Abstract
Frailty is a clinically relevant phenotype with notable gaps in our understanding of its etiology. Using the Hospital Frailty Risk Score (HFRS) to define frailty, we performed a genome-wide association study in FinnGen (N = 500,737), replicated the results in the UK Biobank (N = 407,463) and performed a meta-analysis. We prioritized genes through colocalization with expression, splicing and protein quantitative trait loci and proteomics integration. We identified 53 independent lead variants associated with frailty (P < 5 × 10−8), of which 45 were novel and not previously reported in the GWAS Catalog. Replication at the individual variant and polygenic risk score of the HFRS (P = 1.86 × 10−522) levels and meta-analysis largely confirmed the findings. Colocalization analysis supported a causal role for several genes, including CHST9, C6orf106 (ILRUN), KHK, MET, APOE, CGREF1 and PPP6C. Additionally, plasma levels of MET, CGREF1 and APOE were associated with HFRS. Our results reveal new genetic contributions to frailty and shed light on its biological basis.
Similar content being viewed by others
Main
Aging is a highly complex process with substantial heterogeneity in health trajectories among individuals. Frailty represents a clinically relevant aging phenotype that gauges health in aging1 and predicts various adverse outcomes independent of chronological age2. Frailty describes a syndrome of decreased physiological reserves across multiple homeostatic systems1. Currently, no gold standard exists to measure frailty; instead, several scales with different properties have been developed, each capturing partially different at-risk populations3. Created based on 109 weighted International Classification of Diseases, 10th Revision (ICD-10) codes characterizing older adults with high resource use and diagnoses associated with frailty, the HFRS presents a relatively new scale to measure frailty4. It has a fair overlap with existing frailty definitions based on the deficit accumulation (frailty index (FI)) and phenotypic (frailty phenotype (FP)) models of frailty and has a moderate agreement with the FI4. While the HFRS uses ICD-10 codes for administrative ease, enabling the measurement of frailty in real-world data, the FI5 and FP6 are rooted in clinical and functional data and are often assessed in cohort studies. The FI is a multidimensional measure of frailty, offering a comprehensive view of a person’s overall health5. In contrast, the FP defines frailty through specific physical characteristics: weakness, slowness, exhaustion, low physical activity and weight loss6. While each measure captures distinct aspects of frailty, together they provide a more complete understanding of the condition.
The etiology of frailty remains incompletely understood. Twin studies by us and others suggest that frailty, measured using the FI, is up to 52% heritable7,8, with relatively stable genetic influences across age9. To date, only two previous large-scale genome-wide association studies (GWASs) of frailty exist. Atkins et al. performed a meta-analysis GWAS of FI that identified 34 loci and estimated the single nucleotide polymorphism (SNP) heritability of the FI at 11%10. Ye at al. identified 123 loci for FP and estimated the SNP heritability of the FP at 6%11. However, it is likely that additional genetic signals exist and analyses in other large populations can shed further light on the genetic underpinnings of frailty.
To date, no previous studies into the genetics of frailty using the HFRS exist. To this end, we performed a GWAS of the HFRS in FinnGen (N = 500,737), with replication of the results in the UK Biobank (N = 407,463), both at the individual variant level and through polygenic risk scores (PRSs). We also performed a meta-analysis on the results from both GWASs to capture the totality of the evidence. Given that dementia has the highest weight in the HFRS definition, we performed a sensitivity analysis by excluding dementia from the HFRS definition and similarly replicated the results in the UK Biobank and conducted a meta-analysis on the results. A functional follow-up to identify causal genetic loci was performed through colocalization analysis12 with expression, splicing and protein quantitative trait loci (eQTL, sQTL and pQTL, respectively) and associating measured protein levels with the HFRS in the UK Biobank (N = up to 42,495).
Results
Sample characteristics
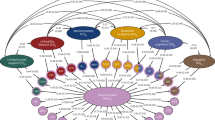
The workflow of the analyses is presented in Fig. 1. In the HFRS GWAS, we included 500,737 (282,202 females, 56.4%) FinnGen and 407,463 UK Biobank participants (220,208 females, 54.1%). Characteristics of the study populations are presented in Table 1.
Discovery GWASs of HFRS and HFRS without dementia were performed in FinnGen to identify genetic variants associated with frailty. The significant variants (P ≺ 5 × 10−8) were then replicated in the UK Biobank, and a meta-analysis of the FinnGen and UK Biobank results was performed. The GWAS summary statistics of FinnGen were used to calculate HFRS-PRSs, which were then assessed for their association with mortality and hospitalizations in the UK Biobank. Finally, protein association and colocalization analyses were performed to prioritize genes and identify causal variants.
Discovery GWAS of HFRS in FinnGen
We identified 1,588 variants associated (P < 5 × 10−8) with the HFRS in the main analysis and 492 variants in the sensitivity analysis, which removed the dementia weights from the HFRS (Fig. 2a,b and Supplementary Tables 1 and 2). Of these, 53 variants (at 50 loci) and 42 variants (at 42 loci) were identified as independent lead variants (r2 < 0.01) for the HFRS and HFRS without dementia, respectively. As dementia diagnosis has the highest weight in the HFRS formula, the most influential peak expectedly resided in the APOE (rs7412) region on chromosome 19 (Fig. 2a). Sensitivity analysis confirmed the expected loss of the APOE peak (Fig. 2b). Of the independent lead variants associated with HFRS and HFRS without dementia, 45/53 and 36/42, respectively, were novel with respect to the GWAS Catalog and previously reported GWAS results of the FI10, FP11 and mvAge13 (Fig. 3a and Supplementary Tables 1 and 2). The variants mapped to 41 (HFRS) and 30 (HFRS without dementia) genes of which 6 and 3, respectively, were novel, that is, previously unreported for any trait at P < 5 × 10−8. The results also demonstrated unique, non-shared associations in both analyses (Fig. 3b and Supplementary Tables 1 and 2). Supplementary Table 3 presents the shared and unique genes between the HFRS, FI and FP GWASs.
a,b, Manhattan plots for the associations with HFRS (a) and HFRS excluding dementia (b) in FinnGen using linear mixed-effects modeling adjusted for birth year, sex and the first ten PCs. The dashed lines indicate the genome-wide significance threshold (P = 5 × 10−8). The annotations represent the independent lead variants associated with frailty.
a, Venn diagram showing the overlap of the lead variants associated with the full HFRS and the HFRS without dementia in FinnGen and those reported in the literature. Previous GWASs refers to genes identified in for the FI10, FP11 and mvAge13. b, Venn diagram showing the overlap of the lead variant genes associated with the full HFRS and the HFRS without dementia in FinnGen and those reported in the literature. c, Genetic correlations between HFRS in FinnGen and other frailty-related traits estimated using linkage disequilibrium score regression. All the correlations were statistically significant at P < 2.2 × 10−16.
