Abstract
The precursor of sterol regulatory element-binding protein-2 (SREBP2) is a membrane-bound transcription factor regulating cholesterol biosynthesis. Under cholesterol-deficient conditions, mature SREBP2 is released from membrane-bound precursors through proteolytic cleavage and enters the nucleus. However, regulation of the transcriptional activity of nuclear SREBP2 (nSREBP2) is poorly understood. In the present study, we reported that nSREBP2 forms nuclear condensates through its amino-terminal, intrinsically disordered region (IDR) and works together with transcription coactivators, partly on superenhancers, for the transcriptional activation of SREBP2 target genes. Substitution of a conserved phenylalanine by alanine within the IDR abolishes the formation of nSREBP2 condensates and reduces its transcriptional activity. This can be effectively rescued by fusion with a phase separation driving FUS-IDR. Knock-in of the phenylalanine-to-alanine substitution in male mice compromises feeding-induced nSREBP2 activity and lowers hepatic and circulating cholesterol levels, underscoring the functional significance of nSREBP2 condensates. Together, the present study reveals that nuclear condensates driven by nSREBP2 N-terminal IDR facilitate the efficient activation of lipogenic genes and play an important role in cholesterol homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Refer to Supplementary Information for the key reagents (Supplementary Table 1), antibodies (Supplementary Table 2), primers for qPCR (Supplementary Table 3), Oligopaint probes (Supplementary Table 4) and MS data (Supplementary Table 5) mentioned in the present study. The MS data have been deposited in the PRIDE database with accession no. PXD061911. The raw sequencing data files generated in the present study have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO) under accession nos. GSE267018, GSE267019 and GSE282800. The data of SEs in HeLa with FBS is from the GEO under accession nos. GSE29611–GSM733684. The list of SREBP target genes was from mouse liver microarray in a published article61. Human SREBF2 SNP information is from UniProt.org. No additional information is required to reanalyse the data reported in this paper. Source data are provided with this paper.
Code availability
This paper does not report original code.
References
Pirillo, A., Casula, M., Olmastroni, E., Norata, G. D. & Catapano, A. L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 18, 689–700 (2021).
Goldstein, J. L., DeBose-Boyd, R. A. & Brown, M. S. Protein sensors for membrane sterols. Cell 124, 35–46 (2006).
Horton, J. D., Goldstein, J. L. & Brown, M. S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131 (2002).
Shao, W. & Espenshade, P. J. Sterol regulatory element-binding protein (SREBP) cleavage regulates Golgi-to-endoplasmic reticulum recycling of SREBP cleavage-activating protein (SCAP). J. Biol. Chem. 289, 7547–7557 (2014).
Jeon, T. I. & Osborne, T. F. SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrin. Met. 23, 65–72 (2012).
Shimano, H. & Sato, R. SREBP-regulated lipid metabolism: convergent physiology—divergent pathophysiology. Nat. Rev. Endocrinol. 13, 710–730 (2017).
Goldstein, J. L. & Brown, M. S. The SREBP pathway: regulation of cholesterol and fatty acid metabolism by proteolysis of a membrane-bound transcription factor. FASEB J. 11, A858–A858 (1997).
Sakai, J. et al. Sterol-regulated release of SREBP-2 from cell membranes requires two sequential cleavages, one within a transmembrane segment. Cell 85, 1037–1046 (1996).
Yang, F. J. et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature 442, 700–704 (2006).
Lukic, S., Nicolas, J. C. & Levine, A. J. The diversity of zinc-finger genes on human chromosome 19 provides an evolutionary mechanism for defense against inherited endogenous retroviruses. Cell Death Differ. 21, 381–387 (2014).
Boija, A. et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell. 175, 1842–1855 (2018).
Basu, S. et al. Unblending of transcriptional condensates in human repeat expansion disease. Cell. 181, 1062–1079 (2020).
Du, M. Y. et al. Direct observation of a condensate effect on super-enhancer controlled gene bursting. Cell 187, 331–344.e17 (2024).
Istvan, E. S. & Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 292, 1160–1164 (2001).
Tang, J. J. et al. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 13, 44–56 (2011).
Wei, M. T. et al. Nucleated transcriptional condensates amplify gene expression. Nat. Cell Biol. 22, 1187–1196 (2020).
