. et al. LKB1 and KEAP1/NRF2 pathways cooperatively promote metabolic reprogramming with enhanced glutamine dependence in KRAS-mutant lung adenocarcinoma. Cancer Res. 79, 3251–3267 (2019).
Ho, C. L., Noji, M., Saito, M., Yamazaki, M. & Saito, K. Molecular characterization of plastidic phosphoserine aminotransferase in serine biosynthesis from Arabidopsis. Plant J. 16, 443–452 (1998).
Singh, R. K., Kumar, D. & Gourinath, S. Phosphoserine aminotransferase has conserved active site from microbes to higher eukaryotes with minor deviations. Protein Pept. Lett. 28, 996–1008 (2021).
Lund, K., Merrill, D. K. & Guynn, R. W. The reactions of the phosphorylated pathway of l-serine biosynthesis: thermodynamic relationships in rabbit liver in vivo. Arch. Biochem. Biophys. 237, 186–196 (1985).
Marchesani, F. et al. A novel assay for phosphoserine phosphatase exploiting serine acetyltransferase as the coupling enzyme. Life 11, 485 (2021).
Luo, Z., Eichinger, K. M., Zhang, A. & Li, S. Targeting cancer metabolic pathways for improving chemotherapy and immunotherapy. Cancer Lett. 575, 216396 (2023).
Zecchini, V. & Frezza, C. Metabolic synthetic lethality in cancer therapy. Biochim. Biophys. Acta Bioenerg. 1858, 723–731 (2017).
Christen, S. et al. Breast cancer-derived lung metastases show increased pyruvate carboxylase-dependent anaplerosis. Cell Rep. 17, 837–848 (2016).
Abla, H., Sollazzo, M., Gasparre, G., Iommarini, L. & Porcelli, A. M. The multifaceted contribution of α-ketoglutarate to tumor progression: an opportunity to exploit? Semin. Cell Dev. Biol. 98, 26–33 (2020).
Hwang, I. Y. et al. Psat1-dependent fluctuations in α-ketoglutarate affect the timing of ESC differentiation. Cell Metab. 24, 494–501 (2016).
Kaushik, A. K. et al. In vivo characterization of glutamine metabolism identifies therapeutic targets in clear cell renal cell carcinoma. Sci. Adv. 8, eabp8293 (2022).
Locasale, J. W. et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 43, 869–874 (2011).
Sullivan, M. R. et al. Increased serine synthesis provides an advantage for tumors arising in tissues where serine levels are limiting. Cell Metab. 29, 1410–1421 (2019).
Mattaini, K. R., Sullivan, M. R. & Vander Heiden, M. G. The importance of serine metabolism in cancer. J. Cell Biol. 214, 249–257 (2016).
Wang, Z. & Zhang, J. Abundant indispensable redundancies in cellular metabolic networks. Genome Biol. Evol. 1, 23–33 (2009).
Sambamoorthy, G. & Raman, K. Understanding the evolution of functional redundancy in metabolic networks. Bioinformatics 34, i981–i987 (2018).
Marx, C. J., Van Dien, S. J. & Lidstrom, M. E. Flux analysis uncovers key role of functional redundancy in formaldehyde metabolism. PLoS Biol. 3, e16 (2005).
Bhatia, S. et al. Patient-derived triple-negative breast cancer organoids provide robust model systems that recapitulate tumor intrinsic characteristics. Cancer Res. 82, 1174–1192 (2022).
Chou, T.-C. The mass-action law based algorithm for cost-effective approach for cancer drug discovery and development. Am. J. Cancer Res 1, 925–954 (2011).
Chou, T.-C. & Martin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values (ComboSyn, 2004).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Peracchi, A. & Polverini, E. Using steady-state kinetics to quantitate substrate selectivity and specificity: a case study with two human transaminases. Molecules 27, 1398 (2022).
Basurko, M.-J., Marche, M., Darriet, M. & Cassaigne, A. Phosphoserine aminotransferase, the second step-catalyzing enzyme for serine biosynthesis. IUBMB Life 48, 525–529 (1999).
Lineweaver, H. & Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 56, 658–666 (1934).
MacKay, G. M., Zheng, L., Van Den Broek, N. J. F. & Gottlieb, E. Analysis of cell metabolism using LC–MS and isotope tracers. Methods Enzymol. 561, 171–196 (2015).
Su, X., Lu, W. & Rabinowitz, J. D. Metabolite spectral accuracy on orbitraps. Anal. Chem. 89, 5940–5948 (2017).