Replication in the UK Biobank and meta-analysis
For HFRS, 1,262/1,588 variants were available for replication and meta-analysis. In the UK Biobank, 73 variants (6%) replicated at P < 5 × 10−8 and 688 (55%) at P < 0.05, while in the meta-analysis, 357 variants (28%) replicated at P < 5 × 10−8 and 1,260 (100%) at P < 0.05 (Supplementary Table 1). Of the 53 lead variants, 36 were available; 2 lead variants (6%) replicated at P < 5 × 10−8 and 14 (39%) at P < 0.05 in the UK Biobank, while 6 (17%) replicated at P < 5 × 10−8 and 35 (97%) at P < 0.05 in the meta-analysis (Supplementary Table 1). For HFRS without dementia, 435/492 variants were available for replication and meta-analysis. In the UK Biobank, 21 variants (5%) replicated at P < 5 × 10−8 and 118 (27%) at P < 0.05, while in the meta-analysis, 50 variants (11%) replicated at P < 5 × 10−8 and 435 (100%) at P < 0.05 (Supplementary Table 1). Of the 42 lead variants, 26 were available; 1 lead variant (4%) replicated at P < 5 × 10−8 and 10 (38%) at P < 0.05 in the UK Biobank, while 4 (17%) replicated at P < 5 × 10−8 and 26 (100%) at P < 0.05 in the meta-analysis (Supplementary Table 2). The effect direction was consistent for all variants that replicated at P < 5 × 10−8 in the meta-analysis (Supplementary Tables 1 and 2).
Genetic correlation and heritability
We observed a lambda genomic control value of 1.27 with an intercept of 1.19 (s.e. = 0.011) for HFRS and 1.11 with an intercept of 1.23 (s.e. = 0.010) for HFRS without dementia (QQ plots provided in Extended Data Fig. 1). Despite the relatively high lambda values, the intercepts suggest that the inflation in test statistics was mainly due to polygenicity, rather than bias due to population stratification. The SNP heritability was 0.06 (s.e. = 0.002) for HFRS and 0.04 (s.e. = 0.002) for HFRS without dementia. Statistically significant and positive genetic correlations (P < 2.2 × 10−16) were observed between HFRS and previous GWASs on frailty and mvAge (Fig. 3c).
Cell-type and pathway enrichment
For HFRS, the top (P < 3.7 × 10−5, corrected for multiple testing) cell types enriched for expression were limbic system neurons in cerebrum, excitatory neurons (Ex6) in visual cortex, oligodendrocyte precursor cells (OPCs) in cerebellar hemisphere and oligodendrocytes in cerebellum (Extended Data Fig. 2 and Supplementary Table 4). For HFRS without dementia, the top cell types were OPCs and astrocytes in cerebellar hemisphere, skeletal muscle satellite cells in muscle and endocrine cells in stromal cells in stomach (Extended Data Fig. 3 and Supplementary Table 5). Enrichr14 pathway analysis (adjusted P < 0.05) showed that the top pathways for the HFRS signals were relevant to the nervous system functions (herpes simplex virus 1 infection, netrin-mediated repulsion signals), cell adhesion and lipid metabolism (Supplementary Table 6). Comparison of the pathways from the HFRS, FI and FP GWASs revealed overlap in herpes simplex virus 1 infection and cell adhesion molecules between HFRS and FI, and in multiple pathways related to lipid and lipoprotein metabolism, cellular interactions and adhesion between HFRS and FP (Supplementary Table 6). Each GWAS also had distinct pathways not shared with the others (Supplementary Table 6). For HFRS without dementia, several functions related to cell cycle were enriched at P < 0.05, although none of the pathways were statistically significant after correction for multiple testing (Supplementary Table 7).
Exploring causal variants through proteomics integration
To identify potentially causal and functional variants (that is, missense, splice region, loss of function and 5′ and 3′ untranslated region variants associated with the HFRS and HFRS without dementia at P < 5 × 10−7; Supplementary Tables 8 and 9), we associated the protein levels of the corresponding genes to HFRS (13 proteins available in UK Biobank Olink platform) and HFRS without dementia (8 proteins available in UK Biobank Olink platform). We adjusted the models for birth year, sex and the first ten principal components (PCs; model 1), as well as batch, baseline assessment center, body mass index (BMI) and smoking (model 2). Significantly associated proteins at a false discovery rate (FDR) < 0.05 in both models 1 and 2 were CGREF1, MET, ALDH2, NECTIN2, APOC1, APOE and FOSB for HFRS, and CDK and POF1B for HFRS without dementia (Fig. 4 and Supplementary Table 10).
a,b, Protein associations (beta coefficients) with the full HFRS (a) and HFRS without dementia (b) the in the UK Biobank using linear regression models (N = 34,879–42,495; exact N for each model is given in Supplementary Table 10). All models were adjusted for birth year, sex and the first ten PCs (model 1), and additionally adjusted for batch, baseline assessment center, BMI and smoking (model 2). Solid dots indicate statistically significant associations at an FDR < 0.05. The bars indicate 95% confidence intervals.
Colocalization analysis
Several gene loci, such as CHST9, C6orf106 (ILRUN), KHK, MET, CGREF1 and PPP6C had shared causal variants (posterior probability for H4 (PP.H4) > 80%) in eQTL and/or sQTL for HFRS. Several colocalized (PP.H4 > 80%) eQTL and/or sQTL loci were also identified for HFRS without dementia, including CHST9, CGREF1, PPP6C, ADARB1 and PSMB7. The full eQTL and sQTL colocalization results for the HFRS and HFRS without dementia are presented in Supplementary Tables 11 and 12, and the colocalization results with a PP.H4 > 80% are summarized by tissue for each gene in Extended Data Fig. 4. In the pQTL analysis, of those genes that had a protein measurement available (that is, the protein was detectable in plasma), a total of 20 loci for HFRS and 9 loci for HFRS without dementia had enough power for the analyses (PP > 88%; Methods). Of them, a colocalized signal (that is, shared single causal variant, PP.H4 > 80%; Methods) was detected within APOE and BRAP genes for HFRS (Supplementary Table 13), whereas no colocalized signal with pQTL was detected within genes for HFRS without dementia. For most of the tested genes, the PP.H3 values were greater than or close to 90%, indicative of distinct causal variants for protein levels and HFRS (Supplementary Tables 13 and 14). Regional association plots of the APOE gene demonstrated that the strongest signal peak rs429358 and variants in high linkage disequilibrium with it fall in the vicinity (Extended Data Fig. 5).