Doerr, A. Proximity labeling with TurboID. Nat. Methods 15, 764–764 (2018).
Cho, K. F. et al. Proximity labeling in mammalian cells with TurboID and split-TurboID. Nat. Protoc. 15, 3971–3999 (2020).
Sabari, B. R. et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 361, eaar3958 (2018).
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013).
Blobel, G. A., Higgs, D. R., Mitchell, J. A., Notani, D. & Young, R. A. Testing the super-enhancer concept. Nat. Rev. Genet. 22, 749–755 (2021).
Whyte, W. A. et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 (2013).
Lovén, J. et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013).
Li, J. et al. Mechanosensitive super-enhancers regulate genes linked to atherosclerosis in endothelial cells. J. Cell Biol. 223, e202211125 (2024).
Guo, X., Olajuyin, A., Tucker, T. A., Idell, S. & Qian, G. Q. BRD4 as a therapeutic target in pulmonary diseases. Int. J. Mol. Sci. 24, 13231 (2023).
Wahi, A., Manchanda, N., Jain, P. & Jadhav, H. R. Targeting the epigenetic reader ‘BET’ as a therapeutic strategy for cancer. Bioorg. Chem. 140, 106833 (2023).
Choo, N. et al. Co-targeting BET, CBP, and p300 inhibits neuroendocrine signalling in androgen receptor-null prostate cancer. J. Pathol. 263, 242–256 (2024).
Kotzka, J. et al. Insulin-activated Erk-mitogen-activated protein kinases phosphorylate sterol regulatory element-binding protein-2 at serine residues 432 and 455. J. Biol. Chem. 279, 22404–22411 (2004).
Luo, J., Yang, H. Y. & Song, B. L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 21, 225–245 (2020).
Yu, X. Y. et al. The STING phase-separator suppresses innate immune signalling. Nat. Cell Biol. 23, 330–U325 (2021).
Kim, N. et al. Intrinsically disordered region-mediated condensation of IFN-inducible SCOTIN/SHISA-5 inhibits ER-to-Golgi vesicle transport. Dev. Cell. 58, 1950–1966 (2023).
Gallo, R., Rai, A. K., Mcintyre, A. B. R., Meyer, K. & Pelkmans, L. DYRK3 enables secretory trafficking by maintaining the liquid-like state of ER exit sites. Dev. Cell 58, 1880–1897 (2023).
Harnish, M. T. et al. Novel covalent modifier-induced local conformational changes within the intrinsically disordered region of the androgen receptor. biology 12, 1442 (2023).
Cai, D. F. et al. Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat. Cell Biol. 21, 1578–1589 (2019).
Shin, Y. et al. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell 168, 159–171 (2017).
Bracha, D. et al. Mapping local and global liquid phase behavior in living cells using photo-oligomerizable seeds. Cell 175, 1467–1480 (2018).
Alberti, S., Gladfelter, A. & Mittag, T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176, 419–434 (2019).
Murthy, A. C. et al. Molecular interactions underlying liquid-liquid phase separation of the FUS low-complexity domain. Nat. Struct. Mol. Biol. 26, 637–648 (2019).
Vergnes, L. et al. SREBP-2-deficient and hypomorphic mice reveal roles for SREBP-2 in embryonic development and SREBP-1c expression. J. Lipid Res. 57, 410–421 (2016).
Kilic, S. et al. Phase separation of 53BP1 determines liquid-like behavior of DNA repair compartments. EMBO J. 38, e101379 (2019).
Ma, L. et al. Co-condensation between transcription factor and coactivator p300 modulates transcriptional bursting kinetics. Mol. Cell 81, 1682–1697 (2021).
Shi, B. et al. UTX condensation underlies its tumour-suppressive activity. Nature. 597, 726–731 (2021).
Jia, L. Y., Gao, S. & Qiao, Y. Optical control over liquid-liquid phase separation. Small Methods 8, e2301724 (2024).
Wang, J. et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell. 174, 688–699 (2018).
Gabryelczyk, B. et al. Hydrogen bond guidance and aromatic stacking drive liquid-liquid phase separation of intrinsically disordered histidine-rich peptides. Nat. Commun. 10, 5465 (2019).