Acknowledgements
We thank all members of the Lukey Laboratory, our colleagues in the Demerec building and C. Thompson for helpful discussions and insights. We are grateful to C. Amor Vegas for providing advice and reagents for senescence staining. We also thank the Cold Spring Harbor Laboratory (CSHL) Cancer Center Animal, Mass Spectrometry, Next-Generation Sequencing and Organoid Shared Resources, which are funded in part by a National Institutes of Health Cancer Center Support Grant (5P30CA045508). This work was supported by grants from the Department of Defense Breast Cancer Research Program (BC200599), National Institutes of Health (R01GM149957 and 5P30CA045508), METAvivor, Simons Foundation and The Elsa U. Pardee Foundation to M.J.L.; the Leslie C. Quick, Jr. Fellowship from the CSHL School of Biological Sciences to J.d.R.S.; National Cancer Institute (5P01CA013106-Project 3) to D.L.S.; and National Institutes of Health NIAID R25 training grant (AI140472) to J.R.C. Schematic images were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Y.Q., O.T.S., Q.H. and J.d.R.S. generated and analysed the majority of data. A.S., A.S.H.C., S.V. and J.R.C. performed MS and data analysis for metabolomics. D.L.S. and S.R. provided patient-derived breast cancer organoids. M.J.L. directed the work, interpreted the data and drafted the manuscript, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Richard Possemato, Mercedes Tome and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Short tandem repeat (STR) profiling.
a, STR profiling report for parental MDA-MB-231 cells. b, STR profiling report for CB839RS MDA-MB-231 cells. c, STR profiling report for GlnIND MDA-MB-231 cells. CB839RS, CB-839-resistant; GlnIND, glutamine-independent.
Extended Data Fig. 2 NEAA dropout analysis.
a, Sensitivity of BT-549 cells to 6 days deprivation of individual NEAAs. Cells were cultured in complete media (parental) or glutamine-free media (GlnIND) lacking individual NEAAs. Viable cell counts under control conditions were set as 100%. Red triangle, dependence unique to GlnIND cells. Green triangle, dependence unique to parental cells. Data are presented as mean values ± SD, n = 3 biological replicates. Two-tailed unpaired t-test. * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001. b, NEAA sensitivity analysis as in panel ‘a’, but with MDA-MB-231 cells cultured in complete media (parental) or complete media supplemented with 500 nM CB-839 (CB839RS). Data are presented as mean values ± SD, n = 3 biological replicates. Two-tailed unpaired t-test. * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001. Parental MDA-MB-231 data are shared between Fig. 1i and Extended Data Fig. 2b for ease of comparison. CB839RS, CB-839 resistant; NA, not applicable; NEAA, nonessential amino acids.
Extended Data Fig. 3 Sequence of GLS protein based on cDNA sequencing data.
The amino acid sequences are translated from sequencing data of full-length GLS cDNA, prepared from parental, GlnIND, and CB839RS MDA-MB-231 cells. The gray box marks a flexible loop located at the dimer-dimer interface of the tetrameric forms of GLS, the binding site of CB-839. CB839RS, CB-839-resistant; GlnIND, glutamine-independent; GLS, glutaminase.
Extended Data Fig. 4 Western blot analysis of adapted and modified breast cancer cells.
a, Western blot analysis of indicated proteins in parental, CB839RS, and GlnIND MDA-MB-231 whole cell lysates (upper panels). [U-13C6]-glucose stable-isotope tracing data for de novo synthesized serine (m + 3) after 16 h (lower panels). Data are presented as mean values ± SD, n = 3 biological replicates. b, Western blot analysis of PHGDH in parental and adapted GlnIND breast cancer cell lines. c, Pearson correlation coefficient analysis of CB-839 sensitivity and serine dependence across cell lines. A value of 1000 nM CB-839 is shown when IC50CB-839 > 1000 nM. Each data point represents the mean from 3 independent experiments. This is a repeat of Fig. 2f, but with the cell lines labeled. d, Sensitivity of BT-549 cells to NCT-503 (upper panel) and BI-4916 (lower panel). Parental cells cultured in complete medium and GlnIND cells in glutamine-free medium. Data are presented as mean values ± SD, n = 3 biological replicates. e, Western blot validation of knockdown of PHGDH or PSAT1 by independent shRNAs. f, Growth of BT-549 cells harboring a control vector, a PHGDH shRNA vector, or a PSAT1 shRNA vector. Parental and GlnIND cells cultured in complete medium or glutamine-free medium, respectively for 6 days. Viable control cell count in each condition was set as 100%. Data are presented as mean values ± SD, n = 3 biological replicates. Two-tailed unpaired t-test. *** P ≤ 0.001; NS, not significant. g, Western blot validation of knockdown of PHGDH or PSAT1 by shRNAs. Tubulin control blots in Fig. 2b, c are shared with Extended Data Fig. 4a because the data were obtained from the same samples. Parental BT-549 NCT-503 dose curve is shared between Fig. 3e and Extended Data Fig. 4d, for different comparisons. CB839RS, CB-839-resistant; GlnIND, glutamine-independent; GLS, glutaminase; GLS2, glutaminase 2; GLUL, glutamine synthetase; PHGDH, D-3-phosphoglycerate dehydrogenase; PSAT1, phosphoserine aminotransferase 1; PSPH, phosphoserine phosphatase; xCT, cystine/glutamate antiporter.