HFRS-PRS analyses in the UK Biobank
The PRSs for HFRS (HFRS-PRSs) were statistically significantly associated with the HFRS in the UK Biobank (β = 0.074 per s.d. increase; P = 1.86 × 10−522) after adjusting for birth year, sex and the first ten PCs (Fig. 5a). Next, using similar adjustments, we analyzed whether the HFRS-PRSs could predict early-onset frailty in the UK Biobank (that is, HFRS > 5 before age 65) and observed an odds ratio of 1.25 (P = 2.0 × 10−322; Fig. 5b). We further examined whether the HFRS-PRSs could predict all-cause mortality and number of hospitalizations and found statistically significant associations with both outcomes (Fig. 5c,d). The estimates of the HFRS-PRSs were similar in men and women compared to the full sample, and also consistent for the HFRS-PRsS excluding dementia (Fig. 5a–d). Numeric estimates for all the HFRS-PRS analyses are presented in Supplementary Table 15. Lastly, we found that adding the HFRS-PRSs to a model with age, sex and the first ten PCs significantly improved model performance on mortality and hospitalizations, as assessed by likelihood-ratio and F-test statistics (Supplementary Table 16).
a–d, Associations of the HFRS-PRSs with the HFRS (a), early-onset frailty (b), all-cause mortality (c) and number of hospitalizations (d) in the UK Biobank (N = 407,463). All models included birth year, birth region, sex and the first ten PCs as covariates. The bars indicate 95% confidence intervals of the beta coefficients, odds ratios (ORs) and hazard ratios (HRs).
Prediction of mortality using HFRS
To assess the validity of HFRS in predicting mortality, we examined its association with all-cause mortality and found that higher HFRSs, both with and without dementia, were associated with mortality in FinnGen (hazard ratio 1.29 for both HFRS and without dementia) and UK Biobank (hazard ratio 1.48 for both HFRS without dementia), independent of age, birth year and sex (Supplementary Table 17).
Discussion
Our study represents a large GWAS of frailty using the HFRS. We identified 1,588 associated variants and 53 lead variants, of which 45 were novel, and not previously reported for any trait. The lead variants mapped to 41 genes, of which 6 were novel. Replication in the UK Biobank and subsequent meta-analysis showed that 28% of all variants and 17% of lead variants replicated at P < 5 × 10−8, while 100% of all variants and 97% of lead variants replicated at P < 0.05 in the meta-analysis. Colocalization analysis identified several causal candidate genes, including CHST9, C6orf106 (ILRUN), KHK, MET, APOE, CGREF1 and PPP6C. Additionally, plasma levels of MET, CGREF1 and APOE were associated with HFRS, further supporting their roles in frailty. We also derived PRSs for HFRS and showed that they predict frailty, early-onset frailty, mortality and hospitalizations in the UK Biobank.
The strongest GWAS signals were observed in the TOMM40/APOE/APOC1/NECTIN2 locus on 19q13.3, a locus in strong linkage disequilibrium and known for its associations with cognitive15 and cardiometabolic16 traits. We observed the strongest signal for the missense variant rs429358 (388 T > C) that, together with rs7412, defines the APOE ε2, ε3 and ε4 haplotypes. The rs7412 was, however, not associated with frailty in our study. A similar finding has been observed for longitudinal weight loss—a feature that also characterizes frailty—where rs429358 increased the risk, while rs7412 did not17. Our sensitivity analysis, which removed dementia from the HFRS, truncated the chromosome 19 peak as expected and revealed additional loci. The HFRS lead variants mapped to 42 genes, 7 of which were shared with HFRS without dementia, while 31 genes were uniquely mapped in HFRS without dementia. The unique lead variant associations for both HFRS and HFRS without dementia are plausible, as dementia had the highest individual weight in the HFRS definition, and for highly polygenic traits like frailty, even small differences in phenotype definitions can influence which variants reach genome-wide significance. Genetic correlation between HFRS and HFRS without dementia was nevertheless almost perfect (0.98), indicating the same underlying genetic construct.
The genes to which the 45 novel lead variants for the HFRS mapped include C6orf106 (ILRUN) and CHST9, both of which also displayed colocalized signals with eQTL across different tissues, supporting their potential causal roles. C6orf106 (ILRUN) is a regulator of inflammation and lipid metabolism18, while CHST9 encodes an enzyme essential for cell–cell interactions and signal transduction19. Notably, several CHST9 variants were also associated with HFRS without dementia and similarly exhibited colocalization with eQTL. CGREF1, a gene linked to cell cycle regulation and adhesion20, and PPP6C, a gene involved in nuclear factor-κB pathway regulation21, showed the same sQTL-colocalized gene–tissue pairs for HFRS and HFRS without dementia, supporting their functional roles in frailty, irrespective of the HFRS definition. While C6orf106 (ILRUN), CHST9, CGREF1 and PPP6C are functionally diverse, they collectively link immunoinflammatory modulation, cellular interactions and adhesion to frailty. Specific to HFRS, we additionally identified multiple colocalized signals in KHK and MET, while for HFRS without dementia, we identified additional colocalized signals in ADARB1 and PSMB7. Aside from a few links to blood pressure, plasma lipids or BMI in the GWAS Catalog, CHST9, CGREF1, PPP6C, KHK, MET, ADARB1 and PSMB7 have no prior GWAS associations with the HFRS conditions, suggesting that HFRS, as a composite measure, can offer insights into frailty beyond its individual components.
Proteomics integration showed that CGREF1, NECTIN2, MET and APOC1 were associated with the HFRS with the largest effect sizes; elevated levels of the first two and lower levels of the latter two were associated with higher HFRS scores. Previous studies have linked elevated circulating NECTIN2 levels to Alzheimer’s disease risk22 and low APOC1 levels to cognitive decline and frailty, as defined using the FP23, which likely explains their associations with the HFRS. In contrast, no prior studies have linked plasma CGREF1 or MET to frailty or HFRS conditions, highlighting a novel association. Additionally, as CGREF1 and MET exhibited eQTL-colocalized and/or sQTL-colocalized signals across multiple tissues, their protein-level associations further support their biological relevance in frailty.