Rekhi, S. et al. Expanding the molecular language of protein liquid-liquid phase separation. Nat. Chem. 16, 1113–1124 (2024).
Leshem, A. B. et al. Biomolecular condensates formed by designer minimalistic peptides. Nat. Commun. 14, 421 (2023).
He, W. J., Li, Q. G. & Li, X. X. Acetyl-CoA regulates lipid metabolism and histone acetylation modification in cancer. Biochem. Biophys. Acta Rev. Cancer 1878, 188837 (2023).
Kusnadi, A. et al. The cytokine TNF promotes transcription factor SREBP activity and binding to inflammatory genes to activate macrophages and limit tissue repair. Immunity 51, 241–257.e249 (2019).
Fowler, J. W. M., Zhang, R., Tao, B., Boutagy, N. E. & Sessa, W. C. Inflammatory stress signaling via NF-kB alters accessible cholesterol to upregulate SREBP2 transcriptional activity in endothelial cells. eLife 11, e79529 (2022).
Ouimet, M. et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 13, 655–667 (2011).
Gu, B. et al. Opposing effects of cohesin and transcription on CTCF organization revealed by super-resolution imaging. Mol. Cell 80, 699–711 (2020).
Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880–887 (2018).
Elias, J. E. & Gygi, S. P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 (2007).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Cox, J. et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 (2011).
Bintu, B. et al. Super-resolution chromatin tracing reveals domains and cooperative interactions in single cells. Science. 362, 419–427 (2018).
Nir, G. et al. Walking along chromosomes with super-resolution imaging, contact maps, and integrative modeling. PLoS Genet. 14, e1007872 (2018).
Lu, X. Y. et al. Feeding induces cholesterol biosynthesis via the mTORC1-USP20-HMGCR axis. Nature. 588, 479–484 (2020).
Gong, X. M. et al. Gpnmb secreted from liver promotes lipogenesis in white adipose tissue and aggravates obesity and insulin resistance. Nat. Metab. 1, 570–583 (2019).
Horton, J. D. et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl Acad. Sci. USA 100, 12027–12032 (2003).
Acknowledgements
We thank the Molecular Imaging Core Facility, Molecular and Cell Biology Core Facility and Laboratory Animal Core Facility (School of Life Science and Technology, ShanghaiTech University), National Center for Protein Science Shanghai and HPC platform of ShanghaiTech University for support. We thank the former student J. Liu for help with KO HeLa cells and J. Tang (Cholesgen Inc.) and X. Lu (Wuhan University) for help with the SREBP2 background and fast–refed animal study. This work was supported by grants from the NNSF (National Natural Science Foundation of China, grant nos. 92253301 and 32270648) and the Ministry of Science and Technology (China; grant nos. 2023YFA0913404 and 2024YFA1306000) to W. Q. This research was also supported by Shanghai Frontiers Science Center for Biomacromolecules and Precision Medicine at ShanghaiTech University. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
W.Q. conceived and designed the study and most of the experiments with the help of H.M. and B.-L.S. M.X. and S-Y.J. performed most of the molecular, cellular and animal experiments. Y.H. performed FISH and constructed the PP7 cell with advice from H.M. S.T. performed the TurboID study. M.Z., C.Q. and Y.A. performed and analysed the ChIP–seq and RNA-seq experiments. J.L., D.T., J.Y. and Y.Q. helped with cellular and animal experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Dmitri Sviridov, Peter Tontonoz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Jean Nakhle and Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 nSREBP2 forms condensates at the target gene locus upon cholesterol depletion.