Extended Data Fig. 5 α-KG is the key SSP product for glutamine-independent growth.
a, Western blot validation of CRISPR/Cas9-mediated knockout of PHGDH or PSAT1 using independent sgRNAs. b, Sensitivity of breast cancer cell lines to 6 days BI-4916 treatment. Data are presented as mean values ± SD, n = 3 biological replicates. c, Western blot validation of knockdown of PHGDH or PSAT1 by independent shRNA constructs. d, Western blot validation of knockdown of PSPH by shRNA. e, Sensitivity of adapted Hs 578T cells to 6 days CB-839 treatment. Data are presented as mean values ± SD, n = 3 biological replicates. f, Growth of GlnIND BT-549 cells treated with 12 µM NCT-503 in glutamine-free medium supplemented with dimethyl α-KG (500 µM) or serine at the indicated concentrations over 6 days. Viable untreated cell count in complete medium was set as 100%. Data are presented as mean values ± SD, n = 3 biological replicates. Two-tailed unpaired t-test. ** P ≤ 0.01; NS, not significant. g, Serine supplementation fails to rescue the growth of CB839RS Hs 578T cells treated with 12 µM NCT-503 or 2 µM BI-4916, in medium also containing 500 nM CB-839, over 6 days. Viable untreated cell count in complete medium was set as 100%. Data are presented as mean values ± SD, n = 3 biological replicates. Two-tailed unpaired t-test. *** P ≤ 0.001; NS, not significant. h, Dimethyl α-KG supplementation rescues the growth of CB839RS Hs 578T cells treated with 12 µM NCT-503 or 2 µM BI-4916, in medium also containing 500 nM CB-839, over 6 days. Viable untreated cell count in complete medium was set as 100%. Data are presented as mean values ± SD, n = 3 biological replicates. i, Growth of adapted BT-549 cells in glutamine-free medium over 6 days. Dimethyl α-KG was used at 500 µM. Viable control cell count in the absence of dimethyl α-KG was set as 100%. Data are presented as mean values ± SD, n = 3 biological replicates. Two-tailed unpaired t-test. ** P ≤ 0.01; *** P ≤ 0.001; NS, not significant. j, Glutamate supplementation fails to rescue the growth of CB839RS Hs 578T cells treated with 12 µM NCT-503 or 2 µM BI-4916, in medium also containing 500 nM CB-839, over 6 days. Viable untreated cell count in complete medium was set as 100%. Data are presented as mean values ± SD, n = 3 biological replicates. Two-tailed unpaired t-test. *** P ≤ 0.001; NS, not significant. k, Supplementation with 500 µM dimethyl α-KG, 4 mM serine, or 4 mM glutamate leads to increased intracellular abundance of these metabolites in GlnIND MDA-MB-231 cells. Data are presented as mean values ± SD, n = 3 biological replicates. Two-tailed unpaired t-test. *** P ≤ 0.001. Parental MDA-MB-231 BI-4916 dose curve is shared between Fig. 3a, b and Extended Data Fig. 5b, and parental BT-549 BI-4916 dose curve is shared between Extended Data Fig. 4d and Extended Data Fig. 5b, for different comparisons. Control and NCT-503 only (no supplementation) data are shared between Extended Data Fig. 5g, h because the data were obtained from the same experiment. Control and BI-4916 only data are shared between Extended Data Fig. 5g, h, j because the data were obtained from the same experiment. CB839RS, CB-839-resistant; GlnIND, glutamine-independent; SSP, serine synthesis pathway; DMα-KG, dimethyl α-ketoglutarate; PHGDH, D-3-phosphoglycerate dehydrogenase; PSPH, phosphoserine phosphatase.
Extended Data Fig. 6 Kinetic analysis of human PSAT1, GOT2, and GPT2.
a, Representative Lineweaver–Burk plot of the inverse initial velocity (1/V0) as a function of the inverse of the glutamate concentration (1/[S]) for PSAT1. b, Representative Lineweaver–Burk plot of the inverse initial velocity (1/V0) as a function of the inverse of the glutamate concentration (1/[S]) for GOT2. c, Representative Lineweaver–Burk plot of the inverse initial velocity (1/V0) as a function of the inverse of the glutamate concentration (1/[S]) for GPT2. d, Representative Lineweaver–Burk plot of the inverse initial velocity (1/V0) as a function of the inverse of the 3-PHP concentration (1/[S]) for PSAT1. PSAT1, phosphoserine aminotransferase 1, GOT2, glutamic-oxaloacetic transaminase 2; GPT2, glutamic–pyruvic transaminase 2; 3-PHP, 3-phosphohydroxypyruvate; Km, Michaelis constant; V0, initial velocity, [S], substrate concentration.