We estimated the SNP heritability of HFRS at 6%, an estimate in the same range as previously reported for the FI (11%)10 and FP (6%)11. Genetic correlations between HFRS, FI and FP were moderate, ranging from 0.54 to 0.63, while gene-level overlap was limited: two shared genes between HFRS and FI and eight between HFRS and FP. The limited gene-level overlap is likely a result of frailty being a highly polygenic trait, where genome-wide significant variants represent only a fraction of the total genetic signal. Genetic correlation, in turn, reflects the combined influence of numerous variants, including those that do not reach the genome-wide significance threshold, but still make a substantial overall contribution to the trait. Moreover, it has been shown that different frailty scales identify only partially overlapping groups of individuals as frail4,24, suggesting that these scales may capture somewhat distinct constructs. In our previous study25, we assessed the phenotypic correlation between HFRS and FI at 0.21 and HFRS and FP at 0.31 in the UK Biobank participants, indicating somewhat lower phenotypic correlations compared to their genotypic counterparts. A possible explanation is that, because most UK Biobank participants are still relatively young, frailty may not yet be fully expressed, leading to many values being 0 and thereby diluting the phenotypic correlations. Additionally, environmental factors, such as physical activity, may directly influence phenotypic frailty, but might not affect the multidimensional FI or HFRS to the same extent, leading to reduced phenotypic correlations. The overall low prevalence of frailty in the UK Biobank participants may also have contributed to the low gene-level overlap between FI, FP and HFRS because both the FI10 and FP11 GWASs included UK Biobank participants. For the same reason, the overall lower HFRS scores in the UK Biobank and differences in the proportions of individuals with certain HFRS conditions between FinnGen and the UK Biobank may have also affected the replication results, potentially leading to underestimated effect sizes in the UK Biobank and the overlooking of some true associations.
Cell-type enrichment indicated enriched expression of the genes associated with the signals in various neuronal cells, such as limbic system neurons, excitatory neurons, OPCs and oligodendrocytes located in the cerebrum, visual cortex, cerebellar hemisphere and cerebellum, respectively. Enrichment of OPCs (cerebellar hemisphere) persisted even after removing the contribution of dementia diagnoses from the HFRS. Expression enrichment in brain tissues was likewise observed the GWAS of FI10, which identified frontal cortex BA9, cerebellar hemisphere, spinal cord cervical C1 and hippocampus as statistically significant. The GWAS on FP11 also identified the genetic signals enriched in brain tissues, such as cerebellar hemisphere, frontal cortex BA9 and cerebellum. It is noteworthy that neither FI nor FP in these GWASs included any items of cognition or dementia diagnosis in the frailty definition. Our findings thus reinforce the role of central nervous system functions in frailty, regardless of the frailty definition.
Our pathway analyses highlighted Herpes simplex virus 1 infection and various cell adhesion and lipid/lipoprotein metabolism pathways relevant to the signals. The first two pathways overlapped with the FI pathways, while lipid metabolism processes were shared with the FP pathways. However, several pathways were unique to each frailty measure: FI was enriched for immunoinflammatory functions, while FP included cardiac and membrane transport processes. These differences likely stem from the varying components of each frailty measure. The HFRS, which includes 109 conditions capturing both multisystem decline and core physiological senescence, showed enrichment in fundamental processes like cell adhesion and lipoprotein metabolism. The FI, also reflecting multisystem decline, appears particularly influenced by immunoinflammatory factors, as seen also in previous associations with GlycA26, a marker of systemic inflammation, including studies supporting a causal link27. Many FI-related conditions, such as cardiovascular disease and diabetes, also have inflammatory components, potentially explaining the connection. The FP, which mostly focuses on physical frailty, was enriched for cardiac function and membrane transport pathways, both essential for muscle activity, ion flux regulation and nutrient uptake.
To assess the usefulness of the HFRS in our samples, we showed that it predicts mortality independent of sex and birth year and performs equally well even when dementia is excluded. Similarly, the HFRS-PRSs, also when dementia was removed, associated with the risk of frailty, early-onset frailty, mortality and hospitalizations. As frailty manifests relatively late in life for most individuals, risk assessment through PRSs may offer possibilities for early intervention to mitigate frailty before it escalates. Future studies are needed to ascertain the clinical utility of such approaches.
Our definition of frailty was based on clinical diagnoses in register data; such an approach has both advantages and disadvantages. A notable advantage is that in Finland and the United Kingdom, public healthcare is primarily tax-funded, and each citizen has equal access. Issues pertinent to self-reported data, such as recall bias and missing information were also avoided. On the other hand, some conditions may be underreported in the registers, while others may have a lag from the onset of symptoms to assigning the diagnosis. We also note that the genetic associations were weaker in the UK Biobank compared to FinnGen, a finding likely explained by healthy selection due to volunteer-based participation in the UK Biobank28 compared to FinnGen, which consists of national cohorts and biobank samples of hospitalized individuals. Also pertinent to all GWASs, the discovery samples tend to have stronger association statistics compared to replication, a phenomenon known as the winner’s curse.
In conclusion, we provide a large GWAS on HFRS and reveal new genetic contributions and causal candidate genes. Overall, the results reinforce previous findings that immunoinflammatory and nervous system functions are relevant to the etiology of frailty, regardless of how frailty is defined. Future studies should thus explore the role of these functions in the development of frailty, including cognitive frailty, to better understand the etiology of frailty.
Methods
This work complies with all relevant ethical regulations. A full list of the ethics boards that approved the study protocols is provided at the end of this section.
Samples
FinnGen is a large national genetic resource (N = 520,210; release 12) established in 2017 and consists of Finnish individuals, aged 18 years and older at study baseline29. FinnGen includes prospective epidemiological and disease-based cohorts, as well as hospital biobank samples. Information on diagnoses since 1969 was linked by the unique national personal identification number to national healthcare, population and cause of death registries and recorded using the ICD Revisions 8–10. Information on dates and causes of death were obtained via linkages to the population and cause of death registers through 30 September 2023 (R12 v1). After excluding individuals with missing information on baseline age, birth year and sex, and samples not passing genotyping quality control (see below), we included 500,737 FinnGen participants in this study.
The UK Biobank includes 502,642 volunteer participants, aged 37 to 73 years old at baseline, recruited through 22 assessment centers across England, Scotland and Wales between 2006 and 2010 (ref. 30). The participants provided self-reported information on demographics, lifestyle and disease history via questionnaire and underwent physiological measurements, including providing a blood sample for genetics data. Hospital inpatient data were sourced from the Hospital Episode Statistics for England, Scottish Morbidity Record and Patient Episode Database for Wales, which contain electronic medical records (that is, ICD-10 codes) for all hospital admissions in England, Scotland and Wales, respectively. The hospital inpatient data were available through 31 October 2022 for England, 31 August 2022 for Scotland and 31 May 2022 for Wales. Death register data contained all deaths in the population through 30 November 2022, including primary and contributory causes of death. Participants in both UK Biobank and FinnGen have not received compensation for their participation.