a,b, Schematic of human SREBP2 domain structure (a) and the process of the activation of nSREBP2 (b). When cells are depleted of sterols, SCAP transports pSREBP2 from the ER to the Golgi apparatus, where two proteases, Site-1 protease (S1P) and Site-2 protease (S2P), act sequentially to release the NH2-terminal nuclear segment (nSREBP2) from the membrane. nSREBP2 enters the nucleus and binds to a sterol response element (SRE) in the enhancer/promoter region of target genes, activating their transcription. c, Total cholesterol content was determined in CHO-K1 cells. After cholesterol depletion for 16 h, CHO-K1 cells were harvested for extracting cholesterol and measuring protein concentration (n = 3 independent biological replicates). Data were shown as mean ± s.d. Statistical significance was calculated with one-way ANOVA followed by Turkey’s multiple comparison. d, Immunofluorescent staining showing nSREBP2 formed puncta under cholesterol depletion condition in SV589 cells. e, Immunofluorescent staining showing nSREBP2 formed puncta under cholesterol depletion condition in HeLa cells. f, Immunofluorescence staining showing that SREBP2 with knocking-in Halo-Tag formed puncta under cholesterol-depleted condition. g, Time-lapse imaging of the fusion behavior of nSREBP2-mEGFP. Images were captured with 5 s between frames. h, Western blotting showing that the expression level of overexpressing nSREBP2-mEGFP was the same as the endogenous level of nSREBP2 under cholesterol-depleted condition. Right panel, quantification of the exogenous and endogenous nSREBP2 expression level (n = 3 independent biological replicates). Data were shown as mean ± s.d. Statistical significance was calculated with unpaired two-tailed Student’s t-test. i, Fluorescence recovery after photo-bleaching imaging of nSREBP2-mEGFP condensates. j, Recovery curve of nSREBP2-mEGFP condensates in FRAP in (i). Data were shown as mean ± s.d. (n = 3 independent biological replicates). k, DNA FISH and immunofluorescent image showing SREBP2 puncta colocalized with INSIG1 gene locus in CHO-K1 cell nucleus under cholesterol depletion condition. Line scan analyses of the merged pictures in the zoomed images in (k) are depicted individually. d-g,i,k, Scale bars for the large images and zoomed images are 10 μm (f), 3 μm and 300 nm. Imaging data represents three independent experiments with similar results.
Extended Data Fig. 2 nSREBP2 interacts with the transcriptional condensates at the target gene loci.
a, Western blotting showing that SREBP2 were successfully labelled with TurboID. b, Western blotting showing that SREBP2 were labelled with 50 μM Biotin for different time after treating with cholesterol depletion for 16 h in HeLa cells. c-e, Immunofluorescence staining showing SREBP2 puncta colocalized with P300 (c), MED15 (d) and histone H3 (e) under cholesterol depletion condition by super resolution imaging. After cholesterol depletion for 16 h, HeLa cells were immunofluorescence stained and then imaged with Nikon SIM super resolution microscope. f, Immunofluorescence staining showing SREBP2 puncta colocalized with BRD4 and Med1 under cholesterol depletion condition by super resolution imaging. After cholesterol depletion for 16 h, 293 T cells were immunofluorescence stained and then imaged with Nikon SIM super resolution microscope. g, Immunofluorescence staining showing SREBP2 puncta colocalized with BRD4 and Med1 under cholesterol depletion condition by super resolution imaging. After cholesterol depletion for 16 h, CHO-K1 cells were immunofluorescence stained and then imaged with Nikon SIM super resolution microscope. h, SREBP2 puncta colocalized with INSIG1 gene locus and BRD4 puncta in the nucleus under cholesterol depletion condition by DNA FISH imaging. After cholesterol depletion for 16 h, CHO-K1 cells were stained and then imaged with Delta Vision Ultra confocal microscope. i, Line scan analyses of the merged pictures in the zoomed images in (h) are depicted individually. c-h, Scale bars for the large images and zoomed images are 10 μm, 3 μm and 300 nm in (c-g), 3 μm and 300 nm in (h). Imaging data represents three independent experiments with similar results.
Extended Data Fig. 3 nSREBP2 contributes to the formation of cholesterol depletion induced super-enhancer.
a, Venn diagram showing the overlapped genes with super-enhancer among HeLa cells treated with FBS, cholesterol-depletion and SREBP target genes from mouse liver microarray. b, Genome browser view of SREBP2 and H3K27Ac ChIP-seq data at the HMGCR, LDLR, SQLE and FASN gene locus in HeLa cells under FBS or cholesterol-depletion condition. c, DNA sequencing results of SREBP2 and SREBP1 knockout HeLa cells. d, Volcano plot showing the up- and down-regulated genes (padj < 0.05, Log2FC > 1 or < -1) from SREBP1 KO HeLa cells with FBS or cholesterol-depletion by RNA-seq. R package DESeq2 calculates two-sided p values by default using the Wald test and performs adjustments for multiple testing. e, Heatmap analysis showing the differential expressed genes of cholesterol synthesis pathway by RNA-seq from WT and SREBP2 KO HeLa cells. f, The top enriched upregulated pathways by GO analysis in Fig. 3d. R package clusterProfiler calculates one-sided p values by hypergeometric test and performs adjustments for multiple testing. g, Motif enrichment from the cholesterol-depletion upregulated genes by RNA-seq using HOMER. A one-sided multiple hypergeometric test for enrichment of motifs in the target sequences compared to the background.