Extended Data Fig. 7 SSP inhibition suppresses de novo serine biosynthesis.
a, [U-13C6]-glucose stable-isotope tracing data showing the effect of 16 h treatment with 12 μM NCT-503 on de novo synthesized serine (m + 3) in parental and CB839RS MDA-MB-231 cells. Data are presented as mean values ± SD, n = 4 biological replicates. Schematic shows incorporation of labeled carbons from glucose into serine and glycine via the SSP. b, [U-13C6]-glucose stable-isotope tracing data showing the effect of 16 h treatment with 10 μM BI-4916 on de novo synthesized serine (m + 3) in parental and GlnIND cells. Data are presented as mean values ± SD, n = 3 biological replicates. CB839RS, CB-839 resistant derivative; GlnIND, glutamine-independent; 3-PG, 3-phosphoglycerate; PHGDH, D-3-phosphoglycerate dehydrogenase.
Extended Data Fig. 8 CB-839 synergizes with PHGDH inhibitors against breast cancer.
a, Drug combination analysis using the Chou-Talalay method to score drug interactions in breast cancer cell lines. b, Drug combination analysis using the Chou-Talalay method to score drug interactions in patient-derived TNBC organoids HCM-CSHL-0366-C50, NH85T, NH93T. Data are presented as mean values ± SD, n = 3 biological replicates.
Extended Data Fig. 9 Combination therapy targeting GLS and the SSP suppresses breast cancer growth.
a, Body weight measurement of tumor-bearing mice in the indicated treatment groups. n = 6 (MDA-MB-231) or n = 7 (BT-549) biological replicates, error bars represent SEM. b, Growth of control and PSAT1-knockout MDA-MB-231 orthotopic breast tumor xenografts in NU/J mice administered the indicated treatments (left panel), and body weight measurements of tumor-bearing mice under the indicated treatment (right panel). Data are presented as mean values ± SEM, n = 3 biological replicates. Two-tailed unpaired t-test. ** P ≤ 0.01. c, Western blot analysis of PSAT1 in cultured cells and orthotopic breast tumor xenografts harvested from untreated or CB-839 treated mice. PSAT1, phosphoserine aminotransferase 1.
Extended Data Fig. 10 AMPK signaling mediates PHGDH upregulation upon glutamine blockade.
a, Western blot analysis of ATF4 and NRF2 in nuclear fractions of parental and GlnIND MDA-MB-231 cells (left panel). Western blot analysis of HIF-1α in whole-cell lysates (right panel). BAY-3827 was used at 5 µM and samples collected at 48 h. b, Western blot analysis of lysates of parental and GlnIND BT-549 cells cultured under the indicated conditions. BAY-3827 was used at 5 µM and samples collected at 48 h. c, Western blot analysis of cultured BT-549 cells and BT-549 orthotopic breast tumor xenografts, harvested from untreated or CB-839-treated mice. d, Western blot analysis of lysates of the indicated breast cancer cell lines and patient-derived TNBC organoids. The Tubulin control blot is shared between Fig. 6c and Extended Data Fig. 10c because the data were obtained from the same experiment.
Supplementary information
Supplementary Table 1
Breast cancer cell lines used in this study, with their receptor status, molecular subtype and IC50 value for CB-839 indicated.
Supplementary Table2
Sequences of primers used in this study.
Supplementary Table 3
Gene expression data from RNA-seq analysis showing differentially expressed genes between parental and GlnIND MDA-MB-231 cells.
Supplementary Table 4
Antibodies used in this study, with dilutions indicated.
Supplementary Table 5
Plasmids generated and/or used in this study.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Figs. 1–7
Unprocessed western blots.
Source Data Extended Data Fig./Table 2
Statistical source data.
Source Data Extended Data Fig./Table 4
Statistical source data.
Source Data Extended Data Fig./Table 5
Statistical source data.
Source Data Extended Data Fig./Table 7
Statistical source data.
Source Data Extended Data Fig./Table 8
Statistical source data.
Source Data Extended Data Fig./Table 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, Y., Stamatatos, O.T., Hu, Q. et al. The unique catalytic properties of PSAT1 mediate metabolic adaptation to glutamine blockade. Nat Metab 6, 1529–1548 (2024). https://doi.org/10.1038/s42255-024-01104-w
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s42255-024-01104-w
This article is cited by
-
Targeting glutamine metabolism as a potential target for cancer treatment
Journal of Experimental & Clinical Cancer Research (2025)
-
PSAT1 regulates hair follicle growth and stem cell behavior in cashmere goats
BMC Veterinary Research (2025)
-
Comparative analysis of the effects of PSPH and PHGDH inhibitors on tumor cell proliferation
Investigational New Drugs (2025)