Assessment of frailty
The HFRS was calculated according to a previously described protocol4 based on 109 weighted ICD-10 codes. The codes were selected through a data-driven approach to include codes that were most prevalent in individuals with frailty and high healthcare resource use4. Each code was assigned with a weight ranging from 0.1 to 7.1, based on its association with frailty and predictive value for frailty-related outcomes4. The weights of all relevant ICD-10 codes present in an individual’s records were then extracted and summed to calculate the HFRS score. The conditions, their respective weights and proportion of individuals with each condition in FinnGen and the UK Biobank are listed in Supplementary Table 18. The HFRS was used as a continuous variable in the GWAS. We also categorized the HFRS into low (<5), intermediate (5–15) and high (>15) risk of frailty as previously described4 and used the cutoff points to describe frailty in our study populations. In the main analysis, we included all available ICD-10 codes for each person from age 30 years to the age at the end of follow-up to calculate the HFRS. As dementia diagnoses have the highest weight in the HFRS, we also calculated the HFRS by excluding dementia weights from the formula and performed sensitivity analyses on all analyses using the HFRS without dementia.
Genotyping and imputation
Genotyping in FinnGen was performed in Illumina and custom AxiomGT1 Affymetrix (Thermo Fisher Scientific) genome-wide arrays and imputed to 16,387,711 (imputation INFO score > 0.6) variants using a population-specific SISu v.3 imputation reference panel as previously described31. Individuals with ambiguous sex and non-Finnish ancestry were excluded. UK Biobank samples (v3 genotyping release) were genotyped on custom Affymetrix microarrays and imputed using the 1000 Genomes and the Haplotype Reference Consortium reference panels to ~93 million variants32. Participants were excluded if they were flagged as having unusually high heterozygosity or missing genotype calls (<5%). Our analysis was restricted to participants with European descent and white British ancestry (N = 407,463). Detailed procedures on genotype calling, quality controls and imputation have been previously described for FinnGen29 and the UK Biobank32.
Statistics and reproducibility
No statistical method was used to predetermine sample size, as the UK Biobank and FinnGen cohorts are sufficiently large and can be anticipated to provide adequate statistical power for the planned analyses. We have sought to include all samples after exclusion based only on incomplete data, such as sex, birth year and genotype quality control as called by the respective cohorts. In the case of the UK Biobank, non-European descent and non-white British ancestry participants were excluded to facilitate the comparison to the homogeneous FinnGen Finnish populations. Our study did not involve randomization/allocation into experimental groups, as it was an observational, hypothesis-free GWAS treating the HFRS as a continuous outcome. Therefore, no experimental manipulation or group assignment was performed. In a hypothesis-free GWAS, blinding is not possible/necessary as the analysis is fully automated and applies standardized statistical tests uniformly across the genome. Data distribution was assumed to be normal, but this was not formally tested.
Discovery GWAS, replication and meta-analysis
The analytical pipeline for GWAS and post-GWAS analyses is presented in Fig. 1. We first performed GWASs of HFRS and HFRS without dementia in FinnGen using the SAIGE33 (v.0.35.8.8) software, which uses linear mixed-effects modeling to account for genetic relatedness and confounding by ancestry34. We included variants (N = 21,294,561) with minor allele frequency > 0.01%, Hardy–Weinberg P value > 1 × 10−9 and imputation INFO score ≥ 0.9. The models were adjusted for birth year, sex and the first ten PCs. The genome-wide significance level was set to 5 × 10−8. The total number of genes to which the variants were mapped was determined by extracting variants with a P < 5 × 10−8, followed by variant mapping and annotation using the Variant Effect Predictor35 in the standard FinnGen GWAS annotation pipeline29. Independent lead variants were identified using the R package gwasRtools36. We used a distance-based loci definition on the genome-wide significant variants (that is, 500 kb from index variant) to estimate the independent genomic loci. Independent lead variants were identified by linkage disequilibrium clumping and defined as those that were independent from each other at r2 < 0.01.
To replicate the findings at the variant level, we performed both HFRS GWASs in the UK Biobank. To account for the related samples in the UK Biobank, we applied a mixed linear model-based GWAS analysis (‘fastGWA’)37, which is an efficient method to control for relatedness by a sparse genetic relationship matrix, without the need of excluding related individuals. The models were adjusted for birth year, sex, genotyping array and the first ten PCs. Finally, to capture the totality of the evidence, we conducted a meta-analysis on the results from FinnGen and the UK Biobank using METAL38. A fixed-effect meta-analysis was performed using the default approach, with P value and direction of effect weighted according to sample size, and with adjustment for genomic control (lambda). Using the NHGRI-EBI GWAS Catalog39 filtered for P < 5 × 10−8 and results of previous GWASs into frailty (using the FP11 and FI10 to measure frailty) and mvAge13, a genomic structural equation modeling-derived composite construct of healthspan, parental lifespan, extreme longevity, frailty and epigenetic aging, we assessed the number of novel and previously unreported associations relative to the FinnGen results.
Genetic correlation and heritability
Using linkage disequilibrium score regression40 (v1.0.1) and linkage disequilibrium merged with the HapMap3 reference panel of ~1.1 million variants, we estimated (1) the potential bias from, for example, population stratification and cryptic heritability in the GWAS results, (2) heritability of HFRS and (3) genetic correlations between HFRS and previous GWASs of FI10, FP11 and mvAge13. As the FI GWAS10 used an opposite effect allele compared to the standard FinnGen workflow, we inverted the genetic correlation coefficient to prevent an artifactual negative correlation and facilitate interpretation.
Functional annotation: cell-type and pathway enrichment
To explore tissue and cell-type specificity of the annotated genes underlying HFRS, we applied WebCSEA, a web platform to derive context-specific expression patterns of genes underlying complex traits, encompassing the Human Cell Atlas and single-cell data resources41,42. Enrichr pathway analysis14 based on KEGG39 and Reactome40 resources was applied to explore enriched pathways of the identified genes (GWAS P < 5 × 10−8). To effectively compare the enriched pathways of the HFRS with those of the FI and FP GWASs, we extracted all genome-wide significant variants from these GWASs and performed KEGG and Reactome pathway analyses using the same (default) settings.