Extended Data Fig. 4 nSREBP2 undergoes phase separation through the N-terminal IDR.
a, Amino acid composition analysis of human nSREBP2. Ticks represent amino acids indicated on the Y axis at the positions indicated on the X axis. b, Net charge per amino acid residue analysis of nSREBP2. c, Schematic of structural model of nSREBP2 by Alphafold2. d, Recovery curve of nSREBP2 IDR-N condensates in FRAP by optoDroplet system in Fig. 4h. Data were shown as mean ± s.d. (n = 3 independent biological replicates). e, Purified nSREBP2 IDR-N-mEGFP showing a concentration-dependent increase in turbidity at increasing levels of large polymeric crowders. f, Recovery curve of nSREBP2 IDR-N-mEGFP droplets in vitro in FRAP in Fig. 4k. Data were shown as mean ± s.d. (n = 3 independent biological replicates). g, In vitro droplet formation analysis of nSREBP2 IDR-N-mEGFP (7.5 μM) with the indicated concentrations of NaCl. Scale bar, 3 μm. Imaging data represents three independent experiments with similar results.
Extended Data Fig. 5 F178 is critical for nSREBP2 phase separation and transactivation of target genes.
a, Representative live cell images of CHO-K1 nuclei expressing nSREBP2 PrLD2 truncation variants and point mutations with light-induced condensation by Corelet system. Scale bar, 3 μm. Imaging data represents three independent experiments with similar results. b, Representative live cell images of CHO-K1 nuclei expressing nSREBP2 PrLD2 truncation variants and point mutations with light-induced condensation by optoDroplet system. Scale bar, 3 μm. Imaging data represents three independent experiments with similar results. c, F178A mutation impaired the expression of SREBP2 target genes in HeLa (SREBP2 KO) cells (n = 3 independent biological replicates). Data were shown as mean ± s.d. Statistical significance was calculated with one-way ANOVA followed by Turkey’s multiple comparison. d, Western blotting showing that HMGCS1 PCP-GFP was expressed the same in overexpressed WT, PrLD2 deletion and F178A mutation in HeLa (SREBP2 KO) cells.
Extended Data Fig. 6 Construction of SREBP2 (F167A) knock-in mice.
a, Alignment of the amino acid sequence of nSREBP2s from different species. Sequence alignment shows that F178 in human SREBP2 is a conserved residue back to killifish. b, Strategy to generate F167A knock-in mice by CRISPR/Cas9 system. c, DNA sequencing results of F167 residue of WT and F167A mice. d, Western blotting showing that F167A mutation did not affect the SREBP2 cleavage process in WT control and F167A knock-in mice primary hepatocytes (n = 3 mice per group). e, Reproductive strategy to generate F167A knock-in homozygous mice. f, Immunofluorescence analysis of SREBP2 WT and F167A mutation mice primary hepatocytes. After cholesterol depletion for 16 h, primary hepatocytes were stained and then imaged with Nikon CSU-W1 confocal microscope. Scale bars for the large images and zoomed images are 25 μm and 5 μm. Imaging data represents three independent experiments. g, Quantification of the percentage of cells that displayed nuclear puncta is shown in (f). Data were shown as mean ± s.d. 100 cells in each group were quantified; n = 3 biologically independent samples. Statistical significance was calculated with unpaired two-tailed Student’s t-test.