Proteomics integration
To prioritize genes and identify potentially functional and causal variants, we narrowed down the association signals to a smaller number of missense, splice region, loss of function and 5′ and 3′ untranslated region variants (the two last mentioned potentially affecting transcript stability, localization and signal response), identified from the Variant Effect Predictor pipeline35, that were associated with the HFRS at a slightly more relaxed threshold (P < 5 × 10−7). Using the Olink proteomics data, we then examined if the protein levels of the variants (at a gene-level resolution) were associated with HFRS in the UK Biobank. Details of the UK Biobank Olink proteomics assay, quality-control and data processing procedures have been described elsewhere43. Briefly, 54,239 UK Biobank participants were selected for the proteomics profiling using EDTA plasma samples collected at the baseline assessment. Of the 54,239 samples, 46,595 were randomly selected, while 6,376 were chosen by UKB-PPP consortium members and 1,268 were from participants in the coronavirus disease 2019 repeat imaging study, resulting in a sample that was predominantly, but not entirely, random. A total of 2,923 proteins were measured across 8 protein panels using the antibody-based Olink Explore 3072 platform. Protein levels were measured in Normalized Protein eXpression values, which represent the relative concentration of proteins on a log2 scale. All the protein levels were scaled to mean = 0 and s.d. = 1 before the association testing. Linear regression models were then performed to assess the associations between the proteins that were available in the Olink platform and HFRS, adjusting for (i) birth year, sex and the first ten PCs and (ii) batch, baseline assessment centers, BMI and smoking. We considered an FDR < 0.05 as statistically significant in the proteomics analysis.
Colocalization analyses
To further prioritize the genes and identify causal variants, we performed a Bayesian-based colocalization analysis with eQTL, sQTL and pQTL, using a flanking window of 1 Mb and default parameters for prior probabilities12. The analysis assumes that only one causal variant exists for each trait in a genomic locus and returns PPs indicating the likelihood that the following hypotheses (H) are true: there is no association at the locus with either expression/splicing/protein level or HFRS (H0); there is an association with expression/splicing/protein level but not HFRS (H1); there is no association with expression/splicing/protein level, but there is an association with HFRS (H2); there is an association with both expression/splicing/protein level and HFRS, but with distinct causal variants (H3); there is an association with both expression/splicing/protein level and HFRS with a shared causal variant (H4). We considered the analysis having enough power if the sum PPs had a distinct or shared causal variant exceeded 88%. A colocalized signal was detected if the PP of a shared causal variant (H4) existence was greater than 80%. The GTEx database44 (v8) was interrogated for eQTL and sQTL, while the UK Biobank Pharma Proteomics Project43 was used for pQTL.
PRS analyses
Using the GWAS summary statistics from FinnGen, we calculated the PRSs for HFRS in the UK Biobank by applying PRSs with continuous shrinkage45,46 and using the European panel from the 1000 Genomes46 linkage disequilibrium reference, where ~1.1 million variants were selected. Using linear regression, we fitted a linear model to assess how the HFRS-PRSs associate with the HFRS score. HFRS was considered as a standardized z-score in the linear regressions. We also performed logistic regressions to assess the associations of the HFRS-PRSs with early-onset frailty, defined as HFRS > 5 before age 65. Age 65 was chosen as the cutoff as it is commonly used to distinguish ‘young’ from ‘old’ in statistical and policy contexts. Our previous work also identified age 65 as the optimal threshold for distinguishing between early-life and late-life frailty47. The PRS was modeled per standard deviation change, and all the models included birth year, sex and the first ten PCs as covariates.
Lastly, as frailty manifests in late life for most individuals, we asked whether the HFRS-PRSs could be used in early risk stratification to identify individuals at risk of adverse outcomes. To this end, Cox models with attained age as the timescale and linear regression models were fitted to assess whether the HFRS-PRSs predict all-cause mortality and number of hospitalizations, respectively. The added value of the HFRS-PRSs beyond age and sex in the prediction was assessed using the F-test for linear regressions and likelihood-ratio test for Cox models. The number of hospitalizations was scaled to a mean = 0 and s.d. = 1 before modeling.
Prediction of mortality using HFRS
Cox models with attained age as the timescale, which inherently adjusts for age, were fitted to assess the association between HFRS, HFRS without dementia and all-cause mortality in FinnGen and the UK Biobank. Two models were fitted for each HFRS definition: one adjusting for sex and birth year, and one without adjustments.
Ethics statements of FinnGen and UK Biobank
FinnGen
Patients and control participants in FinnGen provided informed consent for biobank research, based on the Finnish Biobank Act. Alternatively, separate research cohorts, collected before the Finnish Biobank Act came into effect (in September 2013) and the start of FinnGen (August 2017), were collected based on study-specific consents and later transferred to the Finnish biobanks after approval by the Finnish Medicines Agency, the National Supervisory Authority for Welfare and Health. Recruitment protocols followed the biobank protocols approved by the Finnish Medicines Agency. The Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa (HUS) statement number for the FinnGen study is HUS/990/2017. The FinnGen study is approved by Finnish Institute for Health and Welfare (permit nos. THL/2031/6.02.00/2017, THL/1101/5.05.00/2017, THL/341/6.02.00/2018, THL/2222/6.02.00/2018, THL/283/6.02.00/2019, THL/1721/5.05.00/2019 and THL/1524/5.05.00/2020), Digital and population data service agency (permit nos. VRK43431/2017-3, VRK/6909/2018-3 and VRK/4415/2019-3), the Social Insurance Institution (permit nos. KELA 58/522/2017, KELA 131/522/2018, KELA 70/522/2019, KELA 98/522/2019, KELA 134/522/2019, KELA 138/522/2019, KELA 2/522/2020 and KELA 16/522/2020), Findata (permit nos. THL/2364/14.02/2020, THL/4055/14.06.00/2020, THL/3433/14.06.00/2020, THL/4432/14.06/2020, THL/5189/14.06/2020, THL/5894/14.06.00/2020, THL/6619/14.06.00/2020, THL/209/14.06.00/2021, THL/688/14.06.00/2021, THL/1284/14.06.00/2021, THL/1965/14.06.00/2021, THL/5546/14.02.00/2020, THL/2658/14.06.00/2021 and THL/4235/14.06.00/202), Statistics Finland (permit nos. TK-53-1041-17, TK/143/07.03.00/2020 (earlier TK-53-90-20), TK/1735/07.03.00/2021 and TK/3112/07.03.00/2021) and Finnish Registry for Kidney Diseases permission/extract from the meeting minutes on 4 July 2019.
The Biobank Access Decisions for FinnGen samples and data utilized in FinnGen Data Freeze 9 include: THL Biobank BB2017_55, BB2017_111, BB2018_19, BB_2018_34, BB_2018_67, BB2018_71, BB2019_7, BB2019_8, BB2019_26, BB2020_1, Finnish Red Cross Blood Service Biobank 7.12.2017, Helsinki Biobank HUS/359/2017, HUS/248/2020, Auria Biobank AB17-5154 and amendment no. 1 (17 August 2020), AB20-5926 and amendment no. 1 (23 April 2020) and it’s modification (22 September 2021), Biobank Borealis of Northern Finland_2017_1013, Biobank of Eastern Finland 1186/2018 and amendment 22 § /2020, Finnish Clinical Biobank Tampere MH0004 and amendments (21 February 2020 and 06 October 2020), Central Finland Biobank 1-2017, and Terveystalo Biobank STB 2018001 and amendment 25 August 2020.
UK Biobank
The UK Biobank study was approved by the North West Multi-centre Research Ethics Committee (approval no. 11/NW/03820). All participants provided written informed consent for data collection, analysis and record linkage. We have also obtained ethical approval for the use of UK Biobank data in Sweden (2016/1888-31/1).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Individual-level data cannot be stored in public repositories or otherwise made publicly available due to ethical and data protection restrictions. However, data are available upon request for researchers who meet the criteria for access to confidential data. Data from the UK Biobank are available to bona fide researchers upon application at https://www.ukbiobank.ac.uk/enable-your-research/. The following UK Biobank-associated data were accessed through, and as part of, our UK Biobank accession: Hospital Episode Statistics for England, Scottish Morbidity Record and Patient Episode Database for Wales.
FinnGen results, according to the FinnGen consortium agreement, are subjected to a one-year embargo, and summary statistics are then made available to the scientific community and released two times a year. Information on accessing FinnGen data can be found at https://www.finngen.fi/en/access_results/. The national healthcare, population and cause of death registers were accessed through, and as part of, our FinnGen accession, implemented in the FinnGen pipelines.
Code availability
All the data processing, visualization and statistical analyses were performed using Python 3.8 (2.7 for LDSC) and R v.4.3.2 (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/). Meta-analyses were performed using METAL v.2011-03-25. Independent Genomic loci were identified using the R package gwasRtools36 (version 0.1.7; https://lcpilling.github.io/gwasRtools/). Venn diagrams were created using the R package ggvenn (version 0.1.10; https://cran.r-project.org/web/packages/ggvenn/index.html). Correlation plots were created using the R package corrplot (v.0.92; https://cran.r-project.org/web/packages/corrplot/index.html). Forest plots were created using the R package ggforestplot (v.0.1.0; https://nightingalehealth.github.io/ggforestplot/). The analysis codes are available as follows: FinnGen GWAS via https://github.com/FINNGEN/saige-pipelines/; UK Biobank GWAS via http://cnsgenomics.com/software/gcta/#fastGWA and QTL colocalization analysis via https://github.com/Moritz-JD-Krueger/Colocalization-Analysis/.
References
Clegg, A., Young, J., Iliffe, S., Rikkert, M. O. & Rockwood, K. Frailty in elderly people. Lancet 381, 752–762 (2013).
Kojima, G., Iliffe, S. & Walters, K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing 47, 193–200 (2018).
Theou, O., Brothers, T. D., Mitnitski, A. & Rockwood, K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J. Am. Geriatr. Soc. 61, 1537–1551 (2013).
Gilbert, T. et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet Lond. Engl. 391, 1775–1782 (2018).
Searle, S. D., Mitnitski, A., Gahbauer, E. A., Gill, T. M. & Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 8, 24 (2008).
Fried, L. P. et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A. Biol. Sci. Med. Sci. 56, M146–M156 (2001).
Young, A. C. M., Glaser, K., Spector, T. D. & Steves, C. J. The identification of hereditary and environmental determinants of frailty in a cohort of UK twins. Twin Res. Hum. Genet. 19, 600–609 (2016).
Mak, J. K. L. et al. Sex differences in genetic and environmental influences on frailty and its relation to body mass index and education. Aging 13, 16990–17023 (2021).
Mak, J. K. L. et al. Genetic and environmental influences on longitudinal frailty trajectories from adulthood into old age. J. Gerontol. A. Biol. Sci. Med. Sci. 78, 333–341 (2023).
Atkins, J. L. et al. A genome-wide association study of the frailty index highlights brain pathways in ageing. Aging Cell 20, e13459 (2021).
Ye, Y. et al. A genome-wide association study of frailty identifies significant genetic correlation with neuropsychiatric, cardiovascular, and inflammation pathways. Geroscience 45, 2511–2523 (2023).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Rosoff, D. B. et al. Multivariate genome-wide analysis of aging-related traits identifies novel loci and new drug targets for healthy aging. Nat. Aging 3, 1020–1035 (2023).
Xie, Z. et al. Gene set knowledge discovery with Enrichr. Curr. Protoc. 1, e90 (2021).
Aslam, M. M. et al. Genome-wide analysis identifies novel loci influencing plasma apolipoprotein E concentration and Alzheimer’s disease risk. Mol. Psychiatry 28, 4451–4462 (2023).
Yeh, K. -H. et al. Genetic variants at the APOE Locus predict cardiometabolic traits and metabolic syndrome: a Taiwan Biobank study. Genes 13, 1366 (2022).
Kemper, K. E. et al. Genetic influence on within-person longitudinal change in anthropometric traits in the UK Biobank. Nat. Commun. 15, 3776 (2024).
Ambrose, R. L. et al. Molecular characterisation of ILRUN, a novel inhibitor of proinflammatory and antimicrobial cytokines. Heliyon 6, e04115 (2020).
PubChem. CHST9 - carbohydrate sulfotransferase 9 (human). https://pubchem.ncbi.nlm.nih.gov/gene/CHST9/human
NCBI Gene. CGREF1 cell growth regulator with EF-hand domain 1 (human). https://www.ncbi.nlm.nih.gov/gene/10669
Ziembik, M. A., Bender, T. P., Larner, J. M. & Brautigan, D. L. Functions of protein phosphatase-6 in NF-κB signaling and in lymphocytes. Biochem. Soc. Trans. 45, 693–701 (2017).
Tin, A. et al. Identification of circulating proteins associated with general cognitive function among middle-aged and older adults. Commun. Biol. 6, 1117 (2023).
Lim, S. M. et al. Differential proteomic profiles between cognitive frail and robust older adults from the MELoR cohort. Geroscience https://doi.org/10.1007/s11357-024-01462-z (2024).
Turcotte, L. A. et al. External validation of the Hospital Frailty Risk Score among hospitalised home care clients in Canada: a retrospective cohort study. Age Ageing 52, afac334 (2023).
Mak, J. K. L., Kuja-Halkola, R., Wang, Y., Hägg, S. & Jylhävä, J. Frailty and comorbidity in predicting community COVID-19 mortality in the UK Biobank: the effect of sampling. J. Am. Geriatr. Soc. 69, 1128–1139 (2021).