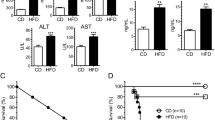
Extended Data Fig. 7 Characteristics of SREBP2 (F167A) knock-in mice.
a–c, Representative images of 8-week-old male WT and F167A mice (a), liver (b) and eWAT (c). d-f, Body weight (d) (n = 15 mice per group), Liver weight (e) (n = 5 mice per group), eWAT weight (f) (n = 5 mice per group) of WT and F167A mice. Data were shown as mean ± s.d. Statistical significance was calculated with unpaired two-tailed Student’s t-test. g, Liver H&E staining of WT and F167A mice within the groups of Non-Fast (NF), fasted for 12 hr (F) and refed for 12 h (R). Scale bar, 100 μm. h, eWAT H&E staining of WT and F167A mice within the groups of Non-Fast (NF), fasted for 12 hr (F) and refed for 12 h (R). Scale bar, 100 μm. i, Volcano plot of the RNA-seq data showing the differentially expressed genes (p < 0.05, Log2FC < -1 or > 1) by comparing the refeeding state with the fasting state from WT liver (left panel) and F167A liver (middle panel), or by comparing the F167A with WT liver under refeeding state (right panel). R package DESeq2 calculates two-sided p values by default using the Wald test and performs adjustments for multiple testing. j, ‘Steroid biosynthetic process’ ranked top in the gene set enrichment analysis (GSEA) analysis of the RNA-seq of F167A liver compared with that of WT liver under refeeding condition. k, The top enriched upregulated pathways in F167A liver compared with WT liver by GO analysis in (i). R package clusterProfiler calculates one-sided p values and performs adjustments for multiple testing.
Extended Data Fig. 8 Effects of nSREBP2 SNP variants and F178Y on the formation of nuclear condensates.
a, Representative live cell images of CHO-K1 cell nuclei expressing nSREBP2 SNP variants with light-induced condensation by optoDroplet system. b, Schematic of nSREBP2 domains and the structural model of F178 and P183 residue by Alphafold2. c, Immunofluorescence analysis of nSREBP2 P183S in HeLa (SREBP2 KO) cells and quantification of the number of condensates per cell. n = 30 cells WT, 28 cells F178A and 29 cells P183S, the cells per component were captured from three different coverslips at random and the condensate numbers were analyzed with manual analysis by Image J. d, Effect of nSREBP2 P183S on the transcriptional activity of SREBP luciferase reporter (n = 3 independent biological replicates). e, Effect of nSREBP2 P183S on the expression of SREBP2 target genes (n = 3 independent biological replicates). f, Representative live cell images of CHO-K1 cell nuclei expressing nSREBP2 P183S mutation with light-induced condensation by optoDroplet. g, Sequence alignment of human nSREBP2 IDR-N and nSREBP1c IDR-N. h, Representative live cell images of CHO-K1 cell nuclei expressing nSREBP1c truncations with light-induced condensation by optoDroplet system. a,c,f,h, Scale bars for the images are 3 μm. Imaging data represents three independent experiments with similar results. c,d,e, Data were shown as mean ± s.d. Statistical significance was calculated with one-way ANOVA followed by Turkey’s multiple comparison.
Extended Data Fig. 9 Graphic summary of transcription activation by nSREBP2 condensates.
A proposed mechanistic model of nSREBP2 condensates regulate the transcriptional activation of lipogenic genes and cholesterol homeostasis.
Supplementary information
Supplementary Information
Images at low magnification for Fig. 5.
Supplementary Tables 1–5
Supplementary Table 1 Reagents. Supplementary Table 2 Antibodies. Supplementary Table 3 Primers for qPCR. Supplementary Table 4 Oligopaint probes for CHO-K1 HMGCS1 and INSIG1. Supplementary Table 5 TurboID-SREBP2 MS protein groups.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, M., Jiang, SY., Tang, S. et al. Nuclear SREBP2 condensates regulate the transcriptional activation of lipogenic genes and cholesterol homeostasis. Nat Metab 7, 1034–1051 (2025). https://doi.org/10.1038/s42255-025-01291-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01291-0
This article is cited by
-
SREBP2-RAB11A-ZDHHC20 axis orchestrates FGFR3 palmitoylation and membrane retention to drive bladder cancer progression
Cellular Oncology (2026)
-
Advanced multifunctional nano-delivery platform focusing on treating diseases related to lipid metabolism via targeted intervention in various lipid metabolic processes
Military Medical Research (2025)