Zhang, S. et al. A metabolomic profile of biological aging in 250,341 individuals from the UK Biobank. Nat. Commun. 15, 8081 (2024).
Mak, J. K. L. et al. Unraveling the metabolic underpinnings of frailty using multicohort observational and Mendelian randomization analyses. Aging Cell 22, e13868 (2023).
Fry, A. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 186, 1026–1034 (2017).
Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613, 508–518 (2023).
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Pärn, K. et al. Genotype imputation workflow v3.0. dx.doi.org/10.17504/protocols.io.xbgfijw (2018).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Zhou, W. et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat. Genet. 50, 1335–1341 (2018).
Loh, P. -R. et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat. Genet. 47, 284–290 (2015).
McLaren, W. et al. The Ensembl Variant Effect Predictor. Genome Biol. 17, 122 (2016).
GitHub. R package for processing of GWAS output. https://lcpilling.github.io/gwasRtools/
Jiang, L. et al. A resource-efficient tool for mixed model association analysis of large-scale data. Nat. Genet. 51, 1749–1755 (2019).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
EMBL-EBI. GWAS Catalog. https://www.ebi.ac.uk/gwas/
Bulik-Sullivan, B. K. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Dai, Y. et al. WebCSEA: web-based cell-type-specific enrichment analysis of genes. Nucleic Acids Res. 50, W782–W790 (2022).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592 (2023).
Sun, B. B. et al. Plasma proteomic associations with genetics and health in the UK Biobank. Nature 622, 329–338 (2023).
GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020).
Ge, T., Chen, C. -Y., Ni, Y., Feng, Y. -C. A. & Smoller, J. W. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat. Commun. 10, 1776 (2019).
Chou, W. -C. et al. A combined reference panel from the 1000 Genomes and UK10K projects improved rare variant imputation in European and Chinese samples. Sci Rep. 6, 39313 (2016).
Bai, G. et al. Is frailty different in younger adults compared to old? Prevalence, characteristics, and risk factors of early-life and late-life frailty in samples from Sweden and UK. Gerontology 69, 1385–1393 (2023).
Acknowledgements
This work was supported by the Swedish Research Council (grant nos. 2018-02077 to J.J., 2019-01272, 2020-06101, 2022-01608), the Research Council of Finland to J.J. (grant no. 3493358), the Sigrid Jusélius Foundation to J.J., the Yrjö Jahnsson Foundation to J.J. (grant no. 20217416), Instrumentarium Science Foundation to J.J. and Signe and Ane Gyllenberg Foundation to J.J. (grant no. 6226). This research was conducted using the UK Biobank resource, as part of the registered project 22224. The analyses of UK Biobank genotypes were enabled by resources in project sens2017519 provided by the National Academic Infrastructure for Supercomputing in Sweden (NAISS) at UPPMAX, funded by the Swedish Research Council through grant agreement no. 2022-06725. The FinnGen project is funded by two grants from Business Finland (HUS 4685/31/2016 and UH 4386/31/2016) and the following industry partners: AbbVie, AstraZeneca, Biogen, Bristol Myers Squibb (and Celgene Corporation & Celgene International II Sàrl), Genentech, Merck Sharp & Dohme, Pfizer, GlaxoSmithKline Intellectual Property Development, Sanofi US Services, Maze Therapeutics, Janssen Biotech and Novartis. The following biobanks are acknowledged for delivering biobank samples to FinnGen: Auria Biobank (https://www.auria.fi/biopankki/), THL Biobank (https://www.thl.fi/biobank/), Helsinki Biobank (https://www.helsinginbiopankki.fi/), Biobank Borealis of Northern Finland (https://www.ppshp.fi/Tutkimus-ja-opetus/Biopankki/Pages/Biobank-Borealis-briefly-in-English.aspx/), Finnish Clinical Biobank Tampere (https://www.tays.fi/en-US/Research_and_development/Finnish_Clinical_Biobank_Tampere/), Biobank of Eastern Finland (https://www.ita-suomenbiopankki.fi/en/), Central Finland Biobank (https://www.ksshp.fi/fi-FI/Potilaalle/Biopankki/), Finnish Red Cross Blood Service Biobank (https://www.veripalvelu.fi/verenluovutus/biopankkitoiminta/) and Terveystalo Biobank (https://www.terveystalo.com/fi/Yritystietoa/Terveystalo-Biopankki/Biopankki/). All Finnish Biobanks are members of BBMRI.fi infrastructure (https://www.bbmri.fi/). The Finnish Biobank Cooperative-FINBB (https://finbb.fi/) is the coordinator of BBMRI-ERIC operations in Finland. The Finnish biobank data can be accessed through the Fingenious services (https://site.fingenious.fi/en/) managed by FINBB. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Consortia
Contributions
J.J. conceived the study plan and designed the proof outline. J.K.L.M., C.Q., M.K., J.L. and A.K. performed the analyses. J.J., J.L. and S.H. were responsible for data acquisition. All authors contributed to the writing of the paper and interpretation of the results. All authors listed under FinnGen contributed to the generation of the primary data of the FinnGen data release 12. FinnGen authors are listed in Supplementary Table 19.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Zuyun Liu, Julian Mutz and Luke Pilling, for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 QQ-plots of association summary statistics for the HFRS and HFRS without dementia.
Panels a and b show results for FinnGen, and panels c and d for the UK Biobank, respectively.
Extended Data Fig. 2 Cell-type enrichment analysis of HFRS.
Top 20 enriched cell types for the variants associated with the HFRS in FinnGen are shown.
Extended Data Fig. 3 Cell-type enrichment analysis of HFRS without dementia.
Top 20 enriched cell types for the variants associated with the HFRS without dementia in FinnGen are shown.
Extended Data Fig. 4 Colocalized expression and splicing quantitative trait loci.
Colocalized eQTL and sQTL by tissue with the genes associated with (a) HFRS and (b) HFRS without dementia. For each gene, the posterior probability for a shared causal variant was >80%.
Extended Data Fig. 5 Regional association plots for gene loci.
Regional association plots for gene loci – panel a for APOE and panel b for BRAP – identified in the colocalization analysis of protein quantitative trait loci (pQTL) and the variants associated with the Hospital Frailty Risk Score (HFRS).
Supplementary information
Supplementary Information
Supplementary Tables 1–19.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mak, J.K.L., Qin, C., Krüger, M. et al. Large-scale genome-wide analyses with proteomics integration reveal novel loci and biological insights into frailty. Nat Aging 5, 1589–1600 (2025). https://doi.org/10.1038/s43587-025-00925-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43587-025-00925-y